Abstract
BACKGROUND: Previous studies have demonstrated some associations between dietary vitamin A intake and ovarian cancer risk with an inconsistent relationship. We therefore performed the present study to further explore the association between them.
METHODS: Databases of PubMed, Embase, and Web of Science were retrieved up to September 1, 2019. Summarized relative risk (RR) with corresponding 95% confidence intervals (CI) were calculated. Stata 14.0 software was used for data analysis.
RESULTS: Fifteen articles involving 4882 cases and 443,179 participants were included in this meta-analysis. A positive association between dietary vitamin A intake and ovarian cancer risk was found (RR = 0.816, 95%CI = 0.723–0.920, I2 = 48.4%, P for heterogeneity = 0.019). Significant association was also found in case–control studies (RR = 0.769, 95%CI = 0.655–0.902), but not in cohort studies. When we performed the analysis between ovarian cancer risk and geographic locations, we found an inverse association in North American populations (RR = 0.825, 95%CI = 0.720–0.946), instead of other populations.
CONCLUSIONS: In summary, findings from the present study suggested that higher dietary intake of vitamin A may contribute to the lower development of ovarian cancer, especially among North Americans.
Keywords: Dietary intake, Meta-analysis, Ovarian cancer, Vitamin A
Introduction
Ovarian cancer is the most deadly gynecological cancer. The American Cancer Society had estimated that there were 22,240 new cases developing in ovarian cancer and 14,070 ovarian cancer cases deaths in 2018 [1]. Efforts to identify lifestyle factors that may affect the risk of ovarian cancer had been ongoing and indicated that some reproductive factors, such as oral contraceptives, carrying children and tubal ligation, may affect disease risk [2,3]. However, these factors usually cannot be changed. Dietary antioxidants, including vitamin A, have been hypothesized to modify cancer risk [4,5]. A previous study had been published to explore the association about vitamin A consumption and ovarian cancer risk, resulted non-significant association [6]. Many articles about vitamin A intake and ovarian cancer risk had been published, with no consistent conclusion. For this reason, this paper increased the sample size and improved the vitamin A efficiency through a meta-analysis to obtain more authentic and reliable analysis results, which is helpful to clarify whether dietary vitamin A intake has some inverse effects on ovarian cancer development, and finally provides evidence of prevention on ovarian cancer.
Methods
Search strategy and inclusion criteria
Three electronic databases (PubMed, Embase and Web of Science) were searched for relevant studies that investigated the association between dietary vitamin A intake and risk of ovarian cancer from inception up to September 1, 2019. The following search terms were used: ‘vitamin A’ OR ‘vitamin*’ OR ‘retinol’ combined with ‘ovarian cancer’ OR ‘ovarian tumor’. The bibliographies of the collected studies and relevant reviews were retrospectively assessed to identify additional articles. All the studies enrolled using this strategy was checked independently by two authors.
Studies were included based on the following criteria: (1) patients were diagnosed of ovarian cancer; (2) observational studies; (3) the interested association was about dietary vitamin A or retinol intake and ovarian cancer; (4) available relative risk (RR) and 95% confidence interval (CI) for ovarian cancer.
The following exclusion criteria were used: (1) reviews or meetings or abstracts or letter to the editors; (2) overlap articles or populations; (3) animal studies; (4) no available data of RR and 95%CI; (5) vitamin supplement.
Data extraction and quality assessment
Two researchers independently reviewed and extracted relevant information from all included studies. These pieces of information included: name of the first author, publishing date, country, ages, study types, vitamin types, sample sizes of the cases and controls, RR and 95%CI for ovarian cancer, adjustment for covariates. The disagreements with these two researchers were resolved by discussion and consensus. The Newcastle–Ottawa Quality Assessment Scale was used to assess the quality of the included studies [7].
Statistical analysis
Data were summarized using a random-effects model for combined RR with its 95%CI [8]. A Q and I2 test were performed to analysis the heterogeneity of the studies that included in this meta-analysis [9]. Moreover, meta-regression was performed to interpret the between-group heterogeneity [10]. Furthermore, sensitivity analyses were conducted to examine the stability of the results by removing each study one by one. Potential publication biases were examined using Begg’s test and Begg’s funnel plots [11]. Statistical analysis was performed using Stata version 14.0. A two-sided P value less than 0.05 was considered statistically significant.
Results
Study selection and study characterization
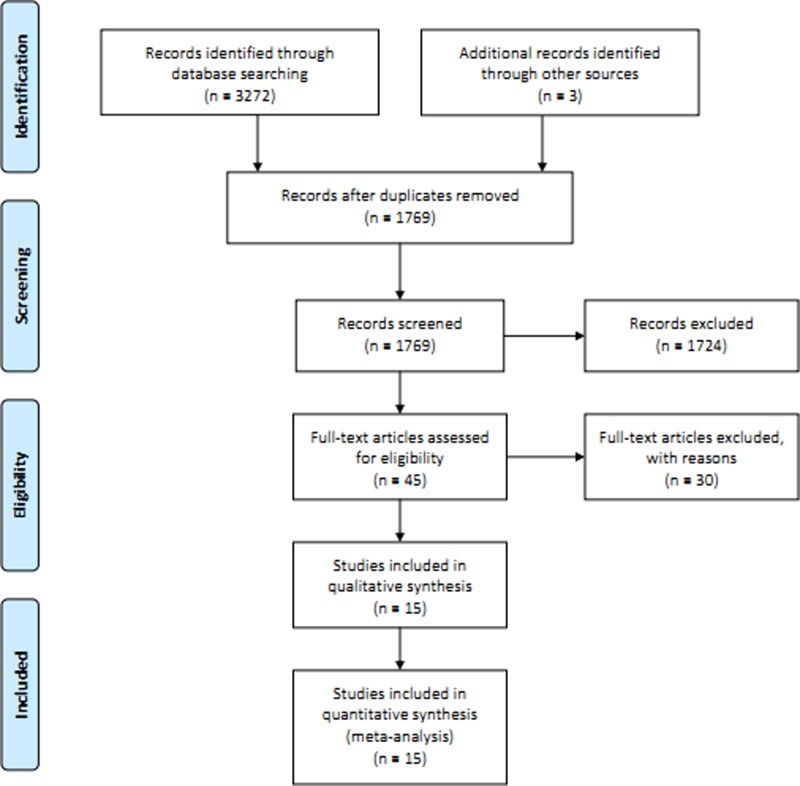
After searching the pre-defined three databases, we got 3272 citations. Through removing the duplicated literatures among databases and those obviously did not meet the criteria by reading the title and abstract, 45 full texts of the papers were downloaded for the further screening. Thirty articles were further excluded due to some reasons (duplicate publication (n = 1); did not report RR (n = 8); reviews (n = 8); vitamin A supplement (n = 2); animal studies (n = 4); serum studies (n = 6); assessment of survival rate of ovarian cancer (n = 1)). Finally, 15 articles [12–26] were included this meta-analysis (Figure 1). The quality evaluation score (Table 1) of each study ranged from 6 to 8 and the methodological quality was higher. The characteristics of the observational studies are shown in Table 1.
Figure 1. Flow chart of meta-analysis for exclusion/inclusion of studies.
Table 1. Characteristics of the included studies about the association of dietary vitamin A intake on ovarian cancer risk.
| Study, year | Design | Age | Participants, Cases | Country | Vitamin A type | Quality score | RR (95%CI) Highest vs. lowest | Adjustment |
|---|---|---|---|---|---|---|---|---|
| Bertone ER, 2001 | PBCC | 50–79 | 3,456, 327 | United States | Vitamin A | 6 | 0.84(0.57–1.20) | Adjusted for age at interview, state, parity, tubal ligation, and family history of ovarian cancer in a first-degree relative. |
| Chang ET, 2007 | Cohort | <84 | 97,275, 280 | United States | Retinol | 7 | 1.17(0.52–2.66) | Adjusted for race, total energy intake, parity, oral contraceptive use, strenuous exercise, wine consumption, and menopausal status/hormone therapy use; stratified by age at baseline. |
| Cramer DW, 2001 | PBCC | >50 | 1,065, 549 | United States | Vitamin A | 7 | 0.60(0.39–0.94) | Adjusted for total caloric intake, age, site, parity, body mass index, oral contraceptive use, family history of breast, ovarian or prostate cancer in a first-degree relative, tubal ligation, education and marital status. |
| Fairfield KM, 2001 | Cohort | 30–55 | 80,326, 301 | United States | Vitamin A | 7 | 0.86(0.60–1.23) | Adjusted for age, body mass index (kg/m2), duration of oral contraception use, smoking history, parity, history of tubal ligation, and caffeine intake. |
| Kushi LH, 1999 | Cohort | 55–69 | 29,083, 139 | United States | Vitamin A | 6 | 1.11(0.65–1.88) | Adjusted for age, total energy intake, number of live births, age at menopause, family history of ovarian cancer in a first-degree relative, hysterectomy/ unilateral oophorectomy status, waist-to-hip ratio, level of physical activity, cigarette smoking (number of pack-years), and educational level. |
| La Vecchia C, 1987 | HBCC | 22–74 | 1,840, 455 | Italy | Vitamin A | 7 | 0.94(0.72–1.22) | Adjusted for age (in cardinal form), interviewer, marital status, social class, education, parity, age at first birth, age at menarche, menopausal status, age at menopause, body mass index, and oral contraceptive and other female hormone use. |
| McCann SE, 2001 | HBCC | 20–87 | 1,921, 496 | United States | Vitamin A | 8 | 0.66(0.45–0.98) | Adjusted for age, education, region of residence, regularity of menstruation, family history of ovarian cancer, parity, age at menarche, oral contraceptive use, and total energy intake. |
| Risch HA, 1994 | PBCC | 35–79 | 1,014, 450 | Canada | Retinol | 7 | 1.00(0.92–1.09) | Adjusted for age at diagnosis/ interview and the continuous variables age, total daily calorie intake, number of full-term pregnancies, and total duration of oral contraceptive use. Each line in this table represents two individual models. |
| Salazar-Martinez E, 2002 | HBCC | 20–79 | 713, 84 | Mexico | Retinol | 8 | 0.52(0.28–0.95) | Adjusted for age, total energy intake, number of live births, recent changes in weight, physical activity and diabetes. |
| Silvera SA, 2006 | Cohort | 40–59 | 89,835, 264 | Canada | Vitamin A | 8 | 0.77(0.52–1.14) | Adjusted for age, menopausal status, use of oral contraceptives, body mass index, education, participation in vigorous physical activity, energy intake at baseline, study center, and randomization group. |
| Slattery ML, 1989 | PBCC | 20–79 | 577, 85 | United States | Vitamin A | 6 | 0.7(0.4–1.3) | Adjusted for age, body mass index of weight/height2, and number of pregnancies. All dietary variables are in separate logistic models. |
| Thomson CA, 2008 | Cohort | 50–79 | 133,614, 451 | United States | Vitamin A | 8 | 0.91(0.62–1.32) | Adjusted for age, log calories, No. breast/ovary cancer relatives, dietary modification randomization arm, hysterectomy status, minority race, pack-years smoking, physical activity, nonsteroidal anti-inflammatory drug use, parity, infertility, duration of oral contraceptive use, lifetime ovulatory cycles, partial oophorectomy, age at menopause, and HT usage at entry. |
| Tung KH, 2005 | PBCC | 45–75 | 1,165, 558 | United States | Vitamin A | 7 | 0.72(0.49–1.07) | Adjusted for age, ethnicity, study site, education, oral contraceptive pill use, pregnancy status, tubal ligation, and energy intake by polytomous logistic regression (histologic type) or unconditional logistic regression (all other variables) |
| Tzonou A, 1993 | HBCC | 18–75 | 389, 189 | Greece | Vitamin A | 7 | 0.87(0.73–1.03) | Adjusted for age, years of schooling, parity, age at first birth, menopausal status as well as for energy intake. |
| Zhang M, 2004 | HBCC | 18–75 | 906, 254 | China | Vitamin A | 8 | 0.41(0.24–0.69) | Adjusted for terms for age, locality, education, family income, BMI, total energy intake, tobacco smoking, alcohol consumption, ovarian cancer in first degree relatives, parity, menopausal status, and oral contraceptive use. |
Abbreviations: CI, confidence interval; HBCC, hospital-based case–control study; PBCC, population-based case–control study; RR, relative risk.
Dietary vitamin A intake and the risk of ovarian cancer
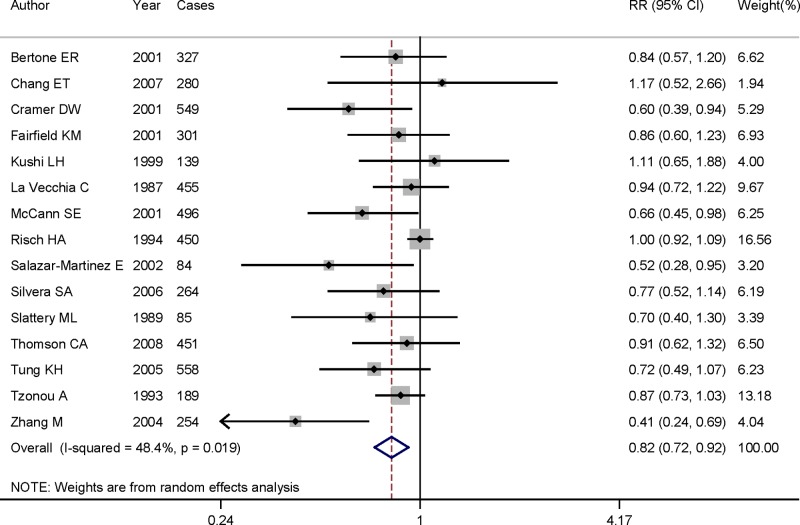
Pooled RR suggested that highest category of dietary vitamin A intake could significantly reduce the risk of ovarian cancer (RR = 0.816, 95%CI = 0.723–0.920, I2 = 48.4%, P for heterogeneity = 0.019) (Figure 2), when compared with the lowest category. In the included studies, five studies were cohort design and the remaining 10 studies were case–control design. Significant association was also found in case–control studies (RR = 0.769, 95%CI = 0.655–0.902), but not in cohort studies (RR = 0.895, 95%CI = 0.736–1.088). When we performed the subgroup analysis between ovarian cancer risk and geographic locations, we found an inverse association in North American populations (RR = 0.825, 95%CI = 0.720–0.946), instead of European populations (RR = 0.890, 95%CI = 0.771–1.028). We did not assess the association between dietary vitamin A intake and the risk of ovarian cancer while only one study was from Asian population. Table 2 shows the results for both whole and subgroup analyses.
Figure 2. The forest plot of the relationship between dietary vitamin A intake and ovarian cancer risk.
Table 2. Summary RR and 95%CI of the association between dietary vitamin A intake and ovarian cancer risk.
| Subgroups | Number of studies | Number of cases | RR(95% CI) | P for trend | Heterogeneity test | |
|---|---|---|---|---|---|---|
| I2 (%) | P | |||||
| Overall | 15 | 4882 | 0.816(0.723–0.920) | 0.001 | 48.4 | 0.019 |
| Study design | ||||||
| Cohort | 5 | 1435 | 0.895(0.736–1.088) | 0.264 | 0.0 | 0.797 |
| Case–control | 10 | 3447 | 0.769(0.655–0.902) | 0.001 | 64.6 | 0.003 |
| PBCC | 5 | 1969 | 0.812(0.651–0.994) | 0.037 | 55.3 | 0.062 |
| HBCC | 5 | 1478 | 0.712(0.551–0.922) | 0.010 | 64.8 | 0.023 |
| Geographic locations | ||||||
| North America | 12 | 3984 | 0.825(0.720-0.946) | 0.006 | 38.4 | 0.088 |
| Europe | 2 | 644 | 0.890(0.771–1.028) | 0.114 | 0.0 | 0.630 |
| Asia | 1 | 254 | – | – | – | – |
Abbreviations: CI, confidence interval; HBCC, hospital-based case–control studies; PBCC, population-based case–control studies; RR, relative risk.
Publication bias and sensitivity analysis
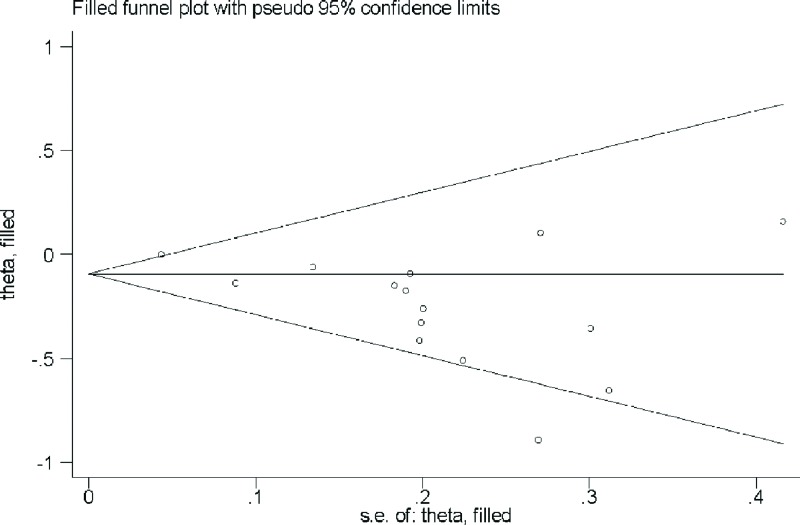
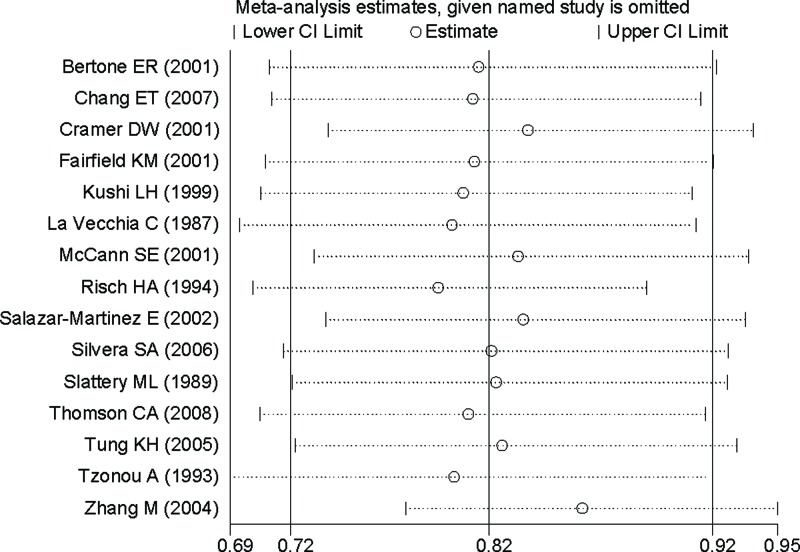
Based on Begg’s test (P = 0.318) and funnel plot (Figure 3), there existed no publication bias. Sensitivity analysis showed that no single study had a potential impact on the pooled RR (Figure 4).
Figure 3. Funnel plot for the analysis of publication bias between dietary vitamin A intake and ovarian cancer risk.
Figure 4. Sensitivity analyses between dietary vitamin A intake and ovarian cancer risk.
Discussion
Our findings based on 15 studies obtained that highest category of dietary vitamin A intake could significantly reduce the risk of ovarian cancer, when compared with the lowest category. We also found a significant association in case–control studies. An inverse association was found in North American populations, instead of other populations.
Our meta-analysis was different from the previous meta-analysis by Koushik et al. [6]. First, although the authors included 10 cohort studies, they said only 4 studies had previously published on vitamin consumption and ovarian cancer risk, in which only 3 studies were about vitamin A and ovarian cancer risk. However, we included 15 articles to explore the association between vitamin A intake and the risk of ovarian cancer. Second, the authors only included cohort studies, which may omit many observational studies. However, we included both cohort studies and case–control studies in our meta-analysis. Third, they concluded that consumption of vitamin A during adulthood does not play a major role in ovarian cancer risk. However, findings from our meta-analysis suggested that dietary vitamin A intake could reduce the risk of ovarian cancer. Interestingly, we obtained a consistent result in the cohort studies although we included 5 cohort studies, which is two more than Koushik et al.
Previous meta-analyses had been published to assess the intake of vitamin A and cancer risk. Huang et al. and Zhang et al. had explored the association between vitamin A intake and pancreatic cancer [27,28], they obtained a consistent result, which might inversely correlate with pancreatic cancer while with vitamin A intake. Yu et al. concluded that higher category of dietary vitamin A intake could reduce the risk of lung cancer [29]. Lv et al. also concluded an inverse association between vitamin A intake and glioma risk [30]. Moreover, Kong et al. found that dietary vitamin A had a significant reduction in the risk of gastric cancer [31]. Our results are all consistent with the previous meta-analyses.
We found significant between-study heterogeneity in the whole pooled results of dietary vitamin A intake and ovarian cancer risk. Between-study heterogeneity in the meta-analysis is common, and it is an essential component to explore the heterogeneity existed in the between-study. Meta-regression was used to explore the causes of heterogeneity for covariates of publication year, vitamins type, study design, geographic locations and number of cases. Results from meta-regression suggested that geographic locations (P = 0.021) was significantly associated with this high heterogeneity. When we performed the subgroup analysis by geographic locations, the I2 was reduced to 38.4% in North American populations and 0.0% in European populations. The result in subgroup of North American populations was consistent with the whole pooled result, while 12 of the 15 included studies were from North America.
However, several limitations should be attention. First, only English language articles were included, which may omit other languages studies. Furthermore, we only searched the articles which had been published in the journal, and did not be able including the meeting articles and some unpublished articles. However, we did not detect any publication bias, suggested our results were stable. Second, 10 of the 15 studies were case–control studies and only 5 were cohort studies. The selection bias, recall bias and some other confounding factors cannot be excluded; for example, some subjects may change their dietary vitamin A intake after the baseline assessment. However, case–control design was a very important epidemiological approach in the observational study. Therefore, it is requirement for evidence from prospective cohort studies. Third, 12 of the 15 studies were from North America, and the result was consistent with the whole pooled result. However, only 2 studies were from Europe and 1 study from Asia. We did not obtain an inverse association between dietary vitamin A intake and the risk of ovarian cancer in Europeans and Asians. Therefore, the result in the present study was more suitable for North America, but not for any populations else. Thus, more studies conducted in some other populations, instead of North Americans, are warranted to further explore the relationship between dietary vitamin A intake and the risk of ovarian cancer.
Conclusions
In summary, our results concluded that dietary intake of vitamin A may contribute to the lower development of ovarian cancer, especially among North Americans. As some limitations existed in our analysis, large scale studies with detailed amount of dietary vitamin A intake are needed to verify our results.
Abbreviations
- CI
confidence interval
- HBCC
hospital-based case–control study
- PBCC
population-based case–control study
- RR
relative risk
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This research is supported by Zhejiang Medical and Health Science and Technology Fund of China [grant number 2015119261].
Author Contribution
Q.Q.W. and C.Y.H. contributed to the conception and design of this research. Q.Q.W. and C.Y.H. contributed to the completion of articles, and the data extraction. Q.Q.W. calculated the data. Q.Q.W. write the manuscript. C.Y.H. reviewed and revised the manuscript. The entire author approved the final manuscript.
References
- 1.Siegel R.L., Miller K.D. and Jemal A. (2018) Cancer statistics, 2018. CA Cancer J. Clin. 68, 7–30 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Edmondson R.J. and Monaghan J.M. (2001) The epidemiology of ovarian cancer. Int. J. Gynecol. Cancer 11, 423–429 10.1046/j.1525-1438.2001.01053.x [DOI] [PubMed] [Google Scholar]
- 3.Riman T., Nilsson S. and Persson I.R. (2004) Review of epidemiological evidence for reproductive and hormonal factors in relation to the risk of epithelial ovarian malignancies. Acta. Obstet. Gynecol. Scand. 83, 783–795 10.1111/j.0001-6349.2004.00550.x [DOI] [PubMed] [Google Scholar]
- 4.Valko M., Rhodes C.J., Moncol J., Izakovic M. and Mazur M. (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160, 1–40 10.1016/j.cbi.2005.12.009 [DOI] [PubMed] [Google Scholar]
- 5.Krinsky N.I. and Johnson E.J. (2005) Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 26, 459–516 10.1016/j.mam.2005.10.001 [DOI] [PubMed] [Google Scholar]
- 6.Koushik A., Wang M., Anderson K.E., van den Brandt P., Clendenen T.V., Eliassen A.H. et al. (2015) Intake of vitamins A, C, and E and folate and the risk of ovarian cancer in a pooled analysis of 10 cohort studies. Cancer Causes Control. 26, 1315–1327 10.1007/s10552-015-0626-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stang A. (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 8.DerSimonian R. and Laird N. (1986) Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 9.Higgins J.P., Thompson S.G., Deeks J.J. and Altman D.G. (2003) Measuring inconsistency in meta-analyses. BMJ 327, 557–560 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins J.P. and Thompson S.G. (2004) Controlling the risk of spurious findings from meta-regression. Stat. Med. 23, 1663–1682 10.1002/sim.1752 [DOI] [PubMed] [Google Scholar]
- 11.Begg C.B. and Mazumdar M. (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 12.Bertone E.R., Hankinson S.E., Newcomb P.A., Rosner B., Willet W.C., Stampfer M.J. et al. (2001) A population-based case-control study of carotenoid and vitamin A intake and ovarian cancer (United States). Cancer Causes Control. 12, 83–90 10.1023/A:1008985015927 [DOI] [PubMed] [Google Scholar]
- 13.Chang E.T., Lee V.S., Canchola A.J., Clarke C.A., Purdie D.M., Reynolds P. et al. (2007) Diet and risk of ovarian cancer in the California Teachers Study cohort. Am. J. Epidemiol. 165, 802–813 10.1093/aje/kwk065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cramer D.W., Kuper H., Harlow B.L. and Titus-Ernstoff L. (2001) Carotenoids, antioxidants and ovarian cancer risk in pre- and postmenopausal women. Int. J. Cancer 94, 128–134 10.1002/ijc.1435 [DOI] [PubMed] [Google Scholar]
- 15.Fairfield K.M., Hankinson S.E., Rosner B.A., Hunter D.J., Colditz G.A. and Willett W.C. (2001) Risk of ovarian carcinoma and consumption of vitamins A, C, and E and specific carotenoids: a prospective analysis. Cancer 92, 2318–2326 [DOI] [PubMed] [Google Scholar]
- 16.Kushi L.H., Mink P.J., Folsom A.R., Anderson K.E., Zheng W., Lazovich D. et al. (1999) Prospective study of diet and ovarian cancer. Am. J. Epidemiol. 149, 21–31 10.1093/oxfordjournals.aje.a009723 [DOI] [PubMed] [Google Scholar]
- 17.La Vecchia C., Decarli A., Negri E., Parazzini F., Gentile A., Cecchetti G. et al. (1987) Dietary factors and the risk of epithelial ovarian cancer. J. Natl. Cancer Inst. 79, 663–669 [PubMed] [Google Scholar]
- 18.McCann S.E., Moysich K.B. and Mettlin C. (2001) Intakes of selected nutrients and food groups and risk of ovarian cancer. Nutr. Cancer 39, 19–28 10.1207/S15327914nc391_3 [DOI] [PubMed] [Google Scholar]
- 19.Risch H.A., Jain M., Marrett L.D. and Howe G.R. (1994) Dietary fat intake and risk of epithelial ovarian cancer. J. Natl. Cancer Inst. 86, 1409–1415 10.1093/jnci/86.18.1409 [DOI] [PubMed] [Google Scholar]
- 20.Salazar-Martinez E., Lazcano-Ponce E.C., Gonzalez Lira-Lira G., Escudero-De los Rios P. and Hernandez-Avila M. (2002) Nutritional determinants of epithelial ovarian cancer risk: a case-control study in Mexico. Oncology 63, 151–157 10.1159/000063814 [DOI] [PubMed] [Google Scholar]
- 21.Silvera S.A., Jain M., Howe G.R., Miller A.B. and Rohan T.E. (2006) Carotenoid, vitamin A, vitamin C, and vitamin E intake and risk of ovarian cancer: a prospective cohort study. Cancer Epidemiol. Biomarkers Prev. 15, 395–397 10.1158/1055-9965.EPI-05-0835 [DOI] [PubMed] [Google Scholar]
- 22.Slattery M.L., Schuman K.L., West D.W., French T.K. and Robison L.M. (1989) Nutrient intake and ovarian cancer. Am. J. Epidemiol. 130, 497–502 10.1093/oxfordjournals.aje.a115363 [DOI] [PubMed] [Google Scholar]
- 23.Thomson C.A., Neuhouser M.L., Shikany J.M., Caan B.J., Monk B.J., Mossavar-Rahmani Y. et al. (2008) The role of antioxidants and vitamin A in ovarian cancer: results from the Women's Health Initiative. Nutr. Cancer 60, 710–719 10.1080/01635580802233983 [DOI] [PubMed] [Google Scholar]
- 24.Tung K.H., Wilkens L.R., Wu A.H., McDuffie K., Hankin J.H., Nomura A.M. et al. (2005) Association of dietary vitamin A, carotenoids, and other antioxidants with the risk of ovarian cancer. Cancer Epidemiol. Biomarkers Prev. 14, 669–676 10.1158/1055-9965.EPI-04-0550 [DOI] [PubMed] [Google Scholar]
- 25.Tzonou A., Hsieh C.C., Polychronopoulou A., Kaprinis G., Toupadaki N., Trichopoulou A. et al. (1993) Diet and ovarian cancer: a case-control study in Greece. Int. J. Cancer 55, 411–414 10.1002/ijc.2910550314 [DOI] [PubMed] [Google Scholar]
- 26.Zhang M., Lee A.H. and Binns C.W. (2004) Reproductive and dietary risk factors for epithelial ovarian cancer in China. Gynecol. Oncol. 92, 320–326 10.1016/j.ygyno.2003.10.025 [DOI] [PubMed] [Google Scholar]
- 27.Huang X., Gao Y., Zhi X., Ta N., Jiang H. and Zheng J. (2016) Association between vitamin A, retinol and carotenoid intake and pancreatic cancer risk: Evidence from epidemiologic studies. Sci. Rep. 6, 38936 10.1038/srep38936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang T., Chen H., Qin S., Wang M., Wang X., Zhang X. et al. (2016) The association between dietary vitamin A intake and pancreatic cancer risk: a meta-analysis of 11 studies. Biosci Rep. 36, pii: e00414 10.1042/BSR20160341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu N., Su X., Wang Z., Dai B. and Kang J. (2015) Association of Dietary Vitamin A and beta-Carotene Intake with the Risk of Lung Cancer: A Meta-Analysis of 19 Publications. Nutrients 7, 9309–9324 10.3390/nu7115463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lv W., Zhong X., Xu L. and Han W. (2015) Association between Dietary Vitamin A Intake and the Risk of Glioma: Evidence from a Meta-analysis. Nutrients 7, 8897–8904 10.3390/nu7115438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kong P., Cai Q., Geng Q., Wang J., Lan Y., Zhan Y. et al. (2014) Vitamin intake reduce the risk of gastric cancer: meta-analysis and systematic review of randomized and observational studies. PLoS One 9, e116060 10.1371/journal.pone.0116060 [DOI] [PMC free article] [PubMed] [Google Scholar]