Abstract
Fire and high summer soil temperatures can break physical seed dormancy in Mediterranean fire-prone ecosystems. Their independent effect is somewhat recognized but both factors may act together with a synergistic effect yet unknown. This study aims to determine the isolated and combined effects of fire and summer temperatures on the release of physical seed dormancy in Cistaceae species. Fire and summer temperature treatments were applied in a factorial experiment to seeds of 12 species of Cistaceae. Seeds previously exposed or not to a heat shock (fire simulation) were kept for 1 or 2 months at constant or alternating temperatures (summer temperatures simulation). Additionally, I compared the effect of exposing the seeds to a heat shock before or after they had been subjected to the summer temperatures. Heat shock increased germination of all species, but summer temperatures produced different results. When seeds were exposed to summer temperatures after heat shock, germination decreased. This negative effect disappeared when heat shock was simulated at the end of the summer temperatures. Fire and summer temperatures modulate timing of germination in Cistaceae with a joint control on post-fire regeneration. Cycling of sensitivity to physical dormancy release may be the mechanism to explain this fine-tuning, which would ensure germination when environmental conditions are suitable for growth. These results contribute to our understanding of vegetation dynamics and postfire regeneration in Mediterranean ecosystems.
Subject terms: Plant ecology, Ecology
Introduction
Cistaceae is a plant family widely distributed in the Mediterranean Basin forming vast areas of shrublands, which dominate the landscape after fire occurrence1. All species of this family are obligated seeders2,3 and consequently their post-fire establishment relies exclusively on seed germination. After fire, Cistaceae germinate massively and their seedlings colonize the new open habitats, which are free of competitors and provided with favourable conditions for establishment4,5.
Cistaceae regeneration associated to fire occurrence is due to the hard coat of seeds that impose physical dormancy6. Seeds with physical dormancy cannot germinate because of palisade cell layer(s) that avoids water intake to the seed7. Seeds remain dormant until some factor(s) make the seed coat permeable to water. Seeds with physical dormancy show specialized structures in the seed coat like the chalazal plug in Cistaceae8. During physical dormancy break, this plug is pushed slightly into the seed and forms a circular opening (oculus) through which the seeds imbibe water9,10. In addition to the unplugging of the seed coat opening, exposure to dry heat also causes cracks in the outer coat layers11–13.
Traditionally, temperature thresholds needed for breaking physical seed dormancy in Cistaceae have been related to fire14,15. However, currently a debate exists about whether fire or summer temperatures are the most appropriate cues for physical dormancy release. The cumulative effect of soil temperatures during summer might provide a similar heat amount to fire and thus, both factors could be equally effective in breaking dormancy. Therefore, the underlying concern is to discern whether the temperature thresholds that break physical seed dormancy have been shaped by fire16 or physical dormancy has evolved associated to the summer temperatures17. According to the summer hypothesis, soil temperatures reached in soil during summer can break physical seed dormancy, especially in bare soils where daily temperatures reach the highest values and fluctuate more widely17–21. These open habitats may be associated to the gaps created after fire but also after other perturbations.
In the Mediterranean Basin, no consistent results have been found for Cistaceae species. While summer treatments had no effect over the seed dormancy of five species (Cistus albidus, C. creticus, C. parviflorus, C. salviifolius and Fumana thymifolia)16, in other case the most influencing factor on seedling emergence of Cistus albidus was the microhabitats opened with fire17. Likewise, unclear results have also emerged for Fabaceae species, other plant family typically with hard seed coats8. In some cases, soil temperature regimes after summer fires play a key role in breaking physical seed dormancy, both in Europe19,21 and in Australia21–23. However, in other cases, seeds show dormancy cues bound to temperatures that only occur during fire22,23.
Another interesting question is to determine whether the break of physical dormancy after summer temperatures is produced by the cumulative heat reached at the soil surface or by the alternating changes of soil temperatures. The mechanism seems to be highly dependent on the species, although there are not enough works to determine which mechanism is the most widely observed and most works have focused mainly on Fabaceae species or on the effects of alternating temperatures22–24. For instance, both mechanisms, high and highly fluctuating temperatures promoted dormancy break in hard seeds of Stylosanthes humilis and S. hamata (Fabaceae) during the hot season in northern Australia25. In the case of two Erodium species (Geraniaceae) and Adenanthera pavonina (Fabaceae), temperature fluctuations were more important than high constant temperatures in overcoming dormancy26,27. However, neither constant high nor alternating temperature treatments were effective in breaking physical dormancy of Senna marilandica (Fabaceae)28.
The combined effect of heat shock and the typical high temperatures of the summer season is even more unknown than their isolated effects17,21,29. In Mediterranean habitats, seed dispersal occurs frequently during the summer, when seeds have to withstand the high soil temperatures before their germination in autumn or winter and eventual wildfire, which typically occurs during the summer in these areas. Cistaceae have frequently extended dispersal times30 and thus, the timing of seed release, soon or later in the season, will determine the duration of exposure to high temperatures on soil. When a fire occurs, seeds suffer not only the short heat shock of the very high temperatures reached during the fire, but also the moderately high summer temperatures throughout the summer days. Depending on the time when fire occurs, two different scenarios are possible, fires occurring at the beginning or at the end of summer season. This involves that seeds could be exposed to the same factors but in different sequences. In the present work, I addressed all these issues by comparing the effects of fire vs. summer, fire plus summer, fire before summer vs. fire after summer, and simulation of summer with different characteristics.
In the Mediterranean region, summer is probably the hardest season for living organisms. The hypothesis tested here is that the temperature thresholds that break physical seed dormancy in Cistaceae have been shaped by fire in combination with high summer temperatures, since typically both are present during the summer. Therefore, it could be expected to find a synergistic effect of fire heat shock and summer temperatures, which would ensure the germination of a greater proportion of seeds when the conditions are favourable for seedling establishment. The main objective of this work was to evaluate the effects of high summer soil temperatures and a short heat shock representative of fire on the release of physical seed dormancy in Cistus and Halimium species. Specifically, we addressed the following questions: (1) is physical dormancy equally released after seed exposure to a heat shock simulating fire than after high summer temperatures? (2) Has the combined effect of heat shock plus summer temperatures a synergistic effect? (3) Can different length of exposure to summer temperatures change the germination response? (4) Is the accumulative effect of high constant temperatures enough for breaking physical dormancy? Or are alternating temperatures required? (5) Finally, does the heat shock have different effects on germination response when is produced before or after the summer temperatures?
Methods
Study species and seed collection
We studied 12 species of the family Cistaceae, belonging to two related genera Cistus and Halimium. The selected species were Cistus albidus L., Cistus clusii Dunnal, Cistus ladanifer L., Cistus laurifolius L., Cistus monspeliensis L., Cistus populifolius L., Cistus psilosepalus Sweet, Cistus salviifolius L., Halimium atriplicifolium (Lam.) Spach, Halimium halimifolium (L.) Willk, Halimium ocymoides (Lam.) Willk and Halimium viscosum (Willk.) P. Silva. All these species are common and widely distributed in the western Mediterranean Basin1.
Mature capsules were collected from at least 30 individuals for each species between July and August 2016 in the centre of the Iberian Peninsula (Supplementary Data Table S1). Fruits were carried to the laboratory where seeds were extracted and cleaned. Seeds were stored in paper bags until the beginning of the experiments the following November.
Fire vs. summer temperatures
Fire and summer temperature treatments were applied in a factorial experiment in such a way that seeds exposed or not to a heat shock simulating fire temperatures were subject or not to the different treatments simulating summer temperatures. That is, seeds were exposed to two factors heat shock (with heat shock or without heat shock) and summer temperatures (1 month at constant 50 °C or at alternating 50/20 °C, 2 months at constant 50° or alternating 50/20 °C). Fire temperatures were simulated by the exposure of seeds to a heat shock of 100 °C for ten minutes in an air-forced oven. Although temperatures reached in the soil during fires vary widely we chose this specific temperature and time of exposure, which have been commonly recorded in Mediterranean fire shrublands31,32, because this temperature seemed to be the optimal for breaking seed dormancy of many Cistaceae species33.
Mediterranean climate is characterized by high summer temperatures, which are usually above 40 °C. When air temperatures are around 20–25 °C, soil temperatures are similar but, when mean of maximum temperatures are around 35 °C, soil temperature can reach 60 °C34. Summer temperatures were simulated by exposing unimbibed seeds to two long duration dry heat treatments for 1 and 2 months at constant temperature (50 °C) or at daily cycles of alternating temperatures (50/20 °C) that might be expected to represent current summer temperatures22. Summer treatments were conducted in a refrigerated precision cabinet (JP Selecta Hotcold-UB).
After these treatments, seeds were sown on two sheets of filter paper moistened with 1.2 ml of distilled water, in plastic Petri dishes of diameter 5.5 cm. For germination tests, four replicates of 25 seeds were used for each species, and seeds incubated at 20 °C and 12 h photoperiod, the optimal germination conditions for many Mediterranean species35,36. Petri dishes were laid at random on a temperature and humidity controlled chamber (Model G-21, Ibercex). Germinated seeds were checked and eliminated weekly over the course of 8 weeks. At the end of the experiment, ungerminated seeds were checked for viability using a cut test. Those firm seeds with white endosperm were considered as viable, while mushy seeds with brown endosperm or affected by fungi were considered as inviable. Germination percentages were corrected by viability, i.e., germination percentages were estimated in relation to viable seeds and not in relation to the total number of seeds.
Fires before and after the summer season
In a second experiment, we tested whether the timing in which seeds are exposed to heat shock, before or after the summer temperatures, affected their germination response. In the first experiment, the 50/20 °C treatment showed highest germination rates than constant 50 °C, and length of exposure (1 or 2 months) had almost no effects. Consequently, in the second experiment I subjected seeds to a heat shock (100 °C for ten minutes) and then they were exposed to 50/20 °C for one month. In the other treatment, seeds were first exposed to summer temperatures (50/20 °C for 1 month) and then were exposed to a similar heat shock. Then, seeds were germinated at the same conditions as in the previous experiment.
Data analysis
For each species, I used generalised linear models (GLMs) with a binomial error distribution and logit link function to compare final germination among the different treatments. First, I analysed the effects of fire vs. summer temperatures on final germination and seed viability. Since germination without heat shock was very low, I analysed the effects of the different summer temperatures (50 vs. 50/20 °C) and different lengths of time (1 vs. 2 months) for the seeds that had been exposed to both heat shock and summer temperatures. Additionally, for the second experiment, the effect of before vs. after summer temperatures heat shock was analysed in the same way.
Results
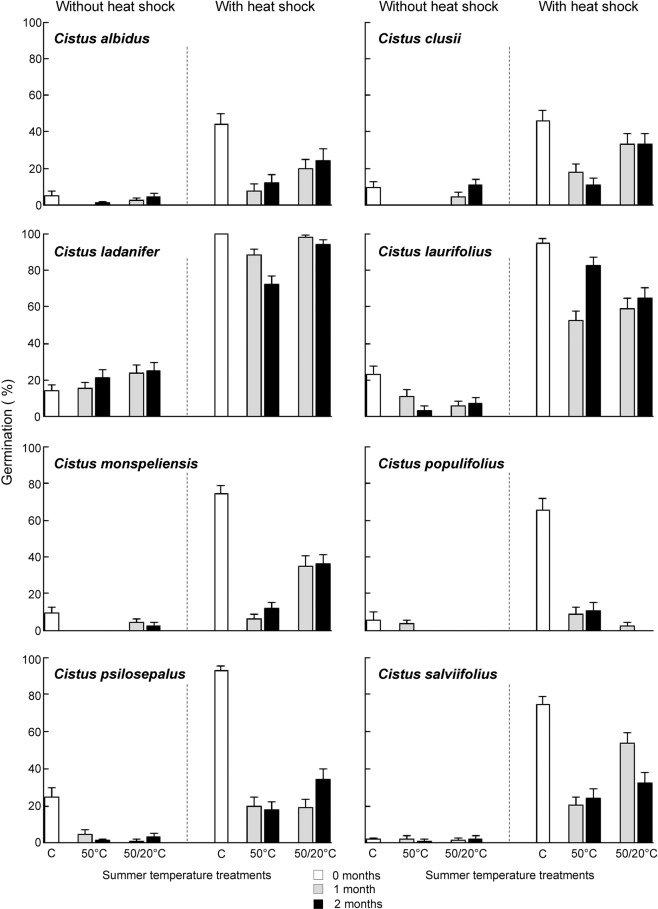
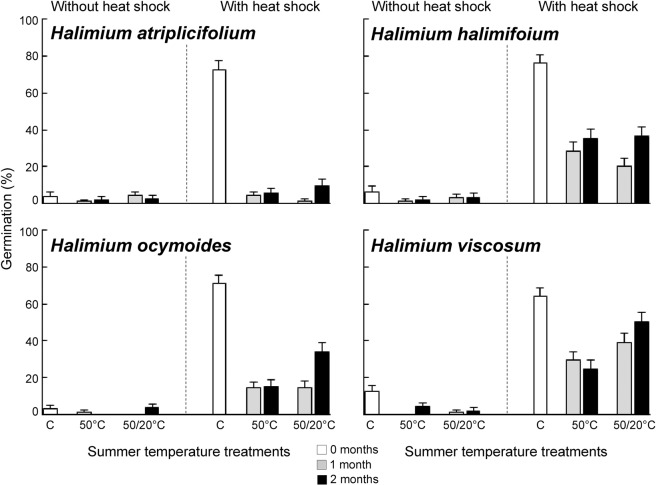
Heat shock simulating fire temperature increased germination of all species, while summer temperatures had the opposite effect by decreasing germination (Table 1, Figs. 1 and 2). Only in the case of C. ladanifer, summer temperatures slightly increased the germination of seeds not exposed to heat shock, such as the significant interaction between both factors shown (Table 1). Additionally, significant interactions between heat shock and summer temperatures also emerged for C. psilosepalus and H. atriplicifolium. In these cases, the decrease of germination produced by summer temperatures was more intense in seeds exposed to heat shock than in non-exposed ones. C. populifolius and H. atriplicifolium were very sensitive to summer temperatures since the treatment strongly lessened germination (Figs. 1 and 2).
Table 1.
Results from GLM for main effects of fire and summer temperature treatments and their interactions on final seed germination of the studied Cistaceae species.
| Fire | Summer | Fire x Summer | ||||
|---|---|---|---|---|---|---|
| χ2 | P | χ2 | P | χ2 | P | |
| Cistus albidus | 50.477 | <0.001 | 12.787 | <0.001 | 0.364 | 0.546 |
| Cistus clusii | 49.274 | <0.001 | 10.834 | 0.001 | 0.092 | 0.762 |
| Cistus ladanifer | 325.653 | <0.002 | 9.539 | 0.002 | 19.111 | <0.001 |
| Cistus laurifolius | 101.235 | <0.001 | 25.574 | <0.001 | 1.903 | 0.168 |
| Cistus monspeliensis | 38.380 | <0.001 | 17.512 | <0.001 | 0.171 | 0.680 |
| Cistus populifolius | 10.170 | 0.001 | 8.997 | 0.003 | 2.080 | 0.149 |
| Cistus psilosepalus | 110.336 | <0.001 | 119.446 | <0.001 | 3.958 | 0.047 |
| Cistus salviifolius | 74.054 | <0.001 | 3.799 | 0.051 | 3.194 | 0.074 |
| Halimium atriplicifolium | 46.080 | <0.001 | 37.079 | <0.001 | 21.720 | <0.001 |
| Halimium halimifolium | 166.490 | <0.001 | 35.038 | <0.001 | 3.768 | 0.052 |
| Halimium ocymoides | 109.385 | <0.001 | 23.958 | <0.001 | 3.361 | 0.067 |
| Halimium viscosum | 72.607 | <0.001 | 20.827 | <0.001 | 1.515 | 0.218 |
Figure 1.
Germination percentages (mean ± standard error) of Cistus species at the different summer temperature treatments without heat shock or previously exposed to heat shock (100 °C for 10 minutes). Control seeds were not exposed to summer temperatures (0 months in white). Summer temperature treatments consisted in the storage at constant 50 °C or alternating 50/20 °C for one month (grey) or two months (black).
Figure 2.
Germination percentages (mean ± standard error) of Halimium species at the different summer temperature treatments without heat shock or previously exposed to heat shock (100 °C for 10 minutes). Control seeds were not exposed to summer temperatures (0 months in white). Summer temperature treatments consisted in the storage at constant 50 °C or alternating 50/20 °C for one month (grey) or two months (black).
Germination without heat shock was very low (Figs. 1 and 2) and consequently, the effects of the different summer temperatures (50 vs. 50/20 °C) and different times (1 vs. 2 months) were analysed just for the seeds that had been previously exposed to heat shock. Overall, different regime of temperatures affected the germination responses more than duration of treatments (Table 2). The negative effect of summer temperatures on seed germination was stronger after constant 50 °C than alternating 50/20 °C. In the cases of C. laurifolius, C. psilosepalus, H. atriplicifolium and H. halimifolium the different summer temperatures had similar effects. Different time of exposure to summer temperatures only had a significant effect for C. laurifolius, H. halimifolium and H. ocymoides (Table 2), with lower germination after one month than after two months of treatment (Figs. 1 and 2).
Table 2.
Results from GLM for main effects of different summer temperatures (50 vs. 50/20 °C), time (1 vs. 2 months) and their interactions for seeds that had been previously exposed to heat shock.
| Temperature | Time | Temp x Time | ||||
|---|---|---|---|---|---|---|
| χ2 | P | χ2 | P | χ2 | P | |
| Cistus albidus | 5.656 | 0.017 | 0.914 | 0.339 | 0.057 | 0.811 |
| Cistus clusii | 14.432 | <0.001 | 0.840 | 0.359 | 0.840 | 0.359 |
| Cistus ladanifer | 6.773 | 0.009 | 2.166 | 0.141 | 0.003 | 0.959 |
| Cistus laurifolius | 0.662 | 0.416 | 4.316 | 0.038 | 2.229 | 0.135 |
| Cistus monspeliensis | 17.130 | <0.001 | 0.740 | 0.390 | 0.584 | 0.445 |
| Cistus populifolius | 20.880 | <0.001 | 2.445 | 0.118 | 3.242 | 0.072 |
| Cistus psilosepalus | 3.273 | 0.070 | 2.096 | 0.148 | 4.240 | 0.039 |
| Cistus salviifolius | 10.827 | 0.001 | 1.193 | 0.275 | 3.672 | 0.055 |
| Halimium atriplicifolium | 0.260 | 0.610 | 2.762 | 0.097 | 1.493 | 0.222 |
| Halimium halimifolium | 0.531 | 0.466 | 4.454 | 0.035 | 0.839 | 0.360 |
| Halimium ocymoides | 6.836 | 0.009 | 9.081 | 0.003 | 6.864 | 0.009 |
| Halimium viscosum | 4.222 | 0.040 | 0.076 | 0.783 | 0.804 | 0.370 |
Finally, the timing in which seeds were exposed to heat shock, before or after the summer temperatures, was determinant of the germination response of all species except C. ladanifer who showed very high germination in any case (Table 3). Exposure to heat shock after summer temperatures improved germination profusely (Fig. 3).
Table 3.
Results from GLM for main effects of the timing of exposure to heat shock, before or after the summer temperatures, on final seed germination of the studied Cistaceae species.
| χ2 | P | |
|---|---|---|
| Cistus albidus | 36.893 | <0.001 |
| Cistus clusii | 50.352 | <0.001 |
| Cistus ladanifer | 1.054 | 0.305 |
| Cistus laurifolius | 6.379 | 0.012 |
| Cistus monspeliensis | 83.970 | <0.001 |
| Cistus populifolius | 15.908 | <0.001 |
| Cistus psilosepalus | 28.338 | <0.001 |
| Cistus salviifolius | 261.677 | <0.001 |
| Halimium atriplicifolium | 42.568 | <0.001 |
| Halimium halimifolium | 57.560 | <0.001 |
| Halimium ocymoides | 25.064 | <0.001 |
| Halimium viscosum | 66.380 | <0.001 |
Figure 3.
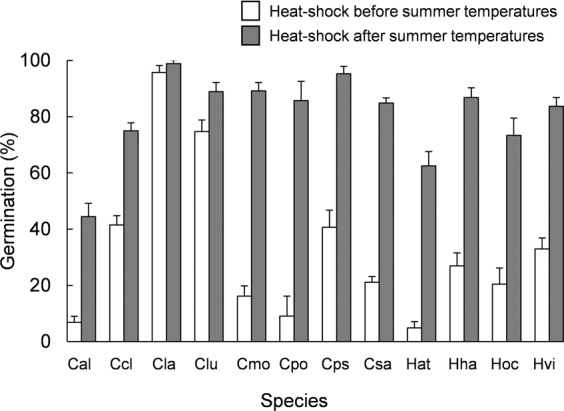
Germination percentages (mean ± standard error) of studied species when were exposed to a heat-shock (100 °C for 10 minutes) before or after the summer temperature treatments (1 month at 50/20 °C). Cal: Cistus albidus, Ccl: Cistus clusii, Cla: Cistus ladanifer, Clu: Cistus laurifolius, Cmo: Cistus monspeliensis, Cpo: Cistus populifolius, Cps: Cistus psilosepalus, Csa: Cistus salviifolius, Hat: Halimium atriplicifolium, Hha: Halimium halimifolium, Hoc: Halimium ocymoides, Hvi: Halimium viscosum.
Discussion
Heat shock by itself was a key factor promoting seed germination of all studied species, which concurs with the massive germination found in mediterranean shrublands after fire4,31,32 and with the results of other laboratory experiments6,14,33,37. In the cases of C. albidus and C. clusii, although germination increased after heat shock it did not reach 50% despite the high viability of their seeds. These results may lead us to think that these species need higher temperatures for breaking dormancy, but most studies report a high variability of temperature thresholds for breaking of physical dormancy14,38–43. Such variability may be explained as a mechanism of diversification in relation to different fire intensities experienced by seeds at the soil surface, as well as variations in burial depth33.
Physical dormancy contributes to maintenance of long-lived soil seed banks, where seeds persist while environmental conditions are unfavourable for establishment. These long-lived soil seed banks confer long-term persistence for the species44 and a bet-hedging strategy, which spreads the risk of extinction34,45. In fire-prone ecosystems, conditions for seedling establishment are particularly favourable just after fire and the temporal window for seedling establishment is usually short32,46. Consequently, many Mediterranean plants produce seeds that are released from dormancy only after being exposed to fire-related factors, such as heat47–49. In species with physical dormancy, once this type of dormancy is broken it cannot be reversed8. Additionally, the embryo is usually non-dormant within the impermeable seed coat and seeds will be ready to germinate when the water is available24. Although in fire-prone habitats, the release from dormancy is usually related to the heat produced during fires6, other cues can act. So, the rupture of physical dormancy can also occur naturally by high summer temperatures or continuous daily fluctuating temperatures50. In this way, Ferrandis et al.51 found that the direct effect of fire was the main responsible for seed germination in three species (C. ladanifer, C. salviifolius and H. ocymoides). However, final germination levels (around 70%) did not correspond to the magnitude of seed bank depletion (>90%). Authors suggested that other environmental factors not exclusively associated to fire, such as temperature fluctuation, might also be involved in softening Cistaceae seeds.
Contrary to fire, in the present study, summer temperatures did not increase germination as expected, but had a significant negative effect on it. In previous works, summer temperatures caused no effects or positive effects on seed germination of Cistaceae species16,17, but these generalized negative effects have not been previously documented. Consequently, the longer and hotter summers may produce negative consequences for regeneration of some plant species. Without simulated fire, seeds showed very low germination levels and summer temperatures caused little effect. However, the negative effect of summer temperatures was much more evident for seeds that had been previously exposed to heat shock. Summer temperatures did not decrease seed viability (Supplementary Data Table S2). The loss of germination after summer temperatures was related with a higher proportion of unimbibed seeds, which may indicate the lack of physical dormancy release. Consequently, one possible explanation for the negative effects of summer temperatures is that Cistaceae may show sensitivity cycling to physical dormancy-break such as it has been described in species of Convolvulaceae and Fabaceae52–55.
According to cycling of sensitivity to physical dormancy-break, dormant seeds can cycle between two states: insensitive and sensitive seeds to physical dormancy break (insensitive ↔ sensitive)56. Insensitive seeds are unable to respond to the dormancy-breaking treatment opposite to sensitive seeds, which can do it56. Previously to cycling sensitivity, some works proposed a cycling of dormancy between seeds with physical dormancy and non-dormant seeds (PY ↔ ND)57,58. However, species with physical dormancy cannot cycle between dormant and non-dormant, because the process of physical dormancy loss is irreversible. That is, once a slit or an opening is formed in the seed coat, a resealing of this opening would not seem possible56.
In a similar work to this, Hagon and Ballard59 made seeds of Trifolium subterraneum permeable by percussion and then germinated at 20 °C obtaining high germination percentages. Authors stated that permeability of seeds was reversed when seeds were stored dry at 5% relative humidity after percussion. However, when percussed seeds were stored at high relative humidity previously to drying at low relative humidity, germination was high. This happened because the palisade layer in the lens of the seeds stored at high humidity had slits through its entire width. However, when seeds were kept at low humidity, the palisade layer had slits but they did not penetrate through its whole width. The authors concluded that the permeability induced by percussion could be reversed by manipulation of relative humidity. According to Jayasuriya et al.56, these results can also be explained from the sensitivity cycling approach. From this perspective, percussion may not have made the seeds permeable, but it might have increased the sensitivity of seeds to dormancy release at 20 °C.
We would need additional work to conclude securely that Cistaceae species show sensitivity cycling to physical dormancy breaking, but here I present the first report of it. When the second experiment finished, I checked the ungerminated seeds that remained as unimbibed hard seeds. I observed a significant higher proportion of hard seeds when heat shock was given before summer than when heat shock was given after summer temperatures (Supplementary Data Fig. S1), supporting the explanation of sensitivity cycling to physical dormancy breaking. Zupo et al.29 also found that seeds of C. albidus exposed to fire plus summer temperatures decreased germination in comparison to seeds only exposed to fire, but results were not explained. Probably, their results can also be explained in terms of sensitivity cycling. The underlying idea is that fire by itself cannot break physical dormancy but improve the sensitivity of seeds to an additional cue which definitively break it. When after heat shock seeds are moistened, the movement of water may facilitate dormancy break by promoting the formation of permanent opening(s) in the seed coat56. However, when after heat shock seeds are kept at moderately high temperatures and/or low humidity, physical dormancy is not released. That is, heat shock is a precondition for physical dormancy break but not the only cue needed for releasing physical dormancy.
Both summer temperature treatments, constant temperatures and fluctuating temperatures, had similar results. However, overall the effects of alternating temperatures allowed more germination than constant temperatures. Seed abundance in the soil decreases with depth so they are mostly located in the upper layer, especially in the first centimetres60,61, where they experience more intense environmental conditions not buffered by soil, such as high fluctuating temperatures. Daily maximum temperatures registered in soil can be very high, up to 50 °C on the soil surface of fire breaks in eastern Spain19 or up to 40–60 °C in south-eastern Australia62 or 60–70 °C in south-west western Australia63. The temperatures and duration of treatments applied in this work have been widely used in other works but with different results20–23,34. The length of the treatments had almost no effects on seed germination, which may be related to the long periods of dispersal from July to January of Cistaceae species30. This would leave seeds in the soil exposed to a variable length of summer temperatures from the first dispersed seeds to the last ones.
Under the current scenario of climate change, seeds in the soil will be exposed to longer dry seasons, increasing summer temperatures, and more frequent, intense and extended heat-wave events64. Consequently, the new temperature conditions in soils may alter the functioning of long-term seed banks by affecting dormancy state and seed viability, and accordingly the seed bank accumulation and persistence as well as its bet-hedging ability and the germination timing20,34,65,66. In this way, those species with lower threshold temperatures for breaking physical seed dormancy are expected to be more affected by climate change because will be released of their dormancy and germinate, altering the dynamics of soil seed banks. In some cases, physical dormancy has been proposed to have evolved to guarantee survival of plants in temporally stochastic and harsh environments8 and thus, it may be hypothesized that plants with physical dormancy would thrive under the warmer and more variable new environment67. However, the reduction in the proportion of dormant and viable seeds could lead to an increased risk of extinction because species may be unable to maintain persistent soil seed banks in the long term22. Although in the present work, summer temperatures by themselves were not enough for breaking physical dormancy or even they prevented germination mostly after fire, these results could change under the scenario of more severe summer conditions.
Very different results emerged when seeds previously subjected to summer temperatures were exposed to heat shock. In this case, a generalized increase of germination was registered. Consequently, timing of fire, at the beginning or at the end of the summer season, determines the promotion or inhibition of Cistaceae germination. A fine-tuning between summer temperatures and timing of fire must control germination of Cistaceae. If fire happens at the beginning of the summer, high summer soil temperatures and low moisture content must lead seeds of Cistaceae to an insensitive state since the environment conditions are not the appropriate for germination. However, when fire happens at the end of summer, previously to autumn rains, it can act as a key signal triggering germination. Additionally, for many species, the combined effect of summer plus fire temperatures was higher than the isolated effect of fire. This synergic effect of summer plus fire temperatures could improve the opportunities of establishment. Summer temperatures by themselves do not break physical dormancy but modulate the response to fire.
These findings can help to take decisions for effective fuel management treatments such as in the case of prescribed burnings. Burnings before summer could reduce germination of all studied species except Cistus ladanifer, which could thrive in absence of competitors. This species forms widespread continuous shrublands very poor in species because it produces phytotoxic active compounds that inhibit the development of other plants68,69 and the resulting landscape accumulates large amounts of standing biomass that produces fine dry fuel, thus increasing the risk of fire. On the contrary, prescribed burnings at the end of the summer season could favour germination of all studied species, which would lead to rich and heterogeneous shrublands and therefore, to reduced fire risk.
In conclusion, in the case of the studied Cistus and Halimium species, the timing of germination must be controlled by fire but also by summer temperatures and probably by available water after fire42, which ensures seedling emergence under favourable conditions for establishment, such as low levels of competition and high availability of resources in postfire environments. However, we should be cautious and avoid generalizations for other genera of Cistaceae such as Fumana, Helianthemum or Tuberaria, since fire and summer temperatures might have played different roles in their evolution and physical dormancy may have diverse origins33,70,71. The onset of the Mediterranean-type climate regions in the Neogene-Quaternary72,73 brought summer high temperatures and a regime of recurrent fire74, and consequently they may have worked simultaneously as evolutionary pressures modulating the suitable time for germination of Cistus and Halimium species in the Mediterranean. Studies like this will help us to reach a better understanding on the dynamics and responses of natural species under the current situation of global warming.
Supplementary information
Acknowledgements
I thank Ángel Velasco and Andrés Lucío for their technical support and to Manuel Hernández, Ailton Rodrigues-Junior and Blanca Céspedes for their suggestive and useful comments. Edmund Gorman is warmly thanked for his effort in the improvement of the writing of the manuscript. Finally, I thank José M. Moreno for placing his trust in me. This study was under the framework of FOCCLIM project (CGL2016-78357-R) and supported by the University of Castilla-La Mancha.
Competing interests
The author declares no competing interests.
Footnotes
This article has been retracted. Please see the retraction notice for more detail: https://doi.org/10.1038/s41598-021-93139-2
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
6/28/2021
A Correction to this paper has been published: 10.1038/s41598-021-93139-2
Supplementary information
is available for this paper at 10.1038/s41598-020-62909-9.
References
- 1.Allen, H. Vegetation and ecosystem dynamics in The physical geography of the Mediterranean (ed. Woodward, J. C.) 203–227 (Oxford University Press, Oxford, 2009).
- 2.Paula S, Pausas JG. Burning seeds: germinative response to heat treatments in relation to resprouting ability. Journal of Ecology. 2008;96:543–552. doi: 10.1111/j.1365-2745.2008.01359.x. [DOI] [Google Scholar]
- 3.Verdú M. Ecological and evolutionary differences between Mediterranean seeders and resprouters. Journal of Vegetation Science. 2000;11:265–268. doi: 10.2307/3236806. [DOI] [Google Scholar]
- 4.Arianoutsou M, Margaris NS. Early stages of regeneration after fire in a phryganic ecosystem (East Mediterranean). I. Regeneration by seed germination. Biologie-Ecologie Méditerraneenne. 1981;8:119–128. [Google Scholar]
- 5.Trabaud, L. Post-fire plant community dynamics in the Mediterranean Basin in The role of fire in Mediterranean-type ecosystems (eds. Moreno, J. M. & Oechel, W. C.) 1–15 (Springer-Verlag, New York, Berlin, Heidelberg, London, Paris, Tokyo, Hong Kong, Barcelona, Budapest, 1994).
- 6.Thanos CA, Georghiou K, Kadis C, Pantazi C. Cistaceae: a plant family with hard seeds. Israel Journal of Botany. 1992;41:251–263. [Google Scholar]
- 7.Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. doi: 10.1079/SSR2003150. [DOI] [Google Scholar]
- 8.Baskin CC, Baskin JM, Li X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biology. 2000;15:139–152. doi: 10.1046/j.1442-1984.2000.00034.x. [DOI] [Google Scholar]
- 9.Gama-Arachchige NS, Baskin JM, Geneve RL, Baskin CC. Identification and characterization of ten new water gaps in seeds and fruits with physical dormancy and classification of water-gap complexes. Annals of Botany. 2013;112:69–84. doi: 10.1093/aob/mct094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geneve RL, Baskin CC, Baskin JM, Jayasuriya KMGG, Gama-Arachchige NS. Functional morpho-anatomy of water-gap complexes in physically dormant seed. Seed Science Research. 2018;28:186–191. doi: 10.1017/S0960258518000089. [DOI] [Google Scholar]
- 11.Aronne G, Mazzoleni S. The effects of heat exposure on seeds of Cistus incanus L. and Cistus monspeliensis L. Giornale Botanico Italiano. 1989;123:283–289. [Google Scholar]
- 12.Ma FS, Cholewa E, Mohamed T, Peterson CA, Gijzen M. Cracks in the palisade cuticle of soybean seed coats correlate with their permeability to water. Annals of Botany. 2004;94:213–228. doi: 10.1093/aob/mch133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black, M. J., Halmer, P. & Bewley, J. D. The Encyclopedia of Seeds: Science, Technology and Uses (CAB International, London, 2006).
- 14.Herranz JM, Ferrandis P, Martínez-Sánchez JJ. Influence of heat on seed germination of nine woody Cistaceae species. International Journal of Wildland Fire. 1999;9:173–182. doi: 10.1071/WF00014. [DOI] [Google Scholar]
- 15.Pérez-García F, González-Benito ME. Effects of temperature and different pre-treatments on seed germination of four Halimium species. Seed Science and Technology. 2005;33:505–509. doi: 10.15258/sst.2005.33.2.24. [DOI] [Google Scholar]
- 16.Moreira, B. & Pausas, J. G. Tanned or Burned: The Role of Fire in Shaping Physical Seed Dormancy. Plos One7 (2012). [DOI] [PMC free article] [PubMed]
- 17.Santana VM, Baeza JM, Blanes CM. Clarifying the role of fire heat and daily temperature fluctuations as germination cues for Mediterranean Basin obligate seeders. Annals of Botany. 2012;111:127–134. doi: 10.1093/aob/mcs238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auld TD, Bradstock RA. Soil temperatures after the passage of a fire: Do they influence the germination of buried seeds? Australian Journal of Ecology. 1996;21:106–109. doi: 10.1111/j.1442-9993.1996.tb00589.x. [DOI] [Google Scholar]
- 19.Baeza MJ, Roy J. Germination of an obligate seeder (Ulex parviflorus) and consequences for wildfire management. Forest Ecology and Management. 2008;256:685–693. doi: 10.1016/j.foreco.2008.05.014. [DOI] [Google Scholar]
- 20.Ooi MKJ, Auld TD, Denham AJ. Projected soil temperature increase and seed dormancy response along an altitudinal gradient: implications for seed bank persistence under climate change. Plant and Soil. 2012;353:289–303. doi: 10.1007/s11104-011-1032-3. [DOI] [Google Scholar]
- 21.Santana VM, et al. Effects of soil temperature regimes after fire on seed dormancy and germination in six Australian Fabaceae species. Australian Journal of Botany. 2010;58:539–545. doi: 10.1071/BT10144. [DOI] [Google Scholar]
- 22.Cochrane A. Are we underestimating the impact of rising summer temperatures on dormancy loss in hard-seeded species? Australian Journal of Botany. 2017;65:248–256. doi: 10.1071/BT16244. [DOI] [Google Scholar]
- 23.Ooi MKJ, Denham AJ, Santana VM, Auld TD. Temperature thresholds of physically dormant seeds and plant functional response to fire: variation among species and relative impact of climate change. Ecology and Evolution. 2014;4:656–671. doi: 10.1002/ece3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baskin, C. & Baskin, J. Seeds: ecology, biogeography, and evolution of dormancy and germination (Academic Press, San Diego, 2014).
- 25.McKeon GM, Mott JJ. The Effect of temperature on the field softening of hard seed of Stylosanthes humilis and Stylosanthes hamata in a dry monsoonal climate. Australian Journal of Agricultural Research. 1982;33:75–85. doi: 10.1071/AR9820075. [DOI] [Google Scholar]
- 26.Rice KJ. Responses of Erodium to varying microsites - the role of germination cueing. Ecology. 1985;66:1651–1657. doi: 10.2307/1938027. [DOI] [Google Scholar]
- 27.Jaganathan, G. K., Yule, K. J. & Biddick, M. Determination of the water gap and the germination ecology of Adenanthera pavonina (Fabaceae, Mimosoideae); the adaptive role of physical dormancy in mimetic seeds. Aob Plants10 (2019). [DOI] [PMC free article] [PubMed]
- 28.Baskin JM, Nan XY, Baskin CC. A comparative study of seed dormancy and germination in an annual and a perennial species of Senna (Fabaceae) Seed Science Research. 1998;8:501–512. doi: 10.1017/S0960258500004475. [DOI] [Google Scholar]
- 29.Zupo T, Jaime Baeza M, Fidelis A. The effect of simulated heat-shock and daily temperature fluctuations on seed germination of four species from fire-prone ecosystems. Acta Botanica Brasilica. 2016;30:514–519. doi: 10.1590/0102-33062016abb0246. [DOI] [Google Scholar]
- 30.Bastida F, Talavera S. Temporal and spatial patterns of seed dispersal in two Cistus species (Cistaceae) Annals of Botany. 2002;89:427–434. doi: 10.1093/aob/mcf065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Céspedes B, Torres I, Luna B, Pérez B, Moreno JM. Soil seed bank, fire season, and temporal patterns of germination in a seeder-dominated Mediterranean shrubland. Plant Ecology. 2012;213:383–393. doi: 10.1007/s11258-011-9983-2. [DOI] [Google Scholar]
- 32.Moreno JM, et al. Rainfall patterns after fire differentially affect the recruitment of three Mediterranean shrubs. Biogeosciences. 2011;8:3721–3732. doi: 10.5194/bg-8-3721-2011. [DOI] [Google Scholar]
- 33.Luna B, Chamorro D, Pérez B. Effect of heat on seed germination and viability in species of Cistaceae. Plant Ecology and Diversity. 2019;12:151–158. doi: 10.1080/17550874.2019.1610916. [DOI] [Google Scholar]
- 34.Ooi MKJ, Auld TD, Denham AJ. Climate change and bet-hedging: interactions between increased soil temperatures and seed bank persistence. Global Change Biology. 2009;15:2375–2386. doi: 10.1111/j.1365-2486.2009.01887.x. [DOI] [Google Scholar]
- 35.Luna B, Pérez B, Torres I, Moreno JM. Effects of incubation temperature on seed germination of Mediterranean plants with different geographical distribution ranges. Folia Geobotanica. 2012;47:17–27. doi: 10.1007/s12224-011-9110-0. [DOI] [Google Scholar]
- 36.Thanos CA, Georghiou K. Ecophysiology of fire-stimulated seed germination in Cistus incanus ssp. creticus (L.) Heywood and C. salvifolius L. Plant, Cell and Environment. 1988;11:841–849. doi: 10.1111/j.1365-3040.1988.tb01910.x. [DOI] [Google Scholar]
- 37.Valbuena L, Tárrega R, Luis E. Influence of heat on seed germination of Cistus laurifolius and Cistus ladanifer. International Journal of Wildland Fire. 1992;2:15–20. doi: 10.1071/WF9920015. [DOI] [Google Scholar]
- 38.Castro J, Romero-García AT. Dormancy and germination in Cistus clusii (Cistaceae): effect of biotic and abiotic factors. Rev. Ecol. (Terre Vie) 1999;54:19–28. [Google Scholar]
- 39.Nadal P, Sanchis E, Pérez-García F, Fos M. Effect of dry-heat, soaking in distilled water and gibberellic acid on the germination of Cistus clusii, C. monspeliensis and C. salvifolius seeds. Seed Science and Technology. 2002;30:663–669. [Google Scholar]
- 40.Reyes O, Trabaud L. Germination behaviour of 14 Mediterranean species in relation to fire factors: smoke and heat. Plant Ecology. 2009;202:113–121. doi: 10.1007/s11258-008-9532-9. [DOI] [Google Scholar]
- 41.Pérez-García, F. & Gonzalez-Benito, M. E. In Xxviii International Horticultural Congress on Science and Horticulture for People 379–384 (Int Soc Horticultural Science, Leuven 1, 2012).
- 42.Luna B, Chamorro D. Germination sensitivity to water stress of eight Cistaceae species from the Western Mediterranean. Seed Science Research. 2016;26:101–110. doi: 10.1017/S096025851600009X. [DOI] [Google Scholar]
- 43.Saura-Mas, S., Saperas, A. & Lloret, F. Climatic and fire determinants of early life-history stages in the Mediterranean shrub Cistus albidus. Journal of Plant Ecology, 10.1093/jpe/rtz040 (2019).
- 44.Fenner, M. & Thompson, K. The ecology of seeds. (Cambridge University Press, Cambridge, 2005).
- 45.Philippi T, Seger J. Hedging one’s evolutionary bets, revisited. Trends in Ecology & Evolution. 1989;4:41–44. doi: 10.1016/0169-5347(89)90138-9. [DOI] [PubMed] [Google Scholar]
- 46.Quintana JR, Cruz A, Fernández-González F, Moreno JM. Time of germination and establishment success after fire of three obligate seeders in a Mediterranean shrubland of Central Spain. Journal of Biogeography. 2004;31:241–249. doi: 10.1111/j.1365-2699.2004.00955.x. [DOI] [Google Scholar]
- 47.Bell DT, Plummer JA, Taylor SK. Seed Germination Ecology in Southwestern Western Australia. The Botanical Review. 1993;59:24–55. doi: 10.1007/BF02856612. [DOI] [Google Scholar]
- 48.Bond, W. J. & Van Wilgen, B. W. Fire and plants (Chapman & Hall, London, 1996).
- 49.Keeley, J. E. & Fotheringham, C. J. In Seeds. The ecology of regeneration in plant communities (ed. Fenner, M.) 311–331 (CAB International, United Kingdom, 2000).
- 50. Probert, R. J. The role of temperature in the regulation of seed dormancy and germination in Seeds. The ecology of regeneration in plant communities (ed. Fenner, M.) 261–292 (CAB International, Wallingford, 2000).
- 51.Ferrandis P, Herranz JM, Martínez-Sánchez JJ. Effect of fire on hard-coated Cistaceae seed banks and its influence on techniques for quantifying seed banks. Plant Ecology. 1999;144:113–114. doi: 10.1023/A:1009816309061. [DOI] [Google Scholar]
- 52.Jayasuriya KMGG, Baskin JM, Baskin CC. Cycling of sensitivity to physical dormancy-break in seeds of Ipomoea lacunosa (Convolvulaceae) and ecological significance. Annals of Botany. 2008;101:341–352. doi: 10.1093/aob/mcm285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jayasuriya KMGG, Baskin JM, Geneve RL, Baskin CC, Chien CT. Physical dormancy in seeds of the holoparasitic angiosperm Cuscuta australis (Convolvulaceae, Cuscuteae): Dormancy-breaking requirements, anatomy of the water gap and sensitivity cycling. Annals of Botany. 2008;102:39–48. doi: 10.1093/aob/mcn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodrigues-Junior AG, Baskin CC, Baskin JM, Garcia QS. Sensitivity cycling in physically dormant seeds of the Neotropical tree Senna multijuga (Fabaceae) Plant Biology. 2018;20:698–706. doi: 10.1111/plb.12719. [DOI] [PubMed] [Google Scholar]
- 55.Taylor GB. Hardseededness in Mediterranean annual pasture legumes in Australia: a review. Australian Journal of Agricultural Research. 2005;56:645–661. doi: 10.1071/AR04284. [DOI] [Google Scholar]
- 56.Jayasuriya KMGG, Baskin JM, Baskin CC. Sensitivity cycling and its ecological role in seeds with physical dormancy. Seed Science Research. 2009;19:3–13. doi: 10.1017/S096025850818730X. [DOI] [Google Scholar]
- 57.Rolston MP. Water Impermeable Seed Dormancy. Botanical Review. 1978;44:365–396. doi: 10.1007/BF02957854. [DOI] [Google Scholar]
- 58.Norsworthy JK, Oliveira MJ. Role of light quality and temperature on pitted morningglory (Ipomoea lacunosa) germination with after-ripening. Weed Science. 2007;55:111–118. doi: 10.1614/WS-06-137.1. [DOI] [Google Scholar]
- 59.Hagon MW, Ballard LAT. Reversibility of strophiolar permeability to water in seeds of subterranean clover (Trifolium subterraneum L). Australian. Journal of Biological Sciences. 1970;23:519–528. [Google Scholar]
- 60.Leck, M. A., Parker, V. T. & Simpson, R. L. Ecology of soil seed banks (Academic Press, San Diego, 1989).
- 61.Traba J, Azcárate FM, Peco B. From what depth do seeds emerge? A soil seed bank experiment with Mediterranean grassland species. Seed Science Research. 2004;14:297–303. doi: 10.1079/SSR2004179. [DOI] [Google Scholar]
- 62.Tieu A, Dixon W, Meney KA, Sivasithamparam K. The interaction of heat and smoke in the release of seed dormancy in seven species from Southwestern Western Australia. Annals of Botany. 2001;88:259–265. doi: 10.1006/anbo.2001.1451. [DOI] [Google Scholar]
- 63.Hnatiuk RJ, Hopkins AJM. An ecological analysis of kwongan vegetation South of Eneabba, Western-Australia. Australian Journal of Ecology. 1981;6:423–438. doi: 10.1111/j.1442-9993.1981.tb01496.x. [DOI] [Google Scholar]
- 64.IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. (Cambridge University Press, Cambridge, UK and New York, 2013).
- 65.Parmesan C, Hanley ME. Plants and climate change: complexities and surprises. Annals of Botany. 2015;116:849–864. doi: 10.1093/aob/mcv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walck JL, Hidayati SN, Dixon KW, Thompson K, Poschlod P. Climate change and plant regeneration from seed. Global Change Biology. 2011;17:2145–2161. doi: 10.1111/j.1365-2486.2010.02368.x. [DOI] [Google Scholar]
- 67.Hudson AR, Ayre DJ, Ooi MKJ. Physical dormancy in a changing climate. Seed Science Research. 2015;25:66–81. doi: 10.1017/S0960258514000403. [DOI] [Google Scholar]
- 68.Herranz JM, Ferrandis P, Copete MA, Duro EM, Zalacaín A. Effect of allelopathic compounds produced by Cistus ladanifer on germination of 20 Mediterranean taxa. Plant Ecology. 2006;184:259–272. doi: 10.1007/s11258-005-9071-6. [DOI] [Google Scholar]
- 69.Sosa T, Valares C, Alías JC, Lobón NC. Persistence of flavonoids in Cistus ladanifer soils. Plant and Soil. 2010;337:51–63. doi: 10.1007/s11104-010-0504-1. [DOI] [Google Scholar]
- 70.Bradshaw SD, Dixon KW, Hopper SD, Lambers H, Turner SR. Little evidence for fire-adapted plant traits in Mediterranean climate regions. Trends in Plant Science. 2010;16:69–76. doi: 10.1016/j.tplants.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 71.Keeley JE, Pausas JG, Rundel PW, Bradstock R. Fire as an evolutionary pressure shaping plant traits. Trends in Plant Science. 2011;16:406–411. doi: 10.1016/j.tplants.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Hernández Fernández M, Álvarez Sierra MA, Pelaez-Campomanes P. Bioclimatic analysis of rodent palaeofaunas reveals severe climatic changes in Southwestern Europe during the Plio-Pleistocene. Palaeogeography Palaeoclimatology Palaeoecology. 2007;251:500–526. doi: 10.1016/j.palaeo.2007.04.015. [DOI] [Google Scholar]
- 73.Suc JP. Origin and Evolution of the Mediterranean Vegetation and Climate in Europe. Nature. 1984;307:429–432. doi: 10.1038/307429a0. [DOI] [Google Scholar]
- 74.Rundel, P. W. et al. Fire and Plant Diversification in Mediterranean-Climate Regions. Frontiers in Plant Science9 (2018). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.