The circulating platelet phenotype may change in disease. We previously demonstrated in a murine model of myocardial infarction (MI) and in patients with acute MI that circulating platelets synthesize and secrete activated matrix metalloproteinase 9 (MMP9) after an ischemic event. Conversely, platelet MMP9 synthesis and secretion was not observed in healthy individuals (1,2). Enhanced platelet reactivity may be involved in abdominal aortic aneurysm (AAA) growth and rupture (3). In a murine model of AAA, treatment with inhibitors of platelet aggregation suppressed aortic MMP activity coincident with protection from rupture (4). Taken together, circulating platelets synthesize and secrete enzymes known to remodel the aorta. This may increase the risk of both aortic dissection and rupture. We hypothesized that pharmacologic agents which suppress platelet reactivity may confer protection against the development and progression of AAA.
The United States National Inpatient Sample (NIS) was queried between 2009 and 2016 for inpatient hospital encounters of AAA, abdominal aortic dissection, and abdominal aortic rupture. The NIS is the largest publicly available all-payer inpatient healthcare database and provides hypothesis-generating information about factors potentially regulating the expression of disease over time.
The following International Classification of Diseases, ninth revision (ICD-9) and tenth revision (ICD-10) codes were used to identify variables in NIS: long-term and current use of anti-platelet drugs (V58.63 and Z79.02), AAA (441.4 ICD9 and 171.4 ICD10), abdominal aortic aneurysm dissection (441.3 ICD-9 and 171.0 ICD-10), and abdominal aortic aneurysm rupture (441.3 ICD-9 and 171.3 ICD-10). We employed multivariate regression analysis to evaluate whether antiplatelet medication use was protective against AAA, aortic dissection, or AAA rupture in patients with established AAA. Our regression model included the following 26 clinical and demographic variables: age, gender, race, history of smoking, hypothyroidism, diabetes, fluid/electrolytes abnormalities, hypertension, history of alcohol abuse, history of drug abuse, chronic liver disease, congestive heart failure (CHF), carotid artery disease (CAD), chronic kidney disease (CKD), chronic lung disease, peripheral artery disease (PAD), anemia, valvular heart disease, obesity, history of percutaneous intervention (PCI), history of coronary artery bypass grafting (CABG), prior MI, hospital bed-size, hospital location, academic hospital location, and hospital region. All analyses were carried out using complex sample analysis to account for hospital clustering using the appropriate weighting samples as recommended by The Healthcare Cost and Utilization Project (HCUP).
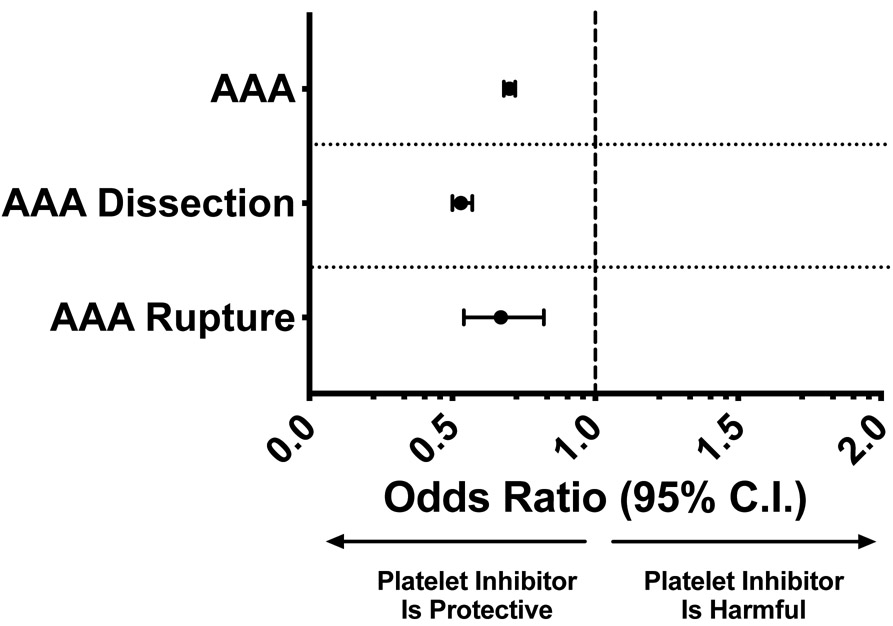
Among 245,195,028 hospitalizations from 2009 – 2016, 3,892,642 had documented long-term and current use of anti-platelet agents; 1,491,372 had AAA, 381,664 had abdominal aortic dissection and 37,398 had abdominal aortic rupture. After multivariate analysis, the use of antiplatelet agents was associated with a lower incidence of AAA (OR = 0.70; 95% CI 0.68 – 0.72, p < 0.001). In patients with AAA, the use of antiplatelet agents was an independent variable conferring protection from both abdominal aortic dissection (OR = 0.53; 95% CI: 0.50 – 0.57, p < 0.001) and rupture (OR = 0.67; 95% CI: 0.54 – 0.82, p < 0.001) (Figure 1). Curiously, using the same model, we did not see a similar protective effect for patients taking long-term anticoagulants (V58.61 ICD-9 and Z79.01 ICD-10) for AAA (adjusted OR=0.97; 95% CI 0.93-1.01, p=0.10) and AAA dissection (adjusted OR=0.99; 95% CI 0.80-1.24, p=0.99). AAA rupture, however, was marginally less in patients taking long-term anticoagulants (adjusted OR=0.92; 95% CI 0.87-0.98, p=0.01).
Figure 1. Platelet inhibitors suppress AAA growth and rupture:
NIS data for 3.8 million patients over 7 years identified anti-platelet agents as an independent predictor of protection from AAA, aortic dissection, and aortic rupture by multivariate regression analysis. The model included the following variables: age, gender, race, tobacco use, hypothyroidism, diabetes, fluid/electrolytes abnormalities, hypertension, alcohol abuse, drug abuse, liver disease, CHF, carotid artery disease, CKD, chronic lung disease, PAD, anemia, valvular heart disease, obesity, prior PCI, prior CABG, prior MI, hospital bed-size, hospital teaching status, and hospital region.
These data have intrinsic limitations. While we show a highly significant signal that antiplatelet medications confer protection from AAA, dissection, and rupture in individuals hospitalized with AAA, the source data relies on accurate coding, and for over-the-counter antiplatelet drugs like aspirin, accurate coding is only implied. In addition, the identity of each antiplatelet class utilized cannot be segregated or verified for the current dataset. The diagnostic codes used for antiplatelet therapy specifically state long-term and current use of antiplatelet medications. This suggests such treatment may protect against acute aortic syndromes, though the possibility exists that patients with aortic rupture never survive to receive this medical therapy since antiplatelet therapy following aortic dissection and rupture is contraindicated. As antiplatelet drug classes display diverse mechanisms of action, it is possible that one class or drug may more benefit than others in the treatment of AAA.
Societal guidelines recommend screening for AAA in patients with risk factors, and then surgical correction once the infrarenal aorta reaches a certain dimension. While platelet reactivity was recently suggested to be augmented in patients with AAA (5), our data imply that antiplatelet drugs may protect against aortic remodeling prior to dissection. Since AAA constitutes a peripheral artery disease (PAD), treatment with antiplatelets drugs conforms to the current guidelines for treating PAD and may protect against rupture in patients identified with AAA. Future studies should focus on prospectively evaluating the role of circulating platelets in the etiology of AAA using orally-available prescription antiplatelet agents which block cyclooxygenase, as well as the platelet P2Y12 receptor inhibitors, and PAR1 antagonists, individually, and in various combinations.
Acknowledgments
Sources of Funding
None of the authors declare conflicts of interest. Financial support from NHLBI 4K08HL128856 and the independent order of Oddfellows fellowship to SJC, and NHLBI 5R00-HL116786-06 and 1R01-HL141401-02 to APOIII.
Footnotes
Disclosures
None of the authors have any relevant conflicting financial, personal or professional relationships.
References
- 1.Cameron SJ, Ture SK, Mickelsen D et al. Platelet Extracellular Regulated Protein Kinase 5 Is a Redox Switch and Triggers Maladaptive Platelet Responses and Myocardial Infarct Expansion. Circulation 2015;132:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt RA, Morrell CN, Ling FS et al. The platelet phenotype in patients with ST-segment elevation myocardial infarction is different from non-ST-segment elevation myocardial infarction. Transl Res 2018;195:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 1995;15:1145–51. [DOI] [PubMed] [Google Scholar]
- 4.Owens AP 3rd, Edwards TL, Antoniak S et al. Platelet Inhibitors Reduce Rupture in a Mouse Model of Established Abdominal Aortic Aneurysm. Arterioscler Thromb Vasc Biol 2015;35:2032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sundermann AC, Saum K, Conrad KA et al. Prognostic value of D-dimer and markers of coagulation for stratification of abdominal aortic aneurysm growth. Blood Adv 2018;2:3088–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]