Abstract
Trimethylation of histone 3 lysine 9 (H3K9me3) at gene promoters is a major epigenetic mechanism that silences gene expression. We have developed a small molecule inhibitor for the H3K9me3-specific histone methyltransferase SUV39H1. We report here that FAS expression is significantly down-regulated and SUV39H1 expression is significantly up-regulated in human colorectal carcinoma (CRC) as compared to normal colon. SUV39H1-selective inhibitor F5446 decreased H3K9me3 deposition at the FAS promoter, increased Fas expression, and increased CRC cell sensitivity to FasL-induced apoptosis in vitro. Furthermore, inhibition of SUV39H1 altered the expression of genes with known functions in DNA replication and cell cycle in the metastatic colon carcinoma cells, which is associated with cell cycle arrest at S phase in the metastatic human colon carcinoma cells, resulting in tumor cell apoptosis and growth inhibition in a concentration-dependent manner in vitro. Moreover, F5446 increased 5-FU-resistant human CRC sensitivity to both 5-FU- and FasL-induced apoptosis and inhibited tumor cell growth in vitro. More importantly, F5446 suppressed human colon tumor xenograft growth in vivo. Our data indicate that pharmacological inhibition of SUV39H1 is an effective approach to suppress human CRC.
Keywords: SUV39H1, H3K9me3, Fas, 5-FU, metastasis, chemoresistance
1. Introduction
5-Fluorouracil (5-FU), in combination with other cytotoxic or targeted agents, is the standard adjuvant therapy for human patients with metastatic colorectal cancer (CRC) [1]. Although recent advance in liver resection, combined with 5-FU-based chemotherapy, have significantly improved the survival of patients with metastatic CRC [2], development of resistance to 5-FU is almost inevitable in CRC patients [3], resulting in tumor recurrence and resultant metastasis to distant organs, primarily to the liver, which accounts for over 90% of human CRC mortality [4]. One of the hallmarks of metastatic human CRC is loss of Fas expression and apoptosis resistance [5–10]. The death receptor Fas mediates the FasL-induced apoptosis pathway, which is one of the two primary effector mechanisms that cytotoxic T lymphocytes (CTLs) use to induce tumor cell apoptosis to suppress tumor development [10–14]. It is likely that metastatic CRC cells using silencing Fas expression as a mechanism to evade host immune surveillance.
p53 is a transcription activator that binds to the p53-binding element at the first intron of the FAS gene to activate FAS transcription in human colon tumor cells [15]. 5-FU is known to induce a DNA damage response that activates p53 [15–17] to up-regulate Fas in colon carcinoma cells [15, 18]. 5-FU chemotherapy may increase colon tumor cell Fas expression to sensitize the tumor cells to host FasL+ CTL-induced apoptosis. It is therefore not surprising that 5-FU chemotherapy may selectively eliminate Fas-sensitive tumor cells to enrich tumor cells with low level of Fas expression, which may underlie CRC immune evasion and progression. Therefore, re-activating FAS expression is an effective approach to suppress chemoresistant and metastatic human CRC.
Covalent modification of histones, one of the two core components of eukaryotic chromatin, is a major mechanism of epigenetic regulation of gene expression. The methylation of lysine residues in histones, particularly in the N-terminal tails of histones H3 and H4 of the chromatin, play a fundamental role in the regulation of gene expression through modulating chromatin structure. Histone methyltransferases (HMTases) catalyze the methylation of histones to modify chromatin structure, thereby influencing gene expression patterns during cellular processes. Unlike genetic mutations of oncogenes and tumor suppressor genes, which are permanent alterations in the cancer genome, histone methylation is a reversible process, which has made HMTases attractive molecular targets for cancer therapy [19, 20]. Genome-wide ChIP-Seq identified H3K9me3 deposition at the FAS promoter [21]. Furthermore, H3K9me3 deposition level is significantly higher in metastatic human colon carcinoma than in primary human colon carcinoma [21]. It is known that H3K9me3 creates a transcriptionally repressive chromatin conformation to repress gene transcription [22, 23]. Consistent with this phenomenon, inhibiting H3K9me3 with a natural histone methyltransferase inhibitor verticillin A decreased H3K9me3 deposition at the FAS promoter and increased FAS expression in the metastatic human colon carcinoma cells [21]. H3K9me3 is catalyzed by HMTase SUV39H1 [24–26]. We have now developed a second generation SUV39H1-selective small molecule inhibitor F5446 [27]. We report here that targeting H3K9me3 with F5446 is effective in re-activating Fas expression and inducing cell cycle arrest to suppress 5-FU-resistant human CRC growth in vitro and in vivo.
2. Materials and Methods
2.1. Mice and cells.
Athymic mice were obtained from the Jackson Laboratory. Seven to eleven weeks old female mice were used. All mice were housed, maintained and studied in accordance with an approved protocol by Augusta University Institutional Animal Use and Care Committee. LS411N, SW620, and CCD841 cells were obtained from American Type Culture Collection (ATCC, Manassas, VA). ATCC characterizes these cells by morphology, immunology, DNA fingerprint, and cytogenetics. LS411N-5FUR and SW620–5FUR cell lines were selected by using increased 5-FU concentrations as previously described [21].
2.2. Reagents.
5-Fluorouracil was obtained from Georgia Cancer Center Pharmacy. F5446 was synthesis in LeadGen Labs LLC (Orange, CT) as previously described [27]. Each lot of F5446 was tested by LC-MS and NMR as quality control. The purity is over 96%. F5446 enzymatic inhibitory activity was quality control tested in Reaction Biology Corp (Malvern, PA). The EC50 of F5446 used in this study in inhibition of SUV39H1 in vitro is 2.03 μM. Mega-Fas Ligand (kindly provided by Dr. Peter Buhl Jensen at Oncology Venture A/S, Denmark) is a recombinant fusion protein that consists of three human FasL extracellular domains linked to a protein backbone comprising the dimer-forming collagen domain of human adiponectin. The Mega-Fas Ligand was produced as a glycoprotein in mammalian cells using Good Manufacturing Practice compliant process in Topotarget A/S (Copenhagen, Denmark).
2.3. TCGA database analysis.
Human datasets of FAS and SUV39H1 expression in human colorectal carcinoma and normal colon tissues were extracted from TCGA Colon and Rectal Cancer (COADREAD) ploy A+ IlluminaHiSeq pancan normalized RNA seq dataset using UCSC Xena Cancer Genomics Browser.
2.4. DNA microarray.
Tumor cells were treated with F5446 at 500 nM for 2 days, Total RNA was isolated and used. The human gene 2.0 ST array (Affymetrix, Santa Clara, CA) was used for the gene expression profiling. Total RNA samples were processed using the Ambion WT expression kit (Life Technologies, Calsbad, CA) and a GeneChip WT terminal labeling kit (Affymetrix). The synthesized sense strand cDNAs were fragmented and biotin-labeled using a GeneChip WT terminal labeling kit. The labeled cDNAs were hybridized onto the arrays using Affymetrix GeneChip fluidics station 450 systems. The expression data were imported into Partek GS version 6.6 using a standard import tool with GeneChip robust multiarray analysis normalization. The differential expressions were calculated using ANOVA of Partek package.
2.5. Chromatin immunoprecipitation (ChIP) assay.
ChIP assays were carried out using anti-H3K9me3 antibody (Abcam) according to the manufacturer’s protocol and as previously described [21]. Fas promoter DNA was detected by PCR using promoter DNA-specific primers as previously described [21]. The primer sequence hFas ChIP-F: 5’- GGTGGACGATGCCAAAGGAATAC −3’; hFas ChIP-B: 5’- CACTCAGAGAAAGACTTGCGGG −3’
2.6. Flow cytometry.
To analyze Fas expression level, tumor cells stained with anti-human Fas antibody (Biolegend). The stained cells were then analyzed by flow cytometry. To analyze apoptotic cell death and apoptosis, tumor cells were treated with F5446, 5-FU, or MegaFasL. Both floating and adherent cells were then collected and incubated with propidium iodide (PI) and Annexin V and analyzed by flow cytometry. The % apoptotic cell death was calculated by the formula: Annexin V+ PI+ cells in the treatment group - Annexin V+ PI+ cells in the control group. To determine Fas expression level in tumor cells of the xenografts, the tumor tissues were dissected, digested with collagenase solution [1 mg/ml Collagenase (Sigma-Aldrich, St Luis, MO), 0.1 mg/ml Hyaluronidase (Sigma-Aldrich), and 30 U/ml DNase I (Thermo Scientific, Waltham, MA)] to make single cells. The tumor digests were then passed through a cell strainer and stained with DAPI, plus anti-mouse CD45 and anti-human Fas (Biolegend). The stained cells were analyzed by flow cytometry. The CD45− live cells were gated for Fas level.
2.7. Cell viability assays.
Cells were seeded in 96-well plates at 1×104 cells/well in 100 μl culture medium for 3 days. Cell viability assays were performed using the MTT cell proliferation assay kit (ATCC, Manassas, VA) according to the manufacturer’s instructions.
2.8. Cell cycle analysis.
Cells were collected and fixed in 70% ethanol for 30 min. The fixed cells were washed in PBS and incubated with DNA extraction buffer [192 mmol/L Na2HPO4, 4 mmol/L citric acid (pH 7.8)] for 5 min. RNase A and propidium iodide (PI) were added to the cells and incubated for 30 min. The cell cycle was analyzed by flow cytometry.
2.9. Human colon tumor xenograft models.
Twenty athymic mice were subcutaneously inoculated with SW620 cells (2.5 × 106 cells/mouse) at the right flanks. At day 8, fifteen tumor-bearing mice with relative similar sizes of tumors were selected and randomly divided into three groups, and treated with solvent [10% cremophor (Sigma-Aldrich, St Luis, MO)](control, n=5), and F5446 at 5 (n=5) and 10 mg/kg (n=5) body weight, respectively, by intraperitoneal injection every two days for 10 times. Fifteen athymic mice were subcutaneously inoculated with SW620–5FUR cells (2.5 × 106 cells/mouse) at the right flanks. At day 8, tumor-bearing mice were randomly divided into three groups and treated as above in the SW620 xenograft. Tumor size was measured in 2 dimensions with a digital micrometer caliper at the indicated time points. Tumor volume was calculated by the formula (tumor length × tumor width2)/2.
2.10. Statistical analysis.
All statistical analysis was performed using SAS 9.4 and statistical significance was assessed using an alpha level of 0.05. Assumptions of normality and equality of variance for analysis of variance (ANOVA) methods were assessed for each outcome. To examine differences in H3K9me3 deposition level at the FAS promoter and FAS MFI between control and F5446 concentration groups, one-way ANOVA was used with a Tukey-Kramer multiple comparison procedure if the overall F-test was statistically significant. To determine effects F5446 and FasL or F5446 and 5-FU on apoptotic cell death in tumor cells, two-factor ANOVA that included the main effects and the two-factor interaction between the main effects was used. A Tukey-Kramer multiple comparison procedure was used on the adjusted least squares means of the two-factor interaction term if the overall F-test for the interaction effect was statistically significant. To examine whether tumor xenograft growth kinetics between F5446 doses and control are different, a repeated-measures mixed model was used with a Kenward-Rodger adjustment to the denominator degrees of freedom and an unstructured correlation matrix to model the correlation within animal between measurement days. To examine differences in tumor size and tumor weight at time of sacrifice between F5446 doses, one-way ANOVA was used with a Tukey-Kramer multiple comparison test if the overall F-test was statistically significant.
3. Results
3.1. FAS is down-regulated and SUV39H1 is up-regulated in human colorectal carcinoma.
Previous studies have shown that Fas protein level is high in normal human colon epithelial cells, but diminished in primary human colorectal carcinoma, and almost completely abolished in metastatic human colorectal carcinoma [6, 8]. Analysis of genomic data set from TCGA database validated that FAS is indeed down-regulated in human colorectal carcinoma as compared to normal colon tissue (Fig. 1A). The Fas-FasL cytotoxicity is one of the two major effector mechanisms that CTLs use to lyse target cells [9–13, 28]. Therefore human colorectal carcinoma cells may use silencing FAS expression as a mechanism to evade host FasL+ tumor-infiltrating CTL-mediated immunosurveillance to progress the disease [8, 29]. FAS is silenced by its promoter H3K9me3 deposition [21]. Analysis of the TCGA human colorectal carcinoma data set revealed that SUV39H1, the histone methyltransferase that catalyzes H3K9me3, is significantly up-regulated in human colorectal carcinoma as compared to the normal colon tissues (Fig. 1B). Linear regression analysis of the expression levels of SUV39H1 and FAS determined that SUV39H1 expression level is inversely correlated with FAS expression level in these human colorectal carcinoma (Fig. 1C). These observations indicate that SUV39H1 represses FAS expression in human colorectal carcinoma cells.
Figure 1. FAS expression is inversely correlated with SUV39H1 expression in human colorectal carcinoma.
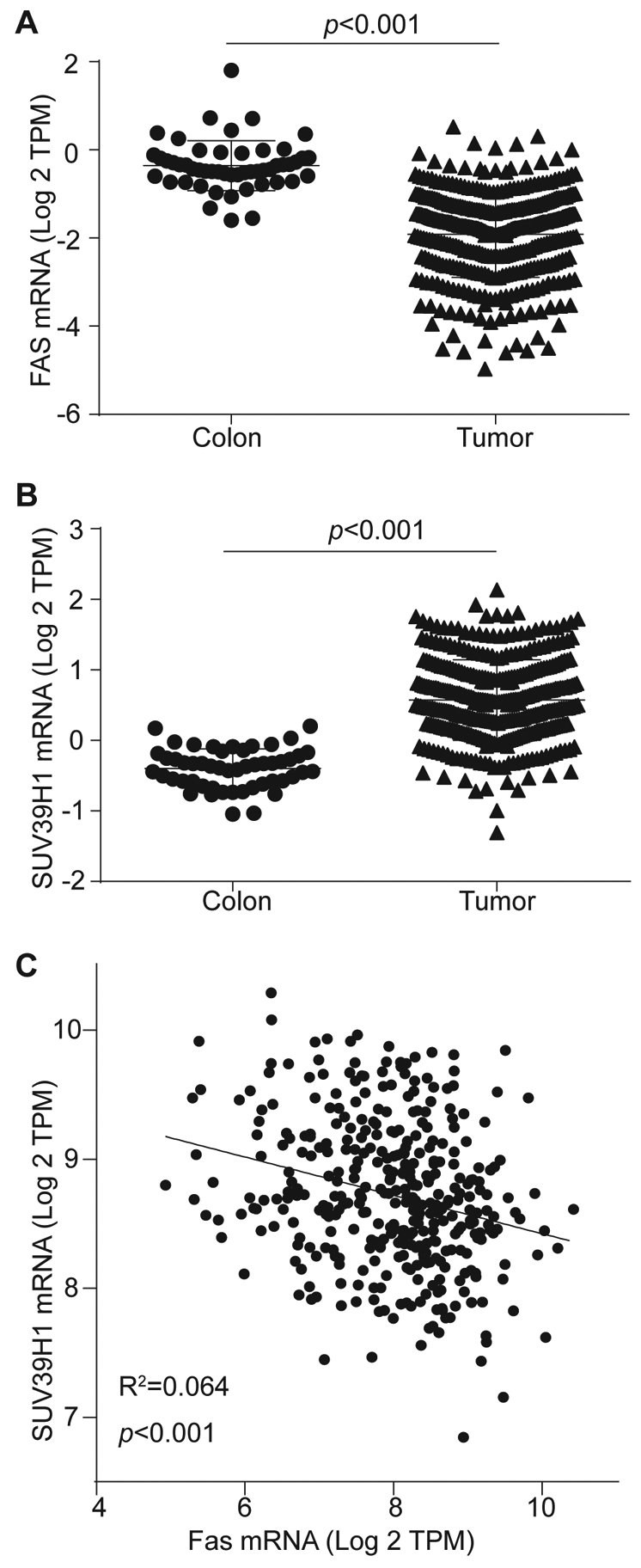
A. FAS and SUV39H1 mRNA expression data sets of human colon carcinoma (n=380) and normal colon tissues (n=51) were extracted from TCGA database and compared. B. The expression levels of FAS and SUV39H1 were then plotted and analyzed for correlation.
3.2. SUV39H1 regulates FAS expression and function in human colon carcinoma cells.
To target SUV39H1, we have developed the SUV39H1-selective small molecule inhibitor F5446 [27]. Because H3K9me3 is highly enriched in the FAS promoter region in the metastatic human colon carcinoma cells [21], we next treated human colon carcinoma cell lines SW620 and LS411N with F5446. SW620 is a metastatic human colon carcinoma cell line that expresses low level of Fas [21, 30] and LS411N is a cell line derived from advanced primary human colon carcinoma that expresses low level of Fas [21, 31]. ChIP analysis indicates that F5446 treatment significantly decreased H3K9me3 deposition level at the FAS promoter in both SW620 and LS411N cells (Fig. 2A & B). Consistent with decreased H3K9me3 deposition at it promoter, FAS expression is significantly increased by F5446 treatment in a concentration-dependent manner on the tumor cells (Fig. 2C). As compared to the tumor cell lines, the normal human colon epithelial cell line CCD841 exhibited significantly less Fas expression increase by F5446 in vitro (Fig. S1). At the functional level, tumor cell sensitivity to FasL-induced apoptosis was significantly increased by a sublethal concentration of F5446 treatment in vitro (Fig. 2D).
Figure 2. SUV39H1 regulates H3K9me3 deposition at the FAS promoter in human colorectal tumor cells.
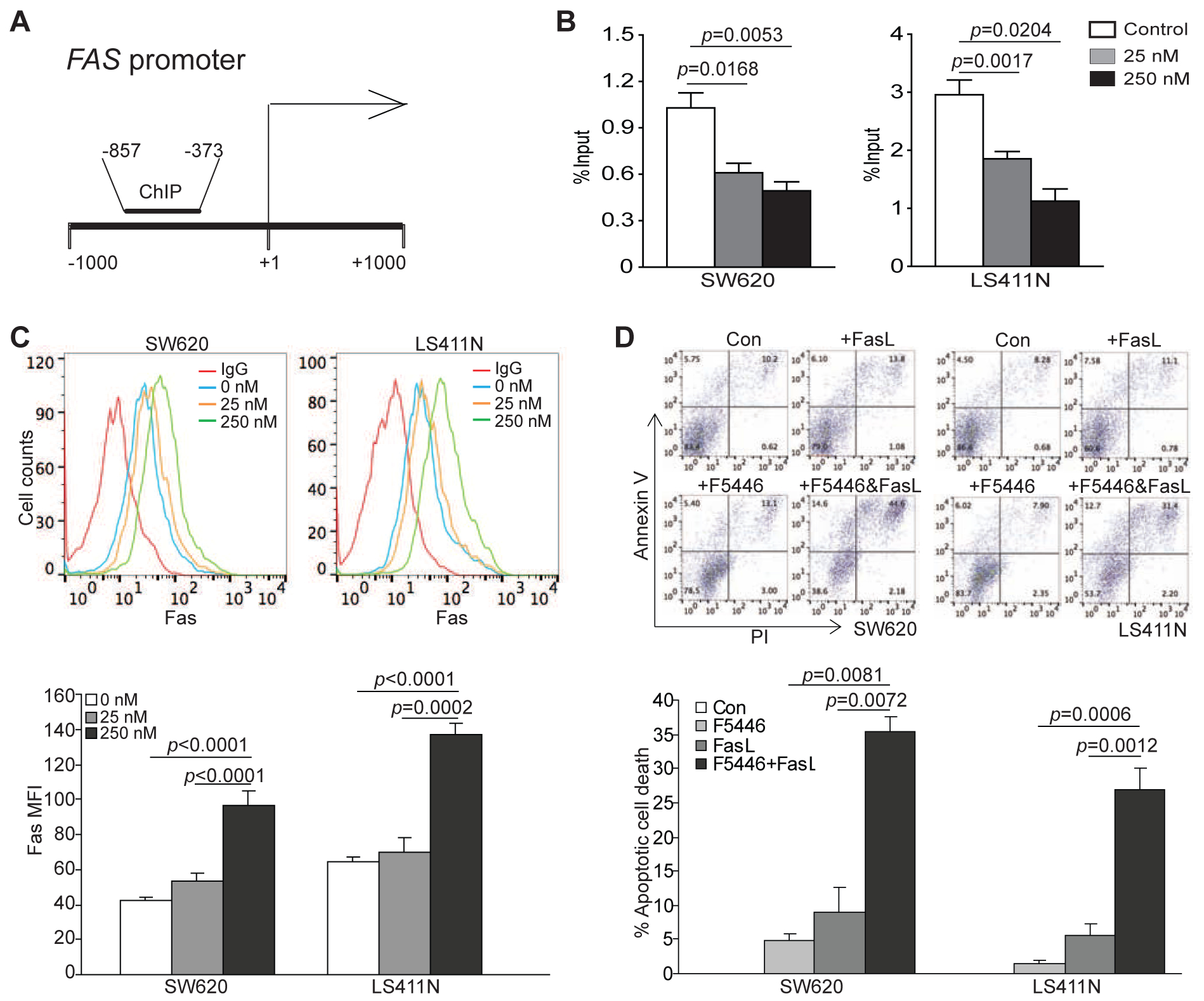
A. The structure of the human Fas promoter showing the ChIP PCR primer location. B. SW620 and LS411N cells were treated with F5446 at the indicated concentrations for two days. Cells were then analyzed by ChIP with anti-H3K9me3 antibody for H3K9me3 deposition at the FAS promoter region. One-way ANOVA was used with a Tukey-Kramer multiple comparison procedure to determine difference in H3K9me3 deposition level between control and treatment groups. Column: mean; Bar: SD. C. Tumor cells as treated in A were stained with IgG or anti-human Fas antibody and analyzed by flow cytometry. Shown are overlays of histograms (top panel). The Fas MFI was quantified and presented at the bottom panel. One-way ANOVA was used with a Tukey-Kramer multiple comparison procedure to determine difference in Fas MFI between control and treatment groups. D. Tumor cells were treated with FasL (100 ng/ml), F5446 (250 nM), or F5446+FasL, respectively, for three days, stained with annexin V and PI and analyzed by flow cytometry. Shown are representative plots of flow cytometry data. The apoptotic cell death and apoptosis were quantified and presented at the bottom panel. % apoptotic cell death was calculated by the formula: Annexin V+ PI+ cells in the treatment group - Annexin V+ PI+ cells in the control group. To determine the effect of F5446 and FasL on cell death, two-factor ANOVA that included the main effects and the two-factor interaction between the main effects was used. A Tukey-Kramer multiple comparison procedure was used on the adjusted least squares means of the two-factor interaction term if the overall F-test for the interaction effect was statistically significant. The p values are shown at top of the bars. Shown are representative data of one of four experiments.
3.3. Inhibition of SUV39H1 induces human colon tumor cell cycle arrest and apoptosis.
To determine the genome-wide gene expression profiles, SW620 cells were treated with F5446 for 2 days and then analyzed for gene expression by DNA microarray using an Affymetrix human gene 2.0 ST array. The entire data set is being deposited to GEO. Genes whose expression was significantly changed as compared with untreated cells were identified by a combination of ANOVA (p < 0.01), intensity changes (≤2.0-fold). These F5446 target genes were then functionally grouped. We identified a large set of genes with known functions in DNA replication and cell cycle (Fig. 3A & B). We then tested the hypothesis that F5446 inhibits SUV39H1 to decrease H3K9me3 level to regulate tumor cell cycle progression. SW620 and LS411N cells were treated with F5446 for 2 days. Cells were then analyzed for cell cycle progression. It is clear that F5446 treatment induced cell cycle arrest at the S phase in a concentration-dependent manner in both SW620 and LS411N cells (Fig. 3C & D). S-phase cell cycle arrest leads to apoptosis [32]. We therefore reasoned that F5446 should be effective as a monotherapeutic agent to induce tumor cell apoptosis. SW620 and LS411N cells were treated with various concentrations of F5446 and analyzed for apoptosis. F5446 induced apoptosis in both tumor cell lines in a concentration-dependent manner in vitro (Fig. 4A). As compared to the tumor cells, the normal human colon epithelial cell line CCD841 is less sensitive to F5446 in apoptotic cell death induction (Fig. 4B). Both SW620 and LS411N cells were also treated with F5446 and measured for cell viability. F5446 effectively suppressed both cell line growth in a concentration-dependent manner and almost completely inhibited both SW620 and LS411N cell growth in vitro at a concentration of 0.25 μM (Fig. 4C).
Figure 3. SUV39H1 regulates cell cycle progression in human colon tumor cells.
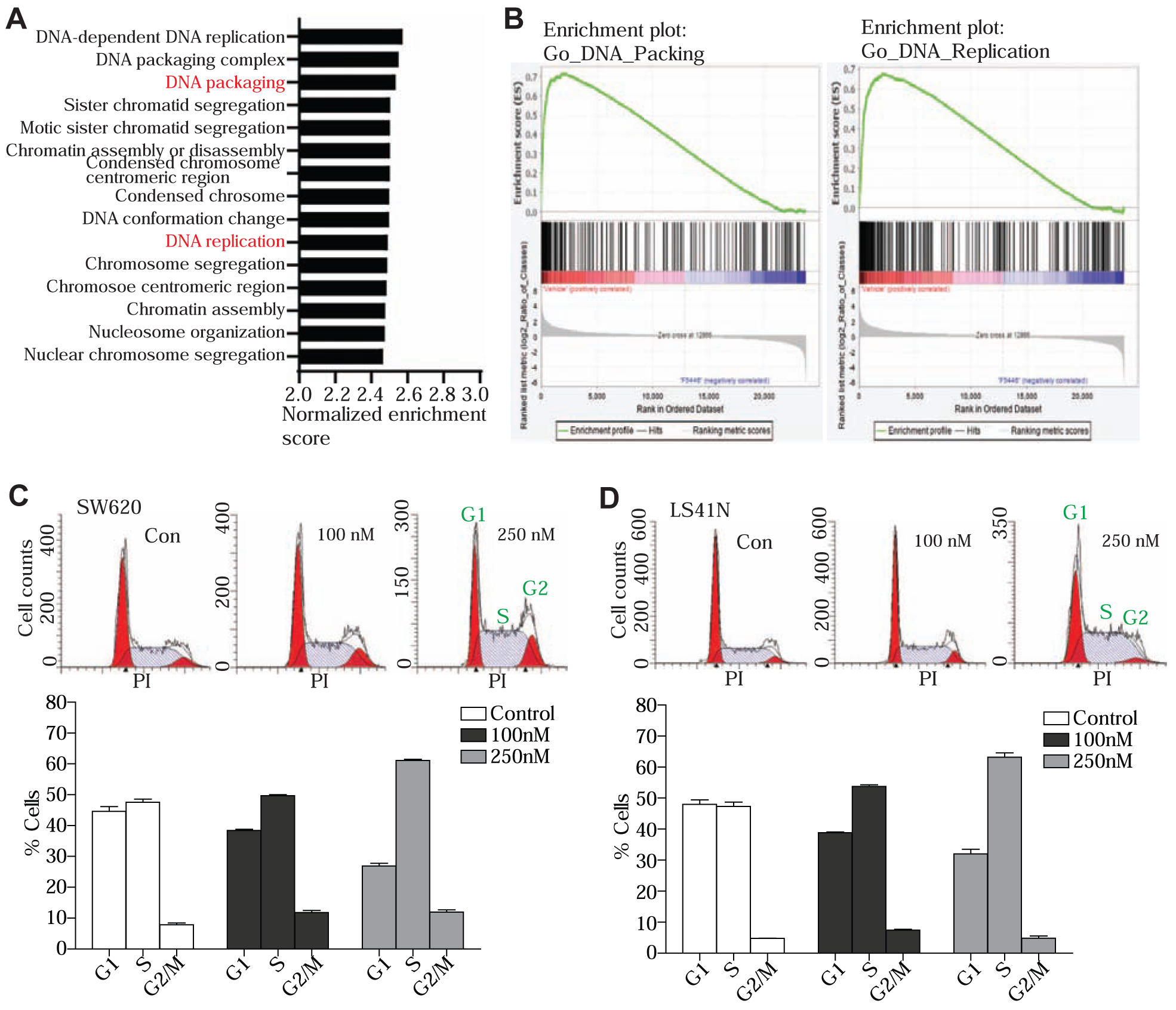
A. SW620 cells were treated with F5446 (500 nM) for 2 days and analyzed for genome-wide gene expression profiles by DNA microarray. The genes whose expression was changed by a 2-fold were selected and fed into the Panther program to group the genes based on their functions in biological process. Shown is the Gene Set Enrichment Analysis (GSEA) of the top fifteen Gene Ontology (GO) terms enriched in untreated cells. All GSEA false discovery rate q-values <0.0001. B. Representative enrichment plots. C. SW620 cells were treated with F5446 at the indicated concentrations for 48 hours. Cells were then fixed, stained with PI, and analyzed by flow cytometry for DNA content. Shown are representative histographs of flow cytometry data (top panel). The % cells at each phase were quantified and presented at the bottom panel. D. LS411N cells were treated and analyzed as in A. Columns, mean; bars, SD. Shown are representative data of one of two experiments.
Figure 4. Inhibition of SUV39H1 induces human colon tumor cell apoptosis in vitro.
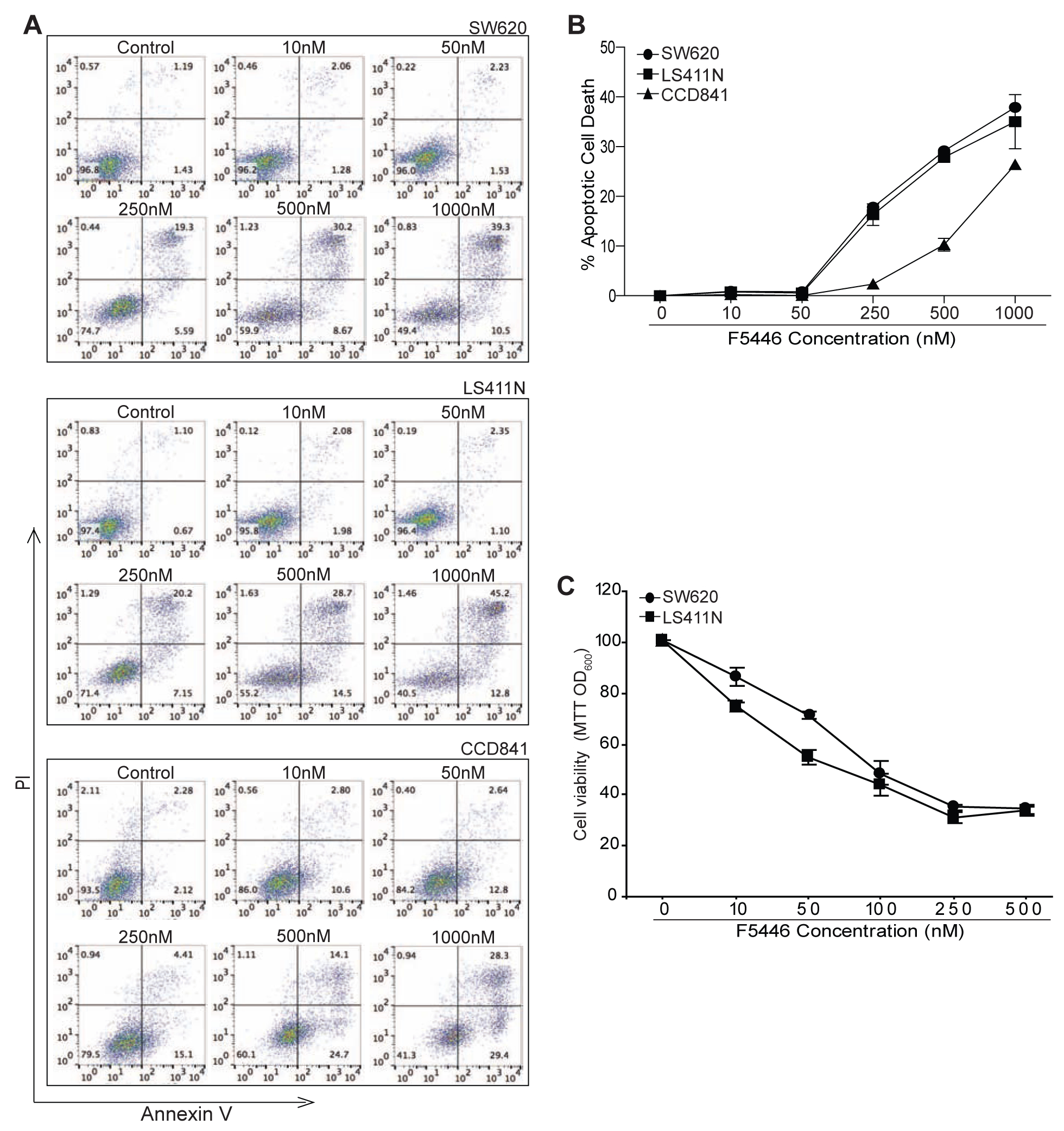
A. SW620, LS411N and CCD841 cells were cultured in the presence of F5446 at the indicated concentrations for three days. Cells were stained with Annexin V and PI and analyzed by flow cytometry. Shown are representative plots of flow cytometry data. B. The F5446-induced cell death as shown in A was quantified and presented at the right panels. The % apoptotic cell death was calculated by the formula: Annexin V+ PI+ cells in the treatment group-Annexin V+ PI+ cells in the control group. C. Tumor cells as indicated were cultured in the presence of F5446 at the indicated concentrations for three days and analyzed by MTT assay for cell viability. Shown are representative data of one of three experiments.
3.4. The SUV39H1-H3K9me3 pathway regulates colon tumor cell sensitivity to 5-FU and FasL.
5-FU can induce a DNA damage response that activates transcription factor p53 in colon tumor cells [15, 18]. It has been shown that 5-FU-activated p53 binds to a p53-binding DNA sequence element in the FAS gene intron 1 to activate FAS expression in colon tumor cells [15–17]. In addition to its direct cytotoxicity, 5-FU therapy therefore may also increase Fas expression on the colon carcinoma cells to sensitize tumor cells to FasL-induced apoptosis. Although the physiological FasL is expressed on T cells [33, 34], it is known that colorectal tumor cells also express FasL [35, 36]. It is therefore possible that tumor cells may secrete FasL to induce tumor cell apoptosis through the 5-FU-activated Fas [37]. As observed above, SUV39H1 regulates Fas expression and function, we next sought to determine whether F5446 can overcome human colon cancer cell resistance to 5-FU. SW620–5FUR and LS411N-5FUR are two 5-FU resistant cell lines established from SW620 and LS411N by increasing concentration of 5-FU selection [21]. These two cell lines were treated with 5-FU, F5446, or both 5-FU and F5446, respectively. A sublethal concentration of F5446 significantly increased LS411N-5FUR cell sensitivity to 5-FU-induced apoptosis (Fig. 5A). Due to the diminished Fas expression, both SW620–5FUR and LS411N-5FUR cells are resistant to FasL-induced apoptosis (Fig. 5B). A sublethal concentration of F5446 significantly increased both SW620–5FUR and LS411N-5FUR cell sensitivity to FasL-induced apoptosis (Fig. 5B).
Figure 5. Inhibition of SUV39H1 increases 5-FU-resistant human colon tumor cell sensitivity to 5-FU and FasL-induced apoptosis.
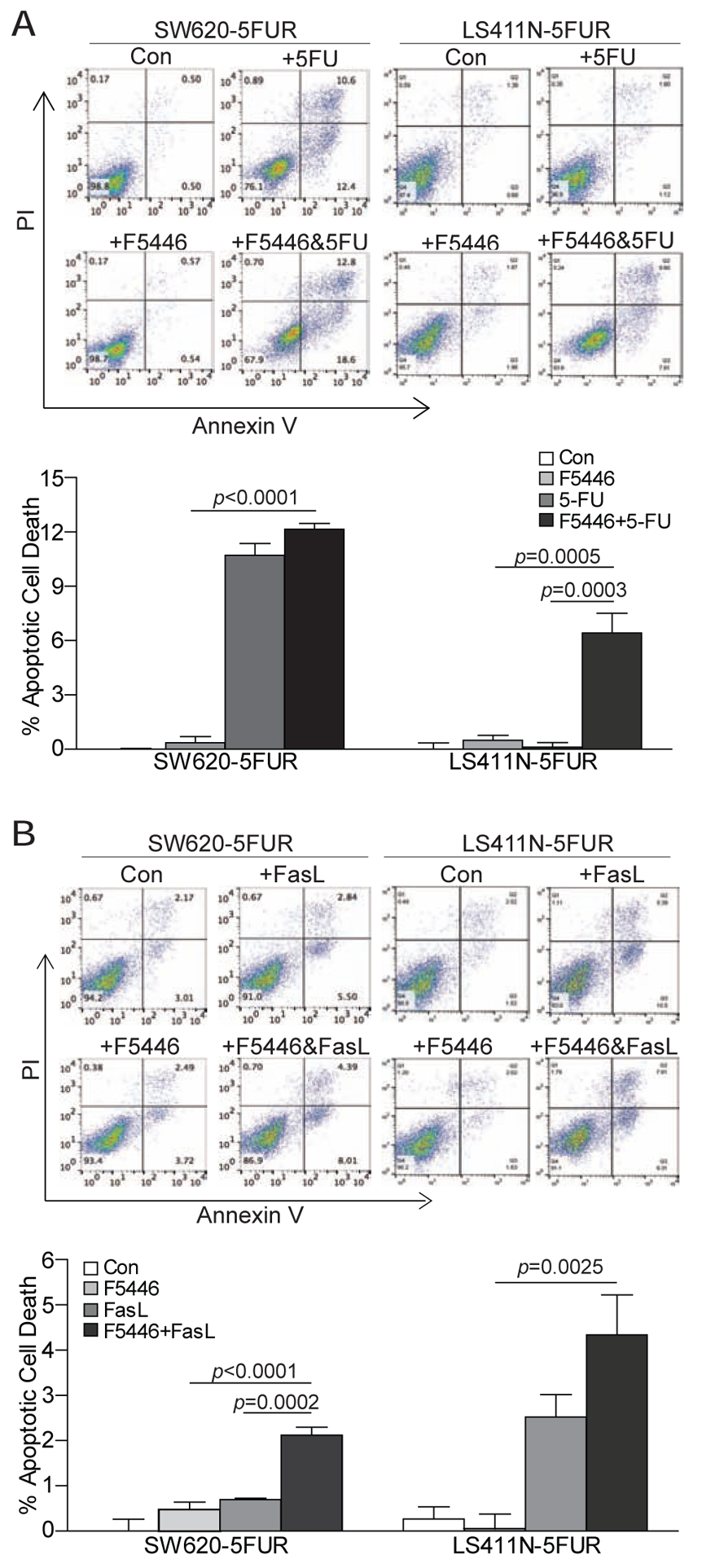
A. The 5-FU-resistant human colon tumor cells were cultured in the presence of 5-FU (2 μg/ml), F5446 (250 nM), or both 5-FU and F5446 for 3 days. The cells were then stained with Annexin V and PI and analyzed by flow cytometry. Shown are representative plots of flow cytometry data (top panel). Cell death was quantified and presented at the bottom panel. The % apoptotic cell death was calculated by the formula: Annexin V+ PI+ cells in the treatment group-Annexin V+ PI+ cells in the control group. Column: mean; Bar: SD. To determine the effect of 5FU and FasL on cell death, two-factor ANOVA that included the main effects and the two-factor interaction between the main effects was used. A Tukey-Kramer multiple comparison procedure was used on the adjusted least squares means of the two-factor interaction term if the overall F-test for the interaction effect was statistically significant. The p values are shown at top of the bars. B. The 5-FU-resistant human colon tumor cells were cultured in the presence of FasL (100 ng/ml), F5446 (250 nM), or both FasL and F5446 for 3 days. The cells were then analyzed as in A. Shown are representative plots of flow cytometry data (top panel). Cell death as shown in the top panel was quantified and presented at the bottom panel. The % apoptotic cell death was calculated by the formula: Annexin V+ PI+ cells in the treatment group-Annexin V+ PI+ cells in the control group. The p values were determined by the same method as in A. Shown are representative data of one of two experiments.
3.5. F5446 induces human colon tumor cell apoptosis and growth arrest in vitro.
To determine the efficacy of F5446 as a monotherapeutic agent in suppression of human colon carcinoma, SW620–5FUR and LS411N-5FUR cells were treated with F5446 at various concentrations in vitro. Analysis of tumor cell apoptosis determined that F5446 induced both 5-FU-resistant tumor cell line apoptosis in a concentration-dependent manner (Fig. 6A). F5446 at a concentration of 1 μM induced about 20% and 60% apoptotic cell death, respectively, in SW620–5FUR and LS411N-5FUR cells (Fig. 6B). Both SW620–5FUR and LS411N-5FUR cells were also treated with F5446 and measured for cell viability. F5446 effectively suppressed both cell line growth in a concentration-dependent manner and almost completely inhibited both SW620–5FUR and LS411N-5FUR cell growth in vitro at a concentration of 0.5 μM (Fig. 6C). These observations suggest that in addition to its function in regulating human colon carcinoma cell sensitivity to apoptosis induction, SUV39H1 plays an essential role in human colon carcinoma cell survival.
Figure 6. Inhibition of SUV39H1 results in 5-FU-resistant tumor cell apoptosis and growth suppression in vitro.
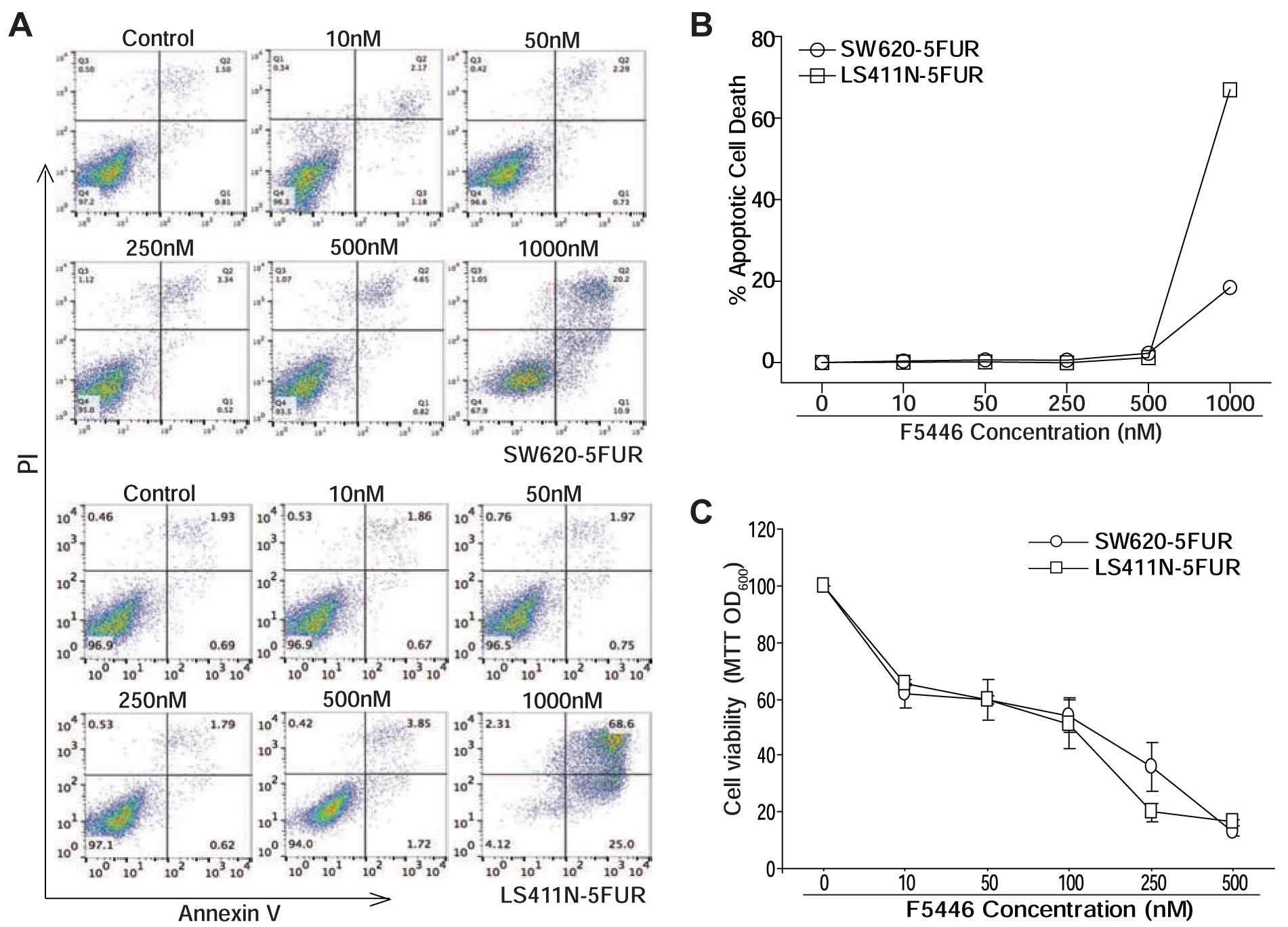
A. The 5-FU-resistant tumor cells were treated with F5446 at the indicated concentrations for 3 days. Cells were stained with annexin V and PI and analyzed by flow cytometry. Shown are representative plots of flow cytometry data (top panel). Cell death was quantified and presented at the bottom panel. The % apoptotic cell death was calculated by the formula: Annexin V+ PI+ cells in the treatment group-Annexin V+ PI+ cells in the control group. B. Tumor cells as indicated were cultured in the presence of F5446 at the indicated concentrations for three days and analyzed by MTT assay for cell viability. Shown are representative data of one of two experiments.
3.6. Targeting SUV39H1 effectively suppresses human colon tumor xenograft growth in vivo.
The above observation indicates that inhibiting SUV39H1 is effective in suppressing metastatic and 5-FU-resistant human colon tumor cell growth in vitro. To determine whether this finding can be translated to tumor growth suppression in vivo, SW620 and SW620–5FUR cells were injected to athymic mice. The tumor-bearing mice were then treated with F5446. For the SW620 xenografts, the pattern of tumor growth across the 26 days was similar in the control and the two F5446 doses (Fig. 7A & B). However, the Tukey-Kramer multiple comparison procedure indicated that an F5446 dose of 10 mg/kg had significantly smaller tumor sizes than the control group (p=0.0042) and the dose of 5 mg/kg (p=0.0135). The Tukey-Kramer multiple comparison procedure indicated that an F5446 dose of 10 mg/kg also had significantly lower tumor weight than the control group (p=0.0105) or the dose of 5 mg/kg (p=0.0204) (Fig. 7A & C).
Figure 7. F5446 suppresses human colon tumor xenograft growth in vivo.
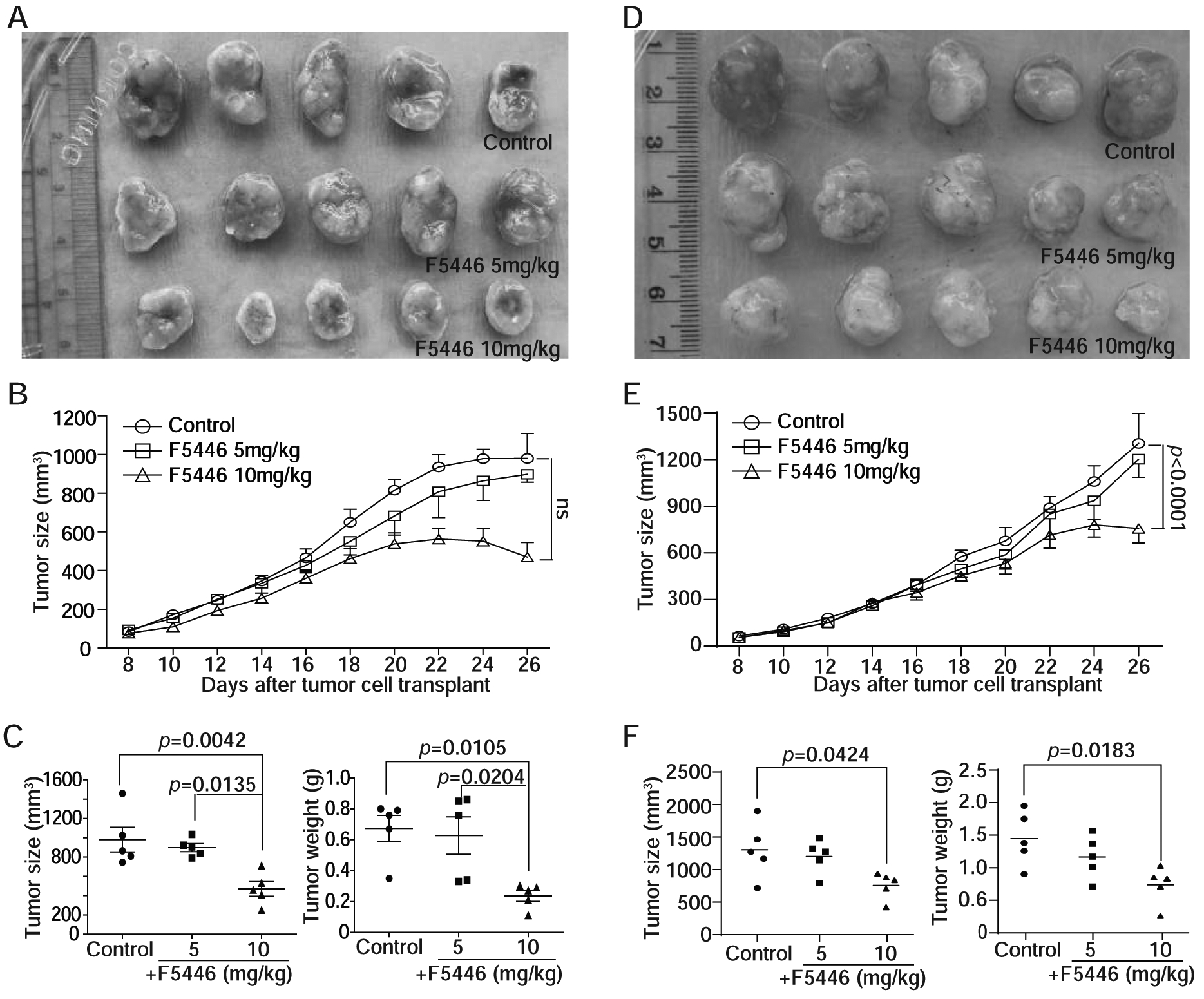
A. SW620 cells (2.5 × 106 cells/mouse) were injected subcutaneously into the right flanks of twenty athymic mice, Fifteen tumor-bearing mice with relative similar sizes of tumors were selected at day 8 after tumor cell injection and randomly grouped into three groups. The mice were then treated with solvent (control, n=5), and F5446 at 5 (n=5) and 10 mg/kg (n=5) body weight, respectively, by intraperitoneal injection every two days for 10 times. Shown are tumor images at the end of the experiment. B. Tumor growth was monitored over time and shown in A. To examine whether growth of tumor size over 26 days between F5446 doses differed, a repeated-measures mixed model was used with a Kenward-Rodger adjustment to the denominator degrees of freedom and an unstructured correlation matrix to model the correlation within animal between measurement days. C. The tumor size and weight at the end of the experiment were quantified. To determine differences in tumor size and weight at the end of the experiment between control and treatment groups, one-way ANOVA was used with a Tukey-Kramer multiple comparison test if the overall F-test was statistically significant. The p values are shown at the top. D. SW620–5FUR cells (2.5 × 106 cells/mouse) were injected subcutaneously into the right flanks of fifteen athymic mice, The tumor-bearing mice were randomly grouped into three groups. The mice were then treated as in A. E. Tumor growth was monitored over time and shown in D. Tumor growth kinetics were analyzed by statistical tools as in B. The p value is shown at the right. F. The tumor size and weight at the end of the experiment were quantified. The differences in tumor size and weight at the end of the experiment were analyzed as in C. The p values are shown at the top.
For the SW620–5FUR xenografts, F5446 at a dose 10 mg/kg had significantly lower tumor growth at day 26 than the control group (p<0.0001) and the dose of 5 mg/kg group (p=0.0035) (Fig. 7D & E). The Tukey-Kramer multiple comparison procedure indicated that a F5446 dose of 10 mg/kg had significantly smaller tumor sizes than the control group (p=0.0424). The Tukey-Kramer multiple comparison procedure indicated that a F5446 dose of 10 mg/kg also had significantly lower tumor weight than the control group (p=0.0183) (Fig. 7D & F).
To determine whether F5446 increased Fas expression in vivo, tumor cells from the SW620 xenografts were stained for Fas and analyzed by flow cytometry. F5446 significantly increased Fas expression in human colon tumor cells in vivo (Fig. S2).
4. Discussion
The H3K9me3-specific histone methyltransferase SUV39H1 plays a key role in regulating constitutive heterochromatin to silence gene expression under physiological conditions [25, 38, 39]. In this study, we observed a significantly elevated SUV39H1 expression level in human colon carcinoma. Furthermore, SUV39H1 expression level is inversely correlated with expression of the death receptor Fas in human colorectal carcinoma. In the functional level, we determined that SUV39H1 not only confers human colon tumor cell resistance to apoptosis induction, but also regulates cell cycle progression to promote human colon tumor cell survival. These observations thus indicate that the human colon carcinoma cells may use the SUV39H1-H3K9me3 axis to silence tumor suppressors to advance tumor progression under pathological conditions [21, 40–44], and suggest that SUV39H1 is a molecular target for suppressing human colorectal carcinoma, particularly the metastatic human colon carcinoma which has a high genome-wide H3K9me3 deposition [21].
Chaetocin and verticillin A are the only two known SUV39H1 inhibitors [21, 45]. Although very potent as histone methyltransferase inhibitors, chaetocin and verticillin A also inhibit several other histone methyltransferases and thus has potential toxicity [21, 45]. Moreover, due to their complicated structures, no chemical synthesis procedure has been developed for chaetocin and verticillin A. To target SUV39H1, we have developed and chemically synthesized the second generation SUV39H1-selective inhibitor F5446. Our previous preliminary toxicity studies determined that F5446 has low toxicity in mouse models in vivo [27]. Consistent with the functions of the SUV39H1-H3K9me3 axis in tumor cell apoptosis and cell cycle regulation, we determined that a sublethal concentration of F5446 is effective in sensitizing human colon carcinoma cells to apoptosis induction by FasL and 5-FU. More importantly, F5446 effectively suppressed 5-FU-resistant human colon carcinoma growth in vitro and human colon carcinoma xenograft growth in vivo. Therefore, F5446 has the great potential to be developed as a monotherapeutic agent to treat 5-FU-resistant human colorectal carcinoma.
Immune checkpoint inhibitor (ICI) immunotherapy, such as anti-PD-1 and anti-PD-L1 blocking antibody immunotherapy, blocks interactions between PD-L1and PD-1 to reverse immune suppression to unleash CTLs to kill tumor cells [46]. CTLs kill tumor cells through inducing tumor cell apoptosis by the perforin-granzyme and Fas-FasL cytotoxic pathways [10–14, 47]. To kill tumor cells, not only CTLs must be activated but also the target tumor cells must be sensitive to apoptosis induction. If tumor cells are not sensitive to apoptosis induction, then regardless how potent the CTLs are, tumor cells are not going to be killed by the CTLs. Resistance to apoptosis is a hallmark of human cancer [5], particularly the metastatic human colorectal cancer [6–10, 29]. We observed here that F5446 is effective in up-regulating Fas expression in human colorectal carcinoma and sensitizing the tumor cells to apoptosis induction. It is therefore expected that F5446 should increase colon carcinoma cells sensitivity to anti-PD-1-based ICI immunotherapy. However, this is not the case. In a recent study, we observed that F5446 and anti-PD-1 immunotherapy have no synergistic or additive efficacy in suppressing colon carcinoma growth in the immune competent syngeneic mouse colon carcinoma tumor model in vivo [27]. Furthermore, even though we demonstrated here that F5446 can effectively suppress human colon carcinoma xenograft growth in vivo in the immune-deficient mice, F5446 showed no direct effect on mouse colon carcinoma growth in vivo since neutralizing CTLs diminished F5446 efficacy in tumor suppression in the immune competent colon tumor mouse model [27]. F5446 was designed based on the human SUV39H1 protein catalytic domain structure [48]. One scenario is that F5446 may have less affinity for mouse Suv39h1 than for human SUV39H1. Nevertheless, F5446 exhibited potent activity in activating tumor-infiltrating CTL effector expression and function to suppress mouse colon carcinoma in preclinical mouse models [27], which suggest that F5446 is potentially effective in targeting both colorectal carcinoma cells and tumor-infiltrating CTLs to enhance the efficacy of ICI immunotherapy to suppress colorectal cancer, particularly the metastatic and 5-FU-resistant colorectal cancer in humans.
Supplementary Material
Highlights.
FAS expression is inversely correlated with SUV39H1 in human CRC.
Gene expression profiling revealed that SUV39H1 regulates cell cycle and apoptosis.
SUV39H1 inhibitor F5446 sensitizes human CRC to apoptosis induction by FasL and 5-FU.
F5446 induces human CRC cell cycle arrest at S phase to suppress tumor growth.
F5446 suppresses 5-FU-resistant human CRC xenograft growth in vivo.
Acknowledgement
Grant support from NIH (CA133085, CA227433, to K.L.), and VA Merit Review (CX001364 to K.L.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have declared that no conflict of interest exists
References
- [1].Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R, Zaniboni A, Tonini G, Buonadonna A, Amoroso D, Chiara S, Carlomagno C, Boni C, Allegrini G, Boni L, Falcone A, Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer, N Engl J Med, 371 (2014) 1609–1618. [DOI] [PubMed] [Google Scholar]
- [2].Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM, McWilliams RR, Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy, J Clin Oncol, 27 (2009) 3677–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Longley DB, Harkin DP, Johnston PG, 5-fluorouracil: mechanisms of action and clinical strategies, Nat Rev Cancer, 3 (2003) 330–338. [DOI] [PubMed] [Google Scholar]
- [4].Longley DB, Johnston PG, Molecular mechanisms of drug resistance, J Pathol, 205 (2005) 275–292. [DOI] [PubMed] [Google Scholar]
- [5].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell, 144 (2011) 646–674. [DOI] [PubMed] [Google Scholar]
- [6].Moller P, Koretz K, Leithauser F, Bruderlein S, Henne C, Quentmeier A, Krammer PH, Expression of APO-1 (CD95), a member of the NGF/TNF receptor superfamily, in normal and neoplastic colon epithelium, Int J Cancer, 57 (1994) 371–377. [DOI] [PubMed] [Google Scholar]
- [7].Krammer PH, CD95(APO-1/Fas)-mediated apoptosis: live and let die, Adv. Immunol, 71 (1999) 163–210. [DOI] [PubMed] [Google Scholar]
- [8].Strater J, Hinz U, Hasel C, Bhanot U, Mechtersheimer G, Lehnert T, Moller P, Impaired CD95 expression predisposes for recurrence in curatively resected colon carcinoma: clinical evidence for immunoselection and CD95L mediated control of minimal residual disease, Gut, 54 (2005) 661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Owen-Schaub LB, Fas function and tumor progression: use it and lose it, Cancer Cell, 2 (2002) 95–96. [DOI] [PubMed] [Google Scholar]
- [10].Owen-Schaub LB, van Golen KL, Hill LL, Price JE, Fas and Fas ligand interactions suppress melanoma lung metastasis, J Exp Med, 188 (1998) 1717–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Golstein P, Griffiths GM, An early history of T cell-mediated cytotoxicity, Nat Rev Immunol, (2018). [DOI] [PubMed] [Google Scholar]
- [12].Afshar-Sterle S, Zotos D, Bernard NJ, Scherger AK, Rodling L, Alsop AE, Walker J, Masson F, Belz GT, Corcoran LM, O’Reilly LA, Strasser A, Smyth MJ, Johnstone R, Tarlinton DM, Nutt SL, Kallies A, Fas ligand-mediated immune surveillance by T cells is essential for the control of spontaneous B cell lymphomas, Nat Med, 20 (2014) 283–290. [DOI] [PubMed] [Google Scholar]
- [13].Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P, Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity, Science, 265 (1994) 528–530. [DOI] [PubMed] [Google Scholar]
- [14].Caldwell SA, Ryan MH, McDuffie E, Abrams SI, The Fas/Fas ligand pathway is important for optimal tumor regression in a mouse model of CTL adoptive immunotherapy of experimental CMS4 lung metastases, J Immunol, 171 (2003) 2402–2412. [DOI] [PubMed] [Google Scholar]
- [15].Muller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman SL, Galle PR, Stremmel W, Oren M, Krammer PH, p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs, J Exp Med, 188 (1998) 2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sun XX, Dai MS, Lu H, 5-fluorouracil activation of p53 involves an MDM2-ribosomal protein interaction, J Biol Chem, 282 (2007) 8052–8059. [DOI] [PubMed] [Google Scholar]
- [17].Ju J, Schmitz JC, Song B, Kudo K, Chu E, Regulation of p53 expression in response to 5-fluorouracil in human cancer RKO cells, Clin Cancer Res, 13 (2007) 4245–4251. [DOI] [PubMed] [Google Scholar]
- [18].Petak I, Tillman DM, Houghton JA, p53 dependence of Fas induction and acute apoptosis in response to 5-fluorouracil-leucovorin in human colon carcinoma cell lines, Clin Cancer Res, 6 (2000) 4432–4441. [PubMed] [Google Scholar]
- [19].Blagitko-Dorfs N, Schlosser P, Greve G, Pfeifer D, Meier R, Baude A, Brocks D, Plass C, Lubbert M, Combination treatment of acute myeloid leukemia cells with DNMT and HDAC inhibitors: predominant synergistic gene downregulation associated with gene body demethylation, Leukemia, 33 (2019) 945–956. [DOI] [PubMed] [Google Scholar]
- [20].Kim G, Kim JY, Lim SC, Lee KY, Kim O, Choi HS, SUV39H1/DNMT3A-dependent methylation of the RB1 promoter stimulates PIN1 expression and melanoma development, FASEB J, 32 (2018) 5647–5660. [DOI] [PubMed] [Google Scholar]
- [21].Paschall AV, Yang D, Lu C, Choi JH, Li X, Liu F, Figueroa M, Oberlies NH, Pearce C, Bollag WB, Nayak-Kapoor A, Liu K, H3K9 Trimethylation Silences Fas Expression To Confer Colon Carcinoma Immune Escape and 5-Fluorouracil Chemoresistance, J Immunol, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T, Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins, Nature, 410 (2001) 116–120. [DOI] [PubMed] [Google Scholar]
- [23].Nicetto D, Donahue G, Jain T, Peng T, Sidoli S, Sheng L, Montavon T, Becker JS, Grindheim JM, Blahnik K, Garcia BA, Tan K, Bonasio R, Jenuwein T, Zaret KS, H3K9me3-heterochromatin loss at protein-coding genes enables developmental lineage specification, Science, 363 (2019) 294–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Peters AH, O’Carroll D, Scherthan H, Mechtler K, Sauer S, Schofer C, Weipoltshammer K, Pagani M, Lachner M, Kohlmaier A, Opravil S, Doyle M, Sibilia M, Jenuwein T, Loss of the Suv39h histone methyltransferases impairs mammalian heterochromatin and genome stability, Cell, 107 (2001) 323–337. [DOI] [PubMed] [Google Scholar]
- [25].Rea S, Eisenhaber F, O’Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, Jenuwein T, Regulation of chromatin structure by site-specific histone H3 methyltransferases, Nature, 406 (2000) 593–599. [DOI] [PubMed] [Google Scholar]
- [26].Pace L, Goudot C, Zueva E, Gueguen P, Burgdorf N, Waterfall JJ, Quivy JP, Almouzni G, Amigorena S, The epigenetic control of stemness in CD8(+) T cell fate commitment, Science, 359 (2018) 177–186. [DOI] [PubMed] [Google Scholar]
- [27].Lu C, Yang D, Klement JD, Oh IK, Savage NM, Waller JL, Colby AH, Grinstaff MW, Oberlies NH, Pearce CJ, Xie Z, Kulp SK, Coss CC, Phelps MA, Albers T, Lebedyeva IO, Liu K, SUV39H1 Represses the Expression of Cytotoxic T-Lymphocyte Effector Genes to Promote Colon Tumor Immune Evasion, Cancer Immunol Res, 7 (2019) 414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Maecker HL, Yun Z, Maecker HT, Giaccia AJ, Epigenetic changes in tumor Fas levels determine immune escape and response to therapy, Cancer Cell, 2 (2002) 139–148. [DOI] [PubMed] [Google Scholar]
- [29].von Reyher U, Strater J, Kittstein W, Gschwendt M, Krammer PH, Moller P, Colon carcinoma cells use different mechanisms to escape CD95-mediated apoptosis, Cancer Res, 58 (1998) 526–534. [PubMed] [Google Scholar]
- [30].Hewitt RE, McMarlin A, Kleiner D, Wersto R, Martin P, Tsokos M, Stamp GW, Stetler-Stevenson WG, Tsoskas M, Validation of a model of colon cancer progression, J Pathol, 192 (2000) 446–454. [DOI] [PubMed] [Google Scholar]
- [31].Paschall AV, Yang D, Lu C, Redd PS, Choi JH, Heaton CM, Lee JR, Nayak-Kapoor A, Liu K, CD133+CD24lo defines a 5-Fluorouracil-resistant colon cancer stem cell-like phenotype, Oncotarget, 7 (2016) 78698–78712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang Y, Rishi AK, Dawson MI, Tschang R, Farhana L, Boyanapalli M, Reichert U, Shroot B, Van Buren EC, Fontana JA, S-phase arrest and apoptosis induced in normal mammary epithelial cells by a novel retinoid, Cancer Res, 60 (2000) 2025–2032. [PubMed] [Google Scholar]
- [33].Strater J, Wellisch I, Riedl S, Walczak H, Koretz K, Tandara A, Krammer PH, Moller P, CD95 (APO-1/Fas)-mediated apoptosis in colon epithelial cells: a possible role in ulcerative colitis, Gastroenterology, 113 (1997) 160–167. [DOI] [PubMed] [Google Scholar]
- [34].LA O, Tai L, Lee L, Kruse EA, Grabow S, Fairlie WD, Haynes NM, Tarlinton DM, Zhang JG, Belz GT, Smyth MJ, Bouillet P, Robb L, Strasser A, Membrane-bound Fas ligand only is essential for Fas-induced apoptosis, Nature, 461 (2009) 659–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Peduto Eberl L, Guillou L, Saraga E, Schroter M, French LE, Tschopp J, Juillerat-Jeanneret L, Fas and Fas ligand expression in tumor cells and in vascular smooth-muscle cells of colonic and renal carcinomas, Int J Cancer, 81 (1999) 772–778. [DOI] [PubMed] [Google Scholar]
- [36].Zhang W, Ding EX, Wang Q, Zhu DQ, He J, Li YL, Wang YH, Fas ligand expression in colon cancer: a possible mechanism of tumor immune privilege, World J Gastroenterol, 11 (2005) 3632–3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Maecker HL, Koumenis C, Giaccia AJ, p53 promotes selection for Fas-mediated apoptotic resistance, Cancer Res, 60 (2000) 4638–4644. [PubMed] [Google Scholar]
- [38].Fodor BD, Shukeir N, Reuter G, Jenuwein T, Mammalian Su(var) genes in chromatin control, Annu Rev Cell Dev Biol, 26 (2010) 471–501. [DOI] [PubMed] [Google Scholar]
- [39].Muller MM, Fierz B, Bittova L, Liszczak G, Muir TW, A two-state activation mechanism controls the histone methyltransferase Suv39h1, Nat Chem Biol, 12 (2016) 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Olcina MM, Leszczynska KB, Senra JM, Isa NF, Harada H, Hammond EM, H3K9me3 facilitates hypoxia-induced p53-dependent apoptosis through repression of APAK, Oncogene, 35 (2016) 793–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Nizialek EA, Sankunny M, Niazi F, Eng C, Cancer-predisposition gene KLLN maintains pericentric H3K9 trimethylation protecting genomic stability, Nucleic Acids Res, 44 (2016) 3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Suryo Rahmanto Y, Jung JG, Wu RC, Kobayashi Y, Heaphy CM, Meeker AK, Wang TL, Shih Ie M, Inactivating ARID1A Tumor Suppressor Enhances TERT Transcription and Maintains Telomere Length in Cancer Cells, J Biol Chem, 291 (2016) 9690–9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Feng Z, Wang L, Sun Y, Jiang Z, Domsic J, An C, Xing B, Tian J, Liu X, Metz DC, Yang X, Marmorstein R, Ma X, Hua X, Menin and Daxx Interact to Suppress Neuroendocrine Tumors through Epigenetic Control of the Membrane Metallo-Endopeptidase, Cancer Res, 77 (2017) 401–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yokoyama Y, Hieda M, Nishioka Y, Matsumoto A, Higashi S, Kimura H, Yamamoto H, Mori M, Matsuura S, Matsuura N, Cancer-associated upregulation of histone H3 lysine 9 trimethylation promotes cell motility in vitro and drives tumor formation in vivo, Cancer Sci, 104 (2013) 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Greiner D, Bonaldi T, Eskeland R, Roemer E, Imhof A, Identification of a specific inhibitor of the histone methyltransferase SU(VAR)3–9, Nat Chem Biol, 1 (2005) 143–145. [DOI] [PubMed] [Google Scholar]
- [46].Wei SC, Duffy CR, Allison JP, Fundamental Mechanisms of Immune Checkpoint Blockade Therapy, Cancer Discov, 8 (2018) 1069–1086. [DOI] [PubMed] [Google Scholar]
- [47].Fingleton B, Carter KJ, Matrisian LM, Loss of functional Fas ligand enhances intestinal tumorigenesis in the Min mouse model, Cancer Res, 67 (2007) 4800–4806. [DOI] [PubMed] [Google Scholar]
- [48].Wang T, Xu C, Liu Y, Fan K, Li Z, Sun X, Ouyang H, Zhang X, Zhang J, Li Y, Mackenzie F, Min J, Tu X, Crystal structure of the human SUV39H1 chromodomain and its recognition of histone H3K9me2/3, PLoS One, 7 (2012) e52977. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


