Abstract
Thrombospondin (TSP)-1 and TSP-2 are matricellular proteins in the extracellular matrix (ECM), which serve a significant role in the pathological processes of various cardiovascular diseases (CVDs). The multiple effects of TSP-1 and TSP-2 are due to their ability to interact with various ligands, such as structural components of the ECM, cytokines, cellular receptors, growth factors, proteases and other stromal cell proteins. TSP-1 and TSP-2 regulate the structure and activity of the aforementioned ligands by interacting directly or indirectly with them, thereby regulating the activity of different types of cells in response to environmental stimuli. The pathological processes of numerous CVDs are associated with the degradation and remodeling of ECM components, and with cell migration, dysfunction and apoptosis, which may be regulated by TSP-1 and TSP-2 through different mechanisms. Therefore, investigating the role of TSP-1 and TSP-2 in different CVDs and the potential signaling pathways they are associated with may provide a new perspective on potential therapies for the treatment of CVDs. In the present review, the current understanding of the roles TSP-1 and TSP-2 serve in various CVDs were summarized. In addition, the interacting ligands and the potential pathways associated with these thrombospondins in CVDs are also discussed.
Keywords: cardiovascular diseases, interacting ligands, pathway, thrombospondin 1, thrombospondin 2
1. Introduction
The extracellular matrix (ECM) serves a significant role in modulating tissue genesis and remodeling not only by connecting cells and providing support for them, but also by regulating connections between the cells, and between the cell and the matrix, inducing cell adhesion, motility and differentiation. In the cardiovascular system, the ECM participates in maintaining the structural continuity of the heart and vessels, providing physical support for cell adhesion, controlling cell growth and death, and regulating diastolic stiffness, as well as tissue repair or remodeling to the cardiovascular damage (1). A number of these functions are performed by a group of non-structural ECM proteins called matricellular proteins, which includes thrombospondins (TSPs), tenascins, periostin, osteopontin, CCN proteins and osteonectin (2).
As a family of matricellular proteins, TSPs may be secreted by various types of cells. A total of 5 members of the TSP family (TSP1-5) have been identified so far, and are divided into two subgroups, subgroup A and subgroup B, according to their structural differences. Subgroup A contains TSP-1 and TSP-2, which are trimeric and similar in structure, and subgroup B consists of TSP-3, TSP-4 and TSP-5, which are pentameric and smaller compared with those in subgroup A (3). TSP-1 and TSP-2 are the most studied thrombospondins. In the present review, the structure and the role of TSP-1 and TSP-2 in cardiovascular diseases (CVDs; Table I), and the potential pathways associated with these TSPs will be discussed.
Table I.
Role of TSP-1 and TSP-2 in various CVDs.
| CVD type | TSP-1 | TSP-2 | ||
|---|---|---|---|---|
|
|
|
|||
| Effect | (Refs.) | Effect | (Refs.) | |
| MI | TSP-1 polymorphism associated with MI | (56–58) | TSP-2 polymorphism promotes MI | (54) |
| TSP-1 expression increases in patients with MI | (47) | Hypoxia induces TSP-2 expression in cardiomyocyte progenitor cells | (52) | |
| TSP-1 protects the myocardium from fibrotic remodeling in MI | (50) | - | - | |
| TSP-1 decreases following PCI associated with adverse cardiac events | (48) | - | - | |
| Ischemia/reperfusion accelerate the induction of TSP-1 in rat MI model | (49) | - | - | |
| Cardiac hypertrophy | TSP-1 protects pressure-overloaded myocardium | (60) | TSP-2 absence leads to age-associated dilated cardiomyopathy | (62) |
| TSP-1 overexpression in the diabetic heart inhibits chamber dilation | (61) | TSP-2 absence enhances cardiomyocyte damage and matrix disruption in cardiomyopathy | (63) | |
| - | - | TSP-2 prevents cardiac injury and dysfunction in viral myocarditis | (64) | |
| - | - | TSP-2 expression marks an early-stage molecular program in hypertrophied hearts that may fail | (65) | |
| Heart failure | TSP-1 expression decreases in failing hearts | (66,67) | Increased TSP-2 related to CHF-associated mortality and all-cause mortality among patients with CAD with CHF | (71) |
| miRNA-18 and miRNA-19 modulate TSP-1 expression in age-associated HF | (69) | High serum TSP-2 levels correlate with poor prognosis in patients with HF | (72,73) | |
| Oral anticoagulation therapy causes the decrease of TSP-1 in patients with HF | (70) | - | - | |
| Valvular disease | Not available | TSP-2 increases in human fibrosclerotic and stenotic aortic valves | (75) | |
| Cerebral and carotid artery disorder | Fluvastatin inhibits intimal hyperplasia after carotid artery ligation in WT but not Thbs1-null mice | (77) | TSP-2 small interfering RNA inhibits vascular response to the injury in rat carotid balloon angioplasty model | (82) |
| TSP-1 expression increases in platelets from patients with CAD | (76) | TSP-2 deficiency leads to an impaired recovery following a stroke | (78,83) | |
| TSP-1 increases following stroke, and TSP-1 deficiency leads | (78,83) | TSP-2 increases in ischemic brain and may lead to spontaneous to an impaired recovery following stroke angiogenesis | (79) | |
| TSP-1 is highly expressed in the ischemic brain | (79) | Altered TSP-2 expression following spontaneous intracerebral hemorrhage is associated with angiogenesis | (81) | |
| Altered TSP-1 expression following spontaneous intracerebral hemorrhage is related to angiogenesis | (81) | - | - | |
| Atherosclerosis | TSP-1 increases in VSMC of human atherosclerotic lesions | (89) | TSP-2 was absent from the endothelium inside the atheromatous plaque | (97) |
| TSP-1 increases in large arteries of diabetic animals and decreases in microvascular ECs | (89,93,94) | - | - | |
| Proatherogenic flow initiates EC apoptosis and arterial stiffening via TSP-1 | (40,84) | - | - | |
| TSP-1 deficiency inhibits atherogenic lesion formation in hyperglycemic ApoE(−/−) mice | (95) | - | - | |
| TSP-1 deficiency promotes atherosclerotic plaque maturation in ApoE(−/−) mice | (85) | - | - | |
| TSP-1 infiltrates into nascent atherosclerotic plaques and promotes atherogenesis | (86,88) | - | - | |
| Injured arteries overexpress TSP-1 in hypercholesterolemic atherosclerotic rabbit | (87) | - | - | |
| TSP-1 modulates SMC migration, promotes the development of atherosclerotic lesions | (90–92) | - | - | |
| TSP-1 increases in hypoxic pulmonary and lead to pulmonary vascular remodeling | (96) | - | - | |
| Angiogenesis | TSP-1 inhibits tumor angiogenesis | (22,100, 101) | TSP-2 inhibits the proliferation of microvascular ECs | (110, 111) |
| TSP-1 overexpression in diabetes leads to an impaired angiogenesis | (102) | TSP-2 deficiency promotes angiogenesis | (112, 113) | |
| TSP-1 may inhibit angiogenic responses in the ischemic retina | (103) | TSP-2 limits angiogenesis by decreasing gelatinolytic activity in situ | (114) | |
| TSP-1 deficiency contributes to enhanced neovascularization in the eye | (104–108) | TSP-2 overexpression result in an inhibition of vascularization in rheumatoid arthritis | (115) | |
| TSP-1 downregulation in EC enhances angiogenesis | (98,99) | Delayed TSP-2 expression in the wounds of aged mice impairs the rate of wound healing | (116) | |
| - | - | Increased vessel density in TSP2−/− mice but not in TSP1−/− animals | (117) | |
| Arterial restenosis | TSP-1 expression by VSMCs is an early response to injury | (118) | TSP-2 silencing of aortic SMCs improved cell attachment but did not affect cell migration or proliferation | (122) |
| TSP-1 is not a major component of the ECM in human restenotic tissues | (121) | - | - | |
| TSP-1 and β1 integrin interaction is related to platelet-stimulated SMC proliferation | (120) | - | - | |
| TSP-1 may reverse the inward remodeling of resistance arteries from hypertension rat | (119) | - | - | |
| Other CVDs | TSP-1 promotes pulmonary hypertension associated with hypoxia | (123) | TSP-2 deficient mice exhibit a bleeding diathesis despite normal blood coagulation | (127) |
| TSP-1 deficiency accelerates aortic aneurysm progression | (124) | TSP-2 deficient mice exhibit an altered foreign body reaction characterized by increased vascularity | (128) | |
| TSP-1 regulates the migration and adhesion of mononuclear cells and contributes to the vascular inflammation in AAA | (125) | TSP-2 elevates in acute Kawasaki disease | (130) | |
| TSP-1-derived peptide RFYVVMWK may enhance the vascular engraftment during autologous proangiogenic cell therapy | (126) | TSP2-knockout leads to decreased fibrosis and increased EC density during cardiac cell transplantation | (131) | |
| TSP-1 increases in the aorta and plasma of patients with acute aortic dissection | (23) | |||
CVD, cardiovascular disease; TSP, thrombospondin; MI, myocardial infarction; PCI, percutaneous coronary intervention; HF, heart failure; CHF, congestive heart failure; CAD, coronary artery disease; WT, wild-type; VSMC, vascular smooth muscle cell; EC, endothelial cell; ECM, extracellular matrix; AAA, abdominal aortic aneurysm; ApoE, apolipoprotein E.
2. The structure of TSP-1 and TSP-2
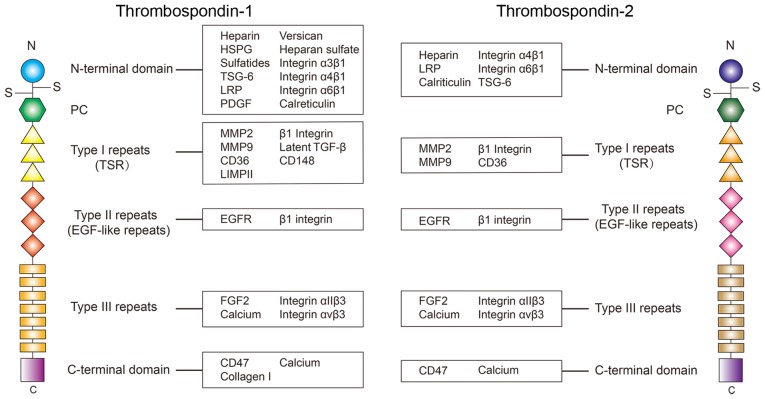
TSP-1 have a complex multidomain structure (Fig. 1), which can interact with various ligands, including ECM structural components, matricellular proteins, growth factors, receptors, proteases and cytokines (4). There is a total of 3 identical chains of TSP-1 or TSP-2 that may form a trimer with a disulfide-bond, which may be critical to some of their functions (5). The monomer of TSP-1 and TSP-2 consists of an N-terminal domain, an interchain disulfide knot, a homologous procollagen region, 3 type I repeats, 3 type II repeats [epidermal growth factor (EGF)-like repeats], 7 type III repeats and a C-terminal domain (3). Each of these domains specifically regulates the cell function via the interaction with various molecules. The N-terminal of TSP-1 may interact with heparin (6,7), heparan sulfate proteoglycan (HSPG) (8), sulfatides (4,8), TNF-inducible gene 6 protein (9), low-density lipoprotein receptor related protein (LRP) (7,10), versican (11), calreticulin (10,12–14), integrin-α3β1 (15–17), -α4β1 (18) and -α6β1 (19). Type I repeats may interact with matrix metallopeptidase (MMP)-2 (20,21), MMP9 (15,22,23), cluster of differentiation 36 (CD36) (24–26), lysosome membrane protein 2 (LIMPII) (27), β1 integrins (28,29), latent transforming growth factor-β (TGF-β) (30–32), and CD148 (33,34). Type II repeats can interact with EGF receptor (EGFR) (35) and β1 integrins (28), while type III repeats have been identified to interact with fibroblast growth factor 2 (FGF2) (36), calcium (36–38), integrin αIIβ3 (39) and integrin αvβ3 (40,41). The C-terminal domain interacts with leukocyte surface antigen CD47 (CD47) (42,43) and calcium (44). Through these different interactions, TSP-1 modulates the activity of these ligands, ultimately inducing the cell response to the environmental stimuli under the circumstances of physiological and pathological processes.
Figure 1.
Structural diagrams of TSP-1 and TSP-2. Ligands demonstrated to interact with each domain are summarized in the boxes. At present, the understanding of the interaction of ligands of each domain in TSP-1 is more advanced compared with that of TSP-2, and numerous molecules that interact with TSP-2 remain to be identified. From the current results, a number of interacting molecules are shared between TSP-1 and TSP-2. However, certain particular ligands, including TGF-β, may only function when it interacts with TSP-1 instead of TSP-2. TSP, thrombospondin; TSG-6, tumor specific glycoprotein; LRP, low-density lipoprotein receptor related protein; PDGF, platelet derived growth factor; MMP, matrix metalloproteinase; CD36, cluster of differentiation 36; TGF-β, transforming growth factor-β; CD148, receptor-type tyrosine-protein phosphatase eta; LIMPII, lysosome membrane protein 2; EGFR, epidermal growth factor receptor; FGF2, fibroblast growth factor 2; CD47, leukocyte surface antigen CD47; LRP, low-density lipoprotein receptor related protein.
TSP-2 shares the same structure with TSP-1, however the amino acid sequences are slightly different. The primary differences in the amino acid sequence are located in the N-terminal domain. Evidence reveals there is only a 32% amino acid sequence similarity in the N-terminal heparin binding domain between TSP-1 and TSP-2 (3). Therefore, the ligands binding TSP-1 and TSP-2 at the N-terminal domain are different in certain circumstances. For example, a previous study identified that recombinant human TSP1 may activate the latent TGF-β, but this phenomenon is not observed in recombinant mouse TSP-2 (45). Therefore, although TSP-1 and TSP-2 share the same structure, their different amino acid sequences, especially in the N-terminal domain, is the cause of their differences in binding ligands.
The interaction between TSP-1 and TSP-2 and their ligands may also affect each other. For example, TSP-1 and TSP-2 are known to compete with each other for degradation through LRP, which contributes to maintaining the levels of TSP-1 and TSP-2 in certain situations, such as wound healing (46).
3. TSP-1 and TSP-2 in CVDs
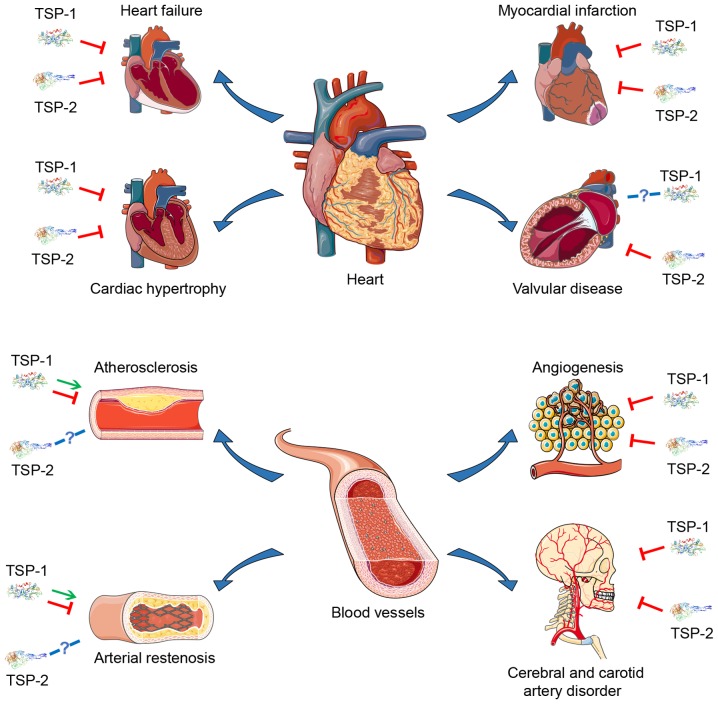
Due to their multidomain structure and the ability to interact with multiple ligands, TSP-1 and TSP-2 are active in various types of physiological and pathological processes. At present, there have been multiple studies concerning the role of TSP-1 and TSP-2 in various CVDs (Table I and Fig. 2), suggesting that they may become potential therapeutic targets.
Figure 2.
Current understanding of the effect of TSP-1 and TSP-2 on various CVDs. The red arrows represent suppression, the green arrows represent promotion and the blue arrows represent not acquired. The primary roles TSP-1 and TSP-2 serve in CVDs are considered to be inhibitory, but there are different hypotheses regarding the contribution of TSP-1 and TSP-2 to certain CVDs, including atherosclerosis and arterial restenosis. However, the association between TSP-1 and TSP-2 and diseases such as valvular disease requires further study. TSP, thrombospondin; CVD, cardiovascular disease.
Myocardial infarction (MI)
MI is myocardial ischemic necrosis caused by a rapid decrease or interruption of coronary blood supply, which leads to high mortality rates worldwide. In patients with acute ST-segment elevation MI, the expression of TSP-1 is significantly increased (47), and the decrease of TSP-1 following percutaneous coronary intervention (PCI) is associated with major adverse cardiac events (48). In rats with infarcted hearts, TSP-1 was transiently induced and located in cells at the border area of the infarction, which can be increased by ischemia/reperfusion (49). In canine and murine models of reperfusion injury, TSP-1 also exhibited a selective distribution in the ECM, the microvascular endothelium, and the mononuclear cells of the infarct border zone. TSP-1-deficient mice have a severe post-infarction remodeling compared with that in wild-type mice, although their infarct size was almost equal, indicating that selective endogenous expression of TSP-1 in the infarct border zone may limit the expansion of the granulation tissue and protect the non-infarcted myocardium from fibrotic remodeling (50).
Following MI, left ventricular (LV) remodeling significantly contributes to LV dilation and dysfunction, which leads to functional damage and poor prognosis. The pathological process of LV remodeling includes scar formation in the infarcted area, as well as hypertrophy and fibrosis in the non-infarcted surrounding area. This is primarily due to the degradation of ECM, the deposition of myofibroblasts, and the rapid increase in the amount of collagen following infarction. Previous studies have identified that extracellular collagen matrix (ECCM) remodeling largely contributes to LV remodeling, and the decrease of ECCM is associated with the increase of LV dilation and rupture, indicating that TSP-1 may participate in the pathology of MI (51). Exposure to hypoxia markedly induced the expression of TSP-2 in cardiomyocyte progenitor cells (hCMPCs), and knockdown of TSP-2 resulted in increased proliferation, migration and MMP activity of hCMPCs, indicating that TSP-2 may participate in controlling the migratory and invasive capacities of hCMPCs under hypoxic conditions (52). However, little is known regarding the specific role of TSP-2 in MI.
Conversely, certain functional variants of the TSP-1 and TSP-2 genes may be associated with MI (53–55). The N700S polymorphism of TSP-1 is a potential genetic risk factor for MI (56–58), as it disrupts calcium-binding sites (59). Those who are homozygous for the minor allele or are heterozygous (GG and TG genotypes, respectively) have a significantly increased risk of MI compared with those who are homozygous (TT genotype) for the major allele (54). These data provide a novel perspective on the TSP-associated therapeutic approach of MI.
Cardiac hypertrophy and heart failure
Cardiac hypertrophy is primarily induced by chronic pressure overload, such as essential hypertension. Pathological features of cardiac hypertrophy include increased growth of the cardiomyocytes, proliferation of the cardiac fibroblasts and increased ECM deposition. There have been some reports on the role of TSP-1 and TSP-2 in cardiac hypertrophy. Compared with wild type mice, TSP-1-deficient mice exhibited enhanced early hypertrophy and late dilation when exposed to pressure overload (60). Despite this, TSP-1 (−/−) mice exhibited increased myocardial MMP-3 and -9 activation following pressure overload (60). In obese diabetic DB/DB mice, myocardial TSP-1 levels are significantly upregulated in the perivascular and interstitial space. In comparison with normal DB/DB mice, DB/DB TSP-1 (−/−) mice exhibited an enhanced LV dilation, which was associated with mild non-progressive systolic dysfunction, and TSP-1 could incorporate into the matrix and inhibit leptin-induced MMP-2 activation (61). These previous studies suggest that TSP-1 is upregulated in the diabetic heart and prevents chamber dilation by exerting matrix-preserving actions on the cardiac fibroblasts.
TSP-2 is also closely associated with cardiac hypertrophy. Data suggests that older TSP-2 (−/−) mice are associated with an enhanced dilated cardiomyopathy characteristic as impaired systolic function as well as increased cardiac dilatation and myocardial fibrosis, indicating that TSP-2 deficiency leads to an age-associated dilated cardiomyopathy (62). Compared to wild-type mice, TSP-2-knockout mice display increased mortality accompanied by decreasing cardiac function, increased cardiomyocyte apoptosis and ECM damage in a doxorubicin-induced cardiomyopathy mouse model (63). The absence of TSP-2 also results in decreased systolic function and enhanced cardiac dilatation in human Coxsackie virus B3 (CVB3)-induced myocarditis (64). Previous data also identified that TSP-2 expression is activated uniquely in hypertrophic hearts that may develop heart failure, which may be an early-stage molecular program of heart failure (65).
Abnormal myocardium remodeling leads to myocardial overload. If not treated promptly, long-term myocardial overload may progress into heart failure. From the perspective of pathology, heart failure is associated with abnormal inflammation, coagulation activation and endothelial dysfunction. TSP-1 and TSP-2 also participate in some of these changes. Previous studies have revealed that TSP-1 expression is decreased in failing hearts, which may be associated with ventricular dilatation (66,67). Treatment of cardiomyocytes with a TSP1-derived peptide that activates CD47 leads to increased cardiomyocyte hypertrophy in a Ca2+ and calmodulin protein kinase II dependent manner, indicating that TSP-1 may contribute to LV hypertrophy and heart failure (68). Using aged mouse models with failure-resistant and failure-prone characteristics, a previous study identified that micro(mi) RNA-18 and miRNA-19 may modulate TSP-1 expression and cardiac ECM protein levels in age-associated heart failure; therefore, decreased miRNA-18/19 and increased TSP-1 levels may contribute to the identification of failure-prone hearts (69). TSP-1 levels in patients with heart failure may also be decreased due to oral anticoagulation therapy, which is used to prevent thromboembolic events (70).
Elevated TSP-2 is primarily associated with poor prognosis in patients with heart failure. Among patients with coronary heart disease with symptomatic congestive heart failure (CHF), circulating TSP-2 is increased, which is associated with increased 3-year CHF-associated death, all-cause mortality and recurrent hospitalization risk (71). In patients with preserved ejection fraction heart failure, high serum levels of TSP-2 are associated with poor prognosis (72,73). TSP-2 overexpression in wild-type mouse hearts led to decreased cardiac inflammation and improved cardiac function after CVB3 infection, suggesting that TSP-2 may mitigate against cardiac injury, inflammation, and dysfunction during acute viral myocarditis (64).
Valvular disease
Calcific aortic valve disease (CAVD) is a progressive disorder manifesting as sclerotic stiffening and valvular thickening, eventually leading to aortic stenosis. The pathological process of CAVD is accompanied by inflammatory cell infiltration, lipid accumulation, fibrosclerosis, ECM disorder, angiogenesis and nodular calcification (74). In fibrotic and stenotic aortic valves, the mRNA levels of TSP-2 are increased 4.9-fold (P=0.037) and 4.8-fold (P=0.001), respectively (75). TSP-1 can also be detected in the fibrotic and stenotic valves, but the expression of TSP-1 is not significantly different, indicating that CAVD was associated with TSP-2 upregulation in aortic cusps (75). However, evidence suggesting an association between TSP-1 and valvular diseases is limited, and the specific role of TSP-2 in the pathological process of valvular disease requires further study.
Cerebral and carotid artery disorder
Cerebral and carotid artery disease are important subgroups of peripheral vascular diseases, which have high mortality rates worldwide. TSP-1 and TSP-2 may also serve a role in cerebral and carotid artery disease. In symptomatic patients with carotid artery diseases, TSP-1 expression on the surface of circulating platelets is significantly increased (76). Compared with wild-type mice, TSP-1 (−/−) mice exhibit a decreased response to fluvastatin in inhibiting intimal hyperplasia following carotid artery ligation, indicating that the statin effect on intimal hyperplasia may be dependent on TSP-1 (77). A previous study identified that the expression of TSP-1 is increased following a stroke, and TSP-1 deficiency leads to impaired recovery (78). High expression levels of TSP-1 and TSP-2 were identified in the ischemic rat brain following cerebral ischemia/reperfusion, which may contribute to spontaneous resolution of postischemic angiogenesis (79). In a spontaneous intracerebral hemorrhage (ICH) rat model, thrombin treatment induced high expression of TSP-1 or TSP-2 in the blood vessels around the damaged brain region. These data provide support the hypothesis that thrombin positively regulates the expression of TSP-1 and TSP-2 following ICH, which may be involved in modulating angiogenesis in injured brains (79–81).
In a rat carotid balloon angioplasty model, intraluminal delivery of TSP-2 small inhibiting (si)RNA inhibited the vascular response to the injury (82). TSP-2 is increased and colocalized to the astrocytes following a stroke, and TSP-2 deficiency leads to an impaired recovery following stroke (78,83). TSP-2 expression was increased in the ischemic brain, which may contribute to the spontaneous resolution of post-ischemic angiogenesis (79). These data suggest that TSP-2 may promote angiogenesis and recovery following cerebral and carotid artery injury.
Atherosclerosis
Atherosclerosis is characterized by thickening, hardening and decreased elasticity of the arterial wall. Lipid levels, endothelial cell injury, inflammation and the migration of vascular smooth muscle cells (VSMC) are considered as several fundamental pathological processes of atherosclerosis. Previous evidence suggested that TSP-1 can interact with some of the aforementioned factors and further regulate the pathological process of atherosclerosis through various mechanisms, while the association between TSP-2 and atherosclerosis requires further investigation. Following partial carotid ligation, disturbed blood flow induced arterial stiffening through collagen deposition. Compared with wild type carotid arteries, TSP-1 knockout animals have significantly decreased arterial stiffening, indicating that disturbed flow may promote arterial stiffening through TSP-1 (84). Conversely, proatherogenic flow conditions may induce endothelial apoptosis via TSP-1 (40). The absence of TSP-1 accelerates the maturation of the atherosclerotic plaque in apolipoprotein E (ApoE−/−) mice, indicating that TSP-1 may function as an inhibitor of atherosclerosis (85,86). TSP-1 may also interact with lipoproteins. In hypercholesterolemic atherosclerotic rabbits, the overexpressed TSP-1 secreted by injured arteries may bind to very-low-density lipoprotein (VLDL), which may promote its incorporation into nascent atherosclerotic plaques, simultaneously delivering VLDL cholesterol into the lesions (87,88). These results indicate that TSP-1 may serve different roles in different pathological stages of atherosclerosis. Therefore, it is necessary to further investigate the specific role of TSP-1 in atherosclerosis.
An important pathological process of atherosclerosis is the migration of media smooth muscle cells (SMCs) into the intima and hyperplasia. The expression of TSP-1 has been demonstrated to increase in VSMC in human atherosclerotic lesions (89), which may contribute to inflammation and atherogenesis. Hypoxia induces the migration of the coronary artery SMCs, which is elicited by TSP-1 (90,91). An additional study identified that TSP modulates SMCs migration, which may accelerate atherosclerotic lesion development during vascular injury or inflammation (92).
TSP-1 may also modulate the interaction between diabetes and atherosclerosis. Evidence reveals that TSP-1 expression is increased in large arteries of diabetic animals however, the protein levels of TSP-1 in microvascular endothelial cells are decreased when exposed to high glucose levels (89,93,94). In a hyperglycemic ApoE (−/−) mouse model, lack of TSP-1 prevented atherogenic lesion formation (95). The expression of TSP-1 is increased in hypoxic pulmonary hypertension rats, which may contribute to the pathogenesis of hypoxic pulmonary vascular remodeling (96).
Compared with TSP-1, there is limited research on the association between TSP-2 and atherosclerosis. In atherosclerotic specimens, TSP-2 mRNA was absent from intraplaque microvessels and endothelial cells lining the atheromatous plaque (97). Therefore, the specific mechanism of TSP-2 in the pathological process of atherosclerosis requires further investigation.
Angiogenesis
Angiogenesis is a fundamental physiological process associated with tissue repair following injury, which also promotes tumor progression. This process is tightly modulated by various growth factors and the interaction between cells and the ECM. TSP-1 and TSP-2 have been revealed to regulate angiogenesis by interacting with specific growth factors, cells and ECM. Previous evidence indicates that downregulation of endothelial cell TSP-1 causes an enhancement of in vitro angiogenesis (98). In vitro and in vivo models indicated that factor XIII, a clotting factor, may also promote angiogenesis by downregulating TSP-1 and stimulate endothelial cell proliferation and migration (99). In TSP-1-deficient animals, tumor burden and vasculature increase markedly, and TSP-1 overexpression resulted in decreased tumor diameter and fewer tumor capillaries, indicating that TSP-1 may inhibit tumor angiogenesis (22,100). The inhibitory effect of TSP-1 on tumors may be accomplished via cross-talk with endothelial cells (101). The overexpression of TSP1-CD47 signaling in diabetes is associated with endothelial cell dysfunction, which leads to impaired angiogenesis (102). In the ischemic retina, glia-derived TSP-1 may inhibit angiogenic responses (103), and deficiency of TSP-1 contributes to enhanced neovascularization in the eye (104–108).
Similar to TSP-1, TSP-2 can also inhibit angiogenesis and tumor growth, even with greater potency compared with that of TSP-1 (109). In vitro experiments indicated that TSP-2 inhibits proliferation of microvascular endothelial cells (110,111), and the absence of TSP-2 is associated with enhanced angiogenesis, partly due to the altered endothelial cell and ECM interactions (112,113). Decreasing gelatinolytic activity in situ leads to TSP-2-limited angiogenesis (114). In rheumatoid arthritis, TSP-2 overexpression also inhibits vascularization (115).
In older mice, the delay of TSP-2 and MMP2 expression in wounds may promote the impaired rate of wound healing (116). TSP-2 gene knockout mice exhibited increased blood vessel density, but no such alteration was observed in TSP-1-deficient animals (117). This evidence indicates the role of TSP-2 in anti-angiogenesis.
Arterial restenosis
Restenosis of the arteries following cardiovascular surgery, such as PCI, is a major problem, which leads to a poor prognosis. The pathological process of arterial restenosis is similar to atherosclerosis to a certain extent, including endothelial injury, migration and proliferating of VSMCs into the intima. Similar to atherosclerosis, the precise role of TSP-1 in the pathological process of arterial restenosis is difficult to define. In the balloon catheter injury rat model, TSP was markedly increased in the thickening arterial wall, and the TSP antigen in thickening arterial wall is primary secreted by VSMCs (118). In rat resistance arteries, TSP-1 was able to reverse the pathological inward remodeling caused by spontaneous hypertension, indicating that TSP-1 may act as an inhibitor of arterial restenosis (119). A previous study identified that the interaction of TSP-1 and β1 integrin is associated with platelet-stimulated SMC proliferation (120). However, there is also evidence revealing that TSP-1 is not a major component of ECM in human restenotic tissues, even in the presence of hypercellularity or ongoing cellular proliferation (121).
In human aortic SMCs, TSP-2 silencing caused by siRNA improves cell attachment but does not affect cell proliferation and migration, suggesting that TSP-2 also participates in the pathological process of arterial restenosis (122), which represents a novel hypothesis.
Other CVDs
In addition to the aforementioned major CVDs, TSP-1 and TSP-2 also serve important roles in a number of other CVDs. Evidence indicated that TSP-1 may contribute to the pathogenesis of pulmonary hypertension associated with hypoxia (123). TSP-1 deficiency contributes markedly to maladaptive remodeling of the ECM, causing an acceleration of aortic aneurysm progression (124). During the abdominal aortic aneurysm development, TSP-1 regulates the adhesion and migration of mononuclear cells and promotes vascular inflammation (125). During autologous proangiogenic cell therapy, TSP-1-derived peptide RFYVVMWK may interact with priming CD34+ cells and enhance the vascular engraftment (126). TSP-2 (−/−) mice exhibit a bleeding diathesis even if they have normal blood coagulation and no thrombocytopenia (127), and an altered foreign body reaction characterized by an enhanced vascularity (128,129). The plasma TSP-2 level is elevated in acute Kawasaki disease, which may be a novel predictor for intravenous immunoglobulin resistance (130). In a TSP-2-knockout mouse model, significantly increased endothelial cell density and reduced fibrosis were observed in the peri-graft region during the cardiac cell transplantation (131). These studies suggest that TSP-1 and TSP-2 also function in other CVDs, such as pulmonary hypertension, aortic aneurysm progression and acute Kawasaki disease.
4. Signal pathways associated with TSP-1 and TSP-2 in CVDs
Due to their multidomain structure, TSP-1 and TSP-2 can specifically bind to numerous types of different ligands. Therefore, they are involved in various signal pathways regulating cellular activities and ECM components in CVDs (Tables II and III). A comprehensive description of these pathways may facilitate the understanding of the role TSP-1 and TSP-2 serve in the pathological processes of multiple CVDs at the molecular level, which may provide certain potential therapeutic strategies.
Table II.
Signal pathways associated with TSP-1 in CVDs.
| Domains | Interacting molecules | Associated signal pathway | Effect | (Refs.) | Inhibitors | (Refs.) |
|---|---|---|---|---|---|---|
| N-terminal domain | Heparan sulfate | Phagosome | Endocytosis of TSP-1 by the vascular endothelial cells | (8) | Heparinase III | (8) |
| HSPG | Phagosome | Mediates binding and degradation of TSP-1 in ECs | (159) | Heparin | (159) | |
| Sulfatides | ECM-receptor interaction | Promotes cell adhesion | (132) | Heparin and dextran sulfates | (129) | |
| TSG-6 | ECM-receptor interaction | Mediates cellular interactions with hyaluronan | (9) | Heparin | (9) | |
| LRP | Phagosome | Internalization and degradation of TSP-1 | (160,161) | - | - | |
| ECM-receptor interaction | Participate in cell signaling with cell surface calreticulin | (10,13,14) | - | - | ||
| Versican | ECM-receptor interaction | Inhibits VSMC inflammatory response | (11) | Heparin | (11) | |
| Integrin α3β1 | ECM-receptor interaction | Inhibits angiogenesis | (17) | - | - | |
| Mediates cell motility | (15) | - | - | |||
| Stimulates cell adhesion and spreading | (16) | - | - | |||
| Integrin α4β1 | ECM-receptor interaction | Mediates adhesion of T cells | (134) | - | - | |
| Integrin α6β1 | ECM-receptor interaction | Mediates adhesion of microvascular endothelial to immobilized TSP-1 | (19) | - | - | |
| Calreticulin | Focal adhesion | Induces focal adhesion disassembly and cell migration | (10,13,14) | - | - | |
| PDGF | PI3K-AKT pathway | Mediates VSMC proliferation and migration | (138) | Protein disulphide isomerase and heparin | (138) | |
| Type I repeats domain | MMP2 | ECM homeostasis | Inhibits MMP2 activity and regulate collagen homeostasis | (20) | - | - |
| MMP9 | ECM homeostasis | Regulates collagen homeostasis | (20) | - | - | |
| Inhibits angiogenesis | (15) | - | - | |||
| PI3K-AKT pathway | Modulates EC invasion and morphogenesis | (139) | - | - | ||
| CD36 | ECM-receptor interaction | Increases EC apoptosis and anti-angiogenic activity | (15,25) | - | - | |
| PI3K-AKT pathway | Promotes cell adhesion of monocytes/macrophages | (147) | - | - | ||
| Phagosome | Inhibits the NO signal transduction | (143) | - | - | ||
| Internalizes the oxidized LDL, fatty acids and anionic phospholipids | (162) | - | - | |||
| LIMPII | ECM-receptor interaction | Promotes cell adhesion in some circumstances | (27) | LIMPII antibody | (27) | |
| β1 integrin | ECM-receptor interaction | Promotes adhesion of cells that express β1 integrin | (28,135) | Alpha-subunit antagonists | (28) | |
| Latent TGF-β | TGF-β pathway | Stimulates endothelial cell tubulogenesis | (153) | Tsp-2 | (45) | |
| Recruits inflammatory cells, stimulate angiogenesis, and deposit new matrix | (45,154,155) | - | - | |||
| Increases myofibroblast differentiation | (156) | - | - | |||
| CD148 | PI3K-AKT pathway | Negative regulation of growth factor signals, suppressing cell proliferation and transformation | (33,34) | - | - | |
| Type II repeats domain | EGFR | PI3K-AKT pathway | Increases cell migration | (140) | - | - |
| β1 integrin | ECM-receptor interaction | Promotes adhesion of the cells that express β1 integrin | (28,135) | B1 blocking antibody, disintegrins | (28) | |
| Type III repeats domain | Integrin αIIβ3 | ECM-receptor interaction | Promotes TSP-1 binding with platelet | (136) | - | - |
| Integrin αvβ3 | PI3K-AKT pathway | Trigger caspase-independent cell death | (142) | - | - | |
| ECM-receptor interaction | Promote TSP-1 binding with the platelets | (136) | - | - | ||
| Promote SMC migration | (141) | - | - | |||
| FGF2 | PI3K-AKT pathway | Inhibits apoptosis | (43) | Calcium and heparin | (6,36) | |
| Triggers caspase-independent cell death | (142) | |||||
| Calcium | Calcium pathway | Inhibits vascular diseases | (38) | |||
| Maintains the balance between vasodilation and vasoconstriction | (163) | |||||
| C-terminal domain | CD47 | ECM-receptor interaction | Promote cell spreading and cell adhesion to immobilized TSP-1 | (18,42) | Heparin | (42) |
| Inhibit cell adhesion of monocytes/macrophages | (147) | - | - | |||
| Promote platelet adhesion on inflamed vascular endothelium | (144) | - | - | |||
| PI3K-AKT pathway | Inhibit cGMP synthesis and NO signaling | (143,145) | - | - | ||
| Promote foam cell formation | (151) | - | - | |||
| Inhibit cell cycle progression and induce senescence in ECs | (146) | - | - | |||
| Inhibit angiogenesis and blood flow | (148–150) | - | - | |||
| Promote pulmonary arterial vasculopathy | (152) | - | - | |||
| Promote left ventricular heart failure | (68) | - | - | |||
| Calcium | Calcium pathway | Prevents vascular diseases | (38) | - | - | |
| Maintains the balance between the vasodilation and the vasoconstriction | (163) | - | - | |||
| Collagen I | ECM homeostasis | Contributes to the fibroblast homeostasis | (156) | - | - | |
| Unknown | DBP | DBP-C5a pathway | Enhances the chemotaxis of the coagulation factor C5a | (164) | - | - |
| ADAMTS1 | ADAMTS1-TSP1 pathway | Promotes wound closure and inhibits angiogenesis | (167) | - | - | |
| ADAMTS7 | ECM homeostasis | Promote atherosclerosis and coronary artery disease | (168–172) | - | - | |
| Inhibit left ventricular reverse remodeling in MI | (177–179) | - | - | |||
| Promote aortic aneurysm | (173) | - | - | |||
| ADAMTS7-TSP1 pathway | Promote vascular remolding | (174–176) | - | - | ||
| Unknown | ECM-receptor interaction | Anti-angiogenesis in diabetes myocardium | (137) | - | ||
| Migration and adhesion of mononuclear cells | (125) | - | - |
HSPG, heparan sulfate proteoglycan; TSG-6, tumor specific glycoprotein; LRP, low-density lipoprotein receptor related protein; PDGF, platelet derived growth factor; MMP, matrix metalloprotein; LIMPII, lysosomal integral membrane protein II; EGFR, epidermal growth factor receptor; FGF2, fibroblast growth factor 2; DBP, vitamin D-binding protein; ADAMTS, disintegrin and metalloproteinase with thrombospondin motifs; TSP-1, thrombospondin-1; ECM, extracellular matrix; cGMP, cyclic guanosine monophosphate; NO, nitric oxide; SMC, smooth muscle cell; VSMC, vascular smooth muscle cell; TGF-β, transforming growth factor-β.
Table III.
Signal pathways associated with TSP-2 in CVDs.
| Domains | Interacting molecules | Associated signal pathways | Effect | (Refs.) | Inhibitors | (Refs.) |
|---|---|---|---|---|---|---|
| N-terminal domain | LRP | Phagosome | Internalization and degradation of TSP-2 | (160,161) | Heparin | (157) |
| Participates in cell signaling with cell surface calreticulin | (10,13,14) | |||||
| Calreticulin | Focal adhesion | Induces focal adhesion disassembly and cell migration | 10,13,14) | |||
| Integrin α4β1 | ECM-receptor interaction | Mediates adhesion of T cells | (134) | |||
| Integrin α6β1 | ECM-receptor interaction | Mediates adhesion of microvascular endothelium to immobilized TSP-2 | (19) | |||
| Versican | ECM-receptor interaction | Inhibits VSMC inflammatory response (significantly weaker compared with TSP-1) | (11) | Heparin | (11) | |
| Type I repeats domain | MMP2 | ECM homeostasis | Inhibition of MMP2 activity | (20) | - | - |
| Mediation of collagen fibrillogenesis | (113) | - | - | |||
| Clearance of extracellular MMP2 by fibroblasts | (157) | - | - | |||
| MMP9 | ECM homeostasis | Regulate collagen homeostasis | (20) | - | - | |
| PI3K-AKT pathway | Inhibit cell invasion | (158) | - | - | ||
| CD36 | PI3K-AKT pathway | Anti-angiogenic activity | (25,26) | - | - | |
| β1 integrin | ECM-receptor interaction | Promotes adhesion of the cells that express β1 integrin | (28) | β1 blocking antibody, disintegrins | (28) | |
| Type II repeats domain | β1 integrin | ECM-receptor interaction | Promotes adhesion of the cells that express β1 integrin | (28) | β1 blocking antibody, disintegrins | (28) |
| EGFR | PI3K-AKT pathway | Increases cell migration | (140) | - | - | |
| Type III repeats domain | Calcium | Calcium pathway | Inhibits vascular diseases | (38) | - | - |
| Maintains the homeostasis between the vasodilation and the vasoconstriction | (163) | - | - | |||
| Integrin αIIβ3 | ECM-receptor interaction | Promotes TSP-1 binding with platelets | (136) | - | - | |
| Integrin αvβ3 | ECM-receptor interaction | Promotes TSP-1 binding with platelets | (136) | - | - | |
| FGF2 | PI3K-AKT pathway | Antiangiogenic activity | (43) | Calcium and heparin | (36) | |
| C-terminal domain | Calcium | Calcium pathway | Inhibits vascular diseases | (38) | - | - |
| Maintains the homeostasis between vasodilation and vasoconstriction | (163) | - | - | |||
| CD47 | ECM-receptor interaction | Promotes the cell adhesion to immobilized TSP-2 | (18) | - | - | |
| Unknown | CYP1B1 | PI3K-AKT pathway | Promotes a pro-angiogenic phenotype via the regulation of the oxidative stress | (165) | - | - |
| NO | PI3K-AKT pathway | Negatively regulates TSP-2 transcription and induces angiogenesis | (166) | - | - | |
| ADAMTS1 | ADAMTS1-TSP2 pathway | Promotes wound closure and inhibits angiogenesis | (167) | - | - |
LRP, lipoprotein receptor-related protein; MMP, matrix metalloprotein; EGFR, epidermal growth factor receptor; FGF2, fibroblast growth factor 2; CYP1B1, cytochrome p450 1b1; NO, nitric oxide; ADAMTS, disintegrin and metalloproteinase with thrombospondin motifs; TSP-2, thrombospondin-2; ECM, extracellular matrix; VSMC, vascular smooth muscle cells; LRP, low-density lipoprotein receptor related protein.
ECM-receptor interaction
Interactions between various cells and the ECM cause direct or indirect modulation of numerous cellular activities, such as proliferation, adhesion, migration, differentiation and apoptosis, which contributes markedly to numerous CVDs. TSP-1 binds to HSPG with high affinity, which promotes human melanoma cell migration (132). At the sites of inflammation, TSP1 binding to tumor-specific glycoprotein 6 may regulate hyaluronan metabolism, indicating a critical role of TSP-1 in mediating cellular interactions with hyaluronan (9). During the vascular smooth muscle inflammatory response, TSP-1 and TSP-2 may bind to versican and negatively modulate the ECM component (11). In certain circumstances, TSP-1 can bind to LIMPII and promote cell adhesion (27). Previous data indicated that TSP-1 may bind to calreticulin (CRT) on the cell surface and induce focal adhesion disassembly, as well as cell migration through the association of CRT with lipoprotein LRP (10,13,133).
Integrins are a family of glycosylated, heterodimeric transmembrane receptors that consist of α and β subunits, which provide a physical link between the ECM and the cytoskeleton. Previous studies identified that TSP-1 and TSP-2 may also interact with various types of integrins. Binding of integrin α3β1 to TSP-1 mediates efficient migration of ECs, indicating that the binding of TSP-1 and integrin α3β1 stimulates cell adhesion and migration (15,16). Despite this, integrin α3β1 binding to TSP-1 can also mediate cell motility and inhibit angiogenesis (15,17). Studies have demonstrated that α4β1 integrin mediates CD47-stimulated sickle red blood cells adhesion to immobilized TSP-1 and modulate T cell behavior (18,134). In addition, the N-terminal domain of TSP-1 is also a ligand for α6β1 integrin, which modulates the adhesion of human microvascular endothelial to immobilized TSP-1 and TSP-2 (19). TSP-1 and TSP-2 may also interact with β1 integrin, contributing to the adhesion of cells that express β1 integrin (28,135). The type III repeats of TSP-1 and TSP-2 may interact with integrin αIIβ3 and αvβ3, promoting their binding with platelets (136). A previous study has demonstrated that TSP-2 may also contribute to anti-angiogenesis in diabetes myocardium (137). These results indicate the important role of the interaction between integrins and TSPs in the ECM-receptor interaction.
PI3K-AKT pathway
The PI3K-Akt pathway can be activated by various cellular stimuli, such as growth factors, and regulates numerous fundamental cellular functions, such as cell proliferation, migration and apoptosis. These cellular activities are critical for the pathological process of CVDs. TSP-1 and TSP-2 also participate in a number of these activities through the PI3K-Akt pathway. The N-terminal domain of TSP-1 can interact with platelet-derived growth factor, leading to mediation of VSMC proliferation and migration (138). In addition to the ability to degrade collagen, studies suggest that MMP-9 may also release vascular endothelial growth factor to participate in modulating the invasion and the morphogenesis of endothelial cells, which can also be modulated by TSP-1 (139). The type II repeats of TSP-1 interacts with EGFR and increases cell migration (140). The type III repeat domain of TSP-1 and TSP-2 may interact with integrin αIIβ3 and αvβ3, promoting SMC migration (141). Binding of TSP-1 and TSP-2 to FGF2 inhibits apoptosis (43) and triggers caspase-independent cell death (142).
CD36 is a multi-ligand receptor that participates in various pathological processes of CVDs, such as the formation of atherosclerosis. CD47 is a glycoprotein on numerous types of cell surfaces, which serves an important regulatory role in immune response and inflammation. The binding of TSP-1 and CD36 inhibits angiogenesis through promoting endothelial cell apoptosis and inhibiting nitric oxide (NO) signal transduction (15,25,143). The C-terminal domain of TSP-1 and TSP-2 can interact with CD47, which may promote cell migration and adhesion (18,42,144), inhibit cyclic guanosine monophosphate synthesis, nitric oxide (NO) signaling (143,145) and cell cycle progression in ECs (146). Previous data revealed that the binding of TSP-1 and CD47 may also inhibit angiogenesis, blood flow, and adhesion of monocytes and macrophages (147–150), which may promote foam cell formation (151), pulmonary arterial vasculopathy (152) and LV heart failure (68). Through these mechanisms, TSP-1 and TSP-2 serve a significant role in numerous CVDs.
TGF-β pathway
A wide range of different cellular functions, such as cell proliferation, differentiation, migration and apoptosis, can also be modulated by TGF-β, a member of the transforming growth factor superfamily, which is a group of secreted cytokines. Studies also revealed that TSP-1 regulates the above cellular activities through the TGF-β pathway. Previous data suggested that the type I repeats of TSP-1 may bind and activate latent TGF-β. The activated TGF-β can further stimulate new matrix deposition and angiogenesis (45,153–155), promote inflammatory response via recruitment of inflammatory cells and increase myofibroblast differentiation (156) through the TGF-β pathway.
TSP-2 may also bind to latent TGF-β. However, TSP-2 cannot activate latent TGF-β. In addition, due to this reason, TSP-1 and TSP-2 can regulate the activity of TGF-β and modulate the downstream pathways by competitively binding to it (45).
ECM homeostasis
Numerous pathological processes of CVDs are accompanied by the destruction of ECM homeostasis. For example, excessive accumulation of type I and type III collagen is a significant feature of cardiac hypertrophy, which is due to the higher collagen synthesis capacity compared with the degradation ability. MMP2 and MMP9 serve crucial roles in maintaining ECM homeostasis. Evidence revealed that TSP-1 and TSP-2 may interact with MMP2 and MMP9, which can inhibit their activity and regulate collagen homeostasis (20,157,158).
In addition, there is also evidence revealing that collagens can interact with TSP-1 directly. The C-terminal domain of TSP-1 may bind to collagen I, contributing to fibroblast homeostasis (156). These results suggest that TSP-1 and TSP-2 contribute markedly to ECM homeostasis.
Phagosome pathway
Phagocytosis of TSP-1 serves a critical role in tissue remodeling and inflammation in CVDs, which is mediated by various ligands. In vascular endothelial cells, the heparan sulfate proteoglycans expressed on the cell surface are associated with the process of binding and endocytosis of TSP-1, which leads to its lysosomal degradation (8). Evidence revealed that the HSPG on the endothelial cells may mediate the binding and degradation of TSP-1 (159). Studies suggest that LRP may also function in mediating phagocytosis of TSP-1 in certain types of cells (160,161), indicating that LRP may serve a significant role in the catabolism of TSP-1 in vivo. The binding of TSP-1 and CD36 has been demonstrated to promote the internalization of oxidized LDL, fatty acids and phospholipids, leading to inhibition of atherosclerosis (162). However, little is known on the specific role of TSP-2 in the phagosome pathway, and requires further study.
Calcium pathway
Calcium is an indispensable ion involved in numerous physiological processes in the human body. It participates in maintaining the biopotentials on both sides of the cell membrane, maintaining normal muscle expansion and relaxation, nerve conduction and vasoconstriction. TSP-1 and TSP-2 can bind to calcium and affect the function of modulating physiological activities. Using a simulated model, previous studies have identified that the change between fully calcium-loaded and calcium-depleted TSP1-Sig1 may modulate its interactions, which may become a novel therapeutic target (38,163). Binding of TSP-2 and FGF2 can be inhibited by calcium, indicating that calcium can affect cell function via intervening in interactions between other molecules (36).
Other pathways
In addition to the aforementioned pathways, TSP-1 and TSP-2 also interact with numerous other ligands. During the coagulation reaction, TSP-1 can interact with the vitamin D-binding protein, contributing to the chemotaxis of coagulation factor C5a (164). TSP-2 can interact with cytochrome p450 1B1, promoting angiogenesis through the regulation of oxidative stress (165). In addition, as an important gas signal in the cardiovascular system, NO can negatively regulate TSP-2 transcription and induce angiogenesis (166).
A disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS) is a type of metalloproteinase which has been demonstrated to be associated with numerous CVDs. Studies have identified that ADAMTS1 contributes to wound closure and inhibits the angiogenesis via interaction with TSP-1 and TSP-2 (167). Evidence has revealed that there is a close association between ADAMTS7 and CVDs. TSP-1 and TSP-2 interaction with ADAMTS7 promotes the pathological processes of atherosclerosis, coronary artery disease (168–172), aortic aneurysm (173) and vascular remodeling (174–176) through interacting with TSP-1 and TSP-2. Conversely, there is also evidence revealing that ADAMTS7 may inhibit LV reverse remodeling following MI (177–179), suggesting ADAMTS7 may be a critical regulator in CVDs.
5. Conclusions
The present review suggests that TSP-1 and TSP-2 serve significant roles in the pathological process of numerous CVDs, and their multi-domain structural features and ability to bind to different ligands may also provide novel targets for the treatment of different CVDs at the molecular level.
However, there are two limitations of the present study. Firstly, although both TSP-1 and TSP-2 have a similar multidomain structure, both bind to different ligands and serve different roles. There is limited research into the specific role of TSP-2 in the pathogenesis of numerous CVDs, indicating that more research is required. Secondly, numerous novel ligands remain to be identified. Fortunately, with the development of new large-scale techniques, including array-based surface plasmon resonance, new-generation yeast two-hybrid and numerous novel computational methods, novel TSP-1 and TSP-2 ligands may be identified (4). Identification of these ligands may contribute to determination of the interaction networks of TSP-1 and TSP-2, which may provide an improved understanding of their role in CVDs.
Acknowledgements
The authors would like to thank Dr Yu Han (Zhejiang University, Hangzhou, China), Dr Xinyi Teng (Zhejiang University, Hangzhou, China) and Dr Xin Guo (School of Medicine, Zhejiang University, Hangzhou, China) for their valuable advice. In addition, the authors acknowledge Dr Yasaman Iran Manesh (Zhejiang University, Hangzhou, China) for her revising the article.
Funding
The present study was supported by the National Natural Science Foundation of China (grant. nos. 81300236, 81670433 and 81970398), the Project of Zhejiang Medical Young Talents 2017 (grant. no. 20190301), the Zhejiang Medical and Health Science and Technology Project (grant. no. 2020RC014) and the Natural Science Foundation of Zhejiang Province (grant. no. LQ20H020008).
Availability of data and materials
Not applicable.
Authors’ contributions
KZ, ML and LY carried out literature search and acquisition of references. KZ, ZL and GF were involved in the conception and design of the manuscript. ZL performed manuscript review and gave final approval of the version to be published. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chistiakov DA, Melnichenko AA, Myasoedova VA, Grechko AV, Orekhov AN. Thrombospondins: A role in cardiovascular disease. Int J Mol Sci. 2017;18:E1540. doi: 10.3390/ijms18071540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bornstein P. Thrombospondins as matricellular modulators of cell function. J Clin Invest. 2001;107:929–934. doi: 10.1172/JCI12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bornstein P. Thrombospondins: Structure and regulation of expression. FASEB J. 1992;6:3290–3299. doi: 10.1096/fasebj.6.14.1426766. [DOI] [PubMed] [Google Scholar]
- 4.Resovi A, Pinessi D, Chiorino G, Taraboletti G. Current understanding of the thrombospondin-1 interactome. Matrix Biol. 2014;37:83–91. doi: 10.1016/j.matbio.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 5.Anilkumar N, Annis DS, Mosher DF, Adams JC. Trimeric assembly of the C-terminal region of thrombospondin-1 or thrombospondin-2 is necessary for cell spreading and fascin spike organisation. J Cell Sci. 2002;115:2357–2366. doi: 10.1242/jcs.115.11.2357. [DOI] [PubMed] [Google Scholar]
- 6.Yu H, Tyrrell D, Cashel J, Guo NH, Vogel T, Sipes JM, Lam L, Fillit HM, Hartman J, Mendelovitz S, et al. Specificities of heparin-binding sites from the amino-terminus and type 1 repeats of thrombospondin-1. Arch Biochem Biophys. 2000;374:13–23. doi: 10.1006/abbi.1999.1597. [DOI] [PubMed] [Google Scholar]
- 7.Elzie CA, Murphy-Ullrich JE. The N-terminus of thrombospondin: The domain stands apart. Int J Biochem Cell Biol. 2004;36:1090–1101. doi: 10.1016/j.biocel.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 8.Feitsma K, Hausser H, Robenek H, Kresse H, Vischer P. Interaction of thrombospondin-1 and heparan sulfate from endothelial cells. Structural requirements of heparan sulfate. J Biol Chem. 2000;275:9396–9402. doi: 10.1074/jbc.275.13.9396. [DOI] [PubMed] [Google Scholar]
- 9.Kuznetsova SA, Day AJ, Mahoney DJ, Rugg MS, Mosher DF, Roberts DD. The N-terminal module of thrombospondin-1 interacts with the link domain of TSG-6 and enhances its covalent association with the heavy chains of inter-alpha-trypsin inhibitor. J Biol Chem. 2005;280:30899–30908. doi: 10.1074/jbc.M500701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan Q, Murphy-Ullrich JE, Song Y. Structural insight into the role of thrombospondin-1 binding to calreticulin in calreticulin-induced focal adhesion disassembly. Biochemistry. 2010;49:3685–3694. doi: 10.1021/bi902067f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuznetsova SA, Issa P, Perruccio EM, Zeng B, Sipes JM, Ward Y, Seyfried NT, Fielder HL, Day AJ, Wight TN, Roberts DD. Versican-thrombospondin-1 binding in vitro and colocalization in microfibrils induced by inflammation on vascular smooth muscle cells. J Cell Sci. 2006;119:4499–4509. doi: 10.1242/jcs.03171. [DOI] [PubMed] [Google Scholar]
- 12.Sweetwyne MT, Pallero MA, Lu A, Van Duyn Graham L, Murphy-Ullrich JE. The calreticulin-binding sequence of thrombospondin 1 regulates collagen expression and organization during tissue remodeling. Am J Pathol. 2010;177:1710–1724. doi: 10.2353/ajpath.2010.090903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Murphy-Ullrich JE, Song Y. Molecular insight into the effect of lipid bilayer environments on thrombospondin-1 and calreticulin interactions. Biochemistry. 2014;53:6309–6322. doi: 10.1021/bi500662v. [DOI] [PubMed] [Google Scholar]
- 14.Orr AW, Pallero MA, Xiong WC, Murphy-Ullrich JE. Thrombospondin induces RhoA inactivation through FAK-dependent signaling to stimulate focal adhesion disassembly. J Biol Chem. 2004;279:48983–48992. doi: 10.1074/jbc.M404881200. [DOI] [PubMed] [Google Scholar]
- 15.Ndishabandi D, Duquette C, Billah GE, Reyes M, Duquette M, Lawler J, Kazerounian S. Thrombospondin-1 modulates actin filament remodeling and cell motility in mouse mammary tumor cells in vitro. Discoveries (Craiova) 2014;2:e31. doi: 10.15190/d.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chandrasekaran L, He CZ, Al-Barazi H, Krutzsch HC, Iruela-Arispe ML, Roberts DD. Cell contact-dependent activation of alpha3beta1 integrin modulates endothelial cell responses to thrombospondin-1. Mol Biol Cell. 2000;11:2885–2900. doi: 10.1091/mbc.11.9.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furrer J, Luy B, Basrur V, Roberts DD, Barchi JJ., Jr Conformational analysis of an alpha3beta1 integrin-binding peptide from thrombospondin-1: Implications for antiangiogenic drug design. J Med Chem. 2006;49:6324–6333. doi: 10.1021/jm060833l. [DOI] [PubMed] [Google Scholar]
- 18.Brittain JE, Han J, Ataga KI, Orringer EP, Parise LV. Mechanism of CD47-induced alpha4beta1 integrin activation and adhesion in sickle reticulocytes. J Biol Chem. 2004;279:42393–42402. doi: 10.1074/jbc.M407631200. [DOI] [PubMed] [Google Scholar]
- 19.Calzada MJ, Sipes JM, Krutzsch HC, Yurchenco PD, Annis DS, Mosher DF, Roberts DD. Recognition of the N-terminal modules of thrombospondin-1 and thrombospondin-2 by alpha-6beta1 integrin. J Biol Chem. 2003;278:40679–40687. doi: 10.1074/jbc.M302014200. [DOI] [PubMed] [Google Scholar]
- 20.Bein K, Simons M. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J Biol Chem. 2000;275:32167–32173. doi: 10.1074/jbc.M003834200. [DOI] [PubMed] [Google Scholar]
- 21.Lee T, Esemuede N, Sumpio BE, Gahtan V. Thrombospondin-1 induces matrix metalloproteinase-2 activation in vascular smooth muscle cells. J Vasc Surg. 2003;38:147–154. doi: 10.1016/S0741-5214(02)75468-2. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci USA. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng T, Yuan J, Gan J, Liu Y, Shi L, Lu Z, Xue Y, Xiong R, Huang M, Yang Z, et al. Thrombospondin 1 is increased in the aorta and plasma of patients with acute aortic dissection. Can J Cardiol. 2019;35:42–50. doi: 10.1016/j.cjca.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silverstein RL, Febbraio M. CD36-TSP-HRGP interactions in the regulation of angiogenesis. Curr Pharm Des. 2007;13:3559–3567. doi: 10.2174/138161207782794185. [DOI] [PubMed] [Google Scholar]
- 26.Simantov R, Febbraio M, Silverstein RL. The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biol. 2005;24:27–34. doi: 10.1016/j.matbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Crombie R, Silverstein R. Lysosomal integral membrane protein II binds thrombospondin-1. Structure-function homology with the cell adhesion molecule CD36 defines a conserved recognition motif. J Biol Chem. 1998;273:4855–4863. doi: 10.1074/jbc.273.9.4855. [DOI] [PubMed] [Google Scholar]
- 28.Calzada MJ, Annis DS, Zeng B, Marcinkiewicz C, Banas B, Lawler J, Mosher DF, Roberts DD. Identification of novel beta1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J Biol Chem. 2004;279:41734–41743. doi: 10.1074/jbc.M406267200. [DOI] [PubMed] [Google Scholar]
- 29.Goel HL, Moro L, Murphy-Ullrich JE, Hsieh CC, Wu CL, Jiang Z, Languino LR. Beta1 integrin cytoplasmic variants differentially regulate expression of the antiangiogenic extracellular matrix protein thrombospondin 1. Cancer Res. 2009;69:5374–5382. doi: 10.1158/0008-5472.CAN-09-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahamed J, Janczak CA, Wittkowski KM, Coller BS. In vitro and in vivo evidence that thrombospondin-1 (TSP-1) contributes to stirring- and shear-dependent activation of platelet-derived TGF-beta1. PLoS One. 2009;4:e6608. doi: 10.1371/journal.pone.0006608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar R, Mickael C, Kassa B, Gebreab L, Robinson JC, Koyanagi DE, Sanders L, Barthel L, Meadows C, Fox D, et al. TGF-β activation by bone marrow-derived thrombospondin-1 causes Schistosoma- and hypoxia-induced pulmonary hypertension. Nat Commun. 2017;8:15494. doi: 10.1038/ncomms15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGillicuddy FC, O’Toole D, Hickey JA, Gallagher WM, Dawson KA, Keenan AK. TGF-beta1-induced thrombospondin-1 expression through the p38 MAPK pathway is abolished by fluvastatin in human coronary artery smooth muscle cells. Vascul Pharmacol. 2006;44:469–475. doi: 10.1016/j.vph.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi K, Mernaugh RL, Friedman DB, Weller R, Tsuboi N, Yamashita H, Quaranta V, Takahashi T. Thrombospondin-1 acts as a ligand for CD148 tyrosine phosphatase. Proc Natl Acad Sci USA. 2012;109:1985–1990. doi: 10.1073/pnas.1106171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi K, Sumarriva K, Kim R, Jiang R, Brantley-Sieders DM, Chen J, Mernaugh RL, Takahashi T. Determination of the CD148-interacting region in thrombospondin-1. PLoS One. 2016;11:e0154916. doi: 10.1371/journal.pone.0154916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garg P, Yang S, Liu A, Pallero MA, Buchsbaum DJ, Mosher DF, Murphy-Ullrich JE, Goldblum SE. Thrombospondin-1 opens the paracellular pathway in pulmonary microvascular endothelia through EGFR/ErbB2 activation. Am J Physiol Lung Cell Mol Physiol. 2011;301:L79–L90. doi: 10.1152/ajplung.00287.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rusnati M, Borsotti P, Moroni E, Foglieni C, Chiodelli P, Carminati L, Pinessi D, Annis DS, Paiardi G, Bugatti A, et al. The calcium-binding type III repeats domain of thrombospondin-2 binds to fibroblast growth factor 2 (FGF2) Angiogenesis. 2019;22:133–144. doi: 10.1007/s10456-018-9644-3. [DOI] [PubMed] [Google Scholar]
- 37.Kvansakul M, Adams JC, Hohenester E. Structure of a thrombospondin C-terminal fragment reveals a novel calcium core in the type 3 repeats. EMBO J. 2004;23:1223–1233. doi: 10.1038/sj.emboj.7600166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta A, Agarwal R, Singh A, Bhatnagar S. Calcium-induced conformational changes of Thrombospondin-1 signature domain: Implications for vascular disease. J Recept Signal Transduct Res. 2017;37:239–251. doi: 10.1080/10799893.2016.1212377. [DOI] [PubMed] [Google Scholar]
- 39.Joo SJ. Mechanisms of platelet activation and integrin αIIβ3. Korean Circ J. 2012;42:295–301. doi: 10.4070/kcj.2012.42.5.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freyberg MA, Kaiser D, Graf R, Buttenbender J, Friedl P. Proatherogenic flow conditions initiate endothelial apoptosis via thrombospondin-1 and the integrin-associated protein. Biochem Biophys Res Commun. 2001;286:141–149. doi: 10.1006/bbrc.2001.5314. [DOI] [PubMed] [Google Scholar]
- 41.Freyberg MA, Kaiser D, Graf R, Vischer P, Friedl P. Integrin-associated protein and thrombospondin-1 as endothelial mechanosensitive death mediators. Biochem Biophys Res Commun. 2000;271:584–588. doi: 10.1006/bbrc.2000.2678. [DOI] [PubMed] [Google Scholar]
- 42.McDonald JF, Dimitry JM, Frazier WA. An amyloid-like C-terminal domain of thrombospondin-1 displays CD47 agonist activity requiring both VVM motifs. Biochemistry. 2003;42:10001–10011. doi: 10.1021/bi0341408. [DOI] [PubMed] [Google Scholar]
- 43.Rath GM, Schneider C, Dedieu S, Rothhut B, Soula-Rothhut M, Ghoneim C, Sid B, Morjani H, El Btaouri H, Martiny L. The C-terminal CD47/IAP-binding domain of thrombospondin-1 prevents camptothecin- and doxorubicin-induced apoptosis in human thyroid carcinoma cells. Biochim Biophys Acta. 2006;1763:1125–1134. doi: 10.1016/j.bbamcr.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Pimanda JE, Annis DS, Raftery M, Mosher DF, Chesterman CN, Hogg PJ. The von Willebrand factor-reducing activity of thrombospondin-1 is located in the calcium-binding/C-terminal sequence and requires a free thiol at position 974. Blood. 2002;100:2832–2838. doi: 10.1182/blood-2002-03-0770. [DOI] [PubMed] [Google Scholar]
- 45.Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem. 1995;270:7304–7310. doi: 10.1074/jbc.270.13.7304. [DOI] [PubMed] [Google Scholar]
- 46.Chen H, Sottile J, Strickland DK, Mosher DF. Binding and degradation of thrombospondin-1 mediated through heparan sulphate proteoglycans and low-density-lipoprotein receptor-related protein: Localization of the functional activity to the trimeric N-terminal heparin-binding region of thrombospondin-1. Biochem J. 1996;318:959–963. doi: 10.1042/bj3180959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Befekadu R, Christiansen K, Larsson A, Grenegard M. Increased plasma cathepsin S and trombospondin-1 in patients with acute ST segment elevation myocardial infarction. Cardiol J. 2019;26:385–393. doi: 10.5603/CJ.a2018.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaiser R, Grotemeyer K, Kalsch T, Graber S, Wilkens H, Elmas E. Decreased TSP-1 following percutaneous coronary intervention is associated with major adverse cardiac events in ST-elevation myocardial infarction. Clin Hemorheol Microcirc. 2013;54:59–73. doi: 10.3233/CH-2012-1565. [DOI] [PubMed] [Google Scholar]
- 49.Sezaki S, Hirohata S, Iwabu A, Nakamura K, Toeda K, Miyoshi T, Yamawaki H, Demircan K, Kusachi S, Shiratori Y, et al. Thrombospondin-1 is induced in rat myocardial infarction and its induction is accelerated by ischemia/reperfusion. Exp Biol Med (Maywood) 2005;230:621–630. doi: 10.1177/153537020523000904. [DOI] [PubMed] [Google Scholar]
- 50.Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 51.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: When is enough enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 52.van Oorschot AA, Smits AM, Pardali E, Doevendans PA, Goumans MJ. Low oxygen tension positively influences cardiomyocyte progenitor cell function. J Cell Mol Med. 2011;15:2723–2734. doi: 10.1111/j.1582-4934.2011.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ashokkumar M, Anbarasan C, Saibabu R, Kuram S, Raman SC, Cherian KM. An association study of thrombospondin 1 and 2 SNPs with coronary artery disease and myocardial infarction among South Indians. Thromb Res. 2011;128:e49–e53. doi: 10.1016/j.thromres.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 54.Koch W, Hoppmann P, de Waha A, Schomig A, Kastrati A. Polymorphisms in thrombospondin genes and myocardial infarction: A case-control study and a meta-analysis of available evidence. Hum Mol Genet. 2008;17:1120–1126. doi: 10.1093/hmg/ddn001. [DOI] [PubMed] [Google Scholar]
- 55.Zhang XJ, Wei CY, Li WB, Zhang LL, Zhou Y, Wang ZH, Tang MX, Zhang W, Zhang Y, Zhong M. Association between single nucleotide polymorphisms in thrombospondins genes and coronary artery disease: A meta-analysis. Thromb Res. 2015;136:45–51. doi: 10.1016/j.thromres.2015.04.019. [DOI] [PubMed] [Google Scholar]
- 56.Zwicker JI, Peyvandi F, Palla R, Lombardi R, Canciani MT, Cairo A, Ardissino D, Bernardinelli L, Bauer KA, Lawler J, Mannucci P. The thrombospondin-1 N700S polymorphism is associated with early myocardial infarction without altering von Willebrand factor multimer size. Blood. 2006;108:1280–1283. doi: 10.1182/blood-2006-04-015701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou X, Huang J, Chen J, Zhao J, Ge D, Yang W, Gu D. Genetic association analysis of myocardial infarction with thrombospondin-1 N700S variant in a Chinese population. Thromb Res. 2004;113:181–186. doi: 10.1016/j.thromres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 58.Abdelmonem NA, Turky NO, Hashad IM, Abdel Rahman MF, El-Etriby A, Gad MZ. Association of thrombospondin-1 (N700S) and thrombospondin-4 (A387P) gene polymorphisms with the incidence of acute myocardial infarction in egyptians. Curr Pharm Biotechnol. 2017;18:1078–1087. doi: 10.2174/1389201019666180115144028. [DOI] [PubMed] [Google Scholar]
- 59.Stenina OI, Ustinov V, Krukovets I, Marinic T, Topol EJ, Plow EF. Polymorphisms A387P in thrombospondin-4 and N700S in thrombospondin-1 perturb calcium binding sites. FASEB J. 2005;19:1893–1895. doi: 10.1096/fj.05-3712fje. [DOI] [PubMed] [Google Scholar]
- 60.Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, Lee DW, Frangogiannis NG. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension. 2011;58:902–911. doi: 10.1161/HYPERTENSIONAHA.111.175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzalez-Quesada C, Cavalera M, Biernacka A, Kong P, Lee DW, Saxena A, Frunza O, Dobaczewski M, Shinde A, Frangogiannis NG. Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation. Circ Res. 2013;113:1331–1344. doi: 10.1161/CIRCRESAHA.113.302593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Swinnen M, Vanhoutte D, Van Almen GC, Hamdani N, Schellings MW, D’hooge J, Van der Velden J, Weaver MS, Sage EH, Bornstein P, et al. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation. 2009;120:1585–1597. doi: 10.1161/CIRCULATIONAHA.109.863266. [DOI] [PubMed] [Google Scholar]
- 63.van Almen GC, Swinnen M, Carai P, Verhesen W, Cleutjens JP, D’hooge J, Verheyen FK, Pinto YM, Schroen B, Carmeliet P, Heymans S. Absence of thrombospondin-2 increases cardiomyocyte damage and matrix disruption in doxorubicin-induced cardiomyopathy. J Mol Cell Cardiol. 2011;51:318–328. doi: 10.1016/j.yjmcc.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 64.Papageorgiou AP, Swinnen M, Vanhoutte D, VandenDriessche T, Chuah M, Lindner D, Verhesen W, de Vries B, D’hooge J, Lutgens E, et al. Thrombospondin-2 prevents cardiac injury and dysfunction in viral myocarditis through the activation of regulatory T-cells. Cardiovasc Res. 2012;94:115–124. doi: 10.1093/cvr/cvs077. [DOI] [PubMed] [Google Scholar]
- 65.Schroen B, Heymans S, Sharma U, Blankesteijn WM, Pokharel S, Cleutjens JP, Porter JG, Evelo CT, Duisters R, van Leeuwen RE, et al. Thrombospondin-2 is essential for myocardial matrix integrity: Increased expression identifies failure-prone cardiac hypertrophy. Circ Res. 2004;95:515–522. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- 66.Batlle M, Perez-Villa F, Lazaro A, García-Pras E, Vallejos I, Sionis A, Castel MA, Roig E. Decreased expression of thrombospondin-1 in failing hearts may favor ventricular remodeling. Transplant Proc. 2009;41:2231–2233. doi: 10.1016/j.transproceed.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 67.Vila V, Martinez-Sales V, Almenar L, Lazaro IS, Villa P, Reganon E. Inflammation, endothelial dysfunction and angiogenesis markers in chronic heart failure patients. Int J Cardiol. 2008;130:276–277. doi: 10.1016/j.ijcard.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 68.Sharifi-Sanjani M, Shoushtari AH, Quiroz M, Baust J, Sestito SF, Mosher M, Ross M, McTiernan CF, St Croix CM, Bilonick RA, et al. Cardiac CD47 drives left ventricular heart failure through Ca2+-CaMKII-regulated induction of HDAC3. J Am Heart Assoc. 2014;3:e000670. doi: 10.1161/JAHA.113.000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Almen GC, Verhesen W, van Leeuwen RE, van de Vrie M, Eurlings C, Schellings MW, Swinnen M, Cleutjens JP, van Zandvoort MA, Heymans S, Schroen B. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell. 2011;10:769–779. doi: 10.1111/j.1474-9726.2011.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vila V, Sales VM, Almenar L, Lazaro IS, Villa P, Reganon E. Effect of oral anticoagulant therapy on thrombospondin-1 and von Willebrand factor in patients with stable heart failure. Thromb Res. 2008;121:611–615. doi: 10.1016/j.thromres.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 71.Berezin AE, Kremzer AA, Samura TA. Circulating thrombospondine-2 in patients with moderate-to-severe chronic heart failure due to coronary artery disease. J Biomed Res. 2015 Mar 2; doi: 10.7555/JBR.29.20140025. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 72.Kimura Y, Izumiya Y, Hanatani S, Yamamoto E, Kusaka H, Tokitsu T, Takashio S, Sakamoto K, Tsujita K, Tanaka T, et al. High serum levels of thrombospondin-2 correlate with poor prognosis of patients with heart failure with preserved ejection fraction. Heart Vessels. 2016;31:52–59. doi: 10.1007/s00380-014-0571-y. [DOI] [PubMed] [Google Scholar]
- 73.Hanatani S, Izumiya Y, Takashio S, Kimura Y, Araki S, Rokutanda T, Tsujita K, Yamamoto E, Tanaka T, Yamamuro M, et al. Circulating thrombospondin-2 reflects disease severity and predicts outcome of heart failure with reduced ejection fraction. Circ J. 2014;78:903–910. doi: 10.1253/circj.CJ-13-1221. [DOI] [PubMed] [Google Scholar]
- 74.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: Pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 75.Pohjolainen V, Mustonen E, Taskinen P, Näpänkangas J, Leskinen H, Ohukainen P, Peltonen T, Aro J, Juvonen T, Satta J, et al. Increased thrombospondin-2 in human fibrosclerotic and stenotic aortic valves. Atherosclerosis. 2012;220:66–71. doi: 10.1016/j.atherosclerosis.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 76.Jurk K, Ritter MA, Schriek C, Van Aken H, Droste DW, Ringelstein EB, Kehrel BE. Activated monocytes capture platelets for heterotypic association in patients with severe carotid artery stenosis. Thromb Haemost. 2010;103:1193–1202. doi: 10.1160/TH09-09-0620. [DOI] [PubMed] [Google Scholar]
- 77.Desai P, Helkin A, Odugbesi A, Stein J, Bruch D, Lawler J, Maier KG, Gahtan V. Fluvastatin inhibits intimal hyperplasia in wild-type but not Thbs1-null mice. J Surg Res. 2017;210:1–7. doi: 10.1016/j.jss.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 78.Liauw J, Hoang S, Choi M, Eroglu C, Choi M, Sun GH, Percy M, Wildman-Tobriner B, Bliss T, Guzman RG, et al. Thrombospondins 1 and 2 are necessary for synaptic plasticity and functional recovery after stroke. J Cereb Blood Flow Metab. 2008;28:1722–1732. doi: 10.1038/jcbfm.2008.65. [DOI] [PubMed] [Google Scholar]
- 79.Lin TN, Kim GM, Chen JJ, Cheung WM, He YY, Hsu CY. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke. 2003;34:177–186. doi: 10.1161/01.STR.0000047100.84604.BA. [DOI] [PubMed] [Google Scholar]
- 80.Yang AL, Zhou HJ, Lin Y, Luo JK, Cui HJ, Tang T, Yang QD. Thrombin promotes the expression of thrombospondin-1 and -2 in a rat model of intracerebral hemorrhage. J Neurol Sci. 2012;323:141–146. doi: 10.1016/j.jns.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 81.Zhou HJ, Zhang HN, Tang T, Zhong JH, Qi Y, Luo JK, Lin Y, Yang QD, Li XQ. Alteration of thrombospondin-1 and -2 in rat brains following experimental intracerebral hemorrhage. Laboratory investigation. J Neurosurg. 2010;113:820–825. doi: 10.3171/2010.1.JNS09637. [DOI] [PubMed] [Google Scholar]
- 82.Bodewes TC, Johnson JM, Auster M, Huynh C, Muralidharan S, Contreras M, LoGerfo FW, Pradhan-Nabzdyk L. Intraluminal delivery of thrombospondin-2 small interfering RNA inhibits the vascular response to injury in a rat carotid balloon angioplasty model. FASEB J. 2017;31:109–119. doi: 10.1096/fj.201600501r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Woo MS, Yang J, Beltran C, Cho S. Cell surface CD36 protein in monocyte/macrophage contributes to phagocytosis during the resolution phase of ischemic stroke in mice. J Biol Chem. 2016;291:23654–23661. doi: 10.1074/jbc.M116.750018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim CW, Pokutta-Paskaleva A, Kumar S, Timmins LH, Morris AD, Kang DW, Dalal S, Chadid T, Kuo KM, Raykin J, et al. Disturbed flow promotes arterial stiffening through thrombospondin-1. Circulation. 2017;136:1217–1232. doi: 10.1161/CIRCULATIONAHA.116.026361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moura R, Tjwa M, Vandervoort P, Van Kerckhoven S, Holvoet P, Hoylaerts MF. Thrombospondin-1 deficiency accelerates atherosclerotic plaque maturation in ApoE−/− mice. Circ Res. 2008;103:1181–1189. doi: 10.1161/CIRCRESAHA.108.185645. [DOI] [PubMed] [Google Scholar]
- 86.Narizhneva NV, Razorenova OV, Podrez EA, Chen J, Chandrasekharan UM, DiCorleto PE, Plow EF, Topol EJ, Byzova TV. Thrombospondin-1 up-regulates expression of cell adhesion molecules and promotes monocyte binding to endothelium. FASEB J. 2005;19:1158–1160. doi: 10.1096/fj.04-3310fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roth JJ, Gahtan V, Brown JL, Gerhard C, Swami VK, Rothman VL, Tulenko TN, Tuszynski GP. Thrombospondin-1 is elevated with both intimal hyperplasia and hypercholesterolemia. J Surg Res. 1998;74:11–16. doi: 10.1006/jsre.1997.5209. [DOI] [PubMed] [Google Scholar]
- 88.Muraishi A, Capuzzi DM, Tuszynski GP. Binding of thrombospondin to human plasma lipoproteins. Biochem Biophys Res Commun. 1993;193:1145–1151. doi: 10.1006/bbrc.1993.1745. [DOI] [PubMed] [Google Scholar]
- 89.Barillari G, Iovane A, Bonuglia M, Albonici L, Garofano P, Di Campli E, Falchi M, Condò I, Manzari V, Ensoli B. Fibroblast growth factor-2 transiently activates the p53 oncosuppressor protein in human primary vascular smooth muscle cells: Implications for atherogenesis. Atherosclerosis. 2010;210:400–406. doi: 10.1016/j.atherosclerosis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 90.Osada-Oka M, Ikeda T, Akiba S, Sato T. Hypoxia stimulates the autocrine regulation of migration of vascular smooth muscle cells via HIF-1alpha-dependent expression of thrombospondin-1. J Cell Biochem. 2008;104:1918–1926. doi: 10.1002/jcb.21759. [DOI] [PubMed] [Google Scholar]
- 91.Takahashi M, Oka M, Ikeda T, Akiba S, Sato T. Role of thrombospondin-1 in hypoxia-induced migration of human vascular smooth muscle cells. Yakugaku Zasshi. 2008;128:377–383. doi: 10.1248/yakushi.128.377. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 92.Yabkowitz R, Mansfield PJ, Ryan US, Suchard SJ. Thrombospondin mediates migration and potentiates platelet-derived growth factor-dependent migration of calf pulmonary artery smooth muscle cells. J Cell Physiol. 1993;157:24–32. doi: 10.1002/jcp.1041570104. [DOI] [PubMed] [Google Scholar]
- 93.Raman P, Krukovets I, Marinic TE, Bornstein P, Stenina OI. Glycosylation mediates up-regulation of a potent antiangiogenic and proatherogenic protein, thrombospondin-1, by glucose in vascular smooth muscle cells. J Biol Chem. 2007;282:5704–5714. doi: 10.1074/jbc.M610965200. [DOI] [PubMed] [Google Scholar]
- 94.Stenina OI, Krukovets I, Wang K, Zhou Z, Forudi F, Penn MS, Topol EJ, Plow EF. Increased expression of thrombospondin-1 in vessel wall of diabetic Zucker rat. Circulation. 2003;107:3209–3215. doi: 10.1161/01.CIR.0000074223.56882.97. [DOI] [PubMed] [Google Scholar]
- 95.Ganguly R, Sahu S, Ohanyan V, Haney R, Chavez RJ, Shah S, Yalamanchili S, Raman P. Oral chromium picolinate impedes hyperglycemia-induced atherosclerosis and inhibits proatherogenic protein TSP-1 expression in STZ-induced type 1 diabetic ApoE−/− mice. Sci Rep. 2017;7:45279. doi: 10.1038/srep45279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang YJ, Cheng DY, Zheng XW, Li F, Yang GL. Expression of thrombospondin-1 in the lung of hypoxic pulmonary hypertension rats. Sichuan Da Xue Xue Bao Yi Xue Ban. 2012;43:19–23. (In Chinese) [PubMed] [Google Scholar]
- 97.Reed MJ, Iruela-Arispe L, O’Brien ER, Truong T, LaBell T, Bornstein P, Sage EH. Expression of thrombospondins by endothelial cells. Injury is correlated with TSP-1. Am J Pathol. 1995;147:1068–1080. [PMC free article] [PubMed] [Google Scholar]
- 98.DiPietro LA, Nebgen DR, Polverini PJ. Downregulation of endothelial cell thrombospondin 1 enhances in vitro angiogenesis. J Vasc Res. 1994;31:178–185. doi: 10.1159/000319585. [DOI] [PubMed] [Google Scholar]
- 99.Dardik R, Solomon A, Loscalzo J, Eskaraev R, Bialik A, Goldberg I, Schiby G, Inbal A. Novel proangiogenic effect of factor XIII associated with suppression of thrombospondin 1 expression. Arterioscler Thromb Vasc Biol. 2003;23:1472–1477. doi: 10.1161/01.ATV.0000081636.25235.C6. [DOI] [PubMed] [Google Scholar]
- 100.Isenberg JS, Hyodo F, Ridnour LA, Shannon CS, Wink DA, Krishna MC, Roberts DD. Thrombospondin 1 and vasoactive agents indirectly alter tumor blood flow. Neoplasia. 2008;10:886–896. doi: 10.1593/neo.08264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tzeng HT, Tsai CH, Yen YT, Cheng HC, Chen YC, Pu SW, Wang YS, Shan YS, Tseng YL, Su WC, et al. Dysregulation of Rab37-mediated cross-talk between cancer cells and endothelial cells via thrombospondin-1 promotes tumor neovasculature and metastasis. Clin Cancer Res. 2017;23:2335–2345. doi: 10.1158/1078-0432.CCR-16-1520. [DOI] [PubMed] [Google Scholar]
- 102.Bitar MS. Diabetes impairs angiogenesis and induces endothelial cell senescence by up-regulating thrombospondin-CD47-dependent signaling. Int J Mol Sci. 2019;20:E673. doi: 10.3390/ijms20030673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yafai Y, Eichler W, Iandiev I, Unterlauft JD, Jochmann C, Wiedemann P, Bringmann A. Thrombospondin-1 is produced by retinal glial cells and inhibits the growth of vascular endothelial cells. Ophthalmic Res. 2014;52:81–88. doi: 10.1159/000362371. [DOI] [PubMed] [Google Scholar]
- 104.Wang S, Sorenson CM, Sheibani N. Lack of thrombospondin 1 and exacerbation of choroidal neovascularization. Arch Ophthalmol. 2012;130:615–620. doi: 10.1001/archopthalmol.2011.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu Z, Wang S, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in transgenic mice over-expressing thrombospondin-1 in the lens. Dev Dyn. 2006;235:1908–1920. doi: 10.1002/dvdy.20837. [DOI] [PubMed] [Google Scholar]
- 106.Wang Y, Wang S, Sheibani N. Enhanced proangiogenic signaling in thrombospondin-1-deficient retinal endothelial cells. Microvasc Res. 2006;71:143–151. doi: 10.1016/j.mvr.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 107.Wang S, Wu Z, Sorenson CM, Lawler J, Sheibani N. Thrombospondin-1-deficient mice exhibit increased vascular density during retinal vascular development and are less sensitive to hyperoxia-mediated vessel obliteration. Dev Dyn. 2003;228:630–642. doi: 10.1002/dvdy.10412. [DOI] [PubMed] [Google Scholar]
- 108.Sheibani N, Sorenson CM, Cornelius LA, Frazier WA. Thrombospondin-1, a natural inhibitor of angiogenesis, is present in vitreous and aqueous humor and is modulated by hyperglycemia. Biochem Biophys Res Commun. 2000;267:257–261. doi: 10.1006/bbrc.1999.1903. [DOI] [PubMed] [Google Scholar]
- 109.Koch M, Hussein F, Woeste A, Gründker C, Frontzek K, Emons G, Hawighorst T. CD36-mediated activation of endothelial cell apoptosis by an N-terminal recombinant fragment of thrombospondin-2 inhibits breast cancer growth and metastasis in vivo. Breast Cancer Res Treat. 2011;128:337–346. doi: 10.1007/s10549-010-1085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tomii Y, Kamochi J, Yamazaki H, Sawa N, Tokunaga T, Ohnishi Y, Kijima H, Ueyama Y, Tamaoki N, Nakamura M. Human thrombospondin 2 inhibits proliferation of microvascular endothelial cells. Int J Oncol. 2002;20:339–342. [PubMed] [Google Scholar]
- 111.Armstrong LC, Bjorkblom B, Hankenson KD, Siadak AW, Stiles CE, Bornstein P. Thrombospondin 2 inhibits microvascular endothelial cell proliferation by a caspase-independent mechanism. Mol Biol Cell. 2002;13:1893–1905. doi: 10.1091/mbc.e01-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kyriakides TR, Zhu YH, Yang Z, Huynh G, Bornstein P. Altered extracellular matrix remodeling and angiogenesis in sponge granulomas of thrombospondin 2-null mice. Am J Pathol. 2001;159:1255–1262. doi: 10.1016/S0002-9440(10)62512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Calabro NE, Kristofik NJ, Kyriakides TR. Thrombospondin-2 and extracellular matrix assembly. Biochim Biophys Acta. 18402014:2396–2402. doi: 10.1016/j.bbagen.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Krady MM, Zeng J, Yu J, MacLauchlan S, Skokos EA, Tian W, Bornstein P, Sessa WC, Kyriakides TR. Thrombospondin-2 modulates extracellular matrix remodeling during physiological angiogenesis. Am J Pathol. 2008;173:879–891. doi: 10.2353/ajpath.2008.080128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Park YW, Kang YM, Butterfield J, Detmar M, Goronzy JJ, Weyand CM. Thrombospondin 2 functions as an endogenous regulator of angiogenesis and inflammation in rheumatoid arthritis. Am J Pathol. 2004;165:2087–2098. doi: 10.1016/S0002-9440(10)63259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Agah A, Kyriakides TR, Letrondo N, Bjorkblom B, Bornstein P. Thrombospondin 2 levels are increased in aged mice: Consequences for cutaneous wound healing and angiogenesis. Matrix Biol. 2004;22:539–547. doi: 10.1016/j.matbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 117.Feige JJ. Thrombospondins: Multimodular proteins with angiostatic function. Pathol Biol (Paris) 1999;47:339–344. (In French) [PubMed] [Google Scholar]
- 118.Raugi GJ, Mullen JS, Bark DH, Okada T, Mayberg MR. Thrombospondin deposition in rat carotid artery injury. Am J Pathol. 1990;137:179–185. [PMC free article] [PubMed] [Google Scholar]
- 119.Lemkens P, Boari G, Fazzi G, Janssen G, Murphy-Ullrich J, Schiffers P, De Mey J. Thrombospondin-1 in early flow-related remodeling of mesenteric arteries from young normotensive and spontaneously hypertensive rats. Open Cardiovasc Med J. 2012;6:50–59. doi: 10.2174/1874192401206010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ichii T, Koyama H, Tanaka S, Shioi A, Okuno Y, Otani S, Nishizawa Y. Thrombospondin-1 mediates smooth muscle cell proliferation induced by interaction with human platelets. Arterioscler Thromb Vasc Biol. 2002;22:1286–1292. doi: 10.1161/01.ATV.0000024684.67566.45. [DOI] [PubMed] [Google Scholar]
- 121.Riessen R, Kearney M, Lawler J, Isner JM. Immunolocalization of thrombospondin-1 in human atherosclerotic and restenotic arteries. Am Heart J. 1998;135:357–364. doi: 10.1016/S0002-8703(98)70105-X. [DOI] [PubMed] [Google Scholar]
- 122.Yoshida S, Nabzdyk CS, Pradhan L, LoGerfo FW. Thrombospondin-2 gene silencing in human aortic smooth muscle cells improves cell attachment. J Am Coll Surg. 2011;213:668–676. doi: 10.1016/j.jamcollsurg.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ochoa CD, Yu L, Al-Ansari E, Hales CA, Quinn DA. Thrombospondin-1 null mice are resistant to hypoxia-induced pulmonary hypertension. J Cardiothorac Surg. 2010;5:32. doi: 10.1186/1749-8090-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Satoh M, Nasu T, Osaki T, Hitomi S. Thrombospondin-1 contributes to slower aortic aneurysm growth by inhibiting maladaptive remodeling of extracellular matrix. Clin Sci (Lond) 2017;131:1283–1285. doi: 10.1042/CS20170275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Z, Morgan S, Ren J, Wang Q, Annis DS, Mosher DF, Zhang J, Sorenson CM, Sheibani N, Liu B. Thrombospondin-1 (TSP1) contributes to the development of vascular inflammation by regulating monocytic cell motility in mouse models of abdominal aortic aneurysm. Circ Res. 2015;117:129–141. doi: 10.1161/CIRCRESAHA.117.305262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cointe S, Rheaume E, Martel C, Blanc-Brude O, Dubé E, Sabatier F, Dignat-George F, Tardif JC, Bonnefoy A. Thrombospondin-1-derived peptide RFYVVMWK improves the adhesive phenotype of CD34(+) cells from atherosclerotic patients with type 2 diabetes. Cell Transplant. 2017;26:327–337. doi: 10.3727/096368916X693329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kyriakides TR, Rojnuckarin P, Reidy MA, Hankenson KD, Papayannopoulou T, Kaushansky K, Bornstein P. Megakaryocytes require thrombospondin-2 for normal platelet formation and function. Blood. 2003;101:3915–3923. doi: 10.1182/blood.V101.10.3915. [DOI] [PubMed] [Google Scholar]
- 128.Kyriakides TR, Leach KJ, Hoffman AS, Ratner BD, Bornstein P. Mice that lack the angiogenesis inhibitor, thrombospondin 2, mount an altered foreign body reaction characterized by increased vascularity. Proc Natl Acad Sci USA. 1999;96:4449–4454. doi: 10.1073/pnas.96.8.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roberts DD, Haverstick DM, Dixit VM, Frazier WA, Santoro SA, Ginsburg V. The platelet glycoprotein thrombospondin binds specifically to sulfated glycolipids. J Biol Chem. 1985;260:9405–9411. [PubMed] [Google Scholar]
- 130.Yang S, Song R, Li X, Zhang T, Fu J, Cui X. Thrombospondin-2 predicts response to treatment with intravenous immunoglobulin in children with Kawasaki disease. BMJ Paediatr Open. 2018;2:e000190. doi: 10.1136/bmjpo-2017-000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Reinecke H, Robey TE, Mignone JL, Muskheli V, Bornstein P, Murry CE. Lack of thrombospondin-2 reduces fibrosis and increases vascularity around cardiac cell grafts. Cardiovasc Pathol. 2013;22:91–95. doi: 10.1016/j.carpath.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roberts DD. Interactions of thrombospondin with sulfated glycolipids and proteoglycans of human melanoma cells. Cancer Res. 1988;48:6785–6793. [PubMed] [Google Scholar]
- 133.Orr AW, Pedraza CE, Pallero MA, Elzie CA, Goicoechea S, Strickland DK, Murphy-Ullrich JE. Low density lipoprotein receptor-related protein is a calreticulin coreceptor that signals focal adhesion disassembly. J Cell Biol. 2003;161:1179–1189. doi: 10.1083/jcb.200302069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Z, Calzada MJ, Sipes JM, Cashel JA, Krutzsch HC, Annis DS, Mosher DF, Roberts DD. Interactions of thrombospondins with alpha4beta1 integrin and CD47 differentially modulate T cell behavior. J Cell Biol. 2002;157:509–519. doi: 10.1083/jcb.200109098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang Z, Newcomb CJ, Lei Y, Zhou Y, Bornstein P, Amendt BA, Stupp SI, Snead ML. Bioactive nanofibers enable the identification of thrombospondin 2 as a key player in enamel regeneration. Biomaterials. 2015;61:216–228. doi: 10.1016/j.biomaterials.2015.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lawler J, Hynes RO. An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood. 1989;74:2022–2027. doi: 10.1182/blood.V74.6.2022.2022. [DOI] [PubMed] [Google Scholar]
- 137.Kong P, Cavalera M, Frangogiannis NG. The role of thrombospondin (TSP)-1 in obesity and diabetes. Adipocyte. 2014;3:81–84. doi: 10.4161/adip.26990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hogg PJ, Hotchkiss KA, Jimenez BM, Stathakis P, Chesterman CN. Interaction of platelet-derived growth factor with thrombospondin 1. Biochem J. 1997;326:709–716. doi: 10.1042/bj3260709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Qian X, Wang TN, Rothman VL, Nicosia RF, Tuszynski GP. Thrombospondin-1 modulates angiogenesis in vitro by up-regulation of matrix metalloproteinase-9 in endothelial cells. Exp Cell Res. 1997;235:403–412. doi: 10.1006/excr.1997.3681. [DOI] [PubMed] [Google Scholar]
- 140.Liu A, Garg P, Yang S, Gong P, Pallero MA, Annis DS, Liu Y, Passaniti A, Mann D, Mosher DF, et al. Epidermal growth factor-like repeats of thrombospondins activate phospholipase Cgamma and increase epithelial cell migration through indirect epidermal growth factor receptor activation. J Biol Chem. 2009;284:6389–6402. doi: 10.1074/jbc.M809198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zheng B, Clemmons DR. Blocking ligand occupancy of the alphaVbeta3 integrin inhibits insulin-like growth factor I signaling in vascular smooth muscle cells. Proc Natl Acad Sci USA. 1998;95:11217–11222. doi: 10.1073/pnas.95.19.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Saumet A, Slimane MB, Lanotte M, Lawler J, Dubernard V. Type 3 repeat/C-terminal domain of thrombospondin-1 triggers caspase-independent cell death through CD47/alphavbeta3 in promyelocytic leukemia NB4 cells. Blood. 2005;106:658–667. doi: 10.1182/blood-2004-09-3585. [DOI] [PubMed] [Google Scholar]
- 143.Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem. 2007;282:15404–15415. doi: 10.1074/jbc.M701638200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lagadec P, Dejoux O, Ticchioni M, Cottrez F, Johansen M, Brown EJ, Bernard A. Involvement of a CD47-dependent pathway in platelet adhesion on inflamed vascular endothelium under flow. Blood. 2003;101:4836–4843. doi: 10.1182/blood-2002-11-3483. [DOI] [PubMed] [Google Scholar]
- 145.Mandler WK, Nurkiewicz TR, Porter DW, Kelley EE, Olfert IM. Microvascular dysfunction following multiwalled carbon nanotube exposure is mediated by thrombospondin-1 receptor CD47. Toxicol Sci. 2018;165:90–99. doi: 10.1093/toxsci/kfy120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gao Q, Chen K, Gao L, Zheng Y, Yang YG. Thrombospondin-1 signaling through CD47 inhibits cell cycle progression and induces senescence in endothelial cells. Cell Death Dis. 2016;7:e2368. doi: 10.1038/cddis.2016.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yamauchi Y, Kuroki M, Imakiire T, Uno K, Abe H, Beppu R, Yamashita Y, Kuroki M, Shirakusa T. Opposite effects of thrombospondin-1 via CD36 and CD47 on homotypic aggregation of monocytic cells. Matrix Biol. 2002;21:441–448. doi: 10.1016/S0945-053X(02)00036-7. [DOI] [PubMed] [Google Scholar]
- 148.Kaur S, Chang T, Singh SP, Lim L, Mannan P, Garfield SH, Pendrak ML, Soto-Pantoja DR, Rosenberg AZ, Jin S, Roberts DD. CD47 signaling regulates the immunosuppressive activity of VEGF in T cells. J Immunol. 2014;193:3914–3924. doi: 10.4049/jimmunol.1303116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rogers NM, Roberts DD, Isenberg JS. Age-associated induction of cell membrane CD47 limits basal and temperature-induced changes in cutaneous blood flow. Ann Surg. 2013;258:184–191. doi: 10.1097/SLA.0b013e31827e52e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rogers NM, Sharifi-Sanjani M, Csanyi G, Pagano PJ, Isenberg JS. Thrombospondin-1 and CD47 regulation of cardiac, pulmonary and vascular responses in health and disease. Matrix Biol. 2014;37:92–101. doi: 10.1016/j.matbio.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Csanyi G, Feck DM, Ghoshal P, Singla B, Lin H, Nagarajan S, Meijles DN, Al Ghouleh I, Cantu-Medellin N, Kelley EE, et al. CD47 and Nox1 mediate dynamic fluid-phase macropinocytosis of native LDL. Antioxid Redox Signal. 2017;26:886–901. doi: 10.1089/ars.2016.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Rogers NM, Sharifi-Sanjani M, Yao M, Ghimire K, Bienes-Martinez R, Mutchler SM, Knupp HE, Baust J, Novelli EM, Ross M, et al. TSP1-CD47 signaling is upregulated in clinical pulmonary hypertension and contributes to pulmonary arterial vasculopathy and dysfunction. Cardiovasc Res. 2017;113:15–29. doi: 10.1093/cvr/cvw218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kellouche S, Mourah S, Bonnefoy A, Schoëvaert D, Podgorniak MP, Calvo F, Hoylaerts MF, Legrand C, Dosquet C. Platelets, thrombospondin-1 and human dermal fibroblasts cooperate for stimulation of endothelial cell tubulogenesis through VEGF and PAI-1 regulation. Exp Cell Res. 2007;313:486–499. doi: 10.1016/j.yexcr.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 154.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: Mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/S1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 155.Schultz-Cherry S, Lawler J, Murphy-Ullrich JE. The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-beta. J Biol Chem. 1994;269:26783–26788. [PubMed] [Google Scholar]
- 156.Rosini S, Pugh N, Bonna AM, Hulmes DJS, Farndale RW, Adams JC. Thrombospondin-1 promotes matrix homeostasis by interacting with collagen and lysyl oxidase precursors and collagen cross-linking sites. Sci Signal. 2018;11:eaar2566. doi: 10.1126/scisignal.aar2566. [DOI] [PubMed] [Google Scholar]
- 157.Yang Z, Strickland DK, Bornstein P. Extracellular matrix metalloproteinase 2 levels are regulated by the low density lipoprotein-related scavenger receptor and thrombospondin 2. J Biol Chem. 2001;276:8403–8408. doi: 10.1074/jbc.M008925200. [DOI] [PubMed] [Google Scholar]
- 158.Nakamura M, Oida Y, Abe Y, Yamazaki H, Mukai M, Matsuyama M, Chijiwa T, Matsumoto H, Ueyama Y. Thrombospondin-2 inhibits tumor cell invasion through the modulation of MMP-9 and uPA in pancreatic cancer cells. Mol Med Rep. 2008;1:423–427. [PubMed] [Google Scholar]
- 159.Murphy-Ullrich JE, Mosher DF. Interactions of thrombospondin with endothelial cells: Receptor-mediated binding and degradation. J Cell Biol. 1987;105:1603–1611. doi: 10.1083/jcb.105.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Godyna S, Liau G, Popa I, Stefansson S, Argraves WS. Identification of the low density lipoprotein receptor-related protein (LRP) as an endocytic receptor for thrombospondin-1. J Cell Biol. 1995;129:1403–1410. doi: 10.1083/jcb.129.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mikhailenko I, Kounnas MZ, Strickland DK. Low density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor mediates the cellular internalization and degradation of thrombospondin. A process facilitated by cell-surface proteoglycans. J Biol Chem. 1995;270:9543–9549. doi: 10.1074/jbc.270.16.9543. [DOI] [PubMed] [Google Scholar]
- 162.Daviet L, McGregor JL. Vascular biology of CD36: Roles of this new adhesion molecule family in different disease states. Thromb Haemost. 1997;78:65–69. doi: 10.1055/s-0038-1657502. [DOI] [PubMed] [Google Scholar]
- 163.Ramanathan S, Mazzalupo S, Boitano S, Montfort WR. Thrombospondin-1 and angiotensin II inhibit soluble guanylyl cyclase through an increase in intracellular calcium concentration. Biochemistry. 2011;50:7787–7799. doi: 10.1021/bi201060c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Trujillo G, Kew RR. Platelet-derived thrombospondin-1 is necessary for the vitamin D-binding protein (Gc-globulin) to function as a chemotactic cofactor for C5a. J Immunol. 2004;173:4130–4136. doi: 10.4049/jimmunol.173.6.4130. [DOI] [PubMed] [Google Scholar]
- 165.Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood. 2009;113:744–754. doi: 10.1182/blood-2008-03-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.MacLauchlan S, Yu J, Parrish M, Asoulin TA, Schleicher M, Krady MM, Zeng J, Huang PL, Sessa WC, Kyriakides TR. Endothelial nitric oxide synthase controls the expression of the angiogenesis inhibitor thrombospondin 2. Proc Natl Acad Sci USA. 2011;108:E1137–E1145. doi: 10.1073/pnas.1104357108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lee NV, Sato M, Annis DS, Loo JA, Wu L, Mosher DF, Iruela-Arispe ML. ADAMTS1 mediates the release of antiangiogenic polypeptides from TSP1 and 2. EMBO J. 2006;25:5270–5283. doi: 10.1038/sj.emboj.7601400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mead TJ, Apte SS. ADAMTS proteins in human disorders. Matrix Biol. 2018;71–72:225–239. doi: 10.1016/j.matbio.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bengtsson E, Hultman K, Duner P, Asciutto G, Almgren P, Orho-Melander M, Melander O, Nilsson J, Hultgardh-Nilsson A, Gonçalves I. ADAMTS-7 is associated with a high-risk plaque phenotype in human atherosclerosis. Sci Rep. 2017;7:3753. doi: 10.1038/s41598-017-03573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pereira A, Palma Dos Reis R, Rodrigues R, Sousa AC, Gomes S, Borges S, Ornelas I, Freitas AI, Guerra G, Henriques E, et al. Association of ADAMTS7 gene polymorphism with cardiovascular survival in coronary artery disease. Physiol Genomics. 2016;48:810–815. doi: 10.1152/physiolgenomics.00059.2016. [DOI] [PubMed] [Google Scholar]
- 171.Bauer RC, Tohyama J, Cui J, Cheng L, Yang J, Zhang X, Ou K, Paschos GK, Zheng XL, Parmacek MS, et al. Knockout of Adamts7, a novel coronary artery disease locus in humans, reduces atherosclerosis in mice. Circulation. 2015;131:1202–1213. doi: 10.1161/CIRCULATIONAHA.114.012669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Reilly MP, Li M, He J, Ferguson JF, Stylianou IM, Mehta NN, Burnett MS, Devaney JM, Knouff CW, Thompson JR, et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: Two genome-wide association studies. Lancet. 2011;377:383–392. doi: 10.1016/S0140-6736(10)61996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Qin W, Cao Y, Li L, Chen W, Chen X. Upregulation of ADAMTS7 and downregulation of COMP are associated with aortic aneurysm. Mol Med Rep. 2017;16:5459–5463. doi: 10.3892/mmr.2017.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kessler T, Zhang L, Liu Z, Yin X, Huang Y, Wang Y, Fu Y, Mayr M, Ge Q, Xu Q, et al. ADAMTS-7 inhibits re-endothelialization of injured arteries and promotes vascular remodeling through cleavage of thrombospondin-1. Circulation. 2015;131:1191–1201. doi: 10.1161/CIRCULATIONAHA.114.014072. [DOI] [PubMed] [Google Scholar]
- 175.Pu X, Xiao Q, Kiechl S, Chan K, Ng FL, Gor S, Poston RN, Fang C, Patel A, Senver EC, et al. ADAMTS7 cleavage and vascular smooth muscle cell migration is affected by a coronary-artery-disease-associated variant. Am J Hum Genet. 2013;92:366–374. doi: 10.1016/j.ajhg.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wang L, Zheng J, Bai X, Liu B, Liu CJ, Xu Q, Zhu Y, Wang N, Kong W, Wang X. ADAMTS-7 mediates vascular smooth muscle cell migration and neointima formation in balloon-injured rat arteries. Circ Res. 2009;104:688–698. doi: 10.1161/CIRCRESAHA.108.188425. [DOI] [PubMed] [Google Scholar]
- 177.Wu W, Li J, Yu C, Gao Y, Fan S, Ye X, Wang Y, Zheng J. Association of serum ADAMTS-7 levels with left ventricular reverse remodeling after ST-elevation myocardial infarction. Eur J Med Res. 2018;23:15. doi: 10.1186/s40001-018-0305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chan K, Pu X, Sandesara P, Poston RN, Simpson IA, Quyyumi AA, Ye S, Patel RS. Genetic variation at the ADAMTS7 locus is associated with reduced severity of coronary artery disease. J Am Heart Assoc. 2017;6:e006928. doi: 10.1161/JAHA.117.006928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wu W, Wang H, Yu C, Li J, Gao Y, Ke Y, Wang Y, Zhou Y, Zheng J. Association of ADAMTS-7 levels with cardiac function in a rat model of acute myocardial infarction. Cell Physiol Biochem. 2016;38:950–958. doi: 10.1159/000443047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.