Abstract
Background:
Sleeve gastrectomy is the most commonly performed weight loss surgery in adolescents with moderate-to-severe obesity. While studies in adults have reported on the deleterious effects of gastric bypass surgery on bone structure and strength estimates, data are lacking for the impact of sleeve gastrectomy on these measures in adolescents.
Objective:
To evaluate the impact of sleeve gastrectomy on bone outcomes in adolescents and young adults over 12 months using dual energy x-ray absorptiometry (DXA) and high resolution peripheral quantitative computed tomography (HRpQCT).
Participants and Methods:
We enrolled 44 youth 14-22 years old with moderate to severe obesity; 22 underwent sleeve gastrectomy and 22 were followed without surgery (16 females and 6 males in each group). At baseline and 12 months, DXA was used to assess areal bone mineral density (aBMD), HRpQCT of the distal radius and tibia was performed to assess bone geometry, microarchitecture and volumetric BMD (vBMD), and finite element analysis to assess strength estimates (stiffness and failure load). These analyses are adjusted for age, sex, race and the bone measure at baseline. Fasting blood samples were assessed for calcium, phosphorus, and 25(OH) vitamin D (250HD) levels.
Results:
Over 12-months, the surgical group lost 27.2% of body weight compared to 0.1% in the non-surgical (control) group. Groups did not differ for changes in 25(OH) vitamin D levels (p=0.186). Compared to controls, the surgical group had reductions in femoral neck and total hip aBMD Z-scores (p≤ 0.0006). At the distal tibia, compared to controls, the surgical group had reductions in cortical area and thickness and trabecular number, and increases in trabecular area and separation (p≤0.026). At the distal radius, the surgical group had greater reductions in trabecular vBMD, than controls (p=0.010). The surgical group had an increase in cortical vBMD at both sites (p≤0.040), possibly from a decrease in cortical porosity (p≤0.024). Most, but not all, differences were attenuated after adjusting for 12-month change in BMI. Groups did not differ for changes in strength estimates over time; except that increases in tibial stiffness were lower in the surgical group (p=0.044) after adjusting for 12-month change in BMI.
Conclusions:
Over 12 months, weight loss associated with sleeve gastrectomy in adolescents had negative effects on areal BMD and certain HRpQCT parameters. However, bone strength estimates remained stable, possibly because of a simultaneous decrease in cortical porosity and increase in cortical volumetric BMD. Additional research is necessary to determine the relative contribution(s) of weight loss and the metabolic effects of surgery, and whether the observed effects on bone stabilize or progress over time.
Keywords: Weight loss surgery, bariatric surgery, adolescents, bone density, bone geometry, bone microarchitecture
1. Introduction
Metabolic and bariatric surgery is an increasingly common treatment strategy in adolescents and young adults with moderate to severe obesity [1-4]. Whereas such surgery is typically associated with a significant improvement in various metabolic parameters, metabolic and bariatric surgery has been associated with deleterious effects on bone health. Several studies in adults indicate that gastric bypass leads to reductions in areal and volumetric bone mineral density (BMD), with negative effects on bone geometry and microarchitecture [5-10]. Similarly, adults undergoing sleeve gastrectomy have a reduction in total hip and femoral neck areal BMD over time [6, 11-13]. Some [6, 8], though not all [12, 13] studies in adults report lesser reductions in total hip and femoral neck BMD following sleeve gastrectomy vs. gastric bypass. A meta-analysis comparing sleeve gastrectomy to bypass reported no differences between groups in postoperative BMD [13]. Metabolic and bariatric surgery has also been associated with an increase in fracture risk [14, 15]. Studies comparing fracture risk following gastric bypass vs. sleeve gastrectomy are conflicting with one reporting increased fracture risk in the bypass group alone [16]. and another reporting no differences between groups [17].
Adolescence is a time of marked increases in bone accrual towards attainment of peak bone mass, a key determinant of bone health and fracture risk in later life [18]. Studies in other populations suggest that suboptimal bone accrual during adolescence can lead to suboptimal peak bone mass, and an increased risk of fractures in later life [18]. The utilization of metabolic and bariatric surgery as a therapeutic strategy in adolescents with obesity has markedly increased in recent times [19, 20], with a 1.8 fold increase reported between 2012-2016 in children’s hospitals in the United States. It is thus important to determine how and to what extent such surgery impacts bone accrual and morphology during the critical teenage years. A few studies have assessed bone outcomes in adolescents undergoing gastric bypass [21, 22], and report significant reductions in whole body bone mineral content and BMD Z-scores following bypass over a two-year period. However, data are lacking regarding the effects of sleeve gastrectomy on bone outcomes in adolescents, particularly data regarding bone structure and strength estimates. Based on some studies in adults, effects may be less severe following sleeve gastrectomy than gastric bypass [6, 8] given that certain factors that drive bone loss (such as malabsorption and hormonal changes) are more pronounced following bypass than sleeve procedures. Conversely, given that sleeve gastrectomy in adolescents results in similar reductions in BMI as gastric bypass [23], effects may be comparable for effects primarily related to skeletal unloading following surgery. It is critical to study the impact of sleeve gastrectomy on bone in youth, given that 1) sleeve gastrectomy is now the most commonly performed bariatric procedure in adolescents, 2) because the massive weight loss associated with surgery may affect load related bone physiology, and 3) the effects of surgery on energy metabolism and nutrition may impact bone and metabolic health in youth differently from adults [3].
In order to address this knowledge gap, we examined bone outcomes in youth aged 14-22 years old with moderate-to-severe obesity undergoing sleeve gastrectomy, as well as non-surgical controls of comparable body size matched for age and sex. This age range was chosen given that this is a critical period of peak bone mass acquisition in youth [18]. We hypothesized that as in adults, adolescents undergoing sleeve gastrectomy would have a reduction in areal BMD measures [assessed using dual energy x-ray absorptiometry (DXA)], and that weight loss after sleeve gastrectomy would have a deleterious effect on high resolution peripheral quantitative computed tomography (HRpQCT) measures of bone geometry, microarchitecture and volumetric BMD, associated with reductions in body mass index (BMI) and lean mass.
2. Participants and Methods
2.1. Participant Selection:
We enrolled 44 adolescents and young adults aged 14-22 years old with moderate to severe obesity, 22 of whom underwent sleeve gastrectomy (16 female and 6 male) and 22 were non-surgical controls (16 female and 6 male). All participants had a BMI of ≥35 kg/m2 with obesity related complications or a BMI of ≥40 kg/m2 (i.e. met criteria for metabolic and bariatric surgery). Exclusion criteria included (i) current pregnancy or breast feeding (in females), (ii) use of oral glucocorticoids and other oral or intravenous medications that may affect bone metabolism (other than calcium, vitamin D or hormonal contraception) within eight weeks of the baseline visit, (iii) use of antipsychotic medications that cause weight gain if treated for less than six months or if the dose was not stable for at least two months preceding study enrollment, (iv) untreated thyroid dysfunction or if the participant was on a stable dose of replacement levothyroxine for less than three months before study enrollment, and (v) history of smoking more than ten cigarettes per day or of substance abuse (per DSM-5). We did not exclude females on oral hormonal contraception because of the large number of females in this age range who are on these medications for management of polycystic ovarian disease or for contraception, but did exclude participants on depot medroxyprogesterone injections, given their known profound effect on bone. Further, we did not exclude patients on the progestogen releasing intrauterine device given its limited systemic effects, or those on progestogenic implants given their limited bone effects.
Participants were recruited from specialized programs focused on providing lifestyle and surgical options for weight regulation and area hospitals. The study was approved by the Partners Institutional Review Board and was Health Insurance Portability and Accountability Act compliant. Informed consent was obtained from participants 18 years and older, or parents of participants < 18 years. Informed assent was obtained from participants < 18 years old.
2.2. Experimental Protocol:
Following a screening visit to confirm eligibility for the study, participants completed a baseline visit for assessment of DXA and HRpQCT bone measures (see subsequent sections). A medical history, physical examination, and anthropometric measurements were obtained in all participants. Height was measured on a wall mounted stadiometer as the average of three measurements, and weight to the nearest 0.1 kg using an electronic scale. Body mass index (BMI) was calculated as weight in kg/(height in meters)2. Height, weight and BMI standard deviation scores (SDS) or z-scores were calculated using CDC 2000 databases [24]. Body composition was assessed using dual energy x-ray absorptiometry (DXA). Fasting blood concentrations of calcium, phosphate, and 25(OH) vitamin D (25OHD) were measured by a reference laboratory (LabCorp, Burlington, NC, USA). Physical activity was assessed using the Paffenbarger questionnaire [25, 26]. The baseline visit was performed within a month preceding surgery for those undergoing sleeve gastrectomy. Participants returned for follow-up 12-months following the baseline visit for non-surgical participants, and 12-months following surgery for those undergoing sleeve gastrectomy. Non-surgical controls received diet and exercise counseling throughout the study from dieticians of our Translational and Clinical Research Center, specialized programs that were enrolled in, or their primary care provider.
2.3. Calcium and vitamin D administration strategy:
All participants completed a calcium and vitamin D food frequency questionnaire to assess their daily intake of these micronutrients [27]. All participants were also offered at least 1200 mg elemental calcium and 800 IUs vitamin D daily to optimize calcium intake and absorption. Additional recommendations for vitamin D supplementation were as follows: for 25-hydroxy vitamin D (25OHD) levels between 20-30 ng/mL: 4000 IUs of vitamin D supplementation daily; for 25OHD levels between 12-20 ng/mL: 50,000 IUs of vitamin D per week for two months followed by 2000 IUs daily; for 25OHD levels <12 ng/mL: 50,000 IUs of vitamin D per week for three months followed by 2000 IUs daily based on recommendations typically provided for those undergoing surgery [28, 29].
2.4. Dual Energy X-Ray Absorptiometry:
Areal BMD (aBMD) at the 1/3 radius, lumbar spine, total hip, femoral neck, whole body and whole body less had was assessed using DXA (Hologic 4500 A, Waltham, MA) on a single instrument at the baseline visit and one-year post gastrectomy in the surgical group, and one year following the baseline visit in the non-surgical control group (least significant change 0.024-0.048 g/cm2, coefficients of variation for areal BMD, fat and lean mass 0.8%– 1.1%, 2.1% and 1.0%, respectively). Areal BMD was adjusted for age, sex and race, and Z-scores are reported using a standardized Hologic dataset. All scans were acquired by an ISCD certified DXA technologist, and analyzed using the same software. DXA was also used to assess total and percent fat mass and lean mass.
2.5. High Resolution Peripheral Quantitative Computed Tomography (HRpQCT) and Microfinite Element Analysis:
HRQCT (XtremeCT; Scanco Medical AG, Bassersdorf, Switzerland) was used to assess bone geometry (cortical perimeter, cortical and trabecular crosssectional area, and cortical thickness), bone microarchitecture (cortical porosity, trabecular number, thickness and separation) and total, cortical and trabecular volumetric BMD (vBMD) at the distal tibia (weight-bearing site) and distal radius (non-weight bearing site). A region of interest spanning 9.02 mm was scanned with an isotropic voxel size of 82 μm. The non-dominant wrist and leg were analyzed unless there was a previous acute fracture at these sites, in which case the non-fractured side was assessed. The same wrist/leg was assessed at the 12-month follow-up visit. CT slices were obtained at 22.5 and 9.5 mm from the tibia and radius endplates, respectively. Fixed sites were used because linear growth was mostly complete in study participants (bone age of 15 or more in girls and 17 or more in boys). Same-day reproducibility for repeated measurements is 0.2 to 1.4% for vBMD values, 0.3 to 8.6% for trabecular microarchitecture parameters, and 0.6 to 2.4% for cortical microarchitecture parameters. For analysis of HRpQCT parameters, seven participants in each group were excluded from analyses for each site due to motion artifact or poor scan quality at the baseline or 12-month visit.
Micro finite element analysis (μFEA) was performed to determine strength estimates (stiffness and failure load) from the CT data in the setting of simulated axial compression [30]. These μFEA estimates correlate strongly with true bone strength assessed using cadaveric bone [31, 32]. Failure load was estimated by scaling the resultant load from a 1% apparent compressive strain until 2% of all elements reached an effective strain > 7000μ strain.
2.6. Statistical Analysis:
Data are presented as mean+/−SEM or median (interquartile range) unless otherwise indicated. JMP Statistical Discovery Software (Version 13, SAS Institute, Carey, NC) was used for statistical analysis. Based on preliminary data, with 44 versus 30 participants (for DXA vs. HRpQCT data), the study was powered at 97 and 90% respectively to detect a 3.5% difference between groups for changes in bone parameters over time at an alpha level of 0.05, based on an estimated SD of change of 2.85%. To compare the surgical vs. non-surgical groups, we used the Student t-test or the Wilcoxon Rank Sum test depending on the data distribution. Within group comparisons were performed using the paired t-test. Multivariable analysis was used to determine differences between groups (least square means +/− SEM) after controlling for possible covariates (age, sex, race and baseline bone measure +/− 12-month change in BMI). Spearman correlations were used to determine associations of covariates known to impact bone (i.e. change in BMI, lean mass, fat mass, 25OHD levels, HbA1C, and physical activity) [33-35] with DXA and HRpQCT parameters. A p value of <0.05 was considered to be statistically significant.
3. Results
3.1. Baseline Characteristics:
The sleeve gastrectomy (surgical) and non-surgical groups did not differ at baseline for age, weight, BMI z-scores, and percent lean and fat mass, although absolute BMI and fat mass were higher in the surgical vs. non-surgical groups (Table 1). The groups did not differ for baseline levels of 25OHD, calcium, phosphorus and HbA1C levels. Further, the number of study participants with HbA1C levels in the normal (<5.7%), prediabetes (5.7-6.4%) and diabetes ranges (≤ 6.5%) did not differ across groups (n=12, 8 and 2 respectively in the surgical group, and 14, 6 and 2 respectively in the non-surgical group). Race was self-reported by study participants and did not differ across groups.
Table 1:
Clinical Characteristics and Body Composition Measures at Baseline and Changes over 12 Months in the Surgical and Non-Surgical Groups
| Clinical Characteristics | Baseline Measure (Mean ± SEM) |
Change over 12-months [Mean (95% CI)] |
P-value comparing changes over 12 months in surgical vs. non-surgical groups |
||
|---|---|---|---|---|---|
| Non-Surgical (n=22) |
Surgical (n=22) |
Non-Surgical (n=22) |
Surgical (n=22) |
||
| Age (years) | 17.0 ± 0.5 | 18.3 ± 0.5 | - | - | - |
| Weight (kg) | 119.5 ± 4.7 | 132.7 ± 4.4 | −0.0 (−4.0, 3.9) | −35.9 (−41.6, −30.2) | <0.0001 |
| BMI (kg/m2) | 42.4 ± 1.3 | 47.0 ± 1.5* | −0.5 (−1.9, 1.0) | −12.8 (−14.8, −10.9) | <0.0001 |
| BMI z-score | 2.5 ± 0.1 | 2.6 ± 0.1 | −0.1 (−0.2, −0.0) | −0.7 (−0.9, −0.6) | <0.0001 |
| 25(OH) vitamin D (ng/ml) | 22.2 ± 1.7 | 23.9 ± 1.9 | −0.6 (−3.7, 2.5) | 2.5 (−3.3, 8.3) | 0.186 |
| Calcium (mg/dl) | 9.3 ± 0.1 | 9.3 ± 0.1 | −0.1 (−0.2, 0.1) | −0.0 (−0.2, 0.1) | 0.460 |
| Phosphorus (mg/dl) | 3.7 ± 0.1 | 3.5 ± 0.2 | 0.0 (−0.3, 0.3) | 0.5 (−0.0, 0.9) | 0.279 |
| HBA1c (%) | 6.1 ± 0.4 | 5.7 ± 0.2 | −0.7 (−1.7, 0.3) | 0.1 (−1.4, 1.6) | 0.355 |
| Moderate to vigorous physical activity (hours/week) | 23.2 ± 4.7 | 31.0 ± 4.7 | 6.7 (−6.5, 19.9) | 5.0 (−7.2, 17.3) | 0.891 |
| DXA Measures of Body Composition |
Non-Surgical (n=22) |
Surgical (n=22) |
Non-Surgical (n=22) |
Surgical (n=22) |
|
| Lean Mass (kg) | 63.9 ± 2.3 | 67.5 ± 2.4 | 2.2 (0.7, 3.6) | −8.5 (−10.9, −6.0) | <0.0001 |
| Lean Mass (%) | 53.2 ± 1.0 | 51.1 ± 1.1 | 1.6 (−0.0, 3.1) | 7.7 (4.8, 10.7) | 0.001 |
| Fat Mass (kg) | 56.7 ± 2.8 | 64.9 ± 2.9* | −1.4 (−4.8, 2.1) | −22.3 (−28.5, −16.2) | <0.0001 |
| Fat Mass (%) | 46.8 ± 1.0 | 48.8 ± 1.1 | −1.6 (−3.1, 0.0) | −7.6 (−10.5, −4.7) | 0.001 |
p<0.05 for baseline comparisons; BMI: body mass index; HbA1C: hemoglobin A1C. Significant changes from baseline and significant p values are bolded. Baseline measures and differences between groups were compared using the Student t-test; Within group changes over 12 months were assessed using the paired t-test.
3.2. Changes in Anthropometric Measures and Body Composition over 12-Months:
Over 12 months, the surgical group had greater reductions in weight, BMI, BMI z-scores, fat and lean mass compared to the non-surgical group (Table 1). Body weight and BMI decreased by 27.2±1.8% and 27.6±1.9% respectively in the surgical group vs. 0.1±1.7% and 1.1±1.7% in the non-surgical group (p<0.0001 for both). Percent fat mass decreased to a greater extent, while percent lean mass increased to a greater extent in the surgical vs. non-surgical groups (Table 1).
3.3. Changes in DXA Measures of Areal Bone Mineral Density:
Table 2 shows baseline aBMD measures for study participants, the within group change over 12 months, and significances for differences between groups after adjusting for age, sex, race, baseline bone measure +/− 12-month BMI change. The surgical and non-surgical groups did not differ for baseline measures of aBMD at any site. The surgical group had significant within group reductions in femoral neck and total hip BMD and BMD Z-scores from baseline, and differed significantly from the non-surgical group for these changes over 12 months (Table 2). The surgical group also had significant within group reductions in lumbar spine BMD Z-scores over 12 months, but did not differ from non-surgical controls for this measure. Groups did not differ for changes in aBMD and corresponding Z-scores for the 1/3 radius and whole body.
Table 2:
Baseline Areal Bone Mineral Density and Changes over 12 Months in the Surgical and Non-Surgical Groups
| Baseline Measures (Mean ± SEM) |
Change over 12-months [Mean (95% CI)] |
P-value comparing changes over 12 months in surgical vs. non-surgical groups |
||||
|---|---|---|---|---|---|---|
| Non-Surgical (n=21) |
Surgical (n=22) |
Non-Surgical (n=21) |
Surgical (n=22) |
Adjusted for age, sex, race and baseline |
Adjusted for age, sex, race, baseline and change in BMI |
|
| 1/3 Radius BMD (g/cm2) | 0.695 ± 0.012 | 0.727 ± 0.013 | 0.021 (−0.007, 0.048) | 0.001 (−0.028, 0.030) | 0.969 | 0.434 |
| 1/3 Radius BMD Z-score | 0.532 ± 0.319 | 0.305 ± 0.267 | −0.224 (−0.471, 0.024) | 0.047 (−0.174, 0.269) | 0.057 | 0.990 |
| Femoral Neck BMD (g/cm2) | 1.082 ± 0.025 | 1.088 ± 0.024 | 0.007 (−0.020, 0.034) | −0.080 (−0.123, −0.037 | 0.0007 | 0.067 |
| Femoral Neck BMD Z-score | 1.755 ± 0.240 | 1.881 ± 0.263 | −0.110 (−0.341, 0.122) | −0.947 (−1.310, −0.584) | 0.0006 | 0.097 |
| Total Hip BMD (g/cm2) | 1.182 ± 0.023 | 1.183 ± 0.031 | 0.001 (−0.021, 0.023) | −0.086 (−0.125, −0.047) | 0.0002 | 0.240 |
| Total Hip BMD Z-score | 1.864 ± 0.220 | 1.776 ± 0.314 | −0.162 (−0.294, −0.030) | −0.858 (−1.155, −0.561) | <0.0001 | 0.161 |
| Lumbar Spine BMD (g/cm2) | 1.098 ± 0.022 | 1.143 ± 0.021 | 0.018 (−0.007, 0.042) | −0.021 (−0.045, 0.002) | 0.244 | 0.185 |
| Lumbar Spine BMD Z-score | 1.064 ± 0.241 | 1.218 ± 0.177 | −0.071 (−0.244, 0.101) | −0.416 (−0.681, −0.150) | 0.147 | 0.315 |
| Whole Body BMD (g/cm2) | 1.095 ± 0.016 | 1.095 ± 0.016 | 0.025 (−0.001, 0.051) | 0.012 (−0.012, 0.035) | 0.723 | 0.030 |
| Whole Body BMD Z-score | 0.282 ± 0.242 | 0.032 ± 0.241 | 0.005 (−0.304, 0.313 | −0.091 (−0.398, 0.217) | 0.613 | 0.030 |
| WBLH BMD (g/cm2) | 0.996 ± 0.014 | 1.000 ± 0.016 | 0.016 (−0.008, 0.040) | −0.006 (−0.033, 0.022 | 0.505 | 0.034 |
BMD: Bone mineral density; WBLH: whole body less head; Significant changes from baseline and significant p values are bolded.
Baseline measures were compared using the Student t-test; Within group changes over 12 months were assessed using the paired t-test
Differences between surgical and non-surgical groups were assessed using multivariable analyses after adjusting for baseline measures, age, sex, race +/− change in BMI over 12 months
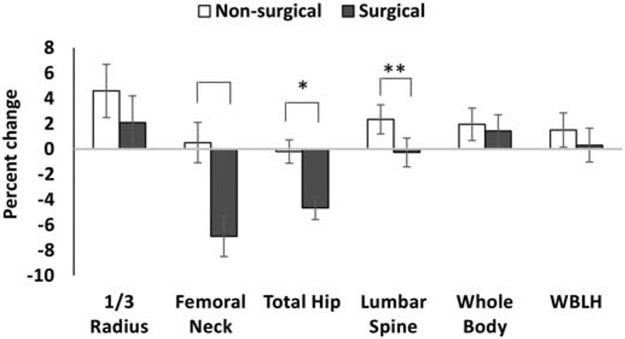
Figure 1 shows differences between groups for percent change in areal BMD after controlling for age, sex and race (percent change takes into account the baseline BMD). Percent change in femoral neck and total hip BMD was −6.9±1.6% and −4.7±0.9% in the surgical group vs. 0.5±1.6% and −0.2±0.9% in the non-surgical group (p=0.0007 and 0.0004 respectively). Percent change in lumbar spine BMD trended lower in the surgical vs. non-surgical group (−0.3±1.1 vs. 2.4±1.2%, p=0.07), but did not reach statistical significance. The groups did not differ for percent change in 1/3 radius and whole body BMD over time. Differences between study groups for changes in femoral neck, total hip and lumbar spine areal BMD and corresponding Z-scores were attenuated and no longer significant after also controlling for 12-month change in BMI (Table 2). In contrast, differences between groups for the 12-month change in whole body BMD measures became significant after also controlling for BMI change. Of note, study participants did not report any new fractures during the study duration.
Figure 1:
Percent change in DXA measures of areal bone mineral density (aBMD) in the non-surgical and surgical groups (after controlling for age, sex and race). The groups differed for percent change in aBMD at the femoral neck and total hip, with a trend observed for changes in aBMD at the lumbar spine. *p<0.05; **p=0.07
3.4. Changes in HRpQCT Measures of Volumetric Bone Mineral Density, Bone Geometry and Microarchitecture:
HRpQCT measures were assessed in a subset of participants that was similar to the larger cohort for clinical characteristics (data not shown). Baseline HRpQCT measures did not differ across surgical and nonsurgical groups, except for tibial trabecular thickness, which was lower in the surgical group (Table 3). Table 3 also shows within group changes over 12 months at the distal tibia and distal radius in the two groups, and significances for comparisons across groups after adjusting for age, sex, race, baseline bone measure +/− 12-month BMI change. Figures 2 (distal tibia) and 3 (distal radius) illustrate the percent change in these measures over 12 months after adjusting for age, sex and race (percent change factors in the baseline bone measure).
Table 3:
HRpQCT and Finite Element Analysis Measures at Baseline and Changes over 12 Months in the Surgical and Non-Surgical Groups at the Distal Tibia and Distal Radius
| Baseline (Mean ± SEM) | Change over 12-months [Mean (95% CI)] | P-value comparing changes over 12 months in surgical vs. non-surgical groups |
||||
|---|---|---|---|---|---|---|
| Distal Tibia | Non-Surgical (n=15) |
Surgical (n=15) |
Non-Surgical (n=15) |
Surgical (n=15) |
Adjusted for age, sex, race and baseline |
Adjusted for age, sex, race, baseline and change in BMI |
| Bone Geometry | ||||||
| Cortical Area (mm2) | 143.6 ± 8.7 | 141.5 ± 5.9 | 6.2 (1.1, 11.2) | −1.3 (−5.0, 2.4) | 0.023 | 0.017 |
| Trabecular Area (mm2) | 603.3 ± 32.2 | 645.3 ± 28.3 | −5.2 (−9.2, −1.2) | 1.6 (−1.9, 5.1) | 0.026 | 0.040 |
| Cortical Thickness (mm) | 1.36 ± 0.08 | 1.31 ± 0.06 | 0.06 (−0.10, −0.01) | 0.01 (−0.02, 0.05) | 0.014 | 0.010 |
| Bone Microarchitecture | ||||||
| Cortical Porosity (%) | 3.98 ± 0.45 | 4.08 ± 0.45 | −0.09 (−0.75, 0.57) | −0.90 (−1.57, −0.22) | 0.012 | 0.123 |
| Trabecular Number (1/mm) | 2.54 ± 0.06 | 2.68 ± 0.08 | −0.06 (−0.13, 0.01) | −0.24 (−0.34, −0.15) | 0.019 | 0.939 |
| Trabecular Separation (mm) | 0.32 ± 0.01 | 0.31 ± 0.01 | 0.01 (−0.00, 0.02) | 0.03 (0.02, 0.05) | 0.008 | 0.440 |
| Trabecular Thickness (mm) | 0.078 ± 0.003 | 0.069 ± 0.002* | 0.003 (0.001, 0.005) | 0.005 (0.001, 0.008) | 0.233 | 0.290 |
| Volumetric BMD (vBMD) | ||||||
| Cortical vBMD (mgHA/cm3) | 856.4 ± 14.2 | 858.9 ± 8.9 | 11.9 (0.1, 23.7) | 19.5 (13.7, 25.4) | 0.040 | 0.192 |
| Trabecular vBMD (mgHA/cm3) | 233.3 ± 7.2 | 220.8 ± 8.6 | 2.5 (−5.8, 0.8) | −6.6 (−3.1, 16.3) | 0.176 | 0.154 |
| Total vBMD (mgHA/cm3) | 359.1 ± 11.9 | 339.9 ± 13.0 | 8.1 (1.5, 14.7) | −3.4 (−14.1, 7.4) | 0.153 | 0.114 |
| Strength Estimates | ||||||
| Stiffness (kN/mm) | 271.6 ± 10.3 | 257.9 ± 9.6 | 7.6 (0.6, 14.6) | 4.8 (−6.2, 15.8) | 0.985 | 0.044 |
| Failure Load (kN) | 13.2 ± 0.5 | 13.1 ± 0.5 | 0.3 (0.0, 0.7) | 0.1 (−0.3, 0.6) | 0.874 | 0.064 |
| Distal Radius | Baseline (Mean ± SEM | Change over 12-months (Mean (95% CI) | P-value comparing changes over 12 months in surgical vs. non-surgical groups |
|||
| Non-Surgical (n=15) |
Surgical (n=15) |
Non-Surgical (n=15) |
Surgical (n=15) |
Adjusted for age, sex, race and baseline |
Adjusted for age, sex, race, baseline and change in BMI |
|
| Bone Geometry | ||||||
| Cortical Area (mm2) | 57.1 ± 4.5 | 58.9 ± 3.9 | 2.6 (−1.2, 6.4) | 1.0 (−1.3, 3.3) | 0.851 | 0.426 |
| Trabecular Area (mm2) | 232.3 ± 11.8 | 235.8 ± 16.2 | −1.8 (−5.0, 1.4) | −0.4 (−2.4, 1.6) | 0.853 | 0.447 |
| Cortical Thickness (mm) | 0.80 ± 0.07 | 0.83 ± 0.06 | 0.04 (−0.0, 0.1) | 0.01 (−0.02, 0.05) | 0.972 | 0.391 |
| Bone Microarchitecture | ||||||
| Cortical Porosity (%) | 2.04 ± 0.35 | 1.79 ± 0.22 | −0.17 (−0.58, 0.24) | −0.54 (−0.96, −0.13) | 0.024 | 0.391 |
| Trabecular Number (1/mm) | 2.26 ± 0.06 | 2.33 ± 0.06 | 0.05 (−0.06, 0.16) | −0.13 (−0.27, 0.00) | 0.078 | 0.357 |
| Trabecular Separation (mm) | 0.37 ± 0.01 | 0.36 ± 0.01 | −0.01 (−0.03, 0.01) | 0.03 (−0.00, 0.06) | 0.054 | 0.214 |
| Trabecular Thickness (mm) | 0.080 ± 0.003 | 0.074 ± 0.003 | −0.002 (−0.006, 0.002) | −0.001 (−0.004, 0.002) | 0.574 | 0.576 |
| Volumetric BMD (vBMD) | ||||||
| Cortical vBMD (mgHA/cm3) | 796.8 ± 25.6 | 807.9 ± 18.8 | 17.7 (0.1, 35.3) | 22.0 (14.2, 29.7) | 0.012 | 0.117 |
| Trabecular vBMD (mgHA/cm3) | 214.9 ± 8.9 | 205.8 ± 6.8 | −0.3 (−2.8, 2.2) | −12.0 (−21.2, −2.8) | 0.010 | 0.191 |
| Total vBMD (mgHA/cm3) | 347.1 ± 12.6 | 344.7 ± 15.8 | 5.4 (−3.1, 13.9) | −3.0 (−10.0, 4.0) | 0.206 | 0.967 |
| Strength Estimates | ||||||
| Stiffness (kN/mm) | 102.1 ± 6.3 | 95.9 ± 4.9 | −1.6 (−5.8, 2.6) | −1.6 (−4.6, 1.3) | 0.823 | 0.779 |
| Failure Load k(N) | 4.8 ± 0.2 | 4.8 ± 0.2 | −0.07 ( −0.24,0.10) | −0.09 (−0.22, 0.05) | 0.768 | 0.651 |
P<0.05 for baseline comparisons; BMD: bone mineral density; Significant changes from baseline and significant p values are bolded
Baseline measures were compared using the Student t-test; Within group changes over 12 months were assessed using the paired t-test
Differences between surgical and non-surgical groups were assessed using multivariable analyses after adjusting for baseline measures, age, sex, race +/− change in BMI over 12 months
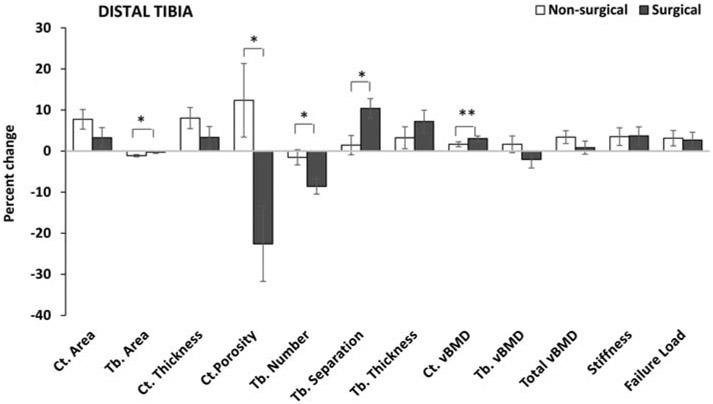
Figure 2:
Percent change in HRpQCT measures at the distal tibia in the non-surgical and surgical groups (after controlling for age, sex and race). The groups differed for percent change in cortical porosity, and trabecular area, number and separation, with a trend observed for change in cortical volumetric bone mineral density. *p <0.05, **p=0.06.
Ct. Cortical; Tb. Trabecular; vBMD volumetric bone mineral density
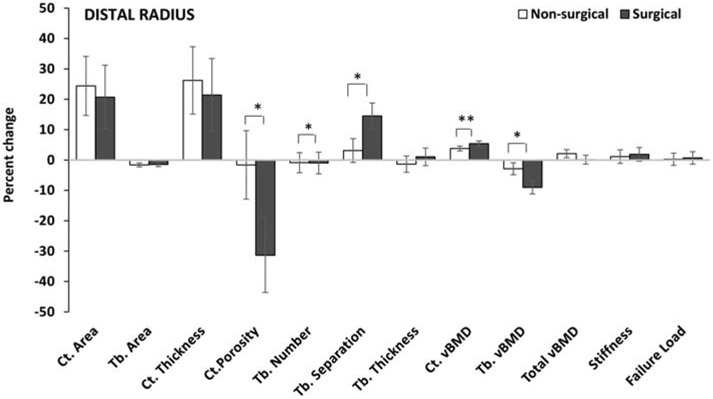
Figure 3:
Percent change in HRpQCT measures at the distal radius in the non-surgical and surgical groups (after controlling for age, sex and race). The groups differed for percent change in cortical porosity, and trabecular number, separation and volumetric bone mineral density, with a trend observed for percent change in cortical volumetric bone mineral density. *p <0.05, **p=0.09.
Ct. Cortical; Tb. Trabecular; vBMD volumetric bone mineral density
Distal Tibia:
At the distal tibia, over the 12-month study duration, the non-surgical group had a significant within group increase in cortical area associated with a decrease in trabecular area, resulting in an increase in cortical thickness; these positive changes in bone geometry were not observed in the surgical group (Table 3). Thus, the groups differed significantly for changes in these measures over time. Differences between groups persisted after also controlling for 12-month change in BMI. For percent change over time, groups differed for trabecular area (−0.3±0.3% vs. −1.1±0.3% in the surgical vs. non-surgical groups, p= 0.020), but not cortical area or thickness (Figure 2), and this difference persisted after adjusting for 12-month BMI change.
For bone microarchitecture, the surgical group had a significant within group decrease (i.e. improvement) in cortical porosity over the study duration that was not seen in the non-surgical group. The surgical group also had a decrease in trabecular number associated with an increase in trabecular separation over time; both groups had a within group increase in trabecular thickness over 12-months. The groups differed significantly for absolute changes in cortical porosity, trabecular number and separation over the study duration; however, these differences were lost after also controlling for the change in BMI over 12 months. Similarly, significant differences were noted between the surgical vs. non-surgical groups for percent change in cortical porosity (−22.6±9.1% vs. 12.4±9.0%, p=0.003), trabecular number (−8.6±1.9% vs. −1.5±1.8%, p=0.004) and trabecular separation (10.4±2.4% vs. 1.4±2.3%, p=0.004) over 12 months (Figure 2); differences were no longer significant after controlling for 12-month BMI change.
Cortical vBMD increased over 12 months in both groups, but demonstrated greater increases in the surgical vs. the non-surgical group (p=0.040 for absolute change (Table 3) with a trend for percent change, p=0.063 (Figure 2)). This difference was no longer significant after also controlling for 12-month change in BMI. The non-surgical group (but not the surgical group) had a 12-month within group increase in total vBMD and strength estimates (stiffness and failure load); however, the groups did not differ from each other for absolute or percent change in these estimates over 12 months. After also controlling for 12-month change in BMI, differences between groups became significant for absolute change in tibial stiffness (Table 3).
Distal Radius:
Neither group had changes in bone geometry over 12 months at the distal radius (Table 3). For microarchitecture, the surgical (but not non-surgical) group demonstrated a significant within group reduction in cortical porosity over 12-months, with a significant difference between groups for both absolute (Table 3) and percent changes (Figure 3) in cortical porosity. Cortical porosity decreased 31.4±12.2% in the surgical group vs. 1.6±11.3% in the non-surgical group (p=0.029). Differences between the groups were no longer significant after also controlling for the 12-month change in BMI. The surgical group, compared to the non-surgical group, had greater percent reduction in trabecular number (−10.0±3.6% vs. −0.9±3.3%, p=0.023) and increase in trabecular separation (14.5±4.2% vs. 3.1±3.9%, p=0.017), with greater percent reductions in trabecular vBMD (−9.0±2.1% vs. −2.9±1.9%, p=0.010) (Figure 3). Both groups had a significant within group absolute increase in cortical vBMD over 12 months, with the increase being greater in the surgical group (p=0.012); significances were lost after also controlling for BMI change over this period. Neither group demonstrated a change in strength estimates.
3.5. Determinants of Changes in Bone Parameters:
For all participants taken together, Table 4 shows associations of changes in BMI z-scores, lean mass, fat mass, 25OHD and physical activity levels with changes in bone parameters that differed (or trended to differ) between surgical and non-surgical groups over time. Changes in BMI z-scores, lean and fat mass were associated positively with changes in femoral neck and total hip (but not lumbar spine) BMD Z-scores. At the distal tibia, a reduction in BMI z-scores and lean mass was associated with a decrease in cortical area and thickness and trabecular number, and an increase in trabecular area and separation. Reductions in fat mass correlated with reductions in tibial trabecular number and radial trabecular vBMD, and increases in tibial trabecular area and separation. Further, a change in 25OHD levels correlated inversely with changes in tibial and radial cortical porosity, and positively with changes in tibial cortical vBMD. Changes in physical activity and HbA1C levels (data not shown) were not associated with bone changes.
Table 4:
Associations of Changes in Anthropometric Measures and Body Composition with Changes in Bone Parameters Over 12 Months
| Δ BMI z-score | Δ Lean Mass | Δ Fat Mass | Δ 25(OH)D | Δ Physical activity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ρ | p | ρ | p | ρ | p | ρ | p | ρ | p | |
| DXA measures | ||||||||||
| Δ FN BMD Z-score | 0.487 | 0.001 | 0.533 | 0.0004 | 0.404 | 0.010 | −0.018 | 0.916 | −0.90 | 0.602 |
| Δ Total Hip BMD Z-score | 0.615 | <0.0001 | 0.715 | <0.0001 | 0.544 | 0.0003 | −0.135 | 0.420 | 0.295 | 0.081 |
| Δ Lumbar Spine BMD Z-score | 0.177 | 0.267 | 0.212 | 0.184 | 0.072 | 0.654 | 0.122 | 0.459 | −0.209 | 0.215 |
| Distal Tibia | ||||||||||
| Δ Cortical Area (mm2) | 0.396 | 0.031 | 0.487 | 0.006 | 0.357 | 0.053 | −0.039 | 0.840 | 0.229 | 0.262 |
| Δ Trabecular Area (mm2) | −0.472 | 0.008 | −0.563 | 0.001 | −0.409 | 0.025 | −0.004 | 0.983 | −0.198 | 0.332 |
| Δ Cortical Thickness (mm) | 0.370 | 0.044 | 0.454 | 0.011 | 0.349 | 0.076 | 0.086 | 0.659 | 0.144 | 0.483 |
| Δ Cortical Porosity (%) | 0.341 | 0.065 | 0.164 | 0.386 | 0.143 | 0.452 | −0.475 | 0.009 | 0.119 | 0.563 |
| Δ Trabecular Number (1/mm) | 0.527 | 0.003 | 0.400 | 0.028 | 0.460 | 0.009 | −0.317 | 0.094 | 0.319 | 0.112 |
| Δ Trabecular Separation (mm) | −0.513 | 0.004 | −0.426 | 0.013 | −0.374 | 0.042 | 0.210 | 0.275 | −0.311 | 0.122 |
| Δ Cortical vBMD (mgHA/cm3) | −0.194 | 0.306 | −0.193 | 0.306 | −0.253 | 0.177 | 0.398 | 0.033 | −0.118 | 0.566 |
| Δ Stiffness (kN/mm) | −0.081 | 0.671 | −0.007 | 0.968 | −0.232 | 0.217 | 0.243 | 0.205 | −0.295 | 0.144 |
| Distal Radius | ||||||||||
| Δ Cortical Porosity (%) | 0.375 | 0.041 | 0.142 | 0.455 | 0.248 | 0.187 | −0.396 | 0.037 | 0.152 | 0.440 |
| Δ Cortical vBMD (mgHA/cm3) | −0.244 | 0.193 | −0.081 | 0.669 | −0.153 | 0.419 | 0.233 | 0.232 | 0.142 | 0.471 |
| Δ Trabecular vBMD (mgHA/cm3) | 0.334 | 0.071 | 0.278 | 0.137 | 0.379 | 0.039 | −0.332 | 0.085 | 0.268 | 0.168 |
Δ: Change; FN: femoral neck; vBMD: volumetric bone mineral density; 25(OH)D: 25-hydroxy vitamin D. Significant p values are bolded; Spearman correlations (ρ) and the corresponding p value are reported
4. Discussion
This is the first report of bone outcomes following sleeve gastrectomy in adolescents and young adults, demonstrating the expected reduction in certain DXA measures of aBMD over 12-months in the surgical group. However, we found no differences between groups for changes in total vBMD and strength estimates using HRpQCT and μFEA.
DXA Measures:
Our data regarding changes in aBMD over a year are consistent with those from studies in adults undergoing sleeve gastrectomy. One meta-analysis of 22 such studies that included data from 1905 adult patients reported reductions in femoral neck and total hip aBMD, but not lumbar spine aBMD over 12-months [11]. We similarly found a reduction in femoral neck and total hip aBMD in the surgical group (6.9 and 4.9% respectively), comparable to that reported in adults after sleeve gastrectomy [6, 11, 36], and also comparable to BMD changes associated with changes in fracture risk in therapeutic trials [37, 38]. Both sites are impacted deleteriously following gastric bypass as well, with variable reports regarding the extent of BMD reduction [6, 8, 12, 39]. Similar to the meta-analysis of adults following sleeve gastrectomy [11], we found no difference between groups for change in spine aBMD over 12 months (or for the 1/3 radius). This is in contrast to adults undergoing gastric bypass in that all aBMD measures decrease over time in most studies following bypass [5, 6], though a few report a sparing of lumbar spine and 1/3 radius aBMD [8]. Of note, one study even reported increases in spine aBMD in adults after sleeve gastrectomy [40]. These contradictory reports may reflect challenges inherent to prospective DXA assessments at the spine because of associated artefacts. In adolescents whole body aBMD Z-scores decreased over two years following gastric bypass [21, 22], and our subjects had a decrease in this measure over a year after adjusting for BMI change. No study thus far has reported on outcomes following sleeve gastrectomy in youth.
HRpQCT Measures:
Few studies have examined changes in HRpQCT measures following weight loss surgery. A study in adults undergoing gastric bypass [5] reported increases in cortical porosity, reductions in cortical thickness, and in cortical, trabecular and total vBMD at the distal radius and tibia following surgery over 5-years. The study also reported a decrease in cortical and increase in trabecular area, and a decrease in trabecular number at the distal radius. Shanbhogue et al. reported similar changes at both skeletal sites over 2-years [10], while Schafer et al. reported changes occurring as early as 6-months following surgery [41]. Frederiksen et al. reported similar changes at the tibia, but not the radius [9] following bypass. Yet another study included 22 women undergoing gastric bypass and eight undergoing sleeve gastrectomy and reported a preferential impact on the tibia (with relative sparing of the radius), and preferential effects on cortical vs. trabecular bone [8]. Other studies examining changes in geometry, microarchitecture and vBMD following sleeve gastrectomy in adults are limited. No studies to date have reported on HRpQCT outcomes in adolescents following any weight loss surgery.
Changes in Bone Geometry:
Adolescence and young adulthood are characterized by an increase in cortical thickness from an increase in cortical and decrease in trabecular crosssectional area subsequent to increased periosteal bone apposition and decreased endocortical resorption [42, 43]. Our non-surgical controls demonstrated similar changes in bone geometry at the distal tibia over 12 months. However, the surgical group did not demonstrate this increase in cortical area and thickness over time, likely because of skeletal unloading following weight loss or associated metabolic and hormonal changes. While changes in BMI and lean mass were associated with changes in bone geometry at the tibia, differences between groups persisted even after controlling for 12-month change in BMI. This suggests that metabolic and hormonal changes following surgery likely contribute to observed changes in bone geometry. Of note, our results differ from those in adults undergoing gastric bypass, who have a decrease in cortical area and thickness over time [5, 8-10] vs. our surgical cohort, which failed to demonstrate the expected increase in these parameters during adolescence/young adulthood (observed in our controls). At the distal radius, neither group demonstrated a change in bone geometry, consistent with some studies in adults after bypass and sleeve procedures [5, 8]. No study thus far has reported on changes in HRpQCT parameters in youth following sleeve gastrectomy.
Changes in Trabecular Microarchitecture and Volumetric BMD Following Sleeve Gastrectomy:
The surgical group had a decrease in trabecular number and an increase in trabecular separation, consistent with reports in adults undergoing gastric bypass [5, 9, 10]. This did not translate to a significant reduction in trabecular vBMD at the distal tibia in the surgical group, although at the distal radius, the surgical group did demonstrate a within and between group reduction in trabecular vBMD. These data are consistent with the greater reductions in trabecular vBMD reported at the distal radius than tibia in adults following gastric bypass [39], although the magnitude of reduction at the distal radius (9%) over a year in our study was similar to that observed after two years in adults following gastric bypass [5, 10, 39] (less marked changes over one year). This is concerning in that a longer duration of follow-up may result in further reductions in trabecular vBMD in youth following sleeve gastrectomy, as noted in a 5-year follow-up study in adults following gastric bypass [5]. Changes in BMI, lean and fat mass were associated positively with changes in trabecular number, and negatively with changes in trabecular separation at the distal tibia. Differences were attenuated after controlling for BMI changes, suggesting that these changes may be consequent to skeletal unloading and associated body composition changes following weight loss. However, reductions in trabecular vBMD at the non-weight bearing radius (which should not be impacted by skeletal unloading) suggest a role for metabolic and hormonal changes resulting either from weight loss following surgery or the procedure per se in mediating bone outcomes.
Changes in Cortical Microarchitecture and vBMD Following Sleeve Gastrectomy:
In contrast to reports of reductions in cortical and total vBMD following gastric bypass in adults [5, 8, 10], we observed an increase in cortical vBMD at the distal radius and tibia in our participants. However, the groups did not differ for total vBMD changes over 12-months at either site, possibly because trabecular vBMD decreased (at least for the radius). A possible explanation for the increase in cortical vBMD is a reduction in cortical porosity after surgery at both sites, given that cortical porosity is associated inversely with cortical vBMD [44, 45]. We have previously reported that adolescent girls with obesity have greater cortical porosity than normal-weight controls at the distal radius and distal tibia [34], and speculate that this may be a consequence of delayed mineralization of the rapidly expanding cortex from increased skeletal loading in youth with obesity. Weight loss and/or metabolic improvement may reverse this effect on bone.
Changes in Strength Estimates Following Sleeve Gastrectomy:
Lindeman et al. [5] reported a reduction in failure load (a bone strength estimate assessed using μFEA) by 20% and 13% at the distal radius and tibia respectively five years after gastric bypass surgery in adults, and others have also reported a reduction in strength estimates following gastric bypass in adults [9, 10]. Consistent with this, Yu et al. [46] reported an increased risk of non-vertebral fractures in those who underwent gastric bypass surgery versus adjustable gastric banding that manifested more than two years post-surgery, with the highest risk evident in the fifth year post-surgery.
In our study, the non-surgical control group had an increase in stiffness and failure load over 12-months at the distal tibia (but not the radius), likely because the tibia represents weightbearing bone more likely to be impacted by skeletal loading from obesity, and consistent with the expected increase in strength estimates through adolescence/young adulthood [42, 43]. In contrast, strength estimates at the distal radius and tibia did not change within the surgical group over 12 months. This is in contrast to data from several studies in adults undergoing gastric bypass [5, 9, 10], but consistent with data reported by Stein et al. [8] in a study that included adults undergoing bypass or sleeve procedures. Given that obesity is a risk factor for wrist and lower leg/ankle fractures [47-50], the fact that sleeve gastrectomy induced weight loss does not worsen strength estimates at these sites over at least 12-months is reassuring. However, longer durations of follow-up after gastric bypass have demonstrated a continued decrease in strength estimates over time in adults [5, 10], concerning for similar decrements in youth following sleeve gastrectomy. Longer-term data are thus necessary to determine whether strength estimates worsen over a prolonged period in youth and how this impacts fracture risk. We did not assess fracture risk (the clinical endpoint of interest) in this study given the relatively small number of participants and short duration of follow-up. At least one study has reported no fractures during a two-year post-surgical period after gastric bypass [22]. It will be important to follow youth over time to determine how they fare for propensity to fracture over a longer duration.
Differences between groups for all DXA and many HRpQCT measures were attenuated after adjusting for BMI changes, suggesting that many observed changes were consequent to either mechanical unloading of the skeleton following surgery from decreases in body mass [7, 8, 51], or metabolic and hormonal changes [10, 52] associated with weight reduction [7].
25OHD Levels and their Relationship to Bone Parameters:
Following gastric bypass, reductions in 25OHD levels consequent to malabsorption are believed to be an important contributor to impaired bone outcomes because of associated reductions in calcium absorption and increases in PTH [7, 52], although some studies have found no association between changes in 25OHD levels and bone parameters [53]. In general, clinical practice has moved towards greater diligence in maintaining post-surgical 25OHD levels in the normal range. In our study, we found no differences within or between groups for changes in calcium, phosphorus or 25OHD levels over time; however, reductions in 25OHD for the group as a whole were associated with increases in cortical porosity at both sites, and decreases in tibial cortical and total vBMD. Given that 25OHD levels did not change during follow-up in our study, a decrease in 25OHD is unlikely to have contributed to post-surgical changes in bone. In fact, a meta-analysis in adults undergoing sleeve gastrectomy reported an increase in serum calcium, phosphate and 25OHD, potentially from rigorous vitamin D supplementation [11]. It will be important in future studies to assess serum PTH and 24-hour urinary calcium excretion.
Study Limitations and Strengths:
Our study has certain limitations, including the relatively small number of participants for whom we had data for HRpQCT measures. However, this is the first report of HRpQCT assessments in this age group, and future studies with a larger number of participants will be necessary to confirm our findings. Another limitation is the lack of data for fractures. One year is a relatively short period over which to report comparisons between groups for fractures, and much larger studies are necessary to determine fracture risk following sleeve gastrectomy in adolescents. Of note, we did not assess levels of gut hormones, gonadal steroids, 1,25(OH)2D or PTH in this study. Changes in gut hormones and gonadal steroids may account in part for changes in bone outcomes following sleeve gastrectomy, particularly at the distal radius, a non-weight bearing site less likely to be impacted by skeletal unloading after weight loss. Given that 25OHD levels did not differ at baseline or over 12 months in the two groups, we do not expect 1,25(OH)2D or PTH levels to differ between groups. However, definitive data confirming this are currently lacking. Our study has many strengths, including a thorough assessment of bone outcomes following sleeve gastrectomy in youth, and that we included a comparison group of non-surgical participants, not available in many studies. This is also the first report of bone outcomes following sleeve gastrectomy in youth.
Conclusion:
Overall, our study indicates that while there is a reduction in aBMD measures following sleeve gastrectomy in youth, and despite the fact that these youth demonstrate deleterious changes in several HRpQCT parameters at the distal tibia and distal radius over a year, these changes do not appear to reduce strength estimates at skeletal sites at particular risk for fractures over this short duration, likely because of a simultaneous increase in cortical vBMD. Further, reduced body mass may compensate for observed deleterious effects on bone following surgery.
Highlights.
Femoral neck and total hip bone mineral density (BMD) (DXA) decreased after surgery
% Change in trabecular number was lower in the surgical group at the radius/tibia
% Change in trabecular separation was higher in the surgical group at both sites
Cortical volumetric BMD increased after surgery at both sites
Strength estimates did not change following surgery at either site
Acknowledgments
Grant Support: This work was supported by the NIH NIDDK R01 DK103946-01A1 (MM, MAB), NIH K23DK110419-01(VS), P30-DK040561 (VS, FCS), K24DK109940 (MAB), K24 HD071843 (MM), L30 DK118710 (FCS), NIH P30-DK057521 (VS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no conflicts of interest to disclose relevant to this paper
References
- [1].Tsai WS, Inge TH, Burd RS, Bariatric surgery in adolescents: recent national trends in use and in-hospital outcome, Arch Pediatr Adolesc Med 161(3) (2007) 217–21. [DOI] [PubMed] [Google Scholar]
- [2].Pratt JSA, Browne A, Browne NT, Bruzoni M, Cohen M, Desai A, Inge T, Linden BC, Mattar SG, Michalsky M, Podkameni D, Reichard KW, Stanford FC, Zeller MH, Zitsman J, ASMBS pediatric metabolic and bariatric surgery guidelines, 2018, Surg Obes Relat Dis 14(7) (2018) 882–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Griggs CL, Perez NP Jr., Goldstone RN, Kelleher CM, Chang DC, Stanford FC, Pratt JS, National Trends in the Use of Metabolic and Bariatric Surgery Among Pediatric Patients With Severe Obesity, JAMA Pediatr 172(12) (2018) 1191–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Armstrong SC, Bolling CF, Michalsky MP, Reichard KW, Pediatric Metabolic and Bariatric Surgery: Evidence, Barriers, and Best Practices, Pediatrics 144(6) (2019). [DOI] [PubMed] [Google Scholar]
- [5].Lindeman KG, Greenblatt LB, Rourke C, Bouxsein ML, Finkelstein JS, Yu EW, Longitudinal 5-Year Evaluation of Bone Density and Microarchitecture After Roux-en-Y Gastric Bypass Surgery, J Clin Endocrinol Metab 103(11) (2018) 4104–4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bredella MA, Greenblatt LB, Eajazi A, Torriani M, Yu EW, Effects of Roux-en-Y gastric bypass and sleeve gastrectomy on bone mineral density and marrow adipose tissue, Bone 95 (2017) 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Stein EM, Silverberg SJ, Bone loss after bariatric surgery: causes, consequences, and management, Lancet Diabetes Endocrinol 2(2) (2014) 165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Stein EM, Carrelli A, Young P, Bucovsky M, Zhang C, Schrope B, Bessler M, Zhou B, Wang J, Guo XE, McMahon DJ, Silverberg SJ, Bariatric surgery results in cortical bone loss, J Clin Endocrinol Metab 98(2) (2013) 541–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Frederiksen KD, Hanson S, Hansen S, Brixen K, Gram J, Jorgensen NR, Stoving RK, Bone Structural Changes and Estimated Strength After Gastric Bypass Surgery Evaluated by HR-pQCT, Calcif Tissue Int 98(3) (2016) 253–62. [DOI] [PubMed] [Google Scholar]
- [10].Shanbhogue VV, Stoving RK, Frederiksen KH, Hanson S, Brixen K, Gram J, Jorgensen NR, Hansen S, Bone structural changes after gastric bypass surgery evaluated by HR-pQCT: a two-year longitudinal study, Eur J Endocrinol 176(6) (2017) 685–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jaruvongvanich V, Vantanasiri K, Upala S, Ungprasert P, Changes in bone mineral density and bone metabolism after sleeve gastrectomy: a systematic review and meta-analysis, Surg Obes Relat Dis 15(8) (2019) 1252–1260. [DOI] [PubMed] [Google Scholar]
- [12].Vilarrasa N, de Gordejuela AG, Gomez-Vaquero C, Pujol J, Elio I, San Jose P, Toro S, Casajoana A, Gomez JM, Effect of bariatric surgery on bone mineral density: comparison of gastric bypass and sleeve gastrectomy, Obes Surg 23(12) (2013) 2086–91. [DOI] [PubMed] [Google Scholar]
- [13].Tian Z, Fan XT, Li SZ, Zhai T, Dong J, Changes in Bone Metabolism After Sleeve Gastrectomy Versus Gastric Bypass: a Meta-Analysis, Obes Surg 30(1) (2020) 77–86. [DOI] [PubMed] [Google Scholar]
- [14].Rousseau C, Jean S, Gamache P, Lebel S, Mac-Way F, Biertho L, Michou L, Gagnon C, Change in fracture risk and fracture pattern after bariatric surgery: nested case-control study, BMJ 354 (2016) i3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yu EW, Kim SC, Sturgeon DJ, Lindeman KG, Weissman JS, Fracture Risk After Roux-en-Y Gastric Bypass vs Adjustable Gastric Banding Among Medicare Beneficiaries, JAMA Surg (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fashandi AZ, Mehaffey JH, Hawkins RB, Schirmer B, Hallowell PT, Bariatric surgery increases risk of bone fracture, Surg Endosc 32(6) (2018) 2650–2655. [DOI] [PubMed] [Google Scholar]
- [17].Javanainen M, Pekkarinen T, Mustonen H, Scheinin T, Leivonen M, Two-Year Nutrition Data in Terms of Vitamin D, Vitamin B12, and Albumin After Bariatric Surgery and Long-term Fracture Data Compared with Conservatively Treated Obese Patients: a Retrospective Cohort Study, Obes Surg 28(9) (2018) 2968–2975. [DOI] [PubMed] [Google Scholar]
- [18].Gordon CM, Zemel BS, Wren TA, Leonard MB, Bachrach LK, Rauch F, Gilsanz V, Rosen CJ, Winer KK, The Determinants of Peak Bone Mass, J Pediatr 180 (2017) 261–269. [DOI] [PubMed] [Google Scholar]
- [19].Humayon S, Altieri MS, Yang J, Nie L, Spaniolas K, Pryor AD, Recent trends of bariatric surgery in adolescent population in the state of New York, Surg Obes Relat Dis 15(8) (2019)1388–1393. [DOI] [PubMed] [Google Scholar]
- [20].Kyler KE, Bettenhausen JL, Hall M, Fraser JD, Sweeney B, Trends in Volume and Utilization Outcomes in Adolescent Metabolic and Bariatric Surgery at Children's Hospitals, J Adolesc Health 65(3) (2019) 331–336. [DOI] [PubMed] [Google Scholar]
- [21].Kaulfers AM, Bean JA, Inge TH, Dolan LM, Kalkwarf HJ, Bone loss in adolescents after bariatric surgery, Pediatrics 127(4) (2011) e956–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Beamish AJ, Gronowitz E, Olbers T, Flodmark CE, Marcus C, Dahlgren J, Body composition and bone health in adolescents after Roux-en-Y gastric bypass for severe obesity, Pediatr Obes 12(3) (2017) 239–246. [DOI] [PubMed] [Google Scholar]
- [23].Inge TH, Courcoulas AP, Jenkins TM, Michalsky MP, Helmrath MA, Brandt ML, Harmon CM, Zeller MH, Chen MK, Xanthakos SA, Horlick M, Buncher CR, Weight Loss and Health Status 3 Years after Bariatric Surgery in Adolescents, N Engl J Med 374(2) (2016)113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL, Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version, Pediatrics 109(1) (2002) 45–60. [DOI] [PubMed] [Google Scholar]
- [25].Paffenbarger RS Jr., Blair SN, Lee IM, Hyde RT, Measurement of physical activity to assess health effects in free-living populations, Med Sci Sports Exerc 25(1) (1993) 60–70. [DOI] [PubMed] [Google Scholar]
- [26].Simpson K, Parker B, Capizzi J, Thompson P, Clarkson P, Freedson P, Pescatello LS, Validity and reliability question 8 of the Paffenbarger Physical Activity Questionnaire among healthy adults, J Phys Act Health 12(1) (2015) 116–23. [DOI] [PubMed] [Google Scholar]
- [27].Taylor C, Lamparello B, Kruczek K, Anderson EJ, Hubbard J, Misra M, Validation of a food frequency questionnaire for determining calcium and vitamin D intake by adolescent girls with anorexia nervosa, J Am Diet Assoc 109(3) (2009) 479–85, 485 e1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Parrott J, Frank L, Rabena R, Craggs-Dino L, Isom KA, Greiman L, American Society for Metabolic and Bariatric Surgery Integrated Health Nutritional Guidelines for the Surgical Weight Loss Patient 2016 Update: Micronutrients, Surg Obes Relat Dis 13(5) (2017) 727–741. [DOI] [PubMed] [Google Scholar]
- [29].Kearns MD, Alvarez JA, Tangpricha V, Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review, Endocr Pract 20(4) (2014) 341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD, Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women, J Bone Miner Res 23(3) (2008) 392–9. [DOI] [PubMed] [Google Scholar]
- [31].Crawford RP, Cann CE, Keaveny TM, Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography, Bone 33(4) (2003) 744–50. [DOI] [PubMed] [Google Scholar]
- [32].Kroker A, Zhu Y, Manske SL, Barber R, Mohtadi N, Boyd SK, Quantitative in vivo assessment of bone microarchitecture in the human knee using HR-pQCT, Bone 97 (2017) 43–48. [DOI] [PubMed] [Google Scholar]
- [33].Muschitz C, Kocijan R, Haschka J, Zendeli A, Pirker T, Geiger C, Muller A, Tschinder B, Kocijan A, Marterer C, Nia A, Muschitz GK, Resch H, Pietschmann P, The Impact of Vitamin D, Calcium, Protein Supplementation, and Physical Exercise on Bone Metabolism After Bariatric Surgery: The BABS Study, J Bone Miner Res 31(3) (2016) 672–82. [DOI] [PubMed] [Google Scholar]
- [34].Singhal V, Sanchita S, Malhotra S, Bose A, Flores LPT, Valera R, Stanford FC, Slattery M, Rosenblum J, Goldstein MA, Schorr M, Ackerman KE, Miller KK, Klibanski A, Bredella MA, Misra M, Suboptimal bone microarchitecure in adolescent girls with obesity compared to normal-weight controls and girls with anorexia nervosa, Bone 122 (2019) 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mitchell DM, Caksa S, Joseph T, Bouxsein ML, Misra M, Elevated HbA1c is associated with altered cortical and trabecular microarchitecture in girls with type 1 diabetes, J Clin Endocrinol Metab (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Adamczyk P, Buzga M, Holeczy P, Svagera Z, Zonca P, Sievanen H, Pluskiewicz W, Body Size, Bone Mineral Density, and Body Composition in Obese Women After Laparoscopic Sleeve Gastrectomy: A 1-Year Longitudinal Study, Horm Metab Res 47(12) (2015) 873–9. [DOI] [PubMed] [Google Scholar]
- [37].Torring O, Effects of denosumab on bone density, mass and strength in women with postmenopausal osteoporosis, Ther Adv Musculoskelet Dis 7(3) (2015) 88–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Boonen S, Reginster JY, Kaufman JM, Lippuner K, Zanchetta J, Langdahl B, Rizzoli R, Lipschitz S, Dimai HP, Witvrouw R, Eriksen E, Brixen K, Russo L, Claessens F, Papanastasiou P, Antunez O, Su G, Bucci-Rechtweg C, Hruska J, Incera E, Vanderschueren D, Orwoll E, Fracture risk and zoledronic acid therapy in men with osteoporosis, N Engl J Med 367(18) (2012) 1714–23. [DOI] [PubMed] [Google Scholar]
- [39].Yu EW, Bouxsein ML, Putman MS, Monis EL, Roy AE, Pratt JS, Butsch WS, Finkelstein JS, Two-year changes in bone density after Roux-en-Y gastric bypass surgery, J Clin Endocrinol Metab 100(4) (2015) 1452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ruiz-Tovar J, Oller I, Priego P, Arroyo A, Calero A, Diez M, Zubiaga L, Calpena R, Short- and mid-term changes in bone mineral density after laparoscopic sleeve gastrectomy, Obes Surg 23(7) (2013) 861–6. [DOI] [PubMed] [Google Scholar]
- [41].Schafer AL, Kazakia GJ, Vittinghoff E, Stewart L, Rogers SJ, Kim TY, Carter JT, Posselt AM, Pasco C, Shoback DM, Black DM, Effects of Gastric Bypass Surgery on Bone Mass and Microarchitecture Occur Early and Particularly Impact Postmenopausal Women, J Bone Miner Res 33(6) (2018) 975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gabel L, Macdonald HM, McKay HA, Sex Differences and Growth-Related Adaptations in Bone Microarchitecture, Geometry, Density, and Strength From Childhood to Early Adulthood: A Mixed Longitudinal HR-pQCT Study, J Bone Miner Res 32(2) (2017) 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nishiyama KK, Macdonald HM, Moore SA, Fung T, Boyd SK, McKay HA, Cortical porosity is higher in boys compared with girls at the distal radius and distal tibia during pubertal growth: an HR-pQCT study, J Bone Miner Res 27(2) (2012) 273–82. [DOI] [PubMed] [Google Scholar]
- [44].Langsetmo L, Peters KW, Burghardt AJ, Ensrud KE, Fink HA, Cawthon PM, Cauley JA, Schousboe JT, Barrett-Connor E, Orwoll ES, Volumetric Bone Mineral Density and Failure Load of Distal Limbs Predict Incident Clinical Fracture Independent HR-pQCT BMD and Failure Load Predicts Incident Clinical Fracture of FRAX and Clinical Risk Factors Among Older Men, J Bone Miner Res 33(7) (2018) 1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Liu CT, Sahni S, Xu H, McLean RR, Broe KE, Hannan MT, Boyd SK, Bouxsein ML, Kiel DP, Samelson EJ, Long-Term and Recent Weight Change Are Associated With Reduced Peripheral Bone Density, Deficits in Bone Microarchitecture, and Decreased Bone Strength: The Framingham Osteoporosis Study, J Bone Miner Res 33(10) (2018) 1851–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yu EW, Lee MP, Landon JE, Lindeman KG, Kim SC, Fracture Risk After Bariatric Surgery: Roux-en-Y Gastric Bypass Versus Adjustable Gastric Banding, J Bone Miner Res 32(6) (2017) 1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Goulding A, Grant AM, Williams SM, Bone and body composition of children and adolescents with repeated forearm fractures, J Bone Miner Res 20(12) (2005) 2090–6. [DOI] [PubMed] [Google Scholar]
- [48].Zonfrillo MR, Seiden JA, House EM, Shapiro ED, Dubrow R, Baker MD, Spiro DM, The association of overweight and ankle injuries in children, Ambul Pediatr 8(1) (2008) 66–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kessler J, Koebnick C, Smith N, Adams A, Childhood obesity is associated with increased risk of most lower extremity fractures, Clin Orthop Relat Res 471(4) (2013) 1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ, Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy x-ray absorptiometry study, J Pediatr 139(4) (2001) 509–15. [DOI] [PubMed] [Google Scholar]
- [51].Fleischer J, Stein EM, Bessler M, Della Badia M, Restuccia N, Olivero-Rivera L, McMahon DJ, Silverberg SJ, The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss, J Clin Endocrinol Metab 93(10) (2008) 3735–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Canales BK, Schafer AL, Shoback DM, Carpenter TO, Gastric bypass in obese rats causes bone loss, vitamin D deficiency, metabolic acidosis, and elevated peptide YY, Surg Obes Relat Dis 10(5) (2014) 878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Yu EW, Bone metabolism after bariatric surgery, J Bone Miner Res 29(7) (2014) 1507–18. [DOI] [PMC free article] [PubMed] [Google Scholar]