Abstract
Background:
Disclosing HIV status to HIV-positive children is a major challenge facing families and healthcare providers. Despite recommendations for disclosure, rates remain low. We tested whether a pediatric HIV disclosure intervention delivered as an integral component of routine HIV healthcare in Ghana would improve disclosure to children.
Methods:
Dyads of HIV-infected children aged 7 to 18 years and their caregivers were enrolled from two HIV clinics in Accra and Kumasi, Ghana. The sites were randomly assigned to one of the two intervention arms to avoid treatment contamination between intervention and control participants. Trained interventionist employed theory-guided therapeutic communication and personalized interaction to promote disclosure. Disclosure outcomes were measured at 12-week intervals. All analyses were completed using a modified intention-to-treat approach.
Results:
We enrolled 446 child-caregiver dyads (N=240 intervention group; N=206 control group); 52% of the children were male, mean age 9.78 (±2.27) years. For disclosure at 1 year, a better overall treatment effect was observed (p<0.001). Children in the treatment group had greater disclosure at each time point (p<0.001) and a higher proportion of them had been disclosed to by 1 year (51.4% vs 16.2%; p<0.001; un-adjusted HR=3.98: 95% CI, 2.63, 6.03) and 3 years (71.3% vs 34.0%; unadjusted HR=4.21: 95% CI, 3.09, 5.72). In the multivariate Cox model, factors associated with disclosure were treatment group (p<0.001), children <11 years of age (p<0.001), HIV-infected caregivers (p=0.015), and caregiver’s with greater education (p=0.022).
Conclusions:
This practical clinic-based disclosure intervention shows excellent promise as a means of improving HIV pediatric disclosure outcomes.
Keywords: HIV, pediatric, disclosure, Ghana, site random assignment, trial, intervention
Introduction
With expanded antiretroviral therapy (ART) coverage in low- and middle-income countries (LMICs), HIV-infected children are living longer and surviving into adulthood.1,2 The World Health Organization (WHO) recommends that children as young as school-age (6-12 years of age) be told of their HIV status in a developmentally appropriate and culturally sensitive manner.3 Available evidence shows that timely and supportive disclosure may confer several benefits including better psychological adjustment, better adherence to therapy, better clinical outcomes, and lowered risk of transmitting HIV when the child becomes sexually active. 4-9 Yet, disclosing an HIV-positive status to a child continues to be a global challenge.10 Parents, caregivers and health care providers are often reluctant to disclose because of uncertainty about how to disclose, concern about the child’s ability to understand and cope with the illness, effect on family relations, shame and fears about stigma and social rejection..8,11-14 In high HIV prevalence settings, it is estimated that as many as 75% of children have not been informed that they have HIV.15
Despite WHO guidelines on disclosure and perceived benefits of disclosure, there is still ongoing debate on ‘the when’, ‘the how’, and ‘the what to inform’. Several interventions based on an education model (a theoretical framework, process-oriented disclosure, and iterative construct) have been deployed to improve the knowledge and skills of caregivers and healthcare workers on disclosure.16 Blasini et al. deployed a five-component intervention in Puerto Rico5. They used audiovisual aids such as an HIV cartoon book and other educational materials portraying HIV as a chronic illness for the educational and intervention sessions. Thailand has a national pediatric disclosure intervention protocol, a four-step counselling-based model to guide health care providers to assist caregivers in the process of disclosing HIV status to infected children (aged 7 years and older). Namibia established a multipronged national pediatric HIV disclosure intervention in 2010.17 Botswana has also adopted a flipbook developed by Baylor International Pediatric AIDS Initiative and Children’s Hospital of Philadelphia. 16 Recently, Vreeman et al. reported findings of an RCT that evaluated the effect of a patient-centered, culturally and age-appropriate disclosure counselling intervention on HIV disclosure rates among children living with HIV in Kenya.18 While the intervention group had greater disclosure than the control group initially, the rate was not maintained over time. Further, the prevalence of depression symptoms was significantly higher in the intervention than in the control group at 6 months.18
Based on our previous findings,12 as well as extant empiric and theoretic knowledge, we developed a clinic-based disclosure intervention that is delivered as an integral component of routine care. The intervention, ‘Sankofa’, is guided by an HIV pediatric disclosure model that incorporates bioecological systems theory 19 and core elements of the Information-Motivation-Behavioral Skills (IMB) model of Health Behavior Change. 13,20-22 The intervention was designed to facilitate the initiation as well as the process of disclosure over time.23
The purpose of this study was to test the efficacy of the Sankofa intervention in Ghana. The primary outcome was the proportion of caregiver disclosure of pediatric HIV at 1-year follow-up.
Methods
Study design and randomization procedures
We conducted a two-arm trial randomized by site.24 The two participating sites were randomly assigned by a coin flip to receive either the treatment (Sankofa intervention) or to continue their current disclosure practice (control group). Site randomization was used to avoid cross-contamination within the same clinic. While randomization by site has well known limitations, it was deemed an acceptable approach in this study because of a very high threat of within site diffusion of the disclosure intervention content and because of the similarity of the participating HIV clinics with respect to profile of the providers, type and quality of services provided, physical structure and size and demographics of the patient population served. 24
Study setting, participants and enrollment procedures
Caregiver-child dyads (n=446) were enrolled at two tertiary HIV clinics in Ghana; Korle-Bu Teaching Hospital (KBTH) (n=206) and Komfo Anokye Teaching Hospital (KATH) (n=240). HIV-infected children receiving care at the two clinics between the ages of 7 to 18 years started on ART within 12 months of study enrollment and who did not know their HIV diagnosis (based on caregiver account and medical records confirmation) and their primary caregivers were eligible to participate in the study. HIV-infected children less than 7 years, children with congenital or developmental disorders, children with comorbidities such as sickle cell disease or diabetes that require frequent clinic visits or hospitalizations, or children with AIDS-defining illness or end stage AIDS regardless of age and their primary caregivers were excluded. We attempted to enroll equal numbers of children aged 7–11 and ≥11 years.
The study protocol was reviewed and approved by the Institutional Review Boards of University of Ghana School of Medicine and Dentistry, Komfo Anokye Teaching Hospital, and Yale University. During regular routine clinic visits, the clinic providers assessed and recorded whether the child had been informed of his or her HIV diagnosis. When the child had not been informed of his or her diagnosis, the provider presented basic information about the Sankofa study to the caregiver/parent and inquired whether the caregiver/parent would like to learn more about it. If so, the designated site project staff explained the study and obtained written informed consent from the caregiver and assent from the child.
Disclosure Intervention
The intervention was designed to facilitate the initiation as well as the process of disclosure over time [fully described previously 23]. Briefly, the patient-centered intervention is delivered by a member of the clinical team who is familiar with the socio-cultural norms of the community and trained to address information, motivation and behavioral skills of caregivers in an individualized manner to facilitate their engagement in the process of disclosure (i.e., predisclosure, disclosure, and post-disclosure phases) in a manner suitable to the age and needs of the child.19-23,25-27
The manualized intervention contained three key elements to target well-documented, modifiable barriers to promote disclosure. First, it used a trained member of the clinical team to serve as the disclosure “point person”. The clinical team member, the interventionist, was familiar with the socio-cultural norms of the community and trained to assist families in the process of disclosure.23 Second, the interventionist used therapeutic communication to facilitate the caregiver’s engagement in the process of disclosure. The interventionist engaged in regular conversations with the caregiver during routine clinic visits to foster development of a trusting relationship and deeper expression of concerns and barriers to disclosure. The interventionist determined whether and when the caregiver was considering disclosing. If the caregiver was ready to disclose, the interventionist facilitated preparation for disclosure appropriate to the developmental age of the child.23 Third, a personalized, process-oriented approach was used throughout the phases of predisclosure, disclosure and post-disclosure. After disclosure, the interventionist continued to meet with the caregiver and child at frequently scheduled intervals to assess post-disclosure problems and coping, provide referral to services that the family may need, and to continue to help the child to understand in an age appropriate manner HIV infection and its implication on his/her day-to-day activities.
The interventionist received competency training until mastery of the manualized intervention protocol was demonstrated (see supplementary material). The interventionist maintained a log of each contact that detailed date, length of visit, whether full intervention protocol was completed, content of visit discussion, difficulties encountered and strategies used to manage them.
Usual Care (control group)
Patients and caregivers in the control arm received the routine care provided in the clinic. The physician provider in the clinic is responsible for assessing the caregiver’s readiness to disclose and give some information to the caregiver as deemed necessary. To provide some control for time/attention of the interventionist, a non-physician clinician who did not receive the disclosure training routinely met with the caregivers and provided general health information (e.g., medication adherence) and answered questions the caregivers might have.
Data Collection and Management
Data were collected at baseline and every 3 months. Data were collected by surveys administered by a trained staff member. The surveys were conducted in English, the official language in Ghana and the language of instruction at schools. To avoid inadvertent disclosure of a child’s status, children were interviewed in the presence of their caregivers while the caregivers were interviewed without their children present. Participants were surveyed using standardized questionnaires that included measures of household and caregiver/child demographic characteristics, caregiver social support (Social Provisions Questionnaire, SPS), 28 caregiver HIV knowledge (HIV-KQ-18), 29 caregiver illness perceptions (Illness Perception Questionnaire (brief IPQ), 30 caregiver perceived stigma (HIV Stigma Scale), 31 and caregiver and child depressive symptoms (Beck Depression Inventory (BDI). 32 Child disclosure status was measured per caregiver self-report and the child’s physician report.33
As previously reported,34 a web-based (http://ccehub.org) data capture system was used for the acquisition, storage, and exploration of data. Study participants were de-identified by auto-assigned alphanumeric identifiers. All electronic personal health information (ePHI) were encrypted for storage. The study investigators and care providers were blinded to outcome data.
Statistical Analyses
Demographic and other baseline data for the caregiver-child dyads, overall and by treatment arm, were calculated using means (standard deviation, SD) or median (interquartile range, IQR) for continuous variables and numbers and percentages for categorical variables. Comparisons between the two sites were carried out using t-test and chi-square techniques, as appropriate (Table 1). Survival analysis (Kaplan-Meier; time as continuous variable) was used to compare time to disclosure between the two interventions using log-rank chi-square test. The unadjusted cumulative incidence curves were plotted to compare the probability of disclosure between the two study arms; univariate and multivariate Cox regression models were used to generate adjusted HR estimates. Variables that were shown, through univariate analysis, to be associated with the outcome (disclosure) (p≤0.1) were then included in the final multivariable analysis. The following algorithm was used to assemble primary outcome data. If at a given protocol time point the primary outcome assessment was missing, the presence of primary outcome assessment at the subsequent or previous time point was evaluated. If the next available assessment indicated ‘No Disclosure’, the missing prior assessment was marked ‘No Disclosure’ as well. If the previous available assessment indicated “Disclosure”, the missing following assessment was marked “Disclosure”. If the previous assessment indicated “No Disclosure” and next available assessment indicated ‘Disclosure’, the missing prior assessment was imputed as follows: (a) if the Date of Disclosure at Follow-up Visit Time, T+1 was after the Date of Follow-up Visit Time Point (T), Disclosure at Time T was imputed as ‘No’; (b) if Date of Disclosure at Follow-up Visit Time T+1 was before the Date of Follow-up Visit Time Point (T), Disclosure at Time T was imputed as ‘Yes’. All analyses and reports were generated using SAS v9.3, SAS Inc.
Table 1.
Demographic and disease characteristics of children enrolled from January 2013 to June 2016
| SANKOFA Study Group | ||||
|---|---|---|---|---|
| Treatment (N = 240) |
Control (N = 206) |
Total (N = 446) |
P Value | |
| Gender | ||||
| Female | 124 (51.67%) | 92 (44.66%) | 216 (48.43%) | |
| Male | 116 (48.33%) | 114 (55.34%) | 230 (51.57%) | 0.14 |
| Child Age (years) | ||||
| Mean (SD) | 10.17 (2.55) | 9.34 (1.82) | 9.78 (2.27) | <0.001*** |
| Child Age (category, years) | ||||
| < 11 | 137 (57.08%) | 147 (71.36%) | 284 (63.68%) | |
| >= 11 | 92 (38.33%) | 59 (28.64%) | 151 (33.86%) | |
| Missing | 11 (4.58%) | 0 (0.00%) | 11 (2.47%) | 0.012* |
| In School | ||||
| Yes | 222 (92.50%) | 205 (99.51%) | 427 (95.74%) | |
| No | 5 (2.08%) | 1 (0.49%) | 6 (1.35%) | |
| Patient refused to answer | 1 (0.42%) | 0 (0.00%) | 1 (0.22%) | |
| Missing | 12 (5.00%) | 0 (0.00%) | 12 (2.69%) | 0.22 |
| HIV Transmission Mode | ||||
| MTCT | 189 (78.75%) | 183 (88.83%) | 372 (83.41%) | |
| Other | 7 (2.92%) | 15 (7.28%) | 22 (4.93%) | |
| Missing | 44 (18.33%) | 8 (3.88%) | 52 (11.66%) | 0.08 |
| Duration of HIV diagnosis (days) | ||||
| Mean (SD) | 1667.19 (1112.44) |
1701.33 (969.41) | 1684.59 (1040.80) |
0.74 |
| Duration of ART treatment (days) | ||||
| Mean (SD) | 1192.62 (1013.08) |
1395.81 (890.74) | 1295.77 (957.13) | 0.035* |
| WHO Staging at time of Diagnosis | ||||
| Stage 1 | 30 (12.50%) | 33 (16.02%) | 63 (14.13%) | |
| Stage 2 | 76 (31.67%) | 58 (28.16%) | 134 (30.04%) | |
| Stage 3 | 68 (28.33%) | 81 (39.32%) | 149 (33.41%) | |
| Stage 4 | 18 (7.50%) | 32 (15.53%) | 50 (11.21%) | |
| Missing | 48 (20.00%) | 2 (0.97%) | 50 (11.21%) | 0.06 |
| HIV+ caregiver | ||||
| Yes | 141 (58.75%) | 124 (60.19%) | 265 (59.42%) | |
| No or Unsure | 94 (39.17%) | 81 (39.32%) | 175 (39.24%) | |
| Missing | 5 (208%) | 1 (0.49%) | 6 (1.35%) | 0.92 |
Notes:
Data are mean (standard deviation, SD) or n/N (%), unless otherwise stated;
KATH, Komfo Anokye Teaching Hospital, Kumasi, Ghana; KBTH, Korle-Bu Teaching Hospital, Accra, Ghana; MTCT, Mother to Child Transmission;
P value less than 0.05
P value less than 0.01
P value less than 0.001
Power Considerations
Initially, the study was designed to enroll a total of 756 caregiver-child dyads. This sample size would afford 90% power (Type I error=0.05) to detect a hazard ratio of 0.77 (i.e. proportion of intervention group being disclosed to =30% and proportion being disclosed to in the control group= 21% 12). In January 2016, it was decided that the sample size target of 756 was not feasible within the planned timeframe because of the slower than expected rate of participant enrollment. This was the consequence of frequent stockouts of reagents for CD4 and viral load determination in the participating clinics (part of the inclusion criteria). Post hoc analyses: Conditional power estimates were carried out using the B-method. The B-method is a trial monitoring tool 35. It utilizes information fraction accumulated at the time of conditional power (CP) estimation, as well as proportion of success/failure, thus allowing for estimating CP under conservative estimates (i.e., worst case scenario of success/failure for those assessments that are missing/not made yet). During an assessment of target sample size achievement, CP estimation was carried out to determine how long, if needed, enrollment should continue. At the time this was carried out, under the worst-case scenario (missing assessments for control were set to ‘Disclosure’ and missing data for intervention were set to ‘No Disclosure’) a sample size of 440 would lead to a z-value was 5.4 and the conditional power was >99%. After verification that the CP was sufficient given the accrued sample size, it was determined that enrollment could stop.
Results
Characteristics of study participants
We screened 606 caregiver-child dyads from January 21, 2013 to June 30, 2016 and 446 met eligibility criteria. Of the 446 caregiver-child dyads, 240 were in the treatment group and 206 were in the control group. There were 15 participants lost to follow up, 11 in the intervention and 4 in the control group (supplementary Figure 1). There were 8 deaths reported in the intervention group and 6 in the usual care group. None of the deaths were attributed to the study intervention.
At baseline, there was no statistically significant difference among the groups of children with regard to gender, school attendance, duration of HIV diagnosis, WHO clinical staging of disease at time of diagnosis, and the HIV status of caregiver (Table 1). The children in the control group were younger (p<0.001) and had been on ART longer (p=0.04) than the children in the intervention group. There was no statistically significant difference in age, gender, marital status, and HIV status of caregivers (Table 2).
Table 2.
Demographic characteristics of caregivers enrolled from January 2013 to June 2016
| SANKOFA Study Group | ||||
|---|---|---|---|---|
| Treatment (N = 240) |
Control (N = 206) |
Total (N = 446) |
P Value | |
| Age caregiver (years) | ||||
| Mean (SD) | 42.81 (10.96) | 41.32 (9.68) | 42.12 (10.41) | 0.13 |
| Marital Status | ||||
| Divorced or separated | 32 (13.33%) | 25 (12.14%) | 57 (12.78%) | |
| Married | 127 (52.92%) | 123 (59.71%) | 250 (56.05%) | |
| Single | 32 (13.33%) | 30 (14.56%) | 62 (13.90%) | |
| Widowed | 49 (20.42%) | 28 (13.59%) | 77 (17.26%) | 0.25 |
| Divorced or separated | 32 (13.33%) | 25 (12.14%) | 57 (12.78%) | |
| Gender | ||||
| Female | 203 (84.58%) | 161 (78.16%) | 364 (81.61%) | |
| Male | 37 (15.42%) | 45 (21.84%) | 82 (18.39%) | 0.08 |
| Caregiver HIV status | ||||
| No or Unsure | 94 (39.17%) | 81 (39.32%) | 175 (39.24%) | |
| Yes | 141 (58.75%) | 124 (60.19%) | 265 (59.42%) | |
| Missing | 5 (208%) | 1 (0.49%) | 6 (1.35%) | 0.92 |
| Education | ||||
| No School | 39 (16.25%) | 33 (16.02%) | 72 (16.14%) | |
| Elementary Education | 162 (67.50%) | 79 (38.35%) | 241 (54.04%) | |
| Secondary & Post-secondary Education | 36 (15.00%) | 93 (45.15%) | 129 (28.92%) | |
| Missing | 3 (1.25%) | 1 (0.49%) | 4 (0.90%) | <0.001*** |
| Employment Status | ||||
| Unemployed | 34 (14.17%) | 27 (13.11%) | 61 (13.68%) | |
| Self-employed | 189 (78.75%) | 156 (75.73%) | 345 (77.35%) | |
| Private/Government Sector | 17 (7.08%) | 23 (11.17%) | 40 (9.0%) | |
| Monthly Household Income | ||||
| < 50 GHS | 41 (17.08%) | 4 (1.94%) | 45 (10.09%) | |
| 50-300 GHS | 155 (64.58%) | 117 (56.80%) | 272 (61.0%) | |
| >300 GHS | 42 (17.50%) | 79 (38.35%) | 121 (2.13%) | |
| Missing | 2 (0.83%) | 6 (2.91%) | 8 (1.79%) | <0.001*** |
Notes:
Data are mean (standard deviation, SD) or n/N (%), unless otherwise stated; Ghana Cedis (GHS): 1 GHS = 0.224 USD;
P value less than 0.05
P value less than 0.01
P value less than 0.001
Proportion of disclosure by 1 year and during the follow up period
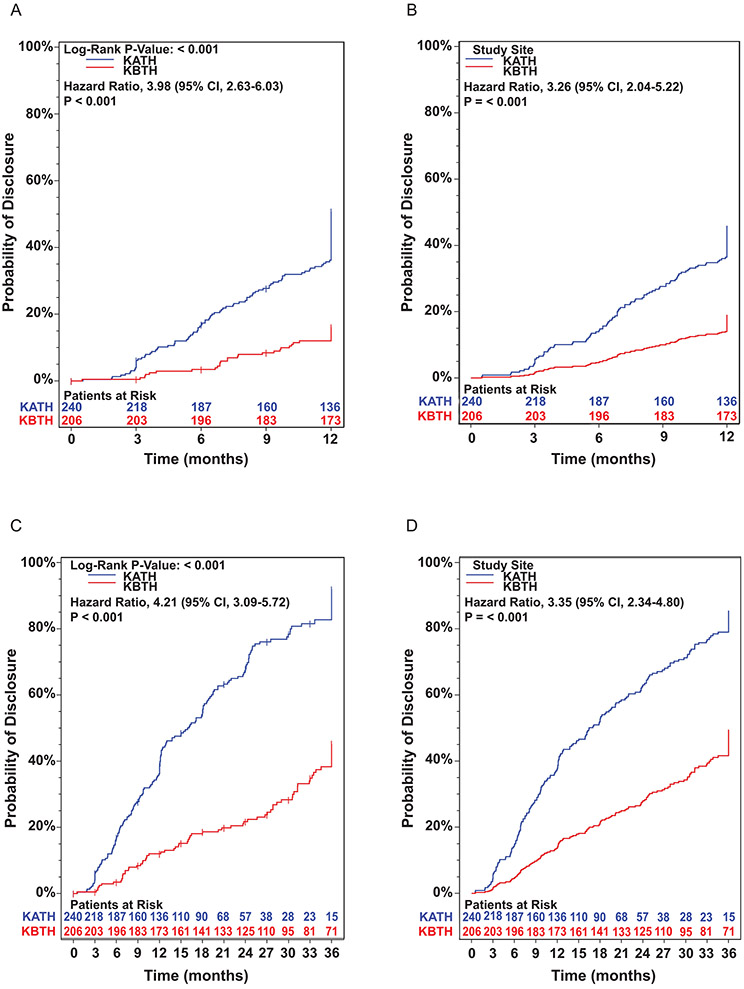
Compared to children in the control group, children in the intervention group were 3.98 times more likely to have been disclosed to by 1 year (95% CI, 2.6, 6.0). As illustrated in Figure 1A, time to disclosure was significantly different among the two groups across all time points, with the intervention group having HIV status disclosure earlier than the control group: 3 months (12.8% vs 0.5%), 6 months (24.6% vs 7.4%), 9 months (32.9% vs 10.0%), and 1 year (51.4% vs 16.2%), all p<0.001. In the adjusted model (child’s age <11 vs ≥11; caregiver’s age; caregiver’s HIV status), time to disclosure remained significantly different among the two groups across all time points (Figure 1B). However, when the analysis was stratified by age, <11 vs ≥11 years, time to disclosure only remained significant between the two groups among children <11 years of age (Supplementary Figures 2A and B).
Figure 1.
Kaplan-Meier plot of probability of disclosure at 1-year follow-up (A) unadjusted model; (B) adjusted model; and 3-year follow up (C) unadjusted model; and (D) adjusted model. KATH, Komfo Anokye Teaching Hospital; KBTH, Korle-Bu Teaching Hospital; CI, Confidence Interval. Inserted numbers represent ‘Patient at Risk’, who have not been disclosed to. Data are Hazard Ratios (HR) with 95% Confidence Interval (CI) unless otherwise noted.
Each caregiver-child dyad was followed for up to 3 years. By 3 years of follow-up, compared to children in the control group, children in the intervention group were 4.21 (95% CI, 3.09, 5.72) and 3.35 times (95% CI, 2.34, 4.80) more likely to have been disclosed to in the unadjusted (Figure 1C) and adjusted (Figure 1D) models, respectively. When the analysis was stratified by age, <11 vs ≥11 years, the rate of disclosure in the intervention group remained statistically significant irrespective of the age bracket (<11, p<0.001 and ≥11, p=0.03) (Supplementary Figures 2C and D). At the end of 3 years of follow up, 171/240 (71.3%) and 70/206 (34.0%) of children have been disclosed in the intervention group and control group, respectively. Among study participants who were disclosed, the mean time (days) to disclosure was 371 (±241) and 560 (±333) in the intervention and control groups, respectively (t-test p<0.001).
Baseline scores of caregivers on Social Provision, HIV Knowledge, HIV Stigma, Beck Depression Inventory, and Illness Perception Questionnaire
The mean (SD) score for caregivers on the various assessment tools were: (i) Social Provision Scale: 71.68 (8.21) (possible range 24 – 96, higher better); (ii) HIV Knowledge Questionnaire (HIV-KQ-18): 14.13 (2.21) (possible range 0 – 18, higher better); (iii). HIV Stigma Scale: 42.55 (6.13) (possible range 18 - 54; higher scores reflect higher level of perceived stigma); (iv) Beck Depression Inventory (BDI):10.54 (10.86) (highest possible score =63, higher worse); (v) Brief Illness Perception Questionnaire (Brief IPQ): 34.10 (11.88) (highest possible =80) (Table 3).
Table 3.
Baseline scores of caregivers on Social provisions, HIV Knowledge, Illness perceptions depression, stigma questionnaire
| SANKOFA Study Group | |||
|---|---|---|---|
| Treatment (N = 240) |
Control (N = 206) |
Total (N = 446) |
|
| Social Provisions Questionnaire (SPS) Overall SPS Score (24-96) | |||
| Mean (SD) | 71.03 (5.78) | 72.43 (10.29) | 71.68 (8.21) |
| Median (Range) | 71 (55 – 93) | 73 (44 – 96) | 71 (44 – 96) |
| N (N Missing) | 238 (2) | 206 (0) | 444 (2) |
| HIV Knowledge (HIV-KQ-18) (0-18) | |||
| Mean (SD) | 14.40 (2.17) | 13.82 (2.22) | 14.13 (2.21) |
| Median (Range) | 15 (7 – 18) | 14 (6 – 18) | 14 (6 – 18) |
| N (N Missing) | 235 (5) | 206 (0) | 441 (5) |
| HIV Stigma Score (18-54) | |||
| Mean (SD) | 40.75 (5.56) | 44.62 (6.11) | 42.55 (6.13) |
| Median (Range) | 42 (22 – 54) | 46 (26 – 54) | 43 (22 – 54) |
| N (N Missing) | 237 (3) | 206 (0) | 443 (3) |
| Beck Depression Inventory (BDI) overall score (0-63) | |||
| Mean (SD) | 7.21 (6.51) | 14.40 (13.34) | 10.54 (10.86) |
| Median (Range) | 6 (0 – 39) | 12 (0 – 62) | 7 (0 – 62) |
| N (N Missing) | 238 (2) | 206 (0) | 444 (2) |
| Illness Perception Score (Brief IPQ) (0-80) | |||
| Mean (SD) | 31.89 (11·86) | 36.64 (11.41) | 34.10 (11.88) |
| Median (Range) | 33 (2 – 60) | 38 (3 – 61) | 35 (2 – 61) |
| N (N Missing) | 238 (2) | 206 (0) | 444 (2) |
Notes:
Data are mean (standard deviation, SD) or median (range), unless otherwise stated
Predictors of disclosure at 1 year
Cox regression analysis techniques were used to assess which caregiver and child characteristics were associated with disclosure at 1 year. All the predictor variables that were associated with disclosure at p≤0.1 in the univariate analyses were included in the final multivariable regression model (Table 4). In the unadjusted analyses, treatment group (p<0.001), age <11 years (p<0.001), higher income levels (p=0.01 and p=0.01), HIV knowledge (p=0.03) and perceived HIV stigma (p=0.02) were associated with disclosure to the child at 1 year. There was no statistically significant association between child’s gender, caregiver’s HIV status, and caregiver’s level of education with disclosure. In the adjusted model, treatment group (p<0.001), HIV-infected caregiver (p=0.014), age<11 years (p<0.001), and caregiver’s education (secondary and postsecondary education vs no school, p=0.02) were significantly associated with disclosure at 1 year.
Table 4.
Child and caregiver characteristics associated with disclosure at 1 year
| Un-adjusted model | Adjusted (multivariable model) | |||||
|---|---|---|---|---|---|---|
| comparison | Hazard Ratio | Standard Error |
P value | Hazard Ratio | Standard Error |
P value |
| Treatment Group | ||||||
| Intervention vs. Control | 3.98 (2.63, 6.03) | 0.21 | <0.001 | 3.26 (2.04, 5.22) | 0.24 | <0.001 |
| Caregiver Age | ||||||
| increase by 1 | 1.02 (1.00, 1.04) | 0.01 | 0.031 | 1.02 (1.00, 1.04) | 0.01 | 0.08 |
| Child Age | ||||||
| < 11 vs. >= 11 | 3.96 (2.69, 5.81) | 0.20 | <0.001 | 3.32 (2.22, 4.96) | 0.20 | <0.001 |
| Gender | ||||||
| Female vs. Male | 1.10 (0.68, 1.77) | 0.24 | 0.70 | |||
| Caregiver HIV Status | ||||||
| Yes vs. No or Unsure | 1.33 (0.89, 1.97) | 0.20 | 0.16 | 1.80 (1.13, 2.88) | 0.24 | 0.014 |
| Caregiver Education | ||||||
| Secondary & Post-secondary Education vs. No School | 1.10 (0.57, 2.12) | 0.33 | 0.77 | 2.37 (1.15, 4.91) | 0.37 | 0.020 |
| Elementary Education vs. No School | 1.54 (0.85, 2.78) | 0.30 | 0.16 | 1.44 (0.77, 2.68) | 0.32 | 0.25 |
| Secondary & Post-secondary Education vs. Elementary Education | 0.72 (0.47, 1.11) | 0.22 | 0.14 | 1.65 (1.00, 2.73) | 0.26 | 0.05 |
| Monthly Household Income (GHS) | ||||||
| >300 GHS vs. <= 50 GHS | 0.38 (0.18, 0.79) | 0.37 | 0.009 | 0.74 (0.34, 1.60) | 0.39 | 0.44 |
| 50 - 300 GHS vs. <= 50 GHS | 0.71 (0.38, 1.33) | 0.32 | 0.28 | 1.30 (0.67, 2.50) | 0.33 | 0.44 |
| >300 GHS vs. 50 - 300 GHS | 0.54 (0.34, 0.86) | 0.24 | 0.010 | 0.57 (0.34, 0.97) | 0.27 | 0.037 |
| Social Provision Scale overall Score | ||||||
| increase by 1 | 1.00 (0.98, 1.02) | 0.01 | 0.71 | |||
| HIV Knowledge Questionnaire (HIV KQ-18) | ||||||
| increase by 1 | 1.11 (1.01, 1.22) | 0.05 | 0.029 | 1.01 (0.91, 1.12) | 0.05 | 0.80 |
| HIV stigma score | ||||||
| increase by 1 | 0.97 (0.94, 1.00) | 0.01 | 0.022 | 0.99 (0.96, 1.03) | 0.02 | 0.67 |
Notes:
Ghana Cedis (GHS): 1 GHS = 0.224 USD
P value less than 0.05
P value less than 0.01
P value less than 0.001
We compared factors that distinguished between disclosed and non-disclosed participants at the intervention site (KATH); we found child’s age and caregiver’s HIV status were statistically significant between the two groups (Supplementary Table 1). Further, we determined factors associated with disclosure at 1 year at the intervention site (KATH). All the predictor variables that were associated with disclosure at p≤0.1 in the univariate analyses were included in the final multivariable regression model. In the adjusted, multivariable regression model, child’s age and caregiver’s HIV status were significant (Supplementary Table 2).
Discussion
In this study, children in Sankofa Pediatric HIV disclosure intervention group were found to be 3.98- and 4.21-times likely to have been disclosed to than children in the control group at 1 and 3 years after initiation of intervention, respectively. Other predictors of disclosure were age <11 years, HIV-infected caregiver, caregiver age, and a higher level of caregiver education. Thus, our findings indicate that caregiver’s preference to disclose at older age11,12,27,36 and unpreparedness to deal with questions and issues on disclosure 11,12,36 are amendable to a structured, culturally-relevant, personalized, and a process-oriented disclosure intervention. When the analysis was stratified by age, <11 vs ≥11 years, the rate of disclosure in the intervention group remained statistically significant irrespective of the age bracket (<11, p<0.001 and ≥11, p=0.02). Thus, appropriate intervention can annul caregiver’s preference to disclose at older age. To the best of our knowledge, our study is the first pediatric HIV disclosure intervention trial in sub-Saharan Africa to provide empiric evidence in support of WHO’s recommendation to disclose HIV status to school-age children (6-12 years) 3.
The intervention is patient-centered with disclosure constructed as a process in a manner suitable to the developmental age and needs of the child.37 There are a few published pediatric disclosure interventions based on a theoretical framework, process-oriented disclosure, and iterative construct like ours. However, most of these interventions have not been tested in prospective trials.5,17,38-40 Beck-Sague et al. evaluated a five-component intervention designed by Blasini et al.5 in the Dominican Republic and Haiti in a quasi- experimental trial and observed a high uptake of disclosure after deploying the intervention.38 Boon-Yasidhi et al. evaluated Thailand’s disclosure model in an observational prospective cohort study using Thailand’s national pediatric disclosure manual.41 O’Malley et al. conducted qualitative interviews with 35 health care workers and 46 caregivers at four high volume HIV clinics to assess the uptake and impact of Namibia’s pediatric HIV disclosure intervention.17 Furthermore, using routinely collected data in Namibia, Beima-Sofie et al. found that among children (7 to 15 years) who participated in the national disclosure intervention, 11% knew their HIV status at enrollment and an additional 38% reached full disclosure after enrollment.10 In each of these studies, the investigators concluded that the interventions were feasible, well accepted, and resulted in satisfactory outcomes, consistent with our findings. In contrast, Vreeman et al. recently reported that a clinic-based intervention in Kenya increased disclosure of HIV status to children living with HIV in intervention compared to control clinics over the first six months, but differences in disclosure were not sustained over the 18 months follow up period.18 In our study, the disclosure benefit of the intervention persisted over 3 years of follow-up.
Although, the Sankofa intervention used a disclosure manual to guide the process and was conceptually similar to the interventions described above, there are some distinct differences. Sankofa was a 2-pronged model and the content of the intervention was personalized based on the interventionist’s assessment of the caregiver’s HIV-disclosure-related knowledge, motivation and behavioral skills needs and neurocognitive development of the child. And more importantly, after disclosure, the interventionist continued to meet with the caregiver and child at frequent and scheduled intervals to assess post-disclosure problems and coping, provide referral to services that the family may need, and continue to help the child to understand in an age-appropriate manner HIV infection and its implication on his/her day-to-day activities. The complexity of disclosure process suggests that having a specific point person who is well prepared to address disclosure is effective. The interventionist is able to build a trusting relationship with the caregiver and child around the sensitive topic of HIV disclosure during routine clinic visits and build skills over time. The skills imported to the caregiver appear to bolster healthy interaction between caregiver-child dyads.
Age remains one of the critical barriers for not disclosing HIV status of infected children to them by both health care providers and caregivers.11,12 Health care providers and caregivers prefer disclosing when the child is 12 years or older.15,42 They cite that children before this age are not cognitively and emotionally mature to understand and handle the process of disclosure.15 Interestingly, it has been shown that adolescents living with HIV prefer to be disclosed to before the age of 12 years.38 As demonstrated by our study, with an appropriate and personalized disclosure intervention, age should not be a barrier to disclosure. In our study, children less than 11 years were 6-times more likely to have been disclosed to than children 11 years or older. Several studies have reported benefits –higher adherence, viral load suppression, delayed disease progression, and improved psychosocial functioning –with disclosing HIV status to children before 12 years of age.5-8 Emerging data support the fact that potential benefits of early disclosure outweighs potential harm. The main reason caregivers give for not disclosing at a young age is immaturity of the child.11,12,27,36 The pre-disclosure sessions of the Sankofa intervention addressed the caregiver’s concerns and developed relevant knowledge, motivation and skills for disclosing.
We found that HIV-infected caregivers were more likely to disclose the child’s status to the child. This is in contrast to prior research that found that HIV-negative caregivers were more likely to have disclosed the child’s status to the child.42 HIV-negative caregivers do not bear the personal guilt of transmitting the infection to the child and, therefore, may be expected to have less emotional and personal challenges compared with HIV-infected caregivers when it comes to disclosing to the child. It is plausible that our finding can be explained by the efficacy of the intervention. One can posit that the intervention most likely effectively addressed HIV-infected caregiver’s concerns and improved their self-efficacy and communication skills. We also found that caregivers with secondary education and post-secondary education were more likely to disclose the child’s status to the child. In contrast, Biadgilign et al. found in a cross-sectional study in Ethiopia that caregivers with primary education and above were less likely to disclose the child’s HIV status to the child than illiterate caregivers.11 Interestingly, other studies did not find any association between the level of education of caregivers and disclosure.12,15,42 One might assume that the level of education of caregivers would be related to their performance on Social Provision, HIV Knowledge, Stigma, and Illness Perception questionnaires. However, in the adjusted model, we did not observe statistically significant association between the scores of caregivers on these questionnaires and disclosure.
Findings from this study must be considered with some caution in view of the limitations. First, randomization was by site. Most randomized trials allocate individual participants to different treatments. However, we were concerned about a substantial threat of within site diffusion of the intervention which would reduce the point estimate of an intervention’s effectiveness and lead to a type II error. Randomization by site was viewed as a desirable alternative given the similarity of the participating HIV clinics with respect to profile of the providers, services, physical structure and size and demographics of the patient population served. As indicated in Table 1, there was no statistically significant difference among the groups with regard to gender, school attendance, duration of HIV diagnosis, WHO clinical staging of disease at time of diagnosis, and the HIV status of caregiver. Moreover, in assessing the baseline characteristics of participants at the two sites, our analysis incorporated adjustment for variables that appear to differ. The study was also limited by our failure to reach our target sample size. However, we then proceeded to carry out conditional power estimates using the commonly employed ‘B-method’ described above. Although we did not accrue the original target sample size, subsequent conditional power estimates informed by the enrollment rate, indicated that a sample size of 440 would afford us enough statistical power. Hence, we believe that the early termination of enrollment did not have a negative impact on the robustness of the results. Furthermore, the generalizability of the study may be limited by a few factors. First, the study clinics were in tertiary care settings, which may limit the extrapolation of our findings to other clinical settings. Second, in the role as disclosure interventionist, a clinic staff member assumed primary responsibility for working with family members on disclosure and became the “point person” and agent for change over time. It is possible that this specialist approach could limit generalizability of the intervention to other sites with more limited resources. However, since the interventionist does not need to be a doctor or a nurse, giving appropriate training to any staff member identified as qualified to be trained to assume this role may override these limitations. Future research should replicate this study in other settings.
In conclusion, while disclosure of a child’s HIV status to the child has beneficial health outcomes and is highly recommended, there is limited evidence-based data on the process of disclosure that considers the socio-cultural context of the caregiver who is expected to disclose the information. We demonstrate that a structured, culturally-relevant, and personalized disclosure intervention can be integrated into routine clinical pediatric HIV care and appears to have good potential to improve disclosure and the welfare of children living with HIV and their caregivers in Ghana and other resource-limited settings.
Supplementary Material
Acknowledgments
We thank the Sankofa Project caregiver and child dyads for their participations. We are grateful to the staff at Pediatric AIDS Clinics at Korle-Bu and Komfo Anokye Teaching Hospitals and the Ghana–Yale Partnership for Global Health for their support.
Funding Source: This study was sponsored by the NIH/NICHD (R01HD074253)
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Presented in part at the 9th International Workshop on HIV Pediatrics, Paris, France 2017; and the 22nd International AIDS Conference (AIDS 2018), Amsterdam, Netherland 2018.
ClinicalTrials.gov Identifier: NCT01701635
References
- 1.Sutcliffe CG, van Dijk JH, Bolton C, Persaud D, Moss WJ. Effectiveness of antiretroviral therapy among HIV-infected children in sub-Saharan Africa. Lancet Infect Dis. 2008;8(8):477–489. [DOI] [PubMed] [Google Scholar]
- 2.Brady MT, Oleske JM, Williams PL, et al. Declines in mortality rates and changes in causes of death in HIV-1-infected children during the HAART era. J Acquir Immune Defic Syndr. 2010;53(1):86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Guideline on HIV Disclosure Counselling for children up to 12 years of age. 2011; www.who.int/hiv/pub/hiv_disclosure/en/. Accessed December 12, 2014, 2014. [PubMed]
- 4.Slavin LA, O’Malley JE, Koocher GP, Foster DJ. Communication of the cancer diagnosis to pediatric patients: impact on long-term adjustment. Am J Psychiatry. 1982;139(2):179–183. [DOI] [PubMed] [Google Scholar]
- 5.Blasini I, Chantry C, Cruz C, et al. Disclosure model for pediatric patients living with HIV in Puerto Rico: design, implementation, and evaluation. J Dev Behav Pediatr. 2004;25(3):181–189. [DOI] [PubMed] [Google Scholar]
- 6.Menon A, Glazebrook C, Campain N, Ngoma M. Mental health and disclosure of HIV status in Zambian adolescents with HIV infection: implications for peer-support programs. J Acquir Immune Defic Syndr. 2007;46(3):349–354. [DOI] [PubMed] [Google Scholar]
- 7.Ferris M, Burau K, Schweitzer AM, et al. The influence of disclosure of HIV diagnosis on time to disease progression in a cohort of Romanian children and teens. AIDS Care. 2007;19(9):1088–1094. [DOI] [PubMed] [Google Scholar]
- 8.Vaz LM, Maman S, Eng E, Barbarin OA, Tshikandu T, Behets F. Patterns of disclosure of HIV status to infected children in a Sub-Saharan African setting. Journal of developmental and behavioral pediatrics : JDBP. 2011;32(4):307–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boon-Yasidhi V, Naiwatanakul T, Chokephaibulkit K, et al. Effect of HIV diagnosis disclosure on psychosocial outcomes in Thai children with perinatal HIV infection. Int J STD AIDS. 2016;27(4):288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beima-Sofie KM, Brandt L, Hamunime N, et al. Pediatric HIV Disclosure Intervention Improves Knowledge and Clinical Outcomes in HIV-Infected Children in Namibia. J Acquir Immune Defic Syndr. 2017;75(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biadgilign S, Deribew A, Amberbir A, Escudero HR, Deribe K. Factors associated with HIV/AIDS diagnostic disclosure to HIV infected children receiving HAART: a multi-center study in Addis Ababa, Ethiopia. PLoS One. 2011;6(3):e17572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kallem S, Renner L, Ghebremichael M, Paintsil E. Prevalence and pattern of disclosure of HIV status in HIV-infected children in Ghana. AIDS Behav. 2011;15(6):1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivet Amico K A situated-Information Motivation Behavioral Skills Model of Care Initiation and Maintenance (sIMB-CIM): an IMB model based approach to understanding and intervening in engagement in care for chronic medical conditions. Journal of health psychology. 2011;16(7):1071–1081. [DOI] [PubMed] [Google Scholar]
- 14.Tran CT, Pham TH, Tran KT, Nguyen TKC, Larsson M. Caretakers’ barriers to pediatric antiretroviral therapy adherence in Vietnam - A qualitative and quantitative study. Appl Nurs Res. 2017;35:1–5. [DOI] [PubMed] [Google Scholar]
- 15.Odiachi A, Abegunde D. Prevalence and predictors of pediatric disclosure among HIV-infected Nigerian children on treatment. AIDS Care. 2016;28(8):1046–1051. [DOI] [PubMed] [Google Scholar]
- 16.Orelly T, Welch H, Machine E, Pameh W, Duke T. Human immunodeficiency virus status disclosure and education for children and adolescents in Papua New Guinea. J Paediatr Child Health. 2018;54(7):728–734. [DOI] [PubMed] [Google Scholar]
- 17.O’Malley G, Beima-Sofie K, Feris L, et al. “If I take my medicine, I will be strong: “ evaluation of a pediatric HIV disclosure intervention in Namibia. J Acquir Immune Defic Syndr. 2015;68(1):e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vreeman RC, Nyandiko WM, Marete I, et al. Evaluating a patient-centred intervention to increase disclosure and promote resilience for children living with HIV in Kenya. AIDS. 2019;33 Suppl 1:S93–S101. [DOI] [PubMed] [Google Scholar]
- 19.Bronfenbrenner U, Ceci SJ. Nature-nurture reconceptualized in developmental perspective: a bioecological model. Psychol Rev. 1994;101(4):568–586. [DOI] [PubMed] [Google Scholar]
- 20.Fisher JD, Fisher WA, Williams SS, Malloy TE. Empirical tests of an information-motivation-behavioral skills model of AIDS-preventive behavior with gay men and heterosexual university students. Health Psychol. 1994;13(3):238–250. [DOI] [PubMed] [Google Scholar]
- 21.Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25(4):462–473. [DOI] [PubMed] [Google Scholar]
- 22.Fisher JD, Amico KR, Fisher WA, Harman JJ. The information-motivation-behavioral skills model of antiretroviral adherence and its applications. Curr HIV/AIDS Rep. 2008;5(4):193–203. [DOI] [PubMed] [Google Scholar]
- 23.Lesch A, Swartz L, Kagee A, et al. Paediatric HIV/AIDS disclosure: towards a developmental and process-oriented approach. AIDS Care. 2007;19(6):811–816. [DOI] [PubMed] [Google Scholar]
- 24.Reynolds NR, Ofori-Atta A, Lartey M, et al. SANKOFA: a multisite collaboration on paediatric HIV disclosure in Ghana. AIDS. 2015;29 Suppl 1:S35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vreeman RC, Gramelspacher AM, Gisore PO, Scanlon ML, Nyandiko WM. Disclosure of HIV status to children in resource-limited settings: a systematic review. Journal of the International AIDS Society. 2013;16:18466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heeren GA. Changing methods of disclosure. Literature review of disclosure to children with terminal illnesses, including HIV. Innovation (Abingdon). 2011;24(1-2):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberdorfer P, Puthanakit T, Louthrenoo O, Charnsil C, Sirisanthana V, Sirisanthana T. Disclosure of HIV/AIDS diagnosis to HIV-infected children in Thailand. J Paediatr Child Health. 2006;42(5):283–288. [DOI] [PubMed] [Google Scholar]
- 28.Cutrona CE RD. The provisions of social relationships and adaptation to stress. Advances in Personal Relationships. 1987;1:37–67. [Google Scholar]
- 29.Carey MP, Schroder KE. Development and psychometric evaluation of the brief HIV Knowledge Questionnaire. AIDS Educ Prev. 2002;14(2):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Broadbent E, Ellis CJ, Thomas J, Gamble G, Petrie KJ. Can an illness perception intervention reduce illness anxiety in spouses of myocardial infarction patients? A randomized controlled trial. J Psychosom Res. 2009;67(1):11–15. [DOI] [PubMed] [Google Scholar]
- 31.Berger BE, Ferrans CE, Lashley FR. Measuring stigma in people with HIV: psychometric assessment of the HIV stigma scale. Res Nurs Health. 2001;24(6):518–529. [DOI] [PubMed] [Google Scholar]
- 32.Beck AT SR, Brown GK. Beck Depression Inventory: Texas Psychological Corporation. New York: Harcourt Brace & Co; 1996. [Google Scholar]
- 33.Kotze M, Visser M, Makin J, Sikkema K, Forsyth B. Psychosocial variables associated with coping of HIV-positive women diagnosed during pregnancy. AIDS Behav. 2013;17(2):498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Catlin AC, Fernando S, Gamage R, et al. Sankofa pediatric HIV disclosure intervention cyber data management: building capacity in a resource-limited setting and ensuring data quality. AIDS Care. 2015;27 Suppl 1:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lan KK, Wittes J. The B-value: a tool for monitoring data. Biometrics. 1988;44(2):579–585. [PubMed] [Google Scholar]
- 36.Vreeman RC, Nyandiko WM, Ayaya SO, Walumbe EG, Marrero DG, Inui TS. The perceived impact of disclosure of pediatric HIV status on pediatric antiretroviral therapy adherence, child well-being, and social relationships in a resource-limited setting. AIDS Patient Care STDS. 2010;24(10):639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cantrell K, Patel N, Mandrell B, Grissom S. Pediatric HIV disclosure: a process-oriented framework. AIDS Educ Prev. 2013;25(4):302–314. [DOI] [PubMed] [Google Scholar]
- 38.Beck-Sague C, Pinzon-Iregui MC, Abreu-Perez R, et al. Disclosure of their status to youth with human immunodeficiency virus infection in the Dominican Republic: a mixed-methods study. AIDS Behav. 2015;19(2):302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandt L, Beima-Sofie K, Hamunime N, et al. Growing-up just like everyone else: key components of a successful pediatric HIV disclosure intervention in Namibia. AIDS. 2015;29 Suppl 1:S81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beima-Sofie K, John-Stewart G, Shah B, Wamalwa D, Maleche-Obimbo E, Kelley M. Using health provider insights to inform pediatric HIV disclosure: a qualitative study and practice framework from Kenya. AIDS Patient Care STDS. 2014;28(10):555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boon-Yasidhi V, Chokephaibulkit K, McConnell MS, et al. Development of a diagnosis disclosure model for perinatally HIV-infected children in Thailand. AIDS Care. 2013;25(6):756–762. [DOI] [PubMed] [Google Scholar]
- 42.John-Stewart GC, Wariua G, Beima-Sofie KM, et al. Prevalence, perceptions, and correlates of pediatric HIV disclosure in an HIV treatment program in Kenya. AIDS Care. 2013;25(9):1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.