Abstract
It has been reported that chitosan has a hemostatic effect and an antibiotic activity. This study aimed to evaluate the efficacy and feasibility of using a chitosan tampon (Hemoblock-Tampon) in preventing hemorrhage and enhancing wound healing after the loop electrosurgical excision procedure (LEEP).This single-blind, prospective, randomized study included 62 consecutive patients who underwent LEEP for cervical intraepithelial neoplasia. A chitosan tampon (31 patients; treatment group), or a general tampon (31 patients; control group) was applied to the uterine cervix immediately after LEEP. One patient in the treatment group declined to participate in this study. Thus, 30 patients in the treatment group and 31 patients in the control group completed this study. For objective analysis of hemorrhage in the postoperative 2 weeks, the amounts of bleeding were checked daily with a pictorial blood assessment chart. We evaluated vaginal discharge, abdominal pain, and impairment in daily living during the postoperative 2 weeks using 5 visual analogue scale questionnaires.The bleeding count was significantly lower in the treatment group than in the control group (21.37 ± 16.86 vs. 40.52 ± 16.55, p = 0.0014). The sum of the scores of the 5 questionnaires was significantly lower in the treatment group than in the control group (6.53 ± 2.84 vs. 8.59 ± 2.88, p = 0.0079). The incidence of vaginal discharge was significantly lower in the treatment group than in the control group (20.0% vs. 48.4%, p = 0.0207). According to logistic regression, only the use of chitosan tampon reduced the risk of moderate to severe vaginal bleeding 2 weeks after surgery (Odd ratio, 0.213; 95% confidence interval, 0.06–0.76; p = 0.0172). Complete healing of the uterine cervix occurred in 86.7% of patients in the treatment group and in 61.3% of patients in the control group at 4 weeks after surgery (p = 0.0255).The use of chitosan tampons can reduce hemorrhage, vaginal discharge, abdominal pain, and impairment of daily living after LEEP. Moreover, chitosan tampon may help enhance wound healing.
Subject terms: Quality of life, Randomized controlled trials
Introduction
The loop electrosurgical excision procedure (LEEP) is a method used for both the diagnosis and treatment of cervical intraepithelial neoplasia (CIN)1. This procedure has been widely used because it is an inexpensive, technically easy-to-perform procedure that requires only local anesthesia and has a low complication rate as well as good specimen quality2,3.
Despite these advantages, complications such as postoperative bleeding, abnormal vaginal discharge, abdominal pain, and infection have been reported2–5. These complications make patients anxious and cause interference in activities of daily living. Moreover, severe hemorrhage requires additional procedures and medical costs. The prevalence rate of LEEP complications was reported to range from 0.8% to 52%5,6.
Chitosan is a promising hemostatic agent because it can adhere to red blood cells and induce platelets to adhere, activate, and aggregate at the site of bleeding7. Furthermore, chitosan has biological properties such as hemostatic activity, antibacterial activity, and ability to accelerate wound healing8. Thereby, it is being used in many medical devices and health-care products9. Recently, several reports have shown the usefulness and effectiveness of chitosan for postpartum hemorrhage10,11. However, chitosan has not been used in the field of gynecologic surgery, especially after LEEP.
The objectives of this pilot study were as follows: (1) to examine the hemostatic efficiency and the reduction of blood clotting time by using an in vitro blood compatibility test; (2) to evaluate whether chitosan tampon could decrease postoperative bleeding, vaginal discharge, pain, and impairment of daily activities; and (3) to assess potential improvement in wound healing and treatment-associated complications such as hypersensitivity after LEEP.
Results
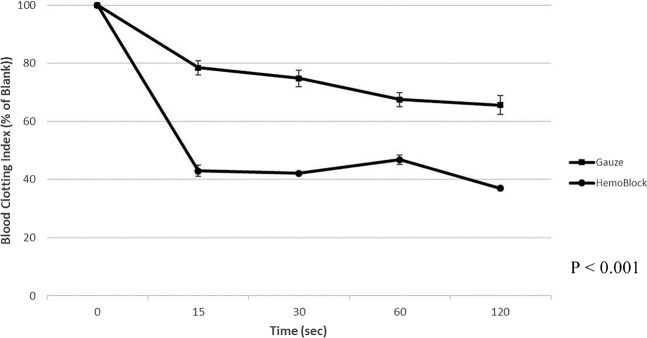
Comparison of blood clotting index (BCI) between chitosan gauze and general gauze
The BCI was measured with reference to the anticoagulant citrate dextrose (ACD)-treated whole blood by using spectrometry. The mean BCIs were significantly lower for chitosan gauze than for general gauze (43.00 ± 1.94 vs. 78.45 ± 2.38, 15 s, p = 0.0026; 42.14 ± 0.55 vs. 74.80 ± 2.88, 30 s, p = 0.0024; 46.85 ± 1.64 vs. 67.52 ± 2.43, 60 s, p = 0.0009; and 36.92 ± 0.61 vs. 65.58 ± 3.22, 120 s, p = 0.0039). On the basis of the BCI results, blood coagulation was more effective with chitosan gauze than with general gauze (Fig. 1).
Figure 1.
Measurement of in vitro blood clotting index.
Patients’ characteristics
The mean age was 43.40 ± 10.27 years in the treatment group and 46.06 ± 7.81 in the control group (p = 0.2576). There was no significant difference in terms of the proportion of menopausal women (23.3% vs. 32.3%, p = 0.4408). The final cervical pathology, and the size and depth of the surgical specimen were similar between the 2 groups (Table 1).
Table 1.
Patients and pathologic characteristics.
| Variables | Treatment group (n = 30) | Control group (n = 31) | p-Value |
|---|---|---|---|
| Age (years) | 43.40 ± 10.27 | 46.06 ± 7.81 | 0.2576 |
| Menopause status, n (%) | 7 (23.3) | 10 (32.3) | 0.4408 |
| LEEP histopathology | |||
| ≤LSIL | 4 (13.3) | 5 (16.1) | 0.7602 |
| ≥HSIL | 226 (86.7) | 22 (83.9) | |
| Surgical specimen | |||
| Size (cm2) | 4.10 ± 1.36 | 4.63 ± 1.77 | 0.1940 |
| Depth (cm) | 1.55 ± 1.17 | 1.76 ± 1.10 | 0.4707 |
LEEP, loop electrosurgical excision procedure; LSIL, low-grade squamous intraepithelial neoplasia; HSIL, high-grade squamous intraepithelial neoplasia.
Complications at 2 and 4 weeks after surgery
At the 2 weeks follow-up visit at the hospital, the sum of PBAC and patient questionnaires, degree of vaginal bleeding, and vaginal discharge were evaluated. The sum of PBAC and questionnaire scores during postoperative 2 weeks were significantly lower in the treatment group than in the control group (21.37 ± 16.86 vs. 40.52 ± 16.55, p = 0.0014; 6.53 ± 2.84 vs. 8.55 ± 2.88, p = 0.0079). The incidence of mild vaginal bleeding was similar between the 2 groups (16.7% vs. 19.4%, p = 0.7866); however, the incidence of moderate to severe vaginal bleeding was significantly lower in the treatment group than in the control group (13.3% vs. 41.9%, p = 0.0135). Moreover, the incidence of vaginal discharge was significantly lower in the treatment group than in the control group (20.0% vs. 48.4%, p = 0.0207).
At the follow-up visit at 4 weeks, vaginal bleeding, vaginal discharge, and healing status of the uterine cervix were evaluated. No significant differences were observed in vaginal bleeding and vaginal discharge between the 2 groups. However, the incidence of complete healing of the uterine cervix was significantly higher in the treatment group than in the control group (86.7% vs. 61.3%, p = 0.0255) (Table 2). No case of hypersensitive reaction occurred in both groups.
Table 2.
Complications at 2 and 4 weeks after surgery.
| Variables | Treatment group (n = 30) | Control group(n = 31) | p-Value |
|---|---|---|---|
| At 2 weeks | |||
| Pictorial blood assessment chart | 21.37 ± 16.86 | 40.52 ± 16.55 | 0.0014 |
| Visual analogue scale score of 5 questionnaires | 6.53 ± 2.84 | 8.55 ± 2.88 | 0.0079 |
| Mild vaginal bleeding, n (%) | 5 (16.7) | 6 (19.4) | 0.7866 |
| Moderate or severe vaginal bleeding, n (%) | 4 (13.3) | 12 (41.9) | 0.0135 |
| Vaginal discharge, n (%) | 6 (20.0) | 15 (48.4) | 0.0207 |
| At 4 weeks | |||
| Mild vaginal bleeding, n (%) | 2 (6.7) | 5 (16.1) | 0.2503 |
| Moderate or severe vaginal bleeding, n (%) | 0 (0) | 3 (9.7) | 0.0831 |
| Vaginal discharge, n (%) | 0 (0) | 1 (3.2) | 0.3252 |
| Complete healing of the cervix, n (%) | 26 (86.7) | 19 (61.3) | 0.0255 |
Logistic regression analysis for moderate to severe vaginal bleeding at 2 weeks after surgery
According to logistic regression, only the use of chitosan tampon reduced the risk of moderate to severe vaginal bleeding 2 weeks after surgery (OR, 0.213; 95% CI, 0.06–0.76; p = 0.0172). However, patient age, size and depth of the specimen, and final pathology were not associated with moderate to severe vaginal bleeding (Table 3).
Table 3.
Logistic regression analysis for moderate to severe vaginal bleeding at 2 weeks after surgery.
| OR | 95% CI | p-Value | |
|---|---|---|---|
| Use of chitosan tampon (yes vs. no) | 0.213 | 0.06–0.76 | 0.0172 |
| Age (>45 years vs. ≤45 years) | 2.064 | 0.66–6.44 | 0.2120 |
| Size (>4 cm2 vs. ≤4 cm2) | 2.400 | 0.72–7.96 | 0.1524 |
| Depth (>1 cm vs. ≤1 cm) | 1.661 | 0.50–5.54 | 0.4085 |
| Pathology (≥HSIL vs. LSIL) | 3.556 | 0.41–30.85 | 0.2499 |
OR, odds ratio; 95% CI, 95% confidence interval; HSIL, high-grade squamous intraepithelial neoplasia; LSIL, low-grade squamous intraepithelial neoplasia.
Discussion
This study investigated the efficacy of chitosan tampon application after LEEP. Chitosan showed effective blood coagulation in the in vitro blood compatibility test. Furthermore, chitosan tampon was effective in reducing postoperative bleeding, vaginal discharge, and impairment of daily activities. Moreover, chitosan tampon may enhance the healing of the uterine cervix.
Chitosan has a positively charged surface and plays a role in attracting the negatively charged red blood cell membranes, resulting in hemagglutination12. Chitosan also induces platelet adhesion and activation and enhances platelet aggregation by absorbing plasma proteins and fibrinogen13,14. Another important property of chitosan that makes it a suitable material for preparation of wound dressing is its inherent antimicrobial activity. Free amine groups present in chitosan provide antimicrobial activity because they bind to the bacterial cell wall, thus causing bacterial cell lysis15. There are many commercially available chitosan-based composites for use in hemostasis and wound healing, including Chitogauze, Celox Gauze, Mini-sponge dressing, Hemcon, TraumaGauze, and ChitoFlex16,17. However, there are no commercially available products for LEEP.
Several methods for preventing hemorrhage after LEEP are described in the literature. Some studies have shown that routine prophylactic application of Mosel’s solution and local anesthesia with epinephrine could reduce postoperative vaginal bleeding18,19. Moreover, several hemostatic agents have been applied for reducing hemorrhage after LEEP; however, their effect on reducing hemorrhage remains controversial. Some studies demonstrated that Tissel (Baxter, Westlake Village, CA, USA) could reduce postoperative vaginal bleeding20,21. However, other reports showed that using hemostats such as Tachosil (Nycomen, Zurich, Switzerland) could not reduce postoperative vaginal bleeding19,22. Because most previous studies evaluated postoperative hemorrhage based on the subjective assessment of symptoms by patients and clinicians, exact evaluation of postoperative hemorrhage was difficult. Therefore, we used the PBAC to objectively measure hemorrhage during the postoperative 2 weeks. In this study, chitosan tampon significantly reduced postoperative vaginal bleeding (21.37 ± 16.86 vs. 40.52 ± 16.55, p = 0.0014).
Because LEEP is a relatively safe procedure, we also focused on self-estimated symptoms that can lead to disability in daily life, by using 5 visual analogue scale questionnaires. In this study, the sum of the scores of the 5 visual analogue scale questionnaires showed that vaginal discharge, abdominal pain, and impairment of daily activities could be reduced by chitosan tampon. Vaginal discharge and wound healing were also improved by chitosan tampon. The antimicrobial activity of chitosan may have influenced these results.
Chitosan has antiviral activity23. Moreover, a recent study reported that sulfated chitosan possesses broad anti-human papilloma virus (HPV) activities in vitro and may possibly inhibit HPV infection by targeting viral capsid protein and host phosphoinositide 2-kinase/Akt/mammalian target of rapamycin pathway24. Therefore, chitosan tampon may have potential as a novel anti-HPV agent. More comprehensive molecular studies should be performed to verify these findings. Furthermore, we plan to evaluate in the future whether the application of chitosan tampon affects disease recurrence and persistence of HPV infection after LEEP.
Our study has some limitations. First, it is a pilot study with a limited number of patients. Second, this study was conducted in a single-blind setting because we could not produce an adequate placebo control.
Despite these limitations, our study offers some unique and significant findings. This is the first study to evaluate the usefulness of chitosan application after LEEP. Moreover, we evaluated postoperative hemorrhage, vaginal discharge, abdominal pain, and impairment of daily activities by using the patients’ self-estimated visual score to reduce bias.
In conclusion, chitosan tampon is effective in reducing vaginal bleeding, vaginal discharge, abdominal pain, and impairment of daily living after LEEP. Moreover, chitosan tampon may help enhance wound healing. However, further studies with a large number of patients should be performed to confirm our findings.
Materials and Methods
Measurement of BCI in vitro
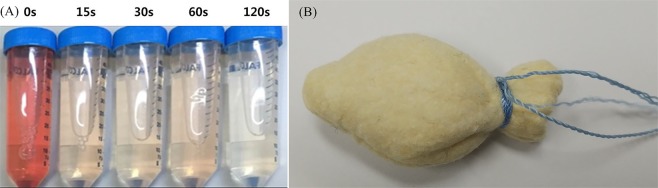
A chitosan gauze and a general gauze of 2 × 2 cm size and 2 mm thickness were placed in a test tube with a flat base. The test tube was boiled in a water bath with the automatic temperature controller set at 37 °C for 5 min. Then, dripping of blood was done carefully to ensure that the surface would be completely covered with 0.27 mL of human blood (whole blood treated with 0.3 mL ACD and added with 0.024 mL calcium chloride). The test tube containing blood was incubated in an incubator with the automatic temperature controller set at 37 °C for 10 min. A 10 mL volume of deionized distilled water was carefully dripped to dissolve the coagulated blood components (Fig. 2A). Subsequently, a 10 mL dissolver contained in a test tube was centrifuged at 100 g for 30 s. Following the centrifugation, the supernatant was placed in a glass tube containing 40 mL deionized distilled water and maintained at 37 °C for 60 min. A blood clotting test was performed according to the relative absorbance measured at a wavelength of 542 nm on a spectrometer. It was hypothesized that the reference absorbance value might be 100 at a wavelength of 542 nm for the solution mixture of 50 mL deionized distilled water and 0.25 mL ACD-treated whole blood. The BCI was measured following previously reported methods, and calculated by the following equation25.
Figure 2.
In vitro blood compatibility test. (A) Chitosan tampon (Hemoblock-Tampon).
Study population
After obtaining approval from the institutional review board of Kyungpook National Univeristiy Chilgok hospital, we recruited the study population. The inclusion criteria were age 20–65 years, biopsy-confirmed CIN grade ≥2, not pregnant status, and provision of informed written consent for participation. Patients with known hypersensitivity to crustacean foods were excluded.
The sample size was estimated, assuming a hypothetical 30% higher overall complication rate in patients who underwent LEEP without the chitosan tampon (control group) compared with those in whom a chitosan tampon was applied (treatment group; 80% power; type I error probability, 0.05; drop rate, 10%). Theoretically, 31 patients were required for each group. Between December 2017 and September 2018, a total of 62 patients underwent LEEP with or without chitosan tampon application. Among these patients, 1 patient in the treatment group declined to participate in this study. Finally, 61 patients were recruited (30 in the treatment group and 31 in the control group) and assigned to LEEP with or without chitosan tampon application.
Chitosan tampon
Chitosan tampon (Hemoblock-Tampon; Incore, Daegu, Korea) contains chitosan fiber, cotton, barium sulfate, and polyamide. The shape of the chitosan tampon was designed for fixing to the wound after LEEP (Fig. 2B).
Study design
This was a single-blind, prospective, randomized study involving 62 consecutive patients undergoing LEEP for biopsy-confirmed CIN grade ≥2. Our committee approved the research and confirmed that all research was performed in accordance with relevant gauidelines and regulation. Informed consent was obtained from all participants. The enrolled patients were randomly allocated (1:1) to a treatment group or control group using a randomized table prepared using a random sequence generator (www.random.org). Among these patients, 61 (30 in the treatment group and 31 in the control group) completed follow-up. The present study was registered at Clinical Research Information Service (CRIS, https://cris.nih.go.kr, KCT0003696, resgisted on April 1, 2019). The study was informed by the CONSORT approach and the results were reported accordingly. The authors confirm that all ongoing and related trials for this intervention are resistered.
All procedures were performed using a right-angled loop carrying high-frequency current (High Frequency; Sometech, Seoul, Korea) with as single pass. We did not use any hemostatic agents during or after LEEP, such as epinephrine or Mosel solution in both groups. Before tampon application, hemostasis was done completely using an electrocautery device. Chitosan tampon was applied to the uterine cervix immediately after LEEP surgery in the treatment group, and general tampon made of cotton was applied to the uterine cervix in the control group for 12 hours. Pateints removed tompons by themselves at home.
Clinical follow-up was performed at 2 and 4 weeks after LEEP. For objective and exact measurement of postoperative bleeding, vaginal discharge, abdominal pain, and impairment of daily living, we used a pictorial blood loss assessment chart (PBAC) and 5 visual analogue scale questionnaires during the 2 weeks after surgery (Supplementary Table 1). When patients returned to the outpatient clinic 2 and 4 weeks after surgery, we evaluated the wound healing process and checked for vaginal bleeding (and determined whether it required intervention) and vaginal discharge. Mild vaginal bleeding was defined as no requirement for intervention, moderate vaginal bleeding as the requirement for intervention (chemical cautery and/or electrocautery) at the outpatient clinic, and severe vaginal bleeding as a visit to the emergency department. Complete wound healing was defined as the appearance of a normal cervix without discharge and bleeding. The details of the study design and protocol are summarized in Fig. 3.
Figure 3.
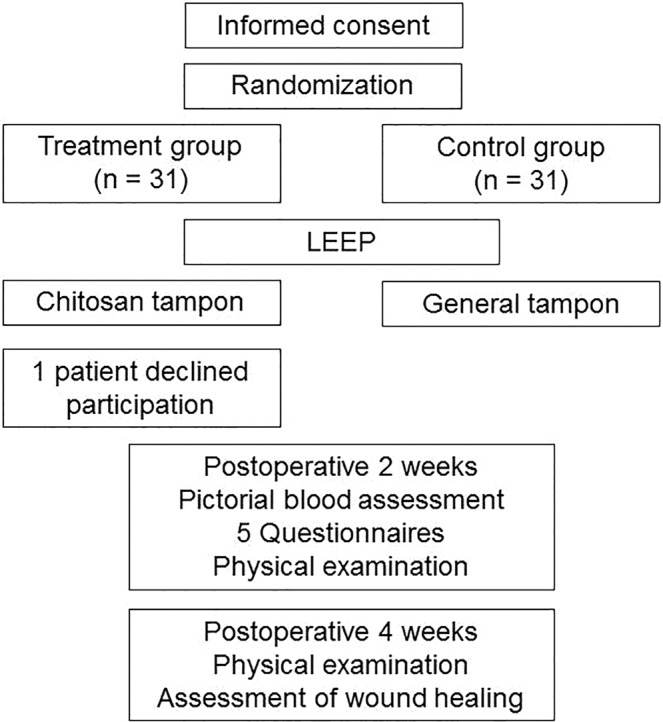
Study design.
Statistical analysis
Continuous data were expressed as mean ± standard deviation and categorical data as frequency and percentage. Differences between subsets were evaluated with Student’s t-test, and differences between proportions were compared with the chi-square test or Fisher’s exact test. A logistic regression model was used to evaluate clinical variables for moderate and severe vaginal bleeding 2 weeks after surgery, and estimated odds ratios (ORs) with 95% confidence intervals (95% CIs) were presented.
All statistical tests were 2-sided, and a p-value of <0.05 was considered significant. Statistical analysis was performed using SPSS software version 22.0 (SPSS, Chicago, IL, USA) and Medcalc version 15.4 (Medcalc Software, Ostend, Belgium).
Supplementary information
Author contributions
Study concept and design: G.O.C., Y.H.L. conduction of experiments: G.O.C., Y.H.L., H.-Y.Y., S.-H.A. acquisition of data: G.O.C., S.Y.J., S.-H.A. analysis and interpretation of data: G.O.C., S.Y.J. drafting of the manuscript: G.O.C., Y.H.L., S.-H.A. critical revision of the manuscript for important intellectual content: G.O.C. study supervision: G.O.C. All authors reviewed the manuscript
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Gun Oh Chong and Yoon Hee Lee.
Supplementary information
is available for this paper at 10.1038/s41598-020-62965-1.
References
- 1.Prendiville W, Cullimore J, Norman S. Large loop excision of the transformation zone (LLETZ). A new method of management for women with cervical intraepithelial neoplasia. Br. J. Obstet. Gynaecol. 1989;96:1054–1060. doi: 10.1111/j.1471-0528.1989.tb03380.x. [DOI] [PubMed] [Google Scholar]
- 2.Wright TC, Jr, Gagnon S, Richart RM, Ferenczy A. Treatment of cervical intraepithelial neoplasia using the loop electrosurgical excision procedure. Obstet. Gynecol. 1992;79:173–178. [PubMed] [Google Scholar]
- 3.Hallam NF, et al. Large loop excision of the transformation zone (LLETZ) as an alternative to both local ablative and cone biopsy treatment: a series of 1000 patients. J. Gynecol. Surg. 1993;9:77–82. doi: 10.1089/gyn.1993.9.77. [DOI] [PubMed] [Google Scholar]
- 4.Keijser KG, et al. Diathermy loop excision in the management of cervical intraepithelial neoplasia: diagnosis and treatment in one procedure. Am. J. Obstet. Gynecol. 1992;166:1281–1287. doi: 10.1016/S0002-9378(11)90622-X. [DOI] [PubMed] [Google Scholar]
- 5.Maleerat P, Chumworathayi B, Kietpeerakool C, Luanratanakorn S, Temtanakitpaisan A. Post-Loop Electrosurgical Excision Procedure Complications in Srinagarind Hospital. Asian Pac. J. Cancer Prev. 2016;17:2211–2215. doi: 10.7314/apjcp.2016.17.4.2211. [DOI] [PubMed] [Google Scholar]
- 6.Woo VG, Cohen CR, Bukusi EA, Huchko MJ. Loop electrosurgical excision procedure: safety and tolerability among human immunodeficiency virus-positive Kenyan women. Obstet. Gynecol. 2011;118:554–559. doi: 10.1097/AOG.0b013e31822b0991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Periayah MH, et al. Chitosan scaffold enhances growth factor release in wound healing in von Willebrand disease. Int. J. Clin. Exp. Med. 2015;8:15611–15620. [PMC free article] [PubMed] [Google Scholar]
- 8.Stricker-Krongrad AH, et al. Efficacy of Chitosan-Based Dressing for Control of Bleeding in Excisional Wounds. Eplasty. 2018;10,18:e14. [PMC free article] [PubMed] [Google Scholar]
- 9.Hattori H, Ishihara M. Feasibility of improving platelet-rich plasma therapy by using chitosan with high platelet activation ability. Exp. Ther. Med. 2017;13:1176–1180. doi: 10.3892/etm.2017.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carles G, et al. Uses of chitosan for treating different forms of serious obstetrics hemorrhages. J Gynecol Obstet. Hum. Reprod. 2017;46:693–695. doi: 10.1016/j.jogoh.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Seidel V, Braun T, Weizsäcker K, Henrich W. Application of chitosan-covered gauze in combination with intrauterine balloon tamponade for postpartum hemorrhage treatment - Case report of a novel “uterine sandwich” approach. Int. J. Surg. Case Rep. 2018;48:101–103. doi: 10.1016/j.ijscr.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ong SY, Wu J, Moochhala SM, Tan MH, Lu J. Development of a chitosan-based wound dressing with improved hemostatic and antimicrobial properties. Biomaterials. 2008;29:4323–4332. doi: 10.1016/j.biomaterials.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 13.Lord MS, Cheng B, McCarthy SJ, Jung M, Whitelock JM. The modulation of platelet adhesion and activation by chitosan through plasma and extracellular matrix proteins. Biomaterials. 2011;32:6655–6662. doi: 10.1016/j.biomaterials.2011.05.062. [DOI] [PubMed] [Google Scholar]
- 14.Chou TC, Fu E, Wu CJ, Yeh JH. Chitosan enhances platelet adhesion and aggregation. Biochem. Biophys. Res. Commun. 2003;302:480–483. doi: 10.1016/s0006-291x(03)00173-6. [DOI] [PubMed] [Google Scholar]
- 15.Sahariah P, et al. The effect of substituent, degree of acetylation and positioning of the cationic charge on the antibacterial activity of quaternary chitosan derivatives. Mar. Drugs. 2014;12:4635–4658. doi: 10.3390/md12084635.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett BL, et al. Management of External Hemorrhage in Tactical Combat Casualty Care: Chitosan-Based Hemostatic Gauze Dressings–TCCC Guidelines-Change 13-05. J. Spec. Oper. Med. 2014;14:40–57. doi: 10.55460/03VO-8FLO. [DOI] [PubMed] [Google Scholar]
- 17.Littlejohn LF, et al. Comparison of Celox-A, ChitoFlex, WoundStat, and combat gauze hemostatic agents versus standard gauze dressing in control of hemorrhage in a swine model of penetrating trauma. Acad. Emerg. Med. 2011;18:340–350. doi: 10.1111/j.1553-2712.2011.01036.x.. [DOI] [PubMed] [Google Scholar]
- 18.Kietpeerakool C, et al. Routine prophylactic application of Monsel’s solution after loop electrosurgical excision procedure of the cervix: is it necessary? J. Obstet. Gynaecol. Res. 2007;33:299–304. doi: 10.1111/j.1447-0756.2007.00528.x. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, et al. Delayed hemorrhage effect of local anesthesia with epinephrine in the loop electrosurgical excisional procedure. Obstet. Gynecol. Sci. 2017;60:87–91. doi: 10.5468/ogs.2017.60.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim K, et al. Efficacy of fibrin sealant in reducing hemorrhage after a loop electrosurgical excision procedure. Gynecol. Obstet. Invest. 2012;74:1–5. doi: 10.1159/000333266. [DOI] [PubMed] [Google Scholar]
- 21.Kim JH, et al. A Pilot Study to Investigate the Efficacy of Fibrin Sealant (Tisseel®) in the Loop Electrosurgical Excision Procedure. Gynecol. Obstet. Invest. 2015;80:21–25. doi: 10.1159/000369391. [DOI] [PubMed] [Google Scholar]
- 22.Kim JJ, et al. Efficacy of TachoSil® in preventing hemorrhage after loop electrosurgical excision procedure. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015;194:245–248. doi: 10.1016/j.ejogrb.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Kulikov SN, Chirkov SN, Il’ina AV, Lopatin SA, Varlamov VP. Effect of the molecular weight of chitosan on its antiviral activity in plants. Appl. Biochem. microbiology. 2006;42:224–228. doi: 10.1134/S0003683806020207. [DOI] [PubMed] [Google Scholar]
- 24.Gao Y, Liu W, Wang W, Zhang X, Zhao X. The inhibitory effects and mechanisms of 3,6-O-sulfated chitosan against human papillomavirus infection. Carbohydr. Polym. 2018;198:329–338. doi: 10.1016/j.carbpol.2018.06.096. [DOI] [PubMed] [Google Scholar]
- 25.Shih MF, et al. Platelet adsorption and hemolytic properties of liquid crystal/composite polymers. Int. J. Pharm. 2006;11:117–125,. doi: 10.1016/j.ijpharm.2006.07.043. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.