Abstract
Melatonin (Mel) elicits beneficial effects on myocardial ischemia/reperfusion injury. However, the underlying mechanism of Mel against oxygen-glucose deprivation/ reperfusion (OGD/R)-induced H9c2 cardiomyocyte damage remains largely unknown. The aim of the present study was to investigate the biological roles and the potential mechanisms of Mel in OGD/R-exposed H9c2 cardiomyocytes. The results of the present study demonstrated that Mel significantly elevated the viability and reduced the activity of lactate dehydrogenase and creatine kinase myocardial band in a doseand time-dependent manner in OGD/R-insulted H9c2 cells. In addition, Mel suppressed OGD/R-induced oxidative stress in H9c2 cells, as demonstrated by the decreased reactive oxygen species and malondialdehyde levels, as well as the increased activities of superoxide dismutase, catalase and glutathione peroxidase. Mel exerted an antioxidant effect by activating the peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α)/nuclear factor erythroid 2-related factor 2 (Nrf2) signaling. Mel reduced the expression of OGD/R-enhanced pro-inflammatory tumor necrosis factor-α (TNF-α), interleukin (IL)-6, IL-1β, IL-8 and monocyte chemotactic protein-1. Mel also abolished the OGD/R-induced increase in H9c2 apoptosis, as evidenced by mitochondrial membrane potential restoration and caspase-3 and caspase-9 inactivation, as well as the upregulation of Bcl-2 and down-regulation of cleaved caspase-3 and Bax. The Mel-induced antiapoptotic effects were dependent on PGC-1α/TNF-α signaling. Overall, the results of the present study demonstrated that Mel alleviated OGD/R-induced H9c2 cell injury via the inhibition of oxidative stress and inflammation by regulating the PGC-1α/Nrf2 and PGC-1α/TNF-α signaling pathways, suggesting a promising role for Mel in the treatment of ischemic heart disease.
Keywords: melatonin, H9c2 cardiomyocytes, oxygen-glucose deprivation, oxidative stress, inflammation, apoptosis, signaling
Introduction
Cardiovascular diseases (CVDs) are a common threat to human health and were the leading cause of human mortality between 2009 and 2011 worldwide (1,2). Ischemic heart disease, which accounts for a large subset of CVDs, leads to hypoxia and, ultimately, cardiomyocyte death (3). Timely reperfusion is the main therapeutic strategy to resuscitate the ischemic or hypoxic myocardium (4). Unfortunately, reperfusion also elicits a number of adverse reactions, including ischemia/reperfusion (I/R) injury (5,6). An increasing body of evidence suggests that apoptosis is initiated shortly after the onset of ischemia and becomes markedly enhanced during reperfusion (7,8). Therefore, strategies aimed at preventing or delaying cardiomyocyte apoptosis may be advisable for the treatment of ischemic heart disease, especially myocardial I/R injury.
Multiple biological processes and cell signaling pathways are involved in myocardial I/R injury. The increased generation of oxygen free radicals is a major contributor to the initiation and development of I/R injury (9). Excessive reactive oxygen species (ROS) production causes the opening of the mitochondrial permeability transition pore, DNA damage and lipid peroxidation, leading to apoptosis (10). Peroxisome proliferator-activated receptor γ (PPARγ) coactivator-1α (PGC-1α) has been demonstrated to suppress oxidative stress and delay the progression of heart failure (11). Nuclear factor erythroid 2-related factor 2 (Nrf2) promotes the transcription of key antioxidant genes, such as heme oxygenase-1 (HO-1) and NADPH quinone oxido reductase 1 (NQO1), to prevent cardiomyocyte damage from oxidative stress (12). PGC-1α/Nrf2 signaling is as a potential target for cardioprotection in myocardial I/R injury (13). Several pro-inflammatory cytokines in the serum have also been demonstrated to be upregulated during myocardial I/R injury, including tumor necrosis factor-α (TNF-α) (14). TNF-α contributes to post-ischemic myocardial dysfunction by directly inhibiting contractility and inducing cardiomyocyte apoptosis (15). The PPARγ/PGC-1α/TNF-α pathway has been implicated in the attenuation of oxygen-glucose deprivation/reperfusion (OGD/R)-induced myocardial injury (16). Therefore, the use of antioxidant and anti-inflammatory compounds is considered to be a promising therapeutic strategy for ischemic heart disease.
Melatonin (Mel; N-acetyl-5-methoxytryptamine), which is mainly produced by the pineal gland, is a powerful endogenous antioxidant due to its direct free-radical scavenging and indirect antioxidant activities (17,18). Due to its amphiphilic property, Mel can easily penetrate all morphophysiological barriers and enter all subcellular compartments to influence the physiological functions of the majority of organs, including the heart (19). Previous studies have indicated that Mel serves a pivotal role in the treatment of myocardial I/R injury (20–24). Mel exerts its cardioprotective effects through its antioxidant and antiapoptotic properties (22–24) and the preservation of mitochondrial function (25). However, the underlying mechanisms through which Mel prevents OGD/R-induced cardiomyocyte damage remain to be further elucidated.
The present study aimed to investigate the potential protective effects of Mel on OGD/R-induced H9c2 cardiomyocyte injury and the underlying molecular mechanisms.
Materials and methods
Cell culture
A H9c2 rat cardiomyocyte cell line was purchased from American Type Culture Collection (cat. no. CRL-1446) and cultured in Dulbecco’s modified Eagle’s medium (DMEM; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Inc.), 2 mM glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin (all from Merck KGaA) at 37°C in a humidified incubator containing 5% CO2.
Cell transfection
The small interfering RNA (siRNA) duplexes corresponding to rat PGC-1α (siPGC-1α), Nrf2 (siNrf2) and the negative control siRNA (siNC) were purchased from Santa Cruz Biotechnology, Inc. siRNAs (100 nM) were transfected into H9c2 cells using Lipofectamine® 2000 reagent (Thermo Fisher Scientific, Inc.), according to the manufacturer’s instructions. In brief, H9c2 cells (5×105) were plated in a 6-well plate and cultured for 24 h. When the cells reached 80% confluence, Lipofectamine 2000 was added to the medium without serum and incubated for 5 min at room temperature. The diluted siRNA was gently mixed with the medium containing Lipofectamine 2000 and incubated for 20 min at room temperature prior to adding the mixture to each well containing cells and medium. The transfected cells were cultured at 37°C in a CO2 incubator for 24 h prior to subsequent experiments. Untransfected cells were used as a blank control, and cells transfected with siNC were used as a negative control.
OGD/R cell model and Mel treatment
H9c2 cells were transfected with siPGC-1α, siNrf2 or siNC mimic 24 h prior to OGD stimulation. For the OGD/R experiments, when the transfected H9c2 cells reached 70–80% confluence, the culture medium was replaced by glucose-free DMEM (Thermo Fisher Scientific, Inc.) and the cells were cultured in an anaerobic chamber (95% N2 and 5% CO2) at 37°C for 4 h. The cells were then incubated with normal culture medium under normoxic conditions (95% air and 5% CO2) at 37°C for the indicated duration (6, 12, 24 and 48 h) as reperfusion. Different concentrations of Mel (0.01, 0.1, 1 and 10 mM; Merck KGaA) or 1 μg/ml TNF-α antibody (cat. no. 11948; Cell Signaling Technology, Inc.) was added into the culture medium at the initiation of reperfusion. Untreated cells were used as the control.
Cell viability assay
Cell viability was measured by Cell Counting Kit-8 (CCK-8; Beyotime Institute of Biotechnology) according to the manufacturer’s instructions. In brief, H9c2 cells were seeded into 96-well plates at a density of 1×104 cells/well. Following treatment, 10 μl CCK-8 solution was added to each well and incubated for 2 h at 37°C. The absorbance was measured at 450 nm using a microplate reader (Bio-Rad Laboratories, Inc.) to calculate the number of viable cells.
Measurement of lactate dehydrogenase (LDH) and creatine kinase myocardial band (CK-MB) activity
The levels of LDH and CK-MB in the supernatant of H9c2 cells were assessed using commercial kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s instructions. Briefly, H9c2 cells were subjected to 4 h of OGD followed by reperfusion for 24 h. Different concentrations of Mel (0.01, 0.1, 1 and 10 mM) were added to the culture medium at the initiation of reperfusion and incubated for the indicated durations (6, 12, 24 and 48 h). The cells were harvested and centrifuged at 250 × g at room temperature for 2 min. A total of 100 μl working solution was added to each well and reacted for 30 min at room temperature. The reaction was stopped by adding 50 μl stop solution to each well. Absorbance was measured at 450 and 340 nm using a microplate reader (Bio-Rad Laboratories, Inc.).
Flow cytometry to determine apoptosis
Apoptosis was measured using an Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) detection kit (BD Biosciences). Briefly, following the indicated treatments, H9c2 cells were harvested, washed thrice with cold PBS and resuspended in binding buffer (BD Biosciences), followed by staining with 5 μl Annexin V-FITC in the dark for 10 min at 37°C. The cells were then incubated with 10 μl PI solution in the dark for 30 min. The apoptotic cells were determined by flow cytometry (BD FACScalibur; BD Biosciences) and analyzed using CellQuest software (BD Biosciences).
Measurement of mitochondrial membrane potential (MMP)
5,5′, 6,6′-Tetracholoro-1,1′3,3′-tetraethybenzimidazole-carbocyanide iodine (JC-1; Beyotime Institute of Biotechnology) was used to measure the MMP of H9c2 cells according to the manufacturer’s instructions. Briefly, following the indicated treatments, H9c2 cells were harvested and resuspended in 1 ml culture medium (DMEM + FBS) with JC-1 fluorescent dye. Following incubation at 37°C for 20 min, the cells were centrifuged at 600 × g at 4°C for 3 min, and the supernatant was removed. The cells were resuspended in 1X JC-1 buffer for observation under a fluorescent microscope at ×100 magnification (Carl Zeiss AG). In each sample, five random fields were selected for observation and analysis.
Measurement of caspase-3 and caspase-9 activity
The activity of caspase-3 and caspase-9 in H9c2 cells subjected to different treatments was measured by Caspase Activity kits (Beyotime Institute of Biotechnology) using the substrate peptides acetyl-Asp-Glu-Val-Asp p-nitroanilide (Ac-DEVD-pNA) and acetyl-Leu-Glu-His-Asp p-nitroanilide (Ac-LEHD-pNA), respectively. In brief, following treatment, the cells were lysed using RIPA lysis buffer (Beyotime Institute of Biotechnology), and the supernatants were mixed with buffer (Beyotime Institute of Biotechnology) containing the substrate peptides and incubated at 37°C for 2 h. The release of pNA was quantified by determining the absorbance at 405 nm using a microplate reader (Bio-Rad Laboratories, Inc.). The caspase activity was expressed as a relative percentage of the control value.
Determination of ROS production
Intracellular ROS levels were monitored using 2′,7′-dichlorofluorescein diacetate (DCFH-DA; Thermo Fisher Scientific, Inc.) according to the manufacturer’s instructions. In brief, following the indicated treatments, H9c2 cells were loaded with 10 μM DCFH-DA at 37°C for 30 min and washed with PBS thrice. Absorbance was measured at 485 and 525 nm using a fluorescent microplate reader (Carl Zeiss AG) to determine the intensity of DCF fluorescence.
Measurement of malondialdehyde (MDA) production and superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) activity levels
The MDA content and SOD, CAT and GSH-Px activity levels were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute) according to the manufacturers’ instructions. In brief, following the indicated treatments, H9c2 cells were lysed using RIPA lysis buffer (Beyotime Institute of Biotechnology) and the supernatants were collected to determine MDA content and SOD, CAT and GSH-Px activity levels. The absorbance was measured at 530 (MDA), 450 (SOD), 405 (CAT) and 412 (GSH-Px) nm using a microplate reader (Bio-Rad Laboratories, Inc.).
Western blot analysis
Following treatment, H9c2 cells were washed twice with PBS, lysed in RIPA buffer (Beyotime Institute of Biotechnology) and centrifuged at 1,000 × g at 4°C for 3 min. The supernatants were collected and quantified for protein concentration using a BCA kit (Beyotime Institute of Biotechnology). Equal amounts (30 μg/lane) of protein from each sample were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (EMD Millipore). The membranes were blocked with 5% non-fat milk in TBST buffer (50 mM Tris, pH 7.4, 250 mM NaCl, 0.1% Tween-20) for 1 h at room temperature and probed with antibodies against PGC-1α (1:1,000; cat. no. ab54481), Nrf2 (1:2,000; cat. no. ab137550), HO-1 (1:1,000; cat. no. ab189491) and NQO1 (1:1,000; cat. no. ab97385; all from Abcam), caspase-3 (1:1,000; cat. no. 14220), cleaved (cl)-caspase-3 (1:1,000; cat. no. 9664), cytochrome c (1:1,000; cat. no. 11940), Bax (1:1,000; cat. no. 5023), Bcl-2 (1:1,000; cat. no. 3498), TNF-α (1:1,000; cat. no. 6945) and β-actin (1:1,000; cat. no. 4967; all from Cell Signaling Technology, Inc.) overnight at 4°C. Following washing with TBST thrice, the membranes were incubated with horseradish peroxidase-conjugated anti-rabbit IgG (1:2,000; cat. no. 7074; Cell Signaling Technology, Inc.) for 1 h at room temperature and visualized by enhanced chemiluminescence reaction reagents (EMD Millipore). Protein band densities were quantified using Quantity One software (Bio-Rad Laboratories, Inc.). The results were normalized to the internal control β-actin and calculated as fold-changes compared with the control group in each experiment.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from H9c2 cells following indicated treatments using TRIzol® reagent (Thermo Fisher Scientific Inc.). Complementary DNA was synthesized by RT reaction with PrimeScript RT Master Mix (Takara Biotechnology Co., Ltd.). The reverse transcription protocol used was 37°C for 15 min and 85°C for 30 sec. qPCR assay was performed using SYBR Premix Ex Taq kit (Takara Biotechnology Co., Ltd.) according to the manufacturer’s instructions. The amplification conditions were as follows: 95°C for 10 min, followed by 40 cycles of 95°C for 10 sec and 60°C for 60 sec. Reactions were conducted on the ABI Prism 7500 Real-Time PCR System (Thermo Fisher Scientific, Inc.). The mRNA levels were calculated using the 2−ΔΔCq method (26). GAPDH was used for normalization. The primers used were as follows: TNF-α forward, 5′-CCTCTTCTCATTCCTGCTCG-3′ and reverse, 5′-GGTATGAAATGGCAAATCGG-3′; interleukin (IL)-6 forward, 5′-CTGCGCAGCTTTAAGGAGTTC-3′ and reverse, 5′-TCTGAGGTGCCCATGCTACA-3′; IL-1β forward, 5′-CAACCAACAAGTGATATTCTCCATG-3′and reverse, 5′-GATCCACACTCTCCAGCTGCA-3′; IL-8 forward, 5′-GGCAGCCTTCCTGATTTCTG-3′ and reverse, 5′-CTTGGCAAAACTGCACCTTCA-3′; monocyte chemotactic protein-1 (MCP-1) forward, 5′-CTCTCGCCTCCAGCATGAA-3′ and reverse, 5′-GGGAATGAAGGTGGCTGCTA-3′; GAPDH forward, 5′-CCATCACCATCTTCCAGGAG-3′ and reverse, 5′-CCTGCTTCACCACCTTCTTG-3′.
Enzyme-linked immunosorbent assay (ELISA)
Following the indicated treatments, the culture media were collected and centrifuged at 600 × g at room temperature for 5 min. The levels of TNF-α, IL-6, IL-1β, IL-8 and MCP-1 in the supernatants were determined using commercially available ELISA kits (R&D systems, Inc.) according to the manufacturer’s instructions and expressed as pg/ml.
Statistical analysis
The data are presented as the mean ± SD from three independent experiments. The results were compared by one-way analysis of variance, followed by least significant difference post-hoc test using SPSS 16.0 software (SPSS, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Mel inhibits OGD/R-induced H9c2 cell injury
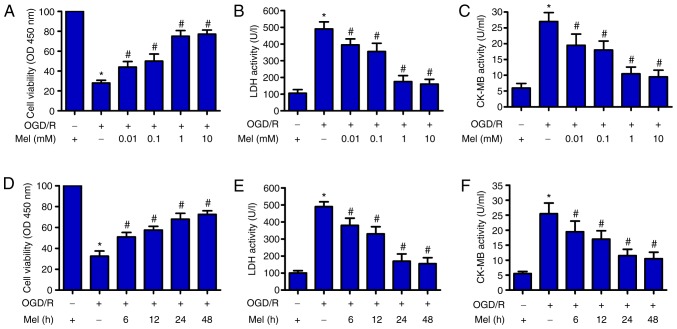
First, the potential effects of Mel on the viability of OGD/R-treated H9c2 cells were explored. CCK-8 assay results demonstrated that OGD/R significantly reduced the viability of H9c2 cells compared with that of the control group. Mel (0.01, 0.1, 1 and 10 mM) significantly attenuated the decrease in OGD/R-stimulated H9c2 cell viability (Fig. 1A). Next, the activity levels of LDH and CK-MB, two indicators of cardiomyocyte injury, were measured using commercial kits. A notable OGD/R-induced increase in the activity levels of LDH and CK-MB was observed in H9c2 cells, whereas Mel (0.01, 0.1, 1 and 10 mM) significantly prevented injury (Fig. 1B and C). In addition, Mel markedly enhanced cell viability (Fig. 1D) and decreased LDH and CK-MB activity (Fig. 1E and F) in OGD/R-exposed H9c2 cells. These results indicated that Mel protected H9c2 cells from OGD/R-induced damage. Mel (1 mM) treatment for 24 h was chosen for subsequent experiments due to the observed effects against OGD/R-induced H9c2 cell injury.
Figure 1.
Mel protects H9c2 cells from OGD/R-induced injury. (A–C) H9c2 cells were subjected to 4 h of OGD followed by reperfusion for 24 h. Mel (0.01, 0.1, 1 and 10 mM) was added to the culture medium at the initiation of reperfusion. (A) The viability of H9c2 cells was measured by CCK-8 assay. H9c2 cell injury was evaluated by measuring (B) LDH and (C) CK-MB activity. (D–F) H9c2 cells were subjected to 4 h of OGD, followed by reperfusion for the indicated durations (6, 12, 24 and 48 h). Mel (1 mM) was added to the culture medium at the initiation of reperfusion. (D) CCK-8 assay was conducted to determine cell viability. The activity of (E) LDH and (F) CK-MB was measured using commercial kits. Data are expressed as the mean ± SD from three independent experiments. *P<0.05 vs. control; #P<0.05 vs. OGD/R. Mel, melatonin; OGD/R, oxygen-glucose deprivation/reperfusion; CCK-8, Cell Counting Kit-8; LDH, lactate dehydrogenase; CK-MB, creatine kinase myocardial band; OD, optical density.
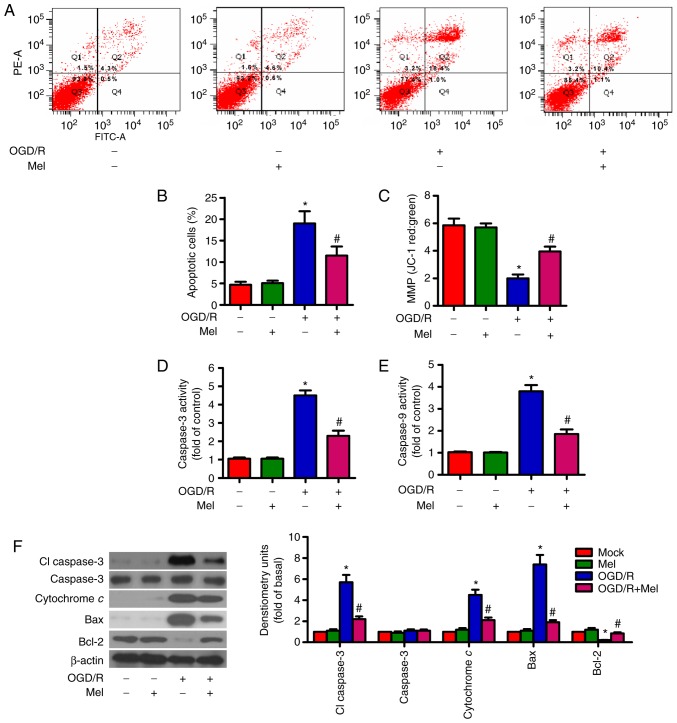
Mel suppresses OGD/R-induced apoptosis of H9c2 cells
OGD/R can result in cardiomyocyte apoptosis (27). Flow cytometry results demonstrated that OGD/R increased the percentage of apoptotic cells compared with that in the control group, whereas Mel reduced the OGD/R-induced apoptosis of H9c2 cells (Fig. 2A and B). The MMP in H9c2 cells subjected to OGD/R was decreased; however, this effect was markedly attenuated in the presence of Mel (Fig. 2C). To assess the involvement of caspase activation in the inhibition of OGD/R-induced apoptosis by Mel, the activity levels of caspase-3 and caspase-9 in the mitochondria-mediated apoptosis pathway were examined. Caspase-3 and caspase-9 activity levels were enhanced by OGD/R exposure compared with the control group (Fig. 2D and E). However, Mel significantly reduced the OGD/R-induced caspase-3 and caspase-9 activity. Consistent with the change in caspase-3 activity, the protein expression levels of cl-caspase-3 and cytochrome c were increased in OGD/R-insulted H9c2 cells. Mel reversed the upregulation of cl-caspase-3 and cytochrome c expression induced by OGD/R (Fig. 2F). Western blot analysis also revealed significant upregulation of Bax and downregulation of Bcl-2 following OGD/R insult in H9c2 cells, which was reversed by Mel treatment (Fig. 2F). These results suggested that Mel inhibited OGD/R-induced H9c2 cell apoptosis.
Figure 2.
Mel prevents OGD/R-induced apoptosis in H9c2 cells. H9c2 cells were subjected to 4 h of OGD, followed by reperfusion for 24 h. Mel (1 mM) was added to the culture medium at the initiation of reperfusion. (A) Flow cytometry was performed to analyze apoptosis. (B) Percentage of apoptotic cells in (A). (C) Measurement of MMP with JC-1 fluorescent staining. (D) Caspase-3 and (E) caspase-9 activities were determined using commercial kits. (F) Representative western blot results of cl-caspase-3, caspase-3, cytochrome c, Bax and Bcl-2. β-actin was used as the loading control. Data are expressed as the mean ± SD from three independent experiments. *P<0.05 vs. control; #P<0.05 vs. OGD/R. Mel, melatonin; OGD/R, oxygen-glucose deprivation/reperfusion; MMP, mitochondrial membrane potential; JC-1, 5,5′,6,6′-tetracholoro-1,1′3,3′-tetraethybenzimidazole-carbocyanide iodine; cl, cleaved.
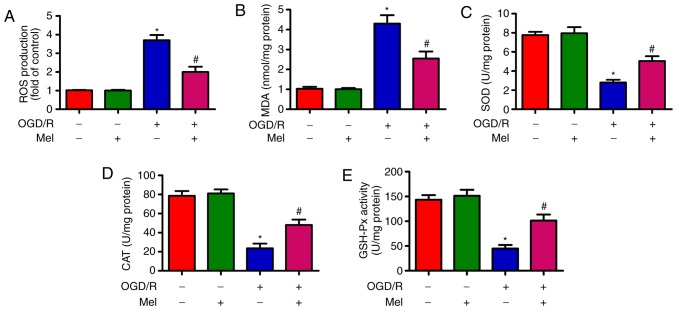
Mel reduces OGD/R-induced oxidative stress in H9c2 cells
OGD/R induces oxidative stress (28). As demonstrated in Fig. 3A, OGD/R promoted intracellular ROS production in H9c2 cells compared with that in the control group. Of note, Mel significantly inhibited OGD/R-induced ROS generation. In addition, the content of MDA, which is an indicator of lipid peroxidation, was significantly reduced by Mel in OGD/R-insulted H9c2 cells (Fig. 3B). Compared with the OGD/R group, Mel also significantly increased the activity of endogenous antioxidant enzymes, including SOD (Fig. 3C), CAT (Fig. 3D) and GSH-Px (Fig. 3E) in H9c2 cells. These results demonstrated that Mel exerted an antioxidant effect in OGD/R-insulted H9c2 cells.
Figure 3.
Mel reduces oxidative stress in OGD/R-exposed H9c2 cells. H9c2 cells were subjected to 4 h of OGD followed by reperfusion for 24 h. Mel (1 mM) was added to the culture medium at the initiation of reperfusion. (A) ROS production was detected using a 2′,7′-dichlorofluorescein diacetate fluorescent probe. (B) MDA content and the activity of (C) SOD, (D) CAT and (E) GSH-Px were measured using commercial kits. Data are expressed as the mean ± SD from three independent experiments. *P<0.05 vs. control; #P<0.05 vs. OGD/R. Mel, melatonin; OGD/R, oxygen-glucose deprivation/reperfusion; ROS, reactive oxygen species; MDA, malondialdehyde; SOD, superoxide dismutase; CAT, catalase; GSH-Px, glutathione peroxidase.
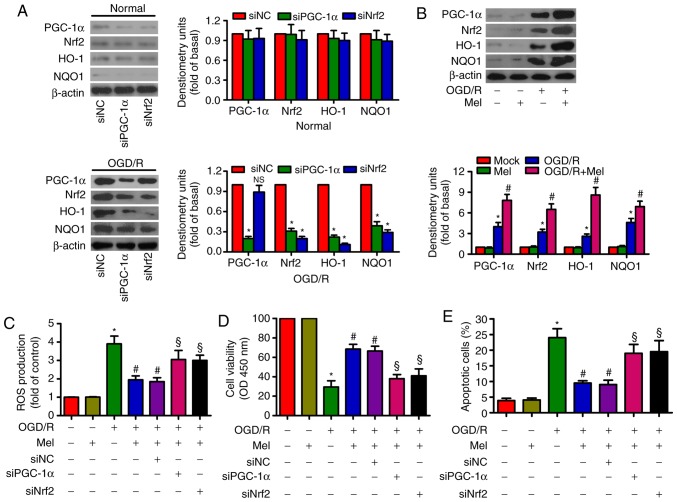
Mel elicits an antioxidant effect via the activation of PGC-1α/Nrf2 signaling in OGD/R-insulted H9c2 cells
PGC-1α knockdown reduced the expression of Nrf2, HO-1 and NQO-1, whereas Nrf2 knockdown suppressed HO-1 and NQO-1 expression in OGD/R-stimulated H9c2 cells (Fig. 4A). OGD/R led to an upregulation of PGC-1α, Nrf2, HO-1 and NOQ1 expression in H9c2 cells. Mel further enhanced the levels of PGC-1α, Nrf2, HO-1and NOQ1 in OGD/R-insulted H9c2 cells (Fig. 4B). In addition, the Mel-induced decrease in ROS generation in OGD/R-exposed H9c2 cells was attenuated by PGC-1α or Nrf2 silencing (Fig. 4C). PGC-1α or Nrf2 knockdown counteracted the Mel-induced increase in the viability of OGD/R-exposed H9c2 cells (Fig. 4D). PGC-1α or Nrf2 knockdown also reversed the Mel-mediated reduction in apoptosis in OGD/R-insulted H9c2 cells (Fig. 4E). These results demonstrated that the antioxidant effects of Mel were dependent on the activation of PGC-1α/Nrf2 signaling in OGD/R-insulted H9c2 cells.
Figure 4.
Mel activates PGC-1α/Nrf2 signaling to exert antioxidant effects on OGD/R-insulted H9c2 cells. (A) H9c2 cells were transfected with 100 nM siNC, siPGC-1αor siNrf2 for 24 h and subjected to 4 h of OGD, followed by reperfusion for 24 h. The expression of PGC-1α, Nrf2, HO-1 and NOQ1 was analyzed by western blotting. β-actin was used as an endogenous control. (B) H9c2 cells were subjected to 4 h of OGD followed by reperfusion for 24 h. Mel (1 mM) was added to the culture medium at the initiation of reperfusion. Western blot analysis was performed to detect the expression of PGC-1α, Nrf2, HO-1and NOQ1. β-actin was used as the endogenous control. (C–E) H9c2 cells were transfected with 100 nM siPGC-1α or siNrf2 for 24 h and subjected to 4 h of OGD, followed by reperfusion for 24 h. Mel (1 mM) was added to the culture medium at the initiation of reperfusion. (C) ROS production was assessed using a 2′,7′-dichlorofluorescein diacetate fluorescent probe. (D) Cell viability and (E) apoptosis were measured by Cell Counting Kit-8 and flow cytometry, respectively. Data are expressed as the mean ± SD from three independent experiments. *P<0.05 vs. control; #P<0.05 vs. OGD/R; §P<0.05 vs. OGD/R + Mel or OGD/R + Mel + siNC. Mel, melatonin; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1α; Nrf2, nuclear factor erythroid 2-related factor 2; HO-1, heme oxygenase-1; OGD/R, oxygen-glucose deprivation/reperfusion; ROS, reactive oxygen species; si, small interfering RNA; NC, negative control.
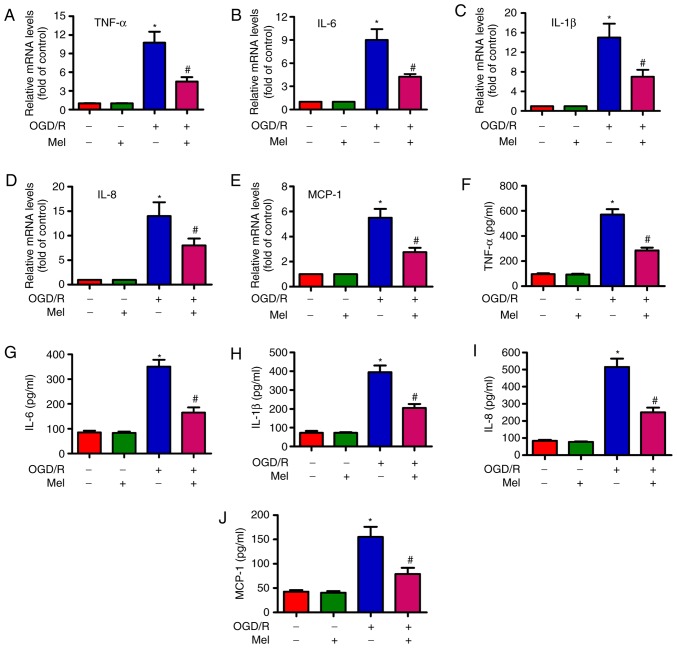
Mel represses the OGD/R-induced expression and release of pro-inflammatory cytokines in H9c2 cells
To examine whether Mel serves an inhibitory role in OGD/R-induced inflammation, the expression and release of several pro-inflammatory cytokines in H9c2 cells was measured by RT-qPCR and ELISA. The RT-qPCR results revealed that the expression of TNF-α (Fig. 5A), IL-6 (Fig. 5B), IL-1β (Fig. 5C), IL-8 (Fig. 5D) and MCP-1 (Fig. 5E) were considerably higher in the OGD/R-exposed H9c2 cells compared with those in the control cells, and that Mel partially reversed the OGD/R-induced increase in the TNF-α, IL-6, IL-1β, IL-8 and MCP-1 levels. Consistently, the production of TNF-α (Fig. 5F), IL-6 (Fig. 5G), IL-1β (Fig. 5H), IL-8 (Fig. 5I) and MCP-1 (Fig. 5J) was significantly suppressed by Mel in OGD/R-treated H9c2 cells. These results demonstrated that Mel reduced the levels of pro-inflammatory cytokines in OGD/R-exposed H9c2 cells.
Figure 5.
Mel reduces the level of pro-inflammatory cytokines in OGD/R-insulted H9c2 cells. H9c2 cells were subjected to 4 h of OGD followed by reperfusion for 24 h. Mel (1 mM) was added to the culture medium at the initiation of reperfusion. (A–E) The mRNA expression levels of (A) TNF-α, (B) IL-6, (C) IL-1β, (D) IL-8 and (E) MCP-1 were analyzed by reverse transcription-quantitative PCR. (F–J) The release of (F) TNF-α, (G) IL-6, (H) IL-1β, (I) IL-8 and (J) MCP-1 in the supernatants was measured by ELISA. Data are expressed as the mean ± SD from three independent experiments. *P<0.05 vs. control; #P<0.05 vs. OGD/R. Mel, melatonin; OGD/R, oxygen-glucose deprivation/reperfusion; TNF-α, tumor necrosis factor-α; IL, interleukin; MCP, monocyte chemotactic protein.
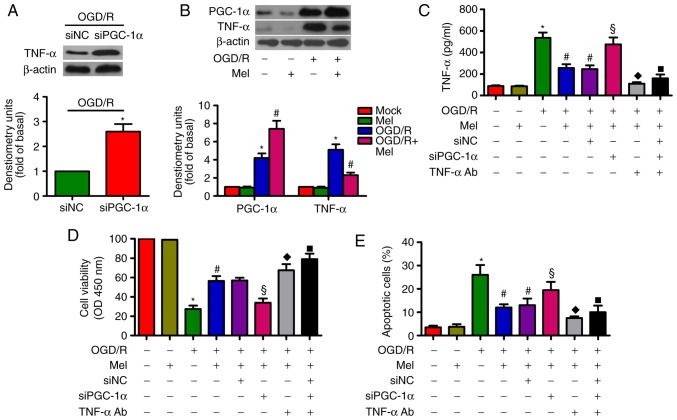
Mel prevents the OGD/R-induced apoptosis of H9c2 cells by regulating the PGC-1α/TNF-α signaling pathway
PGC-1α can decrease TNF-α production in myotubes via TNF-α stimulation (29). TNF-α is implicated in OGD/R-induced H9c2 cell apoptosis (27). As demonstrated in Fig. 6A, siPGC-1α transfection led to an upregulation of TNF-α expression in OGD/R-exposed H9c2 cells. OGD/R induced a significant increase in PGC-1α and TNF-α expression in H9c2 cells, whereas Mel further increased PGC-1α expression but reduced the level of TNF-α (Fig. 6B). In OGD/R-insulted H9c2 cells, the Mel-mediated decrease in the production of TNF-α was reversed by PGC-1α knockdown. However, the addition of the TNF-α antibody reduced TNF-α production (Fig. 6C). In addition, PGC-1α silencing resulted in a decrease in the Mel-induced enhancement in the viability of OGD/R-exposed H9c2 cells, which was rescued by the addition of the TNF-α antibody (Fig. 6D). Incubation with the TNF-α antibody reversed the PGC-1α depletion-induced increase in OGD/R-stimulated H9c2 cell apoptosis (Fig. 6E). These results revealed that Mel exerted an anti-apoptotic effect in OGD/R-treated H9c2 cells via the PGC-1α/TNF-α signaling pathway.
Figure 6.
Mel inhibits apoptosis in OGD/R-treated H9c2 cells by regulating PGC-1α/Nrf2 signaling. (A) H9c2 cells were transfected with 100 nM siNC or siPGC-1α for 24 h and subjected to 4 h of OGD, followed by reperfusion for 24 h. The expression of TNF-α was analyzed by western blot analysis. β-Actin was used as the endogenous control. (B) H9c2 cells were subjected to 4 h of OGD followed by reperfusion for 24 h. Mel (1 mM) was added to the culture medium at the initiation of reperfusion. Western blot analysis was conducted to detect the expression of PGC-1α and TNF-α. β-Actin was used as the endogenous control. (C–E) H9c2 cells were transfected with 100 nM siPGC-1α or treated with a TNF-α antibody (1 μg/ml) for 24 h and subjected to 4 h of OGD, followed by reperfusion for 24 h. Mel (1 mM) was added to the culture medium at the initiation of reperfusion. (C) TNF-α production was measured by ELISA. (D) Cell viability and (E) apoptosis were evaluated by Cell Counting Kit-8 and flow cytometry assays, respectively. Data are expressed as the mean ± SD from three independent experiments. *P<0.05 vs. control; #P<0.05 vs. OGD/R; §P<0.05 vs. OGD/R + Mel or OGD/R + Mel + siNC; ◆P<0.05 vs. OGD/R + Mel or OGD/R + Mel + siNC; ■P<0.05 vs. OGD/R + Mel or OGD/R + Mel + siNC. Mel, melatonin; OGD/R, oxygen-glucose deprivation/reperfusion; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator-1α; Nrf2, nuclear factor erythroid 2-related factor 2; TNF-α, tumor necrosis factor-α; TNF-α Ab, TNF-α antibody; si, small interfering RNA; NC, negative control.
Discussion
The main results of the present study were as follows: i) Mel exhibited cardioprotective effects in OGD/R-stimulated H9c2 cardiomyocytes, as confirmed by the increased cell viability and decreased LDH and CK-MB activity levels; ii) Mel reduced OGD/R-induced H9c2 cell apoptosis via the intrinsic apoptotic pathway; iii) Mel alleviated OGD/R-induced oxidative stress in H9c2 cells; iv) the antioxidant effects of Mel were mediated by activating PGC-1α/Nrf2 signaling in OGD/R-exposed H9c2 cells; v) Mel inhibited OGD/R-induced inflammatory response in H9c2 cells; vi) Mel elicited its antiapoptotic effects in OGD/R-insulted H9c2 cells via the regulation of the PGC-1α/TNF-α signaling pathway. Overall, Mel was demonstrated to inhibit OGD/R-induced H9c2 cardiomyocyte injury by inhibiting oxidative stress and inflammation.
Myocardial I/R injury is mainly caused by cardiomyocyte damage or death. LDH and CK-MB are constitutively expressed in myocardial cells and cannot shuttle across cytoplasmic membranes in the normal physiological state; however, they are released when cells are damaged or dead (30). Therefore, the activity of LDH and CK-MB in the culture media represents the extent of OGD/R-caused H9c2 cell injury. In the present study, decreased viability and increased LDH and CK-MB activity was observed in OGD/R-treated H9c2 cells. Mel protected the H9c2 cells from OGD/R-induced damage. Cardiomyocyte apoptosis serves a primary role in the pathogenesis of myocardial I/R injury (31). Myocardial apoptosis is a complicated process mediated by a series of enzymes and molecules, including the opening of the mitochondrial permeability transition pore, release of cytochrome c and activation of caspases (32). The Bcl-2 family of proteins have emerged as the key regulatory components of the apoptotic process; the Bcl-2 family comprises antiapoptotic proteins (such as Bcl-2 and Bcl-xL) and proapoptotic molecules (such as Bax and Bak), which function primarily to protect or disrupt the integrity of the mitochondrial membrane and control the release of proapoptotic proteins (33). Mitochondrial dysfunction and MMP loss are early events in the apoptotic process (34). I/R stimulation leads to the opening of the mitochondrial permeability transition pore and release of cytochrome c from the intermembrane space of mitochondria (35). Once released, cytochrome c binds to the cytosolic protein Apaf1 facilitating the formation of the apoptosome complex, which results in the activation of caspase-9 and subsequent activation of caspase-3; the activation of caspase-3 triggers apoptosis (36). In the present study, OGD/R led to a significant increase in the activity levels of caspase-3 and caspase-9, loss of MMP and apoptosis in H9c2 cells. However, Mel reduced the apoptotic rate and the activity levels of caspase-3 and caspase-9 and attenuated the loss of MMP. Mel decreased the levels of proapoptotic cl-caspase-3, cytochrome c and Bax and enhanced the antiapoptotic Bcl-2 expression in OGD/R-exposed H9c2 cells. These results suggested that Mel exerted antiapoptotic effects in OGD/R-injured cardiomyocytes.
Myocardial I/R injury induces oxidative stress, which causes apoptosis in cardiovascular cells (37). Excessive amounts of ROS during the reperfusion period may damage cell structure and facilitate myocardial apoptosis (38). MDA, which is a secondary product of lipid peroxidation, is a biomarker of oxidative stress and indicates free radical production and consequent tissue damage (39). Antioxidant enzymes, such as SOD, CAT and GSH-Px, act as a compensatory mechanism for hyperoxidation that protects against oxidative injury (40). However, myocardial I/R leads to the collapse of the antioxidant system and increases the vulnerability of the myocardium to oxygen free radicals (41). The results of the present study demonstrated that the myocardial protective effect of Mel was mediated by the inhibition of oxidative stress, as indicated by the reduction of ROS production and MDA content, and the enhancement of cellular antioxidant enzymes, including CAT, SOD and GSH-Px. Mitochondria are the principal sites of ROS production (42). PGC-1α and Nrf2 are the major regulators of mitochondrial biogenesis and activity (43,44). PGC-1α and Nrf2 regulate the expression of certain antioxidant-related genes, which remove ROS through sequential enzymatic reactions (11,12). A previous study has demonstrated that Mel ameliorated myocardial I/R injury by activating the 5′ AMP-activated protein kinase/PGC-1α/SIRT3 signaling pathway (23). In addition, Mel protected H9c2 cells against OGD/R-induced oxidative damage through the Nrf2/NQO1 pathway (24). A compound hydroxysafflor yellow A that interferes with the PGC-1α/Nrf2 pathway serves an antioxidant role in isoproterenol-damaged H9c2 cells (13). Consistent with these findings, the results of the present study demonstrated that the repressive effects of Mel on ROS generation and apoptosis in OGD/R-insulted H9c2 cells were attenuated by the knockdown of PGC-1α or Nrf2, which suggested that Mel inhibited oxidative stress and apoptosis in H9c2 cells by activating thePGC-1α/Nrf2 signaling pathway.
I/R injury induces the production and release of several pro-inflammatory cytokines and chemokines (27,45,46). TNF-α upregulation in an ischemic region induced TNF-α expression in the neighboring normal myocardium, which resulted in amplified cytokine effects (47). Of note, TNF-α induces the activation of nuclear factor-κB and subsequent upregulation of other inflammatory mediators (48). TNF-α is a pro-inflammatory cytokine that has a potent pro-apoptotic function through binding to its cell surface receptor TNFR1 (49). A neutralizing antibody against TNF-α has been demonstrated to exert antiapoptotic effects in cardiomyocytes (50). OGD/R resulted in a significant increase in the levels of TNF-α, IL-6 and MCP-1 in H9c2 cells (46). Yang et al (16) reported that several pro-inflammatory cytokines, including TNF-α, IL-1β, IL-6 and IL-8, are upregulated by OGD/R in H9c2 cells. Consistently with these results, high levels of TNF-α, IL-6, IL-1β, IL-8 and MCP-1 were observed in OGD/R-treated H9c2 cells. Mel reversed the increase in these pro-inflammatory mediators. Recently, the PPARγ/PGC-1α/TNF-α signaling pathway has been implicated in the inflammatory response of OGD/R-insulted H9c2 cells (16). In the present study, the Mel-mediated reduction in TNF-α release and apoptosis was counteracted by PGC-1α knockdown and rescued by TNF-α antibody treatment in OGD/R-exposed H9c2 cells. These data indicated that Mel suppressed the apoptosis of OGD/R-damaged H9c2 cells through the PGC-1α/TNF-α signaling pathway.
In conclusion, the results of the present study demonstrated that Mel inhibited OGD/R-induced oxidative stress, inflammation and apoptosis in H9c2 cells. Mechanistically, the Mel-induced protection of H9c2 cells was dependent on the PGC-1α/Nrf2 and PGC-1α/TNF-α signaling pathways. Overall, this study provided a deep insight into the protective effects of Mel against myocardial I/R injury.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
YG and ML contributed to the conception and design of the present study. WZ, KL and HW conducted all the experiments. WZ and KL drafted the manuscript and revised it critically for important intellectual content. WZ, HW and ML interpreted and analyzed the data. YG and ML provided final approval of the version to be published. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Steptoe A, Kivimäki M. Stress and cardiovascular disease. Nat Rev Cardiol. 2012;9:360–370. doi: 10.1038/nrcardio.2012.45. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Caspersen CJ. Sedentary behaviour and cardiovascular disease: A review of prospective studies. Int J Epidemiol. 2012;41:1338–1353. doi: 10.1093/ije/dys078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu NB, Wu M, Chen C, Fujino M, Huang JS, Zhu P, Li XK. Novel molecular targets participating in myocardial ischemia-reperfusion injury and cardioprotection. Cardiol Res Pract. 2019;2019 doi: 10.1155/2019/6935147. 6935147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heusch G. Molecular basis of cardioprotection: Signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674–699. doi: 10.1161/CIRCRESAHA.116.305348. [DOI] [PubMed] [Google Scholar]
- 5.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hausenloy DJ, Yellon DM. Targeting myocardial reperfusion injury-the search continues. N Engl J Med. 2015;373:1073–1075. doi: 10.1056/NEJMe1509718. [DOI] [PubMed] [Google Scholar]
- 7.Ibanez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65:1454–1471. doi: 10.1016/j.jacc.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44. doi: 10.1146/annurev.physiol.010908.163111. [DOI] [PubMed] [Google Scholar]
- 9.Cannistra M, Ruggiero M, Zullo A, Gallelli G, Serafini S, Maria M, Naso A, Grande R, Serra R, Nardo B. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int J Surg. 2016;33(Suppl 1):S57–S70. doi: 10.1016/j.ijsu.2016.05.050. [DOI] [PubMed] [Google Scholar]
- 10.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–H2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 11.Kärkkäinen O, Tuomainen T, Mutikainen M, Lehtonen M, Ruas JL, Hanhineva K, Tavi P. Heart specific PGC-1α deletion identifies metabolome of cardiac restricted metabolic heart failure. Cardiovasc Res. 2019;115:107–118. doi: 10.1093/cvr/cvy155. [DOI] [PubMed] [Google Scholar]
- 12.Zhou S, Sun W, Zhang Z, Zheng Y. The role of Nrf2-mediated pathway in cardiac remodeling and heart failure. Oxid Med Cell Longev. 2014;2014 doi: 10.1155/2014/260429. 260429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen M, Wang M, Yang Q, Wang M, Wang Z, Zhu Y, Zhang Y, Wang C, Jia Y, Li Y, Wen A. Antioxidant effects of hydroxysafflor yellow A and acetyl-11-keto-β-boswellic acid in combination on isoproterenol-induced myocardial injury in rats. Int J Mol Med. 2016;37:1501–1510. doi: 10.3892/ijmm.2016.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J, Xia F, Zhao H, Peng K, Liu H, Meng X, Chen C, Ji F. Dexmedetomidine-induced cardioprotection is mediated by inhibition of high mobility group box-1 and the cholinergic anti-inflammatory pathway in myocardial ischemia-reperfusion injury. PLoS One. 2019;14:e0218726. doi: 10.1371/journal.pone.0218726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen ZW, Qian JY, Ma JY, Chang SF, Yun H, Jin H, Sun AJ, Zou YZ, Ge JB. TNF-α-induced cardiomyocyte apoptosis contributes to cardiac dysfunction after coronary microembolization in mini-pigs. J Cell Mol Med. 2014;18:1953–1963. doi: 10.1111/jcmm.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Li J, Hou Z, Yu Y, Yu B. KLF5 overexpression attenuates cardiomyocyte inflammation induced by oxygen-glucose deprivation/ reperfusion through the PPARγ/PGC-1α/TNF-α signaling pathway. Biomed Pharmacother. 2016;84:940–946. doi: 10.1016/j.biopha.2016.09.100. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Sun Y, Yi W, Li Y, Fan C, Xin Z, Jiang S, Di S, Qu Y, Reiter RJ, Yi D. A review of melatonin as a suitable antioxidant against myocardial ischemia-reperfusion injury and clinical heart diseases. J Pineal Res. 2014;57:357–366. doi: 10.1111/jpi.12175. [DOI] [PubMed] [Google Scholar]
- 18.Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 19.Tordjman S, Chokron S, Delorme R, Charrier A, Bellissant E, Jaafari N, Fougerou C. Melatonin: Pharmacology, functions and therapeutic benefits. Curr Neuropharmaco. 2017;15:434–443. doi: 10.2174/1570159X14666161228122115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu L, Sun Y, Cheng L, Jin Z, Yang Y, Zhai M, Pei H, Wang X, Zhang H, Meng Q, et al. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: Role of SIRT1. J Pineal Res. 2014;57:228–238. doi: 10.1111/jpi.12161. [DOI] [PubMed] [Google Scholar]
- 21.Dwaich KH, Al-Amran FG, Al-Sheibani BI, Al-Aubaidy HA. Melatonin effects on myocardial ischemia-reperfusion injury: Impact on the outcome in patients undergoing coronary artery bypass grafting surgery. Int J Cardiol. 2016;221:977–986. doi: 10.1016/j.ijcard.2016.07.108. [DOI] [PubMed] [Google Scholar]
- 22.Yu L, Fan C, Li Z, Zhang J, Xue X, Xu Y, Zhou G, Yang Y, Wang H. Melatonin rescues cardiac thioredoxin system during ischemia-reperfusion injury in acute hyperglycemic state by restoring Notch1/Hes1/Akt signaling in a membrane receptor-dependent manner. J Pineal Res. 2017;62 doi: 10.1111/jpi.12375. [DOI] [PubMed] [Google Scholar]
- 23.Yu L, Gong B, Duan W, Fan C, Zhang J, Li Z, Xue X, Xu Y, Meng D, Li B, et al. Melatonin ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by preserving mitochondrial function: Role of AMPK-PGC-1α-SIRT3 signaling. Sci Report. 2017;7:41337. doi: 10.1038/srep41337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai M, Li B, Duan W, Jing L, Zhang B, Zhang M, Yu L, Liu Z, Yu B, Ren K, et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J Pineal Res. 2017;63 doi: 10.1111/jpi.12419. [DOI] [PubMed] [Google Scholar]
- 25.Gao L, Zhao YC, Liang Y, Lin XH, Tan YJ, Wu DD, Li XZ, Ye BZ, Kong FQ, Sheng JZ, Huang HF. The impaired myocardial ischemic tolerance in adult offspring of diabetic pregnancy is restored by maternal melatonin treatment. J Pineal Res. 2016;61:340–352. doi: 10.1111/jpi.12351. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Wu WY, Wang WY, Ma YL, Yan H, Wang XB, Qin YL, Su M, Chen T, Wang YP. Sodium tanshinone IIA silate inhibits oxygen-glucose deprivation/recovery-induced cardiomyocyte apoptosis via suppression of the NF-κB/TNF-α pathway. Br J Pharmacol. 2013;169:1058–1071. doi: 10.1111/bph.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H, Guan Q, Guo L, Zhang H, Pang X, Cheng Y, Zhang X, Sun Y. Gypenosides alleviate myocardial ischemia-reperfusion injury via attenuation of oxidative stress and preservation of mitochondrial function in rat heart. Cell Stress Chaperones. 2016;21:429–437. doi: 10.1007/s12192-016-0669-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eisele PS, Salatino S, Sobek J, Hottiger MO, Handschin C. The peroxisome proliferator-activated receptor γ coactivator 1α/β (PGC-1) coactivators repress the transcriptional activity of NF-κB in skeletal muscle cells. J Biol Chem. 2013;288:2246–2260. doi: 10.1074/jbc.M112.375253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gürgün C, Ildizli M, Yavuzgil O, Sin A, Apaydin A, Cinar C, Kültürsay H. The effects of short term statin treatment on left ventricular function and inflammatory markers in patients with chronic heart failure. Int J Cardiol. 2008;123:102–107. doi: 10.1016/j.ijcard.2006.11.152. [DOI] [PubMed] [Google Scholar]
- 31.Xia P, Liu Y, Cheng Z. Signaling pathways in cardiac myocyte apoptosis. Biomed Res Int. 2016;2016 doi: 10.1155/2016/9583268. 9583268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He X, Li S, Liu B, Susperreguy S, Formoso K, Yao J, Kang J, Shi A, Birnbaumer L, Liao Y. Major contribution of the 3/6/7 class of TRPC channels to myocardial ischemia/reperfusion and cellular hypoxia/reoxygenation injuries. Proc Natl Acad Sci USA. 2017;114:E4582–E4591. doi: 10.1073/pnas.1621384114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ola MS, Nawaz M, Ahsan H. Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem. 2011;351:41–58. doi: 10.1007/s11010-010-0709-x. [DOI] [PubMed] [Google Scholar]
- 34.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 35.Kinnally KW, Peixoto PM, Ryu SY, Dejean LM. Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim Biophys Acta. 18132011:616–622. doi: 10.1016/j.bbamcr.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–e339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- 37.Lorgis L, Zeller M, Dentan G, Sicard P, Richard C, Buffet P, L’Huillier I, Beer JC, Cottin Y, Rochette L, Vergely C. The free oxygen radicals test (FORT) to assess circulating oxidative stress in patients with acute myocardial infarction. Atherosclerosis. 2010;213:616–621. doi: 10.1016/j.atherosclerosis.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Bartz RR, Suliman HB, Piantadosi CA. Redox mechanisms of cardiomyocyte mitochondrial protection. Front Physiol. 2015;6:291. doi: 10.3389/fphys.2015.00291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ozer MK, Parlakpinar H, Cigremis Y, Ucar M, Vardi N, Acet A. Ischemia-reperfusion leads to depletion of glutathione content and augmentation of malondialdehyde production in the rat heart from overproduction of oxidants: Can caffeic acid phenethyl ester (CAPE) protect the heart? Mol Cell Biochem. 2005;273:169–175. doi: 10.1007/s11010-005-0551-8. [DOI] [PubMed] [Google Scholar]
- 40.Jain AK, Mehra NK, Swarnakar NK. Role of antioxidants for the treatment of cardiovascular diseases: Challenges and opportunities. Curr Pharm Des. 2015;21:4441–4455. doi: 10.2174/1381612821666150803151758. [DOI] [PubMed] [Google Scholar]
- 41.Vijayasarathy K, Shanthi Naidu K, Sastry BK. Melatonin metabolite 6-Sulfatoxymelatonin, Cu/Zn superoxide dismutase, oxidized LDL and malondialdehyde in unstable angina. Int J Cardiol. 2010;144:315–317. doi: 10.1016/j.ijcard.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 42.Kausar S, Wang F, Cui H. The role of mitochondria in reactive oxygen species generation and its implications for neurodegenerative diseases. Cells. 2018;7:E274. doi: 10.3390/cells7120274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gureev AP, Shaforostova EA, Popov VN. Regulation of mitochondrial biogenesis as a way for active longevity: Interaction between the Nrf2 and PGC-1α signaling pathways. Front Genet. 2019;10:435. doi: 10.3389/fgene.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai L, Wang M, Martin OJ, Leone TC, Vega RB, Han X, Kelly DP. A role for peroxisome proliferator-activated receptor γ coactivator 1 (PGC-1) in the regulation of cardiac mitochondrial phospholipid biosynthesis. J Biol Chem. 2014;289:2250–2259. doi: 10.1074/jbc.M113.523654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang R, Xu L, Zhang D, Hu B, Luo Q, Han D, Li J, Shen C. Cardioprotection of ginkgolide B on myocardial ischemia/reperfusion-induced inflammatory injury via regulation of A20-NF-κB pathway. Front Immunol. 2018;9:2844. doi: 10.3389/fimmu.2018.02844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma L, Liu H, Xie Z, Yang S, Xu W, Hou J, Yu B. Ginsenoside Rb3 protects cardiomyocytes against ischemia-reperfusion injury via the inhibition of JNK-mediated NF-κB pathway: A mouse cardiomyocyte model. PLoS One. 2014;9:e103628. doi: 10.1371/journal.pone.0103628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saito Y, Watanabe K, Fujioka D, Nakamura T, Obata JE, Kawabata K, Watanabe Y, Mishina H, Tamaru S, Kita Y, et al. Disruption of group IVA cytosolic phospholipase A(2) attenuates myocardial ischemia-reperfusion injury partly through inhibition of TNF-α-mediated pathway. Am J Physiol Heart Circ Physiol. 2012;302:H2018–H2030. doi: 10.1152/ajpheart.00955.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gray CB, Suetomi T, Xiang S, Mishra S, Blackwood EA, Glembotski CC, Miyamoto S, Westenbrink BD, Brown JH. CaMKIIδ subtypes differentially regulate infarct formation following ex vivo myocardial ischemia/reperfusion through NF-κB and TNF-α. J Mol Cell Cardiol. 2017;103:48–55. doi: 10.1016/j.yjmcc.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qian Q, Cao X, Wang B, Qu Y, Qian Q, Sun Z, Feng F. TNF-α-TNFR signal pathway inhibits autophagy and promotes apoptosis of alveolar macrophages in coal worker’s pneumoconiosis. J Cell Physiol. 2019;234:5953–5963. doi: 10.1002/jcp.27061. [DOI] [PubMed] [Google Scholar]
- 50.Li S, Jiao X, Tao L, Liu H, Cao Y, Lopez BL, Christopher TA, Ma XL. Tumor necrosis factor-alpha in mechanic trauma plasma mediates cardiomyocyte apoptosis. Am J Physiol Heart Circ Physiol. 2007;293:H1847–H1852. doi: 10.1152/ajpheart.00578.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.