Abstract
Background
Prostaglandins have been used for induction of labour since the 1960s. This is one of a series of reviews evaluating methods of induction of labour. This review focuses on prostaglandins given per vaginam, evaluating these in comparison with placebo (or expectant management) and with each other; prostaglandins (PGE2 and PGF2a); different formulations (gels, tablets, pessaries) and doses.
Objectives
To determine the effects of vaginal prostaglandins E2 and F2a for third trimester cervical ripening or induction of labour in comparison with placebo/no treatment or other vaginal prostaglandins (except misoprostol).
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (1 March 2014) and bibliographies of relevant papers.
Selection criteria
Clinical trials comparing vaginal prostaglandins used for third trimester cervical ripening or labour induction with placebo/no treatment, with each other, or other methods listed above it on a predefined list of labour induction methods.
Data collection and analysis
We assessed studies and extracted data independently.
Main results
Seventy randomised controlled trials (RCTs) (11,487 women) are included. In this update seven new RCTs (778 women) have been added. Two of these new trials compare PGE2 with no treatment, four compare different PGE2 formulations (gels versus tablets, or sustained release pessaries) and one trial compares PGF2a with placebo. The majority of trials were at unclear risk of bias for most domains.
Overall, vaginal prostaglandin E2 compared with placebo or no treatment probably reduces the likelihood of vaginal delivery not being achieved within 24 hours. The risk of uterine hyperstimulation with fetal heart rate changes is increased (4.8% versus 1.0%, risk ratio (RR) 3.16, 95% confidence interval (CI) 1.67 to 5.98, 15 trials, 1359 women). The caesarean section rate is probably reduced by about 10% (13.5% versus 14.8%, RR 0.91, 95% CI 0.81 to 1.02, 36 trials, 6599 women). The overall effect on improving maternal and fetal outcomes (across a variety of measures) is uncertain.
PGE2 tablets, gels and pessaries (including sustained release preparations) appear to be as effective as each other, small differences are detected between some outcomes, but these maybe due to chance.
Authors' conclusions
Prostaglandins PGE2 probably increase the chance of vaginal delivery in 24 hours, they increase uterine hyperstimulation with fetal heart changes but do not effect or may reduce caesarean section rates. They increase the likelihood of cervical change, with no increase in operative delivery rates. PGE2 tablets, gels and pessaries appear to be as effective as each other, any differences between formulations are marginal but may be important.
Keywords: Female; Humans; Pregnancy; Administration, Intravaginal; Dinoprost; Dinoprost/administration & dosage; Dinoprostone; Dinoprostone/administration & dosage; Labor, Induced; Labor, Induced/methods; Oxytocics; Oxytocics/administration & dosage; Randomized Controlled Trials as Topic; Term Birth
Plain language summary
Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term
Induction of labour is offered to pregnant women when it is thought the outcome will be better for the mother and/or baby if the baby is born than if the pregnancy continues. Common reasons include prolonged pregnancy, prelabour rupture of the membranes, concerns about the health of the mother such as pre‐eclampsia or the baby such as poor growth. Prostaglandins are hormones, produced throughout the body and can be used to start (induce) labour. They are applied locally to the vagina as tablets, gels, suppositories and pessaries to reduce side‐effects. The dose, number of doses, and time between doses vary considerably. Sustained release pessaries reduce the need for repeat doses and so the number of vaginal examinations.
This review set out to determine the effectiveness and safety of vaginal prostaglandins for third trimester cervical ripening and induction of labour (the cervix softens, shortens and opens, the uterus starts to contract regularly). Eight different comparisons were made, different vaginal prostaglandins were compared with placebos or no treatment, or other vaginal prostaglandins (PGE2, PGF2a, except misoprostol) and different preparations and dosages were compared. We identified 70 studies involving a total of 11,487 women. Vaginal prostaglandins increase the likelihood of vaginal birth within 24 hours, but they can also stimulate the uterus to contract too much and this may cause the baby's heart to slow, however they did not increase the caesarean section rate and may reduce it. Overall, the trials do not show any effect (improvement or worsening) of many important outcomes. Prostaglandin E2 tablets, gels, or pessaries including sustained release preparations appear to be as good as each other or the differences between them are small and have not yet been detected in the trials. Lower‐dose regimens, as defined in the review, appeared to be as good as higher‐dose regimens (eight trials, 1615 women).
Very limited data were available in the included trials on time in labour and patient satisfaction. Few studies have addressed issues relating to the safety of using vaginal prostaglandins for induction of labour as outpatients.
Summary of findings
Summary of findings for the main comparison. PGE2 compared with placebo or no treatment for induction of labour at term (all women).
| PGE2 compared with placebo or no treatment for induction of labour at term (all women) | ||||||
| Patient or population: patients with induction of labour at term Settings: Mainly inpatients Intervention: PGE2 (all regimens) Comparison: placebo or no treatment (all women) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no treatment (all women) | PGE2 (all regimens) | |||||
| Vaginal delivery not achieved within 24 hours | Study population | RR 0.32 (0.02 to 4.83) | 384 (2 studies) | ⊕⊝⊝⊝ very low | Probable reduction in time to delivery using PGE2. Useable data only available in 2 of 15 studies reporting time as an outcome. 39 studies in this comparison. | |
| 989 per 1000 | 317 per 1000 (20 to 1000) | |||||
| Moderate | ||||||
| 950 per 1000 | 304 per 1000 (19 to 1000) | |||||
| Uterine hyperstimulation with FHR changes | Study population | RR 3.16 (1.67 to 5.98) | 1359 (15 studies) | ⊕⊕⊕⊝ moderate | The risk of bias is "unclear" for most quality domain of the 15 RCT's and this may be a serious limitation. | |
| 10 per 1000 | 33 per 1000 (18 to 63) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Caesarean section | Study population | RR 0.91 (0.81 to 1.02) | 6599 (36 studies) | ⊕⊕⊕⊕ high | The risk of bias is unclear for most of the studies, but the largest study with a quarter of the participants) has a low risk of bias. | |
| 148 per 1000 | 134 per 1000 (120 to 151) | |||||
| Moderate | ||||||
| 166 per 1000 | 151 per 1000 (134 to 169) | |||||
| Serious neonatal morbidity or perinatal death | Study population | RR 0.46 (0.09 to 2.31) | 3638 (9 studies) | ⊕⊕⊝⊝ low | Neonatal morbidity or mortality is rare, several studies have no events. Underpowered to detect a difference even if one exists. | |
| 2 per 1000 | 1 per 1000 (0 to 4) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Serious maternal morbidity or death | Study population | RR 2.23 (0.34 to 14.76) | 530 (3 studies) | ⊕⊝⊝⊝ very low | A very rare outcome, so underpowered to detect a difference if one exists. | |
| 4 per 1000 | 9 per 1000 (1 to 57) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2. (4.1) PGE2 gel compared with PGE2 tablet (all women) for induction of labour at term.
| (4.1) PGE2 gel compared with PGE2 tablet (all women) for induction of labour at term | ||||||
| Patient or population: patients with induction of labour at term Settings: Mainly inpatients Intervention: (4.1) PGE2 gel Comparison: PGE2 tablet (all women) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PGE2 tablet (all women) | (4.1) PGE2 gel | |||||
| Vaginal delivery not achieved within 24 hours | Study population | RR 1.03 (0.84 to 1.26) | 566 (3 studies) | ⊕⊕⊕⊝ moderate | Most quality domains unclear or low risk but loss to follow up and reporting bias high in 1 trial. | |
| 369 per 1000 | 380 per 1000 (310 to 464) | |||||
| Moderate | ||||||
| 528 per 1000 | 544 per 1000 (444 to 665) | |||||
| Uterine hyperstimulation with FHR changes | Study population | RR 2 (0.18 to 21.71) | 200 (1 study) | ⊕⊝⊝⊝ very low | Only 1 small trial with an unclear risk of bias reports this outcome. | |
| 10 per 1000 | 20 per 1000 (2 to 217) | |||||
| Moderate | ||||||
| 10 per 1000 | 20 per 1000 (2 to 217) | |||||
| Caesarean section | Study population | RR 0.91 (0.72 to 1.17) | 1046 (6 studies) | ⊕⊕⊕⊕ high | The risk of bias is unclear for most studies, but the largest study has a low risk of bias. | |
| 198 per 1000 | 180 per 1000 (142 to 231) | |||||
| Moderate | ||||||
| 201 per 1000 | 183 per 1000 (145 to 235) | |||||
| Serious maternal morbidity or death | Study population | RR 0.33 (0.01 to 8.09) | 200 (1 study) | See comment | Study far too small to detect a difference. | |
| 10 per 1000 | 3 per 1000 (0 to 81) | |||||
| Moderate | ||||||
| 10 per 1000 | 3 per 1000 (0 to 81) | |||||
| Instrumental vaginal delivery | Study population | RR 0.77 (0.58 to 1.02) | 565 (3 studies) | ⊕⊕⊕⊝ moderate | The largest study has a high risk of bias. This is a secondary outcome in this review. | |
| 287 per 1000 | 221 per 1000 (167 to 293) | |||||
| Moderate | ||||||
| 241 per 1000 | 186 per 1000 (140 to 246) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 3. (7.1) PGE2 (controlled release) compared with all PGE2 delivery systems (all women) for induction of labour at term.
| (7.1) PGE2 (controlled release) compared with all PGE2 delivery systems (all women) for induction of labour at term | ||||||
| Patient or population: patients with induction of labour at term Settings: Mainly inpatients Intervention: (7.1) PGE2 (controlled release) Comparison: all PGE2 delivery systems (all women) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| All PGE2 delivery systems (all women) | (7.1) PGE2 (controlled release) | |||||
| Vaginal delivery not achieved within 24 hours | Study population | RR 1.15 (0.92 to 1.45) | 450 (3 studies) | ⊕⊕⊕⊝ moderate | Although all published after 2002, the risk of bias for most quality domains unclear. | |
| 373 per 1000 | 429 per 1000 (343 to 541) | |||||
| Moderate | ||||||
| 333 per 1000 | 383 per 1000 (306 to 483) | |||||
| Uterine hyperstimulation with FHR changes | Study population | RR 2.15 (0.89 to 5.21) | 643 (5 studies) | ⊕⊕⊕⊝ moderate | 4 of the studies are recent but risk of bias unclear. | |
| 22 per 1000 | 47 per 1000 (20 to 115) | |||||
| Moderate | ||||||
| 18 per 1000 | 39 per 1000 (16 to 94) | |||||
| Caesarean section | Study population | RR 1.02 (0.82 to 1.26) | 1262 (11 studies) | ⊕⊕⊕⊝ moderate | Risk of bias unclear, recent studies poorly reported. | |
| 201 per 1000 | 205 per 1000 (165 to 254) | |||||
| Moderate | ||||||
| 177 per 1000 | 181 per 1000 (145 to 223) | |||||
| Serious neonatal morbidity or perinatal death | Study population | RR 0.31 (0.01 to 7.62) | 320 (2 studies) | ⊕⊕⊝⊝ low | Underpowered to detect effect even if exists. | |
| 6 per 1000 | 2 per 1000 (0 to 49) | |||||
| Moderate | ||||||
| 5 per 1000 | 2 per 1000 (0 to 38) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
Induction of labour is a common intervention in pregnancy, it is undertaken when it is thought that the outcome of the pregnancy will be better for the mother and/or her baby if the baby is born. Approximately one in four or five women in the UK, Europe and USA are induced. Common reasons include prolonged pregnancy, prelabour rupture of the membranes, concerns about fetal well being (for example, poor growth, twin pregnancy) and maternal medical conditions (for example, diabetes, or pre‐eclampsia).The evidence that induction is beneficial in specific clinical situations is not part of this review but is considered in other Cohrane systematic reviews and clinical guidelines (NICE 2008).
The physiological processes surrounding the initiation and promotion of labour are complex. During normal pregnancy the uterus is relaxed and the cervix long, firm and closed. In preparation for labour the cervix "ripens" becoming softer, shorter (effaced) and more open, the uterine smooth muscle begins to respond to stimuli that cause the waves of contractions leading up to and during labour. A variety of physical and pharmacological interventions are or have been used to induce labour. This review is one of a series of reviews of methods for induction of labour that use a standardised published ’generic’ protocol (Hofmeyr 2009). These reviews were initially developed to help inform the recommendations of the NICE clinical practice guidelines on Induction of labour (NICE 2001).
Description of the intervention
Prostaglandins are hormones, produced throughout the body from arachidonic acid via the cyclo‐oxygenase pathway. Their role in cervical ripening and induction of labour was discovered in the 1960s. They have a variety of effects at different sites and receptors in the body that lead to unwanted side‐effects when used. The use of vaginal preparations (rather than oral or intravenous routes) for induction of labour aims to lessen side‐effects. There are a number of different vaginal preparations of prostaglandins used, including gels, tablets, suppositories and pessaries. The induction regimens used vary in the dosage used, the number of applications and time intervals between repeat applications. Sustained release pessaries have been developed to reduce the number of applications (and vaginal examinations) needed during induction of labour.
How the intervention might work
Prostaglandins ripen the cervix and induce uterine contractions. They have been used for induction of labour since the 1960s. Initial work focused on prostaglandin F2a (PGF2a ‐ Dinoprost), but prostaglandin E2 (PGE2, Dinoprostone) is now the most commonly used agent. Prostaglandins are now available in a variety of formulations and may be given by mouth, intravenously, vaginally or intra cervically. Information on the effectiveness of other routes of administration and types of prostaglandin (such as misoprostol) are reviewed separately in the linked reviews. To avoid duplication, the labour induction methods have been listed in a specific order, from one to 27. Each review includes comparisons between one of the methods (from two to 27) with only those methods above it on the list. Thus, this review includes comparison of vaginal prostaglandins to placebo or each other. Comparisons with interventions below it on the list are included in other reviews, for example (4) intravenous oxytocin will include only comparisons with intracervical prostaglandins (3), vaginal prostaglandins (2) or placebo (1). Methods identified in the future will be added to the end of the list. The current list is as follows:
placebo/no treatment;
vaginal prostaglandins (this review);
intracervical prostaglandins (Boulvain 2008);
intravenous oxytocin (Alfirevic 2009);
amniotomy (Bricker 2000);
intravenous oxytocin with amniotomy (Howarth 2001; Bimbashi 2012);
vaginal misoprostol (Hofmeyr 2010);
oral misoprostol (Alfirevic 2014);
mechanical methods including extra‐amniotic Foley catheter (Jozwiak 2012);
membrane sweeping (Boulvain 2005);
extra‐amniotic prostaglandins (Hutton 2001);
intravenous prostaglandins (Luckas 2000);
oral prostaglandins (French 2001);
mifepristone (Hapangama 2009);
oestrogens (Thomas 2001);
corticosteroids (Kavanagh 2006a);
relaxin (Kelly 2001b);
hyaluronidase (Kavanagh 2006b);
castor oil, bath, and/or enema (Kelly 2013);
acupuncture (Smith 2013);
breast stimulation (Kavanagh 2005);
sexual intercourse (Kavanagh 2001);
homoeopathic methods (Smith 2003);
nitric oxide (Kelly 2011);
buccal or sublingual misoprostol (Muzonzini 2004);
hypnosis (Nishi 2013);
other methods for induction of labour.
Why it is important to do this review
These reviews were initially developed to help inform the recommendations of the NICE clinical practice guidelines on Induction of labour (NICE 2001). This review is one of a series of reviews of methods for induction of labour that use a standardised published ’generic’ protocol (Hofmeyr 2009).
Objectives
To determine, from the best available evidence, the effectiveness and safety of vaginal prostaglandin E2 and F2a for third trimester cervical ripening and induction of labour in comparison with placebo/no treatment or other vaginal prostaglandins (except misoprostol).
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials comparing vaginal prostaglandins for cervical ripening or labour induction, with placebo/no treatment or where different formulations of vaginal prostaglandin (either PGE2 or PGF2a) are compared with each other; the trials included some form of random allocation to either group; and they report one or more of the pre‐stated outcomes.
Types of participants
Pregnant women due for third trimester induction of labour, carrying a viable fetus.
Types of interventions
Vaginal prostaglandins E2 and F2a compared with placebo/no treatment or other vaginal prostaglandins (except misoprostol).
Primary comparisons
Prostaglandin E2 versus placebo.
Prostaglandin F2a versus placebo.
Prostaglandin F2a versus prostaglandin E2.
Prostaglandin E2 gel versus prostaglandin E2 tablet.
Prostaglandin E2 gel versus prostaglandin E2 pessary/suppository.
Prostaglandin E2 tablet versus prostaglandin E2 pessary/suppository.
Prostaglandin E2 (sustained release) versus prostaglandin E2 (any vehicle).
Prostaglandin E2 (low dose) versus prostaglandin E2 (high dose).
Dose comparisons of PGE2 into 'low‐dose' and 'high‐dose' categories are made to compare two common clinical practices. 'Low dose': where the maximum possible dose in one arm of the trial protocol was up to 3 mg PGE2. 'High dose': where the maximum possible dose in one arm of the trial protocol was 3 mg PGE2 or more. It was thought that this division would separate those trials using, on the whole, a single or repeated dose protocol. In addition, division at this level allows the largest number of trials to be included in the comparison. Trials comparing doses that both fall into either the high‐ or low‐dose category were excluded.
Subgroup analyses and justifications
In addition to evaluating comparisons for all women entered into the randomised controlled trials, we subdivided the trial participants into four clinical subgroups. These divisions were made prior to examination of the trial data and were thought, by the review authors, to be clinically relevant.
Previous caesarean section or not.
Nulliparity or multiparity.
Membranes intact or ruptured.
Cervix favourable, unfavourable or undefined.
For the comparison prostaglandin E2 versus placebo, subgroup analysis on different vehicle comparisons were included because it was thought that it would not be correct to assume equal effects irrespective of method of application. The three subgroups that were compared to placebo were single‐dose PGE2, repeated dose of PGE2 and sustained release PGE2.
Trial setting
For the updates of the review from 2007, the setting (outpatient or inpatient) of induction of labour, is included. However, this issue is addressed specifically in other Cochrane reviews (Dowswell 2010; Kelly 2013a).
Types of outcome measures
Primary outcomes
Five primary outcomes were chosen as being most representative of the clinically important measures of effectiveness and complications:
(1) vaginal delivery not achieved within 24 hours (or period specified by trial authors); (2) uterine hyperstimulation with fetal heart rate (FHR) changes; (3) caesarean section; (4) serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood); (5) serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicaemia).
Perinatal and maternal morbidity and mortality are infrequent and so composite outcomes have been used. This is not an ideal solution because some components of morbidity are clearly less severe than others and adverse events tend to cluster in individuals, so care is needed to count individuals rather than events. It is also possible for an intervention to cause more deaths but less severe morbidity, however, in the context of labour induction at term, this is unlikely. All of these events will be rare, and a modest change in their incidence will be easier to detect if composite outcomes are presented. The incidence of individual components where available will be explored as secondary outcomes (see below).
Secondary outcomes
Secondary outcomes relate to measures of effectiveness, complications and satisfaction.
Measures of effectiveness
(6) Cervix unfavourable/unchanged after 12 to 24 hours; (7) oxytocin augmentation.
Complications
(8) Uterine hyperstimulation without FHR changes; (9) uterine rupture; (10) epidural analgesia; (11) instrumental vaginal delivery; (12) meconium‐stained liquor; (13) Apgar score less than seven at five minutes; (14) neonatal intensive care unit admission; (15) neonatal encephalopathy; (16) perinatal death; (17) disability in childhood; (18) maternal side‐effects (all); (19) maternal nausea; (20) maternal vomiting; (21) maternal diarrhoea; (22) other maternal side‐effects; (23) postpartum haemorrhage (as defined by the trial authors); (24) serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture); (25) maternal death.
Measures of satisfaction
(26) Woman not satisfied; (27) caregiver not satisfied.
'Uterine rupture' will include all clinically significant ruptures of unscarred or scarred uteri. Asymptomatic scar dehiscence noted incidentally at the time of surgery will be excluded. Additional outcomes may appear in individual reviews. While all the above outcomes will be sought, only those with data will appear in the analysis tables.
The terminology of uterine hyperstimulation is problematic (Curtis 1987). In the reviews we will use the term 'uterine hyperstimulation without FHR changes' to include uterine tachysystole (more than five contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonus (a contraction lasting at least two minutes) and 'uterine hyperstimulation with FHR changes' to denote uterine hyperstimulation syndrome (tachysystole or hypersystole with FHR changes such as persistent decelerations, tachycardia or decreased short‐term variability).
Outcomes will be included in the analysis: if reasonable measures were taken to minimise observer bias; and data were available for analysis according to original allocation.
In more recent reviews and updates, the following outcomes have been added: (28) neonatal infection; (29) neonatal antibiotics; (30) chorioamnionitis; (31) endometritis; (32) maternal antibiotics.
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (1 March 2014)
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of trial reports and reviews by hand.
We did not apply any language restrictions.
The original search was performed simultaneously for all reviews of methods of inducing labour, as outlined in the generic protocol for these reviews. Reviews have been updated individually, in accordance with the generic protocol (Hofmeyr 2009).
Data collection and analysis
For this review (and other linked induction of labour (IOL) methods reviews), the initial data extraction in 2000 was conducted and co‐ordinated by (Josephine Kavanagh (JK),Tony Kelly (TK) Jane Thomas (JT)) at the Clinical Effectiveness Support Unit (CESU) at the Royal College of Obstetricians and Gynaecologists, UK, in co‐operation with the Pregnancy and Childbirth Group of The Cochrane Collaboration. This process allowed the data extraction process to be standardised across all the reviews. For updates, the data extraction has been undertaken by authors of the individual review, for this update it was undertaken by JT and Anna Fairclough (AF).
The trials were initially reviewed on eligibility criteria, using a standardised form and the basic selection criteria specified. A standardised data extraction form was developed and then piloted for consistency and completeness. This pilot process involved the researchers at the CESU and the authors of the initial induction of labour series of reviews. For a description of the methods used to carry out the initial reviews, seeAppendix 1. For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 2. For this update we used the following methods when assessing the reports identified by the updated search.
Selection of studies
For this update two review authors (JT and AF) independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted another author (JK or TK).
Data extraction and management
For this update the same data extraction form was used to extract data. For eligible studies, JT and AF extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, consulted the other authors (TK or JK). We entered data into Review Manager software (RevMan 2012) and checked for accuracy.
When information regarding any of the above was unclear, we contacted the authors of the original reports to provide further details.
Assessment of risk of bias in included studies
For this update two review authors (JT, AF) independently assessed the risk of bias for each study using the criteria outlined in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving another author (JK or TK).
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
No continuous data were analysed in this update (2013). In future updates, if continuous data are analysed, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
Cluster‐randomised trials are eligible for inclusion in the analyses along with individually randomised trials. None have currently been identified. If in future such trials are identified we will adjust their standard errors using the methods described in the Handbook (Higgins 2011), using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If ICCs from other sources are used, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If both cluster‐randomised trials and individually‐randomised trials are identified, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not eligible for inclusion.
Other unit of analysis issues
Trials in pregnancy and childbirth may include outcomes for multiple pregnancies, the generic protocol does not explicitly exclude multiple pregnancies, but the trials identified to date have included singleton pregnancies only. Trials with multiple pregnancy will be included but the outcomes relating to the babies will have to take account of clustering of events. As outlined in the Pregnancy and Childbirth Group Methodological Guidelines and the Handbook (Higgins 2011).
Some trials are multi‐arm studies, where this occurs only the intervention arms relevant to this review are included, where this occurs it is noted in the Included studies table.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 50% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there were 10 or more studies in the meta‐analysis we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). We use fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and the clinical implications of treatment effects differing between trials are discussed. If the average treatment effect was not clinically meaningful we did not combine trials.
If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
Where substantial heterogeneity was identified, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
The following subgroup analyses are included:
previous caesarean section or not;
nulliparity or multiparity;
membranes intact or ruptured;
cervix favourable, unfavourable or undefined.
The following outcomes are used in subgroup analysis:
vaginal delivery not achieved within 24 hours (or period specified by trial authors);
uterine hyperstimulation with fetal heart rate (FHR) changes;
caesarean section;
serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood);
serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicaemia).
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2012). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
Sensitivity analyses were performed for aspects of the review that might affect the results, for example, where there is risk of bias associated with the quality of some of the included trials.
Results
Description of studies
Results of the search
In total, 116 studies were considered; 46 have been excluded, 70 randomised controlled trials with a total of 11,487 women have been included. For further details of study characteristics refer to Characteristics of included studies and Characteristics of excluded studies.
Trial setting
Most of the trials examined outcomes of induction of labour in an inpatient environment. Three trials examined outpatient‐based induction policies (Hage 1993; O'Brien 1995; Ohel 1996).
Included studies
1. Prostaglandin E2 (PGE2) versus placebo or no treatment
Thirty‐nine trials (with 6761 women) compare prostaglandin E2 (PGE2) versus placebo or no treatment (Al Malt 1995; Bezircioglu 2012; Buchanan 1984; Campbell 1984; Cardozo 1986; Chaterjee 1990; Chua 1995; Chung 1992; Curet 1989; Doany 1997; Dommisse 1980; Dunston‐Boone 1991; Egarter 1989; Graves 1985; Hage 1993; Hannah 1996; Hayashi 1983; Liggins 1979; MacKenzie 1979; MacKenzie 1981; Mahmood 1992; Mahmood 1995; McCaul 1997; Newman 1997; O'Brien 1995; Ohel 1996; Poornima 2011; Prasad 1989; Prins 1983; Rayburn 1988; Rayburn 1992; Roach 1997; Sawai 1991; Sawai 1994; Shoaib 1994; Thiery 1984; Ulmsten 1985; Witter 1992; Witter 1996). Two new trials (200 women) have been included in this comparison for this update (Bezircioglu 2012; Poornima 2011).
In most of these studies the comparison was to a placebo, but in 12 trials, the comparison was "expectant management" (no treatment with monitoring for maternal/fetal well being) (Bezircioglu 2012; Cardozo 1986; Egarter 1989; Hannah 1996; Mahmood 1992; Mahmood 1995; McCaul 1997; Newman 1997; Ohel 1996; Poornima 2011; Roach 1997; Shoaib 1994).
Fifteen trials of these trials used prostaglandin gel, the dose used ranged between 0.5 mg to 3 mg (Al Malt 1995; Chaterjee 1990; Graves 1985; Hannah 1996; Hayashi 1983; MacKenzie 1979; Mahmood 1992; Mahmood 1995; McCaul 1997; O'Brien 1995; Poornima 2011; Prins 1983; Rayburn 1988; Sawai 1991; Thiery 1984). In four trials prostaglandin tablets (3 mg or 4 mg) were used (Dommisse 1980; Egarter 1989; Ohel 1996; Shoaib 1994). In 17 trials, the prostaglandin preparation was described as a pessary, the dose given varied from 0.2 mg pessaries (given hourly) (Liggins 1979), a single 2 mg pessary (Doany 1997; Ulmsten 1985 ), repeat 2 mg pessaries (Sawai 1994), single 2.5 mg (MacKenzie 1981), single 3 mg pessaries (Buchanan 1984; Cardozo 1986; Chua 1995; Chung 1992; Curet 1989), repeat 3 mg pessaries (Campbell 1984; Roach 1997), to a single 10 mg from sustained release pessaries, which were used in five trials (Bezircioglu 2012; Dunston‐Boone 1991; Rayburn 1992; Witter 1992; Witter 1996). In one study the prostaglandin was given as a "film" and the dose was 8.5 mg (Prasad 1989).
In 18 studies a single dose of prostaglandin was used, (gel: Al Malt 1995; Chaterjee 1990; Graves 1985; Hayashi 1983; MacKenzie 1979; MacKenzie 1981; Poornima 2011; Prins 1983; Rayburn 1988; Thiery 1984; pessary: Buchanan 1984; Cardozo 1986; Chua 1995; Chung 1992; Curet 1989; Doany 1997; Ulmsten 1985; tablet: Dommisse 1980), and these trials used 2, 2.5 or 3 mg doses, except Poornima 2011 which used 0.5 mg and MacKenzie 1979 which used 5 mg.
Fifteen trials used repeated prostaglandin doses (gel: Hage 1993; Hannah 1996; Mahmood 1992; Mahmood 1995; McCaul 1997; O'Brien 1995; Sawai 1991; pessary: Campbell 1984; Liggins 1979; Roach 1997; Sawai 1994; tablet: Egarter 1989; Ohel 1996; Shoaib 1994); the formulation of PGE2 was unclear in one study: Newman 1997.
2. Prostaglandin F2a (PGF2a) with placebo
Four trials with 435 women compared prostaglandin F2a (PGF2a) with placebo (MacKenzie 1979; MacLennan 1979; MacLennan 1980; Murphy 1980).
3. Prostaglandin F2a versus prostaglandin E2
Two trials with 107 women compared PGF2a with PGE2 (MacKenzie 1979; Neilson 1983).
4. Prostaglandin E2 gel versus prostaglandin E2 tablet
Seven trials with 1086 women compared PGE2: gel (dose varied from 1 mg to 3 mg) with PGE2 tablets (3 mg tablets), single‐dose tablet (Al‐Sebai 1993; Greer 1990; Mahmood 1989; Murray 1995; Rath 1999, or repeat dose 3 mg tablets Payne 1993; Taher 2011). One new trial (Taher 2011) with 165 patients has been added to this section for this update.
5. Prostaglandin E2 gel versus prostaglandin E2 pessary/suppository
Two trials with 159 women compared different PGE2 preparations: gel with pessary or suppository (Perryman 1992; Smith 1990).
6. Prostaglandin E2 tablet versus prostaglandin E2 pessary/suppository
Three trials with 491 women compared PGE2 tablet with pessary/suppository (El‐Mardi 1991; McLaren 1987; Stampe Sorensen 1992).
7. Prostaglandin E2 (sustained release) versus prostaglandin E2 (any vehicle)
Sustained release PGE2 (SR) pessaries were compared with other PGE2 preparations in 13 trials involving 1436 women (Duhl 1997; El Shawarby 2006; Ferraiolo 2010, Glanville 2002; Green 1998; Kalkat 2008; Miller 1991; Mukhopadhyay 2002; Rabl 2002; Smith 1994; Tomlinson 2001, Triglia 2010; Zanconato 2011). In only one trial was the comparison with PGE2 tablet (Rabl 2002), in the remaining 12 trials the comparison was PGE2 gel and the dose varied from 1 mg to 3 mg. In one trial a single dose of PGE2 gel (Miller 1991) was used. The other studies used repeat doses. Three new trials with 333 women have been added to this section for this update (Ferraiolo 2010; Triglia 2010; Zanconato 2011).
8. Prostaglandin E2 (low dose) versus prostaglandin E2 (high dose)
Low dose PGE2 (less than 3 mg) versus high dose PGE2 (more than 3 mg) was compared in eight trials with 1615 women (Ferraiolo 2010; Green 1998: MacKenzie 1997a; McLaren 1987; Miller 1991; Payne 1993; Smith 1990; Tomlinson 2001). Seven of the trials used low dose PGE2 gel; in one trial a single tablet was used (McLaren 1987). The high‐dose comparison was a 10 mg sustained release pessary in four trials (Ferraiolo 2010; Green 1998; Miller 1991; Tomlinson 2001); two trials also used pessaries but in other doses (McLaren 1987; Smith 1990); one used repeat doses of PGE2 tablets (Payne 1993); and the other repeat PGE2 gel (MacKenzie 1997a). A trial previously included in this comparison has been removed in this update because in both arms of the trial women received a low dose (less than 3 mg of PGE2) and so it does not meet the inclusion criteria (Nuutila 1996).One new trial with 151 women has been included (Ferraiolo 2010).
Excluded studies
In 26 studies, primary outcome data were either not reported or extractable (Bamford 1992; Bex 1990; Castle 1983; Danna 1995; De Laat 1991; Dommisse 1981; Fusi 1989; Gordon‐Wright 1979; Greer 1986; Greer 1988; Knogler 1988; Krammer 1995; Lass 1994; Lindblad 1985; MacKenzie 1977; MacKenzie 1997b; MacKenzie 1988; Parker 1990; Ramsey 1998; Sadaty 1998; Sellers 1985; Sorokin 1992; Spitzberg 1991; Tan 1994; Toppozada 1992; Veligati 1998).
Six trials were excluded on grounds of eligibility. Four trials only reported on, or included data on, women undergoing induction of labour who had suffered an intrauterine death (Gauger 1991; Hill 1991; Lorenz 1984; Odum 1993). One study only examined induction in preterm pregnancies (Loria‐Casanova 1989). One study compared three different doses of PGF2a, which was not a prespecified intervention comparison (Tang 1997).
Fourteen trials were excluded that compared prostaglandin E2 with prostaglandin E2 at different doses. These trials were excluded as the dosages used were not comparable within the division made in the review into 'high‐dose' and 'low‐dose' categories (Carlan 1995; Granstrom 1995; Grunstein 1990; Hunter 1982; Hunter 1984; Hunter 1998; Norchi 1993; Seeras 1995; Smith 1996; Tan 1999; Toplis 1979; Varma 1984; Walker 1983; Zanini 1991).
Two studies were excluded because they were not randomised controlled trials (Nikolov 2003; Petrou 2011) and one was an economic analysis (Sorensen 2008).
Risk of bias in included studies
See Figure 1 and Figure 2 for summaries of 'Risk of bias' assessments.
1.
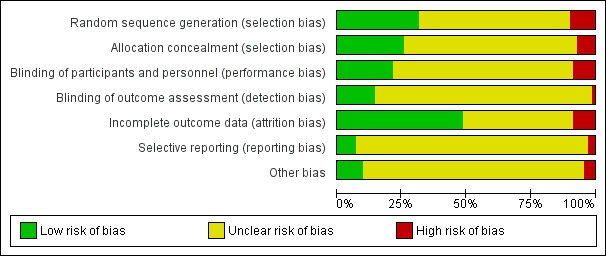
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
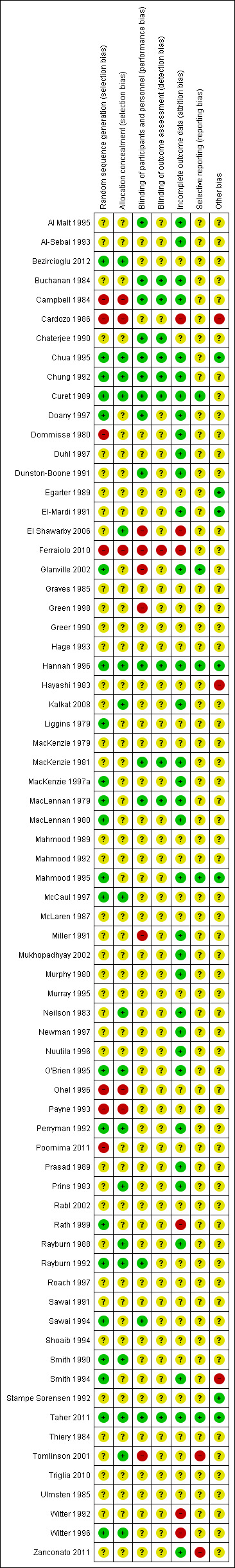
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation and allocation concealment (selection bias)
Fourteen trials employed central/pharmacy preparation of drugs in coded boxes or syringes (Chua 1995; Chung 1992; Curet 1989; Hannah 1996; Liggins 1979; McCaul 1997; Neilson 1983; O'Brien 1995; Perryman 1992; Prins 1983; Rayburn 1992; Smith 1990; Smith 1994; Witter 1996). The randomisation sequences were generated from computer‐generated lists or random number tables.
Thirty‐six trials were unclear on either the method of generation of the randomisation sequence, the method of allocation concealment or both (Al Malt 1995; Al‐Sebai 1993; Buchanan 1984; Chaterjee 1990; Doany 1997; Duhl 1997; Dunston‐Boone 1991; Egarter 1989; El‐Mardi 1991; El Shawarby 2006; Glanville 2002; Graves 1985; Green 1998; Greer 1990; Hage 1993; Hayashi 1983; Kalkat 2008; McLaren 1987; MacKenzie 1979; MacKenzie 1997a; MacKenzie 1981; Miller 1991; Mukhopadhyay 2002; Murphy 1980; Newman 1997; Prasad 1989; Rabl 2002; Rayburn 1988; Sawai 1991; Sawai 1994; Shoaib 1994; Stampe Sorensen 1992; Thiery 1984; Tomlinson 2001; Ulmsten 1985; Witter 1992).
Allocation by alternation was used in one trial (Campbell 1984), two trials allocated using the last digit of the hospital number (Cardozo 1986; Ohel 1996); one trial allocated depending on month of entry into the trial (Payne 1993); and one trial used "year of birth" with even years assigned to group A, odd to group B initially but changing this every five cases (Ferraiolo 2010).
Blinding
A double‐blind approach was used in 21 trials (Al Malt 1995; Buchanan 1984; Campbell 1984; Chaterjee 1990; Chua 1995; Chung 1992; Curet 1989; Doany 1997; Dunston‐Boone 1991; MacKenzie 1981; MacLennan 1979; Prasad 1989; Rayburn 1988; Rayburn 1992; Sawai 1991; Sawai 1994; Smith 1990; Thiery 1984; Ulmsten 1985; Witter 1992; Witter 1996).
Incomplete outcome data
Outcome data were incomplete in five trials (Cardozo 1986; Ferraiolo 2010; Rath 1999; Witter 1992; Witter 1996).
Selective reporting
Selective reporting bias was not assessed in the previous versions of this review. Time from induction to delivery is an important outcome. It is reported in numerous ways in different studies. The choice of endpoint and choice of summary measurement used is a potential source of bias. In this update seven randomised controlled trials have been added. The trials are mainly small single‐centre studies, from a variety of countries. Two trials reported information about trial registration (Taher 2011; Triglia 2010); for the other trials it has not been possible to establish changes and selective reporting of positive findings between protocol and publication. Two of the new studies have a greater risk of selective reporting as they used a variety measures to assess patient satisfaction (Ferraiolo 2010) and pain experienced in labour (average and percentage, repeated measures) (Zanconato 2011), a priori criteria for selecting these are not apparent.
Other potential sources of bias
Other potential sources of bias were not included in earlier versions of this review. Of the seven new trials included in this update only two reported the source of funding (MacLennan 1979; Taher 2011). Two trials included a statement "The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper" (Triglia 2010; Zanconato 2011) and the other states under financial disclosure " the authors have no connection to any companies or products mentioned in this article" (Ferraiolo 2010). Generic names are used in four of the studies (Bezircioglu 2012; MacLennan 1979; Poornima 2011; Taher 2011). Three studies used proprietary names of the preparations (Ferraiolo 2010; Triglia 2010; Zanconato 2011) and in all of these the comparison was sustained release PGE2 (named as Propess by Ferring Pharmaceuticals) versus a vaginal gel. In one of these papers only the generic name of the vaginal gel was used (Triglia 2010). In Zanconato 2011 it is stated that 1 to 2 mg of vaginal PGE2 gel was used but the proprietary name given was"Prepidil", (Pharmacia Upjohn); this is the name of an intracervical preparation. In the third paper (Ferraiolo 2010), intravaginal gel is referred to, (named Prepidil) and the dose of prostaglandin given was 0.5 mg per 3 mg, which is typical of an intracervical preparation doses (Prepidil) and may be less effective than the usual dose given vaginally (making the sustained release preparations appear more effective).
Effects of interventions
See: Table 1; Table 2; Table 3
All the outcomes listed under Types of outcome measures and subgroups defined in Types of participants, were sought. Only those with data appear in the analysis tables.
Data discussed applies to the 'all women' group and, unless stated, there was no difference between any of the prespecified subgroups. This was formally examined by using subgroup interaction tests available within RevMan (RevMan 2012). The results are referred to within the text where relevant.
1. Vaginal prostaglandin E2 versus placebo/no treatment (39 trials, 6761 women)
Primary outcomes
Fifteen trials included some measurement of time from induction to delivery as an outcome but in only two trials (384 women) (Egarter 1989; Ulmsten 1985) is 'Vaginal delivery not achieved within 24 hours' reported in a suitable format to contribute data to this review. Of the trials reporting time from induction to delivery in a format that is not useable in the meta‐analysis, the largest trial (Hannah 1996) (2522 women) compared PGE2 gel with expectant management for women with ruptured membranes (ROM). The authors reported a reduction in the median time from ROM to delivery in the group given PGE2 compared to those in an expectant management. Of the two studies contributing data to this outcome, the larger study by Egarter 1989 (345 women), included both primiparae and multiparae, all with a favourable cervix (Bishop score (BS) > 4), intact membranes and the study used a higher dose of PGE2 (3 mg tablet). They found more women delivered within 24 hours with vaginal PGE2 compared with expectant management (up to 42 weeks' gestation) (12% of the PGE2 group versus 100% expectant management group were undelivered after 24 hours (risk ratio (RR) 0.12, 95% confidence interval (CI) 0.08 to 0.18). The smaller trial by Ulmsten (39 women) included only primiparae with an unfavourable cervix and used a single dose of 2 mg PGE2 suppository compared with a placebo. In this trial (Ulmsten 1985), 79% of the PGE2 group and 90% of the placebo group were undelivered after 24 hours (RR 0.88, 95% CI 0.67 to 1.15). Although both trials' findings are compatible with an increase in vaginal delivery within 24 hours, differences between the interventions used, comparisons groups and characteristics of the participants contribute to the marked heterogeneity observed when the results are combined (I² = 98%). Given the heterogeneity, it is reasonable not to combine these results in meta‐analysis, but if combined, a random‐effects model should be used; the CIs are wider and the difference does not reach statistical significance, (18.1% versus 98.9%, average RR 0.32, 95% CI 0.02 to 4.83, (heterogeneity: Tau² = 3.77; Chi² = 125.43, df = 1 (P < 0.00001); I² = 98%), two trials, 384 women, (test for subgroup differences: Chi² = 43.14, df = 1 (P < 0.00001), I² = 97.7%) (Analysis 1.1). Overall therefore, although not certain, it is likely that vaginal prostaglandin E2 compared with placebo or no treatment reduces the likelihood of vaginal delivery not being achieved within 24 hours.
1.1. Analysis.
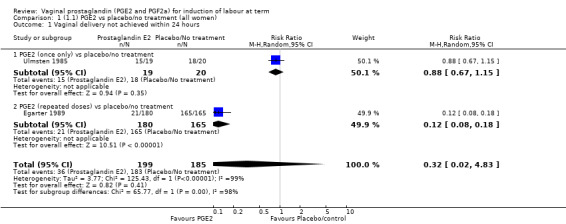
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 1 Vaginal delivery not achieved within 24 hours.
'Uterine hyperstimulation with fetal heart rate (FHR) changes' was reported in 15 studies (Analysis 1.2). An increase in uterine hyperstimulation with FHR changes is seen in association with vaginal PGE2 (4.8% versus 1.0%, RR 3.16, 95% CI 1.67 to 5.98, 15 trials, 1359 women) in comparison with placebo. This RR is reduced compared to previous versions of this review (which was 4.4% versus 0.5%, RR 4.14, 95% CI 1.93 to 8.90), because of a small decrease in event rate in the PGE2 group and an increase event rate in the control group. Uterine hyperstimulation with FHR changes did not occur in either arms of four trials (Chua 1995; Dommisse 1980; O'Brien 1995; Sawai 1991) and is influenced by one trial (Rayburn 1992) where the estimate is a 30‐fold increase in hyperstimulation rates associated with FHR changes (based on 13 events in the PGE2 group and zero in the control group, 12.9% versus 0.0%, RR 30.44, 95% CI 1.83 to 505.65). The new trial (Bezircioglu 2012) had a higher event rate in both arms of the trial. The estimated rates of hyperstimulation with FHR changes seen in either the once only or repeated dose subgroups compared with placebo or no treatment are compatible with no difference between the groups (Analysis 1.2).
1.2. Analysis.
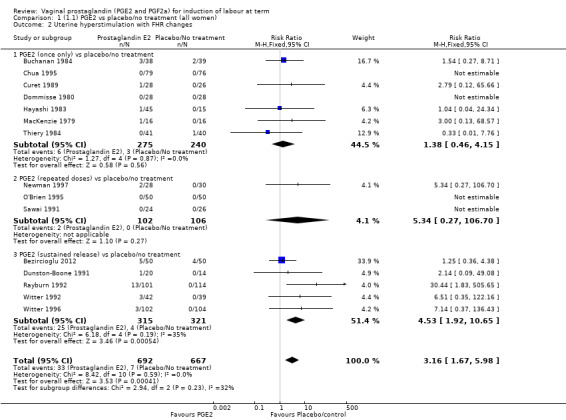
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 2 Uterine hyperstimulation with FHR changes.
The caesarean section rate is lower in the PGE2 groups compared with placebo/expectant management, the estimates are compatible with no difference or a reduction of 10% or more in caesarean section (13.5% versus 14.8%, RR 0.91, 95% CI 0.81 to 1.02, 36 trials, 6599 women) (Analysis 1.3). This finding is mirrored when the data were considered by parity, membrane status or cervical favourability. There was no statistical heterogeneity, and the results were similar when only higher quality studies were considered.
1.3. Analysis.
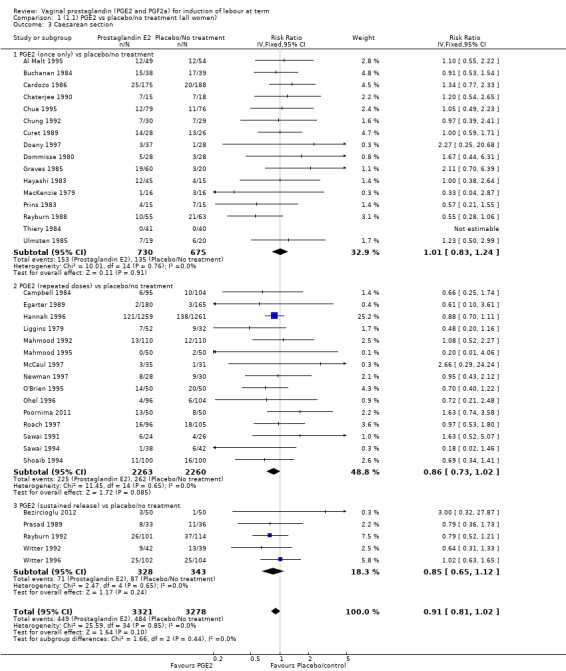
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 3 Caesarean section.
Serious neonatal (Analysis 1.4) or maternal morbidity or death (Analysis 1.5) are rare events and there are insufficient data to make conclusions about the impact of PGE2 on this outcome.
1.4. Analysis.
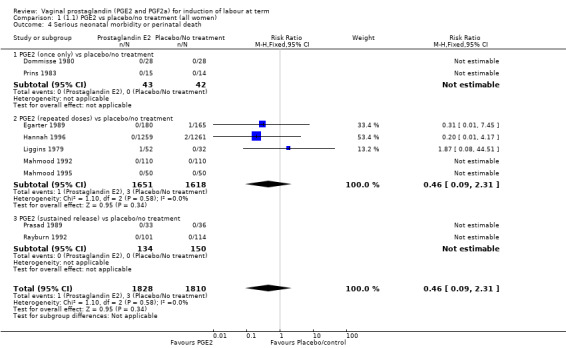
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 4 Serious neonatal morbidity or perinatal death.
1.5. Analysis.
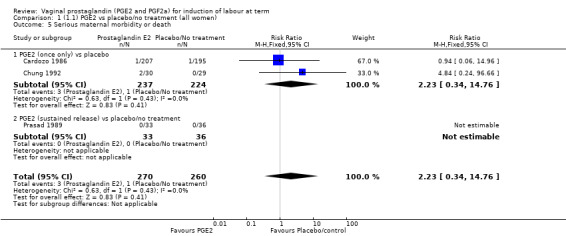
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 5 Serious maternal morbidity or death.
Secondary outcomes
The risk of the cervix remaining unchanged/unfavourable after 12 to 24 hours is reduced with the use of vaginal prostaglandins when compared with placebo (18.9% versus 40.5%, average RR 0.41, 95% CI 0.27 to 0.65, heterogeneity: Tau² = 0.16; Chi² = 11.68, df = 5 (P = 0.04); I² = 57%, six trials, 567 women), test for subgroup differences: Chi² = 5.42, df = 2 (P = 0.07), I² = 63.1% (Analysis 1.6).
1.6. Analysis.
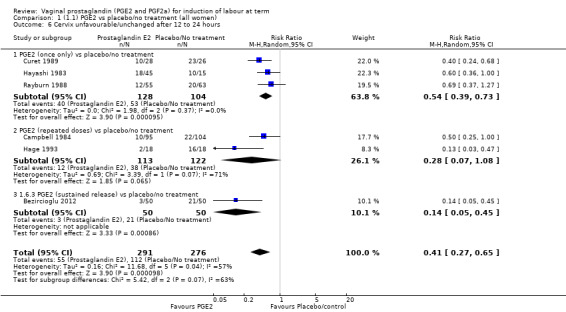
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 6 Cervix unfavourable/unchanged after 12 to 24 hours.
The use of oxytocin augmentation may be reduced if vaginal prostaglandins are used (39.0% versus 47.8%, average RR 0.81, 95% CI 0.63 to 1.05, heterogeneity: Tau² = 0.15; Chi² = 55.20, df = 12 (P < 0.00001); I² = 78%, 13 trials, 1421 women), test for subgroup differences: Chi² = 7.31, df = 2 (P = 0.03), I² = 72.6% (Analysis 1.7). Within the clinical subgroups, the reduction in the use of oxytocin augmentation is apparent in women with an unfavourable cervix (average RR 0.77, 95% CI 0.53 to 1.10, heterogeneity: Tau² = 0.20; Chi² = 27.80, df = 7 (P = 0.0002); I² = 75%. eight trials, 813 women) (Analysis 6.7); where repeated doses or sustained release preparations have been used, in women with a favourable cervix, no reduction is evident (43.7% versus 42.5%, average RR 1.00, 95% CI 0.66 to 1.51, heterogeneity: Tau² = 0.09; Chi² = 7.96, df = 2 (P = 0.02); I² = 75%, three trials, 443 women) (Analysis 7.5).
1.7. Analysis.
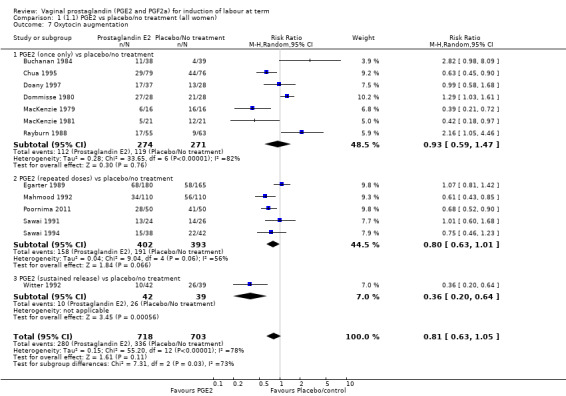
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 7 Oxytocin augmentation.
6.7. Analysis.
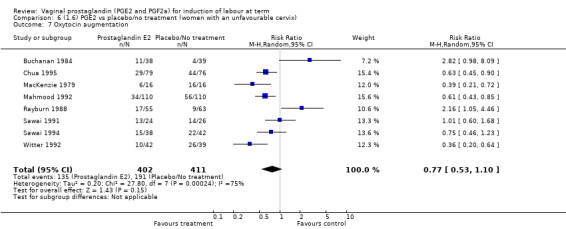
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 7 Oxytocin augmentation.
7.5. Analysis.
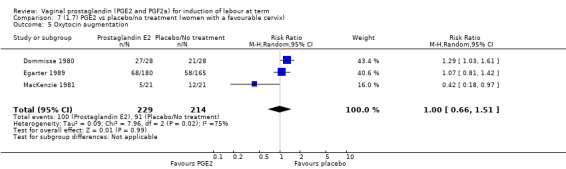
Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 5 Oxytocin augmentation.
Overall, hyperstimulation without FHR changes is increased with PGE2 (1.4% versus 0.4%, RR 2.48, 95% CI 1.17 to 5.26, 13 trials, 3636 women), this effect is greatest in the sustained release subgroup (6.6% versus 0.0%, RR 7.85, 95% CI 1.05 to 58.82) (Analysis 1.8), (test for subgroup differences: Chi² = 2.20, df = 2 (P = 0.33), I² = 9.0%).
1.8. Analysis.
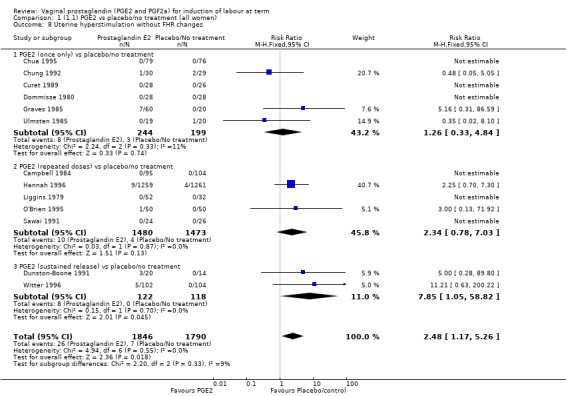
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 8 Uterine hyperstimulation without FHR changes.
There was no increase in the use of epidural anaesthesia when prostaglandins were used (49.6% versus 45.5%, average RR 1.16, 95% CI 0.85 to 1.60, heterogeneity: Tau² = 0.15; Chi² = 50.62, df = 6 (P < 0.000001), I² = 88%, seven trials, 3555 women) (Analysis 1.10). One study (Shoaib 1994) reported a five‐fold increase in epidural rates. The trial compared active and conservative management for ruptured membranes at term. No difference was seen in this study between caesarean section rates or the need for instrumental delivery but the results on epidural use of this study contrast sharply with the other trials. When excluded from the analysis, there is no difference detected between the groups, (48% versus 47%, average RR 0.99, 95% CI 0.87 to 1.12, heterogeneity: Tau² = 0.01; Chi² = 6.85, df = 5 (P = 0.23); I² = 27%). This study also impacts in the analysis of the clinical subgroup of women with ruptured membranes (Analysis 5.5), again when removed the heterogeneity is reduced.
1.10. Analysis.
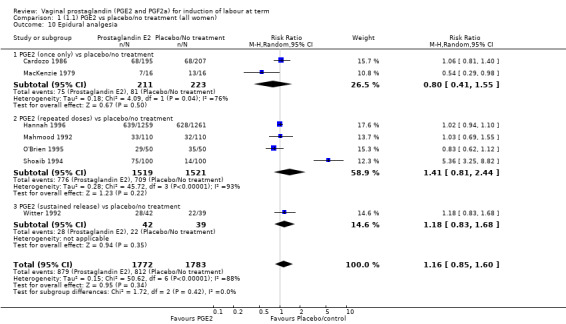
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 10 Epidural analgesia.
5.5. Analysis.
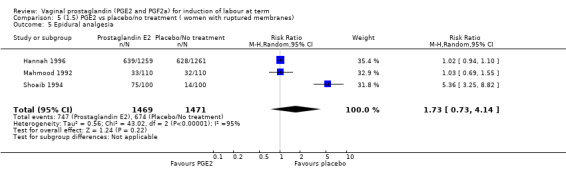
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 5 Epidural analgesia.
There was no evidence on an effect of instrumental vaginal delivery. Meconium‐stained liquor was less likely if induction was undertaken with vaginal prostaglandins (8.5% versus 10.5%, RR 0.82, 95% CI 0.68 to 0.98, 12 trials, 4245 women) (Analysis 1.12).
1.12. Analysis.
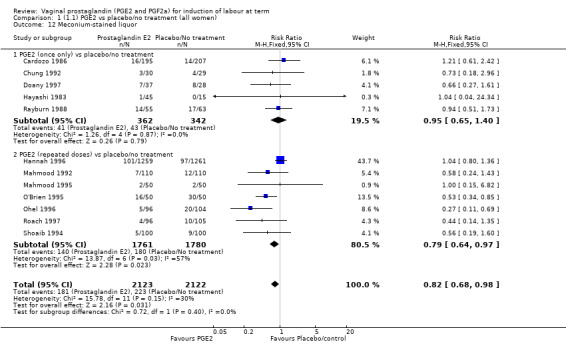
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 12 Meconium‐stained liquor.
There was no evidence of a difference between the two groups in Apgar score less than seven at five minutes (2.2% versus 1.7%, RR 1.28, 95% CI 0.86 to 1.92, 16 trials, 4481 women), (test for subgroup differences: Chi² = 8.05, df = 2 (P = 0.02), I² = 75.2%) (Analysis 1.13) and neonatal intensive care unit admission (8.8% versus 9.4%, RR 0.94, 95% CI 0.78 to 1.14, 12 trials, 4022 women) (Analysis 1.14). Apgar score less than seven at five minutes was not a common event and only 88 cases were reported in the 4481 patients, so the trials may be too small to detect a difference if one exists.
1.13. Analysis.
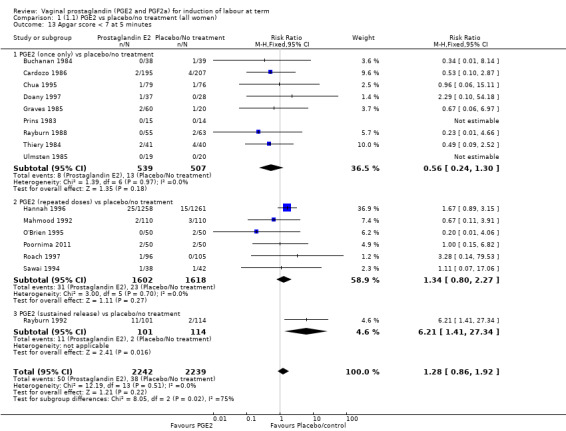
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 13 Apgar score < 7 at 5 minutes.
1.14. Analysis.
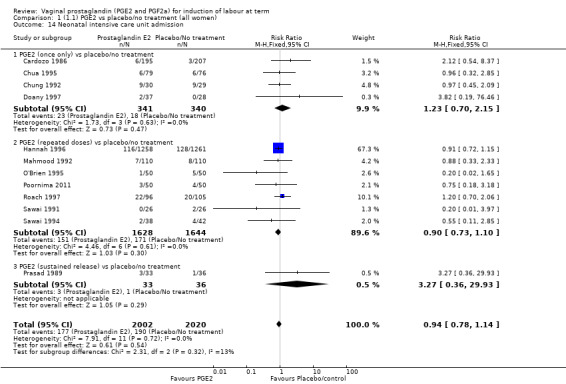
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 14 Neonatal intensive care unit admission.
Maternal side‐effects were not increased with the use of vaginal prostaglandins. The rate of postpartum haemorrhage was increased with the use of prostaglandins (4.1% versus 2.8%, RR 1.47, 95% CI 1.04 to 2.09, nine trials, 3537 women) (Analysis 1.21), the majority of this result is as a result of the increase seen in one study that compared active versus conservative management of ruptured membranes (Hannah 1996).
1.21. Analysis.
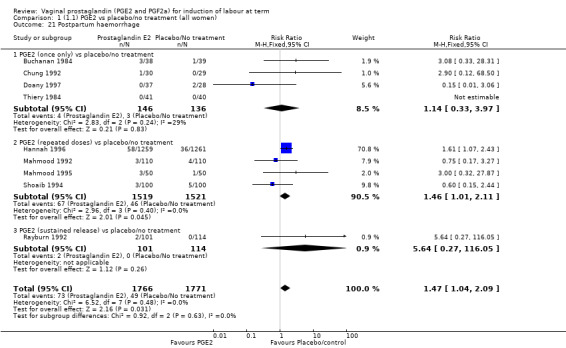
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 21 Postpartum haemorrhage.
Two studies looked at maternal satisfaction with mode of induction (Cardozo 1986; Hannah 1996). In both trials a policy of active induction was compared with expectant management, but the indications for induction were different. In the Cardozo trial, women at 40 + 10 days gestation were offered induction within two to four days or expectant management until labour started spontaneously or monitoring raised concern about either fetal or maternal well being; this could be many days or weeks. Allocation was based on odd or even last digit of hospital number, mothers who did not like their assigned group could request the alternative treatment group. The Hannah trial included women with prelabour rupture of the membranes, expectant management lasted a maximum of four days. The results of the two trials are different, but the larger trial, which is a better quality study with 2520 women (Hannah 1996), has fewer women who are unsatisfied in the PGE2 group. Overall, women were less satisfied with a policy of expectant management (6.4% versus 11.5%, average RR 0.76, 95% CI 0.24 to 2.40, heterogeneity: Tau² = 0.64; Chi² = 14.99, df = 1 (P = 0.0001); I² = 93%, test for subgroup differences: Chi² = 14.97, df = 1 (P = 0.0001), I² = 93.3%, two trials, 2922 women) (Analysis 1.23).
1.23. Analysis.
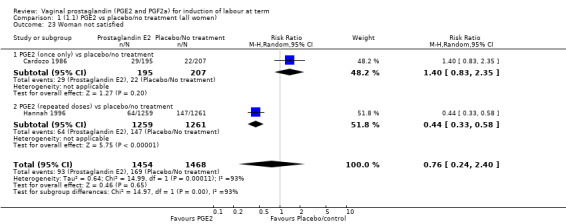
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 23 Woman not satisfied.
There were insufficient data to draw any meaningful conclusions for the remaining outcomes (uterine rupture, neonatal encephalopathy, disability in childhood, perinatal death, postpartum haemorrhage, serious maternal complications or caregiver not satisfied).
2. Vaginal prostaglandin F2a versus placebo (four trials, 435 women)
Three trials included length of labour as an outcome but vaginal delivery not achieved in 24 hours was not reported in a useable format. Uterine hyperstimulation with FHR changes were reported in one small trial of 32 participants, so the effects are uncertain (Analysis 8.1). Caesarean section rates in the trials are lower than are currently found in many countries. The caesarean section rates in the PGF2a group compared with placebo are reduced, this reduction does not reach statistical significance (5.7% versus 9.7%, RR 0.59, 95% CI 0.31 to 1.14, four trials, 467 women) (Analysis 8.2). Instrumental vaginal delivery rates are significantly lower but the rates of operative vaginal delivery are higher than would now be usual in most settings (23.7% versus 37.6%, RR 0.63, 95% CI 0.47 to 0.84, three trials, 435 women) Analysis 8.6. Cervical scores were less likely to be unchanged (12.0% versus 54.2%, RR 0.20, 95% CI 0.11 to 0.37, two trials, 170 women) (MacLennan 1979; MacLennan 1980) (Analysis 8.3). Oxytocin augmentation appeared to be reduced with the use of PGF2a but this does not reach statistical significance (44.0% versus 78.0%, RR 0.59, 95% CI 0.32 to 1.07, three trials, 202 women) (Analysis 8.4), and epidural analgesia (23.1% versus 33.3%, RR 0.74, 95% CI 0.56 to 0.97, four trials, 467 women) (Analysis 8.5).
8.1. Analysis.
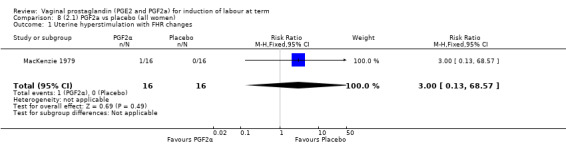
Comparison 8 (2.1) PGF2a vs placebo (all women), Outcome 1 Uterine hyperstimulation with FHR changes.
8.2. Analysis.
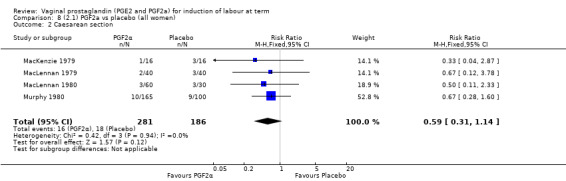
Comparison 8 (2.1) PGF2a vs placebo (all women), Outcome 2 Caesarean section.
8.6. Analysis.
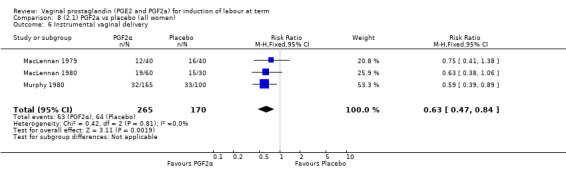
Comparison 8 (2.1) PGF2a vs placebo (all women), Outcome 6 Instrumental vaginal delivery.
8.3. Analysis.
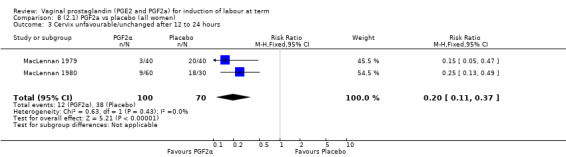
Comparison 8 (2.1) PGF2a vs placebo (all women), Outcome 3 Cervix unfavourable/unchanged after 12 to 24 hours.
8.4. Analysis.
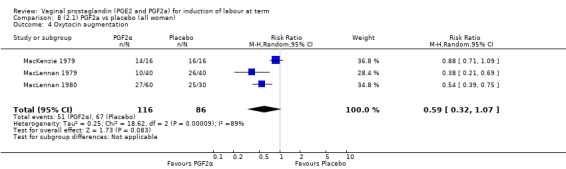
Comparison 8 (2.1) PGF2a vs placebo (all women), Outcome 4 Oxytocin augmentation.
8.5. Analysis.
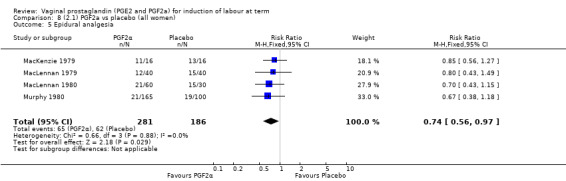
Comparison 8 (2.1) PGF2a vs placebo (all women), Outcome 5 Epidural analgesia.
3. Prostaglandin F2a versus prostaglandin E2 (two trials, 107 women)
Overall, there are insufficient data to make any meaningful conclusions (Analysis 11.1; Analysis 11.2; Analysis 11.3). One trial (MacKenzie 1979) showed a significant increase in the need for oxytocin augmentation with the use of PGF2a (87.5% versus 37.5%, RR 2.33, 95% CI 1.21 to 4.51, one trial, 32 women) (Analysis 11.4), but the numbers in this trial are small (16 in each arm), hence the results must be interpreted with caution. There is no evidence of any difference between any of the other reported outcomes.
11.1. Analysis.
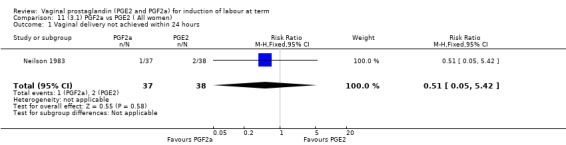
Comparison 11 (3.1) PGF2a vs PGE2 ( All women), Outcome 1 Vaginal delivery not achieved within 24 hours.
11.2. Analysis.
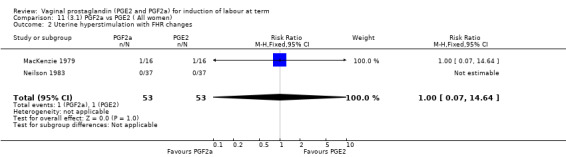
Comparison 11 (3.1) PGF2a vs PGE2 ( All women), Outcome 2 Uterine hyperstimulation with FHR changes.
11.3. Analysis.
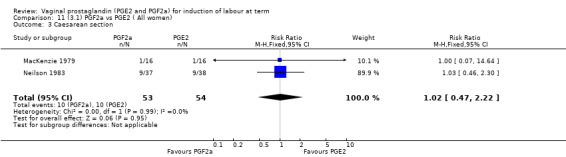
Comparison 11 (3.1) PGF2a vs PGE2 ( All women), Outcome 3 Caesarean section.
11.4. Analysis.
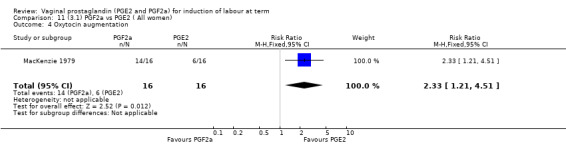
Comparison 11 (3.1) PGF2a vs PGE2 ( All women), Outcome 4 Oxytocin augmentation.
4. Prostaglandin E2 gel versus prostaglandin E2 tablet (seven trials, 1086 women)
Primary outcomes
There is no evidence of a difference in vaginal delivery rates not achieved in 24 hours (37.0% versus 36.9%, RR 1.03, 95% CI 0.84 to 1.26, three trials, 566 women) (Analysis 14.1). There is no evidence of a difference between gel or tablet regarding uterine hyperstimulation with FHR changes (0.5% versus 1.0%, RR 2.00, 95% CI 0.18 to 21.71, one trial, 200 women) (Analysis 14.2), or caesarean section rates (17.9% versus 19.8%, RR 0.91, 95% CI 0.72 to 1.17, six trials 1046 women) (Analysis 14.3). There are insufficient data to make any conclusions regarding serious maternal morbidity or death.
14.1. Analysis.
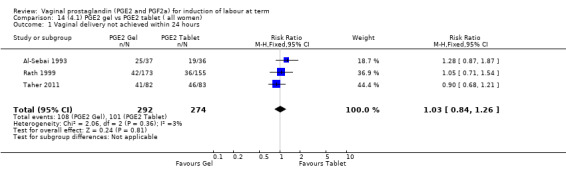
Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 1 Vaginal delivery not achieved within 24 hours.
14.2. Analysis.
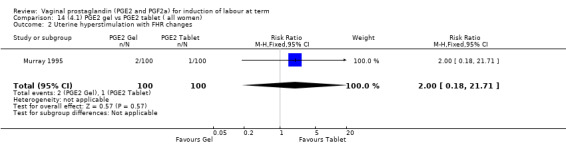
Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 2 Uterine hyperstimulation with FHR changes.
14.3. Analysis.
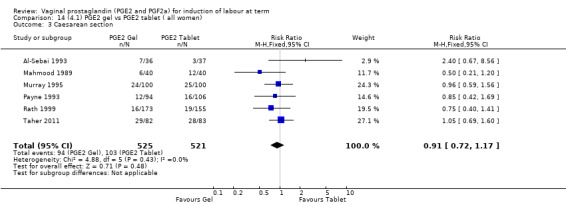
Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 3 Caesarean section.
Secondary outcomes
There is no evidence of a difference between the rates of cervix remaining unfavourable/unchanged at 24 to 48 hours (44.5% versus 51.4%, RR 0.87, 95% CI 0.70 to 1.07, two trials, 365 women) (Murray 1995; Taher 2011) (Analysis 14.5) or in the use of oxytocin augmentation (50.4% versus 58.4%, heterogeneity: Chi² = 12.47, df = 5 (P = 0.03); I² = 60%, six trials, 742 women) (Analysis 14.6). The reduction in oxytocin use with PGE2 gel found in Mahmood 1989 study is not replicated in the other studies. There was no evidence of a difference in epidural use (61.6% versus 56.4%, RR 1.07, 95% CI 0.95 to 1.21, three trials, 565 women) (Analysis 14.7). The findings for instrumental vaginal delivery rates are compatible with a reduction in instrumental delivery, this could be a small difference or a significant reduction of 23% or more (22.5% versus 28.7%, RR 0.77, 95% CI 0.58 to 1.02, three trials, 565 women) (Analysis 14.8); this effect reduction is not seen in the clinical subgroups (Analysis 18.8, Analysis 17.8, Analysis 15.8). The rates of postpartum haemorrhage between the two groups are similar (25.5% versus 27.5%, RR 0.89, 95% CI 0.71 to 1.11, three trials, 445 women) (Analysis 14.12). Except for instrumental vaginal delivery the effects are consistent across clinical groups. One trial reported on neonatal intensive care admissions, there was no difference between the groups but there were insufficient data to make any conclusions regarding neonatal outcomes.
14.5. Analysis.
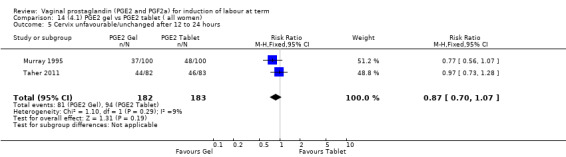
Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 5 Cervix unfavourable/unchanged after 12 to 24 hours.
14.6. Analysis.
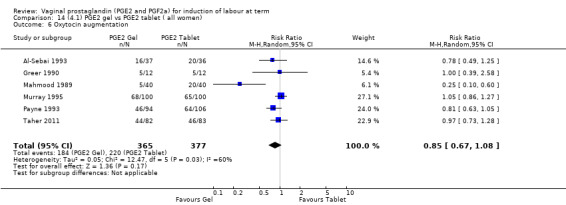
Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 6 Oxytocin augmentation.
14.7. Analysis.
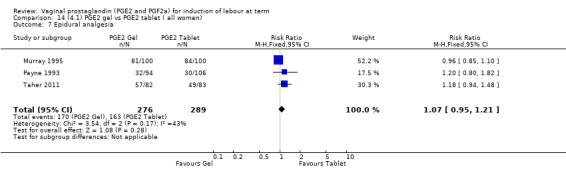
Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 7 Epidural analgesia.
14.8. Analysis.
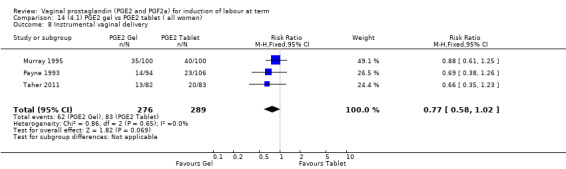
Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 8 Instrumental vaginal delivery.
18.8. Analysis.
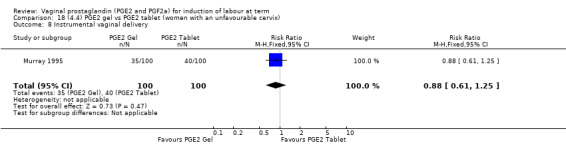
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 8 Instrumental vaginal delivery.
17.8. Analysis.

Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 8 Instrumental vaginal delivery.
15.8. Analysis.
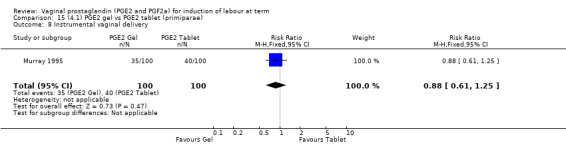
Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 8 Instrumental vaginal delivery.
14.12. Analysis.
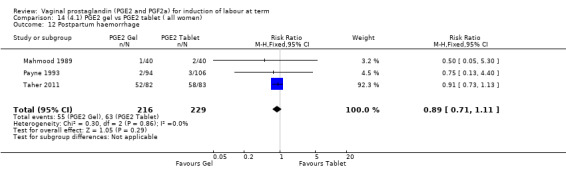
Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 12 Postpartum haemorrhage.
5. Prostaglandin E2 gel versus prostaglandin E2 pessary/suppository (two trials, 159 women)
Primary outcomes
There were no data available regarding vaginal delivery not achieved in 24 hours. A reduction in hyperstimulation with FHR changes was seen in association with PGE2 gel use in comparison with PGE2 pessaries (1.3% versus 11.2%, RR 0.16, 95% CI 0.03 to 0.87, two trials, 159 women) (Perryman 1992; Smith 1990) (Analysis 20.1). The dose used in both arms of one trial (Perryman 1992) was 5 mg of PGE2, which is much higher than commonly used as a single dose. The other trial (Smith 1990) compared 2.5 mg PGE2 gel with a vaginal 'chip' containing 3 mg to 3.5 mg of PGE2. For these reasons, these results should be interpreted with caution. There was no evidence that caesarean section rates differed between the two delivery systems (20.3% versus 31.3%, RR 0.65, 95% CI 0.38 to 1.11, two trials, 159 women) (Analysis 21.2).
20.1. Analysis.
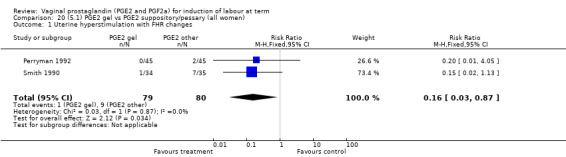
Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 1 Uterine hyperstimulation with FHR changes.
21.2. Analysis.
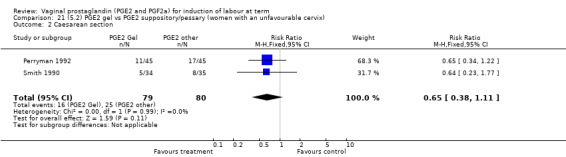
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 2 Caesarean section.
Secondary outcomes
Uterine hyperstimulation rates without FHR changes were not different between gel and pessary groups in the one trial reporting this outcome (Perryman 1992) (0.0% versus 4.4%, RR 0.20, 95% CI 0.01 to 4.05, one trial, 90 women) (Analysis 20.3). There was no evidence of a difference between maternal side‐effects or Apgar scores less than seven at five minutes.
20.3. Analysis.
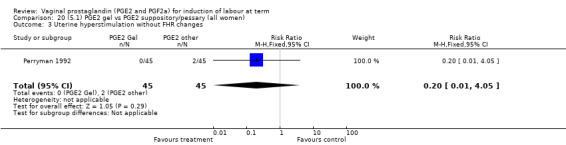
Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 3 Uterine hyperstimulation without FHR changes.
6. Prostaglandin E2 tablet versus prostaglandin E2 pessary/suppository (three trials, 491 women)
Primary outcomes
No evidence of a difference was seen between caesarean section rates between tablet and pessary (9.3% versus 8.1%, RR 1.13, 95% CI 0.64 to 1.99, three trials, 491 women) (Analysis 22.1). No data were available regarding vaginal delivery not achieved in 24 hours.
22.1. Analysis.
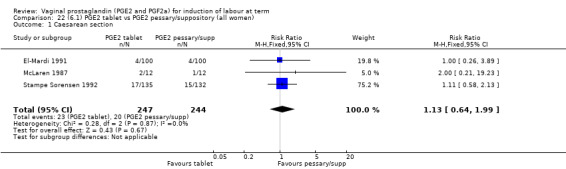
Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 1 Caesarean section.
Secondary outcomes
There was no difference detected in the proportion of women needing oxytocin augmentation between tablet and pessary groups (25.9% versus 35.2%, average RR 0.66, 95% CI 0.31 to 1.40, heterogeneity: Tau² = 0.33; Chi² = 9.31, df = 2 (P = 0.010); I² = 79%, three trials, 491 women) (Analysis 22.2). Marked heterogeneity between the three trials was seen (El‐Mardi 1991; McLaren 1987; Stampe Sorensen 1992) even though all three trials used single applications of PGE2 with similar doses of each medication.
22.2. Analysis.
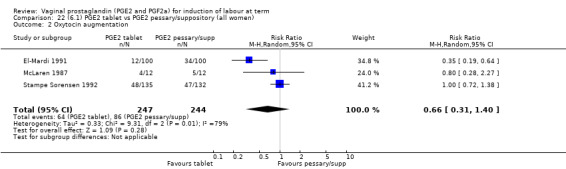
Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 2 Oxytocin augmentation.
Insufficient data were available to comment on uterine hyperstimulation without FHR changes, epidural usage or maternal side‐effects. Instrumental vaginal delivery rates were increased with the use of PGE2 tablets (17.8% versus 10.2%, RR 1.72, 95% CI 1.09 to 2.70, three trials, 491 women) (Analysis 22.5). Postpartum haemorrhage and Apgar scores less than seven at five minutes showed no evidence of a difference.
22.5. Analysis.
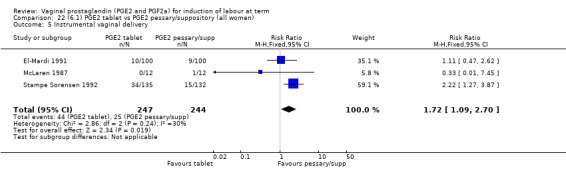
Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 5 Instrumental vaginal delivery.
7. Prostaglandin E2 (sustained release) versus prostaglandin E2 (any vehicle) (13 trials, 1436 women)
Primary outcomes
There is no evidence of a difference between vaginal delivery not achieved in 24 hours (43.1% versus 37.3%, RR 1.15, 95% CI 0.92 to 1.45, three trials, 450 women) (Analysis 26.1). The outcome uterine hyperstimulation rates with FHR changes was reported by five trials and no difference was detected (4.9% versus 2.2%, RR 2.15, 95% CI 0.89 to 5.21, five trials, 643 women) (Analysis 26.2), however, in two of these trials there were no events reported in either arm (El Shawarby 2006; Triglia 2010). In the three remaining trials there was an increase in events in the controlled release arms of the trials but this increase was greatest in the Smith 1994 trial. Caesarean section rates are not different between the two groups (20.4% versus 20.1%, RR 1.02, 95% CI 0.82 to 1.26, 11 trials, 1262 women) (Analysis 26.3). Serious neonatal morbidity or mortality was reported in two trials that included 320 women, poor neonatal outcomes were infrequent so these studies are too small to detect any difference that might exist, and there was a single event in the other PGE2 arm, which may be due to chance (Analysis 26.4). This is also true of maternal morbidity and mortality.
26.1. Analysis.
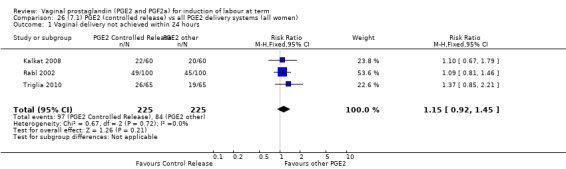
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 1 Vaginal delivery not achieved within 24 hours.
26.2. Analysis.

Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 2 Uterine hyperstimulation with FHR changes.
26.3. Analysis.
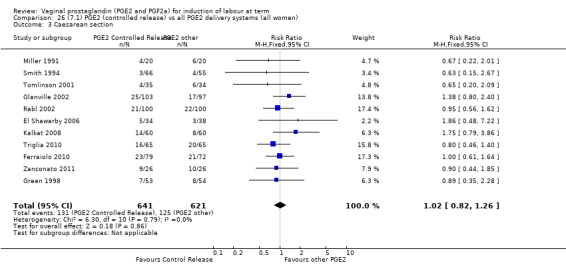
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 3 Caesarean section.
26.4. Analysis.
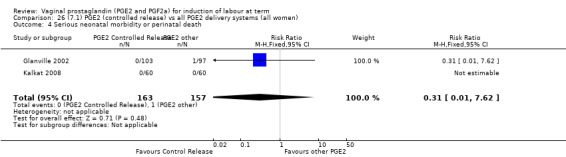
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 4 Serious neonatal morbidity or perinatal death.
Secondary outcomes
PGE2 controlled release pessaries were associated with a reduction in the likelihood that the cervix would remain unfavourable or unchanged (RR 0.61, 95% CI 0.46 to 0.80, two trials, 271 women) (Analysis 26.6). This is also the finding for the single trial that reported on this in the subgroup of women with unfavourable cervix (RR 0.60, 95% CI 0.45 to 0.80, one trial 151 women) (Analysis 29.6). There is no evidence of a difference in the use of oxytocin augmentation (35.0% versus 40.4%, average RR 0.88, 95% CI 0.69 to 1.13, heterogeneity: Tau² = 0.06; Chi² = 15.04, df = 6 (P = 0.02); I² = 60% seven trials, 884 women) (Analysis 26.7). This heterogeneity is caused by just one trial (Smith 1994) and when this trial is removed, there is no difference in oxytocin augmentation and the heterogeneity is reduced (average RR 1.01 95% CI 0.89 to 1.15, heterogeneity: Tau² = 0.00; Chi² = 4.37, df = 5 (P = 0.50); I² = 0%, six trials, 763 women). The Smith 1994 study reported a large reduction in oxytocin use with the sustained release pessary. The Smith 1994 study may differ from others within the group in that they used oxytocin augmentation at 12 hours after the onset of the induction process in both groups. There is also an imbalance in gestational age between the two groups which may have arisen by chance or may reflect bias in the study's methodology. The sustained release insert group has a higher gestational age which may allow potentially for a more straightforward induction process, hence explaining the greater reduction in the use of oxytocin for augmentation in the insert group. The same study reported a reduction in oxytocin use in multiparous women (RR 0.41, 95% CI 0.20 to 0.86, one trial, 66 women) (Analysis 28.4). No difference was detected in the subgroup of women with an unfavourable cervix (average RR 0.81, 95% CI 0.54 to 1.21, heterogeneity: Tau² = 0.14; Chi² = 15.50, df = 4 (P = 0.004); I² = 74%, five trials, 564 women) (Analysis 29.7).
26.6. Analysis.
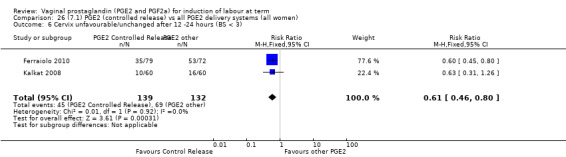
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 6 Cervix unfavourable/unchanged after 12 ‐24 hours (BS < 3).
29.6. Analysis.
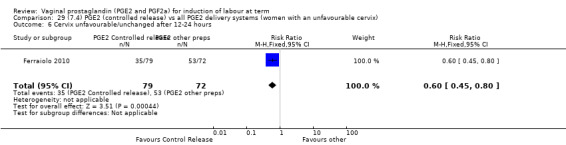
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
26.7. Analysis.
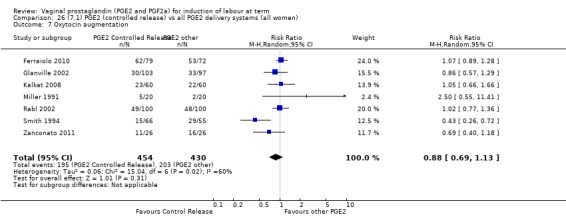
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 7 Oxytocin augmentation.
28.4. Analysis.
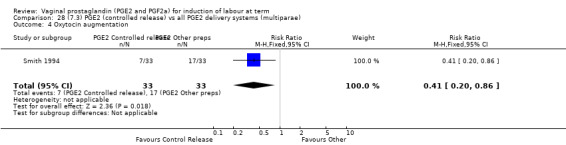
Comparison 28 (7.3) PGE2 (controlled release) vs all PGE2 delivery systems (multiparae), Outcome 4 Oxytocin augmentation.
29.7. Analysis.
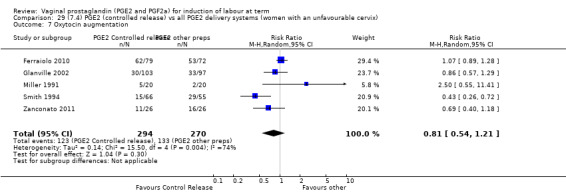
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 7 Oxytocin augmentation.
The rate of uterine hyperstimulation rates without FHR changes may be higher in the sustained release group; the event is infrequent and this does not reach statistical significance (4.2% versus 2.4%, RR 1.59, 95% CI 0.81 to 3.14, eight trials, 908 women) (Analysis 26.8). There is a reduction in instrumental delivery rates associated with the use of the vaginal insert (8.8% versus 18.8%, RR 0.47, 95% CI 0.32 to 0.68, six trials, 791 women) (Analysis 26.11). This effect seems to be greater in the clinical subgroup of women with an unfavourable cervix (7.4% versus 22.0%, RR 0.34, 95% CI 0.20 to 0.59, three trials, 402 women) (Analysis 29.11). There was no evidence of any significant heterogeneity within these results (as compared to those when examining rates of oxytocin augmentation). There were insufficient data regarding neonatal outcomes to draw any conclusions.
26.8. Analysis.
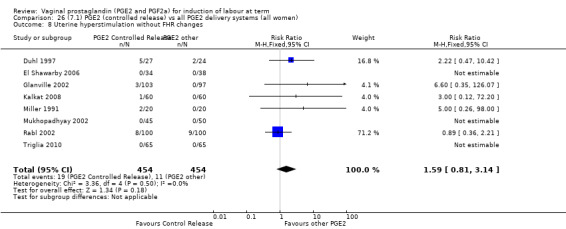
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 8 Uterine hyperstimulation without FHR changes.
26.11. Analysis.
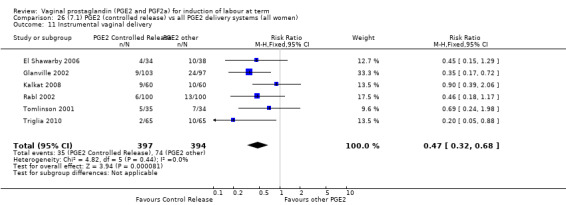
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 11 Instrumental vaginal delivery.
29.11. Analysis.
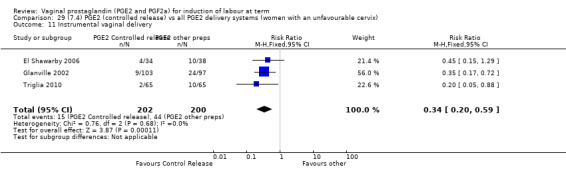
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 11 Instrumental vaginal delivery.
8. Prostaglandin E2 (low dose) versus prostaglandin E2 high dose (eight trials, 1615 women)
Primary outcomes
A reduction in hyperstimulation with FHR changes is seen in association with low‐dose regimens (Smith 1990), but this is a small trial and the reduction does not reach statistical significance (2.9% versus 20.0%, RR 0.15, 95% CI 0.02 to 1.13, one trial, 69 women) (Analysis 30.1). The trial (Smith 1990) used a 3 mg to 3.5 mg pessary in the high‐dose arm. Caesarean section rates do not appear to be different between the two groups (12.1% versus 12.1%, RR 1.02, 95% CI 0.78 to 1.33, seven trials, 1546 women) (Analysis 30.2). There were no neonatal deaths or cases of serious neonatal morbidity in the one trial reporting this outcome (MacKenzie 1997a). No data were available regarding vaginal delivery not achieved in 24 hours.
30.1. Analysis.
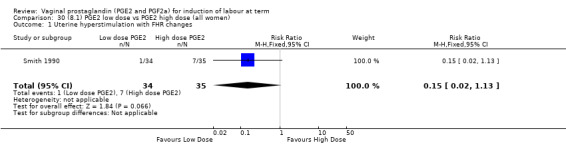
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 1 Uterine hyperstimulation with FHR changes.
30.2. Analysis.
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 2 Caesarean section.
Secondary outcomes
One trial reported on the cervix being unchanged/unfavourable after 12 to 24 hours; this was significantly more likely in the low‐dose group (RR 1.66, 95% CI 1.25 to 2.21, one trial, 151 women) (Analysis 30.4) (Ferraiolo 2010); this study included only primiparous women so this is the effect in this clinical subgroup (Analysis 31.3). In the all women group, overall no evidence of a difference in the use of oxytocin augmentation between the two groups is apparent (47% versus 45%, average RR 0.96, 95% CI 0.77 to 1.20, heterogeneity: Tau² = 0.03; Chi² = 10.47, df = 4 (P = 0.03); I² = 62%, five trials, 1370 women) (Analysis 30.5). However, there is marked heterogeneity between the trials. The heterogeneity in this group is reduced (I² = 0) when the MacKenzie 1997a is excluded, however, this is the only trial to compare the same preparation of PGE2 at different doses (one dose of 2 mg PGE2 gel versus two doses 2 mg PGE2 gel). The other studies compared different PGE2 formulations and doses. MacKenzie 1997a is also the largest study (955 of 1370 women in the meta‐analysis are from this study) and methodologically it has a lower likelihood of bias in comparison to the other studies (Figure 2). MacKenzie 1997a found that oxytocin use was increased when lower doses of PGE2 were used, this effect is mainly in one clinical subgroup ‐ multiparous women, oxytocin use in this group was almost double if the low‐dose regimen was used compared to the high dose (30.5% versus 15.7%, RR 1.94, 95% CI 1.35 to 2.80, one trial, 456 women) (Analysis 32.3). In primiparous women there was no difference detected (RR 1.05, 95% CI 0.93 to 1.18, two studies, 650 women) (Analysis 31.4).
30.4. Analysis.
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 4 Cervix unfavourable/unchanged after 12‐24hrs .
31.3. Analysis.
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 3 Cervix unfavourable/unchanged after 12‐24hrs .
30.5. Analysis.
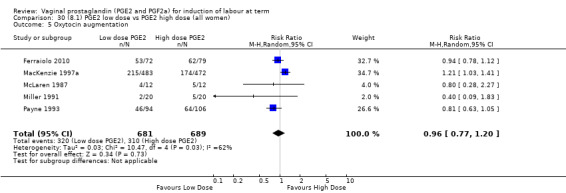
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 5 Oxytocin augmentation.
32.3. Analysis.
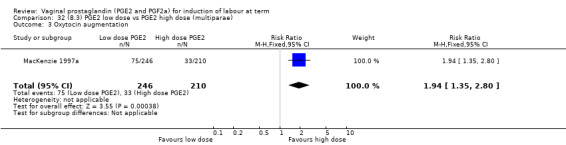
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 3 Oxytocin augmentation.
31.4. Analysis.
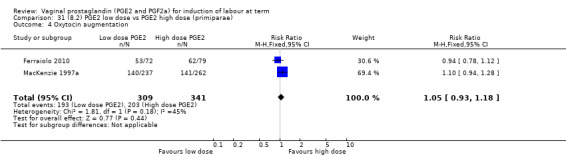
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 4 Oxytocin augmentation.
There was no difference in the following outcomes between low and high doses: Uterine hyperstimulation without FHR changes (Analysis 30.6); epidural analgesia use (Analysis 30.7), instrumental vaginal delivery rates (17.3% versus 19.5%, RR 0.89, 95% CI 0.70 to 1.13, three trials, 1179 women) (Analysis 30.8), meconium‐stained liquor (Analysis 30.9), Apgar score less than seven at five minutes (Analysis 30.10), neonatal intensive care unit admission (Analysis 30.11), maternal side‐effects (Analysis 30.13) or postpartum haemorrhage (Analysis 30.18). However, in the clinical subgroup of multiparae, instrumental delivery rates were increased (5.7% versus 1.0%, RR 5.98, 95% CI 1.37 to 25.99, one trial, 456 women) (Analysis 32.5).
30.6. Analysis.
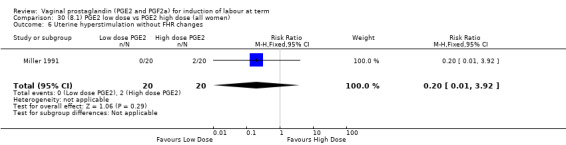
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 6 Uterine hyperstimulation without FHR changes.
30.7. Analysis.
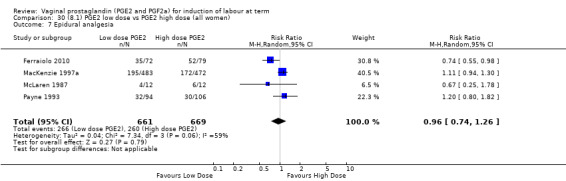
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 7 Epidural analgesia.
30.8. Analysis.
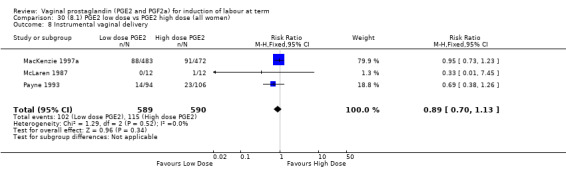
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 8 Instrumental vaginal delivery.
30.9. Analysis.
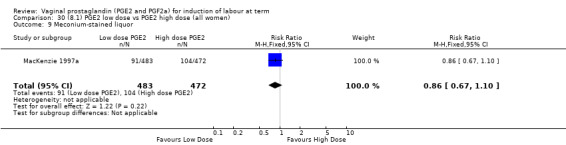
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 9 Meconium‐stained liquor.
30.10. Analysis.
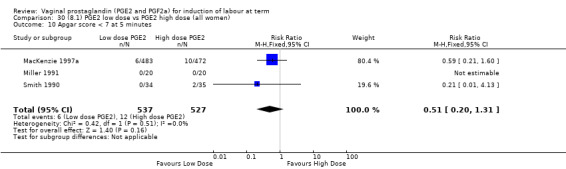
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 10 Apgar score < 7 at 5 minutes.
30.11. Analysis.
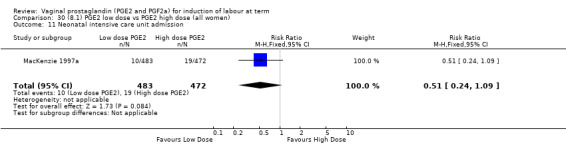
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 11 Neonatal intensive care unit admission.
30.13. Analysis.
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 13 Maternal side‐effects (all).
30.18. Analysis.
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 18 Postpartum haemorrhage.
32.5. Analysis.
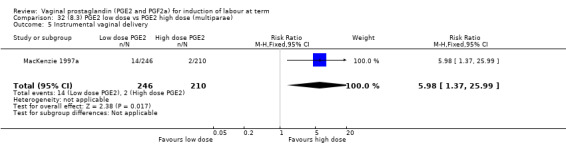
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 5 Instrumental vaginal delivery.
Discussion
Summary of main results
PGE2 versus placebo
Prostaglandins are probably effective at inducing labour: they probably increase the likelihood of delivery within 24 hours (although this does not reach statistical significance in the meta‐analysis), they increase uterine hyperstimulation with fetal heart rate changes, have no effect or may reduce the risk of caesarean section, but there is an absence of evidence on the overall effect on improving maternal and fetal outcomes (across a variety of measures). The reporting of maternal and fetal morbidity is very limited. Induction of labour is a common procedure so even small differences in effects could be important.
Vehicle comparisons
It is not possible to detect a difference in the effectiveness between the gel or tablet forms of PGE2 or between the sustained release pessaries and PGE2 gel/tablets. This is not that surprising as differences between different formulations of the same drug are likely to be small and so the trials in this review may be underpowered to detect such differences if they exist. However, because induction of labour is a frequently used intervention, even small differences could be important.
Dose comparisons
It is not possible to demonstrate a difference in outcomes between low‐ and high‐dose regimens. Accepting the problems with the arbitrary division made in this review, there is no evidence of an advantage of a higher‐dose regimen.
Overall completeness and applicability of evidence
There are numerous trials examining the efficacy of various vaginal prostaglandin (E2) preparations. However, data on the effect of these on important outcomes are limited. There is a particular paucity of comparable data on time in labour/to delivery, and evidence of maternal and neonatal benefits and harms. The evidence is relevant to current clinical practice, and to the one in five pregnant women who undergo induction of labour, because vaginal prostaglandins are the main induction methods recommended in clinical practice guidelines (NICE 2008).
Quality of the evidence
Sensitivity analysis
We attempted to quantify the impact of inadequate or unclear randomisation and/or concealment within the review. The effect of including only those trials with adequate methodology was highlighted in the results. Lower‐quality factors appeared to be responsible for significant heterogeneity in two main areas of the review (caesarean section rates and oxytocin augmentation regarding PGE2 versus placebo), but in neither case was the overall result altered.
Due to the number of agent comparisons in this review and the small number of trials in each group, a more detailed analysis of the impact of trial quality on the results in the other sections of the review could not be undertaken.
Publication bias
We attempted to examine the effect of possible publication bias in this review and its impact on the five primary outcomes. Due to the restriction in data on vaginal delivery rates not achieved in 24 hours and the small amount of data available on serious maternal and neonatal morbidity and mortality, the calculations have been restricted to hyperstimulation rates with fetal heart rate changes and caesarean section. Examination of a standard funnel plot reveals no graphical evidence of publication bias within this review for the two examined outcomes, although the plot does become asymmetrical when only studies of adequate quality are included when examining caesarean section.
Further analysis of publication bias on secondary outcomes was limited to comparisons where more than 10 trials were present. There was evidence of asymmetry within the plot for Apgar score less than seven at five minutes when comparing PGE2 to placebo (Figure 3). However, there was no evidence of similar asymmetry seen for other similar neonatal outcomes.
3.
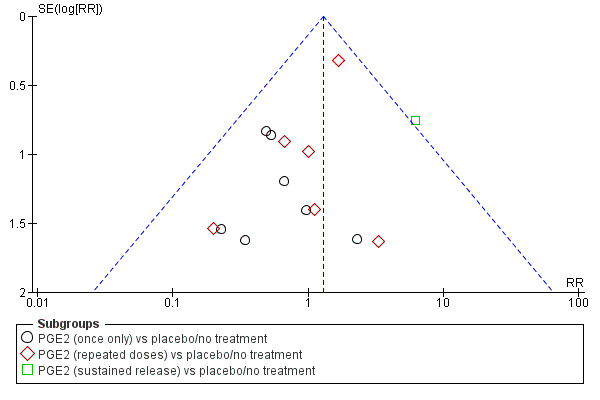
Funnel plot of comparison: 1 (1.1) PGE2 (all regimens) vs placebo/no treatment (all women), outcome: 1.13 Apgar score < 7 at 5 minutes.
Potential biases in the review process
The review includes a measurement of time to delivery (percentage not delivered in 24 hours), although most studies included time to delivery as an outcome, the data are not provided in a way that is usable in this review. The review has five primary outcomes and up to 26 secondary outcomes have been included, so by chance alone we should have at least one significant finding.
Agreements and disagreements with other studies or reviews
Other Cochrane reviews: There are 43 reviews on induction of labour in The Cochrane Library. This review is one in a series of 27 reviews on methods of induction of labour. Four of these linked reviews compare prostaglandins (PGE2/PGF2a) given by alternate routes (intracervical PG (Boulvain 2008), extra‐amniotic PG (Hutton 2001), intravenous PG (Luckas 2000), oral PG (French 2001)) and a further three evaluate misoprostol, a prostaglandin E1 analogue (vaginal misoprostol (Hofmeyr 2010), oral misoprostol (Alfirevic 2014), buccal or sublingual misoprostol (Muzonzini 2004)). Overall, these agents seem to be effective at inducing labour, vaginal delivery within 24 hours is more likely when they are used. In contrast to this review's conclusions, women in trials comparing intracervical PGE2 with placebo were significantly more likely to deliver within 24 hours (Boulvain 2008), and when intracervical and vaginal PGE2 are compared, the findings favour vaginal PGE2. The other reviews include other commonly used interventions that are often used in conjunction with prostaglandins for induction of labour (such as intravenous oxytocin (Alfirevic 2009), amniotomy (Bricker 2000), oxytocin and amniotomy combined (Howarth 2001)).
Two reviews evaluate induction of labour setting ‐ comparing methods of induction of labour in an outpatient setting. Dowswell 2010 includes five trials, also included in this review, but the outcomes considered important differ between the reviews; where the same outcome is considered the findings are consistent. The studies included in outpatient versus inpatient induction (Kelly 2013a) and morning versus evening induction (Bakker 2013) do not overlap with studies in this review because the method of induction is consistent between the groups, but the setting/timing is varied.
Of the 13 reviews on specific indications for induction of labour, reviews comparing induction of labour with expectant management beyond 40 weeks and with ruptured membranes potentially overlap with this review although the outcomes assessed may differ (Dare 2009; Gülmezoglu 2012). Jozwiak 2012a considers methods of induction of labour for women who have had a previous caesarean section, currently none of trials included in that review are eligible for this review, but there is potential for overlap.
Finally searching PROSPERO and Pubmed for reviews including induction of labour using vaginal prostaglandins identified two further non‐Cochrane systematic reviews. A review of reviews on methods of induction of labour by Mozurkewich 2011 summarises the findings of the 27 linked Cochrane reviews and as such, for vaginal prostaglandins its findings are based on the previous version of this review (Kelly 2009). Facchinetti 2012 compares sustained release vaginal PGE2 with repeat PGE2 (both vaginal or intracervical), specifically in a subgroup of women having their first baby, with an unfavourable cervix (Bishop score (BS) < 5) and with intact membranes. Their primary outcome is caesarean section, and although 18 trials are identified, 11 of these are excluded (reasons given included results not stratified into multips/primips, BS > 4, rupture of membranes). In the current version of this review, 13 trials are included in the comparison sustained release vaginal PGE2 versus vaginal PGE2 gel or tablet, 11 of these report caesarean section as an outcome and overall, no difference is detected (Analysis 26.3). Eight trials specify women with an unfavourable cervix and again no difference was detected (Analysis 29.3). If only primips with an unfavourable cervix (five trials) were included, again no difference is found (analysis done but not included for this update as not a prespecified subgroup) and finally the subgroup of women having their first baby, who have an unfavourable cervix and intact membranes, no difference is detected (four trials included) (analysis not included here as not a prespecified subgroup). The same four studies are included in the Facchinetti 2012 review, but two studies using intracervical PGE2 are also included in the meta‐analysis. These trials were not eligible for this review and were excluded from Boulvain 2008 review, both favour sustained release preparations and give 40% of the weight to the result, which is in favour of SR pessaries. Boulvain 2008 identified 29 trials comparing intracervical PGE2 versus vaginal PGE2 (not stratified into gel, tablet, sustained release), so there would appear to be 42 potentially eligible trials, yet Franchetti identified only 18 studies; this is a potential source of bias. In addition, the inclusion/exclusion criteria are not consistently expressed. The review acknowledges sponsorship by Ferring (makers of a sustained release preparation).
29.3. Analysis.
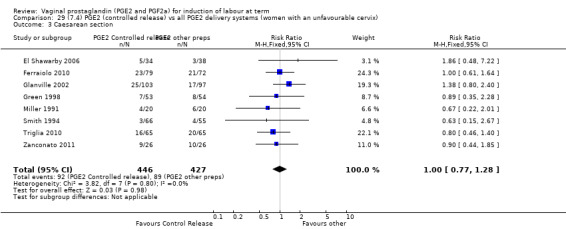
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 3 Caesarean section.
Authors' conclusions
Implications for practice.
Vaginal prostaglandin E2 is probably an effective induction agent, although many trials report a reduction in time to delivery with prostaglandins, in most trials this is not in a useable format and so the likelihood of vaginal birth within 24 hours does not reach statistical significance in the meta‐analysis. Prostaglandins increase uterine hyperstimulation with fetal heart rate changes but do not effect reducing the rate of caesarean section. The overall effect on maternal and neonatal health is not certain. Prostaglandin E2 formulations (either as a gel, tablet or sustained release pessary) should be recommended as effective induction agents. No difference in important maternal and fetal outcomes was detected but there may be marginal differences between preparations (increasing rate of cervical change and operative vaginal delivery). These may be important because induction is a common procedure.
Implications for research.
One of the main limitations of this review was the varied manner in which many of the outcomes were reported. Time from induction to a delivery was measured but not in a format that was useable in this review. Consideration of using other methods to capture this information are needed.
Induction is a common procedure, the differences between different formulations are probably small but these marginal differences could have a big impact on the health of women and babies, of their experience and the costs of health care.
What's new
| Date | Event | Description |
|---|---|---|
| 2 May 2014 | New citation required but conclusions have not changed | Twelve new studies identified and seven included (Bezircioglu 2012; Ferraiolo 2010; MacLennan 1979; Poornima 2011; Taher 2011; Triglia 2010; Zanconato 2011). |
| 1 March 2014 | New search has been performed | Search updated. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 24 February 2012 | Amended | Search updated. Nine reports added to Studies awaiting classification. |
| 9 June 2009 | New citation required but conclusions have not changed | New authors have helped prepare this update. |
| 9 June 2009 | New search has been performed | Six additional trials included: four from new search results (Dommisse 1980; El Shawarby 2006; Kalkat 2008; Mahmood 1995; Rath 1999) and one that was previously awaiting classification (Glanville 2002). One new trial is awaiting classification (Nikolov 2003). Three new trials are excluded (Ramsey 1998; Sadaty 1998; Veligati 1998. Included studies compare sustained release vaginal PGE2 pessaries to standard intermittent dosing. There is new evidence of significant differences between some of the outcomes. |
| 12 November 2008 | Amended | Converted to new review format. |
| 30 May 2003 | New search has been performed | Search updated. |
Acknowledgements
We would like to thank the editorial team in Liverpool for all their help producing this review. Simon Gates for some valuable statistical advice during the peer review process and Caroline Crowther for her patience and attention to detail during the production of earlier versions of the review.
We would like to thank Sidra Malik and Lee Smith for their contribution to previous versions of the review.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Methods used to assess trials included in the initial version of this review
Kelly 2003 The trials included in the primary reviews were extracted from an initial set of trials covering all interventions used in induction of labour (see above for details of search strategy). The data extraction process was conducted centrally. This was co‐ordinated from the Clinical Effectiveness Support Unit (CESU) at the Royal College of Obstetricians and Gynaecologists, UK, in co‐operation with The Pregnancy and Childbirth Group of The Cochrane Collaboration. This process allowed the data extraction process to be standardised across all the reviews.
The trials were initially reviewed on eligibility criteria, using a standardised form and the basic selection criteria specified above. Following this, data were extracted to a standardised data extraction form which was piloted for consistency and completeness. The pilot process involved the researchers at the CESU and previous reviewers in the area of induction of labour.
Information was extracted regarding the methodological quality of trials on a number of levels. This process was completed without consideration of trial results. Assessment of selection bias examined the process involved in the generation of the random sequence and the method of allocation concealment separately. These were then judged as adequate or inadequate using the criteria described in Table 39 for the purpose of the reviews.
1. Methodological quality of trials.
| Methodological item | Adequate | Inadequate |
| Generation of random sequence | Computer‐generated sequence, random number tables, lot drawing, coin tossing, shuffling cards, throwing dice. | Case number, date of birth, date of admission, alternation. |
| Concealment of allocation | Central randomisation, coded drug boxes, sequentially sealed opaque envelopes. | Open allocation sequence, any procedure based on inadequate generation. |
Performance bias was examined with regards to whom was blinded in the trials, i.e. patient, caregiver, outcome assessor or analyst. In many trials the caregiver, assessor and analyst were the same party. Details of the feasibility and appropriateness of blinding at all levels was sought.
Predefined subgroup analyses are: previous caesarean section or not; nulliparity or multiparity; membranes intact or ruptured, and cervix unfavourable, favourable or undefined. Only those outcomes with data appear in the analysis tables.
Individual outcome data were included in the analysis if they meet the prestated criteria in Types of outcome measures. Included trial data were processed as described in the Cochrane Reviewers' Handbook (Clarke 2002). Data extracted from the trials were analysed on an intention to treat basis (when this was not done in the original report, re‐analysis is performed if possible). Where data were missing, clarification was sought from the original authors. If the attrition was such that it might significantly affect the results, these data were excluded from the analysis. This decision rested with the reviewers of primary reviews and is clearly documented. If missing data become available, they will be included in the analyses.
Data were extracted from all eligible trials to examine how issues of quality influence effect size in a sensitivity analysis. In trials where reporting was poor, methodological issues were reported as unclear or clarification sought.
Due to the large number of trials, double data extraction was not feasible and agreement between the three data extractors was therefore assessed on a random sample of trials.
Once the data had been extracted, they were distributed to individual reviewers for entry onto the Review Manager computer software (RevMan 2003), checked for accuracy, and analysed as above using the RevMan software. For dichotomous data, risk ratios and 95% confidence intervals were calculated, and in the absence of heterogeneity, results were pooled using a fixed‐effect model.
The predefined criteria for sensitivity analysis included all aspects of quality assessment as mentioned above, including aspects of selection, performance and attrition bias.
Primary analysis was limited to the prespecified outcomes and subgroup analyses. In the event of differences in unspecified outcomes or subgroups being found, these were analysed post hoc, but clearly identified as such to avoid drawing unjustified conclusions.
Appendix 2. Methods used to assess trials included in previous version of this review
Kelly 2009 The following methods were used to assess: Al Malt 1995; Al‐Sebai 1993; Buchanan 1984; Campbell 1984; Cardozo 1986; Chaterjee 1990; Chua 1995; Chung 1992; Curet 1989; Doany 1997; Dommisse 1980; Duhl 1997; Dunston‐Boone 1991; Egarter 1989; El‐Mardi 1991; El Shawarby 2006; Glanville 2002; Graves 1985; Green 1998; Greer 1990; Hage 1993; Hannah 1996; Hayashi 1983; Kalkat 2008; Liggins 1979; MacKenzie 1979; MacKenzie 1981; MacKenzie 1997a; MacLennan 1980; Mahmood 1989; Mahmood 1992; Mahmood 1995; McCaul 1997; McLaren 1987; Miller 1991; Mukhopadhyay 2002; Murphy 1980; Murray 1995; Neilson 1983; Newman 1997; Nuutila 1996; O'Brien 1995; Ohel 1996; Payne 1993; Perryman 1992; Prasad 1989; Prins 1983; Rabl 2002; Rath 1999; Rayburn 1988; Rayburn 1992; Roach 1997; Sawai 1991; Sawai 1994; Shoaib 1994; Smith 1990; Smith 1994; Stampe Sorensen 1992; Thiery 1984; Tomlinson 2001; Ulmsten 1985; Witter 1992; Witter 1996.
In 2008, the methods and software for carrying out reviews were updated, as a result of which new reviews and updates, where appropriate, will use these new methods (Higgins 2008; RevMan 2008), which will be described in the Methods section of all the individual new and updated reviews.For this update, we used the following methods when assessing the trials identified by the updated search.
Assessment of methodological quality of included studies
We assessed the validity of each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We described methods used for generation of the randomisation sequence for each trial.
(1) Selection bias (randomisation and allocation concealment)
We assigned a quality score for each trial, using the following criteria:
adequate concealment of allocation: such as telephone randomisation, consecutively‐numbered, sealed opaque envelopes;
unclear whether adequate concealment of allocation: such as list or table used, sealed envelopes, or study does not report any concealment approach;
inadequate concealment of allocation: such as open list of random‐number tables, use of case record numbers, dates of birth or days of the week.
(2) Attrition bias (loss of participants, for example, withdrawals, dropouts, protocol deviations)
We assessed completeness to follow up using the following criteria:
(A) less than 5% loss of participants;
(B) 5% to 9.9% loss of participants;
(C) 10% to 19.9% loss of participants;
(D) more than 20% loss of participants.
(3) Performance bias (blinding of participants, researchers and outcome assessment)
We assessed blinding using the following criteria:
blinding of participants (yes/no/unclear);
blinding of caregiver (yes/no/unclear);
blinding of outcome assessment (yes/no/unclear).
Measures of treatment effect
We carried out statistical analysis using the Review Manager software (RevMan 2008). We used fixed‐effect meta‐analysis for combining data in the absence of significant heterogeneity if trials were sufficiently similar. If heterogeneity was found, we explored this by sensitivity analysis, followed by random‐effects if required.
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes are measured in the same way between trials. We used the standardised mean difference to combine trials that measure the same outcome, but use different methods. Where there was evidence of skewness, this has been reported.
Dealing with missing data
We analysed data on all participants with available data in the group to which they were allocated, regardless of whether or not they received the allocated intervention. If, in the original reports, participants were not analysed in the group to which they were randomised and there is sufficient information in the trial report, we attempted to restore them to the correct group.
Assessment of heterogeneity
We applied tests of heterogeneity between trials, if appropriate, using the I² statistic. If we identified high levels of heterogeneity among the trials (exceeding 50%), we explored it by prespecified subgroup analysis and performed sensitivity analysis. We used a random‐effects meta‐analysis as an overall summary if this was considered appropriate.
Subgroup analyses
We conducted planned subgroup analyses as performed for the other reviews in this series (seeData collection and analysis). When assessing differences between subgroups, e.g. within the comparison of vaginal PGE2 versus placebo, we explored this using an inverse variance method of meta‐ analysis and presenting the statistics for subgroup differences using chi² and I² statistics.
Sensitivity analyses
We carried out sensitivity analysis to explore the effect of trial quality assessed by concealment of allocation, by excluding studies with clearly inadequate allocation of concealment.
Data and analyses
Comparison 1. (1.1) PGE2 vs placebo/no treatment (all women).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.02, 4.83] |
| 1.1 PGE2 (once only) vs placebo/no treatment | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.67, 1.15] |
| 1.2 PGE2 (repeated doses) vs placebo/no treatment | 1 | 345 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.08, 0.18] |
| 2 Uterine hyperstimulation with FHR changes | 15 | 1359 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.16 [1.67, 5.98] |
| 2.1 PGE2 (once only) vs placebo/no treatment | 7 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.46, 4.15] |
| 2.2 PGE2 (repeated doses) vs placebo/no treatment | 3 | 208 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.34 [0.27, 106.70] |
| 2.3 PGE2 (sustained release) vs placebo/no treatment | 5 | 636 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.53 [1.92, 10.65] |
| 3 Caesarean section | 36 | 6599 | Risk Ratio (IV, Fixed, 95% CI) | 0.91 [0.81, 1.02] |
| 3.1 PGE2 (once only) vs placebo/no treatment | 16 | 1405 | Risk Ratio (IV, Fixed, 95% CI) | 1.01 [0.83, 1.24] |
| 3.2 PGE2 (repeated doses) vs placebo/no treatment | 15 | 4523 | Risk Ratio (IV, Fixed, 95% CI) | 0.86 [0.73, 1.02] |
| 3.3 PGE2 (sustained release) vs placebo/no treatment | 5 | 671 | Risk Ratio (IV, Fixed, 95% CI) | 0.85 [0.65, 1.12] |
| 4 Serious neonatal morbidity or perinatal death | 9 | 3638 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.09, 2.31] |
| 4.1 PGE2 (once only) vs placebo/no treatment | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 PGE2 (repeated doses) vs placebo/no treatment | 5 | 3269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.09, 2.31] |
| 4.3 PGE2 (sustained release) vs placebo/no treatment | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 3 | 530 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [0.34, 14.76] |
| 5.1 PGE2 (once only) vs placebo | 2 | 461 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [0.34, 14.76] |
| 5.2 PGE2 (sustained release) vs placebo/no treatment | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 to 24 hours | 6 | 567 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.27, 0.65] |
| 6.1 PGE2 (once only) vs placebo/no treatment | 3 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.39, 0.73] |
| 6.2 PGE2 (repeated doses) vs placebo/no treatment | 2 | 235 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.07, 1.08] |
| 6.3 1.6.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.05, 0.45] |
| 7 Oxytocin augmentation | 13 | 1421 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.05] |
| 7.1 PGE2 (once only) vs placebo/no treatment | 7 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.59, 1.47] |
| 7.2 PGE2 (repeated doses) vs placebo/no treatment | 5 | 795 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.01] |
| 7.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.20, 0.64] |
| 8 Uterine hyperstimulation without FHR changes | 13 | 3636 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [1.17, 5.26] |
| 8.1 PGE2 (once only) vs placebo/no treatment | 6 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.33, 4.84] |
| 8.2 PGE2 (repeated doses) vs placebo/no treatment | 5 | 2953 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [0.78, 7.03] |
| 8.3 PGE2 (sustained release) vs placebo/no treatment | 2 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.85 [1.05, 58.82] |
| 9 Uterine rupture | 2 | 2579 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
| 9.1 PGE2 (once only) vs placebo/no treatment | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
| 9.2 PGE2 (repeated doses) vs placebo/no treatment | 1 | 2520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 7 | 3555 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.85, 1.60] |
| 10.1 PGE2 (once only) vs placebo/no treatment | 2 | 434 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.41, 1.55] |
| 10.2 PGE2 (repeated doses) vs placebo/no treatment | 4 | 3040 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.81, 2.44] |
| 10.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.83, 1.68] |
| 11 Instrumental vaginal delivery | 13 | 4219 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 11.1 PGE2 (once only) vs placebo/no treatment | 6 | 721 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.55, 1.28] |
| 11.2 PGE2 (repeated doses) vs placebo/no treatment | 5 | 3348 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.13] |
| 11.3 PGE2 (sustained release) vs placebo/no treatment | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.55, 1.86] |
| 12 Meconium‐stained liquor | 12 | 4245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.68, 0.98] |
| 12.1 PGE2 (once only) vs placebo/no treatment | 5 | 704 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.65, 1.40] |
| 12.2 PGE2 (repeated doses) vs placebo/no treatment | 7 | 3541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.64, 0.97] |
| 13 Apgar score < 7 at 5 minutes | 16 | 4481 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.86, 1.92] |
| 13.1 PGE2 (once only) vs placebo/no treatment | 9 | 1046 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.24, 1.30] |
| 13.2 PGE2 (repeated doses) vs placebo/no treatment | 6 | 3220 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.80, 2.27] |
| 13.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.21 [1.41, 27.34] |
| 14 Neonatal intensive care unit admission | 12 | 4022 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.78, 1.14] |
| 14.1 PGE2 (once only) vs placebo/no treatment | 4 | 681 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.70, 2.15] |
| 14.2 PGE2 (repeated doses) vs placebo/no treatment | 7 | 3272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.73, 1.10] |
| 14.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.36, 29.93] |
| 15 Perinatal death | 7 | 3648 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.14, 2.22] |
| 15.1 PGE2 (once only) vs placebo/no treatment | 2 | 431 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 16.85] |
| 15.2 PGE2 (repeated doses) vs placebo/no treatment | 4 | 3148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.09, 2.31] |
| 15.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal side‐effects (all) | 12 | 6780 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.80, 1.67] |
| 16.1 PGE2 (once only) vs placebo/no treatment | 6 | 577 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [1.02, 3.74] |
| 16.2 PGE2 (repeated doses) vs placebo/no treatment | 5 | 5558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.34] |
| 16.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 645 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Nausea (maternal) | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.1 PGE2 (repeated doses) vs placebo/no treatment | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Vomitting (maternal) | 3 | 2794 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.39, 3.39] |
| 18.1 PGE2 (once only) vs placebo/no treatment | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.15, 6.41] |
| 18.2 PGE2 (repeated doses) vs placebo/no treatment | 1 | 2520 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.34, 4.65] |
| 18.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Diarrhoea (maternal) | 3 | 2819 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.01 [0.36, 135.59] |
| 19.1 PGE2 (repeated doses) vs placebo/no treatment | 2 | 2604 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.01 [0.36, 135.59] |
| 19.2 PGE2 (sustained release) vs placebo/no treatment | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Other maternal side‐effects | 7 | 871 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.62, 1.51] |
| 20.1 PGE2 (once only) vs placebo/no treatment | 4 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [0.97, 8.02] |
| 20.2 PGE2 (repeated doses) vs placebo/no treatment | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.42, 1.15] |
| 20.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Postpartum haemorrhage | 9 | 3537 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.04, 2.09] |
| 21.1 PGE2 (once only) vs placebo/no treatment | 4 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.33, 3.97] |
| 21.2 PGE2 (repeated doses) vs placebo/no treatment | 4 | 3040 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.01, 2.11] |
| 21.3 PGE2 (sustained release) vs placebo/no treatment | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.64 [0.27, 116.05] |
| 22 Serious maternal complication | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
| 22.1 PGE2 (once only) vs placebo/no treatment | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
| 23 Woman not satisfied | 2 | 2922 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.24, 2.40] |
| 23.1 PGE2 (once only) vs placebo/no treatment | 1 | 402 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.83, 2.35] |
| 23.2 PGE2 (repeated doses) vs placebo/no treatment | 1 | 2520 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.33, 0.58] |
1.9. Analysis.
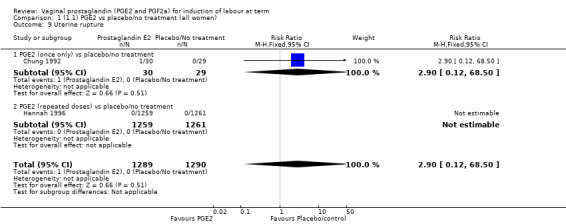
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 9 Uterine rupture.
1.11. Analysis.

Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 11 Instrumental vaginal delivery.
1.15. Analysis.
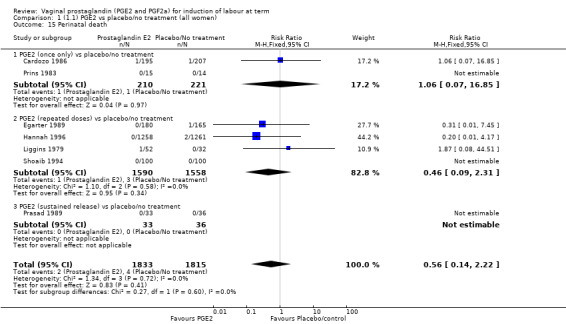
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 15 Perinatal death.
1.16. Analysis.
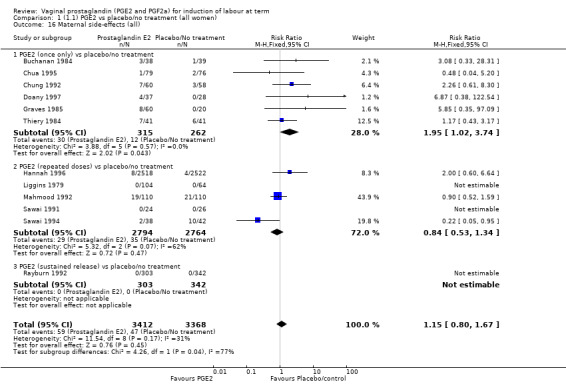
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 16 Maternal side‐effects (all).
1.17. Analysis.
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 17 Nausea (maternal).
1.18. Analysis.
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 18 Vomitting (maternal).
1.19. Analysis.
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 19 Diarrhoea (maternal).
1.20. Analysis.
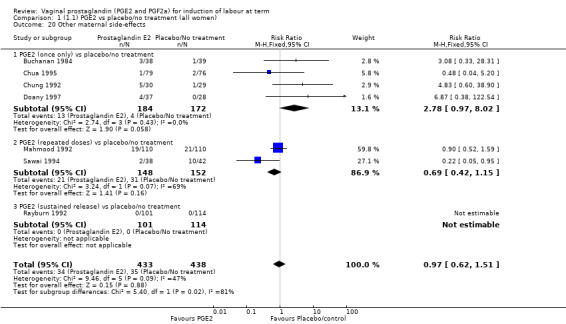
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 20 Other maternal side‐effects.
1.22. Analysis.
Comparison 1 (1.1) PGE2 vs placebo/no treatment (all women), Outcome 22 Serious maternal complication.
Comparison 2. (1.2) PGE2 vs placebo/no treatment (primiparae).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.06, 2.80] |
| 2 Uterine hyperstimulation with FHR changes | 3 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.57] |
| 3 Caesarean section | 10 | 2486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.77, 1.12] |
| 4 Serious neonatal morbidity or perinatal death | 3 | 1796 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.22] |
| 5 Serious maternal morbidity or death | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 to 24 hours | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.47] |
| 7 Oxytocin augmentation | 3 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.47, 0.74] |
| 8 Uterine hyperstimulation without FHR changes | 3 | 1701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.02, 8.10] |
| 9 Uterine rupture | 1 | 1507 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 4 | 1959 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.64, 2.73] |
| 11 Instrumental vaginal delivery | 4 | 1815 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.14] |
| 12 Meconium‐stained liquor | 2 | 420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.29, 1.13] |
| 13 Apgar score < 7 at 5 minutes | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.17, 3.27] |
| 14 Neonatal intensive care unit admission | 3 | 444 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.54, 2.09] |
| 15 Perinatal death | 3 | 1776 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.22] |
| 16 Maternal side‐effects (all) | 3 | 1882 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.63, 1.71] |
| 17 Vomitting (maternal) | 1 | 1507 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.40, 7.00] |
| 18 Diarrhoea (maternal) | 1 | 1507 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.03 [0.24, 104.66] |
| 19 Other maternal side‐effects | 2 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.50, 1.50] |
| 20 Postpartum haemorrhage | 3 | 1927 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.97, 2.34] |
2.1. Analysis.
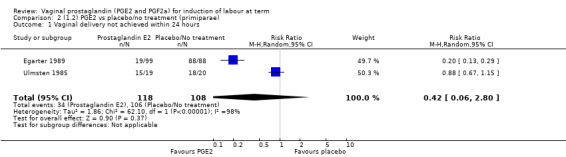
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 1 Vaginal delivery not achieved within 24 hours.
2.2. Analysis.
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 2 Uterine hyperstimulation with FHR changes.
2.3. Analysis.
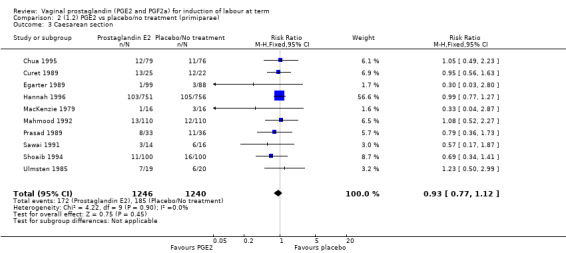
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 3 Caesarean section.
2.4. Analysis.
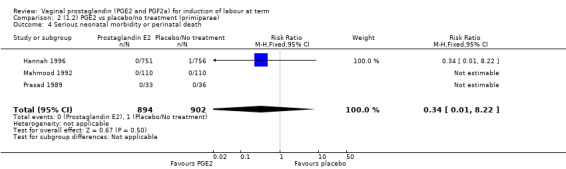
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 4 Serious neonatal morbidity or perinatal death.
2.5. Analysis.
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 5 Serious maternal morbidity or death.
2.6. Analysis.
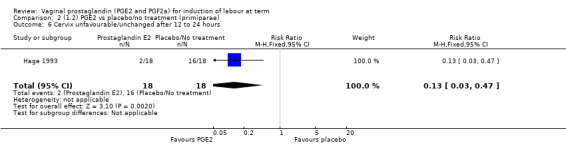
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 6 Cervix unfavourable/unchanged after 12 to 24 hours.
2.7. Analysis.
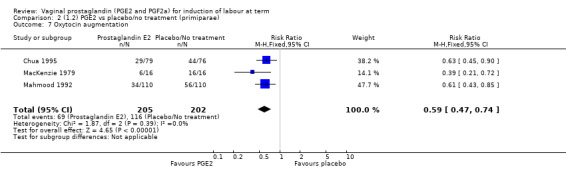
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 7 Oxytocin augmentation.
2.8. Analysis.
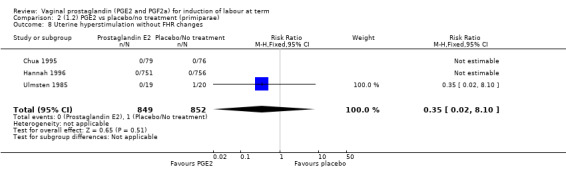
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 8 Uterine hyperstimulation without FHR changes.
2.9. Analysis.
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 9 Uterine rupture.
2.10. Analysis.
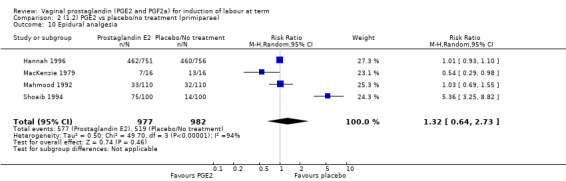
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 10 Epidural analgesia.
2.11. Analysis.
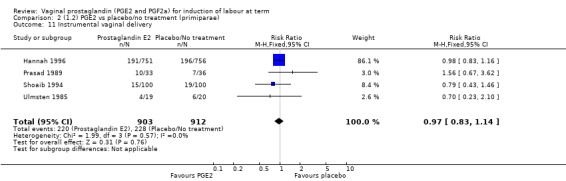
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 11 Instrumental vaginal delivery.
2.12. Analysis.
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 12 Meconium‐stained liquor.
2.13. Analysis.
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 13 Apgar score < 7 at 5 minutes.
2.14. Analysis.
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 14 Neonatal intensive care unit admission.
2.15. Analysis.
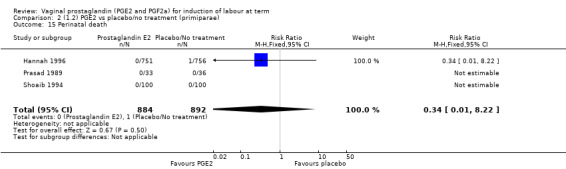
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 15 Perinatal death.
2.16. Analysis.
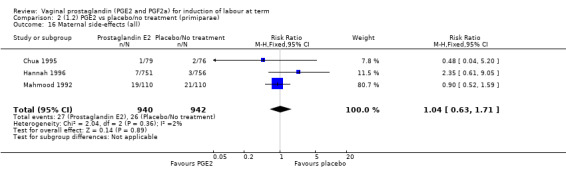
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 16 Maternal side‐effects (all).
2.17. Analysis.
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 17 Vomitting (maternal).
2.18. Analysis.
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 18 Diarrhoea (maternal).
2.19. Analysis.
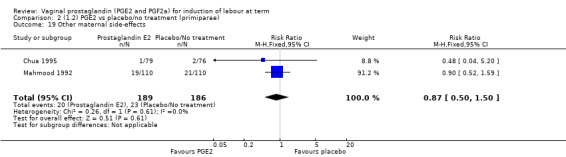
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 19 Other maternal side‐effects.
2.20. Analysis.
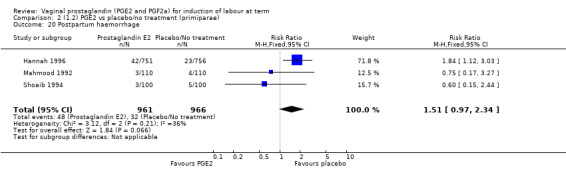
Comparison 2 (1.2) PGE2 vs placebo/no treatment (primiparae), Outcome 20 Postpartum haemorrhage.
Comparison 3. (1.3) PGE2 vs placebo/no treatment (multiparae).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.02, 0.12] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 5 | 1298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.48, 1.42] |
| 4 Serious neonatal morbidity or perinatal death | 2 | 1113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.12] |
| 5 Uterine rupture | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Epidural analgesia | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.88, 1.24] |
| 7 Oxytocin augmentation | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.18, 0.97] |
| 8 Uterine hyperstimulation without FHR changes | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.94 [0.61, 197.24] |
| 9 Instrumental vaginal delivery | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.77, 1.95] |
| 10 Meconium‐stained liquor | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.82] |
| 11 Perinatal death | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.12] |
| 12 Maternal side‐effects (all) | 1 | 2026 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.87] |
| 13 Vomitting (maternal) | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.12] |
| 14 Diarrhoea (maternal) | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.98 [0.12, 73.04] |
| 15 Postpartum haemorrhage | 1 | 1013 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.59, 2.52] |
3.1. Analysis.
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 1 Vaginal delivery not achieved within 24 hours.
3.2. Analysis.
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 2 Uterine hyperstimulation with FHR changes.
3.3. Analysis.
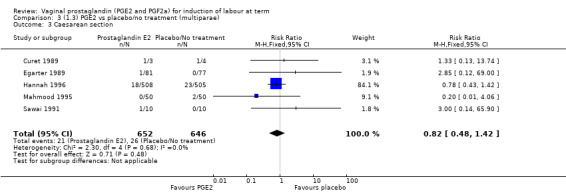
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 3 Caesarean section.
3.4. Analysis.
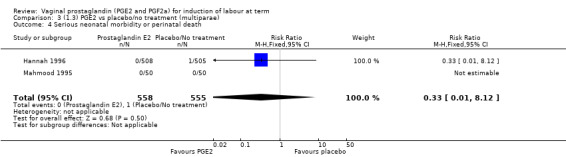
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 4 Serious neonatal morbidity or perinatal death.
3.5. Analysis.
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 5 Uterine rupture.
3.6. Analysis.
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 6 Epidural analgesia.
3.7. Analysis.
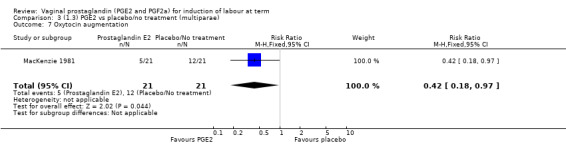
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 7 Oxytocin augmentation.
3.8. Analysis.
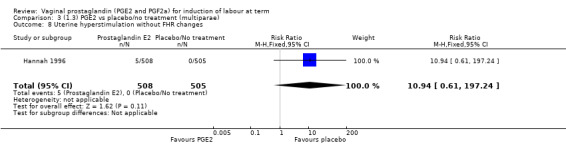
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 8 Uterine hyperstimulation without FHR changes.
3.9. Analysis.
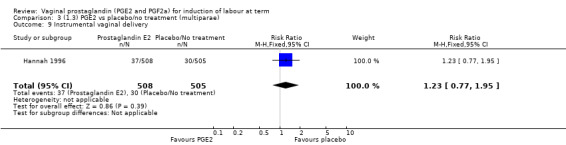
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 9 Instrumental vaginal delivery.
3.10. Analysis.
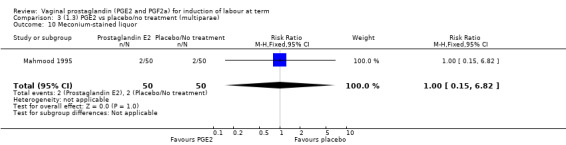
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 10 Meconium‐stained liquor.
3.11. Analysis.
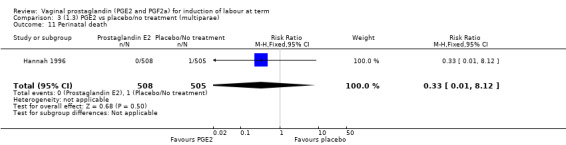
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 11 Perinatal death.
3.12. Analysis.
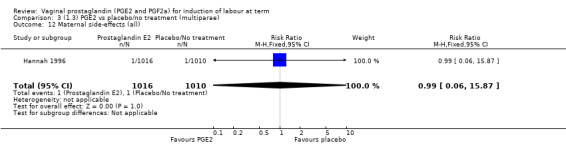
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 12 Maternal side‐effects (all).
3.13. Analysis.
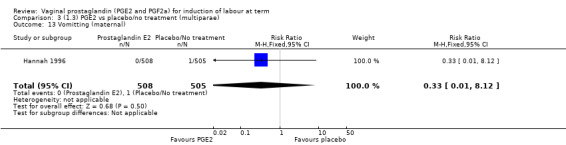
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 13 Vomitting (maternal).
3.14. Analysis.
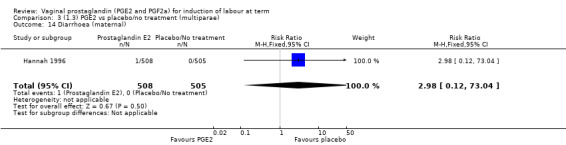
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 14 Diarrhoea (maternal).
3.15. Analysis.
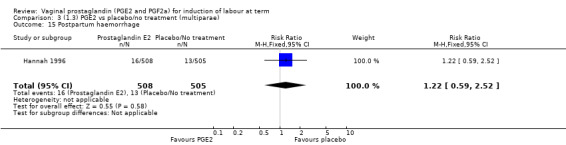
Comparison 3 (1.3) PGE2 vs placebo/no treatment (multiparae), Outcome 15 Postpartum haemorrhage.
Comparison 4. (1.4) PGE2 vs placebo/no treatment (women with intact membranes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.08, 0.18] |
| 2 Uterine hyperstimulation with FHR changes | 5 | 425 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.16 [0.57, 8.21] |
| 3 Caesarean section | 6 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.82, 1.57] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.45] |
| 5 Cervix unfavourable/unchanged after 12 to 24 hours | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.24, 0.68] |
| 6 Oxytocin augmentation | 2 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.83, 1.36] |
| 7 Uterine hyperstimulation without FHR changes | 5 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.76 [1.32, 34.54] |
| 8 Instrumental vaginal delivery | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.28, 5.38] |
| 9 Apgar score < 7 at 5 minutes | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.14, 2.05] |
| 10 Neonatal intensive care unit admission | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.28] |
| 11 Maternal side‐effects (all) | 3 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.66, 4.31] |
| 12 Postpartum haemorrhage | 1 | 81 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Perinatal death | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.45] |
4.1. Analysis.
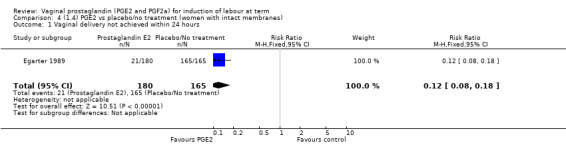
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 1 Vaginal delivery not achieved within 24 hours.
4.2. Analysis.
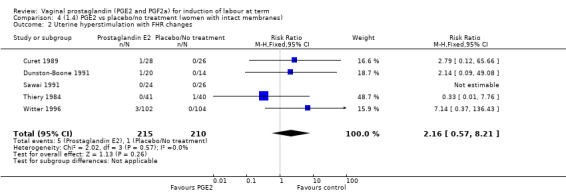
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 2 Uterine hyperstimulation with FHR changes.
4.3. Analysis.
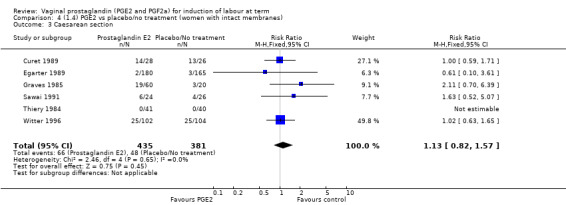
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 3 Caesarean section.
4.4. Analysis.
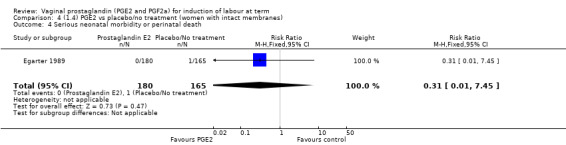
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 4 Serious neonatal morbidity or perinatal death.
4.5. Analysis.
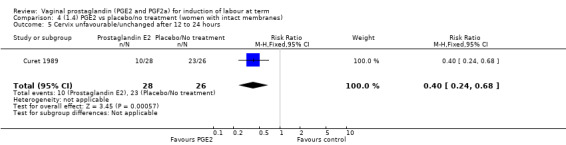
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 5 Cervix unfavourable/unchanged after 12 to 24 hours.
4.6. Analysis.
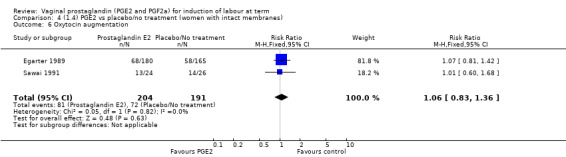
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 6 Oxytocin augmentation.
4.7. Analysis.
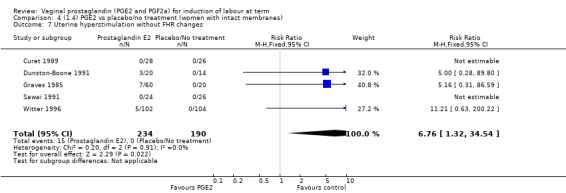
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 7 Uterine hyperstimulation without FHR changes.
4.8. Analysis.
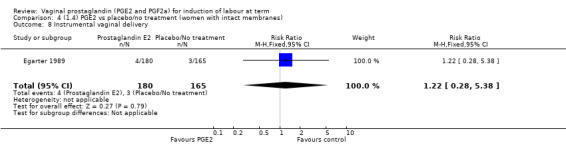
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 8 Instrumental vaginal delivery.
4.9. Analysis.
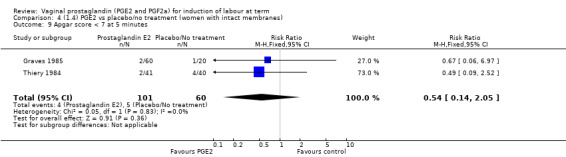
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 9 Apgar score < 7 at 5 minutes.
4.10. Analysis.
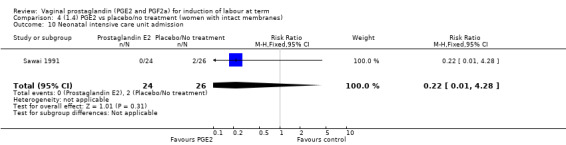
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 10 Neonatal intensive care unit admission.
4.11. Analysis.
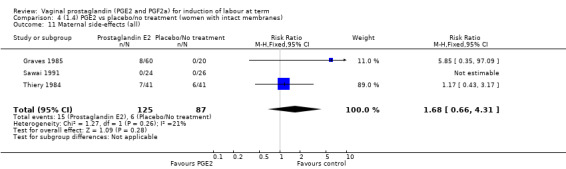
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 11 Maternal side‐effects (all).
4.12. Analysis.
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 12 Postpartum haemorrhage.
4.13. Analysis.
Comparison 4 (1.4) PGE2 vs placebo/no treatment (women with intact membranes), Outcome 13 Perinatal death.
Comparison 5. (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 7 | 3320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.73, 1.08] |
| 2 Serious neonatal morbidity or perinatal death | 3 | 2840 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.17] |
| 3 Uterine hyperstimulation without FHR changes | 3 | 2734 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.61, 4.52] |
| 4 Uterine rupture | 2 | 2579 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
| 5 Epidural analgesia | 3 | 2940 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.73, 4.14] |
| 6 Instrumental vaginal delivery | 3 | 2779 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.81, 1.13] |
| 7 Meconium‐stained liquor | 5 | 3099 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.75, 1.21] |
| 8 Apgar score < 7 at 5 minutes | 3 | 2894 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.83, 2.63] |
| 9 Neonatal intensive care unit admission | 4 | 2953 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.73, 1.13] |
| 10 Perinatal death | 2 | 2719 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.17] |
| 11 Maternal side‐effects (all) | 4 | 5533 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.73, 1.83] |
| 12 Vomitting (maternal) | 2 | 2579 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.39, 3.39] |
| 13 Diarrhoea (maternal) | 1 | 2520 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.01 [0.36, 135.59] |
| 14 Postpartum haemorrhage | 5 | 3099 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.02, 2.13] |
| 15 Woman not satisfied | 1 | 2520 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.33, 0.58] |
| 16 Uterine hyperstimulation with FHR changes | 1 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Serious maternal morbidity or death | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.84 [0.24, 96.66] |
| 18 Oxytocin augmentation | 2 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.49, 0.79] |
| 19 Other maternal side‐effects | 1 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.20] |
5.1. Analysis.
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 1 Caesarean section.
5.2. Analysis.
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 2 Serious neonatal morbidity or perinatal death.
5.3. Analysis.
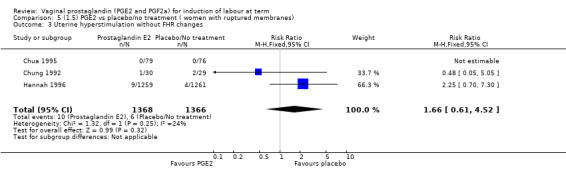
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 3 Uterine hyperstimulation without FHR changes.
5.4. Analysis.
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 4 Uterine rupture.
5.6. Analysis.
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 6 Instrumental vaginal delivery.
5.7. Analysis.
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 7 Meconium‐stained liquor.
5.8. Analysis.
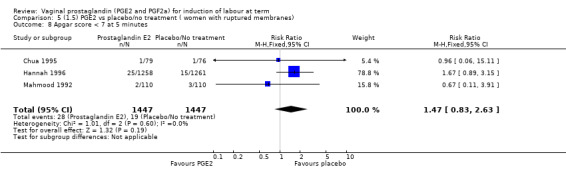
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 8 Apgar score < 7 at 5 minutes.
5.9. Analysis.
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 9 Neonatal intensive care unit admission.
5.10. Analysis.
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 10 Perinatal death.
5.11. Analysis.
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 11 Maternal side‐effects (all).
5.12. Analysis.
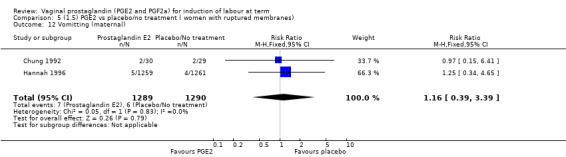
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 12 Vomitting (maternal).
5.13. Analysis.
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 13 Diarrhoea (maternal).
5.14. Analysis.
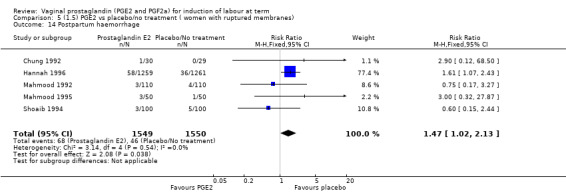
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 14 Postpartum haemorrhage.
5.15. Analysis.
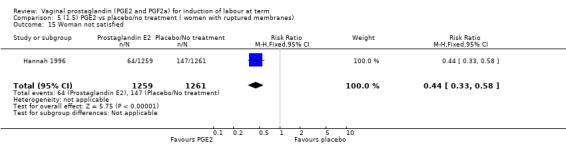
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 15 Woman not satisfied.
5.16. Analysis.
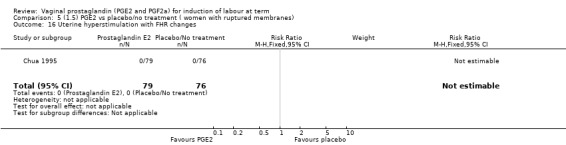
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 16 Uterine hyperstimulation with FHR changes.
5.17. Analysis.
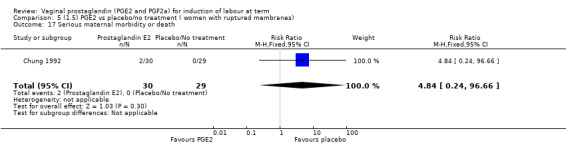
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 17 Serious maternal morbidity or death.
5.18. Analysis.
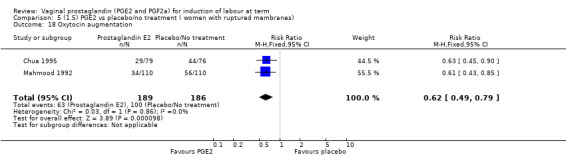
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 18 Oxytocin augmentation.
5.19. Analysis.
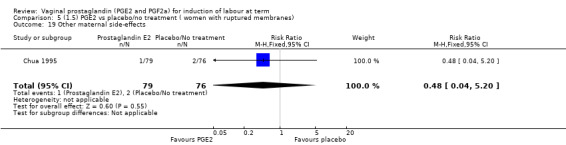
Comparison 5 (1.5) PGE2 vs placebo/no treatment ( women with ruptured membranes), Outcome 19 Other maternal side‐effects.
Comparison 6. (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.67, 1.15] |
| 2 Uterine hyperstimulation with FHR changes | 12 | 1143 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.47 [2.01, 9.93] |
| 3 Caesarean section | 22 | 2173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.75, 1.02] |
| 4 Serious neonatal morbidity or perinatal death | 4 | 533 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.84 [0.24, 96.66] |
| 6 Cervix unfavourable/unchanged after 12 to 24 hours | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.35, 0.79] |
| 7 Oxytocin augmentation | 8 | 813 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.53, 1.10] |
| 8 Uterine hyperstimulation without FHR changes | 9 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [0.99, 7.01] |
| 9 Uterine rupture | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
| 10 Epidural analgesia | 5 | 633 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.63, 2.43] |
| 11 Instrumental vaginal delivery | 7 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.27] |
| 12 Meconium‐stained liquor | 5 | 697 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.47, 0.89] |
| 13 Apgar score < 7 at 5 minutes | 11 | 1194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.59, 1.99] |
| 14 Neonatal intensive care unit admission | 7 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.51, 1.27] |
| 15 Perinatal death | 3 | 298 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal side‐effects (all) | 10 | 1572 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.73, 1.59] |
| 17 Nausea (maternal) | 1 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Vomitting (maternal) | 2 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.15, 6.41] |
| 19 Diarrhoea (maternal) | 2 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Other maternal side‐effects | 7 | 871 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.62, 1.51] |
| 21 Postpartum haemorrhage | 7 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.47, 2.05] |
| 22 Serious maternal complication | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [0.12, 68.50] |
6.1. Analysis.
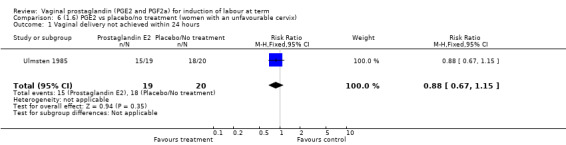
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 1 Vaginal delivery not achieved within 24 hours.
6.2. Analysis.
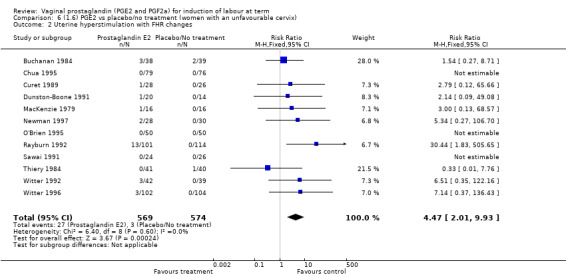
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.
6.3. Analysis.
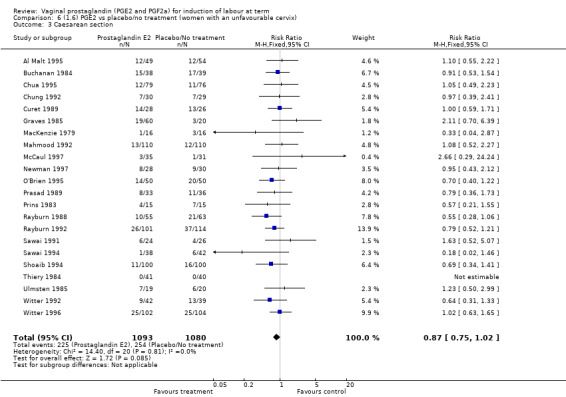
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 3 Caesarean section.
6.4. Analysis.
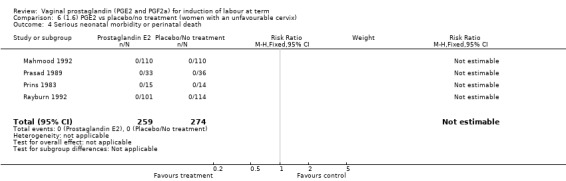
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 4 Serious neonatal morbidity or perinatal death.
6.5. Analysis.
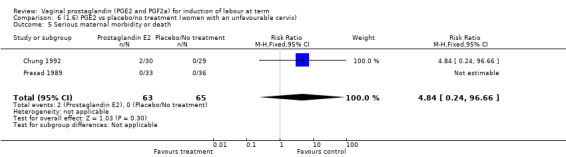
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 5 Serious maternal morbidity or death.
6.6. Analysis.
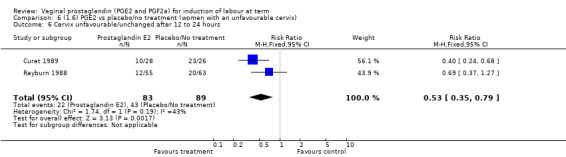
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 6 Cervix unfavourable/unchanged after 12 to 24 hours.
6.8. Analysis.
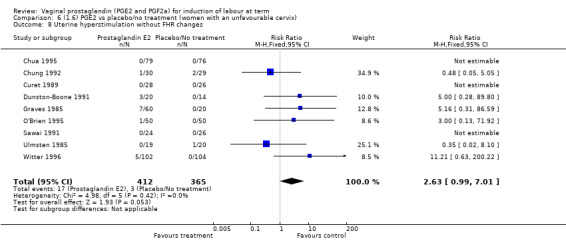
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 8 Uterine hyperstimulation without FHR changes.
6.9. Analysis.
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 9 Uterine rupture.
6.10. Analysis.
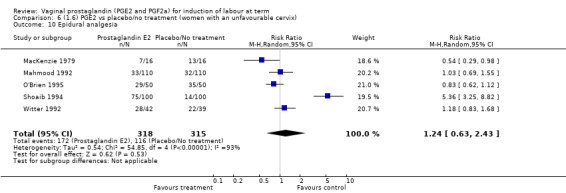
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 10 Epidural analgesia.
6.11. Analysis.

Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 11 Instrumental vaginal delivery.
6.12. Analysis.
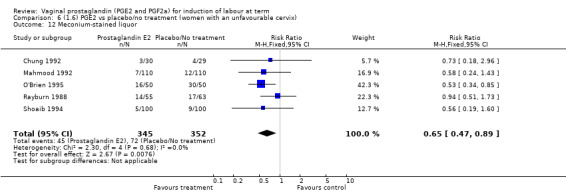
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 12 Meconium‐stained liquor.
6.13. Analysis.
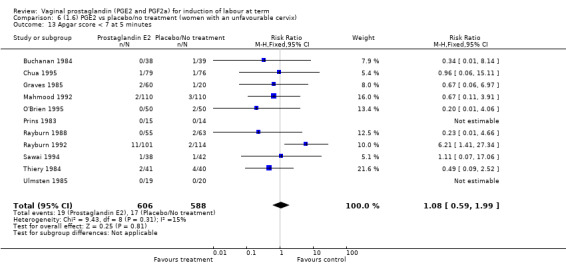
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 13 Apgar score < 7 at 5 minutes.
6.14. Analysis.
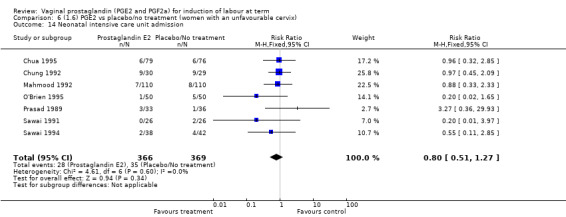
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 14 Neonatal intensive care unit admission.
6.15. Analysis.
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 15 Perinatal death.
6.16. Analysis.
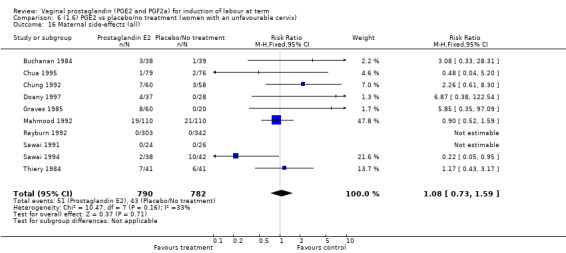
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 16 Maternal side‐effects (all).
6.17. Analysis.
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 17 Nausea (maternal).
6.18. Analysis.
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 18 Vomitting (maternal).
6.19. Analysis.
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 19 Diarrhoea (maternal).
6.20. Analysis.
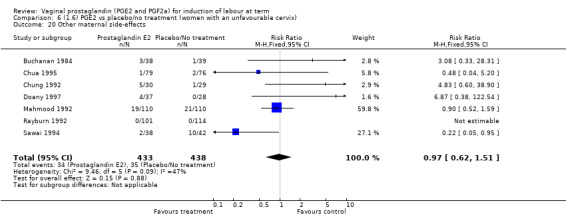
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 20 Other maternal side‐effects.
6.21. Analysis.
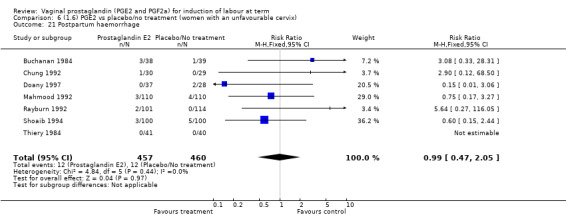
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 21 Postpartum haemorrhage.
6.22. Analysis.
Comparison 6 (1.6) PGE2 vs placebo/no treatment (women with an unfavourable cervix), Outcome 22 Serious maternal complication.
Comparison 7. (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation without FHR changes | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 2 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.40, 3.18] |
| 4 Serious neonatal morbidity or perinatal death | 2 | 401 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.45] |
| 5 Oxytocin augmentation | 3 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.66, 1.51] |
| 6 Vaginal delivery not achieved within 24 hours | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.08, 0.18] |
| 7 Instrumental vaginal delivery | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.28, 5.38] |
| 8 Perinatal death | 1 | 345 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.45] |
7.1. Analysis.
Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 1 Uterine hyperstimulation without FHR changes.
7.2. Analysis.
Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.
7.3. Analysis.
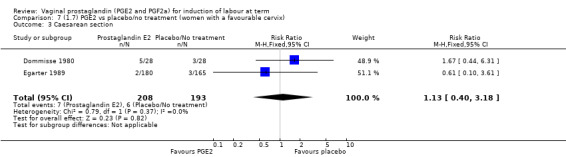
Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 3 Caesarean section.
7.4. Analysis.
Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 4 Serious neonatal morbidity or perinatal death.
7.6. Analysis.
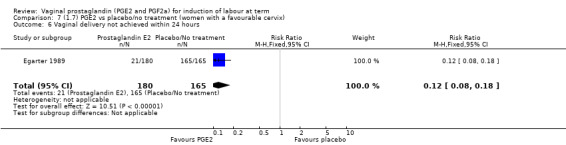
Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 6 Vaginal delivery not achieved within 24 hours.
7.7. Analysis.
Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 7 Instrumental vaginal delivery.
7.8. Analysis.
Comparison 7 (1.7) PGE2 vs placebo/no treatment (women with a favourable cervix), Outcome 8 Perinatal death.
Comparison 8. (2.1) PGF2a vs placebo (all women).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.57] |
| 2 Caesarean section | 4 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.31, 1.14] |
| 3 Cervix unfavourable/unchanged after 12 to 24 hours | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.11, 0.37] |
| 4 Oxytocin augmentation | 3 | 202 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.32, 1.07] |
| 5 Epidural analgesia | 4 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.56, 0.97] |
| 6 Instrumental vaginal delivery | 3 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.47, 0.84] |
Comparison 9. (2.2) PGF2a vs placebo (primiparae).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.57] |
| 2 Caesarean section | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 2.87] |
| 3 Oxytocin augmentation | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.71, 1.09] |
| 4 Epidural analgesia | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.56, 1.27] |
9.1. Analysis.
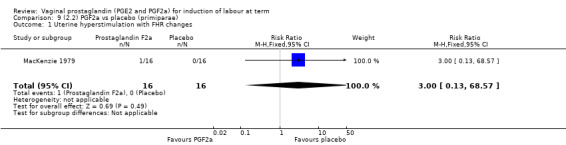
Comparison 9 (2.2) PGF2a vs placebo (primiparae), Outcome 1 Uterine hyperstimulation with FHR changes.
9.2. Analysis.
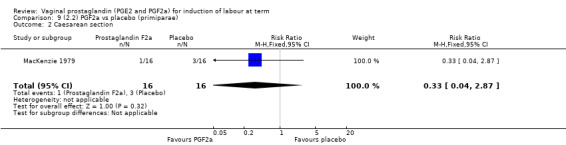
Comparison 9 (2.2) PGF2a vs placebo (primiparae), Outcome 2 Caesarean section.
9.3. Analysis.
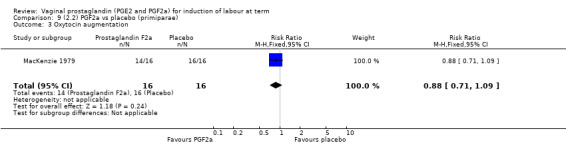
Comparison 9 (2.2) PGF2a vs placebo (primiparae), Outcome 3 Oxytocin augmentation.
9.4. Analysis.
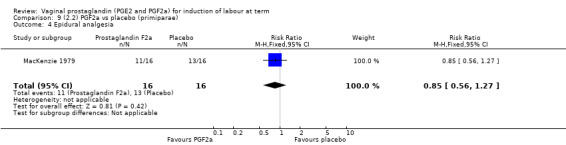
Comparison 9 (2.2) PGF2a vs placebo (primiparae), Outcome 4 Epidural analgesia.
Comparison 10. (2.3) PGF2a vs placebo (women with an unfavourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.05, 0.47] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 68.57] |
| 3 Caesarean section | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.13, 1.90] |
| 4 Instrumental Vaginal Delivery | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.41, 1.38] |
| 5 Oxytocin augmentation | 2 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.17, 2.11] |
| 6 Epidural analgesia | 2 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.56, 1.20] |
10.1. Analysis.
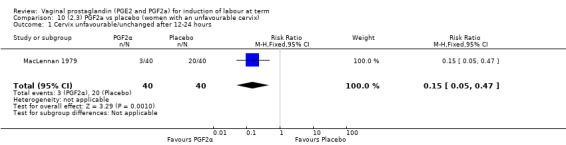
Comparison 10 (2.3) PGF2a vs placebo (women with an unfavourable cervix), Outcome 1 Cervix unfavourable/unchanged after 12‐24 hours.
10.2. Analysis.
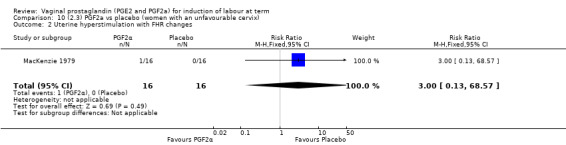
Comparison 10 (2.3) PGF2a vs placebo (women with an unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.
10.3. Analysis.
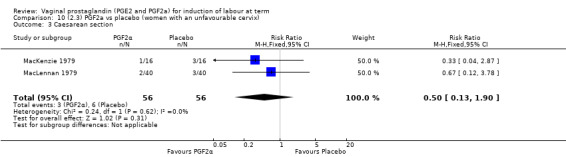
Comparison 10 (2.3) PGF2a vs placebo (women with an unfavourable cervix), Outcome 3 Caesarean section.
10.4. Analysis.
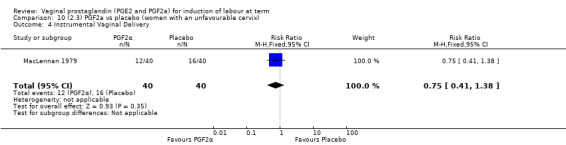
Comparison 10 (2.3) PGF2a vs placebo (women with an unfavourable cervix), Outcome 4 Instrumental Vaginal Delivery.
10.5. Analysis.
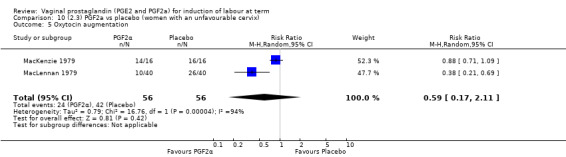
Comparison 10 (2.3) PGF2a vs placebo (women with an unfavourable cervix), Outcome 5 Oxytocin augmentation.
10.6. Analysis.
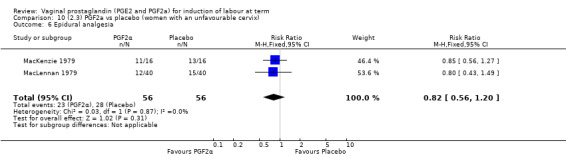
Comparison 10 (2.3) PGF2a vs placebo (women with an unfavourable cervix), Outcome 6 Epidural analgesia.
Comparison 11. (3.1) PGF2a vs PGE2 ( All women).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.42] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.64] |
| 3 Caesarean section | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.47, 2.22] |
| 4 Oxytocin augmentation | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.21, 4.51] |
| 5 Epidural analgesia | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.82, 3.00] |
| 6 Apgar score < 7 at 5 minutes | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.14] |
11.5. Analysis.
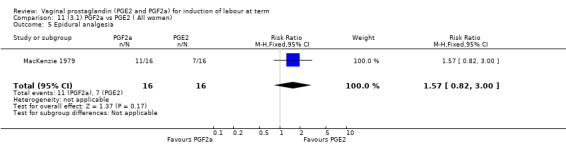
Comparison 11 (3.1) PGF2a vs PGE2 ( All women), Outcome 5 Epidural analgesia.
11.6. Analysis.
Comparison 11 (3.1) PGF2a vs PGE2 ( All women), Outcome 6 Apgar score < 7 at 5 minutes.
Comparison 12. (3.2) PGF2a vs PGE2 (primiparae).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.64] |
| 2 Caesarean section | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.64] |
| 3 Oxytocin augmentation | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.21, 4.51] |
| 4 Epidural analgesia | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.82, 3.00] |
12.1. Analysis.
Comparison 12 (3.2) PGF2a vs PGE2 (primiparae), Outcome 1 Uterine hyperstimulation with FHR changes.
12.2. Analysis.
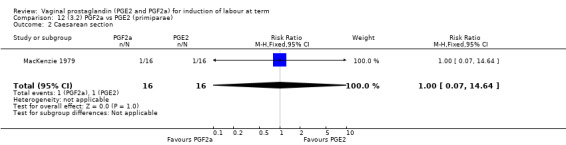
Comparison 12 (3.2) PGF2a vs PGE2 (primiparae), Outcome 2 Caesarean section.
12.3. Analysis.
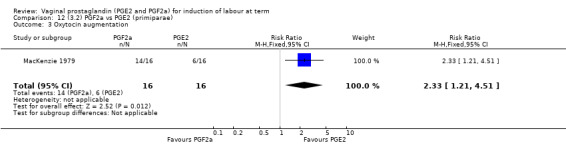
Comparison 12 (3.2) PGF2a vs PGE2 (primiparae), Outcome 3 Oxytocin augmentation.
12.4. Analysis.
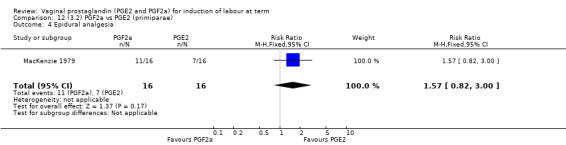
Comparison 12 (3.2) PGF2a vs PGE2 (primiparae), Outcome 4 Epidural analgesia.
Comparison 13. (3.3) PGF2a vs PGE2 (women with an unfavourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.42] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.64] |
| 3 Caesarean section | 2 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.47, 2.22] |
| 4 Oxytocin augmentation | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.21, 4.51] |
| 5 Epidural analgesia | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.82, 3.00] |
| 6 Apgar score < 7 at 5 minutes | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.14] |
13.1. Analysis.
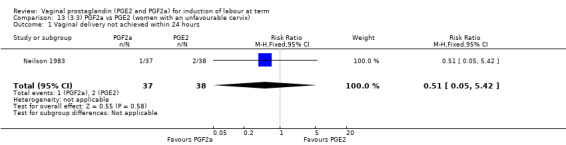
Comparison 13 (3.3) PGF2a vs PGE2 (women with an unfavourable cervix), Outcome 1 Vaginal delivery not achieved within 24 hours.
13.2. Analysis.
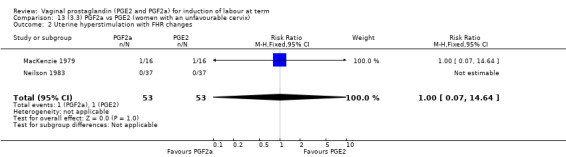
Comparison 13 (3.3) PGF2a vs PGE2 (women with an unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.
13.3. Analysis.
Comparison 13 (3.3) PGF2a vs PGE2 (women with an unfavourable cervix), Outcome 3 Caesarean section.
13.4. Analysis.
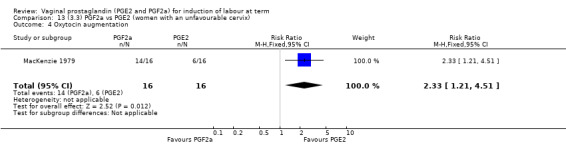
Comparison 13 (3.3) PGF2a vs PGE2 (women with an unfavourable cervix), Outcome 4 Oxytocin augmentation.
13.5. Analysis.
Comparison 13 (3.3) PGF2a vs PGE2 (women with an unfavourable cervix), Outcome 5 Epidural analgesia.
13.6. Analysis.
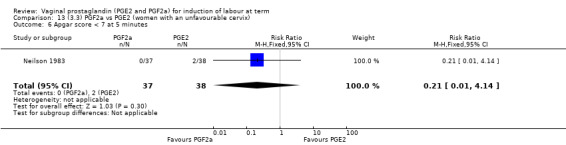
Comparison 13 (3.3) PGF2a vs PGE2 (women with an unfavourable cervix), Outcome 6 Apgar score < 7 at 5 minutes.
Comparison 14. (4.1) PGE2 gel vs PGE2 tablet ( all women).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 3 | 566 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.84, 1.26] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| 3 Caesarean section | 6 | 1046 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.17] |
| 4 Serious maternal morbidity or death | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 5 Cervix unfavourable/unchanged after 12 to 24 hours | 2 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.70, 1.07] |
| 6 Oxytocin augmentation | 6 | 742 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.08] |
| 7 Epidural analgesia | 3 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.95, 1.21] |
| 8 Instrumental vaginal delivery | 3 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.02] |
| 9 Meconium Stained Liquor | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.39, 2.13] |
| 10 Apgar score < 7 at 5 minutes | 4 | 597 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.35, 3.66] |
| 11 Neonatal Intensive Care Unit Admission | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.05, 5.47] |
| 12 Postpartum haemorrhage | 3 | 445 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.11] |
14.4. Analysis.
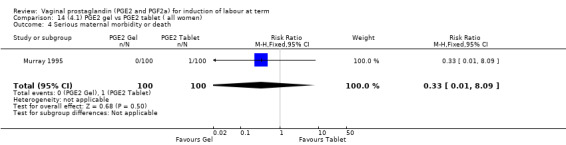
Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 4 Serious maternal morbidity or death.
14.9. Analysis.
Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 9 Meconium Stained Liquor.
14.10. Analysis.
Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 10 Apgar score < 7 at 5 minutes.
14.11. Analysis.
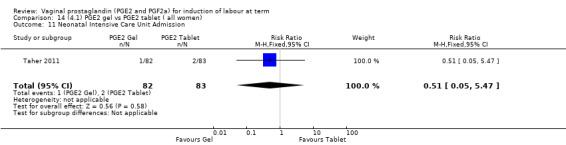
Comparison 14 (4.1) PGE2 gel vs PGE2 tablet ( all women), Outcome 11 Neonatal Intensive Care Unit Admission.
Comparison 15. (4.1) PGE2 gel vs PGE2 tablet (primiparae).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 174 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.72, 1.51] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| 3 Caesarean section | 4 | 454 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.76, 1.34] |
| 4 Serious maternal morbidity or death | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 5 Cervix unfavourable/unchanged after 12 to 24 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.56, 1.07] |
| 6 Oxytocin augmentation | 3 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.34, 1.29] |
| 7 Epidural analgesia | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.85, 1.10] |
| 8 Instrumental vaginal delivery | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.25] |
| 9 Apgar score < 7 at 5 minutes | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 10 Postpartum haemorrhage | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.30] |
15.1. Analysis.
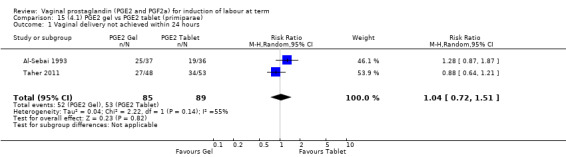
Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 1 Vaginal delivery not achieved within 24 hours.
15.2. Analysis.
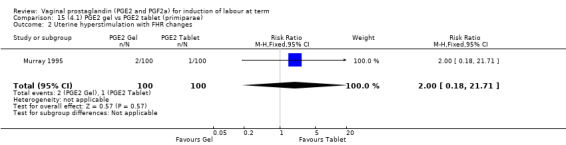
Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 2 Uterine hyperstimulation with FHR changes.
15.3. Analysis.
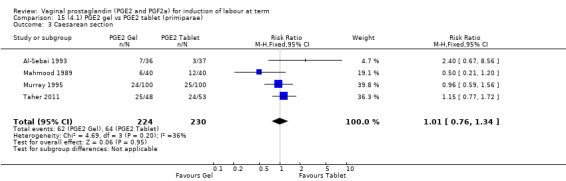
Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 3 Caesarean section.
15.4. Analysis.
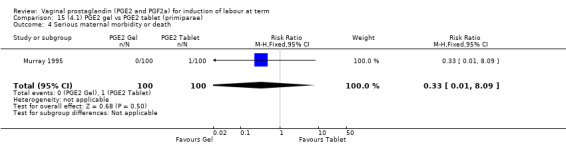
Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 4 Serious maternal morbidity or death.
15.5. Analysis.
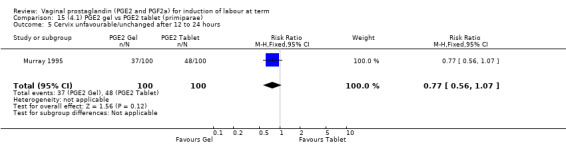
Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 5 Cervix unfavourable/unchanged after 12 to 24 hours.
15.6. Analysis.
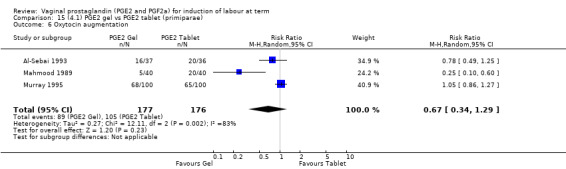
Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 6 Oxytocin augmentation.
15.7. Analysis.
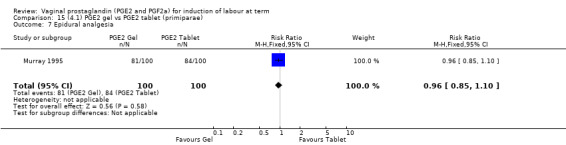
Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 7 Epidural analgesia.
15.9. Analysis.
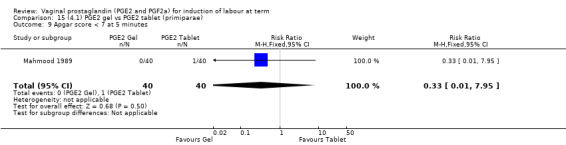
Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 9 Apgar score < 7 at 5 minutes.
15.10. Analysis.
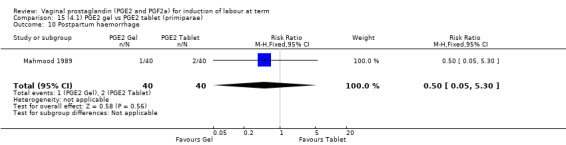
Comparison 15 (4.1) PGE2 gel vs PGE2 tablet (primiparae), Outcome 10 Postpartum haemorrhage.
Comparison 16. (4.2) PGE2 gel vs PGE2 tablet (multiparae).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.57, 1.87] |
| 2 Caesarean section | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.24, 3.22] |
16.1. Analysis.
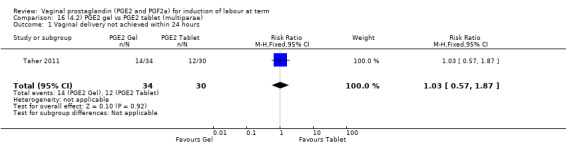
Comparison 16 (4.2) PGE2 gel vs PGE2 tablet (multiparae), Outcome 1 Vaginal delivery not achieved within 24 hours.
16.2. Analysis.
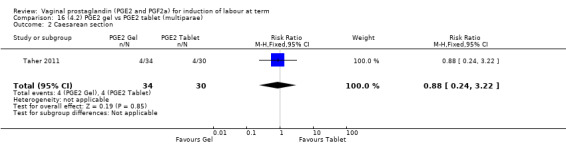
Comparison 16 (4.2) PGE2 gel vs PGE2 tablet (multiparae), Outcome 2 Caesarean section.
Comparison 17. (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.87, 1.87] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| 3 Caesarean section | 3 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.70, 1.49] |
| 4 Serious maternal morbidity or death | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 5 Cervix unfavourable/unchanged after 12 to 24 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.56, 1.07] |
| 6 Oxytocin augmentation | 3 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.78, 1.06] |
| 7 Epidural analgesia | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.89, 1.19] |
| 8 Instrumental vaginal delivery | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.59, 1.10] |
| 9 Postpartum haemorrhage | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.13, 4.40] |
17.1. Analysis.
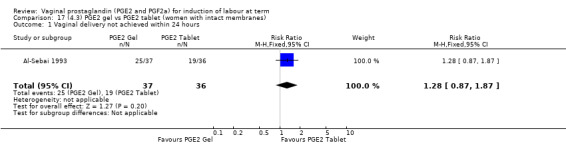
Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 1 Vaginal delivery not achieved within 24 hours.
17.2. Analysis.
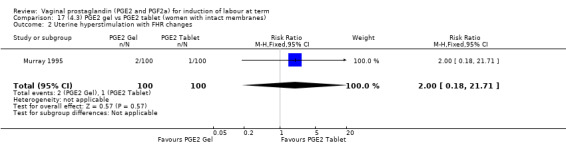
Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 2 Uterine hyperstimulation with FHR changes.
17.3. Analysis.
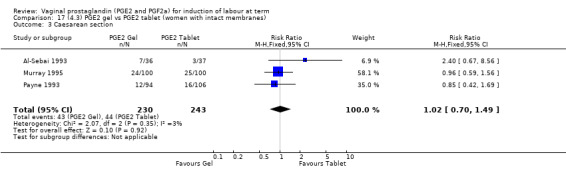
Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 3 Caesarean section.
17.4. Analysis.
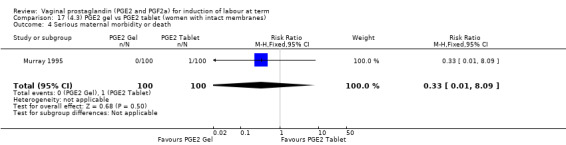
Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 4 Serious maternal morbidity or death.
17.5. Analysis.
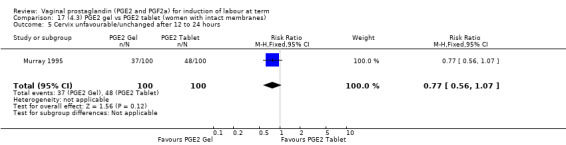
Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 5 Cervix unfavourable/unchanged after 12 to 24 hours.
17.6. Analysis.
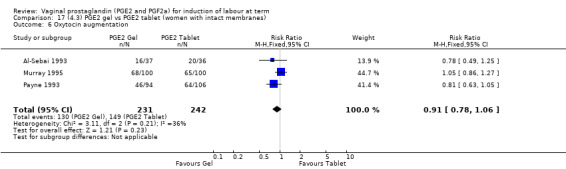
Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 6 Oxytocin augmentation.
17.7. Analysis.
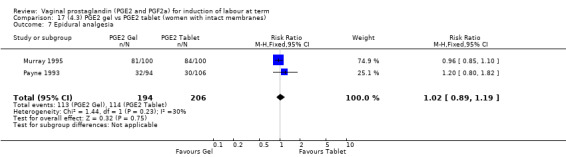
Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 7 Epidural analgesia.
17.9. Analysis.
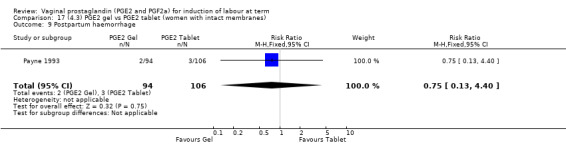
Comparison 17 (4.3) PGE2 gel vs PGE2 tablet (women with intact membranes), Outcome 9 Postpartum haemorrhage.
Comparison 18. (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.62, 1.59] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 21.71] |
| 3 Caesarean section | 3 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.48, 1.86] |
| 4 Serious maternal morbidity or death | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 5 Cervix unfavourable/unchanged after 12 to 24 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.56, 1.07] |
| 6 Oxytocin augmentation | 4 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.43, 1.25] |
| 7 Epidural analgesia | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.85, 1.10] |
| 8 Instrumental vaginal delivery | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.25] |
| 9 Apgar score < 7 at 5 minutes | 2 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |
| 10 Postpartum haemorrhage | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.30] |
18.1. Analysis.
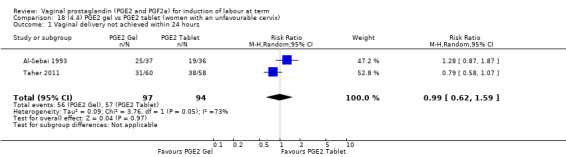
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 1 Vaginal delivery not achieved within 24 hours.
18.2. Analysis.
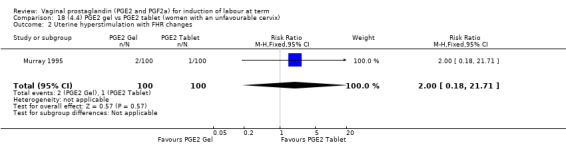
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.
18.3. Analysis.
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 3 Caesarean section.
18.4. Analysis.
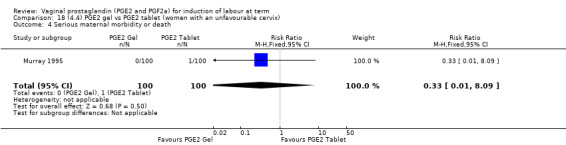
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 4 Serious maternal morbidity or death.
18.5. Analysis.
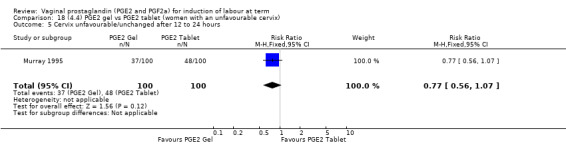
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 5 Cervix unfavourable/unchanged after 12 to 24 hours.
18.6. Analysis.
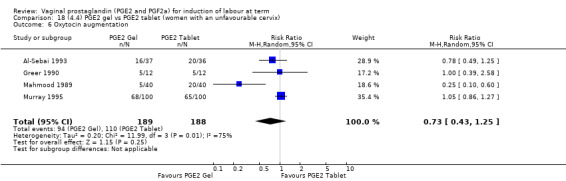
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 6 Oxytocin augmentation.
18.7. Analysis.
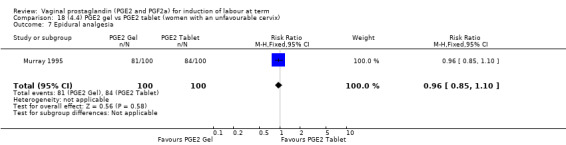
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 7 Epidural analgesia.
18.9. Analysis.
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 9 Apgar score < 7 at 5 minutes.
18.10. Analysis.
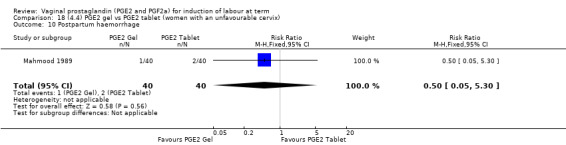
Comparison 18 (4.4) PGE2 gel vs PGE2 tablet (women with an unfavourable cervix), Outcome 10 Postpartum haemorrhage.
Comparison 19. (4.5) PGE2 gel vs PGE2 tablet (women with a favourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.56] |
| 2 Caesarean section | 1 | 328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.40, 1.41] |
| 3 Oxytocin augmentation | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.58] |
| 4 Apgar score < 7 at 5 minutes | 2 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.18, 4.37] |
19.1. Analysis.
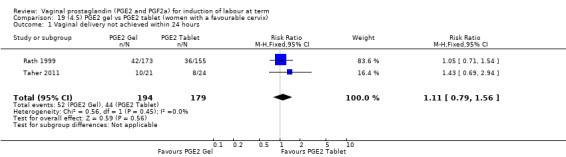
Comparison 19 (4.5) PGE2 gel vs PGE2 tablet (women with a favourable cervix), Outcome 1 Vaginal delivery not achieved within 24 hours.
19.2. Analysis.
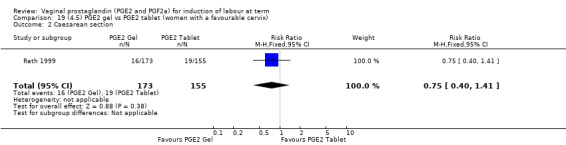
Comparison 19 (4.5) PGE2 gel vs PGE2 tablet (women with a favourable cervix), Outcome 2 Caesarean section.
19.3. Analysis.
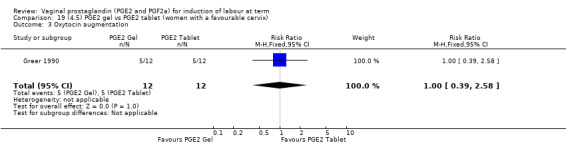
Comparison 19 (4.5) PGE2 gel vs PGE2 tablet (women with a favourable cervix), Outcome 3 Oxytocin augmentation.
19.4. Analysis.
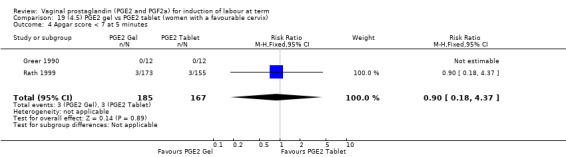
Comparison 19 (4.5) PGE2 gel vs PGE2 tablet (women with a favourable cervix), Outcome 4 Apgar score < 7 at 5 minutes.
Comparison 20. (5.1) PGE2 gel vs PGE2 suppository/pessary (all women).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.03, 0.87] |
| 2 Caesarean section | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.38, 1.11] |
| 3 Uterine hyperstimulation without FHR changes | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.05] |
| 4 Apgar score < 7 at 5 minutes | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.13] |
| 5 Maternal side‐effects (all) | 2 | 460 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.70] |
| 6 Nausea (maternal) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 7 Vomitting (maternal) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 8 Diarrhoea (maternal) | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.97] |
| 9 Other maternal side‐effects | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.97] |
20.2. Analysis.
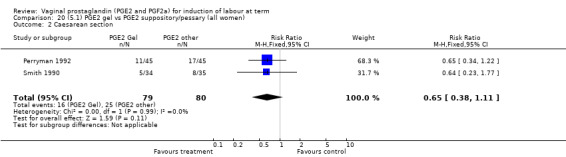
Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 2 Caesarean section.
20.4. Analysis.
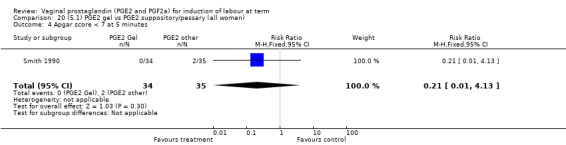
Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 4 Apgar score < 7 at 5 minutes.
20.5. Analysis.
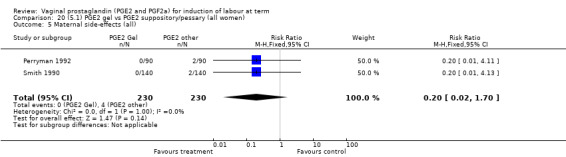
Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 5 Maternal side‐effects (all).
20.6. Analysis.
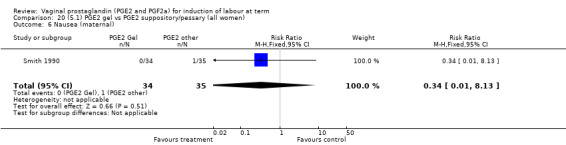
Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 6 Nausea (maternal).
20.7. Analysis.
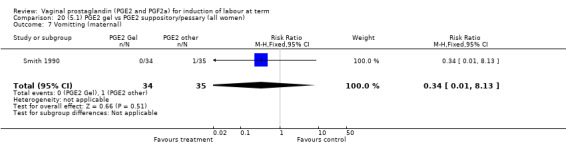
Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 7 Vomitting (maternal).
20.8. Analysis.
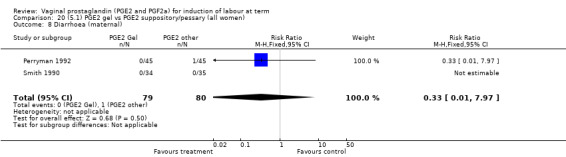
Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 8 Diarrhoea (maternal).
20.9. Analysis.
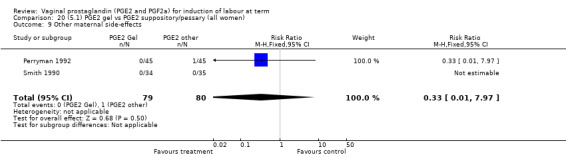
Comparison 20 (5.1) PGE2 gel vs PGE2 suppository/pessary (all women), Outcome 9 Other maternal side‐effects.
Comparison 21. (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.03, 0.87] |
| 2 Caesarean section | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.38, 1.11] |
| 3 Uterine hyperstimulation without FHR changes | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.05] |
| 4 Apgar score < 7 at 5 minutes | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.13] |
| 5 Maternal side‐effects (all) | 2 | 460 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.70] |
| 6 Nausea (maternal) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 7 Vomitting (maternal) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 8 Diarrhoea (maternal) | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.97] |
| 9 Other maternal side‐effects | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.97] |
21.1. Analysis.
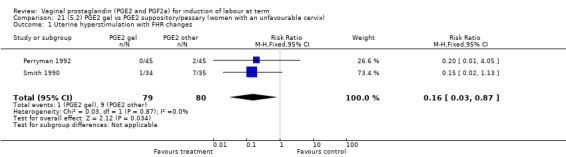
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.
21.3. Analysis.
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 3 Uterine hyperstimulation without FHR changes.
21.4. Analysis.
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 4 Apgar score < 7 at 5 minutes.
21.5. Analysis.
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 5 Maternal side‐effects (all).
21.6. Analysis.
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 6 Nausea (maternal).
21.7. Analysis.
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 7 Vomitting (maternal).
21.8. Analysis.
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 8 Diarrhoea (maternal).
21.9. Analysis.
Comparison 21 (5.2) PGE2 gel vs PGE2 suppository/pessary (women with an unfavourable cervix), Outcome 9 Other maternal side‐effects.
Comparison 22. (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 3 | 491 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.64, 1.99] |
| 2 Oxytocin augmentation | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.31, 1.40] |
| 3 Uterine hyperstimulation without FHR changes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Epidural analgesia | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.25, 1.78] |
| 5 Instrumental vaginal delivery | 3 | 491 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [1.09, 2.70] |
| 6 Apgar score < 7 at 5 minutes | 2 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.58, 3.05] |
| 7 Maternal side‐effects (all) | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Vomitting (maternal) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Diarrhoea (maternal) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Postpartum haemorrhage | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.57, 2.20] |
22.3. Analysis.
Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 3 Uterine hyperstimulation without FHR changes.
22.4. Analysis.
Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 4 Epidural analgesia.
22.6. Analysis.
Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 6 Apgar score < 7 at 5 minutes.
22.7. Analysis.

Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 7 Maternal side‐effects (all).
22.8. Analysis.
Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 8 Vomitting (maternal).
22.9. Analysis.
Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 9 Diarrhoea (maternal).
22.10. Analysis.
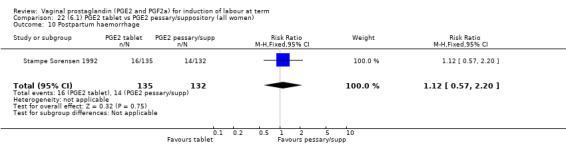
Comparison 22 (6.1) PGE2 tablet vs PGE2 pessary/suppository (all women), Outcome 10 Postpartum haemorrhage.
Comparison 23. (6.2) PGE2 tablet vs PGE2 pessary/suppository (primiparae).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.57, 2.58] |
| 2 Oxytocin augmentation | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.85, 1.88] |
23.1. Analysis.
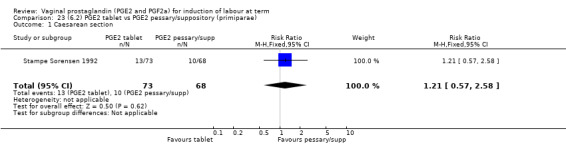
Comparison 23 (6.2) PGE2 tablet vs PGE2 pessary/suppository (primiparae), Outcome 1 Caesarean section.
23.2. Analysis.
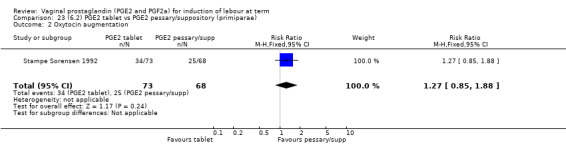
Comparison 23 (6.2) PGE2 tablet vs PGE2 pessary/suppository (primiparae), Outcome 2 Oxytocin augmentation.
Comparison 24. (6.3) PGE2 tablet vs PGE2 pessary/suppository (multiparae).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.23, 2.93] |
| 2 Oxytocin augmentation | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.37, 1.16] |
24.1. Analysis.
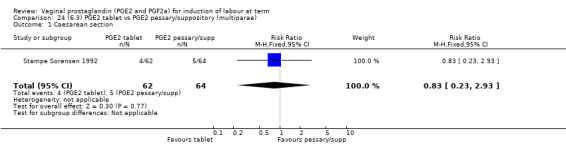
Comparison 24 (6.3) PGE2 tablet vs PGE2 pessary/suppository (multiparae), Outcome 1 Caesarean section.
24.2. Analysis.
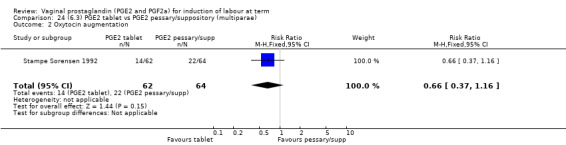
Comparison 24 (6.3) PGE2 tablet vs PGE2 pessary/suppository (multiparae), Outcome 2 Oxytocin augmentation.
Comparison 25. (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.26, 3.89] |
| 2 Oxytocin augmentation | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.19, 0.64] |
| 3 Uterine hyperstimulation without FHR changes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Instrumental vaginal delivery | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.47, 2.62] |
| 5 Apgar score < 7 at 5 minutes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.51, 3.04] |
| 6 Maternal side‐effects (all) | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Vomitting (maternal) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Diarrhoea (maternal) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
25.1. Analysis.
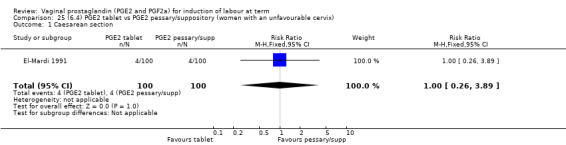
Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 1 Caesarean section.
25.2. Analysis.
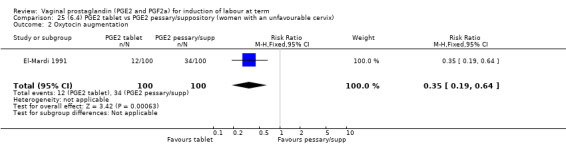
Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 2 Oxytocin augmentation.
25.3. Analysis.
Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 3 Uterine hyperstimulation without FHR changes.
25.4. Analysis.
Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 4 Instrumental vaginal delivery.
25.5. Analysis.

Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 5 Apgar score < 7 at 5 minutes.
25.6. Analysis.
Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 6 Maternal side‐effects (all).
25.7. Analysis.
Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 7 Vomitting (maternal).
25.8. Analysis.
Comparison 25 (6.4) PGE2 tablet vs PGE2 pessary/suppository (women with an unfavourable cervix), Outcome 8 Diarrhoea (maternal).
Comparison 26. (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 3 | 450 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.92, 1.45] |
| 2 Uterine hyperstimulation with FHR changes | 5 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.89, 5.21] |
| 3 Caesarean section | 11 | 1262 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.82, 1.26] |
| 4 Serious neonatal morbidity or perinatal death | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.62] |
| 5 Serious maternal morbidity or death | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 ‐24 hours (BS < 3) | 2 | 271 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.46, 0.80] |
| 7 Oxytocin augmentation | 7 | 884 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.69, 1.13] |
| 8 Uterine hyperstimulation without FHR changes | 8 | 908 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.81, 3.14] |
| 9 Uterine rupture | 2 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 10 Epidural analgesia | 3 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.95, 1.36] |
| 11 Instrumental vaginal delivery | 6 | 791 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.32, 0.68] |
| 12 Postpartum haemorrhage | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.72] |
| 13 Apgar score < 7 at 5 minutes | 3 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
| 14 Vomitting (maternal) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 102.00] |
| 15 Diarrhoea (maternal) | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.02] |
26.5. Analysis.
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 5 Serious maternal morbidity or death.
26.9. Analysis.
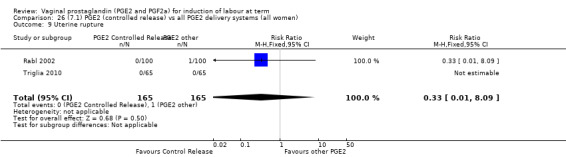
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 9 Uterine rupture.
26.10. Analysis.
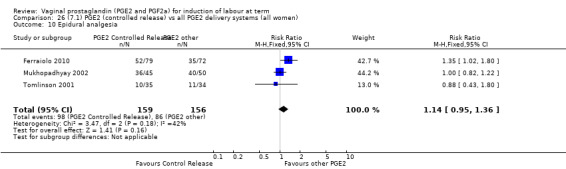
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 10 Epidural analgesia.
26.12. Analysis.
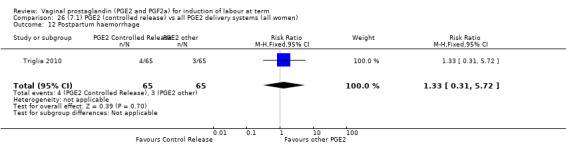
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 12 Postpartum haemorrhage.
26.13. Analysis.
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 13 Apgar score < 7 at 5 minutes.
26.14. Analysis.
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 14 Vomitting (maternal).
26.15. Analysis.
Comparison 26 (7.1) PGE2 (controlled release) vs all PGE2 delivery systems (all women), Outcome 15 Diarrhoea (maternal).
Comparison 27. (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.77, 2.10] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.44 [0.43, 128.16] |
| 3 Caesarean section | 5 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.63, 1.23] |
| 4 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.45, 0.80] |
| 5 Oxytocin augmentation | 3 | 258 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.43, 1.29] |
| 6 Uterine hyperstimulation without FHR changes | 1 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Epidural analgesia | 2 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.83, 1.58] |
| 8 Instrumental vaginal delivery | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.23, 2.13] |
27.1. Analysis.
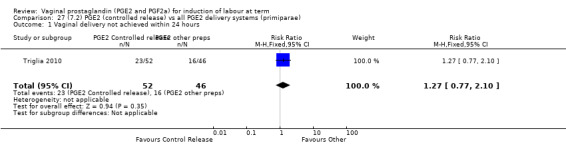
Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 1 Vaginal delivery not achieved within 24 hours.
27.2. Analysis.
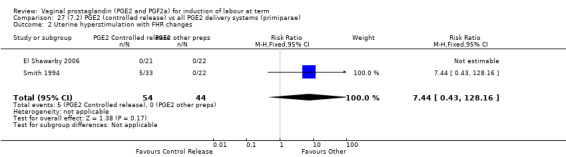
Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 2 Uterine hyperstimulation with FHR changes.
27.3. Analysis.
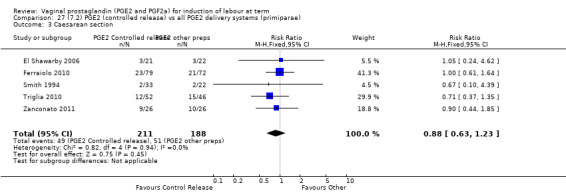
Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 3 Caesarean section.
27.4. Analysis.
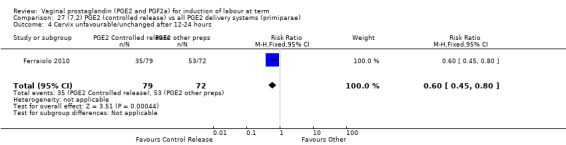
Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 4 Cervix unfavourable/unchanged after 12‐24 hours.
27.5. Analysis.

Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 5 Oxytocin augmentation.
27.6. Analysis.
Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 6 Uterine hyperstimulation without FHR changes.
27.7. Analysis.
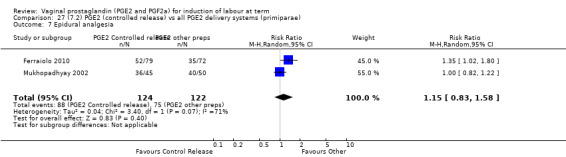
Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 7 Epidural analgesia.
27.8. Analysis.
Comparison 27 (7.2) PGE2 (controlled release) vs all PGE2 delivery systems (primiparae), Outcome 8 Instrumental vaginal delivery.
Comparison 28. (7.3) PGE2 (controlled release) vs all PGE2 delivery systems (multiparae).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.35, 6.15] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 95 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.38] |
| 3 Caesarean section | 3 | 127 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.53, 3.23] |
| 4 Oxytocin augmentation | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.20, 0.86] |
| 5 Instrumental vaginal delivery | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.13, 2.85] |
28.1. Analysis.
Comparison 28 (7.3) PGE2 (controlled release) vs all PGE2 delivery systems (multiparae), Outcome 1 Vaginal delivery not achieved within 24 hours.
28.2. Analysis.
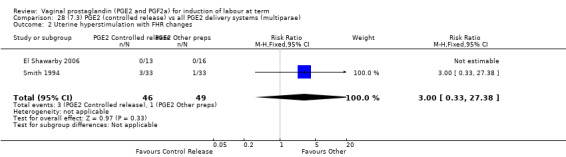
Comparison 28 (7.3) PGE2 (controlled release) vs all PGE2 delivery systems (multiparae), Outcome 2 Uterine hyperstimulation with FHR changes.
28.3. Analysis.
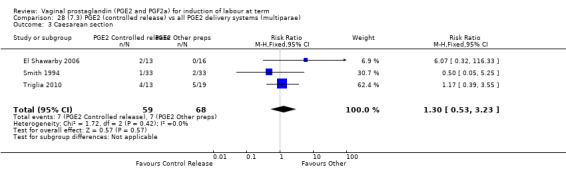
Comparison 28 (7.3) PGE2 (controlled release) vs all PGE2 delivery systems (multiparae), Outcome 3 Caesarean section.
28.5. Analysis.
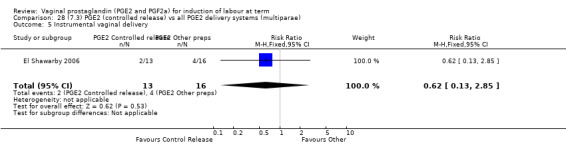
Comparison 28 (7.3) PGE2 (controlled release) vs all PGE2 delivery systems (multiparae), Outcome 5 Instrumental vaginal delivery.
Comparison 29. (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.85, 2.21] |
| 2 Uterine hyperstimulation with FHR changes | 3 | 323 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.67 [0.86, 51.67] |
| 3 Caesarean section | 8 | 873 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.28] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.62] |
| 5 Serious maternal morbidity or death | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.45, 0.80] |
| 7 Oxytocin augmentation | 5 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.54, 1.21] |
| 8 Uterine hyperstimulation without FHR changes | 5 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.81 [0.71, 47.25] |
| 9 Uterine rupture | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 3 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.84, 1.63] |
| 11 Instrumental vaginal delivery | 3 | 402 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.20, 0.59] |
| 12 Apgar score < 7 at 5 minutes | 2 | 170 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Postpartum haemorrhage | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.72] |
29.1. Analysis.
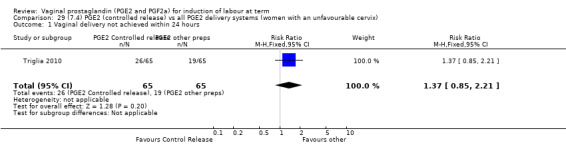
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 1 Vaginal delivery not achieved within 24 hours.
29.2. Analysis.
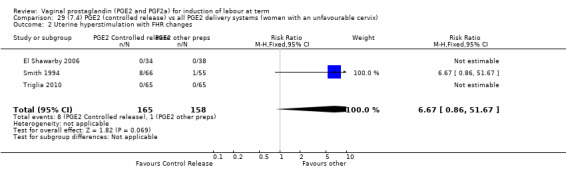
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 2 Uterine hyperstimulation with FHR changes.
29.4. Analysis.
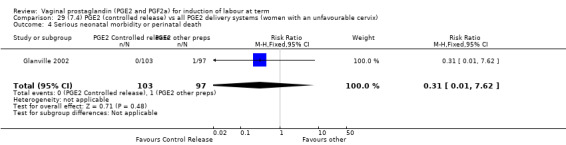
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 4 Serious neonatal morbidity or perinatal death.
29.5. Analysis.
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 5 Serious maternal morbidity or death.
29.8. Analysis.
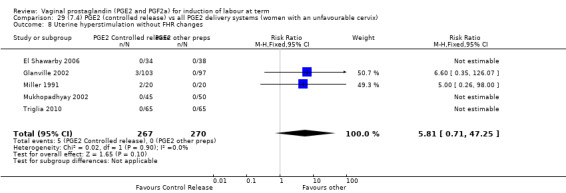
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 8 Uterine hyperstimulation without FHR changes.
29.9. Analysis.
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 9 Uterine rupture.
29.10. Analysis.
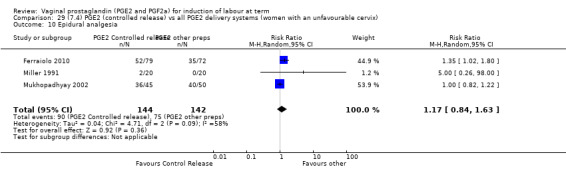
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 10 Epidural analgesia.
29.12. Analysis.
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 12 Apgar score < 7 at 5 minutes.
29.13. Analysis.
Comparison 29 (7.4) PGE2 (controlled release) vs all PGE2 delivery systems (women with an unfavourable cervix), Outcome 13 Postpartum haemorrhage.
Comparison 30. (8.1) PGE2 low dose vs PGE2 high dose (all women).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.13] |
| 2 Caesarean section | 7 | 1546 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.78, 1.33] |
| 3 Serious neonatal morbidity or perinatal death | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Cervix unfavourable/unchanged after 12‐24hrs | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.25, 2.21] |
| 5 Oxytocin augmentation | 5 | 1370 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.77, 1.20] |
| 6 Uterine hyperstimulation without FHR changes | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| 7 Epidural analgesia | 4 | 1330 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.74, 1.26] |
| 8 Instrumental vaginal delivery | 3 | 1179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.70, 1.13] |
| 9 Meconium‐stained liquor | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.67, 1.10] |
| 10 Apgar score < 7 at 5 minutes | 3 | 1064 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.20, 1.31] |
| 11 Neonatal intensive care unit admission | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.24, 1.09] |
| 12 Perinatal death | 1 | 955 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Maternal side‐effects (all) | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.13] |
| 14 Nausea (maternal) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 15 Vomitting (maternal) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 16 Diarrhoea (maternal) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Other maternal side‐effects | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Postpartum haemorrhage | 2 | 1155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.79, 2.09] |
30.3. Analysis.
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 3 Serious neonatal morbidity or perinatal death.
30.12. Analysis.
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 12 Perinatal death.
30.14. Analysis.
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 14 Nausea (maternal).
30.15. Analysis.
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 15 Vomitting (maternal).
30.16. Analysis.
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 16 Diarrhoea (maternal).
30.17. Analysis.
Comparison 30 (8.1) PGE2 low dose vs PGE2 high dose (all women), Outcome 17 Other maternal side‐effects.
Comparison 31. (8.2) PGE2 low dose vs PGE2 high dose (primiparae).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 2 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.83, 1.63] |
| 2 Serious neonatal morbidity or perinatal death | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Cervix unfavourable/unchanged after 12‐24hrs | 1 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.25, 2.21] |
| 4 Oxytocin augmentation | 2 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.93, 1.18] |
| 5 Epidural analgesia | 2 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.93, 1.22] |
| 6 Instrumental vaginal delivery | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.18] |
| 7 Meconium‐stained liquor | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.27] |
| 8 Apgar score < 7 at 5 minutes | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.27] |
| 9 Neonatal intensive care unit admission | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.11, 1.03] |
| 10 Perinatal death | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Postpartum haemorrhage | 1 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.86, 3.05] |
31.1. Analysis.
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 1 Caesarean section.
31.2. Analysis.
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 2 Serious neonatal morbidity or perinatal death.
31.5. Analysis.
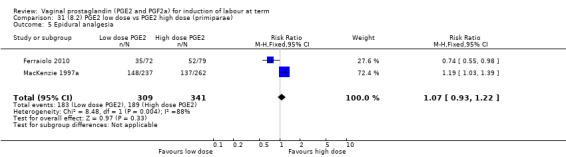
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 5 Epidural analgesia.
31.6. Analysis.
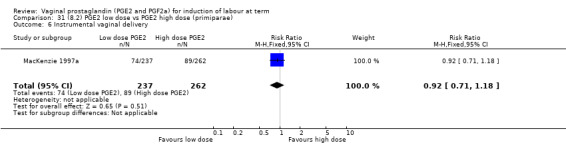
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 6 Instrumental vaginal delivery.
31.7. Analysis.
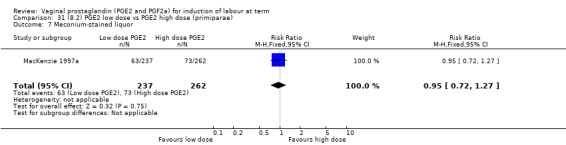
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 7 Meconium‐stained liquor.
31.8. Analysis.
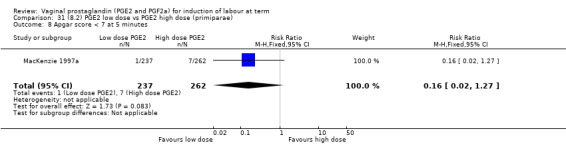
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 8 Apgar score < 7 at 5 minutes.
31.9. Analysis.
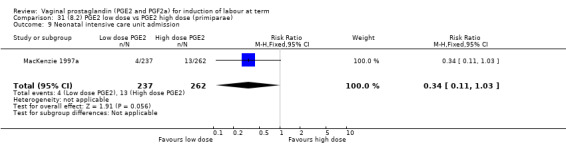
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 9 Neonatal intensive care unit admission.
31.10. Analysis.
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 10 Perinatal death.
31.11. Analysis.
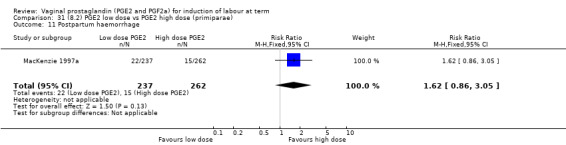
Comparison 31 (8.2) PGE2 low dose vs PGE2 high dose (primiparae), Outcome 11 Postpartum haemorrhage.
Comparison 32. (8.3) PGE2 low dose vs PGE2 high dose (multiparae).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.19, 2.51] |
| 2 Serious neonatal morbidity or perinatal death | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Oxytocin augmentation | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.35, 2.80] |
| 4 Epidural analgesia | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.77, 1.70] |
| 5 Instrumental vaginal delivery | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.98 [1.37, 25.99] |
| 6 Meconium‐stained liquor | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.48, 1.24] |
| 7 Apgar score < 7 at 5 minutes | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.34, 5.88] |
| 8 Neonatal intensive care unit admission | 1 | 492 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.33, 3.06] |
| 9 Perinatal death | 1 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Postpartum haemorrhage | 1 | 456 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.44, 2.47] |
32.1. Analysis.
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 1 Caesarean section.
32.2. Analysis.
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 2 Serious neonatal morbidity or perinatal death.
32.4. Analysis.
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 4 Epidural analgesia.
32.6. Analysis.
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 6 Meconium‐stained liquor.
32.7. Analysis.
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 7 Apgar score < 7 at 5 minutes.
32.8. Analysis.
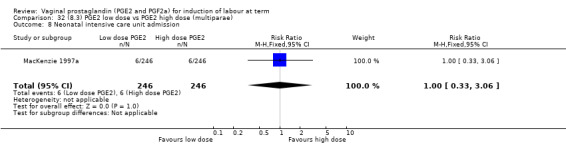
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 8 Neonatal intensive care unit admission.
32.9. Analysis.
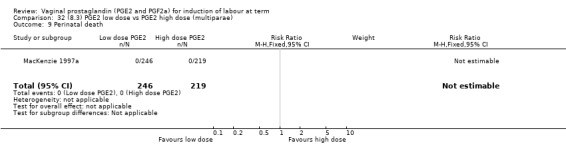
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 9 Perinatal death.
32.10. Analysis.
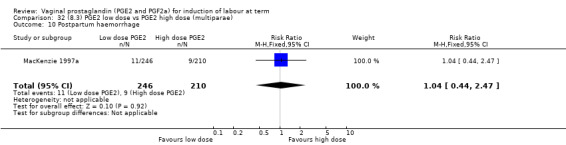
Comparison 32 (8.3) PGE2 low dose vs PGE2 high dose (multiparae), Outcome 10 Postpartum haemorrhage.
Comparison 33. (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 2 | 269 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.44, 1.38] |
| 2 Oxytocin augmentation | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.63, 1.05] |
| 3 Epidural analgesia | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.80, 1.82] |
| 4 Instrumental vaginal delivery | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.38, 1.26] |
| 5 Postpartum haemorrhage | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.13, 4.40] |
| 6 Uterine hyperstimulation with FHR changes | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.13] |
| 7 Apgar score < 7 at 5 minutes | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.13] |
| 8 Maternal side‐effects (all) | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.13] |
| 9 Nausea (maternal) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 10 Vomitting (maternal) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 11 Diarrhoea (maternal) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Other maternal side‐effects | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
33.1. Analysis.
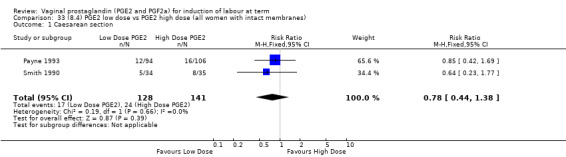
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 1 Caesarean section.
33.2. Analysis.
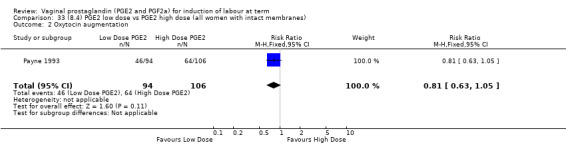
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 2 Oxytocin augmentation.
33.3. Analysis.
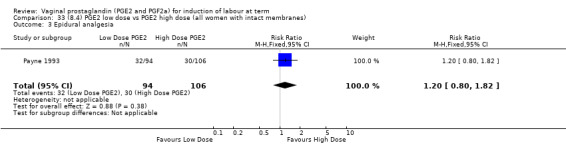
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 3 Epidural analgesia.
33.4. Analysis.
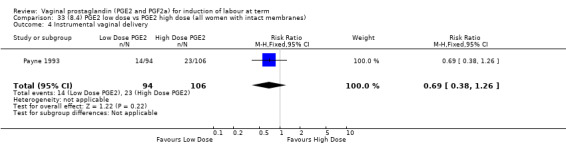
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 4 Instrumental vaginal delivery.
33.5. Analysis.
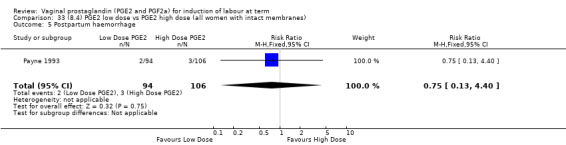
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 5 Postpartum haemorrhage.
33.6. Analysis.
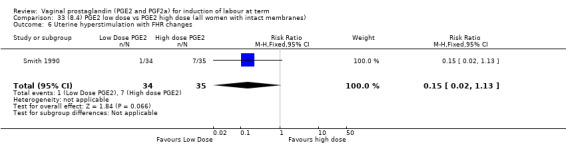
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 6 Uterine hyperstimulation with FHR changes.
33.7. Analysis.
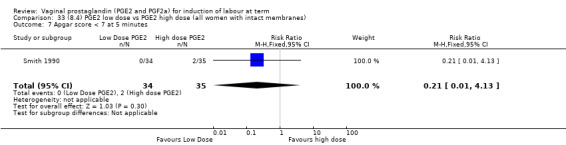
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 7 Apgar score < 7 at 5 minutes.
33.8. Analysis.
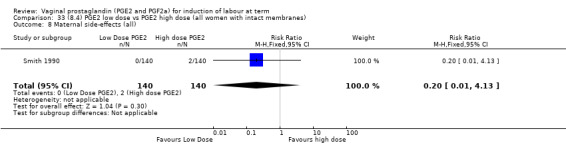
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 8 Maternal side‐effects (all).
33.9. Analysis.
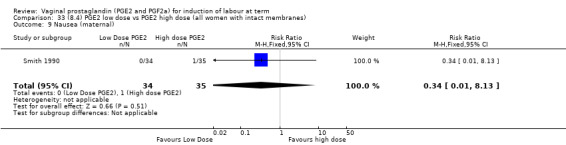
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 9 Nausea (maternal).
33.10. Analysis.
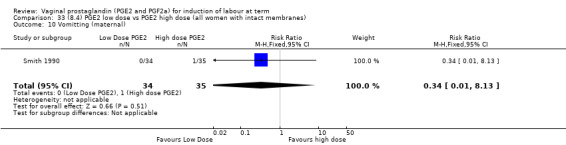
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 10 Vomitting (maternal).
33.11. Analysis.
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 11 Diarrhoea (maternal).
33.12. Analysis.
Comparison 33 (8.4) PGE2 low dose vs PGE2 high dose (all women with intact membranes), Outcome 12 Other maternal side‐effects.
Comparison 34. (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.13] |
| 2 Caesarean section | 4 | 367 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.69, 1.46] |
| 3 Oxytocin augmentation | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.06] |
| 4 Uterine hyperstimulation without FHR changes | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.92] |
| 5 Apgar score < 7 at 5 minutes | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.13] |
| 6 Maternal side‐effects (all) | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.13] |
| 7 Nausea (maternal) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 8 Vomitting (maternal) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 9 Diarrhoea (maternal) | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Other maternal side‐effects | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
34.1. Analysis.
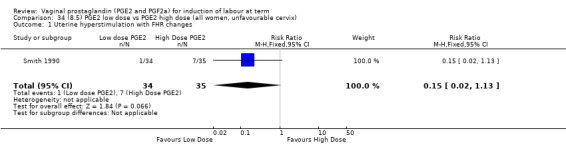
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 1 Uterine hyperstimulation with FHR changes.
34.2. Analysis.
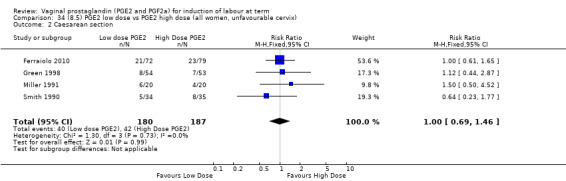
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 2 Caesarean section.
34.3. Analysis.
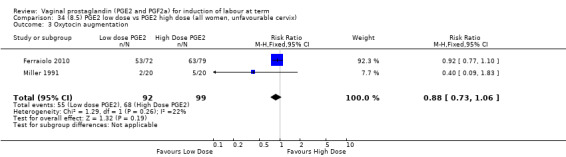
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 3 Oxytocin augmentation.
34.4. Analysis.
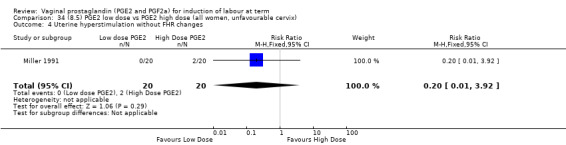
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 4 Uterine hyperstimulation without FHR changes.
34.5. Analysis.
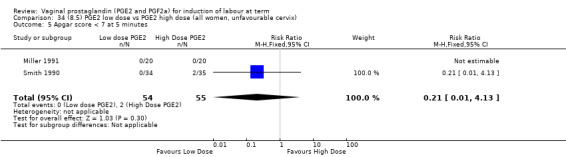
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 5 Apgar score < 7 at 5 minutes.
34.6. Analysis.
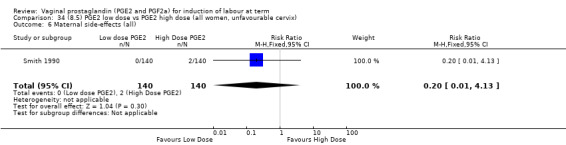
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 6 Maternal side‐effects (all).
34.7. Analysis.
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 7 Nausea (maternal).
34.8. Analysis.
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 8 Vomitting (maternal).
34.9. Analysis.
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 9 Diarrhoea (maternal).
34.10. Analysis.
Comparison 34 (8.5) PGE2 low dose vs PGE2 high dose (all women, unfavourable cervix), Outcome 10 Other maternal side‐effects.
Comparison 35. PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.1 PGE2 (repeated doses) vs placebo/no treatment | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.42, 1.18] |
| 2.1 PGE2 (repeated doses) vs placebo/no treatment | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.42, 1.18] |
| 3 Cervix unfavourable/unchanged after 12 to 24 hours | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.47] |
| 3.1 PGE2 (repeated doses) vs placebo/no treatment | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.47] |
| 4 Uterine hyperstimulation without FHR changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 4.1 PGE2 (repeated doses) vs placebo/no treatment | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 5 Epidural analgesia | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.62, 1.12] |
| 5.1 PGE2 (repeated doses) vs placebo/no treatment | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.62, 1.12] |
| 6 Meconium‐stained liquor | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.28, 0.66] |
| 6.1 PGE2 (repeated doses) vs placebo/no treatment | 2 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.28, 0.66] |
| 7 Apgar score < 7 at 5 minutes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.06] |
| 7.1 PGE2 (repeated doses) vs placebo/no treatment | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.06] |
| 8 Neonatal intensive care unit admission | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.65] |
| 8.1 PGE2 (repeated doses) vs placebo/no treatment | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.65] |
35.1. Analysis.
Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 1 Uterine hyperstimulation with FHR changes.
35.2. Analysis.
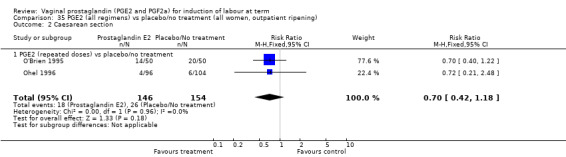
Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 2 Caesarean section.
35.3. Analysis.
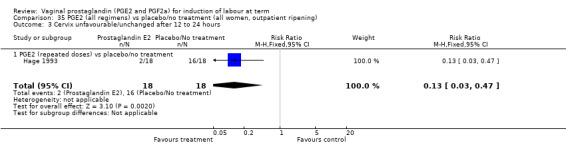
Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 3 Cervix unfavourable/unchanged after 12 to 24 hours.
35.4. Analysis.
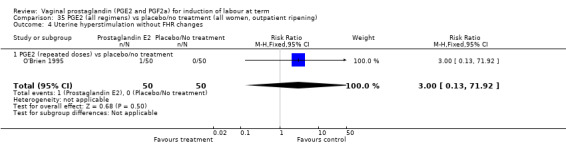
Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 4 Uterine hyperstimulation without FHR changes.
35.5. Analysis.
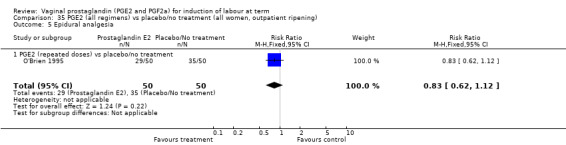
Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 5 Epidural analgesia.
35.6. Analysis.
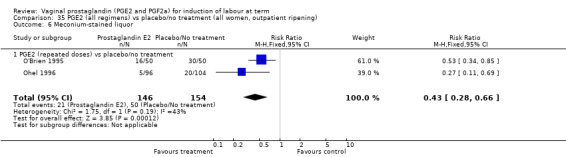
Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 6 Meconium‐stained liquor.
35.7. Analysis.
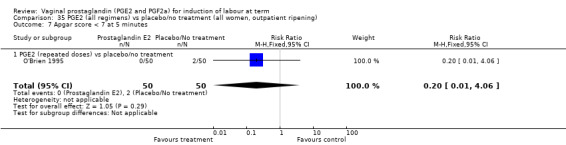
Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 7 Apgar score < 7 at 5 minutes.
35.8. Analysis.
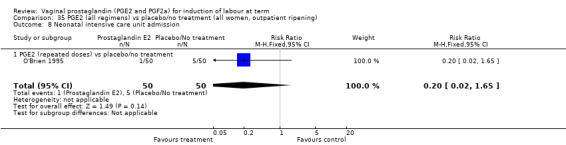
Comparison 35 PGE2 (all regimens) vs placebo/no treatment (all women, outpatient ripening), Outcome 8 Neonatal intensive care unit admission.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al Malt 1995.
| Methods | 'Randomised double‐blind manner.' | |
| Participants | 103 women with indications for labour induction. Inclusion criteria: singleton pregnancy and BS < 5. |
|
| Interventions | 3 mg PGE2 vaginal gel (n = 49) or placebo gel (n = 54). Both 12 hours prior to induction. BS obtained on admission, prior to oxytocin and 10 hours after oxytocin. Patients with BS < 8 to 10 hours after oxytocin had protocol repeated on D2. If BS < 8 after repeat protocol patients released and managed as clinically indicated. |
|
| Outcomes | Primary outcome: change in BS, caesarean section, total deliveries and failed induction. | |
| Notes | Data extracted from abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear, method of random generation not stated. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ unclear, method not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blind." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "double blind." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Limited information in abstract. |
Al‐Sebai 1993.
| Methods | 'Allocated at random.' | |
| Participants | 73 primigravid women. Inclusion criteria: singleton, cephalic, primiparous and > 36 weeks' gestation with a BS < 4. Intact membranes. |
|
| Interventions | 2 mg PGE2 vaginal gel (n = 37) or 3 mg PGE2 vaginal tablet (n = 35). Review at 6 hours after first dose and repeat dose if BS < 7. 2 doses maximum. |
|
| Outcomes | Vaginal delivery not achieved in 24 hours, caesarean section and oxytocin augmentation. | |
| Notes | Staincliffe Maternity Hospital, West Yorkshire, UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, preparations different. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding, outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 73 women recruited, outcomes reported on 72. |
| Selective reporting (reporting bias) | Unclear risk | Uncertain which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Bezircioglu 2012.
| Methods | Computer‐generated random sequence, sealed sequentially numbered envelopes. | |
| Participants | 100 women with singleton, cephalic pregnancy at term with a live fetus, normal CTG and ruptured membranes and decision to induce. Unfavourable cervix with BS < 4. Excluded: multiple pregnancy, previous caesarean section, multiparous women, vaginal bleeding, malpresentations, suspected large baby > 4.5 kg, CPD, BS > 4, chorioamnionitis. |
|
| Interventions | PGE2 sustained release pessary 10 mg (0.3 mg per hour) (n = 50). Removed when in labour or after 12 hrs (n = 50). Comaprison: expectant management (n = 50). |
|
| Outcomes | Duration of latent (to 4 cm) and active phase of labour (from 4 cm), time induction to delivery (mean +/‐SD), caesarean section, uterine hyperstimulation with fetal heart rate changes, decelerations on CTG, cervical ripening at 12 hrs (BS > 9), Apgar score (average). | |
| Notes | Setting Izmir Ataturk training hospital, Izmir, Turkey. May 2009–Dec 2009. Trial registration and source of financial support not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Sealed, sequentially numbered envelopes (may or may not be opaque). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | None. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | None. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Flow diagram is not included. Loss to follow‐up or of outcomes data are not reported. Categorical data (%) suggests all participants included, but many outcomes are reported as mean and SD only. |
| Selective reporting (reporting bias) | Unclear risk | Unclear, no study protocol available. |
| Other bias | Unclear risk | Source of funding not stated. |
Buchanan 1984.
| Methods | 'Randomised.' Case controlled for parity and indication for labour induction. | |
| Participants | 77 women. Inclusion criteria: singleton pregnancy, cephalic presentation, afebrile with a reactive NST. medical or obstetric indications for induction of labour and BS < 4. Exclusion criteria: previous uterine scar, placenta praevia, history of asthma or sickle cell disease, evidence of IUGR, fetal distress or spontaneous uterine contractions. |
|
| Interventions | 3 mg PGE2 vaginal suppository (n = 38) or identical looking glycerin suppository (n = 39). Both suppositories placed the evening prior to induction following a baseline BS. Reassessed the next morning and BS reassessed. Oxytocin then commenced, (started at 0.4 mU/min doubled every 20 to 40 minutes). Failed induction was defined as no change in cervical effacement or dilatation after 8 hours of adequate uterine activity, or after 1 hour of oxytocin at 24 mU/min, these patients underwent caesarean section. |
|
| Outcomes | Primary outcome: percentage requiring oxytocin for induction, caesarean section, instrumental delivery rates, uterine hyperstimulation with and without FHR changes, neonatal Apgar scores, maternal complications, PPH rates. | |
| Notes | 4 protocol violations in placebo group. Reincluded in analysis (3 had LSCS and 1 had NVD). Women's Hospital, Los Angeles County/University of Southern California Medical Center, Los Angeles. November 1981 to December 1982. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method unclear. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double blind." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double blind." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/39 (10%) in placebo group had protocol violations. |
| Selective reporting (reporting bias) | Unclear risk | Uncertain which outcomes prespecified. |
| Other bias | Unclear risk | Small trial, single centre. |
Campbell 1984.
| Methods | Alternation in blocks of 6. | |
| Participants | 199 women with cephalic presentations attending for induction of labour. Exclusion criteria: malpresentations or if inclusion in placebo trial might involve delay and so increase risk to mother or fetus. Indications for induction: post dates (126), hypertension (57), miscellaneous (16). |
|
| Interventions | 3 mg PGE2 vaginal pessaries (n = 95) or placebo pessaries (n = 104). Prepared in batches of 6 pairs. Pessaries were placed on morning of admission, following assignment of baseline BS. Following mean interval of 8 hours if labour had not ensued, patients were reassessed and a further pessary inserted. If no labour by 24 hours after first pessary then trial ended and other methods of induction used. |
|
| Outcomes | Spontaneous labour, change in BS, length of labour, mode of delivery, neonatal Apgar scores, uterine hypertonus. | |
| Notes | 4 post randomisation exclusions for late diagnosed breech presentation. Bangor General Hospital, UK. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Alternation in blocks of six." |
| Allocation concealment (selection bias) | High risk | C ‐ inadequate. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blind" active pessary vs placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double blind." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 (< 2%) post randomisation exclusions. |
| Selective reporting (reporting bias) | Unclear risk | Uncertain which outcomes prespecified. |
| Other bias | Unclear risk | Small trial, single centre. |
Cardozo 1986.
| Methods | Allocation based on even or odd last digit of hospital number. | |
| Participants | 402 women at 40 weeks + 10 days of pregnancy. Gestation calculated from LMP or by ultrasound at 20 weeks if dates uncertain or greater than 7 days difference between estimates by ultrasound and LMP. | |
| Interventions | Active group (even numbers) (n = 195) induced by 3 mg PGE2 pessary followed by amniotomy 3 hours later +/‐ oxytocin where necessary. Induction between 40 weeks + 12 days and 40 weeks + 14 days. Conservative group (odd numbers) (n = 207) had fetal assessment by ultrasound at 40+16 days, daily kick charts and alternate date CTGs. Induced if concern for fetal welfare, abnormality in CTG, ROM. |
|
| Outcomes | Mean gestation at delivery, analgesia used, mode of delivery, Apgar scores, neonatal intensive care unit admission, umbilical cord blood acidosis, patient satisfaction. | |
| Notes | Multiple ITT violations. 19 patients in active group and 20 in conservative group not accounted for in analysis of maternal outcomes. Method of induction in conservative group not specified. Non‐randomised re‐analysis performed to account for women who went into spontaneous labour in active group. Mothers allowed to opt for alternative if allocated group unacceptable. Kings College Hospital, London. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation on last digit of hospital number (odd or even), not random. |
| Allocation concealment (selection bias) | High risk | Allocation not concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding, some subjective outcomes, e.g. analgesia use, patient satisfaction. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | About 10% of recruits not accounted for in analysis of maternal outcomes, but evenly lost across intervention groups ‐19 patients in active group and 20 in conservative group. |
| Selective reporting (reporting bias) | Unclear risk | Uncertain which outcomes prespecified. |
| Other bias | High risk | Multiple ITT violations. Method of induction in conservative group not specified. Non‐randomised re‐analysis performed to account for women who went into spontaneous labour in active group. |
Chaterjee 1990.
| Methods | Randomisation by card shuffling, concealment unclear. | |
| Participants | 38 high‐risk women requiring induction of labour. Indications for induction: diabetes (10), post dates (7), pre‐eclampsia (13), IUGR (4), chronic hypertension (2), rhesus disease (1), others (1). |
|
| Interventions | 2 mg PGE2 vaginal gel (n = 15) or placebo gel (n = 18). BS assigned prior to application and 12 hours after application. Oxytocin started at this point (started at 1mU/min to maximum of 64 mU/min, or until satisfactory contractions observed). If undelivered, patients re‐randomised. If third application needed active gel could be requested. 5 women required multiple applications. |
|
| Outcomes | Mode of delivery, mean Apgar score at 1 and 5 minutes, change in BS. | |
| Notes | Only data from single gel application patients included. University Hospital, New Jersey Medical School. July 1983 to April 1984. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by card shuffling. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double blind." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double blind." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 38 women enrolled, outcome reported on 33, (13% loss to follow‐up). |
| Selective reporting (reporting bias) | Unclear risk | Uncertain which outcomes prespecified. |
| Other bias | Unclear risk | Small trial, single centre. |
Chua 1995.
| Methods | Computer‐generated lists. Centralised preparation of drugs in dark bottles. Master list not available to investigators until end of study. | |
| Participants | 155 nulliparous with ruptured membranes of greater than 2 hours duration and unfavourable cervical scores (mean BS 3). Exclusion criteria: intact membranes, multiple pregnancy, malpresentations, meconium‐stained amniotic fluid or evidence of intrauterine infection. |
|
| Interventions | 3 mg PGE2 vaginal pessary (n = 79) or identical placebo pessary (n = 76). Pessary placed in vagina, reviewed and induced with oxytocin at 14 hours or sooner if signs of infection evident. |
|
| Outcomes | Oxytocin augmentation, admission to study‐onset of labour interval, maternal pyrexia, signs and symptoms of infection, NICU admission rates, Apgar scores at 1 and 5 minutes, mode of delivery, uterine hyperstimulation rates. | |
| Notes | National University Hospital, Singapore. January 1992 to December 1994. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list. |
| Allocation concealment (selection bias) | Low risk | Adequate. Centralised preparation of interventions. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double blind" placebo control. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double blind" placebo control. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients followed up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear what outcomes prespecified. |
| Other bias | Low risk | None. |
Chung 1992.
| Methods | Computer‐generated random number list, allocation known only to trial co‐ordinator, drugs in coded boxes. | |
| Participants | 59 women admitted with pre‐labour rupture of membranes. Inclusion criteria: singleton pregnancy, cephalic presentation, confirmed SROM, BS < 4, no uterine contractions, no signs of maternal infection, clear liquor, absence of medical or obstetric complications. |
|
| Interventions | 3 mg PGE2 vaginal gel (n = 30) or identical placebo gel (n = 29). Instillation of gel post randomisation. Conservative management for subsequent 24 hours. Oxytocin used for augmentation or induction as per departmental protocol. | |
| Outcomes | Interval between SROM and onset of labour and delivery. duration of labour, mode of delivery, need for oxytocin, hyperstimulation rates, febrile episodes, other maternal side‐effects, Apgar scores at 1 and 5 minutes, NICU admission. | |
| Notes | Prince of Wales Hospital, Hong Kong. August 1988 to July 1990. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list. |
| Allocation concealment (selection bias) | Low risk | Allocation concealed, coded boxes ‐ adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double blind." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double blind." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small trial, single centre. |
Curet 1989.
| Methods | Computerised random number tables, allocation unknown to patient or physician. Drugs in coded boxes. | |
| Participants | 54 women undergoing induction of labour. Inclusion criteria: singleton pregnancy, intact membranes and normal placental function. Indications for induction: toxaemia (29), diabetes (9), post term (12), elective (5), oligohydramnios (3), IUGR (3), chronic hypertension (2). |
|
| Interventions | 3 mg PGE2 vaginal gel (n = 28), identical placebo gel (n = 26). Baseline BS assigned, if < 5 patients randomised. oxytocin given if required, but details of time interval not clear. |
|
| Outcomes | Change in BS, incidence of spontaneous labour, mode of delivery, hyperstimulation rates. | |
| Notes | University of Wisconsin Perinatal Cancer at Meriter/Madison General Hospital, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number tables. |
| Allocation concealment (selection bias) | Low risk | Allocation concealed. Drugs in coded boxes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double blind" placebo control. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double blind" placebo control. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Due to the timing of the study (1980) and the limited information in the report, it is not possible to check on the details of recruitment and inclusion. |
Doany 1997.
| Methods | Random number tables, allocation concealment unclear. Factorial design ‐ 4 groups. | |
| Participants | In total 150 women undergoing induction of labour 4 groups only 2 arms of trial (65 women) eligible for inclusion in this review (vaginal PGE2 gel vs placebo). Inclusion criteria: singleton pregnancy, cephalic presentation, reactive NST, AFI between 5 and 25, fetal weight between 2500 g and 4500 g, uterine contractions less frequent than 5 minutes. Exclusion criteria: no prenatal care, previous uterine surgery, acute or chronic medical or psychiatric illness or drug use. |
|
| Interventions | 2 mg PGE2 vaginal gel (n = 37) or identical placebo gel (n = 28). Reassessed 7 days afterwards, then every 2‐4 days after that to a maximum of 307 days. (Outpatient administration.) | |
| Outcomes | Number of fetal surveillance visits, onset of spontaneous labour, incidence of SROM, use of oxytocin, admission to delivery time, total length of labour, mode of delivery, meconium‐stained liquor, 5‐minute Apgar scores, PPH, and amnionitis. | |
| Notes | Only 2 arms of trial analysed here (groups I and II). Additional 2 arms with membrane stripping +/‐ vaginal prostaglandins (groups III and IV) included in review focusing on membrane stripping. UCLA Medical centre, University of California, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double blind" placebo control. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double blind" placebo control. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small single centre RCT. |
Dommisse 1980.
| Methods | Patients were 'randomly' allocated, matched in pairs by parity. | |
| Participants | 56 women with a 'favourable' cervix. Inclusion criteria: singleton pregnancy, cephalic presentation, live fetus greater than 36 weeks' gestation with a BS of greater than 5. |
|
| Interventions | 4 mg PGE2 tablets (8, 0.5 mg tablets or 8 placebo tablets placed in the vagina using a disposable vaginal cream introducer at midday on the day of induction. If not in labour after 18 to 20 hours then referred for surgical induction and oxytocin infusion. | |
| Outcomes | Uterine hyperstimulation with and without FHR changes, caesarean section, serious neonatal morbidity/perinatal death and oxytocin augmentation. | |
| Notes | 2 university hospitals in Cape Town, South Africa. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Method unclear, but possibly alternation if "matched in pairs by parity". |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo used but blinding not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small trial. |
Duhl 1997.
| Methods | 'Randomised.' | |
| Participants | 74 women with medical indication for induction. | |
| Interventions | 3 mg PGE2 vaginal gel (n = 24) every 4 to 6 hours, or PGE2 10 mg vaginal insert (sustained release) (n = 27) or PGE2 0.5 mg intracervical gel every 6 hours. Subsequent management not specified. |
|
| Outcomes | Change in BS, spontaneous labour, need for oxytocin, hyperstimulation. | |
| Notes | Limited data as extracted from abstract. Pennsylvania Hospital, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Were randomised.' |
| Allocation concealment (selection bias) | Unclear risk | Unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | 3 active comparison groups, no blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Dunston‐Boone 1991.
| Methods | 'Blindly randomised.' | |
| Participants | 53 women undergoing induction of labour. Inclusion criteria: intact membranes, no prior uterine surgery, reactive NST, BS < 6. |
|
| Interventions | 10 mg sustained release pessary (1 mg/hr) (n = 39) or placebo pessary (n = 14). Those patients with BS < 4 were randomised. Those with BS > 5 were given active drug. Vaginal examinations repeated at 6 and 12 hours. |
|
| Outcomes | Hyperstimulation rates, change in BS, neonatal outcomes. | |
| Notes | Only randomised patients included in analysis. Outcomes calculated on overall event rate in active arm. Limited data, as extracted from abstract. Medical College of Thomas Jefferson University Hospital, Philadelphia, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method unclear. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double blind" with placebo control. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double blind" with placebo control. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Egarter 1989.
| Methods | Not stated. | |
| Participants | 345 women undergoing induction of labour. Inclusion criteria: singleton pregnancies, cephalic presentation, intact membranes, BS > 4. Exclusion criteria: any pregnancy carrying fetal or maternal risk factors. |
|
| Interventions | 3 mg PGE2 vaginal tablet (n = 180) with a repeat at 6 hours. If not given birth at 24 hours and cervix > 3 cm dilated, a repeat course was given. If < 3 cm dilated no further induction was undertaken. In control group (n = 165) spontaneous labour was awaited until 42 weeks amenorrhoea. |
|
| Outcomes | Spontaneous onset of labour, mode of delivery, use of oxytocin. Subgroup data by parity available on mode of delivery. |
|
| Notes | 8 women in active group refused induction and 3 in control group requested induction. Excluded from analysis. First Department of Obstetrics & Gynaecology, Vienna, Austria. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not clear. |
| Allocation concealment (selection bias) | Unclear risk | Method not clear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 11/345 participants did not want allocated treatment group and were excluded from analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Low risk | None. |
El Shawarby 2006.
| Methods | 'Randomised.' | |
| Participants | 100 women requiring induction of labour. Inclusion criteria: singleton pregnancy, cephalic presentation, greater than 37 weeks' gestation, unfavourable cervix (BS less 7), normal admission CTG. |
|
| Interventions | 10 mg sustained release pessary (n = 34), between 18:00 and 20:00 for primiparous women and at 08.00 the next morning for multiparous women. Repeat examinations at 12 hours, when further insert placed or amniotomy performed versus 1 mg prostin gel for multiparous or 2 mg for primiparous women (n = 38). Similar dosing times as for Propess group. | |
| Outcomes | Uterine hyperstimulation with and without FHR changes, caesarean section and instrumental vaginal delivery. | |
| Notes | Inpatients at Maidstone District Hospital, UK. 28 drop outs split evenly between both arms of trial, most were protocol violations. Impact examined in sensitivity analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given of method of randomisation. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding, active control group with different preparations. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were a large number of drop outs in both arms of the study (27 total). These do not appear to have a significant affect on the outcomes. Additional information was sought from the authors but no response received. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
El‐Mardi 1991.
| Methods | 'Randomly allocated.' | |
| Participants | 200 patients with medical indications for induction. Inclusion criteria: singleton pregnancy, cephalic presentation, unfavourable cervix (BS < 5). Indications for induction: post dates (76), high blood pressure (67), impaired glucose tolerance (19), others (38). |
|
| Interventions | 3 mg PGE2 vaginal pessary (4 x 0.75 mg) (n = 100) or 3 mg PGE2 vaginal tablet (n = 100). Single dose prior to amniotomy at 4 cm, oxytocin as required. |
|
| Outcomes | Successful induction, mode of delivery, delivery intervals, use of oxytocin, Apgar scores, hyperstimulation or maternal side‐effects. | |
| Notes | Maternity Hospital, Safat, Kuwait. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomly allocated" method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation method unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Low risk | None known. |
Ferraiolo 2010.
| Methods | Described as "open label, randomised, prospective study". allocation sequence is "year of birth" with even years assigned to group A, odd to group B initially but changing this every 5 cases. There was no allocation concealment (communication with author). This is a predictable assignment method so is inadequate. | |
| Participants | Outcomes reported on only 151 women. All primiparous, between 36‐42 weeks, with singleton cephalic presentations, intact membranes and a Bishop score less than 4. Excluded women with uterine scars, hypertension, asthma, anhydramnios, vaginal bleeding, pyrexia, glaucoma, signs of fetal distress or spontaneous labour. | |
| Interventions | Intravaginal PGE2 gel (n = 72) 1 mg gel up to 3 applications 6 hours apart, repeatable after a pause of 24 hours. 10 mg Intravaginal sustained release pessary (n = 79). Single application for up to 24 hours, releasing 0.3 mg per hour over 12 hours. |
|
| Outcomes | Caesarean section rate, cervix ripening achieved in less than 12 hours. (Use inverse), oxytocin not used (use inverse). Percentage of women needing epidural analgesia (converted to number), "wouldn't choose treatment again" (as percentage). | |
| Notes | San Martino University Hospital, Genoa, Italy. Jan 2007‐December 2008, 173 women enrolled, Primary purpose of study is to look at "satisfaction" as assessed by before ‐after IOL. Some effectiveness data given. Use proprietary names prepidil (Upjohn) and propess (Ferring pharmaceuticals) and state under financial disclosure " Authors have connection to any companies or products mentioned". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation sequence is "year of birth" with even years assigned to group A, odd to group B initially but changing this every 5 cases. This is could be predictable sequence. |
| Allocation concealment (selection bias) | High risk | Described as "open label, no method of concealment used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | None, open label. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | None, open label. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22 out of 173 didn't complete the questionnaire, no outcomes are reported for these women. All outcomes for remaining 151. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not reported. |
| Other bias | Unclear risk | Source of funding not reported. State under financial disclosure "Authors have connection to any companies or products mentioned". |
Glanville 2002.
| Methods | Computer‐generated randomisation sequence. Allocation concealment unclear. 2‐centre trial, randomisation stratified by centre. | |
| Participants | 200 women undergoing induction at term. Inclusion criteria: singleton, cephalic, greater than 37 completed weeks, unfavourable cervix (BS less than 7), parity less than 4, greater than 18 years of age. Intact membranes. Exclusion criteria: previous caesarean section or other uterine scar or cone biopsy of the cervix, multiple pregnancy, known sensitivity to prostaglandins. |
|
| Interventions | 10 mg sustained release pessary for 24 hours (n = 103), with possible repeat if cervix still unfavourable at 24 hours versus. 1 mg to 2 mg gel every 6 hours to a maximum of 3 insertions in 24 hours (n = 97), repeat after 24 hours if required. Maximum total dose 5 mg. | |
| Outcomes | Caesarean section, serious neonatal morbidity or mortality, serious maternal morbidity or mortality, oxytocin augmentation, uterine hyperstimulation without FHR changes, instrumental vaginal delivery. | |
| Notes | Some differences noted in outcome data between Birmingham and Leeds may be related to variation in in‐house policies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence, stratified by centre. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Suitable dummies were not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding, outcomes not subjective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No post randomisation exclusions. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | There were some differences between the outcomes reported between the 2 centres, which may reflect some bias or just the effect of different in‐house policies. |
Graves 1985.
| Methods | Randomised into groups of 20. | |
| Participants | 80 women requiring induction of labour. Inclusion criteria: singleton pregnancy, BS < 4, para 0‐3. Exclusion criteria: contraindication to vaginal delivery, asthma or prior hypersensitivity to prostaglandins, prior attempt at ripening or induction in index pregnancy, malpresentation or multiple pregnancy, intrauterine death, polyhydramnios, antepartum haemorrhage, SROM, uterine scar. Indications for induction: pre‐eclampsia (35), chronic hypertension (15), prolonged pregnancy (14), diabetes mellitus (6), IUGR (6), other (3). |
|
| Interventions | 1 mg (n = 20), 2 mg (n = 20) or 3 mg (n = 20) PGE2 vaginal gel or identical placebo (n = 20), inserted the evening prior to induction. Re‐examined after 12 to 16 hours, followed by oxytocin and amniotomy when 3 to 4 cm dilated. induction failure is LSCS prior to 5 cm or not in labour after 8 hours of oxytocin. | |
| Outcomes | Change in BS, Oxytocin requirement, mode of delivery, hyperstimulation, gastrointestinal side‐effects, Apgar scores at 1 and 5 minutes. | |
| Notes | 4‐arm trial. 3 PGE2 arms compared to placebo arm. Different dosages not compared as all 3 doses fell into low‐dose category. Dalhousie University and Grace Maternity Hospital, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating random sequence unclear. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealing allocation unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small RCT. |
Green 1998.
| Methods | 'Randomised.' | |
| Participants | 107 women with uncomplicated pregnancies, requiring induction of labour. Inclusion criteria: singleton pregnancy, BS < 6, greater than 36 weeks. |
|
| Interventions | 10 mg PGE2 vaginal pessary (sustained release, Propess) (n = 53) or 1 mg PGE2 vaginal gel (n = 54) which could be repeated after 6 hours. Baseline BS then reassessed after 12 hours. Subsequent management not specified. |
|
| Outcomes | Change in BS, induction to delivery interval, mode of delivery. | |
| Notes | Limited data as extracted from abstract. Leeds General Infirmary, UK. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomised.' |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT abstract only. |
Greer 1990.
| Methods | Randomised by random selection of envelopes. | |
| Participants | 42 women requiring induction of labour. Inclusion criteria: favourable cervix, no previous prostaglandin administration. Indications for induction: post term (20), hypertension (4). |
|
| Interventions | 3 mg PGE2 vaginal tablet (n = 12) or 1 mg PGE2 vaginal gel. Baseline BS prior to instillations with repeat examination 4 hours later. Forewater amniotomy performed at 4 hours and were augmented with escalating doses of oxytocin. |
|
| Outcomes | Endogenous prostaglandin levels, change in BS, delivery intervals, mode of delivery, mean Apgar scores, use of oxytocin. | |
| Notes | University of Edinburgh, UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "random selection of envelopes"‐ method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Unclear ‐"random selection of envelopes" but no further information reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Hage 1993.
| Methods | 'Double blind randomised trial.' | |
| Participants | 36 women with inducible cervices. Inclusion criteria: nulliparous women and BS < 9. |
|
| Interventions | 2.5 mg PGE2 vaginal gel (n = 18) or placebo (n = 18) with repeat at 24 hours (outpatient administration). | |
| Outcomes | Change in BS, length of first stage of labour, mode of delivery, hyperstimulation. | |
| Notes | Limited data available as extracted from abstract. Lutheran Medical Centre, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Double blind." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Double blind." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT, abstract only. |
Hannah 1996.
| Methods | Computer randomisation program. Allocation concealment by touch‐tone telephone access. Randomised to 4 groups (n = 5041), only 2 groups included here (n = 2522) ‐ immediate PGE2 vs expectant oxytocin. | |
| Participants | PROM, GA > 37 weeks, singleton, cephalic, no recent attempt at induction of labour. | |
| Interventions | Immediate 1‐2 mg vaginal PGE2 (n = 1259) repeated after PGE2 after 6 hrs if required and if not in labour, after 4 hrs or more given IV oxytocin versus expectant management (n = 1263) for up to 96 hours, with monitoring as inpatients or outpatients, induced with oxytocin . If complications, induced before. | |
| Outcomes | Time to active labour, time to ROM, time from ROM to delivery, caesarean section, perinatal death, uterine hyperstimulation, uterine rupture, epidural analgesia, instrumental vaginal delivery, meconium‐stained liquor, Apgar < 7 at 5 minutes, admission to NICU, maternal vomiting, maternal diarrhoea, PPH, women not satisfied, chorioamnionitis, maternal antibiotics, endometritis, neonatal infection, fetal distress, duration of hospital stay. | |
| Notes | 4‐arm trial (n = 5041). 2 immediate groups (oxytocin and prostaglandin) versus 2 expectant groups (oxytocin and prostaglandin) Only data relating to use of immediate vaginal PGE2 versus expectant oxytocin management are included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation program. |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding but objective outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding but objective outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported. |
| Other bias | Low risk | Large pragmatic, international RCT. |
Hayashi 1983.
| Methods | 'Assigned in random order.' | |
| Participants | 60 women requiring induction of labour. Indications for induction: depression (2), diabetes mellitus (13), oestradiol decrease (1), fetal abnormality (1), IUGR (1), hypertension (10), hypothyroidism (1), post maturity (31), pre‐eclampsia (20), other (1). |
|
| Interventions | 0.5 mg PGE2 (n = 15), or 1.0 mg PGE2 (n = 15) or 1.5 mg PGE2 (n = 15) vaginal gel or placebo gel (n = 15). Single dose administered following BS, then re‐examined 12 hours later. Subsequent management not specified. |
|
| Outcomes | Change in BS, delivery intervals, maternal side‐effects, mode of delivery, hyperstimulation, Apgar scores, meconium‐stained liquor. | |
| Notes | Unpublished trial. Intra‐prostaglandin comparison not presented as all 3 arms fall into 'low‐dose' category. University of Texas, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method unclear, reported as "assigned in random order". |
| Allocation concealment (selection bias) | Unclear risk | Method unclear, reported as "assigned in random order". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | High risk | Small, single centre RCT, "unpublished", poorly reported. |
Kalkat 2008.
| Methods | 'Randomly allocated'. | |
| Participants | 120 term women requiring induction of labour. No clear inclusion criteria stated. recruitment only included 'high‐risk' women as those deemed low risk were induced in the antenatal clinic and not through the delivery suite where the randomisation process occurred. Exclusion criteria: non‐vertex presentation, previous uterine scar. |
|
| Interventions | 10 mg sustained release pessary or vaginal PGE2 gel. 2 mg to primiparous women with initial BS of < 4, 1 mg to those primiparous women with BS > 4 or if multiparous. Doses were repeated every 6 hours up to maximum dose of 4 mg for primiparous and 3 mg for multiparous women. | |
| Outcomes | Vaginal delivery not achieved in 24 hours, uterine hyperstimulation with and without FHR changes, caesarean section, instrumental vaginal delivery rates, serious neonatal morbidity, maternal complications, syntocinon augmentation. | |
| Notes | Manor Hospital, Walsall, UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated ‐ but method not stated. |
| Allocation concealment (selection bias) | Low risk | Pre‐packed identical sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported. |
| Selective reporting (reporting bias) | Unclear risk | Unclear, study protocol/registration not available. |
| Other bias | Unclear risk | 14 women were excluded prior to randomisation who were eligible and the reasons for exclusion are not noted over and above 'consultant decision'. Source of funding not stated. Small single centre study. |
Liggins 1979.
| Methods | Coded drug boxes in batches of 15, randomisation schedule from random number tables. | |
| Participants | 84 women requiring induction of labour for major or minor complications of pregnancy. Tendency towards those considered unfavourable for surgical induction (high presenting part, low BS, previous failed induction of labour). |
|
| Interventions | 0.2 mg (n = 26) or 0.4 mg (n = 26) PGE2 vaginal suppositories or identical placebo (n = 32) placed at 09:00 then self administered repeat suppositories at 2‐hourly intervals. Rested overnight and continued until 15 suppositories used (maximum 3 mg or 6 mg) or labour ensued. If not in labour after 48 hours patients underwent induction by amniotomy and oxytocin. |
|
| Outcomes | Time to onset of labour, mode of delivery, change in BS, hyperstimulation, maternal side‐effects, meconium‐stained liquor, perinatal mortality. | |
| Notes | 3‐arm trial. Both PG arms compared to placebo. Inter‐prostaglandin arms not compared as both doses in low‐dose category. University of Auckland, NZ. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule from random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Coded drug boxes in batches of 15, (therefore last allocation potentially predictable), not clear if sequentially numbered and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
MacKenzie 1979.
| Methods | 'Randomly allocated.' | |
| Participants | 48 women requiring induction of labour. Inclusion criteria: unfavourable cervix (BS < 3), singleton pregnancy, cephalic presentation. |
|
| Interventions | 5 mg PGE2 vaginal gel (n = 16) or 25 mg PGF2a gel (n = 16) or placebo gel (n = 16). BS assigned prior to instillation, re‐examined 12 to 16 hours later. If not in labour induction with amniotomy and oxytocin. |
|
| Outcomes | Change in BS, length of labour, oxytocin use, epidural anaesthesia, Apgar scores, mode of delivery. | |
| Notes | 3‐arm trial. John Radcliffe Hospital, UK. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly allocated' but method unclear. |
| Allocation concealment (selection bias) | Unclear risk | 'Randomly allocated' but method unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
MacKenzie 1981.
| Methods | 'Double blind.' | |
| Participants | 42 women requiring induction of labour. Inclusion criteria: multigravid, singleton pregnancy, cephalic presentation, favourable cervix (BS > 5). |
|
| Interventions | 2.5 mg PGE2 vaginal suppository (n = 21) or identical placebo (n = 21). Baseline BS prior to instillation at 0600 then amniotomy at 09:00 and oxytocin administered at 14:00. |
|
| Outcomes | Delivery intervals, oxytocin use, Apgar score at 1 minute. | |
| Notes | 2 trials reported second study non‐randomised hence not reported. John Radcliffe Hospital, UK. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method unclear. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 'Double blind.' |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | 'Double blind.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
MacKenzie 1997a.
| Methods | Open randomised parallel group design. Computer‐generated random numbers in blocks of 10. Allocation in opaque sealed envelopes. | |
| Participants | 955 women requiring induction of labour. Inclusion criteria: BS < 8, singleton pregnancy, cephalic presentation. Exclusion criteria: previous caesarean section. Indication for induction: post term (653), hypertensive states (148), fetal concerns (53), maternal health concerns (8), maternal request (78), past obstetric history (15). |
|
| Interventions | 2 mg PGE2 vaginal gel once only (n = 483) or repeated dose (n = 472). | |
| Outcomes | Need for amniotomy prior to labour, oxytocin use, epidural analgesia, fetal blood sampling rates, meconium‐stained liquor, mode of delivery, delivery interval, postpartum haemorrhage, Apgar scores, NICU admission. | |
| Notes | John Radcliffe Hospital, UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers, in blocks of 10. |
| Allocation concealment (selection bias) | Unclear risk | Used "opaque sealed envelopes" but randomisation in blocks of 10, so every 10th allocation potentially predictable. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding "open RCT". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding, may impact on subjective outcomes ‐ e.g. analgesia. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Single centre RCT. |
MacLennan 1979.
| Methods | Randomised double blind placebo controlled. | |
| Participants | Queen Victoria Hospital, Adelaide, South Australia. 80 women with singleton cephalic pregnancy. All over 150 cm tall. | |
| Interventions | PGF2α 50 mg in 10 mL of sterile 8% methylcellulose gel (n = 40) vs placebo (water in 8% methylcellulose gel)(n = 40) via a catheter placed in the posterior vaginal fornix. All women had a cervical sweep and stretch at the time of inserting the liquid. | |
| Outcomes | Length of labour (Mean only), caesarean section, cervix unfavourable after 12‐24 hrs, oxytocin augmentation, epidural analgesia, instrumental vaginal delivery, delivery in under 6 hrs, delivered by 15 hrs, normal delivery, number in labour without further treatment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables used to generate random sequence. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and assessors blind to treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessor blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear. Study protocol not available. |
| Other bias | Unclear risk | Unclear ‐ source of funding not stated, duration of recruitment not stated. Small single centre RCT. |
MacLennan 1980.
| Methods | Random number tables and allocated by sealed envelopes. | |
| Participants | 90 women requiring induction of labour. Inclusion criteria: singleton pregnancy, cephalic presentation, unscarred uterus, maternal height over 150 cm, no history of asthma. |
|
| Interventions | 50 mg PGF2a vaginal gel (n = 30), 25 mg PGF2a vaginal gel (n = 30), or placebo gel (n = 30). Instillation following cervical assessment (modified BS). Reassessed the next morning, subsequent management at obstetrician's discretion. |
|
| Outcomes | Delivery intervals, oxytocin use, mode of delivery, epidural anaesthesia, Apgar scores at 1 and 5 minutes, maternal side‐effects, change in BS. | |
| Notes | 3‐arm trial both active arms combined in analysis and compared to placebo. University of Adelaide, Australia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Unclear risk | Allocated by sealed envelopes, not stated if sequentially numbered, opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Mahmood 1989.
| Methods | Allocation by sealed opaque randomised envelopes. | |
| Participants | 80 women requiring induction of labour. Inclusion criteria: primigravidae, singleton pregnancy, cephalic presentation, unfavourable cervix (BS < 5). Indications for induction: post dates (49), moderate‐severe pre‐eclampsia (26), others (5). |
|
| Interventions | 2 mg PGE2 vaginal gel (n = 40) or 3 mg PGE2 vaginal tablet (n = 40). Baseline BS prior to instillation at 17:00, then reassessed at 09:00. If cervical score > 5 then amniotomy performed. If < 5 repeat instillation. Further reassessment at 17:00 if BS still < 5 then no action for 24 hours. Last assessment at 48 hours since first instillation if cervical score still < 5 then final application made. In total 4 possible applications. Oxytocin started 2 hours after amniotomy in cases where needed. |
|
| Outcomes | Number of applications, change in BS, delivery intervals, oxytocin use, mode of delivery, Apgar scores, postpartum haemorrhage. | |
| Notes | Raigmore Hospital, Scotland, UK. October 1986 to July 1987. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation by sealed opaque randomised envelopes, not stated if sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Mahmood 1992.
| Methods | Randomised by numbered, sealed envelope. | |
| Participants | 220 women with SROM. Inclusion criteria: primigravidae, singleton pregnancies, cephalic presentation, no uterine activity, confirmed SROM. Exclusion criteria: no significant antepartum haemorrhage, IUGR, diabetes mellitus, rhesus disease, moderate pre‐eclampsia, history of venereal disease, temperature of > 37.5C, ruptured membranes > 12 hours or meconium‐stained amniotic fluid on admission. |
|
| Interventions | 2 mg PGE2 vaginal gel (n = 110) with a repeat treatment of 1 mg PGE2 gel at 6 hours if no uterine activity. Oxytocin administered 24 hours after admission if labour had not begun. Conservative group (n = 110) received oxytocin at 24 hours after admission if labour did not ensue. |
|
| Outcomes | Time from admission to onset of labour or delivery, mode of delivery, oxytocin augmentation, epidural anaesthesia, maternal side‐effects, maternal and neonatal infection rates, Apgar scores at 1 and 5 minutes, NICU admission rates. | |
| Notes | Aberdeen Maternity Hospital, Scotland, UK. January 1988 to May 1990. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Numbered, sealed envelopes, not stated if opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Single centre RCT. |
Mahmood 1995.
| Methods | Randomised by using random number list. individual instructions stored in separate envelopes. | |
| Participants | 100 women with SROM. Inclusion criteria: primigravidae, singleton pregnancies, uncomplicated, cephalic presentation, no uterine activity, confirmed SROM. Exclusion criteria: no significant previous antepartum haemorrhage, IUGR, diabetes mellitus, rhesus disease, moderate pre‐eclampsia, history of venereal disease, temperature of > 37.5C, ruptured membranes > 12 hours or meconium‐stained amniotic fluid on admission. |
|
| Interventions | 1 mg PGE2 vaginal gel (n = 50) with a repeat treatment of 1 mg PGE2 gel at 6 hours if no uterine activity. Oxytocin administered 24 hours after admission if labour had not begun. Conservative group (n = 50) remained in the observation ward for 24 hours after admission if labour did not ensue within 24 hours they were treated with IV oxytocin. |
|
| Outcomes | Time from admission to onset of labour or delivery, mode of delivery, oxytocin augmentation, maternal side‐effects, maternal and neonatal infection rates, Apgar scores at 1 and 5 minutes, NICU admission rates, meconium‐stained liquor and postpartum haemorrhage. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number tables. |
| Allocation concealment (selection bias) | Unclear risk | Separate envelopes. Not clear if opaque and sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Unclear which outcomes prespecified. |
| Other bias | Low risk | Small, single centre RCT. |
McCaul 1997.
| Methods | Computer‐generated random‐number tables, with centralised pharmacy allocation. | |
| Participants | 91 women requiring induction of labour. Inclusion criteria: ruptured membranes of less than 24 hours duration, cervix less than 3 cm dilated, < 75% effaced, cephalic presentation, singleton pregnancy, aged 16 to 35 years of age. Exclusion criteria: clinical evidence of chorioamnionitis, antibiotic therapy, regular uterine contractions, meconium‐stained liquor, fetal anomalies, uterine scar, glucocorticoid therapy, active genital herpes, hypertension, diabetes mellitus or placental abruption. |
|
| Interventions | 2 mg PGE2 vaginal gel (n = 35) placed 4 hours after SROM. After 4 additional hours a further 2 mg dose was placed with 2 subsequent doses 6 hours apart, unless in active labour or the cervix > 4 cm dilated. Oxytocin was started 22 hours after the start of therapy. Expectant management group (n = 31), daily NST. Evidence of fetal compromise or chorioamnionitis resulted in induction +/‐ antibiotic therapy. |
|
| Outcomes | Length of first and second stages of labour, mode of delivery, blood loss, maternal fever, 5 minute Apgar, neonatal stay, birth weight. | |
| Notes | 3‐arm trial with additional arm managed with IV oxytocin (n = 25) 4 hours after SROM. These data are analysed in the review focusing on oxytocin alone. 5 patients excluded from analysis (placebo arm) who refused expectant management. University of Mississippi Medical Centre, Jackson, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random‐number tables. |
| Allocation concealment (selection bias) | Low risk | Centralised pharmacy allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
McLaren 1987.
| Methods | 'Randomised.' | |
| Participants | 24 women requiring induction of labour. Indications for induction: prolonged pregnancy (19), pre‐eclampsia (2), IUGR (1), other (2). |
|
| Interventions | 3 mg PGE2 vaginal tablet (n = 12) or 5 mg PGE2 pessary (n = 12). Baseline cervical assessment prior to instillation then reassessment at 4 hours followed by amniotomy and oxytocin if required. |
|
| Outcomes | PGE2 plasma levels, oxytocin use, mode of delivery, analgesic use. | |
| Notes | Glasgow Royal Infirmary, UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Miller 1991.
| Methods | 'Randomised.' | |
| Participants | 40 women requiring induction of labour. Inclusion criteria: singleton pregnancies, BS < 4, uterine activity less than 1 contraction per 10 minutes, greater than 36 weeks. Indications for induction: postdates, hypertension, diabetes and suspected IUGR. |
|
| Interventions | 10 mg PGE2 vaginal pessary (sustained release) (n = 20) or 2.5 mg PGE2 vaginal gel (n = 20). Subsequent management unclear. |
|
| Outcomes | Uterine activity, change in BS, delivery intervals, oxytocin use, mode of delivery, hyperstimulation, neonatal outcomes. | |
| Notes | University of Nebraska College of Medicine, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised" but method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Mukhopadhyay 2002.
| Methods | 'Randomised basis'. Sealed envelopes. | |
| Participants | 95 primiparous women requiring induction of labour. Inclusions criteria: singleton, cephalic, primiparous and BS of less than 6. |
|
| Interventions | 2 mg PGE2 vaginal gel (n = 50) or 10 mg PGE2 vaginal insert (n = 45). Both repeated at 12 hours as necessary. |
|
| Outcomes | Uterine hyperstimulation, epidural analgesia. | |
| Notes | Jessop Hospital for Women, UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised basis". Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Only mentions that envelopes are sealed, not reported if opaque, sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 women were withdrawn from the study following randomisation. All 4 in the vaginal insert arm. 2 who were suitable for amniotomy and 2 requested removal of the insert due to discomfort. Outcome data for these patients were not reported. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Murphy 1980.
| Methods | 'Double blind trial.' | |
| Participants | 265 women requiring induction of labour. | |
| Interventions | 1.5 mg (n = 55) or 3.0 mg (n = 55) or 10 mg (n = 55) PGF2a vaginal gel or placebo gel (n = 100). Gel instilled following BS, re‐examined the following morning prior to amniotomy. |
|
| Outcomes | Change in BS, mode of delivery, epidural anaesthesia, Apgar at 1 minute, spontaneous labour, postpartum haemorrhage. | |
| Notes | All 3 active arms compared to placebo. Royal Women's Hospital, Melbourne, Australia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of concealing allocation not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Murray 1995.
| Methods | Allocation by randomly mixed sealed envelopes. | |
| Participants | 200 women requiring induction of labour. Inclusion criteria: nulliparity, singleton pregnancy, cephalic presentation, BS < 7, intact membranes, no evidence of labour, no previous induction attempt, normal NST, need for delivery within 48 hours. Indications for induction: proteinuric hypertension (68), post dates and oligohydramnios (66), gestational hypertension (26), IUGR (19), maternal disease (21). |
|
| Interventions | 2 mg PGE2 vaginal gel (n = 101) or 3 mg PGE2 vaginal tablet (n = 99). Instillation following baseline BS at 09:00. Reassessed 6 to 8 hours later; if amniotomy not possible, second dose of PG given. Final review at 09:00 on D2; if amniotomy still not possible then third dose of PG given. Following amniotomy, if no evidence of spontaneous labour within 4 hours escalating doses of oxytocin used. |
|
| Outcomes | Delivery intervals, total dose of PG used, analgesic use, mode of delivery, neonatal welfare, hyperstimulation. | |
| Notes | 1 patient excluded post randomisation for hypersensitivity reaction. Wellington Women's Hospital, New Zealand. 1991 to 1994. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | "Allocation by randomly mixed sealed envelopes" not stated if sequentially numbered and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only 1 patient excluded post randomisation, no other loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Neilson 1983.
| Methods | Central pharmacy randomisation with coded drug syringes. | |
| Participants | 75 women requiring induction of labour. Inclusion criteria: unfavourable cervix. |
|
| Interventions | 5 mg PGE2 vaginal gel (n = 38) or 40 mg PGF2a vaginal gel (n = 37). Baseline BS prior to instillation. Re‐examined the following morning prior to amniotomy. |
|
| Outcomes | Change in BS, mode of delivery, vaginal delivery not achieved in 24 hrs, Apgar score at 5 minutes, maternal side‐effects. | |
| Notes | Women's Clinic and Emanuel Hospital, Oregon, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Low risk | Central pharmacy randomisation with coded drug syringes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Newman 1997.
| Methods | 'Prospectively randomised.' | |
| Participants | 58 women requiring induction for post dates pregnancy, or gestational diabetes. Inclusion criteria: unfavourable cervix (BS < 7). |
|
| Interventions | 2 mg PGE2 vaginally (n = 28) followed by repeat doses at 24 and 48 hours. Control group managed expectantly (n = 30) until 44 weeks or if non‐reassuring NST or favourable cervix (BS > 7). |
|
| Outcomes | Rate of spontaneous labour, delivery intervals, mode of delivery, hyperstimulation, neonatal outcomes. | |
| Notes | Limited data available as extracted from abstract. Medical University of South Carolina, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospectively randomised but method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Prospectively randomised but method of concealment of allocation not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT, abstract only. |
Nuutila 1996.
| Methods | Randomisation by sealed opaque envelopes. | |
| Participants | 71 women requiring induction of labour. Inclusion criteria: high‐risk pregnancy, unfavourable cervix (BS < 5), singleton pregnancies, vertex presentation, intact membranes. Exclusion criteria: haemorrhage, asthma, glaucoma, hypersensitivity to prostaglandins. Indications for induction: pre‐eclampsia (23), post dates (19), oligohydramnios (7), diabetes mellitus (9), IUGR (5), macrosomia (2), obstetric cholestasis (2), rhesus disease (1), maternal exhaustion (3). |
|
| Interventions | 1 mg PGE2 vaginal gel (n = 35), or 2 mg PGE2 gel (n = 36) or 0.5 mg PGE2 intracervical gel. Baseline BS prior to application. Gels reapplied maximally twice at 6‐hourly intervals with repeat Bishop scoring. If BS > 5 but no regular contractions, amniotomy +/‐ oxytocin started. If BS < 5 after 18 hours/3 gels then LSCS performed for failed induction. |
|
| Outcomes | Number of gel applications, delivery intervals, mode of delivery, hyperstimulation, maternal side‐effects, neonatal outcomes. | |
| Notes | 3‐arm trial with 110 women in 3 groups ‐ intracervical PGE2 0.5 mg arm (n = 39) not included in this review but is in the review of intracervical prostaglandins. Helsinki University Hospital, Finland. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes used to conceal allocation but not reported if envelopes were opaque or sequentially numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
O'Brien 1995.
| Methods | Randomisation using random number tables using permuted blocks of varying length. Central pharmacy allocation. | |
| Participants | 100 women requiring induction of labour. Inclusion criteria: BS < 6, absence of medical indication for induction, no more than 1 previous caesarean section. Exclusion criteria: non‐reassuring NST, macrosomia, IUGR, oligohydramnios (AFI < 5). |
|
| Interventions | 2 mg PGE2 vaginally (n = 50) or identical placebo (n = 50) every day for 5 consecutive days (outpatient administration). | |
| Outcomes | Hyperstimulation, time interval to spontaneous labour and delivery, mode of delivery, meconium staining of liquor, epidural anaesthesia, NICU admission and 5 minute Apgar < 7. | |
| Notes | University of Tennessee, USA. June 1993 to June 1994. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using random number tables using permuted blocks of varying length. |
| Allocation concealment (selection bias) | Low risk | Central pharmacy allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Ohel 1996.
| Methods | Allocation on case number. Concealment unclear. | |
| Participants | 200 women with post dates pregnancies requiring induction of labour. Inclusion criteria: singleton pregnancies. |
|
| Interventions | 3 mg PGE2 vaginal tablet (n = 70) followed by repeat treatment within 3 to 4 days. Expectant group (n = 104) seen twice weekly until induction at 42 weeks. (outpatient administration). | |
| Outcomes | Delivery intervals, mode of delivery, Apgar score at 5 minutes, meconium‐stained liquor. | |
| Notes | 26 women randomised to treatment arm wished expectant management but are excluded from analysis. University of Tel Aviv, Israel. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Allocation on case number. |
| Allocation concealment (selection bias) | High risk | Concealment unclear.. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 200 women recruited, outcomes reported for 174 (13% loss to follow‐up). |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Payne 1993.
| Methods | Monthly alternation between 2 regimens. | |
| Participants | 200 women requiring induction of labour. Inclusion criteria: singleton pregnancy, cephalic presentation. Exclusion criteria: multiple pregnancy, history of sensitivity to prostaglandins, history of asthma, history of glaucoma, ruptured membranes, previous uterine surgery, grand multiparae, history of precipitate labour, any induction for social reasons. |
|
| Interventions | 3 mg PGE2 vaginal tablets (n = 106) plus a further 3 mg if needed 4 hours later. Or 1 mg PGE2 vaginal gel (n = 94) followed by 2 mg 4 hours later if needed. If no labour after further 4 hours amniotomy undertaken and oxytocin commenced where required. |
|
| Outcomes | Delivery intervals, analgesia used, mode of delivery, postpartum haemorrhage, patient acceptability,. | |
| Notes | Coventry Maternity Hospital and Dudley Road Hospital Birmingham, UK. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Monthly alternation between 2 regimens ‐ not random as predictable. |
| Allocation concealment (selection bias) | High risk | Monthly alternation between 2 regimens ‐ predictable. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Perryman 1992.
| Methods | Central pharmacy randomisation using random number tables. | |
| Participants | 90 women admitted for induction of labour. Inclusion criteria: intact membranes, reactive NST, BS < 5, fewer than 8 contractions per hour. Indications for induction: post dates (23), hypertension (35), diabetes mellitus (8), IUGR (10), macrosomia (6), Rh sensitisation (2), non‐reassuring surveillance (6). |
|
| Interventions | 5 mg PGE2 vaginal gel (n = 45) or 5 mg PGE2 vaginal suppository (n = 45). Instillation following baseline BS, repeat treatment if still met inclusion criteria at 6 hours. If no labour by the following morning oxytocin started. |
|
| Outcomes | Change in BS, number of treatments required, delivery intervals, spontaneous labour, mode of delivery, hyperstimulation. | |
| Notes | Original trial planned for 120 patients, but trial stopped after 90 recruited due to high rates of hyperstimulation in suppository group. St Luke's Perinatal Centre, Missouri, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Central pharmacy randomisation using random number tables. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT, trial stopped early. |
Poornima 2011.
| Methods | This is a prospective study. "....Women were then randomly allotted to either immediate induction group or expectant management group.” | |
| Participants | 100 women with singleton pregnancy more than 36 weeks' pregnant with ruptured membranes, (confirmed on speculum examination). | |
| Interventions | Intervention (n = 50): 0.5 mg PGE2 gel, repeated after 6 hrs if not in labour. If no cervical change at 12 hrs labelled as “failed” if BS > 2 more oxytocin started. Control (n = 50): expectant management for 12 hrs, then oxytocin started. |
|
| Outcomes | Caesarean section, time taken to 3 cm (mean +/‐SD), in active labour and until delivery, need for oxytocin augmentation, fetal distress, analgesia, postpartum fever, Apgar < 7 at 5 minutes, neonatal infection, feeding problems, NICU admission. | |
| Notes | Hospitals attached to J. J. M. Medical College (Bapuji Hospital, Chigateri General Hospital, Women and Child Hospital), India from September 2006 to May 2008. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Women were then randomly allotted", method of random sequence generation not clear. |
| Allocation concealment (selection bias) | Unclear risk | No mention of method if any of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | None reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | None reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None reported. |
| Selective reporting (reporting bias) | Unclear risk | Trial registration not reported. |
| Other bias | Unclear risk | Small, single centre RCT, Source of funding not reported. |
Prasad 1989.
| Methods | 'Random allocation.' | |
| Participants | 69 women requiring induction of labour. Inclusion criteria: primiparous, cephalic presentation, BS < 5. |
|
| Interventions | PGE2 vaginal film (8.5 mg in 24 hours) (n = 33) or identical placebo (n = 36). baseline BS and repeat at 12 and 24 hours. | |
| Outcomes | Change in BS, mode of delivery, NICU admission rates and Apgar scores. | |
| Notes | National University Hospital, Singapore. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Random allocation.' Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding but "identical placebo used". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Prins 1983.
| Methods | Central randomisation by pharmacy with coded syringes. | |
| Participants | 30 patients requiring induction labour. Inclusion criteria: BS < 4. Indications for induction: post dates (20), infant large for gestational age (10), pre‐eclampsia (5), diabetes (1), anencephaly (1). |
|
| Interventions | 2.5 mg PGE2 vaginal gel (n = 15) or identical placebo (n = 15). BS assigned at instillation and the following morning prior to commencement of oxytocin. |
|
| Outcomes | Spontaneous labour, change in BS, mode of delivery, 5 minute Apgar < 6, maternal morbidity and perinatal death. | |
| Notes | 1 perinatal death in experimental group in anencephalic pregnancy excluded from analysis. Report included data on 2 further trials neither of which included a control arm hence data not included. Oregon Health Sciences University, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Low risk | Central randomisation by pharmacy with coded syringes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Rabl 2002.
| Methods | 'Randomised observational study.' | |
| Participants | 200 women requiring induction of labour. Inclusion criteria: singleton, cephalic and > 36 weeks' gestation. Some patients had had previous caesarean sections. Exclusion criteria: IOL for abnormal FHR, oxytocin challenge tests or doppler results. |
|
| Interventions | 3 mg PGE2 vaginal tablet (n = 100) or 10 mg PGE2 vaginal sustained release pessary. Tablets repeated at 6‐hourly interval, maximum of 2 doses. |
|
| Outcomes | Vaginal delivery not achieved in 24 hours, uterine hyperstimulation, caesarean section, oxytocin augmentation, uterine rupture, instrumental vaginal delivery, Apgar score < 7 at 5 minutes. | |
| Notes | University of Vienna, Austria. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised observational study." Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Single centre RCT. |
Rath 1999.
| Methods | Method of generation "Stratified block randomisation". Blinding: unclear. 1001 patients were enrolled in 8 hospitals over 27 months. Information on 204 women is not reported because "data was not‐analysable". 2 arms of RCT are included here. Follow‐up: 2 women were excluded. | |
| Participants | 796 women in RCT‐ women with a BS 5‐7 (n = 326) randomly assigned to PGE2 Gel or tablet and are included in this review. Inclusion criteria: BS 5‐7, singleton, cephalic, live fetus, ruptured membranes after 34 weeks, pregnancy more than 40 +10 days, other maternal medical or fetal reasons for IOL. Exclusion criteria: malpresentations,previous classical caesarean section, multiple births and uterine anomalies. | |
| Interventions | 2 mg PGE2 gel versus 3 mg PGE2 tablet, repeated after 6‐8 hrs, a maximum of 3 doses until BS > 7, then oxytocin used. The applications were repeated at intervals of 6 hours if necessary. | |
| Outcomes | Women: vaginal delivery not achieved within 24 hours caesarean section. Fetal/infant: no outcomes reported. | |
| Notes | The papers are written in German and were translated for this review. Original RCT 1001 women, 204 lost to follow‐up, 326 women randomised to gel or tablet included in this review, 470 women with a BS 3‐4 were randomly assigned to either intracervical PGE2 0.5 mg or 2 mg PGE2 vaginal gel. This arm is included in the intracervical PG review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "stratified block randomisation" by Cologne Institute of Medical Statistics. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1001 women enrolled, 205 not included in report as "data not analysable", so 20% women lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | 20% participants lost to follow‐up. |
| Other bias | Unclear risk | Source of funding unclear. |
Rayburn 1988.
| Methods | Random drawing of cards kept in pharmacy. | |
| Participants | 118 women requiring induction of labour. Inclusion criteria: singleton pregnancies, unfavourable cervix (BS < 5). |
|
| Interventions | 2.5 mg PGE2 vaginal gel (n = 55) or identical placebo (n = 63). Baseline BS prior to instillation. Repeat BS prior to induction with oxytocin 12 hours after instillation. |
|
| Outcomes | Change in BS, oxytocin use, duration of labour, mode of delivery, Apgar scores and meconium staining of the liquor. | |
| Notes | University of Nebraska Medical Centre, USA. December 1985 to February 1987. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random drawing of cards kept in pharmacy. |
| Allocation concealment (selection bias) | Low risk | Randomisation in pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding reported but identical placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding reported but identical placebo used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Rayburn 1992.
| Methods | Centralised computerised randomisation and allocation. Stratified by parity. |
|
| Participants | 215 women requiring induction of labour. Inclusion criteria: BS < 4, singleton pregnancies, cephalic presentation. Exclusion criteria: previous uterine scar, vaginal bleeding, ruptured membranes, asthma or glaucoma, grand multiparity, nonreassuring FHR test. |
|
| Interventions | 10 mg PGE2 vaginal pessary (0.8 mg/hr) (n = 114) or identical placebo (n = 101). Baseline BS at insertion followed by repeat examinations at 6 and 12 hours. Pessary removed at 12 hours. |
|
| Outcomes | Change in BS, hyperstimulation, adverse reactions, need for oxytocin, mode of delivery, postpartum haemorrhage and 5‐minute Apgar score < 7. | |
| Notes | 81 patients in placebo arm crossed over into active treatment arm after initial period. Data for all outcomes reported separately for first 12‐hour period. Multicentre trial, USA. October 1989 to July 1990. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised computerised randomisation. Stratified by parity. |
| Allocation concealment (selection bias) | Low risk | Centralised allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding reported but control is identical placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small study but stated as multicentre, source of funding not reported. |
Roach 1997.
| Methods | Randomised by opening sealed numbered envelope. | |
| Participants | 201 women undelivered at 41 weeks. Exclusion criteria: pre‐eclampsia, gestational diabetes, contraindication to vaginal delivery, placenta praevia, non‐cephalic presentation, evidence of maternal or fetal compromise. |
|
| Interventions | 3 mg PGE2 vaginal pessary (n = 96) 6‐hourly as necessary or expectant management (n = 105) with twice‐weekly assessments. | |
| Outcomes | Spontaneous labour, mode of delivery, Apgar scores, cord blood pH, NICU admission, perinatal mortality. | |
| Notes | Prince of Wales Hospital, Hong Kong. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Sealed numbered envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Sawai 1991.
| Methods | Randomised by drawing of envelopes. | |
| Participants | 50 women requiring induction of labour. Inclusion criteria: unfavourable cervix (BS < 9). Exclusion criteria: diabetes, hypertension, previous uterine surgery, abnormal FHR tracings, vaginal bleeding, SROM, regular uterine contractions, non‐vertex presentation, macrosomia, IUGR, oligohydramnios or multiple gestations. |
|
| Interventions | 2 mg PGE2 vaginal gel (n = 24) or identical placebo (n = 26). Instillation following assignment of BS, repeat treatments and assessments twice weekly (outpatient administration). Induction with oxytocin at 44 weeks if needed. |
|
| Outcomes | Number of gel applications, change in BS, mode of delivery, oxytocin use, hyperstimulation, meconium staining, Apgar scores, cord pH, NICU admission. | |
| Notes | University of South Florida College of Medicine, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised by drawing of envelopes", method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | "Randomised by drawing of envelopes", Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding but "identical placebo used". |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT, source of funding not clear. |
Sawai 1994.
| Methods | Randomised by computer‐generated lists. Concealment unclear. | |
| Participants | 91 women requiring induction of labour. Inclusion criteria: BS < 9. Exclusion criteria: maternal medical problems, previous uterine surgery, previous stillbirth, abnormal FHR, vaginal bleeding, SROM, regular uterine contractions, abnormal ultrasound findings, fetal weight > 4500 g, non‐reactive NST. |
|
| Interventions | 2 mg PGE2 vaginal suppositories (n = 38) or identical placebo (n = 42) daily (outpatient administration). Twice‐weekly assessments until 44 weeks. |
|
| Outcomes | Spontaneous labour, SROM, number of suppositories used, oxytocin use, mode of delivery, Apgar scores, umbilical artery pH, presence of meconium, NICU admission. | |
| Notes | University of South Florida College of Medicine, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer‐generated lists. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding but identical placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT, source of funding for trial not clear. |
Shoaib 1994.
| Methods | 'Allocated at random selection.' | |
| Participants | 200 primigravid women. Inclusion criteria: primigravid, singleton, ruptured membranes and BS < 4. Exclusion criteria: meconium‐stained liquor. |
|
| Interventions | 3 mg PGE2 vaginal tablets repeat after 6 hours, maximum of 3 versus conservative management (n = 100) no details of conservative management given. | |
| Outcomes | Caesarean section, epidural analgesia, instrumental vaginal delivery, meconium‐stained liquor, perinatal death, postpartum haemorrhage. | |
| Notes | Allama Iqbal Medical School, Lahore, Pakistan. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Allocated at random selection' but Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | 'Allocated at random selection' but Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Smith 1990.
| Methods | Central pharmacy allocation using random number tables. | |
| Participants | 69 women requiring induction of labour. Inclusion criteria: unfavourable cervix (BS < 4), intact membranes, cephalic presentation, reactive NST and normal AFI. |
|
| Interventions | 2.5 mg PGE2 vaginal gel (n = 34) with placebo 'chip' vs 3‐3.5 mg PGE2 vaginal chips (n = 35) with placebo gel. Re‐examined after 12 hours and oxytocin started if not in labour, amniotomy performed in labour. |
|
| Outcomes | Change in BS, spontaneous labour, oxytocin use, hyperstimulation, mode of delivery, maternal side‐effects, | |
| Notes | University of Nebraska Medical School, USA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables. |
| Allocation concealment (selection bias) | Low risk | Central pharmacy allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding but identical placebo's used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT, source of funding not reported. |
Smith 1994.
| Methods | Computer‐generated random number tables, central pharmacy preparation. | |
| Participants | 121 women with medical or obstetric indications for induction. Inclusion criteria: intact membranes, unfavourable cervix (BS < 4), cephalic presentation, no spontaneous contractions, reactive NST. |
|
| Interventions | 10 mg PGE2 vaginal pessary (sustained release) (n = 66) or 2.5 mg PGE2 vaginal gel (n = 55). Baseline BS, vaginal gel given up to 2 times during 12‐hour study period. Oxytocin commenced at end of 12 hours if not in labour. |
|
| Outcomes | Change in BS, spontaneous labour rates, oxytocin use, hyperstimulation, mode of delivery, Apgar scores and umbilical artery pH measurements. | |
| Notes | There is a significant difference between the gestational age between the gel and pessary groups where the group receiving gel having a lower gestational age which may disadvantage this group. Also this study generated significant heterogeneity within the results, which could not be explained by the trial conduct alone. University of Nebraska Medical Centre, USA. August 1990 to July 1991. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence. |
| Allocation concealment (selection bias) | Unclear risk | Centrally dispensed, coded drug boxes. There is an imbalance between the groups (which can arise by chance). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding, placebos were not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | High risk | Small, single centre RCT, source of funding not stated. Imbalance in gestational age between the groups, with a significant difference between the gestational age between the gel and pessary groups where the group receiving gel having a lower gestational age which may disadvantage this group. This study generated significant heterogeneity within the results, the reasons for this, are not clear. |
Stampe Sorensen 1992.
| Methods | 'Randomly allocated.' Case‐controlled for parity. | |
| Participants | 267 women requiring induction of labour. Exclusion criteria: breech presentation, haemorrhage, fetal distress, glaucoma. Indications for induction: placental insufficiency (78), pre‐eclampsia (59), pregnancy discomfort (54), prolonged pregnancy (24), rhesus disease (18), others (34). |
|
| Interventions | 3 mg PGE2 vaginal tablet (n = 135) or 3 mg PGE2 vaginal pessary (n = 132). Following baseline BS tablet group received a tablet and a repeat if needed at 6 hours. In pessary group no repeat given. Amniotomy performed at 3 cm with oxytocin as needed. |
|
| Outcomes | Successful induction, oxytocin use, delivery intervals, mode of delivery, postpartum haemorrhage, Apgar scores. | |
| Notes | Multicentre trial, Denmark. October 1987 to January 1989. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly allocated', Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Low risk | Multicentre trial. |
Taher 2011.
| Methods | Computer‐generated random sequence, variable size blocks, stratified for parity. | |
| Participants | Pregnant women with a singleton cephalic presentation after 37 weeks (n = 165). Women with previous caesarean section were excluded. | |
| Interventions | 3 mg PGE2 tablet, repeat at 6 hr, maximum 3 doses until BS > 8 (n = 83) vs PGE2 gel 1‐2 mg (n = 82). 2mg if nulliparous with unfavourable cervix, 1 mg for multiparous women and nulliparous women with favourable cervix given. Repeat 1 mg at 6 hrs and 12 hours if BS > 8. | |
| Outcomes | Time to vaginal delivery (median & IQR), caesarean section, cervix unfavourable or unchanged after 12‐24 hrs, oxytocin augmentation, epidural analgesia, instrumental vaginal delivery, meconium‐stained liquor, Apgar score < 7 at 5 mins, NICU admission, postpartum haemorrhage, uterine hyperstimulation (FHR not specified). | |
| Notes | University Maternity Hospital London, April 2005‐December 2006. Information re: delivery within 24 hrs provided for review by authors. Economic analysis in separate publication. Trial registration: ISRCTN78483537. Clinical trial authorization CTA/MHRA no.: 13690/0212/001‐0001. EudraCT no.: 2004‐003797‐28. Funded by Hammersmith NHS Trust and 1 author funded by NIHR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence using variable blocks and stratification for parity. |
| Allocation concealment (selection bias) | Low risk | Sealed, sequentially numbered identical envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded, probably not important and pragmatic decision. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded, most outcomes objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Protocols and registration accessible. |
| Other bias | Low risk | Source of funding given. |
Thiery 1984.
| Methods | 'Random assignment.' | |
| Participants | 121 women requiring induction of labour, 81 women relevant to this review. Inclusion criteria: singleton pregnancy, cephalic presentation, intact uterus, no contraindications for labour induction or vaginal delivery, not in labour, intact membranes, BS < 5, no contraindications for treatment with prostaglandins. |
|
| Interventions | 3 mg PGE2 vaginal tablet with placebo gel (n = 41) or both placebo treatments (n = 40). Instillation following baseline BS assessment, then reassessed 12 hours later, prior to amniotomy and oxytocin if BS > 6. If BS still < 5 after initial 12‐hour period induced with extra‐amniotic prostaglandin. |
|
| Outcomes | Change in BS, mode of delivery, maternal side‐effects, postpartum haemorrhage, Apgar scores, hyperstimulation. | |
| Notes | 3‐arm trial. Intracervical prostaglandin gel arm presented in reviews focusing on intracervical prostaglandins. University of Gent, Belgium. September 1981 to July 1982. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Random assignment', Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding but placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Tomlinson 2001.
| Methods | 'Randomised non‐blinded controlled trial.' Sealed opaque envelope. | |
| Participants | 69 women requiring induction of labour. Inclusion criteria: intact membranes. Exclusion criteria: ruptured membranes, previous caesarean section. |
|
| Interventions | 1 mg or 2 mg PGE2 vaginal gel (n = 34) or 10 mg sustained release vaginal insert (n = 35). | |
| Outcomes | Caesarean section, epidural analgesia, instrumental vaginal delivery. Patient satisfaction (measured on 6‐point Likert scale). |
|
| Notes | Additional information regarding patient satisfaction requested from authors. raw data awaited. Pinderfields Hospital, Wakefield, UK. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail given. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque sequentially numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Suitable dummies were not used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No blinding. |
| Selective reporting (reporting bias) | High risk | Main focus of paper was maternal satisfaction. Some outcomes not fully reported. |
| Other bias | Unclear risk | Small, single centre RCT, source of funding not reported. |
Triglia 2010.
| Methods | "Randomised controlled trial" no details of given. | |
| Participants | 130 women with singleton cephalic pregnancy, > 36 weeks with intact membranes and a BS < 4. Exclusions: women with malpresentations, previous caesarean section, ruptured membranes. | |
| Interventions | 2 mg PGE2 vaginal gel, repeat 6 hrs for up to 24 hrs (n = 65) versus 10 mg PGE2 SR vaginal pessary for 24 hrs (n = 65) (or until active labour). | |
| Outcomes | Mode of delivery, vaginal delivery not achieved in 24 hrs, uterine hyperstimulation with/without FHR changes, caesarean section, uterine rupture, instrumental vaginal delivery, Apgar score < 7 at 5 mins, postpartum haemorrhage, failed induction (no active labour after 48 hrs). | |
| Notes | University Hospital Brescia, Italy. April 2007 ‐ March 2008. Subgroup primips and multips. Gel source not stated but sustained release named as Propess (Ferring Phamaceuticals). Trial registered in ClinicalTrials.gov with the ID number NCT00843362. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given. |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 person lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified |
| Other bias | Unclear risk | Source of funding not reported but state "The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper." |
Ulmsten 1985.
| Methods | 'Randomly allocated.' | |
| Participants | 58 women requiring induction of labour randomly allocated to 3 groups. 2 groups (n = 39) included in this review. Inclusion criteria: unfavourable cervix (BS < 5). |
|
| Interventions | 2 mg PGE2 vaginal suppository and placebo gel (n = 19) or placebo gel and suppository (n = 20). Baseline BS prior to treatment followed by review at 24 hours. If not in labour induced with oxytocin (started at 2 mU/minute increased to a maximum of 24 mU/minute). |
|
| Outcomes | Change in BS, spontaneous labour, use of oxytocin, hyperstimulation, mode of delivery, maternal side‐effects, Apgar scores. | |
| Notes | 3‐ arm trial. Intracervical prostaglandin gel arm presented in reviews focusing on intracervical prostaglandins. 2‐centre trial, Sweden. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding but placebo controls. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No randomised patients lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small trial, 2 centres, source of funding not clear. |
Witter 1992.
| Methods | 'Randomly assigned.' | |
| Participants | 81 patients requiring induction of labour. Inclusion criteria: singleton pregnancy, cephalic presentation, parity < 3, BS < 4, reactive NST, no previous uterine scars, no vaginal bleeding, intact membranes or SROM < 4 hours duration, no fever, no allergy to prostaglandins, no history of asthma or glaucoma, no fetal distress, not in spontaneous labour, no evidence of clinical hydramnios, no underlying maternal cardiac lesion. |
|
| Interventions | 10 mg PGE2 vaginal pessary (1 mg/hr) (n = 42) or placebo (n = 39). Instillation following baseline BS. Repeat examinations at 6 and 12 hours. Pessary removed at 12 hours. If not in labour after 12 hours, induction with oxytocin, with amniotomy at physicians discretion. |
|
| Outcomes | Change in BS, spontaneous labour, mode of delivery, epidural analgesia, hyperstimulation, oxytocin use. | |
| Notes | 2 active treatment and 9 placebo patients not evaluated with regard to labour parameters. John Hopkins University School of Medicine, USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding but placebo control. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes not reported on 11 of 91 patients (12%), but loss is unbalanced between groups. 9 of these were in placebo group, so 23% of the placebo group was lost to follow‐up and but less than 5% of treatment group. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, single centre RCT. |
Witter 1996.
| Methods | Computer‐generated random number lists with centrally produced coded drug boxes. | |
| Participants | 206 women requiring induction of labour. Inclusion criteria: singleton pregnancy, cephalic presentation, parity < 3, BS < 4, reactive NST, no previous uterine scars, no vaginal bleeding, intact membranes or SROM < 4 hours duration, no fever, no allergy to prostaglandins, no history of asthma or glaucoma, no fetal distress, not in spontaneous labour, no evidence of clinical hydramnios, no underlying maternal cardiac lesion. |
|
| Interventions | 10 mg PGE2 vaginal pessary (1 mg/hr) (n = 102) or identical placebo (n = 104). Instillation following baseline BS, repeat examinations at 6 and 12 hours. Pessary removed after 12 hours. If not in labour after 12 hours, induction with oxytocin with amniotomy at physicians discretion. |
|
| Outcomes | Change in BS, mode of delivery, hyperstimulation, time to delivery. | |
| Notes | 13 patients disqualified for early removal of pessary (some related to hyperstimulation, unclear if these included in analysis or not) 5 for protocol violations. Multicentre trial (10 centres), USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number lists. |
| Allocation concealment (selection bias) | Low risk | Centrally produced coded drug boxes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding but identical placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13 (˜13%) patients disqualified for early removal of pessary (some related to hyperstimulation, unclear if these included in analysis or not) 5 for protocol violations. |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes prespecified. |
| Other bias | Unclear risk | Small, RCT, 10 centres, source of funding not clear. |
Zanconato 2011.
| Methods | "Randomised control trial." | |
| Participants | 52 women attending nulliparous women with singleton, cephalic pregnancy > 38 weeks' gestation with intact membranes, and a BS < 4. | |
| Interventions | 1‐2 mg PGE2, repeat after 6 hrs, max 3 doses (n = 26) versus 10 mg sustained release PGE2 pessary (0.5 mg per hour over 24 hrs) (n = 26). | |
| Outcomes | Cesearean section, oxytocin augmentation, pain ‐ assessed visual analogue, numeric rating and verbal rating, outcomes "mean pain" score and difference in % women with severe score > 5/10 repeated measures. | |
| Notes | University Hospital in Verona between 1 January ‐ 30 June 2010. Gel is named as Prepidil (Upjohn) ‐ this is an intracervical preparation, but in the title and abstract refer to repeat doses of repeat doses of vaginal dinoprostone gel" in the text it is site of administration not stated,the sustained release preparation is named as Propess (Ferring pharmaceuticals). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported. |
| Selective reporting (reporting bias) | High risk | Variety of pains assessment measures and outcomes used. Study protocol not assessed. |
| Other bias | Unclear risk | Source of funding is not reported. "The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article". The sustained release product is named as Propess (Ferring Pharmaceuticals), the proprietary name of the vaginal gel is given as "Prepidil" although this is an intracervical preparation (Pharmacia and Upjohn). |
AFI: amniotic fluid index BS: Bishop score CPD: cephalo‐pelvic disproportion CTG: cardiotocograph FHR: fetal heart rate GA: gestational age IOL: induction of labour ITT: intention‐to‐treat analysis IUGR: intrauterine growth restriction IQR: interquartile range IV: intravenous LMP: last menstrual period LSCS: lower segment caesarean section min: minute(s) NICU: neonatal intensive care unit NST: non‐stress test NVD: normal vaginal delivery PG: prostaglandin PPH: postpartum haemorrhage PROM: premature rupture of membranes RCT: randomised controlled trial Th: rhesus ROM: rupture of membranes SD: standard deviation SROM: spontaneous rupture of the membranes vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bamford 1992 | Comparison of PGE2 gel vs pessary. Unpublished trial, no outcome data available. Recruitment finished 1992. |
| Bex 1990 | Comparison of sustained release prostaglandin pessary to vaginal PGE2 tablets. No primary outcomes reported. |
| Carlan 1995 | Comparison of 2.5.mg PGE2 vaginal gel 1 or 6 hourly. Does not fit into dose comparison as both arms in 'high‐dose' category. |
| Castle 1983 | Study assessing absorption profiles of PG pessaries. No primary outcomes reported. |
| Danna 1995 | Pre‐induction cervical ripening with PGE2 gel. No primary outcomes reported. |
| De Laat 1991 | Absorbtion profiles of PGE2 gel. No primary outcomes reported. |
| Dommisse 1981 | Intrarectal PGE2 suppositories. No primary outcome data. |
| Fusi 1989 | 3‐arm trial comparing PGE2 gel and tablets. No denominator data reported. Limited outcome reporting. |
| Gauger 1991 | Comparison of PGE2 vaginal gel versus suppository. 1 case of IUD included, cannot separate out data. |
| Gordon‐Wright 1979 | PGE2 vaginally for induction of labour. No primary outcomes reported. |
| Granstrom 1995 | Comparison of 3 mg PGE2 gel at 12 or 24 hours post SROM. Does not fit into any comparison groups as treatment effectively the same in both arms. |
| Greer 1986 | Sustained release PGE2 pessaries. No primary outcome data reported. |
| Greer 1988 | Plasma levels of PG metabolites. No primary outcomes reported. |
| Grunstein 1990 | Comparison 3 mg PGE2 6 hourly (maximum 6 mg) or PGE2 varied according to Bishops score. Not possible to accurately compare due to variation in varying arm. Does not fit into dose comparison as both arms in 'high‐dose' category. |
| Hill 1991 | Management of IUDs with PG induction. |
| Hunter 1982 | Comparison of 3 doses of PGE2 gel at 3 different dose intervals. 3 mg 8 hours apart (max 6 mg), 2 mg 4 hours apart (max 6 mg), 0.5 mg 3 hours apart (max 10 mg). Does not fit into dose comparison as both arms in 'high‐dose' category. |
| Hunter 1984 | Comparison of 3 mg PGE2 x2 or 0.5 mg PGE2 3 hourly (max 4 mg) in 48 hours. Does not fit into dose comparison as both arms in 'high‐dose' category. |
| Hunter 1998 | Comparison of PGE2 tablets to Propess (10 mg sustained release pessary). 50% of Propess arm had additional PGE2 tablets. Not possible to dissect out data. |
| Khan 2011 | Compared outcomes from 2 audits in 2005 and 2011. |
| Knogler 1988 | Comparison of vaginal PGE2 tablet and gel. No primary outcomes reported. |
| Krammer 1995 | Outpatient administrated of PGE2 +/‐ oestrogen vs placebo. No primary outcomes reported. |
| Lass 1994 | Variable decelerations during pre‐induction phase, prior to PG induction. No primary outcomes reported. |
| Lindblad 1985 | Fetal and maternal circulation changes during PGE2 induction. No primary outcomes reported. |
| Lorenz 1984 | 44 patients in randomised double‐blind placebo controlled trial comparing 2 mg PGE2 vaginal gel to placebo. Excluded due to 25% of participants being < 20 weeks' gestation and also 1 patient with an IUD was included. Not possible to separate out relevant data. |
| Loria‐Casanova 1989 | Evaluation of PGE2 induction, only in preterm patients. |
| MacKenzie 1977 | 2 trials: 1 small randomised comparison of PGE2 to placebo. No primary outcomes reported. Second non‐randomised cohort not reported. |
| MacKenzie 1988 | Comparison of 2 sustained release vaginal pessaries. No primary outcome data presented. |
| MacKenzie 1997b | Economic analysis of PGE2 induction of labour (see MacKenzie 1997a in included studies) |
| Nikolov 2003 | Translation. Study from Sofia, Bulgaria comparing PGE2 10 mg to placebo. "women who were hospitalized and required IOL...were chosen for the treatment and were divided in 2 groups." |
| Norchi 1993 | Comparison of 2 mg PGE2 gel (max total dose 4 mg) versus 3 mg PGE2 gel (max total dose 6 mg). Does not fit into dose comparison as both arms in 'high‐dose' category. |
| Odum 1993 | Induction with PGE2. Some patients with IUDs included. Not possible to separate these data out. |
| Parker 1990 | Comparison of PGE2 gel to tablet. Unpublished trial. Recruitment started 1990. |
| Petrou 2011 | Economic analysis linked to included trial (Taher 2011). |
| Ramsey 1998 | Comparison of sustained release vaginal PGE2 to vaginal PGE2 to vaginal misoprostol. insufficient information regarding denominators to allow inclusion of outcome data. author contacted. |
| Sadaty 1998 | Comparison of sustained release 10 mg vaginal insert to vaginal PGE2 gel. Relevant outcome data presented within the abstract but not possible to extract due to limited numeric data. author contacted but no reply. |
| Seeras 1995 | Comparison of PGE2 gel. 1 mg followed by 2 mg 6 hourly (maximum 5 mg) vs 2 mg followed by 2 mg 12 hourly (maximum 6 mg). Does not fit into dose comparison as both arms in 'high‐dose' category. |
| Sellers 1985 | Prostaglandin plasma levels in 2nd trimester abortions. No primary outcomes reported. |
| Smith 1996 | Trial comparing 2.5 mg PGE2 gel to 5 mg PGE2 gel maximum of 2 doses. Does not fit into dose comparison as both arms in 'high‐dose' category. |
| Sorensen 2008 | Recent study following up NICE Guideline 2001, conducted in London described as "prospective sequential comparison" (randomisation not mentioned) of 3 vaginal PGE2 preparations ‐ gel, tablet and sustained release pessary. |
| Sorokin 1992 | Effect of PG induction on fetal breathing movements. No primary outcomes reported. |
| Spitzberg 1991 | Controlled release PGE2 for cervical ripening. Cross‐over trial and no primary outcomes reported. |
| Tan 1994 | PGE2 gel vs pessary. No primary outcomes reported. |
| Tan 1999 | Comparison of 3 mg PGE2 24 hourly (6 mg max) or 3 mg PGE2 4 hourly (max 9 mg). Does not fit into dose comparison as both arms in 'high‐dose' category. |
| Tang 1997 | Comparison of 3 doses of PGF2a (0.1, 0.125 and 0.2 mg). Doses comparison of PGF2a not in prespecified intervention comparisons. |
| Toplis 1979 | Comparison of 3 mg PGE2 vaginal pessary to 3 mg PGE2 vaginal paste or 3 mg PGE2 extra‐amniotic paste. Not possible to compare dose as same in both vaginal arms. Does not fall into category for comparison of vehicle. Extra‐amniotic paste comparison reported in extra‐amniotic prostaglandin review. |
| Toppozada 1992 | Effect of vaginal PGE2 on cervical tissues. No primary outcomes reported. |
| Varma 1984 | Dose ranging study of PGE2. Allocation not mentioned. Non‐blinded study. |
| Veligati 1998 | Comparison of 10 mg PGE2 sustained release pessary to 4 mg vaginal PGE2 gel. Relevant outcome data available but details of intervention not detailed enough to allow inclusion. Author contacted. |
| Walker 1983 | Comparison of 4 mg PGE2 gel at 12 or 24 hours. Small trial with limited outcome reporting. Does not fit into dose comparison, due to similarity of both arms. |
| Zanini 1991 | Comparison of 2 mg PGE2 (4 mg max) or 3 mg PGE2 (6 mg max) vaginal gel every 12 hours. Does not fit into dose comparison as both arms in 'high‐dose' category. |
IUD: intrauterine death max: maximum PG: prostaglandin SROM: spontaneous rupture of membranes vs: versus
Differences between protocol and review
Methods updated.
Contributions of authors
For the original review (2001) Anthony Kelly (AK) and Josephine Kavanagh (JK) performed the original data extraction. AK, JK and Jane Thomas (JT) drafted the original review. For this update (2014), additional trials were assessed and the data were extracted by JT and Anna Fairclough (AF), JT and AF redrafted the review and this final draft was reviewed by JK and AK. Changes following editorial review were completed by JT and AF, and reviewed by AK and JK.
Sources of support
Internal sources
Clinical Effectiveness Support Unit, Royal College of Obstetricians and Gynaecologists, London, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Al Malt 1995 {published data only}
- Al‐Malt A, Ashmead G, Amini S. Cervical ripening: effect of vaginal PGE2 on bishop score. American Journal of Obstetrics and Gynecology 1995;172:297. [Google Scholar]
Al‐Sebai 1993 {published data only}
- Al‐Sebai MAH, Manasse PR. Induction of labour in primigravid women with an unfavourable cervix: a prospective comparative study of prostaglandin E2 vaginal tablets and gel. Journal of Obstetrics and Gynaecology 1993;13:112‐3. [Google Scholar]
Bezircioglu 2012 {published data only}
- Bezircioglu I, Akin MK, Baloglu A, Bicer M. The efficacy of dinoprostone vaginal insert for active management of premature rupture of membranes at term: a randomized controlled trial. Clinical and Experimental Obstetrics and Gynecology 2012;39(3):356‐8. [PubMed] [Google Scholar]
Buchanan 1984 {published data only}
- Buchanan D, Macer J, Yonekura ML. Cervical ripening with prostaglandin E2 vaginal suppositories. Obstetrics & Gynecology 1984;63:659‐63. [PubMed] [Google Scholar]
Campbell 1984 {published data only}
- Campbell JM. Induction of labour using prostaglandin E2 pessaries. Clinical and Experimental Obstetrics and Gynecology 1984;11:1‐5. [PubMed] [Google Scholar]
Cardozo 1986 {published data only}
- Cardozo L, Fysh J, Pearce JM. Prolonged pregnancy: the management debate. BMJ 1986;293:1059‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce JM, Cardozo L. Prolonged pregnancy: results of supplemental analysis. BMJ 1988;297:715‐7. [Google Scholar]
Chaterjee 1990 {published data only}
- Chatterjee MS, Ramchandran K, Ferlita J, Mitrik L. Prostaglandin E2 (PGE2) vaginal gel for cervical ripening. European Journal of Obstetrics & Gynecology and Reproductive Biology 1990;38:197‐202. [DOI] [PubMed] [Google Scholar]
Chua 1995 {published data only}
- Chua S, Arulkumaran S, Yap C, Selamat N, Ratnam SS. Premature rupture of membranes in nulliparas at term with unfavorable cervices: a double blind randomized trial of prostaglandin and placebo. Obstetrics & Gynecology 1995;86:550‐4. [DOI] [PubMed] [Google Scholar]
Chung 1992 {published data only}
- Chung T, Rogers MS, Gordon H, Chang A. Prelabour rupture of the membranes at term and unfavourable cervix: a randomized placebo‐controlled trial on early intervention with intravaginal prostaglandin E2 gel. Australian and New Zealand Journal of Obstetrics and Gynaecology 1992;32:25‐7. [DOI] [PubMed] [Google Scholar]
Curet 1989 {published data only}
- Curet LB, Gauger LJ. Cervical ripening with intravaginal prostaglandin E2 gel. International Journal of Gynecology & Obstetrics 1989;28:221‐8. [DOI] [PubMed] [Google Scholar]
Doany 1997 {published data only}
- Doany W. Outpatient management of postdate pregnancy with intravaginal prostaglandin E2 and membrane stripping. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):351. [Google Scholar]
- Doany W, McCarty J. Outpatient management of the uncomplicated postdate pregnancy with intravaginal prostaglandin e2 gel and membrane stripping. Journal of Maternal Fetal Medicine 1997;6:71‐8. [DOI] [PubMed] [Google Scholar]
Dommisse 1980 {published data only}
- Dommisse J, Davey DA, Allerton G. The induction of labour with prostaglandin E2 tablets administered intravaginally. South African Medical Journal 1980;58:518‐9. [PubMed] [Google Scholar]
Duhl 1997 {published data only}
- Duhl A, Tolosa J, Leiva M, Nemiroff R. Randomized trial of intravaginal gel, intravaginal time release insert, and intracervical gel with prostaglandin E2 for induction of labor. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S113. [Google Scholar]
Dunston‐Boone 1991 {published data only}
- Dunston‐Boone G, Turzo E, Wapner RJ. A randomized prospective trial of the slow release prostaglandin E2 (PGE2) vaginal pessary. American Journal of Obstetrics and Gynecology 1991;164:405. [Google Scholar]
Egarter 1989 {published data only}
- Egarter Ch, Kofler E, Fitz R, Husslein P. Is induction of labor indicated in prolonged pregnancy? Results of a prospective randomised trial. Gynecologic and Obstetric Investigation 1989;27:6‐9. [DOI] [PubMed] [Google Scholar]
- Husslein P, Egarter C, Sevelda P, Genger H, Salzer H, Kofler E. Induction of labour with prostaglandin E2 vaginal tablets ‐ a revival of elective induction? Results of a prospective randomised trial. Geburtshilfe Frauenheilkd 1986;46:83‐7. [DOI] [PubMed] [Google Scholar]
El‐Mardi 1991 {published data only}
- El‐Mardi AA, El‐Qarmalawi MA, Siddik M, El‐Haroni A, Ammar A, Madkoor SA. A comparison of single prostaglandin E2 vaginal tablet with prostaglandin E2 vaginal pessaries for induction of labor at term. International Journal of Gynecology & Obstetrics 1991;35:221‐4. [DOI] [PubMed] [Google Scholar]
El Shawarby 2006 {published data only}
- El‐Shawarby SA, Connell RJ. Induction of labour at term with vaginal prostaglandins preparations: A randomised controlled trial of Prostin vs Propess. Journal of Obstetrics and Gynaecology 2006;26(7):627‐30. [DOI] [PubMed] [Google Scholar]
Ferraiolo 2010 {published data only}
- Ferraiolo A, Dellacasa I, Bentivoglio G, Ferrero S, Ragni N. Evaluation of patients' satisfaction of cervical ripening using dinoprostone by either intravaginal gel or pessary: an open‐label, randomized, prospective study. Journal of Reproductive Medicine 2010;55(9‐10):423‐9. [PubMed] [Google Scholar]
Glanville 2002 {published and unpublished data}
- Glanville T, Griffen C, Mason G. A randomised trial of single insertion of a 10mg dinoprostone slow release pessary (Propess) versus multiple insertions of Dinoprostone (prostin) for induction of labour. Personal communication 2008.
- Glanville T, Griffin C, Mason G. A randomised controlled trial of prolonged 10 mg dinoprostone pessary (Propess) vs. dinoprostone gel (Prostin) for induction of labour. Journal of Obstetrics and Gynaecology 2002;22(2 Suppl):S55. [Google Scholar]
Graves 1985 {published data only}
- Graves GR, Baskett TF, Gray JH, Luther ER. The effect of vaginal administration of various doses of prostaglandin E2 gel on cervical ripening and induction of labour. American Journal of Obstetrics and Gynecology 1985;151:178‐81. [DOI] [PubMed] [Google Scholar]
Green 1998 {published data only}
- Green C, Pedder G, Mason G. A randomised trial of propess against prostin gel for induction of labour at term. British Journal of Obstetrics and Gynaecology 1998;105 Suppl 17:82. [Google Scholar]
Greer 1990 {published data only}
- Greer IA, McLaren M, Calder AA. Vaginal administration of PGE2 for induction of labor stimulates endogenous PGF2alpha production. Acta Obstetricia et Gynecologica Scandinavica 1990;69:621‐5. [DOI] [PubMed] [Google Scholar]
Hage 1993 {published data only}
- Hage P, Shaw J, Zarou D, Fleisher J, Wehbeh H. Double blind randomized trial to evaluate the role of outpatient use of PGE 2 in cervical ripening. American Journal of Obstetrics and Gynecology 1993;168:430. [Google Scholar]
Hannah 1996 {published and unpublished data}
- Gafni A, Goeree R, Terri L, Myhr TL, Hannah ME, Blackhouse G, et al. Induction of labour versus expectant management for prelabour rupture of the membranes at term: an economic evaluation. Canadian Medical Association 1997;157(11):1519‐25. [PMC free article] [PubMed] [Google Scholar]
- Hannah ME, Ohlsson A, Farine D, Hewson SA, Hodnett ED, Myhr TL, et al. Induction of labor compared with expectant management for prelabor rupture of the membranes at term. TERMPROM Study Group. New England Journal of Medicine 1996;334(16):1005‐10. [DOI] [PubMed] [Google Scholar]
Hayashi 1983 {published data only}
- Hayashi R, Keirse MJNC. PGE2 gel (Prepidil gel) for preinduction cervical softening. Personal communication 1988.
Kalkat 2008 {published data only}
- Kalkat RK, McMillan E, Cooper H, Palmer K. Comparison of dinoprostone slow release pessary (Propess) with gel (Prostin) for induction of labour at term‐a randomised trial. Journal of Obstetrics and Gynaecology 2008;28(7):695‐9. [DOI] [PubMed] [Google Scholar]
- Kalkat RKB, McMillan E, Cooper H, Palmer K. Comparative study of dinoprostone slow release pessary (propess) versus gel (prostin) for induction of labour [abstract]. 31st British International Congress of Obstetrics and Gynaecology; 2007 July 4‐6; London, UK. 2007:209.
Liggins 1979 {published data only}
- Liggins GC. Controlled trial of induction of labor by vaginal suppositories containing prostaglandin E2. Prostaglandins 1979;18:167‐72. [DOI] [PubMed] [Google Scholar]
MacKenzie 1979 {published data only}
- MacKenzie IZ, Embrey MP. A comparison of PGE2 and PGF2alpha vaginal gel for ripening the cervix before induction of labour. British Journal of Obstetrics and Gynaecology 1979;86:167‐70. [DOI] [PubMed] [Google Scholar]
MacKenzie 1981 {published data only}
- MacKenzie IZ, Bradley S, Embrey MP. A simpler approach to labor induction using lipid‐based prostaglandin E2 vaginal suppository. American Journal of Obstetrics and Gynecology 1981;141:158‐62. [DOI] [PubMed] [Google Scholar]
MacKenzie 1997a {published data only}
- MacKenzie IZ, Burns E. Randomised trial of one versus two doses of prostaglandin E2 for induction of labour: 1. Clinical outcome. British Journal of Obstetrics and Gynaecology 1997;104:1062‐7. [DOI] [PubMed] [Google Scholar]
MacLennan 1979 {published data only}
- MacLennan AH, Green RC. Cervical ripening and induction of labour with intravaginal prostaglandin F2alpha. Lancet 1979;313:117‐9. [DOI] [PubMed] [Google Scholar]
MacLennan 1980 {published data only}
- MacLennan AH, Green RC. A double blind dose trial of intravaginal prostaglandin F2 alpha for cervical ripening and the induction of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1980;20(2):80‐3. [DOI] [PubMed] [Google Scholar]
Mahmood 1989 {published data only}
- Mahmood TA. A prospective comparative study on the use of prostaglandin E2 gel (2 mg) and prostaglandin E2 tablet (3 mg) for the induction of labour in primigravid women with unfavourable cervices. European Journal of Obstetrics & Gynecology and Reproductive Biology 1989;33:169‐75. [DOI] [PubMed] [Google Scholar]
- Mahmood TA. Induction of labour in primigravid with unfavourable cervices: a comparison of PGE2 gel (2mg) with PGE2 pessary (3mg). Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988:149.
Mahmood 1992 {published data only}
- Mahmood TA, Dick MJW, Smith NC. Management of spontaneous rupture of the membranes and no uterine activity in healthy primigravidae after 34 weeks gestation. Lancet 1989;1(8640):721. [DOI] [PubMed] [Google Scholar]
- Mahmood TA, Dick MJW, Smith NC, Templeton A. Management of spontaneous rupture of membranes at term without uterine activity in healthy primigravidae: a prospective study (PGE2 gel vs conservative treatment). Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands. 1991:95.
- Mahmood TA, Dick MJW, Smith NC, Templeton AA. Role of prostaglandin in the management of prelabour rupture of the membranes at term. British Journal of Obstetrics and Gynaecology 1992;99:112‐7. [DOI] [PubMed] [Google Scholar]
Mahmood 1995 {published data only}
- Mahmood TA, Dick MJW. A randomized trial of management of pre‐labour rupture of membranes at term in multiparous women using vaginal prostaglandin gel. Obstetrics & Gynecology 1995;85:71‐4. [DOI] [PubMed] [Google Scholar]
McCaul 1997 {published data only}
- McCaul JF 4th, Rogers LW, Perry KG Jr, Martin RW, Allbert JR, Morrison JC. Premature rupture of membranes at term with an unfavorable cervix: comparison of expectant management, vaginal prostaglandin, and oxytocin induction. Southern Medical Journal 1997;90:1229‐33. [DOI] [PubMed] [Google Scholar]
- McCaul JF, Williams LM, Martin RW, Magann EF, Gallagher L, Morrison JC. Comparison of induction methods for premature rupture of membranes at term. American Journal of Obstetrics and Gynecology 1992;166(1 Pt 2):275. [Google Scholar]
McLaren 1987 {published data only}
- McLaren M, Greer IA, Smith JR, Godfree V, Graham N, Calder AA. Maternal plasma bicycling PGE2 levels following vaginal administration of prostaglandin E2 pessaries in full term pregnancies. Prostaglandins in Clinical Research 1987;242:199‐203. [PubMed] [Google Scholar]
Miller 1991 {published data only}
- Miller AM, Rayburn WF, Smith CV. Patterns of uterine activity after intravaginal prostaglandin E2 during preinduction cervical ripening. American Journal of Obstetrics and Gynecology 1991;165:1006‐9. [DOI] [PubMed] [Google Scholar]
- Miller AM, Rayburn WF, Smith CV, Allen K, Bane T. Uterine activity using ambulatory tocodynamometry after intravaginal prostaglandin E2 (PGE2) for cervical ripening. American Journal of Obstetrics and Gynecology 1991;164:317. [DOI] [PubMed] [Google Scholar]
Mukhopadhyay 2002 {published data only}
- Mukhopadhyay M, Lim K, Fairlie F. Is propess a better method of induction in nulliparous women. Journal of Obstetrics and Gynaecology 2002;22(3):294‐5. [DOI] [PubMed] [Google Scholar]
Murphy 1980 {published data only}
- Murphy AJ, Jalland M, Pepperell RJ, Quinn MA. Use of vaginal prostaglandin gel before induction of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1980;20:84‐6. [DOI] [PubMed] [Google Scholar]
Murray 1995 {published data only}
- Murray HG, Buonocore A, Hawley J. A randomised trial of two dose preparations of vaginal prostaglandin for pre‐induction cervical ripening. Proceedings of the 14th Annual Congress of the Australian Perinatal Society in conjunction with the New Zealand Perinatal Society; 1996 March 24‐27; Adelaide, Australia. 1996:A24.
- Murray HG, Buonocore A, Hawley J. A randomized trial of two preparations of vaginal prostaglandin for pre‐induction cervical ripening. Obstetrics & Gynecology 1995;86:880‐5. [DOI] [PubMed] [Google Scholar]
Neilson 1983 {published data only}
- Neilson DR, Prins RP, Bolton RN, Mark C, Watson P. A comparison of prostaglandin E2 gel and prostaglandin F2alpha gel for preinduction cervical ripening. American Journal of Obstetrics and Gynecology 1983;146:526‐32. [DOI] [PubMed] [Google Scholar]
Newman 1997 {published data only}
- Newman M, Newman R. Multiple‐dose PGE2 cervical ripening on an outpatient basis: safety and efficacy. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S112. [Google Scholar]
Nuutila 1996 {published data only}
- Nuutila M, Kajanoja P. A randomised comparison of intravaginal and intracervical administration of prostaglandin E2 in cervical ripening. Acta Obstetricia et Gynecologica Scandinavica Supplement 1995;73:110‐1. [Google Scholar]
- Nuutila M, Kajanoja P. Local administration of prostaglandin E2 for cervical ripening and labor induction: the appropriate route and dose. Acta Obstetricia et Gynecologica Scandinavica 1996;75:135‐8. [DOI] [PubMed] [Google Scholar]
O'Brien 1995 {published data only}
- O'Brien JM, Mercer B, Cleary N, Sibai BM. Efficacy of outpatient induction with low dose intravaginal prostaglandin E2: a randomised double‐blind placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1995;172:424. [DOI] [PubMed] [Google Scholar]
- O'Brien JM, Mercer BM, Cleary NT, Sibai BM. Efficacy of outpatient induction with low‐dose intravaginal prostaglandin E2: a randomized, double blind, placebo‐controlled trial. American Journal of Obstetrics and Gynecology 1995;173:1855‐9. [DOI] [PubMed] [Google Scholar]
Ohel 1996 {published data only}
- Ohel G, Rahav D, Rothbart H, Ruach M. Ambulatory induction of labor at 40‐41 weeks of gestation. American Journal of Obstetrics and Gynecology 1995;172:425. [DOI] [PubMed] [Google Scholar]
- Ohel G, Rahav D, Rothbart H, Ruach M. Randomised trial of outpatient induction of labor with vaginal PGE2 at 40‐41 weeks of gestation versus expectant management. Archives of Gynecology and Obstetrics 1996;258:109‐12. [DOI] [PubMed] [Google Scholar]
Payne 1993 {published data only}
- Payne E, Reed MF, Cietak KA, Anderson WR, Sant‐Cassia LJ. A comparison of prostaglandin E2 vaginal tablets with vaginal gel for ripening the unfavourable cervix and induction of labour. Journal of Obstetrics and Gynaecology 1993;13:103‐6. [Google Scholar]
Perryman 1992 {published data only}
- Perryman D, Yeast JD, Holst V. Cervical ripening: a prospective, randomised study comparing prostaglandin E2 gel with prostaglandin E2 suppositories. Proceedings of 39th Annual Clinical Meeting of the American College of Obstetricians and Gynecologists; 1991; USA 1991:26.
- Perryman D, Yeast JD, Holst V. Cervical ripening: a randomized study comparing prostaglandin E2 gel to prostaglandin E2 suppositories. Obstetrics & Gynecology 1992;79:670‐2. [PubMed] [Google Scholar]
Poornima 2011 {published data only}
- Poornima B, Dharma DB. Premature rupture of membranes at term: Immediate induction with PGE 2 gel compared with delayed induction with oxytocin. Journal of Obstetrics and Gynecology of India 2011;61(5):516‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prasad 1989 {published data only}
- Prasad RNV, Adaikan PG, Arulkumaran S, Ratnam SS. Preinduction cervical priming with PGE2 vaginal film in primigravidae ‐ a randomised, double blind, placebo controlled study. Prostaglandins, Leukotrienes and Essential Fatty Acids 1989;36:185‐8. [DOI] [PubMed] [Google Scholar]
Prins 1983 {published data only}
- Prins RP, Bolton RN, Mark C, Neilson DR, Watson P. Cervical ripening with intravaginal prostaglandin E2 gel. Obstetrics & Gynecology 1983;61:459‐62. [PubMed] [Google Scholar]
Rabl 2002 {published data only}
- Rabl M, Joura E, Yucel Y, Egarter C. A randomized trial of vaginal prostaglandin E2 for induction of labour. Journal of Reproductive Medicine 2002;47:115‐9. [PubMed] [Google Scholar]
Rath 1999 {published data only}
- Rath W, Heyl W, Kemp B. Intracervical versus intravaginal pge2 gel for induction of labor [Intrazervikalgel versus Vaginalgel zur Geburtseinleitung]. Perinatal Medizin 1998;10:81‐3. [Google Scholar]
- Rath W, Kemp B, Heyl W. Prostaglandin E2 as a vaginal gel, intracervical gel or vaginal tablet for induction of labor: a prospective, randomized, multicenter trial [Prostaglandin E2 Vaginalgel versus Intrazervikalgel und Vaginaltablette in Abhangigkeit vom Zervixstatus ‐ Ergebnisse einer prospecktiven randomisierten Multizenter studie zur Geburtseinleitung]. Geburtshilfe und Frauenheilkunde 1999;59:323‐9. [Google Scholar]
Rayburn 1988 {published data only}
- Rayburn W, Gosen R, Ramadei C, Woods R, Scott J. Outpatient cervical ripening with prostaglandin E2 gel in uncomplicated postdate pregnancies. American Journal of Obstetrics and Gynecology 1988;158:1417‐23. [DOI] [PubMed] [Google Scholar]
Rayburn 1992 {published data only}
- Rayburn W, Barss V, Caritis S, Mandsager N, Molina R, Spitzberg E, et al. A randomized, double‐blind, placebo‐controlled multicenter trial of the efficacy and safety of an intravaginal hydrogel controlled release pessary for the delivery of prostaglandin E2 for cervical ripening prior to induction of labor. Proceedings of 39th Annual Clinical Meeting of the American College of Obstetricians and Gynecologists; 1991; USA. 1991:29.
- Rayburn WF, Wapner RJ, Barss VA, Spitzberg E, Molina RD, Mandsager N, et al. An intravaginal controlled‐release prostaglandin E2 pessary for cervical ripening and initiation of labor at term. Obstetrics & Gynecology 1992;79:374‐9. [DOI] [PubMed] [Google Scholar]
Roach 1997 {published data only}
- Roach, VJ, Rogers, MS. Pregnancy outcome beyond 41 weeks gestation. International Journal of Gynecology & Obstetrics 1997;59:19‐24. [DOI] [PubMed] [Google Scholar]
Sawai 1991 {published data only}
- Sawai SK, Williams MC, O'Brien WF, Angel JL, Mastrogiannis DS, Johnson L. Sequential outpatient application of intravaginal prostaglandin E2 gel in the management of postdates pregnancies. Obstetrics & Gynecology 1991;78:19‐23. [PubMed] [Google Scholar]
- Williams MG, O'Brien WF, Sawai SK, Knuppel RA. Outpatient cervical ripening in the postdates pregnancy. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23‐27; Houston, Texas, USA 1990:533.
Sawai 1994 {published data only}
- Sawai SK, O'Brien WF, Mastrogiannis DS, Krammer J, Mastry MG, Porter GW. Patient‐administered outpatient intravaginal prostaglandin E2 suppositories in post‐date pregnancies: a double‐blind, randomized, placebo‐controlled study. Obstetrics & Gynecology 1994;84:807‐10. [PubMed] [Google Scholar]
- Sawai SK, O'Brien WF, Mastrogiannis MS, Mastry MG, Porter GW, Johnson L. Outpatient prostaglandin E2 suppositories in postdates pregnancies. American Journal of Obstetrics and Gynecology 1992;166:400. [Google Scholar]
Shoaib 1994 {published data only}
- Shoaib F. Management of premature rupture of membranes with unfavourable cervix at term, by prostaglandins. Specialist 1994;10(3):227‐32. [Google Scholar]
Smith 1990 {published data only}
- Smith CV, Rayburn WF, Connor RE, Fredstrom GR, Phillips CB. Double‐blind comparison of intravaginal prostaglandin E2 gel and 'chip' for pre‐induction cervical ripening. Proceedings of 10th Annual Meeting of Society of Perinatal Obstetricians; 1990 Jan 23‐27; Houston, Texas, USA. 1990:134.
- Smith CV, Rayburn WF, Connor RE, Fredstrom GR, Phillips CB. Double‐blind comparison of intravaginal prostaglandin E2 gel and 'chip' for preinduction cervical ripening. American Journal of Obstetrics and Gynecology 1990;163:845‐7. [DOI] [PubMed] [Google Scholar]
Smith 1994 {published data only}
- Smith CV, Rayburn WF, Miller AM. Intravaginal prostaglandin E2 for cervical ripening and initiation of labor: comparison of a multidose gel and single, controlled‐release pessary. Journal of Reproductive Medicine 1994;39:381‐4. [PubMed] [Google Scholar]
Stampe Sorensen 1992 {published data only}
- Sorensen SS, Palmgren N, Andreasson B, Bock JE, Berget A, Schmidt T. PGE2 pessaries versus vaginal tablets for induction of labour. Proceedings of 13th World Congress of Gynaecology and Obstetrics (FIGO); 1991 Sept 15‐20; Singapore. 1991:34.
- Stampe Sorensen S, Colov NP, Andreasson B, Bock JE, Berget A, Schmidt T. Induction of labor by vaginal prostaglandin E2. A randomized study comparing pessaries with vaginal tablets. Acta Obstetricia et Gynecologica Scandinavica 1992;71:201‐6. [DOI] [PubMed] [Google Scholar]
- Stampe Sorenson S, Bock J, Berget A. Pharmacy prepared prostaglandin e2 pessaries versus prostin e2 vaginal tablets for induction of labour. 12th FIGO World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil. 1988:199.
Taher 2011 {published and unpublished data}
- Griffiths AN. Prostaglandin E2 vaginal gel or tablets for the induction of labour at term: a randomised controlled trial. BJOG : an international journal of obstetrics and gynaecology 2011; Vol. 118, issue 13:1679; author reply 1680‐1. [PUBMED: 22077261] [DOI] [PubMed]
- Khan Z, Majoko F. Prostaglandin E2 vaginal gel or tablets for the induction of labour at term: a randomised controlled trial. BJOG : an international journal of obstetrics and gynaecology 2011; Vol. 118, issue 13:1679‐80; author reply 1680‐1. [PUBMED: 22077262] [DOI] [PubMed]
- Taher S, Inder J, Soltan S, Eliahoo J, Edmonds D, Bennett P. Prostaglandin E2 vaginal gel or tablets for the induction of labour at term: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011;118(6):719‐25. [DOI] [PubMed] [Google Scholar]
Thiery 1984 {published data only}
- Thiery M, Decoster JM, Parewijck W, Noah ML, Derom R, Kets H, et al. Endocervical prostaglandin E2 gel for preinduction cervical softening. Prostaglandins 1984;27:429‐39. [DOI] [PubMed] [Google Scholar]
Tomlinson 2001 {published data only}
- Tomlinson A, Archer P, Hobson S. Induction of labour: a comparison of two methods with particular concern to patient acceptability. Journal of Obstetrics and Gynaecology 2001;21(3):239‐41. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Archer P, Hobson S. Prostin or Propess‐ which method of induction of labour do patients prefer. Journal of Obstetrics and Gynaecology 2000;20(Suppl 1):S58. [Google Scholar]
Triglia 2010 {published data only}
- Triglia MT, Palamara F, Lojacono A, Prefumo F, Frusca T. A randomized controlled trial of 24‐hour vaginal dinoprostone pessary compared to gel for induction of labor in term pregnancies with a Bishop score < or = 4. Acta Obstetricia et Gynecologica Scandinavica 2010;89(5):651‐7. [DOI] [PubMed] [Google Scholar]
Ulmsten 1985 {published data only}
- Ulmsten U, Ekman G, Belfrage P, Bygdeman M, Nyberg C. Intracervical vs intravaginal PGE2 for induction of labor at term in patients with an unfavorable cervix. Archives of Gynecology 1985;236:243‐8. [DOI] [PubMed] [Google Scholar]
Witter 1992 {published data only}
- Witter FR, Rocco L, Johnson TRB. A randomised trial of prostaglandin E2 in a controlled release vaginal pessary for cervical ripening at term. American Journal of Obstetrics and Gynecology 1991;164:308. [DOI] [PubMed] [Google Scholar]
- Witter FR, Rocco LE, Johnson TRB. A randomized trial of prostaglandin E2 in a controlled‐release vaginal pessary for cervical ripening at term. American Journal of Obstetrics and Gynecology 1992;166:830‐4. [DOI] [PubMed] [Google Scholar]
Witter 1996 {published data only}
- Witter FR, Mercer BM. Improved intravaginal controlled‐release prostaglandin E2 insert for cervical ripening at term. Prenatal and Neonatal Medicine 1996;1 Suppl 1:249. [DOI] [PubMed] [Google Scholar]
- Witter FR, Mercer BM. Improved intravaginal controlled‐release prostaglandin E2 insert for cervical ripening at term. The Prostaglandin E2 insert Study Group. Journal of Maternal Fetal Medicine 1996;5(2):64‐9. [DOI] [PubMed] [Google Scholar]
Zanconato 2011 {published data only}
- Zanconato G, Bergamini V, Mantovani E, Carlin R, Bortolami O, Franchi M. Induction of labor and pain: a randomized trial between two vaginal preparations of dinoprostone in nulliparous women with an unfavorable cervix. Journal of Maternal‐Fetal and Neonatal Medicine 2011;24(5):728‐31. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bamford 1992 {unpublished data only}
- Bamford PN. Trial to compare prostaglandin gel vs prostaglandin pessary in nulliparous inductions. Personal communication 1992.
Bex 1990 {published data only}
- Bex P, Gunasekera PC, Phipps JH. Difficulties with controlled release prostaglandin E2 pessaries [letter]. Lancet 1990;336:119. [PubMed] [Google Scholar]
Carlan 1995 {published data only}
- Carlan SJ, Danna P, Durkee D, Quinsey C, Lanaris B. Randomized study of pre‐induction cervical ripening with sequential use of intravaginal prostaglandin E2 gel. Obstetrics & Gynecology 1995;85:608‐13. [DOI] [PubMed] [Google Scholar]
Castle 1983 {published data only}
- Castle B, Mountford L, Brennecke S, Embrey MP, MacKenzie IZ. In‐vivo studies using the bicyclo PGEM assay to assess release of PGE2 from vaginal preparations used for labour induction. Proceedings of 23rd British Congress of Obstetrics and Gynaecology; 1983 July 12‐15; Birmingham, UK. 1983:89.
Danna 1995 {published data only}
- Danna P, Carlan S, Logan S, Durkee D, Gushwa J, Fuentes A, et al. Randomised prospective study of preinduction cervical ripening with sequential use of intravaginal prostaglandin E2 gel. American Journal of Obstetrics and Gynecology 1995;172:298. [DOI] [PubMed] [Google Scholar]
De Laat 1991 {published data only}
- Laat WNGM, Egberink J. A highly viscous prostaglandin E2 gel (Cerviprost) for cervical ripening. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands. 1991:98.
Dommisse 1981 {published data only}
- Dommisse J, Davey DA, Martin B, Cohen M. An evaluation of prostaglandin E2 administered intrarectally to induce labour. South African Medical Journal 1981;59:817‐8. [PubMed] [Google Scholar]
Fusi 1989 {published data only}
- Fusi L, MacMaulay J, Gordon H. A prospective and partly randomised trial on the use of vaginal prostaglandin preparations for induction of labour. Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988:107.
- Fusi L, Macaulay J. Induction of labour with vaginal prostaglandins. A random trial comparing pessaries and gels with different concentrations. Journal of Obstetrics and Gynaecology 1989;10:76‐7. [Google Scholar]
Gauger 1991 {published data only}
- Gauger LJ, Curet LB. Comparative efficacy of intravaginal prostaglandin E2 in the gel and suppository forms for cervical ripening. DICP: The Annals of Pharmacotherapy 1991;25:456‐60. [DOI] [PubMed] [Google Scholar]
Gordon‐Wright 1979 {published data only}
- Gordon‐Wright AP, Elder MG. Prostaglandin E2 tablets used intravaginally for the induction of labour. British Journal of Obstetrics and Gynaecology 1979;86:32‐6. [DOI] [PubMed] [Google Scholar]
Granstrom 1995 {published data only}
- Granstrom L, Hammarstrom M, Hjertberg R, Moberger B, Berg A, Norlander E. Expectant management in nulliparous term pregnant women with premature rupture of membranes and an unripe cervix. Journal of Obstetrics and Gynaecology 1995;15:366‐72. [Google Scholar]
Greer 1986 {published data only}
- Greer IA, Smith JR, Godfree V, McLaren M, Graham N, Calder AA. PGE2 absorption after slow release PGE2 pessaries for the induction of labour. Proceedings of the 24th British Congress of Obstetrics and Gynaecology; 1986 April 15‐18; Cardiff, UK. 1986:237.
Greer 1988 {published data only}
- Greer IA, McLaren M, Godfree V, Michie B, Calder AA. The effects of vaginal prostaglandin E2 administration on plasma concentrations of prostaglandin E3 and prostaglandin F2 metabolites. Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria 1988:108.
Grunstein 1990 {published data only}
- Grunstein S, Jaschevatzky OE, Shalit A, Noy Y, Davidson A, Ellenbogen A. A scoring system for induction of labor using prostaglandin E2 vaginal tablets. International Journal of Gynecology & Obstetrics 1990;31:131‐4. [DOI] [PubMed] [Google Scholar]
Hill 1991 {published data only}
- Hill NCW, Selinger M, Ferguson J, MacKenzie IZ. Management of intra‐uterine fetal death with vaginal administration of gemeprost or prostaglandin E2: a random allocation controlled trial. Journal of Obstetrics and Gynaecology 1991;11:422‐6. [Google Scholar]
Hunter 1982 {published data only}
- Hunter IWE, Hammad MK. Induction of labour using prostaglandin pessaries of varying strength. Ulster Medical Journal 1982;51:141‐5. [PMC free article] [PubMed] [Google Scholar]
Hunter 1984 {published data only}
- Hunter IWE, Cato E, Ritchie JWK. Induction of labor using high‐dose or low‐dose prostaglandin vaginal pessaries. Obstetrics & Gynecology 1984;63:418‐20. [PubMed] [Google Scholar]
Hunter 1998 {published data only}
- Hunter G, Parveen R. A comparison of an intravaginal controlled release prostaglandin e2 (10 mg) for cervical ripening and initiation of labour versus prostaglandin e2 (3 mg) vaginal tablet. Journal of Obstetrics and Gynaecology 1998;18:460‐1. [DOI] [PubMed] [Google Scholar]
Khan 2011 {published data only}
- Khan ZA, Abdul B, Majoko F. Induction of labour with vaginal prostaglandin tablet vs gel. Journal of Obstetrics and Gynaecology 2011;31((6)):492‐4. [DOI] [PubMed] [Google Scholar]
Knogler 1988 {published data only}
- Knogler W, Egarter C, Fitz R, Husslein P. Comparison of prostaglandin (PG) E2 vaginal gel and tablet for elective induction of labor. Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988:111.
Krammer 1995 {published data only}
- Krammer J, O'Brien W, Williams M. Outpatient cervical ripening does not affect gestational age at delivery. American Journal of Obstetrics and Gynecology 1995;172:425. [Google Scholar]
Lass 1994 {published data only}
- Lass A, Rosen DJD, Nahum R, Markov S, Kaneti HY, Fejgin MD, et al. Variable decelerations during pre‐induction oxytocin challenge test predict fetal distress during labor in pregnancies with uncomplicated oligohydramnios. Proceedings of 14th European Congress of Perinatal Medicine; 1994 June 5‐8; Helsinki, Finland. 1994:475.
Lindblad 1985 {published data only}
- Lindblad A, Ekman G, Marsal K, Ulmsten U. Fetal circulation 60 to 80 minutes after vaginal prostaglandin E2 in pregnant women at term. Archives of Gynecology 1985;237:31‐6. [DOI] [PubMed] [Google Scholar]
Lorenz 1984 {published data only}
- Lorenz RP, Botti JJ, Chez RA, Bennett N. Variations of biologic activity of low‐dose prostaglandin E2 on cervical ripening. Obstetrics & Gynecology 1984;64:123‐7. [PubMed] [Google Scholar]
Loria‐Casanova 1989 {published data only}
- Loria‐Casanova ML, Lemus‐Maichel M, Kably‐Ambe A. Evaluation of prostaglandin E2 in cervical maturation [Valoracion del uso de prostaglandinas E2 en la maduracion cervical]. Ginecologia y Obstetricia de Mexico 1989;57:193‐5. [PubMed] [Google Scholar]
MacKenzie 1977 {published data only}
- MacKenzie IZ, Embrey MP. Cervical ripening with intravaginal prostaglandin E2 gel. British Medical Journal 1977;2:1381‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacKenzie 1988 {published data only}
- MacKenzie IZ, Annan B, Jackson C, Hurley P, Hey F, Newman M. A randomised trial comparing a non‐biodegradable polymer PGE2 pessary with a glyceride PGE2 pessary for labour induction. 12th World Congress of Gynecology and Obstetrics; 1988 Oct 23‐28; Rio de Janeiro, Brazil, South America. 1988:199‐200.
MacKenzie 1997b {published data only}
- MacKenzie IZ, Magill P, Burns E. Randomised trial of one versus two doses of prostaglandin E2 for induction of labour: 2. Analysis of cost. British Journal of Obstetrics and Gynaecology 1997;104:1068‐72. [DOI] [PubMed] [Google Scholar]
Nikolov 2003 {published data only}
- Nikolov A, Dimitrov A, Krusteva K, Nashar S. Study of the effect of Propess for ripening of the unfavorable cervix for the induction of labor due to medical indications. Akusherstvo i Ginekologiia 2003;42(3):5‐8. [PubMed] [Google Scholar]
Norchi 1993 {published data only}
- Norchi S, Zanini A, Ragusa A, Maccario L, Valle A. Induction of labor with intravaginal prostaglandin E2 gel. International Journal of Gynecology & Obstetrics 1993;42:103‐7. [DOI] [PubMed] [Google Scholar]
Odum 1993 {published data only}
- Odum CU, Isika AN, Lambo AO. Induction of labour with single insertion of vaginal tablet of prostagladin E2 (PGE2), amniotomy, and oxytocin infusion. West African Journal of Medicine 1993;12:153‐7. [PubMed] [Google Scholar]
Parker 1990 {unpublished data only}
- Parker M. Comparison of prostaglandin E2 gel vs vaginal tablet for cervical ripening. Personal communication 1992.
Petrou 2011 {published data only}
- Petrou S, Taher S, Abangma G, Eddama O, Bennett P. Cost‐effectiveness analysis of prostaglandin E2 gel for the induction of labour at term. BJOG : an international journal of obstetrics and gynaecology 2011;118(6):726‐34. [PUBMED: 21332635] [DOI] [PubMed] [Google Scholar]
Ramsey 1998 {published data only}
- Ramsey PS, Harris DY, Ogburn PL, Heise RH, Magtibay PM, Ramin KD. Comparative cost analysis of prostaglandin analogues dinoprostone and misoprostol as labor preinduction agents [abstract]. Primary Care Update for Ob/Gyns 1998;5(4):182. [DOI] [PubMed] [Google Scholar]
Sadaty 1998 {published data only}
- Sadaty A, Pagano M, Greer C, Sison C, Schaffir J. A randomized trial of vaginal prostaglandin E2 gel and dinoprostone vaginal insert for induction of labor at term [abstract]. Primary Care Update for Ob/Gyns 1998;5(4):183. [DOI] [PubMed] [Google Scholar]
Seeras 1995 {published data only}
- Seeras RC, Olatunbosun OA, Pierson RA, Turnell RW. Induction of labor using prostaglandin E2 (PGE2) vaginal gel in triacetin base. Clinical and Experimental Obstetrics and Gynecology 1995;22:105‐10. [PubMed] [Google Scholar]
Sellers 1985 {published data only}
- Sellers S, MacKenzie IZ. Prostaglandin release following vaginal prostaglandin treatment for labour induction. In: Wood C editor(s). The Role of Prostaglandins in Labour. Vol. 92, London: RSM Services Limited, 1985:80‐3. [Google Scholar]
Smith 1996 {published data only}
- Smith CV, Miller A, Livezey GT. Double‐blind comparison of 2.5 and 5.0 mg of prostaglandin E2 gel for preinduction cervical ripening. Journal of Reproductive Medicine 1996;41:745‐8. [PubMed] [Google Scholar]
Sorensen 2008 {published data only}
- Sorensen MB, Evans C, Ekpe A, Cotzias C. Comparison of three modes of administration of prostaglandin for induction of labour. 36th Nordic Congress of Obstetrics and Gynecology; 2008 June 14‐17; Reykjavik, Iceland. 2008:123‐4.
Sorokin 1992 {published data only}
- Sorokin Y, Hallak M, Klein O, Kalderon I, Abramovici H. Effects of induction of labor with prostaglandin E2 on fetal breathing and body movements: controlled, randomized, double‐blind study. International Journal of Gynecology & Obstetrics 1993;42(1):84. [PubMed] [Google Scholar]
- Sorokin Y, Hallak M, Klein O, Kalderon I, Abramovici H. Effects of induction of labor with prostaglandin E2 on fetal breathing and body movements: controlled, randomized, double‐blind study. Obstetrics & Gynecology 1992;80:788‐91. [PubMed] [Google Scholar]
- Sorokin Y, Hallak M, Klein O, Kalderon I, Abramovici H. Prostaglandin and fetal breathing movement: controlled randomized double blind study. Proceedings of 11th European Congress of Perinatal Medicine; 1988; Rome, Italy. 1988:11.
Spitzberg 1991 {published data only}
- Spitzberg E, Yonekura ML. Preinduction cervical ripening with controlled‐release PGE2 pessary. American Journal of Obstetrics and Gynecology 1991;164:313. [Google Scholar]
Tan 1994 {published data only}
- Tan ASA, Abu J, Cheng HH, Liauw P. Comparing the efficacy of prepidil gel vs prostin E2 vaginal pessaries in cervical priming and induction of labour. International Journal of Gynecology & Obstetrics 1994;46:7. [Google Scholar]
Tan 1999 {published data only}
- Tan LK, Tay SK. Two dosing regimens for preinduction cervical priming with intravaginal dinoprostone pessary: a randomised clinical trial. British Journal of Obstetrics and Gynaecology 1999;106:907‐12. [DOI] [PubMed] [Google Scholar]
Tang 1997 {published data only}
- Tang l, Zhu Q, Wang S. Dose study of methyl carboprost suppository for planned delivery at term. Chung‐Hua‐Fu‐Chan‐Ko‐Tsa‐Chih 1997;32:19‐21. [PubMed] [Google Scholar]
Toplis 1979 {published data only}
- Toplis PJ, Sims CD. Prospective study of different methods and routes of administration of prostaglandin E2 to improve the unripe cervix. Prostaglandins 1979;18:127‐36. [DOI] [PubMed] [Google Scholar]
Toppozada 1992 {published data only}
- Toppozada M, El‐Ghazzawi E, Meleis M, Abd‐Rabbo S. Effect of 9‐deoxo‐16,16‐dimethy‐l9‐methylene‐prostaglandin E2 vaginal gel on the tissues of the pregnant unripe cervix at term. Journal of Obstetrics and Gynaecology 1992;12:228‐31. [Google Scholar]
Varma 1984 {published data only}
- Varma TR, Norman J. Comparison of three dosages of prostaglandin E2 pessaries for ripening the unfavourable cervix prior to induction of labor. Acta Obstetricia et Gynecologica Scandinavica 1984;63:17‐21. [DOI] [PubMed] [Google Scholar]
Veligati 1998 {published data only}
- Veligati P, Broekhuizen FF, Kirby RS, Malnory M. A randomized trial comparing prostaglandin E2 vaginal insert (Cervidil) to vaginal gel for cervical ripening before induction of labor [abstract]. Primary Care Update for Ob/Gyns 1998;5(4):182. [DOI] [PubMed] [Google Scholar]
Walker 1983 {published data only}
- Walker E, Gordon AJ. Length of exposure to prostaglandin E2 and cervical ripening in primigravidae. Journal of Obstetrics and Gynaecology 1983;4:88‐9. [Google Scholar]
Zanini 1991 {published data only}
- Zanini A, Norchi S, Ragusa A, Strobelt N, Lissoni A. Induction of labour with vaginal PGE2 gel: a controlled clinical trial. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands. 1991:144.
Additional references
Alfirevic 2009
- Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003246.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alfirevic 2014
- Alfirevic Z, Aflaifel N, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD001338.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bakker 2013
- Bakker JJH, Goes BY, Pel M, Mol BWJ, Post JAM. Morning versus evening induction of labour for improving outcomes. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD007707.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bimbashi 2012
- Bimbashi A, Duley L, Ndoni E, Dokle A. Amniotomy plus intravenous oxytocin for induction of labour. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009821] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulvain 2005
- Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD000451.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulvain 2008
- Boulvain M, Kelly AJ, Irion O. Intracervical prostaglandins for induction of labour. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006971] [DOI] [PubMed] [Google Scholar]
Bricker 2000
- Bricker L, Luckas M. Amniotomy alone for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002862] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 2002
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.1.5 [Updated April 2002]. In: The Cochrane Library, Issue 3, 2002. Oxford: Update Software. Updated quarterly.
Curtis 1987
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91‐5. [PubMed] [Google Scholar]
Dare 2009
- Dare MR, Middleton P, Crowther CA, Flenady V, Varatharaju B. Planned early birth versus expectant management (waiting)for prelabour rupture of membranes at term (37 weeks or more). Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005302.pub2] [DOI] [PubMed] [Google Scholar]
Dowswell 2010
- Dowswell T, Kelly AJ, Livio S, Norman JE, Alfirevic Z. Different methods for the induction of labour in outpatient settings. Cochrane Database of Systematic Reviews 2010, Issue 8. [DOI: 10.1002/14651858.CD007701.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Facchinetti 2012
- Facchinetti F, Fontanesi F, Giovane C. Pre‐induction of labour: comparing dinoprostone vaginal insert to repeated prostaglandin administration: a systematic review and meta‐analysis. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(10):1965–9. [DOI] [PubMed] [Google Scholar]
French 2001
- French L. Oral prostaglandin E2 for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003098] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gülmezoglu 2012
- Gülmezoglu AM, Crowther CA, Middleton P, Heatley E. Induction of labour for improving birth outcomes for women at or beyond term. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD004945.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hapangama 2009
- Hapangama D, Neilson James P. Mifepristone for induction of labour. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002865.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2009
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002074.pub2] [DOI] [Google Scholar]
Hofmeyr 2010
- Hofmeyr GJ, Gülmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD000941.pub2; CD000941] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howarth 2001
- Howarth GR, Botha DJ. Amniotomy plus intravenous oxytocin for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2001
- Hutton E, Mozurkewich E. Extra‐amniotic prostaglandin for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jozwiak 2012
- Jozwiak M, Bloemenkamp KWM, Kelly AJ, Mol BWJ, Irion O, Boulvain M. Mechanical methods for induction of labour. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD001233.pub2] [DOI] [PubMed] [Google Scholar]
Jozwiak 2012a
- Jozwiak M, Dodd JM. Methods of term labour induction for women with a previous caesarean section. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD009792.pub2] [DOI] [PubMed] [Google Scholar]
Kavanagh 2001
- Kavanagh J, Kelly AJ, Thomas J. Sexual intercourse for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003093] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2005
- Kavanagh J, Kelly AJ, Thomas J. Breast stimulation for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003392.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2006a
- Kavanagh J, Kelly AJ, Thomas J. Corticosteroids for induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003100.pub2] [DOI] [PubMed] [Google Scholar]
Kavanagh 2006b
- Kavanagh J, Kelly AJ, Thomas J. Hyaluronidase for cervical priming and induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003097.pub2] [DOI] [PubMed] [Google Scholar]
Kelly 2001b
- Kelly AJ, Kavanagh J, Thomas J. Relaxin for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003103] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2011
- Kelly AJ, Munson C, Minden L. Nitric oxide donors for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD006901.pub2] [DOI] [PubMed] [Google Scholar]
Kelly 2013
- Kelly AJ, Kavanagh J, Thomas J. Castor oil, bath and/or enema for cervical priming and induction of labour. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD003099.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2013a
- Kelly AJ, Alfirevic Z, Ghosh A. Outpatient versus inpatient induction of labour for improving birth outcomes. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD007372.pub3] [DOI] [PubMed] [Google Scholar]
Luckas 2000
- Luckas M, Bricker L. Intravenous prostaglandin for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002864] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mozurkewich 2011
- Mozurkewich EL, Chilimigras JL, Berman DR, Perni UC, Romero VC, King VJ, et al. Methods of induction of labour: a systematic review. BMC Pregnancy and Childbirth 2011;11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muzonzini 2004
- Muzonzini G, Hofmeyr GJ. Buccal or sublingual misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004221.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2001
- Induction of Labour. NICE Clinical Guideline D. http://www.nice.org.uk/guidance/index.jsp?action=byID&o=10909 June 2001.
NICE 2008
- NICE. Induction of Labour. Clinical Guideline CG70. http://guidance.nice.org.uk/CG70 July 2008.
Nishi 2013
- Nishi D, Shirakawa MN, Ota E, Hanada N, Mori R. Hypnosis for induction of labour. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010852] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
RevMan 2008 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Smith 2003
- Smith CA. Homoeopathy for induction of labour. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003399] [DOI] [PubMed] [Google Scholar]
Smith 2013
- Smith CA, Crowther CA, Grant SJ. Acupuncture for induction of labour. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD002962.pub3] [DOI] [PubMed] [Google Scholar]
Thomas 2001
- Thomas J, Kelly AJ, Kavanagh J. Oestrogens alone or with amniotomy for cervical ripening or induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003393] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kelly 2003
- Kelly AJ, Kavanagh J, Thomas J. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003101] [DOI] [PubMed] [Google Scholar]
Kelly 2009
- Kelly AJ, Malik S, Smith L, Kavanagh J, Thomas J. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003101.pub2] [DOI] [PubMed] [Google Scholar]


