Abstract
Physical exercise has a neuroprotective effect and is an important treatment after ischemic stroke. Promoting neurogenesis and myelin repair in the penumbra is an important method for the treatment of ischemic stroke. However, the role and potential mechanism of exercise in neurogenesis and myelin repair still needs to be clarified. The goal of the present study was to ascertain the possible effect of treadmill training on the neuroprotective signaling pathway in juvenile rats after ischemic stroke. The model of middle cerebral artery occlusion (MCAO) in juvenile rats was established and then the rats were randomly divided into 9 groups. XAV939 (an inhibitor of the Wnt/β-catenin pathway) was used to confirm the effects of the Wnt/β-catenin signaling pathway on exercise-mediated neurogenesis and myelin repair. Neurological deficits were detected by modified neurological severity score, the injury of brain tissue and the morphology of neurons was detected by hematoxylin-eosin staining and Nissl staining, and the infarct volume was detected by 2,3,5-triphenyl tetrazolium chloride staining. The changes in myelin were observed by Luxol fast blue staining. The neuron ultrastructure was observed by transmission electron microscopy. Immunofluorescence and western blots analyzed the molecular mechanisms. The results showed that treadmill exercise improved neurogenesis, enhanced myelin repair, promoted neurological function recovery and reduced infarct volume. These were the results of the upregulation of Wnt3a and nucleus β-catenin, brain-derived neurotrophic factor (BDNF) and myelin basic protein (MBP). In addition, XAV939 inhibited treadmill exercise-induced neurogenesis and myelin repair, which was consistent with the downregulation of Wnt3a, nucleus β-catenin, BDNF and MBP expression, and the deterioration of neurological function. In summary, treadmill exercise promotes neurogenesis and myelin repair by upregulating the Wnt/β-catenin signaling pathway, to improve the neurological deficit caused by focal cerebral ischemia/reperfusion.
Keywords: middle cerebral artery occlusion, treadmill exercise, Wnt/β-catenin, neurogenesis, myelin repair, neuroprotection
Introduction
Stroke is one of the main causes of death and permanent disability worldwide (1). Cerebral artery occlusion can cause acute ischemic stroke. Acute ischemic stroke accounts for >80% of all strokes (2). Although some progress has been made in post-stroke treatment, stroke intervention is still insufficient (1). To date, no successful long-term neuroprotective therapy has been identified in clinical trials (3–5). The ischemic core is considered to be an irreparably damaged area. Due to a serious lack of blood flow, numerous nerve cells die within a few minutes after occlusion (6). The ischemic penumbra was first proposed by Astrup et al (7). The penumbra is an area of the brain tissue that is damaged but not yet dead after local ischemia (8). Clinically, the ischemic penumbra is called the low perfusion area around the ischemic core. If the cerebral blood flow is restored in a timely manner, the damaged nerve cells can be saved (9). Neurogenesis (the birth of new neurons) is a process involving the production of functional neurons from precursor cells and occurs throughout the life cycle of the mammalian brain, indicating it is an attractive target for potential intervention (10,11). Most studies have focused on newborn, perinatal and adult rodents, and few have evaluated neurogenesis and myelin repair in adolescents after stroke. Adult neurogenesis is different from developmental neurogenesis (12–14). In the developing brain, immature neurons are extremely sensitive and vulnerable to widespread insults and toxic exposures (15). A recent study reported that 3 to 4-week-old mice have fully developed brains and juvenile mice show mature brain neurons like adult mice, and are not vulnerable to the factors found in neonatal and perinatal brain development (10). Therefore, the juvenile brain is an ideal choice for the study of neurogenesis (10). Elucidation of the signaling molecules and related signaling pathways involved in the protection of nerve cells in the ischemic penumbra after juvenile ischemic stroke is needed.
However, whether in the development of the central nervous system (CNS) or after CNS damage, the Wnt/β-catenin signal transduction pathway plays a key role remains to be elucidated (16). It has been found that Wnt3a is an important protein in the Wnt family. It is involved in neurogenesis in the hippocampus and cortex (17,18). Research showed that intranasal administration of Wnt3a can enhance the neuroprotection and regeneration of the Wnt signaling pathway after focal ischemic stroke in mice (19). Previous studies have shown that Wnt signal transduction is the main regulator of hippocampal neurogenesis in adults (20–23). Activating the Wnt pathway in vivo and in vitro was shown to increase neurogenesis, and blocking the Wnt pathway inhibited the proliferation and differentiation of rat neural progenitor cells (NPCs) (20). Moreover, Wnt signaling promotes functional recovery by increasing neurogenesis (24).
Physical exercise can promote neurogenesis, angiogenesis and enhance dendritic modification and synaptic plasticity (25,26). Promoting brain-derived neurotrophic factor (BDNF) expression during development can regulate the cell signal transduction pathway, promote neuronal regeneration and contribute to synaptic plasticity, learning, memory and sensorimotor recovery (27). Treadmill exercise promotes oligodendrocytes and, thus, myelination. In addition, the improvement of ischemia-induced myelin injury by long-term exercise is related to increased BDNF expression (28). Although the authors’ previous studies (25,29,30) have focused on the neuroprotective effect of treadmill exercise in adult rats its neuroprotective effect on juvenile rats has not been explored.
In this study, it was shown that treadmill exercise promoted neurogenesis and myelin repair in juvenile rats. The inhibitor of the Wnt signaling pathway was used to elucidate the role of the Wnt/β-catenin signaling pathway in treadmill exercise-promoted neurogenesis and myelin repair. It was further clarified that neurogenesis and myelin repair after ischemic stroke are closely related to treadmill exercise and the Wnt/β-catenin signaling pathway.
Materials and methods
Reagents
Anti-Wnt3a (1:1,000; EMD Millipore), anti-β-catenin (1:5,000; Abcam), anti-BDNF (1:1,000; Abcam), anti-myelin basic protein (MBP; 1:1,000; Abcam), anti-Dcx (1:200; Abcam), anti-NESTIN (1:200; Abcam), anti-GAPDH (1:2,000; Abcam), anti-Lamin B1 (1;1,000; Abcam), XAV939 (Selleck Chemicals), 2,3,5-triphenyl tetrazolium chloride (TTC; Sigma-Aldrich; Merck KGaA), phenylmethylsulfonyl-fluoride (Beijing Solarbio Science & Technology Co., Ltd.), Clarity™ Western ECL Substrate kit (Bio-Rad Laboratories, Inc.), Alexa Fluor 488 AffiniPure Donkey Anti-Mouse IgG and Alexa Fluor 594 AffiniPure Goat Anti-Rabbit IgG (1:200; Yesen Bio), 4,6-diamidino-2-phenylindole (DAPI; Beyotime Institute of Biotechnology).
Animals
The Shanghai Experimental Animal Center provided 171 juvenile Sprague-Dawley male rats (weight, 80–90 g; Sprague Dawley rats were weaned for 21 days and matured for 6 to 7 weeks). The Animal Research Committee of Wenzhou Medical University approved the present experimental project and all the experimental followed the guidelines of the National Institutes of Health on the Care and Use of Animals (ethical no. wydw2019–0766). The animals were placed in a suitable environment (4 rats per cage, relative humidity 55±5%, 22°C, 12-h light/dark cycle) and had free access to food and water. The rats were randomly divided into 9 groups: Sham operation group (n=19; S group); model group 7 and 14 days, respectively (M7: n=19 and M14: n=19); treadmill training models (EM7: n=19 and EM14: n=19); inhibitor treatment models (IM7: n=19 and IM14: n=19); and inhibitor treatment and treadmill training models (IEM7: n=19 and IEM14: n=19). The present research was conducted following these criteria (31): i) Randomization, ii) allocation concealment and iii) blinded assessment of outcome.
Middle cerebral artery occlusion (MCAO) model of focal brain ischemia
Briefly, rats were anesthetized with 3% isoflurane vaporized in 30% O2/70% N2 until they were unresponsive to the tail pinch test and then fitted with a nose cone blowing 1.5% isoflurane for anesthesia maintenance (Shenzhen RWD Life Science Co., Ltd.) during surgery. After the rats were anesthetized, they were placed in the supine position on the sterilized operating table and an incision was made in the right side of the neck. The common carotid artery and external carotid artery (ECA) was separated from the peripheral connective tissue without injury to peripheral muscles and nerves. A small gap was opened in the stump of the ECA and then a small silicon-coated surgical nylon monofilament (0.24±0.02 mm/l, 2,400; Guangzhou Jialing Biotechnology Co., Ltd.) was inserted into the lumen of the internal carotid artery (ICA), and the operation was stopped ~17 mm from the far end of the ICA bifurcation to block the middle cerebral artery (MCA). The rats during and after the operation were preserved under a 37°C heating pad. After 1.5 h of occlusion, the filament in the rat ICA was removed under a light microscope (Olympus Corporation) and the blood flow was restored, and the reperfusion was allowed for 7 or 14 days. The rats in the sham operation group were treated with the same surgical method as the experimental group except for the insertion of the surgical nylon monofilament. A total of 59 rats were excluded from the present study, including 14 rats with low Zea Longa score (0 or 4) (32) and 45 rats that died. Among the 45 rats that died, 14 rats died of brain edema, 9 died of subarachnoid hemorrhage, 13 died of massive haemorrhage and 9 died of long operation time. In addition, all juvenile rats were anesthetized by intraperitoneal injection of pentobarbital sodium (65 mg/kg) before sacrifice.
Treadmill exercise and inhibitor
In this study, a small animal electric treadmill was used (XR-PT-10A; Shanghai XinRuan Information Technology Co., Ltd.). Before MCAO, the animals received three days of adaptive treadmill training. The EM group and IEM group underwent treadmill training at 0 slope, 8 m/min, 30 min/d and 5 d/for 7 days or 14 days, respectively.
XAV939, a small molecule inhibitor of Wnt/β-catenin signaling (40 mg/kg; intraperitoneal injection) was used. In short, immediately after MCAO, XAV-939 was injected into the IM7 group and the IEM7 group, and then injected every 24 h for 7 days. Also, in IM14 group and IEM14 group, immediately after MCAO, XAV-939 was injected into IM14 group and IEM14 group, and then injected every 24 h for 14 days. the dose of XAV939 was determined based on dose-response studies and published reports (33,34). In addition, rats of the other groups (S, M and EM) were simultaneously injected with the same amount of physiological saline.
Evaluation of neurologic deficit scores
A total of two independent examiners performed the modified nerve severity score (mNSS) test (35) at 1, 7 and 14 days after MCAO. The researchers were blinded to the treatment group (Table I).
Table I.
Modified neurological severity score points.
| Motor tests | |
| Raising rat by tail | 3 |
| Flexion of forelimb | 1 |
| Flexion of hindlimb | 1 |
| Head moved 10 to vertical axis within 30 sec | 1 |
| Placing rat on floor (normal=0; maximum=3) | 3 |
| Normal walk | 0 |
| Inability to walk straight | 1 |
| Circling toward paretic side | 2 |
| Falls down to paretic side | 3 |
| Sensory tests | 2 |
| Placing test (visual and tactile test) | 1 |
| Proprioceptive test (deep sensation, pushing paw against table edge to stimulate limb muscles) | 1 |
| Beam balance tests (normal=0; maximum=6) | 6 |
| Balances with steady posture | 0 |
| Grasps side of beam | 1 |
| Hugs beam and 1 limb falls down from beam | 2 |
| Hugs beam and 2 limbs fall down from beam, or spins on beam (>60 sec) | 3 |
| Attempts to balance on beam but falls off (>40 sec) | 4 |
| Attempts to balance on beam but falls off (>20 sec) | 5 |
| Falls off; no attempt to balance or hang on to beam (<20 sec) | 6 |
| Reflex absence and abnormal movements | 4 |
| Pinna reflex (head shake when auditory meatus is touched) | 1 |
| Corneal reflex (eye blink when cornea is lightly touched with cotton) | 1 |
| Startle reflex (motor response to a brief noise from snapping a clipboard paper) | 1 |
| Seizures, myoclonus, myodystony | 1 |
| Maximum points | 18 |
One point is awarded for inability to perform the tasks or for lack of a tested reflex: 13–18, severe injury; 7–12, moderate injury; 1–6, mild injury.
Infarct volume assessment
For determination of the volume of cerebral infarction, TTC staining was performed on consecutive sections of bregma +4.0–6.0 mm. the brain was cut with a blade into five consecutive coronal sections separated by 2.0 mm. The obtained brain tissue was frozen at −20°C for 10 min. Then, all brain slices were immediately immersed in 2% TTC solutions, at 37°C for 30 min and fixed at 4°C for 24 h in 4% paraformaldehyde buffer. In 5 sections, the total cerebral infarction volume was equal to the sum of the infarction area. The correction formula for calculating the infarction volume with minimization of the error caused by brain edema is as follows (36): Infarct percentage = [(contralateral hemisphere region-non-infarcted region in the ipsilateral hemisphere)/volume of the contralateral hemisphere] * 100%. Then, the analysis was carried out using ImageJ software (ImageJ bundled with 64-bit Java 1.8.0_112; National Institutes of Health).
Bromodeoxyuridine injection and tissue preparation
Bromodeoxyuridine (BrdU; 50 mg/kg/day) was injected intraperitoneally into rats every day after MCAO for 7 or 14 days to label newly formed cells. The proliferation of neural stem cells (NSCs) and the differentiation of NSCs were observed by double immunofluorescence.
At 7 and 14 days after MCAO, 3 rats in each group were sacrificed. Under deep anesthesia with pentobarbital sodium (65 mg/kg; intraperitoneally), the rats were perfused with 0.9% sodium chloride (4°C) and then perfused with 4% paraformaldehyde (Sigma-Aldrich; Merck KGaA) in 0.1 mol/l phosphate buffer (pH 7.4). The whole brain was collected, fixed at 4°C for 24 h, dehydrated and finally embedded in paraffin wax. Then, coronal sections of 5 μm were sliced by a microtome (Kedee Instrumental Equipment Co. Ltd.) for hematoxylin-eosin (H&E) or Nissl staining. The rat samples used for immunofluorescence labeling were removed and stored in the same fixation solution at 4°C for 24 h and soaked overnight in 0.1 M phosphate buffer (20 and 30% sucrose) at 4°C. The brain tissue was embedded and frozen at −80°C in the optimal cutting temperature complex. Finally, the coronal section of the ischemic penumbra was cut with a cryostat (CM1900; Leica Microsystems GmbH) (5 μm thick).
H&E staining
At least 3 slices were taken from each rat, dewaxed in xylene and then dehydrated in alcohol. Finally, H&E staining was performed at room temperature for 2 h, and observed under a light microscope (Olympus Corporation). Pathological changes in brain tissue were observed, images were captured and recorded.
Toluidine blue (Nissl) staining
The slices were washed 3 times. The slices were dyed at 37°C with 1% toluene blue solution for 30 min. The tissue was decolorized and dehydrated with ethanol and sealed with neutral resin. Under light microscopy (Olympus Corporation), the Nissl bodies of neurons were blue and purple.
Luxol fast blue (LFB) staining
The brain tissue was embedded in paraffin and then, the 5 μm thick coronal slices were stained with 0.1% LFB (cat. no. S3382; Sigma-Aldrich; Merck KGaA) at 60°C for 2 h. The slices were soaked in 0.05% lithium carbonate solution to distinguish white matter from gray matter. Finally, the slices were placed in distilled water, re-dyed at room temperature in cresol purple solution for 30–40 sec and then washed with distilled water.
Immunofluorescence
The slices were dried, then restored to room temperature and soaked in 0.01 M phosphate buffer (PBS, pH 7.6) 3 times for 5 min. The sections were blocked for 1 h (20–22°C) with 10% goat serum (cat. no. C0265; Beyotime Institute of Biotechnology) containing 0.3% Triton X-100. Dcx/BrdU and Nestin/BrdU double immunofluorescence staining was performed. Samples were incubated at 37°C with 1N HCL for 30 min before blocking and then denatured twice for 10 min with borate buffer (pH 8.4). Then mixtures of mouse anti-BrdU antibody (dilution, 1:1,000; cat. no. MAB4072; EMD Millipore) and rabbit anti-Dcx antibody (dilution, 1:200; cat. no. ab207175; Abcam), mixtures of mouse anti-BrdU antibody and rabbit anti-Nestin antibody (dilution, 1:200; cat. no. ab105389; Abcam), and rabbit anti-BDNF antibody (dilution, 1:100; cat. no. ab108319; Abcam), used as antibodies for Dcx/BrdU, Nestin/BrdU and BDNF, respectively, were separately added for incubation overnight at 4°C. Next, the sections were restored to room temperature. Then, the slices were soaked in 0.01 M PBS 3 times for 5 min and performed with the corresponding Alexa Fluor 488 AffiniPure Donkey Anti-Mouse IgG (dilution, 1:200; cat. no. 34106ES60; Shanghai Yeasen Biotechnology Co., Ltd.) and Alexa Fluor 594 AffiniPure Goat Anti-Rabbit IgG (dilution, 1:200; cat. no. 33112ES60; Shanghai Yeasen Biotechnology Co., Ltd.) secondary antibodies for 1 h at room temperature. Nuclei were counterstained at room temperature for 10 min with DAPI. The negative control sections were incubated with 0.01 M PBS instead of primary antibody and no positive fluorescence signal was found. According to the present team’s previous explorations and classical anatomical methods, the area of observation of the ischemic penumbra is as shown in Fig. 1C and D (37). Brain sections were covered and the ischemic penumbra was observed and analyzed by fluorescence microscopy (BX51; Olympus Corporation) in all sections. Counting the positive cells of 5 non-overlapping fields (x400-fold) on one slice using Image-Pro Plus 5.0 analysis software (Media Cybernetics, Inc.).
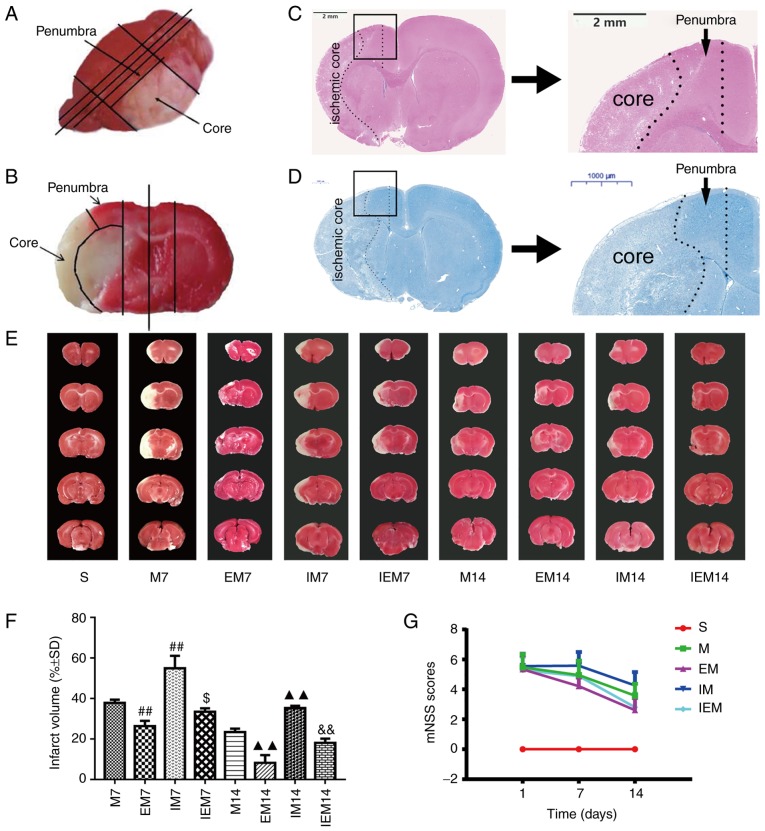
Figure 1.
Treadmill training attenuates neurological deficits and decreases infarct size, while the inhibitor increased infarct injury. (A and B) Site of ischemic core and ischemic penumbra area for the same rat. (A) Overall look for the site of ischemic core and ischemic penumbra area. (B) Local image on site of ischemic core and ischemic penumbra area; picture from the previous paper of the current team (24). (C) Hematoxylin & eosin staining. Scale bars=2 mm. (D) Nissl staining. Scale bars=1,000 μm. (C) and (D) are shown the location of ischemic penumbra. (E) Showed infarct volumes were assessed by 2,3,5-triphenyl tetrazolium chloride. (F) Percentage of infarct volume in each group. Data are presented as the mean ± standard deviation (n=5). ##P<0.001 vs. the M7 group; $P<0.05 vs. the EM7 group; ▲▲P<0.01 vs. the M14 group; &&P<0.01 vs. the EM14 group. (G) Neurological scores in different groups, in which EM7 vs. M7, P<0.01; EM14 vs. M14, P<0.01; IM7 vs. M7, P<0.05; IM14 vs. M14, P<0.01; IEM7 vs. EM7, P<0.01; IEM14 vs. EM14, P>0.05. M group, model group; EM group, treadmill training model group; mNSS, modified nerve severity score.
Western blot analysis
As in the authors’ previous publication (26), coronal sections were performed at 4 and 9 mm from the prefrontal lobe, and then the left and right brains were separated. Finally, sagittal sections were performed on the 1.0–1.5 mm from the midline of the brain. a transverse diagonal cut was then performed at ~’10 o’clock’ to separate the core from the penumbra (38) (Fig. 1A and B). According to the protocol of rat brain tissue extraction kit (Beyotime Institute of Biotechnology), total protein and nuclear protein were extracted respectively. The protein concentration was detected by a BCA protein detection kit (Beyotime Institute of Biotechnology). The same amount of protein (50 μg) was transferred to a polyvinylidene difluoride (EMD Millipore) membrane after 10% SDS-PAGE analysis. The membranes were blocked with 5% skim milk at room temperature for 2 h. Wnt3a, β-catenin, BDNF, MBP, GAPDH and Lamin B1 were incubated on a 4°C shaking table overnight. TBS with 0.05% Tween-20 was washed three times and then, the membranes were soaked in horseradish peroxidase-conjugated secondary antibody (HRP AffiniPure Goat Anti-Rabbit IgG (H+L); dilution, 1:10,000; cat. no. E030120; EarthOx, LLC) at room temperature for 1.5 h. The protein imaging was detected by a Clarity™ Western ECL Substrate kit (cat. no. 1705060; Bio-Rad Laboratories, Inc.). ImageJ software (National Institute of Health) was used for quantitative analysis.
Transmission electron microscopy (TEM)
The rats were sacrificed at 7 and 14 days after MCAO, and ultrastructural changes of neurons in the ischemic penumbra cortex was observed by TEM. The specimens were fixed for at least 2 h with 2.5% (w/v) glutaraldehyde overnight, washed three times for 10 min in PBS and then soaked with 2% (v/v) osmium tetroxide at 37°C for 1 h. The specimens were taken out and rinsed twice, then stained at room temperature for 2 h with 1% uranyl acetate and dehydrated with acetone. After dehydration, the tissue was embedded in the mixture of acetone and entrapped liquid (1:1) for incubation at 37°C for 2 h, followed by maintenance in mixture of acetone and entrapped liquid (1:4) for incubation at 37°C overnight. After that, the tissue was soaked in entrapped liquid for 2 h at 45°C, followed by incubation at 45°C for 3 h and 65°C for 48 h to obtain the coronal sections. Semi-thin sections and toluidine blue staining were used for localization observation. Finally, at least three ultra-thin sections in each sample were observed by TEM (JEM-1200EX; JEOL, Ltd.).
Statistical analysis
All analyses were performed using GraphPad Prism 4.0 (GraphPad Software, Inc.) or IBM SPSS 25.0 statistical software (IBM Corp.). Multiple comparisons were carried out by one-way analysis of variance (ANOVA) with Tukey (when equal variances assumed) or Dunnett’s T3 (when equal variances not assumed) post-hoc analysis. P<0.05 was considered to indicate a statistically significant difference. The data are reported as the mean ± standard deviation.
Results
Treadmill training attenuates neurological deficits, reduces infarct size and decreases the damage of brain tissue and deformation of neurons, while the inhibitor increases infarct injury
Treadmill training attenuates neurologic deficits
As shown in the Table II and Fig. 1G, the S group was normal (the scores=0). One day after MCAO, there were no significant differences in the scores of neurological impairments among the four groups (P>0.05) and the scores decreased gradually with the number of days. The score of the EM group was decreased compared with the M group in the same period (EM7 vs. M7: P<0.01; EM14 vs. M14: P<0.01). The scores of the IM group were increased compared with the M group (IM7 vs. M7: P<0.05; IM14 vs. M14: P<0.01). The score of the EM group was decreased compared with the IEM group (IEM7 vs. EM7: P<0.01; IEM14 vs. EM14: P>0.05).
Table II.
Modified neurological severity scores post middle cerebral artery occlusion.
| Group | N | 1 day | 7 days | 14 days |
|---|---|---|---|---|
| S | 19 | 0 | 0 | 0 |
| M | 38 | 5.47±0.89a | 4.95±0.96a | 3.58±0.79a |
| EM | 38 | 5.34±0.94 | 4.21±0.58b | 2.61±0.82b |
| IM | 38 | 5.55±0.83 | 5.58±0.92b | 4.26±0.89b |
| IEM | 38 | 5.29±0.90 | 4.89±0.83c | 2.81±0.90 |
Data were presented as mean ± standard deviation, ANOVA.
P<0.05 vs. the same period of S.
P<0.05 vs. the same period of M.
P<0.05 vs. the same period of EM.
S group, sham operation group; M group, model group; EM group, treadmill training model group; IM group, inhibitor treatment model group; IEM group, Inhibitor treatment and treadmill training model group.
Treadmill training reduces the infarct volume
As shown in Fig. 1E and F, the white area represents damaged brain cells, and the red area represents functional cells. As expected, no infarction was observed in group S. TTC staining showed that the infarct volume in the EM group was smaller than that in the M group during the same period (P<0.01), while the infarct volume in the IM group was larger than that in the M group during the same period (P<0.01). Compared with the EM group, the IEM group had larger infarct volumes (IEM7 vs. EM7: P<0.05; IEM14 vs. EM14: P<0.01) (Table III).
Table III.
Results of infarct volume in each group after middle cerebral artery occlusion (%).
| Group | N | 7 days | 14 days |
|---|---|---|---|
| S | 3 | 0 | 0 |
| M | 5 | 37.92±1.50a | 23.37±1.75a |
| EM | 5 | 26.28±2.68b | 8.19±3.86b |
| IM | 5 | 54.89±6.21b | 35.25±1.20b |
| IEM | 5 | 33.41±1.77c | 18.05±2.12c |
Data were presented as mean ± standard deviation, ANONE.
P<0.05 vs. the same period of S.
P<0.05 vs. the same period of M.
P<0.05 vs. the same period of EM.
S group, sham operation group; M group, model group; EM group, treadmill training model group; IM group, inhibitor treatment model group; IEM group, Inhibitor treatment and treadmill training model group.
Treadmill training decreases the damage of brain tissue and deformation of neurons
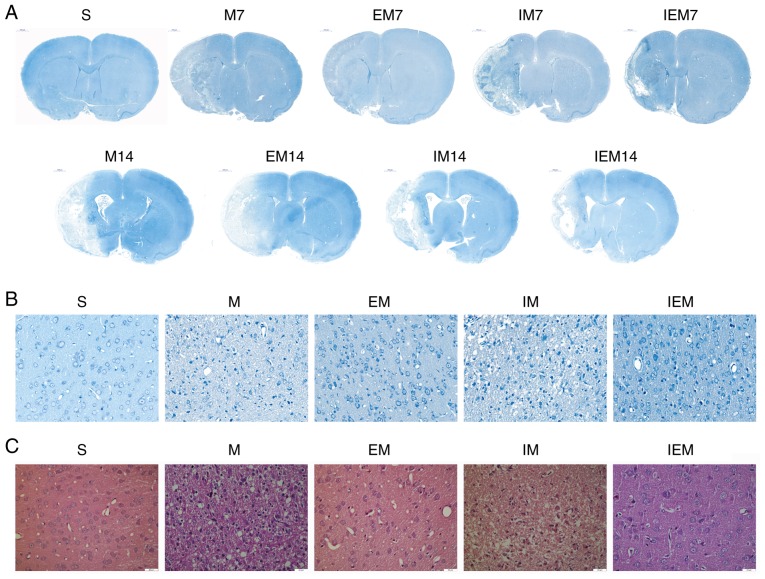
As shown in Fig. 2, no infarction in the S group was found, but infarction was discovered in the ischemic areas of the other groups. In group S, as shown in Fig. 2, the neurons in the cerebral cortex were regular in shape and normal in structure. The nucleolus was clear. In the M and IM groups, numerous neurons in the ischemic penumbra were deformed and necrotic. Increased nuclear pyknosis and nuclear fragmentation occurred compared with in the S group. The Nissl bodies were narrowed, deeply stained and in some instances, disappeared. The cells were swollen, the surrounding space was enlarged and the shape was irregular. In the EM and IEM groups, the cells had a normalized arrangement, and a few cells were deformed and necrotic.
Figure 2.
Treadmill training decreases the damage of tissue structure; reduces the deformation of brain cells and neurons, while inhibitor increased infarct injury. (A) Nissl staining at 7 and 14 days. Scale bars=1,000 μm. (B) Nissl staining of the surviving cells at 7 days. Scale bars=50 μm. (C) Cell morphology by hematoxylin and eosin staining at 7 days. Scale bars=20 μm.
Treadmill training enhances the activation of Wnt/β-catenin signaling pathway in the ischemic penumbra
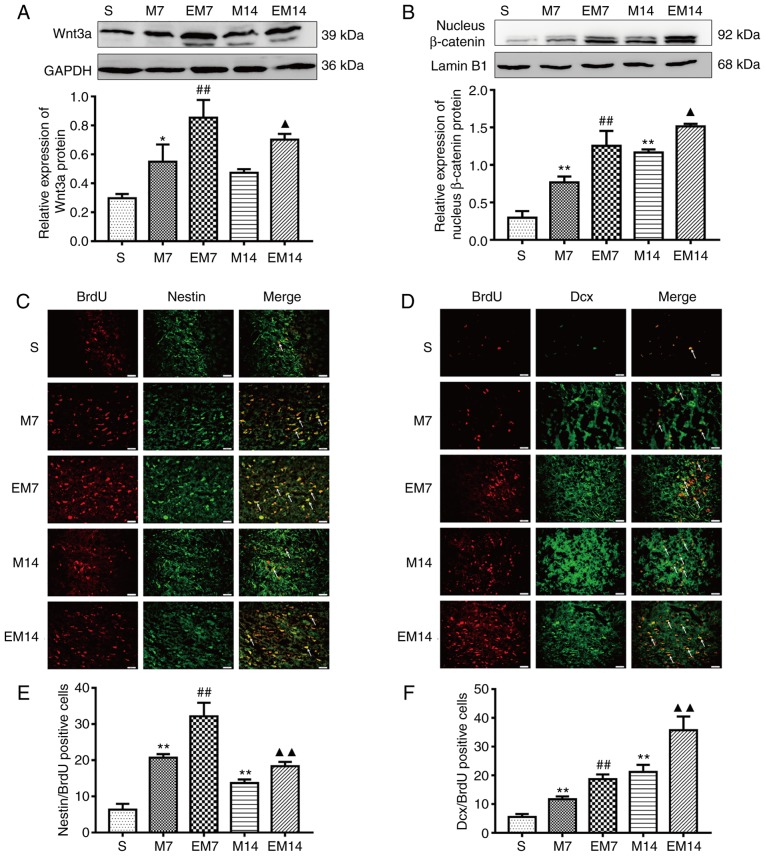
Compared with group M, the expression of Wnt3a and nuclear β-catenin protein in the EM group was increased (Wnt3a, EM7 vs. M7: P<0.01; EM14 vs. M14: P<0.05; nuclear β-catenin, EM7 vs. M7: P<0.01; EM14 vs. M14: P<0.05; Fig. 3A and B). Compared with group S, the expression of Wnt3a and nuclear β-catenin protein in the M group was increased (Wnt3a, S vs. M7: P<0.05; S vs. M14: P>0.05; nuclear β-catenin, S vs. M7: P<0.01; S vs. M14: P<0.01; Fig. 3A and B)
Figure 3.
Treadmill training enhances the activation of the Wnt/β-catenin signaling pathway and improves neurogenesis in the ischemic penumbra. (A) Protein expression levels and western blot analysis of Wnt3a. (B) Protein expression levels and western blot analysis of nucleus β-catenin. Data are presented as the mean ± SD (n=3). (C) IF of Nestin/BrdU-positive cells in the ischemic penumbra. (D) IF of Dcx/BrdU-positive cells in the ischemic penumbra. (E) The number of Nestin/BrdU-positive cells in the ischemic penumbra. (F) The number of Dcx/BrdU-positive cells in the ischemic penumbra. Data are presented as the mean ± SD (n=6). Scale bars=20 μm. *P<0.05 and **P<0.01 vs. the S group; ##P<0.01 vs. the M7 group; ▲P<0.05 and ▲▲P<0.01 vs. the M14 group. SD, standard deviation; BrdU, bromodeoxyuridine; IF, immunofluorescence.
Treadmill training increases the expression level of BDNF in the ischemic penumbra
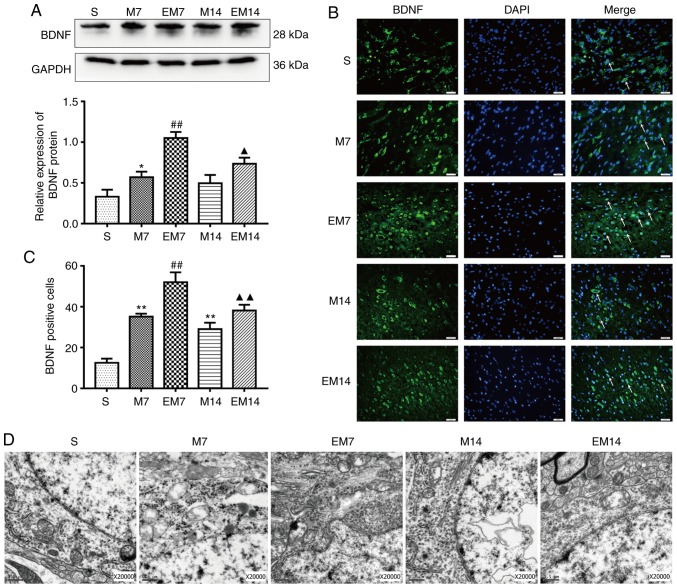
The expression of BDNF protein and the number of BDNF-positive cells in the M groups was increased compared with in the S groups (western blotting: BDNF, S vs. M7: P<0.05; S vs. M14: P>0.05; immunofluorescence: BDNF, S vs. M7: P<0.01; S vs. M14: P<0.01). Similarly, the expression of the BDNF protein and the number of BDNF-positive cells in EM group were increased compared with in the M group (western blotting: BDNF, EM7 vs. M7: P<0.01; EM14 vs. M14: P<0.05; immunofluorescence: BDNF, EM7 vs. M7: P<0.01; EM14 vs. M14: P<0.01; Fig. 4A–C).
Figure 4.
Treadmill training increases the expression level of BDNF and improves the morphology of cortical neurons in the ischemic penumbra after MCAO. (A) Protein expression levels and western blot analysis of BDNF. Data are presented as the mean ± SD (n=3). (B) Immunofluorescence of BDNF-positive cells in the ischemic penumbra. Scale bars=20 μm. (C) The number of BDNF-positive cells in the ischemic penumbra. Data are presented as the mean ± SD (n=6). (D) Transmission electron microscopy showed the neuron nucleus structures (n=3). Scale bars=0.5 μm. *P<0.05 and **P<0.01 vs. the S group; ##P<0.01 vs. the M7 group; ▲P<0.05 and ▲▲P<0.01 vs. the M14 group. S group, sham operation group; M group, model group; MCAO, middle cerebral artery occlusion; SD, standard deviation; BDNF, brain derived neurotrophic protein; DAPI, 4,6-diamidino-2-phenylindole.
Treadmill training improves regenerative neurogenesis in the ischemic penumbra
Nestin/BrdU is a specific marker of proliferating NSCs. Dcx/BrdU is a specific marker of proliferating neuroblasts and differentiating neurons (14). There were almost no Dcx/BrdU-positive cells and Nestin/BrdU-positive cells in the ischemic penumbra of the S group in this study. Compared with that of the sham group, the number of Nestin/BrdU-positive cells in the MCAO group increased, peaked at 7 days and then decreased at 14 days (P<0.01 vs. sham). Similarly, compared with that of the M group, the number of Nestin/BrdU-positive cells in the EM group further increased and the number of cells in the EM group peaked at 7 days and showed a downward trend at 14 days (EM7 vs. M7: P<0.01; EM14 vs. M14: P<0.01; Fig. 3C and E). The number of the Dcx/BrdU-positive cells reached a peak at 14 days in the M group and EM group. The Dcx/BrdU-positive cells in group M were significantly increased compared with that of the sham operation group (P<0.01 vs. sham). Similarly, on the 7 and 14th days, the Dcx/BrdU-positive cells in the EM group were significantly increased compared with in the M group (EM7 vs. M7: P<0.01; EM14 vs. M14: P<0.01; Fig. 3D and F).
The present study used TEM to observe the ultrastructure of the neuron nucleus (Fig. 4D). In the specimens of group S, it was observed that the distribution of nuclear chromatin was uniform, the double nuclear membrane of the neuron was clear and complete. TEM analysis showed that compared with those of group S, the chromatin distribution of group M was irregular, the nuclear membrane structure was blurred and the mitochondrial crest was deformed. In the EM group, the damage of nucleus was alleviated and the contraction of nuclear membrane was not obvious. The edge of the nucleus was smooth and a few of the inner cristae of mitochondria were broken.
Treadmill training increases the repair of myelin in the ischemic penumbra
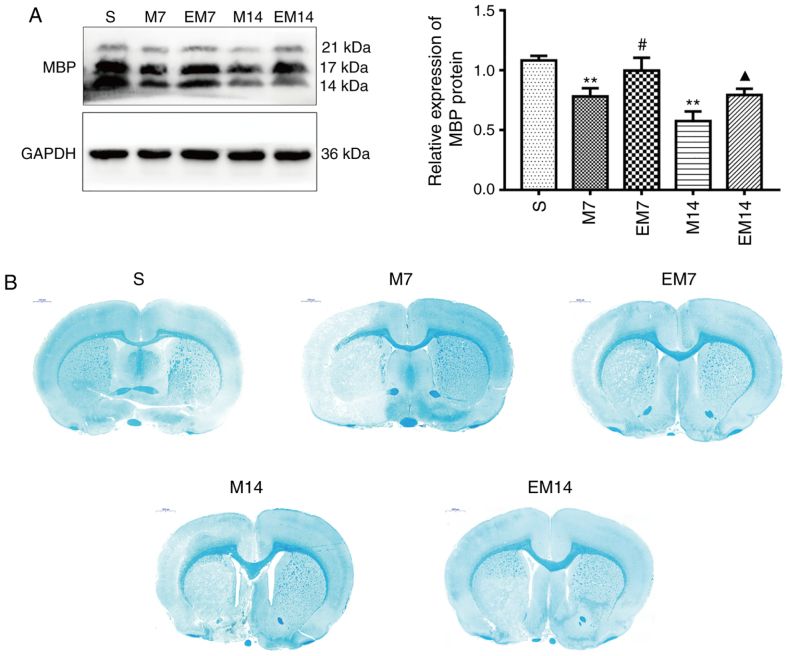
To investigate the effect of treadmill exercise on myelin repair, the expression level of the myelin marker MBP was analyzed in the ischemic penumbra. As shown in Fig. 5A, western blot analysis showed that the expression of MBP in the M group was significantly decreased compared with the S group (P<0.01). At 7 or 14 days after MCAO, compared with M group, the expression of MBP protein in EM group was increased (EM7 vs. M7: P<0.05; EM14 vs. M14: P<0.05). In sham brain sections, the myelinated fibers were strongly stained by LFB, the myelinated fibers were dense and uniform, and the morphology of the myelinated fibers was complete. After 7 and 14 days of MCAO, all groups, except for the S group, showed lesions in the cortex and subcortex. In the M group, LFB staining analysis showed significant demyelination in the ischemic penumbra. Demyelination was significantly decreased in the EM group compared with the group M during the same period (Fig. 5B).
Figure 5.
Treadmill training increases the repair of myelin in the ischemic penumbra. (A) Protein expression levels and western blot analysis of MBP. Data are presented as the mean ± standard deviation (n=3). (B) LFB staining in the ischemic penumbra after middle cerebral artery occlusion. Scale bars=1,000 μm. **P<0.01 vs. the S group; #P<0.05 vs. the M7 group; ▲P<0.05 vs. the M14 group. S group, sham operation group; M group, model group; MBP, myelin basic protein.
Inhibitor abolishes neurogenesis in the ischemic penumbra
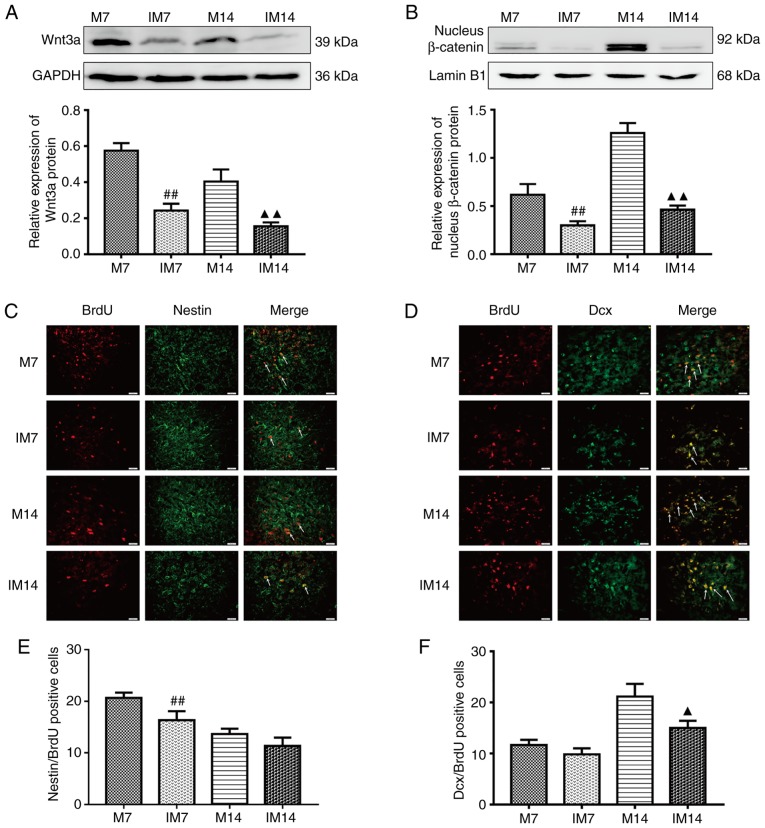
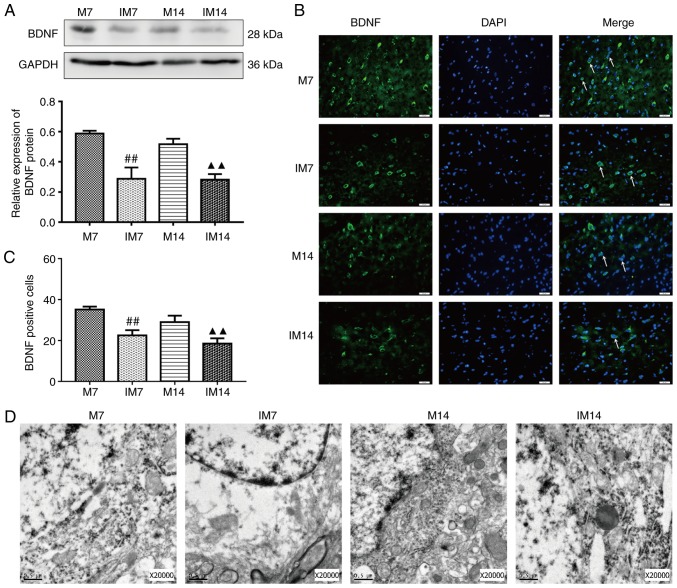
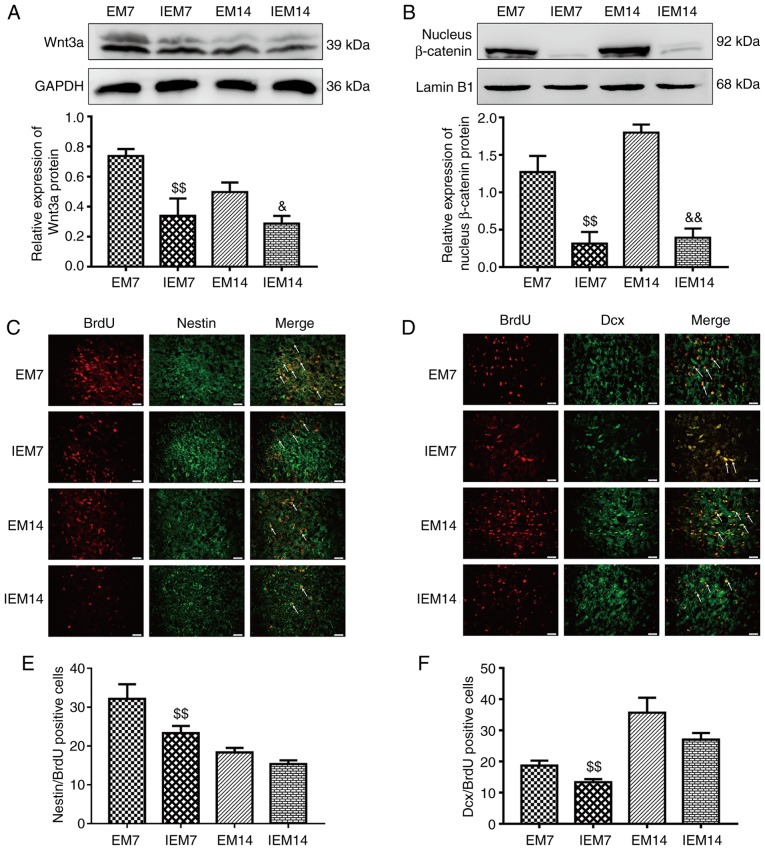
After the addition of the Wnt/β-catenin pathway inhibitor XAV939, compared with the M group, the protein expression of Wnt3a and nuclear β-catenin in the IM group was decreased. (Wnt3a, IM7 vs. M7: P<0.01; IM14 vs. M14: P<0.01; nuclear β-catenin, IM7 vs. M7: P<0.01; IM14 vs. M14: P<0.01; Fig. 6A and B). Compared with the M group, the expression of the BDNF protein in the IM group was decreased during the same period (IM7 vs. M7: P<0.01; IM14 vs. M14: P<0.01; Fig. 7A). The same trend was observed in the BDNF-positive cells (IM7 vs. M7: P<0.01; IM14 vs. M14: P<0.01; Fig. 7B and C). Compared with the M7 group, the number of Dcx/BrdU positive cells in the IM7 group had no significant change, while there was a decrease in the number of Dcx/BrdU-positive cells between group M14 and group IM14 (Dcx/BrdU, IM7 vs. M7: P>0.05; IM14 vs. M14: P<0.05; Fig. 6D and F). There was a difference in the number of Nestin/BrdU-positive cells between group M7 and group IM7. Compared with the M14 group, the number of Nestin/BrdU-positive cells in the IM14 group had no significant change (IM7 vs. M7: P<0.01; IM14 vs. M14: P>0.05; Fig. 6C and E).
Figure 6.
Inhibition of the Wnt/β-catenin pathway abolishes neurogenesis in the ischemic penumbra. (A) Protein expression levels and western blot analysis of Wnt3a. (B) Protein expression levels and western blot analysis of nucleus β-catenin. Data are presented as the mean ± SD (n=3). (C) IF of Nestin/BrdU-positive cells in the ischemic penumbra. (D) IF of Dcx/BrdU-positive cells in the ischemic penumbra. (E) The number of Nestin/BrdU-positive cells in the ischemic penumbra. (F) The number of Dcx/BrdU-positive cells in the ischemic penumbra. Data are presented as the mean ± SD (n=6). Scale bars=20 μm. ##P<0.01 vs. the M7 group; ▲P<0.05 and ▲▲P<0.01 vs. the M14 group. M group, model group; SD, standard deviation; IF, immunofluorescence; BrdU, bromodeoxyuridine.
Figure 7.
Inhibitor downregulates BDNF expression and damages the morphology of cortical neurons in the ischemic penumbra after middle cerebral artery occlusion. (A) Protein expression levels and western blot analysis of BDNF. Data are presented as the mean ± SD (n=3). (B) Immunofluorescence of BDNF-positive cells in the ischemic penumbra. Scale bars=20 μm. (C) The number of BDNF-positive cells in the ischemic penumbra. Data are presented as the mean ± SD (n=6). (D) Transmission electron microscopy showed the neuron nucleus structures (n=3). Scale bars=0.5 μm. ##P<0.01 vs. the M7 group; ▲▲P<0.01 vs. the M14 group. M group, model group; SD, standard deviation; BDNF, brain derived neurotrophic factor; DAPI, 4,6-diamidino-2-phenylindole.
In terms of neuronal structure, TEM showed that compared with group M, the IM group showed dissolved cytoplasm, coacervation of nuclear chromatin and a shrunken or dissolved nuclear membrane. Most of the organelles disappeared and only a small number of denatured mitochondria (with focal cavitation) were present (Fig. 7D).
Inhibitor abolishes myelin repair in the ischemic penumbra
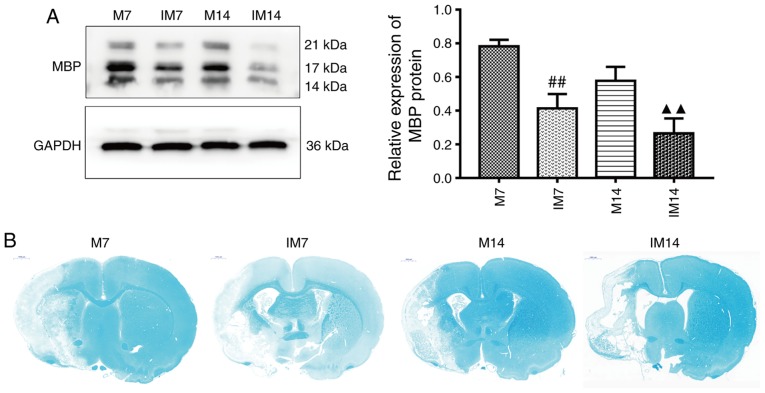
The expression of MBP and the degree of demyelination at 7 and 14 days after injection of the inhibitor was further examined. Compared with group M, the expression of MBP protein in the IM group was decreased (IM7 vs. M7: P<0.01; IM14 vs. M14: P<0.01; Fig. 8A). LFB staining showed that compared with group M, the integrity of the myelin sheath was decreased and demyelination was increased in group IM (Fig. 8B).
Figure 8.
Inhibition of the Wnt/β-catenin pathway abolishes myelin repair in the ischemic penumbra. (A) Protein expression levels and western blot analysis of MBP. Data are presented as the mean ± SD (n=3). (B) Luxol fast blue staining in the ischemic penumbra after middle cerebral artery occlusion. ##P<0.05 vs. the M7 group; ▲▲P<0.01 vs. the M14 group. M group, model group; MCAO, middle cerebral artery occlusion; MBP, myelin basic protein; SD, standard deviation.
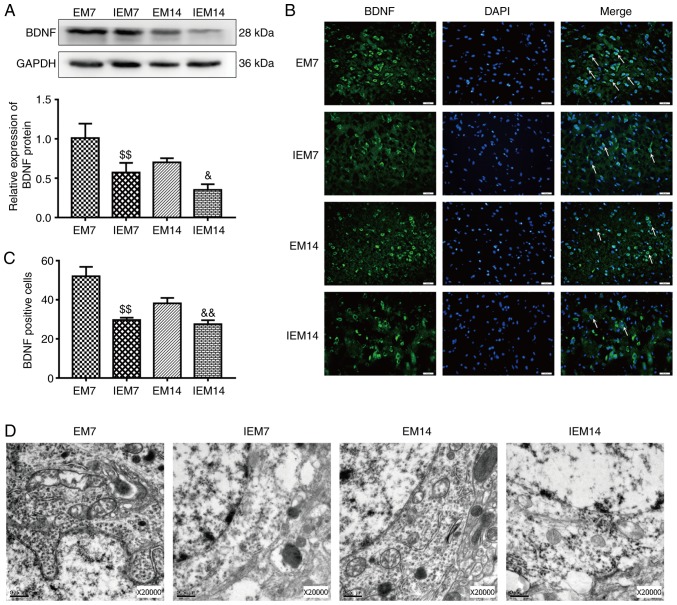
Inhibitor suppresses exercise-promoted neurogenesis in the ischemic penumbra
After 7 and 14 days of MCAO, four groups of EM7, IEM7, EM14 and IEM14 were designed to prove whether the Wnt/β-catenin signaling pathway was involved in exercise-promoted neurogenesis. The results showed that compared with group EM, the expression of Wnt3a and nucleus β-catenin protein in group IEM were decreased (Wnt3a, IEM7 vs. EM7: P<0.01; IEM14 vs. EM14: P<0.05; nucleus β-catenin, IEM7 vs. EM7: P<0.01; IEM14 vs. EM14: P<0.01; Fig. 9A and B). During the same period, compared with group EM, the expression of BDNF protein and the number of BDNF positive cells in group IEM were decreased (western blotting: IEM7 vs. EM7: P<0.01; IEM14 vs. EM14: P<0.05; immunofluorescence: IEM7 vs. EM7: P<0.01; IEM14 vs. EM14: P<0.01; Fig. 10A–C). At 7 and 14 days after MCAO, the expression levels of Dcx/BrdU-and Nestin/BrdU-positive cells in the EM group were different from those in the IEM group (Fig. 9C–F). At 7 days, there was a significant difference in the number of Dcx/BrdU-positive cells between IEM7 and EM7. However, at 14 days, there was no difference in the IEM14 group compared with the EM14 group. Similarly, there was no significant difference in the number of Nestin/BrdU-positive cells between IEM14 and EM14. However, at 7 days, the number of positive cells in the IEM7 group was reduced compared with the EM7 group (Dcx/BrdU, IEM7 vs. EM7: P<0.01; IEM14 vs. EM14: P>0.05; Nestin/BrdU, IEM7 vs. EM7: P<0.01; IEM14 vs. EM14: P>0.05).
Figure 9.
Inhibition of the Wnt/β-catenin signaling pathway suppresses exercise-promoted neurogenesis in the ischemic penumbra. (A) Protein expression levels and western blot analysis of Wnt3a. (B) Protein expression levels and western blot analysis of nucleus β-catenin. Data are presented as the mean ± SD (n=3). (C) IF of Nestin/BrdU-positive cells in the ischemic penumbra. (D) IF of Dcx/BrdU-positive cells in the ischemic penumbra. (E) The number of Nestin/ BrdU-positive cells in the ischemic penumbra. (F) The number of Dcx/BrdU-positive cells in the ischemic penumbra. Data are presented as the mean ± SD (n=6). Scale bars=20 μm. $$P<0.01 vs. the EM7 group; &P<0.05 and &&P<0.01 vs. the EM14 group. EM group, treadmill training model group; IF, immunofluorescence; BrdU, bromodeoxyuridine.
Figure 10.
Inhibition of the Wnt/β-catenin signaling pathway abolishes exercise-promoted BDNF expression and the morphology of cortical neurons in the ischemic penumbra after middle cerebral artery occlusion. (A) Protein expression levels and western blot analysis of BDNF. Data are presented as the mean ± SD (n=3). (B) Immunofluorescence of BDNF-positive cells in the ischemic penumbra. Scale bars=20 μm. (C) The number of BDNF-positive cells in the ischemic penumbra. Data are presented as the mean ± SD (n=6). (D) Transmission electron microscopy showed the neuron nucleus structures (n=3). Scale bars=0.5 μm. $$P<0.05 vs. the EM7 group; &P<0.05 and &&P<0.01 vs. the EM14 group. EM group, treadmill training model group. BDNF, brain derived natriuretic factor; SD, standard deviation.
By observing the ultrastructure of neuronal nuclei under the TEM, the present study found that the IEM group showed edema in the cytoplasm of the neurons, mitochondrial swelling, mitochondrial crest deformation and organelle reduction compared with the EM group (Fig. 10D).
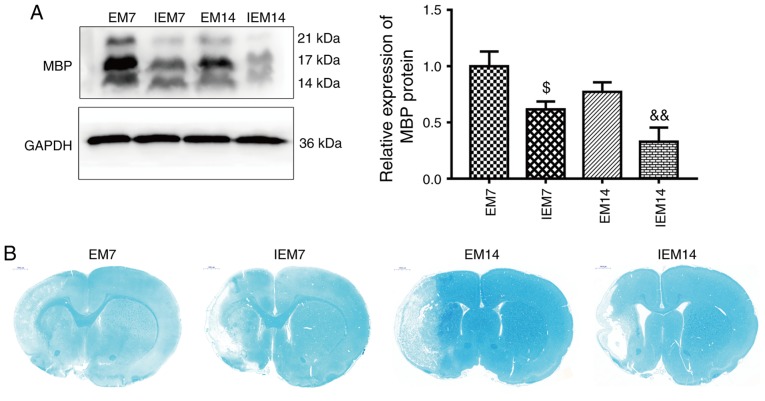
Inhibitor abolishes exercise-promoted myelin repair in the ischemic penumbra
The current study further detected the expression of MBP and the changes in LFB staining at 7 or 14 days in group EM7, IEM7, EM14 and IEM14. Compared with group EM, the expression of MBP protein in group IEM was decreased (IEM7 vs. EM7: P<0.05; IEM14 vs. EM14: P<0.01; Fig. 11A). The results of LFB staining showed that compared with those of the EM group, the myelin staining in the IEM group became lighter, the integrity became worse and the degree of demyelination increased (Fig. 11B).
Figure 11.
Inhibition of the Wnt/β-catenin signaling pathway abolished exercise-promoted myelin repair in the ischemic penumbra. (A) Proteins expression levels and western blot analysis of MBP. Data are presented as the mean ± standard deviation (n=3). (B) Luxol fast blue staining in the ischemic penumbra after middle cerebral artery occlusion. $P<0.05 vs. the EM7 group; &&P<0.01 vs. the EM14 group. MBP, myelin basic protein.
Discussion
The authors’ previous studies have shown that treadmill exercise can promote angiogenesis and neurogenesis in the ischemic penumbra of adult rats. In addition, treadmill exercise can promote dendritic modification and synaptic plasticity (25,29). In this study, the mechanism of neurogenesis and myelin repair after ischemic stroke in juvenile rats was focused on. The results showed that treadmill exercise can promote neurogenesis and myelin repair after ischemic stroke in juvenile rats. In addition, the Wnt/β-catenin signaling pathway was involved in neurogenesis and myelin repair stimulated by treadmill training.
For a long time, the focus of research has been on the use of neurogenesis to promote neuronal replacement and enhance endogenous brain repair. However, studies have found that only a very small number of neurons survive in neurogenesis after adult ischemic stroke (39). The latest research shows that after cerebral ischemia, significant reaggregation of neurons in the juvenile striatum occurs, indicating that the juvenile brain has the ability to repair itself (10). In the present study, Nestin/BrdU- and Dcx/BrdU-positive cells increased in group M at 7 and 14 days after MCAO. Nestin/BrdU-positive cells reached the peak on the 7th day and then decreased gradually. The number of Dcx/BrdU-positive cells increased throughout and reached its peak on the 14th day. In addition, the expression levels of Wnt3a, nuclear β-catenin and BDNF increased on the 7 and 14th days after MCAO. Physical exercise has a neuroprotective effect on both human and animals. A previous study has shown that pre-ischemic treadmill training reduces cerebral infarction volume, brain edema and neurological deficit and improves brain injury after ischemic stroke (40). The authors’ previous studies have shown that after ischemic stroke in adult rats or mice, treadmill exercise enhanced neurogenesis by promoting proliferation, migration and differentiation into mature neurons of NPCs from the subventricular area to the ischemic penumbra (29,30). In the present study, it was found that treadmill exercise promoted the expression of Nestin/BrdU- and Dcx/BrdU-positive cells in the ischemic penumbra of juvenile rats. It is suggested that treadmill exercise enhances neurogenesis by promoting the increment and migration of neuroblasts. More importantly, how to stimulate the neurogenesis of the penumbra and explore its mechanism is the focus of the current research.
After neonatal hypoxia-ischemia injury, treadmill exercise helps improve the recovery of behavior after hypoxia-ischemia via the upregulation of myelin components and neurogenesis (41). In addition, treadmill training combined therapy may improve motor and memory function by increasing the oligodendroglia involved in the cyclic AMP-responsive element-binding protein/BDNF signaling pathway, to restore the myelin components of neonates after hypoxia-ischemia (13). A study has identified that long-term exercise can promote the expression of BDNF and improve the myelin damage caused by ischemia (28). It was found that NPCs could proliferate and differentiate into oligodendrocyte progenitor cells (OPCs) after cerebral ischemia. OPCs can differentiate into mature oligodendrocytes to repair damaged myelin sheaths (42). Oligodendrocyte injury and demyelination can lead to neurological functional deficits (43–45). A previous study has shown that exercise can restore ischemia-induced myelinated hippocampal nerve fiber injury (28). In the present study, treadmill exercise promoted the expression of MBP in the penumbra and reduced demyelination. In this study, treadmill exercise may alleviate brain injury after stroke by promoting neurogenesis and myelin repair. However, the mechanism of brain protection by treadmill training remains to be studied.
A previous study has shown that there is a pathological decrease in Wnt activity in subventricular zone (SVZ) after ischemic stroke (19). More importantly, administration of exogenous Wnt3a can promote the migration and differentiation of newborn neuroblasts to the ischemic cortex (19). It was found that the increased expression of β-catenin could regulate Wnt3a (46). Moreover, it was found that the Wnt pathway is directly involved in the upregulation of BDNF, indicating that the Wnt/β-catenin signaling pathway is one of the upstream pathways of BDNF (19). BDNF plays an important role in brain development and brain injury. BDNF can promote neurogenesis, improve synaptic plasticity, and nourish neurons (47,48). Moreover, BDNF is released by neurons and secreted mainly through dendrites (49–51). It is reported that exogenous BDNF injection can promote neuronal regeneration, while the loss of BDNF inhibited the differentiation of intermediate neurons in mice (52–54). It has been found that activation of the Wnt signaling pathway in SVZ can promote endogenous neurogenesis, increase the number of new neurons and improve nerve function after injury (19). A previous study has shown that neurogenesis in the adult brain may contribute not only to brain repair but also to overall plasticity (55). Interestingly, in this research, it was found that neurogenesis after ischemic stroke in juvenile rats also promotes potential brain repair. Consistent with a previous study (29), at 7 and 14 days after treadmill exercise in juvenile MCAO rats, numerous newborn neuroblasts in the ischemic penumbra were observed. Moreover, an increase in Wnt3a, nucleus β-catenin and BDNF protein levels and BDNF-positive cells were found in the EM7 and EM14 groups.
Rehabilitation training after stroke is a long process, which is consistent with previous studies. (25,29,56). The present study found that compared with 7 days, the volume of cerebral infarction and mNSS score decreased in 14 days. This change may be achieved through some protective responses, strong collateral circulation and favorable external intervention factors (26). These results suggest that treadmill exercise promotes the repair of brain injury by upregulating important molecules in the Wnt signaling pathway and increasing neurogenesis in the ischemic penumbra.
The current study used XAV-939, an inhibitor of the Wnt pathway, to determine the role of the Wnt pathway in the ischemic penumbra. When ischemic stroke occurs, the Wnt signaling pathway is activated. Inhibitors can aggravate neurological deficits and increase the volume of cerebral infarction. The present study found that on the 7 and 14th day after MCAO, the number of Nestin/BrdU- and Dcx/BrdU-positive cells in the IM group was decreased compared with the M group. This finding is consistent with the results of a previous study (19). Nissl staining and TEM results showed that neuronal damage was aggravated in the IM group compared with the M group. LFB staining showed a decrease in myelinated fibers in the IM group compared with the M group. The present results showed that inhibition of Wnt/β-catenin pathway can reduce neurogenesis and myelin repair in the ischemic penumbra.
In addition, inhibitors were used in the treadmill exercise group to explore the relationship between neuroprotection induced by treadmill exercise and Wnt/β-catenin signaling pathway. At 7 and 14 days after MCAO, the number of Nestin/BrdU- and Dcx/BrdU-positive cells in the IEM group was decreased compared with the EM group, which was consistent with the low expression levels of Wnt3a, nuclear β-catenin and BDNF. LFB staining showed that compared with those in the EM group, the myelinated fibers in the IEM group decreased and the staining became lighter, which was consistent with the change in MBP expression. These results suggest that the inhibitor reverses the upregulation of Wnt3a, nuclear β-catenin and BDNF expression after treadmill training, reduces the expression of MBP, and weakens treadmill exercise-induced neurogenesis and myelin repair. It has been found that the neurovascular unit (NVU) is involved in brain injury and brain repair (26). Newly formed neuroblasts after stroke are located in the peri-infarction cortex and are closely related to the vascular endothelium (56). It has been found that the Wnt/β-catenin signaling pathway can regulate vascular development and angiogenesis (57). Activation of the Wnt/β-catenin signaling pathway after MCAO was reported to enhance the angiogenesis of the ischemic core (58). Wnt proteins are important mediators of intercellular communication. Wnt3a is involved in axonal regeneration after CNS injury. The latest studies on the CNS after injury in adults have shown that the classical Wnt signal transduction can induce axonal regeneration and neuronal growth, which indicates that the developmental effect of Wnt can be reused in adults through exogenous stimulation (59,60). It has been found that promoting the expression of Wnt3a can increase vascular regeneration and vascular repair in the peri-infarct region (19). In the model of crush injury of the optic nerve in mice, intravitreal injection of classical Wnt/β-catenin signal activator Wnt3a caused obvious axonal growth beyond the site of axonal lesion (61). Wnt3a signal transduction promotes neurite growth, increases neuronal function and induces repair after spinal cord contusion in adult rats (62). Previous studies have shown that exogenous Wnt3a mediated by lentivirus vectors enhances neurogenesis in vivo (20). In addition, exogenous Wnt3a can also promote the regeneration and differentiation of neural stem cells in vitro (63). In the present study, Wnt3a was focused on because of its established roles in neural development during embryogenesis, as well as in adult neurogenesis (64–66). Moreover, activation of Wnt3a/ β-catenin signaling directly regulates neurogenesis events, which includes a set of transcriptional changes leading to enhanced division and differentiation of Dcx+ neuroblasts out of the SVZ (12,67,68). Furthermore, Wnt3a is neuroprotective and may protect neurons during neurodegenerative processes, such as Alzheimer’s disease and Huntington’s disease (69–71). However, the relationship between neurogenesis and angiogenesis was not investigated in this study. Therefore, the current experiment can only prove that treadmill training promotes neurogenesis and myelin repair through the activation of the Wnt/β-catenin signaling pathway. In contrast, inhibiting the conduction of the Wnt signaling pathway will weaken the neuroprotective effect induced by treadmill training. However, it is unclear whether angiogenesis or pairing of NVUs is directly or indirectly involved in neurogenesis and myelin repair produced by treadmill training through the activation of the Wnt/β-catenin signaling pathway.
The present study has some limitations. First, the conditions to use real-time monitoring of cerebral blood flow unified modeling were not present, which may lead to modeling instability. Second, only a few signal pathway proteins were examined and no in vitro experiments were performed. Third, only neurogenesis in two periods after stroke (7 and 14 days) was studied; neurogenesis over a longer period of time, especially newborn mature neurons should be further studied. Fourth, except for LFB staining to observe the changes of myelin fibers and detect the expression level of the myelin marker protein MBP, no other analyses of proteins and cells related to myelin repair were conducted. In future experiments, the authors will work hard to improve the above deficiencies.
In conclusion, a new role for treadmill training in promoting neurogenesis and myelin sheath repair in juvenile rats was demonstrated. In addition, Wnt/β-catenin signaling pathway was shown to not only mediate neurogenesis and myelin repair, but also participate in exercise-mediated neurogenesis and myelin repair. However, after ischemic stroke, treadmill-induced neurogenesis and myelin repair is a complex process, involving numerous key factors, which need to be fully clarified.
Acknowledgements
Not applicable.
Funding
The present study was funded by a project funded by the Wenzhou Municipal Bureau of Science and Technology (grant nos. Y20170070 and Y20180096).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
XC, HZ and JC conceived and designed the experiment, and JC, QX and QH carried out the experiment. SW, JP, GP and YZ helped in the execution of the experiment; WS and LJ analyzed the data. JC and QX wrote the manuscript. All the authors discussed and suggested the experiment. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All animal procedures in this study were approved by the Experimental Animal Ethics Committee of Wenzhou Medical University (ethical no. Wydw2019-0766) and carried out in accordance with the guidelines for the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interest
The authors declare they have no competing interests.
References
- 1.Feigin VL, Norrving B, George MG, Foltz JL, Roth GA, Mensah GA. Prevention of stroke: A strategic global imperative. Nat Rev Neurol. 2016;12:501–512. doi: 10.1038/nrneurol.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginsberg MD. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology. 2008;55:363–389. doi: 10.1016/j.neuropharm.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minnerup J, Sutherland BA, Buchan AM, Kleinschnitz C. Neuroprotection for stroke: Current status and future perspectives. Int J Mol Sci. 2012;13:11753–11772. doi: 10.3390/ijms130911753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnusson JP, Goritz C, Tatarishvili J, Dias DO, Smith EM, Lindvall O, Kokaia Z, Frisén J. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science. 2014;346:237–241. doi: 10.1126/science.346.6206.237. [DOI] [PubMed] [Google Scholar]
- 6.Ji Y, Teng L, Zhang R, Sun J, Guo Y. NRG-1β exerts neuroprotective effects against ischemia reperfusion-induced injury in rats through the JNK signaling pathway. Neuroscience. 2017;362:13–24. doi: 10.1016/j.neuroscience.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 7.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia-the ischemic penumbra. Stroke. 1981;12:723–725. doi: 10.1161/01.STR.12.6.723. [DOI] [PubMed] [Google Scholar]
- 8.Lo EH. A new penumbra: Transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 9.Jackman K, Iadecola C. Neurovascular regulation in the ischemic brain. Antioxid Redox Signal. 2015;22:149–160. doi: 10.1089/ars.2013.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodgers KM, Ahrendsen JT, Patsos OP, Strnad FA, Yonchek JC, Traystman RJ, Macklin WB, Herson PS. Endogenous neuronal replacement in the juvenile brain following cerebral ischemia. Neuroscience. 2018;380:1–13. doi: 10.1016/j.neuroscience.2018.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Zhao Z, Rege SV, Wang M, Si G, Zhou Y, Wang S, Griffin JH, Goldman SA, Zlokovic BV. 3K3A-activated protein C stimulates postischemic neuronal repair by human neural stem cells in mice. Nat Med. 2016;22:1050–1055. doi: 10.1038/nm.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun FL, Wang W, Zuo W, Xue JL, Xu JD, Ai HX, Zhang L, Wang XM, Ji XM. Promoting neurogenesis via Wnt/β-catenin signaling pathway accounts for the neurorestorative effects of morroniside against cerebral ischemia injury. Eur J Pharmacol. 2014;738:214–221. doi: 10.1016/j.ejphar.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 13.Pak ME, Jung DH, Lee HJ, Shin MJ, Kim SY, Shin YB, Yun YJ, Shin HK, Choi BT. Combined therapy involving electroacupuncture and treadmill exercise attenuates demyelination in the corpus callosum by stimulating oligodendrogenesis in a rat model of neonatal hypoxia-ischemia. Exp Neurol. 2018;300:222–231. doi: 10.1016/j.expneurol.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Hao XZ, Yin LK, Tian JQ, Li CC, Feng XY, Yao ZW, Jiang M, Yang YM. Inhibition of Notch1 signaling at the subacute stage of stroke promotes endogenous neurogenesis and motor recovery after stroke. Front Cell Neurosci. 2018;12:245. doi: 10.3389/fncel.2018.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danzer SC. Postnatal and adult neurogenesis in the development of human disease. Neuroscientist. 2008;14:446–458. doi: 10.1177/1073858408317008. [DOI] [PubMed] [Google Scholar]
- 16.Piccin D, Morshead CM. Wnt signaling regulates symmetry of division of neural stem cells in the adult brain and in response to injury. Stem Cells. 2011;29:528–538. doi: 10.1002/stem.589. [DOI] [PubMed] [Google Scholar]
- 17.Yoshinaga Y, Kagawa T, Shimizu T, Inoue T, Takada S, Kuratsu J, Taga T. Wnt3a promotes hippocampal neurogenesis by shortening cell cycle duration of neural progenitor cells. Cell Mol Neurobiol. 2010;30:1049–1058. doi: 10.1007/s10571-010-9536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munji RN, Choe Y, Li G, Siegenthaler JA, Pleasure SJ. Wnt signaling regulates neuronal differentiation of cortical intermediate progenitors. J Neurosci. 2011;31:1676–1687. doi: 10.1523/JNEUROSCI.5404-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei ZZ, Zhang JY, Taylor TM, Gu X, Zhao Y, Wei L. Neuroprotective and regenerative roles of intranasal Wnt-3a administration after focal ischemic stroke in mice. J Cereb Blood Flow Metab. 2018;38:404–421. doi: 10.1177/0271678X17702669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lie DC, Colamarino SA, Song HJ, Désiré L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, Gage FH. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 21.Qu Q, Sun G, Murai K, Ye P, Li W, Asuelime G, Cheung YT, Shi Y. Wnt7a regulates multiple steps of neurogenesis. Mol Cell Biol. 2013;33:2551–2559. doi: 10.1128/MCB.00325-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Chen T, Shan G. MiR-148b regulates proliferation and differentiation of neural stem cells via Wnt/β-catenin signaling in rat ischemic stroke model. Front Cell Neurosci. 2017;11:329. doi: 10.3389/fncel.2017.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu D, Hou K, Li F, Chen S, Fang W, Li Y. XQ-1H alleviates cerebral ischemia in mice through inhibition of apoptosis and promotion of neurogenesis in a Wnt/β-catenin signaling dependent way. Life Sci. 2019;235:116844. doi: 10.1016/j.lfs.2019.116844. [DOI] [PubMed] [Google Scholar]
- 24.Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 25.Xie Q, Cheng J, Pan G, Wu S, Hu Q, Jiang H, Wang Y, Xiong J, Pang Q, Chen X. Treadmill exercise ameliorates focal cerebral ischemia/reperfusion-induced neurological deficit by promoting dendritic modification and synaptic plasticity via upregulating caveolin-1/VEGF signaling pathways. Exp Neurol. 2019;313:60–78. doi: 10.1016/j.expneurol.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Hu Q, Xie Q, Wu S, Pang Q, Liu M, Zhao Y, Tu F, Liu C, Chen X. Effects of treadmill exercise on motor and cognitive function recovery of MCAO mice through the caveolin-1/VEGF signaling pathway in ischemic penumbra. Neurochem Res. 2019;44:930–946. doi: 10.1007/s11064-019-02728-1. [DOI] [PubMed] [Google Scholar]
- 27.Schabitz WR, Berger C, Kollmar R, Seitz M, Tanay E, Kiessling M, Schwab S, Sommer C. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35:992–997. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- 28.Ahn JH, Choi JH, Park JH, Kim IH, Cho JH, Lee JC, Koo HM, Hwangbo G, Yoo KY, Lee CH, et al. Long-term exercise improves memory deficits via restoration of myelin and microvessel damage, and enhancement of neurogenesis in the aged gerbil hippocampus after ischemic stroke. Neurorehabil Neural Repair. 2016;30:894–905. doi: 10.1177/1545968316638444. [DOI] [PubMed] [Google Scholar]
- 29.Pang Q, Zhang H, Chen Z, Wu Y, Bai M, Liu Y, Zhao Y, Tu F, Liu C, Chen X. Role of caveolin-1/vascular endothelial growth factor pathway in basic fibroblast growth factor-induced angiogenesis and neurogenesis after treadmill training following focal cerebral ischemia in rats. Brain Res. 2017;1663:9–19. doi: 10.1016/j.brainres.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 30.Zhao Y, Pang Q, Liu M, Pan J, Xiang B, Huang T, Tu F, Liu C, Chen X. Treadmill exercise promotes neurogenesis in ischemic rat brains via caveolin-1/VEGF signaling pathways. Neurochem Res. 2017;42:389–397. doi: 10.1007/s11064-016-2081-z. [DOI] [PubMed] [Google Scholar]
- 31.Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M STAIR VI Consortium. Stroke Therapy Academic Industry Roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke. 2009;40:2594–2600. doi: 10.1161/STROKEAHA.109.552554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.STR.20.1.84. [DOI] [PubMed] [Google Scholar]
- 33.Jean LeBlanc N, Menet R, Picard K, Parent G, Tremblay ME, ElAli A. Canonical Wnt pathway maintains blood-brain barrier integrity upon ischemic stroke and its activation ameliorates tissue plasminogen activator therapy. Mol Neurobiol. 2019;56:6521–6538. doi: 10.1007/s12035-019-1539-9. [DOI] [PubMed] [Google Scholar]
- 34.Wang YZ, Yamagami T, Gan Q, Wang Y, Zhao T, Hamad S, Lott P, Schnittke N, Schwob JE, Zhou CJ. Canonical Wnt signaling promotes the proliferation and neurogenesis of peripheral olfactory stem cells during postnatal development and adult regeneration. J Cell Sci. 2011;124:1553–1563. doi: 10.1242/jcs.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. doi: 10.1161/01.STR.32.4.1005. [DOI] [PubMed] [Google Scholar]
- 36.Ding YH, Ding Y, Li J, Bessert DA, Rafols JA. Exercise pre-conditioning strengthens brain microvascular integrity in a rat stroke model. Neurol Res. 2006;28:184–189. doi: 10.1179/016164106X98053. [DOI] [PubMed] [Google Scholar]
- 37.Gao Y, Zhao Y, Pan J, Yang L, Huang T, Feng X, Li C, Liang S, Zhou D, Liu C, et al. Treadmill exercise promotes angiogenesis in the ischemic penumbra of rat brains through caveolin-1/VEGF signaling pathways. Brain Res. 2014;1585:83–90. doi: 10.1016/j.brainres.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 38.Ashwal S, Tone B, Tian HR, Cole DJ, Liwnicz BH, Pearce WJ. Core and penumbral nitric oxide synthase activity during cerebral ischemia and reperfusion in the rat pup. Pediatr Res. 1999;46:390–400. doi: 10.1203/00006450-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Zhang M, Yang SD, Li WB, Ren SQ, Zhang J, Zhang F. Pre-ischemic treadmill training alleviates brain damage via GLT-1-mediated signal pathway after ischemic stroke in rats. Neuroscience. 2014;274:393–402. doi: 10.1016/j.neuroscience.2014.05.053. [DOI] [PubMed] [Google Scholar]
- 41.Kim HN, Pak ME, Shin MJ, Kim SY, Shin YB, Yun YJ, Shin HK, Choi BT. Comparative analysis of the beneficial effects of treadmill training and electroacupuncture in a rat model of neonatal hypoxia-ischemia. Int J Mol Med. 2017;39:1393–1402. doi: 10.3892/ijmm.2017.2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gallo V, Armstrong RC. Myelin repair strategies: A cellular view. Curr Opin Neurol. 2008;21:278–283. doi: 10.1097/WCO.0b013e3282fd1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow Metab. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- 44.Micu I, Jiang Q, Coderre E, Ridsdale A, Zhang L, Woulfe J, Yin X, Trapp BD, McRory JE, Rehak R, et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- 45.McTigue DM, Tripathi RB. The life, death, and replacement of oligodendrocytes in the adult CNS. J Neurochem. 2008;107:1–19. doi: 10.1111/j.1471-4159.2008.05570.x. [DOI] [PubMed] [Google Scholar]
- 46.Yi H, Hu J, Qian J, Hackam AS. Expression of brain-derived neurotrophic factor is regulated by the Wnt signaling pathway. Neuroreport. 2012;23:189–194. doi: 10.1097/WNR.0b013e32834fab06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 48.Bekinschtein P, Oomen CA, Saksida LM, Bussey TJ. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Semin Cell Dev Biol. 2011;22:536–542. doi: 10.1016/j.semcdb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brigadski T, Hartmann M, Lessmann V. Differential vesicular targeting and time course of synaptic secretion of the mammalian neurotrophins. J Neurosci. 2005;25:7601–7614. doi: 10.1523/JNEUROSCI.1776-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuda N, Lu H, Fukata Y, Noritake J, Gao H, Mukherjee S, Nemoto T, Fukata M, Poo MM. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci. 2009;29:14185–14198. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scharfman H, Goodman J, Macleod A, Phani S, Antonelli C, Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp Neurol. 2005;192:348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 53.Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones KR, Farinas I, Backus C, Reichardt LF. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Obernier K, Tong CK, Alvarez-Buylla A. Restricted nature of adult neural stem cells: Re-evaluation of their potential for brain repair. Front Neurosci. 2014;8:162. doi: 10.3389/fnins.2014.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carmichael ST, Ohab J, Nguyen J. Post-stroke neurogenesis and the neurovascular niche: Newly born neuroblasts localize to peri-infarct cortex in close association with the vascular endothelium. J Cereb Blood Flow Metab. 2005;25:S214–S214. doi: 10.1038/sj.jcbfm.9591524.0214. [DOI] [Google Scholar]
- 57.Parmalee NL, Kitajewski J. Wnt signaling in angiogenesis. Curr Drug Targets. 2008;9:558–564. doi: 10.2174/138945008784911822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Y, Zhang G, Kang Z, Xu Y, Jiang W, Zhang S. Cornin increases angiogenesis and improves functional recovery after stroke via the Ang1/Tie2 axis and the Wnt/β-catenin pathway. Arch Pharm Res. 2016;39:133–142. doi: 10.1007/s12272-015-0652-1. [DOI] [PubMed] [Google Scholar]
- 59.Ciani L, Salinas PC. WNTs in the vertebrate nervous system: From patterning to neuronal connectivity. Nat Rev Neurosci. 2005;6:351–362. doi: 10.1038/nrn1665. [DOI] [PubMed] [Google Scholar]
- 60.Salinas PC. Wnt signaling in the vertebrate central nervous system: From axon guidance to synaptic function. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008003. pii: a008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patel AK, Park KK, Hackam AS. Wnt signaling promotes axonal regeneration following optic nerve injury in the mouse. Neuroscience. 2017;343:372–383. doi: 10.1016/j.neuroscience.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin ZS, Zu B, Chang J, Zhang H. Repair effect of Wnt3a protein on the contused adult rat spinal cord. Neurol Res. 2008;30:480–486. doi: 10.1179/174313208X284133. [DOI] [PubMed] [Google Scholar]
- 63.Kalani MY, Cheshier SH, Cord BJ, Bababeygy SR, Vogel H, Weissman IL, Palmer TD, Nusse R. Wnt-mediated self-renewal of neural stem/progenitor cells. Proc Natl Acad Sci USA. 2008;105:16970–16975. doi: 10.1073/pnas.0808616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ikeya M, Takada S. Wnt-3a is required for somite specification along the anteroposterior axis of the mouse embryo and for regulation of cdx-1 expression. Mech Dev. 2001;103:27–33. doi: 10.1016/S0925-4773(01)00338-0. [DOI] [PubMed] [Google Scholar]
- 65.Mastroiacovo F, Busceti CL, Biagioni F, Moyanova SG, Meisler MH, Battaglia G, Caricasole A, Bruno V, Nicoletti F. Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J Cereb Blood Flow Metab. 2009;29:264–276. doi: 10.1038/jcbfm.2008.111. [DOI] [PubMed] [Google Scholar]
- 66.Mussmann C, Hubner R, Trilck M, Rolfs A, Frech MJ. HES5 is a key mediator of Wnt-3a-induced neuronal differentiation. Stem Cells Dev. 2014;23:1328–1339. doi: 10.1089/scd.2013.0557. [DOI] [PubMed] [Google Scholar]
- 67.Giuliani D, Zaffe D, Ottani A, Spaccapelo L, Galantucci M, Minutoli L, Bitto A, Irrera N, Contri M, Altavilla D, et al. Treatment of cerebral ischemia with melanocortins acting at MC4 receptors induces marked neurogenesis and long-lasting functional recovery. Acta Neuropathol. 2011;122:443–453. doi: 10.1007/s00401-011-0873-4. [DOI] [PubMed] [Google Scholar]
- 68.Spaccapelo L, Galantucci M, Neri L, Contri M, Pizzala R, D’Amico R, Ottani A, Sandrini M, Zaffe D, Giuliani D, Guarini S. Up-regulation of the canonical Wnt-3A and Sonic hedgehog signaling underlies melanocortin-induced neurogenesis after cerebral ischemia. Eur J Pharmacol. 2013;707:78–86. doi: 10.1016/j.ejphar.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 69.Inestrosa NC, Urra S, Colombres M. Acetylcholinesterase (AChE)-amyloid-beta-peptide complexes in Alzheimer’s disease. The Wnt signaling pathway. Curr Alzheimer Res. 2004;1:249–254. doi: 10.2174/1567205043332063. [DOI] [PubMed] [Google Scholar]
- 70.Toledo EM, Colombres M, Inestrosa NC. Wnt signaling in neuroprotection and stem cell differentiation. Prog Neurobiol. 2008;86:281–296. doi: 10.1016/j.pneurobio.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 71.Tourette C, Farina F, Vazquez-Manrique RP, Orfila AM, Voisin J, Hernandez S, Offner N, Parker JA, Menet S, Kim J, et al. The Wnt receptor Ryk reduces neuronal and cell survival capacity by repressing FOXO activity during the early phases of mutant huntingtin pathogenicity. PLoS Biol. 2014;12:e1001895. doi: 10.1371/journal.pbio.1001895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.