ABSTRACT
Background
Walnuts have established lipid-/lipoprotein-lowering properties; however, their effect on lipoprotein subclasses has not been investigated. Furthermore, the mechanisms by which walnuts improve lipid/lipoprotein concentrations are incompletely understood.
Objectives
We aimed to examine, as exploratory outcomes of this trial, the effect of replacing SFAs with unsaturated fats from walnuts or vegetable oils on lipoprotein subclasses, cholesterol efflux, and proprotein convertase subtilisin/kexin type 9 (PCSK9).
Methods
A randomized, crossover, controlled-feeding study was conducted in individuals at risk of cardiovascular disease (CVD) (n = 34; 62% men; mean ± SD age 44 ± 10 y; BMI: 30.1 ± 4.9 kg/m2). After a 2-wk run-in diet (12% SFAs, 7% PUFAs, 12% MUFAs), subjects consumed the following diets, in randomized order, for 6 wk: 1) walnut diet (WD) [57–99 g/d walnuts, 7% SFAs, 16% PUFAs [2.7% α-linolenic acid (ALA)], 9% MUFAs]; 2) walnut fatty acid–matched diet [7% SFAs, 16% PUFAs (2.6% ALA), 9% MUFAs]; and 3) oleic acid replaces ALA diet (ORAD) [7% SFAs, 14% PUFAs (0.4% ALA); 12% MUFAs] (all percentages listed are of total kilocalories ). Serum collected after the run-in (baseline) and each diet period was analyzed for lipoprotein classes and subclasses (vertical auto profile), cholesterol efflux, and PCSK9. Linear mixed models were used for data analysis.
Results
Compared with the ORAD, total cholesterol (mean ± SEM −8.9± 2.3 mg/dL; −5.1%; P < 0.001), non-HDL cholesterol (−7.4 ± 2.0 mg/dL; −5.4%; P = 0.001), and LDL cholesterol (−6.9 ± 1.9 mg/dL; −6.5%; P = 0.001) were lower after the WD; no other pairwise differences existed. There were no between-diet differences for HDL-cholesterol or LDL-cholesterol subclasses. Lipoprotein(a) [Lp(a)], cholesterol efflux, and PCSK9 were unchanged after the diets.
Conclusions
In individuals at risk of CVD, replacement of SFAs with unsaturated fats from walnuts or vegetable oils improved lipid/lipoprotein classes, including LDL-cholesterol, non-HDL cholesterol, and total cholesterol, without an increase in Lp(a). These improvements were not explained by changes in cholesterol efflux capacity or PCSK9. This trial was registered at clinicaltrials.gov as NCT01235832.
Keywords: walnut, lipids, lipoproteins, PCSK9, cholesterol efflux
Introduction
Walnut consumption consistently lowers lipids and lipoproteins (1), strong causal risk factors for cardiovascular disease (CVD). A recent meta-analysis of controlled trials showed that 15–108 g walnuts/d significantly lowered total cholesterol (TC), LDL-cholesterol (LDL-C), and triglycerides (TGs) compared with control diets devoid of walnuts (1). However, no randomized controlled trials have investigated how walnut consumption affects lipoprotein subfractions to further our understanding of the antiatherogenic effects of walnuts (2, 3).
Walnuts contain PUFAs, including α-linolenic acid (ALA; 18:3n–3), fiber, and bioactive compounds including phytosterols, tocopherols, and phenolic compounds that may modulate the atherogenic lipoprotein-lowering observed with walnut consumption (4); however, the mechanisms are not fully understood. Evidence shows that walnuts lower cholesterol concentrations to a greater extent than predicted based on their fatty acid composition, suggesting that the non–fatty acid constituents play a role in the observed cholesterol-lowering (5).
Cholesterol efflux and proprotein convertase subtilisin-kexin type 9 (PCSK9) are key components of cholesterol metabolism. Cholesterol efflux capacity is a measure of HDL-cholesterol (HDL-C) functionality and PCSK9 is a protein involved in the degradation of LDL-C receptors; lower HDL-C functionality and higher PCSK9 concentrations are associated with increased CVD risk (6, 7). Whole walnuts and walnut oil have been shown to increase cholesterol efflux (8, 9), and higher intake of PUFAs reduces PCSK9 concentrations (10). Walnut consumption may affect cholesterol efflux and/or PCSK9, which may account for the lipid and lipoprotein benefits that have been reported.
To further understand the effect of replacing SFAs with PUFAs from walnuts or vegetable oils on lipoprotein subfractions, cholesterol efflux, and PCSK9, we conducted exploratory analyses using samples collected during a randomized, crossover, controlled-feeding trial (11). Our objective was to examine how a walnut-rich diet affects cholesterol metabolism compared with isocaloric diets 1) rich in ALA from nonwalnut sources; and 2) higher in MUFAs from oleic acid. It was hypothesized that lipoprotein subfractions, cholesterol efflux, and PCSK9 would be improved to a greater extent after the diet containing whole walnuts than after the other study diets.
Methods
Study design
Serum samples from a previously conducted study (11) (NCT01235832) were analyzed for lipoprotein classes and subclasses, cholesterol efflux capacity, and PCSK9, as exploratory outcomes of the trial. Briefly, a randomized, crossover, controlled-feeding study was conducted at Pennsylvania State University (11). After a 2-wk run-in standard Western diet (SWD), participants were randomly assigned, using a 6-sequence computer-generated randomization scheme (http://www.randomization.com) in blocks of 6, to receive each of the following diets in random order for 6 wk: 1) walnut diet (WD); 2) walnut fatty acid–matched diet (WFMD); and 3) oleic acid replaces ALA diet (ORAD) (Figure 1). The 3 diets had similar carbohydrate, protein, and fat profiles (Table 1; 48% carbohydrate, 17% protein, 35% fat, 7% SFAs). The run-in SWD was designed to represent a typical American diet. The presence or absence of whole walnuts and the fatty acid profile of the study diets were the only differences: WD—18% energy from walnuts (or 57–99 g/d), 16% PUFAs (including 2.7% ALA), 9% MUFAs; WFMD—did not contain walnuts, 16% PUFAs (including 2.6% ALA), 9% MUFAs; and ORAD—did not contain walnuts, 14% PUFAs (including 0.4% ALA), 12% MUFAs. The walnuts were provided by the California Walnut Commission. The primary outcome of the trial was central systolic blood pressure and secondary outcomes included peripheral blood pressure, markers of arterial stiffness, lipids and lipoproteins, glucose, and insulin. Further details about the study design and the results of the primary and secondary outcomes have been described previously (11). The Institutional Review Board at Pennsylvania State University approved the experimental protocol, and all participants gave informed consent.
FIGURE 1.
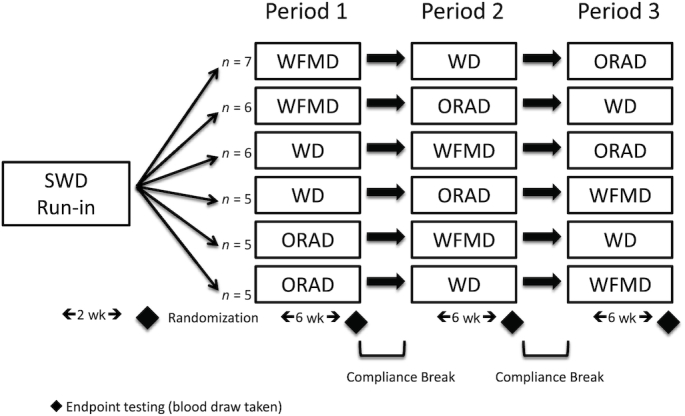
Study design used to examine the effect of replacing SFAs with unsaturated fats from walnuts or vegetable oils on lipoprotein classes and subclasses, cholesterol efflux, and PCSK9 in individuals at risk of cardiovascular disease. ORAD, oleic acid replaces α-linolenic acid diet; SWD, standard Western diet; WD, walnut diet; WFMD, walnut fatty acid–matched diet.
TABLE 1.
Nutrient profile of the run-in and study diets tested in individuals at risk of cardiovascular disease1
| Nutrient2 | SWD (run-in) | WD | WFMD | ORAD |
|---|---|---|---|---|
| Total fat | 34 | 35 | 35 | 35 |
| SFA | 12 | 7 | 7 | 7 |
| MUFA | 12 | 9 | 9 | 12 |
| PUFA | 7 | 16 (ALA 2.7) | 16 (ALA 2.6) | 14 (ALA 0.4) |
| Carbohydrate | 50 | 48 | 48 | 48 |
| Protein | 16 | 17 | 17 | 17 |
All diets used the same 6-d cycle menu, developed using Food Processor SQL software version 10.8 (ESHA Research). ALA, α-linolenic acid; ORAD, oleic acid replaces ALA diet; SWD, standard Western diet; WD, walnut diet; WFMD, walnut fatty acid–matched diet.
Represents percentage of total calories based on a 2100-kcal/d diet.
Participants
A comprehensive description of the participant inclusion and exclusion criteria has been reported elsewhere (11). Briefly, men and women (aged 30–65 y) with overweight and obesity [BMI (in kg/m2): 25–40] with an LDL-C between the 50th and 90th percentiles (men: 128–177 mg/dL; women: 121–172 mg/dL) and/or elevated blood pressure (systolic: 120–159 mm Hg; diastolic: 80–99 mm Hg), and free of chronic disease with no history of CVD, were eligible to participate. In addition, individuals taking medications or supplements known to affect the study outcomes were not eligible (i.e., those with blood pressure–, lipid- or glucose-lowering effects).
Outcome assessment
At baseline and the end of each diet period, subjects visited the Pennsylvania State University Clinical Research Center after a 12-h fast and avoidance of strenuous exercise, caffeine-containing products, and alcohol for 48 h. For these analyses, we used blood samples collected, on 1 d at each time point, by trained research nurses into serum separator vacutainers using standard protocols. The blood was kept at room temperature for 30 min, and then centrifuged at 1590 × g (± 90) for 15 min at room temperature. Serum samples were stored at −80°C from the time of collection until analysis (range: 0.5–4 y).
Serum cholesterol concentrations of major lipids and lipoprotein classes {HDL-C, IDL cholesterol (IDL-C), LDL-C, lipoprotein(a) [Lp(a)], remnant lipoprotein (Lp remnant), TC, TGs, and VLDL cholesterol} and subclasses {HDL2, HDL2A, HDL2B, HDL2C, HDL3, HDL3A, HDL3B, HDL3C, HDL3D, IDL1, IDL2, LDL1, LDL2, LDL3, LDL4, LDLreal [LDL cholesterol minus Lp(a) and IDL cholesterol], VLDL3, VLDL3A, and VLDL3B} were measured by vertical auto profile (VAP) (VAP Diagnostics Lab) (12). Briefly, VAP is an ultracentrifugation technique that quantifies lipoproteins based on flotation rate (a function of size and hydrated density).
Serum HDL-C (apoB-depleted serum) was prepared from individual serum samples by precipitation of apoB-containing lipoproteins using polyethylene glycol. The supernatant containing serum HDL-C was collected and used for analysis of cholesterol efflux capacity [ATP-binding cassette transporter 1 (ABCA1) and global cholesterol efflux] using J774 mouse macrophage cells in the presence of cAMP at a commercial research lab (Vascular Strategies).
Serum PCSK9 concentration was measured using the R&D Systems Human Proprotein Convertase 9 Quantikine® ELISA (R&D Systems, Inc.) in duplicate according to the manufacturers’ instructions at the Biomarker Core Laboratory in the Department of Biobehavioral Health, Penn State University. The CV for the duplicate samples was 3.8%. This assay recognizes free and LDL receptor–bound PCSK9, and recombinant human PCSK9.
Statistical analyses
All statistical analyses were performed with SAS version 9.4 (SAS Institute). For all variables, normality of the residuals was assessed using univariate analysis (PROC UNIVARIATE) to quantitatively evaluate skewness and to visually inspect the distribution and normal probability (Q–Q) plots. Variables with skewed residuals were logarithmically transformed. Change from baseline was calculated by subtracting measurements taken after the run-in diet from post–diet period values. Data are presented as least-squares means ± SEMs unless otherwise indicated.
The mixed-models procedure (PROC MIXED) was used to examine the effect of the study diets on each outcome measurement; subjects were modeled as a repeated factor and diet was included as a fixed factor. Randomization sequence was included as a fixed effect to assess carryover effects. No evidence of carryover effects was detected based on nonsignificant diet × sequence interactions for all outcomes; therefore, sequence was removed from the final model. In the primary analyses, between-diet difference in the mean values for each outcome was assessed. Secondary analyses assessed within- and between-diet change from baseline for all outcome variables. Within-diet changes from baseline were assessed using the mixed-models procedure (PROC MIXED). Selection of model covariance structures was based on optimizing fit statistics (evaluated as the lowest Bayesian Information Criterion). The baseline values (single measurements at the end of the run-in period) for each outcome were included as a covariate for analyses of between-diet mean differences. Tukey–Kramer corrected P values were used to correct for multiple comparisons. Statistical significance was set at P < 0.05.
Results
Participants
Subjects who completed the study and had a complete set of blood samples available for assay were included in these analyses (n = 34); 2 subjects completed the study but did not have a complete set of blood samples available for analysis because of failed blood draws or because no samples remained after analyses of the prespecified secondary outcomes. The sample included 13 women and 21 men with a mean age of 44 ± 10 y at risk of CVD. Participants had overweight or obesity (BMI: 30.1 ± 4.9 kg/m2) and elevated LDL-C (115 ± 30.3 mg/dL) and non-HDL-C (137 ± 31.3 mg/dL) at baseline.
Lipoprotein classes and subclasses measured by VAP
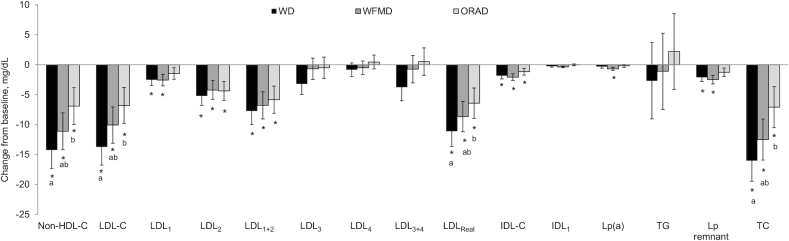
Endpoint-to-endpoint analysis of means showed main diet effects for LDL-C (P = 0.002), LDLreal (P = 0.006), non-HDL-C (P = 0.002), and TC (P = 0.001) (Table 2). Post hoc testing showed mean LDL-C was lower after the WD than after the ORAD (−6.9 ± 1.9 mg/dL; P = 0.001); no differences were observed between the WD and the WFMD (P = 0.1), or the ORAD and the WFMD (P = 0.2). LDLreal was also lower after the WD than after the ORAD (−6.0 ± 1.8 mg/dL; P = 0.005) and there were no differences between the WD and the WFMD (P = 0.09), or the ORAD and the WFMD (P = 0.5). Non-HDL-C was lower after the WD than after the ORAD (−7.3 ± 2.0 mg/dL; P = 0.001), but no other pairwise comparisons were significant. TC was also lower after the WD than after the ORAD (−8.9 ± 2.3 mg/dL; P < 0.001) and there were no differences between the WD and the WFMD (P = 0.3), or the ORAD and the WFMD (P = 0.06). No significant between-diet differences were detected for HDL-C or subfractions, IDL-C or subfractions, Lp(a), remnant lipoproteins, TG, or VLDL-C or subfractions.
TABLE 2.
The effect of the study diets on lipoprotein classes and subclasses, cholesterol efflux, and PCSK9 in individuals at risk of cardiovascular disease1
| Variable | Run-in2 | WD | WFMD | ORAD | Diet P value |
|---|---|---|---|---|---|
| TC, mg/dL | 182 ± 32.3 | 166 ± 2.9a | 170 ± 2.9ab | 175 ± 2.9b | 0.001 |
| HDL-C, mg/dL | 45.0 ± 9.9 | 43.0 ± 0.8 | 43.5 ± 0.8 | 44.7 ± 0.8 | 0.07 |
| HDL2 | 10.7 ± 4.8 | 10.0 ± 0.3 | 10.0 ± 0.3 | 10.5 ± 0.3 | 0.3 |
| HDL2A | 1.5 ± 1.2 | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.4 ± 0.2 | 0.9 |
| HDL2B | 2.3 ± 1.2 | 2.3 ± 0.2 | 2.4 ± 0.2 | 2.6 ± 0.2 | 0.3 |
| HDL2C | 6.8 ± 3.1 | 6.3 ± 0.3 | 6.2 ± 0.3 | 6.5 ± 0.3 | 0.4 |
| HDL3 | 34.4 ± 5.6 | 33.1 ± 0.6 | 33.5 ± 0.6 | 34.3 ± 0.6 | 0.08 |
| HDL3A | 13.0 ± 4.0 | 12.4 ± 0.3 | 12.7 ± 0.3 | 13.1 ± 0.3 | 0.1 |
| HDL3B | 3.8 ± 0.9 | 3.8 ± 0.2 | 3.5 ± 0.2 | 3.6 ± 0.2 | 0.5 |
| HDL3C | 10.3 ± 2.1 | 9.6 ± 0.3 | 10.0 ± 0.3 | 10.3 ± 0.3 | 0.08 |
| HDL3D | 7.4 ± 1.0 | 7.3 ± 0.1 | 7.2 ± 0.1 | 7.2 ± 0.1 | 0.9 |
| Non-HDL-C, mg/dL | 137 ± 31.3 | 123 ± 2.6a | 126 ± 2.6ab | 130 ± 2.6b | 0.002 |
| LDL-C, mg/dL | 115 ± 30.3 | 101 ± 2.4a | 105 ± 2.4ab | 108 ± 2.4b | 0.002 |
| LDL1 | 15.1 ± 6.8 | 12.6 ± 0.8 | 12.6 ± 0.8 | 13.6 ± 0.8 | 0.2 |
| LDL2 | 23.0 ± 14.9 | 17.8 ± 1.4 | 18.8 ± 1.4 | 18.6 ± 1.4 | 0.7 |
| LDL1+2 | 38.1 ± 18.8 | 30.4 ± 1.8 | 31.3 ± 1.8 | 32.3 ± 1.8 | 0.1 |
| LDL3 | 47.8 ± 16.3 | 44.6 ± 1.6 | 47.2 ± 1.6 | 47.3 ± 1.6 | 0.2 |
| LDL4 | 11.6 ± 12.4 | 11.1 ± 0.1 | 11.6 ± 0.1 | 12.7 ± 0.1 | 0.1 |
| LDL3+4 | 59.5 ± 23.5 | 56.0 ± 2.0 | 57.9 ± 2.0 | 59.2 ± 2.0 | 0.3 |
| LDLreal3 | 97.6 ± 25.8 | 86.2 ± 2.2a | 90.1 ± 2.2ab | 92.2 ± 2.2b | 0.006 |
| IDL-C, mg/dL | 12.1 ± 5.3 | 10.3 ± 0.5 | 10.0 ± 0.5 | 10.9 ± 0.5 | 0.3 |
| IDL1 | 3.4 ± 1.5 | 3.2 ± 0.1 | 3.0 ± 0.1 | 3.4 ± 0.1 | 0.6 |
| Lp(a), mg/dL | 5.2 ± 4.9 | 4.8 ± 0.3 | 4.6 ± 0.3 | 5.0 ± 0.3 | 0.2 |
| TG, mg/dL | 111 ± 48.6 | 109 ± 6.3 | 110 ± 6.3 | 114 ± 6.3 | 0.9 |
| VLDL-C, mg/dL | 22.3 ± 6.5 | 21.7 ± 0.8 | 21.6 ± 0.8 | 22.4 ± 0.8 | 0.6 |
| VLDL1+2 | 9.7 ± 4.1 | 9.4 ± 0.5 | 9.4 ± 0.5 | 9.8 ± 0.5 | 0.9 |
| VLDL3 | 12.7 ± 2.5 | 12.4 ± 0.3 | 12.1 ± 0.3 | 12.6 ± 0.3 | 0.4 |
| VLDL3A | 6.4 ± 1.8 | 6.3 ± 0.2 | 6.1 ± 0.2 | 6.3 ± 0.2 | 0.6 |
| VLDL3B | 6.3 ± 1.0 | 6.1 ± 0.1 | 6.0 ± 0.1 | 6.2 ± 0.1 | 0.2 |
| Lp remnant, mg/dL | 24.8 ± 6.7 | 22.7 ± 0.7 | 22.1 ± 0.7 | 23.5 ± 0.7 | 0.2 |
| ABCA1 efflux, % efflux/4 h | 3.7 ± 1.4 | 3.5 ± 0.2 | 3.5 ± 0.2 | 3.8 ± 0.2 | 0.1 |
| Global efflux, % efflux/4 h | 9.2 ± 1.7 | 8.8 ± 0.2 | 8.6 ± 0.2 | 9.1 ± 0.2 | 0.1 |
| PCSK9, ng/mL | 223 ± 75.3 | 227 ± 11.5 | 225 ± 11.5 | 239 ± 11.5 | 0.5 |
Values are least-squares means ± SEMs unless otherwise indicated, n = 34. Statistical analyses were performed with SAS version 9.4 (SAS Institute). The MIXED procedure was used to determine the effect of diet on each outcome measure adjusted for the baseline value. HDL2B, IDL, IDL1, IDL4, Lp(a), and TG were logarithmically transformed. Post hoc tests were adjusted for multiple comparisons using the Tukey–Kramer method. Means in the same row without a common superscript letter significantly differ, P < 0.05. ABCA1, ATP-binding cassette transporter A1; HDL-C, HDL cholesterol; IDL-C, IDL cholesterol; LDL-C, LDL cholesterol; Lp(a), lipoprotein(a); Lp remnant, lipoprotein remnant; ORAD, oleic acid replaces ALA diet; PCSK9, proprotein convertase subtilisin/kexin type 9; TC, total cholesterol; TG, triglyceride; VLDL-C, VLDL cholesterol; WD, walnut diet; WFMD, walnut fatty acid–matched diet.
Baseline measurements were taken after the run-in diet and are presented as mean ± SD.
LDLreal is LDL-C minus Lp(a) and IDL.
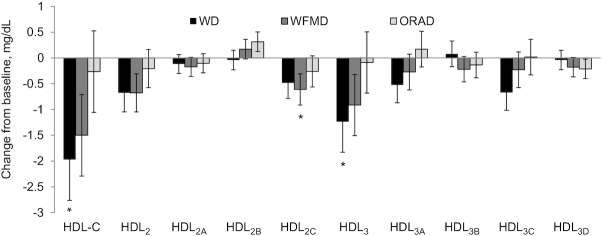
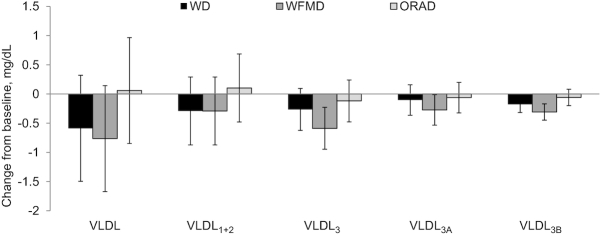
All 3 diets significantly reduced IDL-C, LDL-C, LDL2, LDL1+2, LDLreal, non-HDL-C, and TC from baseline (Figure 2). After the WD and the WFMD, LDL1 and Lp remnant were significantly reduced compared with baseline; Lp(a) was lowered after the WFMD only. A diet effect was detected for the change in LDL-C (P = 0.002) from baseline and this was because of a greater reduction after the WD (−13.7 ± 3.0 mg/dL) than after the ORAD (−6.8 ± 3.0 mg/dL, P = 0.001); no significant differences existed between the WD and the WFMD, or the ORAD and the WFMD. The reduction in LDLreal was also greater after the WD (−11.1 ± 2.5 mg/dL) than after the ORAD (−6.4 ± 2.5 mg/dL, P = 0.01), but no significant differences existed between the WD and the WFMD, or the ORAD and the WFMD. The WD (−14.3 ± 3.1 mg/dL) lowered non-HDL-C to a greater magnitude than the ORAD (−11.1 ± 3.1 mg/dL, P = 0.001); no significant differences were detected between the WD and the WFMD, or the ORAD and the WFMD. The WD (−16.0 ± 3.4 mg/dL) also lowered TC more than the ORAD (−7.1 ± 3.4 mg/dL, P = 0.0007) with no significant differences between the WD and WFMD, or the ORAD and the WFMD. No other between-diet differences in the change from baseline were detected (Figures 3 and 4).
FIGURE 2.
Vertical auto profile–assessed change from baseline for lipids, IDL-C subclasses, LDL-C subclasses, and Lp(a) after each diet in individuals at risk of cardiovascular disease. Data are least-squares means ± SEMs, n = 34. Statistical analyses were performed with SAS version 9.4 (SAS Institute). The MIXED procedure was used to determine the effect of diet on the change from baseline for each outcome measure. *Significant change from baseline (P < 0.05); diets in an outcome group without a common letter are significantly different, P < 0.05. HDL-C, HDL cholesterol; IDL-C, IDL cholesterol; LDL-C, LDL cholesterol; Lp(a), lipoprotein(a); Lp remnant, lipoprotein remnant; ORAD, oleic acid replaces α-linolenic acid diet; TC, total cholesterol; TG, triglyceride; WD, walnut diet; WFMD, walnut fatty acid–matched diet.
FIGURE 3.
Vertical auto profile–assessed change from baseline for HDL-C and subclasses after each diet in individuals at risk of cardiovascular disease. Data are least-squares means ± SEMs, n = 34. Statistical analyses were performed with SAS version 9.4 (SAS Institute). The MIXED procedure was used to determine the effect of diet on the change from baseline for each outcome measure. *Significant change from baseline (P < 0.05). HDL-C, HDL cholesterol; ORAD, oleic acid replaces α-linolenic acid diet; WD, walnut diet; WFMD, walnut fatty acid–matched diet.
FIGURE 4.
Vertical auto profile–assessed change from baseline for VLDL-C and subclasses after each diet in individuals at risk of cardiovascular disease. Data are least-squares means ± SEMs, n = 34. Statistical analyses were performed with SAS version 9.4 (SAS Institute). The MIXED procedure was used to determine the effect of diet on the change from baseline for each outcome measure. ORAD, oleic acid replaces α-linolenic acid diet; VLDL-C, VLDL cholesterol; WD, walnut diet; WFMD, walnut fatty acid–matched diet.
Cholesterol efflux
No difference in means was detected in the endpoint-to-endpoint comparisons for ABCA1-mediated (P = 0.1) or global efflux (P = 0.1) (Table 2). Similarly, between-diet differences in the change from baseline for ABCA1-mediated or global efflux were not observed. Within-diet change from baseline analyses showed a reduction in global efflux after the WFMD only (P = 0.01) (Supplemental Figure 1).
PCSK9
Mean PCSK9 was not different between the diets (P = 0.5) (Table 2). PCSK9 remained unchanged after the 3 study diets, and no significant between-diet difference in the change from baseline was detected.
Discussion
This analysis is the first to our knowledge to examine how walnut consumption affects lipoprotein subfractions. After 6 wk of a healthy walnut-containing diet, LDL-C, LDLreal, non-HDL-C, and TC were significantly lowered compared with a healthy diet matched for SFAs but higher in MUFAs and devoid of walnuts (ORAD). However, no differences in lipoprotein classes or subclasses were observed between the WD and the fatty acid–matched diet (WFMD). Similarly, the WFMD had effects on lipoprotein classes and subclasses comparable with those of the ORAD. This suggests unique benefits of the walnut whole food complex beyond the fatty acid composition, because the presence or lack of ALA did not explain the difference. Our findings also demonstrate no increase in Lp(a), a well-established independent causal risk factor for CVD (13, 14), which has previously been shown to increase when SFA is reduced (15–17). The reduction in atherogenic lipids and lipoproteins without an increase in Lp(a) contributes to our understanding of healthy dietary patterns that lower the risk of CVD by modulating lipoproteins beyond LDL-C.
The primary findings from this clinical trial are reported elsewhere (11) and analyses consistent with intent-to-treat principles showed no between-group differences in lipids or lipoproteins; all 3 study diets reduced LDL-C (estimated using the Friedewald equation), non-HDL-C, and TC from baseline. However, lipoprotein subfractions were not analyzed previously. The results from this secondary analysis corroborate findings from the original study (11) and other trials (1) that show replacing SFAs with walnuts or liquid vegetable oils lowers atherogenic lipoproteins. Furthermore, in the present analyses clinically relevant differences were detected between the diets; the WD significantly reduced non-HDL-C and LDL-C compared with the ORAD. The findings we report herein, from direct measurement of LDL-C, are consistent with the large body of evidence showing that replacement of SFAs with PUFAs confers the greatest lipid-lowering response (18). No between-diet differences were detected in LDL-C subclasses; however, LDL2, and LDL1+2 were reduced from baseline after all 3 diets, which is in contrast to LDL3, LDL4, and LDL3+4 that were unchanged after the diets. After the WD and the WFMD, LDL1 was lower than at baseline, but no change was detected after the ORAD. In sum, the LDL-C-lowering observed was predominately caused by a reduction in large buoyant LDL-C. All LDL-C particles are atherogenic, independent of size or density, although small, dense LDL-C are considered to have greater atherogenic potential because of their longer half-life, increased oxidative susceptibility, and greater endothelial permeability (3). At present, LDL-C and non-HDL-C are the major therapeutic targets for CVD risk reduction (19) and our findings demonstrate that the WD conferred greater improvement in these targets than did the ORAD.
The present study showed no change in Lp(a) and no evidence of a tendency toward increased concentration; after the WFMD, Lp(a) was modestly decreased compared with baseline. This is in contrast to previous studies in which increases in Lp(a) were observed with SFA-lowering (15–17, 20). In the DELTA I and II studies, replacement of SFA with either carbohydrate or MUFA increased Lp(a) (15, 16). Similarly, in the OmniHeart Trial, a Dietary Approaches to Stop Hypertension-style diet rich in unsaturated fats (37% kcal total fat; 21% kcal MUFAs; 10% kcal PUFAs) increased Lp(a) concentrations, but the magnitude of the increase was less than for a protein- or carbohydrate-rich diet (17). In our study, all 3 diets had a higher PUFA composition (14–16% kcal) than those studied in the OmniHeart Trial or DELTA I and II, which may explain the contrasting results. Thus, the lack of any worsening in Lp(a) in our study, and the benefit (albeit very small) after the WFMD are notable because Lp(a) is strongly positively associated with risk of future cardiovascular events (21). Furthermore, Lp(a) contributes to residual risk in patients at target LDL-C concentrations (22). Current evidence suggests replacement of SFAs with unsaturated fats may minimize worsening of Lp(a) compared with protein or carbohydrates. The results of the present study further indicate that replacement of SFAs with food sources of PUFAs including walnuts and vegetable oils may, at the least, result in no change in Lp(a) and may confer small improvements. Further research is required to understand the effect of diet on Lp(a), which is relatively unexplored because of the prevailing perception that diet has no significant effect on Lp(a) (2). However, the clinical significance of Lp(a) lowering, independently of LDL-C, on risk of CVD is currently unclear, but is of significant scientific interest (2).
Based on previous work showing that replacement of SFAs with PUFAs (23), or with PUFA-rich whole walnuts (9) or walnut oil (8), increases cholesterol efflux, we hypothesized that replacement of SFAs with walnuts would improve ex vivo cholesterol efflux and this would, in part, explain the cholesterol-lowering effect of walnuts. However, no change in ex vivo ABCA1 or global efflux was observed in this study. Previously, Berryman et al. (9) observed a 3.3% increase (compared with the premeal value) in ex vivo cholesterol efflux, assessed using the same method as we report herein, 240 min after consumption of 85 g whole walnuts. Similarly, Zhang et al. (8) reported that walnut oil increased in vitro (macrophage-derived foam cells) cholesterol efflux; however, expression of genes related to ABCA1 and ATP-binding cassette transporter G1 (ABCG1) protein expression was unaffected, which is consistent with our findings showing no change in ABCA1-mediated efflux. In addition, walnut oil suppressed stearoyl CoA desaturase 1 (SCD1) transcription and protein expression, and further experiments showed that over-expression of SCD1 inhibited cholesterol efflux (8). In subsequent ex vivo analyses, using serum samples from subjects that consumed 51 g walnut oil, Zhang et al. (8) observed increased cholesterol efflux at 240 min after consumption only in subjects with a C-reactive protein concentration <2 mg/L; SCD1 protein expression was also reduced. Replacement of SFA with PUFA causes upregulation of ABCG1 gene expression (23), which may also explain the lack of change in ABCA1-mediated efflux in our study. In the present study, SCD1 mRNA or protein levels, and ABCG1-mediated efflux were not measured, although because no change in global efflux was observed it is unlikely that walnut consumption induced alterations in these pathways that functionally affected efflux capacity. The discordance in findings from our study compared with previous postprandial studies may be because we assessed cholesterol efflux capacity in the fasted state, which is likely more representative of functional changes in the steady state. Findings from previous in vitro (8) and ex vivo (8, 9) research suggest that walnut consumption increases postprandial cholesterol efflux; however, the results of our current study suggest chronic walnut consumption does not change efflux capacity, but further investigation is warranted.
The present study also showed no change in PCSK9 after replacement of SFAs with unsaturated fats from walnuts or vegetable oils, suggesting that LDL-receptor-mediated cholesterol clearance was not altered by the study diets. Previously, replacement of SFAs with PUFAs has been shown to reduce circulating PCSK9 (10), although increases in PCSK9 have also been observed (23). A 10-wk randomized controlled trial of isocaloric diets enriched in either PUFA or SFA showed PCSK9 concentrations were lowered with the PUFA diet (−36 μg/L) compared with the SFA-rich (+15 μg/L) diet (10). In the study by Bjermo et al. (10), subjects had higher baseline PSCK9 concentrations (∼275 μg/L) and higher LDL-C concentrations (∼130 mg/dL) than the subjects in the present study, and 16% of the subjects were taking lipid-lowering medication, which affects PCSK9 concentrations. Ulven et al. (23) reported increased PCSK9 in response to replacement of SFA with PUFA, which may be because of counter-regulation mechanisms. A decrease in dietary SFA depletes intracellular cholesterol concentrations, causing an upregulation of LDL-receptor and PCSK9 expression by sterol regulatory element binding transcription factor-2. Consistent with this, Simonen et al. (24) observed no change in PCSK9, despite a 10% reduction in LDL-C, in response to a vegetable-oil spread (20 g/d) enriched with plant stanols (3 g/d as ester) compared with an unenriched version after 6 mo. In this study, cholesterol absorption, measured by campesterol and sitosterol, was markedly reduced followed by an increase in cholesterol synthesis.
We did not measure cholesterol absorption in this study, but this is another plausible mechanism that may explain the LDL-C-lowering observed with walnut consumption. Holscher et al. (25) reported lower serum campesterol (−6%) after consumption of 42 g walnuts/d, in a 2-wk controlled feeding study, suggesting a reduction in cholesterol absorption. This may be attributed to the phytosterols present in walnuts, which interfere with intestinal cholesterol absorption (4, 26). Thus, a decrease in cholesterol absorption may be one mechanism by which walnuts lower LDL-C; however, future investigations are required to evaluate this.
Improvements in diet quality, in addition to the replacement of SFAs with walnuts and vegetable oils, may also, in part, explain the observed lipid- and lipoprotein-lowering. The run-in, Western-style diet included 12% energy from SFAs, but the Healthy Eating Index-2015 (HEI) score was 75.5 (out of 100), which is relatively high given Americans, on average, have an HEI of 59 (27). The 3 study diets differed only in the presence or absence of walnuts and the ALA content, and thus there was no difference in the HEI score, which was 98.5 out of 100 (Supplemental Table 1). Replacement of SFA with PUFA or MUFA in the study diets resulted in an 11-point increase in the HEI score. These changes are likely feasible for free-living people; for example, the walnuts [∼28–49 walnut halves/d (57–99 g/d) depending on energy intake] were incorporated in the menu as snacks in place of energy-dense nutrient-poor snack foods typically consumed in the United States. Improving diet quality by replacing SFAs with walnuts or liquid vegetable oils is a relatively simple change that could have substantial health benefits, as demonstrated by the present analyses.
Strengths of the current analyses include the crossover, controlled feeding design of the parent study. In this design, participants serve as their own controls and the influence of other dietary factors that could affect the outcomes is minimized. However, this study was limited by the lack of characterization of the plasma fatty acid profile after consumption of the diets. In addition, chemical analysis of the diets was not done to verify the composition of fatty acids and other constituents. We have reported lipoprotein and subfraction concentrations, measured by VAP; however, quantification by NMR would provide greater detail about lipoprotein particle number/concentration, rather than size and concentration alone. It should also be acknowledged that the risk of type I statistical errors is increased because of the large number of outcome variables assessed in these exploratory analyses. Finally, analyzed serum samples were frozen at −80°C for 0.5–4 y; it is unclear how the storage time and conditions may have affected the integrity of the samples.
In summary, our findings demonstrate that replacement of SFAs with unsaturated fats from walnuts or liquid vegetable oils significantly improves lipids and lipoprotein classes, including LDL-C, LDLreal, non-HDL-C, and TC in the absence of an increase in Lp(a). Replacement of SFAs with walnuts conferred greater reductions in the aforementioned lipids and lipoproteins than a diet with an equivalent SFA composition but lower in ALA. These findings further underscore the importance of replacing SFAs with unsaturated fats in the context of a healthy dietary pattern.
Supplementary Material
Acknowledgments
We thank the Biomarker Core Laboratory in the Department of Biobehavioral Health, Penn State for biological assay data. We also thank the Clinical Research Center staff nurses, Tracey Allen, Cyndi Flanagan, and Christa Oelhaf, for their assistance. The authors’ responsibilities were as follows—KSP and PMK-E: designed the study; AMT: collected the data; AMT and KSP: performed the statistical analyses; and all authors: wrote the paper and read and approved the final manuscript.
Notes
Supported by the California Walnut Commission, the Pennsylvania State University's Clinical and Translational Science Institute supported by the NIH/National Center for Advancing Translational Sciences grant 1UL1TR002014-01.
Author disclosures: PMK-E serves as a consultant for Seafood Nutrition Partnership, Avocado Nutrition Science Advisors, and HUMANn. All other authors report no conflicts of interest.
The California Walnut Commission provided funds for the research conducted. Their staff were not involved with any aspects of conducting the study, data analyses, or interpretation of the results reported in this article.
Supplemental Table 1 and Supplemental Figure 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: ABCA1, ATP-binding cassette transporter A1; ABCG1, ATP-binding cassette transporter G1; ALA, α-linolenic acid; CVD, cardiovascular disease; HEI, Healthy Eating Index; Lp(a), lipoprotein(a); Lp remnant, lipoprotein remnant; ORAD, oleic acid replaces ALA diet; PCSK9, proprotein convertase subtilisin/kexin type 9; SCD1, stearoyl CoA desaturase 1; SWD, standard Western diet; TC, total cholesterol; TG, triglyceride; VAP, vertical auto profile; WD, walnut diet; WFMD, walnut fatty acid–matched diet.
References
- 1. Guasch-Ferré M, Li J, Hu FB, Salas-Salvadó J, Tobias DK. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: an updated meta-analysis and systematic review of controlled trials. Am J Clin Nutr. 2018;108:174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilson DP, Jacobson TA, Jones PH, Koschinsky ML, McNeal CJ, Nordestgaard BG, Orringer CE. Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13(3):374–92. [DOI] [PubMed] [Google Scholar]
- 3. Davidson MH, Ballantyne CM, Jacobson TA, Bittner VA, Braun LT, Brown AS, Brown WV, Cromwell WC, Goldberg RB, McKenney JM. Clinical utility of inflammatory markers and advanced lipoprotein testing: advice from an expert panel of lipid specialists. J Clin Lipidol. 2011;5:338–67. [DOI] [PubMed] [Google Scholar]
- 4. Souza RGM, Gomes AC, Naves MMV, Mota JF. Nuts and legume seeds for cardiovascular risk reduction: scientific evidence and mechanisms of action. Nutr Rev. 2015;73:335–47. [DOI] [PubMed] [Google Scholar]
- 5. Kris-Etherton PM, Yu-Poth S, Sabaté J, Ratcliffe HE, Zhao G, Etherton TD. Nuts and their bioactive constituents: effects on serum lipids and other factors that affect disease risk. Am J Clin Nutr. 1999;70:504s–11s. [DOI] [PubMed] [Google Scholar]
- 6. Rosenson RS, Brewer HB, Ansell B, Barter P, Chapman MJ, Heinecke JW, Kontush A, Tall AR, Webb NR. Translation of high-density lipoprotein function into clinical practice. Circulation. 2013;128:1256–67. [DOI] [PubMed] [Google Scholar]
- 7. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA et al.. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–107. [DOI] [PubMed] [Google Scholar]
- 8. Zhang J, Grieger JA, Kris-Etherton PM, Thompson JT, Gillies PJ, Fleming JA, Vanden Heuvel JP. Walnut oil increases cholesterol efflux through inhibition of stearoyl CoA desaturase 1 in THP-1 macrophage-derived foam cells. Nutr Metab (Lond). 2011;8:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Berryman CE, Grieger JA, West SG, Chen C-YO, Blumberg JB, Rothblat GH, Sankaranarayanan S, Kris-Etherton PM. Acute consumption of walnuts and walnut components differentially affect postprandial lipemia, endothelial function, oxidative stress, and cholesterol efflux in humans with mild hypercholesterolemia. J Nutr. 2013;143:788–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L, Berglund J, Pulkki K, Basu S, Uusitupa M et al.. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr. 2012;95:1003–12. [DOI] [PubMed] [Google Scholar]
- 11. Tindall AM, Petersen KS, Skulas‐Ray AC, Richter CK, Proctor DN, Kris‐Etherton PM. Replacing saturated fat with walnuts or vegetable oils improves central blood pressure and serum lipids in adults at risk for cardiovascular disease: a randomized controlled‐feeding trial. J Am Heart Assoc. 2019;8:e011512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kulkarni KR. Cholesterol profile measurement by vertical auto profile method. Clin Lab Med. 2006;26:787–802. [DOI] [PubMed] [Google Scholar]
- 13. Nordestgaard BG, Langsted A. Lipoprotein(a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res. 2016;57:1953–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS et al.. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ginsberg HN, Kris-Etherton P, Dennis B, Elmer PJ, Ershow A, Lefevre M, Pearson T, Roheim P, Ramakrishnan R, Reed R et al.. Effects of reducing dietary saturated fatty acids on plasma lipids and lipoproteins in healthy subjects: the DELTA Study, protocol 1. Arterioscler Thromb Vasc Biol. 1998;18:441–9. [DOI] [PubMed] [Google Scholar]
- 16. Berglund L, Lefevre M, Ginsberg HN, Kris-Etherton PM, Elmer PJ, Stewart PW, Ershow A, Pearson TA, Dennis BH, Roheim PS et al.. Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: studies in the fasting and postprandial states. Am J Clin Nutr. 2007;86:1611–20. [DOI] [PubMed] [Google Scholar]
- 17. Haring B, von Ballmoos MCW, Appel LJ, Sacks FM. Healthy dietary interventions and lipoprotein(a) plasma levels: results from the Omni Heart Trial. PLoS One. 2014;9:e114859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sacks FM, Lichtenstein AH, Wu JHY, Appel LJ, Creager MA, Kris-Etherton PM, Miller M, Rimm EB, Rudel LL, Robinson JG et al.. Dietary fats and cardiovascular disease: a Presidential Advisory from the American Heart Association. Circulation. 2017;136:e1–e23. [DOI] [PubMed] [Google Scholar]
- 19. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE et al.. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silaste M-L, Rantala M, Alfthan G, Aro A, Witztum JL, Kesäniemi YA, Hörkkö S. Changes in dietary fat intake alter plasma levels of oxidized low-density lipoprotein and lipoprotein(a). Arterioscler Thromb Vasc Biol. 2004;24:498–503. [DOI] [PubMed] [Google Scholar]
- 21. Forbes CA, Quek RGW, Deshpande S, Worthy G, Wolff R, Stirk L, Kleijnen J, Gandra SR, Djedjos S, Wong ND. The relationship between Lp(a) and CVD outcomes: a systematic review. Lipids Health Dis. 2016;15:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Albers JJ, Slee A, O'Brien KD, Robinson JG, Kashyap ML, Kwiterovich PO, Xu P, Marcovina SM. Relationship of apolipoproteins A-1 and B, and lipoprotein(a) to cardiovascular outcomes. J Am Coll Cardiol. 2013;62:1575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ulven SM, Christensen JJ, Nygård O, Svardal A, Leder L, Ottestad I, Lysne V, Laupsa-Borge J, Ueland PM, Midttun Ø et al.. Using metabolic profiling and gene expression analyses to explore molecular effects of replacing saturated fat with polyunsaturated fat—a randomized controlled dietary intervention study. Am J Clin Nutr. 2019;109:1239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Simonen P, Stenman U-H, Gylling H. Serum proprotein convertase subtilisin/kexin type 9 concentration is not increased by plant stanol ester consumption in normo- to moderately hypercholesterolaemic non-obese subjects. The BLOOD FLOW intervention study. Clin Sci. 2015;129:439–46. [DOI] [PubMed] [Google Scholar]
- 25. Holscher HD, Guetterman HM, Swanson KS, An R, Matthan NR, Lichtenstein AH, Novotny JA, Baer DJ. Walnut consumption alters the gastrointestinal microbiota, microbially derived secondary bile acids, and health markers in healthy adults: a randomized controlled trial. J Nutr. 2018;148:861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ros E, Hu FB. Consumption of plant seeds and cardiovascular health: epidemiological and clinical trial evidence. Circulation. 2013;128:553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. USDA. HEI scores for Americans. [Internet] [Accessed 2019 Aug 1]. Alexandria (VA): Food and Nutrition Service; 2019. Available from: https://www.fns.usda.gov/hei-scores-americans. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.