Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited heart muscle disease characterized by progressive fibrofatty replacement of the right ventricular (RV) myocardium which may act as a substrate for ventricular arrhythmias and sudden cardiac death (SCD).1,2 The classic form of ARVC is a genetically determined cardiomyopathy caused by heterozygous or compound mutations in genes encoding proteins of desmosomes, which are specialized intercellular structures providing mechanical attachment of myocytes.3 However, there are other genetic (non-desmosomal) and non-genetic causes of the disease. Biventricular and left-dominant disease variants have been identified and have led some to use the term ‘arrhythmogenic cardiomyopathy’ (ACM) to define the broader spectrum of the disease phenotypic expressions.2–8
However, to avoid confusion of readers, in the present International Expert Report, the original designation of ARVC was maintained because the document is a critical appraisal of the 2010 International Task Force (ITF) criteria that were specifically designed to diagnose the ‘classic’ ARVC phenotype.
The current classification of ARVC includes the following clinical variants: (i) the classic ARVC phenotype, i.e. the originally reported and most common disease variant, characterized by isolated RV involvement (Figure 1); (ii) the ‘biventricular disease variants’, i.e. ‘balanced’, ‘dominant-right’ or ‘dominant-left’, characterized by the parallel, predominant RV, and predominant left ventricular (LV) involvement, respectively; and (iii) the LV phenotype characterized by isolated LV involvement (i.e. without clinically demonstrable RV involvement) (Figure 2).1–8
Figure 1.
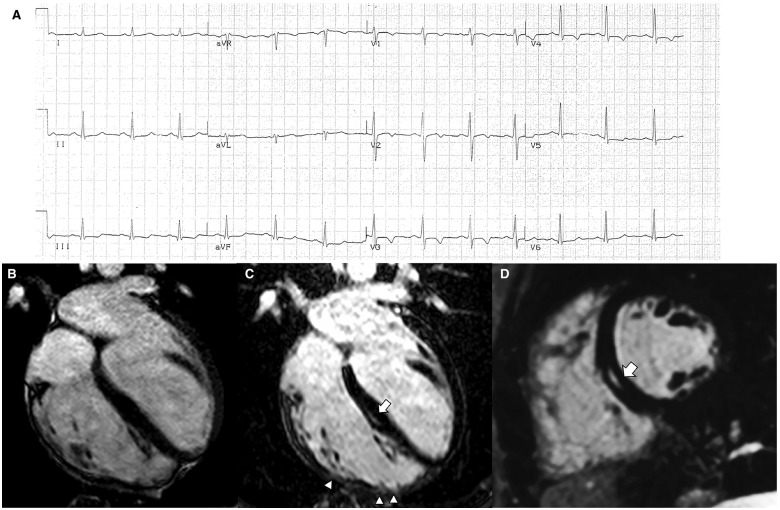
Electrocardiographic and cardiac magnetic resonance features of a representative case of right-dominant (classic) phenotypic variant of arrhythmogenic right ventricular cardiomyopathy. (A) Basal electrocardiographic showing T-wave inversion in right precordial leads (V1–V4). (B) End-diastolic frame of cine cardiac magnetic resonance sequence in long-axis four-chamber view showing a dilated right ventricle (end-diastolic volume, 127 mL/m2) with a severely reduced ejection fraction (25%). The post-contrast orthogonal images in long-axis (C) and short-axis (D) views show late gadolinium enhancement as mid-wall stria in the mid-septum (white arrow). In C, late gadolinium enhancement is also visible in the anterolateral, mid, and apical regions of the right ventricular wall, with segmental transmural involvement (white arrowheads) associated with regional dyskinesia (not shown). From De Lazzari et al.54
Figure 2.
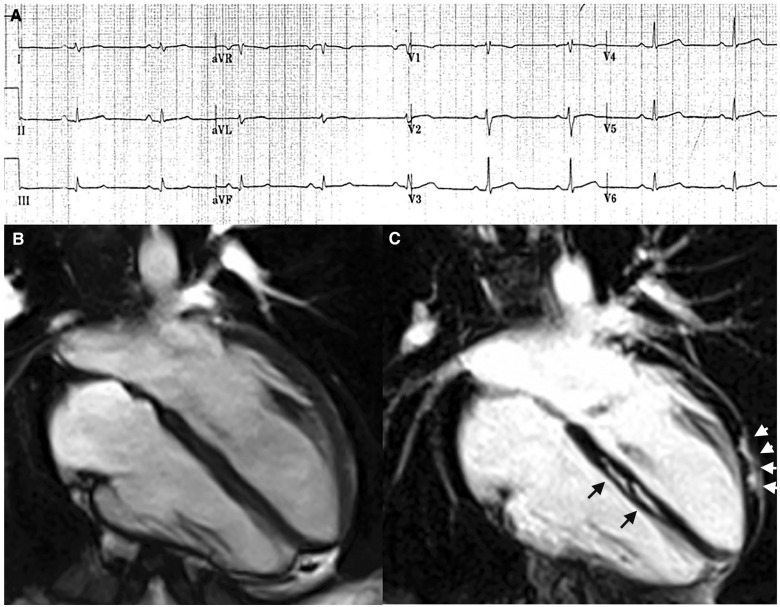
Electrocardiographic and cardiac magnetic resonance findings of a representative case of left-dominant phenotypic variant of arrhythmogenic right ventricular cardiomyopathy in a patient with a DSP-gene mutation and a history of sustained ventricular tachycardia. (A) Basal electrocardiographic showing low QRS voltages (<0.5 mV) in limb leads. (B) End-diastolic frame of cine cardiac magnetic resonance sequence in long-axis four-chamber view showing normal cavity size and function of both ventricles. (C) Post-contrast image showing myocardial fibrosis in the form of stria of late gadolinium enhancement in the epicardium of the left ventricular lateral wall (arrowheads) and mid-mural layer of the interventricular septum (arrows). From De Lazzari et al.54
In 1994, an ITF proposed criteria for diagnosis of ARVC, in the form of a qualitative scoring system which encompassed familial, electrocardiographic, arrhythmic, morpho-functional, and disease features.9 The ITF criteria were revised in 2010 by international consensus with the intention to improve diagnostic accuracy, by providing quantitative criteria for diagnosing structural and functional RV abnormalities, improvement of electrocardiographic criteria, and adding molecular genetic criteria (Supplementary material online, Table S1).10
Clinical experience with the 2010 ITF diagnostic score system has identified limitations on the use of the criteria, potentially resulting in disease misdiagnosis.11,12 The following problems in the use of current ITF criteria have been identified: (i) overdiagnosis due to the inclusion of molecular genetic findings in the diagnostic criteria, misinterpretation of electrocardiographic (ECG) and imaging findings, and misdiagnosis with other diseases mimicking the ARVC phenotype; (ii) underdiagnosis due to absence of cardiac magnetic resonance (CMR) tissue characterization findings.
In recent years, there have been evolving indications for the clinical and imaging tests for reaching definitive diagnosis of ARVC.13–15 Due to technological advances and increased experience in the interpretation of structural, functional and tissue characterization by contrast-enhanced CMR this has become an important imaging technique for the diagnosis of ARVC.14 In the era of CMR, some diagnostic tests have been abandoned because of the non-specific and limited accuracy, while others have been reserved for selected cases because of the invasive nature and the risk of serious complications. Since both the 1994 and 2010 guidelines were developed to diagnose the original right-dominant disease phenotype they did not include specific criteria for diagnosing LV involvement and the more recently recognized left-sided phenotypic variants.7,8 Moreover, peculiarities of diagnosis in the paediatric population, which represents approximately one-sixth of the overall ARVC-population, were not addressed.16
The present international expert report is not intended to redesign the 2010 ITF diagnostic criteria, which in the general view of the authors are still valid and do not need substantial changes. However, the increasing risk of misdiagnosis resulting from the inappropriate use of the criteria has prompted this international expert document aimed to critically review the clinical performance and highlight the potential limitations of current criteria, to propose some solutions for a better clinical use and to identify potential areas of improvement, with particular reference to diagnosis of left-sided phenotypes and identification of early disease in the paediatric population.
Overview of the 2010 diagnostic criteria
(See Supplementary material online, Text).
Current diagnostic criteria: a critical appraisal
The following sections of the document focus on the critical evaluation of each group of current diagnostic criteria and provide key suggestions for improving their use in the clinical practice.
Molecular genetics
Mutations in the genes encoding desmosomal proteins play a key role in the pathogenesis of fibrofatty replacement of the myocardium and the development of the disease phenotype.2,3 Pooled data from major studies on molecular genetic screening for desmosomal gene mutations showed that the overall rate of successful genotyping in patients meeting the ITF diagnostic criteria is approximately 50%.17,18 The most common mutant gene is PKP2 (10–45%), followed by DSP (10–15%), DSG2 (7–10%), and DSC2 (2%).19–21 Screening for non-desmosomal genes marginally increases the rate of detection of gene mutations, even though some mutations in specific genes such as TMEM43 p.P358L22 and PLN p.R14del23 can be highly prevalent in certain populations because of a founder effect (Supplementary material online, Table S2). Compound/digenic heterozygosity has been identified in up to 25% of patients and has been reported to account for both phenotypic variability and more malignant life-time arrhythmic outcome (‘dose-effect’).24–26
Different from all other forms of cardiomyopathy, the ARVC diagnostic criteria include the presence of a pathogenic variant in ARVC related genes as a major criterion to establish the diagnosis.10 However, it has not been defined which mutations have sufficient evidence to be considered as disease-causing and conferring pathogenicity to a variant can be challenging. Moreover, a negative genetic test does not exclude the possibility that the phenotype is due to a mutation in an unknown gene or that the molecular genetic screening technique does not detect all disease-causing variants, including large deletions or duplications.27
An increasingly emerging problem with the use of the current ITF criteria is that the incorrect classification of a desmomosal-gene variant as ‘pathogenic’ (major criterion) or the identification of a pathogenic variant in a gene with insufficient evidence for disease causation may lead to a (mis)-diagnosis of ARVC in probands who otherwise may not fulfil the ITF criteria for a (definite) phenotypic diagnosis (see Supplementary material online, Text).
Key points
The limitations of current understanding of the genetic basis of ARVC and the high genetic noise due to frequent disease-associated genetic variants both in the normal population and other cardiomyopathies are associated with the risk of misdiagnosis if molecular genetic results are integral part of the diagnostic scoring system.
According to the general recommendations for molecular genetic testing in inherited cardiomyopathies,28 genotyping is indicated to identify a pathogenic or likely pathogenic mutation in a proband who already fulfils phenotypic diagnostic criteria for ARVC, and thus to apply mutation-specific cascade genetic testing for detection of gene carriers among family members.
Mutation-specific genetic testing is recommended for family members following the identification of a pathogenic or likely pathogenic mutation in the proband with a clinical diagnosis of definite ARVC, in order to identify genetically affected individuals at a preclinical phase.
Genotyping to achieve a diagnosis in a patient with borderline phenotypic manifestations may be considered in selected cases provided that the results are interpreted by experts on the disease molecular genetics because the high prevalence of variants of uncertain significance may make the genotyping results more confounding than confirming.
Genetic family screening can be also indicated for arrhythmic risk stratification purposes. In fact, compound/digenic heterozygosity of ARVC-causing desmosomal-gene variants predicts a more serious arrhythmic outcome because of a gene dose-effect.
It is important to screen the entire panel of disease-related genes including specific non-desmosomal genes, if founder mutations in these genes are present in specific geographical areas, such as TMEM43 in Newfoundland and PLN in the Netherlands, or when ancestry suggests a relation with these specific geographical areas.
Myocardial tissue characterization
Invasive tissue characterization by transvenous endomyocardial biopsy (EMB) has been part of the diagnostic evaluation of ARVC since 1994.9 This technique offers the potential for an in vivo histologic tissue characterization with demonstration of the hallmark disease lesion, i.e. the loss of myocardium with fibrofatty replacement, that has been considered the gold standard for the clinical diagnosis of ARVC (‘major’ diagnostic criterion).29 Fatty infiltration of the RV myocardium is not specific for ARVC, being reported in normal human hearts, in the elderly and obese people.30 In this regard, the revised ITF criteria introduced the concept that the presence of fibrosis is also essential and provided quantitative parameters for histopathologic evaluation of EMB samples, focusing on the severity of myocyte loss and fibrosis rather than on fatty infiltration of myocardium.10 The diagnosis of ARVC that is achieved for the first time at surgery or autopsy also identifies probands and should enable cascade family screening. The diagnostic tissue characterization can be made by histopathology of either biopsy samples taken during cardiac surgery performed for other reasons or in full hearts obtained at postmortem or cardiac transplantation. The histologic evidence of fibrofatty replacement of the ventricular myocardium with a subepicardial–mid-mural distribution, or with transmural involvement in the absence of obstructive atherosclerotic plaques within the corresponding coronary artery, is a diagnostic criterion for ARVC. An immunohistochemical analysis of EMB samples for the diagnosis of ARVC has been developed with the aim of identifying changes in the localization of desmosomal proteins31 (see Supplementary material online, Text).
Right ventricular endocardial voltage mapping (EVM) is an imaging technique which may be of added value for the diagnosis of ARVC since it has the potential to identify and quantify RV regions of electroanatomic scar with low-amplitude electrical signals, typically showing fractionation, double potentials, or conduction delay.32–34 Right ventricular EVM is an invasive, expensive and highly operator-dependent technique with a significant risk of inaccurate interpretation of low-voltage recordings in areas of normal myocardium due to suboptimal catheter contact. Moreover, a complete EVM should be also obtained from the epicardial side of RV, which implies a pericardial puncture not justifiable solely for diagnostic purposes.
Key points
Endomyocardial biopsy is not indicated as a routine diagnostic test for ARVC. It should be reserved for selected patients such as probands with a sporadic form of ARVC and predominant LV involvement, in whom the final diagnosis depends on histologic exclusion of phenocopies such as chronic myocarditis, sarcoidosis or other heart muscle disorders.
In addition to histopathologic examination of myocardial samples, immunohistochemical analysis for evaluation of desmosomal protein localization within the intercalated disk may provide added diagnostic value.
Right ventricular EVM is not recommended solely for diagnostic purposes. It should be reserved for selected ARVC patients undergoing cardiac catheterization for arrhythmia management and performed in electrophysiologic laboratories with a large experience in electroanatomic mapping.
Endocardial voltage mapping-guided EMB of the RV free wall is not performed in the majority of interventional labs and cannot be proposed for routine diagnosis.
Global or regional dysfunction and structural alterations
Relevant structural and functional abnormalities detectable by imaging techniques include ventricular dilatation, reduced RV ejection fraction, regional wall motion abnormalities, and fibrofatty myocardial replacement.2,3,9,10 Contrast cine-ventriculography, echocardiography, and CMR are the standard imaging techniques for the diagnosis of ARVC.
Cardiac magnetic resonance has become the gold standard method for assessing ventricular volumes, systolic function, and regional wall motion, as well as to characterize myocardial tissue composition. Due to the spatial resolution of CMR voxels and unlimited imaging planes that can be reconstructed, CMR offers the potential to optimally evaluate dilatation/dysfunction, regional wall motion abnormalities, and structural changes of the RV.13–15,35 Cine CMR sequences provide an accurate assessment of RV volume and systolic function. However, these parameters have low sensitivity and specificity for the diagnosis of ARVC35,36 and significant interobserver variability in the interpretation of segmental contraction analysis of the RV free wall has been reported.37–39
Tissue characterization findings by CMR (fibrosis, fatty infiltration, and fibrofatty scar) were not included in the 2010 ITF criteria because of limited experience, difficulty in the interpretation, and low specificity.10 However, recent studies demonstrated the utility of combined regional wall motion assessment and tissue characterization by CMR for the diagnosis of ARVC.4 The best accuracy (98%) was present when wall motion alterations and pre-/post-contrast signal abnormalities [including LV fat infiltration and late gadolinium enhancement (LGE)] were considered together.35
The original concept of ‘triangle of dysplasia’, which referred to the most commonly affected regions of the RV, has evolved to the current perspective of a ‘quadrangle of ARVC’ which also includes the LV infero-lateral wall, which is the most frequently involved LV region. In the early stages of ARVC, the subtricuspid/peritricuspid area and the LV infero-lateral wall may be the only affected regions.4,5,40,41 Thus, LGE imaging technique may increase diagnostic sensitivity for ARVC, even in its early stage, by identifying a myocardial scar in the LV infero-lateral wall, which would otherwise remain undetectable by echocardiography/angiography because it is confined to the subepicardial–mid-myocardial layers and may not be sufficiently large to cause a systolic wall motion abnormality (Figure 2).
Inclusion of criteria for characterization of myocardial fibrofatty replacement offers the potential (i) to enhance the accuracy of the interpretation of RV wall motion abnormalities (which has a high intra-interobserver variability and operator dependence) by demonstrating the underlying myocardial lesion; (ii) to increase the sensitivity for identifying forms of biventricular ARVC which are more easily diagnosed by CMR-imaging with demonstration of the segmental subepicardial LGE in the LV wall which may be the only imaging feature of left-dominant phenotypic variants of ARVC.
Because of its high negative predictive value, contrast-enhanced CMR study has the potential to become the ‘rule-out’ imaging test for evaluation of structural and functional ventricular abnormalities of ARVC (see Supplementary material online, Text).
Key points
Considering the technological advances and improvement of interpretation of CMR tissue characterization images, acquisition and image evaluation of biventricular myocardial fibrosis, and intramyocardial fatty tissue, contrast-enhanced CMR is recommended for definitive diagnosis and better characterization of the disease phenotypic variant.
Transthoracic two-dimensional echocardiography is indicated as part of the initial evaluation of a patient with suspected ARVC. The availability of echocardiographic findings at initial evaluation is important in view of the subsequent serial imaging follow-up. Echocardiographic evaluation should be repeated at 1–3 years intervals, based on the age, genetic status, and clinical features.
To avoid misdiagnosis, demonstration of consistent structural and functional ventricular abnormalities is needed to reach a definite diagnosis in a proband. Instead, overt morpho-functional ventricular alterations may be not detectable in affected family members, because clinical manifestations are more subtle and may occur later over the disease course due to the incomplete and age-dependent penetrance and the variable phenotypic expression.
Given the large variation of geometry and contraction of the normal RV, the presence of both regional wall motion abnormalities and LGE should be confirmed in two orthogonal planes (e.g. horizontal long-axis and short-axis views).
Cardiac magnetic resonance should ideally be performed in high volume centres with particular experience and expertise in imaging acquisition and interpretation of ARVC features.
Because CMR is expensive and time-consuming due to long duration of data acquisition and analysis time, follow-up with systematic serial CMR studies is unpractical. A repeat CMR study should be considered in patients with a diagnosis of definite ARVC who develop over time significant worsening of clinical symptom, ECG abnormalities, arrhythmic events, or echocardiographic findings.
Right ventricular angiography is not of additional diagnostic value and should be reserved to patients in whom EMB is planned. Cardiac catheterization is indicated when oxygen saturation measurement is required for differential diagnosis between ARVC and congenital heart diseases with a left to right shunt.
Arrhythmias
The spectrum of ventricular arrhythmias in ARVC ranges from isolated premature ventricular complexes (PVCs) to sustained ventricular tachycardia (VT) or ventricular fibrillation leading to cardiac arrest.1,4,5,18,42–44 The severity of the arrhythmia is variable between individual patients and during the course of the disease.45,46 The assessment of the morphology of the arrhythmic QRS complexes on 12-lead ECG may allow identification of the ventricular site of origin and the mechanism of the arrhythmia.
According to the 2010 ITF diagnostic criteria, the morphology of VT has an impact on diagnosis.10 Ventricular tachycardia with a left bundle branch block (LBBB)/inferior axis suggesting right (or left) ventricular outflow tract origin is considered a ‘minor’ diagnostic criterion because of its low specificity which may lead to misdiagnosis of ARVC in patients with idiopathic right ventricular outflow tract (RVOT)-VT. However, VT with an LBBB pattern and superior or indeterminate axis suggesting RV free wall origin is more specific for ARVC and this is classified as a ‘major’ diagnostic criterion. VT with a right bundle branch block (RBBB) morphology may occur as a manifestation of additional or predominant LV involvement.10
Studies with 24-h Holter monitoring have shown that most ARVC patients have frequent PVCs, either isolated or coupled, with a mean burden >500 PVC/24 h.47 Current ITF criteria consider the number of PVCs per 24 h without addressing the morphology of the ectopic QRS. This represents a limitation because premature ventricular beats originating from the inferior RV wall have greater specificity for ARVC than the number of premature ventricular beats exceeding 500/24 h or premature ventricular beats from the RVOT. It is diagnostically relevant to record PVCs on 12 ECG leads by exercise testing or 12-lead 24-h Holter monitoring.
The electrophysiologic study has a limited role in the diagnosis of ARVC. The test can provide information regarding the inducibility by programmed ventricular stimulation of one or more VTs with different rates and/or morphologies. This may be useful in differentiating ARVC from idiopathic RVOT-VT which is a benign and non-familial arrhythmic condition characterized by a single morphology (LBBB/inferior axis) and non-inducibility by programmed ventricular stimulation.48,49 Addition of RV EVM may be of incremental diagnostic value for differential diagnosis with idiopathic RVOT-VT, provided that care is taken to obtain adequate sampling and ensure optimal catheter contact using pacing threshold assessment or direct contact force measurements to avoid inappropriate misdiagnosis from low-amplitude recordings. Endocardial unipolar recordings with a large field of view may also indentify the presence of epicardial scar, thus preventing the use of a direct pericardial approach to obtain bipolar epicardial voltage measurements.50
Key points
Twelve-lead 24-h Holter monitoring is indicated to assess the morphology of ventricular arrhythmia, which may suggest the ventricular site of origin.
Electrophysiologic study with programmed ventricular stimulation and RV EVM are not recommended as integral part of the routine diagnostic evaluation of patients with ARVC. Both studies should be limited to selected patients requiring an invasive electrophysiologic evaluation to distinguish ARVC-related VT from idiopathic RVOT tachycardia.
Repolarization and depolarization electrocardiographic abnormalities
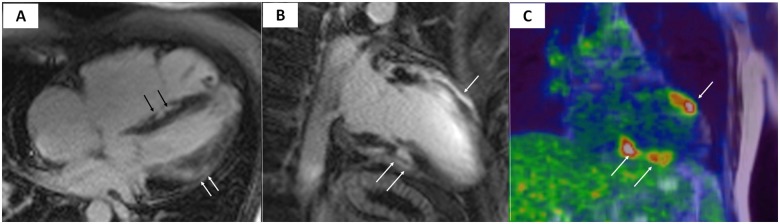
Twelve-lead ECG is a valuable diagnostic test in ARVC and records repolarization and/or depolarization abnormalities in up to 90% of patients with ARVC.51–53 Negative T waves in the right precordial leads are the most common finding (Figure 1).10 Low QRS voltages (<0.5 mV) in the limb leads are frequently observed in ARVC patients with fibrosis/LGE of the LV as evidenced by CMR (Figure 2). Rather than an ECG marker of advanced RV disease, low QRS voltages indicate LV involvement (regardless of the RV disease severity) and reflect loss of myocardium/electrical voltages of the LV wall and replacement by electrically inert fibrofatty scar tissue.54–58
The ECG abnormalities resulting from delayed RV activation/conduction include RBBB (usually incomplete and rarely complete), QRS fragmentation, prolongation of right precordial QRS duration with a delayed S-wave upstroke, terminal activation duration (TAD) ≥55 ms, and epsilon waves. The accuracy of the presence of epsilon waves as a diagnostic tool has been questioned since these discrete signals are related to ECG filtering and sampling rate, giving rise to large interobserver variability.59
Activation delay can also be detected in the form of late potentials in the terminal portion of the QRS complex by signal-averaged ECG (SAECG). The use of SAECG technique for diagnosis of ARVC in probands and family members has been abandoned by most centres because of its non-specific findings and limited diagnostic accuracy (see Supplementary material online, Text).
Key points
The presence of epsilon waves should be evaluated with caution, especially in patients without other diagnostic criteria.
A QRS delayed S-wave upstroke with TAD ≥55 ms in right precordial leads is a specific diagnostic ECG pattern, particularly if followed by negative T waves.
Low QRS voltages (<0.5 mV) in the limb leads can be an ECG marker predictive of LV involvement.
Negative T waves confined to V1 and V2 should be considered as a normal ECG variant in individuals who do not meet other diagnostic criteria.
Left-dominant variants of arrhythmogenic right ventricular cardiomyopathy
Distinctive ECG features of LV involvement in ARVC include T-wave inversion in the infero-lateral leads and low QRS voltages (<0.5 mV) in limb leads, which reflect the loss of myocardium and electrical voltages of the LV wall.55,56 Ventricular tachycardia is characteristically monomorphic, with a RBBB morphology which denotes its origin form the LV.8,60 The typical LV imaging phenotype is characterized by a ventricular remodelling pattern consisting of mild LV dysfunction and no or mild LV dilatation, in association with a significant amount of subepicardial/mid-myocardial (non-ischaemic) LGE affecting multiple LV segments (mostly the inferolateral wall regions) (Figure 2).61 The degree of systolic LV dysfunction appears related to the global extent of LGE which in advanced disease affects multiple septal and LV free wall segments.
In biventricular variants of ARVC, clinically demonstrable RV involvement is an important additional criterion for differential diagnosis with dilated cardiomyopathy (DCM). In the absence of clinically detectable RV involvement, demonstration of a pathogenic mutation of ARVC-related genes, such as DSP, FLNC, and PLN genes, may support the diagnosis of ARVC (see Supplementary material online, Text).
Key points
Criteria for diagnosis of left-sided ARVC phenotypes should include: (i) ECG changes such as low QRS voltages in limb leads and inverted T waves in the inferolateral leads; (ii) ventricular arrhythmias with a right bundle-branch block pattern; and (iii) structural and functional imaging features consistent with a ‘hypokinetic, non-dilated, and fibrotic LV’.
Clinical demonstration of some degree of electrical or structural RV involvement should be considered as an important additional criterion for diagnosis of biventricular or left-dominant phenotypes.
In patients with clinical findings suggestive of left-sided ARVC and no clinically detectable RV involvement, genetic testing for the presence of pathogenic mutations in ARVC-related genes, such as the DSP gene, can confirm the diagnosis (Supplementary material online, Table S2).
Diagnosis of arrhythmogenic right ventricular cardiomyopathy in the paediatric population
Arrhythmogenic RV cardiomyopathy is a genetically determined heart muscle disease characterized by a ‘late-onset phenotype’, which most often becomes clinically overt between the second and fourth decades of life. Clinical manifestations of the disease are very uncommon before pubertal development, whereas up to 15% of affected patients are teenagers.62,63
Arrhythmogenic RV cardiomyopathy is a progressive heart muscle disease with electrical and structural phenotypic manifestations occurring at different times during its natural history. It is noteworthy that progression of structural ventricular alterations can be preceded and predicted by ECG depolarization abnormalities.64 Whereas adult patients more often present with sustained VT, paediatric patients are more likely to experience SCD or resuscitated sudden cardiac arrest.45,63,65 Sudden cardiac death may be the first clinical manifestation of the disease, as it was reported by a study in the Veneto region of Italy, where 20% of SCD in young people and athletes were caused by previously undiagnosed ARVC.66
The diagnosis of ARVC is particularly challenging in children <14 years of age.62 Peculiarities in physiological development as well as differences in disease presentation and progression limit the use of the current criteria for diagnosis of ARVC in this age group.67 Diagnostic clinical work-up should be adapted to this patient population due to the difficulty of some testing modalities in paediatric patients and the low prevalence of manifest disease in very young children. In particular, basal ECG and ambulatory ECG monitoring can be performed at any age, while echocardiography is more easily performed after age 3 years and cardiac CMR after age 8 years (or in younger children but under anaesthesia) (see Supplementary material online, Text).
Key points
An adapted diagnostic clinical work-up using normal ECG and imaging reference values for children should be used in the paediatric population, due to the low prevalence of manifest disease, difficulty of some testing modalities and peculiarity of clinical findings in this age-group.
Invasive studies such as RV angiography and EMB should be reserved to very selected cases until all non-invasive studies have been assessed.
Molecular genetic testing for children of families affected by ARVC is recommended for early, pre-clinical identification of genetically affected individuals as well as for detection of non-genetically affected siblings who can be reassured and not further investigated.
Early detection of genetically affected children allows to establish a focused prevention strategy mostly based on life style changes, including restriction of competitive sports activity which is the most important environmental factor promoting the disease phenotypic expression.
A follow-up by non-invasive clinical evaluation of healthy gene carriers or children with unknown genotype who have a positive family history of ARVC should be performed on a regular basis after puberty (every 1–2 years) to monitor for disease onset and progression.
Phenocopies and differential diagnosis
Conditions that can mimic the ARVC phenotype (phenocopies) and enter into differential diagnosis of ARVC include primary arrhythmia conditions and structural heart muscle diseases affecting the RV, the LV, or both (Table 1).
Table 1.
Differential diagnosis of arrhythmogenic right ventricular cardiomyopathy
| Mimics of right-dominant ARVC |
| Primary arrhythmia conditions |
| Right ventricular outflow tract tachycardia |
| Brugada syndrome |
| Structural diseases |
| Congenital heart diseases (left to right shunt, Ebstein’s anomaly, Uhl’s anomaly) |
| Pulmonary artery hypertension |
| Athlete’s heart |
| Chest deformity and pericardial absence |
| Mimics of left-dominant ARVC |
| Structural diseases |
| Dilated cardiomyopathy |
| Neuromuscular cardiomyopathies (muscular dystrophies and myofibrillar myopathies) |
| Myocarditis |
| Cardiac sarcoidosis |
| Congenital ventricular aneurysms |
| Chagas’ heart disease |
Primary arrhythmia conditions
Primary arrhythmia conditions which may resemble ARVC include idiopathic RVOT-VT and Brugada syndrome (see Supplementary material online, Text).
Key points
Patients with idiopathic RVOT-VT have a normal 12-lead ECG, normal imaging tests, VT induction by isoproterenol test, and VT non-inducible by programmed ventricular stimulation (Table 2).
A septal origin of VT is significantly more often observed in idiopathic RVOT-VT than in ARVC which usually affects the RV free wall and spares the septum.
A single LBBB/inferior axis VT morphology favours the diagnosis of idiopathic RVOT-VT, while multiple VT morphologies, either spontaneous or induced, strongly suggest ARVC.68
Brugada syndrome demonstrates substantial differences from ARVC with respect to involved genes, absence of overt cardiomyopathic changes, autonomic and antiarrhythmic drug modulation of ECG abnormalities, circumstances and mechanisms of arrhythmias and outcome (Table 3).
Table 2.
Idiopathic right ventricular outflow tract tachycardia vs arrhythmogenic right ventricular cardiomyopathy-related ventricular tachycardia
| RVOT-VT | ARVC | |
|---|---|---|
| Disease inheritance | No | Yes (AD) |
| Genetic defect | No | Desmosomal genes-mutations |
| Symptoms | Palpitations, pre-syncope | Palpitations, syncope, cardiac arrest |
| ECG abnormalities | Normal | Right precordial T-wave inversion, ε waves, right precordial QRS prolongation with delayed S-wave upstroke, and terminal activation delay (>55 ms), low QRS voltages |
| Imaging | Normal | Structural and functional RV abnormalities |
| Biopsy | Normal | Fibrofatty myocardial replacement |
| Morphology of VT | NSVT repetitive monomorphic; LBBB usually with inferior QRS axis | LBBB Usually with left deviation |
| Multiple VT morphologies | No | Yes |
| VT mechanism | Enhanced automaticity and triggered activity | Scar-related re-entry |
| Typical site of VT origin | Anteroseptal RVOT | Non-septal RVOT |
| RV EVM | Normal | Low-voltage areas |
| Programmed ventricular stimulation | Non-inducible VT | Inducible VT |
AD, autosomal dominant; EVM, endocardial voltage mapping; LBBB, left bundle branch block; NSVT, non-sustained ventricular tachycardia; RV, right ventricle; RVOT, right ventricular outflow tract; TWI, T-wave inversion; VF, ventricular fibrillation; VT, ventricular tachycardia.
Table 3.
Brugada syndrome vs arrhythmogenic right ventricular cardiomyopathy
| Brugada syndrome | ARVC | |
|---|---|---|
| Age of presentation (years) | 30–40 | 15–30 |
| Gender | M > F (8:1) | M > F (3:1) |
| Distribution | World-wide (predominantly Southeast Asia) | World-wide |
| Inheritance | AD | AD (AR) |
| Predominant pathogenetic genes | SCN5A gene | Desmosomal genes |
| Typical symptoms | Syncope, cardiac arrest Especially nocturnal | Palpitations, syncope, cardiac arrest |
| ECG repolarization | Right precordial ST elevation and TWI | Right precordial TWI |
| ECG depolarization | RBBB/LAD | Right precordial QRS prolongation, ε waves |
| AV conduction times | Prolonged PR/HV interval | Normal |
| Variability of ECG changes | Dynamic | Fixed |
| Imaging | Normal (or mild RVOT dilatation) | Structural RV (and LV) abnormalities with global and regional dilation and dysfunction |
| Biopsy | Normal | Fibrofatty replacement |
| Ventricular arrhythmias | Polymorphic VT, VF | Monomorphic VT, VF |
| Mechanism of VT | Phase 2 re-entry or local micro-reentry | Scar-related macro-reentry |
| Programmed ventricular stimulation | Inducibility of VF | Inducibility of monomorphic VT or VF |
| Natural history | Sudden death | Sudden death, heart failure |
AD, autosomal dominant; AR, autosomal recessive; AV, atrioventricular; LAD, left axis deviation; LV, left ventricle; RBBB, right bundle branch block; RV, right ventricle; RVOT, right ventricular outflow tract; TWI, T-wave inversion; VF, ventricular fibrillation; VT, ventricular tachycardia.
Structural heart muscle diseases mimicking right-dominant arrhythmogenic right ventricular cardiomyopathy
A number of structural conditions may mimic clinical features of right-dominant ARVC including congenital heart diseases, pulmonary artery hypertension, and athlete’s heart (Figure 3 and Table 4) (see Supplementary material online, Text).
Figure 3.
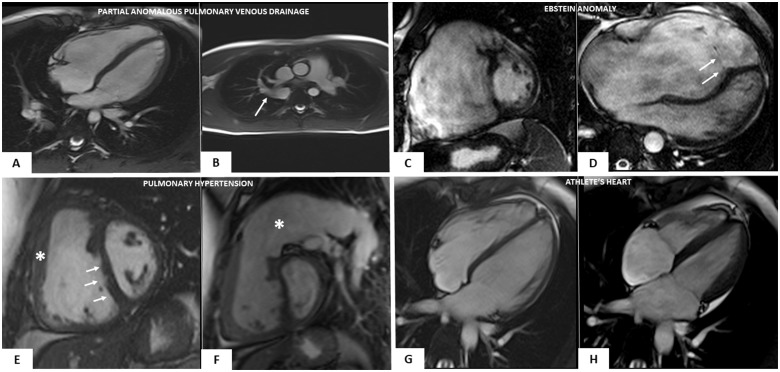
Cardiac magnetic resonance features of heart diseases mimicking right-dominant (classic) phenotypic variant of arrhythmogenic right ventricular cardiomyopathy. Partial anomalous pulmonary vein drainage (A and B): end-diastolic frame of cine cardiac magnetic resonance sequence in long-axis four-chamber view showing moderate right ventricular dilatation (A); cine sagittal view showing the anomalous drainage of the right pulmonary vein in the azygos vein (white arrow) (B). Ebstein anomaly (C and D): end-diastolic frame of cine cardiac magnetic resonance sequence in short-axis view showing a severe right ventricular enlargement due to a large ventricular ‘atrialization’ (C); end-diastolic frame of cine cardiac magnetic resonance sequence in four-chamber view showing a significant apical displacement of the septal leaflet of the tricuspid valve (white arrows) (D). Arterial pulmonary hypertension (E and F): end-diastolic frames of cine cardiac magnetic resonance sequence in short-axis view showing increase of the right ventricular wall thickness (white asterisk) (E), flattening of the interventricular septum (white arrows) (E), and massive pulmonary artery dilatation (white asterisk) (F). Athlete’s heart (G and H): end-diastolic (G) and systolic (H) frames of cine cardiac magnetic resonance sequence in four-chamber view evidencing biventricular dilatation (end-diastolic volume 122 mL/m2) and normal systolic function (ejection fraction 64%), in the absence of wall motion abnormalities (not shown).
Table 4.
Causes of right heart dilatation in adult congenital heart disease
| Left-to-right shunt |
| Atrial septal defects |
| Partial anomalous pulmonary return |
| Extracardial shunts (pulmonary/systemic arteriovenous connections) |
| Coronary artery fistula (to the coronary sinus/right atrium/right ventricle) |
| Valvular dysfunction |
| Tricuspid valve regurgitation |
| Ebstein’s anomaly |
| Pulmonary valve regurgitation |
| Post-operative valvular insufficiency after tetralogy of Fallot repair |
| Myocardial malformation |
| Uhl’s anomaly |
| Ventricular aneurysm/diverticulum |
Chest deformity such as pectus excavatum or carinatum and pericardial absence may cause ECG changes and echocardiographic RV abnormalities mimicking ARVC.69
Key points
Cardiac remodelling due to volume overload secondary to a congenital heart disease consists of global RV dilatation and dysfunction without regional wall motion abnormalities that allow the differential diagnosis with the imaging findings of ARVC characterized by both global and regional RV dilatation and dysfunction with prominent regional akinesia and/or dyskinesia.
RV abnormalities of pulmonary artery hypertension are the result of the elevation of the pulmonary artery pressure that is usually normal or reduced in ARVC.
The clinical phenotype of athlete’s heart differs from that of ARVC with regard to the absence of fibrofatty myocardial replacement which is clinically demonstrable as (i) regional-dyskinesia or bulging on echo and CMR; (ii) RV LGE on CMR; (iii) ECG depolarization abnormalities; and (iv) replacement type-fibrosis (and adiposis) at EMB70 (Table 5).
Table 5.
Athlete’s heart vs. arrhythmogenic right ventricular cardiomyopathy
| Athlete’s heart | ARVC | |
|---|---|---|
| Family history | No | ARVC or SCD |
| ECG abnormalities | Training-related ECG changes such as incomplete right bundle branch block increased in QRS voltages and early repolarization. Right precordial early repolarization variant with negative T-wave preceded by J-point/ST-segment elevation. | Right precordial T-wave inversion, ε waves, right precordial QRS prolongation with delayed S-wave upstroke and terminal activation delay (>55 ms), low QRS voltages |
| Symptoms | No | Palpitations, syncope, cardiac arrest |
| RV dilation | Yes (mainly main RV body) | yes (mainly RVOT) |
| RV/LV dilation ratio | RV/LV <1 | RV/LV >1 |
| Global RV dysfunction | No (or mild) | Yes |
| Regional RV wall motion abnormalities | No | A-dyskinesia; bulging |
| LGE at cardiac magnetic resonance | No (or only junctional) | RV and/or LV (non-ischaemic pattern) |
| Ventricular arrhythmias | No | Yes |
LGE, late gadolinium enhancement; LV, left ventricle; RV, right ventricle; RVOT, right ventricular outflow tract; SCD, sudden cardiac death.
Structural heart muscle diseases mimicking left-dominant arrhythmogenic right ventricular cardiomyopathy
Structural conditions mimicking clinical features of left-dominant ARVC include DCM, cardiac involvement in genetic neuromuscular disorders, myocarditis, sarcoidosis, congenital ventricular diverticulum/aneurysm, and Chagas’ disease (see Supplementary material online, Text).
Key points
At variance with left-sided ARVC, DCM shows more severe LV dilation and dysfunction which are unrelated to the global extent of ventricular scar tissue as evidenced by LGE on CMR (Figure 4).
At variance with left-sided ARVC, DCM shows scarce propensity to life-threatening ventricular arrhythmias that occur late during the disease course and are related to the severity of LV systolic dysfunction (Table 6).
Cardiomyopathy may develop in patients with various genetic neuromuscular disorders and may occur before, during, or after clinical evidence of skeletal muscle dysfunction. While the myocardial involvement is most often part of the spectrum of pathologic features of the disease, ventricular systolic dysfunction and/or rhythm and conduction abnormalities may dominate the clinical presentation or may represent the only phenotypic manifestation of the neuromuscular gene defect (Figure 5).72
Differential diagnosis between the cardiomyopathy occurring in the context of a neuromuscular disease and ARVC relies on recognition of specific phenotypic features of the associated musculo–skeletal involvement.
Identification of isolated neuromuscular cardiomyopathy (without skeletal muscle involvement) requires demonstration of the specific genetic defect by molecular testing.
Differential diagnosis between acute myocarditis or post-myocarditis scar and ARVC requires accurate medical history, clinical family screening, demonstration of ECG and imaging RV involvement, and molecular genetic testing.
Imaging criteria to differentiate post-myocarditis scar from inherited cardiomyopathy have not been defined. Subepicardial/mid-myocardial LV LGE at CMR may suggest priori myocarditis but is not diagnostic and careful family history and screening are needed to exclude left-sided ARVC.
The diagnosis of cardiac sarcoidosis in patients with multisystem involvement relies on consistent clinical and imaging features of the disease (Figure 6) in the presence of histologic evidence of non-caseating epithelial cell granulomas in one or more organs.
Isolated cardiac sarcoidosis is conclusively diagnosed on the basis of EMB, possibly imaging-guided by positron emission tomography or CMR.73,74
The segmental nature of congenital aneurysms with associated normal ventricular myocardium and the predominantly endocardial pattern of myocardial fibrosis are not consistent with the diagnosis of ARVC.
A typical history and a positive Chagas serology allow to differentiate cardiac Chagas disease from ARVC.
Figure 4.
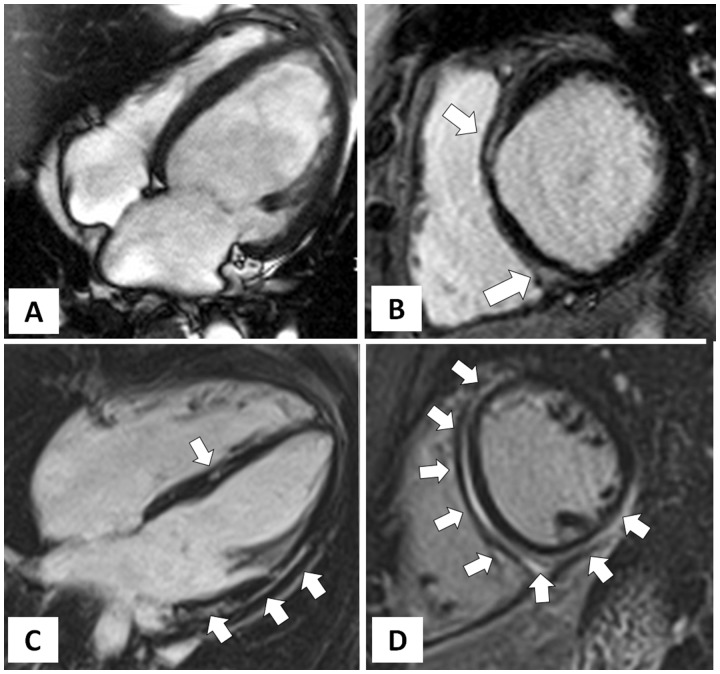
Cardiac magnetic resonance features of dilated cardiomyopathy vs. left-dominant phenotypic variant of arrhythmogenic right ventricular cardiomyopathy. Dilated cardiomyopathy (A and B): end-diastolic frame of cine cardiac magnetic resonance sequence in four-chamber view showing severe left ventricular dilatation (A) with severe systolic dysfunction (not shown); post-contrast T1 inversion recovery sequence in short-axis view showing limited mid-wall late gadolinium enhancement in the anterior interventricular septum and infero-septal junction involving the adjacent infero-basal wall (white arrow) (B). Left-dominant arrhythmogenic right ventricular cardiomyopathy (C and D): post-contrast T1 inversion recovery sequence in four-chamber view showing a non-dilated (C) and hypokinetic (not shown) left ventricle; post-contrast T1 inversion recovery sequence in short-axis view showing a large amount of late gadolinium enhancement involving the interventricular septum and both anterior and inferolateral left ventricular walls (C and D).
Table 6.
Dilated cardiomyopathy vs. left-dominant arrhythmogenic right ventricular cardiomyopathy
| Dilated cardiomyopathy | Left-dominant ARVC | |
|---|---|---|
| Inheritance | ≤35% (AD) | >50% (AD, AR) |
| Predominant genetic background | Mutations of genes encoding for cytoskeleton, muscular sarcomere, and nuclear envelope proteins | Mutations of genes encoding for desmosomal proteins, PLN, or FLN-C |
| Main clinical manifestations | Heart failure, cardiac arrest, palpitations | Palpitations, syncope, cardiac arrest |
| ECG abnormalities | Left ventricular hypertrophy with a strain pattern of ST-segment; left bundle branch block | Low QRS voltages in limb leads; negative T waves in lateral leads; negative T waves in right precordial leads (biventricular form) |
| Echocardiography and cardiac magnetic resonance imaging findings | Dilated and hypokinetic LV with no or patchy non-ischaemic (mid-myocardial) LGE (septum) | Non-dilated and hypokinetic LV with large amount of non-ischemic (subepicardial) LGE (inferolateral LV wall) |
| Regional wall motion abnormalities (uncommon). | Regional wall motion abnormalities (common). | |
| Systolic LV dysfunction unrelated to the global extent of LGE | Systolic LV dysfunction related to the global extent of LGE | |
| EMB features | Non-specific myocardial abnormalities | Fibrofatty myocardial replacement |
| Types of ventricular arrhythmias | PVBs and NSVT (RBBB pattern); sustained VT(uncommon); VF | PVBs, NSVT, and monomorphic sustained VT (RBBB pattern; both LBBB and RBBB patterns in biventricular form); VF |
| Mechanism of VT | Scar-related or functional re-entry (branch to branch re-entry) | Scar-related re-entry |
| Most common site of VT origin | Intramural septum | Subepicardial infero-lateral LV free wall |
AD, autosomal dominant; AR, autosomal recessive; EMB, endomyocardial biopsy; LBBB, left bundle branch block; LV, left ventricle; NSVT, non-sustained ventricular tachycardia; PVBs, premature ventricular beats; RBBB, right bundle branch block; RV, right ventricle; RVOT, right ventricular outflow tract; TWI, T-wave inversion; VF, ventricular fibrillation; VT, ventricular tachycardia.
Figure 5.
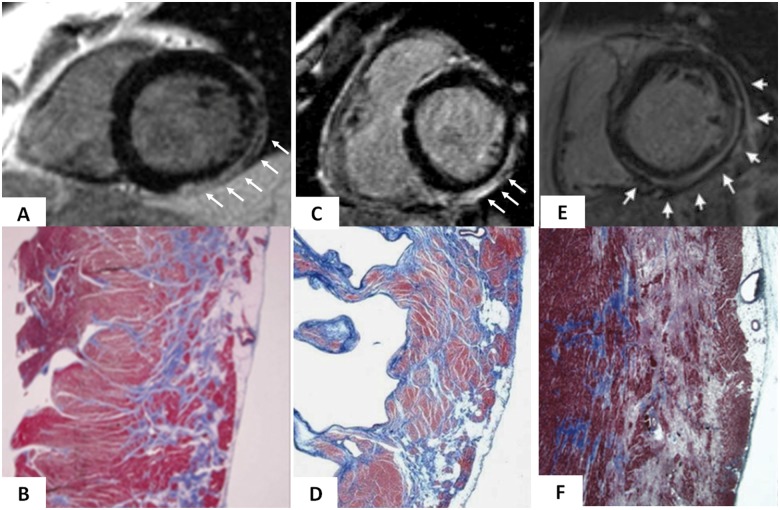
Cardiac magnetic resonance features and histopathologic findings of non-ischaemic left ventricular scar of different aetiologies. Muscular dystrophy (A and B): post-contrast T1 inversion recovery sequence in short-axis view showing a subepicardial stria of late gadolinium enhancement in the left ventricular wall (white arrows) (A); corresponding panoramic histopathologic view of the inferolateral left ventricular wall showing replacement-type fibrosis confined to the outer-mid layer of the musculature (B). Modified from Yilmaz et al.71 Chronic myocarditis (C and D): post-contrast T1 inversion recovery sequence in short-axis view showing subepicardial late gadolinium enhancement of the inferolateral left ventricular wall (C); corresponding panoramic histopathologic view of the inferolateral left ventricular wall showing extensive fibrous tissue replacement in the subepicardial layer of the musculature (D). From Yilmaz et al.71 Desmosomal gene-related, left-sided arrhythmogenic right ventricular cardiomyopathy (E and F): post-contrast T1 inversion recovery sequence in short-axis view showing subepicardial late gadolinium enhancement of the infero-lateral left ventricular wall in a DSP-gene mutation carrier (E). Panoramic histopathologic view showing myocardial replacement of the outer layer of the infero-lateral left ventricular wall in a sudden cardiac death victim carrying a DSP-gene mutation (F). From Zorzi et al.44
Figure 6.
Magnetic resonance and cardiac positron emission tomography features of cardiac sarcoidosis. Post-contrast T1 inversion recovery sequence in four-chamber view showing right ventricular dilatation and late gadolinium enhancement of the interventricular septum (black arrows) and subepicardial lateral left ventricular wall (white arrows) (A). Topographic concordance between the epicardial spot of late gadolinium enhancement involving the anterior (B, single white arrow) and inferior (B, white arrows) left ventricular regions and the areas of fludeoxyglucose uptake on positron emission tomography (C, white arrows pointing to yellow–red areas).
Proposal of an aetiologic classification of arrhythmogenic cardiomyopathies
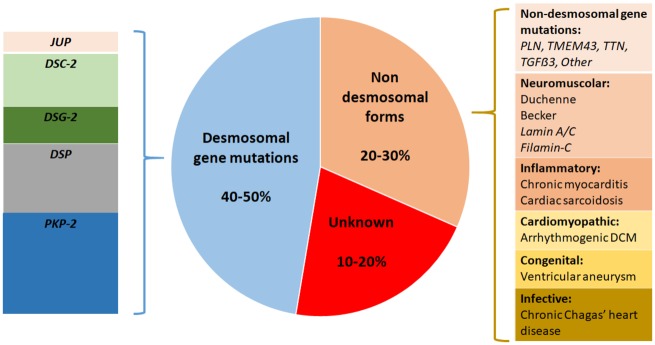
In about 50% of patients, ARVC is a genetic disease caused by a mutation of desmosomal gene. A minority of affected patients may have defective non-desmosomal genes. A phenotype similar to ARVC may also occur in other genetic cardiomyopathies, cardiocutaneous syndromes, or neuromuscular disorders.2,3 Furthermore, a sizeable proportion of patients have non-genetic diseases with a phenotype resembling ARVC and characterized by the distinctive propensity to ventricular arrhythmias which extends beyond the severity of systolic ventricular dysfunction being strongly related to the large amount of myocardial fibrosis which is an independent arrhythmogenic risk factor.
By analogy with current classifications of other cardiomyopathies such as hypertrophic and dilated cardiomyopathy and in keeping with the 2019 HRS Expert Consensus Statement on arrhythmogenic cardiomyopathy,75 it is appropriate to propose a disease classification which under the large umbrella of ‘arrhythmogenic cardiomyopathy’, comprises a spectrum of conditions of different aetiologies involving the RV, the LV or both, either genetic or non-genetic, whose common denominator is the prominent non-ischaemic ventricular myocardial scarring and the scar-related ventricular arrhythmias (Figure 7). All conditions manifesting with the ACM phenotype are associated with a distinctively higher risk of SCD because myocardial fibrosis acts as a substrate of malignant ventricular arrhythmias. Accordingly, in patients affected by ACM, either genetic or non-genetic, the implantation of an ICD for primary prevention should be considered in the presence of large arrhythmogenic ventricular scarring, even if the systolic ventricular function is not severely depressed.76
Figure 7.
Aetiologic classification of arrhythmogenic cardiomyopathies. The most common cause of arrhythmogenic cardiomyopathy is a genetic defect of desmosomal genes, although there are other genetic and non-genetic causes (see the text for details).
Conclusions
A more appropriate use of the current ITF diagnostic criteria and future upgrade/revision of the scoring system for diagnosis of ARVC should take into account: (i) the limitation of current understanding of the genetic background of the disease that translates into the risk of misdiagnosis if molecular genetic test is an integral part of the diagnostic scoring system; (ii) the advances of technology and improvement of interpretation of tissue characterization images by CMR which has become the leading imaging technique for characterization of the disease phenotype; (iii) the broad spectrum of the ARVC phenotype which includes left-dominant disease variants and requires specific diagnostic criteria from different clinical categories; and (iv) the peculiarities of clinical features and diagnostic tests of ARVC in the paediatric population which represents a sizeable proportion of patients due to the increasing clinical and genetic screening of families.
The research should focus on better understanding of the genetic background, improvement of clinical and imaging characterization of the phenotype, with particular reference to left-sided variants and discovery of diagnostic biomarkers.77
The clinical relevance of the proposed classification of arrhythmogenic cardiomyopathy remains to be evaluated by future studies.
Conflict of interest: none declared.
Supplementary Material
Contributor Information
International Experts:
Aris Anastastakis, Angeliki Asimaki, Cristina Basso, Barbara Bauce, Corinna Brunckhorst, Chiara Bucciarelli-Ducci, Hugh Calkins, Domenico Corrado, Firat Duru, Perry Elliott, Robert M Hamilton, Richard N W Hauer, Kristina H Haugaa, Cynthia A James, Daniel Judge, Mark S Link, Francis E Marchlinski, Frank I Marcus, William J McKenna, Andrea Mazzanti, Luisa Mestroni, Antonis Pantazis, Antonio Pelliccia, Martina Perazzolo Marra, Kalliopi Pilichou, Pyotr G A Platonov, Alexandros Protonotarios, Alessandra Rampazzo, Jeffry E Saffitz, Ardan Saguner, Christian Schmied, Sanjay Sharma, Hari Tandri, Anneline S J M Te Riele, Gaetano Thiene, Adalena Tsatsopoulou, Peter J van Tintelen, Thomas Wichter, Wojciech Zareba, and Alessandro Zorzi
This paper is dedicated to the memory of our friends recently passed away, Drs Guy Fontaine, Andrea Nava, and Nikos Protonotarios, who played a pioneering role in the development of the International Task Force criteria for diagnosis of arrhythmogenic right ventricular cardiomyopathy.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Thiene G, Nava A, Corrado D, Rossi L, Pennelli N.. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med 1988;318:129–133. [DOI] [PubMed] [Google Scholar]
- 2. Corrado D, Link MS, Calkins H.. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 2017;376:61–72. [DOI] [PubMed] [Google Scholar]
- 3. Corrado D, Basso C, Judge DP.. Arrhythmogenic cardiomyopathy. Circ Res 2017;121:784–802. [DOI] [PubMed] [Google Scholar]
- 4. Basso C, Thiene G, Corrado D, Angelini A, Nava A, Valente M.. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation 1996;94:983–991. [DOI] [PubMed] [Google Scholar]
- 5. Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, Nava A, Silvestri F, Blomstrom-Lundqvist C, Wlodarska EK, Fontaine G, Camerini F.. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol 1997;30:1512–1520. [DOI] [PubMed] [Google Scholar]
- 6. Corrado D, Thiene G.. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: clinical impact of molecular genetic studies. Circulation 2006;113:1634–1637. [DOI] [PubMed] [Google Scholar]
- 7. Sen-Chowdhry S, Syrris P, Ward D, Asimaki A, Sevdalis E, McKenna WJ.. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation 2007;115:1710–1720. [DOI] [PubMed] [Google Scholar]
- 8. Sen-Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D, Pennell DJ, McKenna WJ.. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol 2008;52:2175–2187. [DOI] [PubMed] [Google Scholar]
- 9. McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom-Lundqvist C, Fontaine G, Camerini F.. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J 1994;71:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W.. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cook TS, Zimmerman SL, Jha S.. Analysis of statistical biases in studies used to formulate guidelines: the case of arrhythmogenic right ventricular cardiomyopathy (ARVC) the case of ARVC. Acad Radiol 2015;22:1010–1015. [DOI] [PubMed] [Google Scholar]
- 12. Femia G, Hsu C, Singarayar S, Sy RW, Kilborn M, Parker G, McGuire M, Semsarian C, Puranik R.. Impact of new task force criteria in the diagnosis of arrhythmogenic right ventricular cardiomyopathy. Int J Cardiol 2014;171:179–183. [DOI] [PubMed] [Google Scholar]
- 13. Te Riele AS, Tandri H, Bluemke DA.. Arrhythmogenic right ventricular cardiomyopathy (ARVC): cardiovascular magnetic resonance update. J Cardiovasc Magn Reson 2014;16:50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Perazzolo Marra M, Rizzo S, Bauce B, De Lazzari M, Pilichou K, Corrado D, Thiene G, Iliceto S, Basso C.. Arrhythmogenic right ventricular cardiomyopathy. Contribution of cardiac magnetic resonance imaging to the diagnosis. Herz 2015;40:600–606. [DOI] [PubMed] [Google Scholar]
- 15. Haugaa KH, Basso C, Badano LP, Bucciarelli-Ducci C, Cardim N, Gaemperli O, Galderisi M, Habib G, Knuuti J, Lancellotti P, McKenna W, Neglia D, Popescu BA, Edvardsen T, Delgado V, Cosyns B, Donal E, Lombardi M, Muraru D, Kauffmann P, Jurcut R, Klein JB, Sade LE.. Comprehensive multi-modality imaging approach in arrhythmogenic cardiomyopathy-an expert consensus document of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2017;18:237–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Te Riele A, James CA, Sawant AC, Bhonsale A, Groeneweg JA, Mast TP, Murray B, Tichnell C, Dooijes D, van Tintelen JP, Judge DP, van der Heijden JF, Crosson J, Hauer RNW, Calkins H, Tandri H.. Arrhythmogenic right ventricular dysplasia/cardiomyopathy in the pediatric population: clinical characterization and comparison with adult-onset disease. JACC Clin Electrophysiol 2015;1:551–560. [DOI] [PubMed] [Google Scholar]
- 17. Marcus FI, Edson S, Towbin JA.. Genetics of arrhythmogenic right ventricular cardiomyopathy: a practical guide for physicians. J Am Coll Cardiol 2013;61:1945–1948. [DOI] [PubMed] [Google Scholar]
- 18. Groeneweg JA, Bhonsale A, James CA, Te Riele AS, Dooijes D, Tichnell C, Murray B, Wiesfeld AC, Sawant AC, Kassamali B, Atsma DE, Volders PG, de Groot NM, de Boer K, Zimmerman SL, Kamel IR, van der Heijden JF, Russell SD, Jan Cramer M, Tedford RJ, Doevendans PA, van Veen TA, Tandri H, Wilde AA, Judge DP, van Tintelen JP, Hauer RN, Calkins H.. Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet 2015;8:437–446. [DOI] [PubMed] [Google Scholar]
- 19. Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, Lerman BB, Markowitz SM, Ellinor PT, MacRae CA, Peters S, Grossmann KS, Drenckhahn J, Michely B, Sasse-Klaassen S, Birchmeier W, Dietz R, Breithardt G, Schulze-Bahr E, Thierfelder L.. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet 2004;36:1162–1164. [DOI] [PubMed] [Google Scholar]
- 20. Syrris P, Ward D, Evans A, Asimaki A, Gandjbakhch E, Sen-Chowdhry S, McKenna WJ.. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am J Hum Genet 2006;79:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hoorntje ET, Te Rijdt WP, James CA, Pilichou K, Basso C, Judge DP, Bezzina CR, van Tintelen JP.. Arrhythmogenic cardiomyopathy: pathology, genetics, and concepts in pathogenesis. Cardiovasc Res 2017;113:1521–1531. [DOI] [PubMed] [Google Scholar]
- 22. Merner ND, Hodgkinson KA, Haywood AF, Connors S, French VM, Drenckhahn JD, Kupprion C, Ramadanova K, Thierfelder L, McKenna W, Gallagher B, Morris-Larkin L, Bassett AS, Parfrey PS, Young TL.. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am J Hum Genet 2008;82:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. van Rijsingen IA, van der Zwaag PA, Groeneweg JA, Nannenberg EA, Jongbloed JD, Zwinderman AH, Pinto YM, Dit Deprez RH, Post JG, Tan HL, de Boer RA, Hauer RN, Christiaans I, van den Berg MP, van Tintelen JP, Wilde AA.. Outcome in phospholamban R14del carriers: results of a large multicentre cohort study. Circ Cardiovasc Genet 2014;7:455–465. [DOI] [PubMed] [Google Scholar]
- 24. Xu T, Yang Z, Vatta M, Rampazzo A, Beffagna G, Pilichou K, Pillichou K, Scherer SE, Saffitz J, Kravitz J, Zareba W, Danieli GA, Lorenzon A, Nava A, Bauce B, Thiene G, Basso C, Calkins H, Gear K, Marcus F, Towbin JA.. Compound and digenic heterozygosity contributes to arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol 2010;55:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rigato I, Bauce B, Rampazzo A, Zorzi A, Pilichou K, Mazzotti E, Migliore F, Marra MP, Lorenzon A, De Bortoli M, Calore M, Nava A, Daliento L, Gregori D, Iliceto S, Thiene G, Basso C, Corrado D.. Compound and digenic heterozygosity predicts lifetime arrhythmic outcome and sudden cardiac death in desmosomal gene-related arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Genet 2013;6:533–542. [DOI] [PubMed] [Google Scholar]
- 26. Cox MG, van der Zwaag PA, van der Werf C, van der Smagt JJ, Noorman M, Bhuiyan ZA, Wiesfeld AC, Volders PG, van Langen IM, Atsma DE, Dooijes D, van den Wijngaard A, Houweling AC, Jongbloed JD, Jordaens L, Cramer MJ, Doevendans PA, de Bakker JM, Wilde AA, van Tintelen JP, Hauer RN.. Arrhythmogenic right ventricular dysplasia/cardiomyopathy: pathogenic desmosome mutations in index-patients predict outcome of family screening: Dutch arrhythmogenic right ventricular dysplasia/cardiomyopathy genotype-phenotype follow-up study. Circulation 2011;123:2690–2700. [DOI] [PubMed] [Google Scholar]
- 27. Pilichou K, Lazzarini E, Rigato I, Celeghin R, De Bortoli M, Perazzolo Marra M, Cason M, Jongbloed J, Calore M, Rizzo S, Regazzo D, Poloni G, Iliceto S, Daliento L, Delise P, Corrado D, Van Tintelen JP, Thiene G, Rampazzo A, Basso C, Bauce B, Lorenzon A, Occhi G.. Large genomic rearrangements of desmosomal genes in Italian arrhythmogenic cardiomyopathy patients. Circ Arrhythm Electrophysiol 2017;10. pii: e005324. [DOI] [PubMed] [Google Scholar]
- 28. Ackerman MJ, Priori SG, Willems S, Berul C, Brugada R, Calkins H, Camm AJ, Ellinor PT, Gollob M, Hamilton R, Hershberger RE, Judge DP, Le Marec H, McKenna WJ, Schulze-Bahr E, Semsarian C, Towbin JA, Watkins H, Wilde A, Wolpert C, Zipes DP.. HRS/EHRA expert consensus statement on the state of genetic testing for the channelopathies and cardiomyopathies: this document was developed as a partnership between the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA). Europace 2011;13:1077–1109. [DOI] [PubMed] [Google Scholar]
- 29. Angelini A, Basso C, Nava A, Thiene G.. Endomyocardial biopsy in arrhythmogenic right ventricular cardiomyopathy. Am Heart J 1996;132:203–206. [DOI] [PubMed] [Google Scholar]
- 30. Basso C, Ronco F, Marcus F, Abudureheman A, Rizzo S, Frigo AC, Bauce B, Maddalena F, Nava A, Corrado D, Grigoletto F, Thiene G.. Quantitative assessment of endomyocardial biopsy in arrhythmogenic right ventricular cardiomyopathy/dysplasia: an in vitro validation of diagnostic criteria. Eur Heart J 2008;29:2760–2771. [DOI] [PubMed] [Google Scholar]
- 31. Asimaki A, Tandri H, Huang H, Halushka MK, Gautam S, Basso C, Thiene G, Tsatsopoulou A, Protonotarios N, McKenna WJ, Calkins H, Saffitz JE.. A new diagnostic test for arrhythmogenic right ventricular cardiomyopathy. N Engl J Med 2009;360:1075–1084. [DOI] [PubMed] [Google Scholar]
- 32. Marchlinski FE, Zado E, Dixit S, Gerstenfeld E, Callans DJ, Hsia H, Lin D, Nayak H, Russo A, Pulliam W.. Electroanatomic substrate and outcome of catheter ablative therapy for ventricular tachycardia in setting of right ventricular cardiomyopathy. Circulation 2004;110:2293–2298. [DOI] [PubMed] [Google Scholar]
- 33. Corrado D, Basso C, Leoni L, Tokajuk B, Bauce B, Frigo G, Tarantini G, Napodano M, Turrini P, Ramondo A, Daliento L, Nava A, Buja G, Iliceto S, Thiene G.. Three-dimensional electroanatomic voltage mapping increases accuracy of diagnosing arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2005;111:3042–3050. [DOI] [PubMed] [Google Scholar]
- 34. Santangeli P, Dello Russo A, Pieroni M, Casella M, Di Biase L, Burkhardt JD, Sanchez J, Lakkireddy D, Carbucicchio C, Zucchetti M, Pelargonio G, Themistoclakis S, Camporeale A, Rossillo A, Beheiry S, Hongo R, Bellocci F, Tondo C, Natale A.. Fragmented and delayed electrograms within fibrofatty scar predict arrhythmic events in arrhythmogenic right ventricular cardiomyopathy: results from a prospective risk stratification study. Heart Rhythm 2012;9:1200–1206. [DOI] [PubMed] [Google Scholar]
- 35. Aquaro GD, Barison A, Todiere G, Grigoratos C, Ait Ali L, Di Bella G, Emdin M, Festa P.. Usefulness of combined functional assessment by cardiac magnetic resonance and tissue characterization versus task force criteria for diagnosis of arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol 2016;118:1730–1736. [DOI] [PubMed] [Google Scholar]
- 36. Vermes E, Strohm O, Otmani A, Childs H, Duff H, Friedrich MG.. Impact of the revision of arrhythmogenic right ventricular cardiomyopathy/dysplasia task force criteria on its prevalence by CMR criteria. JACC Cardiovasc Imaging 2011;4:282–287. [DOI] [PubMed] [Google Scholar]
- 37. Bomma C, Rutberg J, Tandri H, Nasir K, Roguin A, Tichnell C, Rodriguez R, James C, Kasper E, Spevak P, Bluemke DA, Calkins H.. Misdiagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiovasc Electrophysiol 2004;15:300–306. [DOI] [PubMed] [Google Scholar]
- 38. Bluemke DA, Krupinski EA, Ovitt T, Gear K, Unger E, Axel L, Boxt LM, Casolo G, Ferrari VA, Funaki B, Globits S, Higgins CB, Julsrud P, Lipton M, Mawson J, Nygren A, Pennell DJ, Stillman A, White RD, Wichter T, Marcus F.. MR Imaging of arrhythmogenic right ventricular cardiomyopathy: morphologic findings and interobserver reliability. Cardiology 2003;99:153–162. [DOI] [PubMed] [Google Scholar]
- 39. Sievers B, Addo M, Franken U, Trappe HJ.. Right ventricular wall motion abnormalities found in healthy subjects by cardiovascular magnetic resonance imaging and characterized with a new segmental model. J Cardiovasc Magn Reson 2004;6:601–608. [DOI] [PubMed] [Google Scholar]
- 40. Te Riele AS, James CA, Philips B, Rastegar N, Bhonsale A, Groeneweg JA, Murray B, Tichnell C, Judge DP, Van Der Heijden JF, Cramer MJ, Velthuis BK, Bluemke DA, Zimmerman SL, Kamel IR, Hauer RN, Calkins H, Tandri H.. Mutation-positive arrhythmogenic right ventricular dysplasia/cardiomyopathy: the triangle of dysplasia displaced. J Cardiovasc Electrophysiol 2013;24:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marra MP, Leoni L, Bauce B, Corbetti F, Zorzi A, Migliore F, Silvano M, Rigato I, Tona F, Tarantini G, Cacciavillani L, Basso C, Buja G, Thiene G, Iliceto S, Corrado D.. Imaging study of ventricular scar in arrhythmogenic right ventricular cardiomyopathy: comparison of 3D standard electroanatomical voltage mapping and contrast-enhanced cardiac magnetic resonance. Circ Arrhythm Electrophysiol 2012;5:91–100. [DOI] [PubMed] [Google Scholar]
- 42. Marcus FI, Zareba W, Calkins H, Towbin JA, Basso C, Bluemke DA, Estes NA 3rd, Picard MH, Sanborn D, Thiene G, Wichter T, Cannom D, Wilber DJ, Scheinman M, Duff H, Daubert J, Talajic M, Krahn A, Sweeney M, Garan H, Sakaguchi S, Lerman BB, Kerr C, Kron J, Steinberg JS, Sherrill D, Gear K, Brown M, Severski P, Polonsky S, McNitt S.. Arrhythmogenic right ventricular cardiomyopathy/dysplasia clinical presentation and diagnostic evaluation: results from the North American Multidisciplinary Study. Heart Rhythm 2009;6:984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Protonotarios A, Anastasakis A, Panagiotakos DB, Antoniades L, Syrris P, Vouliotis A, Stefanadis C, Tsatsopoulou A, McKenna WJ, Protonotarios N.. Arrhythmic risk assessment in genotyped families with arrhythmogenic right ventricular cardiomyopathy. Europace 2016;8:610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zorzi A, Rigato I, Pilichou K, Perazzolo Marra M, Migliore F, Mazzotti E, Gregori D, Thiene G, Daliento L, Iliceto S, Rampazzo A, Basso C, Bauce B, Corrado D.. Phenotypic expression is a prerequisite for malignant arrhythmic events and sudden cardiac death in arrhythmogenic right ventricular cardiomyopathy. Europace 2016;18:1086–1094. [DOI] [PubMed] [Google Scholar]
- 45. Corrado D, Zorzi A.. Natural history of arrhythmogenic cardiomyopathy: redefining the age range of clinical presentation. Heart Rhythm 2017;14:892–893. [DOI] [PubMed] [Google Scholar]
- 46. Bhonsale A, Groeneweg JA, James CA, Dooijes D, Tichnell C, Jongbloed JD, Murray B, Te Riele AS, van den Berg MP, Bikker H, Atsma DE, de Groot NM, Houweling AC, van der Heijden JF, Russell SD, Doevendans PA, van Veen TA, Tandri H, Wilde AA, Judge DP, van Tintelen JP, Calkins H, Hauer RN.. Impact of genotype on clinical course in arrhythmogenic right ventricular dysplasia/cardiomyopathy-associated mutation carriers. Eur Heart J 2015;36:847–855. [DOI] [PubMed] [Google Scholar]
- 47. Camm CF, Tichnell C, James CA, Murray B, Porterfield F, Te Riele AS, Tandri H, Calkins H.. Premature ventricular contraction variability in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiovasc Electrophysiol 2015;26:53–57. [DOI] [PubMed] [Google Scholar]
- 48. O'Donnell D, Cox D, Bourke J, Mitchell L, Furniss S.. Clinical and electrophysiological differences between patients with arrhythmogenic right ventricular dysplasia and right ventricular outflow tract tachycardia. Eur Heart J 2003;24:801–810. [DOI] [PubMed] [Google Scholar]
- 49. Corrado D, Basso C, Leoni L, Tokajuk B, Turrini P, Bauce B, Migliore F, Pavei A, Tarantini G, Napodano M, Ramondo A, Buja G, Iliceto S, Thiene G.. Three-dimensional electroanatomical voltage mapping and histologic evaluation of myocardial substrate in right ventricular outflow tract tachycardia. J Am Coll Cardiol 2008;51:731–739. [DOI] [PubMed] [Google Scholar]
- 50. Polin GM, Haqqani H, Tzou W, Hutchinson MD, Garcia FC, Callans DJ, Zado ES, Marchlinski FE.. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm 2011;8:76–83. [DOI] [PubMed] [Google Scholar]
- 51. Nasir K, Bomma C, Tandri H, Roguin A, Dalal D, Prakasa K, Tichnell C, James C, Spevak PJ, Jspevak P, Marcus F, Calkins H.. Electrocardiographic features of arrhythmogenic right ventricular dysplasia/cardiomyopathy according to disease severity: a need to broaden diagnostic criteria. Circulation 2004;110:1527–1534. [DOI] [PubMed] [Google Scholar]
- 52. Steriotis AK, Bauce B, Daliento L, Rigato I, Mazzotti E, Folino AF, Marra MP, Brugnaro L, Nava A.. Electrocardiographic pattern in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol 2009;103:1302–1308. [DOI] [PubMed] [Google Scholar]
- 53. Protonotarios N, Anastasakis A, Antoniades L, Chlouverakis G, Syrris P, Basso C, Asimaki A, Theopistou A, Stefanadis C, Thiene G, McKenna WJ, Tsatsopoulou A.. Arrhythmogenic right ventricular cardiomyopathy/dysplasia on the basis of the revised diagnostic criteria in affected families with desmosomal mutations. Eur Heart J 2011;32:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. De Lazzari M, Zorzi A, Cipriani A, Susana A, Mastella G, Rizzo A, Rigato I, Bauce B, Giorgi B, Lacognata C, Iliceto S, Corrado D, Perazzolo Marra M.. Relationship between electrocardiographic findings and cardiac magnetic resonance phenotypes in arrhythmogenic cardiomyopathy. J Am Heart Assoc 2018;7:e009855.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, Pilichou K, Migliore F, Rizzo S, Giorgi B, De Conti G, Sarto P, Serratosa L, Patrizi G, De Maria E, Pelliccia A, Basso C, Schiavon M, Bauce B, Iliceto S, Thiene G, Corrado D.. Nonischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol 2016;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. di Gioia CR, Giordano C, Cerbelli B, Pisano A, Perli E, De Dominicis E, Poscolieri B, Palmieri V, Ciallella C, Zeppilli P, d'Amati G.. Nonischemic left ventricular scar and cardiac sudden death in the young. Hum Pathol 2016;58:78–89. [DOI] [PubMed] [Google Scholar]
- 57. Tzou WS, Zado ES, Lin D, Callans DJ, Dixit S, Cooper JM, Bala R, Garcia F, Hutchinson MD, Riley MP, Deo R, Gerstenfeld EP, Marchlinski FE.. Sinus rhythm ECG criteria associated with basal-lateral ventricular tachycardia substrate in patients with nonischemic cardiomyopathy. J Cardiovasc Electrophysiol 2011;22:1351–1358. [DOI] [PubMed] [Google Scholar]
- 58. Betensky BP, Deyell MW, Tzou WS, Zado ES, Marchlinski FE.. Sinus rhythm electrocardiogram identification of basal-lateral ischemic versus nonischemic substrate in patients with ventricular tachycardia. J Interv Card Electrophysiol 2012;35:311–321. [DOI] [PubMed] [Google Scholar]
- 59. Platonov PG, Calkins H, Hauer RN, Corrado D, Svendsen JH, Wichter T, Biernacka EK, Saguner AM, Te Riele AS, Zareba W.. High interobserver variability in the assessment of epsilon waves: implications for diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm 2016;13:208–216. [DOI] [PubMed] [Google Scholar]
- 60. Oloriz T, Silberbauer J, Maccabelli G, Mizuno H, Baratto F, Kirubakaran S, Vergara P, Bisceglia C, Santagostino G, Marzi A, Sora N, Roque C, Guarracini F, Tsiachris D, Radinovic A, Cireddu M, Sala S, Gulletta S, Paglino G, Mazzone P, Trevisi N, Della Bella P.. Catheter ablation of ventricular arrhythmia in nonischemic cardiomyopathy: anteroseptal versus inferolateral scar sub-types. Circ Arrhythm Electrophysiol 2014;7:414–423. [DOI] [PubMed] [Google Scholar]
- 61. Pinto YM, Elliott PM, Arbustini E, Adler Y, Anastasakis A, Bohm M, Duboc D, Gimeno J, de Groote P, Imazio M, Heymans S, Klingel K, Komajda M, Limongelli G, Linhart A, Mogensen J, Moon J, Pieper PG, Seferovic PM, Schueler S, Zamorano JL, Caforio AL, Charron P.. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J 2016;37:1850–1858. [DOI] [PubMed] [Google Scholar]
- 62. Lee TM, Hsu DT, Kantor P, Towbin JA, Ware SM, Colan SD, Chung WK, Jefferies JL, Rossano JW, Castleberry CD, Addonizio LJ, Lal AK, Lamour JM, Miller EM, Thrush PT, Czachor JD, Razoky H, Hill A, Lipshultz SE.. Pediatric cardiomyopathies. Circ Res 2017;121:855–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Te Riele AS, James CA, Groeneweg JA, Sawant AC, Kammers K, Murray B, Tichnell C, van der Heijden JF, Judge DP, Dooijes D, van Tintelen JP, Hauer RN, Calkins H, Tandri H.. Approach to family screening in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Eur Heart J 2016;37:755–763. [DOI] [PubMed] [Google Scholar]
- 64. Mast TP, James CA, Calkins H, Teske AJ, Tichnell C, Murray B, Loh P, Russell SD, Velthuis BK, Judge DP, Dooijes D, Tedford RJ, van der Heijden JF, Tandri H, Hauer RN, Abraham TP, Doevendans PA, Te Riele AS, Cramer MJ.. Evaluation of structural progression in arrhythmogenic right ventricular dysplasia/cardiomyopathy. JAMA Cardiol 2017;2:293–302. [DOI] [PubMed] [Google Scholar]
- 65. Bhonsale A, Te Riele ASJM, Sawant AC, Groeneweg JA, James CA, Murray B, Tichnell C, Mast TP, van der Pols MJ, Cramer MJM, Dooijes D, van der Heijden JF, Tandri H, van Tintelen JP, Judge DP, Hauer RNW, Calkins H.. Cardiac phenotype and long-term prognosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia patients with late presentation. Heart Rhythm 2017;14:883–891. [DOI] [PubMed] [Google Scholar]
- 66. Corrado D, Basso C, Schiavon M, Thiene G.. Screening for hypertrophic cardiomyopathy in young athletes. N Engl J Med 1998;339:364–369. [DOI] [PubMed] [Google Scholar]
- 67. Etoom Y, Govindapillai S, Hamilton R, Manlhiot C, Yoo SJ, Farhan M, Sarikouch S, Peters B, McCrindle BW, Grosse-Wortmann L.. Importance of CMR within the task force criteria for the diagnosis of ARVC in children and adolescents. J Am Coll Cardiol 2015;65:987–995. [DOI] [PubMed] [Google Scholar]
- 68. Hoffmayer KS, Machado ON, Marcus GM, Yang Y, Johnson CJ, Ermakov S, Vittinghoff E, Pandurangi U, Calkins H, Cannom D, Gear KC, Tichnell C, Park Y, Zareba W, Marcus FI, Scheinman MM.. Electrocardiographic comparison of ventricular arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy and right ventricular outflow tract tachycardia. J Am Coll Cardiol 2011;58:831–838. [DOI] [PubMed] [Google Scholar]
- 69. Quarta G, Husain SI, Flett AS, Sado DM, Chao CY, Tome Esteban MT, McKenna WJ, Pantazis A, Moon JC.. Arrhythmogenic right ventricular cardiomyopathy mimics: role of cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2013;15:16.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. D'Ascenzi F, Solari M, Corrado D, Zorzi A, Mondillo S.. Diagnostic differentiation between arrhythmogenic cardiomyopathy and athlete's heart by using imaging. JACC Cardiovasc Imaging 2018;11:1327–1339. [DOI] [PubMed] [Google Scholar]
- 71. Yilmaz A, Gdynia HJ, Baccouche H, Mahrholdt H, Meinhardt G, Basso C, Thiene G, Sperfeld AD, Ludolph AC, Sechtem U.. Cardiac involvement in patients with Becker muscular dystrophy: new diagnostic and pathophysiological insights by a CMR approach. J Cardiovasc Magn Reson 2008;10:50.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Finsterer J, Stollberger C.. Heart disease in disorders of muscle, neuromuscular transmission, and the nerves. Korean Circ J 2016;46:117–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Corrado D, Thiene G.. Cardiac sarcoidosis mimicking arrhythmogenic right ventricular cardiomyopathy/dysplasia: the renaissance of endomyocardial biopsy? J Cardiovasc Electrophysiol 2009;20:477–479. [DOI] [PubMed] [Google Scholar]
- 74. Nery PB, Keren A, Healey J, Leug E, Beanlands RS, Birnie DH.. Isolated cardiac sarcoidosis: establishing the diagnosis with electroanatomic mapping-guided endomyocardial biopsy. Can J Cardiol 2013;29:1015.e1–1015.e3. [DOI] [PubMed] [Google Scholar]
- 75. Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC, Daubert JP, de Chillou C, DePasquale EC, Desai MY, Estes NAM 3rd, Hua W, Indik JH, Ingles J, James CA, John RM, Judge DP, Keegan R, Krahn AD, Link MS, Marcus FI, McLeod CJ, Mestroni L, Priori SG, Saffitz JE, Sanatani S, Shimizu W, Peter van Tintelen J, Wilde AAM, Zareba W.. 2019 HRS Expert Consensus Statement on Evaluation, Risk Stratification, and Management of Arrhythmogenic Cardiomyopathy. Heart Rhythm 2019. [DOI] [PubMed] [Google Scholar]
- 76. Corrado D, Wichter T, Link MS, Hauer R, Marchlinski F, Anastasakis A, Bauce B, Basso C, Brunckhorst C, Tsatsopoulou A, Tandri H, Paul M, Schmied C, Pelliccia A, Duru F, Protonotarios N, Estes NA 3rd, McKenna WJ, Thiene G, Marcus FI, Calkins H.. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an International Task Force consensus statement. Eur Heart J 2015;36:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chatterjee D, Fatah M, Akdis D, Spears DA, Koopmann TT, Mittal K, Rafiq MA, Cattanach BM, Zhao Q, Healey JS, Ackerman MJ, Bos JM, Sun Y, Maynes JT, Brunckhorst C, Medeiros-Domingo A, Duru F, Saguner AM, Hamilton RM.. An autoantibody identifies arrhythmogenic right ventricular cardiomyopathy and participates in its pathogenesis. Eur Heart J 2018;39:3932–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.