Abstract
The application of photobiomodulation therapy (PBMT) for neuronal stimulation is studied in different animal models and in humans, and has shown to improve cerebral metabolic activity and blood flow, and provide neuroprotection via anti-inflammatory and antioxidant pathways. Recently, intranasal PBMT (i-PBMT) has become an attractive and potential method for the treatment of brain conditions. Herein, we provide a summary of different intranasal light delivery approaches including a nostril-based portable method and implanted deep-nasal methods for the effective systemic or direct irradiation of the brain. Nostril-based i-PBMT devices are available, using either lasers or light emitting diodes (LEDs), and can be applied either alone or in combination to transcranial devices (the latter applied directly to the scalp) to treat a wide range of brain conditions such as mild cognitive impairment, Alzheimer’s disease, Parkinson’s disease, cerebrovascular diseases, depression and anxiety as well as insomnia. Evidence shows that nostril-based i-PBMT improves blood rheology and cerebral blood flow, so that, without needing to puncture blood vessels, i-PBMT may have equivalent results to a peripheral intravenous laser irradiation procedure. Up to now, no studies were conducted to implant PBMT light sources deep within the nose in a clinical setting, but simulation studies suggest that deep-nasal PBMT via cribriform plate and sphenoid sinus might be an effective method to deliver light to the ventromedial part of the prefrontal and orbitofrontal cortex. Home-based i-PBMT, using inexpensive LED applicators, has potential as a novel approach for neurorehabilitation; comparative studies also testing sham, and transcranial PBMT are warranted.
Keywords: Alzheimer’s disease (AD), blood irradiation, brain function, cribriform plate, depression, intranasal photobiomodulation, light emitting diodes (LEDs), nasal cavity, sphenoid sinus
Introduction
Photobiomodulation therapy (PBMT), previously known as low level laser/light therapy, is a promising modality based on the irradiation of tissue with photons in the red to near-infrared (NIR) spectrum (600–1100 nm); various light sources, including lasers and light emitting diodes (LEDs), can be applied (Zein et al., 2018). PBMT is widely used to treat a variety of medical conditions such as wound healing, pain and inflammation, diabetic ulcers, blood disorders, musculoskeletal complications, coronary artery disease as well as tissue repair and regeneration (Chung et al., 2012; Arany et al., 2014). Moreover, PBMT has steadily attracted increasing interest for a wide range of applications to the brain, ranging from neurotrauma, neurodegeneration, and neuropsychiatric disorders to enhance brain functions in healthy individuals (Salehpour et al., 2018b; Caldieraro and Cassano, 2019; Chan et al., 2019).
Brain PBMT is emerging as a potentially effective therapeutic technique in the management of central nervous system (CNS) disorders (Fitzgerald et al., 2013). The application of PBMT for various brain conditions was studied in different animal models and in humans, and the overall results indicate it can improve cerebral metabolic activity and blood flow, stimulate neurogenesis and synaptogenesis, affect neurotransmitters, and provide neuroprotection via anti-inflammatory and antioxidant signaling pathways (Grillo et al., 2013; Hamblin, 2016; Hennessy and Hamblin, 2016; Salehpour et al., 2018b). To date, no serious adverse effects were reported for this modality in literature; however, caution should be used with laser devices due to the risk for macular lesions. Transcranial (Michalikova et al., 2008; Naeser et al., 2014; Thunshelle and Hamblin, 2016; Chang et al., 2018; Salehpour et al., 2018b), intranasal (Pitzschke et al., 2015; Saltmarche et al., 2017), intra-aural (Sun et al., 2016), intraoral (Burchman, 2011; Pitzschke et al., 2015), and intravascular approaches (Maksimovich, 2004) were proposed in literature as potential noninvasive routes to deliver the light in brain PBMT. In addition, recently, the feasibility and safety of more invasive methods for intracranial brain PBMT were demonstrated in rodent and primate models of Parkinson’s disease (PD) (El Massri et al., 2017). In the transcranial PBMT (t-PBMT) method, red/NIR photons must penetrate several types of head tissue including scalp, skull, periosteal, meningeal, subdural space, arachnoid mater, subarachnoid space, and pia mater, respectively, until the light reaches the cerebral cortex (Netter, 2017). In this approach, owing to poor transmission of light through the aforementioned layers, only a small fraction of incident light on the scalp level will actually reach the cortical surface (Salehpour et al., 2018a). It is noteworthy that up to now the transcranial approach was the most commonly studied method for brain PBMT research (Salehpour et al., 2018b); however, its efficiency for delivery of biostimulatory/therapeutic light doses to the deeper structures of the brain (e.g. limbic system and brainstem) remains a challenge (Hamblin, 2016). It should also be considered that due to the exponential attenuation of light transmission through the brain tissues, it is unlikely that biostimulatory light doses could be delivered into deeper regions, without overdosing the overlaying superficial tissues (Caldieraro and Cassano, 2019).
Recently, intranasal PBMT (i-PBMT) was suggested as an alternative to overcome some of the limitations of the t-PBMT to provide effective irradiation of the prefrontal areas and some limbic structures of the brain (Pitzschke et al., 2015; Cassano et al., 2019). i-PBMT is a nose-mediated therapeutic approach that is based on the insertion of one or two small laser/LED equipped portable probes into the nostrils. It is shown that repeated application of nostril-based i-PBMT could potentially improve symptoms associated with neuronal dysfunction caused by cerebral infarction (Xiao et al., 2005), mild traumatic brain injury (TBI) (Bogdanova et al., 2017), mild cognitive impairment (MCI) (Salehpour et al., 2019), Alzheimer’s disease (AD) (Saltmarche et al., 2017; Chao, 2019), PD (Li et al., 1999b), depression and anxiety (Caldieraro et al., 2018), schizophrenia (Liao, 2000), and insomnia (Xu et al., 2001; Saltmarche et al., 2017). Systemic effects of nostril-based intranasal irradiation via the blood cells and components could likely contribute to the observed neurotherapeutic effects (Hennessy and Hamblin, 2016). The tissue around the nasal cavity has abundant blood capillaries with relatively slow blood flow. i-PBMT was shown to improve blood rheology (Liu et al., 2012), reduce blood viscosity (Liu et al., 2012), and improve blood coagulation status (Gao et al., 2008) in various medical conditions. Studies have shown associations of hemorheology and cognitive functions (Elwood et al., 2001) and mood states (Gao et al., 2004). It was suggested that the systemic effects of i-PBMT via the blood irradiation mechanisms could also ultimately produce neuroprotective benefits in the brain (Xiao et al., 2005; Hennessy and Hamblin, 2016; Caldieraro et al., 2018). Studies have shown that intranasal blood irradiation has similar neurological outcomes to the intravenous or intravascular laser therapy (Jing, 1999; Dou et al., 2003). Intravascular laser irradiation has shown to improve regional cerebral blood flow (CBF) and brain function in patients with cerebral infarction (Song-Lin, 1997; Xiao et al., 2001). Therefore, these lines of evidence suggest that the irradiation of blood components and the vascular endothelium could play a role in the systemic effects induced by the intranasal method (Xiao et al., 2005; Va, 2015).
Besides the portable intranasal applicators, i-PBMT using light sources implanted in the deep nasal cavity was recently suggested as a technique to provide sufficient light energy to deep CNS structures (DiMauro et al., 2014, 2018; Pitzschke et al., 2015; Cassano et al., 2019). There is an assumption that because the cribriform plate of the ethmoid bone is substantially permeable to red/NIR light, a therapeutic photon flux could easily reach the prefrontal cortex (PFC) of the brain through this route (Cassano et al., 2019). Most recently, Monte Carlo simulation modeling has shown that the placing of the LEDs source adjacent to the cribriform plate results in the highest fluence values reaching the ventromedial PFC (vmPFC) and ventromedial orbitofrontal cortex (vmOFC) areas, compared to the transcranial positioning of the light source on the head at the Fp1-Fpz-Fp2 points [electroencephalogram (EEG) system] (Cassano et al., 2019). The intranasal positioning of the light near the cribriform plate could also lead to preferential light delivery to the hippocampus and amygdala, compared with when the light probe is placed in the nostril and mid-nose positions (Cassano et al., 2019). Additionally, it was experimentally proven that the transsphenoidal illumination of the substantia nigra pars compacta (SNpc) region is achievable when the light source is placed in the sphenoidal sinus (Pitzschke et al., 2015).
Herein we will provide a summary of the applicability of different intranasal irradiation approaches including the nostril-based portable method and the implanted deep-nasal methods for effective systemic or direct illumination of the brain. For the nostril-based i-PBMT method, the possible proposed mechanisms of action via the blood cells will be described. Furthermore, the light delivery and dosimetry concerns will be discussed for both approaches. We will also summarize the evidence for the effectiveness of i-PBMT for neurological, neurodegenerative, and neuropsychiatric disorders.
Intranasal photobiomodulation therapy
Intranasal photobiomodulation therapy from the nostrils
Nasal delivery is recognized as a promising route for delivering therapeutic agents for the treatment of nasal problems (e.g. asthma, colds, coughs, and sinusitis) (Marianecci et al., 2017). In addition, the nose serves as a direct path to the brain so that the nasal administration is an efficient and noninvasive option for delivering exogenous therapeutic molecules to the CNS (Frey, 1991). The epithelial tissue within the nasal cavity is relatively highly vascularized (Figure 1). Recently, the nasal route has received attention for delivery of systemic therapeutic agents in order to enter the brain via the systemic circulation (Jiang et al., 2015). i-PBMT using the nostril approach involves the simple process of clipping a small laser diode or LED unilaterally/bilaterally to the nose (Figure 2). Investigations into the applications of i-PBMT are becoming increasingly common, and several intranasal portable devices for both clinical and home use have become commercially available. i-PBMT is a low-cost and essentially painless technology that can easily be applied by the subject at home without needing any trained medical personnel, helping to increase patient comfort and compliance. Moreover, commercially available portable i-PBMT devices run on a small size battery and can be rechargeable, which make this technology suitable as a self-applied intervention. These devices are accessible to the public: both with red and NIR diodes, emitting at the wavelength ranges of 600–680 nm and 800–850 nm, respectively. These types of applicators are generally adjustable for the selection of the treatment duration as well as for the choice of continuous or pulsed irradiation mode. In i-PBMT, pulsed wave (PW) mode irradiation has some advantages. First, as a light source in direct contact with the nasal cavity and tissues could cause excessive warming, PW could reduce the heat associated with the illumination, by pausing and allowing the tissue to cool down. Second, recent findings have revealed beneficial neurobiological effects of specific light frequencies such as 10, 40, and 100 Hz (Salehpour et al., 2018b). PBMT at 10-Hz PW is shown to have neurotherapeutic effects in patients with cognitive and mood problems (Morries et al., 2015; Berman et al., 2017; Saltmarche et al., 2017). Third, 40-Hz pulse rate could enhance the brain gamma waves; similar enhancement with visible spectrum was demonstrated and shown to decrease amyloid-β (Aβ) levels and tau phosphorylation in an animal model of AD (Iaccarino et al., 2016). Portable i-PBMT devices, specifically for the 40-Hz PW mode, were tested to improve concentration and cognitive functioning in AD patients (Chao, 2019) and to modulate neural oscillations in healthy individuals (Zomorrodi et al., 2019). Fourth, PD patients treated with 60-Hz frequency deep brain stimulation have shown significant improvements in swallowing and in motor symptoms (Xie et al., 2015). i-PBMT at 60-Hz PW should be further investigated for neurodegenerative diseases in clinical studies.
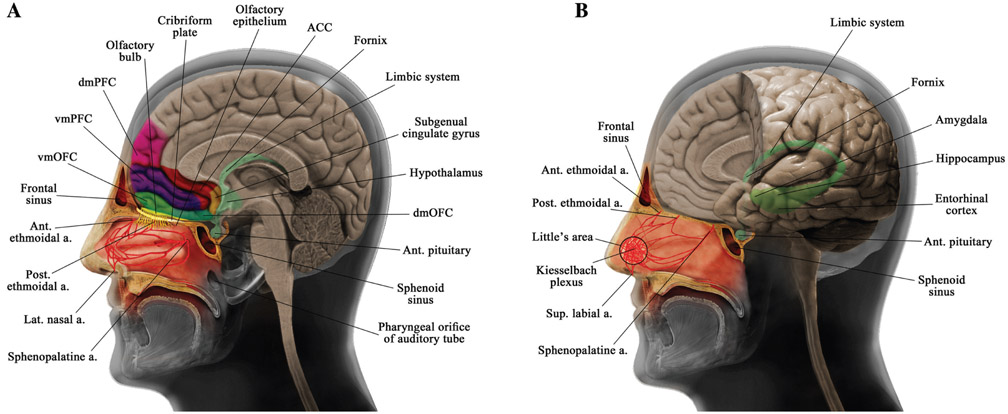
Figure 1:
Schematic representations of the anatomy of the nasal and cerebral structures.
(A) Anatomy of the nasal cavity, lateral nasal blood supply, cribriform plate, frontal and sphenoid sinuses, prefrontal cortex structures, and subgenual cingulate gyrus. (B) Anatomy of nasal septal blood supply and some limbic system structures. ACC, Anterior cingulate cortex; ant., anterior; a., artery; dmOFC, dorsomedial orbitofrontal cortex; dmPFC, dorsomedial prefrontal cortex; lat., lateral; post., posterior; sup., superior; vmOFC, ventromedial orbitofrontal cortex; vmPFC, ventromedial prefrontal cortex.
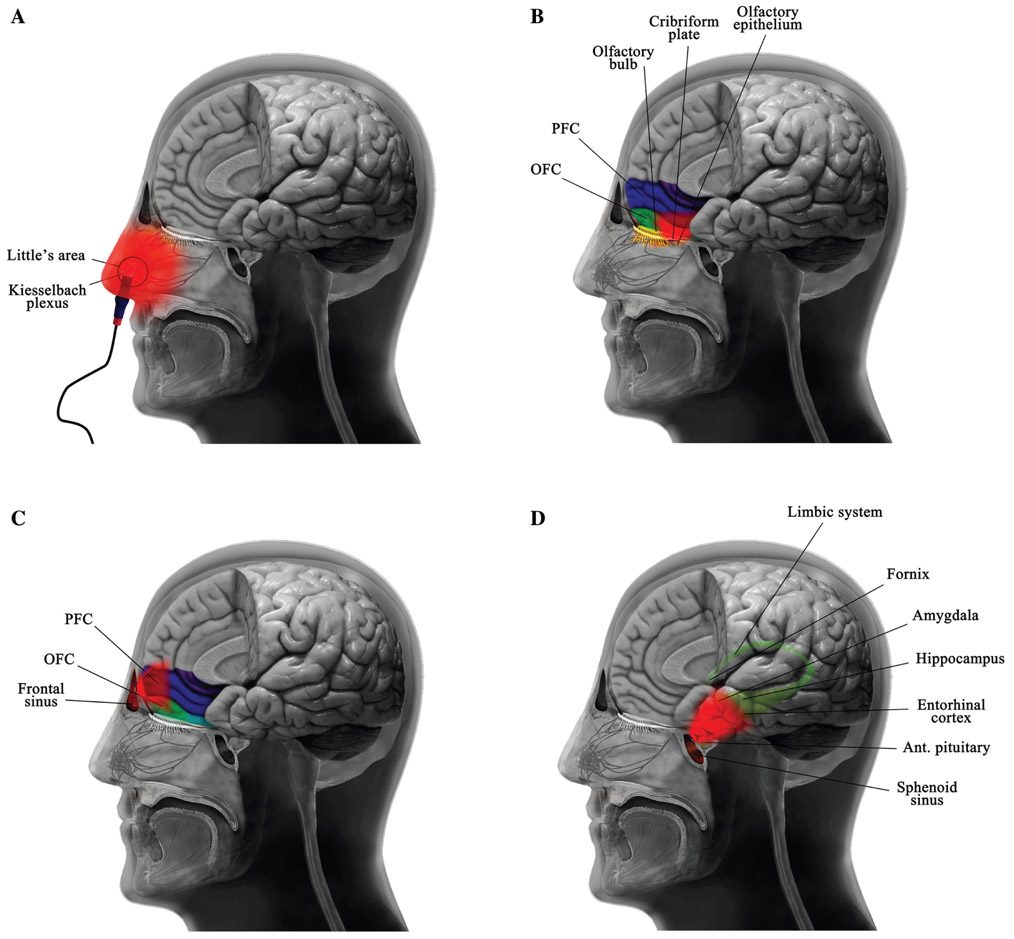
Figure 2:
Different approaches for light delivery to achieve intranasal photobiomodulation therapy.
Intranasal photobiomodulation therapy can be applied using: (A) nostril-based portable applicator or via implanted light sources in the (B) cribriform plate, (C) frontal sinus, and (D) sphenoid sinus. ant., Anterior; OFC, orbitofrontal cortex; PFC, prefrontal cortex.
The portable devices in the market allow clipping a light source into the nostril. Because of the location of the light source at the inside tip of the nose, the light delivered by this technology is expected to be mostly absorbed superficially by the nasal mucosa and surrounding tissues (Figure 2). A simulation study (Cassano et al., 2019) using 810 nm light has shown that among the CNS structures, the vmPFC and vmOFC receive most light, but still only a negligible fraction of the primary photons (~0.001%) is delivered by this method of irradiation. In other words, in terms of peak light fluence with a commercially available device, only 0.014 and 0.025 J/cm2 reached the vmPFC and vmOFC. Further simulations revealed that the photon flux at the amygdala and hippocampus was lower by two orders of magnitude than that of the vmPFC and vmOFC. Taken together, these findings show the inadequacy of the nostril-based i-PBMT for delivery of light to the brain structures, especially limbic structures (Cassano et al., 2019).
The systemic effects of nostril-based i-PBMT via irradiation of blood cells or blood components were suggested as a possible mechanism of action of this intervention (Xiao et al., 2005; Hamblin, 2016; Hennessy and Hamblin, 2016; Caldieraro et al., 2018). Considering its noninvasive and relatively inexpensive nature, i-PBMT may give the same therapeutic outcome as the intravenous blood irradiation approach (Xiao et al., 2005). For instance, it was demonstrated that intranasal laser irradiation was equally effective as intravascular blood irradiation in improving regional CBF and brain function of patients who had suffered a cerebral infarction (Xiao et al., 2005). Most recently, it was shown that 10 days of remote preconditioning PBMT (670 nm, 4.5 J/cm2; delivered to the dorsum and hind limbs) protected against methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced neuropathology in mice through the modulation of various molecular pathways within the brain, including upregulation of cell signaling and migration (including CXCR4 + stem cell and adipocytokine signaling), oxidative stress response pathways, and modulation of the blood-brain barrier (Ganeshan et al., 2019). This is further evidence that blood could be the crucial mechanism responsible for the brain protective effects of nostril-based i-PBMT.
With regard to direct blood irradiation, the nasal cavity has a rich arterial blood supply provided by both the internal and external carotid arteries (Figure 1). Anatomically, the internal carotid artery branches include the anterior and posterior ethmoidal arteries. The ethmoidal arteries are branches of theophthalmicartery descending into the nasal cavity through the cribriform plate supplying the upper nasal septum and nasal sidewalls. The external carotid branches consist of the sphenopalatine artery, greater palatine artery, superior labial artery, and lateral nasal arteries. The sphenopalatine artery, the terminal branch of the maxillary artery, is the main artery of the posterior nasal cavity entering through the sphenopalatine foramen and supplying a substantial portion of the septum and the lateral wall. The superior labial branch of the facial artery supplies the front part of the nose and the nasal septum. Furthermore, the nasal vestibule obtains blood from the lateral nasal artery (Moore et al., 1999; MacArthur and McGarry, 2017). In addition, these arteries make anastomoses with each other, mostly in the anterior portion of the nose, in the area forming the Kiesselbach plexus. The Kiesselbach plexus is an anastomotic-rich vascular region in the anteroinferior quadrant of the nasal septum, over the septal cartilage, where all the above-mentioned arteries anastomose. The veins of the nose follow the arteries and drain into the pterygoid plexus, cavernous sinus, and ophthalmic vein (Ritter, 1970; Koh et al., 2000; Rajagopal and Paul, 2005).
It is known that nostril-based blood i-PBMT improves oxygenation and leads to increased adenosine triphosphate (ATP) levels in various tissues, including the brain. PBMT absorbed by blood using visible as well as NIR light leads to partial photochemical dissociation of hemoglobinligand complexes [e.g. O2, carbon dioxide, nitric oxide (NO)] (Komorowska et al., 2002; Vladimirov et al., 2004; Lohr et al., 2009; Walski et al., 2015). The result of light-induced photodissociation of oxyhemoglobin is a decrease in arterial oxygen saturation (SpO2) in blood capillary vessels followed by significant enrichment of local tissue oxygenation (Asimov et al., 2007; Yesman et al., 2016; Gisbrecht et al., 2017). Evidence also suggests that hemoglobin can absorb 660 nm photons and can amplify the effect of laser irradiation on blood lymphocytes (Stadler et al., 2000). Moreover, the release of small amounts of the NO (one of the most important factors affecting microcirculation) increases vasodilation and consequently improves perfusion, which contributes to improved oxygen delivery to tissues. This suggests nostril-based i-PBM could be an attractive and potentially effective method for treatment of hypoxic-ischemic brain injury, together with neurodegenerative and neuropsychiatric diseases. Indeed, the production of NO from the endothelium is reduced in depressed patients (Chrapko et al., 2006). Likewise, hypertension induces oxidative stress in the endothelial cells and decreases the bioavailability of NO, and subsequently impairs cognitive function (Bomboi et al., 2010). Nostril-based i-PBMT could modulate NO release from either the endothelium or from platelets, and in turn could improve cerebrovascular circulation in the abovementioned diseases. On the other hand, the systemic effect of blood PBMT on the circulation could be a consequence of conformational alterations in the membrane properties of red blood cells (RBCs). Absorption of red/NIR light perturbs hydrogen bonds, which in turn leads to their disruption and increased dissociation of water molecules at the membrane interfaces (Natzle et al., 1981; Natzle and Moore, 1985; Szymborska-Małek et al., 2018) inducing structural changes in RBC membrane proteins and the fluidity of lipid bilayers, thus modulating the activity of membrane ion pumps in the RBCs (Komorowska et al., 2002; Kujawa et al., 2004; Chludzińska et al., 2005; Walski et al., 2015). This in turn, results in an improvement of RBC deformability, ATP content, and normalization of the osmotic properties (Itoh et al., 1996; Walski et al., 2014; Wang et al., 2016). Additionally, an increase in the electrokinetic potential of the membrane could directly contribute to the improvement in blood rheology by reducing the aggregates, which improves circulation. Aggregation causes RBCs to form rouleaux, which resemble stacks of coins that physically jam the capillary flow (Komorowska et al., 2001; Mi et al., 2004; Chludzińska et al., 2005). It was reported that absorption of 810 nm laser by RBCs lead to an increase in the ATPase activity and alteration of the erythrocyte membrane proteins (Kujawa et al., 2004). Transformation of RBC membranes is considered to be a mechanism for the long-term improvement in blood viscosity and circulation after PBMT (Komorowska et al., 2002; Kujawa et al., 2004; Walski et al., 2015).
It is well known that hemoglobin, like other metalloporphyrins, can emit fluorescence when it is excited by visible femtosecond-pulsed laser light. In a study by Zheng et al. (2011), light in a wavelength range from 600 to 750 nm was tested, and the highest hemoglobin fluorescence was emitted when the excitation wavelength was below 700 nm. Blue wavelength biophotons were emitted as bioluminescence from the erythrocytes. It may be possible that blood PBMT using red light may also induce bioluminescence emission of blue light from hemoglobin, which in turn, could significantly increase blood flow, improve flow-mediated dilation, release circulating NO and nitroso compounds as recently shown by Stern et al. (2018).
In addition to the described changes in tissue oxygenation and modifications of the RBC structure, the systemic effect of nostril-based i-PBMT via irradiation of blood cells could be directly related to the mechanism of hemostasis modulation. On one side, reversible suppression of the sensitivity of platelets to different agonists, and reduced activity of enzymes in the arachidonic acid cascade in a dose-dependent manner of PBMT was reported (Brill et al., 2000; Rola et al., 2017; Drohomirecka et al., 2018) leading to the conclusion that the PBMT could prevent platelets from undergoing apoptosis and prolong their lifespan under pathological conditions (Yang et al., 2016; Zhang et al., 2016, 2018). Evidence has shown that the mitochondrial respiratory rate and ATP levels are impaired in the blood platelets of patients with depression (Hroudová et al., 2013). Considering the central mechanism of PBMT via increasing cellular energy metabolism, irradiation of circulating platelet mitochondria via the intranasal route could be a possible explanation for the off-target or abscopal effects seen in a systemic treatment, possibly specifically for improving depression (Sommer and Trelles, 2014).
A wide range of studies on immune cells have demonstrated that direct tissue exposure to red/NIR light can reduce the influx of polymorphonuclear leukocytes into a targeted site of inflammation, thus decreasing the oxidative burst (de Lima et al., 2011; Oliveira et al., 2014; Walski et al., 2018). The remote neuroprotective action of blood-mediated effects of nostril based i-PBMT could be paradoxically related to modulation of reactive oxygen species (ROS) formation (Karu et al., 2005). ROS have an important role in regulation of signal transduction pathways and gene expression. The role of ROS is critical for reprogramming of macrophage polarization to M2 phenotype, which releases anti-inflammatory mediators related to tissue recovery (Zhang et al., 2013; Cheon et al., 2017). This mechanism is in line with previous reports which have shown that in a spinal cord injury rat model, 810 nm PBMT altered the macrophage/microglia polarization state to a M2 phenotype and elevated expression of anti-inflammatory cytokines such as interleukin (IL)-4 and IL-13 (Song et al., 2017), but suppressed pro-inflammatory IL-6 (Byrnes et al., 2005) resulting in alternative activation of macrophages.
Besides systemic effects on blood components, several other possible pathways were proposed that could mediate i-PBMT, including affecting the olfactory nerve and bulb, the olfactory endothelium, autonomic nervous system, and the lymphatic system (Liu et al., 2007, 2010). Systemic activation of mesenchymal stem cells/marrow stromal cells (MSCs) in the nasal bone marrow (Liu et al., 2012) and olfactory ensheathing cells (another type of stem cell) in the nasal mucosa (Hennessy and Hamblin, 2016) were suggested as possible pathways.
Intranasal photobiomodulation therapy from the nasal cavity and from the nasal submucosal space
As mentioned above, the use of nostril-based portable applicators appears to only provide a negligible amount of light energy into the brain deeper structures (Cassano et al., 2019). Moreover, with respect to the transcranial method, limited light penetration through the skull remains a major obstacle to t-PBMT stimulating subcortical neurons. It was reported that only 2% of 1064 nm laser light can pass through the human supraorbital frontal bone (Barrett and Gonzalez-Lima, 2013). Approximately 0.5% of 633 nm and 2.1% of 830 nm LED light were shown to penetrate through 1 cm of frontal skull and overlying tissue, in a cadaver model (Jagdeo et al., 2012). Also, 2.9% of 810 nm light from a high-power laser device was shown to penetrate through 3 cm of scalp, skull and brain tissue (Henderson and Morries, 2015). Given these measurements, the significant attenuation of the light flux will result in an insufficient dose of intracranial light, and subsequent inadequate photostimulation of the subcortical gray and white matter.
Recently, implantable i-PBMT devices were suggested and described to overcome the limitations of portable devices as well as of the transcranial irradiation method. With the use of an implantable intranasal light device, there would be no need for frequent treatment visits in patients requiring long-term treatment protocols. A simple procedure was proposed to implant miniaturized LEDs within submucosal pockets in accessible areas of the nose (Figure 2), this latter procedure would require only local anesthesia, only ambulatory surgery and would allow the placement (as well as removal) of an indwelling submucosal LED (Cassano et al., personal communication). The submucosal placement of miniaturized LEDs is, however, potentially limited by its only relative proximity to the cribriform plate.
The cribriform plate is part of the ethmoid bone that separates the brain from the nasal cavity (Figure 1). It is located between the anterior cranial fossa and the nasal cavity extending anteroposteriorly from the crista galli to the planum sphenoidale. With a thickness of about 1 mm, the cribriform plate sits below the PFC and forms the roof of the nasal cavity. About one-half of its surface comprises very small perforations, providing support through which the fibers of the olfactory nerve enter and exit (Erdem et al., 2004). The olfactory bulb is the part of the brain lying above the nasal cavity on the superior surface of the cribriform plate and inferior to the basal frontal lobe (Masurkar and Chen, 2009). Olfactory dysfunction predicts a risk of occurrence of some neurodegenerative diseases such as MCI, dementia, PD, and the neuropsychiatric complications of PD (Wilson et al., 2010). It is believed that in adult mammals, neurogenesis occurs only in the olfactory bulb and in the dentate gyrus of the hippocampus; and in humans, only in the hippocampus (Bergmann et al., 2015). It was shown that 810 nm t-PBMT remarkably stimulates neurogenesis and upregulates migrating neuroprogenitor cells in the dentate gyrus and subventricular zone in a mouse TBI model (Xuan et al., 2014). Although to date, no study has yet demonstrated induction of neurogenesis in the olfactory bulb after PBMT, the direct irradiation of this area could have the potential to activate neural stem cells.
Mitochondrial complex IV [Cytochrome c oxidase (CCO)] is a primary site for the initial absorption of red/NIR photons (Karu, 2010). It is believed that light absorption by CCO leads to increased ATP production, modulation of NO, and increased tissue oxygenation and blood flow (de Freitas and Hamblin, 2016). The 650 and 850 nm wavelengths delivered by a fiber optic technology, might potentially penetrate deeply through the nasal cavity (Zubia and Arrue, 2001). Visible light in the blue and green spectrum (400–540 nm) has penetration values of <0.1% through the human skull bone, much lower penetration to the brain would be expected if all intervening tissues were considered (Litscher and Litscher, 2013). Therefore, these wavelengths are generally considered not appropriate for t-PBMT in humans; however, the application of blue or green diodes for i-PBMT could be an alternative option due to their beneficial biological effects. A 532 nm laser has a penetration depth of only 0.8 mm in the soft tissue (Te Alexis, 2006). It could be imagined that if the 532 nm laser source is implanted in close proximity of the cribriform plate, green light could adequately irradiate nerve fiber tissue within the cribriform plate (with a thickness of 1 mm). However, it is doubtful that the entire olfactory bulb (with a thickness of 3 mm) could be sufficiently irradiated by this wavelength. Studies have shown that 532 nm green laser can increase ATP levels and cell proliferation in vitro, likely through the modulation of the activity of the mitochondrial complex III (cytochromes b, c1, and c) (Fukuzaki et al., 2013). Irradiation with a 532 nm laser has also been shown to promote the migration of GABAergic neural stem/progenitor cells into deeper layers of the mouse neocortex (Fukuzaki et al., 2015). Moreover, it was suggested that 420 nm blue light could effectively increase ATP synthesis, likely through the regulation of the mitochondria complex I (NADH-dehydrogenase) (Karu, 1988).
As mentioned above, because of the existence of very small perforations in the cribriform plate, it is possible that an unusually large portion of the photons, emitted from a light source positioned in proximity of the cribriform plate, could directly reach olfactory nerve fibers and the olfactory bulb as well as the PFC (Figure 2). The PFC is well connected to cerebral structures involved in memory, sensory perception, and emotion, such as the thalamus, basal ganglia, hypothalamus, amygdala, and hippocampus. The vmPFC is located at the bottom of the cerebral hemispheres inferior to the dorsomedial PFC, and refers to the entire area of PFC both in the ventral and medial position, approximately below the genu of the corpus callosum (Netter, 2017) (Figure 1). There are anatomically and/or functionally specialized subregions within the vmPFC, that are associated with its functional domains. The anterior/pregenual vmPFC and the ventral striatum are involved in decision-making, the posterior/subgenual vmPFC and the amygdala are involved in emotion, and the anterior/pregenual vmPFC and the dorsomedial PFC, precuneus, and temporoparietal cortex are involved in social cognition (Myers-Schulz and Koenigs, 2012; Roy et al., 2012). In fact, the anterior/perigenual subregion of the vmPFC is associated with positive valence such as the value of rewards, and the posterior/subgenual subregion is associated with negative valence such as threat and fear (Hiser and Koenigs, 2018). The OFC is located in the ventral surface of the frontal lobe directly above the orbits, in which the eyes are positioned, and extends posteriorly from the frontal pole to the insula, and ventrally, from the rostral sulcus on the medial wall to the ventrolateral convexity to form the frontal base of the brain. The OFC receives projections from the amygdala, the temporal association cortex, the visual system, taste, olfaction, somatosensory cortex, medial dorsal nucleus of the thalamus, and the hypothalamus (Rudebeck et al., 2013). The OFC is involved in learning (rewards and punishment), emotional control, and emotion-guided behaviors, decision-making, and social and emotional processing (Rolls and Grabenhorst, 2008; Clark et al., 2018).
The olfactory epithelium is a specialized epithelial tissue lining the nasal cavity, about 7 cm above and behind the nostrils. The epithelial layer is about 60 μm thick and is defined by the cribriform plate that separates the nasal cavities from the brain. Given its position inside the nose in close contact with the cribriform plate, it might be proposed as a possible location for implantation of an i-PBMT probe. It is of great importance to note that as this epithelium has sensitive cells involved in olfaction (Choi and Goldstein, 2018), caution should be taken to use this tissue as a location for light probe implantation. If the implantable light device is located at the level of the cribriform plate, the olfactory bulb and vmPFC could be effectively irradiated (Figure 2). Studies on a cadaver-head have shown that intranasal delivery of red/NIR light can potentially illuminate anteromedial and posteromedial portions of the OFC (DiMauro et al., 2018). Monte Carlo simulation has also shown that when the light source is positioned in close proximity to the cribriform plate, the energy deposition on the vmPFC is 46-fold and 658-fold greater than when the light source is implanted in the mid-nose or nostril, respectively (Cassano et al., 2019). Moreover, positioning the light source in the cribriform plate led to a higher delivered light fluence (by at least two orders of magnitude) on the vmPFC and vmOFC, compared to the dorsolateral PFC. The study also suggested that although limbic system structures such as the amygdala and hippocampus receive only negligible light energy from a source positioned in the nostril, both the mid-nose and the cribriform plate locations could allow a slightly higher deposition of light, but still totally insufficient (~0.01% of primary photons), to the aforementioned regions (Cassano et al., 2019).
Intranasal photobiomodulation therapy from the sphenoid sinus
An implantable optical fiber could be advanced through the nasal cavity and positioned in the sphenoidal sinus (DiMauro et al., 2008, 2014, 2018; Pfleiderer et al., 2017) (Figure 2). For instance, the tip of the optical fiber connected to a handheld laser/LED source could be inserted and placed, as an indwelling device, in the sphenoid bone under direct endoscopic visualization (DiMauro et al., 2008). However, this procedure would require a complex surgical nose [ear, nose and throat (ENT)] operation under general anesthesia to place the device in a poorly accessible and very fragile bone structure. The sphenoid sinuses are paired spaces within the body of the sphenoid bone, open into the roof of the nasal cavity via the sphenoethmoidal recessing its anterior wall (Wiebracht and Zimmer, 2014) (Figure 1). It is located superiorly to the ethmoidal sinus roof, cavernous sinus, optic nerve, the olfactory nerve, and sella turcica, and inferiorly to the nasal cavities, anteriorly to the nasal cavities and posteriorethmoid air cells, posteriorly to the contents of the middle cranial fossa, and laterally to the cavernous sinus and cranial cavity (Budu et al., 2013).
The sphenoidal sinus also lies adjacent to important limbic system structures (e.g. pituitary gland, amygdala, hypothalamus, and hippocampus) (Figure 1). The pituitary gland is suspended from the lowermost part of the hypothalamus (underneath the brain) by the pituitary stalk or infundibulum. The pituitary gland sits in the hypophysial fossa of the sphenoid bone and is surrounded by the sella turcica (Amar and Weiss, 2003). The pituitary gland is considered to be the master gland of the body because it regulates the activity of most of the other hormone-secreting glands. The amygdala is located deep and medially within the temporal lobes of the brain and anterior to the hippocampus. The amygdala resides lateral to and somewhat posterior to the sphenoid sinus. The amygdala has projections to several structures including the dorsomedial thalamus, the thalamic reticular nucleus, the hypothalamus, the nuclei of thetrigeminal nerve, and the facial nerve, the locus coeruleus, and the ventral tegmental area, and the laterodorsal tegmental nucleus, allowing it to affect a variety of behavioral functions (Gloor, 1978). The amygdala is involved in the processing of emotional responses such as fear, anxiety, and aggression, memory, perception of facial expressions, and decision-making (Sah et al., 2003; LeDoux, 2007; Ehrlich et al., 2009). The hypothalamus is located adjacent to the pituitary gland on either side of the third ventricle and just above and slightly posterior to the sphenoidal sinus. The hypothalamus is interconnected with the brainstem and reticular formation, limbic structures including the amygdala and septum, and areas of the autonomic nervous system (Freeman, 2003; Parent and Perkins, 2018). The hypothalamus is involved in the regulation of endocrine, body temperature, food and water intake, reproduction and sexual behavior, circadian rhythms, fatigue, sleep, emotional responses, and memory function (Parent and Perkins, 2018). The hippocampus is a convex structure composed of gray matter tissue inside the para-hippocampal gyrus, which is located in the temporal lobe below thecerebral cortex, and forms the medial walls of the inferior horns of the lateral ventricles. The most anterior portion of the hippocampus also extends above and posterior to the sphenoid sinus (Netter, 2017). The hippocampus mediates several higher cognitive functions, such as learning, memory (in particular long-term memory) spatial navigation, regulation of hypothalamic functions, and emotions (Anand and Dhikav, 2012; Insausti and Amaral, 2012).
It is conceivable that the sphenoidal sinus could potentially be used as a location of an implanted light source that could provide therapeutic amounts of light to the limbic structures (DiMauro et al., 2014) (Figure 2). Pitzschke et al. (2015) made an effort to experimentally examine the possibility of light delivery and photon distribution from a transsphenoidal approach, in order to achieve sufficient irradiation of the SNpc in a human cadaver. They coupled an optical fiber-based light diffuser to a laser diode emitting different wavelengths of 671 or 808 nm, and then the probe was introduced into the nasal cavity and placed in the sphenoidal sinus towards the SNpc under endoscopic guidance. Their measurements showed that 0.03% of 671 nm and 0.36% of 808 nm light emitted by the optical fiber could reach the SNpc (Pitzschke et al., 2015). With the use of light sources with sufficient power, these percentages of delivered light could likely provide a sufficient photostimulatory fluence to the target tissue of the SNpc. The SNpc is the main output to the basal ganglia circuit. Dopaminergic neurons of the SNpc project to the dorsal striatum through the nigrostriatal pathway, as well as to the lateral and medial pallidum of the basal ganglia system, the SNpr, and the subthalamic nucleus (Lavoie et al., 1989; Hajos and Greenfield, 1994; Cragg et al., 2004). The SN plays an important role in reward-seeking, motor planning, and movement (Delong et al., 1983; Ilango et al., 2014). It is likely that using transsphenoidal illumination, a reasonable fraction of the primary light energy could be deposited in the pituitary gland, and possibly even to the amygdala, hypothalamus, and anterior portion of the hippocampus, before reaching the SNpc.
Intranasal photobiomodulation therapy for brain conditions
i-PBMT was introduced in 1998 by Chinese researchers who reported the first evidence regarding the therapeutic effects of intranasal red laser irradiation in patients suffering from headache (Li et al., 1998a,b). Most of the clinical studies on i-PBMT for the brain were conducted in China and Russia (Liu et al., 2007). Traditionally, i-PBMT was applied to treat a wide range of brain conditions such as MCI (Jin et al., 2000), AD (Xu et al., 2002b), PD (Li et al., 1999a-c; Xu et al., 2003; Zhao et al., 2003), cerebral thrombosis (Li et al., 1999a), cerebrovascular diseases (Jianbo et al., 1999), and post-stroke depression (Xu et al., 2002a), as well as insomnia (Xu et al., 2001, 2002c), and the overall clinical outcomes appear to be promising. It is worth mentioning that because most of the studies were published in the Chinese language (until the last couple of years). i-PBMT has remained generally unknown in North America and European countries (Jiao et al., 2006). Recently, intranasal delivery of light for PBMT has gained attention among Western researchers as a potential alternative/complementary approach, which is commonly applied either alone or simultaneously with a transcranial approach (using a headpiece probe or a light-helmet). Here, we will review and summarize the most recent available evidence for the clinical application of i-PBMT in brain-related conditions.
Neurodegenerative diseases
Mild cognitive impairment
Recently, Salehpour et al. (2019) reported the rapid reversal of cognitive decline and olfactory dysfunction in a MCI patient following 1 month of transcranial plus intranasal PBMT. MCI is defined as a slight (but noticeable and measurable decline) in cognitive abilities including memory and executive skills (Ritchie and Touchon, 2000). In their study (Salehpour et al., 2019), along with a light helmet, an intranasal device was placed in the left nostril and 10.65 J/cm2 fluence of 810 nm LED light in 10-Hz PW mode was delivered twice daily via the nasal cavity. Cognitive enhancement was observed by improvements in executive function/visuospatial ability, mathematical ability, and orientation as measured by the Montreal Cognitive Assessment. The patient also showed a significant improvement in working memory, attention, and executive domains as assessed by the Working Memory Questionnaire. In addition, data from the Alberta Smell Test and peanut butter odor detection test showed the complete reversal of olfactory impairment.
Alzheimer’s disease
Xu et al. (2002b) demonstrated that i-PBMT using a 633 nm red laser improved cognitive functions as measured by the Mini-Mental State Examination (MMSE) and Wechsler Memory Scale (WMS) in AD patients. Moreover, recently, Lew Lim’s research group from Canada has made strides in treating AD using transcranial and/or intranasal PBMT. In the first report (Lim, 2014), Lim reported that single-modality i-PBMT (810 nm LED, 10-Hz PW) once a day for 1 year, significantly improved cognitive and memory functions of two AD patients as measured by the MMSE. In a recent case series (Saltmarche et al., 2017), they have shown that 3-months of transcranial plus intranasal PBMT significantly enhanced cognitive performance in five mild to moderately severe dementia patients. Along with a wearable transcranial device, they applied an intranasal LED applicator (810 nm, 10-Hz PW) to the left nostril once daily providing a 13.8 J/cm2 fluence into the nasal cavity. Besides the improvement in cognitive abilities as assessed by the MMSE and Alzheimer’s Disease Assessment Scale (ADAS-cog), increased sleep quality and decreased levels of anxiety, of anger outbursts, and of wandering were also observed. In another study (Zomorrodi et al., 2017), they reported that simultaneous application of transcranial and intranasal PBMT (810 nm, 40-Hz PW) for 6 days/week for 2 weeks resulted in a notable improvement of cognitive function in an AD patient as assessed with the MMSE and ADAS-cog scales as well as AD Cooperative Study-Activities of Daily Living Scale. These rapid cognitive enhancements were also accompanied by an overall increase in the absolute power of the EEG signal across all brain oscillations. Likewise, in a most recent study, Chao (2019) also applied the Vielight Neuro Gamma device in four patients diagnosed with dementia or AD. Along with a transcranial headset, the intranasal LED applicator (810 nm, 40-Hz PW, 15 J/cm2) was administered into the left nostril for 3 days/week for 12 weeks at home. According to the ADAS-cog and neuropsychiatric inventory scores, cognitive and behavioral functions were improved. In addition, based on their imaging data, multimodality PBMT increased cerebral perfusion and connectivity between the posterior cingulate cortex and lateral parietal nodes within the default-mode network (DMN) (Chao, 2019). Neuroimaging studies with AD patientshave consistently shown decreased functional connectivity in the DMN, on resting-state functional-connectivity MRI (Greicius et al., 2004). Hence, using t-PBMT to improve functional connectivity in the DMN in AD would be very important. It should be stated that in the studies conducted by Saltmarche et al. (2017), Chao (2019), and Salehpour et al. (2019), one side of the nasal cavity, olfactory system, and possibly of CNS structures was preferentially irradiated by the intranasal device, as the treatment was unilateral (a single nostril). Bilateral application of i-PBMT devices is recommended for uniformity of the treatment in this type of studies.
Parkinson’s disease
Some Chinese researchers have tested the potential neurotherapeutic role of i-PBMT for PD patients. In the first report, Li et al. (1999b) showed that i-PBMT (633 nm laser, 3.5–5.5 mW for 30 min) once daily for 10 days, resulted in a significant decrease of serum cholecystokinin-octapeptide levels accompanied by improvements in PD symptoms for 60% of patients (26 out of 43), as measured by the Webster Scale scores. Likewise, using the same treatment parameters described above (Li et al., 1999b; Zhao et al., 2003) reported reduction in PD symptoms in 89% of the patients (31 out of 36). In these patients, 28% of them showed significant improvements and 58% had gradual improvements. Xu et al. (2003) also showed that i-PBMT (633 nm laser, 3.5–4.5 mW for 30 min) once daily for 20 days, resulted in a decrease in malondialdehyde and an increase of superoxidase dismutase and melatonin levels accompanied by improvements in PD symptoms for 66% of patients (31 out of 47).
Traumatic brain insults
Traumatic brain injury
Dou et al. (2003) administrated an intranasal probe (650 nm laser, 2.4 mW, 30 min) over 10 days in 50 patients with cerebral infarction and TBI and found a significant decrease in the scores of Fugl Meyer Movement Scale and Barthel Index (activities of daily living). Also, along with reduced brain lesion and edema, measurements of cholesterol, triglycerides, lowdensity lipoprotein, erythrocyte sedimentation rate, and hematocrit were significantly decreased. It should be noted that the PFC and the anterior cingulate gyrus are two regions within the frontal lobes that are mainly susceptible to damage during TBI. Damage to the olfactory bulb and tract can also co-occur along with ventral frontal damage in TBI (Levin et al., 1985; Cicerone and Tanenbaum, 1997). Furthermore, in a randomized, double-blind, sham-controlled pilot trial study in eight veterans with mild-TBI. Bogdanova et al. (2017) reported that combined application of the transcranial LED helmet and intranasal LED applicator for 2 days/week for 8 weeks, resulted in significant improvements in attention and executive function as well as sleep quality at 1-week post-treatment.
Ischemic brain injury
Xiao et al. (2005) reported the immediate beneficial effect of i-PBMT on the cerebral function and blood flow using the single photon emission computed tomography cerebral perfusion imaging method in 21 patients with cerebral infarction. They found that a single session of i-PBMT using a 650 nm laser (3.5–4 mW, for 15 min/nostril) significantly improved regional and global CBF. According to the data from one of the patients who had a cerebral infarction accompanied by PD, a 30-min application of i-PBMT increased the blood supply and function of the left temporal lobe and basal ganglia area (Xiao et al., 2005). Intranasal laser irradiation (650 nm, 8.38 mW/cm2, 30 min) for 10 days has also been shown to improve blood and plasma viscosity, RBCs aggregation as well as blood lipid in patients with cerebral infarction (Liu et al., 2012).
Neuropsychiatric disorders and insomnia
Depression and anxiety
More recently, Caldieraro et al. (2018) reported the overall therapeutic effect of long-term PBMT in a patient with major depression and anxiety. During the first 22 months, single-modality i-PBM (810 nm LED, 10-Hz PW) was applied to both nostrils providing a 10.65 J/cm2 fluence per nostril. With the progressive increase of the frequency of i-PBMT sessions, from twice a week sessions to daily and then to twice daily, the anxiety symptoms steadily regressed with approximately a three-fold reduction in Anxiety Symptoms Questionnaire scores. However, i-PBMT alone did not alleviate the depressive symptoms until an additional 830 nm transcranial laser system was applied to the forehead for the next 9 months. According to their speculation, the systemic effects of i-PBMT via the blood cells possibly contributed to the observed anxiolytic effect (Caldieraro et al., 2018).
Insomnia
There is emerging evidence for the therapeutic application of PBMT in sleep disorders. A 2-week program of red-light irradiation (658 nm, 30 J/cm2) using a whole-body treatment machine has shown to improve sleep quality and serum melatonin levels in healthy adults (Zhao et al., 2012). Sleep disruption is the most common side-effect observed in TBI patients. In studies of the application of t-PBMT in TBI patients, improvement in sleep was reported by many of the patients (Naeser et al., 2011, 2014; Morries et al., 2015). A combined PBMT approach using a transcranial device and an intranasal portable applicator also resulted in better sleep in patients diagnosed with AD (Saltmarche et al., 2017) and TBI (Bogdanova et al., 2017). According to individuals’ report, sleepiness is a common side-effect following the use of 10-Hz intranasal portable devices, possibly due to the pulse rate of the light inducing brain alpha wave (8–12 Hz), while also producing neuronal stimulation (Salehpour et al., 2019). Moreover, in a few studies from China, the therapeutic effects of single-modality i-PBMT (632.8 nm) on the sleep quality of patients with insomnia were demonstrated (Xu et al., 2001, 2002c; Chen and Cheng, 2004). Although mechanisms of action involved in PBMT for sleep improvement still remain largely unknown, modulation of circadian rhythms via an increase in serum melatonin levels (Xu et al., 2001; Liu et al., 2010; Zhao et al., 2012) and stimulation of systemic homeostatic response via the blood circulatory system (Moshkovska and Mayberry, 2005) were postulated as possible mechanistic pathways.
Conclusions and future directions
It should come as no surprise, after our comprehensive review of the literature on i-PBMT, that given the limited scientific evidence for the use of i-PBM, there is not yet any approved indication for the clinical use of this modality.
However, i-PBMT devices to be placed in the nostril are currently in the market for wellness (without disease-specific indication). These devices are extremely convenient; they can be easily clipped onto the nostril and are portable, easy-to-use, home-based devices for self-administration of red and NIR light. They are also relatively inexpensive when compared to other devices in the market for t-PBMT use. They represent an ideal first-step for the users of red and NIR light; especially, when users who are interested in leveraging the systemic effects of photobiomodulation for wellness. The actions involved in the neurotherapeutic benefit of nostril-based i-PBMT are only beginning to be understood. A systemic effect via blood cells and components is the most likely mechanism. The release of NO induced in the nasal arteries by intranasal red/NIR light irradiation could increase microvascular circulation by inhibition of platelet aggregation and leukocyte adhesion. Nostril-based i-PBMT also improves hemorheology and local blood O2 saturation, which may ultimately lead to enhanced cerebral circulation and brain function. We have discussed several possible additional pathways including effects on the olfactory nerves and bulb, endothelium, autonomic nervous system, and the lymphatic system. Moreover, systemic activation of the MSCs residing in the nasal bone marrow and olfactory ensheathing cells in the nasal mucosa was speculated. Because of the increasing societal awareness for the need to enhance and maintain brain health – to counteract the threat of neurodegenerative diseases and to offset the disability burden of neuropsychiatric disorders – intranostrili-PBMT for wellness should be appealing to many. Of note, while several systemic effects were postulated there is no yet proof that they would translate in greater mind-body wellbeing or in prevention of brain disorders. Because the nostril-based i-PBMT is so convenient and intuitive to use, it also represents an ideal first intervention when using PBMT for brain disorders. This clinical use is off-label, and should be considered only when patients do not respond or tolerate available approved interventions or decline approved interventions or when no evidence-based interventions exist. The drawback and limitation of intra-nostril i-PBMT is that no direct neuromodulation of the brain is expected, based on dosimetry studies which showed negligible light deposition to the brain.
As concerns indwelling devices for i-PBMT, a primary distinction should be made based on current technological advancements. The most futuristic i-PBMT interventions, such as indwelling devices in the sphenoid sinus or in the submucosal space adjacent to the cribriform plate, are impractical. In fact, both are currently unattainable. The surgical interventions that would be required to insert an LED device to the back of the nose or to the ceiling of the nose are limited by the millimeter-thin, curvy and rigid intranasal cavities which are prohibitive with current surgical tools, unless surgical demolition of bone structures is performed. The nasal bone structures are extremely thin – also millimeter-thin – the space is highly vascularized, which leads to risk of profuse bleedings, of olfactory nerve damage, of leak of cerebrospinal fluids and potentially also of brain structures’ damage (e.g. hypophysis). It is therefore understandable that these futuristic applications of deep i-PBMT are currently prohibitive. It follows that the claims of deep-nose i-PBMT providing superior light fluence to the vmPFC and vmOFC regions, and theoretically even to the limbic system, are still highly speculative.
On the contrary, indwelling mid-nose submucosal i-PBMT devices as well as frontal sinus (Figure 2) i-PBMT devices are realistic and offer several advantages when compared to intra-nostril and t-PBM. Most importantly, these indwelling devices would require minimally invasive ENT surgeries equivalent to septoplasty, therefore they could be placed during an outpatient day-surgery. While the NIR light shedding to vmPFC and vmOFC would be equivalent to depositions achieved by t-PBMT – equivalent to external light sources placed on the skin in proximity of the frontal poles, – i-PBMT indwelling devices have potential advantages when compared to t-PBMT. In fact, some limitations of t-PBMT were already identified thanks to its off-label use in neuropsychiatric and neurorehab PBMT clinics. First, t-PBMT either requires in-office visits for laser light delivery or self-administration with LED devices; both scenarios are time-consuming, as even at home self-administration with LED devices might require up to 60 min of daily sessions to achieve neurotherapeutic effects (as many of the at-home t-PBMT LED devices have low irradiance). This time commitment, required by t-PBMT, is prohibitive to patients who are still highly functioning. Mid-nose or frontal sinus, indwelling i-PBMT would circumvent this limitation as treatments could be programmed and delivered automatically without any interference with user’s routine. Second, in the case of the cognitively or physically impaired patients, t-PBMT – even at home – requires the assistance for placement and monitoring of light delivery by family members or other caregivers. This additional burden on caregivers, typically overwhelmed by the care of developmentally delayed or demented patients, would also be off-set by indwelling i-PBMT devices. Third, a new-generation of smart-neuromodulator devices that can both ‘read’ the state of the brain and ‘write’ into the brain – otherwise respond with adequate stimuli to support brain function – are being developed. t-PBMT is the immediate and most logical modality for this new generation of closed-loop devices integrating both brain activity sensors and red/NIR stimulations to the brain. Nonetheless, i-PBMT indwelling devices offer an additional opportunity as they could allow real-time, automated interventions such as delivery of light when t-PBMT might be impractical or when it requires a conscious effort from the patient. This could include critical scenarios such as the occurrence of sudden cravings for addictive substances or of impulses to commit self-harm or suicide. Of note, despite the aforementioned advantages of i-PBMT over t-PBMT, it is yet to be demonstrated whether the two modalities have equivalent therapeutic effects for brain disorders; some putative mechanisms of action of NIR might exclusively apply to t-PBMT (Herisson et al., 2018).
Overall, while intra-nostril PBMT for wellness is available, its efficacy in achieving or maintaining brain health is still unproven. Submucosal and frontal indwelling i-PBMT has potential as a novel neurotherapeutic modality, however, neither devices are available in the market nor is the scientific evidence to support its use. Deep-nose (cribriform plate or sphenoid sinus), indwelling i-PBMT is still speculative and not yet attainable with current technology and surgical procedures.
Acknowledgments
Conflict of interest statement: F.S. is on the Scientific Advisory Board and consultant of Niraxx Light Therapeutics Inc., and a consultant of ProNeuroLIGHT LLC. T.W. was supported by the European Union’s Horizon 2020 Research and Innovation Programme, under the Marie Skłodowska-Curie Grant Agreement No 713690, and by the Science Foundation Ireland (SFI) and the European Regional Development Fund (Grant Number 13/RC/2073). P.C.’s salary was supported by the Harvard Psychiatry Department (Dupont-Warren Fellowship and Livingston Award), by the Brain and Behavior Research Foundation (NARSAD Young Investigator Award) and by the Photothera Inc. unrestricted grant. Drug donation from TEVA. Travel reimbursement from Pharmacia-Upjohn. P.C. has received consultation fees from Janssen Research and Development. P.C. has filed a provisional patent related to the use of near-infrared light in psychiatry. PhotoMedex, Inc. supplied four devices for a clinical study. P.C. is/has 1. Received unrestricted funding from Litecure Inc. to conduct a study on transcranial photobiomodulation for the treatment of major depressive disorder; 2. Received unrestricted funding from Cerebral Sciences to conduct a study on transcranial photobiomodulation for the treatment of generalized anxiety disorder; 3. Co-founded, member of the board of directors and consultant of Niraxx Light Therapeutics Inc., a company focused on the development of new modalities of treatment based on near-infrared light. M.R.H. was supported by US NIH Grants R01AI050875 and R21AI121700. M.R.H. is on the following Scientific Advisory Boards: Transdermal Cap Inc., Cleveland, OH, USA; Photothera Inc., Carlsbad, CA, USA; BeWell Global Inc., Wan Chai, Hong Kong; Hologenix Inc., Santa Monica, CA, USA; LumiThera, Inc., Poulsbo, WA, USA; Vielight, Toronto, ON, Canada; Bright Photomedicine, São Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA, USA; Global Photon Inc, Bee Cave, TX, USA; Medical Coherence, Boston, MA, USA; NeuroThera, Newark, DE, USA; JOOVV Inc., Minneapolis–Saint Paul, MN, USA; AIRx Medical, Inc., Pleasanton, CA, USA; FIR Industries, Inc., Ramsey, NJ, USA; UVLRx Therapeutics, Oldsmar, FL, USA; Ultralux UV Inc., Lansing, MI, USA; Illumiheal&Petthera, Shoreline, WA, USA; MB Laser Therapy, Houston, TX, USA. M.R.H. has been a Consultant for: Lexington int., Boca Raton, FL, USA; USHIO Corp., Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V.; Johnson & Johnson Inc., Philadelphia, PA; Sanofi-Aventis Deutschland GmbH, Frankfurt, Germany. M.R.H. is a stockholder in Global Photon Inc., Bee Cave, TX, USA and Mitonix, Newark, DE, USA. J.O.D. was a paid business consultant for ProNeuroLIGHT LLC, Kerrville, TX, USA. The other authors have no conflicts of interest to disclose.
Contributor Information
Farzad Salehpour, Neurosciences Research Center, Tabriz University of Medical Sciences, Tabriz 5166614756, Iran.
Sevda Gholipour-Khalili, Neurosciences Research Center, Tabriz University of Medical Sciences, Tabriz 5166614756, Iran.
Fereshteh Farajdokht, Neurosciences Research Center, Tabriz University of Medical Sciences, Tabriz 5166614756, Iran.
Farzin Kamari, Neurosciences Research Center, Tabriz University of Medical Sciences, Tabriz 5166614756, Iran.
Tomasz Walski, Centre for Research in Medical Devices (CÚRAM), National University of Ireland Galway, Galway H91 W2TY, Ireland; and Department of Biomedical Engineering, Wrocław University of Science and Technology, Wrocław 50-370, Poland.
Michael R. Hamblin, Wellman Center for Photomedicine, Massachusetts General Hospital, Harvard Medical School, 50 Blossom Street, Boston, MA 02114, USA; Department of Dermatology, Harvard Medical School, 40 Blossom St, Boston, MA 02114, USA; and Laser Research Centre, Faculty of Health Science, University of Johannesburg, Doornfontein 2028, South Africa
Joseph O. DiDuro, ProNeuroLIGHT LLC, 3504 W Buckhorn Trail, Phoenix, AZ 85083, USA; and Neuropathy Treatment Centers of America LLC, Phoenix, AZ, USA.
Paolo Cassano, Department of Psychiatry, Harvard Medical School, Boston, MA 02114, USA; Depression Clinical and Research Program, Department of Psychiatry, Massachusetts General Hospital, Bowdoin Square, Boston, MA 02114, USA; and Center for Anxiety and Traumatic Stress Disorders, Department of Psychiatry, Massachusetts General Hospital, Boston, MA 02114, USA.
References
- Amar AP and Weiss MH (2003). Pituitary anatomy and physiology. Neurosurg. Clin 14, 11–23. [DOI] [PubMed] [Google Scholar]
- Anand KS and Dhikav V (2012). Hippocampus in health and disease: an overview. Ann. Ind. Acad. Neurol 15, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arany PR, Cho A, Hunt TD, Sidhu G, Shin K, Hahm E, Huang GX, Weaver J, Chen AC-H, Padwa BL, et al. (2014). Photoactivation of endogenous latent transforming growth factor-β1 directs dental stem cell differentiation for regeneration. Sci. Transl. Med 6, 238ra269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asimov M, Korolevich A, and Konstantinova E (2007). Kinetics of oxygenation of skin tissue exposed to low-intensity laser radiation. J. Appl. Spectrosc 74, 133–139. [Google Scholar]
- Barrett DW and Gonzalez-Lima F (2013). Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 230, 13–23. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Spalding KL, and Frisén J (2015). Adult neurogenesis in humans. Cold Spring Harb. Perspect. Biol 7, a018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman MH, Halper JP, Nichols TW, Jarrett H, Lundy A, and Huang JH (2017). Photobiomodulation with near infrared light helmet in a pilot, placebo controlled clinical trial in dementia patients testing memory and cognition. J. Neurol. Neurosci 8, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova Y, Ho V, Martin P, Ho M, Yee M, Hamblin M, and Naeser M (2017). Transcranial LED treatment for cognitive dysfunction and sleep in chronic TBI: randomized controlled pilot trial. Arch. Phys. Med. Rehabil 98, e122–e123. [Google Scholar]
- Bomboi G, Castello L, Cosentino F, Giubilei F, Orzi F, and Volpe M (2010). Alzheimer’s disease and endothelial dysfunction. Neurol. Sci 31, 1–8. [DOI] [PubMed] [Google Scholar]
- Brill AG, Shenkman B, Brill GE, Tamarin I, Dardik R, Kirichuk VF, Savion N, and Varon D (2000). Blood irradiation by He-Ne laser induces a decrease in platelet responses to physiological agonists and an increase in platelet cyclic GMP. Platelets 11, 87–93. [DOI] [PubMed] [Google Scholar]
- Budu V, Mogoanta CA, Fanuta B, and Bulescu I (2013). The anatomical relations of the sphenoid sinus and their implications in sphenoid endoscopic surgery. Rom. J. Morphol. Embryol 54, 13–16. [PubMed] [Google Scholar]
- Burchman MA (2011). Using photobiomodulation on a severe Parkinson’s patient to enable extractions, root canal treatment, and partial denture fabrication. J. Laser Dent 19, 297–300. [Google Scholar]
- Byrnes KR, Waynant RW, Ilev IK, Wu X, Barna L, Smith K, Heckert R, Gerst H, and Anders JJ (2005). Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury. Lasers Surg. Med 36, 171–185. [DOI] [PubMed] [Google Scholar]
- Caldieraro MA and Cassano P (2019). Transcranial and systemic photobiomodulation for major depressive disorder: a systematic review of efficacy, tolerability and biological mechanisms. J. Affect. Disord 243, 262–273. [DOI] [PubMed] [Google Scholar]
- Caldieraro MA, Sani G, Bui E, and Cassano P (2018). Long-term near-infrared photobiomodulation for anxious depression complicated by Takotsubo cardiomyopathy. J. Clin. Psychopharmacol 38, 268–270. [DOI] [PubMed] [Google Scholar]
- Cassano P, Tran AP, Katnani H, Bleier BS, Hamblin MR, Yuan Y, and Fang Q (2019). Selective photobiomodulation for emotion regulation: model-based dosimetry study. Neurophotonics 6, 015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AS, Lee TL, Yeung MK, and Hamblin MR (2019). Photobiomodulation improves the frontal cognitive function of older adults. Int. J. Geriatr. Psychiatry 34, 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Wang R, Li C, Wang Y, and Chu X-P (2018). Transcranial low-level laser therapy for depression and Alzheimer’s disease. Neuropsychiatry 8, 477–483. [Google Scholar]
- Chao LL (2019). Effects of home photobiomodulation treatments on cognitive and behavioral function, cerebral perfusion, and resting-state functional connectivity in patients with dementia: a pilot trial. Photobiomodul. Photomed. Laser Surg 37, 133–141. [DOI] [PubMed] [Google Scholar]
- Chen Y and Cheng H (2004). Clinical observation of the integrated therapy of intranasal low intensity He-Ne laser therapy and herb therapy on insomnia. J. Tradit. Chin. Med 24, 38. [Google Scholar]
- Cheon SY, Kim EJ, Kim JM, Kam EH, Ko BW, and Koo B-N (2017). Regulation of microglia and macrophage polarization via apoptosis signal-regulating kinase 1 silencing after ischemic/hypoxic injury. Front. Mol. Neurosci 10, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chludzińska L, Ananicz E, Jarosawska A, and Komorowska M (2005). Near-infrared radiation protects the red cell membrane against oxidation. Blood Cells Mol. Dis 35, 74–79. [DOI] [PubMed] [Google Scholar]
- Choi R and Goldstein BJ (2018). Olfactory epithelium: cells, clinical disorders, and insights from an adult stem cell niche. Laryngoscope Investig. Otolaryngol 3, 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrapko W, Jurasz P, Radomski MW, Archer SL, Newman SC, Baker G, Lara N, and Le Mellédo J-M (2006). Alteration of decreased plasma NO metabolites and platelet NO synthase activity by paroxetine in depressed patients. Neuropsychopharmacology 31, 1286. [DOI] [PubMed] [Google Scholar]
- Chung H, Dai T, Sharma SK, Huang Y-Y, Carroll JD, and Hamblin MR (2012). The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng 40, 516–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicerone KD and Tanenbaum LN (1997). Disturbance of social cognition after traumatic orbitofrontal brain injury. Arch. Clin. Neuropsychol 12, 173–188. [PubMed] [Google Scholar]
- Clark DL, Boutros NN, and Mendez MF (2018). Frontal lobe In: The Brain and Behavior: An Introduction to Behavioral Neuroanatomy. Clark DL, Mendez MF, and Boutros NN, eds. (Cambridge: Cambridge University Press; ). pp. 73–102. [Google Scholar]
- Cragg SJ, Baufreton J, Xue Y, Bolam JP, and Bevan MD (2004). Synaptic release of dopamine in the subthalamic nucleus. Eur. J. Neurosci 20, 1788–1802. [DOI] [PubMed] [Google Scholar]
- de Freitas LF and Hamblin MR (2016). Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top. Quantum Electron 22, 348–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima FM, Villaverde A, Albertini R, Corrêa J, Carvalho R, Munin E, Araújo T, Silva J, and Aimbire F (2011). Dual effect of low-level laser therapy (LLLT) on the acute lung inflammation induced by intestinal ischemia and reperfusion: action on anti- and pro-inflammatory cytokines. Lasers Surg. Med 43, 410–420. [DOI] [PubMed] [Google Scholar]
- Delong MR, Crutcher MD, and Georgopoulos AP (1983). Relations between movement and single cell discharge in the substantia nigra of the behaving monkey. J. Neurosci 3, 1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro TM, Attawia M, Lilienfeld S, and Holy C (2008). Intranasal red light probe for treating Alzheimer’s disease. Google Patents. [Google Scholar]
- DiMauro TM, Attawia M, Lilienfeld S, and Holy C (2014). Intranasal red light probe for treating Alzheimer’s disease. Google Patents. [Google Scholar]
- DiMauro TM, Wildenhaus K, Pracyk J, and Luedtke M (2018). Intranasal insert for OFC neuroprotection. Google Patents. [Google Scholar]
- Dou Z, Xiquan H, and Zhu H (2003). The effects of two kinds of laser irradiation on patients with brain lesion. Chin. J. Phys. Med. Rehabil 2, 38–43. [Google Scholar]
- Drohomirecka A, Iwaszko A, Walski T, Pliszczak-Król A, Wąż G, Graczyk S, Gałecka K, Czerski A, Bujok J, and Komorowska M (2018). Low-level light therapy reduces platelet destruction during extracorporeal circulation. Sci. Rep 8, 16963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Humeau Y, Grenier F, Ciocchi S, Herry C, and Lüthi A (2009). Amygdala inhibitory circuits and the control of fear memory. Neuron 62, 757–771. [DOI] [PubMed] [Google Scholar]
- El Massri N, Lemgruber AP, Rowe IJ, Moro C, Torres N, Reinhart F, Chabrol C, Benabid A-L, and Mitrofanis J (2017). Photobiomodulation-induced changes in a monkey model of Parkinson’s disease: changes in tyrosine hydroxylase cells and GDNF expression in the striatum. Exp. Brain Res 235, 1861–1874. [DOI] [PubMed] [Google Scholar]
- Elwood PC, Pickering J, and Gallacher JE (2001). Cognitive function and blood rheology: results from the Caerphilly cohort of older men. Age Ageing 30, 135–139. [DOI] [PubMed] [Google Scholar]
- Erdem G, Erdem T, Miman MC, and Ozturan O (2004). A radiological anatomic study of the cribriform plate compared with constant structures. Rhinology 42, 225–229. [PubMed] [Google Scholar]
- Fitzgerald M, Hodgetts S, Van Den Heuvel C, Natoli R, Hart NS, Valter K, Harvey AR, Vink R, Provis J, and Dunlop SA (2013). Red/near-infrared irradiation therapy for treatment of central nervous system injuries and disorders. Rev. Neurosci 24, 205–226. [DOI] [PubMed] [Google Scholar]
- Freeman JL (2003). The anatomy and embryology of the hypothalamus in relation to hypothalamic hamartomas. Epileptic Disord. 5, 177–186. [PubMed] [Google Scholar]
- Frey W (1991). Neurologic agents for nasal administration to the brain. World Intellect. Prop. Organ 5, 89. [Google Scholar]
- Fukuzaki Y, Sugawara H, Yamanoha B, and Kogure S (2013). 532 nm low-power laser irradiation recovers γ-secretase inhibitor-mediated cell growth suppression and promotes cell proliferation via Akt signaling. PLoS One 8, e70737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuzaki Y, Shin H, Kawai HD, Yamanoha B, and Kogure S (2015). 532 nm low-power laser irradiation facilitates the migration of GABAergic neural stem/progenitor cells in mouse neocortex. PLoS One 10, e0123833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganeshan V, Skladnev NV, Kim JY, Mitrofanis J, Stone J, and Johnstone DM (2019). Pre-conditioning with remote photobiomodulation modulates the brain transcriptome and protects against MPTP insult in mice. Neuroscience 400, 85–97. [DOI] [PubMed] [Google Scholar]
- Gao Z-S, Zhang L, and Qin C.-l. (2004). The relationship between hemorheological changes and the anxiety and depression symptoms in schizophrenia. Chin. J. Hemorheol 1. [Google Scholar]
- Gao X, Zhi P, and Wu X (2008). Low-energy semiconductor laser intranasal irradiation of the blood improves blood coagulation status in normal pregnancy at term. Nan Fang Yi Ke Da Xue Xue Bao 28, 1400–1401. [PubMed] [Google Scholar]
- Gisbrecht A, Mamilov S, Esman S, and Asimov M (2017). Estimation of the quantum efficiency of the photodissociation of HbO2 and HbCO. 19th International Conference and School on Quantum Electronics: Laser Physics and Applications International Society for Optics and Photonics, p. 1022619. [Google Scholar]
- Gloor P (1978). Inputs and Outputs of the Amygdala: What the Amygdala is Trying to Tell the Rest of the Brain Limbic Mechanisms (Boston, MA, USA: Springer; ), pp. 189–209. [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, and Menon V (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc. Natl Acad Sci. U.S.A 101, 4637–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo SL, Duggett NA, Ennaceur A, and Chazot PL (2013). Non-invasive infra-red therapy (1072 nm) reduces β-amyloid protein levels in the brain of an Alzheimer’s disease mouse model, TASTPM. J. Photochem. Photobiol. B Biol 123, 13–22. [DOI] [PubMed] [Google Scholar]
- Hajos M and Greenfield S (1994). Synaptic connections between pars compacta and pars reticulata neurones: electrophysiological evidence for functional modules within the substantia nigra. Brain Res. 660, 216–224. [DOI] [PubMed] [Google Scholar]
- Hamblin MR (2016). Shining light on the head: photobiomodulation for brain disorders. BBA Clin. 6, 113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson TA and Morries LD (2015). Near-infrared photonic energy penetration: can infrared phototherapy effectively reach the human brain? Neuropsychiatr. Dis. Treat 11, 2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessy M and Hamblin MR (2016). Photobiomodulation and the brain: a new paradigm. J. Opt 19, 013003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herisson F, Frodermann V, Courties G, Rohde D, Sun Y, Vandoorne K, Wojtkiewicz GR, Masson GS, Vinegoni C, Kim J, et al. (2018). Direct vascular channels connect skull bone marrow and the brain surface enabling myeloid cell migration. Nat. Neurosci 21, 1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiser J and Koenigs M (2018). The multifaceted role of the ventromedial prefrontal cortex in emotion, decision making, social cognition, and psychopathology. Biol. Psychiatr 83, 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hroudová J, Fišar Z, Kitzlerová E, Zvěřová M, and Raboch J (2013). Mitochondrial respiration in blood platelets of depressive patients. Mitochondrion 13, 795–800. [DOI] [PubMed] [Google Scholar]
- Iaccarino HF, Singer AC, Martorell AJ, Rudenko A, Gao F, Gillingham TZ, Mathys H, Seo J, Kritskiy O, and Abdurrob F (2016). Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilango A, Kesner AJ, Keller KL, Stuber GD, Bonci A, and Ikemoto S (2014). Similar roles of substantia nigra and ventral tegmental dopamine neurons in reward and aversion. J. Neurosci 34, 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R and Amaral DG (2012). Chapter 24 – hippocampal formation In: The Human Nervous System (Third Edition). Mai JK and Paxinos G, eds. (San Diego: Academic Press; ). pp. 896–942. [Google Scholar]
- Itoh T, Murakami H, Orihashi K, Sueda T, and Matsuura Y (1996). The protective effect of low power He-Ne laser against erythrocytic damage caused by artificial heart-lung machines. Hiroshima J. Med. Sci 45, 15–22. [PubMed] [Google Scholar]
- Jagdeo JR, Adams LE, Brody NI, and Siegel DM (2012). Transcranial red and near infrared light transmission in a cadaveric model. PLoS One 7, e47460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jianbo X, Qiuyun C, and Jianxin X (1999). Effects of laser irradiation therapy through nasal cavity on viscosity at lower shear rates and hematocrit in patients with acute ischemic cerebrovascular disease (AICVD). J. Binzhou Med. Coll 6, 006. [Google Scholar]
- Jiang Y, Li Y, and Liu X (2015). Intranasal delivery: circumventing the iron curtain to treat neurological disorders. Expert. Opin. Drug Deliv 12, 1717–1725. [DOI] [PubMed] [Google Scholar]
- Jiao J-L, Liu TC-Y, Liu J, Cui L-P, and Liu S-H (2006). Advances in endonasal low intensity laser irradiation therapy. 2004 Shanghai International Conference on Laser Medicine and Surgery International Society for Optics and Photonics, p. 59671C. [Google Scholar]
- Jin L, Shi B, and Zhou C (2000). The effecton serum amyloid bprotein of patients with mild cognitive impairment after semiconductor laser therapy. Acta Acad. Med. Qingdao Univ 36, 175–176. [Google Scholar]
- Jing WYZ (1999). Vascular low level laser irradiation therapy in treatment of brain injury. Acta Laser Biol. Sin 8. [Google Scholar]
- Karu T (1988). Molecular mechanism of the therapeutic effect of low-intensity laser radiation. Lasers Life Sci. 2, 53–74. [Google Scholar]
- Karu TI (2010). Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life 62, 607–610. [DOI] [PubMed] [Google Scholar]
- Karu TI, Pyatibrat LV, and Afanasyeva NI (2005). Cellular effects of low power laser therapy can be mediated by nitric oxide. Lasers Surg. Med 36, 307–314. [DOI] [PubMed] [Google Scholar]
- Koh E, Frazzini VI, and Kagetsu NJ (2000). Epistaxis: vascular anatomy, origins, and endovascular treatment. Am. J. Roentgenol. Radium 174, 845–851. [DOI] [PubMed] [Google Scholar]
- Komorowska M, Cuissot A, Czarnoleski A, and Bialas W (2001). Erythrocyte response to near infrared radiation. Cell. Mol. Biol. Lett 6, 212. [PubMed] [Google Scholar]
- Komorowska M, Cuissot A, Czarnołȩski A, and Białas W (2002). Erythrocyte response to near-infrared radiation. J. Photochem. Photobiol. B Biol 68, 93–100. [DOI] [PubMed] [Google Scholar]
- Kujawa J, Zavodnik L, Zavodnik I, Buko V, Lapshyna A, and Bryszewska M (2004). Effect of low-intensity (3.75–25 J/cm2) near-infrared (810 nm) laser radiation on red blood cell ATPase activities and membrane structure. J. Clin. Laser Med. Surg 22, 111–117. [DOI] [PubMed] [Google Scholar]
- Lavoie B, Smith Y, and Parent A (1989). Dopaminergic innervation of the basal ganglia in the squirrel monkey as revealed by tyrosine hydroxylase immunohistochemistry. J. Comp. Neurol 289, 36–52. [DOI] [PubMed] [Google Scholar]
- LeDoux J (2007). The amygdala. Curr. Biol 17, R868–R874. [DOI] [PubMed] [Google Scholar]
- Levin H, High W, and Eisenberg H (1985). Impairment of olfactory recognition after closed head injury. Brain 108, 579–591. [DOI] [PubMed] [Google Scholar]
- Li Q, Guo K, Kang J, and Jiang B (1998a). Clinic analysis of endonasal low energy He-Ne laser treatment of 39 cases of intractable headache. Acta Acad. Med. Qingdao Univ 1, 53. [Google Scholar]
- Li Q, Guo K, Kang J, Jiang B, and Wang Y (1998b). β endorphin research for endonasal low energy He-Ne laser treatment of ache in head or face. Chin. J. Neurol 31, 91. [Google Scholar]
- Li Q, Kang J, Song Y, Shen L, Liu Z, and Tan J (1999a). Electron microscope observation of erythrocytes of cerebral thrombosis treated by endonasal low energy He-Ne laser. Shandong Med. Pharm 39, 3–4. [Google Scholar]
- Li Q, Song L, Guo K, Yu Y, Ma S, and Shen L (1999b). The effect of endonasal low energy He-Ne laser treatment of Parkinson’s disease on CCK-8 content in blood. Chin. J. Neurol 32, 364. [Google Scholar]
- Li Q-M, Guo K, and Tang J-Q (1999c). Effects on plasma CCK-8 content of low power laser nasal cavity directional irradiation in treatment on Parkinson. Chin. J. Neurol 32, 364. [Google Scholar]
- Liao Z (2000). Nursing patients of schizophrenia treated by intranasal low energy He-Ne laser therapy. J. Jiangxi Uni. Tradit. Chin. Med 12, 140. [Google Scholar]
- Lim L (2014). Intranasal Photobiomodulation Improves Cognitive and Memory Performance of Alzheimer’s Disease Patients in Case Studies (NAALT/WALT, Arlington, Virginia, USA: ). [Google Scholar]
- Litscher D and Litscher G (2013). Laser therapy and stroke: quantification of methodological requirements in consideration of yellow laser. Int. J. Photoenergy 575798, 1–4. [Google Scholar]
- Liu TC-Y, Jiaob J-L, Lianga J, and Liu S-H (2007). Mechanism of Itranasal Low Intensity Laser Irradiation Therapy. World Symposium on TCM Acupuncture and Moxibustion, Tarragona, Spain. [Google Scholar]
- Liu TC-Y, Wu D-F, Gu Z-Q, and Wu M (2010). Applications of intranasal low intensity laser therapy in sports medicine. J. Innov. Opt. Health Sci 3, 1–16. [Google Scholar]
- Liu TC-Y, Cheng L, Su W-J, Zhang Y-W, Shi Y, Liu A-H, Zhang L-L, and Qian Z-Y (2012). Randomized, double-blind, and placebo-controlled clinic report of intranasal low-intensity laser therapy on vascular diseases. Int. J. Photoenergy 489713, 1–5. [Google Scholar]
- Lohr NL, Keszler A, Pratt P, Bienengraber M, Warltier DC, and Hogg N (2009). Enhancement of nitric oxide release from nitrosyl hemoglobin and nitrosyl myoglobin by red/near infrared radiation: potential role in cardioprotection. J. Mol. Cell. Cardiol 47, 256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacArthur FJ and McGarry GW (2017). The arterial supply of the nasal cavity. Eur. Arch. Otorhinolaryngol 274, 809–815. [DOI] [PubMed] [Google Scholar]
- Maksimovich IV (2004). Transluminal laser angioplasty in treatment of ischemic lesions of a brain MD Dissertation (Russian University of Friendship of the People, Moscow: ). [Google Scholar]
- Marianecci C, Rinaldi F, Hanieh PN, Di Marzio L, Paolino D, and Carafa M (2017). Drug delivery in overcoming the blood–brain barrier: role of nasal mucosal grafting. Drug Des. Devel. Ther 11, 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masurkar AV and Chen WR (2009). Olfactory bulb physiology In: Encyclopedia of Neuroscience. Squire LR, ed. (Oxford: Academic Press; ). pp. 77–86. [Google Scholar]
- Mi X, Chen J, Cen Y, Liang Z, and Zhou L (2004). A comparative study of 632.8 and 532 nm laser irradiation on some rheological factors in human blood in vitro. J. Photochem. Photobiol. B, Biol 74, 7–12. [DOI] [PubMed] [Google Scholar]
- Michalikova S, Ennaceur A, van Rensburg R, and Chazot PL (2008). Emotional responses and memory performance of middle-aged CD1 mice in a 3D maze: effects of low infrared light. Neurobiol. Learn. Mem 89, 480–488. [DOI] [PubMed] [Google Scholar]
- Moore K, Dalley A, and Agur A (1999). Clinically Oriented Anatomy (Philadelphia: Lippincott, Williams & Wilkins; ). [Google Scholar]
- Morries LD, Cassano P, and Henderson TA (2015). Treatments for traumatic brain injury with emphasis on transcranial near-infrared laser phototherapy. Neuropsychiatr. Dis. Treat 11, 2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshkovska T and Mayberry J (2005). It is time to test low level laser therapy in Great Britain. Postgrad. Med. J 81, 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers-Schulz B and Koenigs M (2012). Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol. Psychiatr 17, 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Saltmarche A, Krengel MH, Hamblin MR, and Knight JA (2011). Improved cognitive function after transcranial, light-emitting diode treatments in chronic, traumatic brain injury: two case reports. Photobiomodul. Photomed. Laser Surg 29, 351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser MA, Zafonte R, Krengel MH, Martin PI, Frazier J, Hamblin MR, Knight JA, Meehan III WP, and Baker EH (2014). Significant improvements in cognitive performance post-transcranial, red/near-infrared light-emitting diode treatments in chronic, mild traumatic brain injury: open-protocol study. J. Neurotrauma 31, 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natzle WC and Moore CB (1985). Recombination of hydrogen ion (H+) and hydroxide in pure liquid water. J. Phys. Chem 89, 2605–2612. [Google Scholar]
- Natzle WC, Moore CB, Goodall D, Frisch W, and Holzwarth J (1981). Dissociative ionization of water induced by single-photon vibrational excitation. J. Phys. Chem 85, 2882–2884. [Google Scholar]
- Netter FH (2017). Atlas of Human Anatomy E-Book (Elsevier Health Sciences, Philadelphia, PA, USA: ), pp. 61–64. [Google Scholar]
- Oliveira MC Jr, Greiffo FR, Rigonato-Oliveira NC, Custódio RWA, Silva VR, Damaceno-Rodrigues NR, Almeida FM, Albertini R, Lopes-Martins RÁB, and de Oliveira LVF (2014). Low level laser therapy reduces acute lung inflammation in a model of pulmonary and extrapulmonary LPS-induced ARDS. J. Photochem. Photobiol. B, Biol 134, 57–63. [DOI] [PubMed] [Google Scholar]
- Parent AD and Perkins E (2018). Chapter 30 – the hypothalamus In: Fundamental Neuroscience for Basic and Clinical Applications (Fifth Edition). Haines DE and Mihailoff GA, eds. (Elsevier; ). pp. 442–456. [Google Scholar]
- Pfleiderer M, Tardy YS, and Lovisa B (2017). Transnasal delivery of low level light via the sphenoidal sinus to irradiate the substantia nigra. Google Patents. [Google Scholar]
- Pitzschke A, Lovisa B, Seydoux O, Zellweger M, Pfleiderer M, Tardy Y, and Wagnières G (2015). Red and NIR light dosimetry in the human deep brain. Phys. Med. Biol 60, 2921. [DOI] [PubMed] [Google Scholar]
- Rajagopal M and Paul J (2005). Applied anatomy and physiology of the airway and breathing. Ind. J. Anaesth 49, 251–256. [Google Scholar]
- Ritchie K and Touchon J (2000). Mild cognitive impairment: conceptual basis and current nosological status. Lancet 355, 225–228. [DOI] [PubMed] [Google Scholar]
- Ritter FN (1970). The vasculature of the nose. Ann. Otol. Rhinol. Laryngol 79, 468–474. [DOI] [PubMed] [Google Scholar]
- Rola P, Doroszko A, Szahidewicz-Krupska E, Rola P, Dobrowolski P, Skomro R, Szymczyszyn A, Mazur G, and Derkacz A (2017). Low-level laser irradiation exerts antiaggregative effect on human platelets independently on the nitric oxide metabolism and release of platelet activation markers. Oxid. Med. Cell. Longev 2017, [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET and Grabenhorst F (2008). The orbitofrontal cortex and beyond: from affect to decision-making. Prog. Neurobiol 86, 216–244. [DOI] [PubMed] [Google Scholar]
- Roy M, Shohamy D, and Wager TD (2012). Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn. Sci 16, 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Mitz AR, Chacko RV, and Murray EA (2013). Effects of amygdala lesions on reward-value coding in orbital and medial prefrontal cortex. Neuron 80, 1519–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber EL, Lopez de Armentia M, and Power J (2003). The amygdaloid complex: anatomy and physiology. Physiol. Rev 83, 803–834. [DOI] [PubMed] [Google Scholar]
- Salehpour F, De Taboada L, Cassano P, Kamari F, Mahmoudi J, Ahmadi-Kandjani S, Rasta SH, and Sadigh-Eteghad S (2018a). A protocol for transcranial photobiomodulation therapy in mice. J. Vis. Exp e59076. [DOI] [PubMed] [Google Scholar]
- Salehpour F, Mahmoudi J, Kamari F, Sadigh-Eteghad S, Rasta SH, and Hamblin MR (2018b). Brain photobiomodulation therapy: a narrative review. Mol. Neurobiol 55, 6601–6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehpour F, Hamblin MR, and DiDuro JO (2019). Rapid reversal of cognitive decline, olfactory dysfunction, and quality of life using multi-modality photobiomodulation therapy: case report. Photobiomodul. Photomed. Laser Surg 37, 159–167. [DOI] [PubMed] [Google Scholar]
- Saltmarche AE, Naeser MA, Ho KF, Hamblin MR, and Lim L (2017). Significant improvement in cognition in mild to moderately severe dementia cases treated with transcranial plus intranasal photobiomodulation: case series report. Photobiomodul. Photomed. Laser Surg 35, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer AP and Trelles MA (2014). Light pumping energy into blood mitochondria: a new trend against depression? Photobiomodul. Photomed. Laser Surg 32, 59–60. [DOI] [PubMed] [Google Scholar]
- Song JW, Li K, Liang ZW, Dai C, Shen XF, Gong YZ, Wang S, Hu XY, and Wang Z (2017). Low-level laser facilitates alternatively activated macrophage/microglia polarization and promotes functional recovery after crush spinal cord injury in rats. Sci. Rep 7, 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song-Lin X (1997). Laser medicine. Chin. Med. J 76, 939–940. [Google Scholar]
- Stadler I, Evans R, Kolb B, Naim JO, Narayan V, Buehner N, and Lanzafame RJ (2000). In vitro effects of low-level laser irradiation at 660 nm on peripheral blood lymphocytes. Lasers Surg. Med 27, 255–261. [DOI] [PubMed] [Google Scholar]
- Stern M, Broja M, Sansone R, Gröne M, Skene SS, Liebmann J, Suschek CV, Born M, Kelm M, and Heiss C (2018). Blue light exposure decreases systolic blood pressure, arterial stiffness, and improves endothelial function in humans. Eur. J. Prev. Cardiol 25, 1875–1883. [DOI] [PubMed] [Google Scholar]
- Sun L, Peräkylä J, Kovalainen A, Ogawa KH, Karhunen PJ, and Hartikainen KM (2016). Human brain reacts to transcranial extraocular light. PLoS One 11, e0149525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymborska-Małek K, Komorowska M, and Gąsior-Głogowska M (2018). Effects of near infrared radiation on DNA. DLS and ATR-FTIR study. Spectrochim. Acta A Mol. Biomol. Spectrosc 188, 258–267. [DOI] [PubMed] [Google Scholar]
- Te Alexis E (2006). The next generation in laser treatments and the role of the green light high-performance system laser. Rev. Urol 8, S24. [PMC free article] [PubMed] [Google Scholar]
- Thunshelle C and Hamblin MR (2016). Transcranial low-level laser (light) therapy for brain injury. Photobiomodul. Photomed. Laser Surg 34, 587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Va M (2015). The use of intravenous laser blood irradiation (ILBI) at 630-640 nm to prevent vascular diseases and to increase life expectancy. Laser Ther 24, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimirov YA, Osipov A, and Klebanov G (2004). Photobiological principles of therapeutic applications of laser radiation. Biochemistry (Mosc) 69, 81–90. [DOI] [PubMed] [Google Scholar]
- Walski T, Chludzińska L, Komorowska M, and Witkiewicz W (2014). Individual osmotic fragility distribution: a new parameter for determination of the osmotic properties of human red blood cells. Biomed. Res. Int 2014, 162102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walski T, Dyrda A, Dzik M, Chludzińska L, Tomków T, Mehl J, Detyna J, Gałecka K, Witkiewicz W, and Komorowska M (2015). Near infrared light induces post-translational modifications of human red blood cell proteins. Photochem. Photobiol. Sci 14, 2035–2045. [DOI] [PubMed] [Google Scholar]
- Walski T, Drohomirecka A, Bujok J, Czerski A, Wąż G, Trochanowska N, Gorczykowski M, Cichoń R, and Komorowska M (2018). Low-level light therapy protects red blood cells against oxidative stress and haemolysis during extracorporeal circulation. Front. Physiol 9, 647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Deng J, Tu W, Zhang L, Chen H, Wu X, Li Y, and Sha H (2016). The hematologic effects of low intensity 650 nm laser irradiation on hypercholesterolemia rabbits. Am. J. Transl. Res 8, 2293. [PMC free article] [PubMed] [Google Scholar]
- Wiebracht ND and Zimmer LA (2014). Complex anatomy of the sphenoid sinus: a radiographic study and literature review. J. Neurol. Surg. B Skull Base 75, 378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Yu L, and Bennett DA (2010). Odor identification and mortality in old age. Chem. Senses 36, 63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X-C, Jia S-W, and Zheng X-Y (2001). Study on SPECT for intravascular laser irradiation treatment on cerebral infarction. Chin. J. Phys. Ther 24, 133–135. [Google Scholar]
- Xiao X, Guo Y, Chu X, Jia S, Zheng X, and Zhou C (2005). Effects of low power laser irradiation in nasal cavity on cerebral blood flow perfusion of patients with brain infarction. Chin. J. Phys. Med 27, 418–420. [Google Scholar]
- Xie T, Vigil J, MacCracken E, Gasparaitis A, Young J, Kang W, Bernard J, Warnke P, and Kang UJ (2015). Low-frequency stimulation of STN-DBS reduces aspiration and freezing of gait in patients with PD. Neurology 84, 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Wang L, Liu J, Tan Y, and Li Q (2001). Endonasal low energy He-Ne laser treatment of insomnia. Qian. Wei. J. Med. Pharm 18, 337–338. [Google Scholar]
- Xu C, Wang L, and Lu C (2002a). Endonasal low energy He-Ne laser treatment of poststroke depression. Prac. J. Med. Pharm 19, 893. [Google Scholar]
- Xu C, Wang L, Shang X, and Li Q (2002b). Endonasal low energy He-Ne laser treatment of Alzheimer’s disease. Prac. J. Med. Pharm 19, 647–648. [Google Scholar]
- Xu C, Wu Z, Wang L, Shang X, and Li Q (2002c). The effects of endonasal low energy He–Ne laser treatment of insomnia on sleep EEG. Prac. J. Med. Pharm 19, 407–408. [Google Scholar]
- Xu C, Lu C, Wang L, and Li Q (2003). The effects of endonasal low energy He-Ne laser therapy on antioxydation of Parkinson’s disease. Prac. J. Med. Pharm 20, 816–817. [Google Scholar]
- Xuan W, Vatansever F, Huang L, and Hamblin MR (2014). Transcranial low-level laser therapy enhances learning, memory, and neuroprogenitor cells after traumatic brain injury in mice. J. Biomed. Opt 19, 108003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang Q, Li P, Dong T, and Wu MX (2016). Low-level light treatment ameliorates immune thrombocytopenia. Sci. Rep 6, 38238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesman S, Mamilov S, Veligotsky D, and Gisbrecht A (2016). Local changes in arterial oxygen saturation induced by visible and near-infrared light radiation. Lasers Med. Sci 31, 145–149. [DOI] [PubMed] [Google Scholar]
- Zein R, Selting W, and Hamblin MR (2018). Review of light parameters and photobiomodulation efficacy: dive into complexity. J. Biomed. Opt 23, 120901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Choksi S, Chen K, Pobezinskaya Y, Linnoila I, and Liu Z-G (2013). ROS play a critical role in the differentiation of alternatively activated macrophages and the occurrence of tumor-associated macrophages. Cell Res. 23, 898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Dong T, Li P, and Wu MX (2016). Noninvasive low-level laser therapy for thrombocytopenia. Sci. Transl. Med 8, 349ra101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Lu M, and Wu MX (2018). Prolonging shelf-life of platelets by low-level laser. Mechanisms of Photobiomodulation Therapy XIII Proc SPIE, International Society for Optics and Photonics; 10477, 104770D. [Google Scholar]
- Zhao G, Guo K, and Dan J (2003). Case analysis of Parkinson’s disease treated by endonasal low energy He-Ne laser. Acta. Acad. Med. Q+ ingdao Univ 39, 398. [Google Scholar]
- Zhao J, Tian Y, Nie J, Xu J, and Liu D (2012). Red light and the sleep quality and endurance performance of Chinese female basketball players. J. Athl. Train 47, 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Li D, Zeng Y, Luo Y, and Qu JY (2011). Two-photon excited hemoglobin fluorescence. Biomed. Opt. Exp 2, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomorrodi R, Saltmarche AE, Loheswaran G, Ho KF, and Lim L (2017). Complementary EEG evidence for a significantly improved Alzheimer’s disease case after photobiomodulation treatment. Alzheimer’s Association International Conference p. P621. [Google Scholar]
- Zomorrodi R, Genane L, Abhiram P, and Lew L (2019). Pulsed near infrared transcranial and intranasal photobiomodulation significantly modulates neural oscillations: a pilot exploratory study. Sci. Rep 9, 6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubia J and Arrue J (2001). Plastic optical fibers: an introduction to their technological processes and applications. Opt. Fiber Technol 7, 101–140. [Google Scholar]