Abstract
This investigation examined anthropometric, hormonal, and physiological differences between advanced (ADV; n = 8, 27.8 ± 4.2 years, 170 ± 11 cm, 79.8 ± 13.3 kg) and recreational (REC; n = 8, 33.5 ± 8.1 years, 172 ± 14 cm, 76.3 ± 19.5 kg) CrossFit (CF) trained participants in comparison to physically-active controls (CON; n = 7, 27.5 ± 6.7 years, 171 ± 14 cm, 74.5 ± 14.3 kg). ADV and REC were distinguished by their past competitive success. REC and CON were resistance-trained (>2 years) and exercised on 3–5 days·wk-1 for the past year, but CON utilized traditional resistance and cardiovascular exercise. All participants provided a fasted, resting blood sample and completed assessments of resting metabolic rate, body composition, muscle morphology, isometric mid-thigh pull strength, peak aerobic capacity, and a 3-minute maximal cycle ergometer sprint across two separate occasions (separated by 3–7 days). Blood samples were analyzed for testosterone, cortisol, and insulin-like growth factor-1. Compared to both REC and CON, one-way analysis of variance revealed ADV to possess lower body fat percentage (6.7–8.3%, p = 0.007), greater bone and non-bone lean mass (12.5–26.8%, p ≤ 0.028), muscle morphology characteristics (14.2–59.9%, p < 0.05), isometric strength characteristics (15.4–41.8%, p < 0.05), peak aerobic capacity (18.8–19.1%, p = 0.002), and 3-minute cycling performance (15.4–51.1%, p ≤ 0.023). No differences were seen between REC and CON, or between all groups for resting metabolic rate or hormone concentrations. These data suggest ADV possess several physiological advantages over REC and CON, whereas similar physiological characteristics were present in individuals who have been regularly participating in either CF or resistance and cardiovascular training for the past year.
Introduction
CrossFit® (CF) is a form of high-intensity functional training that combines resistance exercises, gymnastics, and traditional aerobic modalities (e.g., cycling, rowing, running) into single workouts that vary by day to elicit general physical preparedness [1, 2]. This training form is enjoyed recreationally by participants of varying levels of fitness, training experience, age, and lifestyles [3] and also exists as its own sport. The primary CF competition is the Reebok CrossFit Games™ (the Games) which awards individual winners the title of “Fittest on Earth™”. Historically, this competition has consisted of several stages designed to narrow the initial participant pool down to the top athletes. Although the competition’s structure has changed over time [4, 5], the presence of an initial online qualifying round (e.g., the CrossFit OpenTM) has remained. This round typically involves multiple workout challenges that are completed over the course of several weeks. Competitors who complete all workouts and rank high enough will progress to the next stage of the competition. Regardless of which stage, it is expected that each workout will consist of a set of challenges that will require some combination of strength, power, endurance, and/or sport-specific skill [1]. However, little is known about which physiological characteristics of competitors who progress beyond the opening round of the competition.
Body mass [6], strength and anaerobic power [6–10], aerobic capacity [9], sport-specific skill [8, 10], and experience [9] have all been associated with either CF workout performance or competitive ranking. Collectively, these data imply that athletes must train to be proficient in each to perform well in competition. However, several limitations exist among these studies that prevent making such a conclusion. For instance, Serafini et al. (2018) reported that higher ranking competitors of the 2016 Open were stronger, more powerful, and more proficient at short-duration, sprint-type CF workouts. Among regional competitors, final ranking was positively related to 400-m sprint time and time-to-completion in longer, benchmark workouts (i.e., Filthy-50) (r = 0.69–0.77), and negatively related to maximal weight lifted in the Olympic lifts (r = -0.39 to -0.42) [10]. Although these studies involved participants who have successful competitive records, the measures used to distinguish rank were all self-reported. As such, the authenticity and actual data of measurement (self-reported data were obtained from an online resource) cannot be verified. In contrast, others have measured a variety of physical parameters and related them to CF-style workouts performed in a controlled, laboratory setting [6, 7, 9]. While these studies have also included successful CF athletes, laboratory workouts do not adequately emulate the competitive setting and may influence the physiological response to CF training [11–14]. Thus, questions remain about the distinguishing characteristics of successful CF athletes.
In more traditional sports (e.g., football, baseball, basketball, etc.), identifying the key physiological and athletic characteristics that distinguish performance is common [15–18]. The practice enables strength and conditioning professionals to develop sport-specific training programs that are more effective in translating adaptations to in-game performance. However, CF is unique in that typical training session workouts mirror those that appear in competition. Moreover and consistent with its primary purpose [1, 2], chronic participation in CF training has been documented to improve a variety of fitness parameters [19]. Though it might be assumed that CF training represents an ideal training strategy for developing the physiological characteristics present in successful competitors, such a conclusion would be premature based on the available data.
Evidence of CF training being more advantageous towards developing a variety of fitness outcomes in comparison to alternative training strategies (e.g., resistance training, high-intensity interval training) is equivocal [19–25]. This is likely because most comparative training studies have utilized untrained or novice (to CF) participants, which is problematic because they do not require a very specific or intense training stimulus to elicit adaptations compared to experienced trainees [26]. It is possible that either a longer training duration or more advanced participants are necessary to observe the advantages or disadvantages of the CF strategy. Unfortunately, elite competitors rarely share their training strategies and anecdotal evidence suggests that they incorporate more than what commonly occurs during a typical CF training session. To the best of our knowledge, only one well-controlled study exists where a variety of physiological parameters were examined between CF-trained participants and those trained in more traditional exercise modalities (e.g., resistance training) [27]. In that cross-sectional investigation, men with at least on year of CF training experience outperformed their resistance-trained (> 1 year) counterparts in a multi-stage shuttle run test and possessed a higher aerobic capacity; all other measures were statistically similar. While this study provides evidence in favor of CF training, there was no aerobic training requirement for the resistance-trained group, and the actual experience of the CF group was unclear beyond their having participated in the strategy for at least one year. It is possible that multiple physiological differences exist when experience is considered. Therefore, the purpose of this study was to examine anthropometric, hormonal, and physiological differences between advanced CF athletes, recreational CF practitioners, and physically-active adults who regularly participate in both resistance and cardiovascular training. Since adaptations are specific to the training modality and effort [26], we hypothesized that body composition, muscle morphology, aerobic and anaerobic performance, and strength would be different between groups. Specifically, the advanced CF athletes would outperform the other groups whereas recreational CF practitioners and physically-active adults would be similar. However, because resting hormonal concentrations do not typically change through training [14], it was hypothesized that these would be similar between groups.
Materials and methods
Experimental design
For this cross-sectional study, physically-active adults were recruited and assigned into groups based on their experience with CF training and performance during specific CF competitions. Participants who possessed CF training experience (> 2 years) were classified as advanced (ADV) if they had previously qualified for the regional round of the Games competition. Otherwise, they were classified as recreational (REC) because they had never progressed beyond the opening round of the competition (i.e., The Open) but still trained on 3–5 days per week for at least the previous year. Individuals who did not possess CF training experience but possessed resistance training experience (> 2 years) and participated in both resistance and cardiovascular training on 3–5 days per week for at least the previous year, were assigned to the physically-active control (CON) group. All participants reported to the Exercise Physiology Laboratory on two separate occasions, within one month of the onset of the Open, to complete all testing. During the first visit, each participant provided a fasted blood sample before completing assessments of muscle morphology and then a graded exercise test to measure peak aerobic capacity. Participants returned to the Exercise Physiology Lab for the second visit (within 3–7 days of the first visit) to complete assessments of resting metabolic rate, body composition, and strength before finishing the study with a 3-minute all-out cycling test. All testing sessions occurred in the morning (~6:00–10:00 a.m.) with the participants having abstained from unaccustomed physical activity and alcohol for 24 hours, caffeine for 12 hours, and fasted for 8 hours. Participants completed all measurements while wearing comfortable athletic clothing and were able to consume a light snack prior to performance testing (i.e., peak aerobic capacity, strength, and 3-minute cycling performance). Prior to leaving the laboratory on the first visit, participants were asked to complete a 24-hour dietary recall, retain a copy, and follow a similar diet prior to their second visit. Comparisons were made between groups for all anthropometric, biochemical, and physiological measures.
Participants
A priori analysis was based on published [8, 28] and related unpublished data collected by our laboratory where comparisons were made between competitive levels and ranks for self-reported measures of strength and power in CF athletes. The effect sizes produced from group comparisons (partial eta squared > 0.485), standard alpha (p = 0.05), and minimum beta (ß = 0.80) were input into statistical software (G*Power, v. 3.1.9.4, Heinrich-Heine-Universität, Germany). It was determined that a minimum of 20 participants was needed to obtain sufficient power to observe differences between sexes and groups. Consequently, twenty-three physically-active adults (29.7 ± 6.8 years, 171 ± 12 cm, 76.9 ± 15.4 kg) agreed to participate in this study. All participants were free of any physical limitations (determined by medical and physical-activity history questionnaire and PAR-Q+) and had been regularly participating (at the time of recruitment) in their chosen exercise form (i.e., CrossFit training or Resistance/Cardiovascular training) for a minimum of 2 years. Participants in ADV (n = 8 [men = 4, women = 4], 27.8 ± 4.2 years, 170 ± 11 cm, 79.8 ± 13.3 kg) reported having regularly participated in resistance training for 11.5 ± 5.8 years and CF training for 6.4 ± 5.6 years (6–7 sessions·week-1). As individual competitors, the highest rank these participants ever achieved in the Open was 659th ± 991st (range: 19th– 3,052nd) within their respective divisions worldwide. While each of these athletes qualified for this study by having competed as members of a team in regional (highest average rank = 11th ± 13th) and Games competition (highest average rank = 20th ± 9th), three competed individually in their respective regions with one having progressed to the Games on multiple occasions. REC participants (n = 8 [men = 4, women = 4], 33.5 ± 8.1 years, 172 ± 14 cm, 76.3 ± 19.5 kg) reported having regularly participated in resistance training for 8.1 ± 7.9 years and CF training for 3.3 ± 1.7 years (4–5 sessions·week-1). The highest rank these participants had ever achieved in the Open was 22,306th ± 14,028th (range: 5,466th– 44,315th) within their respective divisions worldwide. Participants in CON (n = 7 [men = 4, women = 3], 27.5 ± 6.7 years, 171 ± 14 cm, 74.5 ± 14.3 kg) reported having 7.6 ± 4.8 years of regular resistance training experience and incorporated 3.7 ± 1.3 sessions and 3.6 ± 1.0 sessions of resistance and cardiovascular training per week. Although two participants in CON reported having previously participated in CF-style workouts, these did not occur with regularity (< 3 sessions·week-1) or for an extended duration (< 1 year) and they had never competed in the Open at the time of data collection. Following an explanation of all procedures, risks and benefits, each participant provided his or her written informed consent to participate in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Kennesaw State University Institutional Review Board (#17–501).
Blood sampling and biochemical analysis
Blood samples were obtained on the first visit prior to any physical activity. All samples were obtained from an antecubital vein using a needle by a research team member who was trained and experienced in phlebotomy. Approximately 15 mL of blood was drawn into SST tubes (for serum collection) and EDTA-treated Vacutainer® tubes (for plasma). SST tubes were allowed to clot for 10 minutes prior to centrifugation, while EDTA treated tubes were centrifuged immediately for 10 minutes at 3600 rpms at 4 ºC. The resulting serum and plasma were aliquoted and stored at -80ºC until analysis.
Circulating concentrations of testosterone (T; in ng·dL-1), cortisol (C; in μg·dL-1), and insulin-like growth factor (IGF-1; in ng·mL-1) were assessed via enzyme-linked immunosorbent assays (ELISA) via a 96-well spectrophotometer (BioTek, Winooski, VT) using commercially available kits. To eliminate inter-assay variance, all samples for each assay were thawed once and analyzed in duplicate in the same assay run by a single technician. Samples were analyzed in duplicate, with an average coefficient of variation of 1.63% for T, 6.88% for C, and 2.00% IGF-1.
Muscle morphology
Non-invasive skeletal muscle ultrasound images were collected from the right thigh and arm locations of all participants. Prior to image collection, all anatomical locations of interest were identified using standardized landmarks for the rectus femoris (RF), vastus medialis (VM), vastus lateralis (VL), biceps brachii (BB), and triceps brachii (TB) muscles. The landmarks for the thigh musculature were identified along the longitudinal distance over the femur. The RF and VM were respectively assessed at 50% and 20% of the distance from the proximal border of the patella to the anterior, inferior suprailiac crest. The VL was assessed at 50% of the distance from the lateral condyle of the tibia to the most prominent point of the greater trochanter of the femur. VL measurement required the participant to lay on their side. Landmark identification of the BB and TB required the participant to sit upright on the examination table and extend their arm to rest upon the shoulder of the researcher. Both muscles were assessed along the humerus at a position equal to 40% of the distance from the lateral epicondyle to the acromion process of the scapula [29]. Subsequently, the participant resumed laying supine on the examination table for a minimum of 5–10 minutes to allow fluid shifts to occur before images were collected [30]. The same investigator performed all landmark measurements for each participant.
A 12 MHz linear probe scanning head (General Electric LOGIQ S7 Expert, Wauwatosa, WI, USA) was coated with water soluble transmission gel to optimize spatial resolution and used to collect all ultrasound images. Collection of each image began with the probe being positioned on (and perpendicular to) the surface of the skin to provide acoustic contact without depressing the dermal layer. Subsequently, two consecutive images were collected in the extended field of view mode (Gain = 50 dB; Image Depth = 5–6 cm) using a cross-sectional sweep in the axial plane to capture panoramic images of each muscle. At the same sites, two consecutive images were collected with the probe oriented longitudinal to the muscle tissue interface using Brightness Mode (B-mode) ultrasound [31]. Each of these images included a horizontal line (approximately 1 cm), located below the image, which was used for calibration purposes when analyzing the images offline [32]. To capture images of the RF and VM, the participant remained in the supine position, with their legs extended but relaxed. A rolled towel was placed beneath the popliteal fossa of the dominant leg, allowing for a 10° bend in the knee as measured by a goniometer, and the dominant foot secured [33]. For the VL, the participant was placed on their side with their legs together and the rolled towel between their needs. Once again, the legs were positioned to allow a 10° bend in the knees, as measured by a goniometer [33]. Measurement of the BB and TB required the participant to sit upright with their arm extended, resting on the shoulder of the researcher. The same investigator positioned each participant and collected all images.
After all images were collected, the ultrasound data were transferred to a personal computer for analysis via Image J (National Institutes of Health, Bethesda, MD, USA, version 1.45s) by the same technician. All panoramic images were used to measure cross-sectional area (CSA) and echo intensity. For these measures, the polygon tracking tool in the ImageJ software was used to isolate as much lean muscle as possible without any surrounding bone or fascia [31]. Subsequently, Image J calculated the area contained within the traced muscular image and reported this value in centimeters squared (± 0.1cm2). Concurrently, echo intensity was determined by grayscale analysis using the standard histogram function in ImageJ [31] and expressed as an arbitrary unit (au) value between 0–255 (0: black; 255: white) with lower values reflecting more contractile tissue within each muscle [31, 34]. Mean echo intensity values were then corrected for subcutaneous fat thickness (SFT; averaged from the SFT values obtained at the medial, midline, and lateral sites of each muscle) using Eq 1 [35]. All B-mode images were used to measure muscle thickness (± 0.01 cm; perpendicular distance between the superficial and deep aponeuroses) and pennation angle (± 0.1°; intersection of the fascicles with the deep aponeurosis). Fascicle length (± 0.1 cm) across the deep and superficial aponeuroses was estimated from muscle thickness and pennation angle using Eq 2. Intraclass correlation coefficients (ICC3,k = 0.77–0.99) for determining muscle thickness, pennation angle, CSA and echo intensity was previously determined in ten active, resistance-trained men (25.3 ± 2.0 years, 180 ± 7 cm, 90.8 ± 6.8 kg) using the methodology described above. The methodology for determination of FL has a reported estimated coefficient of variation of 4.7% [36].
| Eq 1 |
| Eq 2 |
Graded exercise testing
Peak aerobic capacity (VO2peak; ml·kg-1·min-1), respiratory compensation threshold (RCT; ml·kg-1·min-1), and gas exchange threshold (GET; ml·kg-1·min-1) were assessed using a continuous, ramp exercise protocol performed on an electromagnetic-braked cycle ergometer (Lode Excalibur Sport, Lode., B.V., Groningen, The Netherlands). Prior to testing, each participant completed a standardized warm-up that consisted of riding a cycle ergometer for 5 minutes at the participant’s preferred resistance and cadence followed by 10 body weight squats, 10 alternating lunges, 10 walking knee hugs and 10 walking butt kicks. Participants were then permitted to continue their warm-up with any additional practices that would help them feel comfortable entering the test. Participants were fitted with a heart rate (HR) monitor (Team2, Polar, Lake Success, NY), a nose clip, and a 2-way valve mask connected to a metabolic measurement system (True One 2400, ParvoMedics Inc., Salt Lake City, UT) to measure expired gases. The cycle ergometer seat height and handlebar distance were adjusted to the participant’s comfort. The participants initially completed a 3-minute warm-up period with the resistance set at 50 W before starting the test at 75 W. During testing, the participants were asked to maintain a self-selected pedaling rate (> 50 rpm’s) while power output was increased by 25 W every minute until volitional fatigue or pedaling rate dropped below 50 rpm’s for longer than 15 seconds. Upon completion of the test, each participant immediately progressed to a 3-minute active recovery period where they continued to pedal at their own cadence against a 50 W load. HR was assessed on each minute of the 3-minute recovery period. Participants were then removed from the cycle ergometer and asked to rest in a chair for an additional two minutes.
Relative oxygen consumption values (i.e., VO2·kg-1) collected on each breath were averaged using the 11-breath averaging technique [37] and used to determine the highest value achieved during the test (i.e., VO2peak). RCT, also known as the second ventilatory threshold, was identified as the VO2 value at which the increase in ventilation-VO2 relationship was accompanied by an increase in the ventilation-VCO2 relationships [38]. The GET was determined using the V-slope method described by Beaver et al. [39]. The GET was defined as the VO2 value corresponding to the intersection of two linear regression lines derived separately from the data points below and above the breakpoint in the CO2 produced (VCO2) versus the VO2 relationship [40].
Dietary recall
Participant’s dietary intake was tracked for the 24-hour period preceding each visit via a paper dietary food recall form. All participants were instructed on how to properly log their food, snacks and drinks via the paper form. Specifically, following their enrollment on their first visit, participants were asked to record their food intake (breakfast, lunch, dinner, drinks and snacks) for the previous 24 hours prior. Prior to leaving the laboratory on the first visit, the participants were given a copy of their food recall form and asked to consume a similar diet during the 24 hours prior to their second visit. Each form was visually inspected to confirm dietary compliance.
Resting metabolic rate assessment
Resting metabolic rate (RMR, kcals·day-1) assessment was completed in a quiet room with minimal lighting (e.g., only light from the RMR machine) located within the Exercise Physiology Laboratory. Prior to their arrival, participants were informed of all pre-test guidelines as outlined by Compher et al. [41]. These included: 1) avoiding alcohol consumption 24 hours prior to testing, 2) no food or caffeine ingestion 8 and 12 hours prior to testing, respectively, and 3) discontinuing unaccustomed physical activity 24 hours prior to testing. Resting metabolic rate was measured via a metabolic measurement system (Parvo Medics TrueOne 2400, ParvoMedics Inc., Salt Lake City, UT) utilizing a ventilated hood. Participants were asked to rest in the supine position with the ventilated hood placed over their face and neck for a maximum of 30 minutes. RMR determination was based on a 5-minute interval of measured volume of oxygen consumption (VO2) with a coefficient of variation less than 10% [41]. The average coefficient of variation was 6.36%.
Body composition assessments
Initially, height (± 0.1 cm) and body mass (± 0.1 kg) were determined using a stadiometer (WB-3000, TANITA Corporation, Tokyo, Japan) with the participants standing barefoot, with feet together, in their normal daily attire. Subsequently, body composition was assessed by three common methods (i.e., dual energy X-ray absorptiometry [iDXA, Lunar Corporation, Madison, WI], air displacement plethysmography [BodPod, COSMED USA Inc., Chicago, IL], and bioelectrical impedance analysis [770 Body Composition and Body Water Analyzer, InBody, Seoul, South Korea]) using standardized procedures. Briefly, iDXA scanning required participants to remove any metal or jewelry and lay supine on the iDXA table prior to an entire body scan in “standard” mode using the company’s recommended procedures and supplied algorithms. Quality assurance was assessed by daily calibrations performed prior to all scans using a calibration block provided by the manufacturer. All iDXA measurements were performed by the same researcher using standardized subject positioning procedures. For air displacement plethysmography, the device and associated scale were calibrated daily using a known volume and mass provided by the manufacturer. During testing, participants were asked to wear a tight-fitting bathing suit or compression shorts and swim cap before entering the device. Two trials were performed for each participant to obtain two measurements of body volume within 150 mL. A third trial was performed if body volume estimates from the first two trials were not within 150 mL, and values from the two closest trials were averaged. Thoracic lung volume was estimated [42]. Bioelectrical impedance analysis required participants to stand barefoot on two metal sensors located at the base of the device and hold two hand grips for approximately 30–60 seconds. Prior to stepping onto the device, participants cleaned the soles of their feet with alcohol wipes provided by the manufacturer.
Following testing, body mass, bone mineral content (BMC; from iDXA), body volume (from BodPod), and total body water (from bioelectrical impedance analysis) were entered into a 4-compartment model, Eq 3 to estimate body fat percentage (BF%) [43], fat mass (± 0.1 kg), and fat-free mass (± 0.1 kg). These values, along with regional (arms [sum of each arm], legs [sum of each leg], and trunk [sum of spine and pelvis]) estimates of bone mineral content (± 0.1 kg) and non-bone lean mass (± 0.1 kg) obtained from iDXA following manual demarcation of these regions of interest were used for all group comparisons. Intraclass correlation coefficients (ICC3,1 = 0.74–0.99) for manually determining regional estimates of bone mineral content and non-bone lean mass had been previously found in 10 healthy, physically-active adults (25.1 ± 2.4 years; 176 ± 7 cm, 81.1 ± 18.5 kg).
| Eq 3 |
Strength assessment
Following RMR and body composition assessments, strength was assessed by an isometric mid-thigh pull test. Prior to testing, each participant completed the same standardized warm-up described for the first visit (i.e., 5 minutes of cycling, dynamic stretching, additional self-selected warm-up practices) followed by a protocol specific to the isometric mid-thigh pull test. The specific component included three isometric efforts on an immobilized barbell positioned at approximately the mid-thigh using a perceived intensity of 50, 70, and 90% of maximum effort, interspersed with a one-minute recovery. The specific warm-up and isometric mid-thigh pull test were completed within a power rack (Rogue Fitness, Columbus, OH) while standing upon a portable force plate (Accupower, AMTI, Watertown, MA). While standing on the force plate, the mid-thigh position was determined for each participant before testing by marking the midpoint distance between the knee and hip joints. Each participant was instructed to assume their preferred second pull power-clean position by self-selecting their hip and knee angles. The height of the barbell was adjusted to a position approximately equal (± 2.54 cm) to the mid-thigh. The participants were then asked to use an overhand, hooked grip on the barbell. The hook grip was selected for this test because all participants reported having had experience with the technique and it is commonly used among CF athletes during competition. Participants were also allowed to wrap their thumbs with athletic training tape and use chalk. Upon the researcher’s “3, 2, 1, Go!” command, the participants were instructed to pull upwards on the barbell as hard and as fast as possible and to continue their maximal effort for 6 seconds. All participants were instructed to relax before the command “GO!” to avoid precontraction and were allotted three maximal attempts. The portable force plate measured the ground reaction forces, imposed onto the plate by the participant, as he/she pulled upon the bar. Peak force (F; in N) production, peak and average rate of force development (RFDPEAK, RFDAVG; in N·s-1), and F and RFD across specific time bands (i.e., 0–30, 0–50, 0–90, 0–100, 0–150, 0–200, and 0–250 milliseconds) were subsequently calculated, as previously described [44].
3-minute all-out cycling test
Following the strength assessment, performance was assessed during a 3-minute maximal sprint on an electromagnetic-braked cycle ergometer (Lode Excalibur Sport, Lode., B.V., Groningen, The Netherlands). Prior to the test, seat height and handlebar positions were adjusted to mirror their positions during the peak aerobic capacity test, and participants were provided with time (~3–5 minutes) to acclimate to the cycle ergometer. A 5-minute rest period was then allotted before initiating the testing protocol, which has been previously described in detail elsewhere [45]. Briefly, the test began with a 1-minute baseline period that involved 55 seconds of unloaded cycling at 90 rpm and then accelerating up to approximately 110 rpm over the last 5 seconds of the minute. The protocol immediately transitioned to the 3-minute testing period where the participants attempted to maintain cadence as high as possible throughout its entirety. Resistance for the test was set using the linear mode of the cycle ergometer (linear factor = power / [preferred cadence]2). That is, the linear factor was calculated as the power output halfway between the VO2peak and GET, divided by the preferred cadence of untrained cyclists (70 rpm2) [46–48]. To prevent pacing and ensure an all-out effort, participants were not informed of the elapsed time and strong verbal encouragement was provided. After 3 minutes, the participants progressed to a 3-minute recovery stage at 50 Watts at their preferred cadence. Peak power (± 1 W), critical power (CP; average power over the final 30 seconds of the test; ± 1 W) [47], and anaerobic work capacity (AWC; work done above CP; (± 0.1 kJ) (48) were calculated based upon performance during the 3-minute sprint test.
Statistical analysis
Data were modeled using both a frequentist and Bayesian approach. The frequentist approach involved a two-tailed, two-way (Group x Sex) analysis of variance (ANOVA) for each dependent variable. Since no between-group differences were observed, age was not included in the model as an additional factor or covariate. Assumptions of normality and equal variance were verified by Shapiro-Wilk and Levene’s tests, respectively. Significant interactions and main effects were further examined using Tukey’s post-hoc analysis. Criterion alpha was set at p ≤ 0.05. To further assess the likelihood (or the effect of group and/or sex) of the data under the alternative hypothesis compared to the null hypothesis, a two-way Bayesian ANOVA was performed with default prior scales [49]. Likelihood was represented in the form of Bayes factors (i.e., BF10) and were interpreted according to the recommendations of Wagenmakers et al. [50]. That is, data were interpreted as evidence in favor of the null hypothesis when BF10 < 1. Otherwise, it was interpreted as “anecdotally” (1 < BF10 < 3), “moderately” (3 < BF10 < 10), “strongly” (10 < BF10 < 30), “very strongly” (30 < BF10 < 100), or “extremely” (BF10 > 100) in favor of the alternative hypothesis. All statistical analyses were performed using JASP 0.10.2 (Amsterdam, the Netherlands). All data are reported as mean ± standard deviation.
Results
Resting hormone concentrations
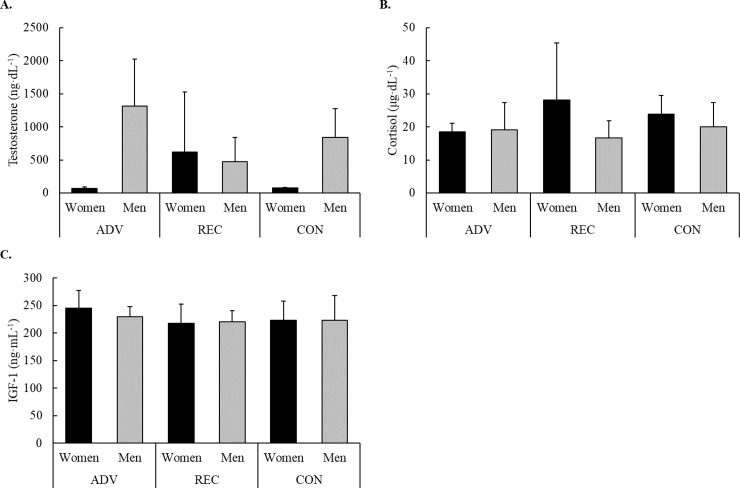
No interactions were observed for T, C, IGF-1. However, a trend for an interaction (F = 2.87, p = 0.090) driven by a main sex effect was seen for T (F = 6.11, p = 0.027) with anecdotal differences between sexes being 2.058 times likely compared to the null hypothesis. Specifically, women in ADV tended to exhibit lower T concentrations (p = 0.083) than ADV men. Male and female hormone concentrations are illustrated in Fig 1.
Fig 1.
Male and female resting concentrations in A) testosterone, B) cortisol, and C) IGF-1.
Muscle morphology
Measures of muscle morphology for each group and sex are presented in Table 1. Significant (p < 0.05) group x sex interactions were observed for BB fascicle length and EI for each muscle, though the likelihood of these interactions favored the null hypotheses (BF10 < 1). Rather, the observed interactions were primarily driven by anecdotal-to-strong evidence (1.7 < BF10 < 30.0) of main effects for sex and group. The observed interaction for BB fascicle length was primarily driven by a main effect for sex where women were 8.8 times more likely to possess shorter fascicles than men, specifically REC women compared to the men of REC (p = 0.029) and CON (p = 0.012). Though the underlying causes for the interactions seen for EI varied with each muscle, anecdotal-to-moderate evidence indicated that men were 1.7–5.5 times more likely to possess a lower EI than women. Specifically, women in REC possessed higher EI (p < 0.05) than men in ADV (RF, VL, and TB; a trend [p = 0.056] for VM) and REC (RF, VM, VL, and TB; a trend [p = 0.087] was noted for BB), and tended (p < 0.10) to be higher than men in CON (RF, VL, and TB). Even though a main effect was not seen, the effect of group was 2.4–30.0 times likely to influence EI. Specifically, post-hoc analysis of the interaction showed that women in REC possessed higher EI than their counterparts in ADV (RF, VM, VL, and TB).
Table 1. Measures of muscle morphology by group and sex.
| ADV | REC | CON | Group | Sex | Group x Sex | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | p | BF10 | p | BF10 | p | BF10 | ||
| Muscle thickness (cm) | Rectus femoris | 2.48 ± 0.36 | 3.28 ± 0.50 | 2.32 ± 0.27 | 2.86 ± 0.20 | 2.46 ± 0.25 | 2.61 ± 0.43 | 0.155 | 8.2 | 0.004 | 6.7 | 0.248 | 0.5 |
| Vastus medialis | 3.44 ± 0.84 | 4.35 ± 0.58 | 2.77 ± 0.08 | 4.28 ± 0.61 | 3.41 ± 0.62 | 4.26 ± 0.22 | 0.439 | >100 | 0.001 | 39.8 | 0.502 | 0.3 | |
| Vastus lateralis | 1.92 ± 0.49 | 2.47 ± 0.39 | 1.49 ± 0.25 | 1.97 ± 0.19 | 1.63 ± 0.19 | 1.67 ± 0.28 | 0.009 | 11.9 | 0.017 | 7.9 | 0.288 | 1.7 | |
| Biceps brachii | 3.32 ± 0.60 | 4.29 ± 0.87 | 2.62 ± 0.19 | 3.74 ± 0.71 | 2.43 ± 0.08 | 3.31 ± 0.17 | 0.013 | >100 | 0.001 | 40.6 | 0.910 | 1.3 | |
| Triceps brachii | 2.51 ± 0.45 | 3.05 ± 0.76 | 2.44 ± 0.64 | 2.91 ± 0.58 | 1.98 ± 0.24 | 3.05 ± 0.43 | 0.674 | 7.1 | 0.008 | 2.1 | 0.541 | 0.3 | |
| Pennation angle (°) | Rectus femoris | 12.7 ± 3.5 | 15.5 ± 0.5 | 10.0 ± 3.0 | 14.5 ± 1.2 | 13.3 ± 5.6 | 16.4 ± 5.5 | 0.375 | 2.9 | 0.036 | 1.4 | 0.894 | 0.5 |
| Vastus medialis | 19.2 ± 3.9 | 26.2 ± 9.3 | 17.2 ± 3.4 | 24.9 ± 6.0 | 27.7 ± 10.8 | 24.0 ± 3.5 | 0.417 | 0.7 | 0.216 | 0.4 | 0.229 | 0.2 | |
| Vastus lateralis | 14.3 ± 3.1 | 14.8 ± 2.9 | 10.7 ± 3.4 | 12.4 ± 5.8 | 12.3 ± 0.4 | 13.2 ± 3.5 | 0.286 | 0.6 | 0.502 | 0.5 | 0.947 | 0.1 | |
| Biceps brachii | 13.6 ± 3.1 | 17.5 ± 2.2 | 12.8 ± 2.1 | 12.3 ± 5.0 | 10.5 ± 2.2 | 9.7 ± 1.5 | 0.009 | 7.0 | 0.489 | 3.2 | 0.249 | 0.4 | |
| Triceps brachii | 17.9 ± 5.1 | 26.8 ± 7.8 | 11.1 ± 3.2 | 16.8 ± 4.5 | 14.2 ± 2.6 | 20.3 ± 4.1 | 0.012 | 34.5 | 0.004 | 13.8 | 0.791 | 2.4 | |
| Fascicle length (cm) | Rectus femoris | 12.2 ± 5.1 | 12.3 ± 2.2 | 14.1 ± 3.8 | 11.5 ± 0.8 | 12.8 ± 7.6 | 10.3 ± 4.5 | 0.852 | 0.6 | 0.365 | 0.3 | 0.786 | 0.1 |
| Vastus medialis | 10.6 ± 2.4 | 11.2 ± 4.8 | 9.6 ± 1.8 | 10.6 ± 2.7 | 7.9 ± 2.4 | 10.6 ± 1.1 | 0.552 | 0.6 | 0.280 | 0.3 | 0.758 | 0.1 | |
| Vastus lateralis | 7.7 ± 0.4 | 9.9 ± 2.1 | 8.3 ± 1.5 | 10.5 ± 4.6 | 7.6 ± 0.6 | 7.7 ± 2.2 | 0.399 | 0.8 | 0.163 | 0.4 | 0.643 | 0.2 | |
| Biceps brachii | 14.5 ± 2.8 | 14.2 ± 1.3 | 12.0 ± 1.1df | 19.0 ± 4.8c | 13.8 ± 2.8 | 19.9 ± 2.7c | 0.271 | 9.8 | 0.002 | 8.8 | 0.043 | 0.5 | |
| Triceps brachii | 8.7 ± 2.8 | 7.3 ± 2.9 | 12.8 ± 1.7 | 10.5 ± 3.1 | 8.2 ± 0.9 | 9.1 ± 2.2 | 0.018 | 4.3 | 0.370 | 2.4 | 0.448 | 0.5 | |
| Cross-sectional area (cm2) | Rectus femoris | 10.8 ± 2.4 | 17.8 ± 3.3 | 8.8 ± 1.5 | 15.6 ± 1.8 | 11.2 ± 1.3 | 15.0 ± 2.8 | 0.216 | >100 | 0.000 | >100 | 0.364 | 0.4 |
| Vastus medialis | 24.3 ± 6.6 | 29.8 ± 4 | 17.0 ± 3.9 | 27.8 ± 6.4 | 18.2 ± 2.1 | 23.7 ± 1.2 | 0.046 | 22.3 | 0.002 | 12.0 | 0.441 | 0.8 | |
| Vastus lateralis | 29.8 ± 4.1 | 44.9 ± 4.1 | 24.4 ± 2.6 | 38.2 ± 4.5 | 24.1 ± 1.7 | 37.9 ± 3.0 | 0.004 | >100 | 0.001 | >100 | 0.912 | 0.5 | |
| Biceps brachii | 8.4 ± 1.7 | 17.7 ± 9.2 | 7.4 ± 2.0 | 14.9 ± 2.5 | 7.9 ± 0.2 | 12.9 ± 1.0 | 0.464 | 77.5 | 0.001 | 32.6 | 0.622 | 0.3 | |
| Triceps brachii | 10.5 ± 1.2 | 18 ± 4.3 | 7.0 ± 1.4 | 17.0 ± 5.7 | 8.9 ± 1.4 | 14.0 ± 3.6 | 0.273 | >100 | 0.000 | >100 | 0.417 | 0.3 | |
| Echo intensity (au) | Rectus femoris | 116 ± 26c | 113 ± 11c | 174 ± 33abd | 97 ± 14ce | 151 ± 16d | 129 ± 14 | 0.061 | 30.0 | 0.001 | 5.5 | 0.008 | 0.6 |
| Vastus medialis | 105 ± 16c | 108 ± 11 | 153 ± 39ad | 104 ± 12c | 116 ± 11 | 134 ± 13 | 0.093 | 2.4 | 0.289 | 0.8 | 0.012 | 0.4 | |
| Vastus lateralis | 111 ± 20c | 113 ± 15c | 171 ± 42abd | 107 ± 19c | 134 ± 16 | 123 ± 10 | 0.087 | 4.3 | 0.023 | 1.7 | 0.028 | 0.7 | |
| Biceps brachii | 123 ± 26 | 140 ± 9 | 170 ± 45 | 115 ± 29 | 148 ± 9 | 142 ± 17 | 0.585 | 0.6 | 0.206 | 0.5 | 0.044 | 0.2 | |
| Triceps brachii | 83 ± 17c | 89 ± 18c | 145 ± 43abd | 78 ± 13c | 114 ± 16 | 100 ± 6 | 0.079 | 7.2 | 0.014 | 1.8 | 0.012 | 0.6 | |
a = Significantly (p < 0.05) different from ADV women
b = Significantly (p < 0.05) different from ADV men
c = Significantly (p < 0.05) different from REC women
d = Significantly (p < 0.05) different from REC men
e = Significantly (p < 0.05) different from CON women
f = Significantly (p < 0.05) different from CON men.
Significant group effects were found for muscle thickness (VL and BB), pennation angle (BB and TB), fascicle length of TB, and CSA (VM and VL). Compared to CON, ADV possessed greater muscle thickness in VL (p = 0.013, BF10 = 3.0) and in BB (p = 0.012, BF10 = 2.2), larger BB pennation angle (p = 0.007, BF10 = 21.9), and greater CSA in VM (p = 0.050, BF10 = 2.1) and VL (p = 0.009, BF10 = 0.7). Compared to REC, ADV possessed greater muscle thickness in VL (p = 0.026, BF10 = 1.9), larger pennation angle in TB (p = 0.009, BF10 = 3.2), longer fascicles in TB (p = 0.019, BF10 = 3.9), and greater CSA in VL (p = 0.009, BF10 = 0.8); a tendency for greater muscle thickness in BB was also noted for ADV compared to REC (p = 0.086, BF10 = 0.9). No differences were seen between REC and CON. Morphological comparisons are presented in Table 1.
Graded exercise test
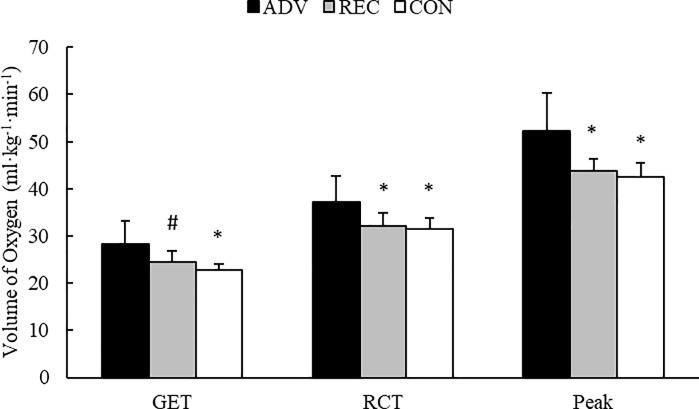
No significant group x sex interactions were observed for VO2peak (F = 1.09, p = 0.358, BF10 = 10.1), RCT (F = 0.32, p = 0.730, BF10 = 1.7), or GET (F = 0.05, p = 0.949, BF10 = 1.1). However, moderate-to-strong evidence were found in favor of main group effects for each variable. VO2peak (F = 9.10, p = 0.002, BF10 = 17.0) and RCT (F = 5.56, p = 0.014, BF10 = 4.5) were significantly greater in ADV compared to REC (p ≤ 0.039) and CON (p ≤ 0.020), while GET (F = 5.29, p = 0.016, BF10 = 5.7) was significantly greater in ADV compared to CON (p = 0.016) and tended to be greater compared to REC (p = 0.087). No differences were seen between REC and CON. Further, the percentage of VO2peak for GET and RCT were similar between ADV (GET = 55.2 ± 11.2%; RCT = 71.7 ± 7.5%), REC (GET = 55.9 ± 6.8%; RCT = 73.5 ± 5.9%), and CON (GET = 53.9 ± 4.3%; RCT = 74.6 ± 7.7%). Group differences in measures of aerobic performance are illustrated in Fig 2.
Fig 2. Group differences in aerobic performance measures.
* = Significantly (p < 0.05) different from ADV. # = Different (p < 0.10) from ADV.
Resting metabolic rate
Neither a group x sex interaction (F = 0.21, p = 0.817, BF10 = 0.2) or main group effect (F = 1.67, p = 0.220, BF10 = 0.1) was observed for RMR recordings in ADV (1788 ± 232 kcal·day-1), REC (1768 ± 407 kcal·day-1), and CON (1572 ± 356 kcal·day-1).
Body composition
No significant group x sex interactions were observed for any measure of body composition (presented in Table 2). However, the evidence was strongly-to-extremely in favor of main group effects for body density, regional and total BMC, regional and total lean mass, and BF%. Compared to the REC, ADV possessed greater body density (p = 0.004), greater BMC of the arms (p = 0.009), greater lean mass (i.e., total and regional; p ≤ 0.035), lower BF% (p = 0.009), and tended to possess more BMC (total-body: p = 0.066; legs: p = 0.060) and less fat mass (p = 0.064). Compared to CON, ADV possessed greater body density (p = 0.006), greater BMC throughout the body (p ≤ 0.024), lean mass throughout the body (p ≤ 0.009), and lower BF% (p = 0.023). No differences were observed between REC and CON.
Table 2. Group differences in measures of body composition.
| ADV | REC | CON | Group | Group x Sex | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women | Men | Total | Women | Men | Total | Women | Men | Total | p | BF10 | p | BF10 | |
| Anthropometric | |||||||||||||
| Height (cm) | 160 ± 13 | 177 ± 3 | 170 ± 11 | 161 ± 4 | 183 ± 8 | 172 ± 14 | 158 ± 4 | 180 ± 9 | 171 ± 14 | 0.785 | >100 | 0.526 | 0.3 |
| Weight (kg) | 68.3 ± 5.0 | 91.5 ± 5.1 | 79.8 ± 13.3 | 59.0 ± 2.0 | 93.5 ± 9.5 | 76.3 ± 19.5 | 60.8 ± 6.3 | 84.9 ± 7.3 | 74.5 ± 14.3 | 0.127 | >100 | 0.169 | 0.3 |
| BMI (kg·m-2) | 26.0 ± 3.5 | 29.2 ± 1.9 | 27.6 ± 3.1 | 22.9 ± 1.2 | 28.0 ± 3.4 | 25.5 ± 3.6 | 24.4 ± 3.4 | 26.1 ± 0.7 | 25.4 ± 2.2 | 0.163 | 6.6 | 0.456 | 0.6 |
| Density (kg·L-1) | 1.07 ± 0.01 | 1.07 ± 0.01 | 1.07 ± 0.01 | 1.05 ± 0.01 | 1.06 ± 0.01 | 1.05 ± 0.01* | 1.04 ± 0.02 | 1.06 ± 0.01 | 1.05 ± 0.02* | 0.002 | 13.8 | 0.159 | 1.1 |
| Bone Mineral Content (kg) | |||||||||||||
| Total | 3.05 ± 0.38 | 3.75 ± 0.13 | 3.45 ± 0.44 | 2.42 ± 0.16 | 3.62 ± 0.41 | 3.02 ± 0.70# | 2.43 ± 0.14 | 3.29 ± 0.41 | 2.92 ± 0.55* | 0.012 | >100 | 0.299 | 0.7 |
| Arms | 0.45 ± 0.07 | 0.62 ± 0.05 | 0.55 ± 0.11 | 0.32 ± 0.03 | 0.57 ± 0.05 | 0.44 ± 0.14* | 0.30 ± 0.01 | 0.48 ± 0.06 | 0.40 ± 0.11* | 0.001 | >100 | 0.266 | 1.2 |
| Legs | 1.12 ± 0.13 | 1.44 ± 0.11 | 1.30 ± 0.20 | 0.82 ± 0.05 | 1.38 ± 0.17 | 1.10 ± 0.32# | 0.81 ± 0.03 | 1.31 ± 0.22 | 1.09 ± 0.31* | 0.022 | >100 | 0.255 | 0.5 |
| Trunk | 0.95 ± 0.11 | 1.16 ± 0.03 | 1.07 ± 0.13 | 0.79 ± 0.11 | 1.11 ± 0.14 | 0.95 ± 0.21 | 0.82 ± 0.08 | 0.97 ± 0.09 | 0.90 ± 0.11* | 0.028 | >100 | 0.271 | 0.8 |
| Non-bone fat-free mass (kg) | |||||||||||||
| Arms | 7.15 ± 0.89 | 11.12 ± 1.22 | 9.42 ± 2.35 | 4.87 ± 0.49 | 10.02 ± 0.56 | 7.45 ± 2.79* | 4.83 ± 0.42 | 9.04 ± 1.14 | 7.24 ± 2.40* | 0.001 | >100 | 0.400 | 0.6 |
| Legs | 18.4 ± 1.4 | 25.4 ± 1.6 | 22.4 ± 4.0 | 14.3 ± 1.0 | 24.4 ± 1.1 | 19.3 ± 5.4* | 14.7 ± 0.7 | 22.5 ± 3.2 | 19.2 ± 4.7* | 0.008 | >100 | 0.252 | 0.5 |
| Trunk | 27.7 ± 2.9 | 35.2 ± 2.0 | 32.0 ± 4.5 | 20.3 ± 1.2 | 33.5 ± 1.4 | 26.9 ± 7.2* | 21.4 ± 2.2 | 30.1 ± 3.7 | 26.4 ± 5.5* | 0.001 | >100 | 0.073 | 0.8 |
| 4-compartment model | |||||||||||||
| Body fat percentage (%) | 11.9 ± 2.4 | 11.0 ± 2.6 | 11.4 ± 2.3 | 23.3 ± 2.4 | 16.1 ± 6.2 | 19.7 ± 5.8* | 23.9 ± 8.4 | 13.7 ± 3.2 | 18.1 ± 7.6* | 0.007 | 16.1 | 0.183 | 2.7 |
| Fat-free mass (kg) | 60.2 ± 3.5 | 81.3 ± 3.4 | 72.3 ± 11.7 | 45.2 ± 2.3 | 78.1 ± 5.3 | 61.7 ± 18.0* | 45.9 ± 1.6 | 73.3 ± 8.4 | 61.6 ± 15.9* | 0.001 | >100 | 0.097 | 0.5 |
| Fat mass (kg) | 8.2 ± 2.1 | 10.2 ± 2.7 | 9.3 ± 2.5 | 13.8 ± 1.4 | 15.4 ± 7.0 | 14.6 ± 4.7# | 14.9 ± 6.7 | 11.5 ± 2.2 | 13.0 ± 4.5 | 0.069 | 1.5 | 0.436 | 0.3 |
* = Significantly (p < 0.05) different from ADV
# = Different (p < 0.10) from ADV.
Strength
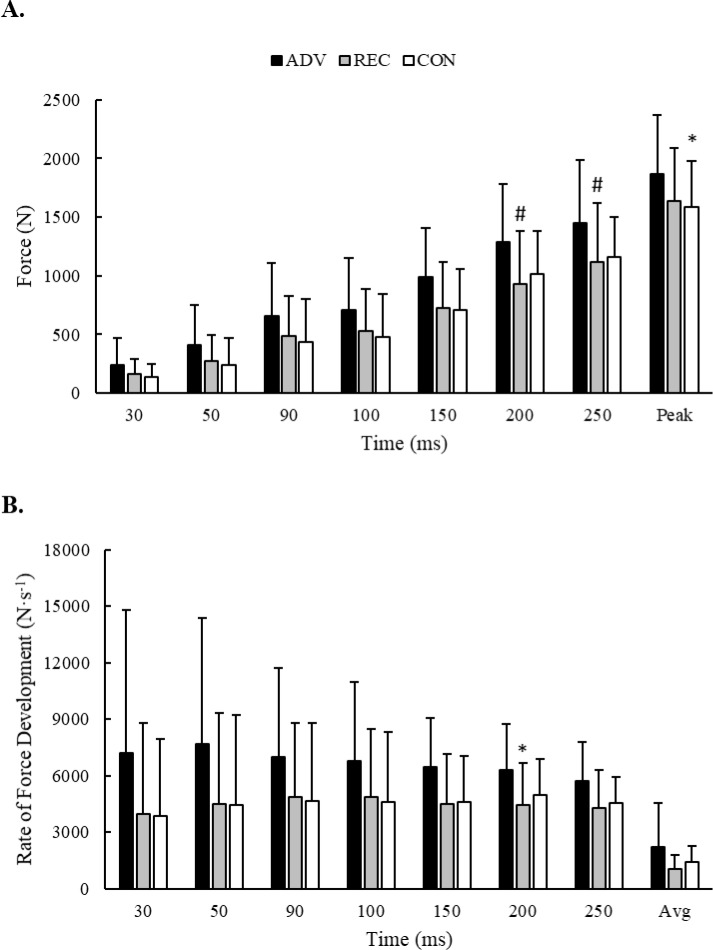
No significant group x sex interactions were observed for variables obtained from the isometric mid-thigh pull assessment. Extreme evidence suggested significant main group effects for F (F = 3.89, p = 0.042, BF10 = 667,577) and RFD at 200 ms (F = 3.67, p = 0.049, BF10 = 12,676), as well as tendencies for group differences in F at 150 ms (F = 2.80, p = 0.091, BF10 = 1,898), F at 200 ms (F = 3.50, p = 0.055, BF10 = 17,296), F at 250 ms (F = 3.14, p = 0.071, BF10 = 21524), RFD at 150 ms (F = 2.94, p = 0.082, BF10 = 1,868), and RFD at 250 ms (F = 3.37, p = 0.060, BF10 = 20,187). According to post-hoc analysis, ADV produced a higher peak F than CON (p = 0.036) and expressed greater RFD at 200 ms than REC (p = 0.049). ADV also tended to produce greater F at 200 ms (p = 0.062) and 250 ms (p = 0.097) compared to REC. No other specific differences were seen between groups. Group differences in F and RFD production across time are illustrated in Fig 3.
Fig 3.
Group differences in A) force and B) rate of force production during an isometric mid-thigh pull. * = Significantly (p < 0.05) different from ADV. # = Different (p < 0.10) from ADV.
3-minute all-out cycling test
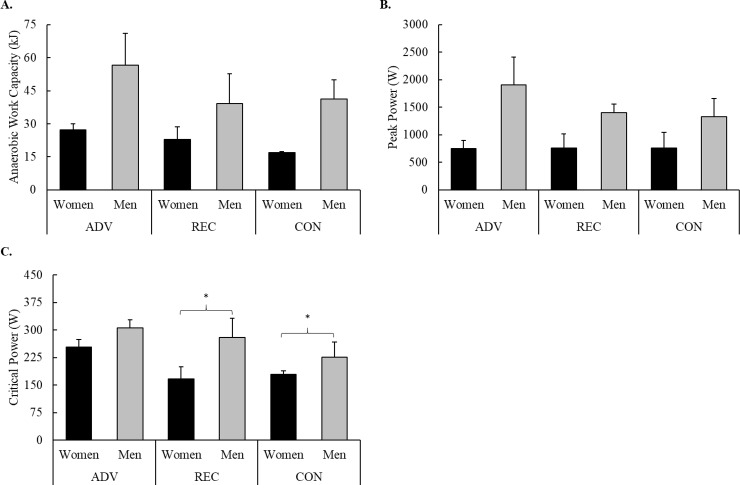
No significant group x sex interactions were observed for measures collected from the 3-minute all-out cycling test. Extreme evidence in favor of a significant group main effect for CP (F = 7.56, p = 0.005, BF10 = 267) indicated that ADV possessed a higher CP than REC (p = 0.029) and CON (p = 0.005). Although extreme evidence was also seen for AWC (F = 4.79, p = 0.023, BF10 = 247), post-hoc analysis did not reveal specific group differences. No other differences were observed. Group differences in measures of performance during the 3-minute all-out cycling test are illustrated in Fig 4.
Fig 4.
Group differences in A) anaerobic work capacity, B) peak power, and C) critical power. * = Significantly (p < 0.05) different than ADV.
Discussion
The primary objectives of this study were to examine anthropometric, hormonal, and physiological differences between advanced CF athletes, recreational CF participants, and resistance and cardiovascular trained adults. Previously, only one other cross-sectional investigation has made physiological comparisons between individuals with at least one year of CF or resistance-training experience [27]. The authors reported no differences between the groups except for the CF-trained group possessing greater aerobic ability. This outcome, however, is not surprising considering that the resistance-trained group was not required to also have been performing aerobic exercise. Typical CF training workouts will concurrently incorporate strength and conditioning elements into training [1, 2, 51]. Although the conditioning component varies in intensity and duration for each workout, it is important that alternative exercise strategies include both elements to make a fair comparison. The present study builds upon this limitation by having required participants in the CON group to have been participating in both resistance and cardiovascular training on at least 3 days per week each; a similar training frequency was expected of the recreational CF group (i.e., training on at least 3 days per week). Another important aspect of CF training worth consideration is that it includes a wide variety of traditional resistance and aerobic training exercises, along with simple-to-complex gymnastic movements. Proficiency in these movements cannot be assumed after only a year of training and would likely necessitate frequent workout modification. Recently, our group has reported different physiological responses and recovery rates to CF workouts that are completed as prescribed versus those that are modified (i.e., scaled) [11]. Thus, CF-trained participants were required to possess at least two years of experience and they were further divided into ADV and REC based upon evidence of their skill as CF athletes (i.e., their previous success in CF competition). Within these contexts, advanced CF athletes were observed to have a more favorable body composition and muscular morphological characteristics, as well as greater aerobic capacity, strength, and ability to sustain high-intensity effort compared to recreational CF participants and physically-active adults. In contrast, no differences were observed between recreational CF participants and physically-active adults in any measure and no differences were seen in resting hormone concentrations or metabolic rate across all groups. This is the first investigation to make comparisons among CF practitioners based on their competitive rank and relative to resistance- and cardiovascular-trained, active adults.
Most competitive CF workouts require athletes to perform 2 or more exercises in a circuit or listwise fashion for several repetitions and rounds, and to do so as quickly as possible or to complete as much work as possible within a given time limit [1, 2, 51]. Athletes who can maintain a faster pace or rapidly recover between minimal rest periods would appear to be best positioned to excel in this sport. A recent study in advanced CF athletes, as determined by their performance in a common benchmark workout (i.e., “Fran”), supports this idea [52]. Feito et al. (2018) found that the best predictor of repetitions completed during a 15-minute CF workout was the amount of work the athletes could perform on the final trial of four maximal Wingate sprints separated by 90 seconds of rest. In the present study, the ADV group possessed a lower percentage of body fat and greater non-bone fat-free mass compared to the REC and CON groups. In sports, possessing an ideal ratio of skeletal muscle to fat mass may offer a competitive advantage by improving efficiency, thermoregulation, and the ability to sustain effort [53]. Aside from their historical success in CF competition, the ADV group’s performance during testing provide evidence of this ability. ADV participants possessed a higher VO2peak than the other groups, which would imply that they were able to perform aerobic work throughout a greater range of workloads [54, 55] but it does not completely explain their ability to sustain effort at higher intensities [56]. As the oxygen requirements of a workload exceed an athlete’s capacity to efficiently deliver oxygen, the ability to sustain effort may be further explained by measures of anerobic performance and specific threshold points indicative of the onset of fatigue (i.e., GET, RCT, and CP) [48, 56, 57]. Participants in the ADV group were also found to possess a higher GET, RCT, and CP, which are all strongly correlated [57] and thought (specifically RCT and CP) to demarcate the point in which exercise transitions from ‘heavy’ to ‘severe’ [57, 58]. Together, these data suggest that the ADV athletes in this study had a greater capacity to produce energy aerobically, and that they were better equipped to maintain efforts at higher absolute workloads and thus, be successful in their sport.
Skeletal mass and the morphological characteristics of muscle are suggestive of a greater ability to produce force [59–62]. That is, the size, architecture and quality of skeletal muscle reflect the capability of activated muscle to produce force, whereas bone mass provides the structural support and stability needed to effectively translate force production into human movement. In the present study, ADV athletes possessed greater bone and muscle mass/size, larger pennation angles, shorter fascicles, and better quality in the arm and quadriceps musculature compared to the other groups. However, these only partially translated to greater force production by ADV group participants during the IMTP test. IMTP performance was highly variable until 0 to 200–250 ms, upon which ADV clearly produced greater force and at a faster rate. The lack of uniformity across all strength measures might be explained by testing specificity and the skillset of our sample. The importance of being able to rapidly activate muscle (i.e., higher RFD) and the magnitude of IMTP force production varies across sports and athletic activities. In weightlifters, significant relationships have been reported between one-repetition maximums in the Olympic lifts and IMTP force (peak and from 0 to 100–250 ms) [63] but relationships to RFD have either been limited to specific time bands (from 0 to 200–250 ms) [63] or remain unclear in other athletes [64, 65]. Although maximal strength in the Olympic and power lifts can distinguish competitive ranking in CF athletes [8, 10], it is not a common requisite of CF competition to maximally perform these lifts. Rather, most competitive workouts either utilize submaximal loads that are performed for several repetitions or they require the athlete to perform maximal (or near maximal) lifts after a fatiguing task (i.e., not a true measure of maximal strength) [51]. It is also possible that the composition of the ADV group may help explain the variability observed prior to 200 ms. While all ADV group participants ranked higher than REC in the Open, their participation in later rounds of the Games competition had primarily occurred as part of a team. Within this capacity, team members may be included based on their skill set (e.g., strong/powerful athletes, gymnastically-skilled athletes, endurance athletes) to minimize team weaknesses. This differs from individual competitors who must be proficient in a broader set of skills to be competitive [8, 10]. Currently, evidence documenting the physiological differences between high-ranking individual and team competitors does not exist.
There is little evidence to suggest that consistent alterations will occur to resting concentrations in T, C, or IGF-1 as a result of chronic training [14]. Rather, their concentrations generally reflect the current status of muscle tissue in response to the demands of training. Transient changes in T, IGF-1, and C may occur following acute and prolonged overreaching (or overstress) periods that could negatively impact anabolic status [14]. CF training is characterized by an effort to maximize training density (i.e., complete a set amount of work as quickly as possible, or maximize work completed within given time frame) within an unplanned (i.e., non-periodized) training structure to promote general physical preparedness [1, 2]. Further, the 5-week Open is the most common avenue used by athletes to qualify for the Games [4, 5]. Prior to an important competitive event, athletes may elevate training intensity to promote peak performance [66]. Thus, the combination of the CF training strategy and the approach of an important, extended competitive event could increase the likelihood of a prolonged period of overstress. The occurrence of which might be identified by changes in resting hormonal concentrations, resting metabolic rate, performance, as well as a variety of other factors [14, 67, 68]. However, the present investigation did not reveal any evidence of prolonged stress or negative adaptations to training. Resting hormone concentrations and metabolic rates were similar between groups and the physiological advantages demonstrated by the ADV group appeared to reflect their reported training habits over the past six months (via medical and physical activity history questionnaire). Excluding the conditioning component typically present in CF workouts, members from each group reported using a similar number of sets per muscle group (3–6), repetitions (3–12), and rest intervals (60–90 seconds) during the strength component of their workouts. Only training frequency was reported to be different with the ADV group utilizing a form of resistance exercise on approximately 5.3 days per week whereas the REC and CON groups averaged 4.6 days per week and 3.7 days per week, respectively. Although the greater training frequency seen in ADV would have theoretically provided more of an opportunity to accumulate training volume and promote adaptations, it could have also interfered with their recovery. Nevertheless, ADV possessed a more favorable body composition and generally outperformed the other groups in each performance measure. Therefore, as of one-month prior to competition, adequate recovery appeared to be present in this group. Likewise, the lack of differences seen between REC and CON, who were not actively training for the Open, also provides evidence of adequate recovery. Future investigations can expand on this by more closely monitoring training and performance surrounding the extended Open competition.
The findings of this study suggest that advanced CF athletes possess a more favorable body composition, greater bone and muscle mass, greater muscle quality and strength, greater aerobic capacity, and a greater ability to sustain effort than recreational CF participants and physically-active adults. The reasons for these differences remain unclear due to the cross-sectional design of this study but may be related to differences in training experience and recent training habits. Although all participants in this study could be considered well-trained [69], ADV group participants reported having more resistance training experience and having been training more frequently over the past 6 months than the other groups. It is possible that their advantages are simply the result of training for a longer amount of time or creating more opportunities to increase their volume load throughout the week. Without documentation (i.e., extensive, detailed training logs), however, it is only possible to speculate upon their potential influence as unknown factors (e.g., training quality, genetic predisposition) would certainly modulate resultant adaptations. It may be worthwhile for future investigations to make comparisons between advanced CF athletes and non-CF individuals with comparable training experience and habits to better determine whether an advantage exists.
It is also interesting to note that despite the superficial differences in each strategy (i.e., CF versus traditional resistance and cardiovascular training), REC and CON were found to possess similar physiological characteristics. It is possible that this was the consequence of our sample size being sufficient to observe the large differences that existed between ADV and the other groups but not for the smaller differences that existed between REC and CON. However, it may also be the consequence of effort and volume load during training being similar between these groups. To be included in the study, both REC and CON had to have been regularly participating in their chosen training strategy on 3–5 days per week for at least the past year. Beyond this requirement, however, our ability to quantify training volume load was limited to the participants’ recall over the past 6 months. Future longitudinal investigations that document both the quality and quantity of these training forms may help to provide insight into whether an advantage exists between these strategies or if they promote comparable adaptations among recreationally-active adults. Nevertheless, the present findings represent a starting point for future comparisons between experienced CF participants (athletes and recreational) and resistance-trained and cardiovascular-trained adults, as well as between sexes across these populations.
Supporting information
(CSV)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Glassman G. CrossFit training guide level 1.: The CrossFit Journal; 2011. [Google Scholar]
- 2.Feito Y, Heinrich K, Butcher S, Poston W. High-Intensity Functional Training (HIFT): Definition and Research Implications for Improved Fitness. Sports. 2018;6(3):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thompson WR. Worldwide survey of fitness trends for 2018: the CREP edition. ACSM's Health & Fitness Journal. 2017;21(6):10–9. [Google Scholar]
- 4.CrossFit. Finding the Fittest on Earth. CrossFit Games [Internet]. 2016 August 29, 2019. Available from: https://games.crossfit.com/workouts/open/2016.
- 5.CrossFit. Welcome to the 2019 CrossFit Games Season. CrossFit Games [Internet]. 2016 August 29, 2019.
- 6.Butcher SJ, Neyedly TJ, Horvey KJ, Benko CR. Do physiological measures predict selected crossFit® benchmark performance? Open Access Journal of Sports Medicine. 2015;6:241 10.2147/OAJSM.S88265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martínez-Gómez R, Valenzuela PL, Barranco-Gil D, Moral-González S, García-González A, Lucia A. Full-Squat as a Determinant of Performance in CrossFit. International journal of sports medicine. 2019;40(09):592–6. [DOI] [PubMed] [Google Scholar]
- 8.Serafini PR, Feito Y, Mangine GT. Self-reported measures of strength and sport-specific skills distinguish ranking in an international online fitness competition. Journal of strength and conditioning research. 2018;32(12):3474–84. 10.1519/JSC.0000000000001843 [DOI] [PubMed] [Google Scholar]
- 9.Bellar D, Hatchett A, Judge L, Breaux M, Marcus L. The relationship of aerobic capacity, anaerobic peak power and experience to performance in CrossFit exercise. Biology of Sport. 2015;32(4):315–20. 10.5604/20831862.1174771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbieri JF, Correia RF, Castaño LAA, Brasil DVC, Ribeiro AN. Comparative and correlational analysis of the performance from 2016 crossfit games high-level athletes. Manual Therapy, Posturology & Rehabilitation Journal = Revista Manual Therapy. 2017;15 10.4081/ejtm.2019.8331 [DOI] [Google Scholar]
- 11.Mangine GT, Kliszczewicz BM, Boone JB, Williamson-Reisdorph CM, Bechke EE. Pre-anticipatory anxiety and autonomic nervous system response to two unique fitness competition workouts. Sports. 2019;7(9):199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casto KV, Edwards DA. Testosterone, cortisol, and human competition. Horm Behav. 2016;82:21–37. 10.1016/j.yhbeh.2016.04.004 [DOI] [PubMed] [Google Scholar]
- 13.Kivlighan KT, Granger DA. Salivary α-amylase response to competition: Relation to gender, previous experience, and attitudes. Psychoneuroendocrinology. 2006;31(6):703–14. 10.1016/j.psyneuen.2006.01.007 [DOI] [PubMed] [Google Scholar]
- 14.Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports medicine. 2005;35(4):339–61. 10.2165/00007256-200535040-00004 [DOI] [PubMed] [Google Scholar]
- 15.McGee KJ, Burkett LN. The National Football League Combine: A Reliable Predictor of Draft Status? The Journal of Strength & Conditioning Research. 2003;17(1):6–11. [DOI] [PubMed] [Google Scholar]
- 16.Mangine GT, Hoffman JR, Vazquez J, Pichardo N, Fragala MS, Stout JR. Predictors of fielding performance in professional baseball players. International Journal of Sports Physiology and Performance. 2013;8(5):510–6. 10.1123/ijspp.8.5.510 [DOI] [PubMed] [Google Scholar]
- 17.Hoffman JR, Vazquez J, Pichardo N, Tenenbaum G. Anthropometric and performance comparisons in professional baseball players. Journal of Strength & Conditioning Research. 2009;23(8):2173–8. [DOI] [PubMed] [Google Scholar]
- 18.Mangine GT, Hoffman JR, Wells AJ, Gonzalez AM, Rogowski JP, Townsend JR, et al. Visual Tracking Speed Is Related to Basketball-Specific Measures of Performance in NBA Players. The Journal of Strength & Conditioning Research. 2014;28(9):2406–14. [DOI] [PubMed] [Google Scholar]
- 19.Feito Y, Brown C, Olmos A. A content analysis of the High-Intensity Functional Training Literature: a look at the past and directions for the future. Human Movement. 2019;20(2):1–15. [Google Scholar]
- 20.Heinrich KM, Spencer V, Fehl N, Carlos Poston WS. Mission essential fitness: comparison of functional circuit training to traditional Army physical training for active duty military. Military Medicine. 2012;177(10):1125–30. 10.7205/milmed-d-12-00143 [DOI] [PubMed] [Google Scholar]
- 21.Barfield J, Anderson A. Effect of CrossFit™ on health-related physical fitness: A pilot study. Journal of Sport and Human Performance. 2014;2(1). [Google Scholar]
- 22.Barfield J, Channell B, Pugh C, Tuck M, Pendel D. Format of basic instruction program resistance training classes: Effect on fitness change in college students. Physical Educator. 2012;69(4):325. [Google Scholar]
- 23.Feito Y, Patel P, Sal Redondo A, Heinrich KM. Effects of Eight Weeks of High Intensity Functional Training on Glucose Control and Body Composition among Overweight and Obese Adults. Sports. 2019;7(2):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buckley S, Knapp K, Lackie A, Lewry C, Horvey K, Benko C, et al. Multimodal high-intensity interval training increases muscle function and metabolic performance in females. Applied Physiology, Nutrition, and Metabolism. 2015;40(11):1157–62. 10.1139/apnm-2015-0238 [DOI] [PubMed] [Google Scholar]
- 25.Carnes AJ, Mahoney SE. Polarized Versus High-Intensity Multimodal Training in Recreational Runners. International journal of sports physiology and performance. 2019;14(1):105–12. [DOI] [PubMed] [Google Scholar]
- 26.Ratamess N, Alvar B, Evetoch T, Housh T, Kibler W, Kraemer W. Progression models in resistance training for healthy adults [ACSM position stand]. Medicine and Science in Sports and Exercise. 2009;41(3):687–708. 10.1249/MSS.0b013e3181915670 [DOI] [PubMed] [Google Scholar]
- 27.de Sousa AF, dos Santos GB, dos Reis T, Valerino AJ, Del Rosso S, Boullosa DA. Differences in Physical Fitness between Recreational CrossFit® and Resistance Trained Individuals. Journal of Exercise Physiology Online. 2016;19(5). [Google Scholar]
- 28.Mangine GT, Cebulla B, Feito Y. Normative values for self-reported benchmark workout scores in crossfit® practitioners. Sports medicine-open. 2018;4(1):39 10.1186/s40798-018-0156-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichinose Y, Kanehisa H, Ito M, Kawakami Y, Fukunaga T. Morphological and functional differences in the elbow extensor muscle between highly trained male and female athletes. European Journal of Applied Physiology and Occupational Physiology. 1998;78(2):109–14. 10.1007/s004210050394 [DOI] [PubMed] [Google Scholar]
- 30.Arroyo E, Stout JR, Beyer KS, Church DD, Varanoske AN, Fukuda DH, et al. Effects of supine rest duration on ultrasound measures of the vastus lateralis. Clinical physiology and functional imaging. 2018;38(1):155–7. 10.1111/cpf.12403 [DOI] [PubMed] [Google Scholar]
- 31.Cadore EL, Izquierdo M, Conceição M, Radaelli R, Pinto RS, Baroni BM, et al. Echo intensity is associated with skeletal muscle power and cardiovascular performance in elderly men. Experimental Gerontology. 2012;47(6):473–8. 10.1016/j.exger.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 32.Chapman DW, Newton M, McGuigan MR, Nosaka K. Comparison between old and young men for responses to fast velocity maximal lengthening contractions of the elbow flexors. European Journal of Applied Physiology. 2008;104(3):531–9. 10.1007/s00421-008-0806-7 [DOI] [PubMed] [Google Scholar]
- 33.Bemben M. Use of diagnostic ultrasound for assessing muscle size. Journal of Strength & Conditioning Research. 2002;16(1):103–8. [PubMed] [Google Scholar]
- 34.Scanlon TC, Fragala MS, Stout JR, Emerson NS, Beyer KS, Oliveira LP, et al. Muscle architecture and strength: Adaptations to short‐term resistance training in older adults. Muscle & Nerve. 2013. [DOI] [PubMed] [Google Scholar]
- 35.Young HJ, Jenkins NT, Zhao Q, Mccully KK. Measurement of intramuscular fat by muscle echo intensity. Muscle & nerve. 2015;52(6):963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumagai K, Abe T, Brechue WF, Ryushi T, Takano S, Mizuno M. Sprint performance is related to muscle fascicle length in male 100-m sprinters. Journal of Applied Physiology. 2000;88(3):811–6. 10.1152/jappl.2000.88.3.811 [DOI] [PubMed] [Google Scholar]
- 37.Astorino TA, Robergs RA, Ghiasvand F, Marks D, Burns S. Incidence of the oxygen plateau at VO2max during exercise testing to volitional fatigue. Journal of exercise physiology online. 2000;3(4):1–12. [Google Scholar]
- 38.Wasserman K, McIlroy MB. Detecting the threshold of anaerobic metabolism in cardiac patients during exercise. The American journal of cardiology. 1964;14(6):844–52. [DOI] [PubMed] [Google Scholar]
- 39.Beaver WL, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. Journal of applied physiology. 1986;60(6):2020–7. 10.1152/jappl.1986.60.6.2020 [DOI] [PubMed] [Google Scholar]
- 40.Wasserman K, Whipp BJ, Koyl S, Beaver W. Anaerobic threshold and respiratory gas exchange during exercise. Journal of applied physiology. 1973;35(2):236–43. 10.1152/jappl.1973.35.2.236 [DOI] [PubMed] [Google Scholar]
- 41.Compher C, Frankenfield D, Keim N, Roth-Yousey L, Group EAW. Best practice methods to apply to measurement of resting metabolic rate in adults: a systematic review. Journal of the American Dietetic Association. 2006;106(6):881–903. 10.1016/j.jada.2006.02.009 [DOI] [PubMed] [Google Scholar]
- 42.McCrory MA, Molé PA, Gomez TD, Dewey KG, Bernauer EM. Body composition by air-displacement plethysmography by using predicted and measured thoracic gas volumes. Journal of Applied Physiology. 1998;84(4):1475–9. 10.1152/jappl.1998.84.4.1475 [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Deurenberg P, Guo SS, Pietrobelli A, Wang J, Pierson R Jr, et al. Six-compartment body composition model: inter-method comparisons of total body fat measurement. International journal of obesity. 1998;22(4):329 10.1038/sj.ijo.0800590 [DOI] [PubMed] [Google Scholar]
- 44.Haff GG, Ruben RP, Lider J, Twine C, Cormie P. A comparison of methods for determining the rate of force development during isometric midthigh clean pulls. The Journal of Strength & Conditioning Research. 2015;29(2):386–95. [DOI] [PubMed] [Google Scholar]
- 45.Bergstrom HC, Housh TJ, Zuniga JM, Traylor DA, Lewis RW Jr, Camic CL, et al. Differences among estimates of critical power and anaerobic work capacity derived from five mathematical models and the three-minute all-out test. The Journal of Strength & Conditioning Research. 2014;28(3):592–600. [DOI] [PubMed] [Google Scholar]
- 46.Marsh AP, Martin PE. Effect of cycling experience, aerobic power, and power output on preferred and most economical cycling cadences. Medicine and Science in Sports and Exercise. 1997;29(9):1225–32. 10.1097/00005768-199709000-00016 [DOI] [PubMed] [Google Scholar]
- 47.Burnley M, Doust JH, Vanhatalo A. A 3-min all-out test to determine peak oxygen uptake and the maximal steady state. Medicine & Science in Sports & Exercise. 2006;38(11):1995–2003. [DOI] [PubMed] [Google Scholar]
- 48.Vanhatalo A, Doust JH, Burnley M. Determination of critical power using a 3-min all-out cycling test. Medicine and science in sports and exercise. 2007;39(3):548–55. 10.1249/mss.0b013e31802dd3e6 [DOI] [PubMed] [Google Scholar]
- 49.Rouder JN, Morey RD, Speckman PL, Province JM. Default Bayes factors for ANOVA designs. Journal of Mathematical Psychology. 2012;56(5):356–74. [Google Scholar]
- 50.Wagenmakers E-J, Love J, Marsman M, Jamil T, Ly A, Verhagen J, et al. Bayesian inference for psychology. Part II: Example applications with JASP. Psychonomic bulletin & review. 2018;25(1):58–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.CrossFit. Open Workouts. CrossFit Games [Internet]. 2019; (May 1). Available from: https://games.crossfit.com/workouts/open/2019.
- 52.Feito Y, Giardina MJ, Butcher S, Mangine GT. Repeated anaerobic tests predict performance among a group of advanced CrossFit-trained athletes. Applied Physiology, Nutrition, and Metabolism. 2018;44(7):727–35. 10.1139/apnm-2018-0509 [DOI] [PubMed] [Google Scholar]
- 53.O’Connor H, Slater G. Losing, gaining and making weight for athletes Sport and Exercise Nutrition West Sussex, UK: Wiley-Blackwell; 2011:210–32. [Google Scholar]
- 54.Hawkins MN, Raven PB, Snell PG, Stray-Gundersen J, Levine BD. Maximal oxygen uptake as a parametric measure of cardiorespiratory capacity. Med Sci Sports Exerc. 2007;39(1):103–7. 10.1249/01.mss.0000241641.75101.64 [DOI] [PubMed] [Google Scholar]
- 55.Day JR, Rossiter HB, Coats EM, Skasick A, Whipp BJ. The maximally attainable VO2 during exercise in humans: the peak vs. maximum issue. Journal of applied physiology. 2003;95(5):1901–7. 10.1152/japplphysiol.00024.2003 [DOI] [PubMed] [Google Scholar]
- 56.Medbo JI, Tabata I. Relative importance of aerobic and anaerobic energy release during short-lasting exhausting bicycle exercise. Journal of Applied Physiology. 1989;67(5):1881–6. 10.1152/jappl.1989.67.5.1881 [DOI] [PubMed] [Google Scholar]
- 57.Bergstrom HC, Housh TJ, Zuniga JM, Traylor DA, Camic CL, Lewis RW Jr, et al. The relationships among critical power determined from a 3-min all-out test, respiratory compensation point, gas exchange threshold, and ventilatory threshold. Research quarterly for exercise and sport. 2013;84(2):232–8. 10.1080/02701367.2013.784723 [DOI] [PubMed] [Google Scholar]
- 58.Chicharro JL, Hoyos J, Lucía A. Effects of endurance training on the isocapnic buffering and hypocapnic hyperventilation phases in professional cyclists. British journal of sports medicine. 2000;34(6):450–5. 10.1136/bjsm.34.6.450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller TA. NSCA's Guide to Tests and Assessments: Human Kinetics; 2012. [Google Scholar]
- 60.Schipilow J, Macdonald H, Liphardt A, Kan M, Boyd S. Bone micro-architecture, estimated bone strength, and the muscle-bone interaction in elite athletes: an HR-pQCT study. Bone. 2013;56(2):281–9. 10.1016/j.bone.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 61.Lieber RL, Fridén J. Functional and clinical significance of skeletal muscle architecture. Muscle & Nerve: Official Journal of the American Association of Electrodiagnostic Medicine. 2000;23(11):1647–66. [DOI] [PubMed] [Google Scholar]
- 62.Stock MS, Mota JA, Hernandez JM, Thompson BJ. Echo intensity and muscle thickness as predictors Of athleticism and isometric strength in middle‐school boys. Muscle & nerve. 2017;55(5):685–92. [DOI] [PubMed] [Google Scholar]
- 63.Beckham G, Mizuguchi S, Carter C, Sato K, Ramsey M, Lamont H, et al. Relationships of isometric mid-thigh pull variables to weightlifting performance. J Sports Med Phys Fitness. 2013;53(5):573–81. [PubMed] [Google Scholar]
- 64.McGuigan MR, Winchester JB. The relationship between isometric and dynamic strength in college football players. Journal of sports science & medicine. 2008;7(1):101. [PMC free article] [PubMed] [Google Scholar]
- 65.Stone MH, Sanborn K, O'BRYANT HS, HARTMAN M, STONE ME, PROULX C, et al. Maximum strength-power-performance relationships in collegiate throwers. The Journal of Strength & Conditioning Research. 2003;17(4):739–45. [DOI] [PubMed] [Google Scholar]
- 66.Haff GG. Periodization for Tactical Populations In: Alvar BA, Sell K, Deuster PA, editors. NSCA's Essentials of Tactical Strength and Conditioning. 1st ed Champaign, IL: Human Kinetics, Inc.; 2015. p. 181–205. [Google Scholar]
- 67.Bahr R, Opstad P, Medbø J, Sejersted O. Strenuous prolonged exercise elevates resting metabolic rate and causes reduced mechanical efficiency. Acta Physiologica Scandinavica. 1991;141(4):555–63. 10.1111/j.1748-1716.1991.tb09117.x [DOI] [PubMed] [Google Scholar]
- 68.Lee EC, Fragala MS, Kavouras SA, Queen RM, Pryor JL, Casa DJ. Biomarkers in sports and exercise: tracking health, performance, and recovery in athletes. Journal of strength and conditioning research. 2017;31(10):2920 10.1519/JSC.0000000000002122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheppard JM, Triplett NT. Program Design for Resistance Training In: Haff GG, Triplett NT, editors. Essentials of Strength Training and Conditioning. 4th ed: Human Kinetics; 2015. p. 439–69. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(CSV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.