Abstract
Co-morbid chronic musculoskeletal pain (CMSP) and posttraumatic stress symptoms (PTSS) are frequent sequelae of motor vehicle collision, are associated with greater disability than either outcome alone, and are more prevalent in women than men. In the current study we assessed for evidence that gene transcripts originating from the X chromosome contribute to sex differences in vulnerability to CMSP and PTSS after motor vehicle collision. Nested samples were drawn from a longitudinal study of African American individuals, and CMSP (0–10 Numeric Rating Scale) and PTSS (Impact of Events Scale, Revised) outcomes were assessed six months following motor vehicle collision. Blood RNA were sequenced (n=101) and the relationship between X chromosome mRNA expression levels and co-morbid CMSP and PTSS outcomes was evaluated using logistic regression analyses. A disproportionate number of peritraumatic X chromosome mRNA predicting CMSP and PTSS in women were genes previously found to escape X chromosome inactivation (11/40, z=−2.9, p=0.004). Secondary analyses assessing gene ontology relationships between these genes identified an enrichment in genes known to influence neuronal plasticity. Further, the relationship of expression of two critical regulators of X chromosome inactivation, X-inactive specific transcript (XIST) and Yin Yang 1 (YY1), was different in women developing CMSP and PTSS. Together, these data suggest that X chromosome genes that escape inactivation may contribute to sex differences in vulnerability to CMSP and PTSS after motor vehicle collision.
Keywords: sex differences, XIST, anxiety, hyperalgesia, RNA
Introduction
Exposure to traumatic events is common in life.(Norris, 1992) In industrialized nations, motor vehicle collisions are one of the most common types of trauma(Norris, 1992), with over 50 million motor vehicle collisions occurring worldwide each year(Niska, Bhuiya, & Xu, 2010). The great majority of individuals experiencing motor vehicle collision do not have serious injury; in the US, more than 90% of individuals seen in the emergency department after motor vehicle collision are discharged home after evaluation(LF, 2008). A substantial proportion of such individuals develop chronic musculoskeletal pain (CMSP)(Holm et al., 2009; McLean, Clauw, Abelson, & Liberzon, 2005; Suissa, Harder, & Veilleux, 2001) and/or posttraumatic stress symptoms (PTSS)(Kuch, Cox, & Evans, 1996; Mayou, Ehlers, & Bryant, 2002; Ursano et al., 1999). Some individuals develop both CMSP and PTSS; such individuals experience greater mental and physical disability than those with either outcome alone(Geisser, Roth, Bachman, & Eckert, 1996; Hickling & Blanchard, 1992; Sherman, Turk, & Okifuji, 2000; Toomey et al., 1997; Turk & Okifuji, 1996), and are an important outcome group for further study(Otis, Keane, & Kerns, 2003).
Most individuals who develop CMSP and PTSS following motor vehicle collision are women.(Madsen et al., 2018; McLean et al., 2014; Ryb, Dischinger, Read, & Kufera, 2009) This fact is consistent with the marked increase in CMSP and PTSS burden experienced by women vs. men in other settings.(Bartley & Fillingim, 2013; Breslau, Davis, Andreski, Peterson, & Schultz, 1997; Craft, Mogil, & Aloisi, 2004; Fillingim & Ness, 2000; Greenspan et al., 2007; Mogil, 2012; Tolin & Foa, 2006) Additionally, data from animal models suggest that there are sex differences in response to stress/priming events that lead to increased hyperalgesia and deficits in fear extinction(Baran, Armstrong, Niren, Hanna, & Conrad, 2009; Fontella et al., 2005; Joseph, Parada, & Levine, 2003; Keller, Schreiber, Staib, & Knox, 2015; Yamaura et al., 2013). Despite substantial sex differences, genetic/molecular mechanisms responsible for increased CMSP and PTSS vulnerability in women remain poorly understood.
One biological mechanism that may contribute to increased CMSP and PTSS vulnerability in women is altered expression of messenger RNA (mRNA) originating from the X chromosome. Women have two copies of the X chromosome (XX), while men have only one copy (XY). In women, one copy of each X chromosome gene is silenced via a process termed X chromosome inactivation. Mechanisms important to X chromosome silencing include epigenetic/chromatin modification and the coating of one X chromosome by the long non-coding RNA (lncRNA), X-inactive specific transcript (XIST) (Allen, Zoghbi, Moseley, Rosenblatt, & Belmont, 1992; Csankovszki, Nagy, & Jaenisch, 2001; Penny, Kay, Sheardown, Rastan, & Brockdorff, 1996; Plath, Mlynarczyk-Evans, Nusinow, & Panning, 2002). The latter mechanism, XIST RNA coating, requires the Ying Yang 1 (YY1) protein to activate the XIST RNA (Makhlouf et al., 2014) and tether it to the X chromosome(Jeon & Lee, 2011). Interestingly, despite such inactivation mechanisms, 10–15% of X chromosome genes escape X chromosome inactivation and are bi-allelically expressed(Berletch, Yang, Xu, Carrel, & Disteche, 2011). X chromosome genes that escape X chromosome inactivation have been shown to play potentially pathogenic roles in a number of diseases, such as major psychiatric disorders, systemic lupus erythematosus, Rett syndrome, and thyroid autoimmunity (Brix et al., 2005; Gibson, Williamson, Arbuckle, & Christodoulou, 2005; Ji, Higa, Kelsoe, & Zhou, 2015; Ji, Kelsoe, & Zhou, 2014).
Increasing evidence suggests that altered expression of mRNA originating from the X chromosome in general, and escape from X chromosome inactivation in particular, may be an important mechanism contributing to sex differences in CMSP and PTSS susceptibility. XIST RNA has been found to be dysregulated in women with CMSP and PTSS-related major affective disorders(Ji et al., 2015; Ji et al., 2014), sex choromosome abnormalities contribute to a range of brain-health disorders(Zhang, Yang, Li, Ma, & Li, 2017), X chromosome inactivation mechanisms have been implicated in nervous system development and function(H. Wu et al., 2014), and in mice, YY1 expression is associated with inflammatory pain and stress-induced analgesia(Sorge et al., 2013).
In this study we assessed for evidence that expression differences in mRNA originating from the X chromosome contribute to the increased incidence of CMSP and PTSS in women experiencing motor vehicle collision. We hypothesized that altered expression of mRNA originating from the X chromosome would predict the development of co-morbid CMSP and PTSS in such women. In addition, we hypothesized that X chromosome genes known to escape from X chromosome inactivation would predict CMSP and PTSS development, and play an important role. In addition, we evaluated whether the peritraumatic expression of critical regulators of X chromosome inactivation, XIST and YY1, might also predict CMSP and PTSS outcomes.
Materials/Subjects and Methods
Parent longitudinal cohort study of women and men experiencing motor vehicle collision
This prospective longitudinal study enrolled African American individuals ≥ 18 and ≤ 65 years of age who presented within 24 hours of motor vehicle collision to one of eleven emergency departments (EDs) in six states/districts (Michigan, Pennsylvania, Florida, Alabama, Massachusetts, and Washington D.C.) between July 2012 and July 2015. The study only enrolled African Americans because of the pressing need for pain studies that focus on such understudied, high risk groups.(Campbell, Edwards, & Fillingim, 2005; Edwards, Moric, Husfeldt, Buvanendran, & Ivankovich, 2005; Portenoy, Ugarte, Fuller, & Haas, 2004) This study has been described in detail previously(Linnstaedt et al., 2016). In brief, individuals who did not have a fracture or other injury requiring hospital admission were screened for eligibility. Patients who were not alert and oriented were excluded, as were patients who did not self-identify as African American, pregnant patients, prisoners, patients unable to read and understand English, or patients taking opioids above a total daily dose of 30 mg of oral morphine or equivalent. The study was approved by the institutional review boards of all participating hospitals. Each participant provided written informed consent before enrollment.
Motor vehicle collision-related pain intensity and distribution in the past week was assessed six months following motor vehicle collision using the modified Regional Pain Scale.(Wolfe, 2003) Overall pain intensity was evaluated via a 0 (no pain) to 10 (maximum possible pain) numeric rating scale (NRS).(Farrar, Young, LaMoreaux, Werth, & Poole, 2001) Motor vehicle collision-related PTSS was assessed six months following motor vehicle collision using the Impact of Event Scale: Revised (IESR).(Weiss, 2007) This 22-item questionnaire assesses symptoms such as physical reactions, dreams, and reminders of the stressful event on a scale of 0–4. The questionnaire also includes assessments for avoidance, intrusion and hyperarousal subscales; total scores can range from 0–88.
Sample selection and outcome definitions
To increase power, samples were not selected from the parent cohort study for analyses at random. Rather, individuals with relatively high levels and relatively low levels of pain and PTSS over time after motor vehicle collision were selected from the overall cohort for gene expression analyses (more extreme phenotypes). In addition, only parent cohort participants who at the time of the emergency department visit reported little or no pain in the weeks prior to motor vehicle collision were selected, and only participant samples with an RNA integrity score of 7 or greater were selected for sequencing. This selection occurred intermittently throughout the parent study by a single investigator, when samples were selected for gene expression analyses, and well prior to planning of these specific analyses.
Individuals with co-morbid CMSP and PTSS were defined based on previously validated cut-offs for moderate-severe pain (NRS ≥ 4) and PTSS (IES-R score ≥ 33)(Creamer, Bell, & Failla, 2003). Individuals with co-morbid CMSP and PTSS were compared to controls (individuals reporting neither CMSP nor PTSS). The average NRS and IES-R scores of women who developed co-morbid CMSP and PTSS was 7.98 (SD 1.71) and 59.33 (SD 15.79), respectively, while the NRS and IES-R scores of women who recovered was 0.71 (SD 1.10) and 8.51 (SD 8.51), respectively.
RNA collection and isolation from motor vehicle collision study participants
Research assistants collected blood samples in the ED at the time of enrollment using PAXgene RNA tubes. Total RNA was isolated using the PAXgene blood miRNA kit (QIAGEN) and RNA was stored at −80˚C until use. RNA concentration and purity were measured using a NanoDrop One (Nanodrop Technologies, Wilmington, DE), and RNA integrity was measured using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA).
Next Generation Sequencing and RNA read count normalization
Template libraries for total RNA sequencing were produced from 600ng total RNA using Ovation Human Blood RNA-Seq Library Systems kit (NuGen, San Carlos, CA) according to manufacturer’s specifications. Libraries were multiplexed in groups of six and sequenced on a HiSeq 2500 at the University of North Carolina at Chapel Hill High Throughput Sequencing Facility. Raw sequencing reads were aligned to the human hg19 genome assembly using STAR (version 2.4.2a).(Dobin et al., 2013) Expression levels of each transcript (n=20,353) were estimated via RSEM(Li & Dewey, 2011) using University of California Santa Cruz (UCSC) known gene transcript and gene definitions. Raw RSEM read counts for all samples were normalized to the overall upper quartile(Bullard, Purdom, Hansen, & Dudoit, 2010) before comparison and visualization. Consistent with study goals, only non-pseudoautosomal region (non-PAR) messenger RNA (mRNA) originating from the X chromosome (n=783) with mean read counts ≥ 10 (n=724) were included in analyses. As a quality control measure and verification of appropriate male/female categorization based on genetic attributes (vs. self-reported gender), median XIST RNA read counts were compared between women and men in this cohort: median XIST RNA read counts were 99,806 in women and 339 in men.
Statistical analyses
Sample sociodemographic characteristics were summarized using standard descriptive statistics. Logistic regression analyses adjusted for ED study site, education level, and participant age were used to assess the relationship between X chromosome gene expression levels and co-morbid CMSP and PTSS six months following motor vehicle collision, and to derive β coefficients, Z-score, standard error and p-value significance. RNA expression levels were scaled using the ‘Scale’ function in R. For this exploratory study, a p-value significance threshold for each of the different hypotheses assessed was set at p < 0.05. Adjustment for multiple hypothesis testing was explored using methods that account for correlation between expression values for each mRNA tested (Conneely & Boehnke, 2007). Gene expression values meeting the p < 0.05 threshold were used to generate a heatmap comparing relative expression levels of significant genes in women who develop CMSP and PTSS vs recovery following motor vehicle collision.
To determine whether there was a statistically significant difference in proportions of genes from two different groups, a z score and p value were calculated by comparing the proportions between the two groups using a two-tailed significance test.
Competitive gene set analyses (CGSA) were performed using the limma package in R/Bioconductor, version 3.34.9(Ritchie et al., 2015). The Camera function was used to test whether specified sets of genes (e.g. EscapeSet) were more highly ranked in an ordered list of genes than would be expected by chance. While some CGSA methods (e.g., WilcoxGST) assume that each gene is conditionally independent of other genes, Camera adjusts for inter-gene correlation and is thus considered a more rigorous analysis for large sets of related genes(D. Wu & Smyth, 2012).
The relationship between XIST RNA expression levels and YY1 mRNA expression levels with CMSP or PTSS was assessed using bivariate analyses (Spearman Correlation) in stratified outcome groups (women who developed co-morbid CMSP and PTSS vs those who recovered). All statistical analyses were carried out using SPSS software v24.0, SAS software v9.4, or R Studio v3.3.3.
Pathway analyses
The Ingenuity Pathway Analysis (Qiagen, 2018) software was used to assess functional relationships between X chromosome genes that predicted CMSP and PTSS, X chromosome genes known to escape X chromosome inactivation that predicted CMSP and PTSS (“EscapeSet”, n=9) and X chromosomeSet (n=31) separately. A core expression anlysis was done to assess the similar functionalities between genes in the context of curated Canonical pathways in the Ingenuity Pathway Analysis library. Functional relationships identified using Ingenuity Pathway Analysis were validated across three other freely-available pathway analysis tools available online: DAVID(Huang, Sherman, & Lempicki, 2008a, 2008b), PANTHER(Mi, Muruganujan, & Thomas, 2012), and GATHER(Chang & Nevins, 2006).
Results
Study participants / Cohort
Study analyses were performed on a subsample of participants from a parent prospective observational study of African American women and men who presented to the ED for evaluation after motor vehicle collision. This subsample was comprised of participants who were selected to have gene expression analyses performed on RNA blood samples obtained from them in the ED. Participants who reported little or no pain in the weeks prior to motor vehicle collision and who had more extreme outcome phenotypes (little or no symptoms, or more substantial persistent symptoms) were selected for gene expression analyses. Samples with an RNA integrity score of 7 or greater were used in the present analyses. This method removed those with prior pain and enriched the study subsample for the outcome of interest (i.e., 25% of parent cohort (n=930) had co-morbid CMSP and PTSS six months following motor vehicle collision, vs. 51% of the study subsample (n = 101)). The majority of these study subsample participants were between twenty and forty years of age and had not completed college; a little more than 60% were female. The majority of female participants were drivers, and more than half reported severe damage to their vehicle (Table 1). Peritraumatic pain and distress were common in the cohort, and consistent with selection critieria, pain symptoms in the weeks prior to motor vehicle collision were mild (Table 1).
Table 1.
Baseline characteristics of female study participants (n=65)
| Recover (n=35) | CMSP and PTSS (n=30) | t-test*, t (p-value) | |
|---|---|---|---|
| Age, years, mean (SD) | 28.9 (10.1) | 30.5 (10.9) | 0.62 (0.54) |
| BMI, mean (SD) | 30.0 (9.7) | 30.3 (9.7) | 0.13 (0.90) |
| Education, n (%) | |||
| HS or less | 9 (25.7) | 11 (36.7) | 0.95 (0.35) |
| Post-HS not college | 2 (5.7) | 2 (6.7) | 0.16 (0.88) |
| Some college | 13 (37.1) | 13 (43.3) | 0.50 (0.62) |
| College | 8 (22.9) | 4 (13.3) | −0.98 (0.33) |
| Post-college | 3 (8.6) | 0 (0.0) | −1.79 (0.08) |
| Collision characteristics | |||
| Driver, n (%) | 30 (85.7) | 19 (63.3) | −2.13 (0.04) |
| Severe vehicle damage, n (%) | 6 (17.1) | 11 (36.7) | 1.80 (0.08) |
| Seatbelt worn, n (%) | 32 (91.4) | 25 (83.3) | −0.98 (0.33) |
| Airbag deployed, n (%) | 14 (40.0) | 14 (46.7) | 0.53 (0.60) |
| Pain level prior to motor vehicle collision (0–10 NRS), mean (SD) | 1.0 (1.9) | 1.4 (2.9) | 0.59 (0.56) |
| Overall pain in the ED (0–10 NRS), mean (SD) | 5.6 (2.4) | 8.4 (1.3) | 5.84 (<0.001) |
| Number of previous life traumas, mean (SD) | 2.9 (2.6) | 3.1 (2.8) | 0.32 (0.75) |
| Peritraumatic distress level, mean (SD) | 21.7 (12.7) | 31.3 (10.4) | 3.30 (0.002) |
Student’s t-test to measure the statistical difference between participant characteristics in women who recover vs develop CMSP and PTSS following motor vehicle collision trauma.
Associations between altered peritraumatic expression of X chromosome mRNA and CMSP and PTSS outcomes in women vs. men
In logistic regression analyses adjusted for participant age, education level, and enrollment study site, forty mRNA originating from the X chromosome were differentially expressed in the early aftermath of motor vehicle collision among women who developed CMSP and PTSS (n=30) vs those who recovered (n=35) (p < 0.05; Figure 1 and Table 2). Differences in expression of these RNA transcripts ranged from −4 to 4 fold at the individual level (Figure 1) and 0.64 to 1.58 fold at the group level (Table 2). These mRNAs clustered into two main groups, one group showing predominately increased expression of differentially expressed X chromosome mRNA in women who developed CMSP and PTSS (n=23 mRNA) and another group with predominately decreased expression in women who developed CMSP and PTSS (n=17 mRNA) (Figure 1). These RNA transcripts have been identified as having a variety of broad ranging functions such as regulation of transcription/translation (e.g. EIF1AX, EIF2S3), immune cell function (e.g. CD40LG, TLR8, SH2D1A), and cell communication/signaling (e.g. GPR174, GPRASP1) (Supplementary Table 1). When adjusting for multiple hypothesis testing, SH2D1A and RPS4X were still significant at the p < 0.05 level. In parallel logistic regression analyses conducted in men who developed CMSP and PTSS (n=21) vs those who recovered (n=15), twenty-five mRNA were differentially expressed; these mRNAs did not cluster into well-defined groups as they did in women (Supplementary Figure 1). Differences in expression of these RNA transcripts ranged from 0.58 to 15.71 fold at the group level (Supplementary Table 2). Similar analyses of CMSP and PTSS-associated X chromosome genes in men indicated that differentially expressed X chromosome mRNA were distinct from those identified in women (only RBBP7 and SEPT6 that were associated with CMSP and PTSS in both sexes).
Figure 1.
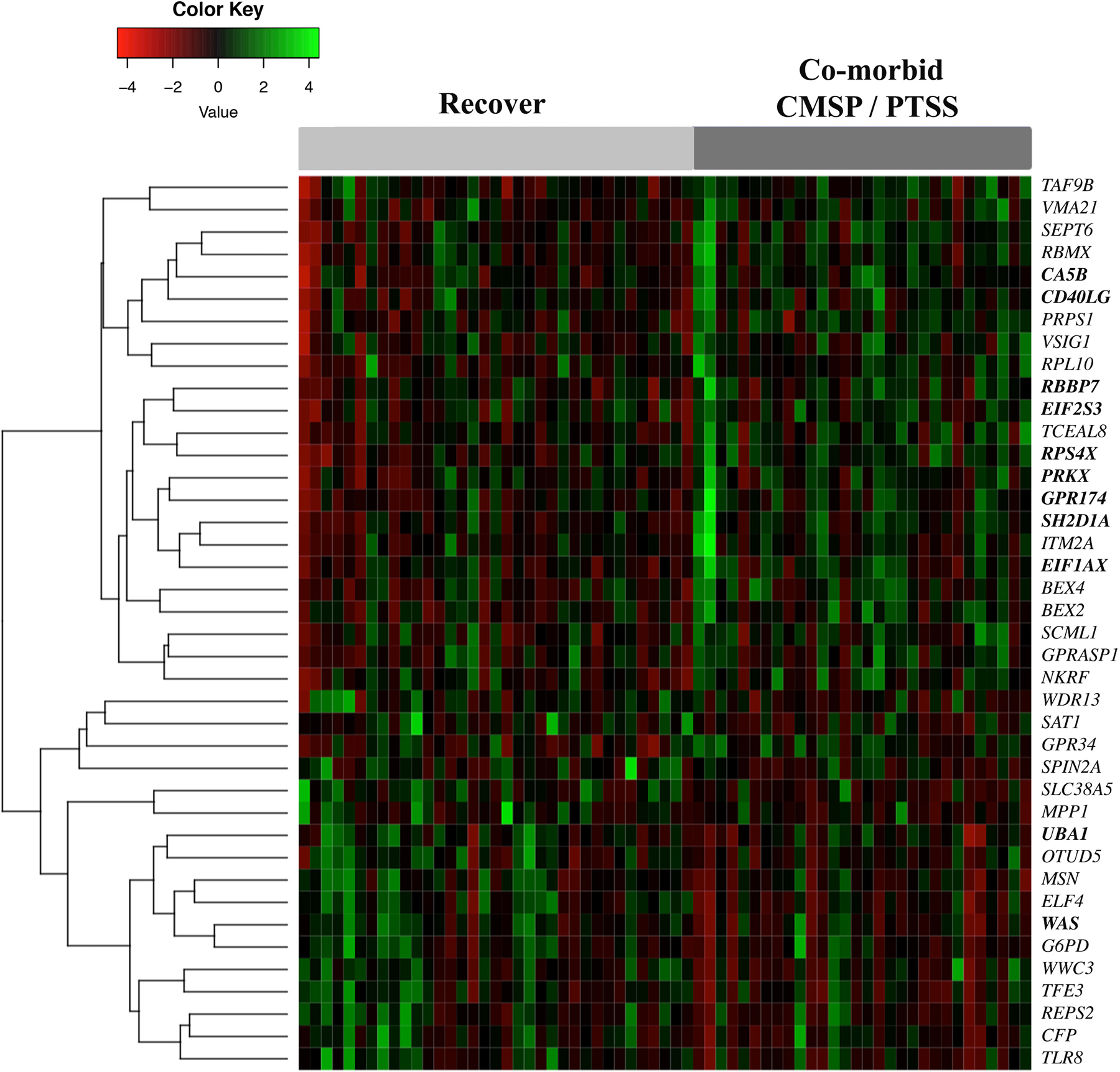
Heatmap illustrating X chromosome genes that are differentially expressed in women who recover vs develop co-morbid CMSP and PTSS six months following motor vehicle collision (motor vehicle collision) (n=65). Each column represents a female participant from the study, with individuals who recovered on the left (light gray bar) and individuals who developed CMSP and PTSS on the right (dark gray bar). Each row represents an X chromosome mRNA (with the corresponding official gene symbol to the right) that significantly predicts CMSP and PTSS development following motor vehicle collision. Bolded mRNA represent genes that have been shown previously to escape X chromosome inactivation. The dendrogram on the left of the heatmap indicates the hierarchical clustering of X chromosome genes associated with CMSP and PTSS. CMSP = chronic musculoskeletal pain, PTSS = posttraumatic stress symptoms.
Table 2.
X Chromosome mRNA that predict co-morbid CMSP and PTSS in women six months following motor vehicle collision (n=65).
| RNA | Fold Differencea | β Value | Std. Error | Z-score | p Valueb |
|---|---|---|---|---|---|
| SH2D1A | 1.58 (655.1/413.7) | 1.55 | 0.49 | 3.15 | 0.002 |
| RPS4X | 1.29 (17512.3/13585.3) | 1.28 | 0.41 | 3.15 | 0.002 |
| ELF4 | 0.82 (4319.1/5236.8) | −1.39 | 0.46 | −3.04 | 0.002 |
| TCEAL8 | 1.47 (217.9/147.7) | 1.06 | 0.38 | 2.75 | 0.006 |
| SEPT6 | 1.18 (12224.9/10333.6) | 1.02 | 0.38 | 2.72 | 0.007 |
| GPR174 | 1.35 (533.6/394.3) | 1.13 | 0.44 | 2.59 | 0.010 |
| SLC38A5 | 0.64 (3491.9/5445) | −1.03 | 0.40 | −2.57 | 0.010 |
| PRPS1 | 1.18 (554.8/468.2) | 0.91 | 0.35 | 2.57 | 0.010 |
| PRKX | 1.14 (2237.1/1954.2) | 0.99 | 0.39 | 2.56 | 0.010 |
| TFE3 | 0.85 (3400.6/4004.5) | −0.91 | 0.36 | −2.52 | 0.012 |
| MSN | 0.83 (24891.8/30062.4) | −0.99 | 0.40 | −2.52 | 0.012 |
| WAS | 0.8 (9341.2/11624.8) | −1.02 | 0.41 | −2.50 | 0.012 |
| RPL10 | 1.27 (22451.2/17620) | 0.99 | 0.40 | 2.49 | 0.013 |
| BEX4 | 1.33 (304.2/228.9) | 0.83 | 0.34 | 2.46 | 0.014 |
| TAF9B | 1.19 (615.4/518.2) | 0.85 | 0.35 | 2.45 | 0.014 |
| RBMX | 1.16 (4245.2/3666) | 1.05 | 0.43 | 2.44 | 0.015 |
| CA5B | 1.18 (1412.8/1196.6) | 0.95 | 0.39 | 2.42 | 0.016 |
| GPRASP1 | 1.25 (943.2/755.2) | 0.83 | 0.35 | 2.35 | 0.019 |
| REPS2 | 0.76 (1989.7/2607.2) | −0.75 | 0.33 | −2.31 | 0.021 |
| CD40LG | 1.15 (1303.5/1132) | 0.80 | 0.35 | 2.26 | 0.024 |
| SPIN2A | 0.69 (44.2/64.4) | −0.78 | 0.35 | −2.22 | 0.027 |
| BEX2 | 1.49 (120.5/80.6) | 0.82 | 0.37 | 2.21 | 0.027 |
| MPP1 | 0.79 (7647.5/9736.2) | −0.83 | 0.38 | −2.20 | 0.028 |
| EIF1AX | 1.3 (1458.3/1119.9) | 0.88 | 0.40 | 2.20 | 0.028 |
| TLR8 | 0.78 (6849.1/8826.4) | −0.74 | 0.34 | −2.19 | 0.028 |
| VSIG1 | 1.18 (477.8/403.9) | 0.71 | 0.32 | 2.19 | 0.029 |
| VMA21 | 1.19 (823.4/694.8) | 0.74 | 0.34 | 2.18 | 0.029 |
| OTUD5 | 0.91 (2165.2/2378.1) | −0.72 | 0.33 | −2.15 | 0.032 |
| GPR34 | 1.28 (240.1/187.2) | 0.69 | 0.32 | 2.14 | 0.033 |
| G6PD | 0.85 (3445.5/4054.8) | −0.80 | 0.38 | −2.13 | 0.033 |
| WDR13 | 0.89 (803.1/899.7) | −0.64 | 0.30 | −2.12 | 0.034 |
| SAT1 | 0.79 (2970.5/3756.3) | −0.73 | 0.35 | −2.11 | 0.035 |
| RBBP7 | 1.18 (882.8/745.3) | 0.74 | 0.36 | 2.09 | 0.036 |
| SCML1 | 1.2 (273.1/227) | 0.69 | 0.33 | 2.09 | 0.037 |
| ITM2A | 1.32 (1274/966) | 0.90 | 0.44 | 2.06 | 0.039 |
| UBA1 | 0.91 (5976.2/6559.7) | −0.78 | 0.38 | −2.03 | 0.042 |
| WWC3 | 0.87 (3304.1/3805.4) | −0.64 | 0.32 | −2.00 | 0.045 |
| EIF2S3 | 1.11 (5000.1/4511.8) | 0.69 | 0.34 | 2.00 | 0.045 |
| CFP | 0.84 (4061.4/4819.9) | −0.69 | 0.34 | −1.99 | 0.046 |
| NKRF | 1.17 (554.5/472.7) | 0.64 | 0.33 | 1.98 | 0.048 |
Fold difference was calculated by dividing the average sequencing read counts for women who developed co-morbid CMSP and PTSS by the average sequencing read counts for women who recovered following motor vehicle collision.
p values were calculated using logistic regression analyses adjusted for participant age, enrollment study site, and education level. Bolded mRNA have been defined previously as genes that escape X chromosome inactivation.
In women, but not in men, X chromosome gene transcripts that predict post-motor vehicle collision co-morbid CMSP and PTSS are enriched in gene transcripts that have been shown previously to escape X chromosome inactivation
In women, 11/40 (28%) of X chromosome mRNA differentially expressed in those who subsequently developed CMSP and PTSS have been shown in previous literature to escape X chromosome inactivation (Balaton, Cotton, & Brown, 2015; Carrel & Willard, 2005a; Cotton et al., 2013; Cotton et al., 2014) (Figure 2). When compared to the total number of X chromosome genes shown in previous literature to escape X chromosome inactivation (Balaton et al., 2015) (93/783, 12%, Supplementary Table 3), the percentage of escape genes predicting CMSP and PTSS in women was much higher than would be expected due to chance (z = −2.9, p = 0.004, Figure 2A). In contrast, in men the twenty-five X chromosome mRNA differentially expressed in those who did vs. did not develop CMSP and PTSS were not enriched for genes that escape X chromosome inactivation (3/25, 12%; z = −0.0187, p = 0.984).
Figure 2.
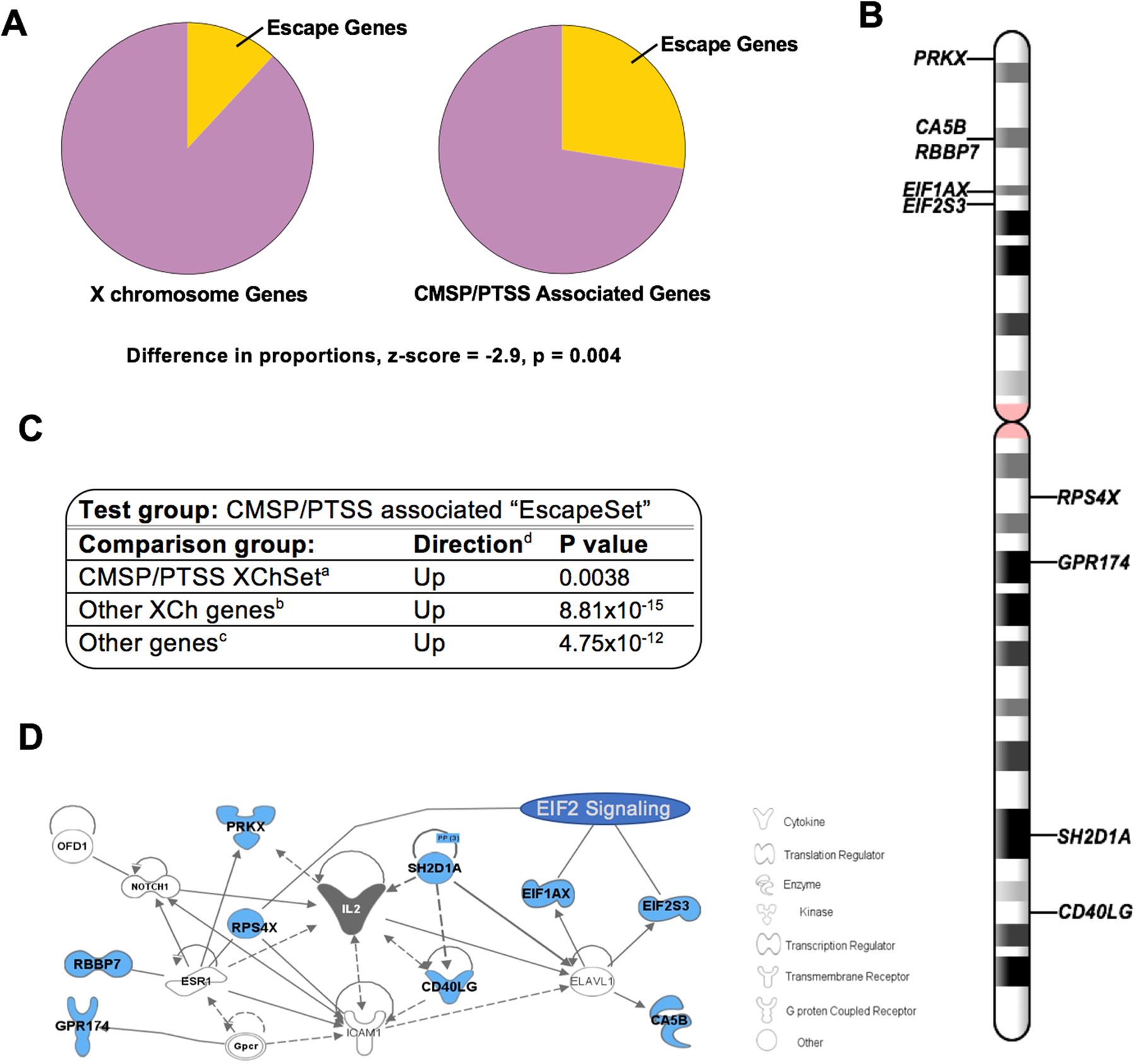
In women, gene transcripts previously shown to escape X chromosome inactivation (X chromosome inactivation) are important predictors of co-morbid CMSP and PTSS. A) Escape genes are over-represented in the list of 40 X chromosome (X chromosome) genes that significantly predict co-morbid CMSP and PTSS in women following motor vehicle collision. The pie chart on the left represents the proportion of all X chromosome genes that have been shown to escape X chromosome inactivation (n=93, yellow wedge) out of the total number of non-PAR X chromosome genes (n=783); the pie chart on the right represents the proportion of genes that have been shown to escape X chromosome inactivation (n=11) out of the total number of X chromosome genes that predict CMSP and PTSS in women following motor vehicle collision. B) Schematic representation of the location of “EscapeSet” genes on the X chromosome. C) Results of competitive gene set analyses indicate that EscapeSet genes are more differentially expressed in women who develop co-morbid CMSP and PTSS vs recover following motor vehicle collision than aX chromosomeSet genes, bany other set of nine X chromosome genes, and cany other set of nine randomly selected genes from the full transcriptome. dDirection of expression of genes in women who developed co-morbid CMSP and PTSS relative to expression of the same genes in women who recover following motor vehicle collision (i.e. “Up” indicates the genes are higher expressed in women with co-morbid CMSP and PTSS). D) Network analysis results based on EscapeSet genes using Ingenuity design software (IPA, Qiagen).
The great majority of X chromosome gene transcripts found in previous literature to escape X chromosome inactivation and that were differentially expressed in the early aftermath of motor vehicle collision among women developing CMSP and PTSS were differentially expressed in a direction consistent with escape from X chromosome inactivation
Consistent with the direction of effect that would be expected of escape gene transcripts, nine out of eleven X chromosome gene transcripts found in previous literature to escape X chromosome inactivation and that were differentially expressed in the early aftermath of motor vehicle collision among women developing CMSP and PTSS were expressed at higher levels in women who developed CMSP and PTSS vs those who recovered. This set of nine genes was the focus of the following analyses and are herein referred to as “EscapeSet” genes. Consistent with the chromosomal location of most genes that escape X chromosome inactivation(Carrel & Willard, 2005b), the majority of EscapeSet transcripts originate from the distal end of the short arm of the X chromosome (Figure 2B).
EscapeSet gene transcripts demonstrated greater differential expression in women who developed CMSP and PTSS than both other differentially expressed X chromosome genes and other sets of genes
In competitive gene set analyses (CGSA) that further account for inter-gene correlation (Camera, Bioconductor/R), EscapeSet genes were more differentially expressed (in the direction of increased expression) in women who developed CMSP and PTSS vs those who recovered than the other 31 differentially expressed X chromosome genes (the “X chromosomeSet”, p=0.004, Figure 2C). The EscapeSet was also more differentially expressed in women who developed CMSP and PTSS vs recovered in comparison to other randomly selected sets of nine genes from the X chromosome (Direction: up-regulated, p= 8.81×10−15, Figure 2C) and when compared to any other set of nine genes from the full transcriptome (Directoin: up-regulated, p =4.75×10−12, Figure 2C).
EscapeSet pathway analyses indicate an enrichment for genes involved in protein translation.
Shared relationships between EscapeSet genes were evaluated via pathway analysis (Ingenuity Pathway Analysis software). An enrichment of the Eukaryotic Initiation Factor 2 (EIF2) Signaling/Protein Biosynthesis Pathway was identified (Canonical pathway enrichment function, p=9.9×10−5), a pathway which regulates translation in response to a range of stress-related signals(Pakos‐Zebrucka et al., 2016). Enrichment of this pathway was mainly driven by differential expression of escape genes EIF1AX, EIF2S3, and RPS4X, which have been shown to play a role in the regulation of transcription and translation. Additionally, using the Network analysis function in Ingenuity Pathway Analysis, we found that EscapeSet genes are all connected to IL2 regulation/function/signaling (Figure 2D). The association between EscapeSet genes and translational regulation was confirmed with additional pathway analysis software including GATHER (enrichment in protein biosynthesis (GO:0006412, Bayes Factor = 3, p value = 0.002)), PANTHER (translation initiation” (GO:0003743, >100 fold enrichment, p = 1.65×10−4)), and DAVID (enrichment in translational initiation (GO:0006413, p value = 1.8×10−3)).
Expression levels of the non-coding RNA XIST and the transcription factor YY1 were differentially associated with each other and with CMSP and PTSS outcomes when comparing women who developed CMSP and PTSS vs women who recovered following motor vehicle collision.
Based on our results showing that women who develop CMSP and PTSS following motor vehicle collision over-express more genes that escape X chromosome inactivation than expected based on chance, we hypothesized that these at-risk women would also exhibit further evidence of altered X chromosome inactivation. Therefore, we examined the relationship between the expression of two major regulators of X chromosome inactivation, XIST and YY1, and CMSP or PTSS outcomes in women who recovered and in women who developed co-morbid CMSP and PTSS following motor vehicle collision. We first assessed a relationship between XIST RNA expression and PTSS severity and found that in women who recovered there was no statistically significant relationship (r = −0.113, p = 0.520, Figure 3A). However, in women who developed co-morbid CMSP and PTSS, we found a more negative but still non-significant relationship between XIST RNA and PTSS severity (r = −0.294, p = 0.114, Figure 3A). While the correlations in these two groups differed in magnitude, the statistical difference between their slopes was non-significant (Fisher’s r-to-z; z=0.725, p=0.234). Similar relationships were observed when assessing the correlation between XIST RNA expression levels and CMSP severity in women (Supplementary Figure 2) but no such relationships were observed in men (Supplementary Figure 3A).
Figure 3.
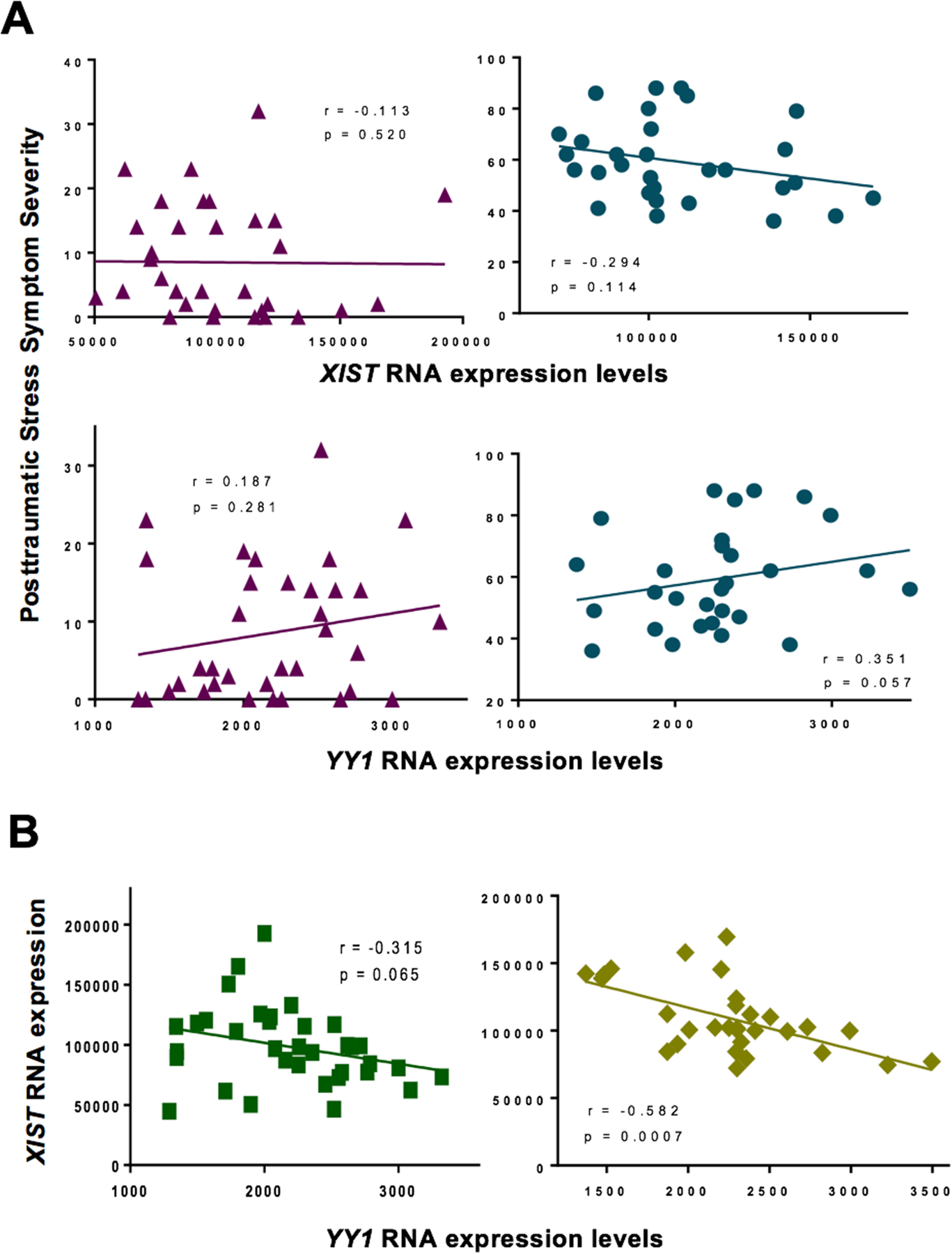
Relationship between RNA expression levels of key regulators of X Chromosome inactivation, XIST and YY1, and posttraumatic stress symptom (PTSS) severity scores in women following motor vehicle collision trauma. A) XIST RNA expression levels were examined in relation to PTSS severity scores in women who recovered following motor vehicle collision (top left, purple triangles, n=35), and in women who developed co-morbid CMSP and PTSS following motor vehicle collision (top right, teal circles, n=30). YY1 RNA expression levels were also analyzed in relation to PTSS severity scores in women who recovered following motor vehicle collision (bottom left, purple triangles, n=35), and in women who developed co-morbid CMSP and PTSS following motor vehicle collision (bottom right, teal circles, n=30). B) The relationship between XIST RNA expression levels and YY1 RNA expression levels in women who recovered following motor vehicle collision (left, green squares, n=35) and in women who developed co-morbid CMSP and PTSS following motor vehicle collision (right, yellow diamonds, n=30). RNA expression levels were measured via RNA sequencing of blood collected in the early aftermath of motor vehicle collision. Bivariate analyses were used to derive Spearman correlation coefficients and corresponding p values.
We next examined whether there was a relationship between YY1 mRNA expression and PTSS and found that in women who recovered, there was no statistically significant relationship (r = 0.187, p = 0.281, Figure 3A). However, in women who developed co-morbid CMSP and PTSS, we found a positive relationship between YY1 mRNA expression and PTSS severity, with trend level statistical significance (r = 0.351, p = 0.057, Figure 3A). Again, while the correlations in these two groups differed in magnitude, the statistical difference between their slopes was non-significant (Fisher’s r-to-z; z=−0.679, p=0.249). The relationship between YY1 mRNA expression and CMSP severity in women was not as pronounced as was observed for PTSS severity (Supplementary Figure 2) and no statistically significant relationships were observed in men (Supplementary Figure 4).
To further extend these analyses, we examined whether there were different relationships between XIST RNA and YY1 RNA expression in women who recovered vs women who developed co-morbid CMSP and PTSS following motor vehicle collision. In women who recovered, we detected a negative correlation with trend level statistical significance (r = −0.315, p = 0.065, Figure 3B). In comparison, in women who developed co-morbid CMSP and PTSS, we detected a strong and highly significant negative correlation between these RNA molecules (r = −0.582, p = 0.0007, Figure 3B). The difference in slopes for the correlations in these two groups were statistically significant at the trend level (Fisher’s r-to-z; z=1.299, p=0.097). In contrast, in men, we detected positive correlations between XIST and YY1 RNA expression levels in both outcome groups (Supplementary Figure 3B).
Discussion
In the current study, we found that forty genes originating from the X chromosome were differentially expressed in the early aftermath of motor vehicle collision among women who subsequently developed CMSP and PTSS vs those who recovered, and twenty five X chromosome genes were differentially expressed in men. Differentially expressed X chromosome genes in women demonstrated greater change than men, were distinct from men, clustered into two well-defined groups, and unlike men were greatly enriched for genes previously shown to escape X chromosome inactivation. In addition, the expression of X chromosome genes known to escape X chromosome inactivation appeared to be particularly dysregulated, as this subset of nine genes exhibited greater differential expression than any other nine gene set from the X chromosome or the full transcriptome. Importantly, consistent with potential escape from X chromosome inactivation, in women who developed CMSP and PTSS, these genes exhibited increased expression. These results, together with data indicating that women who developed CMSP and PTSS following motor vehicle collision exhibited altered relationships between XIST and YY1 RNA expression levels, support the hypothesis that genes that escape X chromosome inactivation might contribute to the increased vulnerability to CMSP and PTSS in women experiencing motor vehicle collision.
Pathway analyses to look for clues regarding mechanisms through which differentially expressed X chromosome genes that escape X chromosome inactivation might contribute to increased CMSP and PTSS vulnerability in women implicated EIF2 signaling. The EIF2 pathway has been shown to be involved in the cellular response to diverse stressors(Leitman et al., 2014; Pakos‐Zebrucka et al., 2016; Wek, Jiang, & Anthony, 2006). More recently, the EIF2 pathway in neurons has also been shown to be involved in processes related to learning and neuroplasticity(Bellato & Hajj, 2016; Kapur, Monaghan, & Ackerman, 2017; Trinh & Klann, 2013); processes central to the alterations in central and peripheral nervous system function that mediate the development of CMSP and PTSS. Of note, a recent study assessing gene expression patterns associated with adverse neuropsychiatric outcomes in trauma exposed individuals also observed enrichment in genes involved in EIF2 signaling(Flory et al., 2017). Pathway analyses also indicated that differentially expressed X chromosome genes that escape X chromosome inactivation influence IL-2 signaling/function. IL-2 has been implicated in the pathogenesis of both chronic pain(Parkitny et al., 2013; Üçeyler et al., 2006) and PTSS(Gill, Saligan, Woods, & Page, 2009; Guo et al., 2012; Smith et al., 2011; Song, Zhou, Guan, & Wang, 2007). Further studies evaluating the potential contribution of EIF2 and IL-2 signaling to sex differences in pain and PTSS outcomes after motor vehicle collision and other stressors are warrented.
On the individual gene level, gene transcripts shown to be associated with co-morbid CMSP and PTSS in the current study have previously been shown to play a role in a variety of cellular, systemic, and disease associated roles, but consistent with the majority of gene transcripts originating from the X chromosome, are mostly associated with immune and cognitive/neuronal processes(Bianchi, Lleo, Gershwin, & Invernizzi, 2012; Skuse, 2005). For instance, the RNA transcript with the most significant association with CMSP and PTSS in this study, SH2D1A has been shown previously to play a role in the stimulation of T and B cells (Hron, Caplan, Gerth, Schwartzberg, & Peng, 2004; Morra et al., 2005), to be associated with primary immunodeficiency (Jin, Zhou, Tian, & Chen, 2016), and to play a role in lymphocyte response and cytokine production (Czar et al., 2001). CD40LG, another RNA transcript identified in this study, is expressed on the surface of T cells and regulates B cell function. It has shown to be associated with female predominant disorders such as lupus (Lu et al., 2007) and rheumatoid arthritis(Lee et al., 2014). Finally, EIF2S3 mRNA, also shown to be associated with CMSP and PTSS in this study, is a subunit of the EIF2 protein initiation complex, and has been shown previously to play a direct role in cognitive/intellectual impairment (Buffington, Huang, & Costa-Mattioli, 2014; Skopkova et al., 2017), and a role in nociception via EIF2 (Khoutorsky et al., 2016). How increased expression levels of these gene transcripts in women who develop CMSP and PTSS might contribute to the development of these adverse outcomes following trauma exposure is not known but further investigation on the role of individual gene transcripts (or the full set of gene transcripts escaping X chromosome inactivation identified to be associated with CMSP and PTSS in this study) is warranted.
Our results demonstrating a negative correlation between XIST RNA expression levels and PTSS or CMSP severity in women who developed co-morbid CMSP and PTSS was interesting in light of our consistent results showing that genes known to escape from X chromosome inactivation predict CMSP and PTSS development in women following motor vehicle collision. These data suggest that low levels of XIST RNA expression (and thus potentially less coating of the inactive X chromosome) may allow certain X chromosome RNA to escape X chromosome inactivation in women who go on to develop CMSP and PTSS. Of note, in addition to the amount of XIST RNA available to silence the X chromosome, available evidence suggest that regulation of intracellular location of XIST RNA may also be important. For instance, in an elegant study by Wang et al, women with systemic lupus erythematosus (SLE) exhibited dispersed localization of XIST in mature naïve lymphocytes, leading to gene escape from X chromosome inactivation in these cells(Wang et al., 2016). This finding is of interest because our data are consistent with the mechanism, and like CMSP and PTSS, SLE is a disorder that disproportionately affects women. Wang et al. also examined expression of the YY1 protein and its ability to recruit XIST to the inactive X chromosome, and showed that YY1 was important for XIST localization. Interestingly, we found that the relationship between YY1 and PTSS severity (but not CMSP severity) and the relationship between YY1 and XIST is more pronounced in women who developed CMSP and PTSS, indicating that the amount and the relationship between these RNA molecules might contribute to gene escape from X chromosome inactivation in these women. However, these results should be interpreted with caution as they are only correlative, and further mechanistic studies are needed.
The strengths of this study included our ability to obtain nested samples from a prospective study of trauma survivors with high follow-up rates, our ability to increase power via selection of more extreme phenotypes (reduced type II error), the quality of the RNA data used in the study, and our ability to look for evidence that genes that escape X chromosome inactivation contribute to sex differences in vulnerability to post-motor vehicle collision CMSP and PTSS using multiple complementary approaches in both women and men (i.e., gene expression analyses, pathway analyses, XIST and YY1 RNA association analyses). However, several limitations should also be considered when interpreting our results. First, the main findings of the study have not been replicated in a second cohort of post-trauma survivors. This is an essential next step for future studies. Second, this study did not rigorously adjust for multiple hypothesis testing. This has the disadvantage of producing false positive associations (i.e. Type I error), but is optimal given the exploratory nature of the study and our relatively small sample size. Third, this work was performed in only African American individuals. Therefore, whether there are also ethnic differences in the association between X chromosome genes and CMSP and PTSS is not known. Fourth, we relied on previous studies for the identification of escape genes and did not assess escape status in our cohort. However, most of the escape genes identified as predictors of CMSP and PTSS have been shown across a number of studies to escape X chromosome inactivation(Balaton et al., 2015). Fifth, RNA expression levels were measured in blood tissue (vs neuronal tissue that might be more relevant to CMSP and PTSS pathophysiology). However, a number of previous studies have linked RNA expression in the blood with neurological disease outcomes (Breen et al., 2018; Scherzer et al., 2007; Segman et al., 2005), and central to the current study, patterns of X chromosome inactivation have been shown to be mostly consistent across all tissue types, likely due to early developmental processes that control X chromosome inactivation(Tukiainen et al., 2017). Finally, we have not performed mechanistic studies to more fully explicate relationships between XIST and YY1 RNA expression levels in women who develop CMSP and PTSS vs recover. Further studies are needed to replicate these findings and, if present, to better understand the underlying mechanisms driving these differences.
In conclusion, we report the first evidence suggesting that differential expression of genes originating from the X chromosome, including genes that escape X chromosome inactivation, may contribute to the increased vulnerability to co-morbid CMSP and PTSS in women vs. men experiencing motor vehicle collision. The expression of a set of X chromosome genes known to escape X chromosome inactivation appeared to be particularly dysregulated/influential; pathway analyses indicate that the effect of these genes may influence the EIF2 pathwway, which is involved in neuronal neuroplasticity. Further suggesting a role in CMSP and PTSS pathogenesis of X chromosome genes that escape inactivation, women who developed CMSP and PTSS following motor vehicle collision exhibited altered relationships between XIST and YY1 RNA expression levels. Future studies are needed to further understand the potential role of X chromosome gene expression in general, and X chromosome genes that escape X chromosome inactivation in particular, in mediating sex differences in vulnerability to CMSP and PTSS after motor vehicle collision.
Supplementary Material
Acknowledgements
Funding for this study was provided by the National Institute of Arthritis, Musculoskeletal, and Skin Diseases (R01AR060852, PI: McLean; K01AR071504, PI: Linnstaedt), by the Mayday Fund (PIs: McLean and Linnstaedt), and by a Future Leaders in Pain Grant from The American Pain Society (PI: Linnstaedt). None of the above funding agencies had any role in the design and conduct of the study, in the collection, management, analysis and interpretation of the data, or in the preparation, review, or approval of the manuscript.
The authors would like to acknowledge the University of North Carolina BioSpecimen Facility for the storage, accessioning and disbursement of biological samples.
The authors would also like to thank the participants for taking part in this study.
Footnotes
Conflict of Interest
Authors declare no conflict of interest.
References
- Allen RC, Zoghbi H, Moseley A, Rosenblatt H, & Belmont J (1992). Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. American journal of human genetics, 51(6), 1229. [PMC free article] [PubMed] [Google Scholar]
- Balaton BP, Cotton AM, & Brown CJ (2015). Derivation of consensus inactivation status for X-linked genes from genome-wide studies. Biology of sex differences, 6(1), 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, & Conrad CD (2009). Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiology of learning and memory, 91(3), 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley EJ, & Fillingim RB (2013). Sex differences in pain: a brief review of clinical and experimental findings. British journal of anaesthesia, 111(1), 52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellato HM, & Hajj GNM (2016). Translational control by eIF2α in neurons: Beyond the stress response. Cytoskeleton, 73(10), 551–565. [DOI] [PubMed] [Google Scholar]
- Berletch JB, Yang F, Xu J, Carrel L, & Disteche CM (2011). Genes that escape from X inactivation. Human genetics, 130(2), 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi I, Lleo A, Gershwin ME, & Invernizzi P (2012). The X chromosome and immune associated genes. Journal of autoimmunity, 38(2–3), J187–J192. [DOI] [PubMed] [Google Scholar]
- Breen MS, Tylee DS, Maihofer AX, Neylan TC, Mehta D, Binder EB, … Risbrough VB (2018). PTSD blood transcriptome mega-analysis: shared inflammatory pathways across biological sex and modes of trauma. Neuropsychopharmacology, 43(3), 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL, & Schultz LR (1997). Sex differences in posttraumatic stress disorder. Archives of general psychiatry, 54(11), 1044–1048. [DOI] [PubMed] [Google Scholar]
- Brix TH, Knudsen GPS, Kristiansen M, Kyvik KO, Ørstavik KH, & Hegedüs L (2005). High frequency of skewed X-chromosome inactivation in females with autoimmune thyroid disease: a possible explanation for the female predisposition to thyroid autoimmunity. The Journal of Clinical Endocrinology & Metabolism, 90(11), 5949–5953. [DOI] [PubMed] [Google Scholar]
- Buffington SA, Huang W, & Costa-Mattioli M (2014). Translational control in synaptic plasticity and cognitive dysfunction. Annual review of neuroscience, 37, 17–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard JH, Purdom E, Hansen KD, & Dudoit S (2010). Evaluation of statistical methods for normalization and differential expression in mRNA-Seq experiments. BMC bioinformatics, 11(1), 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell CM, Edwards RR, & Fillingim RB (2005). Ethnic differences in responses to multiple experimental pain stimuli. Pain, 113(1), 20–26. [DOI] [PubMed] [Google Scholar]
- Carrel L, & Willard HF (2005a). X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature, 434(7031), 400. [DOI] [PubMed] [Google Scholar]
- Carrel L, & Willard HF (2005b). X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature, 434(7031), 400–404. [DOI] [PubMed] [Google Scholar]
- Chang JT, & Nevins JR (2006). GATHER: a systems approach to interpreting genomic signatures. Bioinformatics, 22(23), 2926–2933. [DOI] [PubMed] [Google Scholar]
- Conneely KN, & Boehnke M (2007). So many correlated tests, so little time! Rapid adjustment of P values for multiple correlated tests. The American Journal of Human Genetics, 81(6), 1158–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton AM, Ge B, Light N, Adoue V, Pastinen T, & Brown CJ (2013). Analysis of expressed SNPs identifies variable extents of expression from the human inactive X chromosome. Genome biology, 14(11), R122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton AM, Price EM, Jones MJ, Balaton BP, Kobor MS, & Brown CJ (2014). Landscape of DNA methylation on the X chromosome reflects CpG density, functional chromatin state and X-chromosome inactivation. Human molecular genetics, 24(6), 1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM, Mogil JS, & Aloisi AM (2004). Sex differences in pain and analgesia: the role of gonadal hormones. European journal of pain, 8(5), 397–411. [DOI] [PubMed] [Google Scholar]
- Creamer M, Bell R, & Failla S (2003). Psychometric properties of the impact of event scale—revised. Behaviour research and therapy, 41(12), 1489–1496. [DOI] [PubMed] [Google Scholar]
- Csankovszki G, Nagy A, & Jaenisch R (2001). Synergism of Xist RNA, DNA methylation, and histone hypoacetylation in maintaining X chromosome inactivation. The Journal of cell biology, 153(4), 773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, … Ahmed R (2001). Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proceedings of the National Academy of Sciences, 98(13), 7449–7454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, … Gingeras TR(2013). STAR: ultrafast universal RNA-seq aligner. Bioinformatics, 29(1), 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RR, Moric M, Husfeldt B, Buvanendran A, & Ivankovich O (2005). Ethnic similarities and differences in the chronic pain experience: a comparison of African American, Hispanic, and white patients. Pain Medicine, 6(1), 88–98. [DOI] [PubMed] [Google Scholar]
- Farrar JT, Young JP Jr., LaMoreaux L, Werth JL, & Poole RM (2001). Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain, 94(2), 149–158. [DOI] [PubMed] [Google Scholar]
- Fillingim RB, & Ness T (2000). Sex-related hormonal influences on pain and analgesic responses. Neuroscience & Biobehavioral Reviews, 24(4), 485–501. [DOI] [PubMed] [Google Scholar]
- Flory J, Donohue D, Muhie S, Yang R, Miller S, Hammamieh R, … Yehuda R (2017). Gene expression associated with suicide attempts in US veterans. Translational psychiatry, 7(9), e1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontella FU, Bruno AN, Balk RS, Rücker B, Crema LM, Corrêa MD, … Dalmaz C (2005). Repeated stress effects on nociception and on ectonucleotidase activities in spinal cord synaptosomes of female rats. Physiology & behavior, 85(2), 213–219. [DOI] [PubMed] [Google Scholar]
- Geisser ME, Roth RS, Bachman JE, & Eckert TA (1996). The relationship between symptoms of post-traumatic stress disorder and pain, affective disturbance and disability among patients with accident and non-accident related pain. PAIN®, 66(2–3), 207–214. [DOI] [PubMed] [Google Scholar]
- Gibson JH, Williamson SL, Arbuckle S, & Christodoulou J (2005). X chromosome inactivation patterns in brain in Rett syndrome: implications for the disease phenotype. Brain and Development, 27(4), 266–270. [DOI] [PubMed] [Google Scholar]
- Gill JM, Saligan L, Woods S, & Page G (2009). PTSD is associated with an excess of inflammatory immune activities. Perspectives in psychiatric care, 45(4), 262–277. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Craft RM, LeResche L, Arendt-Nielsen L, Berkley KJ, Fillingim RB, … Mayer EA (2007). Studying sex and gender differences in pain and analgesia: a consensus report. Pain, 132, S26–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Liu T, Guo J-C, Jiang X-L, Chen F, & Gao Y-S (2012). Study on serum cytokine levels in posttraumatic stress disorder patients. Asian Pacific journal of tropical medicine, 5(4), 323–325. [DOI] [PubMed] [Google Scholar]
- Hickling EJ, & Blanchard EB (1992). Post-traumatic stress disorder and motor vehicle accidents. Journal of anxiety disorders, 6(3), 285–291. [Google Scholar]
- Holm LW, Carroll LJ, Cassidy JD, Hogg-Johnson S, Côté P, Guzman J, … van der Velde G. (2009). The burden and determinants of neck pain in whiplash-associated disorders after traffic collisions: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Journal of manipulative and physiological therapeutics, 32(2), S61–S69. [DOI] [PubMed] [Google Scholar]
- Hron JD, Caplan L, Gerth AJ, Schwartzberg PL, & Peng SL (2004). SH2D1A regulates T-dependent humoral autoimmunity. Journal of Experimental Medicine, 200(2), 261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, & Lempicki RA (2008a). Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic acids research, 37(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, & Lempicki RA (2008b). Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols, 4(1), 44. [DOI] [PubMed] [Google Scholar]
- Jeon Y, & Lee JT (2011). YY1 tethers Xist RNA to the inactive X nucleation center. Cell, 146(1), 119–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Higa KK, Kelsoe JR, & Zhou X (2015). Over-expression of XIST, the master gene for X chromosome inactivation, in females with major affective disorders. EBioMedicine, 2(8), 909–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji B, Kelsoe J, & Zhou X (2014). Abnormal X Chromosome Inactivation in Females with Major Psychiatric Disorders. bioRxiv, 009555. [Google Scholar]
- Jin Y-Y, Zhou W, Tian Z-Q, & Chen T-X (2016). Variable clinical phenotypes of X-linked lymphoproliferative syndrome in China: Report of five cases with three novel mutations and review of the literature. Human immunology, 77(8), 658–666. [DOI] [PubMed] [Google Scholar]
- Joseph EK, Parada CA, & Levine JD (2003). Hyperalgesic priming in the rat demonstrates marked sexual dimorphism. Pain, 105(1–2), 143–150. [DOI] [PubMed] [Google Scholar]
- Kapur M, Monaghan CE, & Ackerman SL (2017). Regulation of mRNA translation in neurons—a matter of life and death. Neuron, 96(3), 616–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller SM, Schreiber WB, Staib JM, & Knox D (2015). Sex differences in the single prolonged stress model. Behavioural brain research, 286, 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoutorsky A, Sorge RE, Prager-Khoutorsky M, Pawlowski SA, Longo G, Jafarnejad SM, … Gkogkas CG (2016). eIF2α phosphorylation controls thermal nociception. Proceedings of the National Academy of Sciences, 113(42), 11949–11954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuch K, Cox BJ, & Evans RJ (1996). Posttraumatic stress disorder and motor vehicle accidents: a multidisciplinary overview. The Canadian Journal of Psychiatry, 41(7), 429–434. [DOI] [PubMed] [Google Scholar]
- Lee S-H, Lee E-B, Shin E-S, Lee J-E, Cho S-H, Min K-U, & Park H-W (2014). The interaction between allelic variants of CD86 and CD40LG: a common risk factor of allergic asthma and rheumatoid arthritis. Allergy, asthma & immunology research, 6(2), 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman J, Barak B, Benyair R, Shenkman M, Ashery U, Hartl FU, & Lederkremer GZ (2014). ER stress-induced eIF2-alpha phosphorylation underlies sensitivity of striatal neurons to pathogenic huntingtin. PLoS One, 9(3), e90803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LF M (2008). National Center for Health Statistics. Retrieved from [Google Scholar]
- Li B, & Dewey CN (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC bioinformatics, 12(1), 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnstaedt SD, Hu J, Liu AY, Soward AC, Bollen KA, Wang HE, … Velilla M-A (2016). Methodology of AA CRASH: a prospective observational study evaluating the incidence and pathogenesis of adverse post-traumatic sequelae in African-Americans experiencing motor vehicle collision. BMJ open, 6(9), e012222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Q, Wu A, Tesmer L, Ray D, Yousif N, & Richardson B (2007). Demethylation of CD40LG on the inactive X in T cells from women with lupus. The Journal of Immunology, 179(9), 6352–6358. [DOI] [PubMed] [Google Scholar]
- Madsen TE, McLean S, Zhai W, Linnstaedt S, Kurz MC, Swor R, … Pearson C (2018). Gender Differences in Pain Experience and Treatment after Motor Vehicle Collisions: A Secondary Analysis of the CRASH Injury Study. Clinical Therapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhlouf M, Ouimette J-F, Oldfield A, Navarro P, Neuillet D, & Rougeulle C (2014). A prominent and conserved role for YY1 in Xist transcriptional activation. Nature communications, 5, 4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayou R, Ehlers A, & Bryant B (2002). Posttraumatic stress disorder after motor vehicle accidents: 3-year follow-up of a prospective longitudinal study. Behaviour research and therapy, 40(6), 665–675. [DOI] [PubMed] [Google Scholar]
- McLean SA, Clauw DJ, Abelson JL, & Liberzon I (2005). The development of persistent pain and psychological morbidity after motor vehicle collision: integrating the potential role of stress response systems into a biopsychosocial model. Psychosomatic medicine, 67(5), 783–790. [DOI] [PubMed] [Google Scholar]
- McLean SA, Ulirsch JC, Slade GD, Soward AC, Swor RA, Peak DA, … Domeier RM (2014). Incidence and predictors of neck and widespread pain after motor vehicle collision among US litigants and nonlitigants. PAIN®, 155(2), 309–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Muruganujan A, & Thomas PD (2012). PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic acids research, 41(D1), D377–D386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil JS (2012). Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nature Reviews Neuroscience, 13(12), 859–866. [DOI] [PubMed] [Google Scholar]
- Morra M, Barrington RA, Abadia-Molina AC, Okamoto S, Julien A, Gullo C, … Spolski R (2005). Defective B cell responses in the absence of SH2D1A. Proceedings of the National Academy of Sciences, 102(13), 4819–4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niska R, Bhuiya F, & Xu J (2010). National hospital ambulatory medical care survey: 2007 emergency department summary. Natl Health Stat Report, 26(26), 1–31. [PubMed] [Google Scholar]
- Norris FH (1992). Epidemiology of trauma: frequency and impact of different potentially traumatic events on different demographic groups. Journal of consulting and clinical psychology, 60(3), 409. [DOI] [PubMed] [Google Scholar]
- Otis JD, Keane TM, & Kerns RD (2003). An examination of the relationship between chronic pain and post-traumatic stress disorder. Journal of rehabilitation research and development, 40(5), 397. [DOI] [PubMed] [Google Scholar]
- Pakos‐Zebrucka K, Koryga I, Mnich K, Ljujic M, Samali A, & Gorman AM (2016). The integrated stress response. EMBO reports, e201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkitny L, McAuley JH, Di Pietro F, Stanton TR, O’Connell NE, Marinus J, … Moseley GL (2013). Inflammation in complex regional pain syndrome A systematic review and meta-analysis. Neurology, 80(1), 106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny GD, Kay GF, Sheardown SA, Rastan S, & Brockdorff N (1996). Requirement for Xist in X chromosome inactivation. Nature, 379(6561), 131–137. [DOI] [PubMed] [Google Scholar]
- Plath K, Mlynarczyk-Evans S, Nusinow DA, & Panning B (2002). Xist RNA and the mechanism of X chromosome inactivation. Annual review of genetics, 36(1), 233–278. [DOI] [PubMed] [Google Scholar]
- Portenoy RK, Ugarte C, Fuller I, & Haas G (2004). Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. The Journal of Pain, 5(6), 317–328. [DOI] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, & Smyth GK (2015). limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids research, 43(7), e47–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryb GE, Dischinger PC, Read KM, & Kufera JA (2009). PTSD after severe vehicular crashes. Paper presented at the Annals of Advances in Automotive Medicine/Annual Scientific Conference. [PMC free article] [PubMed] [Google Scholar]
- Scherzer CR, Eklund AC, Morse LJ, Liao Z, Locascio JJ, Fefer D, … Vance JM (2007). Molecular markers of early Parkinson’s disease based on gene expression in blood. Proceedings of the National Academy of Sciences, 104(3), 955–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segman R, Shefi N, Goltser-Dubner T, Friedman N, Kaminski N, & Shalev A (2005). Peripheral blood mononuclear cell gene expression profiles identify emergent post-traumatic stress disorder among trauma survivors. Molecular psychiatry, 10(5), 500. [DOI] [PubMed] [Google Scholar]
- Sherman JJ, Turk DC, & Okifuji A (2000). Prevalence and impact of posttraumatic stress disorder-like symptoms on patients with fibromyalgia syndrome. The Clinical journal of pain, 16(2), 127–134. [DOI] [PubMed] [Google Scholar]
- Skopkova M, Hennig F, Shin BS, Turner CE, Stanikova D, Brennerova K, … Müller U (2017). EIF2S3 Mutations Associated with Severe X‐Linked Intellectual Disability Syndrome MEHMO. Human mutation, 38(4), 409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuse DH (2005). X-linked genes and mental functioning. Human molecular genetics, 14(suppl_1), R27–R32. [DOI] [PubMed] [Google Scholar]
- Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B, … Ressler KJ (2011). Differential immune system DNA methylation and cytokine regulation in post‐traumatic stress disorder. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 156(6), 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Zhou D, Guan Z, & Wang X (2007). Disturbance of serum interleukin-2 and interleukin-8 levels in posttraumatic and non-posttraumatic stress disorder earthquake survivors in northern China. Neuroimmunomodulation, 14(5), 248–254. [DOI] [PubMed] [Google Scholar]
- Sorge R, LaCroix‐Fralish M, Tuttle A, Khoutorsky A, Sotocinal S, Austin JS, … Wood J (2013). The Yin and Yang of pain: variability in formalin test nociception and morphine analgesia produced by the Yin Yang 1 transcription factor gene. Genes, Brain and Behavior, 12(4), 405–413. [DOI] [PubMed] [Google Scholar]
- Suissa S, Harder S, & Veilleux M (2001). The relation between initial symptoms and signs and the prognosis of whiplash. European Spine Journal, 10(1), 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, & Foa EB (2006). Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychological bulletin, 132(6), 959. [DOI] [PubMed] [Google Scholar]
- Toomey TC, Seville JL, Finkel AG, Mann JD, Abashian SW, & Klocek JW (1997). Circumstances of chronic pain onset: Relationship to pain description, coping and psychological distress. Pain Clinic, 10(1), 19–26. [Google Scholar]
- Trinh MA, & Klann E (2013). Translational control by eIF2α kinases in long-lasting synaptic plasticity and long-term memory. Neurobiology of learning and memory, 105, 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukiainen T, Villani A-C, Yen A, Rivas MA, Marshall JL, Satija R, … Kirby A (2017). Landscape of X chromosome inactivation across human tissues. Nature, 550(7675), 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk DC, & Okifuji A (1996). Perception of traumatic onset, compensation status, and physical findings: impact on pain severity, emotional distress, and disability in chronic pain patients. Journal of Behavioral Medicine, 19(5), 435–453. [DOI] [PubMed] [Google Scholar]
- Üçeyler N, Valenza R, Stock M, Schedel R, Sprotte G, & Sommer C (2006). Reduced levels of antiinflammatory cytokines in patients with chronic widespread pain. Arthritis & Rheumatology, 54(8), 2656–2664. [DOI] [PubMed] [Google Scholar]
- Ursano RJ, Fullerton CS, Epstein RS, Crowley B, Kao T-C, Vance K, … Baum A (1999). Acute and chronic posttraumatic stress disorder in motor vehicle accident victims. American Journal of Psychiatry, 156(4), 589–595. [DOI] [PubMed] [Google Scholar]
- Wang J, Syrett CM, Kramer MC, Basu A, Atchison ML, & Anguera MC (2016). Unusual maintenance of X chromosome inactivation predisposes female lymphocytes for increased expression from the inactive X. Proceedings of the National Academy of Sciences, 113(14), E2029–E2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DS (2007). The impact of event scale: revised In Cross-cultural assessment of psychological trauma and PTSD (pp. 219–238): Springer. [Google Scholar]
- Wek R, Jiang H-Y, & Anthony T (2006). Coping with stress: eIF2 kinases and translational control. In: Portland Press Limited. [DOI] [PubMed] [Google Scholar]
- Wolfe F (2003). Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. J Rheumatol, 30(2), 369–378. [PubMed] [Google Scholar]
- Wu D, & Smyth GK (2012). Camera: a competitive gene set test accounting for inter-gene correlation. Nucleic acids research, 40(17), e133–e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Luo J, Yu H, Rattner A, Mo A, Wang Y, … Nathans J (2014). Cellular resolution maps of X chromosome inactivation: implications for neural development, function, and disease. Neuron, 81(1), 103–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaura K, Bi Y, Ishiwatari M, Oishi N, Fukata H, & Ueno K (2013). Sex differences in stress reactivity of hippocampal BDNF in mice are associated with the female preponderance of decreased locomotor activity in response to restraint stress. Zoological science, 30, 1019–1024. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yang J, Li Y, Ma X, & Li R (2017). Sex chromosome abnormalities and psychiatric diseases. Oncotarget, 8(3), 3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


