Abstract
Background
Women living with HIV (WLHIV) have a high risk of developing invasive anal cancer. Anal cancer may be prevented with early detection and treatment of anal histologic high-grade squamous intraepithelial lesions (HSIL). However, there is limited data on the efficacy of anal HSIL treatment in WLHIV.
Study Design
We conducted a retrospective study of WLHIV treated for anal HSIL under high resolution anoscopy (HRA) guidance from January 1st, 2007 to December 31st, 2017 with at least one post treatment visit at an urban tertiary care hospital.
Results
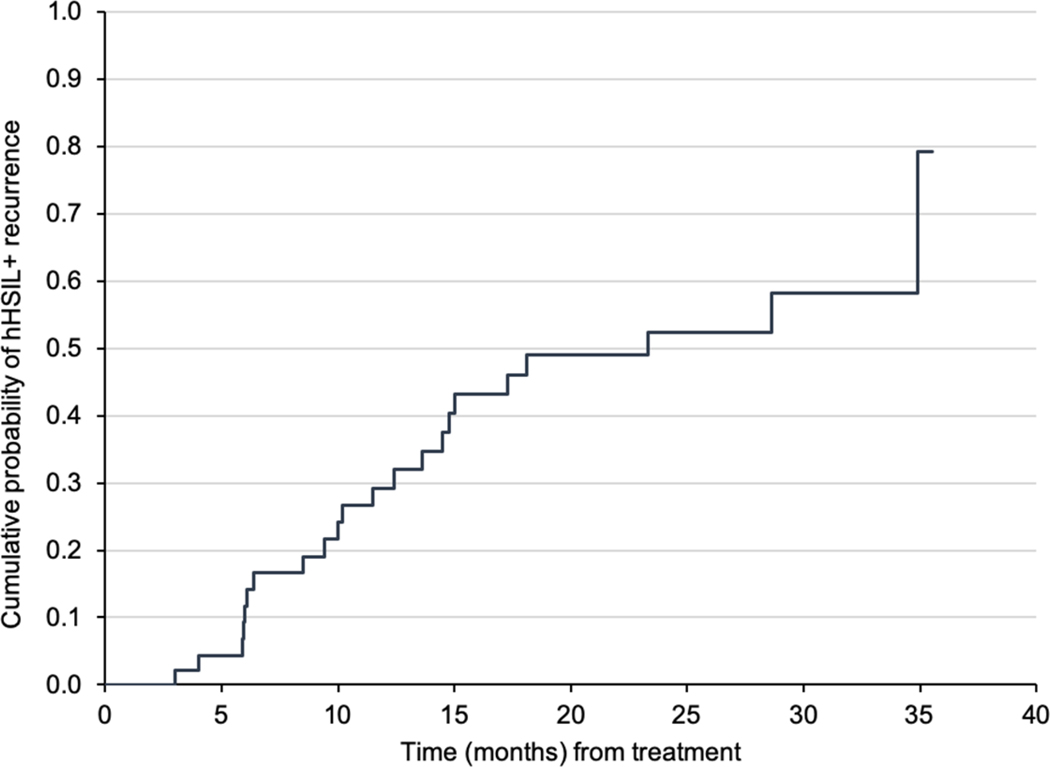
45 WLHIV women with at least 1 follow-up evaluation after treatment for anal HSIL were identified. The median age was 46 years (range 35–66 years), 63% were African American, 27% were Hispanic/Latino, and 53% were current smokers. The mean absolute CD4+ T-cell count was 516 cells/mm3; 50% and 24% of the cohort had a history of cervical or vulvar HSIL respectively. The cumulative probability of anal HSIL recurrence was 29% at 12 months, 52% at 24 months, and 79% at 36 months post-treatment
Conclusion
The majority of WLHIV treated for anal HSIL recurred within 3 years suggesting need for continued surveillance following treatment. Our data contributes to the information needed to develop effective anal cancer prevention guidelines in WLHIV.
Keywords: HIV, Anal HSIL, Women living with HIV
INTRODUCTION
Squamous cell carcinoma of the anus (SCCA) incidence and mortality rates have dramatically increased (over 3% per year) in the United States, with notable rise among women. 1 In particular, women living with HIV (WLHIV) have an over ten-fold elevated risk of SCCA compared to the general population of women.2 The rates are particularly striking among older age groups (>33 per 100,000 among WLHIV aged 45 years or older) and in WLHIV with AIDS (34 per 100,000 person-years).2
High-grade squamous intraepithelial lesions (HSIL) are anal cancer precursor lesions that are common among WLHIV. Notably, a recent national AIDS Malignancy Consortium (AMC084) trial study revealed that 28% of WLHIV have HSIL.3 HSIL treatment using ablative therapies (i.e., infrared coagulation [IRC], electrocautery), topical treatments (i.e., 5% imiquimod or 5% fluorouracil cream) and continued surveillance, where HSIL recurrence may prompt further treatment, could prevent progression to SCCA.4 Yet, to the best of our knowledge, recurrence rates (treatment effectiveness measure) among WLHIV remain unknown.
Determination of anal HSIL recurrence rate could help inform optimal HSIL surveillance recommendations for WLHIV, similar to studies focused on HIV-infected men who have sex with men (individuals with highest SCCA risk and known HSIL recurrence rates [over 50% within a year of treatment]).5 Therefore, we determined anal HSIL recurrence rates among WLHIV with the objective to inform optimal surveillance recommendations for these women.
METHODS
Boston University School of Medicine’s institutional review board approved this retrospective chart review. Through the Boston Medical Center’s (BMC’s) Clinical Data Warehouse, we identified WLHIV who underwent treatment for anal HSIL between 2007 and 2017 and had at least one follow-up high-resolution anoscopy (HRA) 3 to 36 months after anal HSIL treatment. Anal HSIL was defined as biopsy-proven AIN2 and/or AIN3. Ninety WLHIV met this criterion. In preliminary review, we excluded 6 study participants: 4 had anal cancer concurrent with anal HSIL for their initial pathology report, 1 did not have HSIL on pathology, and 1 was a male-to-female transgender patient. Of the remaining participants, 24 were excluded due to no history of anal HSIL treatment and 12 were excluded due to no record of HRA. An additional 3 patients were excluded because the first follow-up HRA was more than 3 years post treatment. The final analytic sample consisted of 45 patients who met the study criteria.
Demographic, medical and surgical history data were obtained by chart review. Demographic information included age, ethnicity, race, and marital status. Baseline clinical information included the year of HIV diagnosis, history of AIDS-defining illnesses, nadir and current CD4 counts, prescribed combination anti-retroviral therapy (cART), smoking history, and HPV medical history (history of HPV vaccine, cervical and vaginal cytology, HPV testing).
Clinical data were collected from their initial visit, to either (1) the first post-treatment anal HSIL+ diagnosis, or (2) the last HRA within 3 years of treatment. Treatment and follow-up data including subsequent anal cytology, HRA, and histology were collected by chart review. First, we identified date of the initial HSIL treatment. The 45 patients included were considered to have had “lesion clearance” at the time of their treatment. The duration of follow-up was determined to be the interval from the date of treatment to either (1) the first post-treatment anal HSIL diagnosis, or (2) the last HRA within 3 years of treatment. Recurrence was defined as the first post-treatment diagnosis of anal HSIL (regardless of whether the newly diagnosed anal HSIL occurred in the same or different location as the initially treated anal HSIL). For time-to-event analysis, time to recurrence was measured from the date of treatment to recurrence.
Statistical analyses were performed using the Statistical Analysis System (SAS) v 9.3 (SAS Corp., Cary, NC, USA). Chi-square tests or Fisher’s exact test were used for discrete outcomes and Student’s t-test was used for continuous outcomes. A p-value of ≤ 0.05 was considered significant. The cumulative risk of recurrence was determined by the Kaplan-Meier method. Patients lost to follow up or patients who did not recur until their last visit were censored.
RESULTS
The median age of participants was 46 years (range 35–66 years), the majority were African American (63%) and current/past smokers (53% and 22% respectively). Almost half of the women were born outside of North America and 12 women (26.7%) identified as Hispanic. Nearly all of the women were on cART (95.6%) and 8 women (17.8%) had a history of an AIDS-defining illness. Only 1 woman had received the quadrivalent HPV vaccine [data not presented]. At the age of anal HSIL diagnosis, participants had been living with HIV for an average of 14 years and 71% of participants had a nadir CD4+ T-cell count of less than 200 cells/mm3. At their baseline visit, study participants had an average absolute CD4+ T-cell count of 516 cells/mm3. Many women had a history of other HPV-associated diseases of the anogenital tract: 20% had vulvar HSIL, 51% had cervical HSIL and 13% had genital warts.
The majority of WLHIV were treated in the operating room (OR) with ablation and/or excision (53%); 35% were treated with office ablative procedures (hyfrecator (20%) and IRC (15%) and 10% were treated with topical therapies [data not presented].
The cumulative probability of anal HSIL recurrence was 29% at 12 months, 52% at 24 months, 79% at 36 months post-treatment (Figure 1). Women with recurrence were diagnosed at a mean duration of 148 days (interquartile ±242 days) or 2.8 HRA evaluations after treatment. Women without recurrence had an average of 5 HRA evaluations by 3 years. Women without recurrence were more likely to have a history of anogenital condyloma compared with those with recurrence (Table 1). There was no significant difference in age at HSIL diagnosis, years of HIV diagnosis, nadir CD4 count, CD4 count at anal HSIL diagnosis, smoking history, or history of other HPV-associated anogenital diseases observed among women with or without recurrent HSIL (Table 1). Of the 45 women, one was diagnosed with an invasive anal cancer 3 months following an operating room procedure with HRA-guided excisional biopsies and ablation of extensive anal HSIL lesions.
Figure 1.
Kaplan-Meier curves for probability of recurrence of anal histologic high-grade squamous intra-epithelial (HSIL) lesions or cancer over time
Table 1.
Patient characteristics at baseline and comparing those with recurrent anal HSIL vs. no recurrent HSIL at 3 years of follow-up post treatment
| Patient characteristic | All patients (n=45) Number (%) or Mean ± SD |
Recurrence (n= 22) Number (%) or Mean ± SD |
No recurrence (n= 23) Number (%) or Mean ± SD |
P Value (recurrence vs. no recurrence) |
|---|---|---|---|---|
| Age, years | 46.0 ± 7.8 | 46.0 ± 8.2 | 46.0 ± 7.5 | 0.86 |
| Years of HIV at hHSIL Diagnosis | 14.04 ± 7.93 | 14.6 ± 8.1 | 13.6 ± 8.0 | 0.61 |
| Nadir CD4 count | ||||
| Mean | 198.4 ± 202.1 | 164.6 ± 139.7 | 230.3 ± 247.1 | |
| ≥ 200 cells/mm3 | 13 (28.9) | 5 (22.7) | 8 (34.8) | 0.72 |
| < 200 cells/mm3 | 32 (71.1) | 17 (77.3) | 15 (65.2) | 0.51 |
| CD4 at hHSIL Diagnosis | ||||
| Mean | 516.1 ± 333.2 | 504.1 ± 399.8 | 527.4 ± 266.1 | |
| ≥ 200 cells/mm3 | 33 (73.3) | 15 (68.2) | 18 (78.3) | 0.39 |
| < 200 cells/mm3 | 12 (26.7) | 7 (31.8) | 5 (21.7) | 0.51 |
| History of Genital Dysplasia | ||||
| Cervical hHSIL | 23 (51.1) | 13 (59.1) | 10 (43.5) | 0.29 |
| Vulvar hHSIL | 9 (20.0) | 6 (27.3) | 3 (13.0) | 0.28 |
| Vaginal hHSIL | 2 (4.44) | 1 (4.6) | 1 (4.4) | 1.00 |
| Genital Warts | 6 (13.33) | 0 (0.0) | 6 (26.1) | 0.02a |
| Smoking History | 0.85b | |||
| Never Smoker | 10 (22.2) | 6 (27.3) | 4 (17.4) | |
| Current Smoker | 24 (53.3) | 11 (50.0) | 13 (56.5) | |
| Past Smoker | 10 (22.2) | 5 (22.7) | 5 (21.7) | |
| Unknown | 1 (2.2) | 0 (0.0) | 1 (4.4) |
Data are presented as mean +/− standard deviation or as N (%)
Significant at ≤ 0.05
Never smoker compared with all other smoking categories
hHSIL = histologic high-grade squamous intraepithelial lesion
DISCUSSION
This is the first study to report anal HSIL recurrence following treatment among WLHIV. We report that 79% of WLHIV experienced anal HSIL recurrence within 3 years of follow-up and the majority of recurrences were identified within the first year.
The recurrence rate of our cohort of WLHIV (79% at 3 years) is similar to that reported by prior studies with predominantly HIV infected men-who-have-sex-with-men (MSM) cohorts (53–68% at 1–2 years). Goldstone et al reported a median time to recurrence of 11 months and the recurrence rates of 58%, 67% and 77% at 1, 2 and 3 years respectively.6 Another retrospective study by Marks et al reported 61% recurrence rate with a mean follow-up of 29 months.7 Prospective studies showed 53–67% recurrence rate over 18 to 24 months.8,9 In a prospective randomized trial of IRC treatment among PLWH (90% of enrolled participants were MSM), Goldstone et al found a recurrence rate of 68% at 2 years.10 In comparison with our study that found only a history of anogenital condyloma associated with lack of recurrence, these studies identified the following risk factors for recurrence: lower nadir or current CD4+ T-cell count or higher number or larger size of treated lesions.6,8,9,10
The strengths of our study include a single provider performing HRA on all of the patients, the overall frequency of HRA evaluations (averaging every 6 months), and the largest (and only) cohort to date of WLHIV treated for anal HSIL with up to 3 years of follow-up. The longer duration of follow-up allowed the inclusion of patients with intermittent follow-up (with intervals from a few months to a few years). Limitations, which may limit the generalizability of our findings, include retrospective single-site study design, heterogeneity of treatments (office vs. operating room; ablative procedures vs. patient applied topical medications), absence of information on initial HSIL lesion(s) size and number, and variable intervals for follow-up evaluations. In addition, as a significant portion of the study period preceded The Lower Anogenital Squamous Terminology Standardization project,11 p16 immunohistochemistry staining confirmation for AIN2 diagnoses was not routinely done.
In conclusion, nearly 30% of WLHIV experienced anal HSIL recurrence within one year of treatment and 80% within three years, suggesting the need for continued surveillance for these women. High prevalence of anal HSIL and SCCA among WLHIV calls for optimizing screening for HSIL detection and surveillance for this risk group. However, careful consideration towards anal HSIL recurrence, its impact on health-related quality of life, and risk of progression to SCCA should be given to inform anal HSIL screening and surveillance recommendations.
ACKNOWLEDGEMENTS
The authors thank Linda Rosen at Boston University School of Medicine for her electronic medical records search.
FINANCIAL SUPPORT.
This work was supported by the National Cancer Institute of the National Institutes of Health (UM1CA121947, (principal investigator: Dr Ronald Mitsuyasu)); and from Boston University, CTSI 1UL1TR001430).
Contributor Information
Elizabeth A Stier, Department of Obstetrics and Gynecology, Boston Medical Center/Boston University School of Medicine, Boston, MA, USA.
Wafaa Abbasi, Department of Obstetrics and Gynecology, Boston Medical Center/Boston University School of Medicine, Boston, MA, USA.
Amma F. Agyemang, Department of Obstetrics and Gynecology, Boston Medical Center/Boston University School of Medicine, Boston, MA, USA.
Eduardo Amílkar Valle Álvarez, Department of Obstetrics and Gynecology, Boston Medical Center/Boston University School of Medicine, Boston, MA, USA.
Elizabeth Y Chiao, Section Infectious Diseases, Department of Medicine, Baylor College of Medicine, Houston, TX, USA; Center for Innovations in Quality, Effectiveness, and Safety, Michael E. DeBakey VA Medical Center, Houston, TX, USA.
Ashish A Deshmukh, Department of Management Policy and Community Health, The University of Texas Health Science Center at Houston, School of Public Health, Houston, TX, USA.
REFERENCES
- 1.Deshmukh AA, Suk R, Shiels MS, et al. Recent trends in squamous cell carcinoma of the anus incidence and mortality in the United States, 2001–2015. JNCI J Natl Cancer Inst. November 2019. doi: 10.1093/jnci/djz219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colón-López V, Shiels MS, Machin M, et al. Anal cancer risk among people with HIV infection in the United States. J Clin Oncol. 2018;36(1):68–75. doi: 10.1200/JCO.2017.74.9291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stier EA, Lensing SY, Darragh TM, et al. Prevalence of and Risk Factors for Anal High-grade Squamous Intraepithelial Lesions in Women Living with Human Immunodeficiency Virus. Clin Infect Dis. July 2019. doi: 10.1093/cid/ciz408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Revollo B, Videla S, Llibre JM, et al. Routine screening of anal cytology in HIV-infected subjects and the impact on invasive anal cancer. A prospective cohort study. Clin Infect Dis. September 2019. doi: 10.1093/cid/ciz831 [DOI] [PubMed] [Google Scholar]
- 5.Deshmukh AA, Chiao EY, Cantor SB, et al. Management of precancerous anal intraepithelial lesions in human immunodeficiency virus-positive men who have sex with men: Clinical effectiveness and cost-effectiveness. Cancer. 2017;123(23):4709–4719. doi: 10.1002/cncr.31035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstone SE, Johnstone AA, Moshier EL. Long-term outcome of ablation of anal high-grade squamous intraepithelial lesions: Recurrence and incidence of cancer. Dis Colon Rectum. 2014;57(3):316–323. doi: 10.1097/DCR.0000000000000058 [DOI] [PubMed] [Google Scholar]
- 7.Marks DK, Goldstone SE. Electrocautery ablation of high-grade anal squamous intraepithelial lesions in HIV-negative and HIV-positive men who have sex with men. J Acquir Immune Defic Syndr. 2012;59(3):259–265. doi: 10.1097/QAI.0b013e3182437469 [DOI] [PubMed] [Google Scholar]
- 8.Richel O, de Vries HJ, van Noesel CJ, Dijkgraaf MG, Prins JM. Comparison of imiquimod, topical fluorouracil, and electrocautery for the treatment of anal intraepithelial neoplasia in HIV-positive men who have sex with men: an open-label, randomised controlled trial. Lancet Oncol. 2013;14(4):346–353. doi: 10.1016/S1470-2045(13)70067-6 [DOI] [PubMed] [Google Scholar]
- 9.Burgos J, Curran A, Landolfi S, et al. Risk factors of high-grade anal intraepithelial neoplasia recurrence in HIV-infected MSM. AIDS. 2017;31(9):1245–1252. doi: 10.1097/QAD.0000000000001433 [DOI] [PubMed] [Google Scholar]
- 10.Goldstone SE, Lensing SY, Stier EA, et al. A Randomized Clinical Trial of Infrared Coagulation Ablation Versus Active Monitoring of Intra-anal High-grade Dysplasia in Adults With Human Immunodeficiency Virus Infection: An AIDS Malignancy Consortium Trial. Clin Infect Dis. 2019;68(7):1204–1212. doi: 10.1093/cid/ciy615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darragh T, Colgan T, Thomas Cox J, Heller D, Henry M, Luff R. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136(10):1266–1297. [DOI] [PubMed] [Google Scholar]