ABSTRACT
Background
Iron deficiency anemia affects hundreds of millions of women and children worldwide and is associated with impaired infant outcomes. Small-quantity lipid-based nutrient supplement (LNS) have been found to reduce the prevalence of anemia and iron deficiency in some trials.
Objectives
We evaluated the effectiveness of daily LNS supplementation on child anemia and micronutrient status in Madagascar within the context of an existing, scaled-up nutrition program.
Methods
We cluster-randomized 125 communities to (T0) a routine program with monthly growth monitoring and nutrition education; (T1) T0 + home visits for intensive nutrition counselling; (T2) T1 + LNS for children aged 6–18 mo; (T3) T2 + LNS for pregnant/lactating women; or (T4) T1 + parenting messages. Pregnant women and infants aged <12 mo were enrolled in 2014 and followed for 2 y. Child outcome measures included hemoglobin and anemia assessed using the HemoCue 301 system (n = 3561), and serum ferritin and soluble transferrin receptor as markers of iron status, retinol-binding protein as a marker of vitamin A status, and C-reactive protein and α-1 acid glycoprotein from a finger stick blood draw among a subsample (n = 387). We estimated mean difference using linear regression and prevalence ratios using modified Poisson regression accounting for the clustered design. All analyses were intention-to-treat.
Results
Children in the LNS groups (T2 and T3) had ∼40% lower prevalence of anemia and iron deficiency anemia and 25% lower prevalence of iron deficiency than children in the control group (T0) (P < 0.05 for all). There were no differences in any of the biomarkers when comparing children in the T4 group with those in T0; nor were there differences between T3 and T2.
Conclusions
Our findings suggest the provision of LNS in the context of a large-scale program offers significant benefits on anemia and iron status in young children.
This trial was registered at www.isrctn.com as ISRCTN14393738.
Keywords: lipid-based nutrient supplement, anemia, iron deficiency, Madagascar, children, program evaluation
Introduction
Hundreds of millions of the world's children suffer from poor nutrition, and as a consequence they experience delays in physical and mental development (1, 2). Micronutrient deficiencies, and iron deficiency in particular, contribute to these poor developmental outcomes (3, 4). It is estimated that anemia affects 43% of children <5 y old in low- and middle-income countries, 38% of pregnant women, and 29% of nonpregnant women globally (5). Iron deficiency is the most prominent cause of anemia, although other micronutrient deficiencies, infections, and inherited hemoglobin disorders are also important causal factors.
Many approaches have been evaluated to treat and prevent iron deficiency and anemia in young children in low-income countries, including supplementation, food fortification, and dietary diversification (6–8). Small-quantity lipid-based nutrient supplement (LNS), which include micronutrients with protein and essential fatty acids, have been found to improve growth, reduce the prevalence of anemia, and improve iron status in a number of studies (9–13). Some, but not all, have also reported improvements in child development outcomes (14–20). However, to our knowledge there have been no evaluations of the effect of LNS on micronutrient status or anemia within the context of a large-scale program.
Madagascar is marked by widespread and deep poverty, with 70% of the population identified as poor (living below $1.90 per person per day) (1). The prevalence of stunting [height-for-age z score (HAZ) <−2] in Madagascar is among the highest in the world: 49% of all children aged 0–5 y are severely or moderately stunted (21). The prevalence of anemia (hemoglobin <11 g/dL) among children <5 y old is ∼45%, and >55% among children <2 y old (22). To address poor nutrition in Madagascar, the National Nutrition Office (ONN) implemented a large-scale community-based nutrition program in 1999, which is still operational throughout the country.
A multiyear evaluation of the long-term effects of the existing program showed no effects on HAZ or stunting prevalence (23). As a result, the ONN partnered with the research team to implement a multiarm cluster-randomized controlled trial (RCT) to test the effectiveness and cost-effectiveness of different packages of interventions that have shown evidence for improving linear growth in other low-income settings within a large-scale program. The multiarm, cluster-randomized trial tested the value added of preventive home visits to intensify nutrition counseling, of providing LNS, as well as integrating parenting support over and above the group-level growth monitoring approach. The overall objective of the Mahay (meaning “smart” and “skilled” in Malagasy) trial was to determine whether the set of selected intervention packages could significantly improve child growth and development (24). Overall, the interventions had limited impact on these outcomes, except in growth among the youngest children in the T2 and T3 groups, who had the greatest potential period of exposure (25). In the present article, the objective is to evaluate the impact of LNS supplementation on iron deficiency and anemia in a subsample of children who had just stopped supplementation and were targeted for additional collection of biomarkers.
Methods
Study design, setting, and participants
A detailed description of the study design and rationale for the selection of the interventions has been published elsewhere (24, 25). The trial (ISRCTN14393738) was conducted in 5 regions with some of the highest prevalence of linear growth retardation and food insecurity in Madagascar: Amoron'i Mania, Androy, Atsimo Atsinanana, Haute Matsiatra, and Vatovavy-Fitovinany (Supplemental Figure 1), regions that were prioritized for World Bank service delivery support after a political and economic crisis in 2009–2012. Sites eligible for inclusion in the RCT were all active government community nutrition program sites (n = 1476) in these 5 regions as of January 2014. Each site covers an average of 2–3 small communities, with an eligible population of ∼200 households with children aged 0–24 mo. Selection of 125 sites and site random assignment to intervention arm were carried out by the research team using administrative data. Because of regional differences in prevalence of malnutrition, food security, and income opportunities, the sample was stratified at the regional level.
Randomization and masking
Using a random number generator, the study team block-randomized 5 sites per treatment arm per region into the current status quo group (T0, control group) or to 1 of 4 arms with additions to the status quo program: 1) intensive nutrition counselling (T1), 2) intensive nutrition counselling + LNS to children aged 6–18 mo (T2), 3) intensive nutrition counselling + LNS to pregnant and lactating women and children aged 6–18 mo (T3), and 4) intensive nutrition counselling with parenting messages for early stimulation (T4). Owing to the nature of the interventions, it was impossible to blind participants to their intervention group assignment. Participants were informed about their assignment status after the baseline assessments were completed.
Within-cluster household sampling
Within the sampled sites, 30 households were sampled at each site, stratified by children's age cohort: 10 households with a pregnant woman in her second or third trimester (cohort A), 10 households with a child aged 0–5 mo (cohort B), and 10 households with a child aged 6–11 mo (cohort C). Supplemental Figure 2 describes the timeline of the trial in detail. Households and children sampled at baseline (2014) were tracked longitudinally at 1-y intervals (midline in 2015 and endline in 2016). Mothers or children who died before final assessment were not replaced. Children who had moved outside the program site catchment area before final assessment were each replaced with a randomly drawn child from the catchment area within the same age range. Children and their households who returned to the site between the baseline and final assessment were re-interviewed. The periods of data collection were as follows: baseline survey from June to August 2014, midline survey from August to October 2015, and endline survey from September to November 2016. The micronutrient subsample data collection occurred between August and October 2015.
Biochemical subsample
The sample size for the biochemical subsample was based on an expected reduction in the prevalence of iron deficiency from 38.6% to 22.4% as observed in a similar study (26), an intracluster correlation coefficient of 0.04, α of 0.05, and power of 80%; we estimated a sample size of 16 clusters per arm with 6 children per cluster. With this, we would expect to be able to detect a difference of 0.45–0.50 SD in the micronutrient biomarkers. A subset of 64 sites was randomly sampled among the list of sites that were accessible via a paved road (82 out of the 125). A random draw of 16 sites each from T0, T2, T3, and T4 was selected for the collection of blood samples to estimate the impact of the interventions on micronutrient status (T1 was excluded owing to cost constraints). Within each of the selected sites, a random sample of 6 children in the youngest age cohort (18–24 mo at endline) was selected for the assessment of micronutrient biomarkers. The roster of children alive and measured at midline was used as a sampling frame for this selection. The rationale for these selection criteria was to align the biospecimen collection closely with the timing of the end of LNS supplementation. The biochemical data collection took place in June 2016, 1 mo after the end of LNS supplementation. Selected participants were invited to a central location in the community for blood collection.
Ethical approval
Study protocols were reviewed and approved by the local Ethics Committee in Madagascar, as well as the Institutional Review Board at the University of California, Davis. Mothers provided verbal consent before study enrolment.
Interventions
The status quo treatment arm was based on the standard-of-care in Madagascar of growth monitoring and nutrition education. Key messages included information about maternal nutrition, early initiation of breastfeeding, exclusive breastfeeding for the first 6 mo, continued breastfeeding through 2 y, and age-appropriate complementary feeding and hygiene practices. Community nutrition workers led cooking demonstrations for complementary foods using local ingredients. In addition, the government administers biannual vitamin A distribution campaigns for children <5 y old. Pregnant women are also offered iron–folic acid supplements through antenatal care consultations at the health clinics. All pregnant women and women with age-eligible children (0–2 y) living in the catchment area of a project site were eligible to participate in these services. The services were delivered monthly in a group setting at a community center located at each site. The treatment arms were as follows.
T1: intensive nutrition counseling
An added community nutrition worker, additional to the existing nutrition worker, visited homes to provide intensive personalized counseling. The added workers provided advice on diversified diets for the pregnant women and children, small-scale livestock rearing, and gardening to address food security. The added workers were instructed to make home visits to all children in the site catchment area ≤2 y of age, with 1 visit during pregnancy, monthly visits during the first 8 mo, bimonthly visits during the window of 9–12 mo, and quarterly visits from 12 to 24 mo.
T2: intensive nutrition counseling + LNS for children
In addition to the intensive nutrition counseling, caregivers were provided with LNS (Nutriset) to feed to their 6- to 18-mo-old children. The LNS was provided in 10-g sachets to be provided twice daily. The manufacturer worked with ONN to define the content of the supplements and develop culturally appropriate brand names and packages. The micronutrient composition of the LNS was based on the composition being used in the International Lipid-Based Nutrient Supplement Project conducted in Burkina Faso, Ghana, and Malawi (27). The supplements provided 118 kcal/d, 52% of the iron daily needs, and ∼100% of the recommended micronutrient intakes for young children (Supplemental Table 1). The LNS was to be stored by the community nutrition worker, either at the site or at her home. Fourteen sachets were delivered to each child (Kalina Zaza) per week. Families were instructed to mix 10-g sachets of supplement into their children's typical food twice per day. The LNS was available to all children in the target age ranges in the participating communities. No nutritional screening criteria were set for eligibility; however, severely malnourished children identified through growth monitoring were referred for treatment according to national guidelines. The LNS was securely stored at the district level and transported monthly to local nongovernmental organizations responsible for community nutrition worker supervision and delivery of LNS to the project sites. Empty sachets had to be returned to the nutrition worker to minimize selling of the products.
T3: intensive nutrition counseling + LNS for children + LNS for pregnant and lactating women
Supplements were provided to pregnant women and women within the first 6 mo postpartum, in addition to intensive nutrition counseling and LNS for children. The supplement for women was 40 g/d, providing ∼200 kcal/d, 50% of the iron daily needs, and 1–2 times the recommended daily amount of micronutrients for pregnant women (27). Seven sachets per week were delivered to pregnant and lactating women (to be consumed once a day, with breakfast). As with the LNS for children, there were no exclusion criteria for pregnant women and women ≤6 mo postpartum. The same storage, distribution, and returning of empty sachets applied for the women's LNS.
T4: intensive nutrition counseling + early childhood stimulation
A structured curriculum on early childhood stimulation was added to the intensive nutrition counseling sessions during home visits (28, 29).
Potential program exposure to LNS
There were 3 age cohorts at baseline, ranging from in utero (cohort A: −6 to 0 mo old), to birth through 5 mo (cohort B), and 6 through 11 mo (cohort C). After the first year of program implementation, target children were 6 to <24 mo old; after the second year at endline, they were 18 to <36 mo old. Owing to their differing ages at the start of the trial, varying age eligibility by treatment arm, and the timing of data collection, children experienced different durations of exposure to the interventions (see Supplemental Figure 2).
In T2, LNS was delivered when children were aged 6–18 mo. Children in cohorts A and B (−6 to <6 mo old at baseline) were eligible to receive the full 12 mo of supplementation. However, children in cohort C (6 to <12 mo old at baseline) had ≤12 mo of supplementation and stopped receiving supplements a year or more before the endline survey. In T3, LNS was also provided to mothers during pregnancy and lactation. Only the youngest cohort, A, maximally benefited from the maternal supplements, whereas cohort B benefited very little and cohort C not at all. The biochemical subsample focused only on children from cohort A, who had maximal potential exposure to both T2 and T3.
Outcomes
Anemia was assessed using the portable HemoCue 301 system. Hemoglobin was measured in capillary blood collected from one-third of the sample children randomly selected at baseline and midline because of logistical limitations, and from all children at endline. In the selected biochemical subsample, blood samples were collected for the assessment of hemoglobin and micronutrient status during a separate visit scheduled close in time to the end of LNS supplementation in the index child. Finger-prick blood samples were collected into serum collection tubes by a mobile team composed of a survey firm supervisor, a lab technician from the Laboratoire National de la Reference (Institute Pasteur), and 2 child development interviewers. Samples were placed on ice and then within 60 min of collection, they were centrifuged at ambient temperature for 15 min at 2200 g and 50 μ/L serum was divided into aliquots in each of 2 tubes. The samples collected in the field were transported to the central laboratory in Antananarivo, stored at −80°C, and then shipped to the VitMin Laboratory in Germany for analysis using established ELISA methods (30). Serum ferritin, soluble transferrin receptor (sTfR), and retinol-binding protein (RBP) concentrations were corrected for inflammation using the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia methods (31–33). Definitions of deficiency were as follows: anemia, hemoglobin <11 g/dL (34); iron deficiency, low ferritin (<12 μg/L) or high sTfR (>8.3 mg/L) (32, 33); iron deficiency anemia, hemoglobin <11 g/dL plus ferritin <12 μg/L or sTfR >8.3 mg/L; and vitamin A deficiency, RBP <0.83 μmol/L (35).
Statistical analysis
Analyses followed a complete-case intention-to-treat framework. For the purpose of this analysis, the planned primary inference compared the prevalence of iron deficiency or anemia in each intervention arm with that in the T0 control arm. Other outcome measures were considered exploratory. We also performed analyses in which LNS treatment arms were compared with non-LNS groups, by comparing T2 and T3 pooled with T0, T1, and T4 pooled. For each comparison we estimated the mean difference for continuous outcomes using linear regression and prevalence ratios using modified Poisson regression (36, 37), each with robust sandwich variance estimation to account for the site-level clustering. All models controlled for region, age of measurement, and sex. Covariate-adjusted analyses were also performed. Potential covariates included maternal age, height, parity, and education, along with household membership size, dwelling quality, wealth index, food security assessed using the Household Food Insecurity Access Scale (38), animal ownership, and month of measurement. Variables that were associated with the outcome (P < 0.1 in bivariate models) were included in multivariable-adjusted models. Adjusted models did not differ substantively from the unadjusted models, however, and are therefore not reported here. No adjustments were made for multiple comparisons.
Heterogeneous treatment effects were explored by interacting the treatment status with prespecified variables of interest (24): child age (dichotomized below and above the median values at time of measurement), sex, food insecurity (any food insecurity), maternal education [below secondary (low) compared with above secondary (high)], maternal age (≤19 y compared with >19 y), and household wealth [first 3 quintiles (low) compared with top 2 quintiles (high)]. Interaction tests with a P < 0.1 were considered significant. Post hoc exploratory analysis examined the prevalence of anemia over age of assessment by group using lowess plots combining data from all survey rounds. We further explored differences between the T3 and T2 intervention groups.
Results
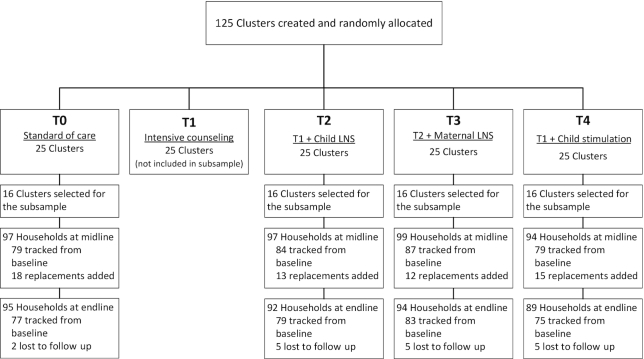
For the main sample, a total of 3561 children participated in the assessment of hemoglobin concentrations, equally balanced across all treatment arms and including all age cohorts. A total of 387 children in the subsample participated in the micronutrient status assessments, equally balanced between the T0, T2, T3, and T4 groups (Figure 1). The subsample children were a mean age of ∼21 mo at the time of sample collection (Table 1). Their mothers were a mean of ∼26 y of age and between 70% and 80% had completed primary schooling or less. This subsample differed from the larger cohort in a number of important ways (Supplemental Table 2). Children in the subsample were selected from the youngest cohort A, hence they were ∼8 mo younger. They were more likely to have been selected from the Androy and Atsimo Atsinanana regions and less likely to have been selected from the Haute Matsiatra and Vatovavy-Fitovinany regions; and they were more likely to reside in homes with improved-quality walls or flooring.
FIGURE 1.
Study flow diagram. The sampling frame for the subsample cohort included children alive and observed at the midline visit. Losses to follow-up are reported from that sampling frame. LNS, lipid-based nutrient supplement.
TABLE 1.
Micronutrient subsample: characteristics by intervention group in the Mahay Trial in Madagascar, 2014–20161
| Variables | T0 | T2 | T3 | T4 |
|---|---|---|---|---|
| Children, n | 95 | 92 | 94 | 89 |
| Child age, mo | 20.8 ± 2.2 | 21.1 ± 2.1 | 21.3 ± 2.0 | 21.2 ± 1.9 |
| Male | 42.1 | 50.0 | 44.7 | 60.7 |
| Region | ||||
| Amoron'i Mania | 23.2 | 25.0 | 18.1 | 25.8 |
| Androy | 31.6 | 30.4 | 31.9 | 33.7 |
| Atsimo Atsinanana | 25.3 | 26.1 | 10.6 | 5.6 |
| Haute Matsiatra | 12.6 | 10.9 | 24.5 | 29.2 |
| Vatovavy-Fitovinany | 7.4 | 7.6 | 14.9 | 5.6 |
| Maternal characteristics | ||||
| Age, y | 25.4 ± 7.7 | 25.9 ± 7.1 | 26.2 ± 7.2 | 25.7 ± 6.9 |
| Maternal height, cm | 153.4 ± 6.0 | 153.4 ± 5.9 | 153.1 ± 8.7 | 153.2 ± 5.7 |
| Prior children | 1.7 ± 1.3 | 1.7 ± 1.2 | 1.9 ± 1.2 | 1.7 ± 1.2 |
| Maternal education—primary or less | 71.6 | 81.3 | 77.4 | 69.7 |
| Household characteristics | ||||
| People per household, n | 5.2 ± 2.2 | 5.9 ± 3.3 | 5.9 ± 2.6 | 5.4 ± 2.9 |
| Children aged <18 y in the household, n | 3.0 ± 1.8 | 3.4 ± 2.5 | 3.6 ± 2.3 | 3.2 ± 2.3 |
| Any food insecurity | 27.3 | 29.1 | 20.5 | 25.3 |
| Above median distance to water | 52.6 | 47.8 | 63.8 | 43.8 |
| Water source protected | 17.9 | 11.2 | 4.3 | 16.9 |
| Floor improved | 33.3 | 39.6 | 23.9 | 46.0 |
| Wall improved | 25.3 | 21.7 | 20.2 | 19.1 |
| Roof improved | 31.0 | 19.3 | 11.8 | 29.9 |
| Top 2 wealth quintiles | 49.4 | 44.3 | 30.5 | 54.7 |
| Poultry | 1.8 ± 1.8 | 2.1 ± 1.8 | 2.1 ± 2.0 | 1.9 ± 1.8 |
| Cows or zebus | 0.9 ± 1.4 | 0.9 ± 1.4 | 1.2 ± 1.4 | 0.8 ± 1.5 |
Values are percentages or means ± SDs. Trial arms were as follows: T0, standard of care control group; T2, intensive nutrition counseling plus LNS for children aged 6–18 mo; T3, intensive nutrition counseling plus LNS for children plus LNS for mothers during pregnancy through 6 mo postpartum; and T4, intensive nutrition counseling plus child stimulation. LNS, lipid-based nutrient supplement.
In the subsample control group, the prevalence of anemia (hemoglobin <11 g/dL) was 40%, vitamin A deficiency (RBP <0.83 μmol/L) was 13.7%, iron deficiency (ferritin <12 μg/mL or sTfR >8.3 mg/L) was 63.2%, and iron deficiency anemia was 29.5% (Table 2). In T2 and T3, there was ∼40% lower prevalence of anemia and iron deficiency anemia than among children in the control group. Correspondingly, they also had higher hemoglobin and inflammation-corrected ferritin concentrations and lower sTfR concentrations (Table 3). There were no significant differences in RBP, C-reactive protein (CRP), or α-1-acid glycoprotein (AGP) between any of the intervention groups and the T0 control group. There were no differences in any of the biomarkers when comparing the children in the T4 group with those in the control group. There also were no differences in the mean concentrations of any of the biomarkers when comparing the children in the T3 group with those in the T2 group (data not shown).
TABLE 2.
Impact on anemia and micronutrient deficiency prevalence in the subsample of the Mahay Trial in Madagascar, 2014–20161
| Outcome | n | Prevalence (%) | Prevalence ratio vs. T0 (95% CI)2 |
|---|---|---|---|
| Anemia (hemoglobin <11 g/dL) | |||
| T0 | 95 | 40.0 | Ref. |
| T2 | 92 | 23.9 | 0.58 (0.39, 0.87)** |
| T3 | 94 | 22.3 | 0.60 (0.45, 0.80)*** |
| T4 | 89 | 36.0 | 0.97 (0.72, 1.31) |
| Vitamin A deficiency3 (RBP <0.83 μmol/L) | |||
| T0 | 95 | 13.7 | Ref. |
| T2 | 92 | 15.2 | 1.01 (0.52, 1.94) |
| T3 | 94 | 8.5 | 0.44 (0.18, 1.09) |
| T4 | 89 | 21.3 | 1.78 (0.88, 3.60) |
| Low ferritin3 (<12 μg/L) | |||
| T0 | 95 | 44.2 | Ref. |
| T2 | 92 | 20.7 | 0.48 (0.29, 0.82)** |
| T3 | 94 | 22.3 | 0.48 (0.28, 0.82)** |
| T4 | 89 | 40.4 | 0.86 (0.58, 1.26) |
| High sTfR3 (>8.3 mg/L) | |||
| T0 | 95 | 53.7 | Ref. |
| T2 | 92 | 40.2 | 0.75 (0.54, 1.03) |
| T3 | 94 | 42.6 | 0.79 (0.56, 1.10) |
| T4 | 89 | 51.7 | 0.96 (0.72, 1.28) |
| Iron deficiency3 (ferritin <12 μg/L or sTfR >8.3 mg/L) | |||
| T0 | 95 | 63.2 | Ref. |
| T2 | 92 | 47.8 | 0.75 (0.58, 0.97)* |
| T3 | 94 | 46.8 | 0.72 (0.53, 0.97)* |
| T4 | 89 | 59.6 | 0.94 (0.75, 1.19) |
| Iron deficiency anemia3 (anemic and iron deficient) | |||
| T0 | 95 | 29.5 | Ref. |
| T2 | 92 | 16.3 | 0.54 (0.33, 0.91)* |
| T3 | 94 | 16.0 | 0.57 (0.37, 0.89)* |
| T4 | 89 | 23.6 | 0.84 (0.48, 1.44) |
| High AGP (>1 g/L) | |||
| T0 | 95 | 38.9 | Ref. |
| T2 | 92 | 52.2 | 1.28 (0.96, 1.71) |
| T3 | 94 | 40.4 | 1.05 (0.78, 1.42) |
| T4 | 89 | 37.1 | 0.96 (0.70, 1.32) |
| High CRP (>5 mg/L) | |||
| T0 | 95 | 20.0 | Ref. |
| T2 | 92 | 14.1 | 0.69 (0.35, 1.38) |
| T3 | 94 | 23.4 | 1.09 (0.62, 1.92) |
| T4 | 89 | 15.7 | 0.71 (0.36, 1.38) |
Trial arms were as follows: T0, standard of care control group; T2, intensive nutrition counseling plus LNS for children aged 6–18 mo; T3, intensive nutrition counseling plus LNS for children plus LNS for mothers during pregnancy through 6 mo postpartum; and T4, intensive nutrition counseling plus child stimulation. *,**,***Significant difference: *P < 0.05, **P < 0.01, ***P < 0.001. AGP, α-1 acid glycoprotein; CRP, C-reactive protein; LNS, lipid-based nutrient supplement; RBP, retinol-binding protein; sTfR, soluble transferrin receptor.
95% CIs in parentheses, based on robust SEs, clustered at the village level. All analyses adjusted for region, age of measurement, and child sex.
Ferritin, sTfR, and RBP concentrations corrected for inflammation using the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia method (31–33).
TABLE 3.
Impact on biomarker concentrations in the subsample of the Mahay Trial in Madagascar, 2014–20161
| Outcome | n | Median (IQR) | Difference vs. T0 (95% CI)2 |
|---|---|---|---|
| Hemoglobin, g/dL | |||
| T0 | 95 | 11.3 (10.6–11.8) | Ref. |
| T2 | 92 | 11.7 (11.0–12.2) | 0.44 (0.08, 0.80)* |
| T3 | 94 | 11.6 (11.1–12.1) | 0.32 (0.05, 0.59)* |
| T4 | 89 | 11.5 (10.7–12.0) | 0.18 (−0.09, 0.44) |
| RBP,3 μmol/L | |||
| T0 | 95 | 1.09 (0.91–1.26) | Ref. |
| T2 | 92 | 1.10 (0.92–1.32) | 0.05 (−0.02, 0.12) |
| T3 | 94 | 1.18 (0.95–1.32) | 0.07 (−0.01, 0.14) |
| T4 | 89 | 1.03 (0.87–1.24) | −0.04 (−0.11, 0.04) |
| Ferritin,3,4 μg/L | |||
| T0 | 95 | 14.1 (8.8–20.9) | Ref. |
| T2 | 92 | 17.3 (12.9–23.6) | 25.9 (4.6, 51.5)* |
| T3 | 94 | 17.3 (13.2–27.7) | 48.4 (18.7, 85.5)*** |
| T4 | 89 | 14.3 (8.6–21.9) | 14.8 (−8.0, 43.4) |
| sTfR,3, 4 mg/L | |||
| T0 | 95 | 8.75 (7.17–12.55) | Ref. |
| T2 | 92 | 7.89 (6.36–10.30) | −14.3 (−23.2, −4.4)** |
| T3 | 94 | 7.73 (6.53–10.17) | −12.3 (−21.0, −2.7)* |
| T4 | 89 | 8.53 (7.05–12.72) | −5.4 (−15.0, 5.3) |
| AGP,4 g/L | |||
| T0 | 95 | 0.83 (0.67–1.50) | Ref. |
| T2 | 92 | 1.08 (0.74–1.67) | 10.9 (−6.3, 31.2) |
| T3 | 94 | 0.91 (0.70–1.38) | −3.0 (−17.8, 14.6) |
| T4 | 89 | 0.85 (0.65–1.43) | −7.7 (−22.0, 9.3) |
| CRP,4 mg/L | |||
| T0 | 95 | 1.31 (0.58–2.99) | Ref. |
| T2 | 92 | 1.14 (0.54–3.03) | −0.5 (−37.2, 57.6) |
| T3 | 94 | 1.05 (0.44–3.57) | −3.5 (−36.1, 45.6) |
| T4 | 89 | 0.92 (0.41–2.10) | −29.9 (−54.3, 7.4) |
Trial arms were as follows: T0, standard of care control group; T2, intensive nutrition counseling plus LNS for children aged 6–18 mo; T3, intensive nutrition counseling plus LNS for children plus LNS for mothers during pregnancy through 6 mo postpartum; and T4, intensive nutrition counseling plus child stimulation. *,**,***Significant difference: *P < 0.05, **P < 0.01, ***P < 0.001. AGP, α-1 acid glycoprotein; CRP, C-reactive protein; LNS, lipid-based nutrient supplement; RBP, retinol-binding protein; sTfR, soluble transferrin receptor.
95% CIs in parentheses, based on robust SEs, clustered at the village level. All analyses adjusted for region, age of measurement, and sex. Hemoglobin and RBP: mean difference; ferritin, sTfR, AGP, and CRP: percentage difference.
Ferritin, sTfR, and RBP concentrations corrected for inflammation using the Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia method (31–33).
Log transformed for analysis.
In the full study sample, where only hemoglobin concentrations were measured, the prevalence of anemia in the control group at midline was 65.4% and at endline was 41.7%, when children were a mean age of 17.5 mo and 30.1 mo, respectively (Supplemental Table 3). In contrast to the subsample, there were no significant differences between groups in mean hemoglobin concentration or prevalence of anemia at midline or endline of the study. However, when the 2 LNS groups (T2 and T3) were compared with all non-LNS groups combined (T0, T1, and T4), there was a significant 22–23% reduction in the prevalence of anemia at midline only.
When the prevalence of anemia was examined by group over age (Figure 2), the effect of the intervention appeared to be confined to the period of LNS supplementation (6–18 mo of age) and shortly thereafter. The curves converged by ∼22 mo of age. However, when age was tested as a dichotomous interaction term, we found no significant interaction in either the main sample or subsample groups (Supplemental Table 4). We did find a significant interaction with child sex, with a larger effect on hemoglobin and sTfR in T3 among boys in the subsample (Supplemental Table 5). There were also significant interactions with food security status, with generally greater improvements in hemoglobin concentrations in the food-secure strata in the T2 and T3 groups and no impacts in the food-insecure strata (Supplemental Table 6). There was no significant interaction with maternal education (Supplemental Table 7) or age (Supplemental Table 8), but a significant interaction with wealth on RBP only (Supplemental Table 9). There was a significant increase in RBP among the lower wealth quintiles in the T3 group.
FIGURE 2.
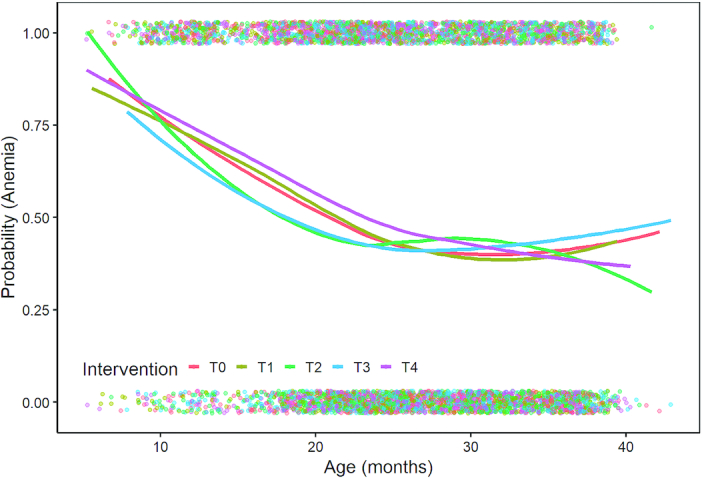
The prevalence of anemia by intervention group over child age. Dots represent individual data points, whereas lines represent lowess plots by group combining data from all survey rounds.
Discussion
Our key findings were that children in the groups that received LNS (T2 and T3) had ∼40% lower prevalence of anemia and iron deficiency anemia than children in the control group. Children in T2 and T3 also had significantly higher concentrations of inflammation-corrected ferritin and lower sTfR concentrations than those in T0. The observed effects in the 2 groups were of a similar magnitude and they were not different from each other. There were no differences in any of the biomarkers when comparing the children in the group receiving intensive nutrition counselling plus parenting education (T4) with those in the control group. When the prevalence of anemia was examined by group over age, the effects of the intervention appeared to be confined to the period of LNS supplementation (6–18 mo of age) and shortly thereafter.
These biochemical results provide strong evidence that LNS affects iron deficiency anemia. Both T2 and T3 increased hemoglobin, increased ferritin, and reduced sTfR, a biomarker that is elevated during iron deficiency. There was no detectable difference in vitamin A status (as measured by RBP) or in acute or chronic inflammation (as measured by the 2 indicators of CRP and AGP) comparing each of the intervention groups to the T0 control group.
The hemoglobin effects in the subsample were larger than those observed in the main sample, mainly due to the fact that the biochemical sample included a narrow age group with full exposure to the supplementation at endline, and with a shorter time having elapsed since their last supplementation. At a median age of 21 mo, half the subsample children were within 3 mo of having last received LNS. In the main study sample, the children were a median age of 30 mo at endline, a full year beyond the last point of supplementation. At midline, the median age of the main study sample was 17.5 mo, and we would have expected to see a larger impact of the LNS supplementation. Although there was a 12% lower prevalence of anemia comparing the LNS groups with the non-LNS groups, the difference was of a smaller magnitude than what was seen in the subsample (∼40% reduction). This discrepancy may be due to selection criteria for the subsample areas, which were chosen for logistical reasons to be closer to main roads and towns with reliable electricity. Notably, the prevalence of anemia in the subsample area was also much lower than in the main study sample at midline (40% compared with 65%). The predominant causes of anemia might have been different in the subsample area than in the more remote areas of the study population. For example, remote areas may have had a greater burden of infections such as malaria or intestinal helminths, which could have limited the potential for children to benefit from the nutritional supplements.
The results of this study are similar to a number of other recent reports of LNS supplementation on anemia and micronutrient status (39). A recently published study in Bangladesh similarly reported significantly higher hemoglobin concentrations and iron status biomarkers in the groups of children who received LNS than in control group children (12). In Burkina Faso, a trial of LNS plus the screening and treatment of malaria and diarrheal disease also reported lower prevalence of anemia and iron deficiency and increased RBP concentrations compared with a no-intervention control group (9). Three recent trials of water, sanitation, and hygiene + infant and young child feeding (IYCF) interventions in Zimbabwe, Kenya, and Bangladesh reported effects on anemia (11, 40, 41). Similarly to our study in Madagascar, all 3 trials provided LNS together with counseling on IYCF. The trials reported reductions in anemia of ∼25–40%. The Kenya and Bangladesh studies also measured biomarkers of iron and vitamin A status. Both reported significant improvements in iron status and, in Kenya, there were also significant improvements in vitamin A status (11). These effects are unlikely to have been due to the IYCF counseling alone. In our study, we did not observe any effect of the T4 intervention, which included a package of IYCF messages comparable with that which was delivered in the T2 and T3 groups. The consistency in findings across a variety of contexts suggests that LNS may be a successful strategy to improve iron status and reduce iron deficiency anemia in infants and toddlers. Observed effect sizes are comparable with those reported for multiple micronutrient powders (42), although few studies have performed direct comparisons (12).
This study presents an important contribution to the literature on the effects of LNS within the context of a large-scale program on nutritional status of young children. In this community-level cluster-randomized controlled trial, it is unlikely that there would have been substantial spillover from any of the intervention groups to the control group. Caregivers had to return empty sachets to the health worker to minimize sharing or sales. There are a number of important limitations, however. The subsample was of small sample size and not randomly selected from the full study population. The timing of sample collection also occurred after children had aged out of the age eligibility window for supplementation. Further, we lacked data on other micronutrients, such as vitamin B-12, folate, or zinc, that might have been influenced by the intervention and also could contribute to anemia. We also lack data on adherence or the total number of supplements consumed or whether the supplements were mixed with other foods. These decisions were made for logistical feasibility. It is likely that the effects would have been larger if the timing of sample collection had coincided with LNS delivery. Nevertheless, the consistency in findings between this study and other studies of the effect of LNS on anemia and iron deficiency (39) suggests that these results are broadly generalizable to populations in which anemia and micronutrient deficiency are public health problems.
We conclude that the provision of LNS in the context of a large-scale program offers significant benefits on anemia and iron status. While these factors have been associated with child development in other studies, we did not find any impacts on development in this study (25). However, there was evidence that the benefits of supplementation on anemia washed out within months after the end of supplementation. It is unknown if these short-term improvements in micronutrient status and anemia will translate into lasting effects on child health and development beyond the period of supplementation. This remains an important question for programs focused on the first 1000 d and an important area for future research.
Supplementary Material
Acknowledgments
We thank Kodjo Aflagah, Maria Dieci, Ling Hsin, and Xiuping Tan for providing research assistance. We thank Mamane Zeilani (LNS-Nutriset) for technical support on the LNS composition, Robert Ackatia-Armah for implementing the LNS acceptability trial, and Kara Breshanan as the field coordinator of the biomarker data collection. We are grateful to the Statistical Institute of Madagascar for providing supporting material for fieldwork, and the LNR-Institute Pasteur for the storage of the serum samples. The authors’ responsibilities were as follows—CPS, EG, and LCHF: drafted the manuscript with input from all co-authors; CPS: developed the nutrition interventions and the protocols for biochemical assays; EG, AMW, and CPS: oversaw the piloting and study implementation, contributed to refinements in interventions and measurements, and responded to threats to validity; EG, AMW, and CA: developed the analytical approach; CA: conducted the statistical analysis and constructed the tables and figures with input from co-authors; and all authors: read, contributed to, and approved the final manuscript.
Notes
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development grant 1R03HD091636-01A1 (to LCHF), the World Bank Strategic Impact Evaluation Fund (to EG), the World Bank Early Learning Partnership Program (to EG), a World Bank Innovation Grant (to EG), Grand Challenges Canada (to EG), World Bank Research Committee, Japan Nutrition Trust Fund (to EG), and Power of Nutrition Trust Fund (to EG).
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–9 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn.
Abbreviations used: AGP, α-1 acid glycoprotein; CRP, C-reactive protein; HAZ, height for age z score; IYCF, infant and young child feeding; LNS, lipid-based nutrient supplement; ONN, Office National de Nutrition [National Nutrition Office]; RBP, retinol-binding protein; RCT, randomized controlled trial; sTfR, soluble transferrin receptor.
References
- 1. UNICEF. The state of the world's children 2016: a fair chance for every child. [Internet] New York (NY): UNICEF; 2016; [cited 2016 May 5]. Available from: http://www.unicef.org/sowc2016. [Google Scholar]
- 2. Grantham-McGregor SM, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B; International Child Development Steering Group . Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369(9555):60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prado EL, Dewey KG. Nutrition and brain development in early life. Nutr Rev. 2014;72(4):267–84. [DOI] [PubMed] [Google Scholar]
- 4. Murray-Kolb LE. Iron and brain functions. Curr Opin Clin Nutr Metab Care. 2013;16(6):703–7. [DOI] [PubMed] [Google Scholar]
- 5. WHO. The global prevalence of anaemia in 2011. Geneva (Switzerland): World Health Organization; 2015. [Google Scholar]
- 6. Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, Haider BA, Kirkwood B, Morris SS, Sachdev HP et al.. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371(9610):417–40. [DOI] [PubMed] [Google Scholar]
- 7. Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4:24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ten Year Strategy to Reduce Vitamin and Mineral Deficiences, Maternal, Infant, and Young Child Nutrition Working Group: Formulation Subgroup. Formulations for fortified complementary foods and supplements: review of successful products for improving the nutritional status of infants and young children. Food Nutr Bull. 2009;30(2):S239–S55. [PubMed] [Google Scholar]
- 9. Abbeddou S, Yakes Jimenez E, Some JW, Ouedraogo JB, Brown KH, Hess SY. Small-quantity lipid-based nutrient supplements containing different amounts of zinc along with diarrhea and malaria treatment increase iron and vitamin A status and reduce anemia prevalence, but do not affect zinc status in young Burkinabe children: a cluster-randomized trial. BMC Pediatr. 2017;17(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Siega-Riz AM, Estrada Del Campo Y, Kinlaw A, Reinhart GA, Allen LH, Shahab-Ferdows S, Heck J, Suchindran CM, Bentley ME. Effect of supplementation with a lipid-based nutrient supplement on the micronutrient status of children aged 6–18 months living in the rural region of Intibuca, Honduras. Paediatr Perinat Epidemiol. 2014;28(3):245–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stewart CP, Dewey KG, Lin A, Pickering AJ, Byrd KA, Jannat K, Ali S, Rao G, Dentz HN, Kiprotich M et al.. Effects of lipid-based nutrient supplements and infant and young child feeding counseling with or without improved water, sanitation, and hygiene (WASH) on anemia and micronutrient status: results from 2 cluster-randomized trials in Kenya and Bangladesh. Am J Clin Nutr. 2019;109(1):148–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Matias SL, Mridha MK, Young RT, Khan MSA, Siddiqui Z, Ullah MB, Vosti SA, Dewey KG. Prenatal and postnatal supplementation with lipid-based nutrient supplements reduces anemia and iron deficiency in 18-month-old Bangladeshi children: a cluster-randomized effectiveness trial. J Nutr. 2018;148(7):1167–76. [DOI] [PubMed] [Google Scholar]
- 13. Adu-Afarwuah S, Young RT, Lartey A, Okronipa H, Ashorn P, Ashorn U, Oaks BM, Arimond M, Dewey KG. Maternal and infant supplementation with small-quantity lipid-based nutrient supplements increases infants’ iron status at 18 months of age in a semiurban setting in Ghana: a secondary outcome analysis of the iLiNS-DYAD randomized controlled trial. J Nutr. 2019;149(1):149–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stewart CP, Kariger P, Fernald L, Pickering AJ, Arnold CD, Arnold BF, Hubbard AE, Dentz HN, Lin A, Meerkerk TJ et al.. Effects of water quality, sanitation, handwashing, and nutritional interventions on child development in rural Kenya (WASH Benefits Kenya): a cluster-randomized controlled trial. Lancet Child Adolesc Health. 2018;2(4):269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tofail F, Fernald LC, Das KK, Rahman M, Ahmed T, Jannat KK, Unicomb L, Arnold BF, Ashraf S, Winch PJ et al.. Effect of water quality, sanitation, hand washing, and nutritional interventions on child development in rural Bangladesh (WASH Benefits Bangladesh): a cluster-randomised controlled trial. Lancet Child Adolesc Health. 2018;2(4):255–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matias SL, Mridha MK, Tofail F, Arnold CD, Khan MS, Siddiqui Z, Ullah MB, Dewey KG. Home fortification during the first 1000 d improves child development in Bangladesh: a cluster-randomized effectiveness trial. Am J Clin Nutr. 2017;105(4):958–69. [DOI] [PubMed] [Google Scholar]
- 17. Gladstone MJ, Chandna J, Kandawasvika G, Ntozini R, Majo FD, Tavengwa NV, Mbuya MNN, Mangwadu GT, Chigumira A, Chasokela CM et al.. Independent and combined effects of improved water, sanitation, and hygiene (WASH) and improved complementary feeding on early neurodevelopment among children born to HIV-negative mothers in rural Zimbabwe: substudy of a cluster-randomized trial. PLoS Med. 2019;16(3):e1002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prado EL, Adu-Afarwuah S, Lartey A, Ocansey M, Ashorn P, Vosti SA, Dewey KG. Effects of pre- and post-natal lipid-based nutrient supplements on infant development in a randomized trial in Ghana. Early Hum Dev. 2016;99:43–51. [DOI] [PubMed] [Google Scholar]
- 19. Prado EL, Maleta K, Ashorn P, Ashorn U, Vosti SA, Sadalaki J, Dewey KG. Effects of maternal and child lipid-based nutrient supplements on infant development: a randomized trial in Malawi. Am J Clin Nutr. 2016;103(3):784–93. [DOI] [PubMed] [Google Scholar]
- 20. Prado EL, Phuka J, Maleta K, Ashorn P, Ashorn U, Vosti SA, Dewey KG. Provision of lipid-based nutrient supplements from age 6 to 18 months does not affect infant development scores in a randomized trial in Malawi. Matern Child Health J. 2016;20(10):2199–208. [DOI] [PubMed] [Google Scholar]
- 21. UNICEF. The state of the world's children 2019: children, food and nutrition. New York (NY): UNICEF; 2019. [Google Scholar]
- 22. Institut National de la Statistique (INSTAT), Programme National de lutte contre le Paludisme (PNLP), Institut Pasteur de Madagascar (IPM), and ICF International. Enquête sur les Indicateurs du Paludisme 2016. Calverton (MD): INSTAT, PNLP, IPM, and ICF International; 2016. [Google Scholar]
- 23. Weber AM, Galasso E, Fernald LCH. Perils of scaling up: effects of expanding a nutrition programme in Madagascar. Matern Child Nutr. 2019;15(Suppl 1):e12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fernald LCH, Galasso E, Qamruddin J, Ranaivoson C, Ratsifandrihamanana L, Stewart CP, Weber AM. A cluster-randomized, controlled trial of nutritional supplementation and promotion of responsive parenting in Madagascar: the MAHAY study design and rationale. BMC Public Health. 2016;16:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Galasso E, Weber AM, Stewart CP, Ratsifandrihamanana L, Fernald LCH. Effects of nutritional supplementation and home visiting on growth and development in young children in Madagascar: a cluster-randomised controlled trial. Lancet Glob Health. 2019;7(9):e1257–e68. [DOI] [PubMed] [Google Scholar]
- 26. Suchdev PS, Ruth LJ, Woodruff BA, Mbakaya C, Mandava U, Flores-Ayala R, Jefferds ME, Quick R. Selling Sprinkles micronutrient powder reduces anemia, iron deficiency, and vitamin A deficiency in young children in Western Kenya: a cluster-randomized controlled trial. Am J Clin Nutr. 2012;95(5):1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arimond M, Zeilani M, Jungjohann S, Brown KH, Ashorn P, Allen LH, Dewey KG. Considerations in developing lipid-based nutrient supplements for prevention of undernutrition: experience from the International Lipid-Based Nutrient Supplements (iLiNS) project. Matern Child Nutr. 2015;11(Suppl 4):31–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walker SP, Chang SM, Powell CA, Grantham-McGregor SM. Effects of early childhood psychosocial stimulation and nutritional supplementation on cognition and education in growth-stunted Jamaican children: prospective cohort study. Lancet. 2005;366:1804–7. [DOI] [PubMed] [Google Scholar]
- 29. Gertler P, Heckman J, Pinto R, Zanolini A, Vermeersch C, Walker S, Chang SM, Grantham-McGregor S. Labor market returns to an early childhood stimulation intervention in Jamaica. Science. 2014;344(6187):998–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2004;134(11):3127–32. [DOI] [PubMed] [Google Scholar]
- 31. Larson LM, Namaste SM, Williams AM, Engle-Stone R, Addo OY, Suchdev PS, Wirth JP, Temple V, Serdula M, Northrop-Clewes CA. Adjusting retinol-binding protein concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(Suppl 1):390S–401S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Namaste SM, Rohner F, Huang J, Bhushan NL, Flores-Ayala R, Kupka R, Mei Z, Rawat R, Williams AM, Raiten DJ et al.. Adjusting ferritin concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(Suppl 1):359S–71S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rohner F, Namaste SM, Larson LM, Addo OY, Mei Z, Suchdev PS, Williams AM, Sakr Ashour FA, Rawat R, Raiten DJ et al.. Adjusting soluble transferrin receptor concentrations for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project. Am J Clin Nutr. 2017;106(Suppl 1):372S–82S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva (Switzerland): World Health Organization; 2011. [Google Scholar]
- 35. Engle-Stone R, Haskell MJ, Ndjebayi AO, Nankap M, Erhardt JG, Gimou MM, Brown KH. Plasma retinol-binding protein predicts plasma retinol concentration in both infected and uninfected Cameroonian women and children. J Nutr. 2011;141(12):2233–41. [DOI] [PubMed] [Google Scholar]
- 36. Petersen MR, Deddens JA. A comparison of two methods for estimating prevalence ratios. BMC Med Res Methodol. 2008;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 38. Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for measurement of food access: indicator guide. Washington (DC): Academy for Educational Development, Food and Nutrition Technical Assistance Project (FANTA); 2007. [Google Scholar]
- 39. Das JK, Salam RA, Hadi YB, Sadiq Sheikh S, Bhutta AZ, Weise Prinzo Z, Bhutta ZA. Preventive lipid-based nutrient supplements given with complementary foods to infants and young children 6 to 23 months of age for health, nutrition, and developmental outcomes. Cochrane Database Syst Rev. 2019;5:CD012611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Humphrey JH, Mbuya MN, Ntozini R, Moulton LH, Stoltzfus RJ, Tavengwa NV, Mutasa K, Majo F, Mutasa B, Mangwadu G et al.. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on child stunting and anaemia in rural Zimbabwe: a cluster-randomised trial. Lancet Glob Health. 2019;7(1):e132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prendergast AJ, Chasekwa B, Evans C, Mutasa K, Mbuya MNN, Stoltzfus RJ, Smith LE, Majo FD, Tavengwa NV, Mutasa B et al.. Independent and combined effects of improved water, sanitation, and hygiene, and improved complementary feeding, on stunting and anaemia among HIV-exposed children in rural Zimbabwe: a cluster-randomised controlled trial. Lancet Child Adolesc Health. 2019;3(2):77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. De-Regil LM, Suchdev PS, Vist GE, Walleser S, Pena-Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database Syst Rev. 2011;(9):CD008959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.