Abstract
Background
Resveratrol is an antiaging, antioxidant, anti‐inflammatory, and insulin‐sensitizing natural polyphenolic compound. Growing evidence indicates that resveratrol has potential therapeutic effects in infertile women with diminished ovarian function, polycystic ovary syndrome (PCOS), or endometriosis. However, only one clinical trial in women undergoing in vitro fertilization (IVF) cycles using resveratrol has ever been reported. This review focuses on the potential therapeutic effects of resveratrol on pregnancy and on its advantages and disadvantages in pregnancy outcomes during infertility treatment.
Methods
We performed a literature review to describe the known impacts of resveratrol on the ovary and endometrium.
Results
Resveratrol upregulates sirtuin (SIRT)1 expression in ovaries, which is associated with protection against oxidative stress. It leads to the activation of telomerase activity and mitochondrial function, improving ovarian function. In the endometrium, resveratrol downregulates the CRABP2‐RAR pathway leading to suppressing decidual and senescent changes of endometrial cells, which is essential for embryo implantation and placentation. Moreover, resveratrol may also induce deacetylation of important decidual‐related genes.
Conclusions
Resveratrol has potential therapeutic effects for improving ovarian function; however, it also has anti‐deciduogenic actions in uterine endometrium. In addition, its teratogenicity has not yet been ruled out; thus, resveratrol should be avoided during the luteal phase and pregnancy.
Keywords: aging, assisted reproductive technology, infertility, resveratrol, sirtuin
Resveratrol, an antiaging natural polyphenolic compound, has therapeutic effects for improving ovarian function, but it also has anti‐deciduogenic actions in endometrium.
1. INTRODUCTION
Resveratrol (trans‐3, 5, 40‐trihydroxystilbene) is a natural polyphenolic compound, detected in a variety of plants, foods, and drinks, such as grapes, nuts, cranberries, and red wine.1 Resveratrol has beneficial effects on human health, including antiaging, antioxidant, anti‐inflammatory, insulin‐sensitizing, cardioprotective, vasodilating, and anti‐neoplastic properties2 (Figure 1). Therefore, resveratrol intake can improve metabolic diseases, such as obesity, diabetes mellitus, and hypertension, and reduce the risk of cardiovascular diseases and malignant neoplasm.3 Growing evidence indicates that resveratrol has potential therapeutic effects in women with diminished ovarian function, polycystic ovary syndrome (PCOS), endometriosis, or uterine fibroids.4, 5, 6, 7 In addition, resveratrol may improve testicular function and sperm quality.8, 9 Therefore, resveratrol supplementation may help to treat both male and female infertility based on animal studies. However, in humans, only a single clinical trial on resveratrol for women undergoing in vitro fertilization (IVF) cycles has been carried out.10 Our review discusses the potential therapeutic effects of resveratrol on pregnancy and its advantages and disadvantages for pregnancy outcomes during infertility treatment.
Figure 1.
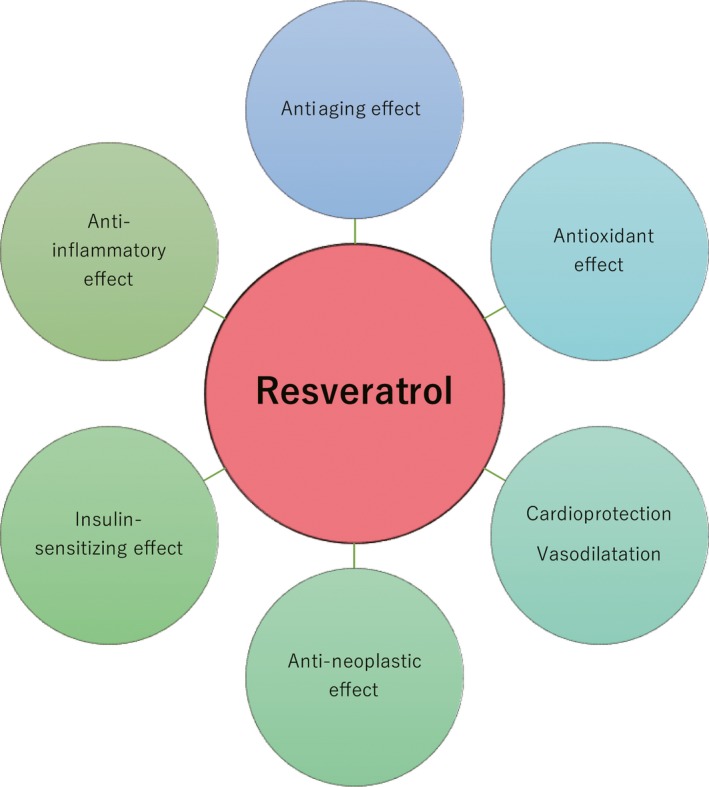
Effects of resveratrol on human health. Resveratrol has beneficial effects on human health, including antiaging, antioxidant, anti‐inflammatory, insulin‐sensitizing, cardioprotective, vasodilating, and anti‐neoplastic properties
2. IMPACT ON OVARY FOR PREGNANCY
2.1. Aging
Aging is a major detrimental factor for achieving pregnancy11, 12, 13 due to ovarian aging leading to mitochondrial dysfunction, telomere shortening, cohesion dysfunctions, and spindle instability. Both genetic and environmental factors contribute to aging damage, but the major mechanism underlying ovarian deterioration is chronic damage by reactive oxygen species (ROS).11, 14, 15, 16 Reactive oxygen species can damage mitochondria DNA, promoting mutations, and induce telomere shortening and cellular senescence.17 Resveratrol is a natural activator of sirtuin, the NAD+‐dependent deacetylase. Sirtuins are emerging molecules in aging diseases.18 In mice oocytes, sirtuin (SIRT)1 is upregulated in response to oxidative stress, whereas a SIRT1 inhibitor increases intracellular ROS.19 SIRT1 may protect mitochondria against oxidative stress (Figure 2). However, aged oocytes have undetectable SIRT1 expression levels and low ability to regulate SIRT1.19, 20 Therefore, aged oocytes may be susceptible to the effects of oxidative stress through their decreased ability to produce SIRT1. Resveratrol may compensate for the decreased SIRT1 expression in aged oocytes, leading to the inhibition of age‐associated ovarian aging changes. In rats, resveratrol intake increased the number of follicles and had ovary life‐extending effects.4 Moreover, resveratrol improved the number of follicle in aged mice ovaries.21 Therefore, resveratrol may protect the ovarian reserve against aging via SIRT1 activation, resulting in prolonged ovarian life span.
Figure 2.
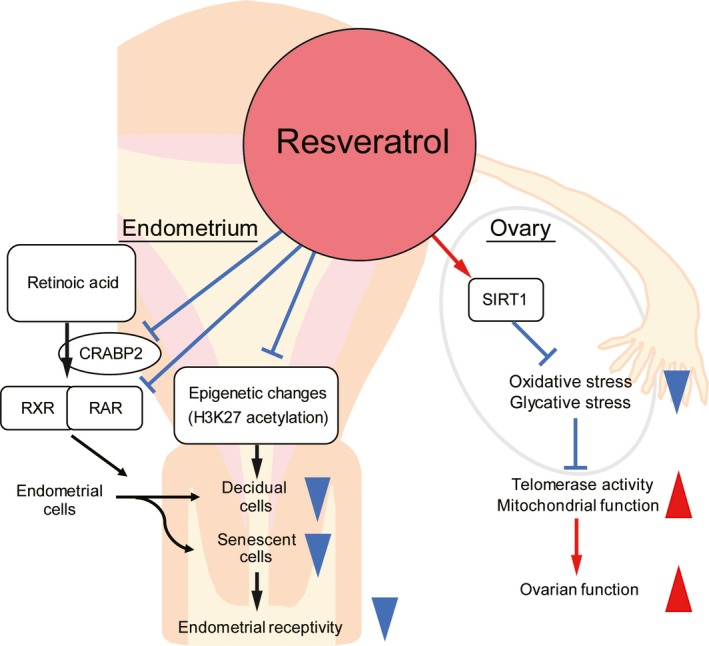
Effects of resveratrol on ovaries and endometrium. Resveratrol upregulates ovarian SIRT1 expression, which is associated with protection against oxidative stress and glycation stress. Moreover, it activates telomerase activity and mitochondrial function, improving ovarian function. In the decidualized endometrium, resveratrol accelerates downregulation of CRABP2‐RAR pathway, inhibiting decidual senescence and decidualization. In addition, resveratrol may also induce deacetylation of important decidual‐related genes. Up‐ and down‐arrowheads: promotive and inhibitive actions
2.2. Primary ovarian insufficiency (POI)
Primary ovarian insufficiency is an ovarian dysfunction with amenorrhea and sex steroid deficiency in women younger than 40 years.22 It can be caused by genetic abnormalities and ovarian damage due to chemotherapy, radiotherapy, or surgery. However, in most cases, the cause of premature depletion of primordial follicles is unknown.23 During oocyte maturation and folliculogenesis, the phosphatidylinositol‐4,5‐bisphosphate 3 kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) and nuclear factor‐κ light‐chain enhancer of activated B cell (NF‐κB) signaling pathway play roles in the development of primordial follicles and oocytes and in the proliferation and differentiation of granulosa cells.24, 25 In a rat POI model induced by chemotherapy or radiotherapy, resveratrol inhibited oxidative stress and inflammatory events in ovaries by activating the PI3K/Akt/mTOR and NF‐κB signaling pathways.26, 27, 28 Resveratrol also improved loss of the oogonial stem cells through antiapoptotic effects in POI model mice.29 Therefore, resveratrol may help as a therapeutic POI supplement; however, this has not yet been proven in human studies.
2.3. PCOS and obesity‐related infertility
Polycystic ovary syndrome is characterized by enlarged polycystic ovaries with a hyperplastic theca compartment and clinical and/or biochemical signs of hyperandrogenism, resulting in ovulation disorders.30 In rat studies, resveratrol has antiproliferative effects on thecal interstitial cells via inhibition of the mevalonate pathway, involved in cholesterol production and steroidogenesis.31 Moreover, resveratrol can also suppress cellular expression of the Cyp17α1 (17α‐hydroxylase) that catalyzes various reactions, including androgen production.32, 33 In a rat model of PCOS, resveratrol intake improved the increased number of secondary and atretic follicles and the reduced number of Graafian follicles through antioxidant and anti‐inflammatory effects, reducing aberrant elevated levels of testosterone, luteinizing hormone, and anti‐Müllerian hormones.34, 35
Advanced glycation end products (AGEs) are generated by the reaction between reducing sugars and proteins, lipids, or nucleic acids, and their accumulation in tissues has been involved in the pathogenesis of various diseases, including diabetes mellitus and PCOS.36 Methylglyoxal is the most powerful precursor of AGEs. SIRT1 is associated with the response to methylglyoxal‐dependent glycation stress, and it may have a positive effect on the ovarian function in PCOS by interfering with AGEs.37
In an obese mouse model study on IVF, resveratrol supplementation increased the number of oocytes collected after ovarian hyperstimulation via anti‐inflammatory, insulin‐sensitizing, and anti‐hyperandrogenism effects.38
According to animal experiments, resveratrol is a candidate novel treatment against PCOS. In humans, a randomized double‐blind clinical trial in women with PCOS showed that high‐dose resveratrol administration (1.5 g per day) significantly decreased the levels of total testosterone, dehydroepiandrosterone sulfate, and fasting insulin and increased the insulin sensitivity index.39 However, the fertility outcome following the treatment of resveratrol in women with PCOS has not been evaluated. Further clinical trials are warranted.
2.4. In vitro maturation (IVM) and IVF
Resveratrol has a therapeutic direct effect on oocytes in in vitro culture. Treating the culture media with resveratrol improves oocyte maturation and the developmental competence of embryos to blastocysts in both animals and humans.40, 41, 42, 43, 44 Moreover, during IVF treatments, minimization of the time‐dependent deterioration after ovulation (postovulatory oocyte aging) is a key for successful pregnancies. Resveratrol treatment can protect against postovulatory oocyte aging in vivo in middle‐aged mice.45 This effect is attributed to the intracellular ROS level reduction and mitochondrial function improvement by resveratrol via SIRT1 activation. The quality and quantity of mitochondria are associated with the balance of mitochondrial biogenesis and autophagy. Resveratrol has effects on mitochondrial biogenesis as well as autophagy in the process of oocyte development, leading to remaining homeostasis in oocytes and granulosa cells through the clearance of damaged mitochondria.44 Resveratrol also protects postovulatory human granulosa cells from apoptosis by activating the ERK pathway associated with the follicular antiapoptotic effect and by suppressing inflammatory functions of NF‐κB signaling.46
Taken together, the addition of resveratrol to in vitro culture media may have local beneficial impacts on human oocytes, leading to improved oocyte maturation and developmental competence of embryos. However, data from human studies are still limited.
3. IMPACT ON ENDOMETRIUM FOR PREGNANCY
3.1. Effects on human endometrium in vitro
Successful pregnancy requires endometrial receptivity with optimal decidualization and synchronization with developmentally competent embryos.47 Decidualization consists in cellular morphological changes of the human endometrial stromal cells (HESCs) accompanied by integrated gene expression alterations, such as those of prolactin (PRL) and insulin‐like growth factor‐binding protein‐1 (IGFBP‐1), generating an implantation window.47, 48 During decidualization, the endometrium is receptive for implantation and maintenance of pregnancy, resists oxidative stress, and promotes immune tolerance by modulating a local inflammatory reaction to allow trophoblast invasion. By contrast, impaired decidualization causes a variety of pre‐ and post‐pregnancy disorders, such as implantation failure, pregnancy loss, and uteroplacental dysfunction.47, 49, 50, 51
The evidence for the effects of resveratrol on the human endometrium is still limited. Resveratrol promotes calcium‐dependent, cell adhesion–related gene E‐cadherin expression via increased expression of SIRT1 in Ishikawa cells, which means it can induce embryo attachment to the endometrium.52 Also, resveratrol has antiapoptosis and antiproliferative effects, and it can inhibit progression of ectopic endometrium (endometriosis).6 These reports suggest beneficial therapeutic effects of resveratrol on infertility with endometriosis.
However, implantation requires an inflammatory reaction with local secretion of proinflammatory cytokines and prostaglandins from the decidualized endometrium.53, 54, 55 Resveratrol has anti‐inflammatory actions that may suppress embryo implantation directly. The decidualization of endometrial stromal cells does not entail only cellular differentiation; the alteration requires a combination of differentiation and apoptosis/senescence.56, 57, 58 In fact, decidual cells secrete proapoptotic factors during the decidualization of HESCs.58 In in vitro primary cultures, the decidualization of HESCs induces senescence‐associated β‐galactosidase (SAβG) activity and increases expressions of major senescent markers, including p16 and p53.56, 57 Decidual cells secrete inflammatory mediators associated with endometrial receptivity, through acute cellular senescence. In addition, pro‐senescent decidual cells are cleared by uterine natural killer (uNK) cells (the most abundant immune cells in the decidualized endometrium) for remodeling and rejuvenating the environment.57 A suppressor of cellular senescence, the mTOR inhibitor rapamycin inhibits SAβG‐positive cells, expression of senescent markers, and decidualization, leading to reduced expression of decidual marker genes, PRL and IGFBP1.57 Resveratrol as an anti‐senescence agent also has potential adverse effects on the decidua of humans and may impair implantation and pregnancy.
Decidual changes of HESCs depend on orchestrated reprogramming of various pathways, including that of retinoic acid (RA) signaling.47, 59 Retinoic acid regulates two opposing cell fates, differentiation and apoptosis, by binding to cellular RA‐binding proteins, CRABP2 or FABP5, and getting activated by the nuclear receptors (retinoic acid receptor, RAR) to promote the activation of genes involved in apoptotic machinery and cell cycle arrest, or by peroxisome the proliferator‐activated receptor (PPAR) β/δ to promote cell differentiation. Decidualization of HESCs downregulates RA signaling via a decrease in cytoplasmic binding proteins, CRABP2 and FABP5. Moreover, RA‐binding receptors, RAR and PPARβ/δ, are induced and suppressed by decidualization, respectively. Therefore, decidualization of HESCs requires an appropriate suppression of the proapoptotic CRABP2‐RAR signaling pathway.59 SIRT1 is an important modulator of RA signaling, and it interacts with and deacetylates CRABP260 and also inhibits the transcriptional activity of RAR.61 Our previous study demonstrated that resveratrol treatment accelerates downregulation of the CRABP2‐RAR pathway in decidualized HESCs, leading to decreasing SAβG activity and expression of p53.56 In addition, decidual markers were inhibited in decidual cells treated with resveratrol 56 (Figure 2). Impaired decidualization causes implantation failure and pregnancy loss.49
Moreover, decidualization of HESCs is associated with epigenetic changes, including H3K27 acetylation of promoter regions in decidual markers PRL and IGFBP1.62, 63, 64, 65, 66 SIRT1 has histone deacetylation effects on decidual‐related genes (PRL, IGFBP1, p53, and FOXO‐family).60, 67, 68, 69, 70 Thus, resveratrol supplementation may inhibit decidual senescence and induce deacetylation of important decidual‐related genes, leading to decreasing endometrial receptivity, in the clinical practice (Figure 2).
3.2. Clinical study on IVF treatment with resveratrol supplementation
Resveratrol has potential therapeutic effects in women with diminished ovarian reserve and function through its suppression of oxidative stress and its stimulation of mitochondrial biogenesis, but it also has adverse effects on implantation and endometrial decidualization. Is resveratrol “a friend or a foe” for pregnancy?
We evaluated the impact of resveratrol on human pregnancy outcomes during IVF‐embryo transfer (ET) cycles for the first time and found poor outcomes.10 The retrospective cross‐sectional study was carried out to compare the pregnancy outcomes after ET cycles between women with infertility using resveratrol (200 mg per day) continuously (RES group) and women in a control group not using any (non‐RES group). Our multivariate logistic regression analysis demonstrated that resveratrol intake was significantly associated with low clinical pregnancy rates (post‐adjusted odds ratio [OR] 0.539, 95% confidence interval [CI] 0.341‐0.853) and high miscarriage rates (OR 2.602, 95% CI 1.070‐6.325). In agreement with the effects of the senescence suppressor on decidualization in primary cultures, resveratrol intake may adversely impact pregnancy outcomes following ET cycles. In our clinical study, the patients in the RES group had poor pregnancy outcomes after ET even though embryos with good quality were transferred. However, in some patients with impaired ovarian reserve, resveratrol supplementation may improve oocyte quality and quantity leading to the collection of competent embryos. Our study focused on pregnancy outcomes after ET, and we had no data on ovarian function before and after resveratrol intake. Resveratrol treatment has been shown to protect against ovarian aging and to improve PCOS and endometriosis;4, 5, 6 therefore, resveratrol intake may have benefits for some patients.
Based on these data, when using resveratrol during infertility treatments, if the tissue level of resveratrol vanishes in the endometrium before decidualization, it may not adversely affect implantation or pregnancy. In humans, the half‐life of resveratrol is only 9‐10 hours.71, 72 We tested this hypothesis on decidualized primary HESCs with or without treatment with 100 μmol/L of resveratrol or pre‐treating cells with resveratrol for 48 hours followed by wash‐off and decidualization without resveratrol treatment (Figure 3); our results showed that resveratrol treatment suppressed PRL and IGFBP1 expression, but the pre‐treatment had no impact on the induction of decidual markers.56 Thus, in the clinical practice, discontinuation of resveratrol intake at the beginning of the luteal phase (the day of ovulation) or cryopreservation of all embryo (freeze‐all policy) and warmed ET without supplementation (Figure 4) should help overcome these adverse effects. In all, a randomized controlled trial is needed to evaluate the use of resveratrol as an infertility treatment.
Figure 3.
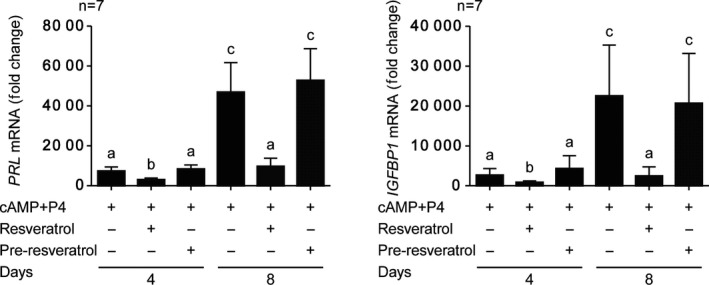
Pre‐treatment with resveratrol does not inhibit decidual marker genes in the decidualized endometrium. Real‐time quantitative PCR analysis of decidual markers PRL and IGFBP1 in human endometrial stromal cells (treated with 8‐bromoadenosine‐cAMP and progesterone (P4) with or without 100 μmol/L of resveratrol for 4 or 8 days and pre‐treatment of the cells with resveratrol for 48 h followed by wash‐off and decidualization without resveratrol treatment) showing fold changes (mean ± SEM) in PRL and IGFBP1 transcript levels relative to negative control (from four independent primary cultures). Different letters above the error bars suggest groups that are significantly different from each other at P < .05. This is a modified graph from our previous reports (Ochiai et al 56)
Figure 4.
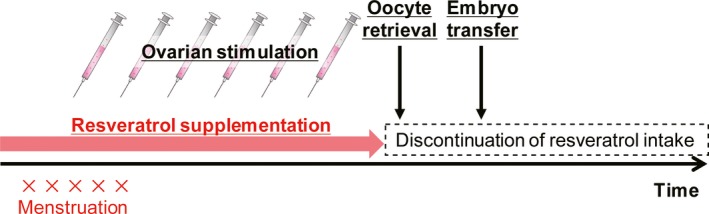
Recommendations for resveratrol supplementation in IVF treatment. We recommend discontinuation of resveratrol intake at the beginning of the luteal phase (the day of ovulation) or cryopreservation of all embryos (freeze‐all policy) and vitrified and warmed ET without supplementation
4. POSSIBLE SIDE EFFECTS
Resveratrol does not appear to produce severe side effects at doses <1.0 g/day in various in vivo and in vitro studies with a wide range of resveratrol doses, whereas doses ≥1.0 g may produce side effects, including headache, dizziness, nausea, diarrhea, and liver dysfunction.73, 74, 75, 76 In a phase 2 clinical study, resveratrol intake at doses of 5.0 g in patients with refractory multiple myeloma caused severe renal failure in 5 of 24 patients before the study was stopped.77 However, other clinical studies in patients with colorectal cancer and healthy volunteers did not show any nephrotoxicity at the same dose of resveratrol.78, 79 The renal failure may have been caused by the progression of multiple myeloma, but as a precaution, high‐dose resveratrol should not be administered to infertile women. Although little is known about the maternal and fetal effects of resveratrol, its supplementation should be discontinued during pregnancy, based on the adverse decidualization effects in the human endometrium.
5. CONCLUSIONS AND FUTURE PERSPECTIVES
Women with advanced age face difficulties in getting pregnant due to decreased quality and quantities in their oocytes. Resveratrol has potential therapeutic effects for improving ovarian function; however, it also has anti‐deciduogenic actions. Moreover, its teratogenicity has not yet been ruled out; therefore, resveratrol should be avoided during the luteal phase and pregnancy. Further clinical studies are needed to establish optimal doses and periods of resveratrol intake while preventing adverse effects on implantation, subsequent pregnancy, and the fetus.
CONFLICT OF INTEREST
All authors declare having no conflicts of interest.
DISCLOSURES
Human/Animal rights: This article does not contain any studies with human and animal subjects performed by any of the authors.
ACKNOWLEDGEMENTS
We thank the people at the Laboratory of Molecular and Biochemical Research, Research Support Center, Juntendo University Graduate School of Medicine, Tokyo, Japan, for their technical assistance. Keiji Kuroda was supported by JSPS KAKENHI Grant Number 18K09273.
Ochiai A, Kuroda K. Preconception resveratrol intake against infertility: Friend or foe? Reprod Med Biol. 2020;19:107–113. 10.1002/rmb2.12303
REFERENCES
- 1. Hasan M, Bae H. An overview of stress‐induced resveratrol synthesis in grapes: perspectives for resveratrol‐enriched grape products. Molecules. 2017;22:E294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Neves AR, Lucio M, Lima JL, Reis S. Resveratrol in medicinal chemistry: a critical review of its pharmacokinetics, drug‐delivery, and membrane interactions. Curr Med Chem. 2012;19:1663‐1681. [DOI] [PubMed] [Google Scholar]
- 3. Singh AP, Singh R, Verma SS, et al. Health benefits of resveratrol: evidence from clinical studies. Med Res Rev. 2019;39:1851‐1891. [DOI] [PubMed] [Google Scholar]
- 4. Ortega I, Duleba AJ. Ovarian actions of resveratrol. Ann N Y Acad Sci. 2015;1348:86‐96. [DOI] [PubMed] [Google Scholar]
- 5. Aquino CI, Nori SL. Complementary therapy in polycystic ovary syndrome. Transl Med UniSa. 2014;9:56‐65. [PMC free article] [PubMed] [Google Scholar]
- 6. Kolahdouz Mohammadi R, Arablou T. Resveratrol and endometriosis: in vitro and animal studies and underlying mechanisms (Review). Biomed Pharmacother. 2017;91:220‐228. [DOI] [PubMed] [Google Scholar]
- 7. Ho Y, Sh Yang YC, Chin YT, et al. Resveratrol inhibits human leiomyoma cell proliferation via crosstalk between integrin alphavbeta3 and IGF‐1R. Food Chem Toxicol. 2018;120:346‐355. [DOI] [PubMed] [Google Scholar]
- 8. Garcez ME, dos Santos BC, Lara LV, Pasqualotto FF, Salvador M. Effects of resveratrol supplementation on cryopreservation medium of human semen. Fertil Steril. 2010;94:2118‐2121. [DOI] [PubMed] [Google Scholar]
- 9. Branco CS, Garcez ME, Pasqualotto FF, Erdtman B, Salvador M. Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology. 2010;60:235‐237. [DOI] [PubMed] [Google Scholar]
- 10. Ochiai A, Kuroda K, Ikemoto Y, et al. Influence of resveratrol supplementation on IVF‐embryo transfer cycle outcomes. Reprod Biomed Online. 2019;39(2):205‐210. [DOI] [PubMed] [Google Scholar]
- 11. Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L. Impact of maternal age on oocyte and embryo competence. Front Endocrinol (Lausanne). 2018;9:327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crawford NM, Steiner AZ. Age‐related infertility. Obstet Gynecol Clin North Am. 2015;42:15‐25. [DOI] [PubMed] [Google Scholar]
- 13. Liu K, Case A, Reproductive Endocrinology and Infertility Committee . Advanced reproductive age and fertility. J Obstet Gynaecol Can. 2011;33:1165‐1175. [DOI] [PubMed] [Google Scholar]
- 14. Keefe DL, Marquard K, Liu L. The telomere theory of reproductive senescence in women. Curr Opin Obstet Gynecol. 2006;18:280‐285. [DOI] [PubMed] [Google Scholar]
- 15. Seidler EA, Moley KH. Metabolic determinants of mitochondrial function in oocytes. Semin Reprod Med. 2015;33:396‐400. [DOI] [PubMed] [Google Scholar]
- 16. Hamatani T, Falco G, Carter MG, et al. Age‐associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet. 2004;13:2263‐2278. [DOI] [PubMed] [Google Scholar]
- 17. Passos JF, Saretzki G, von Zglinicki T. DNA damage in telomeres and mitochondria during cellular senescence: is there a connection? Nucleic Acids Res. 2007;35:7505‐7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morris BJ. Seven sirtuins for seven deadly diseases of aging. Free Radic Biol Med. 2013;56:133‐171. [DOI] [PubMed] [Google Scholar]
- 19. Di Emidio G, Falone S, Vitti M, et al. SIRT1 signalling protects mouse oocytes against oxidative stress and is deregulated during aging. Hum Reprod. 2014;29:2006‐2017. [DOI] [PubMed] [Google Scholar]
- 20. Ma R, Zhang Y, Zhang L, Han J, Rui R. Sirt1 protects pig oocyte against in vitro aging. Anim Sci J. 2015;86:826‐832. [DOI] [PubMed] [Google Scholar]
- 21. Liu M, Yin Y, Ye X, et al. Resveratrol protects against age‐associated infertility in mice. Hum Reprod. 2013;28:707‐717. [DOI] [PubMed] [Google Scholar]
- 22. Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604‐606. [PubMed] [Google Scholar]
- 23. Rebar RW, Connolly HV. Clinical features of young women with hypergonadotropic amenorrhea. Fertil Steril. 1990;53:804‐810. [PubMed] [Google Scholar]
- 24. Sobinoff AP, Sutherland JM, McLaughlin EA. Intracellular signalling during female gametogenesis. Mol Hum Reprod. 2013;19:265‐278. [DOI] [PubMed] [Google Scholar]
- 25. Paciolla M, Boni R, Fusco F, et al. Nuclear factor‐kappa‐B‐inhibitor alpha (NFKBIA) is a developmental marker of NF‐kappaB/p65 activation during in vitro oocyte maturation and early embryogenesis. Hum Reprod. 2011;26:1191‐1201. [DOI] [PubMed] [Google Scholar]
- 26. Li N, Liu L. Mechanism of resveratrol in improving ovarian function in a rat model of premature ovarian insufficiency. J Obstet Gynaecol Res. 2018;44:1431‐1438. [DOI] [PubMed] [Google Scholar]
- 27. Said RS, El‐Demerdash E, Nada AS, Kamal MM. Resveratrol inhibits inflammatory signaling implicated in ionizing radiation‐induced premature ovarian failure through antagonistic crosstalk between silencing information regulator 1 (SIRT1) and poly(ADP‐ribose) polymerase 1 (PARP‐1). Biochem Pharmacol. 2016;103:140‐150. [DOI] [PubMed] [Google Scholar]
- 28. Said RS, Mantawy EM, El‐Demerdash E. Mechanistic perspective of protective effects of resveratrol against cisplatin‐induced ovarian injury in rats: emphasis on anti‐inflammatory and anti‐apoptotic effects. Naunyn Schmiedebergs Arch Pharmacol. 2019;392:1225‐1238. [DOI] [PubMed] [Google Scholar]
- 29. Wu M, Ma L, Xue L, et al. Resveratrol alleviates chemotherapy‐induced oogonial stem cell apoptosis and ovarian aging in mice. Aging (Albany N Y). 2019;11:1030‐1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nandi A, Chen Z, Patel R, Poretsky L. Polycystic ovary syndrome. Endocrinol Metab Clin North Am. 2014;43:123‐147. [DOI] [PubMed] [Google Scholar]
- 31. Wong DH, Villanueva JA, Cress AB, Sokalska A, Ortega I, Duleba AJ. Resveratrol inhibits the mevalonate pathway and potentiates the antiproliferative effects of simvastatin in rat theca‐interstitial cells. Fertil Steril. 2011;96:1252‐1258. [DOI] [PubMed] [Google Scholar]
- 32. Ortega I, Villanueva JA, Wong DH, et al. Resveratrol potentiates effects of simvastatin on inhibition of rat ovarian theca‐interstitial cells steroidogenesis. J Ovarian Res. 2014;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ortega I, Villanueva JA, Wong DH, et al. Resveratrol reduces steroidogenesis in rat ovarian theca‐interstitial cells: the role of inhibition of Akt/PKB signaling pathway. Endocrinology. 2012;153:4019‐4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Furat Rencber S, Kurnaz Ozbek S, Eraldemir C, et al. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: an experimental study. J Ovarian Res. 2018;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ergenoglu M, Yildirim N, Yildirim AG, et al. Effects of Resveratrol on ovarian morphology, plasma anti‐mullerian hormone, IGF‐1 levels, and oxidative stress parameters in a rat model of polycystic ovary syndrome. Reprod Sci. 2015;22:942‐947. [DOI] [PubMed] [Google Scholar]
- 36. Rutkowska AZ, Diamanti‐Kandarakis E. Do advanced glycation end products (AGEs) contribute to the comorbidities of polycystic ovary syndrome (PCOS)? Curr Pharm Des. 2016;22:5558‐5571. [DOI] [PubMed] [Google Scholar]
- 37. Di Emidio G, Santini SJ, D'Alessandro AM, et al. SIRT1 participates in the response to methylglyoxal‐dependent glycative stress in mouse oocytes and ovary. Biochim Biophys Acta Mol Basis Dis. 2019;1865:1389‐1401. [DOI] [PubMed] [Google Scholar]
- 38. Cabello E, Garrido P, Moran J, et al. Effects of resveratrol on ovarian response to controlled ovarian hyperstimulation in ob/ob mice. Fertil Steril. 2015;103:570.e1‐579.e1. [DOI] [PubMed] [Google Scholar]
- 39. Banaszewska B, Wrotynska‐Barczynska J, Spaczynski RZ, Pawelczyk L, Duleba AJ. Effects of resveratrol on polycystic ovary syndrome: a double‐blind, randomized, placebo‐controlled trial. J Clin Endocrinol Metab. 2016;101:4322‐4328. [DOI] [PubMed] [Google Scholar]
- 40. Liu MJ, Sun AG, Zhao SG, et al. Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil Steril. 2018;109:900‐907. [DOI] [PubMed] [Google Scholar]
- 41. Zabihi A, Shabankareh HK, Hajarian H, Foroutanifar S. Resveratrol addition to in vitro maturation and in vitro culture media enhances developmental competence of sheep embryos. Domest Anim Endocrinol. 2019;68:25‐31. [DOI] [PubMed] [Google Scholar]
- 42. Itami N, Shirasuna K, Kuwayama T, Iwata H. Resveratrol improves the quality of pig oocytes derived from early antral follicles through sirtuin 1 activation. Theriogenology. 2015;83:1360‐1367. [DOI] [PubMed] [Google Scholar]
- 43. Santos E, Appeltant R, Dang‐Nguyen TQ, et al. The effect of resveratrol on the developmental competence of porcine oocytes vitrified at germinal vesicle stage. Reprod Domest Anim. 2018;53:304‐312. [DOI] [PubMed] [Google Scholar]
- 44. Sugiyama M, Kawahara‐Miki R, Kawana H, Shirasuna K, Kuwayama T, Iwata H. Resveratrol‐induced mitochondrial synthesis and autophagy in oocytes derived from early antral follicles of aged cows. J Reprod Dev. 2015;61:251‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liang QX, Lin YH, Zhang CH, et al. Resveratrol increases resistance of mouse oocytes to postovulatory aging in vivo. Aging (Albany N Y). 2018;10:1586‐1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Han Y, Luo H, Wang H, Cai J, Zhang Y. SIRT1 induces resistance to apoptosis in human granulosa cells by activating the ERK pathway and inhibiting NF‐kappaB signaling with anti‐inflammatory functions. Apoptosis. 2017;22:1260‐1272. [DOI] [PubMed] [Google Scholar]
- 47. Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev. 2014;35:851‐905. [DOI] [PubMed] [Google Scholar]
- 48. Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology. 1999;140:4809‐4820. [DOI] [PubMed] [Google Scholar]
- 49. Kuroda K. Impaired endometrial function and unexplained recurrent pregnancy loss. Hypertens Res Pregnancy. 2019;7:16‐21. [Google Scholar]
- 50. Salker M, Teklenburg G, Molokhia M, et al. Natural selection of human embryos: impaired decidualization of endometrium disables embryo‐maternal interactions and causes recurrent pregnancy loss. PLoS ONE. 2010;5:e10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weimar C, Kavelaars A, Brosens JJ, et al. Endometrial stromal cells of women with recurrent miscarriage fail to discriminate between high‐and low‐quality human embryos. PLoS ONE. 2012;7:e41424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shirane A, Wada‐Hiraike O, Tanikawa M, et al. Regulation of SIRT1 determines initial step of endometrial receptivity by controlling E‐cadherin expression. Biochem Biophys Res Commun. 2012;424:604‐610. [DOI] [PubMed] [Google Scholar]
- 53. Chard T. Cytokines in implantation. Hum Reprod Update. 1995;1:385‐396. [DOI] [PubMed] [Google Scholar]
- 54. Sharkey A. Cytokines and implantation. Rev Reprod. 1998;3:52‐61. [DOI] [PubMed] [Google Scholar]
- 55. Kelly RW, King AE, Critchley H. Cytokine control in human endometrium. Reproduction. 2001;121:3‐19. [DOI] [PubMed] [Google Scholar]
- 56. Ochiai A, Kuroda K, Ozaki R, et al. Resveratrol inhibits decidualization by accelerating downregulation of the CRABP2‐RAR pathway in differentiating human endometrial stromal cells. Cell Death Dis. 2019;10:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Brighton PJ, Maruyama Y, Fishwick K, et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. Elife. 2017;6:e31274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leno‐Duran E, Ruiz‐Magana MJ, Munoz‐Fernandez R, Requena F, Olivares EG, Ruiz‐Ruiz C. Human decidual stromal cells secrete soluble pro‐apoptotic factors during decidualization in a cAMP‐dependent manner. Hum Reprod. 2014;29:2269‐2277. [DOI] [PubMed] [Google Scholar]
- 59. Ozaki R, Kuroda K, Ikemoto Y, et al. Reprogramming of the retinoic acid pathway in decidualizing human endometrial stromal cells. PLoS ONE. 2017;12:e0173035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tang S, Huang G, Fan W, et al. SIRT1‐mediated deacetylation of CRABPII regulates cellular retinoic acid signaling and modulates embryonic stem cell differentiation. Mol Cell. 2014;55:843‐855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kang MR, Lee SW, Um E, et al. Reciprocal roles of SIRT1 and SKIP in the regulation of RAR activity: implication in the retinoic acid‐induced neuronal differentiation of P19 cells. Nucleic Acids Res. 2010;38:822‐831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Grimaldi G, Christian M, Steel JH, Henriet P, Poutanen M, Brosens JJ. Down‐regulation of the histone methyltransferase EZH2 contributes to the epigenetic programming of decidualizing human endometrial stromal cells. Mol Endocrinol. 2011;25:1892‐1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tamura I, Jozaki K, Sato S, et al. The distal upstream region of insulin‐like growth factor‐binding protein‐1 enhances its expression in endometrial stromal cells during decidualization. J Biol Chem. 2018;293:5270‐5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tamura I, Sato S, Okada M, et al. Importance of C/EBPbeta binding and histone acetylation status in the promoter regions for induction of IGFBP‐1, PRL, and Mn‐SOD by cAMP in human endometrial stromal cells. Endocrinology. 2014;155:275‐286. [DOI] [PubMed] [Google Scholar]
- 65. Tamura I, Asada H, Maekawa R, et al. Induction of IGFBP‐1 expression by cAMP is associated with histone acetylation status of the promoter region in human endometrial stromal cells. Endocrinology. 2012;153:5612‐5621. [DOI] [PubMed] [Google Scholar]
- 66. Katoh N, Kuroda K, Tomikawa J, et al. Reciprocal changes of H3K27ac and H3K27 me3 at the promoter regions of the critical genes for endometrial decidualization. Epigenomics. 2018;10:1243‐1257. [DOI] [PubMed] [Google Scholar]
- 67. Sin TK, Yung BY, Siu PM. Modulation of SIRT1‐Foxo1 signaling axis by resveratrol: implications in skeletal muscle aging and insulin resistance. Cell Physiol Biochem. 2015;35:541‐552. [DOI] [PubMed] [Google Scholar]
- 68. Kim HN, Han L, Iyer S, et al. Sirtuin1 suppresses osteoclastogenesis by deacetylating FoxOs. Mol Endocrinol. 2015;29:1498‐1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Feige JN, Auwerx J. Transcriptional targets of sirtuins in the coordination of mammalian physiology. Curr Opin Cell Biol. 2008;20:303‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vaziri H, Dessain SK, Ng Eaton E, et al. hSIR2(SIRT1) functions as an NAD‐dependent p53 deacetylase. Cell. 2001;107:149‐159. [DOI] [PubMed] [Google Scholar]
- 71. Goldberg DM, Yan J, Soleas GJ. Absorption of three wine‐related polyphenols in three different matrices by healthy subjects. Clin Biochem. 2003;36:79‐87. [DOI] [PubMed] [Google Scholar]
- 72. Gescher AJ, Steward WP. Relationship between mechanisms, bioavailibility, and preclinical chemopreventive efficacy of resveratrol: a conundrum. Cancer Epidemiol Biomarkers Prev. 2003;12:953‐957. [PubMed] [Google Scholar]
- 73. Brown VA, Patel KR, Viskaduraki M, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin‐like growth factor axis. Cancer Res. 2010;70:9003‐9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Almeida L, Vaz‐da‐Silva M, Falcao A, et al. Pharmacokinetic and safety profile of trans‐resveratrol in a rising multiple‐dose study in healthy volunteers. Mol Nutr Food Res. 2009;53(Suppl 1):S7‐S15. [DOI] [PubMed] [Google Scholar]
- 75. Chow HH, Garland LL, Heckman‐Stoddard BM, et al. A pilot clinical study of resveratrol in postmenopausal women with high body mass index: effects on systemic sex steroid hormones. J Transl Med. 2014;12:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Goh KP, Lee HY, Lau DP, Supaat W, Chan YH, Koh AF. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int J Sport Nutr Exerc Metab. 2014;24:2‐13. [DOI] [PubMed] [Google Scholar]
- 77. Popat R, Plesner T, Davies F, et al. A phase 2 study of SRT501 (resveratrol) with bortezomib for patients with relapsed and or refractory multiple myeloma. Br J Haematol. 2013;160:714‐717. [DOI] [PubMed] [Google Scholar]
- 78. Howells LM, Berry DP, Elliott PJ, et al. Phase I randomized, double‐blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases–safety, pharmacokinetics, and pharmacodynamics. Cancer Prev Res (Phila). 2011;4:1419‐1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pignitter M, Schueller K, Burkon A, et al. Concentration‐dependent effects of resveratrol and metabolites on the redox status of human erythrocytes in single‐dose studies. J Nutr Biochem. 2016;27:164‐170. [DOI] [PubMed] [Google Scholar]


