Abstract
Background
Several rehabilitation programmes are available for individuals after lumbar disc surgery.
Objectives
To determine whether active rehabilitation after lumbar disc surgery is more effective than no treatment, and to describe which type of active rehabilitation is most effective. This is the second update of a Cochrane Review first published in 2002.
First, we clustered treatments according to the start of treatment. 1. Active rehabilitation that starts immediately postsurgery. 2. Active rehabilitation that starts four to six weeks postsurgery. 3. Active rehabilitation that starts longer than 12 months postsurgery.
For every cluster, the following comparisons were investigated. A. Active rehabilitation versus no treatment, placebo or waiting list control. B. Active rehabilitation versus other kinds of active rehabilitation. C. Specific intervention in addition to active rehabilitation versus active rehabilitation alone.
Search methods
We searched CENTRAL (2013, Issue 4) and MEDLINE, EMBASE, CINAHL, PEDro and PsycINFO to May 2013.
Selection criteria
We included only randomised controlled trials (RCTs).
Data collection and analysis
Pairs of review authors independently assessed studies for eligibility and risk of bias. Meta‐analyses were performed if studies were clinically homogeneous. The GRADE approach was used to determine the overall quality of evidence.
Main results
In this update, we identified eight new studies, thereby including a total of 22 trials (2503 participants), 10 of which had a low risk of bias. Most rehabilitation programmes were assessed in only one study. Both men and women were included, and overall mean age was 41.4 years. All participants had received standard discectomy, microdiscectomy and in one study standard laminectomy and (micro)discectomy. Mean duration of the rehabilitation intervention was 12 weeks; eight studies assessed six to eight‐week exercise programmes, and eight studies assessed 12 to 13‐week exercise programmes. Programmes were provided in primary and secondary care facilities and were started immediately after surgery (n = 4) or four to six weeks (n = 16) or one year after surgery (n = 2). In general, the overall quality of the evidence is low to very low. Rehabilitation programmes that started immediately after surgery were not more effective than their control interventions, which included exercise. Low‐ to very low‐quality evidence suggests that there were no differences between specific rehabilitation programmes (multidisciplinary care, behavioural graded activity, strength and stretching) that started four to six weeks postsurgery and their comparators, which included some form of exercise. Low‐quality evidence shows that physiotherapy from four to six weeks postsurgery onward led to better function than no treatment or education only, and that multidisciplinary rehabilitation co‐ordinated by medical advisors led to faster return to work than usual care. Statistical pooling was performed only for three comparisons in which the rehabilitation programmes started four to six weeks postsurgery: exercise programmes versus no treatment, high‐ versus low‐intensity exercise programmes and supervised versus home exercise programmes. Very low‐quality evidence (five RCTs, N = 272) shows that exercises are more effective than no treatment for pain at short‐term follow‐up (standard mean difference (SMD) ‐0.90; 95% confidence interval (CI) ‐1.55 to ‐0.24), and low‐quality evidence (four RCTs, N = 252) suggests that exercises are more effective for functional status on short‐term follow‐up (SMD ‐0.67; 95% CI ‐1.22 to ‐0.12) and that no difference in functional status was noted on long‐term follow‐up (three RCTs, N = 226; SMD ‐0.22; 95% CI ‐0.49 to 0.04). None of these studies reported that exercise increased the reoperation rate. Very low‐quality evidence (two RCTs, N = 103) shows that high‐intensity exercise programmes are more effective than low‐intensity exercise programmes for pain in the short term (weighted mean difference (WMD) ‐10.67; 95% CI ‐17.04 to ‐4.30), and low‐quality evidence (two RCTs, N = 103) shows that they are more effective for functional status in the short term (SMD ‐0.77; 95% CI ‐1.17 to ‐0.36). Very low‐quality evidence (four RCTs, N = 154) suggests no significant differences between supervised and home exercise programmes for short‐term pain relief (SMD ‐0.76; 95% CI ‐2.04 to 0.53) or functional status (four RCTs, N = 154; SMD ‐0.36; 95% CI ‐0.88 to 0.15).
Authors' conclusions
Considerable variation was noted in the content, duration and intensity of the rehabilitation programmes included in this review, and for none of them was high‐ or moderate‐quality evidence identified. Exercise programmes starting four to six weeks postsurgery seem to lead to a faster decrease in pain and disability than no treatment, with small to medium effect sizes, and high‐intensity exercise programmes seem to lead to a slightly faster decrease in pain and disability than is seen with low‐intensity programmes, but the overall quality of the evidence is only low to very low. No significant differences were noted between supervised and home exercise programmes for pain relief, disability or global perceived effect. None of the trials reported an increase in reoperation rate after first‐time lumbar surgery. High‐quality randomised controlled trials are strongly needed.
Keywords: Female, Humans, Male, Exercise Therapy, Lumbar Vertebrae, Diskectomy, Diskectomy/methods, Diskectomy/rehabilitation, Intervertebral Disc, Intervertebral Disc/surgery, Laminectomy, Laminectomy/rehabilitation, Postoperative Period, Randomized Controlled Trials as Topic, Recovery of Function
Plain language summary
Rehabilitation after surgery for herniation of the lumbar disc
Review question
We reviewed the evidence on the effects of rehabilitation programmes on pain, recovery, function and return to work in people who have had lumbar disc surgery.
Background
A 'slipped' or 'herniated' disc is thought to be the most common cause of leg pain associated with a 'pinched' or compressed nerve in the lower back. Many patients are treated with a combination of non‐surgical measures such as medication or physiotherapy. Patients with persistent symptoms may undergo surgery. Although 78% to 95% of patients will improve after surgery, some will continue to have symptoms. It is estimated that 3% to 12% of patients who have disc surgery will have recurrent symptoms, and most of these patients will have surgery again.
Rehabilitation programmes, such as exercise therapy by a physiotherapist and advice to return to normal activities like returning to work, are common approaches after surgery.
Study characteristics
This updated review evaluated the effectiveness of various rehabilitation programmes for patients who had lumbar disc surgery for the first time. We included 22 randomised controlled trials with 2503 participants, both men and women, between the ages of 18 and 65 years. The evidence is current to May 2013. Most commonly, treatment started four to six weeks after surgery, but the start of treatment ranged from two hours to 12 months after surgery. Considerable variation in the content, duration and intensity of treatments (i.e. exercise programmes) has been noted. The duration of the interventions varied from two weeks to one year; most programmes lasted six to 12 weeks. Participants reported on average serious pain intensity (56 points on a zero to 100 scale, with 100 being the worst possible pain). Most studies compared (1) exercise versus no treatment, (2) high‐intensity exercise versus low‐intensity exercise or (3) supervised exercise versus home exercise, most commonly starting four to six weeks after surgery. Comparisons in this review included (1) exercise versus no treatment, (2) high‐intensity versus low‐intensity exercise and (3) supervised versus home exercise.
Key results
Patients who participated in exercise programmes four to six weeks after surgery reported slightly less short‐term pain and disability than those who received no treatment. Patients who participated in high‐intensity exercise programmes reported slightly less short‐term pain and disability than those participating in low‐intensity exercise programmes. Patients in supervised exercise programmes reported little or no difference in pain and disability compared with those in home exercise programmes. Here it was difficult to draw firm conclusions in the absence of high‐quality evidence.
None of the trials reported an increase in reoperation rate after first‐time lumbar surgery.
The evidence does not show whether all patients should be treated after surgery or only those who still have symptoms four to six weeks later.
Quality of the evidence
Limitations in the methods of half of the trials suggest that the results should be read with caution. Most of the treatments were assessed in only one trial. Therefore for most of the interventions, only low‐ to very low‐quality evidence indicates that no firm conclusions can be drawn regarding their effectiveness.
Summary of findings
Summary of findings for the main comparison. Exercise therapy versus no treatment four to six weeks after lumbar disc surgery.
| Exercise compared with no treatment for patients after lumbar disc surgery | |||||
|
Patient or population: patients four to six weeks after lumbar disc surgery Settings: primary care facilities and outpatient clinics Intervention: exercise Comparison: no treatment | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| No treatment | Exercise | ||||
| Pain (post‐treatment) VAS or LBPRS Follow‐up: mean three months | Mean pain (post‐treatment) ranged across control groups from 3.25 to 42.9 VAS points or 12.13 LBPRS points | Mean pain (post‐treatment) in the intervention groups was 0.90 standard deviations lower (1.55 to 0.42 lower)1 | 272 (five studies) | ⊕⊝⊝⊝ very low2,3,4 | |
| Functional status (post‐treatment) ODI or LBPRS Follow‐up: mean three months | Mean functional status (post‐treatment) ranged across control groups from 15.1 to 23 ODI points or 10.95 LBPRS points |
Mean functional status (post‐treatment) in the intervention groups was 0.67 standard deviations lower (1.22 to 0.12 lower)5 | 252 (four studies) | ⊕⊕⊝⊝ low2,4 | |
| Functional status (long term) ODI or LBPRS Follow‐up: mean one year | Mean functional status (long term) ranged across control groups from 12 to 28 ODI points or 11.37 LBPRS points |
Mean functional status (long term) in the intervention groups was 0.22 standard deviations lower (0.49 lower to 0.04 higher)6 | 226 (three studies) | ⊕⊕⊝⊝ low2,4 | |
| CI: Confidence interval; VAS: Visual analogue scale; LBPRS: Low Back Pain Rating Scale; ODI: Oswesty Disabiliy Index | |||||
| Grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. No evidence: No RCTs were identified that addressed this outcome. | |||||
1Large effect size. 2Less than 75% of participants are from low risk of bias studies. 3Statistical inconsistency. 4Number of participants smaller than optimal information size. 5Medium effect size. 6Small effect size.
Summary of findings 2. High‐intensity exercise versus low‐intensity exercise programmes four to six weeks after lumbar disc surgery.
| High‐intensity exercise compared with low‐intensity exercise for participants four to six weeks after lumbar disc surgery | |||||
|
Patient or population: patients four to six weeks after lumbar disc surgery Settings: primary care facilities and outpatient clinics Intervention: high‐intensity exercise Comparison: low‐intensity exercise | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Low‐intensity exercise | High‐intensity exercise | ||||
| Pain (short term) VAS Follow‐up: mean three months | Mean pain (short term) in the control groups was 25.64 VAS points | Mean pain (short term) in the intervention groups was 10.67 lower (17.04 to 4.3 lower)1 | 103 (two studies) | ⊕⊝⊝⊝ very low2,3,4 | |
| Function (short term) RDQ or ODI Follow‐up: mean three months | Mean function (short term) ranged across control groups from 6.1 RDQ points to 11.65 ODI points | Mean function (short term) in the intervention groups was 0.77 standard deviations lower (1.17 to 0.36 lower)5 | 103 (two studies) | ⊕⊕⊝⊝ low2,4 | |
| Functional status (long term) | NA | NA | NA | no evidence | This outcome was not measured |
| CI: Confidence interval; VAS: Visual analogue scale; RDQ: Roland‐Morris Disability QuestionnaireODI: Oswestry Disability Questionnaire | |||||
| Grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. No evidence: No RCTs were identified that addressed this outcome. | |||||
1Lower than clinical significance level (30 mm). 2Less than 75% of participants are from low risk of bias studies. 3Statistical inconsistency. 4Number of participants smaller than optimal information size. 5Small effect size.
Summary of findings 3. Supervised programmes versus home exercises four to six weeks after lumbar disc surgery.
| Supervised programmes compared with home exercises for participants four to six weeks after lumbar disc surgery | |||||
|
Patient or population: patients four to six weeks after lumbar disc surgery Settings: primary care facilities and outpatient clinics Intervention: supervised exercise programmes Comparison: home exercises | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Home exercises | Supervised programmes | ||||
| Pain (short term) VAS or five‐point box scale Follow‐up: mean three months | Mean pain (short term) ranged across control groups from 4.3 to 29.3 VAS points or 2.4 on a five‐point box scale |
Mean pain (short term) in the intervention groups was 0.76 standard deviations lower (2.04 lower to 0.53 higher)1 | 229 (five studies) | ⊕⊝⊝⊝ very low2,3,4 | |
| Functional status (short term) ODI or 12‐item scale Follow‐up: mean three months | Mean functional status (short term) ranged across control groups from 11.65 to 30.6 ODI points or zero on a 12‐item scale |
Mean functional status (short term) in the intervention groups was 0.36 standard deviations lower (0.88 lower to 0.15 higher)5 | 229 (five studies) | ⊕⊝⊝⊝ very low2,3,4 | |
| Functional status (long term) | NA | NA | NA | no evidence | This outcome was not measured |
| CI: Confidence interval; VAS: Visual analogue scale; ODI: Oswestry Disability Index | |||||
| Grades of evidence.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. No evidence: No RCTs were identified that addressed this outcome. | |||||
1Medium effect size. 2Less than 75% of participants are from low risk of bias studies. 3Statistical inconsistency. 4Number of participants smaller than optimal information size. 5Small effect size.
Background
Description of the condition
The lumbosacral radicular syndrome (LRS) is characterised by lower limb pain radiating in an area of the leg served by one or more lumbosacral nerve roots. Sometimes neurological phenomena such as sensory and motor deficits are present. The prevailing view is that the condition is most commonly caused by a lumbar disc herniation; however, other pathologies may also cause LRS. In the Netherlands, the incidence of sciatica has increased from 75,000 to 85,000 cases per year over the past decade (HCN 1999; van Beek 2010). Direct and indirect costs of patients suffering from sciatica approximate EUR 1.2 billion per year (HCN 1999). Many patients with LRS are treated conservatively, but surgery is a common option in patients with persistent symptoms. Surgery rates vary across countries. In the Netherlands, with a population of about 16 million people, it is estimated that about 12,000 operations for herniated lumbar discs are performed each year (van Beek 2010). In the UK, lumbar disc excisions were performed 9694 times in 2011‐2012 in National Health Service (NHS) hospitals (HESonline 2012) that serve an estimated English population of 53 million (ONS 2011). In the United States—population about 287 million (USCB 2002)—an estimated 287,122 lumbar discectomies were performed (Sherman 2010). But even within one country, considerable regional variations are reported (van Beek 2010; Weinstein 2006).
The reported success rate of lumbar disc surgery varies from 78% to 95% at one to two years postoperatively (Arts 2009; Hoogland 2006; Peul 2007; Rasmussen 2008; Ruetten 2008; Weinstein 2006b) and from 46% to 75% at six to eight weeks postoperatively (Arts 2009; Peul 2007; Weinstein 2006b). Differences between these studies with regard to inclusion criteria, indications for surgery and operationalisation of success may account for the wide range in success rate. Still, these figures show that at long‐term follow‐up in up to 22% of patients, the results of surgery are unsatisfactory, and patients still have symptoms. These persisting symptoms mainly consist of pain, motor deficits, a decreased functional status, not being able to return to work or any combination. In 3% to 12% of patients who undergo disc surgery for the first time, a recurrent herniated lumbar disc occurs, for which almost all patients undergo a reoperation (CBO 2008). Recently, the role of magnetic resonance imaging (MRI) assessment of disc herniation performed at one‐year follow‐up in patients who had been treated for sciatica and lumbar disc herniation has been criticised, as the MRI did not distinguish between those with a favourable outcome and those with an unfavourable outcome. So MRI evidence of reherniation should be interpreted with caution (el Barzouhi 2013).
Description of the intervention
For our review, active rehabilitation programmes after lumbar disc surgery include exercise therapy, strength and mobility training, physiotherapy and multidisciplinary programmes, which may include elements of back schools and ergonomics aiming at, for example, motor control modification, resumption of activities of daily living including work and physical activity and enhancement of pain coping strategies. These programmes may consist of individual sessions, group training or education or a combination of these.
How the intervention might work
The mechanisms explaining the effects of exercise therapy remain largely unclear. Local biomechanical changes and more central mechanisms may play a role. Central effects include changes due to correction of a distorted body schema or altered cortical representation of the back, as well as modification of motor control patterns. Other factors that may affect outcome include the therapist–patient relationship, changes in fear‐avoidance beliefs, catastrophising and self efficacy regarding pain control (Steiger 2012).
Why it is important to do this review
Further treatment is often recommended after lumbar disc surgery (e.g. physiotherapy, rehabilitation programmes), but persistent controversies are ongoing about many issues related to postsurgical rehabilitation. First of all, the necessity and duration of activity restrictions after lumbar disc surgery remain controversial. Second, the question continues regarding whether all patients should receive further treatment immediately after surgery, or only those patients who still suffer from persisting symptoms six to eight weeks after surgery. A diversity of rehabilitation programmes are available, but a systematic overview is lacking. In this updated review, we therefore systematically evaluated the effectiveness of active treatments used in rehabilitation after first‐time lumbar disc surgery.
Objectives
To determine whether active rehabilitation after lumbar disc surgery is more effective than no treatment, and to describe which type of active rehabilitation is most effective. This is the second update of a Cochrane Review first published in 2002.
First, we clustered treatments according to the start of treatment. 1. Active rehabilitation that starts immediately postsurgery. 2. Active rehabilitation that starts four to six weeks postsurgery. 3. Active rehabilitation that starts longer than 12 months postsurgery.
For every cluster, the following comparisons were investigated. a. Active rehabilitation versus no treatment, placebo or waiting list control. b. Active rehabilitation versus other kinds of active rehabilitation. c. Specific intervention in addition to active rehabilitation versus active rehabilitation alone.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) were included, and non‐randomised controlled trials (CCTs) or quasi‐RCTs were excluded.
Types of participants
Participants aged between 18 and 65 years who had first‐time lumbar disc surgery because of a lumbar disc prolapse were included. All types of surgical techniques for lumbar disc herniation (e.g. standard discectomy, microdiscectomy, laser discectomy, chemonucleolysis) were included.
Types of interventions
For our review, active rehabilitation programmes after lumbar disc surgery include exercise therapy, strength and mobility training, physiotherapy and multidisciplinary programmes, which may include elements of back schools and ergonomics aiming at, for example, motor control modification, resumption of activities of daily living including work and physical activity and enhancement of pain coping strategies. These programmes may consist of individual sessions, group training or education or a combination of these.
Types of outcome measures
Trials were included if they used at least one of the four primary outcome measures that we considered to be important, that is, pain (e.g. visual analogue scale (VAS)), a global measure of improvement (overall improvement, proportion of participants recovered, subjective improvement of symptoms), back pain–specific functional status (e.g. Roland‐Morris Disability Questionnaire (RDQ), Oswestry Disability Index (ODI)) and return to work (return‐to‐work status, days off work). Outcomes of physical examination (e.g. spinal range of motion, straight‐leg raise range of motion, muscle strength), behavioural outcomes (e.g. anxiety, depression, pain behaviour) and generic functional status (Short Form (SF)‐36, Nottingham Health Profile, Sickness Impact Profile) were considered as secondary outcomes. Other outcomes such as medication use, reherniation, reoperation and adverse effects were also considered.
Search methods for identification of studies
All relevant trials meeting our inclusion criteria were identified by:
a search of CENTRAL (2013, Issue 4);
a computer‐aided search of MEDLINE (from 1966 to June 2013), EMBASE (from 1988 to June 2013), CINAHL (from 2000 to June 2013), PsycINFO (from 1984 to June 2013) and PEDro (from 1965 to June 2013) databases using the search strategy recommended by the Editorial Board of the Cochrane Back Review Group (Furlan 2009). Specific search terms for low back pain, leg pain, lumbar disc surgery and postsurgery treatment were added. No language restriction was used. The complete search strategies for the five databases are outlined in Appendix 1, Appendix 2, Appendix 3, Appendix 4 and Appendix 5,
screening of references given in relevant reviews and identified trials; and
screening of personal bibliographies and communication with experts in the field.
Data collection and analysis
Selection of studies
Pairs of review authors (initial review MWvT, HCWdV; first update LOPC, RWJGO; second update LOPC, TO) independently selected the studies to be included in this systematic review by applying the selection criteria to studies that were retrieved by the literature search. Consensus was used to resolve disagreements concerning selection and inclusion of studies, and a third review author (initial review P Leffers, first update CGM, second update RWJGO) was consulted if disagreements persisted.
Data extraction and management
Pairs of review authors (initial review MR Kerckhoffs, RWJGO; first update LOPC, CGM, RWJGO, MWvT; second update LOPC, TO) independently extracted data from the studies using a standardised form. All pairs of review authors who extracted data first piloted the data extraction form by using two RCTs on back pain without surgery. The domains assessed for data extraction were characteristics of participants and interventions, as well as results on primary and secondary outcome measures. Appendix 6 shows the questions used to extract data to assess clinical relevance. The results of the clinical relevance assessment are presented in Table 4.
1. Results of clinical relevance assessment.
| Study | Patients | Interventions | Relevant outcomes | Size of effect | Benefits and harms |
| Alaranta 1986 | N | N | Y | N | N |
| Choi 2005 | Y | ? | Y | N | N |
| Danielsen 2000 | Y | Y | Y | Y | Y |
| Dolan 2000 | Y | Y | Y | Y | ? |
| Donaldson 2006 | Y | Y | Y | N | N |
| Donceel 1999 | Y | Y | ? | Y | ? |
| Erdogmus 2007 | Y | Y | Y | N | N |
| Filiz 2005 | Y | Y | Y | N | N |
| Hakkinen 2005 | N | Y | Y | N | N |
| Johannsen 1994 | Y | Y | Y | N | N |
| Johansson 2009 | Y | Y | Y | N | N |
| Kjellby‐Wendt 1998 | Y | Y | Y | N | N |
| Kulig 2009 | Y | Y | N | ? | ? |
| Manniche 1993a | Y | Y | Y | ? | ? |
| Manniche 1993b | Y | Y | Y | ? | ? |
| McGregor 2011 | Y | Y | Y | N | N |
| Newsome 2009 | Y | Y | Y | N | N |
| Ostelo 2003 | Y | Y | Y | N | N |
| Scrimshaw 2001 | Y | Y | Y | N | N |
| Timm 1994 | Y | Y | N | ? | ? |
| Yilmaz 2003 | Y | N | Y | Y | ? |
Assessment of risk of bias in included studies
The risk of bias of the included studies was assessed by using the criteria recommended in the updated method guidelines of the Cochrane Back Review Group (Furlan 2009) (see Appendix 7, for criteria). Pairs of review authors (initial review MRK, RWJGO; first update LOPC, CGM, RWJGO, MWvT; second update LOPC, TO) independently assessed the risk of bias of included studies. For each study, the risk of bias criteria were rated as high, low or unclear and were entered into the risk of bias table. Studies with a low risk of bias were defined as RCTs that fulfilled six or more of the risk of bias criteria.
RWJGO and CGM were not involved in the methodological quality assessment or any other decision regarding the trials (Ostelo 2003; Scrimshaw 2001) on which they served as authors. We decided not to blind studies for authors, institution or journal because the review authors who assessed the risk of bias were familiar with the literature. A consensus method was used to resolve disagreements, and a third review author (initial review PL, first update CGM, second update RWJGO) was consulted if disagreements persisted. If the article did not contain enough information to assess all risks of bias (i.e. if one or more criteria were scored as "unclear"), the study authors were contacted for additional information. The risk of bias assessment form was mailed to all study authors, and they were asked whether they agreed with the risk of bias assessment.
Assessment of heterogeneity
Assessment of heterogeneity was based on I2 tests. Results were combined in a meta‐analysis if I2 ≤ 50%. If I2 > 50%, we assessed how serious heterogeneity was by inspecting the forest plots (opposite directions of effect, too little or no overlap in confidence intervals). If the heterogeneity was thought not to be too serious, a random‐effects model was used to pool the data, to take heterogeneity into account. If substantial statistical or clinical heterogeneity (study population, types of treatments, outcomes and measurement instruments) was present, the results were not combined but were presented by a narrative synthesis and description of characteristics in the table showing the studies included.
Data synthesis
If studies were clinically homogeneous regarding study population, types of treatment and reference treatment and outcomes and measurement instruments, a meta‐analysis was performed. If possible, we calculated the weighted mean difference (WMD) because this improves the interpretability of the results. If a WMD was not possible, the standardised mean difference (SMD) was calculated. If studies were clinically too heterogeneous, no meta‐analysis was performed. We used the GRADE approach to assess the overall quality of the evidence per outcome (Guyatt 2011). Factors that may decrease the quality of the evidence include study design and risk of bias, inconsistency of results, indirectness (not generalisable), imprecision (sparse data: A general rule of thumb suggests an optimal information size of n > 300 for dichotomous data and n > 400 for continuous data (Higgins 2011)) and other factors (e.g. reporting bias). The quality of the evidence for a specific outcome was reduced by a level according to the performance of the studies against these five factors. We slightly modified the cutoff point for reducing one level based on the risk of bias assessment. We used the limit of at least 75% of participants coming from low risk of bias studies, rather than 75% of RCTs having low risk of bias.
High‐quality evidence: Consistent findings were reported among at least 75% of participants from low risk of bias studies; consistent, direct and precise data were obtained, and no known or suspected publication bias was detected. Further research is unlikely to change the estimate or our confidence in the results.
Moderate‐quality evidence: One of the domains is not met. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low‐quality evidence: Two of the domains are not met. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low‐quality evidence: Three of the domains are not met. We are very uncertain about the results.
No evidence: No RCTs were identified that addressed this outcome.
Single studies with a sample size smaller than the optimal information size of n > 300 for dichotomous data and n > 400 for continuous data are considered to yield very low‐ (in the case of high risk of bias trials) or low‐ (in the case of low risk of bias trials) quality evidence. To improve the readability of this review, a GRADE table was completed only when we had completed a meta‐analysis. If only one study was present for a given comparison, the results are described in the text and in the Characteristics of included studies table. The results presented for all comparisons in the 'Effects of interventions' section are the results as reported by the study authors, unless stated otherwise.
Results
Description of studies
In total, 22 RCTs were included in this updated systematic review, eight of which were added since the last update. Both men and women were included—in total 2503 participants; overall mean age was 41.4 years. Sixteen studies reported a mean pain intensity score at baseline ranging from 20.5 to 82.8/100. The mean pain intensity score of these 16 studies at baseline was 56.65/100. Four RCTs assessed the effectiveness of programmes that started immediately after surgery: One RCT compared an exercise programme versus no treatment (Ju 2012), another RCT investigated the effectiveness of mobilisation starting two hours after surgery (Newsome 2009), one RCT focused on neural mobilisation (Scrimshaw 2001) and another RCT assessed the effectiveness of intensive exercise (Kjellby‐Wendt 1998). Most trials focused on treatments that started four to six weeks postsurgery. Participants visited a therapist in a primary care setting or at an outpatient clinic, one to three times a week, 30 to 90 minutes per session. Two trials ( Filiz 2005; Yilmaz 2003) included three arms, one of which was a no treatment arm, yielding two comparisons per RCT. One RCT comprised four arms (McGregor 2011), and of these, three arms yielding two comparisons were included in this review in two separate meta‐analyses. For three comparisons assessing the effectiveness of interventions starting four to six weeks after surgery, a meta‐analysis could be performed: exercise programme versus no treatment (comparison 2a); high‐intensity programme versus low‐intensity programme (comparison 2b.1) and supervised exercise programme versus home exercise programme (comparison 2b.2). For all other types of interventions (or programmes) that started four to six weeks after surgery, only one study was identified per comparison. Finally, two studies assessed treatment regimens that started longer than 12 months after surgery.
Results of the search
The search for the original review (until 2000) yielded 427 hits in MEDLINE, 414 in EMBASE and 135 in CENTRAL. Selection resulted in the inclusion of nine RCTs and four CCTs in the original review. For the first update, we searched the same databases plus CINAHL from 2000 until May 2007, yielding a total of 3059 hits. In line with the updated guidelines, only RCTs were included. Selection resulted in five new RCTs. Four studies that were included in the original review were excluded because they were not randomised. Therefore, the first update of this systematic review included a total of 14 RCTs. For the current update, we searched all aforementioned databases and PEDro until May 2013, yielding 2023 references in MEDLINE, 1591 in EMBASE, 978 in CENTRAL, 2021 in CINAHL, 101 in PsycINFO and 598 in PEDro. Removing duplicates resulted in a total of 5202 unique papers. The first selection, based on title and abstract, resulted in 15 new RCTs. For one potentially eligible study, no full‐text paper could be retrieved (Ishida 2010). After reading the full‐text papers, the review authors excluded four RCTs because participants were not randomly assigned (Imamovic 2010; Kim 2010; Kim 2010b; Millisdotter 2007). One study was excluded because the predefined upper age limit was exceeded (Mannion 2007). Finally, one RCT was excluded because it was unclear whether participants had a disc herniation, and the presurgery treatment programmes in this study differed between the two groups (Nielsen 2010). Therefore, the current update of this systematic review included eight new trials (i.e. 22 RCTs in total).
Risk of bias in included studies
About half of the included studies (10 out of 22) had a low risk of bias. Care providers could not be blinded because of the nature of the interventions. Intention‐to‐treat analysis including correct handling of missing data was performed in only four studies. In 13 studies, compliance with the rehabilitation programme was inadequate or was not assessed. Published details concerning co‐interventions were lacking: Only eight studies explicitly provided information on co‐interventions. Four studies scored positive on this item. In eleven studies, the randomisation procedure and concealment of treatment allocation were not described adequately. Eight studies had a high percentage of dropouts, and the numbers were unclear in five studies. Selective reporting bias was present in six studies because of differences between the protocol and the published report, or because pain and function were not reported. These methodological shortcomings in the conduct and reporting of studies suggest considerable potential for bias in more than half of the included trials (see Figure 1 for results of individual trials). The overall judgement of quality of evidence, according to the five domains as described by GRADE, can be found in Table 1; Table 2; Table 3. Authors of five of the eight new RCTs responded to the risk of bias assessment, and their comments were taken into account in the final judgement.
1.
Effects of interventions
See: Table 1; Table 2; Table 3
Effectiveness of rehabilitation programmes
1. Comparisons among rehabilitation programmes that start immediately after surgery
1a. Treatment versus no treatment, placebo or waiting list control
Very low‐quality evidence, based on one very small (N = 14) RCT with a high risk of bias (Ju 2012), suggests that there is no difference in pain posttreatment (12 weeks postoperative) between an exercise programme and no rehabilitation (Analysis 1.1). The intervention group had significantly lower scores than the control group for function post‐treatment (mean difference ‐3.99; 96% confidence interval (CI) ‐4.95 to ‐3.03; Analysis 1.2). Data on reoperations were not presented.
1.1. Analysis.
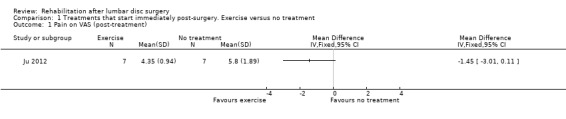
Comparison 1 Treatments that start immediately post‐surgery. Exercise versus no treatment, Outcome 1 Pain on VAS (post‐treatment).
1.2. Analysis.
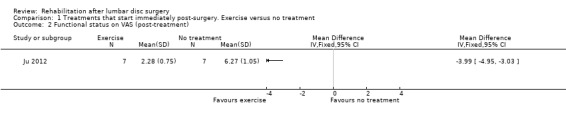
Comparison 1 Treatments that start immediately post‐surgery. Exercise versus no treatment, Outcome 2 Functional status on VAS (post‐treatment).
1b. Treatment versus other kinds of treatment
Very low‐quality evidence, based on one small (N = 60) RCT with a high risk of bias (Kjellby‐Wendt 1998), suggests that there is no difference over the long term in global perceived effect, pain (Analysis 2.1; Analysis 2.2) or return to work (Analysis 2.3) between an intensive exercise programme and a less active programme. One reoperation (3.4%) was reported in the intervention group and two reoperations (6.5%) in the reference group.
2.1. Analysis.
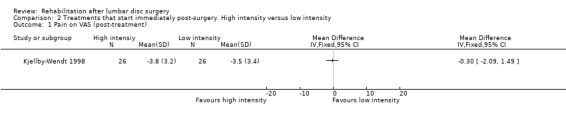
Comparison 2 Treatments that start immediately post‐surgery. High intensity versus low intensity, Outcome 1 Pain on VAS (post‐treatment).
2.2. Analysis.
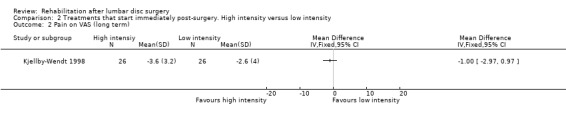
Comparison 2 Treatments that start immediately post‐surgery. High intensity versus low intensity, Outcome 2 Pain on VAS (long term).
2.3. Analysis.
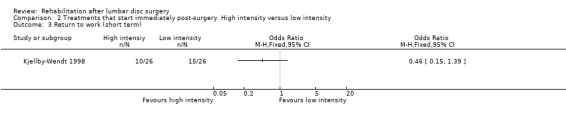
Comparison 2 Treatments that start immediately post‐surgery. High intensity versus low intensity, Outcome 3 Return to work (short term).
Very low‐quality evidence, based on one very small RCT (N = 30) with a high risk of bias (Newsome 2009), shows that immediate physiotherapy, starting two hours postsurgery (consisting of 10 times flexion of knee and hip and the advice to repeat this every 30 minutes), and usual care do not significantly differ at four weeks and three months in terms of function (Analysis 3.1; Analysis 3.2), back pain (Analysis 3.3; Analysis 3.4), leg pain and McGill pain scores. The intervention group returned to work earlier (median six weeks vs control eight weeks) (median difference two weeks, 95% confidence interval (CI) zero to six). In each group, one recurrent disc protrusion was reported; data on reoperations were not presented.
3.1. Analysis.
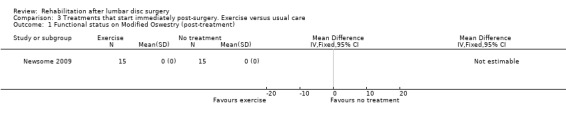
Comparison 3 Treatments that start immediately post‐surgery. Exercise versus usual care, Outcome 1 Functional status on Modified Oswestry (post‐treatment).
3.2. Analysis.
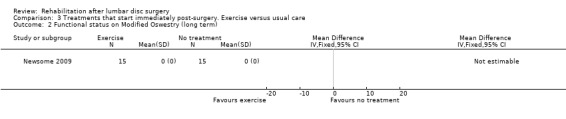
Comparison 3 Treatments that start immediately post‐surgery. Exercise versus usual care, Outcome 2 Functional status on Modified Oswestry (long term).
3.3. Analysis.
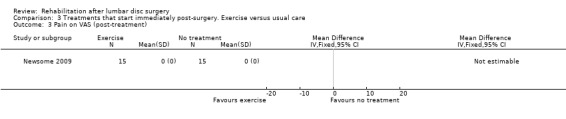
Comparison 3 Treatments that start immediately post‐surgery. Exercise versus usual care, Outcome 3 Pain on VAS (post‐treatment).
3.4. Analysis.
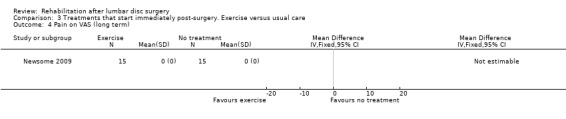
Comparison 3 Treatments that start immediately post‐surgery. Exercise versus usual care, Outcome 4 Pain on VAS (long term).
1c. Specific intervention in addition to a treatment programme versus treatment alone
Low‐quality evidence from one RCT (N = 59) (Scrimshaw 2001) with a low risk of bias shows that neural mobilisation is not effective as an adjunct to standard postoperative care in terms of functional status (Analysis 4.1) and pain (Analysis 4.3) after six weeks of follow‐up. For these outcome measures (Analysis 4.2; Analysis 4.4), as well as for overall improvement (Analysis 4.5), no differences were noted after 12 months. No data on reoperation rates were presented.
4.1. Analysis.
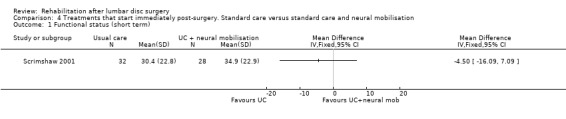
Comparison 4 Treatments that start immediately post‐surgery. Standard care versus standard care and neural mobilisation, Outcome 1 Functional status (short term).
4.3. Analysis.
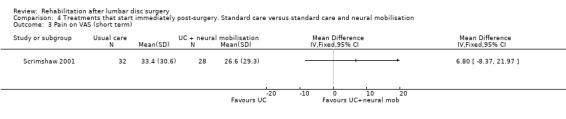
Comparison 4 Treatments that start immediately post‐surgery. Standard care versus standard care and neural mobilisation, Outcome 3 Pain on VAS (short term).
4.2. Analysis.
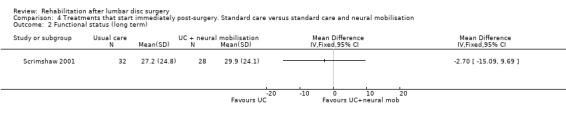
Comparison 4 Treatments that start immediately post‐surgery. Standard care versus standard care and neural mobilisation, Outcome 2 Functional status (long term).
4.4. Analysis.
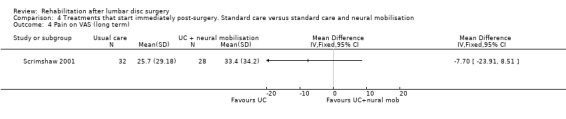
Comparison 4 Treatments that start immediately post‐surgery. Standard care versus standard care and neural mobilisation, Outcome 4 Pain on VAS (long term).
4.5. Analysis.
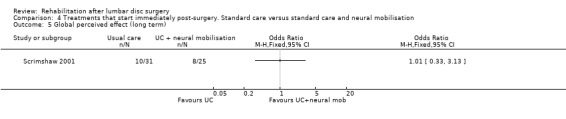
Comparison 4 Treatments that start immediately post‐surgery. Standard care versus standard care and neural mobilisation, Outcome 5 Global perceived effect (long term).
2. Comparisons among rehabilitation programmes that start four to six weeks postsurgery
2a. Exercise programmes versus no treatment
For exercise programmes that start four to six weeks postsurgery, very low‐quality evidence (five RCTs, N = 272; Dolan 2000;Erdogmus 2007; Filiz 2005; Filiz 2005 (1); McGregor 2011; Yilmaz 2003; Yilmaz 2003 (1)) shows that exercise programmes are more effective than no treatment in terms of short‐term follow‐up for pain (SMD ‐0.90, 95% CI ‐1.55 to ‐0.24; Analysis 5.1); low‐quality evidence (four RCTs, N = 252;Erdogmus 2007; Filiz 2005; Filiz 2005 (1); McGregor 2011; Yilmaz 2003; Yilmaz 2003 (1)) has been found to favour exercise programmes for functional status on short‐term follow‐up (SMD ‐0.67; 95% CI ‐1.22 to ‐0.12; Analysis 5.2); and low‐quality evidence (three RCTs, N = 226; Donaldson 2006; Erdogmus 2007; McGregor 2011) shows no difference in functional status on long‐term follow‐up (SMD ‐0.22; 95% CI ‐0.49 to 0.04; Analysis 5.3). None of the included studies reported on reoperations.
5.1. Analysis.
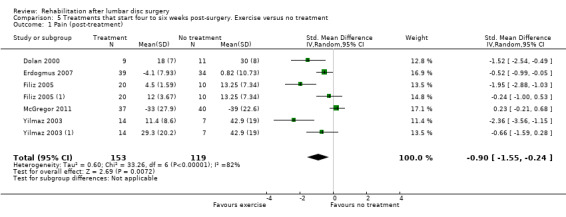
Comparison 5 Treatments that start four to six weeks post‐surgery. Exercise versus no treatment, Outcome 1 Pain (post‐treatment).
5.2. Analysis.
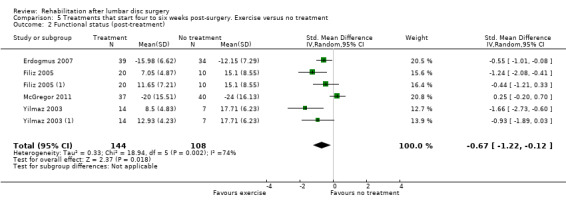
Comparison 5 Treatments that start four to six weeks post‐surgery. Exercise versus no treatment, Outcome 2 Functional status (post‐treatment).
5.3. Analysis.
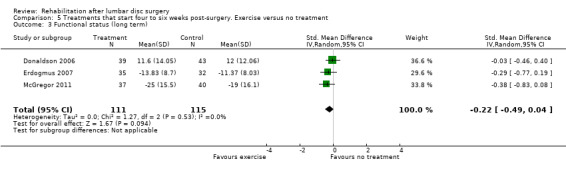
Comparison 5 Treatments that start four to six weeks post‐surgery. Exercise versus no treatment, Outcome 3 Functional status (long term).
Low‐quality evidence from one RCT with low risk of bias (N = 80; Erdogmus 2007) suggests that the effectiveness of physiotherapy is not significantly different from no treatment regarding long‐term pain (Analysis 5.4) and is not significantly different from that of sham neck massage regarding function (Analysis 5.5; Analysis 5.6) and pain (Analysis 5.7; Analysis 5.8). No data on reoperation rates were presented.
5.4. Analysis.
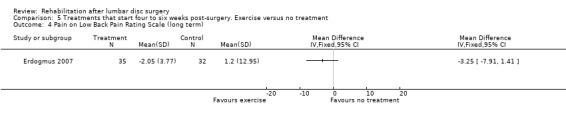
Comparison 5 Treatments that start four to six weeks post‐surgery. Exercise versus no treatment, Outcome 4 Pain on Low Back Pain Rating Scale (long term).
5.5. Analysis.
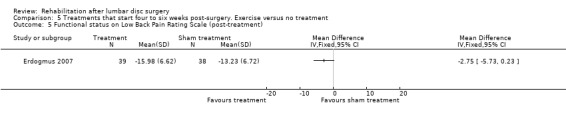
Comparison 5 Treatments that start four to six weeks post‐surgery. Exercise versus no treatment, Outcome 5 Functional status on Low Back Pain Rating Scale (post‐treatment).
5.6. Analysis.
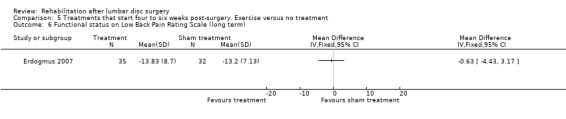
Comparison 5 Treatments that start four to six weeks post‐surgery. Exercise versus no treatment, Outcome 6 Functional status on Low Back Pain Rating Scale (long term).
5.7. Analysis.
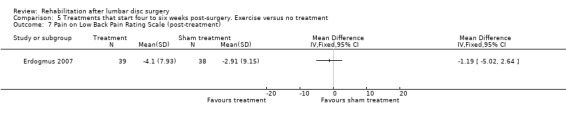
Comparison 5 Treatments that start four to six weeks post‐surgery. Exercise versus no treatment, Outcome 7 Pain on Low Back Pain Rating Scale (post‐treatment).
5.8. Analysis.
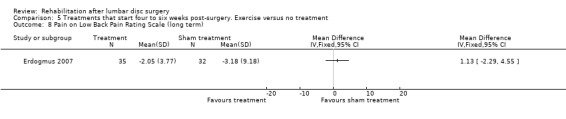
Comparison 5 Treatments that start four to six weeks post‐surgery. Exercise versus no treatment, Outcome 8 Pain on Low Back Pain Rating Scale (long term).
2b. Treatment versus other kinds of treatment
2b1. High‐intensity exercise programmes versus low‐intensity exercise programmes
Very low‐quality evidence (two RCTs, N = 103; Danielsen 2000; Filiz 2005) shows that high‐intensity exercise programmes are slightly more effective for pain in the short term compared with low‐intensity exercise programmes (WMD ‐10.67; 95% CI ‐17.04 to ‐4.30 on a zero to 100 VAS; Analysis 6.1), and low‐quality evidence (two RCTs, N = 103; Danielsen 2000; Filiz 2005) favours high‐intensity exercise programmes compared with low‐intensity exercise programmes in terms of functional status in the short term (SMD ‐0.77; 95% CI ‐1.17 to ‐0.36; Analysis 6.2). Long‐term follow‐up results for both pain and functional status showed no significant differences between groups. One RCT (Manniche 1993a) reports no statistically significant differences in overall improvement at short‐term and long‐term follow‐up (Analysis 6.3; Analysis 6.4; Analysis 6.5; Analysis 6.6; we used the sample sized presented for 52 weeks of follow‐up for all analyses of this study), and one RCT (Danielsen 2000) reports no significant differences in long‐term function (Analysis 6.7) and pain (Analysis 6.8). Results for sick leave, which could not be pooled, were also contradictory: Danielsen 2000 reported no significant differences in sick leave during one‐year follow‐up (high intensity: mean 18.5 weeks (SD 14.3) vs 22.0 weeks (SD 18.6) for low intensity); Manniche 1993a reported no differences in return to work (16% difference between groups; Analysis 6.9) and Filiz 2005 reported that participants in high‐intensity programmes returned to work more quickly (mean after 56 days, SD 18.6) as compared with low‐intensity programmes (mean after 75 days, SD 24.9). Danielsen 2000 reported one‐year reoperative rates of two/39 in the exercise group versus two/24 in the control group. Manniche 1993a reported no adverse effects or complications.
6.1. Analysis.
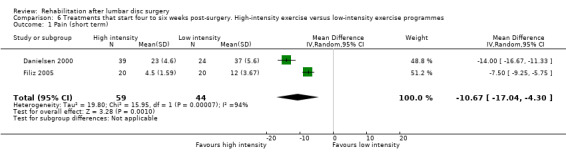
Comparison 6 Treatments that start four to six weeks post‐surgery. High‐intensity exercise versus low‐intensity exercise programmes, Outcome 1 Pain (short term).
6.2. Analysis.
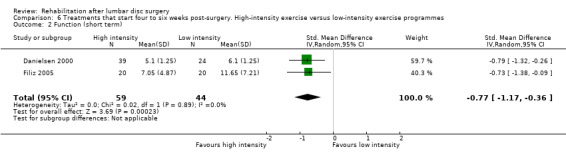
Comparison 6 Treatments that start four to six weeks post‐surgery. High‐intensity exercise versus low‐intensity exercise programmes, Outcome 2 Function (short term).
6.3. Analysis.
Comparison 6 Treatments that start four to six weeks post‐surgery. High‐intensity exercise versus low‐intensity exercise programmes, Outcome 3 Functional status on Low Back Pain Rating Scale (post‐treatment).
6.4. Analysis.
Comparison 6 Treatments that start four to six weeks post‐surgery. High‐intensity exercise versus low‐intensity exercise programmes, Outcome 4 Functional status on Low Back Pain Rating Scale (long term).
6.5. Analysis.
Comparison 6 Treatments that start four to six weeks post‐surgery. High‐intensity exercise versus low‐intensity exercise programmes, Outcome 5 Pain on Low Back Pain Rating Scale (post‐treatment).
6.6. Analysis.
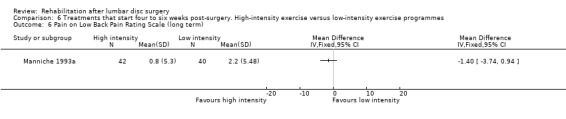
Comparison 6 Treatments that start four to six weeks post‐surgery. High‐intensity exercise versus low‐intensity exercise programmes, Outcome 6 Pain on Low Back Pain Rating Scale (long term).
6.7. Analysis.
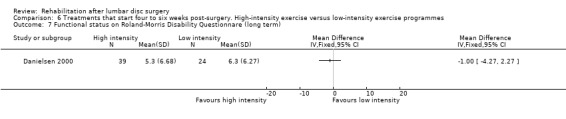
Comparison 6 Treatments that start four to six weeks post‐surgery. High‐intensity exercise versus low‐intensity exercise programmes, Outcome 7 Functional status on Roland‐Morris Disability Questionnare (long term).
6.8. Analysis.
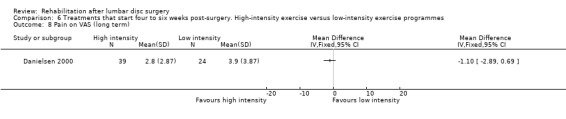
Comparison 6 Treatments that start four to six weeks post‐surgery. High‐intensity exercise versus low‐intensity exercise programmes, Outcome 8 Pain on VAS (long term).
6.9. Analysis.
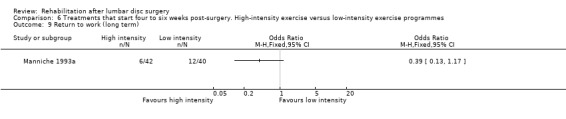
Comparison 6 Treatments that start four to six weeks post‐surgery. High‐intensity exercise versus low‐intensity exercise programmes, Outcome 9 Return to work (long term).
2b2. Supervised exercise programme versus home exercise programme
Very low‐quality evidence (four RCTs, N = 154; Filiz 2005; Johannsen 1994; Johansson 2009; Yilmaz 2003) shows no significant differences between supervised exercise programmes and home exercise programmes in terms of short‐term pain relief (pooled SMD ‐0.76, 95% CI ‐2.04 to 0.53; Analysis 7.1). One RCT (N = 75) with high risk of bias (Choi 2005) showed no difference in pain (VAS) post‐treatment. One trial (Johannsen 1994) showed no differences in global perceived effect (four‐point scale) post‐treatment (Analysis 7.3) and at three‐month follow‐up (Analysis 7.4). The data from two trials (Choi 2005; Johannsen 1994) show no differences between groups in long‐term pain relief (Analysis 7.5; Analysis 7.6). For functional status, very low‐quality evidence (four RCTs, N = 154;Filiz 2005; Johannsen 1994; Johansson 2009; Yilmaz 2003) shows no short‐term differences between supervised exercise programmes and home exercise programmes (pooled SMD ‐0.36, 95% CI ‐0.88 to 0.15; Analysis 7.2). One additional RCT (Choi 2005) showed no difference post‐treatment. Over the long term, only sparse data (Johannsen 1994) reported no significant differences between groups (Analysis 7.7). One RCT (Choi 2005) showed that more participants in the supervised group (87%) returned to work within four months than participants in the home‐based exercise group (24%). One small (N = 40) trial (Johannsen 1994) reported one reoperation in the intervention group. The other RCTs reported no data on reoperations.
7.1. Analysis.
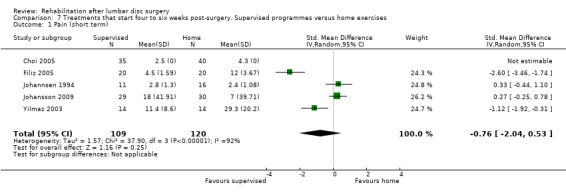
Comparison 7 Treatments that start four to six weeks post‐surgery. Supervised programmes versus home exercises, Outcome 1 Pain (short term).
7.3. Analysis.

Comparison 7 Treatments that start four to six weeks post‐surgery. Supervised programmes versus home exercises, Outcome 3 Global perceived effect (short term) (four‐point scale).
7.4. Analysis.

Comparison 7 Treatments that start four to six weeks post‐surgery. Supervised programmes versus home exercises, Outcome 4 Global perceived effect (long term) (four‐point scale).
7.5. Analysis.

Comparison 7 Treatments that start four to six weeks post‐surgery. Supervised programmes versus home exercises, Outcome 5 Pain (long term) (five‐point scale).
7.6. Analysis.

Comparison 7 Treatments that start four to six weeks post‐surgery. Supervised programmes versus home exercises, Outcome 6 Pain (long term) (VAS).
7.2. Analysis.
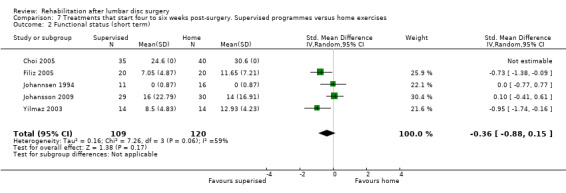
Comparison 7 Treatments that start four to six weeks post‐surgery. Supervised programmes versus home exercises, Outcome 2 Functional status (short term).
7.7. Analysis.

Comparison 7 Treatments that start four to six weeks post‐surgery. Supervised programmes versus home exercises, Outcome 7 Functional status (long term) (12‐point scale).
2b3. Exercise and education versus education
Very low‐quality evidence (one RCT, N = 98; Kulig 2009) suggests that functional status post‐treatment was significantly better for exercise plus one educational session (mean change score ‐18.4, 95% CI ‐22.5 to ‐14.3) than for education only (mean change score ‐9.4, 95% CI ‐13.0 to ‐5.8; Analysis 8.1). From the education‐only group, 19/32 participants sought usual physiotherapy care. One reoperation occurred; it was not reported in which group the reoperation was performed.
8.1. Analysis.
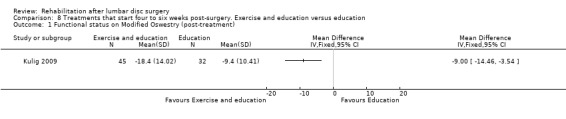
Comparison 8 Treatments that start four to six weeks post‐surgery. Exercise and education versus education, Outcome 1 Functional status on Modified Oswestry (post‐treatment).
2b4. Exercise and booklet versus exercise
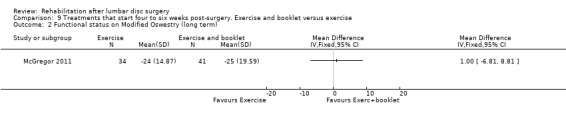
Low‐quality evidence from one RCT (N = 75; McGregor 2011) indicates that functional status was not statistically different between exercise and booklet versus exercise alone post‐treatment (Analysis 9.1) or at one‐year follow‐up (Analysis 9.2). Leg pain did not differ between exercise and booklet and exercise alone post‐treatment (Analysis 9.3) or at one‐year follow‐up (Analysis 9.4). A cost‐effectiveness analysis of this trial showed no significant differences in costs between interventions (Morris 2011). Reoperative rates were not reported.
9.1. Analysis.
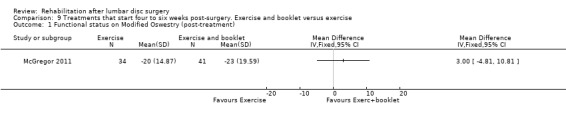
Comparison 9 Treatments that start four to six weeks post‐surgery. Exercise and booklet versus exercise, Outcome 1 Functional status on Modified Oswestry (post‐treatment).
9.2. Analysis.
Comparison 9 Treatments that start four to six weeks post‐surgery. Exercise and booklet versus exercise, Outcome 2 Functional status on Modified Oswestry (long term).
9.3. Analysis.
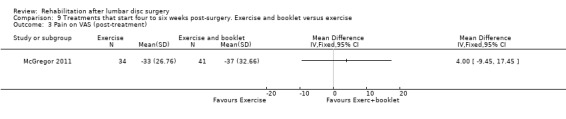
Comparison 9 Treatments that start four to six weeks post‐surgery. Exercise and booklet versus exercise, Outcome 3 Pain on VAS (post‐treatment).
9.4. Analysis.
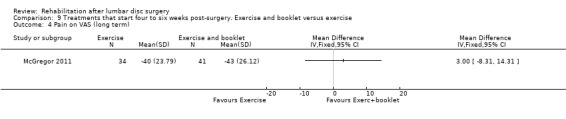
Comparison 9 Treatments that start four to six weeks post‐surgery. Exercise and booklet versus exercise, Outcome 4 Pain on VAS (long term).
2b5. Multidisciplinary rehabilitation programme
Very low‐quality evidence from one RCT (N = 212; Alaranta 1986) shows that at one‐year follow‐up, no statistically significant differences were noted between multidisciplinary rehabilitation and usual care for global perceived effect (Analysis 10.1), sick leave (Analysis 10.2) or reoperative rates (3.7% in both groups).
10.1. Analysis.
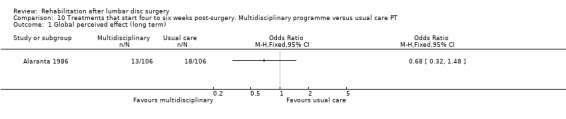
Comparison 10 Treatments that start four to six weeks post‐surgery. Multidisciplinary programme versus usual care PT, Outcome 1 Global perceived effect (long term).
10.2. Analysis.
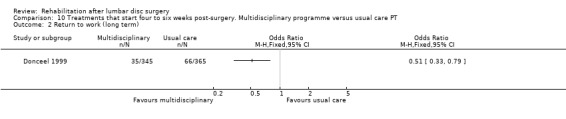
Comparison 10 Treatments that start four to six weeks post‐surgery. Multidisciplinary programme versus usual care PT, Outcome 2 Return to work (long term).
2b6. Rehabilitation in the occupational setting
Low‐quality evidence from one RCT (N = 710; Donceel 1999) shows that a rehabilitation‐oriented approach by the medical advisors of a social security fund (a fund covering people with mandatory insurance) was more effective than usual care on return to work at long‐term follow‐up. Data on reoperative rates were not reported.
2b7. Behavioural treatment
Low‐quality evidence (one RCT, N = 105; Ostelo 2003) shows that in the short term, global perceived recovery was better after a standard physiotherapy programme than after a behavioural graded activity programme (Analysis 11.1), but no differences were noted in the long term (Analysis 11.2). Low‐quality evidence also suggests no differences (short‐term or long‐term) in functional status (RDQ) (Analysis 11.3; Analysis 11.4), pain (VAS) (Analysis 11.5; Analysis 11.6) or return to work. This trial also included a cost‐effectiveness analysis, which suggested that the behavioural programme was associated with higher costs during one‐year follow‐up. Reoperative rates were not reported.
11.1. Analysis.
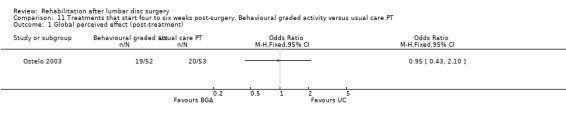
Comparison 11 Treatments that start four to six weeks post‐surgery. Behavioural graded activity versus usual care PT, Outcome 1 Global perceived effect (post‐treatment).
11.2. Analysis.
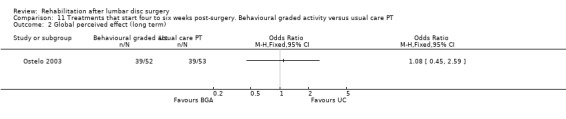
Comparison 11 Treatments that start four to six weeks post‐surgery. Behavioural graded activity versus usual care PT, Outcome 2 Global perceived effect (long term).
11.3. Analysis.
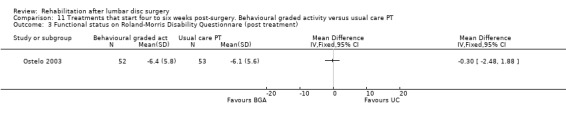
Comparison 11 Treatments that start four to six weeks post‐surgery. Behavioural graded activity versus usual care PT, Outcome 3 Functional status on Roland‐Morris Disability Questionnare (post treatment).
11.4. Analysis.
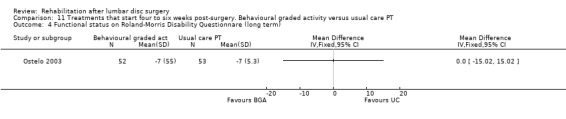
Comparison 11 Treatments that start four to six weeks post‐surgery. Behavioural graded activity versus usual care PT, Outcome 4 Functional status on Roland‐Morris Disability Questionnare (long term).
11.5. Analysis.
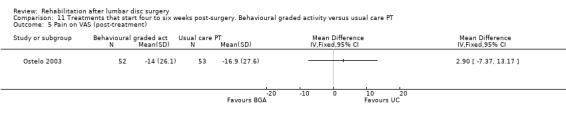
Comparison 11 Treatments that start four to six weeks post‐surgery. Behavioural graded activity versus usual care PT, Outcome 5 Pain on VAS (post‐treatment).
11.6. Analysis.
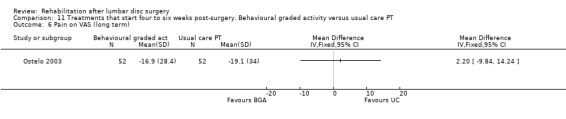
Comparison 11 Treatments that start four to six weeks post‐surgery. Behavioural graded activity versus usual care PT, Outcome 6 Pain on VAS (long term).
2b8. Stretching and strength training
Very low‐quality evidence shows that after 12 months, no clinically relevant or statistically significant differences in disability (ODI) (Analysis 12.1) and pain (VAS) (Analysis 12.2) were noted between combined strength training and stretching, and strength training alone (Hakkinen 2005). Reoperative rates were not reported.
12.1. Analysis.
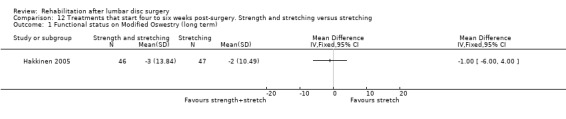
Comparison 12 Treatments that start four to six weeks post‐surgery. Strength and stretching versus stretching, Outcome 1 Functional status on Modified Oswestry (long term).
12.2. Analysis.
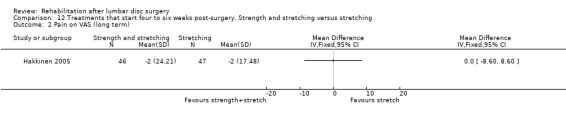
Comparison 12 Treatments that start four to six weeks post‐surgery. Strength and stretching versus stretching, Outcome 2 Pain on VAS (long term).
2c. Specific intervention in addition to a treatment programme versus treatment alone
No RCTs were identified.
3. Comparisons among rehabilitation programmes that start longer than 12 months postsurgery
3a. Treatment versus no treatment, placebo or waiting list control
No RCTs were identified.
3b. Treatment versus other kinds of treatment
Very low‐quality evidence (one RCT, N = 150, three arms; Timm 1994) shows that low‐tech and high‐tech exercise might be more effective in improving low back functional status as compared with no treatment (Analysis 13.1; Analysis 14.1; Analysis 15.1). Data on reoperative rates were not reported.
13.1. Analysis.
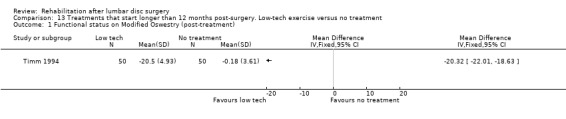
Comparison 13 Treatments that start longer than 12 months post‐surgery. Low‐tech exercise versus no treatment, Outcome 1 Functional status on Modified Oswestry (post‐treatment).
14.1. Analysis.
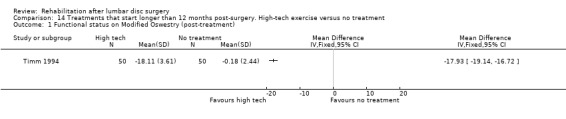
Comparison 14 Treatments that start longer than 12 months post‐surgery. High‐tech exercise versus no treatment, Outcome 1 Functional status on Modified Oswestry (post‐treatment).
15.1. Analysis.
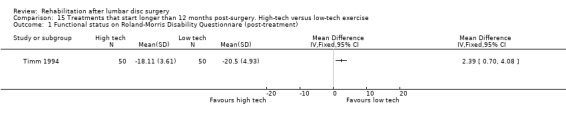
Comparison 15 Treatments that start longer than 12 months post‐surgery. High‐tech versus low‐tech exercise, Outcome 1 Functional status on Roland‐Morris Disability Questionnare (post ‐treatment).
3c. Specific intervention in addition to a treatment programme versus treatment alone
Low‐quality evidence from one small (N = 62) RCT with a low risk of bias (Manniche 1993b) suggests that adding hyperextension to an intensive exercise programme might not be more effective than intensive exercise alone for functional status (Analysis 16.1) or pain (Analysis 16.2) outcomes. Reoperative rates were not reported.
16.1. Analysis.
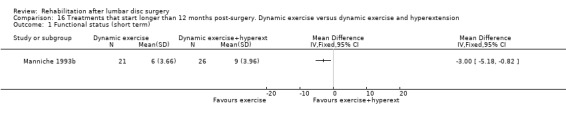
Comparison 16 Treatments that start longer than 12 months post‐surgery. Dynamic exercise versus dynamic exercise and hyperextension, Outcome 1 Functional status (short term).
16.2. Analysis.
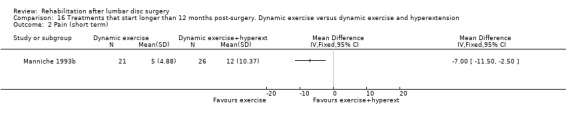
Comparison 16 Treatments that start longer than 12 months post‐surgery. Dynamic exercise versus dynamic exercise and hyperextension, Outcome 2 Pain (short term).
Discussion
Twenty‐two RCTs were included in this systematic review. The studies were heterogeneous with regard to timing, content, duration and intensity of treatment and also regarding the comparison. Moreover, some studies did not describe whether any treatment was provided in the first few weeks, or whether an intervention in the first few weeks was offered to all participants (such as advice and an exercise sheet). We did not identify high‐ or moderate‐quality evidence for any of the investigated interventions. Overall the results of this review seem to suggest that exercise programmes mainly in the first few months after surgery contribute a small benefit to recovery, but because of the low to very low quality underpinning this evidence, this finding should be interpreted cautiously.
Seven studies specified adverse events, and none of those studies reported that active programmes increase the reoperative rate. Therefore, we concluded that it seems not to be harmful to return to activity after lumbar disc surgery, and consequently, that it is not necessary for patients to stay passive after lumbar disc surgery. This is in line with Carragee 1996, who concluded that lifting postoperative restrictions after limited discectomy led to shortened sick leave without increased complications.
An interesting observation is that adherence to treatment or compliance is hardly addressed in the included studies. Johansson 2009 reports that 25/29 (86%) participants attended all clinic sessions of the eight‐week programme and that 25/30 (83%) participants did home exercises during this period. Erdogmus 2007 found that during the intervention period of three months, 50% of the total sample regularly performed exercises at home and 25% at 1.5 years. The proportion of adherers was similar in all groups, irrespective of their received intervention. Hakkinen 2005 assessed adherence rates to home exercise programmes that lasted 12 months and demonstrated that after two months, adherence rates dropped to 50% to 60% of the target, with a further decline to 30% in the last six months. This seems to suggest that more intensive supervision needs to be in place for long‐term rehabilitation to maintain patients' motivation. Another potential patient‐related issue is the need to consider patient preferences for treatment. Kulig 2009 showed that most participants (n = 21/32) allocated to an intervention that included only one educational session crossed‐over to the education plus exercise group (n = 2) or sought usual care physiotherapy (n = 19) during the trial period. Therefore, one could argue that the type of intervention needs to match patients’ expectations. On the other hand, RCTs are artificial environments wherein the differences between treatment options are articulated; consequently, participants in this trial were aware of the existence of both intervention options, which possibly increased cross‐over. A Zelen design (Zelen 1979) may be useful in trials comparing treatment versus no or minimal treatment to adequately assess the effectiveness of treatment.
One RCT with a low risk of bias (Donceel 1999) assessed an intervention of medical advisors of a social security sickness fund on a patient population with mandatory insurance. These medical advisors co‐ordinated a multidisciplinary rehabilitation‐oriented approach. The results of this study indicate that an intervention aimed at an active rehabilitation policy, encompassing gradual work resumption, information, early mobilisation and early contact with the medical advisor, increased the probability of return to work for these participants. Although this is only one RCT in a specific setting (approaches like this are probably dependent on the social security system), these results look promising. Furthermore, this study highlights the need for more than just exercise if an intervention aims to ensure early return to work. Further research is needed to assess whether these types of interventions are (cost‐)effective.
Regarding biopsychosocial aspects of postsurgery rehabilitation, it has been suggested that high‐intensity programmes confront patients with their fears and insecurities, and that they learn that symptoms related to training are not necessarily dangerous (Manniche 1993b). One RCT with a low risk of bias assessed the effectiveness of a behavioural graded activity (BGA) programme that focused on biopsychosocial aspects of recovery (Ostelo 2003). Results of this study show no differences between the BGA programme and standard physiotherapy. As of yet, no convincing evidence has been found regarding use of specific biopsychosocial‐oriented approaches in the rehabilitation of patients after first‐time disc surgery.
In this systematic review, any type of surgical technique was included a priori. All participants included in the studies had received standard discectomy, microdiscectomy and, in one study (Erdogmus 2007), standard laminectomy and either discectomy or microdiscectomy. A recent systematic Cochrane review showed no significant differences in effectiveness between these approaches (Jacobs 2012). Another important issue regarding surgery needs to be discussed. Although it was not the main focus of the current systematic review, it is important to know the indication for surgery because indications might change over time, with potential consequences for rehabilitation.
Authors' conclusions
Implications for practice.
In clinical practice, considerable variation is seen in the content, duration and intensity of rehabilitation programmes. Based on this review, because of lack of high‐ or moderate‐quality evidence, no firm conclusion can be drawn regarding their effectiveness, and consequently, no strong recommendations can be made for clinical practice. Taking this caution into account, it seems that exercise programmes starting four to six weeks postsurgery lead to a faster decrease in pain and disability than no treatment, and that high‐intensity exercise programmes lead to a slightly faster decrease in pain and disability than low‐intensity programmes. No evidence suggests that these active programmes increase the reoperation rate or that patients need to have their activities restricted after first‐time lumbar disc surgery.
Implications for research.
Based on this review, we suggest the following directions for future research. First, future research should focus on the implementation of rehabilitation programmes in daily practice. Should all patients be treated postsurgery? Or is minimal intervention with the message "return to an active lifestyle" sufficient, with only patients who still have symptoms four to six weeks postsurgery requiring rehabilitation programmes? The cost‐effectiveness of this approach needs to be investigated. Second, it is still unclear which exact components should be included in rehabilitation programmes. High‐intensity programmes seem to be slightly more effective, but they could also be more expensive. Prognostic variables for poor outcome, including psychosocial factors (den Boer 2010), may be taken into account when the content of rehabilitation programmes is determined. Finally, as the quality of evidence in this review is low to very low, larger high‐quality RCTs are warranted.
Feedback
Comments on version of review published in The Cochrane Library 2002, Issue 2
Summary
January 2005
Feedback 1: The results are described almost entirely in terms of whether they were statistically significant, and very few numbers are presented. It would be much more informative to state how big the differences between groups were (risk ratio or mean difference, or other measure of the size of the difference), along with a confidence interval to indicate the uncertainty around the estimate. The statistical significance of a result is of little importance and alone is very uninformative. Feedback 2: Thanks for this. I just wanted to correct a possible misunderstanding. I was not criticising the lack of meta‐analysis and overall effect estimate. This is a very reasonable position when the trials are heterogeneous. My criticism was about the description of the results of included trials as "statistically significant" or not, which does not give much idea of the size of the differences found. It would be much better to quote risk ratios or differences and confidence intervals.
Reply
Response 1: It is always more informative if one can calculate an overall effect size ... provided data are sufficient and it makes clinical sense to do so. However, you have identified the challenges facing authors who try to synthesise the data in this field. The review authors found only 13 studies that met the inclusion criteria and believed that for studies that did include sufficient data, too much heterogeneity in the duration and intensity of the interventions and in the timing of outcome measures was noted to allow pooling of the data. You also comment on the lack of clinical relevance. Our guidelines have changed and ask authors to include this parameter in their reviews. Because this review is due for updating, I would anticipate the review authors including this in the updated review. I will pass on your comments to the review authors, so that they can consider them as they complete the update. Response 2: Thanks for clarifying. And the editorial board does agree with you! As I'm sure you are aware, authors often describe their results as 'statistically' significant because they are, but for a variety of reasons, their results really may mean little or nothing from a clinical perspective. The rheumatology field is ahead of the back pain field in definitions, calculations and reporting of minimum clinically important differences. At our last international forum on back pain research (Edmonton, Canada, October 2004), a lot of discussion focused on this, but no consensus has been reached yet. Some consensus has been reached on participant‐centred outcomes of import, but again, the older trials do not necessarily follow this. The other really unfortunate thing is that much of the literature just does not give any stats at all! The newer trials are better, but it's still an uphill battle, despite widespread acceptance of CONSORT and TREND statements.
Contributors
Dr Simon Gates, Trials Researcher/Statistician Victoria Pennick, Back Group Co‐ordinator
What's new
| Date | Event | Description |
|---|---|---|
| 3 October 2013 | New search has been performed | We included eight new studies and used the updated method guidelines by the Cochrane Back Review Group (Furlan 2009). We assessed risk of bias using the 12 items recommended by the Back Group. |
| 3 October 2013 | New citation required but conclusions have not changed | The conclusions of this review have not changed as a result of this update. |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 2, 2002
| Date | Event | Description |
|---|---|---|
| 19 January 2011 | Amended | Contact details updated. |
| 23 November 2009 | Amended | Contact details updated. |
| 28 November 2008 | Amended | Description of ROB and clinical relevance assessment criteria moved to appendices; minor correction of data, with no changes to results |
| 9 September 2008 | Amended | literature search dates edited (history & text) |
| 23 June 2008 | Amended | Converted to new review format. |
| 19 March 2008 | New citation required and conclusions have changed | In contrast to the original review, we only included RCTs in this update. In total, five new RCTs were included, yielding a total of 14 included RCTs. Therefore, it was now possible to pool the data in three comparisons. In addition, in this update we used the GRADE approach, as recommended by the Editorial Board of the Cochrane Back Review Group (CBRG), while in the original review the 'levels of evidence' approach was used. The following 'new' results were found, none of which are supported by high quality evidence 1) Adding neural mobilization to an exercise program is not effective on pain and functional disability in the short‐term and the long‐term. 2) Exercise programs that start four to six weeks post‐surgery lead to a faster decrease in pain and functional disability as compared to no treatment 3) A behavioural graded activity program is not more effective than a standard physiotherapy program 4) Supervised training does not seem to be more effective than home‐based training in the short‐term. |
| 19 May 2007 | New search has been performed | The literature search was updated to May 19, 2007. |
Acknowledgements
We would like to thank Teresa Marin, Vicki Pennick, Heather Widdrington, Rachel Couban and Marie‐Andree Nowlan of the Cochrane Back Review Group for their support.
We would also like to thank Ana Marin from the University of Split, School of Medicine, for her help with the translation of a Croatian language article; Maria Kerckhoffs, Pieter Leffers and Gordon Waddell for their contributions to the original review and Claus Manniche for his critical appraisal of the original review.
Appendices
Appendix 1. MEDLINE search strategy
exp "Clinical Trial [Publication Type]"/
randomized.ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐7
Animals/
Humans/
9 not (9 and 10)
8 not 11
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatica/
spondylosis.ti,ab.
lumbago.ti,ab.
or/13‐22
exp Spine/
discitis.ti,ab.
exp Spinal Diseases/
(disc adj degeneration).ti,ab.
(disc adj prolapse).ti,ab.
(disc adj herniation).ti,ab.
spinal fusion.sh.
spinal neoplasms.sh.
(facet adj joints).ti,ab.
intervertebral disk.sh.
postlaminectomy.ti,ab.
arachnoiditis.ti,ab.
(failed adj back).ti,ab.
or/24‐36
Oswestry.tw.
Roland‐Morris.tw.
or/38‐39
23 or 37 or 40
exp Physical Therapy Modalities/
physiotherapy.mp.
exp Rehabilitation/
rehabilitation.mp.
exp Exercise/
exp Exercise Movement Techniques/
exercise.mp.
or/42‐48
12 and 41 and 49
Appendix 2. EMBASE search strategy
Clinical article/
clinical study/
Clinical trial/
controlled study/
randomized controlled trial/
major clinical study/
double blind procedure/
multicenter study/
single blind procedure/
phase 3 clinical trial/
phase 4 clinical trial/
crossover procedure/
placebo/
or/1‐13
allocat$.mp.
assign$.mp.
blind$.mp.
(clinica$ adj25 (study or trial)).mp.
compar$.mp.
control$.mp.
cross?over.mp.
factorial$.mp.
follow?up.mp.
placebo$.mp.
prospectiv$.mp.
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
trial.mp.
(versus or vs).mp.
or/15‐29
14 or 30
human/
nonhuman/
animal/
animal experiment/
33 or 34 or 35
32 not 36
31 not 36
31 and 37
38 not 39
dorsalgia.mp.
exp back pain/
backache.mp.
(lumbar adj pain).mp.
coccyx.mp.
coccydynia.mp.
sciatica.mp.
sciatica/
spondylosis.mp.
lumbago.mp.
or/41‐50
exp spine/
discitis.mp.
exp spinal diseases/
(disc adj degeneration).mp.
(disc adj prolapse).mp.
(disc adj herniation).mp.
spinal fusion.mp.
spinal neoplasms.mp.
(facet adj joints).mp.
intervertebral disk.mp.
postlaminectomy.mp.
arachnoiditis.mp.
(failed adj back).mp.
or/52‐64
Oswestry.mp.
roland‐morris.mp.
66 or 67
51 or 65 or 68
exp PHYSIOTHERAPY/
exp REHABILITATION/
exp EXERCISE/
physical therapy.mp.
exercise.mp.
rehabilitation.mp.
physiotherapy.mp.
or/70‐76
40 and 69 and 77
Appendix 3. CINAHL search strategy
Randomized Controlled Trials.mp.
clinical trial.pt.
exp Clinical Trials/
(clin$ adj25 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw.
exp PLACEBOS/
placebo$.tw.
random$.tw.
exp Study Design/
(latin adj square).tw.
exp Comparative Studies/
exp Evaluation Research/
Follow‐Up Studies.mp.
exp Prospective Studies/
(control$ or prospectiv$ or volunteer$).tw.
Animals/
or/1‐15
17 not 16
dorsalgia.mp.
exp Back Pain/
backache.mp.
(lumbar adj pain).mp. [mp=title, subject heading word, abstract, instrumentation]
exp COCCYX/
exp SCIATICA/
coccyx.mp.
sciatica.mp.
exp Low Back Pain/
coccydynia.mp.
sciatica.mp. or exp SCIATICA/
exp Lumbar Vertebrae/ or exp Spondylolisthesis/ or exp Spondylolysis/
lumbago.mp.
or/19‐31
exp SPINE/
exp Intervertebral Disk/
exp Spinal Diseases/
(disc adj degeneration).mp. [mp=title, subject heading word, abstract, instrumentation]
(disc adj prolapse).mp. [mp=title, subject heading word, abstract, instrumentation]
(disc adj herniation).mp. [mp=title, subject heading word, abstract, instrumentation]
exp Spinal Fusion/
(facet adj joint$).mp. [mp=title, subject heading word, abstract, instrumentation]
exp Laminectomy/
exp KYPHOSIS/
(failed adj back).mp. [mp=title, subject heading word, abstract, instrumentation]
or/33‐43
oswestry.mp.
roland‐morris.mp.
or/45‐46
32 or 44 or 47
exp Physical Therapy/
physiotherapy.mp.
exp REHABILITATION/
rehabilitation.mp.
exp EXERCISE/
exercise.mp.
or/49‐54
18 and 48 and 55
Appendix 4. PsycINFO search strategy
(KW=(Randomized controlled trial?) or KW=(clinical trial?) or KW=(clin* within 25 trial*) or kw=(sing* within 25 blind*) or kw=(sing* within 25 mask*) or kw=(doubl* within 25 blind*) or kw=(doubl* within 25 mask*) or kw=(trebl* within 25 blind) or kw=(trebl* within 25 mask*) or kw=(tripl* within 25 blind*) or kw=(tripl* within 25 mask*) or KW=(placebo*) or KW=(random*) or DE=(Research Design) or KW=(Latin square) or KW=(comparative stud*) or KW=(evaluation stud*) or kw=(follow up stud*) or DE=(Prospective studies) or KW= (control*) or KW=(prospective*) or KW=(volunteer*)) and (DE=(back) or DE = (back pain) or DE=(neck)) and (KW=(physiotherapy) or DE=(rehabilitation) or DE=(exercise) or DE=(physical therapy) or KW=(lumbar diskectomy)or KW=(post operative) or KW=(discectomy) or KW=(back surgery) or KW=(lumbar surgery)or KW=(lumbar disk herniation))
Appendix 5. PEDro search strategy
Body part: lumbar spine, sacro‐iliac joint or pelvis
Method: clinical trial
Appendix 6. Clinical relevance assessment questions
Based on the data provided, can you determine whether the results will be clinically relevant?
Are participants described in detail so that you can decide whether they are comparable with patients that you see in your practice?
Are the interventions and treatment settings described well enough that you can provide the same for your patients?
Were all clinically relevant outcomes measured and reported?
Is the size of the effect clinically important?
Are the likely treatment benefits worth the potential harms?
Appendix 7. Criteria for the risk of bias assessment
Random sequence generation (selection bias)
Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence
Risk of selection bias is low if the investigators describe a random component in the sequence generation process, such as referring to a random number table, using a computer random number generator, performing coin tossing, shuffling cards or envelopes, throwing dice, drawing lots, and minimising (minimisation may be implemented without a random element, and this is considered to be equivalent to being random).
Risk of selection bias is high if the investigators describe a non‐random component in the sequence generation process, such as sequence generated by odd or even date of birth, date (or day) of admission or hospital or clinic record number or allocation by judgement of the clinician, preference of the participant, results of a laboratory test or series of tests or availability of the intervention.
Allocation concealment (selection bias)
Selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment
Risk of selection bias is low if participants and investigators enrolling participants could not foresee the assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance or sequentially numbered, opaque, sealed envelopes.
Risk of bias is high if participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on using an open random allocation schedule (e.g. a list of random numbers); using assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or were not sequentially numbered) or using alternation or rotation; date of birth; case record number or other explicitly unconcealed procedures.
Blinding of participants
Performance bias due to knowledge of allocated interventions by participants during the study
Risk of performance bias is low if blinding of participants was ensured and it was unlikely that blinding could have been broken; or if blinding was absent or incomplete, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of personnel/care providers (performance bias)
Performance bias due to knowledge of allocated interventions by personnel/care providers during the study
Risk of performance bias is low if blinding of personnel was ensured and it was unlikely that the blinding could have been broken; or if blinding was absent or incomplete, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of outcome assessor (detection bias)
Detection bias due to knowledge of allocated interventions by outcome assessors
Risk of detection bias is low if blinding of the outcome assessment was ensured and it was unlikely that the blinding could have been broken; or if blinding was absent or incomplete, but the review authors judge that the outcome is not likely to be influenced by lack of blinding or:
for participant‐reported outcomes in which the participant was the outcome assessor (e.g. pain, disability): Risk of bias for outcome assessors is low if risk of bias for participant blinding is low (Boutron 2005);
for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between participants and care providers (e.g. co‐interventions, length of hospitalisation, treatment failure), in which the care provider is the outcome assessor: Risk of bias is low for outcome assessors if risk of bias is low for care providers (Boutron 2005); or
for outcome criteria that are assessed from data from medical forms: Risk of bias is low if the treatment or adverse effects of the treatment could not be noticed in the extracted data (Boutron 2005).
Incomplete outcome data (attrition bias)
Attrition bias due to quantity, nature or handling of incomplete outcome data
Risk of attrition bias is low if no outcome data were missing; reasons for missing outcome data were unlikely to be related to the true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data were balanced in numbers, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes was not enough to have a clinically relevant impact on observed effect size, or missing data were imputed using appropriate methods (if dropouts are very large, imputation using even "acceptable" methods may still suggest a high risk of bias) (van Tulder 2003). The percentage of withdrawals and dropouts should not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and should not lead to substantial bias (these percentages are commonly used but are arbitrary, not supported by the literature) (van Tulder 2003).
Selective reporting (reporting bias)
Reporting bias due to selective outcome reporting
Risk of reporting bias is low if the study protocol is available and all of the study's prespecified (primary and secondary) outcomes of interest in the review have been reported in the prespecified way, or if the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). For this review, at least pain and function need to be reported.
Risk of reporting bias is high if not all of the study's prespecified primary outcomes have been reported; one or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified; one or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; or the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Group similarity at baseline (selection bias)
Bias due to dissimilarity at baseline for the most important prognostic indicators.
Risk of bias is low if groups are similar at baseline for demographic factors, value of main outcome measure(s) and important prognostic factors (examples in the field of back and neck pain are duration and severity of complaints, vocational status and percentage of participants with neurological symptoms) (van Tulder 2003).
Co‐interventions (performance bias)
Bias because co‐interventions were different across groups
Risk of bias is low if no co‐interventions were provided or if they were similar between index and control groups (van Tulder 2003).
Compliance (performance bias)
Bias due to inappropriate compliance with interventions across groups
Risk of bias is low if compliance with the interventions was acceptable, based on reported intensity/dosage, duration, number and frequency for both index and control intervention(s). For single‐session interventions (e.g. surgery), this item is irrelevant (van Tulder 2003).
Intention‐to‐treat‐analysis
Risk of bias is low if all randomly assigned participants were reported/analysed in the group to which they were allocated by randomisation, regardless of the intervention they actually received; if outcome data were measured in all participants and if all randomly assigned participants were included in the analysis.
Timing of outcome assessments (detection bias)
Bias because important outcomes were not measured at the same time across groups
Risk of bias is low if all important outcome assessments for all intervention groups were measured at the same time (van Tulder 2003).
Other bias
Bias due to problems not covered elsewhere in the table
Risk of bias is low if the study appears to be free of other sources of bias not addressed elsewhere (e.g. study funding).
Data and analyses
Comparison 1. Treatments that start immediately post‐surgery. Exercise versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain on VAS (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Functional status on VAS (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Treatments that start immediately post‐surgery. High intensity versus low intensity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain on VAS (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Pain on VAS (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Return to work (short term) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Treatments that start immediately post‐surgery. Exercise versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional status on Modified Oswestry (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Functional status on Modified Oswestry (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Pain on VAS (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Pain on VAS (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 4. Treatments that start immediately post‐surgery. Standard care versus standard care and neural mobilisation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional status (short term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Functional status (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Pain on VAS (short term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Pain on VAS (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Global perceived effect (long term) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Treatments that start four to six weeks post‐surgery. Exercise versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (post‐treatment) | 7 | 272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.55, ‐0.24] |
| 2 Functional status (post‐treatment) | 6 | 252 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.22, ‐0.12] |
| 3 Functional status (long term) | 3 | 226 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.49, 0.04] |
| 4 Pain on Low Back Pain Rating Scale (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Functional status on Low Back Pain Rating Scale (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Functional status on Low Back Pain Rating Scale (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Pain on Low Back Pain Rating Scale (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Pain on Low Back Pain Rating Scale (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 6. Treatments that start four to six weeks post‐surgery. High‐intensity exercise versus low‐intensity exercise programmes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (short term) | 2 | 103 | Mean Difference (IV, Random, 95% CI) | ‐10.67 [‐17.04, ‐4.30] |
| 2 Function (short term) | 2 | 103 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.17, ‐0.36] |
| 3 Functional status on Low Back Pain Rating Scale (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Functional status on Low Back Pain Rating Scale (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Pain on Low Back Pain Rating Scale (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Pain on Low Back Pain Rating Scale (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Functional status on Roland‐Morris Disability Questionnare (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Pain on VAS (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Return to work (long term) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Treatments that start four to six weeks post‐surgery. Supervised programmes versus home exercises.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (short term) | 5 | 229 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐2.04, 0.53] |
| 2 Functional status (short term) | 5 | 229 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.88, 0.15] |
| 3 Global perceived effect (short term) (four‐point scale) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Global perceived effect (long term) (four‐point scale) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Pain (long term) (five‐point scale) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Pain (long term) (VAS) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Functional status (long term) (12‐point scale) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 8. Treatments that start four to six weeks post‐surgery. Exercise and education versus education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional status on Modified Oswestry (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 9. Treatments that start four to six weeks post‐surgery. Exercise and booklet versus exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional status on Modified Oswestry (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Functional status on Modified Oswestry (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Pain on VAS (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Pain on VAS (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 10. Treatments that start four to six weeks post‐surgery. Multidisciplinary programme versus usual care PT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global perceived effect (long term) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Return to work (long term) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 11. Treatments that start four to six weeks post‐surgery. Behavioural graded activity versus usual care PT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global perceived effect (post‐treatment) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Global perceived effect (long term) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Functional status on Roland‐Morris Disability Questionnare (post treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Functional status on Roland‐Morris Disability Questionnare (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Pain on VAS (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Pain on VAS (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 12. Treatments that start four to six weeks post‐surgery. Strength and stretching versus stretching.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional status on Modified Oswestry (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Pain on VAS (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 13. Treatments that start longer than 12 months post‐surgery. Low‐tech exercise versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional status on Modified Oswestry (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 14. Treatments that start longer than 12 months post‐surgery. High‐tech exercise versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional status on Modified Oswestry (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 15. Treatments that start longer than 12 months post‐surgery. High‐tech versus low‐tech exercise.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional status on Roland‐Morris Disability Questionnare (post ‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 16. Treatments that start longer than 12 months post‐surgery. Dynamic exercise versus dynamic exercise and hyperextension.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Functional status (short term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Pain (short term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alaranta 1986.
| Methods | Patients were randomly assigned with stratification on sex and age (older than 40 years) before the operation | |
| Participants | 212 patients after first‐time disc surgery performed for lumbar prolapse: operation that was usually carried out through an interlaminar trepanation and sequesters and any loose nucleus pulposus material was removed | |
| Interventions | Immediate postoperative care the same in both groups: out of bed day after surgery, two one‐hour health education lessons. (I) Start four weeks after surgery (N = 106): multifactorial rehabilitation (physiatrist, physical and occupational therapists, psychologist, social worker) for two weeks, "Intensive Back School". Encouraging physical activities. (C) Normal care: not described | |
| Outcomes | All numbers: one‐year follow‐up. Global perceived effect (five‐point scale). "Much better" or "Better": (I) 88%, (C) 83% not statically significant (NB: includes surgery!). Occupational handicap (WHO scales) and total sick leave during one‐year follow‐up period. No significant differences between groups. Reoperations: (I) 4/106, (C) 4/106: no difference | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear from text |
| Allocation concealment (selection bias) | Unclear risk | Unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Rehabilitation versus UC |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Rehabilitation versus UC |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Participant reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Data on all participants reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Unclear from text |
| Selective reporting (reporting bias) | High risk | WHO scale handicap and sick leave, pain not reported |
| Similarity of baseline characteristics? | Unclear risk | Unclear from text |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text |
| Compliance acceptable? | Unclear risk | Unclear from text |
| Timing outcome assessments similar? | Low risk | One‐year follow‐up |
Choi 2005.
| Methods | Randomised | |
| Participants | 75 patients, mean age 46.09 years, male and female, with primarily leg pain not responding to conservative treatment, who had undergone discectomy | |
| Interventions | Intervention (I: n = 35): advice, lumbar extension exercise handout, home exercise for six weeks, then intensive training for 12 weeks. MedX system, which restricts hip and pelvic motion Control (C: n = 40): advice, lumbar extension exercise handout, six weeks of home‐based exercise, continued for another 12 weeks | |
| Outcomes | Pain intensity (VAS): largely decreased in both exercise and control groups after 12 weeks of extension exercise. (I) 2.51 and (C) 4.3 (P < 0.05) post‐treatment, (I) 1.5 and (C) 1.3 at one year (no significant difference). Functional status (ODI): Postoperative ODI scores were improved compared with preoperative ODI scores (I) 24.6 and (C) 30.6 post‐treatment (no significant difference). Return to work: More than 92% returned to work within six months after surgery. (I) 87% and (C) 24% returned within four months | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned/participants and methods paragraph one |
| Allocation concealment (selection bias) | Unclear risk | Participants were randomly assigned/participants and methods paragraph one |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Home based versus clinic based |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Home based versus clinic based |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Participants were not blinded, patient reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | Five of 40 IG dropout (patient and methods paragraph two and Table 1), less than 20% but not similar in groups |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | Five dropouts |
| Selective reporting (reporting bias) | Low risk | Pain VAS, function ODI |
| Similarity of baseline characteristics? | Unclear risk | Only age reported, 51.05 versus 42.02, = significantly different |
| Co‐interventions avoided or similar? | Unclear risk | Not described |
| Compliance acceptable? | Unclear risk | Not described |
| Timing outcome assessments similar? | Low risk | Patients and methods paragraph four: baseline six weeks, paragraph six: follow‐up at 12 weeks, one year |
Danielsen 2000.
| Methods | Randomisation "by random number table" | |
| Participants | 63 patients aged 22 to 58 years (range), four weeks after operation for lumbar disc herniation (arcotomy in 36 patients, microsurgical in 27 patients, N = 3 at L3‐L4, N = 34 at L4‐L5, N = 24 L5‐S1) | |
| Interventions | (I) Rehabilitation programme (N = 39): from week 4 to week 12, three times per week (40 minutes a session) exercise therapy; exclusively active, no manual intervention or physical therapist, strengthen muscles (various apparatus), participant tailored. (C) (N = 24): weeks 1 through 3: standard programme, then follow‐up consultation (info about clinical course and clinical examination) with physical therapist every two weeks for eight weeks, formula with mild home exercise programme, relaxing and resting the back, and resuming daily activities gradually, avoiding any kind of heavy work at home | |
| Outcomes | Pain intensity (VAS) absolute values (abs) and mean improvement (MI) (95% CI) at six months: (I) abs 2.3 (1.5 to 3.1) (MI) 3.7 (2.7 to 4.7), (C) abs 3.6 (2.5 to 4.7) (MI) 2.0 (0.7 to 3.3); for functional status (RDQ) (I) abs 5.1 (3.1 to 7.1) (MI) 8.9 (7.0 to 10.8), (C) abs 6.2 (4.1 to 8.4) (MI) 5.4 (3.0 to 7.8). For pain at 12 months: (I) abs.2.8 (1.9 to 3.7) (MI) 3.2 (2.1 to 4.3), (R) abs 3.9 (2.6 to 5.7) (MI) 1.8 (0.5 to 3.1); (RDQ) (I) abs 5.3 (3.2 to 7.4) (MI) 8.7 (6.8 to 10.6), (C) abs 6.3 (3.8 to 8.8) (MI): 5.3 (2.6 to 8.0). Absolute RDQ values minor advantage for (I): 6 and 12 months, on MI significantly larger scores for (I). Pain, both abs. MI significantly better for (I), 12 months no differences between groups. Significantly more participants in (I) participation in daily activities (subscale WONCA) at six months. At 6 and 12 months, no significant differences for overall health or sick leave. No significant changes for analysis with only complete follow‐up | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Result of the allocation was handed over to the project co‐ordinator |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Active rehabilitation versus mild programme at home |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Active rehabilitation versus mild programme at home |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Training three/39 = 8%, five/39 = 13% Control two/24 = 8% |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | ITT, imputation |
| Selective reporting (reporting bias) | Low risk | RDQ, Wonca and VAS |
| Similarity of baseline characteristics? | High risk | Roland and sick leave differences, Table 2 |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text |
| Compliance acceptable? | Unclear risk | Unclear from text |
| Timing outcome assessments similar? | Low risk | Six, 12 months |
Dolan 2000.
| Methods | Blindly randomised | |
| Participants | 20 patients between 18 and 60 years of age (18 men, three women) with radiological evidence of disc prolapse associated with sciatica of less than 12 months' duration (N = 5 L4‐L5), (N = 15 L5‐S1). Type of surgery: microdiscectomy, followed by six weeks of normal postoperative care by physical therapy: advice about exercise and return to normal activities | |
| Interventions | (I) (N = 9) received an exercise programme by experienced physiotherapist, two one‐hour sessions per week for four weeks (start six weeks after surgery); progress at own pace, general aerobic exercises, stretching exercises, extension exercises, strength and endurance exercises (back and abdominal). (C) (N = 11) no further treatment | |
| Outcomes | Pain intensity (VAS) and (pain diary): significant reduction in both groups six weeks after surgery, but (I) showed further decrease (within group) compared with (C). Between groups (12 months) pain (diary): significantly less pain (P < 0.05) in favor of (I) and for pain (VAS) not significant (P value 0.08). Functional status (range 0 to 75, high scores: good status): improvement in both groups after surgery: mean (SD) (I) 54 (24), (C) 50 (25). On 12 months, no between‐group analysis. Behavioural outcomes: little change postsurgery and during follow‐up. ROM and muscle endurance: no differences | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blindly randomised, personal communication |
| Allocation concealment (selection bias) | Low risk | Not described, personal communication |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Exercise versus no treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Exercise versus no treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Results, disability, last par: exercise group: All but one obtained score of 74 to 75, control n = 11, scores presented for n = 3, 2, 5, 1 |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | One participant excluded, personal communication |
| Selective reporting (reporting bias) | Low risk | VAS, low back outcome score |
| Similarity of baseline characteristics? | Low risk | Table 3 |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text |
| Compliance acceptable? | Low risk | Personal communication: All participants in exercise group attended all sessions |
| Timing outcome assessments similar? | Low risk | 10, 26, 52 weeks |
Donaldson 2006.
| Methods | Randomised | |
| Participants | 93 patients, aged 17 to 63 years (mean 41, range 17 to 63, and 42, range 25 to 63) male and female, who had standard open lumbar discectomy (Sprengler technique) | |
| Interventions | Intervention (I, n = 47): six‐month progressive training, start six weeks postop, three sets of repetitions per exercise. Three phases: conditioning, hypertrophy and strength. Abdominals and lifting technique. Control (C: n = 46): surgical advice | |
| Outcomes | Participants in both groups improved significantly from the six‐week baseline measures to the 58‐week outcome measures. No significant between‐group differences. Functional status (ODI): at 58 weeks, mean score and SD (I) 11.66 (2.25) and (C) 12.00 (1.84); P = 0.90. Functional status (RMDQ): at 58 weeks, mean score and SD (I) 4.03 (.91) and (C) 4.53 (.74); P = 0.83. Differences in SF36 not significant: physical category (P = 0.19) and mental category (P = 0.85). Median time to return to work (I) 35 days and (C) 37 days (P = 0.65). Intervention started six weeks postsurgery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated/methodology paragraph one |
| Allocation concealment (selection bias) | Unclear risk | Blinded assessor made use of computer‐generated randomisation |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Treatment versus no treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Treatment versus no treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Participant not blinded, patient reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | One lost per group, two and seven did not complete 58‐week measures = 6.5% and 17%, 11 non‐completers intervention (results par 2 & 4) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | Dropouts, also Fig. 1 |
| Selective reporting (reporting bias) | High risk | No protocol, introduction last par: levels of pain; levels of function/methodology par 2: RMDQ, ODI, pain not mentioned/results: RMDQ, ODI no pain measures. Abstract mentions pain |
| Similarity of baseline characteristics? | Low risk | Age, gender, history, ODI similar table 2/neurological symptoms and pain not reported |
| Co‐interventions avoided or similar? | Low risk | GP eight‐two, therapist seven‐five non‐significant differences (results last paragraph) |
| Compliance acceptable? | Low risk | 36 (77%) completed intervention, four partially (results paragraph three) |
| Timing outcome assessments similar? | Low risk | Study design paragraph one: six‐weeks, paragraph two: three years + annual questionnaire |
Donceel 1999.
| Methods | Randomisation "by computer‐generated random number" | |
| Participants | 710 patients (workers) who have mandatory insurance that introduced a benefit claim after open lumbar discectomy. Age between 15 and 64 years and no longer than one year off work before surgery. Interventions start six weeks postsurgery | |
| Interventions | (I) (N = 345) Rehabilitation‐oriented approach (in insurance medicine) by medical advisor (MA). First visit six weeks postsurgery, functional evaluation, information on medicolegal aspects, rehabilitation, natural history and expected work incapacity period. Encourage and stimulate personal activities and early mobilisation. MA asks treating physician for information regarding diagnosis and treatment, encourages rehabilitation measures, promotes multidisciplinary approach. (C) (N = 365) MA: usual care | |
| Outcomes | On return to work at follow‐up (52 weeks): (I) 89.9% (C) 81.9%. Statistically significant | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number |
| Allocation concealment (selection bias) | High risk | Medical advisors were randomly assigned |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Rehabilitation versus usual care |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Rehabilitation versus usual care |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Unclear risk | Unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Results last paragraph: No participants dropped out |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Personal communication |
| Selective reporting (reporting bias) | High risk | RTW only |
| Similarity of baseline characteristics? | Low risk | Results paragraph two: significant difference in gender only |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text |
| Compliance acceptable? | Low risk | Personal communication |
| Timing outcome assessments similar? | Low risk | 52 weeks |
Erdogmus 2007.
| Methods | Randomised, three groups | |
| Participants | 120 patients, male and female, aged 41.8 ± 10.4; 42.3 ± 9.8; 39.8 ± 10.5. Standard laminectomy and (micro)discectomy after mean 5.8 to 6.5 weeks of complaints. Level of surgery: L3–L4: 7; L4–L5: 45; L5–S1: 57 | |
| Interventions | Intervention (PT: n = 40): physiotherapy‐based rehabilitation start one week postoperatively, 12 weeks, 20 sessions of 30 minutes, strength, stretching, ergonomics, improvement in general mobility of the spine, improving muscle coordination and automatic muscle response time. Sham therapy (S: n = 40): 20 sessions “sham” neck massage of 30 minutes’ duration each. Control (C: n = 40): no treatment | |
| Outcomes | Post‐treatment scores (mean, 95% CI) on functional status (LBPRS) were (PT) ‐15.98 (‐18.02 to ‐13.9), (S) ‐13.23 (‐15.35 to ‐11.1) and (C) ‐12.15 (‐14.59 to ‐9.71). Significant difference PT versus C: ‐3.82 (‐6.96 to ‐.69), P = 0.017. No difference PT versus S: ‐2.75 (‐5.65 to 0.15), P = .063. At one year, scores were (PT) ‐13.83 (‐16.71 to ‐10.94), (S) ‐13.2 (‐15.66 to ‐10.74) and (C) ‐11.37 (‐14.16 to ‐8.58). No significant differences: PT versus C: ‐2.45 (‐6.41 to 1.50), P = 0.220 and PT versus S: ‐.63 (‐4.36 to 3.11), P = 0.74. Post‐treatment scores (mean, 95% CI) on pain (LBPRS) were (PT) ‐4.1 (‐6.59 to ‐1.61), (S) ‐2.91 (‐6.53 to 0.7) and (C) 0.82 (‐2.8 to 4.43). Significant difference between PT and C: ‐4.92 (‐9.23 to ‐.60), P = 0.026. No difference between PT and S: ‐1.19 (‐5.51 to 3.14), P = 0.59. At one year, scores were (PT) ‐2.05 (‐6.27 to 2.17), (S) ‐3.81 (‐8.18 to 0.56) and (C) 1.2 (‐3.29 to 5.68). No differences between PT and C: ‐3.24 (‐9.31 to 2.82), P = 0.29 and PT versus S: 1.77 (‐4.21 to 7.74), P = 0.56. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocks |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes/randomisation and blinding |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | PT, massage, no treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | PT, massage, no treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Participant not blinded, patient reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | One PT, two sham, six control dropouts, later lost four, six, two/12.5%, 20%, 20% (results/Figure 1) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | ITT (statistical analysis paragraph two), but dropouts |
| Selective reporting (reporting bias) | Low risk | No protocol found, pain, disability: LBP‐RS outcome measures paragraph two. Results presented Table 4 |
| Similarity of baseline characteristics? | Low risk | Demographics, history, lasegue Table 2, LBPRS Table 3 + 4 |
| Co‐interventions avoided or similar? | Unclear risk | Personal communication: type/frequency of medication: no difference at the end of therapy. Main comparison of PT versus no therapy: P value 0.8 at three months Other interventions not reported |
| Compliance acceptable? | Low risk | Personal communication: The weekly amount of home exercise was about 20 minutes and did not differ between groups: Results of main comparison (PT vs no therapy): P value 0.8 at three months and 0.4 at 1.5 years |
| Timing outcome assessments similar? | Low risk | Outcome measures paragraph one, Tables 3 and 4 |
Filiz 2005.
| Methods | Randomised by opaque envelopes prepared by independent person | |
| Participants | 60 patients (three arms) included one month after first‐time lumbar disc surgery. Aged between 20 and 50 years. Only short‐term follow‐up | |
| Interventions | (I1, N = 20) intensive exercise programme and back school education under supervision for eight weeks; three days a week with sessions of 1.5 hours each. (I2, N = 20) back education and McKenzie and Williams exercise in home programme for eight weeks; advice to practice three days/wk. (C, N = 20) no treatment | |
| Outcomes | RTW in days (I1) 56.07 (18.66) versus (I2) 75.0 (24.9) versus (C) 86.2 (27.1). Pain (post‐treatment score on VAS): (I1) 4.5 (1.6) versus (I2) 12.0 (3.7) versus (C) 13.3 (7.3). Functional status (post‐treatment scores on Modified Oswestry): (I1) 7.1 (4.9) versus (I2) 11.7 versus (C) 15.1 (8.6) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised opaque envelopes prepared by independent person |
| Allocation concealment (selection bias) | Low risk | Sheets of opaque paper, folded and taped from the corners, put in a box |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Intensive exercise versus home exercise |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Intensive exercise versus home exercise |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | Unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Dropouts, if any, not reported |
| Selective reporting (reporting bias) | Low risk | VAS, ODI, LBPRS |
| Similarity of baseline characteristics? | Low risk | Table 1 |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text |
| Compliance acceptable? | Unclear risk | Unclear from text |
| Timing outcome assessments similar? | Low risk | Eight weeks (post‐treatment) |
Filiz 2005 (1).
| Methods | See Filiz 2005 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Hakkinen 2005.
| Methods | Randomly assigned | |
| Participants | 126 patients included two months after their first lumbar disc surgery and not pain free (VAS > 10 mm) | |
| Interventions | (I) Home exercise programme after one instruction session for 12 months. Instructions for stretching and stabilisation exercises, instructed to stretch three times AND strength training, instructed to perform two series of exercises twice a week (C) Home exercise programme after one instruction session for 12 months. Instructions for stretching and stabilisation exercises, instructed to stretch three times | |
| Outcomes | At 12 months' follow‐up: improvement in back pain (100‐mm VAS): (I) 4 mm (IQR: ‐11 to 5) versus (C) 1 mm (IQR : ‐7 to 9); leg pain (100‐mm VAS): (I) ‐2 (IQR: ‐7 to 7) versus (C) ‐2 (IQR: ‐7 to 3). Improvement in disability (ODI) (I): 3 mm (IQR: ‐6 to 1) versus (C): ‐2 (‐5 to 1) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomly assigned |
| Allocation concealment (selection bias) | Unclear risk | Unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | Unclear risk | Unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | Unclear risk | Unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | Unclear risk | Unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | Unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Unclear from text |
| Selective reporting (reporting bias) | Low risk | VAS, Oswestry and Million’s Disability Indices (outcome measurements/results) |
| Similarity of baseline characteristics? | Low risk | Table 1 |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text |
| Compliance acceptable? | High risk | Results paragraph two, Figure 2 |
| Timing outcome assessments similar? | Low risk | Six, 12 months |
Johannsen 1994.
| Methods | Randomised by minimisation and stratified for sex, age (cutoff 40 years) ± preoperative hospitalisation ± postoperative complications | |
| Participants | 40 patients undergoing a first lumbar discectomy (L4‐L5) for classic nerve root compression symptoms without cauda equina and confirmatory imaging; at least two weeks of unsuccessful conservative therapy; aged between 18 and 65 years, employed were included. Excluded: specific other diseases of spine or hip or system diseases. Interventions start within four to six weeks after surgery | |
| Interventions | (I) (N = 20) supervised group training (max 10 participants) one hour twice a week for three months. Session: 10 minutes warming up bicycle, dynamic exercises (endurance) for low and high back, buttock and abdominal muscles supervised by PT. (C) (N = 20) individual training at home with two hours of instruction by PT plus written instructions. Same exercises as (I) | |
| Outcomes | Back pain (five‐point scale): T0, three, six months; median and 12.5 percentiles: (I): 4.1 (2.5 to 6.0), 2.8 (1.8 to 4.8), 2.8 (1.8 to 4.2), (C) 4.0 (2.0 to 5.9), 2.4 (1.7 to 4.2), 2.5 (1.8 to 5.8) Global Perceived Effect (four‐point scale, 0 = good, 3 = bad): (I) 1.6 (0.8 to 2.5), 1.1 (0.7 to 1.9), 1.0 (0.6 to 1.5), (C) 1.4 (0.7 to 2.2), 1.2 (0.7 to 2.0), 1.3 (0.7 to 2.9). No differences except extension strength at three months for (C). ROM (sum‐score in cm) (I): 12 (‐3 to 26), 26 (19 to 41), 27 (8 to 37), (C) 16 (2 to 29), 23 (17 to 30), 26 (15 to 41). Disability (12‐point scale with 12 = maximum disability) (I) 3 (0 to 4), 0 (0 to 2), 0 (0 to 3), (C) 2 (0 to 5), 0 (0 to 2), 0 (0 to 2) (NS). Isokinetic trunk extension strength: (I) 36 (13 to 48), 45 (23 to 57), 50 (34 to 77), (C) 47 (12 to 59), 54 (35 to 69), 64 (45 to 73). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by minimisation and stratified for sex |
| Allocation concealment (selection bias) | Low risk | Personal communication |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Supervised versus home exercises |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Supervised versus home exercises |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | Nine/20 = 45% and four/20 = 20% dropouts |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | Dropouts |
| Selective reporting (reporting bias) | Low risk | Pain and function in methods, reported in Table 2 |
| Similarity of baseline characteristics? | Unclear risk | Unclear, baseline dropouts not reported |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text |
| Compliance acceptable? | Unclear risk | Unclear from text |
| Timing outcome assessments similar? | Low risk | Three, six months |
Johansson 2009.
| Methods | Randomised | |
| Participants | 59 patients, aged 18 to 60 (median 43 (IQR 35 to 47) and 38 (IQR 31 to 43) years), male and female, who had standard microdiscectomy after a median of 10 (IQR 6 to 24) versus six (IQR 4 to 17) months. Level of surgery: L5‐S1: 30; L4‐5: 26; L2‐3: 2; L3‐4: 1. | |
| Interventions | Both groups: first day after surgery start: stabilisation of the back and hip mobility, activation back, abdominal and buttock muscles, transfers. A written exercise programme to follow at least once a day. Clinic‐based training (I1: n = 29): start three weeks postop: clinic‐based training (including recommended home exercises), exercises, BGA for eight weeks, 1/wk. Home‐based training (I2; n = 30): continue at three weeks postoperative: recommended exercises at home. | |
| Outcomes | Functional status and pain improved but did not differ significantly between groups. Functional status (ODI) post‐treatment, median difference and IQR (I1) ‐20 (‐5 to ‐36) and (I2) ‐20 (‐13 to ‐36), P = 0.49. At 12 months, (I1) ‐23 (‐9 to ‐38) and (I2) ‐32 (‐17 to ‐42), P = 0.09. Leg pain (VAS) post‐treatment (I1) ‐32 (‐14 to ‐71) and (I2) ‐53 (‐23 to ‐77), P = 0.34. At 12 months, (I1) ‐23 (‐11 to ‐67) and (I2) ‐58 (‐32 to ‐80), P = 0.06. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, blocks of four/methods/participants paragraph four |
| Allocation concealment (selection bias) | Low risk | Numbered concealed envelopes/methods/participants paragraph four |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Clinic versus home |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Clinic versus home |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Participants not blinded, participant‐reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Missing data at 12 months: one per group, Figure 1 |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | ITT (statistics paragraph four), Figure 1: missing data |
| Selective reporting (reporting bias) | Low risk | No protocol found, ODI and VAS for pain (OM), results and Table 2 |
| Similarity of baseline characteristics? | Low risk | Gender, duration, Table 1, ODI, pain, Table 2. Neurological symptoms not reported |
| Co‐interventions avoided or similar? | High risk | Healthcare provider home clinic: four to one = 13% to 3% (paragraph on treatment by other healthcare providers) |
| Compliance acceptable? | Low risk | 25/29 all clinic sessions, 25/30 did home exercises (compliance paragraphs one + two) |
| Timing outcome assessments similar? | Low risk | Outcome measures paragraph one: baseline, three, 12 months/Figure 1 |
Ju 2012.
| Methods | Randomised | |
| Participants | 14 patients who underwent lumbar disc surgery, aged 45.2 ± 3.96 years and 46.2 ± 5.3 years | |
| Interventions | I: Medx lumbar extension programme and progressive resistance exercise, 12‐week programme three times/week, 70 minutes per session, postoperative conservative treatment period was 15.57 ± 2.94 days. C: no exercise rehabilitation programme, postoperative conservative treatment period was 15.43, SD 3.74 days. | |
| Outcomes | I: VAS scores: back pain preoperative 5.11 ± 1.10, postoperative 4.35 ± 0.94, handicap/functional status preoperative 5.27 ± 1.68, postoperative 2.28 ± 0.75. C: VAS scores: back pain preoperative 5.42 ± 1.61, postoperative 5.80 ± 1.89, handicap/functional status preoperative 6.55 ± 0.92, postoperative 6.27 ± 1.05 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Subjectsts and methods: randomly allocated, not described how |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Exercise versus no treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Exercise versus no treatment |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | No dropouts |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Unclear from text |
| Selective reporting (reporting bias) | Low risk | No protocol, methods mentions VAS for pain and function, reported in results and in Table 2 |
| Similarity of baseline characteristics? | Low risk | Subjects and methods, Tables 1 + 2 |
| Co‐interventions avoided or similar? | Unclear risk | Not described |
| Compliance acceptable? | Unclear risk | Not described |
| Timing outcome assessments similar? | Low risk | Table 2: pretreatment and post‐treatment measures |
Kjellby‐Wendt 1998.
| Methods | Randomised according to a table of random numbers | |
| Participants | 60 patients (aged 16 to 70 years), microdiscectomy after not responding to conservative treatment. Patients with reoperation other surgery as microdiscectomy without microscope (e.g. laminectomy). Interventions start immediately after surgery | |
| Interventions | (I) (N = 29) Total duration is 12 weeks, starting directly after surgery: out of bed from prone position, increased ADL and lumbar support (sitting). First six weeks home training (five to six times per day) with mobilisation of neural structures and low back, increased trunk strength (functional positions), correct work posture and pain coping. Second six weeks (five to six times per day) mainly intensive muscle strength and flexion exercises and cardiovascular exercises (in total: four instruction sessions). (C) Total duration 12 weeks, starting directly after surgery out of bed from side position, no increase in ADL and no lumbar support (sitting). First six weeks: abdominal exercises (once a day) lying position. Second six weeks: more intensive strength exercises, mobilisation of spine. No promotion of cardiovascular exercises (total: three instruction sessions) | |
| Outcomes | At two years: satisfaction (I) 88%, (C) 67%. Percentage of positive SLR (three weeks): (I) 0 (C) 7, significant difference. At 6, 12, 52 weeks: no significant differences. At 52 weeks: (I) 30 (14.8) extension increased and (C) flexion 42 (11.5) significantly increased. Patients pain free at 6 weeks: (I) 8 and (C) 4; no differences at 12, 52, 104 weeks. Leg pain intensity (VAS) (in patients with sciatica) at 6, 12, 52 weeks (mean, SD): (I) 1.0 (0.6), 1.5 (0.9) 2.7 (0.5), (C) 4.1 (2.9), 3.4 (2.2), 3.0 (1.9); statistically significant at 6 weeks, 12 weeks, not at 52 weeks. At five to seven years' follow‐up: (I) 52%, (C) 50% pain (leg); (I) 73% (C) 60% pain (back). Participants on sick leave at 12 weeks: (I) 10 (C) 15 (NS). At 52 weeks: (I) 120 (75) days and (C) 153 (107) days on sick leave. At two years: (I) 88% (C) 67% satisfied with result. (I) 10 = 40% (C) 8 = 33% no pain. During two to five years after surgery, no differences in days of sick leave: (I) 146 (SD: 243 days) (C) 157 days (SD: 203). On five to seven years' follow‐up, no differences in numbers of participants with leg pain: (I) 16/30 (C) 15/30 or in numbers of participants with back pain: (I) 22/30, (C) 18/30 | |
| Notes | Unpublished data were used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Low risk | Personal communication: nurse allocated participants |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Intensive versus less intensive |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Intensive versus less intensive |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | 5/23 = 22% 12‐week control |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | Dropouts |
| Selective reporting (reporting bias) | High risk | Pain, not function, reported on a VAS |
| Similarity of baseline characteristics? | Low risk | Tables 1, 2, 3 |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text |
| Compliance acceptable? | Unclear risk | Uunclear, response low |
| Timing outcome assessments similar? | Low risk | 12, 52 weeks |
Kulig 2009.
| Methods | Randomised | |
| Participants | 98 participants, aged 18 to 60 years (mean age 39.2 (10.2) and 41.4 (9.9) years), male and female who underwent microdiscectomy after first sciatica 33.1 (67.6) to 38.7(69.8) months ago. Level of surgery: L2‐3: 1, L4‐5: 43, L5‐S1: 54 | |
| Interventions | Intervention (I: n = 51): USC Spine Exercise Programme + one back care education session. Back extensor strength and endurance training (using a variable‐angle Roman chair) and mat and therapeutic exercise training. 12 weeks of training, 3/wk, start four to six weeks postoperatively. Control (C: n = 47): a one‐hour back care education single session, four to six weeks postoperatively. | |
| Outcomes | Functional status (ODI) post‐treatment, mean change score and 95% CI: (I) ‐18.4 (‐22.5 to ‐14.3) and (C) ‐9.4 (‐13.0 to ‐5.8); significant difference P < 0.001 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation/method interventions paragraph one |
| Allocation concealment (selection bias) | Low risk | Personal communication: data management provided the random participant number to the study coordinator, who provided it to the blinded tester and interventionist |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Education versus education + exercise |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Education versus education + exercise |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Participant was not blinded, patient reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | Dropouts 11.8%, 31.9% (Figure 2) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | ITT and as treated, but dropouts |
| Selective reporting (reporting bias) | High risk | Protocol: ODI/RDQ/VAS: 'administered immediately after sitting for 10 min, after 5MWT' (POM paragraph five, p 10). Reported: ODI, not pain (OM) ODI, not pain (results, Tables 2 + 3) |
| Similarity of baseline characteristics? | Low risk | Demographics, history, neurological symptoms, ODI Table1 |
| Co‐interventions avoided or similar? | High risk | Education: 19/32 to PT Figure 2, results, recruitment and retention paragraph one. Analysis post hoc: three groups |
| Compliance acceptable? | High risk | Cross‐over to PT or other group (results, recruitment and retention paragraph one) |
| Timing outcome assessments similar? | Low risk | Outcome measures paragraph one: four to six weeks postop/Abstract I&O: four to six weeks, 12 weeks later. Protocol: methods/design: pre/post interv outcome measures: pre/post interv, one year Q/VAS: every six months for four years |
Manniche 1993a.
| Methods | Randomised by drawing of lots | |
| Participants | 96 patients (49 men, 47 women) who had undergone first‐time discectomy for lumbar disc protrusion, aged 18 to 70 years. Interventions start five weeks after surgery | |
| Interventions | Both groups: (in classes of two to six patients) in total 14 hours, including five instruction and ergonomics sessions. (I) Intensive exercises: (start session hot packs and five heavy exercises: (1) leg lifting, (2) trunk lifting (one and two from 45 degrees flexion to 0 degrees), (3) abdominal exercise, (4) leg abduction, (5) leg adduction (10 repetitions each). End of session: six minutes submaximal bicycle training and five stretching exercises. Six one‐hour sessions, twice a week; next three weeks, six 30‐minute sessions in water (same principles, no limits to range of motion) (including rotatory elements). Pain was no reason for stopping. (C) 15 mild general mobilisation exercises, 10 repetitions each, programme started with six 30‐minute sessions (twice a week) in water. Next three weeks, same principles in gymnasium. If pain occurred: stop. | |
| Outcomes | Overall improvement at 52 weeks: (I) 76% (C) 70% "very satisfactory" or "satisfactory, little discomfort". Not significantly different. Medians: pretreatment post‐treatment: 6, 12, 26, 52 weeks: on low back pain scale 0 to 30: (I) 5.5, 2.0, 1.8, 5.2, 3.7; (C) 7.1, 3.4, 2.4, 5.5, 6.5, no significant differences; on leg pain scale (0 to 30): (I) 4.5, 2.2, 3.0, 3.0, 0.8, (C) 4.8, 3.2, 3.0, 5.0, 2.2; no significant differences; on disability scale (0 to 30): (I) 10.8, 4.5, 4.4, 4.0, 4.2, (C) 11.5, 6.1, 4.3, 6.5, 6.0, statistically significant at 26 weeks. Physical impairment scale (0 to 40): pretreatment, post‐treatment and 6 weeks: (I) 16.2, 11.8, 12.5, (C) 16.8, 11.8, 12.3, no significant difference. All scales are subscales of Low‐Back Pain Rating Scale (high scores denote poor outcome). Days off work in (I) significantly less on 26 and 52 weeks. Number of participants not returned to work: (I) 14.3% (C) 30%: statistically significant. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by drawing of lots |
| Allocation concealment (selection bias) | Low risk | Personal communication |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | High‐ versus low‐intensity programme |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | High‐ versus low‐intensity programme |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | Size of intervention and control groups unclear |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | Dropouts |
| Selective reporting (reporting bias) | Low risk | Pain and function in methods (last paragraph) and reported in results, Table 3 |
| Similarity of baseline characteristics? | Low risk | Results paragraph two |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text |
| Compliance acceptable? | Low risk | Personal communication |
| Timing outcome assessments similar? | Low risk | 6, 26, 52 weeks |
Manniche 1993b.
| Methods | Randomised by drawing of envelopes | |
| Participants | 62 patients (30 men, 32 women) with chronic low back pain occurring 14 to 60 months after first‐time discectomy for lumbar disc protrusion; participants' self reported global assessment of operation outcome was "good", "fair" or "unchanged". Interventions start 14 to 60 months post‐surgery | |
| Interventions | (I) (N = 31) Intensive dynamic exercise with hyperextension, start session with hot pack (optional) (20 minutes), followed by (1) trunk lifting, (2) leg lifting: one and two with greatest possible extension, (3) abdominal exercise (all in series of 10; one minute rest in between), (4) pull to neck (50 times). Two sessions a week (one session: 60 to 90 minutes), total of 24 sessions in three months. (C) (N = 31) exactly the same procedure, but in the 1st and 2nd exercise, the movement range of the back and hip is only from 90 degrees flexion to 0 degrees. No hyperextension allowed. | |
| Outcomes | Overall improvement post‐treatment, 3 months and one year (at one year) (I) 38%, (C) 61% scored "very satisfactory" or "satisfactory, little discomfort" not statistically significant. Improvement functional status (low back pain rating scale 0 to 130) post‐treatment, 3, 12 months (Median 10th to 90th percentile): (I) 10 (0 to 31), 8 (‐15 to 28), 3 (‐11 to 23), (C) 7 (‐13 to 22), 1 (‐14 to 9), 0 (‐26 to 9), statistically significant at 3 months only. Post‐treatment, both groups significantly improved | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by drawing envelopes |
| Allocation concealment (selection bias) | Low risk | Personal communication |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | With versus without hyperextension |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | With versus without hyperextension |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | 5/31 = 16%, 10/31 = 32% |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | Dropouts |
| Selective reporting (reporting bias) | Low risk | Pain and function in methods, reported in Table 4 |
| Similarity of baseline characteristics? | Low risk | Tables 3, 4 |
| Co‐interventions avoided or similar? | Low risk | Personal communication: no problems with compliance observed |
| Compliance acceptable? | Low risk | 47 = 76% completed intervention, results |
| Timing outcome assessments similar? | Low risk | Three, 12 months |
McGregor 2011.
| Methods | Randomised, four groups | |
| Participants | 140 participants, mean (SD) age 43 (11); 44 (10); 44 (11); 46 (11) years, male and female, with root symptoms and signs and MRI confirmation of lumbar disc herniation, primarily leg pain for a median of 20 to 32 months, who had discectomy, according to the surgeons’ routine practice | |
| Interventions | Rehabilitation (I1, n = 37): start six to eight weeks postop rehab programme, 12 one‐hour classes, aerobic fitness; stretching; stability exercises; strengthening and endurance training for the back, abdominal, and leg muscles; ergonomic training; advice on lifting and setting targets; and self motivation. Rehabilitation and booklet (I2, n = 42): same programme and educational booklet ‘Your Back Operation’. Control (C, n = 40): managed according to the relevant surgeon’s usual practice, which varied and was limited |
|
| Outcomes | Functional status (ODI) post‐treatment, mean change and 95% CI: (I1) ‐20 (‐14 to ‐25), (I2) ‐23 (‐16 to ‐29) and (C) ‐24 (‐19 to ‐29). At one year: (I1) ‐24 (‐18 to ‐29), (I2) ‐25 (‐20 to ‐31) and (C) ‐26 (‐21 to ‐31). Leg pain (VAS) post‐treatment, mean change and 95% CI: (I1) ‐33 (‐24 to ‐42), (I2) ‐37 (‐27 to ‐47) and (C) ‐39 (‐33 to ‐46). At one year: (I1) ‐40 (‐32 to ‐48), (I2) ‐43 (‐36 to ‐51) and (C) ‐41 (‐33 to ‐48) | |
| Notes | Only data from three of four groups are used for this review and are presented here | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central telephone randomisation, random permuted blocks/methods paragraph two |
| Allocation concealment (selection bias) | Unclear risk | Not described how/methods paragraph two |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Exercise versus no exercise, booklet versus no booklet |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Exercise versus no exercise, booklet versus no booklet |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Participant was not blinded, patient reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | 3/37 = 8.1% dropouts rehab, 1/42 = 2.4% dropouts rehab + booklet, 4/40 = 10% usual care |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Intention‐to‐treat (statistical analysis paragraph two)/imputation (paragraph three) |
| Selective reporting (reporting bias) | Low risk | ODI, VAS for pain (OM), reported in analysis of trial interventions + supplement tables |
| Similarity of baseline characteristics? | Low risk | Supplement Table 1 |
| Co‐interventions avoided or similar? | Low risk | Personal communication: Participants were asked if they instigated any treatments; this was minimal if any |
| Compliance acceptable? | High risk | Relatively high % non‐compliance |
| Timing outcome assessments similar? | Low risk | Methods last line: preop, six weeks, 3/6/9/12 months, tables |
Newsome 2009.
| Methods | Randomised | |
| Participants | 30 participants, aged 21 to 72 years (median age 38 (IQR 27 to 43.5) and 37 (IQR 30.5 to 45) years), male and female, who had complaints for a median of 10 (IQR 7 to 16) to 12 (IQR 8.5 to 13.5) months and underwent microdiscectomy (Caspar) | |
| Interventions | Intervention (I, n = 15): immediate physiotherapy (two hours post‐surgery) consisting of 10 times flexion of knee/hip, out of bed after four to five hours, advice + exercise sheet, additional physiotherapy after four weeks if < 10% improvement ODI. Control (C, n = 15): standard physiotherapy care, which is out of bed after four to five hours, advice + exercise sheet. Additional physiotherapy after four weeks if < 10% improvement ODI | |
| Outcomes | At four weeks after surgery, a significant reduction for all participants in ODI score (P < 0.001), VAS back (P < 0.001), VAS leg (P < 0.001) and McGill pain scores (P < 0.001). No significant differences between groups were noted at four weeks or at three months. Return to work (I) median six weeks and (C) eight weeks, median difference two weeks, 95% CI 0 to 6, P = 0.002 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers/materials and methods paragraph one |
| Allocation concealment (selection bias) | Unclear risk | Participants were allocated/materials and methods paragraph one |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Immediate start or not |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Immediate start or not |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Participants were not blinded, patient reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | Dropouts = 26.6%, 20% (Figure 1) |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | Participants with recurrences and missing data not included (results paragraph one) |
| Selective reporting (reporting bias) | Low risk | No protocol, ODI, VAS + McGill for pain (materials and methods paragraph three) as secondary outcomes measure, all reported (results) |
| Similarity of baseline characteristics? | Low risk | Demographics, history Table 1, before surgery no differences ODI and VAS/McGill (results paragraph four). Neurological symptoms not reported |
| Co‐interventions avoided or similar? | Unclear risk | Additional rehab similar (Figure 1) but no reports on consultation with others outside the study |
| Compliance acceptable? | Unclear risk | Hospital‐based intervention: not reported (two lost to 4‐week follow‐up (results/Figure 1)) |
| Timing outcome assessments similar? | Low risk | Materials and methods paragraph three: preop, 4 weeks, 3 months postop |
Ostelo 2003.
| Methods | Randomised by a priori prepared, opaque and sealed envelopes | |
| Participants | 105 patients still suffering complaints six weeks post‐surgery | |
| Interventions | (I: N = 52) Behavioral graded activity (operant therapy) using graded activity and positive reinforcement, time‐contingency management. Based on baseline measurements, an individually graded exercise training programme was established, using quota setting. In total, 18 sessions (30 minutes a session) over a three‐month period (R: N = 53) Physiotherapiy programme: ADL instructions, exercise trunk muscles (increase strength and stability). Mobilisation exercises. Number of sessions (of 30 minutes each) at the discretion of therapists (max 18 sessions) |
|
| Outcomes | Global perceived effect: I: 48% recovered versus R: 67% (three short‐term) and 75% (I) versus 73% on one‐year follow‐up Functional status (24‐item RDQ): I: mean improvement (–5.2, SD: 5.9) versus (5.6, SD: 5.3) for R on the short term, long term: I: (7.0, SD: 5.5) versus R: (7.0, SD: 5.3) Pain back (VAS): mean improvement: I: (9.3, SD: 27.8) versus R: (16.0, SD: 25.3) short term; one‐year follow‐up: I: (17.6, SD: 32.5) versus R: (22.4, SD: 33.0) Cost‐effectiveness analysis: Total direct costs in behavioural graded activity are EUR 639 (95% CI 91 to 1368) higher than physiotherapy costs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | A priori prepared opaque and sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | BGA versus UC PT |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | BGA versus UC PT |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | High risk | Number of dropouts not similar in the two groups: 1/53 = 2% and 7/52 = 14% short term, 2/53 = 4% and 10/52 = 19% |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Low risk | Figure 1 |
| Selective reporting (reporting bias) | Low risk | VAS for pain and RDQ in outcome measurements and reported in results |
| Similarity of baseline characteristics? | Low risk | Table 1 |
| Co‐interventions avoided or similar? | High risk | Number of visits to allied health professionals higher in BGA (economic evaluation article, results) |
| Compliance acceptable? | Low risk | UC on average 15.5 treatments, BGA 14.8 |
| Timing outcome assessments similar? | Low risk | 3,6,12 months |
Scrimshaw 2001.
| Methods | Randomisation by random numbers table, unclear concealment | |
| Participants | 81 patients undergoing spinal surgery randomly assigned, 59 of whom underwent laminectomy or discectomy. Others were operated for fusion | |
| Interventions | (I) (N = 32) Standard postoperative care (isometric and dynamic exercises, progress as tolerated) AND active and passive exercises for neural mobilisation (six days in hospital, encouraged to continue for at least six weeks) (C) Standard postoperative care ONLY (isometric and dynamic exercises, progress as tolerated) | |
| Outcomes | Overall improvement at 12 months (I) 67.7% versus (C) 68.9% Pain (VAS) six weeks' score (I) 26.6 (SD: 29.3) versus (C) 33.4 (SD: 30.6); at 12 months (I) 33.4 (SD: 34.2) versus (C) 25.7 (SD: 29.18) Functional status (QBPQ) at six weeks (I) 34.9 (SD: 22.9) versus (C) 30.4 (SD: 22.8), at 12 months (I) 29.9 (SD: 24.1) versus (C) 27.2 (SD: 24.8) | |
| Notes | Unpublished data used for analyses so that only the 59 participants who underwent laminectomy or discectomy were included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Addition of neural mobilisation |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Addition of neural mobilisation |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Low risk | Results paragraph 1 |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | High risk | Dropouts |
| Selective reporting (reporting bias) | Low risk | VAS, McGill, Quebec, reported in outcome measures and in results |
| Similarity of baseline characteristics? | Low risk | Table 1 |
| Co‐interventions avoided or similar? | Low risk | Equal exercise programme |
| Compliance acceptable? | High risk | 37/46 control and 28/34 intervention compliant, results |
| Timing outcome assessments similar? | Low risk | 6 weeks, 6 and 12 months |
Timm 1994.
| Methods | Randomised | |
| Participants | 250 employers (68 females) in manufacturing segment of automobile industry, aged 34 to 51 years, with chronic low back pain for at least six months following a single‐level lumbar laminectomy (L5 segment) performed at least one year before start of the experiment | |
| Interventions | (I1) (N = 50) Physical agents: three sessions/wk for eight weeks (24 sessions), hot packs (20 minutes), ultrasound (paravertebral musculature 1.5 W/cm2, six minutes), TENS in non‐clinical setting (100‐millisecond pulse, 100 pulses/s, "to tolerance"); (I2) (N = 50) Joint manipulation: large‐amplitude low‐velocity manual therapy procedures (Maitland grade III or IV) combined with oscillations or sustained stretches; (I3) Low‐tech exercise: McKenzie under supervision (plus spinal stabilisation); (I4) (N = 50) High‐tech exercise: cardiovascular (bicycle), isotonic trunk muscle training (DAPRE), isokinetic exercises flexion/extension left/right rotation (Cybex TEF and TORSO); (C) (N = 50) no treatment | |
| Outcomes | Functional status (Oswestry): mean (SD) pretest (I1) (I2) (I3) (I4) (C): 37 (2.6), 36 (4.1), 35 (4.0), 33 (4.7) 37 (1.8); post‐test 37 (1.7), 32 (5.1), 14 (4.9), 15 (3.6) 37 (2.4). (I3) and (I4) significantly improved; (I1), (I2) and (C) did not. No significant differences between (I3) and (I4). ROM flexion (modified‐modified Schober in cm): mean (SD) pretest (I1) (I2) (I3) (I4) (C): 6.4 (1.5), 6.3 (1.4), 6.3 (1.4), 6.3 (1.4) 6.3 (1.5); post‐test: 6.3 (1.5), 6.5 (2.2), 8.8 (2.4), 9.1 (2.6), 6.2 (1.5). (I3) and (I4) significantly improved; (I1), (I2) and (C) did not. No significant differences between (I3) and (I4). Lifting force output (in N): mean (SD) pretest (I1) (I2) (I3) (I4) (C): 374 (107), 387 (80), 352 (98), 356 (111), 360 (102); post‐test: 378 (98), 382 (87), 627 (117), 705 (108), 363 (94). (I3) and (I4) significantly improved; (I1), (I2) and (C) did not. No significant differences between (I3) and (I4). Weeks to reentry into treatment (I1) (I2) (I3) (I4) (C); mean (SD): 2.0 (0.5), 5.7 (1.3), 91.4 (60.1), 52.8 (3.6), 1.6 (0.2). (I3) significantly better than others. (I3) significantly more cost‐effective than others | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, not described how |
| Allocation concealment (selection bias) | Unclear risk | Unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Low tech versus high tech versus control |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Low tech versus high tech versus control |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | Unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Unclear from text |
| Selective reporting (reporting bias) | High risk | Function reported (ODI), not pain, Tables 2 and 6 |
| Similarity of baseline characteristics? | Low risk | Table 1 |
| Co‐interventions avoided or similar? | High risk | |
| Compliance acceptable? | Low risk | |
| Timing outcome assessments similar? | Low risk | Eight weeks, follow‐up one year |
Yilmaz 2003.
| Methods | Randomisation or concealment not described | |
| Participants | 42 patients (22 male, 20 female), age (range 22 to 60) included one month after first‐time lumbar disc surgery. Only short‐term follow‐up | |
| Interventions | (I1, N = 14) Dynamic lumbar stabilisation exercise for eight weeks under supervision; (I2, N = 14) Flexion‐extension programme (Williams‐McKenzie) home programme for eight weeks; (C) no treatment | |
| Outcomes | Pain (VAS scores at post‐treatment) (I1) 1.14 (0.86) versus (I2) 2.93 (2.02) versus (C) 4.29 (1.90). Functional status (scores on Modified Oswestry at post‐treatment) (I1) 8.5 (4.8) versus (I2) 12.93 (4.23) versus (C) 17.71 (6.23) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in text |
| Allocation concealment (selection bias) | Unclear risk | Unclear from text |
| Blinding (performance bias and detection bias) All outcomes ‐ patients? | High risk | Supervised versus home versus no exercise |
| Blinding (performance bias and detection bias) All outcomes ‐ care providers? | High risk | Supervised versus home versus no exercise |
| Blinding (performance bias and detection bias) All outcomes ‐ outcome assessors? | High risk | Patient reported |
| Incomplete outcome data (attrition bias) All outcomes ‐ drop‐outs? | Unclear risk | Unclear from text |
| Incomplete outcome data (attrition bias) All outcomes ‐ ITT analysis? | Unclear risk | Unclear from text |
| Selective reporting (reporting bias) | Low risk | VAS for pain, Oswestry, methods last paragraph, reported in results |
| Similarity of baseline characteristics? | Low risk | Table 1 |
| Co‐interventions avoided or similar? | Unclear risk | Unclear from text |
| Compliance acceptable? | Unclear risk | Unclear from text |
| Timing outcome assessments similar? | Low risk | Three months |
Yilmaz 2003 (1).
| Methods | See Yilmaz 2003 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brennan 1994 | Not randomised |
| Burke 1994 | Not randomised |
| Imamovic 2010 | Not randomised |
| Ishida 2010 | No full text |
| Kim 2010 | Participants were pseudorandomly divided into three groups |
| Kim 2010b | Not randomised |
| Kitteringham 1996 | Not randomised |
| Lavyne 1992 | No active rehabilitation but epidural corticosteroids |
| Le Roux 1999 | No active rehabilitation but Ketorolac during wound closure |
| Mannion 2007 | Stenosis + HNP, no separate data on HNP because of unclear diagnosis |
| Millisdotter 2007 | Not randomised |
| Nielsen 2010 | Unclear population, probably not HNP |
| Rotthaupt 1997 | No adequate randomisation (date of birth) |
| Woischnek 2000 | Descriptive pilot study |
Contributions of authors
Pairs of review authors identified and selected all studies, assessed the methodological quality of studies and performed data extraction (for the current version, Teddy Oosterhuis and Leo Costa). Raymond Ostelo, Teddy Oosterhuis, Henrica de Vet and Maurits van Tulder conducted the data analyses. All review authors were involved in writing the review and read and approved the final version of the review.
Declarations of interest
Raymond Ostelo, Riekie de Vet and Chris Maher, authors of this second update of the review, were authors of one of the included studies. As this is a potential conflict of interest, they were not involved in the methodological quality assessment, in data extraction or in any other decision regarding these trials.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Alaranta 1986 {published data only}
- Alaranta H, Hurme M, Einola S, Kallio V, Knuts LR, Torma T. Rehabilitation after surgery for lumbar disc herniation: results of a randomized clinical trial. International Journal of Rehabilitation Research 1986;9(3):247‐57. [DOI] [PubMed] [Google Scholar]
Choi 2005 {published data only}
- Choi G, Raiturker PP, Kim MJ, Chung DJ, Chae YS, Lee SH. The effect of early isolated lumbar extension exercise program for patients with herniated disc undergoing lumbar discectomy. Neurosurgery 2005;57(4):764‐72; discussion 764‐72. [DOI] [PubMed] [Google Scholar]
Danielsen 2000 {published data only}
- Danielsen JM, Johnsen R, Kibsgaard SK, Hellevik E. Early aggressive exercise for postoperative rehabilitation after discectomy. Spine 2000;25(8):1015‐20. [DOI] [PubMed] [Google Scholar]
Dolan 2000 {published data only}
- Dolan P, Greenfield K, Nelson RJ, Nelson IW. Can exercise therapy improve the outcome of microdiscectomy?. Spine 2000;25:1523‐32. [DOI] [PubMed] [Google Scholar]
Donaldson 2006 {published data only}
- Donaldson BL, Shipton EA, Inglis G, Rivett D, Frampton C. Comparison of usual surgical advice versus a nonaggravating six‐month gym‐based exercise rehabilitation program post‐lumbar discectomy: results at one‐year follow‐up. The Spine Journal 2006;6(4):357‐63. [DOI] [PubMed] [Google Scholar]
Donceel 1999 {published data only}
- Donceel P, Du Bois M, Lahaye D. Return to work after surgery for lumbar disc herniation. A rehabilitation‐oriented approach in insurance medicine. Spine 1999;24(9):872‐6. [DOI] [PubMed] [Google Scholar]
Erdogmus 2007 {published data only}
- Erdogmus CB, Resch KL, Sabitzer R, Muller H, Nuhr M, Schoggl A, et al. Physiotherapy‐based rehabilitation following disc herniation operation: results of a randomized clinical trial. Spine 2007;32(19):2041‐9. [DOI] [PubMed] [Google Scholar]
Filiz 2005 {published data only}
- Filiz M, Cakmak A, Ozcan E. The effectiveness of exercise programmes after lumbar disc surgery. Clinical Rehabilitation 2005;19:4‐11. [DOI] [PubMed] [Google Scholar]
Filiz 2005 (1) {published data only}
- Filiz M, Cakmak A, Ozcan E. The effectiveness of exercise programmes after lumbar disc surgery. Clinical Rehabilitation 2005;19:4‐11. [DOI] [PubMed] [Google Scholar]
Hakkinen 2005 {published data only}
- Hakkinen A, Ylinen J, Kautiainen H, et al. Effects of home strength training and stretching program versus stretching alone after lumbar disk surgery. Archives of Physical Medicine and Rehabilitation 2005;86:865‐70. [DOI] [PubMed] [Google Scholar]
Johannsen 1994 {published data only}
- Johannsen F, Remvig L, Kryger P, Beck P, Larsen LH, Warming S, et al. Supervised endurance exercise training compared to home training after first lumbar diskectomy: a clinical trial. Clinical and Experimental Rheumatology 1994;12(6):609‐14. [PubMed] [Google Scholar]
Johansson 2009 {published data only}
- Johansson AC, Linton SJ, Bergkvist L, Nilsson O, Cornefjord M. Clinic‐based training in comparison to home‐based training after first‐time lumbar disc surgery: a randomised controlled trial. European Spine Journal 2009;18(3):398‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ju 2012 {published data only}
- Ju S, Park G, Kim E. Effects of an exercise treatment program on lumbar extensor muscle strength and pain of rehabilitation patients recovering from lumbar disc herniation surgery. Journal of Physical Therapy Science 2012;24(6):515‐8. [Google Scholar]
Kjellby‐Wendt 1998 {published data only}
- Kjellby Wendt G, Styf J. Early active training after lumbar discectomy. A prospective, randomized, and controlled study. Spine 1999;23(21):2345‐51. [DOI] [PubMed] [Google Scholar]
- Kjellby‐Wendt G, Carlsson S, Styf J. Results of early active rehabilitation 5‐7 years after surgical treatment for lumbar disc herniation. Journal of Spinal Disorders and Techniques 2002;15:404‐9. [DOI] [PubMed] [Google Scholar]
- Kjellby‐Wendt G, Styf J, Carlsson S. Early active rehabilitation after surgery for lumbar disc herniation. Acta Orthopaedica Scandinavica 2001;72:518‐24. [DOI] [PubMed] [Google Scholar]
Kulig 2009 {published data only}
- Kulig K, Beneck GJ, Selkowitz DM, Popovich JM Jr, Ge TT, Flanagan SP, et al. An intensive, progressive exercise program reduces disability and improves functional performance in patients after single‐level lumbar microdiskectomy. Physical Therapy 2009;89(11):1145‐57. [DOI] [PubMed] [Google Scholar]
Manniche 1993a {published data only}
- Manniche C, Skall HF, Braendholt L, Christinsen BH, Christophersen L, Ellegaard B, et al. Clinical trial of postoperative dynamic back exercises after first lumbar discectomy. Spine 1993;18(1):92‐7. [DOI] [PubMed] [Google Scholar]
Manniche 1993b {published data only}
- Manniche C, Asmussen K, Lauritsen B, Vinterberg H, Karbo H, Abildstrup S, et al. Intensive dynamic back exercises with or without hyperextension in chronic back pain after surgery for lumbar disc protrusion. A clinical trial. Spine 1993;18(5):560‐7. [DOI] [PubMed] [Google Scholar]
McGregor 2011 {published data only}
- McGregor AH, Dore CJ, Morris TP, Morris S, Jamrozik K. ISSLS prize winner: function after spinal treatment, exercise, and rehabilitation (FASTER): a factorial randomized trial to determine whether the functional outcome of spinal surgery can be improved. Spine 2011;36(21):1711‐20. [DOI] [PubMed] [Google Scholar]
- Morris S, Morris TP, McGregor AH, Doré CJ, Jamrozik K. Function after spinal treatment, exercise, and rehabilitation: cost‐effectiveness analysis based on a randomized controlled trial. Spine 2011;36(21):1807‐14. [DOI] [PubMed] [Google Scholar]
Newsome 2009 {published data only}
- Newsome RJ, May S, Chiverton N, Cole AA. A prospective, randomised trial of immediate exercise following lumbar microdiscectomy: a preliminary study. Physiotherapy 2009;95(4):273‐9. [DOI] [PubMed] [Google Scholar]
Ostelo 2003 {published data only}
- Ostelo RW, Goossens ME, Vet HC, Brandt PA. Economic evaluation of a behavioral‐graded activity program compared to physical therapy for patients following lumbar disc surgery. Spine 2004;29(6):615‐22. [DOI] [PubMed] [Google Scholar]
- Ostelo RW, Vet HC, Berfelo MW, Kerckhoffs MR, Vlaeyen JW, Wolters PM, et al. Effectiveness of behavioral graded activity after first‐time lumbar disc surgery: short term results of a randomized controlled trial. European Spine Journal 2003;12(6):637‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostelo RW, Vet HC, Vlaeyen JW, Kerckhoffs MR, Berfelo WM, Wolters PM, et al. Behavioral graded activity following first‐time lumbar disc surgery: 1‐year results of a randomized clinical trial. Spine 2003;28(16):1757‐65. [DOI] [PubMed] [Google Scholar]
Scrimshaw 2001 {published and unpublished data}
- Scrimshaw S, Maher C. Randomized controlled trial of neural mobilization after spinal surgery. Spine 2001;26:2647‐52. [DOI] [PubMed] [Google Scholar]
- Scrimshaw S, Maher C. Randomized controlled trial of neural mobilization after spinal surgery. Unpublished data. [DOI] [PubMed]
Timm 1994 {published data only}
- Timm KE. A randomized‐control study of active and passive treatments for chronic low back pain following L5 laminectomy. The Journal of Orthopaedic and Sports Physical Therapy 1994;20(6):276‐86. [DOI] [PubMed] [Google Scholar]
Yilmaz 2003 {published data only}
- Yilmaz F, Yilmaz A, Merdol F, Parlar D, Sahin, F, Kuran B. Efficacy of dynamic lumbar stabilization exercise in lumbar microdiscectomy. Journal of Rehabilitation Medicine 2003;35:163‐7. [DOI] [PubMed] [Google Scholar]
Yilmaz 2003 (1) {published data only}
- Yilmaz F, Yilmaz A, Merdol F, Parlar D, Sahin, F, Kuran B. Efficacy of dynamic lumbar stabilization exercise in lumbar microdiscectomy. Journal of Rehabilitation Medicine 2003;35:163‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Brennan 1994 {published data only}
- Brennan GP, Shultz BB, Hood RS, Zahniser JC, Johnson SC, Gerber AH. The effects of aerobic exercise after lumbar microdiscectomy. Spine 1994;19(7):735‐9. [DOI] [PubMed] [Google Scholar]
Burke 1994 {published data only}
- Burke SA, Harms Constas CK, Aden PS. Return to work/work retention outcomes of a functional restoration program. A multi‐center, prospective study with a comparison group. Spine 1994;19(17):1880‐5. [DOI] [PubMed] [Google Scholar]
Imamovic 2010 {published data only}
- Imamovic MZ, Hodzic M, Durakovic SK, Basic NK, Cickusic A, Imamovic G. [Functional status of patients after lumbar disc herniation surgery] [Funkcionalni status bolesnika nakon operacije lumbalne diskus hernije.]. Reumatizam 2010;57(1):21‐5. [PubMed] [Google Scholar]
Ishida 2010 {published data only}
- Ishida K, Umeno Y, Yoshimoto H, Satoh S. Early active rehabilitation after surgery for lumbar disc herniation: a prospective, randomized control trial. Spine Affiliated Society Meeting Abstracts 2010:Suppl 259. [Google Scholar]
Kim 2010 {published data only}
- Kim YS, Park J, Shim JK. Effects of aquatic backward locomotion exercise and progressive resistance exercise on lumbar extension strength in patients who have undergone lumbar diskectomy. Archives of Physical Medicine and Rehabilitation 2010;91(2):208‐14. [DOI] [PubMed] [Google Scholar]
Kim 2010b {published data only}
- Kim YS, Park J, Hsu J, Cho KK, Kim YH, Shim JK. Effects of training frequency on lumbar extension strength in patients recovering from lumbar dyscectomy. Journal of Rehabilitation Medicine 2010;42(9):839‐45. [DOI] [PubMed] [Google Scholar]
Kitteringham 1996 {published data only}
- Kitteringham C. The effect of straight leg raise exercises after lumbar decompression surgery ‐ A pilot study. Physiotherapy 1996;82(2):115‐23. [Google Scholar]
Lavyne 1992 {published data only}
- Lavyne MH, Bilsky MH. Epidural steroids, postoperative morbidity, and recovery in patients undergoing microsurgical lumbar discectomy. Journal of Neurosurgery 1992;77(1):90‐5. [DOI] [PubMed] [Google Scholar]
Le Roux 1999 {published data only}
- Roux PD, Samudrala S. Postoperative pain after lumbar disc surgery: a comparison between parenteral ketorolac and narcotics. Acta Neurochirurgica 1999;141(3):261‐7. [DOI] [PubMed] [Google Scholar]
Mannion 2007 {published data only}
- Mannion AF, Denzler R, Dvorak J, Grob D. Five‐year outcome of surgical decompression of the lumbar spine without fusion. European Spine Journal 2010 Nov;19(11):1883‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion AF, Denzler R, Dvorak J, Muntener M, Grob D. A randomised controlled trial of post‐operative rehabilitation after surgical decompression of the lumbar spine. European Spine Journal 2007;16(8):1101‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Millisdotter 2007 {published data only}
- Millisdotter M, Strömqvist B. Early neuromuscular customized training after surgery for lumbar disc herniation: a prospective controlled study. European Spine Journal 2007;16(1):19‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 2010 {published data only}
- Nielsen PR, Andreasen J, Asmussen M, Tønnesen H. Costs and quality of life for prehabilitation and early rehabilitation after surgery of the lumbar spine. BMC Health Services Research 2008 Oct 9;8:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen PR, Jorgensen LD, Dahl B, Pedersen T, Tonnesen H. Prehabilitation and early rehabilitation after spinal surgery: randomized clinical trial. Clinical Rehabilitation 2010;24(2):137‐48. [DOI] [PubMed] [Google Scholar]
Rotthaupt 1997 {published data only}
- Rothaupt D, Laser T, Ziegler H, Liebig K. Orthopedic hippotherapy in postoperative rehabilitation of lumbar intervertebral disk patients. A prospective, randomized therapy study [Die Orthopädische Hippotherapie in der postoperativen Rehabilitation von lumbalen Bandscheibenpatienten. Eine prospektive, randomisierte Therapiestudie]. Sportverletzung Sportschaden 1997;11:63‐9. [DOI] [PubMed] [Google Scholar]
Woischnek 2000 {published data only}
- Woisschnek D, Hussein S, Ruckert N, Heissler H. Criteria for the initiation of rehabilitation after lumbar disc surgery: pilot study. Rehabilitation 2000;39:88‐92. [DOI] [PubMed] [Google Scholar]
Additional references
Arts 2009
- Arts MP, Brand R, Akker ME, Koes BW, Bartels RH, Peul WC, Leiden‐The Hague Spine Intervention Prognostic Study Group (SIPS). Tubular diskectomy vs conventional microdiskectomy for sciatica: a randomized controlled trial. Journal of the American Medical Association 2009;302(2):149‐58. [DOI] [PubMed] [Google Scholar]
Boutron 2005
- Boutron I, Moher D, Tugwell P, Giraudeau B, Poiraudeau S, Nizard R, et al. A checklist to evaluate a report of a non pharmacological trial (CLEAR NPT) was developed using consensus. Journal of Clinical Epidemiology 2005;58:1233‐40. [DOI] [PubMed] [Google Scholar]
Carragee 1996
- Carragee EJ, Helms E, O'Sullivan G. Are postoperative activity restrictions necessary after posterior lumbar discectomy?. Spine 1996;21:1893‐7. [DOI] [PubMed] [Google Scholar]
CBO 2008
- The Dutch Institute for Healthcare Improvement (CBO). The lumbosacral radicular syndrome [Richtlijn Lumbosacraal Radiculair Syndroom]. Report. Utrecht, 2008.
den Boer 2010
- Boer JJ, Oostendorp RA, Evers AW, Beems T, Borm GF, Munneke M. The development of a screening instrument to select patients at risk of residual complaints after lumbar disc surgery. European Journal of Physical and Rehabilitation Medicine 2010;46(4):497‐503. [PubMed] [Google Scholar]
el Barzouhi 2013
- Barzouhi A, Vleggeert‐Lankamp CL, Lycklama à Nijeholt GJ, Kallen BF, Hout WB, Jacobs WC, et al. Leiden‐The Hague Spine Intervention Prognostic Study Group. Magnetic resonance imaging in follow‐up assessment of sciatica. New England Journal of Medicine 2013;368(11):999‐1007. [DOI] [PubMed] [Google Scholar]
Furlan 2009
- Furlan AD, Pennick V, Bombardier C, Tulder M, Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34(18):1929‐41. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
HCN 1999
- Health Council of the Netherlands. Management of the lumbosacral radicular syndrome (sciatica). The Hague: Health Council of the Netherlands. Publication no. 1999/18.
HESonline 2012
- The Health and Social Care Information Centre, Hospital Episode Statistics for England. Inpatient statistics, 2011‐12. www.hscic.gov.uk/hes 01.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [Google Scholar]
Hoogland 2006
- Hoogland T, Schubert M, Miklitz B, Ramirez A. Transforaminal posterolateral endoscopic discectomy with or without the combination of a low‐dose chymopapain: a prospective randomized study in 280 consecutive cases. Spine 2006;31(24):E890‐7. [DOI] [PubMed] [Google Scholar]
Jacobs 2012
- Jacobs WC, Arts MP, Tulder MW, Rubinstein SM, Middelkoop M, Ostelo RW, et al. Surgical techniques for sciatica due to herniated disc, a systematic review. European Spine Journal 2012;21(11):2232‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2011
- Morris S, Morris TP, McGregor AH, Doré CJ, Jamrozik K. Function after spinal treatment, exercise, and rehabilitation: cost‐effectiveness analysis based on a randomized controlled trial. Spine 2011;36(21):1807‐14. [DOI] [PubMed] [Google Scholar]
ONS 2011
- Office for National Statistics. Table P07 2011 census: number of usual residents living in households and communal establishments, local authorities in England and Wales. www.ons.gov.uk/ons/publications/re‐reference‐tables.html?edition=tcm%3A77‐257414.
Peul 2007
- Peul WC, Houwelingen HC, Hout WB, Brand R, Eekhof JA, Tans JT, et al. Leiden‐The Hague Spine Intervention Prognostic Study Group. Surgery versus prolonged conservative treatment for sciatica. New England Journal of Medicine 2007;356(22):2245‐56. [DOI] [PubMed] [Google Scholar]
Rasmussen 2008
- Rasmussen S, Krum‐Møller DS, Lauridsen LR, Jensen SE, Mandøe H, Gerlif C, et al. Epidural steroid following discectomy for herniated lumbar disc reduces neurological impairment and enhances recovery: a randomized study with two‐year follow‐up. Spine 2008;33(19):2028‐33. [DOI] [PubMed] [Google Scholar]
Ruetten 2008
- Ruetten S, Komp M, Merk H, Godolias G. Full‐endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine 2008;33(9):931‐9. [DOI] [PubMed] [Google Scholar]
Sherman 2010
- Sherman J, Cauthen J, Schoenberg D, Burns M, Reaven NL, Griffith SL. Economic impact of improving outcomes of lumbar discectomy. The Spine Journal 2010;10:108–116. [DOI] [PubMed] [Google Scholar]
Steiger 2012
- Steiger F, Wirth B, Bruin ED, Mannion AF. Is a positive clinical outcome after exercise therapy for chronic non‐specific low back pain contingent upon a corresponding improvement in the targeted aspect(s) of performance?. European Spine Journal 2012;21(4):575‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
USCB 2002
- U.S. Agency for International Development Bureau for Global Health Office of Population and Reproductive Health / U.S. Department of Commerce, Economics, Statistics Administration. U.S. CENSUS BUREAU. Global Population Profile: 2002 Issued March 2004 WP/02. www.census.gov/prod/2004pubs/wp‐02.pdf 2004.
van Beek 2010
- Beek E, Lemmens K, Schooten G, Vlieger EJ. Reduceren van praktijkvariatie: budgettaire effecten van scherpere indicatiestelling. Reduceren van praktijkvariatie: budgettaire effecten van scherpere indicatiestelling. Breukelen: Plexus, 2010. [Google Scholar]
van Tulder 2003
- Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the Cochrane Collaboration Back Review Group. Spine 2003;28(12):1290‐9. [DOI] [PubMed] [Google Scholar]
Weinstein 2006
- Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States' trends and regional variations in lumbar spine surgery: 1992‐2003. Spine 2006;31:2707‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinstein 2006b
- Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Hanscom B, Skinner JS, et al. Surgical vs nonoperative treatment for lumbar disk herniation: the Spine Patient Outcomes Research Trial (SPORT): a randomized trial. Journal of the American Medical Association 2006;296(20):2441‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zelen 1979
- Zelen M. A new design for randomized clinical trials. New England Journal of Medicine 1979;300(22):1242‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ostelo 2003a
- Ostelo RWJG, Vet HCW, Waddell G, Kerckhoffs MR, Leffers P, Tulder MW. Rehabilitation after lumbar disc surgery. Spine 2003;28(3):209‐18. [DOI] [PubMed] [Google Scholar]
Ostelo 2004
- Ostelo RW, Vet HC, Waddell G, Kerckhoffs MR, Leffers P, Tulder MW. Rehabilitation after lumbar disc surgery. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD003007] [DOI] [PubMed] [Google Scholar]
Ostelo 2008
- Ostelo RW, Costa LO, Maher CG, Vet HC, Tulder MW. Rehabilitation after lumbar disc surgery. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003007.pub2] [DOI] [PubMed] [Google Scholar]
Ostelo 2009
- Ostelo RW, Costa LO, Maher CG, Vet HC, Tulder MW. Rehabilitation after lumbar disc surgery: an update Cochrane review. Spine 2009;34(17):1839‐48. [DOI] [PubMed] [Google Scholar]


