Abstract
Objective—
To examine associations among race, the accumulation of multiple forms of discriminatory experiences (i.e., “pervasive discrimination”) and allostatic load (AL) in African-Americans and Whites in mid-life.
Methods—
Using data collected in 2004–2006 from 226 African-American and 978 White adults (57% female; mean age=54.7 years (SD=0.11)) in the Midlife in the United States II (MIDUS II) Biomarker Project, a pervasive discrimination score was created by combining three discrimination scales, and an AL score was created based on 24 biomarkers representing 7 physiological systems. Linear regression models were conducted to examine the association between pervasive discrimination and AL, adjusting for demographics and medical, behavioral, and personality covariates. A race by pervasive discrimination interaction was also examined in order to determine whether associations varied by race.
Results—
African-Americans had higher pervasive discrimination and AL scores than Whites. In models adjusted for demographics, socioeconomic status, medications, health behaviors, neuroticism and negative affect, a pervasive discrimination score of 2 vs. 0 was associated with a greater AL score (b=0.30; SE=0.07, p<.001). While associations appeared to be stronger among African-Americans as compared to Whites, associations did not statistically differ by race.
Conclusions—
More pervasive discrimination was related to greater multisystemic physiological dysregulation in a cohort of African-American and White adults. Measuring discrimination by combining multiple forms of discriminatory experiences may be important for studying the health effects of discrimination
Keywords: African-Americans, discrimination, allostatic load, social determinants of health, health disparities
INTRODUCTION
On almost every major indicator of poor health, African-Americans fare worse than their White counterparts. From diabetes mellitus (1, 2) and stroke (3) incidence and mortality to pre-term birth and infant mortality (4), as well as mortality from heart disease (5) and cancer (1), racial disparities have been documented. Although factors including socioeconomic status (SES) (6), access to and receipt of medical care (7), and behavioral risk factors (1) have been cited as contributors to these disparities, they do not fully account for health gaps between African-Americans and Whites (6).
Researchers have proposed that experiences of discrimination may be important to consider in studies designed to advance understanding of racial disparities in health (8, 9). Across cohorts, African-Americans report more discrimination than Whites (10, 11), and self-reported experiences of discrimination have been associated with outcomes such as breast cancer (12), incident cardiovascular disease (13), incident diabetes (14) and metabolic syndrome (15), asthma (16), and poor sleep (17–19). However, although African-Americans typically report discrimination for different reasons than Whites (e.g. “race/ethnicity” versus “sex/gender” or “appearance”) (20, 21), to date, studies have not consistently found evidence to support stronger associations among African-Americans (or other minority groups) compared to Whites (9, 13, 18, 22, 23); nor have studies consistently found that one attribute (e.g. race) more negatively affects outcomes than another (15, 17, 24–26).
Some have argued that the lack of observed racial differences in the association between discrimination and health is largely due to the possibility that interpersonal mistreatment impacts individuals similarly, irrespective of their racial/ethnic backgrounds (8). However, it could also potentially be due to the fact that the majority of prior studies have focused on discrimination assessed using a single scale. Both national and self-reported data indicate that relative to Whites, African-Americans are exposed to discrimination across a wider variety of situations and settings in life, including while shopping (27), at work (28) and when seeking housing (29), employment (30) and healthcare (31), as well as in interactions with the criminal justice system (32). For African-Americans in particular, the pervasiveness of discrimination in everyday life may be stressful, unavoidable and ultimately detrimental to health. This is consistent with the “weathering hypothesis,” which posits that cumulative exposure to chronic stressors such as discrimination in the context of overall racial disadvantage leads to accelerated physiological aging, or “weathering” among African-Americans, relative to their White counterparts (33, 34). However, the impact of pervasive discrimination on health, and any potential racial differences in its association with outcomes, has been underexplored.
In the present study, we examined associations between self-reported “pervasive” discrimination—assessed by combining multiple scales that assess experiences of discrimination across a range of settings and situations-- and an indicator of impaired physiological functioning, allostatic load (AL), among African-Americans and Whites. We chose AL because it summarizes overall systemic dysregulation, i.e. physiological “wear and tear” on the body (35), and may be one pathway through which discrimination affects a variety of health outcomes. Prior studies have documented positive associations between self-reported discrimination measured via a single scale and AL (36–42); however experiences of discrimination assessed using a combination of multiple scales may better capture the level of pervasiveness by which it permeates the lives of certain groups. Additionally, to our knowledge, only one of these prior studies of discrimination and AL examined racial differences (39), but that study focused on weight discrimination specifically, which is actually underreported by African-Americans, relative to Whites (43). Consequently, we further examined whether the association between pervasive discrimination and AL was stronger among African-Americans than Whites, given the particularly ubiquitous nature of discrimination in the lives of African-Americans as compared to Whites and the potential role of weathering.
METHODS
Sample
The sample was drawn from the Midlife in the United States II (MIDUS II) Biomarker Project, a national assessment of long-term change in the relationships between sociodemographic and psychosocial variables with biological functioning among non-institutionalized adults living in the 48 contiguous states. The original MIDUS I participants, aged 25–74 (N=7,108), were interviewed using random digit dialing between 1995–1996 and were contacted again for MIDUS II between 2004–2006 (N=5,895). The response rate in the MIDUS II follow-up survey was 70%. A subset of 1,255 MIDUS II participants provided further information through a physical exam, medical history, medication regime, sleep assessment, laboratory challenge of physical functioning, and a comprehensive array of biomarkers during a 2-day clinic visit. A more detailed description of the procedures and methods in the MIDUS II Biomarker project has been previously published (44). The current study focused on data from the 2004–2006 study visit as it was the only study visit where information on both biomarkers and psychosocial factors was collected. Of the original 1,255 participants included in the MIDUS II Biomarkers Project, 51 were excluded from this analysis because they were not either White or African American. A total sample of 1,204 was used in this analysis and was based on 10 multiple imputed data sets. Multiple imputation was used to minimize bias from the missing data for a few variables (i.e., among variables with missing data, missingness ranged from <1% −8.5% of the sample).
Measures
Pervasive Discrimination
Pervasive discrimination was assessed using a combination of three discrimination scales: Everyday Discrimination Scale (45), Lifetime Discrimination Scale (20), and Workplace Discrimination Scale (46).
Everyday Discrimination and Lifetime Discrimination Scales
The introduction to the self-administered questions for both the Everyday and Lifetime Discrimination scales asked respondents “How many times in your life have you been discriminated against in each of the following ways because of such things as your race, ethnicity, sex, age, religion, physical appearance, sexual orientation, or other characteristics?” Participants were instructed to only report experiences due to discrimination and not experiences due to other reasons.
Everyday discrimination, defined as “chronic, routine, and relatively minor experiences of unfair treatment” was measured using a nine-item self-administered scale (Cronbach’s alpha=0.91) (45). The scale asked participants: “In your day-to-day life, how often do any of the following things happen to you?” The following list included nine situations, such as having been treated with less courtesy than other people or having received poorer service than other people at restaurants or stores. Participants could respond if they “often,” “sometimes,” “rarely,” or “never” experienced these situations. The coded responses for each of the nine items were summed so that a higher score indicated a higher level of everyday discrimination.
Lifetime discrimination, defined as events that occurred over the life course, but had greater potential consequences for the individual’s socioeconomic position, was measured using an 11-item self-administered index (Cronbach’s alpha=0.85) (20). Participants were asked if they had ever experienced major events such as being “discouraged by a teacher or advisor,” “not hired for a job,” “prevented from renting or buying a home in the neighborhood [they] wanted,” or “denied or provided inferior medical care.” Each time the respondent answered “yes” to one of the 11 questions a score of 1 was assigned. Scores were summed, with a higher score indicating a higher level of lifetime discrimination.
Workplace Discrimination Scale
Workplace discrimination, defined as job harassment and unfair treatment at work, was measured using a 6-item self-administered scale (Cronbach’s alpha = 0.79) (46), with Likert-style items such as: “how often are you watched more closely than other workers?” or “how often has a co-worker with less experience and qualifications gotten promoted before you?” Participants who reported working in the last 10 years were asked to indicate whether they experienced one of the six scenarios “once a week or more,” “a few times a month,” “a few times a year,” “less than once a year,” or “never.” The scale was scored by summing the responses of each of the 6 items and coded so that a higher score indicated a higher level of workplace discrimination.
Pervasive Discrimination Score
The Everyday Discrimination Scale, Lifetime Discrimination Scale, and Workplace Discrimination Scale were moderately correlated among African-Americans (rho range: 0.22–0.48) and Whites (rho range: 0.21–0.42) (all p<0.0001). Because we were particularly interested in the unavoidable aspects of pervasive discrimination, we wanted to capture those who reported relatively high exposure to discrimination across multiple contexts (rather than high in one context, but moderate in others). Consequently, we created a pervasive discrimination score by combining the three discrimination scales, using the following procedure. First, for each of the three scales, respondents were categorized into tertiles based on the sample distribution. Second, the pervasive discrimination score was computed as follows: 1) participants in the lowest tertile on all three discrimination scales were given a “0” for the pervasive discrimination score; 2) participants in the highest tertile for only one of the three discrimination scales were given a “1”; and 3) participants in the highest tertile for two or three of the discrimination scales were given a “2.” This last group was combined given the small number (8.4%) of participants in the highest tertile for all three of the scales. In supplemental analyses, we also examined each individual discrimination scale continuously in separate models.
Allostatic load
Using established criteria, an overall AL score was created from seven AL subscales based on a set of 24 biomarkers collected from individuals in the MIDUS II Biomarker Project. The inter-assay and intra-assay variation of the set of biomarkers ranged from 0.85% to 13.0% and from 0.8% to 7.9%, respectively (47). The 24 biomarkers were chosen to describe physiological dysregulation across multiple systems (47–50). The seven AL subscales created from these 24 biomarkers included: sympathetic system functioning (urine epinephrine and norepinephrine, both adjusted for urine creatinine), parasympathetic system functioning (heart rate variability measures), hypothalamic pituitary adrenal-axis functioning (urine cortisol adjusted for urine creatinine and blood DHEAS), inflammation (Interleukin-6, fibrinogen, C-reactive protein, E-selectin, and ICAM), cardiovascular system functioning (systolic blood pressure, pulse pressure, heart rate), glucose metabolism (HbA1c, blood glucose, insulin resistance), and lipid metabolism (body mass index (BMI), waist to hip ratio, triglycerides, high-density lipoproteins and low-density lipoproteins). The distributions of each of the 24 biomarkers were split into risk quartiles. Values in the quartile with highest risk received a risk-score of 1 and all other values received a risk-score of 0. For each participant with valid measurements for at least half of the biomarkers in a given subscale, the risk-scores for each biomarker in the seven subscales were averaged to create a summary score. For example, risk-scores for systolic blood pressure, heart rate, and pulse pressure were averaged together to create a cardiovascular summary score. If an individual ranked in the highest quartile of risk for only one of these three biomarkers, the cardiovascular summary score was 0.33; if two of the three biomarkers fell within the highest quartile of risk, the cardiovascular summary score was 0.66; all three biomarkers within the highest quartile of risk would produce a cardiovascular summary score of 1.00. The seven subscale scores were summed to compute a final overall AL score that ranged between 0 and 7 for all participants with valid scores for at least six of the subscales (47–50).
Demographics
Age, sex, marital status, self-reported race, employment status, education, and total household income were chosen as demographic covariates based on previous literature (36–42). Age was determined by participant birth date. Sex was coded as male or female. Marital status was either currently married, formerly married, or never married. Race was non-Hispanic White or non-Hispanic African-American. Employment status was coded as currently employed or not currently employed. SES was measured using education coded as a high school diploma or less, some college, and an Associate’s or Bachelor’s degree or more, and total household income was reported in dollars per year.
Covariates
Oral steroid, beta-blocker, SSRI (selective serotonin reuptake inhibitor), and cholesterol medications were self-reported and included as covariates. Based on previous literature (47), health behavior covariates included self-reported smoking status, past month alcohol usage, and physical activity. Current smoking was coded as either current smoker or not currently a smoker. Alcohol usage was split into three categories of past month usage: never or not in the past month, less than once per week, at least once per week in the past month. Physical activity was measured using a summary weighted score based on weekly frequency and intensity of household, leisure, and occupational physical activity (47). The scores ranged from 9 to a possible maximum of 54. To minimize bias that could potentially be linked to personality factors, neuroticism and negative affect were included as covariates. Neuroticism was measured using four self-report items (51). Negative affect was measured using six self-report Likert style items combined into a scale, taken from the Positive and Negative Affect Scale (52).
Statistical Analyses
All statistical analyses were performed using SAS 9.4 (Cary, NC). Descriptive statistics were utilized to summarize participant characteristics for the overall sample and by race. Linear regression analyses were conducted to examine associations between pervasive discrimination and overall AL. Models were sequentially adjusted for demographics (race, age, sex, marital status, and employment status), SES (educational attainment and total household income), medications (oral steroid, SSRI, beta-blocker, and cholesterol use), health behaviors (current smoking, physical activity score, past month alcohol use), and personality covariates (neuroticism and negative affect). We also ran sensitivity analyses to adjust for anti-hypertensives and diabetes medication. To be consistent with prior studies, these were not included in our primary models. Similarly, models were run with and without BMI as a covariate (only the lipid metabolism subscale included BMI). Sensitivity analyses with anti-hypertensives, diabetes medication, and BMI did not alter our findings, thus they were excluded from final models.
The interaction between race and pervasive discrimination was formally assessed in minimally and fully-adjusted non-stratified linear regression models by creating cross-product terms. We did not observe a significant interaction (p-values >.05); nonetheless, we ran race-stratified models in exploratory analyses. This was done for two reasons: 1) to obtain race-specific effect sizes of the association between pervasive discrimination and AL in our cohort, given our original hypothesis, and emerging consensus across fields for the importance of effect sizes over p-values;(53–55) as well as 2) conceptual arguments for the importance of stratifying by race in order to account for differential confounding of associations of interest within each racial group (56).
Following these primary and exploratory analyses, supplementary analyses were conducted to: 1) examine the association between pervasive discrimination and each of the seven AL subscales separately; 2) examine the association between each individual discrimination scale (modeled continuously) and overall AL; and 3) examine the associations between pervasive discrimination and AL using a continuous version of the pervasive discrimination score, in order to retain those individuals who scored in the moderate range across the three scales. The continuous version of the pervasive discrimination score was created by standardizing aggregated z-scores from the three individual discrimination scales. Our analyses of continuous pervasive discrimination z-scores were conducted in both the overall sample and then separately for African-Americans and Whites, in order to more effectively compare any observed results to those in our primary and exploratory analyses.
RESULTS
Participant Characteristics
Descriptive statistics of the analytic sample are presented in Table 1. African-Americans reported more pervasive discrimination than Whites (p<0.001), with 42.7% of African-Americans receiving a score of 2 (i.e. reporting discrimination in the highest tertile for 2 or 3 of the 3 discrimination scales) relative to 20.1% of Whites. On average, African-Americans also had a higher AL score (mean=1.93, standard deviation (SD)=1.01) than Whites (mean=1.71, SD=1.05) (p=.005). Additionally, in comparison to Whites, African-Americans were younger, less likely to be male, less likely to be currently employed or married, and reported less education and lower total household incomes. Although African-Americans reported less alcohol use than their White counterparts, they were more likely to be current smokers than Whites.
Table 1.
Characteristics of African-American and White adults in the MIDUS II Biomarker Project (n=1,204)
| All (1,204) | African-Americans (n=226) | Whites (n=978) | p-value | |
|---|---|---|---|---|
| % or mean (SD) | % or mean (SD) | |||
| Age (years) | 54.68 (11.77) | 50.90 (10.59) | 55.54 (11.87) | <.001 |
| Male (%) | 43.02 | 32.30 | 45.50 | <.001 |
| Marriage Status (%) | ||||
| Currently married | 64.83 | 28.32 | 73.27 | <.001 |
| Formerly married | 22.96 | 39.38 | 19.16 | |
| Never married | 12.21 | 32.30 | 7.57 | |
| Currently Employed (%) | 70.62 | 62.39 | 72.53 | .003 |
| Education (%) | ||||
| HS or less | 28.07 | 46.90 | 23.72 | <.001 |
| Some College | 22.48 | 27.88 | 21.24 | |
| At least Associates/Bachelors | 49.44 | 25.22 | 55.04 | |
| Total Household Income | 69960.70 (58260.01) | 38192.50 (34567.70) | 77301.80 (60129.00) | <.001 |
| Pervasive Discrimination Score (%) | ||||
| 0 | 44.18 | 30.75 | 47.28 | <.001 |
| 1 | 31.51 | 26.59 | 32.65 | |
| 2 or 3 | 24.31 | 42.65 | 20.07 | |
| Allostatic Load Score | 1.75 (1.05) | 1.93 (1.01) | 1.71 (1.05) | .005 |
| Neuroticism1 | 2.03 (0.63) | 2.10 (0.69) | 2.02 (0.62) | .11 |
| Negative affect2 | 1.53 (0.62) | 1.77 (0.83) | 1.48 (0.54) | <.001 |
| Oral Steroid Use (%) | 1.16 | 2.21 | 0.92 | <.001 |
| Beta Blocker Use (%) | 14.53 | 15.04 | 14.42 | .45 |
| SSRI Use (%) | 8.14 | 2.65 | 9.41 | <.001 |
| Cholesterol Med Use (%) | 28.41 | 21.24 | 30.06 | <.001 |
| Current Smoker (%) | 13.54 | 27.88 | 10.22 | <.001 |
| Alcohol Use (%) | ||||
| No alcohol in past month | 34.14 | 44.48 | 31.78 | .002 |
| <Once per week in past month | 31.15 | 27.57 | 31.98 | |
| ≥Once per week in past month | 34.70 | 28.05 | 36.24 | |
| Physical Activity Score3 | 29.72 (10.90) | 29.72 (12.65) | 29.72 (10.45) | .98 |
| Body Mass Index | 29.78 (6.62) | 32.80 (8.33) | 29.08 (5.94) | <.001 |
Percentages or mean (SD=standard deviation) presented.
P-values determined from chi-square and t-tests across racial groups.
Out of a possible 4 points
Out of a possible 6 points
Weighted score based on 3 questions (range: 9 to 54).
SSRI= selective serotonin reuptake inhibitor; MIDUS=Midlife in the United States.
Primary Analyses
Among the full sample, in models adjusted for demographics and SES only, a pervasive discrimination score of 1 as compared to 0 was not associated with AL (b=0.07, standard error (SE)= 0.07, p=0.33), although a score of 2 compared to 0 was associated with greater AL (b=0.35, SE=0.07, p<.001) (Table 2). These relationships were observed in models further adjusted for medications, health behaviors, and personality characteristics (pervasive discrimination score of 1 vs. 0 b=0.04, SE=0.06, p=0.50; score of 2 vs. 0 b=0.30, SE=0.07, p<.001).
Table 2.
Associations between pervasive discrimination and allostatic load in the MIDUS II Biomarker Project (n=1,204)
| Model 1a | Model 2b | Model 3c | Model 4d | Model 5e | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta (SE) | p | Beta (SE) | p | Beta (SE) | p | Beta (SE) | p | Beta (SE) | p | |
| 0 (ref) | -- | -- | -- | -- | -- | |||||
| 1 | 0.07 (0.07) | 0.33 | 0.06 (0.07) | 0.32 | 0.07 (0.07) | 0.28 | 0.05 (0.06) | 0.44 | 0.04 (0.06) | 0.50 |
| 2 | 0.35 (0.07) | <.001 | 0.34 (0.07) | <.001 | 0.33 (0.07) | <.001 | 0.33 (0.07) | <.001 | 0.30 (0.07) | <.001 |
SE= standard error; MIDUS=Midlife in the United States.
Adjusted for demographics (race, age, sex, marital status, employment status)
Model 1 + socioeconomic status (education, total household income)
Model 2 + medications (oral steroid, beta-blocker, selective serotonin reuptake inhibitor, cholesterol)
Model 3 + health behaviors covariates (current smoker, physical activity score, past month alcohol usage)
Model 4 + psychosocial characteristics (neuroticism, negative affect)
Exploratory Analyses
Table 3 presents exploratory, race-stratified models. In fully adjusted linear regression models among Whites, a pervasive discrimination score of 1 as compared to 0 was not significantly associated with AL (b=0.02, SE= 0.07, p=0.74), although a score of 2 compared to 0 was significantly associated with greater AL (b=0.29, SE=0.09, p<.001). Similarly, among African-Americans in fully-adjusted models, a pervasive discrimination score of 1 as compared to 0 was not significantly associated with AL (b=0.26, SE= 0.17, p=0.12), although a score of 2 compared to 0 was significantly associated with greater AL (b=0.44, SE=0.15, p=0.004). The race by pervasive discrimination interaction was not statistically significant (p=.18 for 0 vs 1 and p=.19 for 0 vs 2), although the effect size among African-Americans comparing a score of 2 to 0 was 52% larger than that among Whites. Figure 1 displays the race-specific least square means for African-Americans and Whites separately, based on the fully-adjusted models. In addition to the magnitude of the association between pervasive discrimination and AL appearing larger among African-Americans as compared to Whites, the association between reports of pervasive discrimination seemed to be dose-response in nature among African-Americans, but not Whites. Of note, within group associations also differed. SES variables appeared to strengthen the association between pervasive discrimination and AL for African-Americans, but not for Whites (Model 2, Table 3). In fully-adjusted models, among African-Americans only, sex (female), SSRI use and negative affect were also significantly associated with a higher AL score; whereas among Whites, alcohol and beta-blocker use were significantly associated with a higher AL score.
Table 3.
Associations between pervasive discrimination and allostatic load by race in the MIDUS II Biomarker Project
| Model 1a | Model 2b | Model 3c | Model 4d | Model 5e | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Beta (SE) | p | Beta (SE) | p | Beta (SE) | p | Beta (SE) | p | Beta (SE) | p | |
| African-Americans (n=226) | ||||||||||
| 0 (ref) | -- | -- | -- | -- | -- | |||||
| 1 | 0.19 (0.17) | 0.26 | 0.26 (0.17) | 0.12 | 0.29 (0.17) | 0.08 | 0.25 (0.17) | 0.14 | 0.26 (0.17) | 0.12 |
| 2 | 0.41 (0.16) | 0.01 | 0.52 (0.16) | 0.002 | 0.48 (0.16) | 0.002 | 0.46 (0.16) | 0.003 | 0.44 (0.15) | 0.004 |
| Whites (n=978) | ||||||||||
| 0 (ref) | -- | -- | -- | -- | -- | |||||
| 1 | 0.04 (0.07) | 0.56 | 0.03 (0.07) | 0.62 | 0.04 (0.07) | 0.57 | 0.03 (0.07) | 0.72 | 0.02 (0.07) | 0.74 |
| 2 | 0.33 (0.09) | <.001 | 0.31 (0.09) | <.001 | 0.30 (0.09) | <.001 | 0.30 (0.09) | <.001 | 0.29 (0.09) | <.001 |
SE= standard error; MIDUS=Midlife in the United States.
Adjusted for demographics (age, sex, marital status, employment status)
Model 1 + socioeconomic status (education, total household income)
Model 2 + medications (oral steroid, beta-blocker, selective serotonin reuptake inhibitor, cholesterol)
Model 3 + health behaviors covariates (current smoker, physical activity score, past month alcohol usage)
Model 4 + psychosocial characteristics (neuroticism, negative affect)
Figure 1. Associations between pervasive discrimination and allostatic load by race in the MIDUS II Biomarker Project (n=1,204).
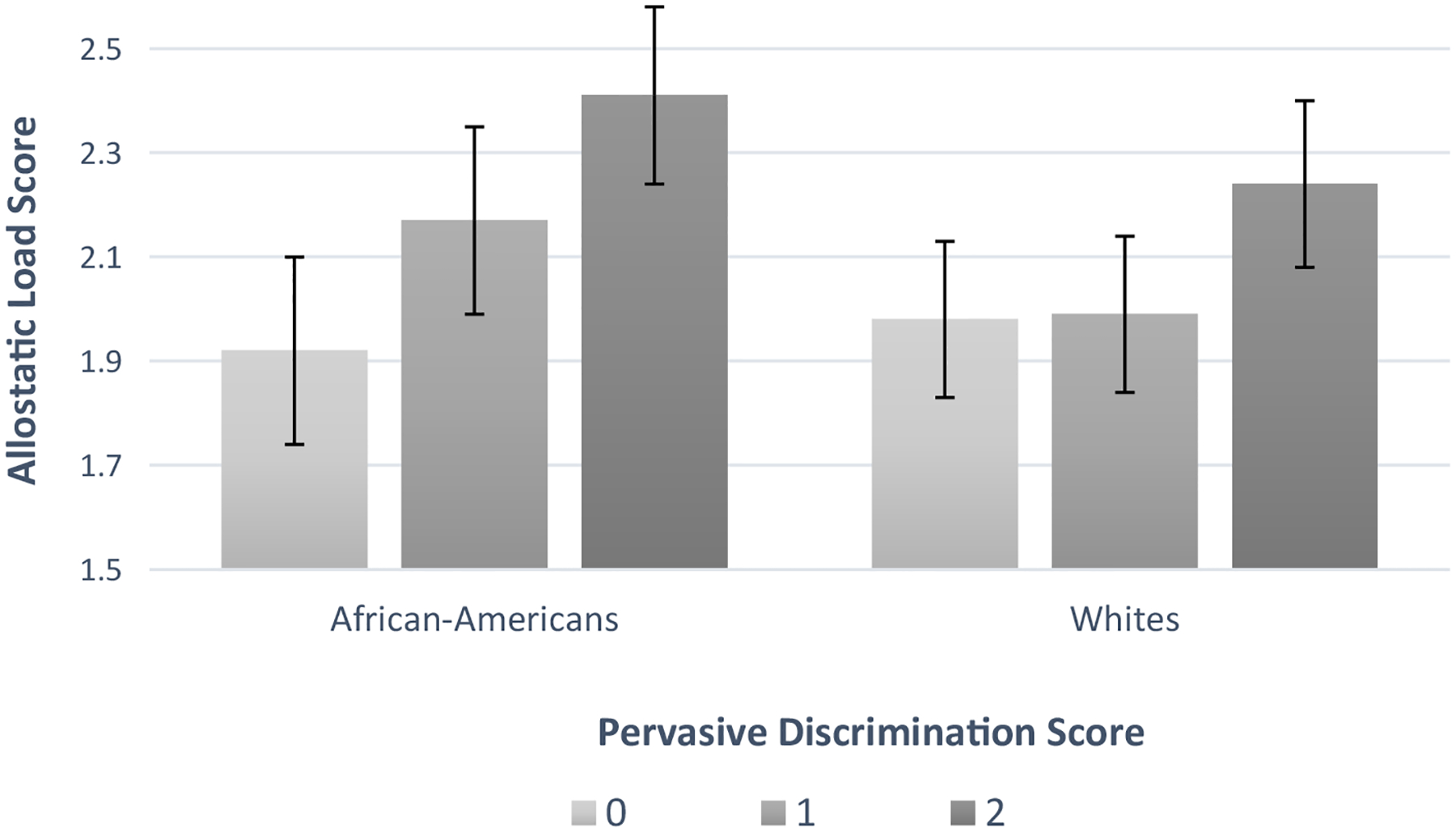
MIDUS=Midlife in the United States; Values are estimated marginal means from linear regression models adjusted for demographics (age, sex, marital status, and employment status), socioeconomic status (educational attainment and total household income), medications (oral steroid, SSRI, beta-blocker, and cholesterol use), health behaviors (current smoking, physical activity score, past month alcohol use), and personality covariates (neuroticism and negative affect). Error bars represent standard errors. Sample based on 10 multiple imputed data sets.
Supplementary Analyses
In supplementary analyses, we examined associations between pervasive discrimination and each of the seven AL subscales (Table S1, Supplemental Digital Content), to document whether pervasive discrimination was associated with each of the individual system subscales included in the overall AL score. In the full cohort, positive associations were observed for glucose metabolism, lipid metabolism, and inflammation summary scores.
Supplementary analyses were also conducted examining associations between each individual discrimination scale modeled continuously and overall AL. Among the full sample in minimally and fully-adjusted models, Everyday Discrimination and Lifetime Discrimination (but not Workplace Discrimination) were associated with the AL score (Fully adjusted models: Everyday Discrimination: b=0.02, SE=0.01, p<.001; Lifetime Discrimination: b=0.05, 0.02, p=0.001; Workplace Discrimination: b=0.01, SE=0.01, p=0.27).
Greater pervasive discrimination, in the form of a continuous score created by standardizing aggregated z-scores from the three individual discrimination scales, was also associated with a higher AL score in the full sample in minimally- and fully-adjusted models (b=0.14, SE=0.03, p<.001; b=0.12, SE=0.03, p=0.001; Table S2, Supplemental Digital Content). In fully-adjusted race-stratified models, associations were observed among both African-Americans (b=0.13, SE=0.05, p<.001), and Whites (b=0.12, SE=0.04, p=.003), and the effect sizes appeared to be comparable. There were also no significant race by pervasive discrimination interactions using the continuous pervasive discrimination score.
DISCUSSION
In this cohort of African-American and White adults, reporting higher levels of pervasive discrimination—i.e. the experience of multiple forms of discriminatory experiences— was more common among African-Americans compared to Whites. Among both African-Americans and Whites, reports of pervasive discrimination were independently associated with greater overall AL after adjusting for sociodemographics, health behaviors and psychosocial risk factors. Findings from this study are a contribution to a literature that includes studies that have simultaneously examined multiple scales of discrimination (11, 13, 57), but have done so by examining these scales individually, thereby potentially not fully realizing the effects of the pervasiveness of discrimination for some groups. Additionally, because few studies have examined racial differences in the association between overall discrimination (pervasive or individual scales) and AL, these analyses further add to our understanding of whether and how various forms of discrimination might be contextualized by race to contribute to adverse health outcomes.
Although we did not observe a statistically significant race by pervasive discrimination interaction, the magnitude of the pervasive discrimination and AL association in our exploratory race-stratified analyses appeared to be stronger and had more of a dose-response pattern among African-Americans in comparison to Whites. Although not conclusive, the race-specific findings in this exploratory analysis suggests that the measurement of discrimination using a combination of multiple scales instead of a single scale could be particularly important for studying the health effects of discrimination within specific racial groups. This could be one potential reason for why prior studies that focused on a single scale of discrimination did not observe more pronounced, or in some instances any, associations between discrimination and health among African-Americans (23, 58, 59).
However, it is important to note that the apparently stronger within-group findings for African-Americans were only observed with the pervasive discrimination score that compared relatively high levels of discrimination across scales to relatively low levels. Our supplemental analyses that focused on continuous scores found similar within group associations for both African-Americans and Whites. This suggests that experiencing discrimination past a “threshold” or at a relatively higher level than others across multiple domains in life may be particularly detrimental for health among African-Americans as compared to Whites. It is possible that the qualitative experience of pervasive discrimination is different for these two groups, such that the consequences of experiencing such discrimination may be more severe for African-Americans than Whites. For example, a primary stressor related to discrimination (i.e., discrimination at work, school, or when seeking housing, etc.) could give rise to other types of secondary stressors (i.e., financial strain, relational strain, etc.) which African-Americans may have fewer additional resources to manage (60, 61). Further, it is plausible that African-Americans in particular who experience high levels of pervasive discrimination develop heightened levels of vigilance or anticipatory stress (62, 63) around discrimination which may result in differential health effects by race.
In the current study, both neuroticism and negative affect were included as covariates in models. Yet, associations between pervasive discrimination and AL among both African-Americans and Whites in our cohort persisted after adjusting for these factors. Thus, our findings were independent of two important psychosocial risk factors that may be important to consider in the relationship between self-reported experiences of discrimination and health (8). However, there may be other personality characteristics not included in this study with known linkages to discrimination, such as hostility, that should be considered in future research (64).
In supplementary analyses, we examined the relationship between pervasive discrimination and the individual system subscales included in the overall AL score to gain additional insight into whether certain indicators of impaired physiological functioning were more strongly associated with our measure of pervasive discrimination (65). In our study, pervasive discrimination was positively associated with the glucose metabolism and the lipid metabolism scores. These findings are in line with another study using the MIDUS cohort which found that weight discrimination was associated with the dysregulation of lipid and glucose metabolism (39). In addition to these system subscales, pervasive discrimination was also positively associated with the inflammation summary score. Additional research is needed to understand the mechanisms through which pervasive discrimination negatively affects physiological systems.
There are limitations to consider in the interpretation of this study. First, we had a relatively small number of African-Americans compared to Whites in our cohort, which may have limited our power to detect a race by pervasive discrimination interaction. The results of our exploratory analyses are suggestive of the possibility of an association among African-Americans that could potentially be stronger than that observed in Whites. However, additional research in cohorts with larger numbers of African-Americans is needed to draw definitive conclusions. Second, Whites in our cohort were of higher SES than Whites nationally, and the African-Americans in our cohort were primarily from a community-based sample of African-Americans residing in one of the most highly segregated cities in the United States. Thus, our data are not completely generalizable. However, while the African American sample is not representative, it nonetheless captures the experience of a large segment of African-Americans who reside in urban, economically disadvantaged communities. Future studies should examine the relationship between pervasive discrimination and AL among other racial/ethnic groups in varying geographic areas. Third, the cross-sectional nature of our study limits the ability of causal inference of the effect of pervasive discrimination on AL. Fourth, our overall AL score included BMI, which might temporally precede the development of many physiological disorders captured by AL. However, BMI was only included in the lipid metabolism subscale, and we observed associations with two additional subscales (inflammation and glucose metabolism). This suggests that BMI alone is not the primary driver of our associations. Fifth, the three scales used to assess pervasive discrimination were measured by self-report questionnaires, which could be subject to multiple forms of bias (i.e., recall and reporting bias). Finally, because these data were collected in the mid-2000’s, it is unclear whether our findings would generalize to populations from older or more recent cohorts.
Despite these limitations, this study has a number of strengths. This study, which combined multiple scales to assess the pervasive nature of discrimination, adds to a literature that has primarily focused on capturing experiences of discrimination using a single scale; and while this study relied on self-report questionnaires to assess discrimination, this methodology is the most widely used. Moreover, findings in this study were robust to important personality confounders, including negative affect and neuroticism. Lastly, the examination of AL and its individual system components as health outcomes provides an opportunity to examine the effects of pervasive discrimination using a multi-system approach which recognizes that the cumulative toll of the adaptation to stressful life experiences (i.e., discrimination) may manifest in several physiological systems (35).
In conclusion, to our knowledge, this is the first study to examine the association between pervasive discrimination-- measured by combining multiple scales that assess experiences of discrimination across a range of settings and situations-- and AL. In this sample of African-American and White adults, more pervasive discrimination was associated with greater multisystemic physiological dysregulation in both African-Americans and Whites. Future research on the measurement of pervasive discrimination and its effects on health is warranted.
Supplementary Material
Conflicts of Interest and Source of Funding:
The Midlife in the United States II (MIDUS II) Study was supported by the National Institutes of Health (Grants: U19 AG 051426; R01 AG 047154). M. Van Dyke received funding from National Heart, Lung, and Blood Institute (Grant 1 T32 HL130025-01A1) and a Howard Hughes Medical Institute Gilliam Fellowship for Advanced Study. N.K. Baumhofer received funding from Queenʻs Health System Health Equity Fellowship. For the remaining authors none were declared.
Abbreviations
- AL
allostatic load
- MIDUS II
Midlife in the United States II
- SSRI
selective serotonin reuptake inhibitor
- BMI
body mass index
REFERENCES
- 1.Cunningham TJ, Croft JB, Liu Y, Lu H, Eke PI, Giles WH. Vital Signs: Racial Disparities in Age-Specific Mortality Among Blacks or African Americans - United States, 1999–2015. MMWR Morb Mortal Wkly Rep. 2017;66:444–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. JAMA. 2000;283:2253–9. [DOI] [PubMed] [Google Scholar]
- 3.Howard G, Moy CS, Howard VJ, McClure LA, Kleindorfer DO, Kissela BM, Judd SE, Unverzagt FW, Soliman EZ, Safford MM, Cushman M, Flaherty ML, Wadley VG, Investigators* R. Where to Focus Efforts to Reduce the Black-White Disparity in Stroke Mortality: Incidence Versus Case Fatality? Stroke. 2016;47:1893–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schempf AH, Branum AM, Lukacs SL, Schoendorf KC. The contribution of preterm birth to the Black-White infant mortality gap, 1990 and 2000. Am J Public Health. 2007;97:1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Dyke ME, Greer S, Odom E, Schieb L, Vaughan A, Kramer MR, Casper M. Heart Disease Death Rates Among Blacks and Whites Aged ≥35 Years - United States, 1968–2015. MMWR CDC Surveill Summ. 2018;67:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams DR, Mohammed SA, Leavell J, Collins C. Race, socioeconomic status, and health: complexities, ongoing challenges, and research opportunities. Ann N Y Acad Sci. 2010;1186:69–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purnell TS, Calhoun EA, Golden SH, Halladay JR, Krok-Schoen JL, Appelhans BM, Cooper LA. Achieving Health Equity: Closing The Gaps In Health Care Disparities, Interventions, And Research. Health Aff (Millwood). 2016;35:1410–5. [DOI] [PubMed] [Google Scholar]
- 8.Lewis TT, Cogburn CD, Williams DR. Self-reported experiences of discrimination and health: scientific advances, ongoing controversies, and emerging issues. Annual review of clinical psychology. 2015;11:407–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paradies Y, Ben J, Denson N, Elias A, Priest N, Pieterse A, Gupta A, Kelaher M, Gee G. Racism as a Determinant of Health: A Systematic Review and Meta-Analysis. PloS one. 2015;10:e0138511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Dyke ME, Vaccarino V, Quyyumi AA, Lewis TT. Socioeconomic status discrimination is associated with poor sleep in African-Americans, but not Whites. Soc Sci Med. 2016;153:141–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krieger N, Waterman PD, Kosheleva A, Chen JT, Carney DR, Smith KW, Bennett GG, Williams DR, Freeman E, Russell B, Thornhill G, Mikolowsky K, Rifkin R, Samuel L. Exposing racial discrimination: implicit & explicit measures--the My Body, My Story study of 1005 US-born black & white community health center members. PloS one. 2011;6:e27636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor TR, Williams CD, Makambi KH, Mouton C, Harrell JP, Cozier Y, Palmer JR, Rosenberg L, Adams-Campbell LL. Racial discrimination and breast cancer incidence in US Black women: the Black Women’s Health Study. Am J Epidemiol. 2007;166:46–54. [DOI] [PubMed] [Google Scholar]
- 13.Everson-Rose SA, Lutsey PL, Roetker NS, Lewis TT, Kershaw KN, Alonso A, Diez Roux AV. Perceived Discrimination and Incident Cardiovascular Events: The Multi-Ethnic Study of Atherosclerosis. American journal of epidemiology. 2015;182:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitaker KM, Everson-Rose SA, Pankow JS, Rodriguez CJ, Lewis TT, Kershaw KN, Diez Roux AV, Lutsey PL. Experiences of Discrimination and Incident Type 2 Diabetes Mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA). Am J Epidemiol. 2017;186:445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beatty Moody DL, Chang Y, Brown C, Bromberger JT, Matthews KA. Everyday Discrimination and Metabolic Syndrome Incidence in a Racially/Ethnically Diverse Sample: Study of Women’s Health Across the Nation. Psychosom Med. 2018;80:114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coogan PF, Yu J, O’Connor GT, Brown TA, Cozier YC, Palmer JR, Rosenberg L. Experiences of racism and the incidence of adult-onset asthma in the Black Women’s Health Study. Chest. 2014;145:480–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slopen N, Williams DR. Discrimination, other psychosocial stressors, and self-reported sleep duration and difficulties. Sleep. 2014;37:147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis TT, Troxel WM, Kravitz HM, Bromberger JT, Matthews KA, Hall MH. Chronic exposure to everyday discrimination and sleep in a multiethnic sample of middle-aged women. Health Psychol. 2013;32:810–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Owens SL, Hunte HER, Sterkel A, Johnson DA, Johnson-Lawrence V. Association Between Discrimination and Objective and Subjective Sleep Measures in the Midlife in the United States Study Adult Sample. Psychosom Med. 2017;79:469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kessler RC, Mickelson KD, Williams DR. The prevalence, distribution, and mental health correlates of perceived discrimination in the United States. J Health Soc Behav. 1999;40:208–30. [PubMed] [Google Scholar]
- 21.Brown C, Matthews KA, Bromberger JT, Chang Y. The Relation between Perceived Unfair Treatment and Blood Pressure in a Racially/Ethnically Diverse Sample of Women. Am J Epidemiol. 2006;164:257–62. [DOI] [PubMed] [Google Scholar]
- 22.Dolezsar CM, McGrath JJ, Herzig AJ, Miller SB. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol. 2014;33:20–34 %8 Jan %! Perceived racial discrimination and hypertension: a comprehensive systematic review %@ 1930–7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascoe EA, Richman LS. Perceived Discrimination and Health: A Meta-Analytic Review. Psychological bulletin. 2009;135:531–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis TT, Everson-Rose SA, Powell LH, Matthews KA, Brown C, Karavolos K, Sutton-Tyrrell K, Jacobs E, Wesley D. Chronic exposure to everyday discrimination and coronary artery calcification in African-American women: the SWAN Heart Study. Psychosomatic medicine. 2006;68:362–8. [DOI] [PubMed] [Google Scholar]
- 25.Roberts CB, Vines AI, Kaufman JS, James SA. Cross-Sectional Association between Perceived Discrimination and Hypertension in African-American Men and Women: The Pitt County Study. Am J Epidemiol. 2008;167:624–32. [DOI] [PubMed] [Google Scholar]
- 26.Chae DH, Drenkard CM, Lewis TT, Lim SS. Discrimination and Cumulative Disease Damage Among African American Women With Systemic Lupus Erythematosus. American journal of public health. 2015;105:2099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewis TT, Yang FM, Jacobs EA, Fitchett G. Racial/ethnic differences in responses to the everyday discrimination scale: a differential item functioning analysis. Am J Epidemiol. 2012;175:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pew Research Center. Racial, gender wage gaps persist in U.S. despite some progress 2016. URL: https://www.pewresearch.org/fact-tank/2016/07/01/racial-gender-wage-gaps-persist-in-u-s-despite-some-progress/ Date of Access: March 14, 2020
- 29.US Department of Housing and Urban Development. Housing Discrimination against Racial and Ethnic Minorities 2012. 2013. URL: https://www.huduser.gov/portal/publications/fairhsg/hsg_discrimination_2012.html Date of Access: March 14, 2020
- 30.Bertrand M, Mullainathan S. Are Emily and Greg More Employable than Lakisha and Jamal? A Field Experiment on Labor Market Discrimination. American Economic Review. 2004;94:991–1013. [Google Scholar]
- 31.Ben J, Cormack D, Harris R, Paradies Y. Racism and health service utilisation: A systematic review and meta-analysis. PLoS One. 2017;12:e0189900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.New York Civil Liberties Union. Stop-and-Frisk During the Bloomberg Administration (2002–2013). 2014. URL: https://www.nyclu.org/en/publications/stop-and-frisk-during-bloomberg-administration-2002-2013-2014 Date of Access: March 14, 2020
- 33.Geronimus AT. Understanding and eliminating racial inequalities in women’s health in the United States: the role of the weathering conceptual framework. J Am Med Womens Assoc. 2001;56:133–6, 49–50. [PubMed] [Google Scholar]
- 34.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American journal of public health. 2006;96:826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation--allostatic load and its health consequences. MacArthur studies of successful aging. Arch Intern Med. 1997;157:2259–68. [PubMed] [Google Scholar]
- 36.Brody GH, Lei MK, Chae DH, Yu T, Kogan SM, Beach SR. Perceived discrimination among African American adolescents and allostatic load: a longitudinal analysis with buffering effects. Child development. 2014;85:989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ong AD, Williams DR, Nwizu U, Gruenewald TL. Everyday unfair treatment and multisystem biological dysregulation in African American adults. Cultur Divers Ethnic Minor Psychol. 2017;23:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomfohr LM, Pung MA, Dimsdale JE. Mediators of the relationship between race and allostatic load in African and White Americans. Health Psychol. 2016;35:322–32. [DOI] [PubMed] [Google Scholar]
- 39.Vadiveloo M, Mattei J. Perceived Weight Discrimination and 10-Year Risk of Allostatic Load Among US Adults. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2017;51:94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zilioli S, Imami L, Ong AD, Lumley MA, Gruenewald T. Discrimination and anger control as pathways linking socioeconomic disadvantage to allostatic load in midlife. J Psychosom Res. 2017;103:83–90. [DOI] [PubMed] [Google Scholar]
- 41.Upchurch DM, Stein J, Greendale GA, Chyu L, Tseng CH, Huang MH, Lewis TT, Kravitz HM, Seeman T. A Longitudinal Investigation of Race, Socioeconomic Status, and Psychosocial Mediators of Allostatic Load in Midlife Women: Findings From the Study of Women’s Health Across the Nation. Psychosom Med. 2015;77:402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cuevas AG, Wang K, Williams DR, Mattei J, Tucker KL, Falcon LM. The Association Between Perceived Discrimination and Allostatic Load in the Boston Puerto Rican Health Study. Psychosom Med. 2019;81:659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dutton GR, Lewis TT, Durant N, Halanych J, Kiefe CI, Sidney S, Kim Y, Lewis CE. Perceived weight discrimination in the CARDIA study: differences by race, sex, and weight status. Obesity (Silver Spring, Md). 2014;22:530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dienberg Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS national study: protocol, measures, sample, and comparative context. J Aging Health. 2010;22:1059–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams DR, Yan Y, Jackson JS, Anderson NB. Racial Differences in Physical and Mental Health: Socio-economic Status, Stress and Discrimination. J Health Psychol. 1997;2:335–51. [DOI] [PubMed] [Google Scholar]
- 46.Sternthal MJ, Slopen N, Williams DR. Racial disparities in health. Du Bois Review: Social Science Research on Race. 2011;8:95–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gruenewald TL, Karlamangla AS, Hu P, Stein-Merkin S, Crandall C, Koretz B, Seeman TE. History of socioeconomic disadvantage and allostatic load in later life. Soc Sci Med. 2012;74:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brooks KP, Gruenewald T, Karlamangla A, Hu P, Koretz B, Seeman TE. Social relationships and allostatic load in the MIDUS study. Health Psychol. 2014;33:1373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Slopen N, Chen Y, Priest N, Albert MA, Williams DR. Emotional and instrumental support during childhood and biological dysregulation in midlife. Prev Med. 2016;84:90–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carroll JE, Gruenewald TL, Taylor SE, Janicki-Deverts D, Matthews KA, Seeman TE. Childhood abuse, parental warmth, and adult multisystem biological risk in the Coronary Artery Risk Development in Young Adults study. Proc Natl Acad Sci U S A. 2013;110:17149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zimprich D, Allemand M, Lachman ME. Factorial structure and age-related psychometrics of the MIDUS personality adjective items across the life span. Psychol Assess. 2012;24:173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mroczek DK, Kolarz CM. The effect of age on positive and negative affect: a developmental perspective on happiness. J Pers Soc Psychol. 1998;75:1333–49. [DOI] [PubMed] [Google Scholar]
- 53.Fricker RD, Burke K, Han X, Woodall WH. Assessing the Statistical Analyses Used in Basic and Applied Social Psychology After Their p-Value Ban. The American Statistician. 2019;73:374–84. [Google Scholar]
- 54.Wasserstein RL, Lazar NA. The ASA Statement on p-Values: Context, Process, and Purpose. The American Statistician. 2016;70:129–33. [Google Scholar]
- 55.Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, Altman DG. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. European journal of epidemiology. 2016;31:337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones CP. Invited Commentary: “Race,” Racism, and the Practice of Epidemiology. Am J Epidemiol. 2001;154:299–304. [DOI] [PubMed] [Google Scholar]
- 57.Sims M, Diez-Roux AV, Dudley A, Gebreab S, Wyatt SB, Bruce MA, James SA, Robinson JC, Williams DR, Taylor HA. Perceived discrimination and hypertension among African Americans in the Jackson Heart Study. Am J Public Health. 2012;102 Suppl 2:S258–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Albert MA, Ravenell J, Glynn RJ, Khera A, Halevy N, de Lemos JA. Cardiovascular risk indicators and perceived race/ethnic discrimination in the Dallas Heart Study. American heart journal. 2008;156:1103–9. [DOI] [PubMed] [Google Scholar]
- 59.Hickson DA, Lewis TT, Liu J, Mount DL, Younge SN, Jenkins WC, Sarpong DF, Williams DR. The associations of multiple dimensions of discrimination and abdominal fat in African American adults: the Jackson Heart Study. Ann Behav Med. 2012;43:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pearlin LI, Schieman S, Fazio EM, Meersman SC. Stress, health, and the life course: some conceptual perspectives. J Health Soc Behav. 2005;46:205–19. [DOI] [PubMed] [Google Scholar]
- 61.Williams DR, Mohammed SA. Discrimination and racial disparities in health: evidence and needed research. J Behav Med. 2009;32:20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lewis TT, Cogburn CD, Williams DR. Self-Reported Experiences of Discrimination and Health: Scientific Advances, Ongoing Controversies, and Emerging Issues. Annu Rev Clin Psychol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lewis TT, Lampert R, Charles D, Katz S. Expectations of Racism and Carotid Intima-Media Thickness in African American Women. Psychosom Med. 2019;81:759–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hill LK, Sherwood A, McNeilly M, Anderson NB, Blumenthal JA, Hinderliter AL. Impact of Racial Discrimination and Hostility on Adrenergic Receptor Responsiveness in African American Adults. Psychosom Med. 2018;80:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gallo LC, Fortmann AL, Mattei J. Allostatic load and the assessment of cumulative biological risk in biobehavioral medicine: challenges and opportunities. Psychosom Med. 2014;76:478–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


