Abstract
With more than 900,000 confirmed cases worldwide and nearly 50,000 deaths during the first 3 months of 2020, the coronavirus disease 2019 (COVID-19) pandemic has emerged as an unprecedented health care crisis. The spread of COVID-19 has been heterogeneous, resulting in some regions having sporadic transmission and relatively few hospitalized patients with COVID-19 and others having community transmission that has led to overwhelming numbers of severe cases. For these regions, health care delivery has been disrupted and compromised by critical resource constraints in diagnostic testing, hospital beds, ventilators, and health care workers who have fallen ill to the virus exacerbated by shortages of personal protective equipment. Although mild cases mimic common upper respiratory viral infections, respiratory dysfunction becomes the principal source of morbidity and mortality as the disease advances. Thoracic imaging with chest radiography and CT are key tools for pulmonary disease diagnosis and management, but their role in the management of COVID-19 has not been considered within the multivariable context of the severity of respiratory disease, pretest probability, risk factors for disease progression, and critical resource constraints. To address this deficit, a multidisciplinary panel comprised principally of radiologists and pulmonologists from 10 countries with experience managing patients with COVID-19 across a spectrum of health care environments evaluated the utility of imaging within three scenarios representing varying risk factors, community conditions, and resource constraints. Fourteen key questions, corresponding to 11 decision points within the three scenarios and three additional clinical situations, were rated by the panel based on the anticipated value of the information that thoracic imaging would be expected to provide. The results were aggregated, resulting in five main and three additional recommendations intended to guide medical practitioners in the use of chest radiography and CT in the management of COVID-19.
Abbreviations: COVID-19, coronavirus disease 2019; PPE, personal protection equipment; RT-PCR, reverse-transcriptase polymerase chain reaction; SARS-CoV2, severe acute respiratory syndrome coronavirus 2
Summary
Structured around three scenarios and three key situations, this Fleischner statement provides context for the use of imaging to direct patient management during the coronavirus disease 2019 pandemic in different practice settings, different phases of epidemic outbreak, and environments of varying critical resource availability.
Essentials.
-
•
Imaging is not indicated in patients suspected of having coronavirus disease 2019 (COVID-19) and mild clinical features unless they are at risk for disease progression.
-
•
Imaging is indicated in a patient with COVID-19 and worsening respiratory status.
-
•
In a resource-constrained environment, imaging is indicated for medical triage of patients suspected of having COVID-19 who present with moderate-to-severe clinical features and a high pretest probability of disease.
On March 11, 2020, the World Health Organization (WHO) officially characterized the rapid global spread of coronavirus disease 2019 (COVID-19) as a pandemic and called for urgent international action in four key areas: to prepare and be ready; to detect, protect, and treat; to reduce transmission; and to innovate and learn.1 At the time of writing (April 1, 2020), there are more than 900,000 confirmed COVID-19 cases and nearly 50,000 deaths in 205 countries around the world, with the majority of cases concentrated in four countries: United States, Italy, Spain, and China.2 , 3 With sustained community transmission now established in multiple countries on multiple continents, the WHO public health goal has changed from containment to mitigation of the pandemic’s impact. Consequently, strategies are now focused on efforts to reduce the incidence, morbidity, and mortality of COVID-19 by breaking the chain of human transmission through social distancing and imposed quarantine.
Diagnostic Testing
Early detection and containment of infection caused by the novel coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) has been hindered by the need to develop, mass produce, and widely disseminate the required molecular diagnostic test, a real-time reverse-transcriptase polymerase chain reaction (RT-PCR) assay. Early reports of test performance in the Wuhan, China, outbreak showed variable sensitivities ranging from 37% to 71%.4 , 5 While laboratory-based performance evaluations of RT-PCR tests show high analytical sensitivity and near-perfect specificity with no misidentification of other coronaviruses or common respiratory pathogens, test sensitivity in clinical practice may be adversely affected by a number of variables, including adequacy of specimen, specimen type, specimen handling, and stage of infection when the specimen is acquired (Centers of Disease Control guidelines for in-vitro diagnostics).6 , 7 False-negative RT-PCR tests have been reported in patients with CT findings of COVID-19 who eventually tested positive with serial sampling.8 Limited testing capacity due to insufficient specimen collection kits, laboratory test supplies, and testing equipment precluded early widespread testing and is believed to have contributed to rapid and unchecked transmission of infection within communities by undetected individuals with milder, limited, or no symptoms.9 , 10 For example, CT screening of 82 asymptomatic individuals with confirmed COVID-19 from the cruise ship “Diamond Princess” showed findings of pneumonia in 54%.11
Imaging Logistics During Pandemic
Provision of diagnostic imaging services to large numbers of patients suspected of having or confirmed to have COVID-19 during an outbreak can be challenging, as each study is lengthened and complicated by the need for strict adherence to infection control protocols designed to minimize risk of transmission and protect health care personnel.12 Droplet transmission followed by contaminated surfaces are believed to be the main modes of spread for SARS-CoV2 in radiology suites; all patients undergoing imaging should be masked and imaged by using dedicated equipment that is cleaned and disinfected after each patient encounter.13 Although personal protection equipment (PPE) recommendations vary between countries, the current Centers of Disease Control and Prevention guidelines recommend radiology staff wear a mask, goggles or face shield, gloves, and an isolation gown. In countries with more stringent PPE protocols, a surgical cap and shoe covers may be added, while a surgical mask and goggles or face shield are suggested in some countries with less stringent PPE protocols.14 Additional precautions are required for specific situations that are more likely to generate aerosols, including patients receiving noninvasive ventilation, during intubation or extubation, throughout bronchoscopy, or when patients are receiving nebulized therapies. Portable imaging, including imaging patients through glass walls, has been used in some hospitals to further reduce the chance of spreading infection.
Written from multidisciplinary and multinational perspectives, this Fleischner statement is intended to provide context for the use of imaging to direct patient management during the COVID-19 pandemic in different practice settings, different phases of epidemic outbreak, and environments of varying critical resource availability. This document is structured around three clinical scenarios and three additional situations in which chest imaging is often considered in the evaluation of patients with potential COVID-19 infection. The committee elected to present this document as a consensus statement rather than a guideline given the limited evidence base and the urgent need for direction on this topic for the medical community.
Materials and Methods
This consensus statement is based on expert opinion among a panel of 15 thoracic radiologists, 10 pulmonologists and/or intensivists (including one anesthesiologist), and one pathologist, as well as additional experts in emergency medicine, infection control, and laboratory medicine. The panel included individuals from the United States, Italy, China, Germany, France, the United Kingdom, the Netherlands, South Korea, Canada, and Japan, representing nine of the 15 countries with the highest number of confirmed COVID-19 cases reported worldwide as of April 1, 2020.2 The panel possessed experience managing patients during periods of local viral amplification and critical resource constraints in Wuhan, China, Northern Italy, and New York City.
A subcommittee composed of five radiologists, four pulmonologists and/or intensivists (including one anesthesiologist), and one emergency medicine physician identified and iteratively developed three scenarios that illustrate imaging-related dilemmas occurring in common clinical presentations and across varying risk factors, community conditions, and resource constraints. These scenarios included 11 distinct nodes where imaging potentially provides clinically actionable information (Figure 1, Figure 2, Figure 3 ), with three additional situations identified in which chest imaging is also often considered (Fig 4 ). The entire panel was convened during a single session by using a live audio and video interface (Zoom Video Communications). The three scenarios and three additional situations were presented, discussed, and refined. The panel independently and anonymously rated the appropriateness of imaging with chest radiography or CT at each of these decision points on a five-point scale. At least 70% agreement on the direction of a recommendation was considered consensus. The scenarios are intended to support the management of adults only. Children, who are typically spared from severe infections,15 merit separate consideration—particularly with regard to use of radiation-associated procedures—and are beyond the scope of the current document.
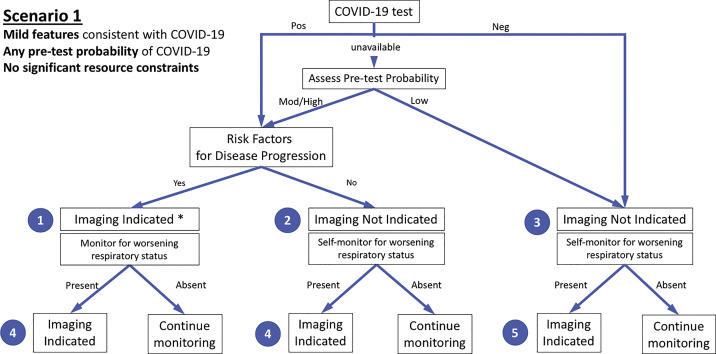
Figure 1.
Diagram illustrates the first of three clinical scenarios presented to the panel with final recommendations. Mild features refer to absence of significant pulmonary dysfunction or damage. Pretest probability is based on background prevalence of disease and may be further modified by individual’s exposure risk. The absence of resource constraints corresponds to sufficient availability of personnel, personal protective equipment, coronavirus disease 2019 (COVID-19) testing, hospital beds, and/or ventilators with the need to rapidly triage patients. Numbers in blue circles indicate key questions referenced in the text and presented in Figure 4. Contextual detail and considerations for imaging with chest radiography versus CT are presented in the text. Although not covered by this scenario and not shown in the figure, in the presence of substantial resources constraints, there is no role for imaging of patients with mild features of COVID-19. ∗ = Clinical judgment should dictate the use of imaging through consideration of patient risk factors and local resources. Mod = moderate; Neg = negative; Pos = positive.
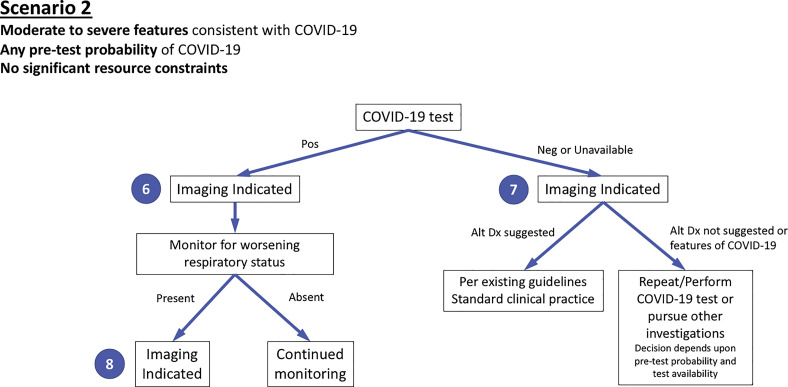
Figure 2.
Diagram illustrates the second of three clinical scenarios presented to the panel with final recommendations. Moderate-to-severe features refer to evidence of significant pulmonary dysfunction or damage. Pretest probability is based on background prevalence of disease and may be further modified by individual’s exposure risk. The absence of resource constraints corresponds to sufficient availability of personnel, personal protective equipment, coronavirus disease 2019 (COVID-19) testing, hospital beds, and/or ventilators with the need to rapidly triage patients. Numbers in blue circles indicate key questions referenced in the text and presented in Figure 4. Contextual detail and considerations for imaging with chest radiography versus CT are presented in the text. Alt Dx = alternate diagnosis; Neg = negative; Pos = positive.
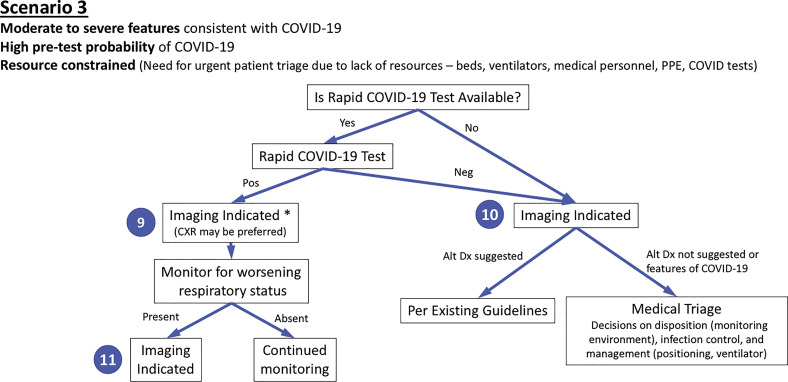
Figure 3.
Diagram illustrates the third of three clinical scenarios presented to the panel with final recommendations. Moderate-to-severe features refer to evidence of significant pulmonary dysfunction or damage. High pretest probability is based on high background prevalence of disease associated with community transmission. Rapid coronavirus disease 2019 (COVID-19) test is a point-of-care test with a turnaround time of less than 1 hour. Numbers in blue circles indicate key questions referenced in the text and presented in Figure 4. Contextual detail and considerations for imaging with chest radiography (CXR) versus CT are presented in the text. ∗ = Lower priority if severely resource constrained, relative to question 10 or 11. Alt Dx = alternate diagnosis; Neg = negative; Pos = positive; PPE = personal protection equipment.
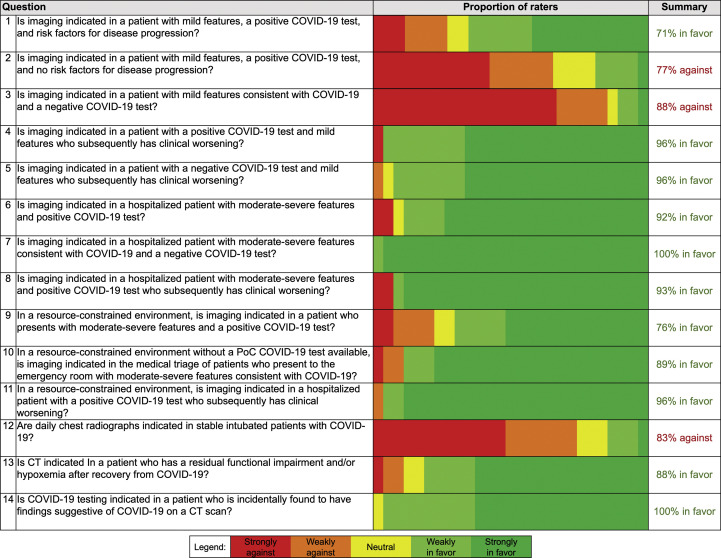
Figure 4.
Panel members (n = 27) developed 14 key questions used to support creation of common scenarios and recommendations related to the use of chest imaging in patients with features of coronavirus disease 2019 (COVID-19). The proportion of panel member votes for each question is presented on a 5-point scale as well as a summary column that shows the total percentage of committee members who voted for or against imaging for each key question, excluding those members who were neutral or who abstained (one panel member abstained for questions 1 and 2). Numbers in left column correspond to question numbers in text and Figure 1, Figure 2, Figure 3. PoC = point of care.
The final document was supported by a comprehensive literature search for relevant articles. Using the search terms “((coronavirus OR COVID OR SARS-CoV OR ∗nCoV∗) AND (CT OR Computed Tomography OR Radio∗ OR Imag∗)),” a total of 137 English articles published between December 1, 2019, and March 23, 2020, were identified. Each article was assessed for relevance to the primary objective, and a summary of key findings from relevant articles was created.
Use of Imaging in COVID-19
The value of an imaging test relates to the generation of results that are clinically actionable either for establishing a diagnosis or for guiding management, triage, or therapy. That value is diminished by costs that include the risk of radiation exposure to the patient, risk of COVID-19 transmission to uninfected health care workers and other patients, consumption of PPE, and need for cleaning and downtime of radiology rooms in resource-constrained environments. The appropriate use of imaging in each of the scenarios was considered on this basis.
This statement focuses exclusively on the use of chest radiography and CT of the thorax. Although US has been suggested as a potential triage and diagnostic tool for COVID-19 given the predilection for the disease in subpleural regions, there is limited experience at this time16 as well as infection control issues.
Chest radiography is insensitive in mild or early COVID-19 infection.17 However, with respect to the relative value of chest radiography or CT for detecting the presence of viral pneumonia, the experience is vastly different depending on community norms and public health directives. When patients are encouraged to present early in the course of their disease, as was the case in Wuhan, China, chest radiography has little value. The greater sensitivity of CT for early pneumonic changes is more relevant in the setting of a public health approach that required isolation of all infected patients within an environment where the reliability of COVID-19 testing was limited and turnaround times were long.4 Alternatively, in New York City, where patients were instructed to stay at home until they experienced advanced symptoms, chest radiographs were often abnormal at the time of presentation. Equipment portability with imaging performed within an infected patient’s isolation room is another factor that may favor chest radiography in selected populations, effectively eliminating the risk of COVID-19 transmission along the transport route to a CT scanner and within the room housing a CT scanner, particularly in environments lacking PPE. In hospitalized patients, chest radiography can be useful for assessing disease progression and alternative diagnoses (eg, lobar pneumonia suggestive of bacterial superinfection, pneumothorax, and pleural effusion).
CT is more sensitive for early parenchymal lung disease, disease progression, and alternative diagnoses including acute heart failure from COVID-19 myocardial injury18 and, when performed with intravenous contrast material, pulmonary thromboembolism. Leveraging these superior capabilities depends on the availability of CT capacity, particularly considering the potential reduction in CT scanner availability due to the additional time required to clean and disinfect equipment after imaging of patients suspected of having COVID-19. Some centers rely on the improved depiction of COVID-19 findings with CT relative to chest radiography19 and their association with clinical worsening to determine patient disposition to home, hospital admission, or intensive care. In recognition of variance among local practice patterns and resource availability, it is important to state at the outset that the scenarios specify the use of imaging but do not articulate the relative merit of chest radiography versus CT. Ultimately, the choice of imaging modality is left to the judgment of clinical teams at the point of care, accounting for the differing attributes of chest radiography and CT, local resources, and expertise.
Overview of Clinical Scenarios
The scenarios apply only to patients presenting with features consistent with COVID-19 infection. The severity of respiratory disease and pretest probability of COVID-19 infection are specified for each scenario, with additional key considerations including the presence of risk factors for disease progression, evidence of disease progression, and the presence of substantial critical resource constraints (Table 1 ). The scenarios help distinguish mild respiratory disease from moderate-to-severe respiratory disease on the basis of the absence versus presence of significant pulmonary dysfunction or damage. Pretest probability is defined by the background prevalence of infection and can be estimated with observed transmission patterns, as follows: low with sporadic transmission, moderate with clustered transmission, and high with community transmission.20 Individual pretest probability is further modified if there is known exposure through contact with a person confirmed to have COVID-19.21 For health care providers, the Centers for Disease Control and Prevention22 categorizes medical-related exposures into low-, medium-, and high-risk groups. Within a diagnostic radiology department, brief (a few minutes or less) unprotected interaction with a patient with COVID-19 and prolonged close contact with a masked, infected patient by a medical provider wearing PPE are categorized as low-risk exposures.21 , 22 Risk factors for poor outcomes in patients with COVID-19 infection are considered separately from pretest probability, with common risk factors including age older than 65 years, cardiovascular disease, diabetes, chronic respiratory disease, hypertension, and immune-compromised status.23 Identifying a patient as being at high risk for COVID-19 progression is not necessarily a feature of any single risk factor but is rather a clinical judgment based on the combination of underlying comorbidities and general health status that suggests a higher level of clinical concern. Where appropriate, management variations based on risk factors for disease progression are called out explicitly, as in scenario 1. All clinical scenarios begin by characterizing COVID-19 status based on the availability of laboratory test results.
Table 1.
Definitions and Criteria for Key Components of Common Clinical Scenarios
| Severity of respiratory disease |
| Mild: no evidence of significant pulmonary dysfunction or damage (eg, absence of hypoxemia, no or mild dyspnea) |
| Moderate to severe: evidence of significant pulmonary dysfunction or damage (eg, hypoxemia, moderate-to-severe dyspnea) |
| Pretest probability |
| Based on background prevalence of disease as estimated by observed transmission patterns. May be further modified by individual’s exposure risk. Subcategorized as: |
| Low: sporadic transmission |
| Medium: clustered transmission |
| High: community transmission |
| Risk factors for disease progression |
| Present: clinical judgment regarding combination of age >65 years and presence of comorbidities (eg, cardiovascular disease, diabetes, chronic respiratory disease, hypertension, immune-compromised) |
| Absent: defined by the absence of risk factors for disease progression |
| Disease progression |
| Progression of mild disease to moderate-to-severe disease as defined above |
| Progression of moderate-to-severe disease with worsening objective measures of hypoxemia |
| Resource constraints |
| Limited access to personnel, personal protective equipment, COVID-19 testing ability (including swabs, reagent, or personnel), hospital beds, and/or ventilators with the need to rapidly triage patients |
COVID-19 = coronavirus 2019.
Scenario 1: Mild Features of COVID-19
The first scenario (Fig 1) addresses a patient presenting for evaluation at an outpatient clinic or via telehealth with mild respiratory features consistent with COVID-19 infection, any pretest probability of COVID-19 infection, and no significant critical resource constraints. When COVID-19 test results are unavailable, patients with moderate-to-high pretest probability should be initially managed as if COVID-19 testing is positive, whereas patients with low pretest probability should be initially managed as if COVID-19 testing is negative. Imaging is advised for patients with risk factors for COVID-19 progression and either positive COVID-19 testing or moderate-to-high pretest probability in the absence of COVID-19 testing (Fig 1, question 1). Imaging provides a baseline for future comparison, may establish manifestations of important comorbidities in patients with risk factors for disease progression (Table 1), and may influence the intensity of monitoring for clinical worsening. Imaging is not advised for patients with mild features who are COVID-19 positive without accompanying risk factors for disease progression or for patients with mild features who are COVID-19 negative (Fig 1, questions 2 and 3). The panel believed that the yield of imaging in these settings would be very low and that it was safe for most patients to self-monitor for clinical worsening. Regardless of COVID-19 test results and risk factors, imaging is advised for patients with mild clinical features who subsequently develop clinical worsening (Fig 1, questions 4 and 5). In the absence of clinical worsening, management involves support and isolation of patients with positive COVID-19 testing or patients with moderate-to-high pretest probability without COVID-19 test results available.
Although not specifically addressed with this scenario, in the presence of substantial resources constraints there is no role for imaging of patients with mild features of COVID-19.
Scenario 2: Moderate-to-Severe Features of COVID-19
The second scenario (Fig 2) addresses a patient presenting with moderate-to-severe features consistent with COVID-19 infection, any pretest probability of COVID-19 infection, and no substantial critical resource constraints. Separate ratings were obtained for COVID-19-positive patients and either COVID-19-negative patients or patients for whom COVID-19 testing is unavailable (Fig 2, questions 6 and 7). Imaging is advised regardless of the results or availability of COVID-19 testing given the impact of imaging in both circumstances.
For COVID-19-positive patients, imaging establishes baseline pulmonary status and helps identify underlying cardiopulmonary abnormalities that may facilitate risk stratification for clinical worsening. In the presence of clinical worsening, imaging is again advised to assess for COVID-19 progression or secondary cardiopulmonary abnormalities such as pulmonary embolism, superimposed bacterial pneumonia, or heart failure that can potentially be secondary to COVID-19 myocardial injury (Fig 2, question 8).
For COVID-19-negative patients or any patient for whom testing is not performed, imaging may reveal an alternative diagnosis to explain the patient’s clinical features. This should direct patient care as per existing clinical guidelines or standard clinical practice. If an alternative diagnosis is not revealed or images demonstrate features of COVID-19 infection, then subsequent clinical evaluation would depend on the pretest probability of COVID-19 infection and COVID-19 test availability. Falsely negative COVID-19 testing is more prevalent in high pretest probability circumstances, and repeat COVID-19 testing is therefore advised if available. Depending on the imaging findings, other clinical investigations may be pursued.
Scenario 3: Moderate-To-Severe Features of COVID-19 in a Resource-constrained Environment
The third scenario (Fig 3) addresses a patient presenting with moderate-to-severe features consistent with COVID-19 infection within an environment of high community disease burden and critical resource limitations as seen in Wuhan, China, in regions of Italy and Spain, and in New York City. Because health care personnel and infrastructure may be overwhelmed by a high influx of new patients and resources are limited to provide critical care, urgent decision-making and triage are of primary importance. At the time of this writing, turnaround times for COVID-19 test results range from 6 to over 48 hours, with most sites waiting at least 12 hours for results. This is an impractically long time period to consider triage to limited hospital beds and ventilators. However, rapid point-of-care COVID-19 tests are expected to be released into clinical environments during the 1st week of April 2020, providing routine turnaround times of less than an hour and potentially as little as 5 minutes.24, 25, 26, 27 Although the initial availability and sample processing capacity of point-of-care COVID-19 testing is expected to be limited, this should increase over time.
The third scenario first considers the potential availability of point-of-care COVID-19 testing. Imaging is advised when point-of-care COVID-19 testing is available and results are positive (Fig 3, question 9) for the same reasons as described for scenario 2. On the basis of imaging findings and clinical features, patients are subsequently supported and monitored with a level of intensity consistent with clinical features. Imaging is again indicated if patients subsequently clinically worsen (Fig 3, question 11).
Imaging is advised to support more rapid triage of patients in a resource-constrained setting when point-of-care COVID-19 testing is not available or results are negative (Fig 3, question 10). Imaging may reveal features of COVID-19, which within this scenario may be taken as a presumptive diagnosis of COVID-19 for medical triage and associated decisions regarding disposition, infection control, and clinical management. In this high pretest probability environment, and as described for scenario 2, the possibility of falsely negative COVID-19 testing creates a circumstance where a COVID-19 diagnosis may be presumed when imaging findings are strongly suggestive of COVID-19 despite negative COVID-19 testing. This guidance represents a variance from other published recommendations that advise against the use of imaging for the initial diagnosis of COVID-1928 and was supported by direct experience among panelists providing care within the conditions described for this scenario. The relationship between disease severity and triage may need to be fluid depending on resources and case load. When imaging reveals an alternative diagnosis to COVID-19, management is based on established guidelines or standard clinical practice.
Additional Key Questions
Daily Chest Radiographs Are Not Indicated in Stable Intubated Patients with COVID-19 (Question 12)
Multiple studies have shown no difference in important outcomes (mortality, length of stay, and ventilator days) for patients in the intensive care unit imaged on-demand as compared with a daily routine protocol.29, 30, 31, 32 Avoidance of non-value-added imaging is particularly important in the COVID-19 patient population to minimize exposure risk of radiology technologists and to conserve PPE.
CT Is Indicated in a Patient with Functional Impairment and/or Hypoxemia after Recovery from COVID-19 (Question 13)
With the recent emergence of SARS-CoV2 as a human pathogen, there are no long-term follow-up studies of survivors. Postmortem evaluation of a patient who succumbed to severe COVID-19 showed pathologic findings consistent with diffuse alveolar damage, similar to findings previously described with severe acute respiratory syndrome and Middle East respiratory syndrome.33 Patients with functional impairment after recovery from COVID-19 should undergo imaging to differentiate between expected morphologic abnormalities as sequelae of infection, mechanical ventilation, or both versus a different and potentially treatable process.
COVID-19 Testing Is Indicated in a Patient Who Is Found Incidentally to Have Typical Findings of COVID-19 at CT (Question 14)
Although CT findings of COVID-19 infection are nonspecific, their presence in an asymptomatic patient with no or mild respiratory symptoms is concerning in a setting of known community transmission, particularly if there is no better alternative diagnosis. Asymptomatic carriers of COVID-19 have been estimated to comprise 17.9%-33.3% of all infected cases.34 , 35 Asymptomatic infection with CT findings suggestive of COVID-19 in the lung has been documented in screened cruise ship passengers.11 It is believed that the presence of undetected infected and mildly symptomatic or asymptomatic individuals may be contributing to the rapid geographic spread of SARS-CoV2 (9). RT-PCR testing in this scenario is important to potentially identify an occult infection and limit further transmission both within the community and in the environment where the patient is receiving medical care. In highly prevalent areas, an additional uncertainty is whether CT should be used as a screening tool either as a stand-alone or as an adjunct to RT-PCR to exclude occult infection before surgery or intensive immunosuppressive therapies.
The panel’s ratings are provided in Figure 4, and a summary of all recommendations is provided in Table 2 .
Table 2.
Summary of Recommendations for Imaging
| Main recommendations |
| Imaging is not routinely indicated as a screening test for COVID-19 in asymptomatic individuals |
| Imaging is not indicated for patients with mild features of COVID-19 unless they are at risk for disease progression (scenario 1) |
| Imaging is indicated for patients with moderate to severe features of COVID-19 regardless of COVID-19 test results (scenarios 2 and 3) |
| Imaging is indicated for patients with COVID-19 and evidence of worsening respiratory status (scenarios 1, 2, and 3) |
| In a resource-constrained environment where access to CT is limited, chest radiography may be preferred for patients with COVID-19 unless features of respiratory worsening warrant the use of CT (scenarios 2 and 3) |
| Additional recommendations |
| Daily chest radiographs are NOT indicated in stable intubated patients with COVID-19 |
| CT is indicated in patients with functional impairment and/or hypoxemia after recovery from COVID-19 |
| COVID-19 testing is indicated in patients incidentally found to have findings suggestive of COVID-19 on a CT scan |
See Table 1 legend for expansion of abbreviation.
Additional Resources
For purposes of image interpretation and reporting, readers are referred to a recently published systematic review of imaging findings of COVID-1936 and a multisociety consensus paper on reporting chest CT findings related to COVID-19.37 As an aid to improving radiologist and pulmonologist familiarity with the imaging findings of COVID-19, an educational repository of proven COVID-19 cases can be found at the Fleischner Society website (https://www.fleischner-covid19.org).
Conclusion
This statement is intended to offer guidance to physicians on the use of thoracic imaging across a breadth of health care environments. It represents the collective opinions and perspectives of thoracic radiology, pulmonology, intensive care, emergency medicine, laboratory medicine, and infection control experts practicing in 10 countries, representative of the highest burden of coronavirus disease 2019 (COVID-19) worldwide. It also represents opinion at a moment in time within a highly dynamic environment where the status of regional epidemics and the availability of critical resources to combat those epidemics vary daily. The evidence base supporting the use of imaging across the scenarios presented is scant and the advice presented herein may undergo refinement through rigorous scientific investigation, exposing nuances of image interpretation that may lead to prognostic information and guide management decisions. At the time of this writing, no therapy has been confirmed to alter the course of COVID-19, there is no known cure, and there is no vaccine for prevention. As effective treatments are developed, thoracic imaging may find new roles by establishing treatment response or characterizing patients as likely responders to novel therapies.
Acknowledgments
Author contributions: G. D. R., M. H., C. M. S.-P., and A. N. L. are guarantors of integrity of the entire study. All authors contributed to study concepts/study design or data acquisition or data analysis/interpretation, contributed to manuscript drafting or manuscript revision for important intellectual content, approved the final version of the submitted manuscript, agree to ensure any questions related to the work are appropriately resolved, and contributed to manuscript editing. G. D. R., C. J. R., L. B. H., N. S., S. R., N. W. S., J.-J. Y., D. J. A., C. K., A. B., S. R. D., J. G., M. H., Y. I., H.-U. K., F. L., M. R.-J., C. M. S.-P., and A. N. L. contributed to the literature research. J. P. K., N. W. S., A. V., A. B., H.-U. K., F. L., and N. T. contributed to the clinical studies. I. B. K. M., Y. I., and A. N. L. contributed to the experimental studies. G. D. R. contributed to the statistical analysis.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. P. K. is a consultant for Parexel International, outside the submitted work. D. J. A. received grants from AHRQ and CDC and receives royalties from Up to Date, outside the submitted work. T. A. received speakers fees from Philips and is a paid consultant for Vertex, outside the submitted work. J. M. G. received research grants from Infinitt Healthcare and Dongkook Lifescience, outside the submitted work. H.-U. K. received a grant and nonfinancial support from Siemens; received a grant and personal fees from Philips; received nonfinancial support from Bayer; and received fees for speakers bureau from Boehringer Ingelheim, MSD, and Astra Zeneca, outside the submitted work. M. P. received a grant and speakers fees from Siemens Healthineers, received a grant and speakers fees from Canon Medical Systems, and received speakers fees from Bracco and Bayer, outside the submitted work. M. R.-J. received clinical research support from Siemens Healthineers, outside the submitted work. L. R. is a paid consultant for Biogen, ImmuneWorks, Celgen, and Nitto; received a grant from Roche; received a grant from Boehringer Ingelheim; is paid by Roche, Boehringer Ingelheim, and FibroGen to be a member of the advisory board; and is paid to be on the steering committee at Boehringer Ingelheim, outside the submitted work. C. M. S.-P. is a member of the Dutch COVID Working Group, outside the submitted work. None declared (G. D. R., C. J. R., L. B. H., N. S., S. R., N. W. S., A. V., J.-J. Y., I. B. K. M., C. K., A. B., S. R. D., J. G., M. H., Y. I., F. L., P. J. M., N. T., A. U. W., A. N. L.).
Footnotes
Editor’s note: This article is being simultaneously published in Radiology.
References
- 1.World Health Organization Director-General’s opening remarks at the media briefing on COVID-19 - March 11, 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020
- 2.World Health Organization Coronavirus disease (COVID-19) situation dashboard. https://experience.arcgis.com/experience/685d0ace521648f8a5beeeee1b9125cd
- 3.Johns Hopkins University Coronavirus COVID-19 global cases by the Center for Systems Science and Engineering (CSSE) https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- 4.Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases [published online ahead of print February 26, 2020]. Radiology. https://doi.org/10.1148/radiol.2020200642. [DOI] [PMC free article] [PubMed]
- 5.Li Y, Yao L, Li J, et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19 [published online ahead of print March 26, 2020]. J Med Virol. https://doi.org/10.1002/jmv.25786. [DOI] [PMC free article] [PubMed]
- 6.Wang W., Xu Y., Gao R., et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou L., Ruan F., Huang M., et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382(12):1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online ahead of print February 19, 2020]. Radiology. https://doi.org/10.1148/radiol.2020200432. [DOI] [PMC free article] [PubMed]
- 9.Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2). Science. In press. [DOI] [PMC free article] [PubMed]
- 10.Rothe C., Schunk M., Sothmann P., et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inui S., Fujikawa A., Jitsu M., et al. Findings in cases from the cruise ship “Diamond Princess” with coronavirus disease 2019 (COVID-19) Radiol Cardiothorac Imaging. 2020;2(2) doi: 10.1148/ryct.2020204002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mossa-Basha M, Meltzer CC, Kim DC, Tuite MJ, Kolli KP, Tan BS. Radiology department preparedness for COVID-19: Radiology Scientific Expert Panel [published online ahead of print March 16, 2020]. Radiology. https://doi.org/10.1148/radiol.2020200988. [DOI] [PMC free article] [PubMed]
- 13.Kooraki S., Hosseiny M., Myers L., Gholamrezanezhad A. Coronavirus (COVID-19) outbreak: what the department of radiology should know. J Am Coll Radiol. 2020;17(4):447–451. doi: 10.1016/j.jacr.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19) interim infection prevention and control recommendations. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/control-recommendations.html#adhere
- 15.Dong Y, Mo X, Hu Y, et al. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. In press.
- 16.Soldati G, Smargiassi A, Inchingolo R, et al. Is there a role for lung ultrasound during the COVID-19 pandemic [published online ahead of print March 20, 2020]? J Ultrasound Med. https://doi.org/10.1002/jum.15284. [DOI] [PMC free article] [PubMed]
- 17.Wong HYF, Lam HYS, Fong AH, et al. Frequency and distribution of chest radiographic findings in COVID-19 positive patients [published online ahead of print March 27, 2019]. Radiology. https://doi.org/10.1148/radiol.2020201160.
- 18.Driggin E., Madhavan M.V., Bikdeli B., et al. Cardiovascular considerations for patients, health care workers, and health systems during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orsi M.A., Oliva A.G., Cellina M. Radiology department preparedness for COVID-19: facing an unexpected outbreak of the disease. Radiology. 2020;295(3):E8. doi: 10.1148/radiol.2020201214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Critical preparedness, readiness and response actions for COVID-19. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/critical-preparedness-readiness-and-response-actions-for-covid-19
- 21.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19) public health recommendations for community-related exposure. https://www.cdc.gov/coronavirus/2019-ncov/php/public-health-recommendations.html [PubMed]
- 22.Centers for Disease Control and Prevention Interim U.S. guidance for risk assessment and public health management of healthcare personnel with potential exposure in a healthcare setting to patients with coronavirus disease (COVID-19) https://www.cdc.gov/coronavirus/2019-ncov/hcp/guidance-risk-assesment-hcp.html
- 23.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention [published online ahead of print February 24, 2020]. JAMA. https://doi.org/10.1001/jama.2020.2648. [DOI] [PubMed]
- 24.DiaSorin Molecular Simplexa COVID-19 Direct Kit. https://molecular.diasorin.com/us/kit/simplexa-covid-19-direct-kit/
- 25.bioMérieux. First of 3 diagnostic tests for SARS-CoV-2 coronavirus available from bioMérieux. https://www.biomerieux.com/en/novel-coronavirus-covid-19
- 26.Cepheid. Xpert Xpress SARS-CoV-2 has received FDA emergency use authorization. https://www.cepheid.com/coronavirus
- 27.Abbott I.D. NOW COVID-19 Molecular. In minutes. On the front line. https://www.alere.com/en/home/product-details/id-now-covid-19.html
- 28.American College of Radiology ACR recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection. https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection
- 29.Oba Y., Zaza T. Abandoning daily routine chest radiography in the intensive care unit: meta-analysis. Radiology. 2010;255(2):386–395. doi: 10.1148/radiol.10090946. [DOI] [PubMed] [Google Scholar]
- 30.Hejblum G., Chalumeau-Lemoine L., Ioos V., et al. Comparison of routine and on-demand prescription of chest radiographs in mechanically ventilated adults: a multicentre, cluster-randomised, two-period crossover study. Lancet. 2009;374(9702):1687–1693. doi: 10.1016/S0140-6736(09)61459-8. [DOI] [PubMed] [Google Scholar]
- 31.Lakhal K., Serveaux-Delous M., Lefrant J.Y., Capdevila X., Jaber S. AzuRéa network for the RadioDay study group. Chest radiographs in 104 French ICUs: current prescription strategies and clinical value (the RadioDay study) Intensive Care Med. 2012;38(11):1787–1799. doi: 10.1007/s00134-012-2650-9. [DOI] [PubMed] [Google Scholar]
- 32.Suh R.D., Genshaft S.J., Kirsch J., et al. ACR Appropriateness Criteria® intensive care unit patients. J Thorac Imaging. 2015;30(6):W63–W65. doi: 10.1097/RTI.0000000000000174. [DOI] [PubMed] [Google Scholar]
- 33.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizumoto K., Kagaya K., Zarebski A., Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10) doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishiura H., Kobayashi T., Suzuki A., et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19) Int J Infect Dis. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients [published online ahead of print March 14, 2020]. AJR Am J Roentgenol. https://doi.org/10.2214/AJR.20.23034. [DOI] [PubMed]
- 37.Simpson S., Kay F.U., Abbara A., et al. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA. Radiol Cardiothorac Imaging. 2020;2(2) doi: 10.1148/ryct.2020200152. [DOI] [PMC free article] [PubMed] [Google Scholar]