Abstract
Objective:
To investigate the relationship between waist circumference as a measure of abdominal obesity and brain responses to stress among patients with coronary artery disease (CAD)
Methods:
Patients with CAD (N=151) underwent acute mental stress tasks in conjunction with High Resolution Positron Emission Tomography (HR-PET) and radiolabelled water imaging of the brain. Brain responses to mental stress were correlated with waist circumference.
Results:
Waist circumference was positively correlated with increased activation in right and left frontal lobes (beta values ranging from 2.81 to 3.75 in the paracentral, medial, and superior gyri), left temporal lobe, left hippocampal, left amygdala, left uncus, and left anterior and posterior cingulate gyri (beta values ranging from 2.93 to 3.55). Waist circumference was also negatively associated with left and right parietal lobe, right superior temporal gyrus, and right insula and precuneus (beta values ranging from 2.82 to 5.20).
Conclusion:
Increased brain activation in the brain regions involved in the stress response and autonomic regulation of the cardiovascular system during psychological stress may underlie stress-induced overeating and abdominal obesity in patients with CAD.
Keywords: coronary artery disease, mental stress, body mass index, waist circumference
Introduction
Coronary artery disease (CAD) remains a major cause of morbidity and mortality worldwide with a prevalence that is increasing worldwide (1). Excess abdominal fat has shown to be an important risk factor for CAD (2-5). Precise measurements of abdominal fat content requires the use of radiological imaging techniques such as computerized tomography, magnetic resonance imaging or dual energy X-ray absorptiometry (6, 7). However, given that these imaging methods are not practical in large-scale field epidemiology, waist circumference (WC) has often been used as a surrogate to determine abdominal obesity. WC has increased significantly in the United States in both women and men in recent decades, (8). Few treatments for obesity have been shown to result in lasting weight loss (9). Likewise, most prevention programs have resulted in only modest and short-term changes in obesity-related metabolic abnormalities and have not been able to prevent the development of obesity (9, 10). Given the increasing societal burden of abdominal obesity and CAD, understanding the complex mechanisms that drive this condition is necessary in order to optimize and target interventions that could prevent obesity or result in lasting weight loss in obese individuals.
One factor that may play a role in the development and maintenance of obesity is psychological stress. Evidence from population-based and clinical studies have shown a strong association between chronic stress and increased adiposity and weight gain (11-14). A number of potential mechanisms for this have been proposed, including changes in eating patterns and increased food cravings (15-17). These behavioral changes may be driven by alterations in neuropeptides and neurohormones that play a role in both stress and appetite / metabolism (18). Stress is associated with a preferential deposition of abdominal fat (19-22). Stress-related psychiatric disorders have been linked to obesity as evidenced by studies that showed an increase in depression in obese individuals (20) and increased obesity in patients with childhood abuse-related PTSD (23-26). These disorders may be independently associated with increased rates of obesity through adverse lifestyle behaviors, neurohormonal and/or metabolic dysregulation (27-29).Diet can also affect mood, including deficiencies of specific nutrients including Vitamin B12 and folate. (30-35), while diets that are high in saturated fats can increase the risk for CAD.
In addition to diet-related effects, central insulin resistance has been suggested as an important factor contributing to altered motivation for food as well as changes in brain regions involved in reward pathways (36, 37). In obese individuals, insulin levels have been shown to correlate positively with the neural activity in corticolimbic-striatal brain regions during favorite-food and stress cues (16). These data suggest that insulin sensitivity may affect specific human brain responses during exposure to stress. However, the mechanisms by which this occurs are not known
The purpose of the present study was to investigate the relationship between WC as a measure of abdominal obesity and brain responses to stress among patients with CAD. We hypothesized that among patients with CAD, those with increased WC would show increased stress-induced changes in brain areas involved in the regulation of memory, emotion, and/or peripheral cardiovascular responses to stress, including hippocampus, insula, and cingulate gyrus.
Methods
Study Cohort
All participants were participants recruited from the Mental Stress Ischemia Mechanisms and Prognosis Study (MIPS) (38-41). Recruitment methods for MIPS cohort were described elsewhere (41). Briefly, between the years of 2011 and 2014, 695 patients between 30 and 80 years of age with confirmed stable CAD were prospectively enrolled from Emory-affiliated hospitals and clinics. Patients were excluded if they had unstable angina, myocardial infarction, or decompensated heart failure within one week of their baseline visit; if they had any severe comorbidities expected to decrease their life expectancy to the next 5 years; or if they had systolic or diastolic blood pressure above 190 and 115 mmHg, respectively, on the day of testing. Patients were also excluded if they had a history of alcohol or substance abuse in the past year, severe psychiatric disorders other than depression, inflammatory diseases, malignancies, organ transplants, permanent atrial fibrillation, and severe neurological disorders such as dementia or Parkinson’s disease. Pregnant women, patients with chronic steroid use above 1500 meg per day, and patients who were unable to complete the mental stress testing were also excluded from study participation. From this initial cohort of 695 patients, a random sample of 151 patients were selected and underwent brain scans. Data regarding the sociodemographic characteristics, medical history, and medication history of all participants were collected by a research nurse using standard questionnaires, chart reviews and in-person interviews and were verified using medical records. Height, weight, and waist circumference were measured for each individual. These measurements were used to calculate the body mass index (BMI; in kg·m−2). All study participants provided informed consent and the study was approved by the Emory University Institutional Review Board.
Mental Stress Testing
All patients underwent mental stress testing using a standardized protocol, as previously described (41, 42). Briefly, following 30 minutes of resting in a quiet room, participants were asked to perform two control tasks, followed by two mentally stressful conditions, each lasting approximately 2 minutes. All participants were scanned twice during each of the 4 testing conditions. During the control conditions, participants were tasked with counting out loud and discussing a neutral event. The stress conditions included arithmetic and public speaking tasks.
The mental arithmetic task included multiplication, division, addition and subtraction. Patients were required to listen to a recorded message that provided instructions for the tasks. All tasks were performed under time pressure and negative feedback regarding the performance and the time spent in the task given. For the mental arithmetic task the level of difficulty was increased until participants were unable to successfully complete the 3 consecutive arithmetic problems. The purpose of calibrating difficulty to individual performance is to have a similar level of stress for all participants regardless of ability. The negative feedback was standardized by having the physician use a stop watch to time the performance and paper and pencil to score performance, and to provide standardized negative feedback. This type of negative feedback has shown to consistently result in an increased stress level. PET data recorded from the mental arithmetic task was compared to neutral counting.
In the public speaking task, two scripted scenarios of stressful interpersonal situations were provided to each participant and they were asked to develop a speech regarding these events. Each participant was given 2 minutes to prepare each speech and 3 minutes to present it in front of an audience and camera. Participants were told that their speeches would later be evaluated. Data from the public speaking tasks was only gathered during the speech. During the neutral recall, each participant was asked to recall a recent neutral memory. PET data recorded from the speech task was compared to neutral recall. These tasks were randomized.
All participants were required to hold beta-adrenergic antagonists and nitrate and calcium channels blockers for a minimum of 24 and 12 hours, respectively, prior to mental stress testing.
Brain imaging during mental stress
High Resolution-Positron Emission Tomography (HR-PET) brain imaging was conducted using the High Resolution Research Tomograph (HRRT) (Siemens, Inc., Knoxville, TN), with a spatial resolution of 2 mm (43). Brain perfusion was investigated by quantifying the regional activation in response to both stressful and control tasks. Ten seconds into each HR-PET scan, 20 mCi of radio-labeled water (H2[O15]), was injected to measure blood perfusion within the brain. Each scan lasted for approximately two minutes and electrocardiogram and vital signs were continuously monitored with physician present. A total of eight brain scans were performed for each individual with two scans during each of the 2 control (counting aloud and recalling a neutral event) and 2 scans for the stress (arithmetic and public speaking) conditions.
Data Analysis
Analysis of HR-PET images was performed using statistical parametric mapping (SPM8) software, as previously described (44, 45). Pre-processing of HR-PET images, including realigning, normalizing, and smoothing images, was completed identical to previous research (44, 46). HR-PET imaging analysis was completed on two levels. The first level consisted of creating individual contrast maps to determine brain areas with increased regional blood flow indicating activation (Blood FlowNet = Blood FlowMentai stress Task – Blood Flow control Task). These individual contrast maps were then used within the second-level voxel-wise regression analysis (SPM8; www.fil.ion.ucl.ac.uk/spm). Voxel-wise regression models were completed for brain activations and waist circumference with both positive and negative correlations assessed. . In order to average the contrasts, we first computed the contrast between mental arithmetic vs neutral counting, and second between the speech preparation vs neutral memory recall, and subsequently averaged these two contrasts. For the voxel-wise regression analysis, significant cluster peaks were identified using a threshold of p < 0.005 and a minimum cluster size of 11 voxels to balance Type I and II error as had been previously determined (47) Contrast maps were created for second level regression analyses in order to identify areas with increased and decreased blood flow following mental stress. Dice coefficient was computed using a built-in MATLAB function. The Dice coefficient was calculated to quantify the amount of overlap between two stress tasks. This index is calculated as the ratio of twice the intersection of two volumes divided by the sum of the two volumes, where A and B are the two volumes to be compared (DS-Coeffs(A, B) = 2(A ⋂ B)/(A + B)). Values range between 0 and 1 with a value of 1 indicating a total concordance between the two volumes, whereas a value of 0 indicating no overlap between the two volumes.
Differences in demographic, clinical and behavioral variables between BMI groups were assessed using ANOVA for continuous variables and Chi-square tests for categorical variables. All analyses were done using SPSS version 22.
Results
Table 1 presents the demographic characteristics of all 151 participants. Overall, the study sample consisted of 68.2% male with a mean age of 62 ± 8 years old and a mean BMI of 30.17 ± 6.0. The most common comorbidity in the cohort was dyslipidemia (82.1%), followed by hypertension (76.2%), and history of prior myocardial infarction (35.8%). As shown in Table 2, WC was positively correlated with male sex and presence of diabetes. No significant correlation was observed between age and WC (B −0.04, 95% CI (−0.15, 0.05)).
Table 1.
Baseline demographics of the study cohort
| All Patients (N=151) |
|
|---|---|
| Demographics | |
| Age, y ± SD | 62 (8) |
| Male, % | 103 (68.2) |
| African American, % | 54 (35.8) |
| Comorbidities, | |
| Diabetes, % | 52 (34.4) |
| Dyslipidemia, % | 124 (82.1) |
| Heart Failure, % | 22 (14.6) |
| Hypertension, % | 115 (76.2) |
| History of myocardial infarction, % | 54 (35.8) |
| Depression, % | 79 (52.3) |
| History of smoking, % | 23 (15.2) |
| Medication Use | |
| ACE Inhibitors, % | 68 (44.7) |
| Antidepressants, % | 53 (34.7) |
| Aspirin, % | 132 (87.3) |
| Beta Blockers, % | 111 (73.3) |
| Diuretics, % | 47 (30.7) |
| Statins, % | 136 (90.0) |
ACE: Angiotensin converting enzyme
Table 2.
Differences in waist circumference between participants based on clinical and demographic variables.
| Variable | Waist Circumference (Mean ± SD) | P- Value | |
|---|---|---|---|
| Absent | Present | ||
| Male | 39 ± 5 | 41 ± 5 | 0.02 |
| African American | 41 ± 5 | 40 ± 5 | 0.21 |
| Comorbidities | |||
| Diabetes | 40 ± 4.8 | 42 ± 6 | 0.003 |
| Dyslipidemia | 40 ± 7 | 41 ± 5 | 0.67 |
| Heart Failure | 41 ± 5 | 39 ± 5 | 0.21 |
| Hypertension | 40 ± 6 | 41 ± 5 | 0.44 |
| History of myocardial infarction | 40 ± 5 | 41 ± 5 | 0.70 |
| Depression | 40 ± 5 | 41 ± 6 | 0.15 |
| History of smoking | 41 ± 5 | 39 ± 5 | 0.10 |
| Medication Use | Not Taking | Taking | |
| ACE Inhibitors | 41.2 ± 5 | 41.1 ± 5 | 0.93 |
| Antidepressants | 40 ± 5 | 41 ±6 | 0.41 |
| Aspirin | 42 ± 5 | 41 ± 5 | 0.21 |
| Beta Blockers | 41.6 ± 5 | 41.0 ± 6 | 0.54 |
| Diuretics | 41.0 ± 6 | 41.7 ± 5 | 0.68 |
| Statins | 42.2 ± 6 | 41.0 ± 5 | 0.45 |
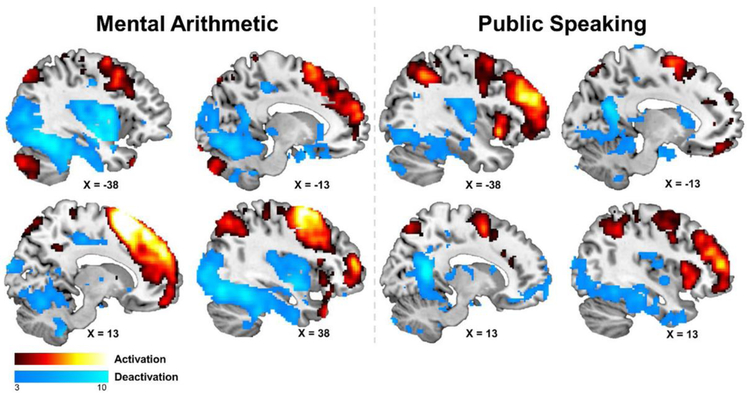
Overall, as shown in Figure 1,in response to mental stress testing, large areas of the bilateral frontal lobe including the inferior, medial, and superior gyri, bilateral temporal lobe, parietal lobe, bilateral anterior cingulate, and the bilateral posterior cerebellum showing increased activation. There were large overlaps between the mental arithmetic and public speaking tasks with a dice coefficient of 0.55.
Figure 1:
Overall brain activation for mental arithmetic (levt), and public speaking (right), for both stress-induced activation and deactivation.
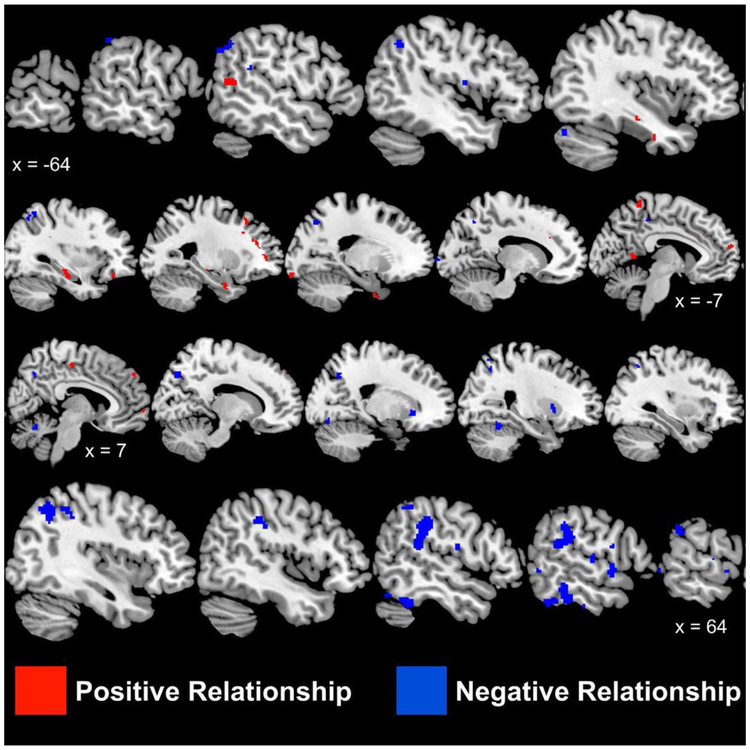
As shown in Table 3 and Figure 2 and 3, waist circumference was positively correlated with increased activation in right and left frontal lobes (paracentral, medial, and superior gyri), left temporal lobe, left parahippocampal, left amygdala, left uncus, left limbic lobe, left occipital lobe and left anterior cingulate gyri (Table 3 and Figure 2). WC was negatively associated with right and left insula, left and right parietal lobe, right and left superior temporal gyri, and right and left cerebellum (Table 4, and Figure 4).
Table 3:
Associations between Areas of Increased Activation During Mental Stress Testing and Waist Circumference.
| Talairach | ||||||
|---|---|---|---|---|---|---|
| Voxel Number | Brain Region | Brodmann Area | X | Y | Z | Z Score |
| 41 | R Frontal Lobe, Paracentral Lobule | 31 | 2 | −17 | 44 | 3.75 |
| 96 | L Parahippocampal Gyrus | −34 | −18 | −14 | 3.55 | |
| L Temporal Lobe, Sub-Gyral | −30 | −31 | −4 | 3.16 | ||
| L Parahippocampal Gyrus | 36 | −42 | −20 | −11 | 2.91 | |
| 32 | L Temporal Lobe, Superior Gyrus | 22 | −51 | −56 | 14 | 3.38 |
| 37 | L Limbic Lobe, Posterior Cingulate | 29 | −8 | −50 | 7 | 3.38 |
| 58 | L Limbic Lobe, Uncus | −24 | −1 | −25 | 3.38 | |
| L Limbic Lobe, Uncus | 38 | −16 | 6 | −34 | 2.96 | |
| L Limbic Lobe, Uncus | 36 | −20 | −2 | −32 | 2.76 | |
| 12 | L Frontal Lobe, Paracentral Lobule | 6 | −2 | −28 | 55 | 3.28 |
| 11 | L Frontal Lobe, Middle Gyrus | 11 | −34 | 34 | −15 | 3.26 |
| 21 | L Amygdala | −30 | −5 | −15 | 3.23 | |
| 31 | L Frontal Lobe, Medial Gyrus | 10 | 0 | 66 | −2 | 3.21 |
| 27 | L Anterior Cingulate | 32 | −12 | 28 | 22 | 3.17 |
| 13 | L Occipital Lobe, Inferior Gyrus | 17 | −20 | −94 | −9 | 3.17 |
| 19 | L Frontal Lobe, Sub-Gyral | −24 | 45 | 5 | 3.15 | |
| 32 | L Frontal Lobe, Paracentral Lobule | 5 | −8 | −42 | 57 | 3.13 |
| 23 | R Frontal Lobe, Superior Gyrus | 9 | 10 | 54 | 34 | 3.13 |
| 11 | L Frontal Lobe, Cingulate Gyrus | 32 | 0 | 23 | 36 | 3.10 |
| 15 | L Temporal Lobe, Middle Gyrus | 21 | −38 | −7 | −25 | 3.07 |
| 17 | R Frontal Lobe, Medial Gyrus | 0 | 52 | 1 | 3.06 | |
| 11 | R Frontal Lobe, Paracentral Lobule | 5 | 10 | −44 | 61 | 3.04 |
| 19 | L Frontal Lobe, Middle Gyrus | −26 | 32 | 19 | 2.98 | |
| L Frontal Lobe, Middle Gyrus | −28 | 37 | 10 | 2.68 | ||
| 16 | R Frontal Lobe, Superior Gyrus | 9 | 22 | 48 | 35 | 2.97 |
| 14 | R Parietal Lobe, Superior Lobule | 7 | 22 | −55 | 61 | 2.93 |
| 15 | L Frontal Lobe, Medial Gyrus | 10 | −6 | 61 | 12 | 2.88 |
| 20 | L Frontal Lobe, Middle Gyrus | 8 | −26 | 27 | 37 | 2.85 |
| 13 | L Frontal Lobe, Middle Gyrus | 9 | −28 | 23 | 27 | 2.81 |
Brain Regions with significant (p < 0.005) positive relationships between waist circumference (cm) and brain activation as measured with [15O]H2O positron emission tomography following mental stress (mental arithmetic, public speaking). The XYZ coordinates identify the cluster peak with sub-cluster peaks occurring > 8mm away also presented. Voxel number refers to number of voxels in cluster with Z-score of cluster/sub-cluster peak also presented.
Figure 2:
Sagittal brain slices showing areas with significant (p < 0.005) positive relationships between waist circumference (cm; red) and brain activation
Sagittal brain slices showing areas with significant (p < 0.005) positive relationships between waist circumference (cm; red) and brain activation as measured with as measured with [15O]H2O positron emission tomography following mental stress (mental arithmetic, public speaking). Values underneath brains present Talairach x axis values (negative = left, positive = right hemisphere).
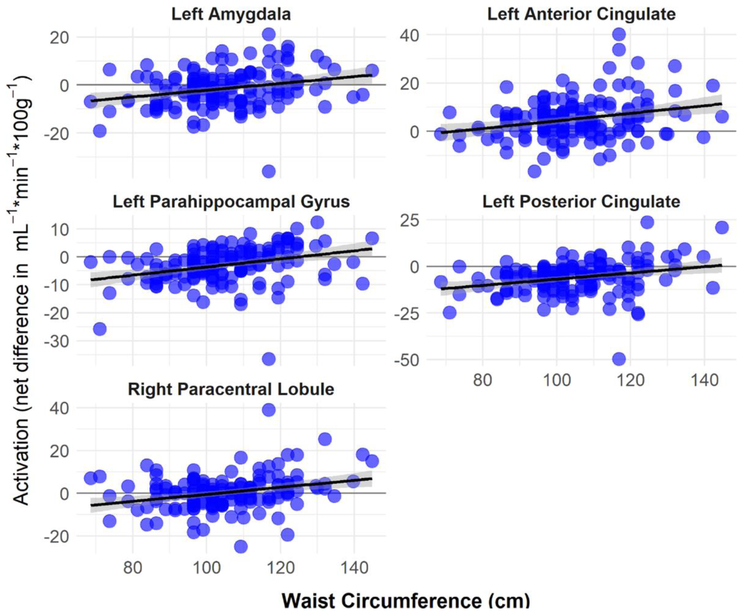
Figure 3:
Positive correlations between brain activations (net difference in blood flow in mL−1·min−1·100g−1) and waist circumference (cm). Brain activations were computed from mean activity within significant (p < 0.005) clusters as identified with voxel-wise regression.
Table 4:
Associations between Areas of Decreased Activation During Mental Stress Testing and Waist Circumference.
| Talairach | ||||||
|---|---|---|---|---|---|---|
| Voxel Number |
Brain Region | Brodmann Area | X | Y | Z | Z Score |
| 450 | R Insula | 13 | 53 | −36 | 20 | 5.20 |
| R Parietal Lobe, Inferior Lobule | 40 | 48 | −29 | 33 | 4.25 | |
| R Parietal Lobe, Inferior Lobule | 40 | 55 | −32 | 27 | 4.14 | |
| 127 | R Parietal Lobe, Precuneus | 7 | 12 | −70 | 39 | 4.32 |
| R Parietal Lobe, Superior Lobule | 7 | 24 | −58 | 41 | 3.27 | |
| R Parietal Lobe, Precuneus | 7 | 22 | −60 | 48 | 2.95 | |
| 49 | R Cerebellum | 24 | −53 | −13 | 4.29 | |
| 40 | L Parietal Lobe, Precuneus | 7 | −20 | −64 | 42 | 4.17 |
| L Parietal Lobe, Precuneus | 7 | −12 | −56 | 43 | 2.88 | |
| 86 | R Cerebellum | 53 | −48 | −21 | 4.02 | |
| 40 | L Parietal Lobe, Superior Lobule | 7 | −36 | −54 | 50 | 3.80 |
| 145 | R Parietal Lobe, Inferior Lobule | 40 | 40 | −54 | 41 | 3.73 |
| 100 | R Temporal Lobe, Fusiform Gyrus | 20 | 57 | −34 | −18 | 3.70 |
| R Temporal Lobe, Middle Gyrus | 21 | 59 | −36 | −12 | 3.41 | |
| 82 | R Lentiform Nucleus | 18 | 21 | −4 | 3.69 | |
| R Lentiform Nucleus | 24 | 12 | −1 | 3.18 | ||
| 24 | R Parietal Lobe, Precuneus | 7 | 4 | −58 | 38 | 3.58 |
| 30 | R Temporal Lobe, Superior Gyrus | 22 | 59 | −11 | 7 | 3.57 |
| 70 | L Parietal Lobe, Angular Gyrus | 39 | −51 | −64 | 37 | 3.43 |
| L Parietal Lobe, Inferior Lobule | 40 | −44 | −50 | 39 | 3.28 | |
| L Parietal Lobe, Inferior Lobule | 40 | −53 | −54 | 38 | 3.12 | |
| 45 | R Temporal Lobe, Superior Gyrus | 22 | 57 | 2 | −3 | 3.40 |
| 12 | R Temporal Lobe, Inferior Gyrus | 20 | 67 | −17 | −23 | 3.38 |
| 19 | R Frontal Lobe, Inferior Gyrus | 44 | 59 | 5 | 15 | 3.33 |
| 20 | R Frontal Lobe, Precentral Gyrus | 43 | 51 | −7 | 14 | 3.23 |
| 15 | L Temporal Lobe, Superior Gyrus | 22 | −63 | −31 | 8 | 3.22 |
| 13 | L Parietal Lobe, Precuneus | 7 | −8 | −33 | 42 | 3.17 |
| 21 | L Temporal Lobe, Superior Gyrus | 13 | −53 | −42 | 22 | 3.16 |
| 13 | L Cerebellum | −42 | −75 | −18 | 3.13 | |
| 11 | L Insula | 13 | −44 | −3 | 12 | 3.13 |
| 17 | R Parietal Lobe, Inferior Lobule | 40 | 53 | −42 | 46 | 3.07 |
| 14 | R Temporal Lobe, Middle Gyrus | 59 | −56 | 3 | 3.05 | |
| 12 | R Temporal Lobe, Fusiform Gyrus | 37 | 50 | −63 | −15 | 3.04 |
| 12 | R Frontal Lobe, Inferior Gyrus | 47 | 34 | 29 | 5 | 2.98 |
| 18 | R Cerebellum | 6 | −61 | −14 | 2.98 | |
| 15 | R Occipital Lobe, Lingual Gyrus | 18 | 18 | −74 | −8 | 2.95 |
| 11 | R Temporal Lobe, Fusiform Gyrus | 20 | 55 | −21 | −26 | 2.91 |
| 12 | L Parietal Lobe, Inferior Lobule | 40 | −55 | −41 | 44 | 2.88 |
| 19 | L Occipital Lobe, Cuneus | 17 | −8 | −95 | 5 | 2.83 |
| L Occipital Lobe, Cuneus | 18 | −14 | −101 | 7 | 2.82 | |
Brain Regions with significant (p < 0.005) negative relationships between waist circumference (cm) and brain activation as measured with [15O]H2O positron emission tomography following mental stress (mental arithmetic, public speaking). The XYZ coordinates identify the cluster peak with sub-cluster peaks occurring > 8mm away also presented. Voxel number refers to number of voxels in cluster with Z-score of cluster/sub-cluster peak also presented.
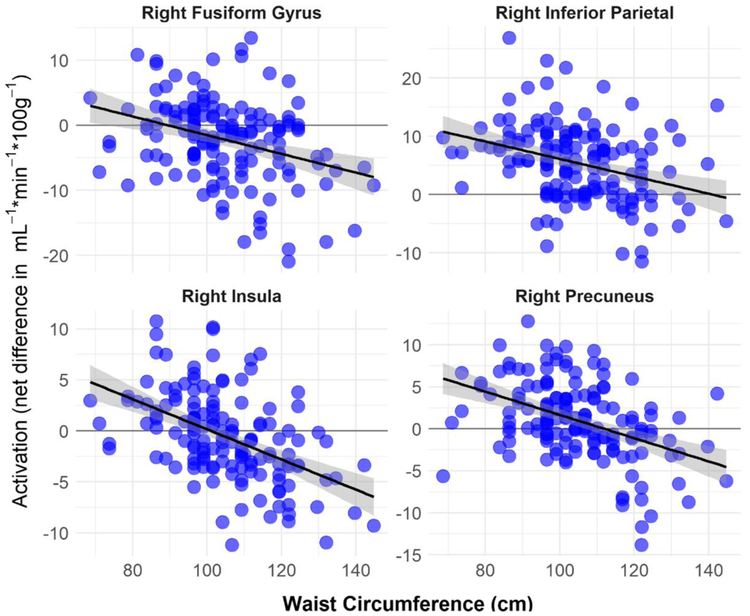
Figure 4:
Negative correlations between brain activations (net difference in blood flow in mL−1·min−1·100g−1) and waist circumference (cm). Brain activations were computed from mean activity within significant (p < 0.005) clusters as identified with voxel-wise regression.
Discussion
The results of the present study revealed that waist circumference in CAD patients were associated with exacerbated neural responses in the left temporal lobe, left occipital lobe, left anterior and posterior cingulate gyrus, right paracentral lobule of the frontal lobe, and increased activation in hippocampus and amygdala. The brain regions found by our analysis have also been shown to be involved in pathways influenced by eating behaviors, regions affected by hyperinsulinemia, and areas controlling the central processing of autonomic function. It is important to point out that in our study links between the identified brain areas and their function were not directly investigated. However, our findings may suggest that differences in brain responsivity to stress could play a role in the link between stress and vulnerability to obesity.
While stress has been associated with obesity and abdominal obesity (11-14, 48), the mechanisms underlying this process are not fully elucidated (49). One of the proposed pathways is through the influence of stress on eating behaviors (50, 51). Previous studies have shown that both acute and chronic stress increase food intake, particularly high-fat and high-sugar foods (52, 53). Food cravings have also shown to partly mediate the relationship between chronic stress and increased BMI (15). Previous studies in participants without CAD have shown higher corticolimbic activations in obese, but not lean, individuals in response to favorite-food cue and stress conditions. These regions included the inferior and superior frontal gyrus, middle temporal gyrus, inferior occipital gyrus, and parahippocampus. In addition, the magnitude of insulin resistance, has shown to positively correlate with the activation of these same regions in response to both favorite-food cue and stress conditions in obese but not lean individuals (16, 54). In our study, we found strong correlations between similar regions that were more activated in response to stress among those with higher measures of abdominal obesity. These findings suggest that among obese individuals, insulin resistance may accentuate stress- and food cue–related neural responses by impairing insulin’s ability to suppress pathways implicated in reward-motivation. Therefore, it is a possibility that in fact hyperinsulinemia is a factor influencing the brain responses to mental stress. Therefore, brain responses to stress are not necessarily the cause of increased abdominal obesity and increases in WC. As our study was cross-sectional in nature, the casual relationship between brain activation and increase in WC could not be clearly investigated.
In addition to hyperinsulinemia, abdominal obesity is highly associated with an increase in inflammatory markers including C-reactive protein and interleukin 6 (55, 56). There is evidence that inflammation causes mood disturbances that correlate with brain activity in anterior cingulate cortex- a brain region implicated in the etiology of depression (57). Brain regions implicated in stressor-evoked blood pressure reactivity have also been shown to mediate the relationship between inflammation and blood pressure in healthy participants (58). These data suggest that it could be abdominal obesity that influences brain responses to stress and not necessarily brain activation that causes changes in WC in response to stress.
Accumulating data from animal and human studies suggest that dysregulation of the autonomic nervous system activity plays a pivotal role in the etiology of metabolic abnormalities including obesity, central obesity and insulin resistance (59, 60). The results from brain-imaging studies in humans have proposed a central autonomic network (CAN) designed to modulate autonomic outputs (61-67). Our results showed that several of the regions identified in the CAN were more activated in patients with increased BMI and waist circumference. These regions included the anterior cingulate, and the paracentral lobe of the frontal lobe which showed higher activation with increases in both BMI and waist circumference. Other regions of the CAN including the hippocampus, and parts of ventromedial prefrontal cortex (Brodmann’s Area 10) were also positively correlated with increases in waist circumference, while activation of the right insula and cerebellum were seen in patients with elevated BMI. These results suggest that altered CAN activity to stress may play an important role in higher susceptibility of patients to obesity.
In the present study, we found strong associations between waist circumference and brain activity in the left hippocampus. Increases in waist circumference, which is a measure of central obesity has shown to better assess body fat distribution in CAD patients. Compared to BMI, measurement of waist circumference is a stronger predictor of future development of CAD (68). Prior studies have also shown that patients with CAD with normal BMI but who are centrally obese have the highest cardiovascular mortality compared with other patterns of adiposity (4, 5). The hippocampus, has been implicated in the regulation of body weight through functions including memory coding and inhibitory processes related to food intake (69). It has been shown that increased activity of the hippocampus correlates with increased emotional and uncontrolled eating (70) and because of its role in memory, the hippocampus may also be an important structure to guide food decisions. Our findings expand upon previous work showing the importance of these structures following an acute stress as a possible neurobiological signature in individuals with increased waist circumference. Future studies are needed to assess whether the observed brain-evoked patterns of activation also predict future outcomes in this vulnerable patient population.
The present study had a number of limitations. First, the cross-sectional nature of the study precluded any assessment of causality. Second, all individuals were selected from a cohort of patients with stable CAD, which may limit the generalizability of our findings to individuals without CAD. Finally, women comprised only 32.3% of the total participants. Therefore, while no sex differences were seen in the patterns of brain activation in response to stress, our sample of women was small. In addition, the PET imaging was acquired during speech delivery which could potentially confound the results. Finally, mental stress testing was only performed during a single day, and therefore we could not ascertain whether the brain responses to stress seen in our study are stable and trait-like throughout life.
In summary, we found that obese individuals with CAD exhibit exaggerated neural responses in regions involved in stress, memory and emotion. These neural responses may underlie eating patterns and food cravings which could subsequently lead to increases in waist circumference. Our findings underscore the importance of better understanding environmental exposures such as psychological stress in the pathophysiology of obesity in order to design more effective treatment and prevention strategies. It is however unclear whether the brain responses to mental stress are similar between patients with CAD and the healthy participants. Future studies are required to explore the similarities and differences in the patterns of brain reactivity to mental stress between papulations with and without coronary disease.
ACKNOWLEDGMENTS:
This study was supported by NIH research grants P01 HL101398, HL088726, MH076955, MH067547-01, MH56120, RR016917, HL077506, HL068630, HL109413, HL125246, and HL127251. We wish to acknowledge Margie Jones, C.N.M.T., for assistance with imaging and analysis procedures and Nancy Murrah, R.N., Janice Parrott, R.N., Karen Sykes, and Steve Rhodes, R.N., for assistance with patient assessments and clinical research.
Footnotes
Publisher's Disclaimer: This manuscript has been accepted by the editors of Psychosomatic Medicine, but it has not yet been copy-edited; information within these pages is therefore subject to change. During the copy-editing and production phases, language usage and any textual errors will be corrected, and pages will be composed into their final format.
References
- 1.Writing Group M, Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics C, Stroke Statistics S. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38–360. [DOI] [PubMed] [Google Scholar]
- 2.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R, Association for Weight M, Obesity P, Naaso, Obesity S, American Society for N, American Diabetes A. Waist circumference and cardiometabolic risk: a consensus statement from shaping America's health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care. 2007;30:1647–52. [DOI] [PubMed] [Google Scholar]
- 3.Sharma S, Batsis JA, Coutinho T, Somers VK, Hodge DO, Carter RE, Sochor O, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Lopez-Jimenez F. Normal-Weight Central Obesity and Mortality Risk in Older Adults With Coronary Artery Disease. Mayo Clin Proc. 2016;91:343–51. [DOI] [PubMed] [Google Scholar]
- 4.Coutinho T, Goel K, Correa de Sa D, Carter RE, Hodge DO, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F. Combining body mass index with measures of central obesity in the assessment of mortality in subjects with coronary disease: role of "normal weight central obesity". J Am Coll Cardiol. 2013;61:553–60. [DOI] [PubMed] [Google Scholar]
- 5.Coutinho T, Goel K, Correa de Sa D, Kragelund C, Kanaya AM, Zeller M, Park JS, Kober L, Torp-Pedersen C, Cottin Y, Lorgis L, Lee SH, Kim YJ, Thomas R, Roger VL, Somers VK, Lopez-Jimenez F. Central obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol. 2011;57:1877–86. [DOI] [PubMed] [Google Scholar]
- 6.Neeland IJ, Grundy SM, Li X, Adams-Huet B, Vega GL. Comparison of visceral fat mass measurement by dual-X-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: the Dallas Heart Study. Nutr Diabetes. 2016;6:e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klopfenstein BJ, Kim MS, Krisky CM, Szumowski J, Rooney WD, Purnell JQ. Comparison of 3 T MRI and CT for the measurement of visceral and subcutaneous adipose tissue in humans. Br J Radiol. 2012;85:e826–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. Jama. 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galani C, Schneider H. Prevention and treatment of obesity with lifestyle interventions: review and meta-analysis. Int J Public Health. 2007;52:348–59. [DOI] [PubMed] [Google Scholar]
- 10.Lemmens VE, Oenema A, Klepp KI, Henriksen HB, Brug J. A systematic review of the evidence regarding efficacy of obesity prevention interventions among adults. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2008;9:446–55. [DOI] [PubMed] [Google Scholar]
- 11.Block JP, He Y, Zaslavsky AM, Ding L, Ayanian JZ. Psychosocial stress and change in weight among US adults. American journal of epidemiology. 2009;170:181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain, behavior, and immunity. 2005;19:275–80. [DOI] [PubMed] [Google Scholar]
- 13.Torres SJ, Nowson CA. Relationship between stress, eating behavior, and obesity. Nutrition. 2007;23:887–94. [DOI] [PubMed] [Google Scholar]
- 14.Adam TC, Epel ES. Stress, eating and the reward system. Physiology & behavior. 2007;91:449–58. [DOI] [PubMed] [Google Scholar]
- 15.Chao A, Grilo CM, White MA, Sinha R. Food cravings mediate the relationship between chronic stress and body mass index. J Health Psychol. 2015;20:721–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN. Neural correlates of stress- and food cue-induced food craving in obesity: association with insulin levels. Diabetes care. 2013;36:394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chao AM, Jastreboff AM, White MA, Grilo CM, Sinha R. Stress, cortisol, and other appetite-related hormones: Prospective prediction of 6-month changes in food cravings and weight. Obesity. 2017;25:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bremner JD, Pearce B. Neurotransmitter, neurohormonal, and neuropeptidal function in PTSD In: Bremner JD, editor. Posttraumatic Stress Disorder: From Neurobiology to Treatment. Hoboken, New Jersey: Wiley-Blackwell; 2016. p. 181–232. [Google Scholar]
- 19.Ladabaum U, Mannalithara A, Myer PA, Singh G. Obesity, abdominal obesity, physical activity, and caloric intake in US adults: 1988 to 2010. Am J Med. 2014;127:717–27 e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markowitz S, Friedman MA, Arent SM. Understanding the relation between obesity and depression: Causal mechanisms and implications for treatment. Clinical Psychology: Science and Practice. 2008;15:1–20. [Google Scholar]
- 21.Rosmond R, Chagnon YC, Holm G, Chagnon M, Perusse L, Lindell K, Carlsson B, Bouchard C, Bjorntorp P. A glucocorticoid receptor gene marker is associated with abdominal obesity, leptin, and dysregulation of the hypothalamic-pituitary-adrenal axis. Obes Res. 2000;8:211–8. [DOI] [PubMed] [Google Scholar]
- 22.Shively CA, Register TC, Clarkson TB. Social stress, visceral obesity, and coronary artery atherosclerosis: product of a primate adaptation. Am J Primatol. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't Review]. 2009;71:742–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–6. [DOI] [PubMed] [Google Scholar]
- 24.Su S, Jimenez MP, Roberts CT, Loucks EB. The role of adverse childhood experiences in cardiovascular disease risk: a review with emphasis on plausible mechanisms. Curr Cardiol Rep. 2015;17:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anda RF, Felitti VJ, Walker J, Whitfield C, Bremner JD, Perry BD, Dube SR, Giles WH. The enduring effects of childhood abuse and related experiences in childhood: A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256:174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bremner D, Vermetten E, Kelley ME. Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. J Nerv Ment Dis. 2007;195:919–27. [DOI] [PubMed] [Google Scholar]
- 27.Farr OM, Sloan DM, Keane TM, Mantzoros CS. Stress- and PTSD-associated obesity and metabolic dysfunction: a growing problem requiring further research and novel treatments. Metabolism. 2014;63:1463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Reedt Dortland AK, Vreeburg SA, Giltay EJ, Licht CM, Vogelzangs N, van Veen T, de Geus EJ, Penninx BW, Zitman FG. The impact of stress systems and lifestyle on dyslipidemia and obesity in anxiety and depression. Psychoneuroendocrinology. 2013;38:209–18. [DOI] [PubMed] [Google Scholar]
- 29.Yu J, Fei K, Fox A, Negron R, Horowitz C. Stress eating and sleep disturbance as mediators in the relationship between depression and obesity in low-income, minority women. Obes Res Clin Pract. 2016;10:283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin P-Y, Su K-P. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–61. [DOI] [PubMed] [Google Scholar]
- 31.Lewis SJ, Lawlor DA, Davey Smith G, Araya R, Timpson N, Day INM, Ebrahim S. The thermolabile variant of MTHFR is associated with depression the British Women's Heart and Health Study and a meta-analysis. Mol Psychiatry. 2006;11:352–60. [DOI] [PubMed] [Google Scholar]
- 32.McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006;354:2764–72. [DOI] [PubMed] [Google Scholar]
- 33.Penninx BW, Guralnik JM, Ferrucci L, Fried LP, Allen RH, Stabler SP. Vitamin B(12) deficiency and depression in physically disabled older women: epidemiological evidence from the Women's Health and Aging Study. Am J Psychiatry. 2000;157:715–21. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds EH, Carney MWP, Toone BK. Methylation and mood. Lancet. 1984;ii:196–8. [DOI] [PubMed] [Google Scholar]
- 35.Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–61. [DOI] [PubMed] [Google Scholar]
- 36.Anthony K, Reed LJ, Dunn JT, Bingham E, Hopkins D, Marsden PK, Amiel SA. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: the cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes. 2006;55:2986–92. [DOI] [PubMed] [Google Scholar]
- 37.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain research. 2003;964:107–15. [DOI] [PubMed] [Google Scholar]
- 38.Hammadah M, Alkhoder A, Al Mheid I, Wilmot K, Isakadze N, Abdulhadi N, Chou D, Obideen M, O'Neal WT, Sullivan S, Samman Tahhan A, Kelli HM, Ramadan R, Pimple P, Sandesara P, Shah AJ, Ward L, Ko Y-A, Sun Y, Uphoff I, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Sheps DS, Raggi P, Vaccarino V, Quyyumi AA. Hemodynamic, catecholamine, vasomotor and vascular responses: Determinants of myocardial ischemia during mental stress. Int J Cardiol. 2017;243:47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaccarino V, Wilmot K, Al Mheid I, Ramadan R, Pimple P, Shah AJ, Garcia EV, Nye J, Ward L, Hammadah M, Kutner M, Long Q, Bremner JD, Esteves F, Raggi P, Quyyumi AA. Sex differences in mental stress-Induced myocardial ischemia in patients with coronary heart disease. JAHA. 2016;5:pii: e003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bremner JD, Campanella C, Khan Z, Shah M, Hammadah M, Wilmot K, Al Mheid I, Lima BB, Garcia EV, Nye J, Ward L, Kutner MH, Raggi P, Pearce BD, Shah AJ, Quyyumi AA, Vaccarino V. Brain correlates of mental stress-induced myocardial ischemia. Psychosom Med. 2018;80:515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Shah AJ, Sun Y, Pearce B, Garcia EV, Kutner M, Bremner JD, Esteves F, Raggi P, Sheps DS, Vaccarino V, Quyyumi AA. The Mental Stress Ischemia Prognosis Study: Objectives, Study Design, and Prevalence of Inducible Ischemia. Psychosom Med. 2017;79:311–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sheps DS, McMahon RP, Becker L, Carney RM, Freedland KE, Cohen JD, Sheffield D, Goldberg AD, Ketterer MW, Pepine CJ, Raczynski JM, Light K, Krantz DS, Stone PH, Knatterud GL, Kaufmann PG. Mental stress-induced ischemia and all-cause mortality in patients with coronary artery disease: Results from the Psychophysiological Investigations of Myocardial Ischemia study. Circulation. 2002;105:1780–4. [DOI] [PubMed] [Google Scholar]
- 43.Weinhard K, Schmand M, Casey ME, Baker K, Bao J, Eriksson L, Jones WF, Knoess C, Lenox M, Lercher M, Luk P, Michel C, Reed JH, Richerzhagen N, Treffert J, Vollmar S, Young JW, Heiss WD, Nutt R. The ECAT HRRT: Performance and first clinical application of the new high resolution research tomograph. IEEE Trans Med Imaging. 2000;3:17/2–/6. [Google Scholar]
- 44.Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry. 1999;156:1787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Staib LH, Soufer R, Charney DS. Neural correlates of declarative memory for emotionally valenced words in women with posttraumatic stress disorder related to early childhood sexual abuse. Biological psychiatry. 2003;53:879–89. [DOI] [PubMed] [Google Scholar]
- 46.Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Duncan JS, Charney DS. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry. 2003;160:924–32. [DOI] [PubMed] [Google Scholar]
- 47.Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997;154:926–33. [DOI] [PubMed] [Google Scholar]
- 48.Kraynak TE, Marsland AL, Gianaros PJ. Neural Mechanisms Linking Emotion with Cardiovascular Disease. Curr Cardiol Rep. 2018;20:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gianaros PJ, Wager TD. Brain-Body Pathways Linking Psychological Stress and Physical Health. Curr Dir Psychol Sci. 2015;24:313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of "comfort food". Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aroniadis OC, Drossman DA, Simren M. A Perspective on Brain-Gut Communication: The American Gastroenterology Association and American Psychosomatic Society Joint Symposium on Brain-Gut Interactions and the Intestinal Microenvironment. Psychosom Med. 2017;79:847–56. [DOI] [PubMed] [Google Scholar]
- 52.Tryon MS, Carter CS, Decant R, Laugero KD. Chronic stress exposure may affect the brain's response to high calorie food cues and predispose to obesogenic eating habits. Physiology & behavior. 2013;120:233–42. [DOI] [PubMed] [Google Scholar]
- 53.Rudenga KJ, Sinha R, Small DM. Acute stress potentiates brain response to milkshake as a function of body weight and chronic stress. International journal of obesity. 2013;37:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soreca I, Rosano C, Jennings JR, Sheu LK, Kuller LH, Matthews KA, Aizenstein HJ, Gianaros PJ. Gain in adiposity across 15 years is associated with reduced gray matter volume in healthy women. Psychosom Med. 2009;71:485–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rana JS, Arsenault BJ, Despres JP, Cote M, Talmud PJ, Ninio E, Wouter Jukema J, Wareham NJ, Kastelein JJ, Khaw KT, Boekholdt SM. Inflammatory biomarkers, physical activity, waist circumference, and risk of future coronary heart disease in healthy men and women. Eur Heart J. 2011;32:336–44. [DOI] [PubMed] [Google Scholar]
- 56.Thorand B, Baumert J, Doring A, Herder C, Kolb H, Rathmann W, Giani G, Koenig W, Group K. Sex differences in the relation of body composition to markers of inflammation. Atherosclerosis. 2006;184:216–24. [DOI] [PubMed] [Google Scholar]
- 57.Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry. 2009;66:407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Harrison NA, Cooper E, Voon V, Miles K, Critchley HD. Central autonomic network mediates cardiovascular responses to acute inflammation: relevance to increased cardiovascular risk in depression? Brain Behav Immun. 2013;31:189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thorp AA, Schlaich MP. Relevance of Sympathetic Nervous System Activation in Obesity and Metabolic Syndrome. J Diabetes Res. 2015;2015:341583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ginty AT, Kraynak TE, Fisher JP, Gianaros PJ. Cardiovascular and autonomic reactivity to psychological stress: Neurophysiological substrates and links to cardiovascular disease. Auton Neurosci. 2017;207:2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Critchley HD, Mathias CJ, Josephs O, O'Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ. Human cingulate cortex and autonomic control: converging neuroimaging and clinical evidence. Brain. 2003;126:2139–52. [DOI] [PubMed] [Google Scholar]
- 62.Gianaros PJ, Van Der Veen FM, Jennings JR. Regional cerebral blood flow correlates with heart period and high-frequency heart period variability during working-memory tasks: Implications for the cortical and subcortical regulation of cardiac autonomic activity. Psychophysiology. 2004;41:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nugent AC, Bain EE, Thayer JF, Sollers JJ, Drevets WC. Sex differences in the neural correlates of autonomic arousal: a pilot PET study. Int J Psychophysiol. 2011;80:182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Morree HM, Szabo BM, Rutten GJ, Kop WJ. Central nervous system involvement in the autonomic responses to psychological distress. Neth Heart J. 2013;21:64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gianaros PJ, Onyewuenyi IC, Sheu LK, Christie IC, Critchley HD. Brain systems for baroreflex suppression during stress in humans. Hum Brain Mapp. 2012;33:1700–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jennings JR, van der Molen MW, Somsen RJ, Graham R, Gianaros PJ. Vagal function in health and disease: studies in Pittsburgh. Physiol Behav. 2002;77:693–8. [DOI] [PubMed] [Google Scholar]
- 67.Gianaros PJ, May JC, Siegle GJ, Jennings JR. Is there a functional neural correlate of individual differences in cardiovascular reactivity? Psychosom Med. 2005;67:31–9. [DOI] [PubMed] [Google Scholar]
- 68.Dimitriadis K, Tsioufis C, Mazaraki A, Liatakis I, Koutra E, Kordalis A, Kasiakogias A, Flessas D, Tentolouris N, Tousoulis D. Waist circumference compared with other obesity parameters as determinants of coronary artery disease in essential hypertension: a 6-year follow-up study. Hypertens Res. 2016;39:475–9. [DOI] [PubMed] [Google Scholar]
- 69.Coppin G The anterior medial temporal lobes: Their role in food intake and body weight regulation. Physiol Behav. 2016;167:60–70. [DOI] [PubMed] [Google Scholar]
- 70.Wang GJ, Yang J, Volkow ND, Telang F, Ma Y, Zhu W, Wong CT, Tomasi D, Thanos PK, Fowler JS. Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:15641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]