Abstract
Objective:
Reactivation viremia is associated with adverse clinical outcomes and immune dysfunction in adults with sepsis. We determined the incidence of viremia and its association with clinical outcomes and immune paralysis phenotype in children with severe sepsis.
Design:
Prospective cohort study
Setting:
Single academic pediatric intensive care unit from September 2016 to March 2018
Patients:
Fifty-nine patients 2-17 years old treated for severe sepsis
Measurements and Results:
We performed real-time polymerase chain reaction (PCR) assays on whole blood specimens to determine the incidence of cytomegalovirus (CMV). CMV was detected in three (5%) patients. All patients with CMV viremia were seropositive, with an incidence of 13% in this subset. We additionally performed Epstein Barr virus (EBV) and human herpesvirus-6 (HHV-6) PCR assays on last available specimens and detected EBV in 4% and HHV-6 in 30% of the study population. Overall, viremia was not associated with clinical outcomes or immune function in univariable analyses. However, viremia was associated with lower odds of complicated course (defined as death within 28 days or ≥2 organ dysfunctions at seven days) after controlling for age, PRISM III score, and blood transfusion (aOR 0.08, 95% CI 0.01,0.84, p=0.04).
Conclusions:
Children with severe sepsis had low rates of detectable viremia, which limited analyses of its association with clinical outcomes or immune paralysis phenotype. Given the rare occurrence of CMV viremia, in particular, our study does not support a role for viremia as a biomarker of illness severity or as a modifiable risk factor of clinical outcomes for most patients. Future studies on the role of viremia in pediatric sepsis will need to consider the challenges posed by low rates of viremia in this population.
Keywords: sepsis, cytomegalovirus, viremia, immune suppression, pediatric, mortality
INTRODUCTION
Sepsis remains a leading cause of morbidity, mortality, and healthcare costs for children and adults despite improvements in resuscitation and supportive therapies [1, 2]. Some patients develop a sepsis-associated immune paralysis phenotype that has been linked to adverse clinical outcomes including prolonged infection, secondary infections, and viral reactivation [3-8].
Reactivation of herpes viruses, including cytomegalovirus (CMV), Epstein-Barr virus (EBV), and human herpesvirus 6 (HHV-6), is associated with morbidity and mortality in adult sepsis [9-17]. CMV, in particular, has promise as a biomarker of immune paralysis in sepsis, since viremia in seropositive patients signals a suppressed host immune system unable to limit viral replication. The reactivation of CMV and other previously latent viruses may also propagate the systemic inflammatory response in sepsis along with, or perhaps even accelerating, the inciting infection. However, the significance of viral reactivation in sepsis remains unclear [18]. For example, a trial of ganciclovir prophylaxis in adult sepsis reduced CMV reactivation and increased ventilator-free days, but had no impact on systemic inflammation as measured by interleukin-6 levels [19].
In pediatric sepsis, there are limited data on the incidence and clinical significance of viral reactivation. One study reported a combined rate of CMV, EBV, herpes simplex virus (HSV), HHV-6, and adenovirus DNA detection in 38% of children and showed that viral DNAemia was associated with pre-existing immune suppression, lower innate immune function, and secondary infections [20]. However, the study relied on secondary analysis of residual plasma samples from an unrelated parent study and a lack of serologic data precluded differentiation of primary viral infection from latent viral reactivation.
Additional understanding of the incidence and significance of viral reactivation in pediatric sepsis is needed. Certain viruses, such as CMV, are amenable to targeted antiviral therapies if proven to contribute to the pathobiology of sepsis, while detection of viremia could provide a pragmatic biomarker to identify children who may benefit from immunomodulatory therapies [7, 21-28]. We therefore sought to determine the incidence of viremia and its association with clinical outcomes and immune paralysis phenotype in children with severe sepsis. Given the emphasis on CMV reactivation in adult sepsis and its potential as a therapeutic target, we focused primarily on CMV and secondarily on EBV and HHV-6.
MATERIALS AND METHODS
Study Design and Population
We conducted a prospective observational study of children admitted to the pediatric intensive care unit (PICU) at the Children’s Hospital of Philadelphia from September 30, 2016 to March 22, 2018. All consecutive admissions during the study period were screened for eligibility. Patients were eligible for inclusion if they were aged 2-17 years and treated for severe sepsis based on published consensus definitions [29]. Severe sepsis was defined as 1) presence of ≥2 systemic inflammatory response syndrome criteria, 2) suspected or documented invasive infection, and 3) cardiovascular organ dysfunction or ≥2 non-cardiovascular organ dysfunctions. Severe sepsis definition also included septic shock, defined as severe sepsis with cardiovascular dysfunction. Study day 0 was defined as day of severe sepsis recognition. Patients were excluded if they had received viral prophylaxis or treatment within seven days of severe sepsis recognition or if they were previously enrolled.
This study was approved by the Institutional Review Board at the Children’s Hospital of Philadelphia. Written informed consent was obtained from all parents/guardians with a waiver of patient assent due to level of illness severity.
Study Procedures
One mL of blood was collected on study day 2 (with acceptable window of study day 2-3) for CMV serology. An additional one mL of blood was collected on study days 2 (window 2-3), 6 (window 5-7), 10 (window 10-14), and 22 (window 22-28) for whole blood CMV real-time polymerase chain reaction (PCR) assay, with early cessation if the patient died or was discharged from the hospital. CMV PCR was performed for all blood and tracheal aspirate samples, while EBV and HHV-6 PCR measurements were limited to the last available blood specimen due to funding limitations. Given prior adult studies demonstrating pulmonary CMV reactivation and its association with increased morbidity, tracheal aspirates were also collected if patients had an artificial airway (endotracheal or tracheostomy tube) in place at time of blood collection [30-32]. After collection, blood and tracheal samples were immediately transferred to the hospital’s Infectious Disease Diagnostics Laboratory (IDDL), a Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory. All processing and testing were performed by the IDDL.
Serology samples were centrifuged and sera were isolated for measurement of CMV immunoglobulin (Ig)-G. CMV IgG was measured with the Capitia™ CMV IgG Enzyme-Linked Immunosorbent Assay kit (Trinity Biotech) as per package insert with a negative control, a calibrator, and a positive control.
Blood and tracheal aspirate samples for PCR assay were processed and stored at −80°C for batched analyses. Quantitative PCR was performed for blood samples and qualitative PCR was performed for tracheal aspirate samples per the IDDL’s clinical testing protocols. Viral DNA was detected using laboratory-developed real-time PCR assays validated for clinical testing. Total nucleic acid was extracted from 200 μL of whole blood or tracheal aspirates using MagNA Pure LC and Total Nucleic Acid Isolation kit (Roche Diagnostics) with 66 μL elution volume. Real-time PCR for CMV, EBV, and HHV-6 was performed on 10 μL of extracted product using Quanta PerfeCTa qPCR master mix (Qiagen). Primer and probe sequences have been previously published [33-35]. Amplification and detection was performed on a QuantStudio DX (ThermoFisher Scientific) in conjunction with positive and negative controls. Quantitative PCR assays were performed (limit of detection of 800 IU/mL for CMV, 125 copies/mL for HHV-6, and 630 IU/mL for EBV). Binary results of detected or not detected were reported for qualitative PCR assays performed on tracheal aspirate samples.
Immune function assessments were obtained for exploratory analysis of a subgroup of study patients separately consented for a companion study. An additional one mL of blood was collected on study days 1 (window 1-2), 3 (window 3-5), and 8 (window 8-14) for measurement of monocyte human leukocyte antigen (HLA)-DR expression and ex vivo lipopolysaccharide (LPS)-stimulated whole blood tumor necrosis factor (TNF)-α production. The percentage of HLA-DR positive cells among the CD-14 positive monocyte population was determined using flow cytometry, as previously described [36]. Ex vivo LPS-stimulated whole blood TNF-α was measured by mixing 50 μL heparinized whole blood with 500 μL (250 pg) of phenol-extracted LPS from Salmonella enterica abortus equi (Sigma-Aldrich, L5886) within 60 minutes of blood collection. After centrifugation, the supernatant was stored at −80°C for batched analysis and TNF-α measured using a commercially-available ELISA kit (Invitrogen KHC3011C).
Clinical data from the electronic medical record (EMR) were collected onto a dedicated case report form using the Research Electronic Data Capture (REDCap). Variables included patient demographics, comorbid conditions, laboratory data, source of primary infection, specific therapies (corticosteroids, immunomodulatory agents, blood products), length of PICU and hospital stay, and vital status at PICU and hospital discharge. Severity of illness was determined using the Pediatric Index of Mortality (PIM)-2 and Pediatric Risk of Mortality (PRISM) III scores ascertained from the institution’s Virtual PICU Systems database [37, 38].
Outcomes
The primary outcome was the cumulative incidence of CMV DNA detection, defined as the proportion of patients with positive qualitative or quantitative PCR assay from blood or tracheal aspirate on at least one time point divided by total number of patients at risk. Given the lack of data in pediatric sepsis, we did not pre-specify a minimal clinically significant viral load to define viral positivity in our primary outcome. To differentiate potential primary infection from latent viral reactivation, we also determined the cumulative incidence of CMV DNA detection in patients stratified by CMV IgG seropositivity. Missing blood samples were presumed negative for CMV if either a subsequent sample from that patient was negative or if the patient clinically improved to the point of hospital discharge. Secondarily, the proportion of EBV and HHV-6 viremia was defined as the number of patients with a positive quantitative blood PCR assay divided by total number of patients with residual samples available for testing.
The main clinical outcome was complicated course, a composite endpoint defined as ≥2 organ system dysfunction at seven days or death within 28 days from severe sepsis recognition. Additional clinical outcomes included 28-day mortality, hospital and PICU length of stay, and health care-associated infections (HAI). HAIs were defined according to CDC criteria and included bacterial, viral, or fungal infections occurring after 48 hours of hospitalization [39].
Immune paralysis was primarily defined as lymphopenia with absolute lymphocyte count (ALC) <1,000 cells/uL obtained from clinical complete blood counts at any time point within 7 days of sepsis recognition. In a separate exploratory analysis, the definition of immune paralysis was expanded to include whole blood ex vivo LPS-induced TNF-α <200 pg/mL or monocyte HLA-DR expression <30% at any time point within 8 days of sepsis recognition [7, 8, 36, 40, 41].
Statistical Analysis
Statistical analyses were performed using Stata software version 14.2 (StataCorp, College Station, TX). Descriptive data are presented as medians with interquartile range (IQR) for continuous variables and frequencies with percentages for categorical variables. Patient characteristics and clinical outcomes were compared using Wilcoxon rank sum and Fisher’s exact tests as appropriate. CMV and viremia incidences are presented as proportions with 95% confidence intervals determined using exact estimates. In addition, the cumulative incidence of CMV, accounting for a change in the number at risk at each time point is presented as a proportion with 95% confidence intervals.
Multivariable logistic regression analysis was used to compare the independent association of viremia with immune paralysis and complicated course. We a priori identified the following covariates as potential confounders based on biological plausibility, available data, and past studies: age, sex, number of comorbid conditions, race, PRISM III score, cancer, active chemotherapy with white blood cell count (WBC) <1,000 cells/μL, and blood product transfusion within seven days of sepsis recognition. Covariates were individually tested in separate bivariable models that included exposure, outcome, and the covariate. Covariates that changed the base model OR by 10% or greater were considered to be true confounders and included in the multivariable models [42]. Although PRISM III did not meet our pre-specified criteria as a confounder, it was forced into final models given its strong association with outcomes in critically ill children [38, 43].
Based on adult studies of CMV reactivation available at the time of study inception, we hypothesized that 30% of pediatric sepsis patients would have CMV DNA detection [9, 10, 12-14, 44]. However, because we expected a lower rate of CMV seropositivity in pediatric compared to adult patients—and therefore a reduced potential for viral reactivation—we considered a minimal clinically significant CMV detection rate to be 15% [45-47]. In order to achieve an effect estimate with a lower boundary of the 95% confidence interval of at least 15%, we originally calculated a sample size of 50 patients. After interim analysis demonstrated lower than anticipated cumulative incidence of CMV DNA detection, we increased our sample size to 60 evaluable patients to provide 81% power to determine that an observed incidence of 5% was indeed significantly lower than our previously hypothesized incidence of 15% using a one-sided Fisher’s exact test. To ensure balanced enrollment of younger versus older patients (with expected lower versus higher CMV seropositivity rate, respectively) and patients with versus without pre-existing immune suppression, enrollment of patients 2-5 years old and patients receiving chemotherapy who had WBC <1,000 cells/μL were limited to 15 for each group. To test the potential impact of a pre-existing immune suppressed state on outcomes, we performed sensitivity analysis which excluded patients receiving chemotherapy who had WBC <1,000 cells/μL.
RESULTS
Sixty-five patients were enrolled. After six patients were excluded (five due to inability to collect study day 2 samples and one patient who subsequently was noted to have met exclusion criteria), 59 patients were available for the final analysis (Supplemental Figure 1). Blood samples were available for all 59 patients and tracheal aspirates were available for 32 patients (Supplemental Figure 2). Thirteen patients were aged 2-5 years and nine were receiving chemotherapy and had WBC <1,000 cells/μL. Patient characteristics are shown in Table 1. The median age was 11 (IQR 6-13.5) years, 56% were male, and 63% had ≥3 comorbid conditions. The median PIM2 risk of mortality and PRISM III score were 3.7% (IQR 1.3-48%) and 11 (IQR 8-16), respectively. Sixty-six percent of patients were treated with corticosteroids, 12% with immunomodulatory therapies including monoclonal antibodies, granulocyte-monocyte colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (G-CSF), and 47% with blood product transfusions within 7 days of sepsis recognition. Overall 28-day mortality was 5%.
Table 1.
Patient characteristics
| Variablea | Value |
|---|---|
| N | 59 |
| Age, years | 11 (6-13.5) |
| 2-5 years, n (%) | 13 (22) |
| 6-17 years, n (%) | 46 (78) |
| Male sex, n (%) | 33 (56) |
| Race, n (%) | |
| White | 32 (54) |
| Black | 6 (10) |
| Other | 21 (36) |
| PIM2 risk of mortality | 3.7 (1.3-4.8) |
| PRISM III score | 11 (8-16) |
| Source of infection, n (%) | |
| Primary bacteremia | 6 (10) |
| Respiratory | 27 (46) |
| Abdominal | 7 (12) |
| Genitourinary | 6 (10) |
| Other | 13 (22) |
| Bacterial or fungal pathogen | 30 (51) |
| ≥ 3 comorbid conditions, n (%) | 37 (63) |
| Cancer, n (%) | 12 (20) |
| Active chemotherapy and leukopenia, n (%) | 9 (15) |
| Lymphopenia on study day 0-2, n (%) | 32 (54) |
| Corticosteroidsb, n (%) | 39 (66) |
| Hydrocortisone | 25 (42) |
| Dexamethasone | 7 (12) |
| Methylprednisolone | 11 (19) |
| Immunomodulator therapyb,c, n (%) | 7 (12) |
| Immunoglobulinb, n (%) | 4 (7) |
| Blood product transfusionb, n (%) | 28 (47) |
| PRBC | 24 (41) |
| Platelet | 15 (25) |
| FFP | 11 (19) |
| Cryoprecipitate | 2 (3) |
| 28-day mortality, n (%) | 3 (5) |
PIM2, pediatric index of mortality-2; PRISM, pediatric risk of mortality; PRBC, packed red blood cell; FFP, fresh frozen plasma
Medians with interquartile range reported unless otherwise specified
Therapies administered at any point during study day 0-7
canakinumab, anakinra, tocilizumab, granulocyte-colony stimulating factor, granulocyte-macrophage colony-stimulating factor
Three patients tested positive for CMV, all in blood samples and at low levels of viremia (detected but below level of quantification). CMV was detected on study day 6, 10, and 22 for these three patients. The clinical characteristics of the three patients with CMV viremia are shown in Table 2. All tracheal aspirate CMV PCR tests were negative.
Table 2.
Characteristics for patients with CMV viremia
| Variable | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age, years | 2.1 | 9.9 | 11.1 |
| Sex | Female | Female | Male |
| Race | Other | Other | Other |
| PIM2 risk of mortality | 8.2 | 5.5 | 0.8 |
| PRISM III score | 15 | 9 | 3.0 |
| Number of comorbid conditions | >5 | 2 | 1 |
| Cancer | No | AML | No |
| Active chemotherapy and leukopenia | No | Yes | No |
| Corticosteroids | Dexamethasone | No | Methylprednisolone |
| Immunomodulator therapy | No | GM-CSF | No |
| Immunoglobulin therapy | No | Yes | No |
| Blood product transfusion | PRBC | PRBC, Platelet, FFP | PRBC, Platelet |
| 28-day mortality | No | No | No |
PIM2, pediatric index of mortality-2; PRISM, pediatric risk of mortality; AML, acute myeloid leukemia; GM-CSF, granulocyte-macrophage colony-stimulating factor; PRBC, packed red blood cell; FFP, fresh frozen plasma
The total cumulative incidence of CMV DNA detection was 5.1% (95% CI 1.1,14.1%) over the three week study period following sepsis recognition, which is lower than our a priori hypothesized minimal clinically significant CMV detection rate of 15% (p=0.06). All three patients with CMV viremia were CMV IgG seropositive, making the cumulative incidence of CMV viremia 13.0% (95% CI 2.8, 3.4%) in the subset of 23 CMV seropositive patients. The cumulative incidence function of CMV DNA detection in all patients and in the CMV IgG seropositive subset at each of the four study days is shown in Figure 1.
Figure 1: Cumulative incidence function of CMV DNA detection.
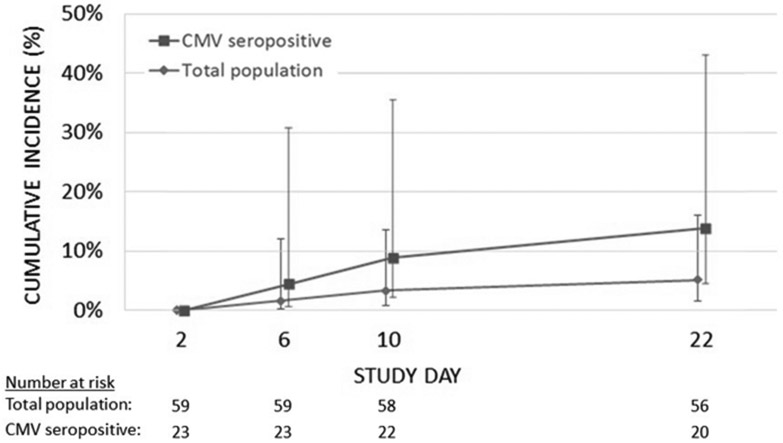
The cumulative incidence of CMV DNA detection at each study time point is shown for all patients (n=59, diamonds) and the subset with CMV IgG sero-positivity (n=23, squares). Error bars indicate the 95% confidence interval.
Fifty-four patients had residual samples from study day 2 (20%), study day 6 (24%), study day 10 (26%), or study day 22 (30%) available for EBV and HHV-6 quantification. EBV was detected in two (frequency 4%, 95% CI 1, 9%) and HHV-6 in 16 (frequency 30%, 95% CI 18, 42%) patients. One patient had both CMV and HHV-6 detected. Viral DNA was detected at low levels in both EBV patients and 11 of the sixteen HHV-6 patients. Overall, 20 patients (34%, 95% CI 24, 50%) had detectable viremia of either CMV, EBV, or HHV-6, including 22% of patients with and 36% of patients without cancer on chemotherapy with WBC <1,000 cells/μL.
The detection of viremia (with any virus) was not associated with complicated course or other clinical outcomes in univariable analyses (Table 3). Of the patients with viremia, only one had complicated course; this patient was CMV positive (Supplemental Table 1). None of the patients with viremia died. The three deaths occurred at 19, 28, and 28 days, with 2, 3, and 4 samples available for viral DNA detection in each patient, respectively, prior to death. In multivariable analysis, viremia was associated with a lower odds of complicated course after controlling for age, PRISM III score, and blood transfusion within 7 days of sepsis recognition (aOR 0.08, 95% CI 0.01,0.84, p=0.04).
Table 3.
Clinical outcomes in patients with and without viremia
| Outcomea | Viremia (n=20) |
No Viremia (n=39) |
p |
|---|---|---|---|
| Complicated course | 1 (5) | 10 (26) | 0.08 |
| 28 day mortality | 0 | 3 (8) | 0.54 |
| Immune paralysis | 12 (60) | 30 (77) | 0.23 |
| Hospital acquired infection | 2 (10) | 6 (15) | 0.70 |
| PICU LOSb | 11 (7.5-14.5) | 10 (5-18) | 0.55 |
| survivors only | 11 (7.5-14.5) | 10 (5-17) | 0.48 |
| Hospital LOSb | 20 (11-29) | 19 (11-30) | 0.96 |
| survivors only | 20 (11-29) | 18 (11-32) | 0.97 |
LOS, length of stay in days
Frequencies with percentages in parentheses reported unless otherwise specified
Medians with interquartile ranges in parentheses reported
ALC was available for all patients. Eleven (55%) patients with viremia had immune paralysis, as defined by ALC <1,000 cells/μL, compared to 28 (72%) patients without viremia (p=0.25). Viremia was not associated with immune paralysis in multivariable analysis after controlling for age, PRISM III score, and platelet transfusion within 7 days of sepsis recognition (aOR 0.68, 95% CI 0.2,2.35, p=0.54).
LPS-stimulated TNF-α production test results were available for 33 (56%) patients and HLA-DR expression results were available for 14 (24%) patients for an exploratory analysis of immune paralysis using expanded criteria. Twelve (60%) patients with versus 30 (77%) patients without viremia had immune paralysis as defined by ALC <1,000 cells/μL, ex vivo LPS-induced TNF-α <200 pg/mL, or monocyte HLA-DR expression <30% (p=0.23). There were no significant differences in ALC, LPS-stimulated TNF-α, or HLA-DR expression between patients with versus without viremia, though LPS-stimulated TNF-α and HLA-DR expression trended lower in patients with virema (Figure 2).
Figure 2: Association between markers of immune paralysis and viral reactivation.
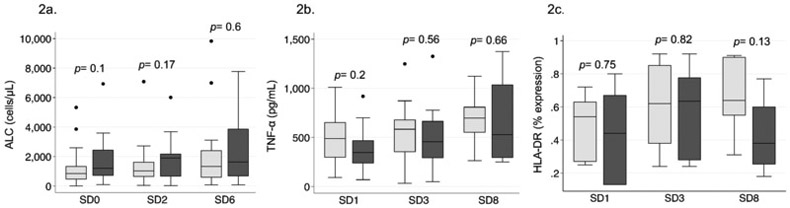
2a. Box plots comparing median absolute lymphocyte count (ALC) with IQR at study days 0, 2-3, and 5-7 between patients with (black boxes) and without (gray boxes) reactivation viremia (including CMV, EBV, and HHV-6).
2b and 2c. Box plots comparing median ex vivo lipopolysaccharide (LPS)-stimulated whole blood tumor necrosis factor (TNF)-α production with IQR and median monocyte human leukocyte antigen (HLA)-DR expression with IQR, respectively, at study days 1, 3, and 8 between patients with (black boxes) and without (gray boxes) reactivation viremia (including CMV, EBV, and HHV-6).
In sensitivity analysis excluding 9 patients receiving chemotherapy who had WBC <1,000 cells/μL, 18 (36%) patients had viremia and 32 (64%) did not. No patient with viremia versus 8 patients without viremia had complicated course (p=0.04).
DISCUSSION
In this prospective study we found a low incidence of CMV, EBV, and HHV-6 viremia in children with severe sepsis. Viremia was not associated with worse clinical outcomes or immune paralysis phenotype in univariable analysis, but was associated with lower odds of complicated course in multivariable analysis. However, these findings must be interpreted with caution given the overall low viremia incidence, especially with CMV. Our findings highlight the challenge of testing whether viral DNA may serve as a pathogen that contributes to worse clinical outcomes or can be used as a biomarker to identify high-risk patients in pediatric severe sepsis.
We demonstrated a cumulative CMV incidence of 5.1% in our total population, similar to the 5% CMV incidence reported by Davila et al in a pediatric sepsis cohort, and 13% in our subset of CMV seropositive patients [20]. The detection of CMV viremia among CMV seropositive patients suggests reactivation, rather than primary infection, as a possible mechanism, but this remains speculative given the few cases of CMV viremia in this study. One possible explanation for the lower incidence in pediatric sepsis is that children have lower rates of primary CMV exposure and are therefore at lower risk for CMV reactivation [45-47].
Proportions of patients with EBV and HHV-6 in this cohort were similar to the prior pediatric study by Davila et al, which observed HHV-6 to be more common than CMV viremia. The difference in incidence rates of individual viruses reflects the epidemiology of viral infections in pediatric populations. While children may have lower rates of CMV and EBV exposure when compared to adults, their exposure to HHV-6 may be higher. As such, future studies on viremia and immune paralysis in pediatric sepsis should include evaluation of common viruses present in children rather than a sole focus on CMV as in the majority of adult studies.
We tested the association of overall viremia, as opposed to each virus, with clinical outcomes given low incidence rates of individual viruses. We did not identify an association of overall viremia with clinical outcomes in univariable analyses, and unexpectedly found a protective association with complicated course in multivariable analyses. These findings are in contrast to adult studies demonstrating association of viremia with increased hospital and ICU length of stay, ventilator days, and 28-day mortality [9-11, 13, 14, 44]. The difference in findings may be secondary to the low viral loads detected in our study, as adult studies have shown stronger association of clinical outcomes with increasing viral load, or to the small sample size in this study [13]. Our findings also differ from the report by Davila et al that showed an association between viral DNAemia and HAIs in pediatric sepsis [20]. Davila et al reported an HAI rate of 42%, while our most recent study had an HAI rate of only 14%. This difference may reflect an increasing emphasis in quality improvement initiatives focused on reducing HAIs over time.
Viremia was not associated with immune paralysis in our study in univariable or multivariable analyses using a definition of immune paralysis that included lymphopenia only or in univariable analysis using an expanded definition including lymphopenia, TNF-α production, and HLA-DR expression. This is in contrast to prior studies that have observed a link between viral reactivation and sepsis-induced immune paralysis [17, 48]. For example, Davila et al demonstrated that immune function was significantly lower in patients with viral DNAemia when TNF-α production was analyzed as a continuous variable, though very few patients approached their threshold for immune paralysis regardless of DNAemia status [20]. We did, however, observe a similar trend of lower LPS-stimulated TNF-α production in the patients with viremia. Our analysis of the association between viremia and immune paralysis, especially our analysis including TNF-α production and HLA-DR expression, was exploratory and limited by small numbers. Additionally, our analysis tested for association between overall viremia and immune paralysis, limiting our ability to evaluate the causal pathway for individual viruses. Future studies are needed to investigate the potential causal association between sepsis-induced immune paralysis and primary viral infection and/or viral reactivation in children.
The test the incidence of viremia associated with sepsis, we limited enrollment of patients receiving chemotherapy who had WBC <1,000 cells/μL whose pre-existing immune suppressed state may increase risk of viremia and poor clinical outcomes independent of sepsis. In sensitivity analysis that excluded 9 patients receiving chemotherapy who had WBC <1,000 cell/μL, viremia had a protective association with complicated course. While it is theoretically possible that low-level viremia could bolster the host response to sepsis by providing a secondary immunological stimulus, we cannot exclude that our statistical finding is due to the small sample size in this study.
There are several limitations to this study. First, we determined the sample size using reported CMV reactivation rates in adult studies given lack of pediatric data at time of study design. The lower than anticipated CMV incidence in pediatric sepsis limited our analysis of CMV with clinical outcomes. Future studies should consider including only seropositive children to enrich the study population. Additionally, the study cohort was heterogeneous with the majority of patients having 3 or more comorbid conditions. Future larger studies are needed to test reproducibility and generalizability of our findings. Third, we had fewer tracheal aspirates than blood samples given many of our patients did not have an artificial airway at time of blood collection. It is possible that we missed detection of pulmonary CMV in our cohort due to low rate of tracheal aspirate sampling and testing. Fourth, we were only able to perform EBV and HHV-6 PCR assays on a single sample per patient due to funding limitations. Serial testing and inclusion of other viruses may have increased the yield of viremia in our cohort. Additionally, many of the study patients received blood product transfusions which may carry a risk of viral transmission. We were not able to test blood products for viral DNA and therefore could not exclude transfusions as a possible etiology of viremia. Lastly, while PCR is a widely accepted test for viremia in clinical use, it cannot differentiate between DNA detected from live or killed viruses.
CONCLUSION
Children with severe sepsis had low rates of detectable CMV, EBV and HHV-6. Although the low incidence limited statistical power, we did not find evidence that viremia was useful as a biomarker of adverse clinical outcomes. Low incidence rates of viremia must be considered in future studies evaluating utility of viremia as a biomarker or modifiable risk factor in pediatric severe sepsis.
Supplementary Material
Supplemental Figure 1: Flow diagram showing screening and enrollment of subjects
Supplemental Figure 2: Proportion of patients with collected blood and tracheal aspirate samples by study day
Acknowledgments
Financial Disclosure and Conflicts of Interest:
This study was performed at the Children’s Hospital of Philadelphia and funded by the Foerderer Grant from the Children’s Hospital of Philadelphia Research Institute and NIGMS K23GM110496 (SLW).
Footnotes
For the remaining authors none were declared.
Copyright form disclosure: Dr. Han’s institution received funding from Foerderer Grant from the Children’s Hospital of Philadelphia Research Institute. Dr. Weiss’s institution received funding from the National Institute of General Medical Sciences, and he received support for article research from the National Institutes of Health. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Hartman ME, Linde-Zwirble WT, Angus DC, et al. : Trends in the epidemiology of pediatric severe sepsis*. Pediatr Crit Care Med 2013, 14(7):686–693. [DOI] [PubMed] [Google Scholar]
- 2.Weiss SL, Fitzgerald JC, Pappachan J, et al. : Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med 2015, 191(10):1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boomer JS, To K, Chang KC,et al. : Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011, 306(23):2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Monneret G, Payen D: Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013, 13(12):862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang H, Xu R, Lin F, et al. : High circulating CD39(+) regulatory T cells predict poor survival for sepsis patients. Int J Infect Dis 2015, 30:57–63. [DOI] [PubMed] [Google Scholar]
- 6.Felmet KA, Hall MW, Clark RS, et al. : Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol 2005, 174(6):3765–3772. [DOI] [PubMed] [Google Scholar]
- 7.Hall MW, Knatz NL, Vetterly C, et al. : Immunoparalysis and nosocomial infection in children with multiple organ dysfunction syndrome. Intensive Care Med 2011, 37(3):525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muszynski JA, Nofziger R, Greathouse K, et al. : Early adaptive immune suppression in children with septic shock: a prospective observational study. Crit Care 2014, 18(4):R145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heininger A, Haeberle H, Fischer I, et al. : Cytomegalovirus reactivation and associated outcome of critically ill patients with severe sepsis. Crit Care 2011, 15(2):R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heininger A, Jahn G, Engel C, et al. : Human cytomegalovirus infections in nonimmunosuppressed critically ill patients. Crit Care Med 2001, 29(3):541–547. [DOI] [PubMed] [Google Scholar]
- 11.Jaber S, Chanques G, Borry J, et al. : Cytomegalovirus infection in critically ill patients: associated factors and consequences. Chest 2005, 127(1):233–241. [DOI] [PubMed] [Google Scholar]
- 12.Kutza AS, Muhl E, Hackstein H, et al. : High incidence of active cytomegalovirus infection among septic patients. Clin Infect Dis 1998, 26(5):1076–1082. [DOI] [PubMed] [Google Scholar]
- 13.Limaye AP, Kirby KA, Rubenfeld GD, et al. : Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 2008, 300(4):413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Muller L, Klemm A, Weiss M, et al. : Active cytomegalovirus infection in patients with septic shock. Emerg Infect Dis 2006, 12(10):1517–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libert N, Bigaillon C, Chargari C, et al. : Epstein-Barr virus reactivation in critically ill immunocompetent patients. Biomed J 2015, 38(1):70–76. [DOI] [PubMed] [Google Scholar]
- 16.Ong DSY, Bonten MJM, Spitoni C, et al. : Epidemiology of Multiple Herpes Viremia in Previously Immunocompetent Patients With Septic Shock. Clin Infect Dis 2017, 64(9):1204–1210. [DOI] [PubMed] [Google Scholar]
- 17.Walton AH, Muenzer JT, Rasche D, et al. : Reactivation of multiple viruses in patients with sepsis. PLoS One 2014, 9(2):e98819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansfield S, Griessl M, Gutknecht M, et al. : Sepsis and cytomegalovirus: foes or conspirators? Med Microbiol Immunol 2015, 204(3):431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Limaye AP, Stapleton RD, Peng L, et al. : Effect of Ganciclovir on IL-6 Levels Among Cytomegalovirus-Seropositive Adults With Critical Illness: A Randomized Clinical Trial. JAMA 2017, 318(8):731–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davila S, Halstead ES, Hall MW, et al. , Eunice Kennedy Shriver Collaborative Pediatric Critical Care Research Network I: Viral DNAemia and Immune Suppression in Pediatric Sepsis. Pediatr Crit Care Med 2018, 19(1):e14–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meisel C, Schefold JC, Pschowski R, et al. : Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med 2009, 180(7):640–648. [DOI] [PubMed] [Google Scholar]
- 22.Unsinger J, Burnham CA, McDonough J, et al. : Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J Infect Dis 2012, 206(4):606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Unsinger J, McGlynn M, Kasten KR, et al. : IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol 2010, 184(7):3768–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellegrini M, Calzascia T, Toe JG, et al. : IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 2011, 144(4):601–613. [DOI] [PubMed] [Google Scholar]
- 25.Venet F, Foray AP, Villars-Mechin A, et al. : IL-7 restores lymphocyte functions in septic patients. J Immunol 2012, 189(10):5073–5081. [DOI] [PubMed] [Google Scholar]
- 26.Chang KC, Burnham CA, Compton SM, et al. : Blockade of the negative co-stimulatory molecules PD-1 and CTLA-4 improves survival in primary and secondary fungal sepsis. Crit Care 2013, 17(3):R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang X, Venet F, Wang YL, et al. : PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A 2009, 106(15):6303–6308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brahmamdam P, Inoue S, Unsinger J, et al. : Delayed administration of anti-PD-1 antibody reverses immune dysfunction and improves survival during sepsis. J Leukoc Biol 2010, 88(2):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein B, Giroir B, Randolph A, International Consensus Conference on Pediatric S: International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005, 6(1):2–8. [DOI] [PubMed] [Google Scholar]
- 30.Chiche L, Forel JM, Roch A, et al. : Active cytomegalovirus infection is common in mechanically ventilated medical intensive care unit patients. Crit Care Med 2009, 37(6):1850–1857. [DOI] [PubMed] [Google Scholar]
- 31.Coisel Y, Bousbia S, Forel JM, et al. : Cytomegalovirus and herpes simplex virus effect on the prognosis of mechanically ventilated patients suspected to have ventilator-associated pneumonia. PLoS One 2012, 7(12):e51340. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Papazian L, Fraisse A, Garbe L, et al. : Cytomegalovirus. An unexpected cause of ventilator-associated pneumonia. Anesthesiology 1996, 84(2):280–287. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez JL, Storch GA: Multiplex, quantitative, real-time PCR assay for cytomegalovirus and human DNA. J Clin Microbiol 2002, 40(7):2381–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niesters HG, van Esser J, Fries E, et al. : Development of a real-time quantitative assay for detection of Epstein-Barr virus. J Clin Microbiol 2000, 38(2):712–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gautheret-Dejean A, Manichanh C, Thien-Ah-Koon F, et al. : Development of a real-time polymerase chain reaction assay for the diagnosis of human herpesvirus-6 infection and application to bone marrow transplant patients. J Virol Methods 2002, 100(1-2):27–35. [DOI] [PubMed] [Google Scholar]
- 36.Monneret G, Lepape A, Voirin N, et al. : Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med 2006, 32(8):1175–1183. [DOI] [PubMed] [Google Scholar]
- 37.Slater A, Shann F, Pearson G, Paediatric Index of Mortality Study G: PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med 2003, 29(2):278–285. [DOI] [PubMed] [Google Scholar]
- 38.Pollack MM, Patel KM, Ruttimann UE: PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med 1996, 24(5):743–752. [DOI] [PubMed] [Google Scholar]
- 39.Horan TC, Andrus M, Dudeck MA: CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008, 36(5):309–332. [DOI] [PubMed] [Google Scholar]
- 40.Drewry AM, Samra N, Skrupky LP, et al. : Persistent lymphopenia after diagnosis of sepsis predicts mortality. Shock 2014, 42(5):383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ertel W, Kremer JP, Kenney J, et al. : Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood 1995, 85(5):1341–1347. [PubMed] [Google Scholar]
- 42.Maldonado G, Greenland S: Simulation study of confounder-selection strategies. Am J Epidemiol 1993, 138(11):923–936. [DOI] [PubMed] [Google Scholar]
- 43.Hamshary A, Sherbini SAE, Elgebaly HF, et al. : Prevalence of multiple organ dysfunction in the pediatric intensive care unit: Pediatric Risk of Mortality III versus Pediatric Logistic Organ Dysfunction scores for mortality prediction. Rev Bras Ter Intensiva 2017, 29(2):206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Domart Y, Trouillet JL, Fagon JY, et al. : Incidence and morbidity of cytomegaloviral infection in patients with mediastinitis following cardiac surgery. Chest 1990, 97(1):18–22. [DOI] [PubMed] [Google Scholar]
- 45.Bate SL, Dollard SC, Cannon MJ: Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988-2004. Clin Infect Dis 2010, 50(11):1439–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cannon MJ, Schmid DS, Hyde TB: Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 2010, 20(4):202–213. [DOI] [PubMed] [Google Scholar]
- 47.Staras SA, Dollard SC, Radford KW, et al. : Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis 2006, 43(9):1143–1151. [DOI] [PubMed] [Google Scholar]
- 48.Hotchkiss RS, Coopersmith CM, McDunn JE, et al. : The sepsis seesaw: tilting toward immunosuppression. Nat Med 2009, 15(5):496–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Flow diagram showing screening and enrollment of subjects
Supplemental Figure 2: Proportion of patients with collected blood and tracheal aspirate samples by study day


