Abstract
Objective
The purpose of this study was to investigate perceptual and electrophysiological encoding of complex periodic signals as a function of age.
Design
Two groups of adults completed three listening tasks: a behavioural task of detection of a mistuned harmonic component in a complex tone, an electrophysiological measure of speech-evoked auditory brainstem response (sABR), and a speech-in-noise measure. Between group comparisons were undertaken for each task as well as pairwise correlation analyses for all tasks.
Study sample
One group of younger adults (n = 20) and one group of older adults (n = 20) participated. All listeners had relatively normal audiometric thresholds (≤ 20 dB HL) from 250–4000 Hz.
Results
Younger adults had better results than the older adults on all three tasks: sensitivity for detecting a mistuned harmonic, spectral encoding for sABR, and release from masking for the speech-in-noise test. There were no significant correlations between measures when evaluating the older adults in isolation.
Conclusions
The results are consistent with the body of literature that demonstrates reduced temporal processing abilities for older adults. The combined method approach undertaken in this investigation did not result in correlations between the perceptual and electrophysiological measures of temporal processing.
Keywords: Aging, temporal processing, speech-evoked ABR, periodicity coding
Introduction
Older adults with normal audiometric thresholds have often been shown to perform more poorly than younger adults on measures of speech perception in complex listening backgrounds (Goossens et al. 2017, Helfer and Freyman 2014, Rajan and Cainer 2008, Tun, O’Kane, and Wingfield 2002). Poor speech perception abilities in the presence of clinically normal audiometric thresholds have often been attributed to deficits in auditory temporal processing (Dubno, Horwitz, and Ahlstrom 2002, 2003, Gifford, Bacon, and Williams 2007, Gordon-Salant 2006, Grose, Mamo, and Hall 2009, Strouse et al. 1998). Temporal processing has been evaluated with psychophysical and electrophysiological methodologies, and both approaches demonstrate age-related deficits in the coding of transient (Harris et al. 2010, Poth et al. 2001, Schneider and Hamstra 1999, Walton, Orlando, and Burkard 1999) and sustained stimulus components (Grose, Mamo, and Hall 2009, He et al. 2008, Leigh-Paffenroth and Fowler 2006, Purcell et al. 2004, Takahashi and Bacon 1992). While both transient and sustained portions of speech stimuli contribute important temporal information to speech understanding, the current investigation focuses on the temporal processing of sustained – nominally steady state – stimuli. Specifically, it measures perceptual sensitivity to changes in an on-going periodic temporal envelope.
Processing of temporal envelopes is important for speech understanding in noise in part because it affects the listener’s ability to make use of the temporal fluctuations in a noisy background in order to ‘glimpse’ more speech cues (Cooke 2006). Studies have shown that, while older adults can perform similarly to younger adults on sentence measures of speech understanding in steady background noise, they often do not experience as much benefit when amplitude modulation is introduced to the background noise (e.g., Grose, Mamo, and Hall 2009, Stuart and Phillips 1996). This benefit can be summarized with a derived measure of masking release (MR) which is the difference in the speech reception threshold (SRT) measured in the steady masker and the modulated masker. There are likely multiple – and not mutually exclusive – factors that contribute to this reduced MR. For example, masking period patterns indicate that elevated levels of non-simultaneous masking in older listeners can obscure the temporal contours of the modulated masker (Grose et al. 2016). Additionally, deficient temporal fine structure coding (Grose and Mamo 2010) coupled with increased neural ‘jitter’ (Pichora-Fuller et al. 2007) can render the speech segments available during the masker minima less salient for older listeners.
The encoding of steady state, periodic stimuli is also important to masked speech perception because it contributes to pitch tracking. The ability to differentiate, or segregate, the voice pitch of a target speaker against a competing background enables the listener to selectively attend to that target speaker (Lee and Humes 2012). The fidelity with which periodic information is encoded in the auditory system can be assessed electrophysiologically. A common approach is to measure the speech-evoked auditory brainstem response (sABR) which uses a simple consonant-vowel token such as a /da/ to elicit a synchronized neural response. Studies of the sABR in older listeners have shown an age-related deficit for the steady-state portion of the /da/ stimulus when analyzed in terms of spectral magnitude or phase locking (Anderson et al. 2012, Mamo, Grose, and Buss 2016, Ruggles, Bharadwaj, and Shinn-Cunningham 2012). The results are consistent with tonal envelope following response (EFR) studies that have shown poorer temporal envelope coding for rapid modulation rates in older adults (Grose, Mamo, and Hall 2009, Leigh-Paffenroth and Fowler 2006, Purcell et al. 2004).
Envelope cues also feature in the processing of complex sounds consisting of unresolved harmonics. The internal representations of stimuli comprising linearly spaced, but unresolved, spectral components convey temporal information that is dominated by the envelope. The envelope rate reflects the frequency difference between the unresolved components which, for harmonic stimuli, is the fundamental frequency (F0). Shifts away from linear spacing among the unresolved components, such as mistuning one of the components, therefore affects the resultant envelope pattern. Previous studies related to mistuned harmonics have generally focused on frequency discrimination tasks and concurrent sound segregation (Micheyl and Oxenham 2010). There have been few studies investigating the perception of mistuning in older adults. Grube and colleagues (Grube, von Cramon, and Rubsamen 2003) found that older adults had higher thresholds for detecting a mistuned harmonic compared to younger adults for higher harmonic components (i.e., 4th and 8th harmonic), but not for lower harmonic components (i.e., 1st and 2nd harmonics). In addition, Alain and colleagues (Alain et al. 2001) found that older adults were poorer than younger adults at detecting a mistuned harmonic, particularly for short duration stimuli. These sparse findings suggest that older adults may exhibit reduced sensitivity to harmonic mistuning for conditions where envelope cues are dominant, especially for shorter-duration stimuli.
In summary, age-related deficits in temporal envelope processing are known to have several functional and perceptual consequences including a reduced benefit of modulated maskers for speech-in-noise perception and a reduced fidelity of envelope information encoded in the sABR. It is also possible that age-related deficits in temporal envelope processing can result in a reduced sensitivity to envelope changes associated with harmonic mistuning. It was the purpose of this study to test this latter possibility within the context of a three-pronged approach to assessing envelope processing. Three tasks were employed: (a) a speech-in-noise task, (b) an electrophysiological sABR task, and (c) a psychophysical harmonic mistuning task. The purpose of choosing the three measures investigated in the current study was to relate a ‘real world’ measure involving envelope processing (i.e., speech-in-modulated-noise) to both a well-known complex electrophysiolocial meaure (i.e., sABR) and a novel psychophysical measure of temporal envelope processing. The speech-in-noise task measured MR for speech as derived by the SRT difference between a steady and a temporally modulated masker. The electrophysiological task was a /da/-evoked sABR with a particular focus on the response to the periodic portion the stimulus. The psychophysical task assessed envelope sensitivity based on the processing of unresolved harmonic complexes. The over-arching hypothesis was that older adults exhibit envelope processing deficits relative to their younger counterparts, and that performance would therefore correlate among the three tasks. In an effort to isolate effects of age from effects of hearing loss, all participants in this study had audiometrically normal hearing.
Methods
Participants
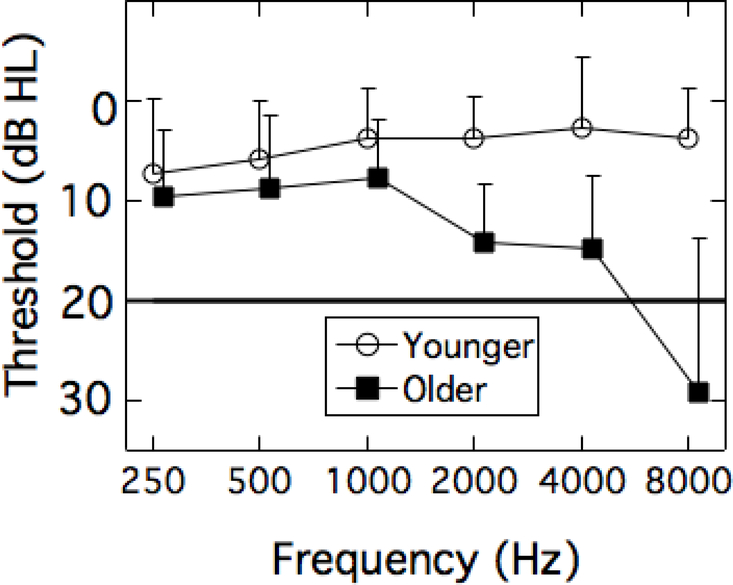
Two groups of listeners participated in this study: (1) younger adults (n = 20; 17 women; age range = 18–30 yrs; mean = 23.4 yrs, s.d. = 3.3 yrs) and (2) older adults (n = 20; 13 women; age range = 65–80 yrs; mean = 70.0 yrs, s.d. = 4.7 yrs). All listeners had normal audiometric thresholds (≤ 20 dB HL) from 250–4000 Hz, with four exceptions in the older adult group (Figure 1). Specifically, one listener had a threshold of 25 dB HL at 2000 Hz, two listeners had thresholds of 25 dB HL at 4000 Hz, and one listener had a threshold of 30 dB HL at 4000 Hz. For the older adult group, the ear with better hearing thresholds was tested (right = 10); for the younger adults, a test ear was assigned to balance the number of right and left ears tested (right = 11). All older adults scored ≥ 24 on the Montreal Cognitive Assessment (MoCA (Nasreddine et al. 2005)), which uses a screening cutoff score of 26 out of a maximum of 30 as a clinical indication of possible mild cognitive impairment.1 No one was excluded based on their MoCA score, and there was no association between MoCA score and any of the test measures in this project. All participants signed a consent form approved by the UNC-Chapel Hill Institutional Review Board and were paid for their participation.
Figure 1.
Group mean audiograms for younger (open circles) and older (filled squares) adults. Error bars are +1 standard deviation. Data are offset for better visualization.
Speech-in-noise task
The purpose of this task was to assess speech recognition in both a steady and a modulated masker. The goal was to obtain a profile of the speech-in-noise capabilities of this cohort of listeners, with a particular focus on the magnitude of modulation benefit (i.e., MR).
Stimuli
The speech test comprised HINT sentences presented in steady and amplitude modulated (AM) speech-shaped noise. The HINT is a common clinical measure that consists of 12 lists of 20 sentences per list (Nilsson, Soli, and Sullivan 1994). The sentences are syntactically correct and contain three to five key words that are expected to be familiar to school-age children. The sentences were presented in the presence of one of two maskers. One masker was a steady speech-shaped noise with a fixed level of 65 dB SPL. The other masker was the same noise but square-wave modulated at a rate of 10 Hz between the 65 dB SPL level and a ‘floor’ level of 30 dB SPL. The stimuli were controlled via a custom MATLAB (Mathworks, Natick, MA) program and output through a Tucker-Davis-Technologies (TDT; Alachua, FL) digital signal processor. The stimuli were presented monaurally through a Sennheiser HD 580 (Old Lyme, CT) headphone in a sound-attenuated booth.
Procedure
The initial sentence presented was selected randomly for each listener, and subsequent sentences progressed sequentially through the lists for all trials and conditions. Sentence-level scoring was implemented, such that each key word in the sentence had to be repeated back correctly for the sentence to be scored as correct. The listener repeated aloud what was heard, and the researcher monitored responses over headphones via a talk-back loop. The researcher entered the score via a mouse click on the computer, and the adaptive program adjusted the sentence presentation level accordingly. The presentation level of the sentence adaptively varied with a two-down, one-up stepping rule to approximate 70.7% correct performance. The adaptive step-size was 2 dB SPL, and the trial ended after six reversals. One practice trial in the modulated masker was presented. Two threshold estimates per condition were collected first, and then each condition was repeated once. If these three threshold estimates differed by more than 3 dB, a fourth threshold was collected. Final threshold was the average of these three or four threshold estimates.
Analysis
The MR was calculated by subtracting the SRT in the AM masker condition from the SRT in the steady masker condition. Group data were compared via t-tests for the derived MR as well as for the SRTs measured separately in the steady masker and the AM masker. The association between MR and the other experimental results was assessed using Pearson correlations. The hypothesis was that older adults would have a reduced MR compared to the younger adults. Further, it was expected that older adult performance would be similar to that of younger adults in the steady masker, but not in the AM masker.
Speech-evoked ABR task
The purpose of this task was to derive an objective electrophysiological measure of neural synchrony associated with speech processing. This was obtained by assessing the spectral magnitude of the harmonic structure of the response evoked by the periodic portion of a speech token.
Stimulus
A synthetic speech stimulus provided by the Auditory Neuroscience Laboratory at the Northwestern University School of Communication was employed. The 170-ms /da/ contained a stop burst, a formant transition, and a 120-ms steady vowel. The F0 was 100 Hz throughout the stimulus. The first three formants shifted during the 50-ms transition portion of the stimulus. Formant 1 ramped up from 400 to 720 Hz, formant 2 fell from 1700 to 1240 Hz, and formant 3 fell from 2580 to 2500 Hz; subsequently, formants 1–3 remained constant during the steady vowel. Formants 4–6 were fixed throughout the stimulus at 3300, 3750, and 4900, respectively (Anderson et al. 2012). Presentation level was 80 dB peak-equivalent SPL. Stimuli were presented monaurally with alternating polarity at a rate of 3.9/second. The stimulus was presented through a shielded insert earphone (ER2; Etymotic Research, Inc., Elk Grove Village, IL). Mu-metal enclosures and electrical shielding tape (3M; Moncure, NC) were used to shield the transducers, as well as the cables within the sound booth (Campbell et al. 2012). The stimulus was controlled via a custom MATLAB program and output through a TDT digital signal processor, which also sent a time-locked trigger to a Neuroscan SynAmpsRT recording system (Compumedics; Charlotte, NC).
Recording
Electrophysiological recordings were collected using the Neuroscan system. All reported findings were measured via a single, bipolar channel with a midline electrode montage: hairline at top of forehead (non-inverting) to nape of neck (inverting), with the ground at the eyebrows. The continuous EEG recording was filtered online from 0.5–3000 Hz and subsequently digitally filtered offline from 100–3000 Hz with a 12-dB/octave roll-off. Recordings were digitized at a sampling rate of 20,000 Hz. Artifact rejection was applied to any epoch exceeding ±35 μV. Approximately 3000 sweeps per stimulus polarity were collected. To reduce effects based on changes in the participant’s resting state, the 6000 total sweeps were collected in blocks of 2000 sweeps and then combined using the weighted average transform in the Neuroscan editing software. The weighted average transform accounts for the number of sweeps contributing to each averaged response. Responses to alternating polarities were added together to emphasize the response to the temporal envelope of the stimulus (Aiken and Picton 2008). The participant rested in a recliner in a sound-attenuated booth and watched a silent movie of choice with subtitles. The participant was allowed to sleep and was provided with breaks per request. Recordings were made during one or two sessions lasting no more than 2 hours each. The extended duration was because additional evoked potential conditions not reported in this study were collected at the same time.
Analysis
All analyses were done offline. For the averaged waveform from each individual, the segment associated with the steady-state portion of the stimulus (60–180 ms post-stimulus onset) was submitted to a Fast Fourier Transform (FFT). After zero-padding, the resulting bin width in the frequency domain was 8.3 Hz. The metric used for analysis was the signal-to-noise ratio (SNR) for the first six harmonic components (F0 plus harmonics 2–6). The SNR (dB) was calculated based on the amplitude of the respective response component compared to the surrounding noise floor. A noise floor value was calculated for each response component by averaging the amplitude from four frequency bins over a +/− 25 Hz range, excluding the frequency bins immediately adjacent to that containing the component of interest. A criterion SNR of 3 dB was used to determine whether a response was reliably present. If the SNR was < 3 dB, a value of 0 dB was used for all statistical analysis.
Responses were analyzed using a repeated-measures analysis of variance (ANOVA) with harmonic component as a within-subjects factor and age group as a between-subjects factor. There were six levels of the within-subjects factor (F0 and harmonics 2 – 6) and two levels of the between-subjects age group factor. Interactions were probed with pairwise comparisons and Bonferroni corrections for multiple comparisons. In cases of unequal variance among independent variables, Greenhouse-Geisser corrections for violations of sphericity were applied. The hypothesis was that older adults would have a lower amplitude response for the sABR components than the younger adults. The same repeated-measures ANOVA approach was used to assess the noise floor level associated with each harmonic component across the two age groups.
Mistuned harmonic detection task
The purpose of this task was to obtain a psychophysical measure of envelope processing for envelope rates within the fundamental frequency range of voiced speech. By using stimuli with unresolved harmonics, the goal was to maximize the probability that envelope cues dominated performance. The possibility that temporal fine structure (TFS) cues contributed to performance, however, cannot be entirely excluded; in studies that have used similar stimuli comprising unresolved harmonics, the role of TFS cues has been debated (e.g., Oxenham, Micheyl, and Keebler 2009, Jackson and Moore 2014).
Stimuli
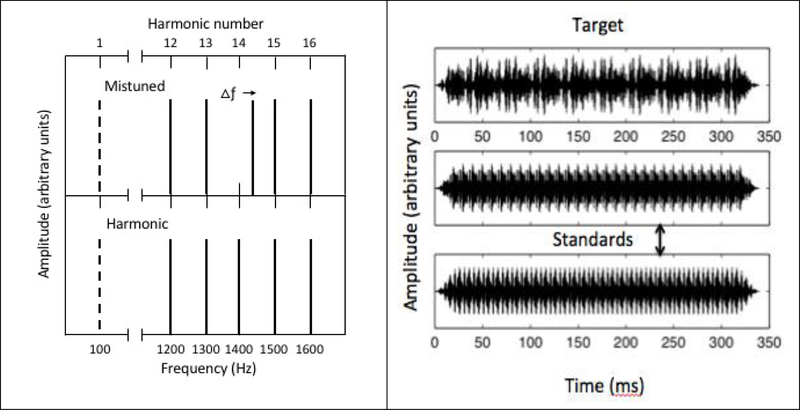
The stimulus was a five-component complex tone comprising (unresolved) harmonic numbers 12–16 for each of two different F0s: 100 Hz [i.e., 1200, 1300, 1400, 1500, 1600 Hz] and 200 Hz [i.e., 2400, 2600, 2800, 3000, 3200 Hz]. Three different stimulus durations were tested: 170, 340, and 680 ms. The shortest duration matched the duration of the sABR speech-token stimulus. The two longer stimuli (each reflecting a doubling of temporal cycles) were selected based on the expectation that older adults require a longer temporal integration period to detect the inharmonicity of the complex tone (Alain, McDonald, and Van Roon 2012, Alain et al. 2001). The complex tone had an overall level of 70 dB SPL, and was presented to the test ear through a Sennheiser HD 580 headphone. A continuous low-pass noise was presented to mask distortion products (Pressnitzer and Patterson 2001). That noise had a cutoff frequency of 100 Hz (15-dB/octave roll-off) and was presented at a pressure spectrum level of 50 dB SPL. Each harmonic component was generated with a random starting phase for each presentation. Because of the random starting phase, the stimuli presented in the two standard intervals of the three-alternative, forced choice (3AFC) procedure (described in detail below) were not identical but did have uniform periodicity at the rate of the F0. In the target interval, an upward frequency shift was applied to the 14th harmonic component. The intended effect of shifting only the center component of the complex tone was to disrupt the periodic temporal envelope while minimizing spectral cues. Figure 2 shows schematic stimulus spectra (left panel) and examples of a target and two standard waveforms (right panels). Note that only the center harmonic component (#14) is shifted in the target stimulus. The F0 is shown as a dashed line, but the F0 is not present in the complex tone. In the time domain example (right panel of Figure 2), the envelope periodicity of the target stimulus is perturbed as a result of the mistuning of the 14th harmonic by 5%. All stimuli were digitally generated at a sampling rate of 24,414 Hz using a TDT digital signal-processing platform interfaced with custom MATLAB code.
Figure 2.
Stimulus example. Left Panel—Top: Schematic of the stimulus spectrum for target in which the 14th harmonic has been upshifted in frequency. The missing F0 is shown as a dashed line. Bottom: Stimulus spectrum for the standard complex showing harmonics 12–16 of 100 Hz. Right Panel—Example time waveforms of the target stimulus and two standard foils for the 340 ms, 100-Hz F0 condition.
Procedure
The 3AFC procedure incorporated a three-down, one-up adaptive rule that converged on 79.4% correct. Lights on a response box marked intervals, and the listener selected the target interval with a button press. Visual feedback was provided after each response. At the start of each adaptive threshold estimation track, the 14th harmonic component was mistuned by either 1, 3, or 5 % in the target interval. This frequency offset was then adjusted by a factor of 2 at each of the first two reversals in the direction of mistuning; thereafter, the step size for adjusting the frequency offset was reduced to a factor of √2. A threshold estimation track was terminated after eight reversals, and the resulting threshold estimate was taken as the geometric mean of the final six reversal frequencies. The harmonic was always shifted upwards in frequency, and the adaptive track was not allowed to exceed 7.14% mistuning because beyond that point, the mistuned component would have a frequency higher than the next higher harmonic of the complex tone.
Listeners were provided with one to two practice runs per condition, with practice conditions sampled randomly. Following practice, three to five thresholds were collected for each condition in randomized blocks. Each threshold estimation track began with training trials in which the listener knew that the target signal always occurred in the second interval. The listener could continue the training trial sequence as long as he/she wanted in order to become familiar with the perceptual distinction between signal and standard before initiating the adaptive track. There were five younger and six older adults who showed a substantial improvement in thresholds during data collection (i.e., beyond the practice conditions); for these subjects, blocks of three to five additional threshold estimates were collected, with final threshold based on these latter estimates, to ensure that analyses were based on stable thresholds. Testing was typically completed over two or three one-hour sessions.
Analysis
Responses were analyzed using a repeated-measures ANOVA with two within-subjects factors (F0 and duration) and one between-subjects factor of age group. For the within-subjects factors, there were two levels of F0 (100 and 200 Hz), and three levels of duration (170, 340, and 680 ms). There were two levels for the between-subjects age group factor. Interactions were probed with pairwise comparisons incorporating Bonferroni corrections for multiple comparisons. Greenhouse-Geisser corrections for violations of sphericity were applied where appropriate. The hypothesis was that older adults would have higher thresholds (poorer responses) for detecting the mistuned harmonic component and that there would be an interaction of group and stimulus duration, with the older adults being more affected by the shorter duration stimulus.
Results
Audiometric sensitivity
Analysis of the audiometric thresholds showed a significant within-subject main effect of frequency (F(2.8,104.9) = 12.45, p < 0.001) and a significant between-subjects main effect of age group (F(1,38) = 50.36, p < 0.001), as well as a significant interaction of frequency and age group (F(2.8,104.9) = 17.29, p < 0.001). Further analysis of the interaction via pairwise comparisons with Bonferroni adjustments for multiple comparisons revealed no difference between age groups for audiometric thresholds at 250 and 500 Hz and significant differences between age groups for all test frequencies from 1000–8000 Hz (p < 0.05). Despite an age-related elevation in thresholds at octave frequencies from 1000–8000 Hz, the mean thresholds for the older adults were still within normal audiometric limits (≤ 20 HL) for 1000–4000 Hz (Figure 1). Given the presentation level of all the stimuli, audibility was not likely to be a limiting factor in performance.
Speech perception in noise
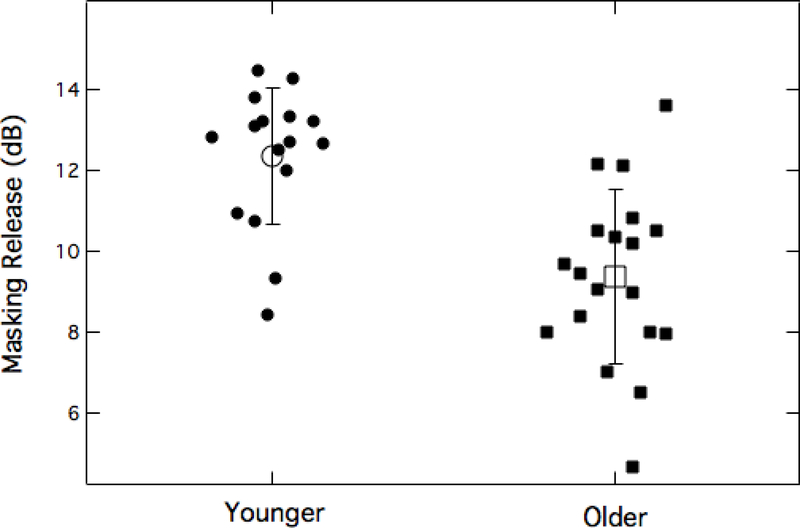
Sixteen of the younger and 19 of the older adults completed the HINT sentence testing in the steady and AM maskers. Figure 3 shows the group mean and individual MR values. Threshold values for 70.7% correct sentence repetition in the 65-dB-SPL steady masker were 62.3 dB SPL and 62.8 dB SPL for the younger and older adults, respectively. These thresholds did not differ significantly (t(33) = −1.94, p = 0.06). Complementary thresholds in the modulated masker were 50.0 dB SPL and 53.5 dB SPL for the younger and older adults, respectively. The derived MR for each group was therefore 12.3 dB SPL (younger) and 9.3 dB SPL (older); these MR magnitudes differed significantly between the two age groups (t(33) = 4.50, p < 0.001). The results of this test indicate that, whereas performance in steady noise was equivalent between the two age groups, the older adults experienced less benefit from modulations in the background noise.
Figure 3.
MR plotted for younger and older adults. Small filled symbols represent individual thresholds; larger open symbols show group mean thresholds. Error bars are +/− 1 standard deviation.
Speech-evoked ABR
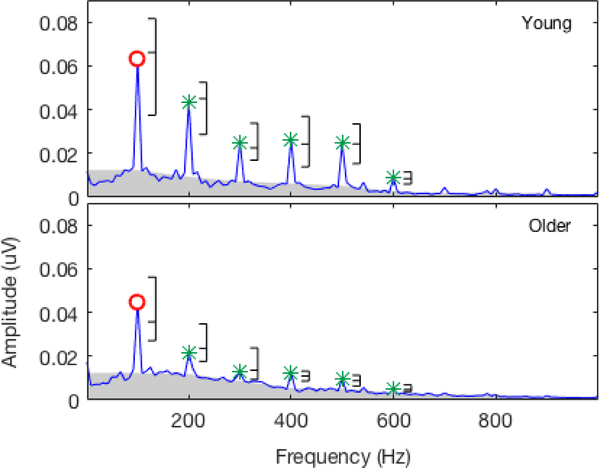
For the sABR, the younger adults had more robust harmonic components than the older adults in the magnitude spectrum for the steady, periodic portion of the response (Figure 4). A repeated-measures ANOVA analysis confirmed a within-subjects main effect of frequency (F(3.6,136.3) = 7.04, p < 0.001) and a between-subjects main effect of age group (F(1,38) = 58.12, p < 0.001). There was no significant interaction of frequency and age group (F(3.6,136.3) = 1.66, p = 0.17). These findings indicate that older adults had less robust encoding of the periodic portion of the sABR and that this effect was not frequency dependent.
Figure 4.
Grand mean magnitude spectra for the sABR response to the steady-state segment (60–180 ms) for younger (top panel) and older (bottom panel) adults. The open circles mark the F0, and the asterisks mark the expected harmonic components. Brackets beside each component display the distribution of the data in which the bottom and top dashes indicate the 25th and 75th percentiles, respectively, and the middle dash indicates the median. The gray shading provides an illustration of the noise floor and is extrapolated from the point estimates of noise used to analyze the noise floor.
Analysis of the noise floor revealed no effect of age group (F(1,38) = 0.39, p = 0.54). There was a significant effect of frequency (F(3.4,130) = 100.48, p < 0.001), which was expected due to the rising noise floor with decreasing frequency in EEG recordings. There was no interaction between frequency and age group (F(3.4,130) = 2.24, p = 0.08). This result indicates that the poorer representation of the spectral content of the stimulus in the older listeners was not due to an increased noise floor.
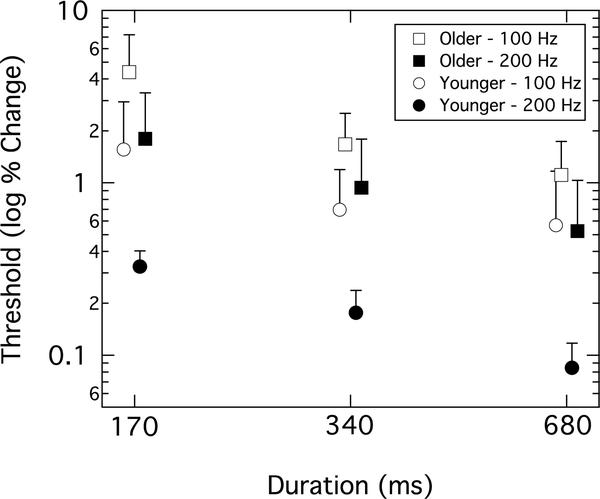
Mistuned harmonic detection
Younger adults were more sensitive to mistuning of the 14th harmonic than older adults for all test conditions (Figure 5). Results were analyzed in terms of percent change in frequency of the 14th harmonic component. If a listener performed at ceiling (i.e., was unable to achieve a threshold at the maximum frequency shift), a value of 7.14% was entered for analysis purposes; out of the 240 individual thresholds submitted for analysis, the older adult group had 11 ceiling thresholds and the younger adult group had one. Both groups improved as the stimulus duration increased, and both groups performed better for the fundamental frequency of 200 Hz as compared to 100 Hz. The log transformed thresholds were used because log units are thought to reflect perceptually equivalent changes in frequency (Micheyl et al. 2006). A repeated-measures ANOVA included two within-subjects factors of F0 and duration as well as the between-subjects factor of age group. There were significant within-subject main effects of F0 (F(1,38) = 178.84, p < 0.001) and duration (F(1.5,57.6) = 215.31, p < 0.001), and a between-subjects main effect of age group (F(1,38) = 60.29, p < 0.001). There was a significant interaction of F0 by duration (F(1.8,69) = 3.39, p = 0.04). There were no significant interactions for duration by age group (F(1.5,57.6) = 0.04, p = 0.92), or the three-way interaction of F0 by duration by age group (F(1.8,69) = 0.10, p = 0.88).
Figure 5.
Group mean thresholds for detection of the mistuned harmonic. Response reported as log percent change in the shifted component. Results are shown for younger (circles) and older (squares) adults. Thresholds were collected at two F0s—100 Hz (open symbol) and 200 Hz (filled symbol)—and three different durations, indicated on the abscissa. Error bars represent +1 standard deviation. Symbols are offset for better visualization.
Relations among measures
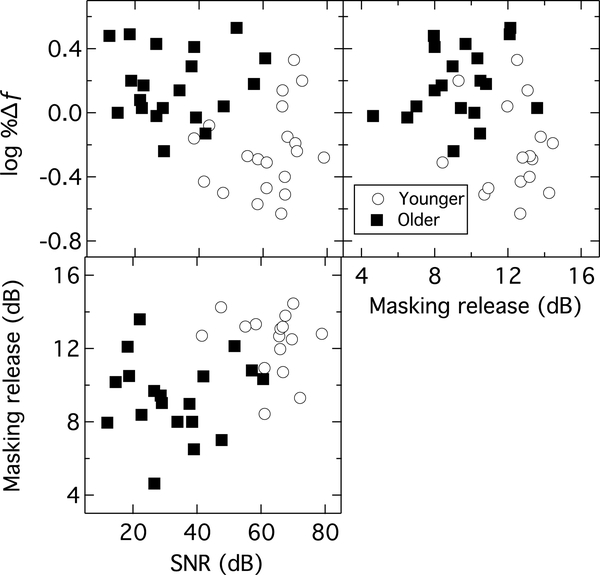
Metrics from the three tasks were compared using correlational analyses. To limit the number of comparisons, one measure was selected to best represent each task. For the speech-in-noise task, the magnitude of MR was selected as the measure. For the sABR task, a summed SNR value for the first five spectral components was selected (F0 [H1] through the fifth harmonic [H5]). This measure was chosen with the intention of capturing the overall response to the complex temporal envelope. Visual inspection of the sABR data plotted in the frequency domain showed that, particularly for the younger adults, there was a substantial drop in response magnitude after the 5th harmonic component. For the mistuned harmonic detection task, the log percent change threshold for the F0=100 Hz, 340-ms duration condition was selected. There was a frequency effect for the mistuning task such that listeners performed better for the conditions that had a fundamental frequency of 200 Hz; however, a fundamental frequency of 100 Hz was chosen for the association measures because the fundamental frequency of the /da/ used in the sABR measures was also 100 Hz. Although the stimulus in the F0=100 Hz, 170-ms duration stimulus condition most closely matched the /da/ stimulus in terms of F0 and duration, thresholds were at ceiling in this condition for 10 of 20 older adults and 1 of 20 younger adults. All subjects yielded a valid behavioral threshold for the F0=100 Hz, 340-ms duration condition. Pearson correlations (two-tailed) indicated significant associations between the summed SNR of the sABR and the mistuned harmonic threshold (r(38) = −0.43, p = 0.01), as well as between the summed SNR of the sABR and the MR for the HINT sentences (r(33) = 0.47, p < 0.01; Figure 6). There was a borderline significant correlation between the harmonic mistuning threshold and MR (r(33) = −0.34, p = 0.05; Figure 6).
Figure 6.
Scatterplots showing individual pairwise associations between the sABR (summed SNR dB), the log transformed mistuned harmonic thresholds (100 Hz F0 and 340-ms condition), and the MR for HINT sentences. Symbols indicate either younger (open circles) or older (filled squares) individuals.
Although there was a significant correlation between the sABR response and both behavioral measures, as well as a significant correlation between the behavioral temporal processing task and the speech perception measure, a visual inspection of the data in Figure 6 suggests that these correlations are due to group effects, with a cluster of well-performing younger adults dominating the correlation analyses. The older adults’ data in isolation do not approach a significant correlation for the sABR and the mistuned harmonic threshold (r(18) = 0.07, p = 0.78), the sABR and the MR value (r(17) = 0.02, p = 0.93), or the mistuned harmonic threshold and the MR value (r(17) = 0.25, p = 0.29). Despite positive correlations when all participants are included, the variability of the older adults does not suggest any inherent associations. Although the small sample size when restricting the correlational analyses to only the older adults likely underpowers the tests, visual inspection of the data supports the conclusion that there is no trend toward a pattern of associations between measures for the older adults. Other measures thought to potentially contribute to the summed SNR of the sABR, the mistuned harmonic task, and MR for the HINT sentences were considered for the older group only (i.e., threshold at 8000 Hz, pure-tone average (PTA) for 1000–4000 Hz or 4000–8000 Hz, age, and MoCA score), but none of these yielded significant correlations.
Discussion
The purpose of this study was to gain further insights into age-related deficits in the processing of temporal envelopes. The results indicated that the younger adults performed better on all test measures. For the speech-in-noise task, it was expected that older adults would perform similarly to the younger adults for sentences in steady noise but poorer than the younger adults in the AM masker, leading to a smaller MR in the older group. This was, in fact, observed and is consistent with previous findings (e.g., Grose, Mamo, and Hall 2009). For the sABR measure it was hypothesized that the older adults would exhibit less robust encoding of the components of the complex temporal envelope, and this also was observed, consistent with earlier reports (e.g., Anderson et al. 2012). These findings establish that the older adults with relatively normal audiograms tested here exhibit the expected profile of envelope processing deficits.
For the novel mistuned harmonic task, the hypothesis was that the detection of a change in the temporal envelope periodicity of a complex tone would be poorer for the older adults. Indeed, the older adults had higher (i.e., poorer) thresholds for detecting the target mistuned stimulus for all test conditions. Both groups showed poorer performance for the shorter duration stimuli and the F0 of 100 Hz. This finding suggests that more temporal modulation cycles improves the sensitivity for detection of periodicity in these stimuli. These findings are consistent with studies that have shown poorer temporal resolution and integration for older adults with normal or near-normal hearing sensitivity (He et al. 2008, Schneider and Hamstra 1999, Strouse et al. 1998).
Another pattern consistent with reduced temporal integration is that the thresholds for the older adults in the 200-Hz F0 condition were similar to the thresholds for the younger adults in the 100-Hz F0 condition (see Figure 5). This suggests that the older adults needed twice as many temporal modulations to encode the temporal envelope and detect a change at the same level of sensitivity as the younger adults. Perhaps the older adults need more “looks” at the temporal modulation of the signal to determine the periodicity. These findings are consistent with previous work showing that older adults require longer duration stimuli to perform like younger adults on tasks of temporal resolution (Schneider and Hamstra 1999). Moreover, a magnetoencephalography (MEG) study that used harmonic mistuning to evaluate age-related effects in concurrent sound segregation found a duration-by-age interaction (Alain, McDonald, and Van Roon 2012). The authors suggested that this indicates a general slowing of auditory processing and/or longer temporal integration windows for older adults.
A key interest of this investigation was the association among measures of envelope processing. This interest was examined by using an approach that included both behavioral and electrophysiological measures in the same individuals. Such an approach can help isolate factors associated with the perceptual and sensory encoding of the acoustic features of speech and lead to an improved understanding of the mechanisms underlying the auditory processing of complex stimuli. Studies in which psychophysical and electrophysiological measures demonstrate parallel results within the same group of subjects offer insight into the sensory encoding underlying perceptual deficits (e.g., Purcell et al. 2004; Ross, Tremblay, and Picton 2007) However, such parallels are not always found. For example, associations between the frequency following response (FFR) and frequency discrimination measures have yielded inconsistent results in terms of associations between measures (Clinard, Tremblay, and Krishnan 2010, Marmel et al. 2013). The approach of parallel behavioral and electrophysiological measures has also been applied to speech processing. Recent investigations of age-related deficits in neural phase locking have employed complex stimuli to elicit responses, such as speech tokens used in sABR testing, and draw correlations with measures of speech perception (Anderson et al. 2012, Ruggles, Bharadwaj, and Shinn-Cunningham 2012). An inherent challenge to combining these methods effectively is to use complementary stimuli in the two measures. Otherwise, it is challenging to differentiate between stimulus and processing factors when interpreting incongruent patterns of results.
In the current study, the intention of the combined-method design was to have an opportunity to determine whether listeners with robust sensory encoding for the sABR also have superior perception for mistuning of a similar complex acoustic signal. When considering the older adults in isolation in the current investigation, there was not a significant correlation between the electrophysiological response and the behavioral performance. Although an insignificant correlation between the two measures could be interpreted as indicating that someone with good sensory encoding is, nevertheless, poor at the perceptual task, with perception presumably affected by more central or cognitive processes, there are many other factors to consider as well. For example, the lack of an association between the sABR and either behavioral measure for the older adult group raises questions as to the complementary nature of the different stimuli used in each measure. The stimulus created for the behavioral task was designed to have the same envelope periodicity as the synthetic speech token (/da/) used to elicit the sABR. However, the spectra of the two stimuli are different in that the sABR token (when considering only the steady portion of the stimulus) is dominated by harmonic energy from approximately 100–1500 Hz, while the behavioral stimulus was composed of the harmonics between 1200–1600 Hz for the 100 Hz F0 condition. Nevertheless, evidence that neural encoding of the upper harmonics dominates the FFR for a complex tone (Zhu et al. 2013) suggests that there could be associations between the recorded sABR and the perceptual processing of a complex tone comprised only of upper harmonics.
The behavioral stimulus for the mistuned harmonic task was also limited to unresolved harmonic frequencies in order to enhance reliance on temporal envelope cues. Although the dominant cue in the target stimulus of the psychophysical task was a change in perceptible roughness, typically associated with envelope cues, it is possible that participants also made use of TFS cues. Moore and Sek (2009) have demonstrated that TFS cues can be used to detect a change in harmonicity by holding the temporal envelope constant while shifting all the components of a complex tone, even when the components are unresolved. If, in fact, the listener was able to improve their behavioral performance through the use of TFS cues in addition to the change in envelope periodicity, this may not have been reflected in the recorded sABR, which was averaged using alternating polarity, thus, enhancing the envelope response. As noted earlier, the relative role of envelope and TFS cues in processing stimuli comprising unresolved harmonics has been a topic of debate (e.g., Oxenham, Micheyl, and Keebler 2009, Jackson and Moore 2014).
To further consider the lack of association between physiological and behavioral measures of temporal processing, it is interesting to consider a few individual participants. Table 1 presents the mean responses from the older adult group for all three study measures, as well as individual thresholds from the older individual with the most robust F0 encoding and the three older adults with an absent F0 for the sABR. There is no dependent variable (e.g., mistuned harmonic threshold or masking release), nor any particular subject characteristic (e.g., PTA, age, cognitive score), that differentiates these participants. In fact, the individual with the best F0 SNR also had the worst threshold for the harmonic mistuning task within the older adult group.
Table 1.
Thresholds from Exemplar Older Adults. All measures collected on three subjects with absent F0 in the sABR (O-1 to O-3) as well as the older adult with the strongest F0 (O-4) and the group means and standard deviations.
| Subject | F0 SNR | Summed SNRa | Mistuned Harmonic | Masking Release | PTAb | 8000 Hz Threshold | Age | Cognitive Screenc |
|---|---|---|---|---|---|---|---|---|
| O-1 | 0 | 12.01 | 3.00 | 7.95 | 16.7 | 35 | 69 | 25 |
| O-2 | 0 | 14.58 | 1.00 | 10.17 | 13.3 | 35 | 71 | 29 |
| O-3 | 0 | 28.67 | 1.08 | 9.43 | 11.7 | 15 | 67 | 30 |
| O-4 | 17.38 | 51.69 | 3.40 | 12.13 | 6.7 | 30 | 66 | 27 |
| O-Mean (s.d.) | 9.52 (5.65) | 34.49 (14.61) | 1.66 (0.85) | 9.15 (2.23) | 12.0 (4.4) | 29.6 (15.5) | 69.7 (4.4) | 27.1 (1.8) |
The summed SNR for the sABR response was the sum of the first five spectral components, H1-H5.
PTA = Pure tone average of audiometric thresholds 1000, 2000, and 4000 Hz.
All cognitive screen results presented here were obtained using the Montreal Cognitive Assessment.
This combined-method investigation of temporal envelope processing did not yield associations between test responses for the older adults. Nevertheless, better understanding of the relationship between sensory, perceptual, and central processing of signals may allow better understanding of the mechanistic source driving an individual’s speech-in-noise difficulties. In the context of assessing age-related effects in temporal envelope processing, future work will continue to refine the parallels between behavioral and electrophysiological tasks – particularly in regards to stimulus characteristics.
Conclusion
This study adds to a body of literature that demonstrates that older adults have poorer temporal envelope processing than younger adults. The motivation for this study was to investigate individual associations between objective and behavioral measures of temporal processing. Although the temporal envelope measures employed here were not associated within subjects in a manner that explained the variance within the older adult group, the need remains to find measures that do explain performance at an individual level in order to advance our understanding of the underlying factors contributing to speech perception difficulties. The sABR is powerful in that its elicitation relies on multiple temporal processing mechanisms in one measure (e.g., abrupt onsets, sustained envelopes, fine structure); however, the variety of metrics available and analyzed throughout the literature adds to the challenge of interpreting the sABR. The current study sought to capitalize on one temporal aspect of the sABR stimulus, and to measure perceptual sensitivity to a shift in the periodicity of a complex tone. Future investigations may benefit from parametrically considering different complex stimuli that can be tested in parallel with psychophysical measures. In particular, stimuli that allow for evaluation of neural synchrony concurrently at multiple levels (e.g., 8th nerve, brainstem, and cortex) would allow for a wider range of within-subject comparisons to improve understanding of what generators are dominating the response and what metrics are best associated with functional communication.
Acknowledgements
Support for this research was provided by grants from the National Institutes of Health (NIDCD) under Grants 1-F32-DC012217 (SKM) and 5-R01-DC001507 (JHG).
Funding details: Support for this research was provided by grants from the National Institutes of Health (NIDCD) under Grants 1-F32-DC012217 (SKM) and 5-R01-DC001507 (JHG).
Footnotes
The MoCA was not administered to the first four older adults enrolled in the study. They completed the Mini-Mental Status Examination (MMSE) and scored ≥ 29 out of 30.
References
- Aiken SJ, and Picton TW. 2008. “Envelope and spectral frequency-following responses to vowel sounds.” Hear Res 245 (1–2):35–47. doi: 10.1016/j.heares.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Alain C, McDonald KL, Ostroff JM, and Schneider B. 2001. “Age-related changes in detecting a mistuned harmonic.” J Acoust Soc Am 109 (5 Pt 1):2211–6. [DOI] [PubMed] [Google Scholar]
- Alain C, McDonald K, and Van Roon P. 2012. “Effects of age and background noise on processing a mistuned harmonic in an otherwise periodic complex sound.” Hear Res 283 (1–2):126–35. doi: 10.1016/j.heares.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, and Kraus N. 2012. “Aging affects neural precision of speech encoding.” J Neurosci 32 (41):14156–64. doi: 10.1523/JNEUROSCI.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell T, Kerlin JR, Bishop CW, and Miller LM. 2012. “Methods to eliminate stimulus transduction artifact from insert earphones during electroencephalography.” Ear Hear 33 (1):144–50. doi: 10.1097/AUD.0b013e3182280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinard CG, Tremblay KL, and Krishnan AR. 2010. “Aging alters the perception and physiological representation of frequency: evidence from human frequency-following response recordings.” Hear Res 264 (1–2):48–55. doi: 10.1016/j.heares.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke M 2006. “A glimpsing model of speech perception in noise.” J Acoust Soc Am 119 (3):1562–73. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Horwitz AR, and Ahlstrom JB. 2002. “Benefit of modulated maskers for speech recognition by younger and older adults with normal hearing.” J Acoust Soc Am 111 (6):2897–907. [DOI] [PubMed] [Google Scholar]
- Dubno JR, Horwitz AR, and Ahlstrom JB. 2003. “Recovery from prior stimulation: masking of speech by interrupted noise for younger and older adults with normal hearing.” J Acoust Soc Am 113 (4 Pt 1):2084–94. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, and McHugh PR. 1975. ““Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician.” J Psychiatr Res 12 (3):189–98. [DOI] [PubMed] [Google Scholar]
- Gifford RH, Bacon SP, and Williams EJ. 2007. “An examination of speech recognition in a modulated background and of forward masking in younger and older listeners.” J Speech Lang Hear Res 50 (4):857–64. doi: 10.1044/1092-4388(2007/060). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens T, Vercammen C, Wouters J, and van Wieringen A. 2017. “Masked speech perception across the adult lifespan: Impact of age and hearing impairment.” Hear Res 344:109–124. doi: 10.1016/j.heares.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S 2006. “Speech perception and auditory temporal processing performance by older listeners: implications for real-world communication.” Seminars in Hearing 27 (4):264–268. [Google Scholar]
- Grose JH, and Mamo SK. 2010. “Processing of temporal fine structure as a function of age.” Ear Hear 31:755–760. doi: 10.1097/AUD.0b013e3181e627e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Mamo SK, and Hall JW 3rd. 2009. “Age effects in temporal envelope processing: speech unmasking and auditory steady state responses.” Ear Hear 30 (5):568–75. doi: 10.1097/AUD.0b013e3181ac128f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grose JH, Menezes DC, Porter HL, and Griz S. 2016. “Masking Period Patterns and Forward Masking for Speech-Shaped Noise: Age-Related Effects.” Ear Hear 37 (1):48–54. doi: 10.1097/AUD.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube M, von Cramon DY, and Rubsamen R. 2003. “Inharmonicity detection. Effects of age and contralateral distractor sounds.” Exp Brain Res 153 (4):637–42. doi: 10.1007/s00221-003-1640-0. [DOI] [PubMed] [Google Scholar]
- Harris KC, Eckert MA, Ahlstrom JB, and Dubno JR. 2010. “Age-related differences in gap detection: effects of task difficulty and cognitive ability.” Hear Res 264 (1–2):21–9. doi: 10.1016/j.heares.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He NJ, Mills JH, Ahlstrom JB, and Dubno JR. 2008. “Age-related differences in the temporal modulation transfer function with pure-tone carriers.” J Acoust Soc Am 124 (6):3841–9. doi: 10.1121/1.2998779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer KS, and Freyman RL. 2014. “Stimulus and listener factors affecting age-related changes in competing speech perception.” J Acoust Soc Am 136 (2):748–59. doi: 10.1121/1.4887463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson HM, and Moore BC. 2014. “The role of excitation-pattern, temporal-fine-structure, and envelope cues in the discrimination of complex tones.” J Acoust Soc Am 135 (3):1356–70. doi: 10.1121/1.4864306. [DOI] [PubMed] [Google Scholar]
- Lee JH, and Humes LE. 2012. “Effect of fundamental-frequency and sentence-onset differences on speech-identification performance of young and older adults in a competing-talker background.” J Acoust Soc Am 132 (3):1700–17. doi: 10.1121/1.4740482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh-Paffenroth ED, and Fowler CG. 2006. “Amplitude-modulated auditory steady-state responses in younger and older listeners.” J Am Acad Audiol 17 (8):582–97. [DOI] [PubMed] [Google Scholar]
- Mamo SK, Grose JH, and Buss E. 2016. “Speech-evoked ABR: Effects of age and simulated neural temporal jitter.” Hear Res 333:201–209. doi: 10.1016/j.heares.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmel F, Linley D, Carlyon RP, Gockel HE, Hopkins K, and Plack CJ. 2013. “Subcortical neural synchrony and absolute thresholds predict frequency discrimination independently.” J Assoc Res Otolaryngol 14 (5):757–66. doi: 10.1007/s10162-013-0402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheyl C, Delhommeau K, Perrot X, and Oxenham AJ. 2006. “Influence of musical and psychoacoustical training on pitch discrimination.” Hear Res 219 (1–2):36–47. doi: 10.1016/j.heares.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Micheyl C, and Oxenham AJ. 2010. “Objective and subjective psychophysical measures of auditory stream integration and segregation.” J Assoc Res Otolaryngol 11 (4):709–24. doi: 10.1007/s10162-010-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore BC, and Sek A. 2009. “Development of a fast method for determining sensitivity to temporal fine structure.” Int J Audiol 48 (4):161–71. doi: 10.1080/14992020802475235. [DOI] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, and Chertkow H. 2005. “The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment.” J Am Geriatr Soc 53 (4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Soli SD, and Sullivan JA. 1994. “Development of the Hearing in Noise Test for the measurement of speech reception thresholds in quiet and in noise.” J Acoust Soc Am 95 (2):1085–99. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, Micheyl C, and Keebler MV. 2009. “Can temporal fine structure represent the fundamental frequency of unresolved harmonics?” J Acoust Soc Am 125 (4):2189–99. doi: 10.1121/1.3089220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Schneider BA, Macdonald E, Pass HE, and Brown S. 2007. “Temporal jitter disrupts speech intelligibility: a simulation of auditory aging.” Hear Res 223 (1–2):114–21. doi: 10.1016/j.heares.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Poth EA, Boettcher FA, Mills JH, and Dubno JR. 2001. “Auditory brainstem responses in younger and older adults for broadband noises separated by a silent gap.” Hear Res 161 (1–2):81–6. [DOI] [PubMed] [Google Scholar]
- Pressnitzer D, and Patterson RD. 2001. “Distortion products and the pitch of harmonic complex tones” In Proceedings of the 12th International Symposium on Hearing: Physiological and Psychophysical Bases of Auditory Function, edited by Breebaart DJ, Houtsma AJM, Kohlrausch A, Prijs VF and Schoonhoven R, 97–104. The Netherlands: Shaker. [Google Scholar]
- Purcell DW, John SM, Schneider BA, and Picton TW. 2004. “Human temporal auditory acuity as assessed by envelope following responses.” J Acoust Soc Am 116 (6):3581–93. [DOI] [PubMed] [Google Scholar]
- Rajan R, and Cainer KE. 2008. “Ageing without hearing loss or cognitive impairment causes a decrease in speech intelligibility only in informational maskers.” Neuroscience 154 (2):784–95. doi: 10.1016/j.neuroscience.2008.03.067. [DOI] [PubMed] [Google Scholar]
- Ross B, Tremblay KL, and Picton TW. 2007. “Physiological detection of interaural phase differences.” J Acoust Soc Am 121 (2):1017–27. [DOI] [PubMed] [Google Scholar]
- Ruggles D, Bharadwaj H, and Shinn-Cunningham BG. 2012. “Why middle-aged listeners have trouble hearing in everyday settings.” Curr Biol 22 (15):1417–22. doi: 10.1016/j.cub.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BA, and Hamstra SJ. 1999. “Gap detection thresholds as a function of tonal duration for younger and older listeners.” J Acoust Soc Am 106 (1):371–80. [DOI] [PubMed] [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN, and Grantham DW. 1998. “Temporal processing in the aging auditory system.” J Acoust Soc Am 104 (4):2385–99. [DOI] [PubMed] [Google Scholar]
- Stuart A, and Phillips DP. 1996. “Word recognition in continuous and interrupted broadband noise by young normal-hearing, older normal-hearing, and presbyacusic listeners.” Ear Hear 17 (6):478–89. [DOI] [PubMed] [Google Scholar]
- Takahashi GA, and Bacon SP. 1992. “Modulation detection, modulation masking, and speech understanding in noise in the elderly.” J Speech Hear Res 35 (6):1410–21. [DOI] [PubMed] [Google Scholar]
- Tun PA, O’Kane G, and Wingfield A. 2002. “Distraction by competing speech in young and older adult listeners.” Psychol Aging 17 (3):453–67. [DOI] [PubMed] [Google Scholar]
- Walton J, Orlando M, and Burkard R. 1999. “Auditory brainstem response forward-masking recovery functions in older humans with normal hearing.” Hear Res 127 (1–2):86–94. [DOI] [PubMed] [Google Scholar]
- Zhu L, Bharadwaj H, Xia J, and Shinn-Cunningham B. 2013. “A comparison of spectral magnitude and phase-locking value analyses of the frequency-following response to complex tones.” J Acoust Soc Am 134 (1):384–95. doi: 10.1121/1.4807498. [DOI] [PMC free article] [PubMed] [Google Scholar]