Abstract
Increasing evidence reveals that a broad spectrum of environmental chemicals and pharmaceutical compounds cause female ovarian toxicity (ovotoxicity). The current gold standard of ovotoxicity testing largely relies on whole laboratory animals, but in vivo models are time consuming, costly, and present animal welfare concerns. We previously demonstrated that the 3D encapsulated in vitro follicle growth (eIVFG) is a robust in vitro model for ovotoxicity testing. However, the follicle preparation process is complex and highly dependent on technical skills. Here, we aimed to use vitrification method to cryopreserve murine immature follicles for a high-content eIVFG, chemical exposure, and ovotoxicity screening. Results indicated that a closed vitrification system combined with optimized vitrification protocols preserved mouse follicle viability and functionality and vitrified follicles exhibited comparable follicle and oocyte reproductive outcomes to freshly harvested follicles during eIVFG, including follicle survival and development, ovarian steroidogenesis, and oocyte maturation and ovulation. Moreover, vitrified follicles consistently responded to ovotoxic chemical, doxorubicin (DOX). We further used vitrified follicles to test the response of microcystins (MCs), an emerging category of environmental contaminants produced by cyanobacteria associated with harmful algal blooms (HABs), and found that different congeners of MCs exhibited differential ovotoxicities. In summary, our study demonstrates that vitrification enables a long-term-storage and ready-to-use ovarian follicle bank for high-throughput ovotoxicity screening, which identifies endocrine disrupting effects of MCs.
Keywords: Vitrification, in vitro follicle growth, high-throughput, ovotoxicity, microcystin
Introduction
The ovary is the primary female reproductive organ and consists of various developmental stages of follicles as the basic functional unit. Each follicle contains a central germ cell oocyte and the surrounding somatic cells, and functions to secrete steroid and peptide hormones and to mature oocytes for ovulation and fertilization. It is believed that the ovarian follicle pool is established prior to birth and is non-renewable [1]. Thus, chemicals or other factors that compromise the quality and quantity of follicles will result in ovarian toxicity (ovotoxicity) [2]. For instance, increasing evidence reveals that a broad spectrum of both environmental contaminants (e.g., endocrine disrupting chemicals, EDCs) and pharmaceutical compounds (e.g., chemotherapeutics) cause ovotoxicity and increase women’s risks of premature ovarian failure (POF), hormonal imbalance, and infertility [3-5].
Thus far, the gold standard for testing the ovotoxicity of chemicals largely relies on the use of whole laboratory animals. However, in vivo models are time consuming, costly, and harmful to animals. Additionally, the specific reproductive endpoints in the ovary such as follicle development, oocyte maturation, and ovulation are difficult to monitor in real time without dissecting animals. Furthermore, over 84,000 chemicals are registered and used daily in consumer products, and about 2,000 new chemicals are being introduced each year, making the safety evaluation of all chemicals using whole animals not feasible [6]. These facts indicate that there is an urgent and unmet need to develop an efficient and reliable in vitro ovotoxicity screening platform.
In our previous studies, we have used the alginate hydrogel encapsulation method to grow both mouse and human immature follicles in vitro, termed encapsulated in vitro follicle growth (eIVFG) [7-9]. The eIVFG maintains the 3D architecture of follicles and support follicle development for maturation, ovarian steroidogenesis, and ovulation, as well as the acquisition of both oocyte meiotic and developmental competence. We further demonstrated that eIVFG is a robust in vitro model to screen for the ovotoxicity of xenobiotic exposures [10, 11]. Unlike a complex ovary containing various developmental stages of follicles in vivo, the follicle culture using eIVFG allows us to individually and accurately assess the effect of xenobiotic exposures on follicle development, particularly for growing follicles. However, the follicle preparation procedure for eIVFG needs further optimization as it is a complex process and requires multiple rounds of follicle isolation and culture. Therefore, the ovotoxicity screening costs several weeks or months to complete the chemical exposure and ovotoxicity assessment with multiple concentrations, times, and replicates. These shortfalls make the eIVFG not optimal for a high-throughput ovotoxicity screening, particularly when a rapid screening is desired in the face of unpredictable and emerging environmental threats such as the Deepwater Horizon oil spill in Gulf of Mexico.
Cryopreservation is a process by which cells, tissues, and organs are stored at deep cryogenic temperatures (e.g., −196 °C) for years, including slow freezing and vitrification. Compared to slow freezing, vitrification, a process that allows biological samples to be transformed into a glass-like state at ultra-rapid cooling rate without both extra- and intra-cellular ice formation, is simple, cost-effective, and requires less procedural time.
Previous studies have used vitrification method to cryopreserve preantral follicles from various species as an additional option of female fertility preservation [12-18]. However, the systemic evaluation of folliculogenesis and oogenesis has not been performed using recovered follicles after vitrification in previous studies, or the compromised quality of follicles and/or oocytes was observed. Additionally, it is also largely unknown whether these vitrified follicles will consistently respond to ovotoxic chemicals compared to freshly harvested follicles.
In the present study, we used a closed vitrification system combined with optimized vitrification protocols to cryopreserve individual murine preantral follicles and demonstrated that vitrification preserved follicle viability and functionality, and vitrified follicles exhibited comparable follicle/oocyte reproductive outcomes to freshly harvested follicles. Moreover, we used doxorubicin (DOX), a commonly used chemotherapeutic chemical that has ovotoxicity [10, 19-21], as the positive control to compare the response of vitrified and fresh follicles during eIVFG. Further, we used vitrified follicles and eIVFG to screen for the ovotoxicity of microcystins (MCs), an emerging category of environmental contaminants associated with harmful algal blooms (HABs) which can present transformational threats to the environment and public health [22]. Our results demonstrate that vitrification enables a long-term-storage and ready-to-use ovarian follicle bank for high-throughput ovotoxicity screening; different MC congeners have differential ovotoxic effects on ovarian follicles, and MC-LF may exhibit endocrine disrupting effects on the ovary.
Materials and Methods
Animals and follicle isolation
Animals were treated according to the NIH Guideline for the Care and Use of Laboratory Animals and the approved Institutional Animal Care and Use Committee (IACUC) protocol at the University of South Carolina (UofSC). The CD-1 mouse breeding colony (Charles River Laboratory, Wilmington, MA) was housed in a temperature- and light-controlled animal facility (22°C ± 1°C; 12 h light: 12 h dark) with 40-60% humidity and provided with food and water ad libitum. Pregnant females were checked daily to record the accurate date of birth of pups. Ovaries were removed from 15- or 16-day-old female mice and multilayered secondary follicles were mechanically isolated using insulin syringe needles. Morphologically normal follicles with three to five layers of granulosa cells, intact basement membrane and theca cell layers, and 130-160 μm in diameters were distributed into different experimental groups.
Vitrification protocol development, follicle vitrification and warming
To develop a vitrification protocol that can efficiently and effectively cryopreserve individual follicles, we performed a systemic review of previous studies that vitrified both ovarian tissues or individual follicles of multiple species (Supplemental Table 1). It was found that various types of cryo-devices, compositions of CPAs, equilibration time, cooling and warming procedure, and animal species have been used and inconsistent follicle and oocyte reproductive outcomes were observed, indicating that there is no standardized follicle vitrification protocol thus far. For the cryo-device and cooling and warming procedures, here, we elected to use a closed vitrification system with cooling in liquid nitrogen (LN2) vapor and a two-phase warming procedure, because Ting et al. used this method to cryopreserve macaque ovarian cortex tissues and demonstrated that the ovarian tissues after vitrification and warming maintained follicle integrity and the isolated macaque secondary follicles were able to survive and grow to antral stage using eIVFG [23-25].
To establish an effective murine follicle vitrification protocol, two recipes of vitrification solutions were tested because both of them have shown promising results on cryopreserving ovarian tissues [23-27]. The first vitrification solution used ethylene glycol (EG) and glycerol (G) as CPAs and synthetic polymers of PXZ (polyvinylpyrrolidone (PVP) K-12; Acros Organics, New Jersey, polyvinylalcohol and polyvinylacetate (PVA); Supercool X-1000™, and polyglycerol; Supercool Z-1000™, PXZ; 21st Century Medicine, Fontana, CA) to further inhibit ice nucleation and formation, which was referred to EG+PXZ below. The second vitrification solution used EG, dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO), and sucrose as CPAs, which was referred to EDS below. The EDS vitrification solution has been used to vitrify mouse ovarian tissues and obtained live pups after ovarian tissue transplant, and this recipe has also been clinically applied for cryopreserving ovarian tissues from cancer patients [27, 28]. The detailed recipes of EG+PXZ and EDS vitrification solutions are shown in Table 1.
Table 1.
Recipes of vitrification and warming solutions.
| Vitrification solutions (EG+PXZ) | ||||
|---|---|---|---|---|
| Solution 1 | Solution 2 | Solution 3 | Solution 4 | |
| Total CPA concentration | 5% | 15% | 30% | 50% |
| CPA composition | 5% G | 7.5% G + 7.5% EG | 15% G + 15% EG | 25% G +25% EG |
| PXZ polymers | NA | NA | NA | 3% |
| Base medium | L-15 supplemented with 15% FBS and 1 μM L-Ascorbic acid 2-phosphate sesquimagnesium salt hydrate (AA2P) | |||
| Vitrification solutions (EDS) | |||
|---|---|---|---|
| Solution 1 | Solution 2 | Solution 3 | |
| Total CPA concentration | 0% | 15% | 40% |
| CPA composition | NA | 7.5% EG + 7.5% DMSO | 20% EG + 20% DMSO + 0.5 M sucrose |
| Base medium | L-15 supplemented with 20% FBS | ||
| Warming solutions (EG+PXZ and EDS) | |||||
|---|---|---|---|---|---|
| Solution 1 | Solution 2 | Solution 3 | Solution 4 | Solution 5 | |
| Sucrose concentration | 1 M | 0.75 M | 0.5 M | 0.25 M | 0 M |
| Base medium | L-15 supplemented 15% FBS and 1 μM AA2P | ||||
Notes: G: glycerol; EG: ethylene glycol; P: polyvinylpyrrolidone (PVP); X: Supercool X-1000™; Z: Supercool Z-1000™; DMSO: dimethyl sulfoxide; M: mol/L; NA: not applicable.
Since the volume of individual follicles are significantly smaller than ovarian cortex tissues, we first performed a preliminary experiment by testing different equilibration times in both vitrification solutions, which allowed us to gain complete follicle vitrification as well as to minimize CPA-induced cytotoxicity. The follicle vitrification procedure and equilibration time are shown in Fig. 1. Specifically, for the EG+PXZ vitrification protocol, freshly harvested follicles were sequentially equilibrated in four vitrification solutions containing increasing concentrations of CPAs for 1, 1, and 1 minutes (min) and 10 seconds (sec), respectively (Fig. 1). For the EDS vitrification protocol, follicles were sequentially equilibrated in three vitrification solutions containing increasing concentrations of CPAs for 1 min, 3 min, and 10 sec, respectively (Fig. 1). Following equilibration, 10-15 follicles were loaded into each 0.25 cc straw (Agtech Inc., Manhattan, KS) containing 100 μL of the final vitrification solution and then heat sealed of both ends and cooled in LN2 vapor for 2 min. Finally, the straws with vitrified follicles were immediately plunged into LN2 for long-term storage.
Figure. 1.
Procedures of ovarian follicle vitrification and warming. Freshly harvested follicles were sequentially equilibrated in EG+PXZ and EDS vitrification solutions containing increasing concentrations of CPAs. Follicles were next loaded into 0.25 cc straw, cooled in LN2 vapor, and plunged into LN2 for long-term storage. The follicle retrieval was performed using a two-phase warming procedure followed by five sequential incubations in warming solutions with decreasing concentrations of sucrose. CPA, cryoprotective agents; PXZ, polyvinylpyrrolidone (PVP) K-12, Supercool X-1000™, and Supercool Z-1000™; LN2, liquid nitrogen.
For both EG+PXZ and EDS vitrification protocols, follicles were warmed using a two-phase warming procedure because it has been demonstrated that this method can effectively avoid devitrification or re-crystallization, a major lethal mechanism to vitrified cells during warming, and can preserve ovarian cell viability and functionality [23]. Briefly, straws containing vitrified follicles were removed from LN2, exposed to room temperature air at 25°C for 5 sec, and then immediately plunged into a 42°C water bath with gentle shaking for 5 sec. Follicles were released by cutting both straw ends and serially incubated in 5 warming solutions with decreasing concentrations of sucrose for 1, 2, 3, 3, and 5 min, respectively (Fig. 1). The detailed recipes of warming solutions are shown in Table 1. Warmed follicles were transferred and placed in dissection media containing L-15 media with 1% FBS for alginate encapsulation and eIVFG.
Follicle encapsulation and eIVFG
Both freshly harvested follicles and follicles after vitrification and warming were encapsulated for eIVFG as we previously described [7-9]. Briefly, follicles were individually encapsulated in 0.5% alginate hydrogel (Sigma-Aldrich). Alginate beads were placed into maintenance media containing minimum essential medium (αMEM Glutamax; Gibco, Grand Island, NY) with 1% FBS for 30 min at 37°C in 5% CO2 incubator. Then, encapsulated follicles were placed individually in 96-well plate, with each well containing 100 μl growth media (50% αMEM Glutamax and 50% F-12 Glutamax supplemented with 3 mg/μL bovine serum albumin (BSA; Fisher Scientific, Denver, CO), 10 mIU/mL recombinant follicle-stimulating hormone (rFSH; from A.F. Parlow, National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD), 1 mg/mL bovine fetuin (Sigma-Aldrich), 5 μg/mL insulin, 5 μg/mL transferrin, and 5 ng/mL selenium (ITS; Sigma-Aldrich). Follicles were cultured at 37°C in 5% CO2 for 8 days and half of the culture media (50 μl) was exchanged every other day. Follicles were imaged at each media change for survival and diameter assessments using an Olympus inverted microscope with 10x objective (Olympus Optical Co Ltd, Tokyo, Japan). Follicles were considered dead if they had unhealthy appearing oocytes and/or granulosa cells, or if the integrity of the oocyte and somatic cell interface was visibly compromised. The follicle diameter was calculated by averaging 2 perpendicular measurements from one side to another side of basement membrane per follicle using ImageJ software (National Institutes of Health, Bethesda, MD).
In vitro maturation
On day 8 of eIVFG, follicles grown to antral stage were removed from alginate beads and treated with αMEM-based maturation media containing 10% FBS, 1.5 IU/mL human chorionic gonadotropin (hCG), and 10 ng/mL epidermal growth factor (EGF; BD Biosciences, Franklin Lakes, NJ) for 14-16 h at 37°C in 5% CO2 to trigger in vitro ovulation and oocyte maturation. Then, follicles were incubated in L-15 media supplemented with 0.3% hyaluronidase (Sigma-Aldrich) for 3 min at 37 °C and the oocytes were denuded from surrounding cumulus cells by gentle aspiration. The oocytes were considered as metaphase II (MII) oocytes if a polar body was present in the perivitelline space and the MII oocyte percentage was calculated.
Oocyte diameter and polar body size measurement
After in vitro maturation, about 30 MII oocytes in each group was randomly selected to detect the oocyte diameter as we previously described [21]. Briefly, the oocyte diameter was obtained from two perpendicular measurements, including the zona pellucida. The first measurement detected the widest diameter of oocyte and the second measurement originated at a right angle from the midpoint of the first measurement. Next, the final oocyte diameter was calculated by averaging these two diameters. Twenty-four to 32 MII oocytes were randomly selected to quantify the first polar body size for the fresh and vitrification groups. The polar body area was measured using ImageJ software (National Institutes of Health, Bethesda, MD) to indicate the polar body size.
Oocyte spindle and chromosome structure analysis
Oocytes retrieved after in vitro maturation were fixed in 4% paraformaldehyde (PFA; Sigma-Aldrich) for 30 min and incubated with 0.5% Triton X-100 (Sigma-Aldrich) for 20 min at RT. After incubation in the blocking solution containing 1 x PBS with 0.3% BSA and 0.01% Tween-20 for 1 h, oocytes were incubated with anti-α-tubulin antibody (1:100, Cell Signaling Technology, Danvers, MA) in blocking solution overnight, and the blocking solution without antibody was used as negative control. Oocytes were washed 3 times with blocking solution and mounted using Vectashield containing DAPI (Vector Laboratories, Burlingame, CA). Finally, oocytes were examined using a laser scanning confocal microscope (Carl Zeiss LSM 700, Oberkochen, Germany). Oocytes with barrel-shaped bipolar spindles and well-organized microtubule fibers, along with tightly aligned chromosomes on the metaphase plate was indicated as normal. All other configurations were considered as abnormal.
Hormone assay
The concentration of 17β-estradiol (E2) in the follicle culture media was measured using an ELISA kit (Calbiotech, Spring Valley, CA) according to manufacturer’s instructions. Briefly, the anti-estradiol capture antibodies pre-coated wells were incubated with E2 standards, conditioned culture media, and Estradiol Biotin Reagent for 45 min. Next, the Estradiol Enzyme Reagent was added and incubated for another 45 min. After washing the wells with washing buffer three times, the solution of TMB reagent was added and incubated at room temperature for 20 min, resulting in the development of blue color. Last, the reaction was stopped by the addition of Stop Solution and the absorbance was measured using a BioTek Synergy HT microplate reader (BioTek Instruments, Inc., Winooski, VT) at 450 nm within 15 min. The inter-assay coefficients of variability were less than 15% and intra-assay coefficients of variability were less than 10% for all assays. The detection limit of the assay was 70 ng/ml.
Quantitative RT-PCR
Both freshly harvested and vitrified follicles were collected on day 8 of eIVFG for examining the expression of genes involved in ovarian steroidogenesis and oocyte-specific genes by quantitative reverse transcription PCR (qRT-PCR). The whole follicles were used to perform the gene expression analysis for both ovarian steroidogenesis and oocyte-specific genes. Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions with 20-30 follicles in each group. Total RNA was then reverse transcribed into cDNA using Superscript III reverse transcriptase with random hexamer primers (Invitrogen, Carlsbad, CA) and stored at −80°C. qPCR was performed in 384-well plate using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) on Bio-Rad CFX384 real time system (Bio-Rad, Hercules, CA). qPCR thermos cycle was programmed for 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 40 s at 60 °C, and finally a melting stage to determine the specificity of primers. The standard curve method was used to analyze qRT-PCR results and the mRNA expression levels of each genes were normalized by the expression of glyceraldehyde-3-phosphate dehydrogenase (Gapdh). The primer sequences were: Gapdh forward: CATCACTGCCACCCAGAAGACTG, Gapdh reversed: ATGCCAGTGAGCTTCCCGTTCAG; Gdf9 forward: GATGGGACTGACAGGTCTGG, Gdf9 reversed: CAGCGGTCCTGTCACCTG; Bmp15 forward: AAGGGAGAACCGCACGATTG, Bmp15 reversed: TGCTTGGTCCGGCATTTAGG; Zp1 forward: TACAAAGGCTGCCACGTTCTA, Zp1 reversed: TTGGGACAGATCAGTGTGACA; Zp2 forward: AAGCCTTCCTCAGTCCGAGAA, Zp2 reversed: CCAGAGCATAAGTGCAGTTCA; Zp3 forward: ATGGCGTCAAGCTATTTCCTC, Zp3 reversed: CGTGCCAAAAAGGTCTCTACT; Star forward: TTGGGCATACTCAACAACCA, Star reversed: CCTTGACATTTGGGTTCCAC, Cyp11a1 forward: TCAAAGCCAGCATCAAGGAGA, Cyp11a1 reversed: TGGCAAAGCTAGCCACCTGTA; Hsd3b1 forward: AGTGATGGAAAAAGGGCAGGT, Hsd3b1 reversed: GCAAGTTTGTGAGTGGGTTAG; Cyp17a1 forward: AGTCAAAGACACCTAATGCCAAG, Cyp17a1 reversed: ACGTCTGGGGAGAAACGGT; Hsd17b1 forward: ACTGTGCCAGCAAGTTTGCG, Hsd17b1 reversed: AAGCGGTTCGTGGAGAAGTAG; Cyp19a1 forward: CATGGTCCCGGAAACTGTGA, Cyp19a1 reversed: GTAGTAGTTGCAGGCACTTC.
Histology and TUNEL assay
Follicles were fixed in 4% PFA for 2 h at RT and processed for paraffin embedding and serial sectioning at 5 μm. Follicle sections were stained with hematoxylin and eosin (H&E, ThermoFisher Scientific, Waltham, MA) for histological evaluation between freshly harvested follicles and vitrified-warmed follicles. After DOX exposure, follicle apoptosis was determined using Terminal Deoxynucleotidyl Transferase (TdT) – mediated dUTP Nick-End Labeling (TUNEL) assay using the DeadEnd™ Fluorometric TUNEL System (G3250, Promega, Madison, MI) following manufacturer’s instructions. TUNEL detects DNA breaks formed when DNA fragmentation occurs in the last phase of cell apoptosis and has been widely used as a reliable marker for detecting cell apoptosis.
DOX and MCs exposure
Isolated multilayered secondary follicles were vitrified using EDS based vitrification protocol and were stored in LN2 tank for 2-7 days. The EDS but not EG+PXZ protocol was selected because it produced higher percentage of follicles with normal morphology after warming. Upon chemical exposure and eIVFG, follicles were warmed as described in Fig. 1. To investigate whether vitrified follicles consistently respond to ovotoxic chemicals compared to freshly harvested follicle, both freshly harvested and vitrified follicles were treated with DOX (Sigma-Aldrich) at 100 nM, a clinically relevant but ovotoxic concentration as we previously demonstrated [10]. As we have found that DOX at 100 nM resulted in a time and cell type-dependent toxic manner and the majority of DOX-treated follicles completely died at 24 h [10], follicles were collected at 6 h and 12 h post treatment and fixed in 4% PFA for paraffin processing and cytotoxicity assessments.
For the ovotoxicity screening of MCs, vitrified follicles were warmed and exposed to four most common types of MCs, including MC-LA (Cayman Chemicals, Ann Arbor, MI), MC-LF (Enzo Life Sciences, Farmingdale, NY), MC-LR (Cayman Chemicals), and MC-LY (Enzo Life Sciences). All the MCs were dissolved in DMSO to obtain the 10 mM stock solution and stored at −20°C. Previous studies reported that the serum concentration of MC-LR, the most potent congener of MCs, was at 0.05-1.8 nM in fishermen in Lake Chaohu, China, and 7.6-31.4 nM in patients treated with contaminated dialysate fluids in Caruaru, Brazil [29, 30]. Unfortunately, the effects of MC-LR on female reproductive health, particularly on the ovary, have been scarcely investigated [31]. Thus, we decided to include a broad range of exposure concentrations to gain the first insight into the response of ovarian follicles to MC-LR, including a human relevant exposure concentration at 0.1 μM and two higher concentrations at 1 and 10 μM during the entire period of eIVFG. Because the in vivo exposure data for the other types of MCs are very limited but have been reported to be at the similar order of magnitude to MC-LR [32, 33], we chose the same exposure concentration range from 0 to 10 μM for the MC-LA, MC-LF, and MC-LY. The reproductive endpoints of follicle survival and development, E2 secretion, in vitro ovulation, and oocyte meiosis were evaluated as described above.
Statistical Analyses
One-way ANOVA followed by a Tukey’s multiple comparisons test was performed to analyze the follicle growth, survival, oocyte and polar body size, and E2 concentration in comparing the freshly isolated follicles and vitrified follicles and in different MC congeners-treated groups at a single time point. Kruskal-Wallis with a Dunn’s multiple comparisons test was performed to analyze the steroidogenesis related genes and oocyte-specific genes expression, MII oocyte percentage, and percentage of MII oocytes with normal spindle and chromosome structure. P < 0.05 was considered statistically significant.
Results
Vitrified follicles displayed normal follicle morphology after warming
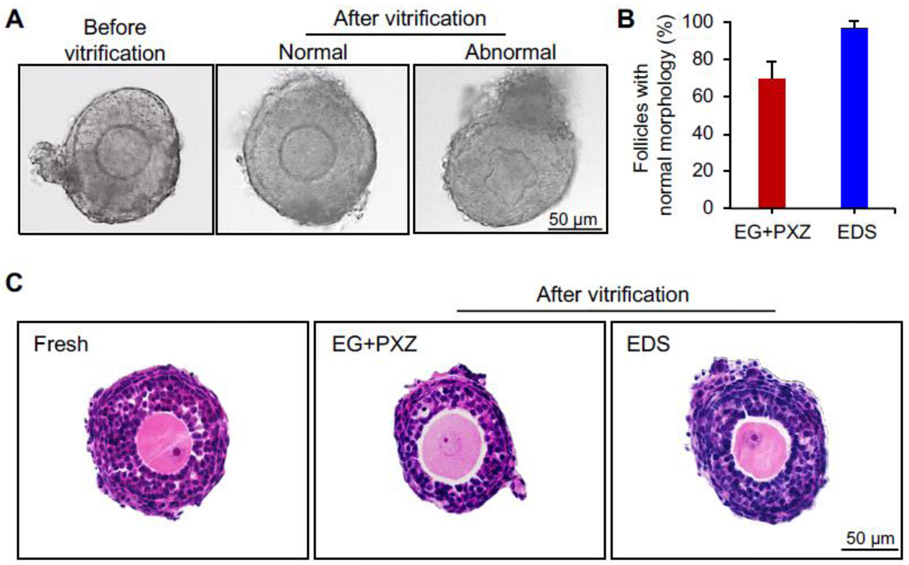
The morphology of vitrified follicles was first examined to determine the impact of vitrification on overall follicle and oocyte health. The morphologically normal follicles were defined by the complete granulosa cell and theca cell layers, intact basement membrane, and round shape of central germ cell oocytes. After warming, 69 ± 10% and 97 ± 3% of vitrified follicles showed normal morphological appearance using EG+PXZ and EDS vitrification solutions, respectively (Fig. 2A and 2B). For vitrified follicles with abnormal morphology, > 90% of them showed distorted oocytes but with normal somatic cell layers (Fig. 2A). Consistently, the morphologically normal vitrified follicles also had normal histological appearance of the somatic cells, oocytes, and entire follicle architecture compared to freshly harvested follicles (Fig. 2C).
Figure 2.
Follicle morphology after vitrification and warming. (A) Representative images of freshly harvested follicles and vitrified follicles immediately after warming. (B) Percentage of vitrified follicles with normal morphology using EG+PXZ and EDS vitrification solutions. Error bar: standard deviation. (C) Representative histological images of freshly harvested and vitrified follicles with normal morphology. N=20-40 follicles in each experimental group and 5 replicates were performed.
Vitrified follicles had comparable survival rate and development pattern to freshly harvested follicles during eIVFG
We next included vitrified follicles with normal morphology after warming for eIVFG and compared their follicle and oocyte reproductive outcomes with freshly harvested follicles.
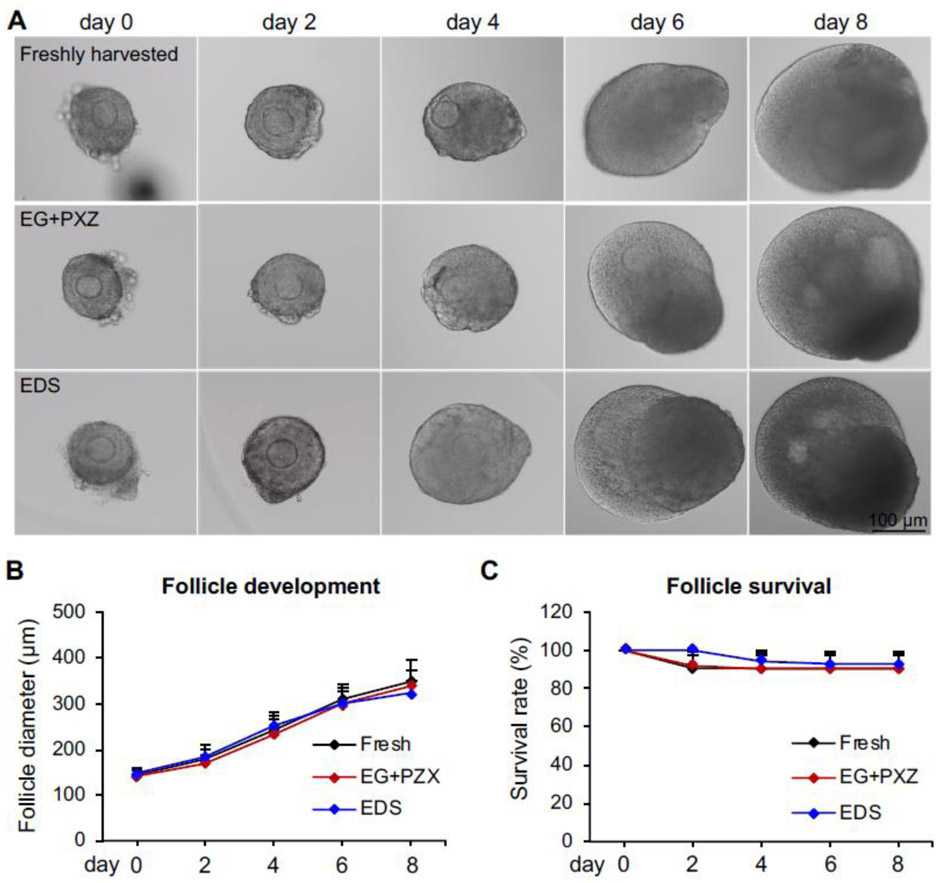
The results of eIVFG indicated that the alginate hydrogel encapsulation maintained the 3D architecture of both freshly harvested and vitrified follicles, and supported follicle growth and development from multilayered secondary stage on day 0 to antral stage on day 8 (Fig. 3A). For freshly harvested follicles, the follicle diameter increased from 144 ± 12 μm on day 0 to 349 ± 46 μm on day 8 (Fig. 3B) and the follicle survival rate was 91 ± 7% on day 8 (Fig. 3C). For vitrified follicles using EG+PXZ and EDS vitrification solutions, the follicle terminal diameters on day 8 were 339 ± 34 μm and 324 ± 27 μm and the survival rates were 90 ± 8 % and 93 ± 6 %, respectively, which were comparable to freshly harvested follicles (Fig. 3B and 3C). These results indicate that vitrification preserves follicle viability and vitrified follicles have normal survival rate and developmental pattern during eIVFG.
Figure 3.
Effect of vitrification on follicle growth and survival during encapsulated in vitro follicle growth (eIVFG). (A) Representative images of freshly harvested and vitrified follicles on days 0, 2, 4, 6, and 8 of eIVFG. (B) Follicle diameters and (C) follicle survival rates from day 0 to 8 during eIVFG. Error bar: standard deviation. N=15-20 follicles for each experimental group and 3 replicates of eIVFG were performed.
Vitrified follicles exhibited comparable ovarian steroidogenesis to freshly harvested follicles during eIVFG
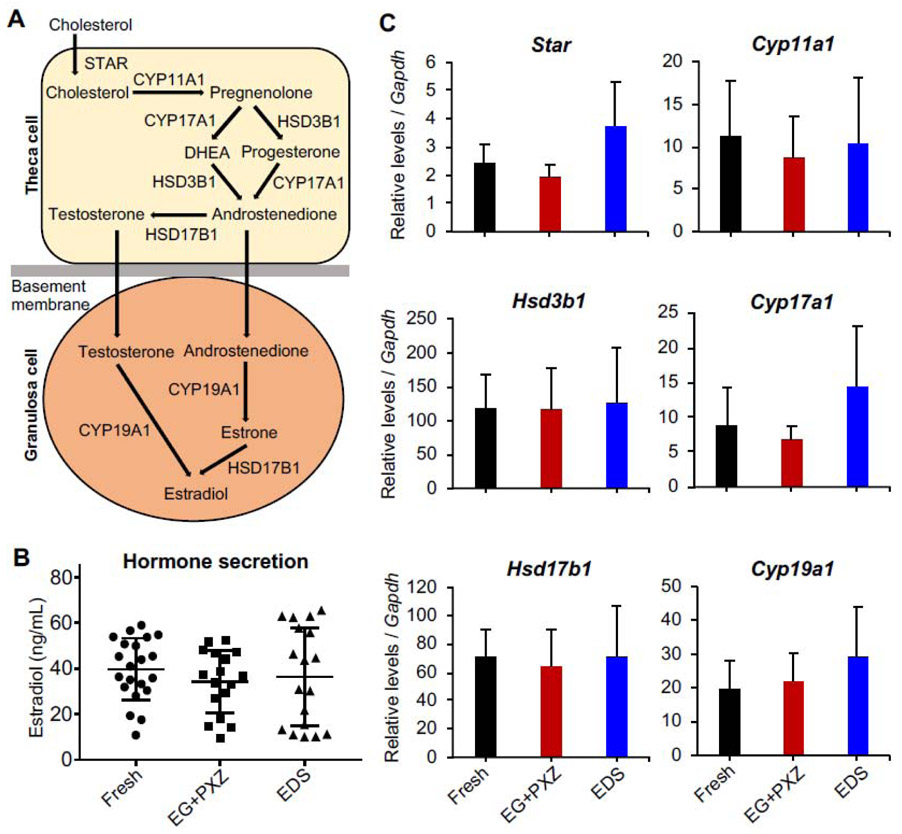
To evaluate the ovarian steroidogenesis of vitrified follicles during eIVFG, the expression of steroidogenesis-related genes and hormonal secretion of E2 were examined. Compared to freshly harvested follicles on day 8, qRT-PCR results showed that vitrified follicles using both EG+PXZ and EDS vitrification protocols had comparable mRNA expression levels of genes that are critical for ovarian steroidogenesis, including Star, Cyp11a1, Hsd3b1, Cyp17a1, Hsd17b1, and Cyp19a1 (Fig. 4A and 4C). At hormonal secretion level, there was no significant difference for the E2 concentrations in follicle culture media on day 8 of eIVFG between freshly harvested follicles and vitrified follicles using both vitrification solutions (Fig. 4B). These results suggest that vitrified follicles have normal ovarian steroidogenesis during eIVFG.
Figure 4.
Effect of vitrification on the mRNA expression of ovarian steroidogenesis related genes and 17β-estradiol (E2) secretion. (A) Classic ovarian steroidogenesis pathway in follicular cells. (B) Concentration of E2 in follicle culture media on day 8 of eIVFG measured by ELISA. (C) mRNA expression levels of steroidogenesis related genes in freshly harvested and vitrified follicles on day 8 of eIVFG. Error bar: standard deviation. For E2 concentration analysis, N=6-7 follicles in each group for each replicate and three replicates was performed. For mRNA expression analysis, N=20-30 follicles in each group for each replicate and three replicates were included.
Vitrified follicles exhibited normal in vitro ovulation and oocyte meiotic maturation
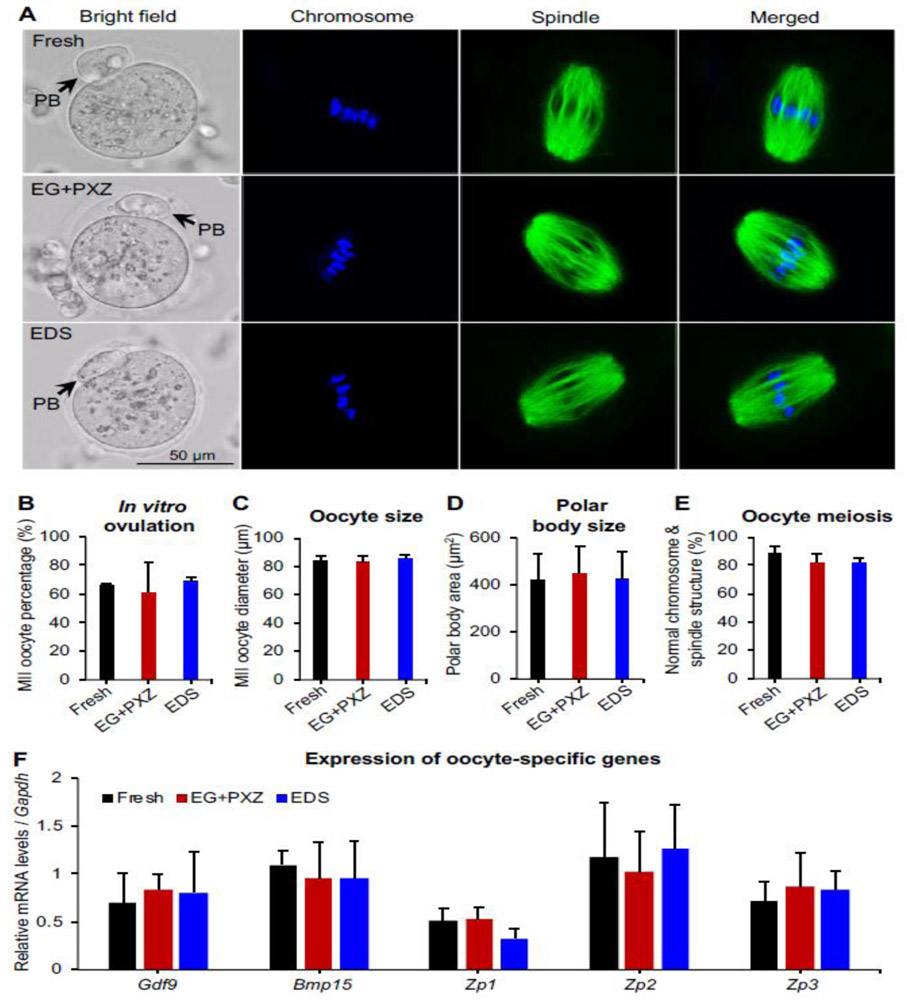
On day 8 of eIVFG, grown antral follicles were treated with hCG to trigger in vitro ovulation and oocyte meiotic division. The ovulated MII oocytes from vitrified follicles had normal appearance (Fig. 5A, bright field) and the MII oocyte percentages of vitrified follicles using EG+PXZ and EDS vitrification solutions were 61 ± 21% and 69 ± 3%, respectively, which were comparable to the MII oocyte percentage of freshly harvested follicles at 66 ± 1% (Fig. 5B). Moreover, there was no significant difference for the size of both entire MII oocytes and extruded first polar bodies among different groups (Fig. 5C and 5D).
Figure 5.
Effect of vitrification on oocyte meiotic maturation after in vitro maturation. (A) Representative images of metaphase II (MII) oocyte morphology, chromosome distribution (blue) and spindle morphology (green) from freshly harvested and vitrified follicles. PB: polar body. Blue: DAPI; green: α-tubulin. (B) MII oocyte percentage after in vitro oocyte maturation. (C) MII oocyte diameter after in vitro oocyte maturation. (D) The size of first polar body after in vitro oocyte maturation. (E) Percentage of oocytes with normal spindle morphology and chromosome alignment. (F) mRNA expression of oocyte specific genes in freshly harvested and vitrified follicles on day 8 of eIVFG. For all examined parameters in A-E, there were N=7-20 follicles in each experimental group and replicate and 3 replicates were included. For mRNA expression analysis, N=20-30 follicles in each group for each replicate and three replicates were included.
Because we and others have previously demonstrated that the oocyte polar body extrusion is not a sufficient indicator for oocyte quality, and that a functional spindle is also essential for proper chromosome segregation and producing a haploid gamete [7, 8, 10, 34], we therefore further examined oocyte spindle morphology and chromosome alignment by immunofluorescent staining of oocyte α-tubulin and chromosomes. Results indicated that 89 ± 5% of MII oocytes from freshly harvested follicles had barrel-shaped bipolar spindles and tightly aligned chromosomes on the metaphase plate (Fig. 5A and 5E), suggesting normal oocyte meiotic division upon in vitro maturation. The MII oocytes from vitrified follicles using EG+PXZ and EDS vitrification solutions had the percentages of normal spindle morphology and chromosome alignment at 82 ± 6% and 82 ± 4%, respectively, which were slightly decreased compared to freshly harvested follicles but with the difference not statistically significant (Fig. 5A and 5E). Taken together, these results indicate that vitrified follicles maintain normal functions of in vitro ovulation and oocyte meiotic division.
Vitrified follicles had comparable expression of oocyte-specific genes to freshly harvested follicles
At mRNA level, we further performed qRT-PCR to investigate the expression of 5 oocyte-specific genes, including Gdf9 and Bmp15, two oocyte-derived growth factors in transforming growth factor-β (TGF-β) superfamily that are essential for folliculogenesis and oogenesis [35, 36], and Zp1, Zp2, and Zp3, three oocyte zona pellucida-specific genes that are critical for oogenesis, fertilization, and preimplantation embryo development [37, 38]. Results showed that the mRNA expression of all examined oocyte-specific genes were comparable between freshly harvested follicles and vitrified follicles (Fig. 5F). These results demonstrate that vitrification preserve normal transcription of oocyte specific genes that are essential for folliculogenesis and oogenesis.
DOX exhibited consistent ovotoxicities in vitrified and freshly isolated follicles
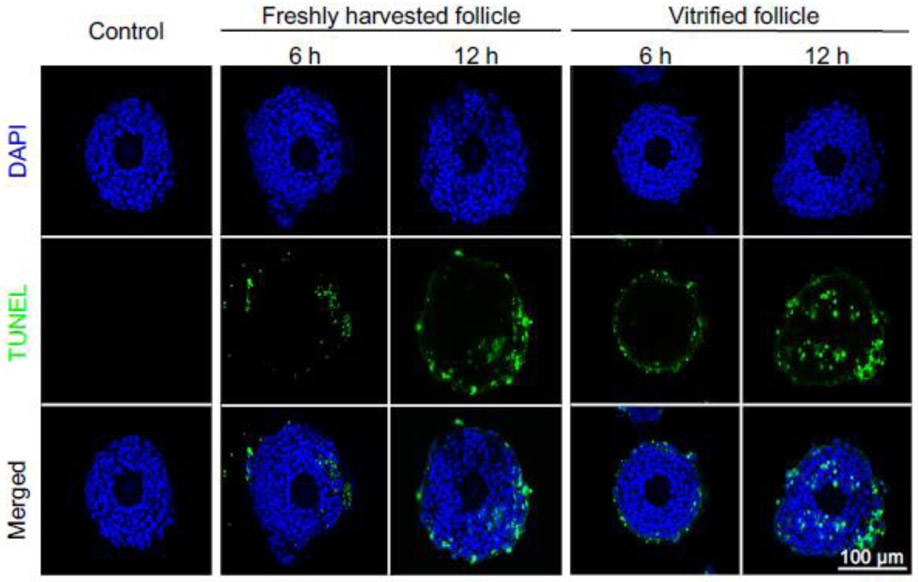
Using both eIVFG and in vivo animal models, we have recently demonstrated that the clinically relevant exposure of DOX has a dose-dependent ovotoxicity [10, 11]. At the cellular level, DOX primarily induces DNA damage and apoptosis of granulosa cells and then results in entire follicle apoptosis. Here, we used DOX as a positive control to determine whether vitrified follicles consistently respond to ovotoxic chemicals [10]. TUNEL staining results showed that DOX at 100 nM primarily induced theca cell apoptosis at 6 h post-DOX treatment and more positive signals were found in both granulosa cell and theca cell layers at 12 h in both vitrified and freshly harvested follicles (Fig. 6). These results reveal that DOX consistently induces follicular cell DNA damage and apoptosis in vitrified follicles, providing an efficient and reliable model for eIVFG and in vitro ovotoxicity screening.
Figure 6.
Ovotoxicity testing of doxorubicin (DOX) using vitrified and warmed follicles, with representative TUNEL images of follicles treated with DOX at 100 nM for 0, 6, and 12 hours. Isolated multilayered secondary follicles were vitrified using EDS based vitrification protocol. Blue: DAPI; green: DNA fragmentation revealed by TUNEL staining. N=5-6 follicles in each experimental group for each time point and replicate and three replicates were performed.
eIVFG using vitrified follicles discovered differential ovotoxicities of different MC congeners
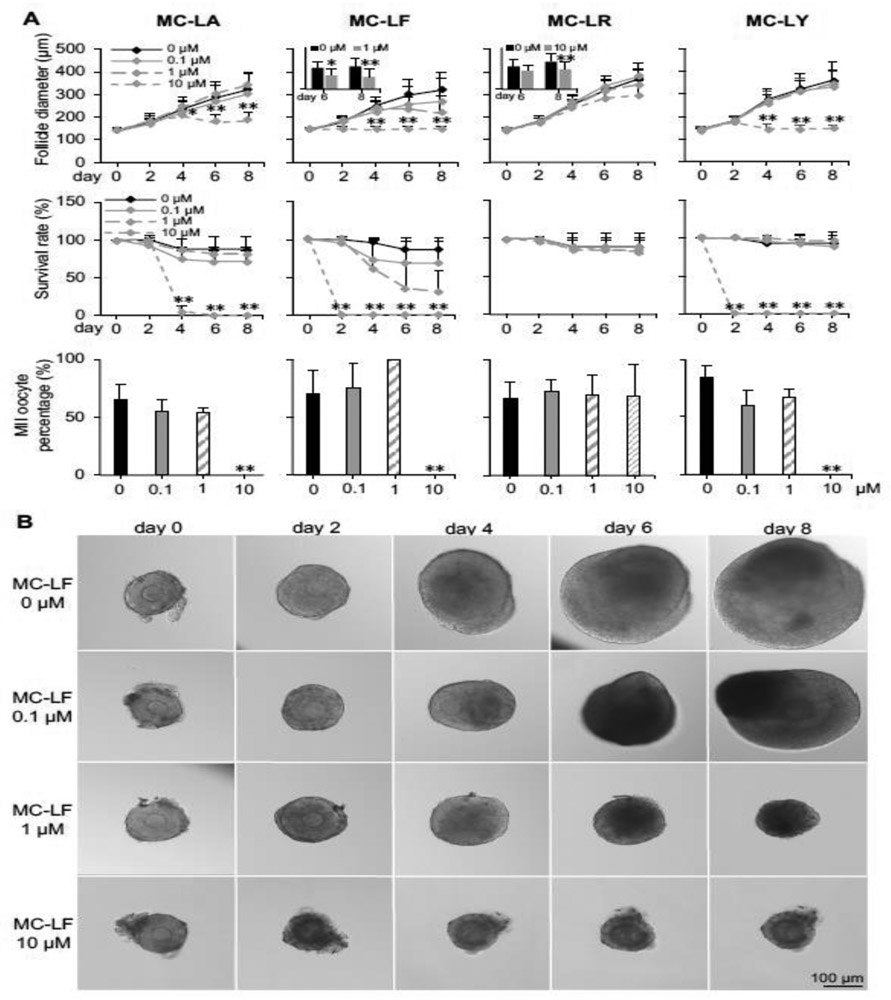
We next warmed vitrified follicles for eIVFG and screened for the potential ovotoxicity of MCs, including MC-LA, LF, LR, and LY, by investigating the follicle survival and development, E2 secretion, in vitro ovulation, and oocyte meiotic maturation. Ovotoxicity screening results indicated that MC-LR was the least ovotoxic MC with comparable follicle survival rates at all exposure concentrations but significantly decreased follicle terminal diameter at 10 μM on day 8 of eIVFG (Fig. 7A and Supplemental Fig. 2). MC-LA, LF, and LY significantly decreased follicle survival rates and inhibited follicle growth at 10 μM, however, there was no significant difference when the exposure levels of MC-LA and -LY were at 0.1 and 1 μM (Fig. 7A and Supplemental Fig. 1 and 3). MC-LF was found to be the most ovotoxic MC congener and exhibited dose-dependent ovotoxicity (Fig. 7). Specifically, MC-LF at 0.1 μM did not significantly impact follicle survival and development during eIVFG. However, 69 ± 28% of follicles were dead on day 8 when the exposure level was at 1 μM, and the average follicle diameter was significantly decreased to 219 ± 77 μm compared to the average follicle diameter at 319 ± 79 μm in the control group (Fig. 7). At 10 μM, MC-LF resulted in 100% follicle death on day 2, which were characterized by the dark granulosa cell layers and compromised follicle integrity (Fig. 7). We next treated all survived follicles with hCG to trigger in vitro ovulation and found that all tested MC congeners did not significantly affect follicle ovulation and oocyte meiotic division if treated follicles could survive and develop to antral stage on day 8 (Fig. 7A). In addition, we further treated fresh follicles with MC-LA, LF, LR, and LY, at the same concentrations to determine whether the vitrified and fresh follicles consistently respond to xenobiotic exposures. The follicle development and survival results showed that all 4 tested MC congeners exhibited comparable ovotoxicities between vitrified and fresh follicles, suggesting that vitrification provides us with an efficient and reliable follicle resource for eIVFG and ovotoxicity screening (Supplemental Fig. 4).
Figure 7.
Effect of different congeners of microcystins (MCs) on follicle growth, survival, and oocyte maturation during encapsulated in vitro follicle growth (eIVFG) using vitrified and warmed follicles. Isolated multilayered secondary follicles were vitrified using EDS based vitrification protocol. (A) Follicle diameter, survival rate, and MII oocyte percentage after in vitro oocyte maturation upon different congeners and concentrations of MCs exposure. (B) Representative images of follicles treated with different concentrations of MC-LF on days 0, 2, 4, 6, and 8 during elVFG. Error bar: standard deviation; *p< 0.05 and **p<0.01. N=8-10 follicles in each group for each replicate and 3 replicates were performed.
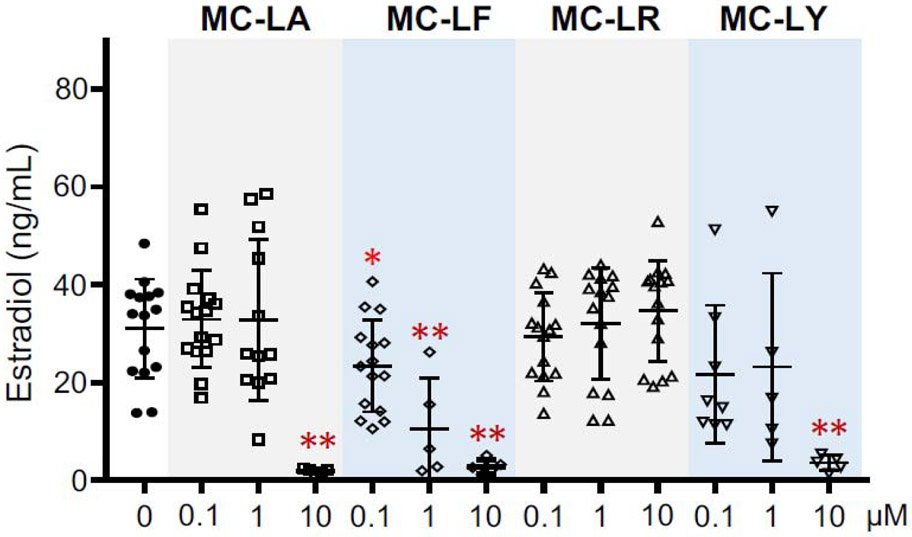
Estradiol is primarily produced in growing follicles and is essential for follicle development and oocyte maturation as well as for the functions of downstream reproductive tract organs such as the uterus and systemic health. Therefore, we next measured the E2 secretion in the conditioned follicle culture media on day 8. ELISA results showed that there was no significant difference for the E2 secretion between control group and follicles treated with MC-LA, LR, and LY at 0.1 and 1 μM (Fig. 8). The extremely low concentrations of E2 from follicles treated with MC-LA and LY at 10 μM and MC-LF at 1 and 10 μM (Fig. 8) reflected the fact that most of follicles were dead on day 8 (Fig. 7). Surprisingly, follicles treated with MC-LF at 0.1 μM did not affect follicle survival and development (Fig. 7). However, the E2 secretion was significantly decreased compared to control group (Fig. 8). In summary, these results demonstrate that different congeners of MCs exhibit differential ovotoxicities, and MC-LF has dose-dependent toxicities on follicle survival, follicle development, and hormone secretion.
Figure 8.
Effect of different congeners and concentrations of microcystins (MCs) on 17β-estradiol (E2) secretion of in vitro cultured vitrified and warmed follicles on day 8 of eIVFG. Error bar: standard deviation; *p<0.05 and **p<0.01. N=5-15 follicles in each group and 3 replicates were performed.
Discussion
In the present study, we aimed to cryopreserve mouse preantral follicles through vitrification to establish a long-term-storage and ready-to-use ovarian follicle bank, which can be used for a high-throughput in vitro ovotoxicity screening. We tested two commonly used vitrification protocols for cryopreserving ovarian tissues or individual follicles with minor modifications. For the EG+PXZ vitrification protocol, Ting et al. used this method to cryopreserve macaque ovarian cortex tissues and demonstrated that the isolated secondary follicles were able to grow to antral stage [23]. Compared to individual cells, larger volume of tissues/organs usually requires longer exposure time and higher concentrations of CPAs to facilitate CPA penetration and avoid intra- and extra-cellular ice formation [39, 40]. However, CPAs are also cytotoxic [41]. Since individual follicles are much smaller than ovarian cortical pieces, we decreased the equilibration time in vitrification solutions to minimize CPA-induced cytotoxicity. For the EDS-based vitrification solution, it has been widely used to cryopreserve ovarian tissues or follicles (Supplemental Table 1). Lee et al. compared four different compositions of CPAs and found that the EDS-based vitrification solution produced the best follicle survival rates after warming (96%) as well as after an 8-day 2D in vitro follicle culture (88%). However, they also discovered that the follicle growth and oocyte maturation outcomes were significantly compromised [42]. Similarly, another three studies also reported that the EDS vitrification protocol was able to result in > 85% follicle survival rate after warming and > 70% survival rate after 2D in vitro follicle culture, however, the follicle growth or MII oocyte percentage after in vitro maturation was decreased [15, 43, 44]. Compared to their vitrification solution recipes, we increased the final concentration of EG and DMSO from 30% to 40%. As we did in the EG+PXZ vitrification protocol, we also used a closed vitrification system with cooling in LN2 vapor and a two-phase warming procedure, and further included one more step of equilibration in HM without CPA to decrease the potential osmotic shock induced by high concentration of FBS in the following vitrification solutions [45]. After integrating the optimized cryo-device, vitrification solutions, and cooling and warming procedures, our results demonstrate that both EG+PXZ and EDS protocols can successfully vitrify mouse preantral follicles and the follicle survival rate and developmental pattern during eIVFG are comparable to freshly harvested follicles.
Ovarian steroidogenesis requires bi-communications between theca cells and granulosa cells and multiple steps of enzymatic conversion to produce the final sex steroid hormone, E2 (Fig. 4A) [46]. Most of previously published results showed that vitrified follicles from multiple species had significantly decreased secretion of E2 during in vitro follicle/tissue culture, suggesting that the ovarian steroidogenesis pathway was interfered by vitrification (Supplemental Table 1). Our study revealed that vitrified follicles using both modified EG+PXZ and EDS vitrification protocols had comparable E2 secretion and mRNA expression of all examined steroidogenesis related genes on day 8 of eIVFG compared to freshly harvested follicles. Therefore, our optimized vitrification protocols maintain hormone synthesis and secretion of follicular somatic cells and the vitrified follicles are good models to study the effect of xenobiotic exposure on ovarian steroidogenesis.
The normal spindle assembly and chromosome alignment during oocyte meiotic division are essential for the formation of a haploid gamete to ensure the success of subsequent fertilization, embryo development, and full-term pregnancy [47]. A majority of previous studies did not examine the spindle and chromosome organization after in vitro oocyte maturation (Supplemental Table 1). For the three studies which did, no defect was found [15, 17, 42]. Consistently, our results also showed that vitrified follicles using both EG+PXZ and EDS vitrification protocols produced MII oocytes with normal MII percentage, oocyte and polar body size, and normal spindle and chromosome organization compared to freshly harvested follicles, suggesting a good in vitro model to study the impact of environmental factors on oocyte meiotic maturation.
Although vitrified preantral mouse follicles can produce live birth following in vitro follicle growth, oocyte maturation, IVF, and embryo transfer, oocytes derived from vitrified follicles had lower developmental competence compared to oocytes grown in vivo [16]. Moreover, vitrification has been found to induce oocyte zona pellucida hardening which might be caused by the presence of calcium in vitrification solution [48, 49]. Our results revealed that vitrification maintain normal expression of two key oocyte-derived growth factors, Gdf-9 and Bmp-15, as well as three zona pellucida related genes, Zp1, Zp2, and Zp3, indicating that the optimized vitrification protocols and eIVFG might be able to maintain oocyte developmental competence. However, future studies such as IVF and in vitro embryo development are necessary to obtain more promising evidence before we use vitrified follicles to study the effect of environmental exposures on oocyte developmental competence during folliculogenesis and oogenesis.
Thus far, there has been no study investigating whether the vitrified-warmed follicles consistently respond to ovotoxic chemicals. We previously demonstrated that DOX induced DNA damage in follicles in a time and cell type dependent manner using cultured freshly isolated follicles [10]. Compared to freshly isolated follicles, our results showed that vitrified follicles had consistent toxic responses after DOX treatment, which enables us to use vitrified follicles for a high-content eIVFG, chemical exposure, and ovotoxicity screening.
HABs have increased significantly and globally in magnitude, frequency, and duration over the past few decades [50, 51]. People can be exposed to HAB toxins through seafood, drinking water, swimming, and contaminated medical solutions [52]. HAB toxins have been demonstrated to exhibit neurotoxicity, hepatotoxicity, and dermal toxicity, etc [31, 53, 54]. However, their impacts on female reproductive health and fertility have not been well studied. MCs are a family of cyclic heptapeptides produced from cyanobacteria (also termed blue-green algae) and are the most common and hazardous type of HAB toxins. The general chemical structure of MCs is cyclo-(D-Ala1-X2-D-MeAsp3-Z4-Adda5-D-Glu6-Mdha7), with two variable L-amino acids at the positions two (X) and four (Z) [55]. MCs has been well-characterized as a selective inhibitor of protein serine/threonine phosphatases 2A (PP2A) [56, 57], which results in hyperphosphorylation of proteins, leading to cytoskeleton disruption, oxidative stress, mitochondria dysfunction, endoplasmic reticulum (ER) stress, and DNA damage [58, 59].
Among different MC congeners, MC-LR has been reported to be the most potent PP2A inhibitor [60, 61]. Therefore, most of previous studies only used MC-LR for toxicity testing. However, it is poorly understood whether the other types of MC congeners exhibit similar toxicity or they can disrupt cellular functions through other mechanisms. Our dose-response ovotoxicity screening results revealed that MC- LF, but not the more commonly studied MC-LR, was the most ovotoxic MC congener. More surprisingly, compared to MC-LA, LF, and LY, MC-LR even showed the least adverse impact on follicle survival and development. Similar to our results, MC-LF has been shown to have a more pronounced cytotoxic effects on Caco-2 cells and neuronal cells than MC-LR [53, 62]. These results suggest that, at these higher and pharmacological concentrations, MC-LA, LF, and LY may cause follicle death through other molecular mechanisms besides the inhibition of PP2A. However, the underlying mechanism of MCs-induced differential ovotoxicities is still unknown and further studies are required. For example, the organic acid transporter polypeptides (OATPs) are the primary transporters to facilitate the uptake and export of MCs in multiple tissues [63]. Therefore, it is worth investigating the expression profile and function of OATPs in the ovary and whether OATPs exert different transporting activities to different MC congeners, which have been poorly studied so far.
Although we found toxic effects of MCs on follicle survival and development when the exposure concentrations were at 1-10 μM, the reported highest human serum level of MCs was at 31.4 nM in patients treated with contaminated dialysate fluids [30], which are 30- to 300-fold lower compared to the ovotoxic concentrations we discovered here. These results suggest that the human relevant exposure level of MCs may not severely affect ovarian follicle survival and development. However, considering that humans are exposed to MCs at lower concentrations but over a much longer period of time, we still need to investigate the effects of MCs on the follicle development using in vivo animal models. However, our ovotoxicity screening results also discovered that MC-LF at 0.1 μM, which is only about 3-fold higher than the peak MC serum concentration in humans, significantly inhibit E2 secretion during eIVFG, indicating that the human relevant exposure of MC-LF may interfere with ovarian steroidogenesis and exert an endocrine disrupting effect on the ovary. However, these in vitro ovotoxicity screening results will need to be validated using in vivo animal models and even human epidemiological studies and the underlying molecular mechanism are necessary to be defined in our future studies. In addition, the effects of ovotoxicants found here should be evaluated in other species such as human to determine the potential inter-species differences. As all vitrified follicles for eIVFG and ovotoxicity testing were from the EDS vitrification protocol, it is also worth to further investigate if there is any difference for the response to the ovotoxicants between different vitrification protocols. Furthermore, more well-identified ovotoxic chemicals, such as endocrine disrupting chemicals (EDCs), can be tested to further validate the consistent response between vitrified and fresh follicles. To explore whether the tested chemicals result in similar ovotoxic patterns between vitrified and fresh follicles, more in-depth investigations can also be further included, such as the steroid hormone signaling pathways in somatic cells and the acquisition of oocyte developmental competence during eIVFG.
In summary, our optimized vitrification protocols can be used to establish a long-term-storage and ready-to-use follicle bank, enabling for a high-throughput in vitro follicle culture, chemical exposure, and ovotoxicity screening. This platform is not meant to replace in vivo animal models, but it can efficiently and significantly help prioritize chemicals with high ovotoxicity concern for more targeted and mechanistic in vitro and in vivo toxicity assessments. Additionally, our ovotoxicity screening results show that MCs exhibit endocrine disrupting effect on the ovary. Furthermore, our optimized follicle vitrification protocol provides an additional fertility preservation option, particularly for prepubertal cancer patients who have no mature gametes or embryos available and are also concerned for the risk of metastasis after ovarian tissue transplantation [64].
Supplementary Material
Highlights.
Optimized vitrification protocols preserve follicle viability and functionality
Vitrified follicles show normal follicle and oocyte reproductive outcomes
Vitrified follicles consistently respond to ovotoxic chemical, doxorubicin
Different microcystin congeners exhibit differential ovotoxicities
Vitrification enables a follicle bank for high-throughput ovotoxicity screening
Acknowledgments
Funding and acknowledgments: This work was supported by the Arnold School of Public Health Start Up Fund and Advanced Support for Innovative Research Excellence (ASPIRE) from the Office of the Vice President for Research (OVPR) at the University of South Carolina, National Science Foundation (NSF 1832910) to S. Xiao, National Institutes of Health (NIH P01ES028942) to GI. Scott, S. Chatterjee, S. Xiao, and BW. Brooks, NIH K01ES030014 to S. Xiao, NIH R01 HD083930 to MB. Zelinski, and NIH P51OD011092 to the Oregon National Primate Research Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: The authors declare no conflict of interest.
References
- [1].Grive KJ, Freiman RN, The developmental origins of the mammalian ovarian reserve, Development 142(15) (2015) 2554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Goswami D, Conway GS, Premature ovarian failure, Human reproduction update 11(4) (2005) 391–410. [DOI] [PubMed] [Google Scholar]
- [3].Bhattacharya P, Keating AF, Impact of environmental exposures on ovarian function and role of xenobiotic metabolism during ovotoxicity, Toxicology and applied pharmacology 261(3) (2012) 227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Spears N, Lopes F, Stefansdottir A, Rossi V, De Felici M, Anderson RA, Klinger FG, Ovarian damage from chemotherapy and current approaches to its protection, Human reproduction update 25(6) (2019) 673–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vabre P, Gatimel N, Moreau J, Gayrard V, Picard-Hagen N, Parinaud J, Leandri RD, Environmental pollutants, a possible etiology for premature ovarian insufficiency: a narrative review of animal and human data, Environmental health : a global access science source 16(1) (2017) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].R. Roundtable on Environmental Health Sciences, and Medicine; Board on Population Health and Public Health Practice; Institute of Medicine., Identifying and reducing environmental health risks of chemicals in our society: Workshop summary- the challenge: Chemicals in today's society., Identifying and Reducing Environmental Health Risks of Chemicals in Our Society: Workshop Summary, Washington (DC), 2014. [PubMed] [Google Scholar]
- [7].Xiao S, Duncan FE, Bai L, Nguyen CT, Shea LD, Woodruff TK, Size-specific follicle selection improves mouse oocyte reproductive outcomes, Reproduction 150(3) (2015) 183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Xiao S, Zhang J, Romero MM, Smith KN, Shea LD, Woodruff TK, In vitro follicle growth supports human oocyte meiotic maturation, Sci Rep 5 (2015) 17323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xiao S, Coppeta JR, Rogers HB, Isenberg BC, Zhu J, Olalekan SA, McKinnon KE, Dokic D, Rashedi AS, Haisenleder DJ, Malpani SS, Arnold-Murray CA, Chen K, Jiang M, Bai L, Nguyen CT, Zhang J, Laronda MM, Hope TJ, Maniar KP, Pavone ME, Avram MJ, Sefton EC, Getsios S, Burdette JE, Kim JJ, Borenstein JT, Woodruff TK, A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle, Nat Commun 8 (2017) 14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Xiao S, Zhang J, Liu M, Iwahata H, Rogers HB, Woodruff TK, Doxorubicin Has Dose-Dependent Toxicity on Mouse Ovarian Follicle Development, Hormone Secretion, and Oocyte Maturation, Toxicological sciences : an official journal of the Society of Toxicology 157(2) (2017) 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang Y, Liu M, Zhang J, Liu Y, Kopp M, Zheng W, Xiao S, Multidrug Resistance Protein 1 Deficiency Promotes Doxorubicin-Induced Ovarian Toxicity in Female Mice, Toxicological sciences : an official journal of the Society of Toxicology 163(1) (2018) 279–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bian J, Li T, Ding C, Xin W, Zhu B, Zhou C, Vitreous cryopreservation of human preantral follicles encapsulated in alginate beads with mini mesh cups, The Journal of reproduction and development 59(3) (2013) 288–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Camboni A, Van Langendonckt A, Donnez J, Vanacker J, Dolmans MM, Amorim CA, Alginate beads as a tool to handle, cryopreserve and culture isolated human primordial/primary follicles, Cryobiology 67(1) (2013) 64–9. [DOI] [PubMed] [Google Scholar]
- [14].Asgari F, Valojerdi MR, Ebrahimi B, Fatehi R, Three dimensional in vitro culture of preantral follicles following slow-freezing and vitrification of mouse ovarian tissue, Cryobiology 71(3) (2015) 529–36. [DOI] [PubMed] [Google Scholar]
- [15].Trapphoff T, El Hajj N, Zechner U, Haaf T, Eichenlaub-Ritter U, DNA integrity, growth pattern, spindle formation, chromosomal constitution and imprinting patterns of mouse oocytes from vitrified pre-antral follicles, Hum Reprod 25(12) (2010) 3025–42. [DOI] [PubMed] [Google Scholar]
- [16].dela Pena EC, Takahashi Y, Katagiri S, Atabay EC, Nagano M, Birth of pups after transfer of mouse embryos derived from vitrified preantral follicles, Reproduction 123(4) (2002) 593–600. [DOI] [PubMed] [Google Scholar]
- [17].Desai N, AbdelHafez F, Ali MY, Sayed EH, Abu-Alhassan AM, Falcone T, Goldfarb J, Mouse ovarian follicle cryopreservation using vitrification or slow programmed cooling: assessment of in vitro development, maturation, ultra-structure and meiotic spindle organization, The journal of obstetrics and gynaecology research 37(1) (2011) 1–12. [DOI] [PubMed] [Google Scholar]
- [18].Lunardi FO, de Aguiar FL, Duarte AB, Araujo VR, de Lima LF, Ribeiro de Sa NA, Vieira Correia HH, Domingues SF, Campello CC, Smitz J, de Figueiredo JR, Ribeiro Rodrigues AP, Ovine secondary follicles vitrified out the ovarian tissue grow and develop in vitro better than those vitrified into the ovarian fragments, Theriogenology 85(7) (2016) 1203–10. [DOI] [PubMed] [Google Scholar]
- [19].Ben-Aharon I, Bar-Joseph H, Tzarfaty G, Kuchinsky L, Rizel S, Stemmer SM, Shalgi R, Doxorubicin-induced ovarian toxicity, Reproductive biology and endocrinology : RB&E 8 (2010) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Roti Roti EC, Leisman SK, Abbott DH, Salih SM, Acute doxorubicin insult in the mouse ovary is cell- and follicle-type dependent, PLoS One 7(8) (2012) e42293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang Y, Liu M, Johnson SB, Yuan G, Arriba AK, Zubizarreta ME, Chatterjee S, Nagarkatti M, Nagarkatti P, Xiao S, Doxorubicin obliterates mouse ovarian reserve through both primordial follicle atresia and overactivation, Toxicology and applied pharmacology 381 (2019) 114714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Brooks BW, Lazorchak JM, Howard MD, Johnson MV, Morton SL, Perkins DA, Reavie ED, Scott GI, Smith SA, Steevens JA, Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems?, Environmental toxicology and chemistry 35(1) (2016) 6–13. [DOI] [PubMed] [Google Scholar]
- [23].Ting AY, Yeoman RR, Campos JR, Lawson MS, Mullen SF, Fahy GM, Zelinski MB, Morphological and functional preservation of pre-antral follicles after vitrification of macaque ovarian tissue in a closed system, Hum Reprod 28(5) (2013) 1267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ting AY, Yeoman RR, Lawson MS, Zelinski MB, In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification, Hum Reprod 26(9) (2011) 2461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ting AY, Yeoman RR, Lawson MS, Zelinski MB, Synthetic polymers improve vitrification outcomes of macaque ovarian tissue as assessed by histological integrity and the in vitro development of secondary follicles, Cryobiology 65(1) (2012) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nikiforov D, Russo V, Nardinocchi D, Bernabo N, Mattioli M, Barboni B, Innovative multi-protectoral approach increases survival rate after vitrification of ovarian tissue and isolated follicles with improved results in comparison with conventional method, J Ovarian Res 11(1) (2018) 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kagawa N, Silber S, Kuwayama M, Successful vitrification of bovine and human ovarian tissue, Reproductive biomedicine online 18(4) (2009) 568–77. [DOI] [PubMed] [Google Scholar]
- [28].Kagawa N, Kuwayama M, Nakata K, Vajta G, Silber S, Manabe N, Kato O, Production of the first offspring from oocytes derived from fresh and cryopreserved pre-antral follicles of adult mice, Reproductive biomedicine online 14(6) (2007) 693–9. [DOI] [PubMed] [Google Scholar]
- [29].Chen J, Xie P, Li L, Xu J, First identification of the hepatotoxic microcystins in the serum of a chronically exposed human population together with indication of hepatocellular damage, Toxicol Sci 108(1) (2009) 81–9. [DOI] [PubMed] [Google Scholar]
- [30].Hilborn ED, Carmichael WW, Yuan M, Azevedo SM, A simple colorimetric method to detect biological evidence of human exposure to microcystins, Toxicon 46(2) (2005) 218–21. [DOI] [PubMed] [Google Scholar]
- [31].Chen L, Chen J, Zhang X, Xie P, A review of reproductive toxicity of microcystins, Journal of hazardous materials 301 (2016) 381–99. [DOI] [PubMed] [Google Scholar]
- [32].Meneely JP, Elliott CT, Microcystins: measuring human exposure and the impact on human health, Biomarkers 18(8) (2013) 639–49. [DOI] [PubMed] [Google Scholar]
- [33].Haddad SP, Bobbitt JM, Taylor RB, Lovin LM, Conkle JL, Chambliss CK, Brooks BW, Determination of microcystins, nodularin, anatoxin-a, cylindrospermopsin, and saxitoxin in water and fish tissue using isotope dilution liquid chromatography tandem mass spectrometry, J Chromatogr A 1599 (2019) 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li Y, Feng HL, Cao YJ, Zheng GJ, Yang Y, Mullen S, Critser JK, Chen ZJ, Confocal microscopic analysis of the spindle and chromosome configurations of human oocytes matured in vitro, Fertility and sterility 85(4) (2006) 827–32. [DOI] [PubMed] [Google Scholar]
- [35].Su YQ, Wu X, O'Brien MJ, Pendola FL, Denegre JN, Matzuk MM, Eppig JJ, Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop, Dev Biol 276(1) (2004) 64–73. [DOI] [PubMed] [Google Scholar]
- [36].de Castro FC, Cruz MH, Leal CL, Role of Growth Differentiation Factor 9 and Bone Morphogenetic Protein 15 in Ovarian Function and Their Importance in Mammalian Female Fertility - A Review, Asian-Australas J Anim Sci 29(8) (2016) 1065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wassarman PM, Jovine L, Litscher ES, Mouse zona pellucida genes and glycoproteins, Cytogenet Genome Res 105(2-4) (2004) 228–34. [DOI] [PubMed] [Google Scholar]
- [38].Epifano O, Liang LF, Familari M, Moos MC Jr., Dean J, Coordinate expression of the three zona pellucida genes during mouse oogenesis, Development 121(7) (1995) 1947–56. [DOI] [PubMed] [Google Scholar]
- [39].Gao D, Critser JK, Mechanisms of cryoinjury in living cells, ILAR journal 41(4) (2000) 187–96. [DOI] [PubMed] [Google Scholar]
- [40].Bakhach J, The cryopreservation of composite tissues: Principles and recent advancement on cryopreservation of different type of tissues, Organogenesis 5(3) (2009) 119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Elliott GD, Wang S, Fuller BJ, Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures, Cryobiology 76 (2017) 74–91. [DOI] [PubMed] [Google Scholar]
- [42].Lee J, Kim EJ, Kong HS, Youm HW, Kim SK, Lee JR, Suh CS, Kim SH, Establishment of an improved vitrification protocol by combinations of vitrification medium for isolated mouse ovarian follicles, Theriogenology 121 (2018) 97–103. [DOI] [PubMed] [Google Scholar]
- [43].Oryan Abkenar Z, Ganji R, Eghbal Khajehrahimi A, Bahadori MH, Vitrification and subsequent in vitro maturation of mouse preantral follicles in presence of growth factors, Cell journal 16(3) (2014) 271–8. [PMC free article] [PubMed] [Google Scholar]
- [44].Taghavi SA, Valojerdi MR, Moghadam MF, Ebrahimi B, Vitrification of mouse preantral follicles versus slow freezing: Morphological and apoptosis evaluation, Anim Sci J 86(1) (2015) 37–44. [DOI] [PubMed] [Google Scholar]
- [45].Gstraunthaler G, Alternatives to the use of fetal bovine serum: Serum-free cell culture, Altex-Altern Tierexp 20(4) (2003) 275–281. [PubMed] [Google Scholar]
- [46].Segawa T, Teramoto S, Omi K, Miyauchi O, Watanabe Y, Osada H, Changes in estrone and estradiol levels during follicle development: a retrospective large-scale study, Reproductive biology and endocrinology : RB&E 13 (2015) 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bennabi I, Terret ME, Verlhac MH, Meiotic spindle assembly and chromosome segregation in oocytes, The Journal of cell biology 215(5) (2016) 611–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wiesak T, Wasielak M, Zlotkowska A, Milewski R, Effect of vitrification on the zona pellucida hardening and follistatin and cathepsin B genes expression and developmental competence of in vitro matured bovine oocytes, Cryobiology 76 (2017) 18–23. [DOI] [PubMed] [Google Scholar]
- [49].Larman MG, Sheehan CB, Gardner DK, Calcium-free vitrification reduces cryoprotectant-induced zona pellucida hardening and increases fertilization rates in mouse oocytes, Reproduction 131(1) (2006) 53–61. [DOI] [PubMed] [Google Scholar]
- [50].EPA, Climate Change and Harmful Algal Blooms, 2011. https://www.epa.gov/nutrientpollution/climate-change-and-harmful-algal-blooms.
- [51].CDC, Harmful algal blooms (HABs)-Associated Illness, 2018. https://www.cdc.gov/habs/index.html.
- [52].Drobac D, Tokodi N, Simeunovic J, Baltic V, Stanic D, Svircev Z, Human exposure to cyanotoxins and their effects on health, Arh Hig Rada Toksikol 64(2) (2013) 119–30. [DOI] [PubMed] [Google Scholar]
- [53].Hinojosa MG, Gutierrez-Praena D, Prieto AI, Guzman-Guillen R, Jos A, Camean AM, Neurotoxicity induced by microcystins and cylindrospermopsin: A review, The Science of the total environment 668 (2019) 547–565. [DOI] [PubMed] [Google Scholar]
- [54].Zhou M, Tu WW, Xu J, Mechanisms of microcystin-LR-induced cytoskeletal disruption in animal cells, Toxicon 101 (2015) 92–100. [DOI] [PubMed] [Google Scholar]
- [55].Rastogi RP, Sinha RP, Incharoensakdi A, The cyanotoxin-microcystins: current overview, Rev Environ Sci Bio 13(2) (2014) 215–249. [Google Scholar]
- [56].MacKintosh C, Beattie KA, Klumpp S, Cohen P, Codd GA, Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants, Febs Lett 264(2) (1990) 187–92. [DOI] [PubMed] [Google Scholar]
- [57].Runnegar MT, Kong S, Berndt N, Protein phosphatase inhibition and in vivo hepatotoxicity of microcystins, Am J Physiol 265(2 Pt 1) (1993) G224–30. [DOI] [PubMed] [Google Scholar]
- [58].Meng G, Sun Y, Fu W, Guo Z, Xu L, Microcystin-LR induces cytoskeleton system reorganization through hyperphosphorylation of tau and HSP27 via PP2A inhibition and subsequent activation of the p38 MAPK signaling pathway in neuroendocrine (PC12) cells, Toxicology 290(2-3) (2011) 218–29. [DOI] [PubMed] [Google Scholar]
- [59].Wang H, Liu J, Lin S, Wang B, Xing M, Guo Z, Xu L, MCLR-induced PP2A inhibition and subsequent Rac1 inactivation and hyperphosphorylation of cytoskeleton- associated proteins are involved in cytoskeleton rearrangement in SMMC-7721 human liver cancer cell line, Chemosphere 112 (2014) 141–53. [DOI] [PubMed] [Google Scholar]
- [60].Ikehara T, Imamura S, Oshiro N, Ikehara S, Shinjo F, Yasumoto T, A protein phosphatase 2A (PP2A) inhibition assay using a recombinant enzyme for rapid detection of microcystins, Toxicon 51(8) (2008) 1368–73. [DOI] [PubMed] [Google Scholar]
- [61].Ikehara T, Imamura S, Sano T, Nakashima J, Kuniyoshi K, Oshiro N, Yoshimoto M, Yasumoto T, The effect of structural variation in 21 microcystins on their inhibition of PP2A and the effect of replacing cys269 with glycine, Toxicon 54(4) (2009) 539–44. [DOI] [PubMed] [Google Scholar]
- [62].Vesterkvist PS, Misiorek JO, Spoof LE, Toivola DM, Meriluoto JA, Comparative cellular toxicity of hydrophilic and hydrophobic microcystins on Caco-2 cells, Toxins 4(11) (2012) 1008–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].EPA, Drinking Water Health Advisories for the Cyanobacterial Microcystin Toxins, in: Water O.o. (Ed.) Washington, DC, 2015. [Google Scholar]
- [64].Meirow D, Hardan I, Dor J, Fridman E, Elizur S, Ra'anani H, Slyusarevsky E, Amariglio N, Schiff E, Rechavi G, Nagler A, Ben Yehuda D, Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients, Hum Reprod 23(5) (2008) 1007–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.