ABSTRACT
Background
Serum folate forms were measured in the US population during recent NHANES to assess folate status.
Objective
We describe post-folic acid–fortification concentrations of serum folate forms in the fasting US population ≥1 y from the NHANES 2011–2016.
Methods
We measured 5 biologically active folates and 1 oxidation product (MeFox) of 5-methyltetrahydrofolate (5-methyl-THF). We calculated geometric means of 5-methyl-THF, unmetabolized folic acid (UMFA), nonmethyl folate (sum of tetrahydrofolate, 5-formyltetrahydrofolate, and 5,10-methenyltetrahydrofolate), total folate (sum of above biomarkers), and MeFox by demographic, physiologic, and lifestyle variables; estimated the magnitude of variables on biomarker concentrations after covariate adjustment; and determined the prevalence of UMFA >2 nmol/L.
Results
After demographic adjustment, age, sex, and race-Hispanic origin were significantly associated with most folate forms. MeFox increased with age, while 5-methyl-THF, UMFA, and nonmethyl folate displayed U-shaped age patterns. Compared with non-Hispanic whites, non-Hispanic blacks had 23% lower predicted 5-methyl-THF but comparable UMFA; non-Hispanic Asians had comparable 5-methyl-THF but 28% lower UMFA; Hispanics, non-Hispanic Asians, and non-Hispanic blacks had ∼20% lower MeFox. After additional physiologic and lifestyle adjustment, predicted UMFA and MeFox concentrations were 43% and 112% higher, respectively, in adults with chronic kidney disease and 17% and 15% lower, respectively, in adults consuming daily 1–<2 alcoholic beverages; 5-methyl-THF concentrations were 20% lower in adult smokers. The prevalence of UMFA >2 nmol/L was highest in persons aged ≥70 y (9.01%) and lowest in those aged 12–19 y (1.14%). During 2011–2014, the prevalence was 10.6% in users and 2.22% in nonusers of folic acid–containing supplements.
Conclusions
In fasting persons ≥1 y, the demographic, physiologic, and lifestyle characteristics observed with serum total folate differed among folate forms, suggesting biological and/or genetic influences on folate metabolism. High UMFA was mostly observed in supplement users and older persons.
Keywords: NHANES, 5-methyltetrahydrofolate, unmetabolized folic acid, folate oxidation product, MeFox, LC-MS/MS
Introduction
The folate status of the US population has been continuously assessed through the NHANES7 since the 1970s by measuring serum and red blood cell “total” folate (1). During the post-folic acid–fortification period, clinical folate deficiency (i.e., megaloblastic anemia) has almost been eradicated (2). However, folate insufficiency in women of reproductive age is still a public health concern because this increases the risk of neural tube birth defects in their offspring (3). Furthermore, there is interest in assessing concentrations of serum folate forms, including unmetabolized folic acid (UMFA) (1). This form appears in circulation when intake of folic acid exceeds the limited ability of the human gut to reduce and methylate folic acid (4–6).
The introduction of liquid-chromatography tandem-mass-spectrometry (LC-MS/MS) to public health laboratories allowed measurement of 5-methyltetrahydrofolate (5-methyl-THF) and UMFA in serum samples from NHANES 2007–2008 (1/3 subset) (7). UMFA was detected in almost all samples and concentrations >1 nmol/L were largely explained by fasting status and by total folic acid intake from diet and supplements. During 2011–2012, additional nonmethyl folate forms [tetrahydrofolate (THF), 5-formyltetrahydrofolate (5-formyl-THF), and 5,10-methenyltetrahydrofolate (5,10-methenyl-THF)] and an oxidation product of 5-methyl-THF (pyrazino-s-triazine derivative of 4α-hydroxy-5-methyl-THF, so called MeFox) were measured for the first time in a full set of NHANES (8). We observed mostly higher concentrations of serum folate forms in nonfasting individuals. Given that plasma folate concentrations respond rapidly to dietary intake, nonfasting concentrations must be interpreted with caution (9). In NHANES 2011–2012, the associations between each folate form and demographic, physiologic, and lifestyle variables were similar to serum total folate except for MeFox, suggesting the possibility of altered folate metabolism dependent on biological characteristics (e.g., BMI, kidney function, smoking status) (8). The same serum folate forms were measured during 2 recent NHANES survey cycles: 2013–2014 and 2015–2016. We later discovered that the UMFA measurements in 2011–2012 and 2013–2014 were biased ∼25% higher because of issues with folic acid calibrator solubility (10). The UMFA calibration bias was corrected mathematically in NHANES 2011–2014 before data release (11) and UMFA results produced during NHANES 2015–2016 are based on a modified procedure that avoided the calibration bias (10).
Our objectives were to describe post-fortification concentrations of serum folate forms for the first time in the fasting US population ≥1 y by various demographic, physiologic, and lifestyle variables with a large data set from NHANES 2011–2016. We also estimated the prevalence of high UMFA concentrations (>2 nmol/L) overall and stratified by use of folic acid–containing supplements (limited to 2011–2014). Lastly, we defined a serum total folate cutoff value associated with high UMFA to aid investigations that may not have UMFA measurements.
Methods
Participants and study design
The National Center for Health Statistics at the CDC conducts the NHANES. The continuous survey collects cross-sectional data on the health and nutritional status of the civilian noninstitutionalized US population through home-based interviews combined with medical and physical examinations at a Mobile Examination Center (MEC). NHANES uses a stratified, multistage, probability sample designed to represent the US population. Interview and examination response rates for each survey period are publicly available (12). All respondents gave their informed consent, and the National Center for Health Statistics Research Ethics Review Board approved the NHANES protocol.
Biomarker measurements
The CDC laboratory analyzed NHANES 2011–2016 serum samples from participants ≥1 y for 5 biologically active folate forms and MeFox through use of LC-MS/MS (13). Serum total folate was defined as the sum of the 5 biologically active folate forms excluding MeFox which is biologically inactive. Imputed values [limit of detection (LOD) divided by the √2] were used if any folate form result was <LOD. No serum total folate was calculated when 1 of the folate form results was missing. Long-term quality control CVs were <3% for 5-methyl-THF, and mostly <10% for other folate forms (Supplemental Table 1). For information on the UMFA calibration bias, see Supplemental Text 1.
Study variables
We categorized the demographic variables as follows: 7 age groups (1–5, 6–11, 12–19, 20–39, 40–59, 60–69, and ≥70 y), sex (males and females), and 4 race-Hispanic origin groups [Hispanic, non-Hispanic Asian (NHA), non-Hispanic black (NHB), and non-Hispanic white (NHW)]. We categorized physiologic and lifestyle variables as follows: inflammation as determined by C-reactive protein [CRP; <5 mg/L no inflammation and ≥5 mg/L inflammation (14); only available in NHANES 2015–2016 for persons ≥1 y and measured as high-sensitivity CRP]; kidney function as determined by estimated glomerular filtration rate [eGFR; 0–<60 chronic kidney disease (moderate or severe decrease in eGFR, including kidney failure), 60–<90 mild decrease in eGFR, and ≥90 mL/(min × 1.73 m2) normal eGFR (15, 16); available for persons ≥12 y]; BMI [<18.5 underweight, 18.5–<25 normal, 25–<30 overweight, and ≥30 kg/m2 obese (17); available for persons ≥2 y]; body surface area [calculated as square root of (height in cm × weight in kg/3600); <1.5 generally represents children, 1.5–<1.8, 1.8–<2.0, and ≥2.0 m2 generally represents adult men (18); available for persons ≥2 y]; smoking status as determined by serum cotinine [≤10 µg/L nonsmoker and >10 µg/L smoker (19); available for persons ≥3 y]; and alcohol intake as determined by average daily number of “standard” drinks (∼15 g alcohol/drink) [no drinks, <1 (not 0), 1–<2, and ≥2 drinks/d (20); available for persons ≥18 y]. In analyses limited to NHANES 2011–2014, we categorized data by use of folic acid–containing dietary supplements (self-reported use during the 24 h before visiting the MEC; yes and no; supplement use information not available for 2015–2016).
Statistical analyses
Statistical analyses were performed with SAS for Windows software version 9.4 (SAS Institute) and SAS callable SUDAAN software version 11 (RTI) to account for the complex survey design. We calculated nonmethyl folate as the sum of 3 minor forms (THF, 5-formyl-THF, and 5,10-methenyl-THF; nonmethyl folate <LOD if all minor forms <LOD and ≥LOD if at least 1 minor form ≥LOD). With use of descriptive analysis, we first assessed the concentration distribution of folate forms during each survey cycle for all participants ≥1 y via the 2-y MEC survey weights to account for unequal probabilities of selection and adjustment for nonresponse (Supplemental Figure 1). We found comparable median concentrations for total folate and 5-methyl-THF and only small significant differences for the minor forms present in low concentrations (UMFA, nonmethyl folate, and MeFox) (Supplemental Table 2 and Supplemental Methods 1) and thus decided to combine the data for the 3 survey cycles.
Because we noted differences in concentrations of some serum folate forms by fasting status in our previous analysis limited to 2011–2012 (8), we focused the current combined 2011–2016 analysis on samples from fasting persons (no food intake for the past ≥8 h before blood draw). Our analytical sample (n = 10,070) consisted of fasting persons ≥1 y with complete data for all serum folate forms (including MeFox) and excluded pregnant and lactating women (n = 274) (Supplemental Figure 1). We created combined 6-y MEC survey weights to calculate geometric mean concentrations (95% CI) for total folate and each folate form overall and by demographic, physiologic, and lifestyle variables. We assessed unadjusted differences for each variable with a Wald F test. We used multiple linear regression after log-transforming the dependent variable, to estimate the magnitude of demographic differences after adjusting for age, sex, and race-Hispanic origin (in fasting persons ≥1 y) and physiologic and lifestyle differences after additionally adjusting for demographic variables and eGFR, BMI, serum cotinine, and alcohol intake (in fasting persons ≥20 y). We limited the model including physiologic and lifestyle variables to adults because certain variables were unavailable for children (e.g., alcohol intake, creatinine) and we did not include inflammation because CRP data were only available for 2015–2016. To facilitate interpretation of the log-transformed model, we back-transformed the estimated β coefficients, which can be interpreted as the difference between a pair of predicted values. We assessed the significance of each variable in the model with a Satterthwaite F test. We report adjusted pairwise comparisons to the reference category; to account for multiple comparisons, the type-I error (α = 0.05) was controlled with the sequentially rejective Bonferroni procedure of Hochberg (21) with use of the Wald F P values from the regression coefficients for each comparison.
We estimated the mean % contribution of 5-methyl-THF, UMFA, and nonmethyl folate to serum total folate by demographic variables by first calculating the % contribution for each participant. We also calculated the mean % contribution of MeFox to serum total folate plus MeFox. Furthermore, we calculated the mean absolute concentrations and mean % contributions of 5-methyl-THF, UMFA, and nonmethyl folate to serum total folate by weighted decile of serum total folate. The decile categories were: 1st (<19.2); 2nd (19.2–<24.3); 3rd (24.3–<29.2); 4th (29.2–<33.9); 5th (33.9–<39.2); 6th (39.2–<45.1); 7th (45.1–<52.4); 8th (52.4–<62.3); 9th (62.3–<76.2); and 10th (≥76.2).
We determined the prevalence of high UMFA concentrations (>2 nmol/L) in our analytical sample by demographic characteristics. We chose this UMFA concentration because it was the 95th percentile in fasting persons ≥1 y in NHANES 2007–2008 and thus represents an unusual concentration (7). The 2007–2008 data were not affected by the UMFA calibration bias and thus did not require adjustment (22). To estimate a cutoff for serum total folate that is associated with high UMFA concentrations (>2 nmol/L), we conducted a receiver operating characteristic (ROC) analysis with sample data from fasting persons ≥1 y. To identify an optimal cutoff, we minimized the Euclidean distance between the ROC curve and the (0,1) point. To assess the consistency of the serum total folate cutoff, we repeated the ROC analysis in the overall population ≥1 y as a sensitivity analysis.
In exploratory analyses, we limited the NHANES data to 2011–2014 to assess another variable, namely use of folic acid–containing supplements. All statistical comparisons were evaluated at a 2-sided significance level of α = 0.05.
Results
Folate biomarker concentrations by demographic characteristics
Of the 23,682 persons ≥1 y with complete serum folate forms data and no missing fasting status data, 42.5% (unweighted; 10,070 persons) reported to have been fasting for at least 8 h before the blood draw (Table 1). There was no significant difference between fasting and nonfasting persons with regards to sex and race-Hispanic origin; however, there was a significant difference in age distribution driven by children <12 y who are not requested to fast. There was also a significant difference with regards to all physiologic and lifestyle characteristics except for alcohol intake, likely for the same reason.
TABLE 1.
Participant characteristics by variable categories in the US population ≥1 y, NHANES 2011–20161
| Persons ≥1 y | Fasting persons ≥1 y | Nonfasting persons ≥1 y | |||||
|---|---|---|---|---|---|---|---|
| Sample size | Estimate | Sample size | Estimate | Sample size | Estimate | ||
| Variables | n | % | n | % | n | % | P value2 |
| Overall | 23,682 | 100 | 10,070 | 100 | 13,612 | 100 | NA |
| Age group, y | <0.0001 | ||||||
| 1–5 | 2118 | 4.5 | 265 | 1.1 | 1853 | 7.4 | |
| 6–11 | 3021 | 7.0 | 706 | 3.2 | 2315 | 10.1 | |
| 12–19 | 3435 | 10.9 | 1660 | 11.3 | 1775 | 10.5 | |
| 20–39 | 5017 | 27.1 | 2475 | 28.6 | 2542 | 25.8 | |
| 40–59 | 5145 | 29.2 | 2545 | 31.8 | 2600 | 27.0 | |
| 60–69 | 2552 | 11.5 | 1293 | 13.9 | 1259 | 9.5 | |
| ≥70 | 2394 | 9.8 | 1126 | 10.1 | 1268 | 9.6 | |
| Sex | 0.88 | ||||||
| Male | 11,840 | 49.6 | 5028 | 49.7 | 6812 | 49.6 | |
| Female | 11,842 | 50.4 | 5042 | 50.3 | 6800 | 50.4 | |
| Race-Hispanic origin | 0.23 | ||||||
| Hispanic | 6646 | 17.0 | 2725 | 16.4 | 3921 | 17.4 | |
| Non-Hispanic Asian | 2599 | 5.1 | 1174 | 5.2 | 1425 | 5.0 | |
| Non-Hispanic black | 5475 | 11.5 | 2359 | 11.6 | 3116 | 11.4 | |
| Non-Hispanic white | 7948 | 63.0 | 3467 | 63.7 | 4481 | 62.5 | |
| Inflammation3 | 0.0195 | ||||||
| CRP <5 | 6364 | 81.4 | 2583 | 79.8 | 3781 | 82.7 | |
| CRP ≥5 | 1371 | 18.6 | 625 | 20.2 | 746 | 17.3 | |
| Kidney function4 | 0.0215 | ||||||
| eGFR 0–<60 | 1326 | 6.4 | 579 | 5.7 | 747 | 7.1 | |
| eGFR 60–<90 | 4691 | 29.3 | 2229 | 29.4 | 2462 | 29.3 | |
| eGFR ≥90 | 12,368 | 64.3 | 6213 | 64.9 | 6155 | 63.6 | |
| BMI5 | <0.0001 | ||||||
| Underweight | 3867 | 10.1 | 913 | 5.3 | 2954 | 14.2 | |
| Normal weight | 7036 | 29.9 | 3168 | 30.1 | 3868 | 29.8 | |
| Overweight | 5741 | 28.5 | 2745 | 30.1 | 2996 | 27.1 | |
| Obese | 6356 | 31.5 | 3118 | 34.5 | 3238 | 28.9 | |
| Body surface area,6 cm × kg | <0.0001 | ||||||
| <1.5 | 5724 | 14.8 | 1499 | 8.5 | 4225 | 20.1 | |
| 1.5–1.8 | 6204 | 26.7 | 2958 | 28.0 | 3246 | 25.5 | |
| 1.8–2 | 4963 | 24.6 | 2433 | 26.3 | 2530 | 23.2 | |
| ≥2 | 6109 | 33.9 | 3054 | 37.1 | 3055 | 31.1 | |
| Smoking status7 | 0.0003 | ||||||
| Cotinine ≤10 | 18,605 | 79.8 | 7916 | 77.6 | 10,689 | 81.6 | |
| Cotinine >10 | 3952 | 20.2 | 2001 | 22.4 | 1951 | 18.4 | |
| Alcohol intake8 | 0.25 | ||||||
| No drinks | 4823 | 26.3 | 2362 | 26.5 | 2461 | 26.1 | |
| <1 (not 0) | 7992 | 59.2 | 3969 | 59.3 | 4023 | 59.2 | |
| 1–<2 | 1007 | 8.9 | 490 | 8.4 | 517 | 9.4 | |
| ≥2 | 656 | 5.5 | 352 | 5.8 | 304 | 5.3 | |
Estimates are weighted percentages. Fasting refers to no food intake for the past ≥8 h before blood draw; only participants ≥12 y were requested to fast before blood draw. The table is limited to participants with no missing fasting status data and with complete serum folate forms data to allow calculation of serum total folate, excluding pregnant women and women who were lactating and/or breastfeeding. CRP, C-reactive protein; eGFR, estimated glomerular filtration rate.
Chi-square Wald F P value tests the null hypothesis of no difference in distribution of the proportions for each variable between fasting and nonfasting persons.
CRP (mg/L) used to assess inflammation; measured as serum high-sensitivity CRP in participants ≥1 y; only available in NHANES 2015–2016; CRP <5 (no inflammation), CRP ≥5 (inflammation).
eGFR [mL/(min × 1.73 m2)] used to assess kidney function; available for persons ≥12 y; eGFR 0–<60 (chronic kidney disease), eGFR 60–90 (mild decrease in kidney function); eGFR ≥90 (normal kidney function).
BMI (kg/m2): calculated for participants ≥2 y; <18.5 (underweight); 18.5–25 (normal weight); 25–<30 (overweight); and ≥30 (obese).
Body surface area used to assess body size; calculated as square root of [(height in cm × weight in kg)/3600] or square root of [(height in inches × weight in pounds)/3131]; calculated for participants ≥2 y.
Serum cotinine (µg/L) used as biomarker of tobacco smoke exposure; calculated for participants ≥3 y; cotinine ≤10 (nonsmoker), cotinine >10 (smoker).
Calculated for participants ≥18 y as average daily number of “standard” drinks [(quantity × frequency)/365.25]; 1 drink ≈15 g ethanol.
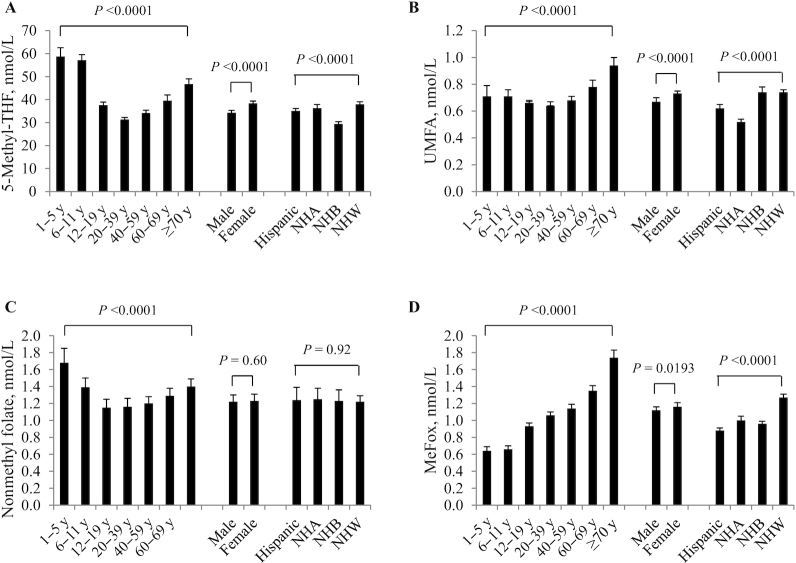
In fasting persons, age, sex, and race-Hispanic origin were significantly associated with the various folate forms before (Figure 1) and after controlling for demographic variables (Table 2), except for nonmethyl folate (no significant association with sex and race-Hispanic origin) and MeFox (no significant association with sex after covariate adjustment). We noted U-shaped age patterns for all serum folate forms where concentrations decreased from the 1–5 y age group through the 20–39 y age group and then increased with age. The only exception was for MeFox where concentrations increased with age. Percentiles presented by age, sex, and race-Hispanic origin generally showed the greatest variation by age group (Supplemental Table 3). The overall central 95% reference intervals (2.5th–97.5th percentile, nmol/L) were: serum total folate 13.3–103; 5-methyl-THF 11.7–96.6; UMFA 0.27–3.24; nonmethyl folate <LOD–4.71; and MeFox 0.38–4.39.
FIGURE 1.
Weighted geometric mean concentrations of serum 5-methyl-THF (A), UMFA (B), nonmethyl folate (C), and MeFox (D) by age, sex, and race-Hispanic origin for the fasting US population ≥1 y, NHANES 2011–2016. Error bars represent 95% CI. Sample sizes by age group, sex, and race-Hispanic origin are as listed in Table 1, fasting persons ≥1 y. Nonmethyl folate represents the sum of 3 minor forms: tetrahydrofolate, 5-formyltetrahydrofolate, and 5,10-methenyltetrahydrofolate. MeFox, pyrazino-s-triazine derivative of 4α-hydroxy-5-methyl-THF; NHA, non-Hispanic Asian; NHB, non-Hispanic black; NHW, non-Hispanic white; UMFA, unmetabolized folic acid; 5-methyl-THF, 5-methyltetrahydrofolate.
TABLE 2.
Model-adjusted % difference in biomarker concentrations for demographic variables in the fasting US population ≥1 y, NHANES 2011–20161
| Variable | Total folate | 5-Methyl-THF | UMFA | Nonmethyl folate | MeFox |
|---|---|---|---|---|---|
| Age group, y | |||||
| 1–5 | 90 (78, 102)* | 92 (81, 105)* | 11 (−1.8, 26) | 45 (32, 59)* | −36 (−41, −30)* |
| 6–11 | 81 (73, 89)* | 85 (77, 94)* | 12 (4.7, 20)* | 20 (13, 27)* | −35 (−39, −31)* |
| 12–19 | 19 (15, 23)* | 21 (17, 25)* | 2.6 (−1.9, 7.3) | −0.2 (−5.4, 5.4) | −12 (−16, −7.7)* |
| 20–392 | Reference | Reference | Reference | Reference | Reference |
| 40–59 | 7.7 (4.3, 11)* | 7.7 (4.2, 11)* | 3.4 (−1.5, 8.6) | 4.2 (−0.5, 9.0) | 5.3 (1.6, 9.2)* |
| 60–69 | 23 (16, 31)* | 23 (15, 31)* | 19 (11, 28)* | 12 (5.7, 19)* | 22 (15, 28)* |
| ≥70 | 44 (37, 51)* | 44 (37, 52)* | 41 (31, 51)* | 22 (15, 29)* | 54 (45, 64)* |
| Pmodel3 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Sex | |||||
| Male2 | Reference | Reference | Reference | Reference | Reference |
| Female | 10 (7.4, 14)* | 11 (7.8, 14)* | 7.0 (3.2, 11)* | 0.0 (−2.2, 2.3) | 3.0 (−0.2, 6.4) |
| Pmodel3 | <0.0001 | <0.0001 | 0.0004 | 0.99 | 0.06 |
| Race-Hispanic origin | |||||
| Hispanic | −6.5 (−9.7, −3.2)* | −6.8 (−10, −3.2)* | −12 (−17, −7.8)* | 3.1 (−6.4, 14) | −24 (−27, −21)* |
| Non-Hispanic Asian | −2.1 (−6.3, 2.2) | −1.9 (−6.4, 2.8) | −28 (−31 −24)* | 4.1 (−2.9, 12) | −18 (−22, −14)* |
| Non-Hispanic black | −21 (−24, −18)* | −23 (−26, −20)* | 3.2 (−2.0, 8.6) | 1.9 (−5.9, 10) | −20 (−23, −16)* |
| Non-Hispanic white2 | Reference | Reference | Reference | Reference | Reference |
| Pmodel3 | <0.0001 | <0.0001 | <0.0001 | 0.76 | <0.0001 |
| R2 (%)4 | 10 | 10 | 5.1 | 1.9 | 14 |
Values are weighted % difference (95% CI) in the adjusted geometric mean (or predicted value) relative to the reference category. Fasting refers to no food intake for the past ≥8 h before blood draw. Total folate is the sum of biologically active folate forms (5-methyl-THF, UMFA, and nonmethyl folate), not including MeFox. Nonmethyl folate is the sum of 3 minor forms (tetrahydrofolate, 5-formyltetrahydrofolate, and 5,10-methenyltetrahydrofolate). MeFox, pyrazino-s-triazine derivative of 4α-hydroxy-5-methyl-THF; UMFA, unmetabolized folic acid; 5-methyl-THF, 5-methyltetrahydrofolate.
For every comparison to the reference category, the type-I error was controlled with use of the sequentially rejective Bonferroni procedure of Hochberg (21) with the Wald F P values from the regression coefficients; asterisked when P ≤ 0.05.
P model is the Satterthwaite F P value adjusted for age, sex, and race-Hispanic origin, but not for multiple comparisons; tests the null hypothesis of no difference in the geometric means across the categories for each demographic variable.
The model R2 indicates the % of the biomarker's (on the log scale) total variability explained by a model that includes age, sex, and race-Hispanic origin.
The 3 demographic variables explained a small portion of the biomarker's total variability: serum total folate (R2 = 10%), 5-methyl-THF (R2 = 10%), UMFA (R2 = 5.1%), nonmethyl folate (R2 = 1.9%), and MeFox (R2 = 14%) (Table 2). After controlling for demographic variables, children <12 y had ∼80–90% higher serum total folate and 5-methyl-THF concentrations, while they had ∼10% higher UMFA, 20–45% higher nonmethyl folate, and ∼35% lower MeFox compared with the reference group. Predicted serum total folate, 5-methyl-THF, UMFA, and MeFox concentrations were ∼20% higher in persons 60–69 y but ∼40–50% higher in persons ≥70 y compared with the reference group. Females had significantly higher predicted serum total folate (10%), 5-methyl-THF (11%), and UMFA (7%) concentrations, but comparable nonmethyl folate and MeFox to males. NHB persons had 21% and 23% lower predicted serum total folate and 5-methyl-THF concentrations, respectively, but comparable UMFA with NHW persons. NHA persons, on the other hand, had comparable predicted serum total folate and 5-methyl-THF concentrations but 28% lower UMFA compared with NHW persons. We observed no race-Hispanic origin differences for nonmethyl folate. Hispanic, NHA, and NHB persons all had ∼20% lower predicted MeFox concentrations compared with NHW persons. An exploratory analysis limited to NHANES 2011–2014 in which we additionally adjusted for use of folic acid–containing supplements, produced similar results to those mentioned above except that Hispanic persons no longer had significantly higher predicted total folate, 5-methyl-THF, and UMFA concentrations compared with NHW persons (data not shown). Controlling for supplement use in addition to demographic variables explained approximately twice the variability in biomarker concentration around its mean except for MeFox where we observed no change (data not shown).
Folate biomarker concentrations by physiologic and lifestyle characteristics
In fasting persons ≥1 y, all physiologic and lifestyle variables were significantly associated with total folate, 5-methyl-THF, nonmethyl folate, and MeFox, while only kidney function and alcohol intake were significantly associated with UMFA (Supplemental Table 4). After controlling for covariates (age, sex, race-Hispanic origin, kidney function, BMI, smoking, and alcohol intake) in persons ≥20 y, kidney function was no longer significantly associated with total folate and 5-methyl-THF; BMI, smoking, and alcohol intake were no longer significantly associated with nonmethyl folate; and the association between alcohol intake and UMFA became significant (Table 3). In adults with chronic kidney disease, predicted UMFA (43%) and MeFox (112%) concentrations were substantially higher compared with adults with normal kidney function. In obese adults, predicted total folate and 5-methyl-THF concentrations were ∼14% lower, while MeFox was 13% higher compared with adults with normal BMI. In smokers, predicted total folate and 5-methyl-THF concentrations were ∼20% lower, while MeFox was 8.4% higher compared with nonsmokers. Daily consumption of 1–<2 alcoholic beverages resulted in lower predicted UMFA (17%) and MeFox (15%) concentrations compared with no alcohol consumption. The addition of physiologic and lifestyle variables to the model explained only slightly more of the biomarker's total variability compared to demographic variables only: serum total folate (R2 = 11%), 5-methyl-THF (R2 = 11%), UMFA (R2 = 7.3%), nonmethyl folate (R2 = 1.7%), and MeFox (R2 = 18%).
TABLE 3.
Model-adjusted % difference in biomarker concentrations for physiological and lifestyle variables in the fasting US population ≥20 y, NHANES 2011–20161
| Variable | Total folate | 5-Methyl-THF | UMFA | Nonmethyl folate | MeFox |
|---|---|---|---|---|---|
| Kidney function2 | |||||
| eGFR 0–<60 | 2.9 (−6.8, 14) | 0.8 (−8.6, 11) | 43 (28, 60)* | 12 (3.2, 21) | 112 (90, 138)* |
| eGFR 60–<90 | −0.6 (−5.2, 4.2) | −1.1 (−5.8, 3.8) | 8.0 (2.0, 14)* | 4.3 (−0.8, 9.7) | 14 (9.4, 20)* |
| eGFR ≥903 | Reference | Reference | Reference | Reference | Reference |
| Pmodel4 | 0.72 | 0.84 | <0.00001 | 0.0302 | <0.00001 |
| BMI5 | |||||
| Underweight | −3.6 (−13, 7.0) | −3.1 (−13, 8.2) | 1.6 (−7.9, 12) | 2.1 (−7.6, 13) | −6.4 (−17, 5.4) |
| Normal weight3 | Reference | Reference | Reference | Reference | Reference |
| Overweight | −5.4 (−9.4, −1.1) | −5.4 (−9.6, −0.9) | −1.5 (−5.9, 3.1) | 2.5 (−1.2, 6.3) | 3.7 (−2.4, 10) |
| Obese | −13 (−16, −9.9)* | −14 (−17, −10)* | −5.2 (−10, −0.0) | 4.8 (−0.4, 10) | 13 (7.4, 20)* |
| Pmodel4 | <0.00001 | <0.00001 | 0.08 | 0.24 | <0.00001 |
| Smoking status6 | |||||
| Cotinine ≤103 | Reference | Reference | Reference | Reference | Reference |
| Cotinine >10 | −19 (−22, −15)* | −20 (−24, −17)* | 0.8 (−4.8, 6.8) | −2.3 (−7.1, 2.8) | 8.4 (2.7, 14)* |
| P model 4 | <0.00001 | <0.00001 | 0.78 | 0.37 | 0.0042 |
| Alcohol intake7 | |||||
| No drinks3 | Reference | Reference | Reference | Reference | Reference |
| <1 (not 0) | −3.4 (−6.5, −0.1) | −3.4 (−6.6, −0.0) | −9.3 (−13, −5.2)* | −0.3 (−4.9, 4.5) | −9.9 (−13, −6.6)* |
| 1–<2 | −9.6 (−16, −3.3)* | −10 (−17, −3.8)* | −17 (−25, −9.0)* | 4.3 (−3.6, 13) | −15 (−20, −11)* |
| ≥2 | −2.5 (−10, 6.2) | −2.5 (−11, 6.8) | −18 (−26, −9.6)* | 7.3 (−2.0, 18) | −12 (−19, −3.9)* |
| Pmodel4 | 0.0323 | 0.0254 | <0.0001 | 0.26 | <0.00001 |
| R2 (%)8 | 11 | 11 | 7.3 | 1.7 | 18 |
Values are weighted % difference (95% CI) in the adjusted geometric mean (or predicted value) relative to the reference category. Fasting refers to no food intake for the past ≥8 h before blood draw. Total folate is the sum of biologically active folate forms (5-methyl-THF, UMFA, and nonmethyl folate), not including MeFox. Nonmethyl folate is the sum of 3 minor forms (tetrahydrofolate, 5-formyltetrahydrofolate, and 5,10-methenyltetrahydrofolate). eGFR, estimated glomerular filtration rate; MeFox, pyrazino-s-triazine derivative of 4α-hydroxy-5-methyl-THF; UMFA, unmetabolized folic acid; 5-methyl-THF, 5-methyltetrahydrofolate.
eGFR [mL/(min × 1.73 m2)] used to assess kidney function; available for persons ≥12 y; eGFR 0–<60 (chronic kidney disease), eGFR 60–90 (mild decrease in kidney function); eGFR ≥90 (normal kidney function).
For every comparison to the reference category, the type-I error was controlled with use of the sequentially rejective Bonferroni procedure of Hochberg (21) with the Wald F P values from the regression coefficients; asterisked when P ≤ 0.05.
P model is the Satterthwaite F P value adjusted for age, sex, race-Hispanic origin, eGFR, BMI, serum cotinine, and alcohol intake, but not for multiple comparisons; tests the null hypothesis of no difference in the geometric means across the categories for each demographic variable.
BMI (kg/m2): calculated for participants ≥2 y; <18.5 (underweight); 18.5–25 (normal weight); 25–<30 (overweight); and ≥30 (obese).
Serum cotinine (µg/L) used as biomarker of tobacco smoke exposure; calculated for participants ≥3 y; cotinine ≤10 (nonsmoker), cotinine >10 (smoker).
Calculated for participants ≥18 y as average daily number of “standard” drinks [(quantity × frequency)/365.25]; 1 drink ≈15 g ethanol.
The model R2 indicates the % of the biomarker's (on the log scale) total variability explained by a model that includes age, sex, race-Hispanic origin, eGFR, BMI, serum cotinine, and alcohol intake.
Contribution of folate forms to serum total folate
In fasting persons, 5-methyl-THF (93.7%) was the biggest contributor to serum total folate, whereas UMFA (2.4%) and nonmethyl folate (3.9%) contributed only small amounts (Supplemental Table 5). Although there was a significant age effect for the relative contribution of folate forms to serum total folate, only MeFox showed a clear age pattern with increasing contribution from 1.3% (children 1–5 y) to 4.2% (persons ≥70 y) to total folate plus MeFox. There was no significant sex difference in the relative contribution of UMFA to serum total folate and of MeFox to serum total folate plus MeFox. Although the relative contribution varied significantly by race-Hispanic origin, differences were small.
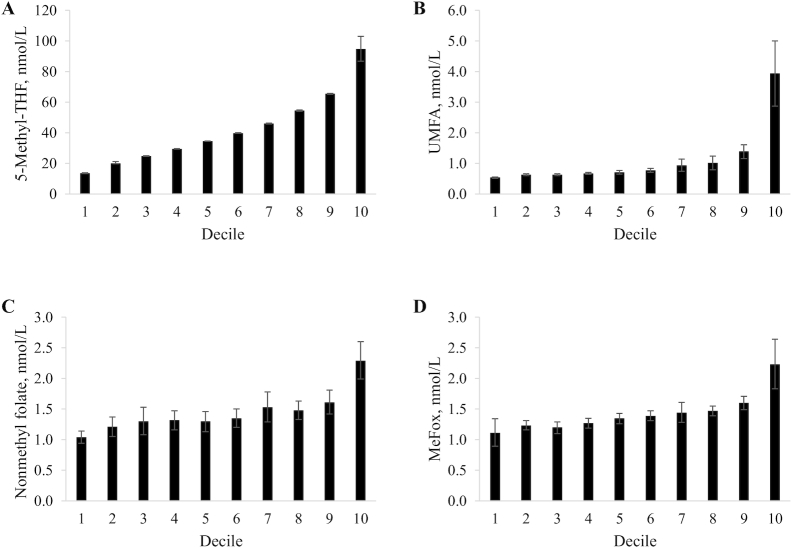
When we categorized concentrations of folate forms by decile of serum total folate, we noted a mostly linear increase in 5-methyl-THF concentrations, while UMFA, nonmethyl folate, and MeFox concentrations were mostly constant up to the 8th or 9th decile but showed a clear increase in the 10th decile (Figure 2 and Supplemental Table 6). The relative contribution of folate forms to serum total folate by decile showed some fluctuations for 5-methyl-THF (89.2–95.6%) and UMFA (1.8–3.7%), while nonmethyl folate showed a decrease with increasing decile (7.1–2.3%) (Supplemental Table 6). In NHANES 2011–2014 (Supplemental Figure 2), about half of the people in the 10th decile of serum total folate were ≥60 y (48%, Supplemental Figure 2A) and about two-thirds were supplement users (64%, Supplemental Figure 2D) compared to only 19% and 7.4% in the first decile, respectively.
FIGURE 2.
Weighted mean absolute concentration of 5-methyl-THF (A), UMFA (B), nonmethyl folate (C), and MeFox (D) by weighted decile of serum total folate in the fasting US population ≥1 y, NHANES 2011–2016. Error bars represent 95% CI. Sample sizes by decile are as listed in Supplemental Table 6 (between 961 and 1041 persons per decile). Serum total folate represents the sum of 5-methyl-THF, UMFA, and nonmethyl folate. Nonmethyl folate represents the sum of 3 minor forms: tetrahydrofolate, 5-formyltetrahydrofolate, and 5,10-methenyltetrahydrofolate. UMFA, unmetabolized folic acid; 5-Methyl-THF, 5-methyltetrahydrofolate.
Prevalence of high UMFA and cutoff for serum total folate to define high UMFA
The overall prevalence of UMFA concentrations >2 nmol/L was 4.18% in the fasting US population ≥1 y in 2011–2016 and 4.67% in 2011–2014 (Table 4). While the prevalence was comparable in males and females (∼4%), it differed by age group (from ∼1% in persons 12–19 y to ∼10% in persons ≥70 y) and race-Hispanic origin (from ∼2% in Hispanic persons to ∼5% in NHW persons). In 2011–2014, ∼10% of supplement users compared to only ∼2% of nonusers had high UMFA and the prevalence in older supplement users (≥70 y) was 20.3% (Table 4).
TABLE 4.
Prevalence of high serum UMFA concentrations by demographic variables and stratified by folic acid–containing supplement use for the fasting US population ≥1 y, NHANES 2011–20161
| 2011–2016 | 2011–2014 | |||
|---|---|---|---|---|
| Variable | Overall | Overall | Supplement users | Supplement nonusers |
| Overall | 4.18 (3.63, 4.81) 10,070 | 4.67 (3.96, 5.49) 6781 | 10.6 (8.98, 12.6) 1705 | 2.22 (1.65, 2.97) 5076 |
| Age group, y | ||||
| 1–5 | 4.08 (1.75, 9.22) 265 | 5.57 (2.15, 13.7) 165 | 4.22 (1.04, 15.6) 41 | 5.98 (1.85, 17.6) 124 |
| 6–11 | 3.48 (1.93, 6.20) 706 | 4.41 (2.24, 8.50) 481 | 6.52 (2.19, 17.8) 97 | 3.74 (1.91, 7.21) 384 |
| 12–19 | 1.14 (0.70, 1.84) 1660 | 1.15 (0.61, 2.16) 1140 | 2.04 (0.44, 8.95) 168 | 0.99 (0.53, 1.84) 972 |
| 20–39 | 2.99 (2.12, 4.21) 2475 | 3.59 (2.41, 5.31) 1708 | 9.46 (5.99, 14.6) 384 | 1.70 (0.93, 3.11) 1324 |
| 40–59 | 4.27 (3.26, 5.59) 2545 | 5.00 (3.61, 6.89) 1724 | 10.2 (7.38, 13.9) 497 | 2.59 (1.53, 4.34) 1227 |
| 60–69 | 5.55 (3.71, 8.21) 1293 | 4.56 (2.66, 7.71) 833 | 9.38 (5.71, 15.0) 253 | 1.60 (0.46, 5.47) 580 |
| ≥70 | 9.01 (6.87, 11.70) 1126 | 11.1 (8.08, 15.0) 739 | 20.3 (14.3, 28.0) 265 | 4.53 (2.31, 8.68) 465 |
| P value2 | <0.0001 | <0.0001 | 0.0004 | 0.0004 |
| Sex | ||||
| Male | 4.45 (3.67, 5.39) 5028 | 4.81 (3.80, 6.07) 3379 | 11.9 (9.28, 15.2) 782 | 2.22 (1.51, 3.24) 2597 |
| Female | 3.91 (3.27, 4.68) 5042 | 4.52 (3.77, 5.41) 3402 | 9.58 (7.33, 12.4) 923 | 2.21 (1.56, 3.14) 2479 |
| P value2 | 0.30 | 0.64 | 0.25 | 0.10 |
| Race-Hispanic origin | ||||
| Hispanic | 1.72 (1.26, 2.35) 2725 | 1.96 (1.39, 2.75) 1672 | 5.74 (3.47, 9.35) 304 | 1.08 (0.63, 1.85) 1368 |
| Non-Hispanic Asian | 2.33 (1.52, 3.56) 1174 | 2.53 (1.54, 4.11) 814 | 10.7 (7.27, 15.4) 345 | 1.18 (0.53, 2.59) 594 |
| Non-Hispanic black | 4.46 (3.58, 5.54) 2359 | 4.32 (3.16, 5.87) 1628 | 11.8 (9.71, 14.2) 779 | 2.60 (1.67, 4.04) 1283 |
| Non-Hispanic white | 5.02 (4.24, 5.94) 3467 | 5.67 (4.71, 6.83) 2453 | 6.12 (3.42, 10.7) 220 | 2.63 (1.88, 3.67) 1674 |
| P value2 | <0.0001 | <0.0001 | 0.0054 | 0.0071 |
Values are weighted % (95% CI) and sample size (in italics). Fasting refers to no food intake for the past ≥8 h before blood draw. UMFA, unmetabolized folic acid.
Chi-square P value tests the null hypothesis of no difference in prevalence across the categories for each demographic variable.
In the fasting population ≥1 y, the optimal cutoff for serum total folate obtained by ROC analysis was 56 nmol/L, with a sensitivity of 78% and a specificity of 76%; the AUC was 0.834 (data not shown). When we repeated the analysis with the overall population ≥1 y, we obtained very similar results: serum total folate optimal cutoff of 54 nmol/L, sensitivity of 78%, specificity of 76%, and AUC of 0.848 (data not shown).
Discussion
The uniqueness of this paper is that it describes nationally representative estimates for post-fortification concentrations of serum folate forms and total folate measured by LC-MS/MS in the fasting US population over a 6-y period of NHANES (2011–2016). The demographic, physiologic, and lifestyle characteristics noted for serum total folate differed among folate forms.
Most folate forms displayed a U-shaped age pattern, except for MeFox where concentrations increased with age, suggesting more MeFox generation or accumulation in older compared with younger persons. After we adjusted for the effect of age in our model, we saw that impaired kidney function was still associated with higher MeFox concentrations (nearly double in adults with chronic kidney disease), which may suggest impaired clearance as a reason for high MeFox concentrations in older persons. Given that predicted MeFox concentrations were also higher in obese adults and smokers, and that unadjusted MeFox concentrations were higher in inflammation, MeFox may be an indicator of negative health factors. The lower predicted MeFox in Hispanic and NHA persons compared to NHW persons relative to the similar 5-methyl-THF concentrations in these 3 groups may indicate different metabolism possibly as a result of genetic differences. These findings, while mostly consistent with our previous report (8), can be interpreted more reliably as they represent a large fasting data set.
We are unaware of other reports discussing MeFox by various characteristics. Of note is a recent case-control study of daily supplementation with 5 mg folic acid in Brazilian patients with hereditary spherocytosis, which found a much higher ratio of MeFox to 5-methyl-THF in supplemented patients (∼8 nmol/L to ∼60 nmol/L) compared to healthy controls (∼1 nmol/L to ∼30 nmol/L, which was similar to our study) (23). These observations strengthen our working hypothesis that MeFox in serum may not be entirely generated post-blood collection from 5-methyl-THF oxidation. We therefore question the approach of use of a sum indicator called “methylated folate” (sum of 5-methyl-THF and MeFox) to interpret folate status (24). Instead, we suggest reporting results separately for 5-methyl-THF and MeFox to provide broader utility to investigators. In addition to providing relevant information regarding the quality of sample handling, MeFox data may also provide insights into folate metabolism. Although it is still debatable whether to include MeFox as part of the total folate, it only contributes 3.6% and not including MeFox represents a more conservative approach to folate status assessment because it avoids underestimating the prevalence of low folate concentrations.
UMFA concentrations were not proportional to total folate concentrations across race-Hispanic origin groups. Of note are the lower predicted UMFA concentrations in NHA compared with NHW persons relative to the similar total folate in these 2 groups; this holds true even after additionally adjusting for supplement use in NHANES 2011–2014 (data not shown). Also of note are the comparable predicted UMFA concentrations in NHB compared with NHW persons despite NHB persons having lower total folate, which also holds true after supplement use adjustment. These race-Hispanic origin differences in UMFA seem to be independent of supplement use and may point to differences in uptake, reduction, and/or methylation of folic acid in different population groups. Previous studies found a limited ability of the human gut to reduce and methylate folic acid (4), human liver has been shown to have low and variable dihydrofolate reductase activity (25), and Kalmbach et al. found a polymorphism of dihydrofolate reductase that may limit folic acid assimilation into cellular folate stores (26). The higher predicted UMFA concentrations with decreasing kidney function likely indicate impaired clearance, whereas the lower predicted UMFA and MeFox concentrations with increasing alcohol intake may be a result of increased clearance and/or altered metabolism (27).
Only limited UMFA data are available from other populations. In the UK National Diet and Nutrition Survey Rolling Programme (Years 1 to 4), the median (95% central reference interval; % undetectable) UMFA concentration in those aged 19–64 y was 0.33 nmol/L [0.06–1.12 nmol/L; ∼30%; (28)] compared with 0.65 nmol/L (0.27–3.24 nmol/L; ∼1%) in fasting persons ≥1 y in our study. Other reports of small convenience samples also showed lower median (% undetectable) UMFA concentrations: 0.34 nmol/L (6%) in fasting older Irish persons [voluntary fortification; (29)]; 0.10 nmol/L (80%) in nonpregnant German women who were not taking supplements [no fortification; (30)]; and 0.08 nmol/L (74%) in fasting older German persons who were not taking supplements [no fortification; (31)].
Interestingly nonmethyl folate did not show sex or race-Hispanic origin differences, nor did it show an association with BMI, smoking status, or alcohol intake after covariate adjustment; it only showed a significant positive association with kidney function. This folate component is mostly composed of THF. If the THF is produced from UMFA reduction during absorption, one may expect higher THF with higher UMFA concentrations. Earlier we found higher UMFA concentrations in nonfasting compared with fasting persons at the upper tail of the distribution [95th percentile of 13.7 compared with 2.47 nmol/L in persons ≥1 y, (8)], but comparable nonmethyl folate concentrations [5.29 compared with 4.99 nmol/L, (8)], suggesting that the appearance of nonmethyl folate may be rate-limited.
The prevalence of high UMFA was ∼10% or less regardless of demographic subgroup and it was highest among supplement users ≥70 y (∼20% in 2011–2014). In 2011–2016, 47.3% of persons with high UMFA belonged to the 10th decile of serum total folate and 79.2% belonged to the 8th decile or higher (data not shown). Our serum total folate cutoff of 56 nmol/L associated with high UMFA (>2 nmol/L) was similar to the cutoff found in a recent case-control study in Brazilian patients with hereditary spherocytosis supplemented with a daily 5 mg dose of folic acid [54 nmol/L, (23)]. The availability of such a cutoff may help other investigators predict the proportion of participants with high UMFA in the absence of measured UMFA data. However, the sensitivity (78%) and specificity (76%) found in our study indicate that there are some false positives and false negatives with this approach. The higher sensitivity (100%) and specificity (91.7%) found in the Brazilian study may have been a result of the larger proportion of high UMFA because of the high folic acid dose (23). Confirmation from other studies would be desirable.
The major strength of this study is the use of a large nationally representative, racially and ethnically diverse survey spanning 6 y of NHANES. Because of the large sample size, we were able to focus on the fasting US population and explore folate status across a variety of variables, which we could not do previously because of sample size limitations (8). For example, in our previous report (overall population) UMFA was significantly associated with BMI and smoking status (8), whereas in the current analysis (fasting persons) it is not, likely because of the clearance of the circulating UMFA compared to the nonfasting state. Furthermore, MeFox is significantly associated with smoking status in current reports, but was not previously (8), likely because of the confounding effect of nonfasting on the association between MeFox and smoking. These examples show that in some instances the interpretation based on the overall population can be misleading. Another strength of this study is that it used accurately calibrated or adjusted UMFA data. While it would be of interest to characterize concentrations of folate forms by intake and/or supplement use, we conducted exploratory analyses by supplement use where this variable was available. Although the clinical significance of serum total folate is well understood, the clinical interpretation of folate forms is yet to be defined. In conclusion, these findings identify population groups susceptible to higher concentrations of folate forms, including UMFA. This in turn may help identify at-risk populations for potential cause-and-effect relations between excess folate and adverse health outcomes.
Supplementary Material
Acknowledgments
We acknowledge contribution from the following laboratory members: Donna LaVoie, Ralph D. Whitehead, Bridgette Haynes, and Daniel Rabinowitz, (CDC's National Center for Environmental Health) for performing the serum folate forms analysis by LC-MS/MS. The authors’ responsibilities were as follows—CMP: primary responsibility for content; CMP: developed the study concept; MRS: performed the statistical data analysis: ZF: responsible for drafting the manuscript; ZF, CMP, and MRS: participated in the study design; all authors: participated in the interpretation of the data and were responsible for critical revision of the manuscript; and all authors: read and approved the final manuscript.
Notes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, the National Institutes of Health, the Food and Drug Administration, or the Department of Health and Human Services.
This work was performed under employment of the U.S. Federal government and the authors did not receive any outside funding.
Author disclosures: The authors report no conflicts of interest.
Supplemental Text 1, Supplemental Methods 1, Supplemental Tables 1–6 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; LOD, limit of detection; MEC, Mobile Examination Center; MeFox, pyrazino-s-triazine derivative of 4α-hydroxy-5-methyl-THF; NHA, non-Hispanic Asian; NHB, non-Hispanic black; NHW, non-Hispanic white; ROC, receiver operating characteristic; THF, tetrahydrofolate; UMFA, unmetabolized folic acid; 5-formyl-THF, 5-formyltetrahydrofolate; 5,10-methenyl-THF, 5,10-methenyltetrahydrofolate; 5-methyl-THF, 5-methyltetrahydrofolate.
References
- 1. Yetley EA, Pfeiffer CM, Phinney KW, Fazili Z, Lacher DA, Bailey RL, Blackmore S, Bock JL, Brody LC, Carmel R et al.. Biomarkers of folate status in the National Health and Nutrition Examination Survey (NHANES): a roundtable summary. Am J Clin Nutr. 2011;94:303S–12S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pfeiffer CM, Sternberg MR, Zhang M, Fazili Z, Storandt RJ, Crider KS, Yamini S, Gahche JJ, Juan WY, Wang C-Y et al.. Folate status in the US population 20 y after the introduction of folic acid fortification. Am J Clin Nutr. 2019;110(5):1088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tinker SC, Hamner HC, Qi YP, Crider KS. U.S. women of childbearing age who are at possible increased risk of a neural tube defect-affected pregnancy due to suboptimal red blood cell folate concentrations, National Health and Nutrition Examination Survey 2007 to 2012. Birth Defects Res A Clin Mol Teratol. 2015;103:517–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patanwala I, King MJ, Barrett DA, Rose J, Jackson R, Hudson M, Philo M, Dainty JR, Wright AJA, Finglas PM et al.. Folic acid handling by the human gut: implications for food fortification and supplementation. Am J Clin Nutr. 2014;100:593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kelly P, McPartlin J, Goggins M, Weir DG, Scott JM. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. Am J Clin Nutr. 1997;65:1790–5. [DOI] [PubMed] [Google Scholar]
- 6. Sweeney MR, McPartlin J, Weir DG, Daly L, Scott JM. Postprandial serum folic acid response to multiple doses of folic acid in fortified bread. Br J Nutr. 2006;95:145–51. [DOI] [PubMed] [Google Scholar]
- 7. Pfeiffer CM, Sternberg MR, Fazili Z, Yetley EA, Lacher DA, Bailey RL, Johnson CL. Unmetabolized folic acid is detected in nearly all serum samples from US children, adolescents, and adults. J Nutr. 2015;145:520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pfeiffer CM, Sternberg MR, Fazili Z, Lacher DA, Zhang M, Johnson CL, Hamner HC, Bailey RL, Rader JI, Yamini S et al.. Folate status and concentrations of serum folate forms in the US population: National Health and Nutrition Examination Survey 2011–2. Br J Nutr. 2015;113:1965–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF III, Mills JL, Pfeiffer CM, Fazili Z, Zhang M et al.. Biomarkers of nutrition for development – folate review. J Nutr. 2015;145:1636S–80S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fazili Z, Sternberg MR, Paladugula N, Pfeiffer CM. Two international Round Robin studies showed good comparability of 5-methyltetrahydrofolate, but poor comparability of folic acid measured in serum by different HPLC-MS/MS methods. J Nutr. 2017;147:1815–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. [Internet] 2011–2012 Data documentation, codebook, and frequencies. Folate forms – Total & Individual – Serum (FOLFMS_G). Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2011-2012/FOLFMS_G.htm(cited 2019 Aug 20). [Google Scholar]
- 12. Centers for Disease Control and Prevention, National Center for Health Statistics. Response rates & CPS population totals, National Health and Nutrition Examination Survey. [Internet] Available from: https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx(cited 2019 Aug 20). [Google Scholar]
- 13. Fazili Z, Whitehead RD Jr., Paladugula N, Pfeiffer CM. A high-throughput LC-MS/MS method suitable for population biomonitoring measures five serum folate vitamers and one oxidation product. Anal Bioanal Chem. 2013;405:4549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dati F, Schumann G, Thomas L, Aguzzi F, Baudner S, Bienvenu J, Blaabjerg O, Blirup-Jensen S, Carlström A, Petersen PH et al.. Consensus of a group of professional societies and diagnostic companies on guidelines for interim reference ranges for 14 proteins in serum based on the standardization against the IFCC/BCR/CAP reference material (CRM 470). Eur J Clin Chem Clin Biochem. 1996;34:517–20. [PubMed] [Google Scholar]
- 15. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F et al.. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function – measured and estimated glomerular filtration rate. N Engl J Med. 2006;354;2473–83. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series no. 894. Geneva: WHO; 2000. [PubMed] [Google Scholar]
- 18. Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. [DOI] [PubMed] [Google Scholar]
- 19. Pirkle JL, Flegal KM, Bernert JT, Brody DJ, Etzel RA, Maurer KR. Exposure of the US population to environmental tobacco smoke. J Am Med Assoc. 1996;275:1233–40. [PubMed] [Google Scholar]
- 20. Pfeiffer CM, Sternberg MR, Schleicher RL, Rybak ME. Dietary supplement use and smoking were strongly related to biomarkers of water-soluble vitamin status after adjusting for socioeconomic and lifestyle factors in a representative sample of US adults. J Nutr. 2013;143:957S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):d800–2. [Google Scholar]
- 22. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. [Internet]. 2007–2008 Data documentation, codebook, and frequencies. Folate forms – Individual – Serum (FOLFMS_E). Available from: https://wwwn.cdc.gov/Nchs/Nhanes/2007-2008/FOLFMS_E.htm(cited 2019 Aug 20). [Google Scholar]
- 23. Paniz C, Lucena MR, Bertinato JF, Lourenco FR, Barros BC, Gomes GW, Figueiredo MS, Cancado R, Davila VL, Pfeiffer CM et al.. Daily supplementation with 5 mg of folic acid in Brazilian patients with hereditary spherocytosis. J Invest Med. 2019; pii: jim-2019-001025. doi: 10.1136/jim-2019-001025. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rees JR, Morris CB, Peacock JL, Ueland PM, Barry EL, McKeown-Eyssen GE, Figueiredo JC, Snover DC, Baron JA. Unmetabolized folic acid, tetrahydrofolate and colorectal adenoma risk. Cancer Prev Res (Phila). 2017;10:451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bailey SW, Ayling JE.. The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake. Proc Natl Acad Sci USA. 2009;106:15424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kalmbach RD, Choumenkovitch SF, Troen AP, Jacques PF, D'Agostino R, Selhub J. A 19-base pair deletion polymorphism in dihydrofolate reductase is associated with increased unmetabolized folic acid in plasma and decreased red blood cell folate. J Nutr. 2008;138:2323–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roberts KE. Mechanism of dehydration following alcohol ingestion. Arch Intern Med. 1963;112:154–7. [DOI] [PubMed] [Google Scholar]
- 28. Public Health England. National Diet and Nutrition Survey Rolling Programme (NDNS RP). [Internet] Supplementary report: blood folate results for the UK as a whole, Scotland, Northern Ireland (Years 1 to 4 combined) and Wales (Years 2 to 5 combined). Revised November 2017. Available from: https://www.gov.uk/government/statistics/national-diet-and-nutrition-survey-supplementary-report-blood-folate(cited 2019 Aug 20). [Google Scholar]
- 29. Boilston A, Staines A, Kelleher CC, Daly L, Shirley I, Shrivastava A, Bailey SW, Alverson PB, Ayling JE, McDermott AP et al.. Unmetabolized folic acid prevalence is widespread in the older Irish population despite the lack of a mandatory fortification program. Am J Clin Nutr. 2012;96:613–21. [DOI] [PubMed] [Google Scholar]
- 30. Obeid R, Kasoha M, Kirsch SH, Munz W, Herrmann W. Concentrations of unmetabolized folic acid and primary folate forms in pregnant women at delivery and in umbilical cord blood. Am J Clin Nutr. 2010;92:1416–22. [DOI] [PubMed] [Google Scholar]
- 31. Obeid R, Kirsch SH, Kasoha M, Eckert R, Herrmann W. Concentrations of unmetabolized folic acid and primary folate forms in plasma after folic acid treatment in older adults. Metabolism. 2011;60:673–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.