Abstract
Purpose
To evaluate the outcomes of embryo transfer (ET) and to identify the parameters influencing pregnancy outcomes.
Methods
This study included 938 ET cycles involving single frozen and thawed good‐quality blastocyst (Gardner grade ≥3BB) between August 2017 and January 2018. The significance of several parameters including endometrial thickness, position of the transferred air bubble, self‐evaluation score by physicians, and uterus direction at ET as predictors of clinical pregnancy was evaluated using univariate and multivariate analyses.
Results
Among 938 ET cycles, 462 (49.3%) resulted in a clinical pregnancy. Endometrial thickness was positively associated with clinical pregnancy in a linear trend. Between the variable position of the transferred air bubble and clinical pregnancy rate showed a curvilinear relationship. Clinical pregnancy rate was higher in cases with good self‐evaluation score, whereas there was no difference between groups with different uterus directions. Univariate analysis of predictive parameters identified endometrial thickness, self‐evaluation score by physicians, and position of air bubbles as significant predictors of clinical pregnancy, of which endometrial thickness and position of air bubbles appeared to be independently related to clinical pregnancy.
Conclusion
Endometrial thickness and the position of transferred air bubbles influenced clinical pregnancy in ET cycles.
Keywords: assisted reproductive technique, clinical pregnancy rate, endometrial thickness, frozen‐thawed embryo transfer, predictive factors
1. INTRODUCTION
Embryo transfer (ET) is one of the most important parts of assisted reproductive technology (ART), directly influencing the cumulative pregnancy rate (PR). Various factors are known to affect the outcomes of ET, including embryo quality, 1 , 2 female age, 1 endometrial thickness, 3 and the technique of the transfer procedure. 4
Among these factors, higher female age is the most frequently mentioned factor known to be negatively associated with ART outcomes. 5 , 6 However, current techniques of preimplantation genetic testing revealed that a chromosomally normal embryo eliminates the impact of advancing female age on implantation rate, 7 indicating that the importance of age is mainly related to embryo formation and not to the ET procedure.
Endometrial thickness is a second frequently mentioned factor during ET, which can be measured by transvaginal ultrasound and has been evaluated as a possible predictor of pregnancy in multiple studies with conflicting results. 8 Some investigators have reported endometrial thickness as a significant factor influencing PR, 9 , 10 whereas other studies could not confirm such a relationship. 11 , 12 Therefore, there is no solid consensus on the clinical significance of endometrial thickness as predicting factor for ET outcome.
The technique for ET is influenced by physicians, which directly affects the overall outcome of ART. Therefore, there has been considerable interest in improving several aspects of ET. The use of transabdominal ultrasonographic guidance during the transfer procedure is widespread, which offers the opportunity to visualize the transfer catheter, transferred air bubble, and aspects of the endometrium, resulting in better ET outcomes. 13 , 14 The use of transvaginal ultrasound has been reported to improve PR compared with transabdominal ultrasound, because of its clear view and real‐time monitoring during the procedure. 4 Real‐time monitoring during ET makes it possible to place the transferred air bubble indicating the position of the embryo as intended. Coroleu et al 13 recommended that the tip of the catheter should be positioned 15‐20 mm from the fundus of the uterine cavity to avoid placement of the embryo too close to the fundus. Similarly, a number of studies analyzed the relationship between catheter position and PR and found that catheter position influences PR. 15 , 16 Moreover, Lambers et al 17 reported that positioning of the air bubble closer to the fundus resulted in better ET outcomes. These objective indexes can help to standardize the ET procedure and contribute to minimize the discrepancies in ET outcomes among physicians.
For such reasons, it is important for physicians to know which factors and how much they affect ET outcomes during the ET procedure. This study was designed to evaluate the importance of variables in ET and to identify the factors influencing PR.
2. MATERIALS AND METHODS
This was a retrospective study that included 938 cases of frozen ET (FET) cycles performed by ten expert reproductive endocrinologists using same procedure at our institution between August 2017 and January 2018. In order to exclude the influence of embryo quality, analyses were limited to FET with a single good‐quality blastocyst. Blastocysts were graded based on Gardner's classification, 18 and blastocysts grade of 3BB or better at day 5 was considered as good quality. The vitrification/warming protocol was performed according to the method described previously. 19 Briefly, embryos were first incubated in equilibration solution at room temperature. After having observed cellular shrinkage, embryos were aspirated and placed on the tip of the Cryotop® (Kitazato) and directly contacted in liquid nitrogen. On the day of FET, the embryos were exposed to thawing solution at 37.0℃ temperature and transferred into dilution solution. Additionally, in order to exclude the influence of the maternal factor, five patients with congenital uterine anomalies were excluded from this study. Patients with endometrial polyp or leiomyoma require surgical excision, and inappropriate endometrial flora or huge hydrosalpinx were treated properly before FET. This study was approved by our Institutional Ethical Committee in accordance with ethical principles that have their origin in the Declaration of Helsinki. All patients were well informed, and written informed consent was obtained prior to the treatment period before FET.
Frozen ET was performed with either a hormone replacement cycle or natural cycle protocol. Hormone replacement cycle FET was performed after ovarian suppression using a similar method as reported previously. 20 Briefly, transdermal estradiol (E2) patches (Estrana® TAPE 0.72 mg; Hisamitsu Pharmaceutical Co.) were started with three patches every other day from day 2 of the menstrual cycle. And oral E2 administration (Progynova® 2 mg; Bayer Holding Ltd.) was started with two tablets every day from day 2 of the menstrual cycle. Transvaginal ultrasound and E2 follow‐up were performed after 13‐15 days. Transvaginal progesterone (P) supplementation was then started if the endometrial thickness was >8 mm and E2 level was at least 200 pg/mL. Five days after the initiation of P supplementation, frozen embryos were thawed and used for FET. In cases of patients who underwent the natural cycle protocol, transdermal E2 patches and vaginal P were administered after ovulation and FET was performed at 5 days after ovulation.
Frozen ET was performed using a transvaginal ultrasound‐guided method. 4 The patients were positioned in the lithotomy position, and transvaginal ultrasound was performed to explore uterine direction and endometrial thickness. Then, the cervix was exposed using a bivalve speculum and swabbed with cotton after irrigation. The hard outer catheter (Kitazato ET catheter ET‐G3017ART‐30®, Kitazato) was inserted blindly until the stopper touched the cervix. The position of the stopper was arranged in accordance with the uterine length to place the tip of the outer catheter at 2‐3 cm from the fundal myometrium. After the transvaginal ultrasound probe was reinserted, the inner stylet was removed. An embryologist first aspirated 10 μl medium, then about the same amount of air, 10 μl of medium and the embryo, and finally air into the inner soft catheter (Kitazato ET catheter ET‐TUC3040SM5‐17®, Kitazato). The inner catheter was inserted into the outer sheath. Then, the embryo was slowly released into the uterine cavity, and the distance between the uterine fundus and the transferred air bubble was measured.
The self‐evaluation rating was categorized into three levels (A: excellent, B: moderate, and C: poor), based on the impression of the physician who performed FET; this comprised the smoothness of the procedure, the absence of pain or bleeding, avoiding the use of hard uterine forceps, and whether the position of the air bubble was as planned. Based on the previous reports, the targeted air bubble position was set between 5 and 15 mm from the fundus. 21 , 22 , 23
The primary outcome of this study was clinical pregnancy. Serum human chorionic gonadotropin was evaluated 10 days after FET. Pregnancy was monitored using transvaginal ultrasound at 5 weeks gestational age, and detection of a fetal sac in uterus was defined as clinical pregnancy.
Statistical analysis of clinical PR associated with clinical parameters was evaluated using scatter plots and chi‐square test. A clinical threshold was calculated using a receiver operator characteristic curve. Univariate and multivariate logistic regression analyses were conducted to identify the regulators of clinical pregnancy from several parameters. All statistical analyses were performed with EXCEL (2016, Microsoft) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for statistical Computing). 24 P‐values <.05 were considered to be significant in this study.
3. RESULTS
The characteristics of 938 FET cycles are presented in Table 1. Among 938 FET cycles, 462 (49.3%) resulted in a clinical pregnancy. Endometrial thickness at the time of FET was > 8.0 mm in most cases (mean 10.8 mm, range 5.5‐21.8 mm). The number of hormone replacement cycle FET (751 cases) was more prevalent than natural cycle FET (187 cases), due to the ease of scheduling. The position of the air bubble was mostly within 5.0‐10.0 mm from fundus endometrium. Similarly, the self‐evaluation rating was mostly A (88.1%), and ratings B and C were rarely given, indicating that most FET was performed as planned. There were 655 cases (69.8%) of anteflexion uterine direction, which was similar to the normal population.
Table 1.
Characteristics of 938 patients who underwent frozen‐thawed embryo transfer
| Mean ± SD | Range | |
|---|---|---|
| Age (y) | 36.6 ± 4.3 | 25‐52 |
| Endometrial thickness (mm) | 10.8 ± 5.3 | 5.5‐21.8 |
| Position of transferred air bubble (mm) | 7.3 ± 3.2 | 0‐14.7 |
| Self‐evaluation (cases) | ||
| A | 827 | ‐ |
| B | 107 | ‐ |
| C | 4 | ‐ |
| Uterus direction (cases) | ||
| Anteflexion | 655 | ‐ |
| Retroflexion | 227 | |
| Straight | 56 | |
Figure 1A shows the relationship between the clinical PR and endometrial thickness by scatter plot, showing a positive correlation with a linear trend. A receiver operator characteristic curve was constructed to assess the effectiveness of predicting clinical pregnancy. A clinically acceptable threshold was calculated when sensitivity plus specificity was at its maximum, and this correlated with 10.0 mm (Figure 1B).
Figure 1.
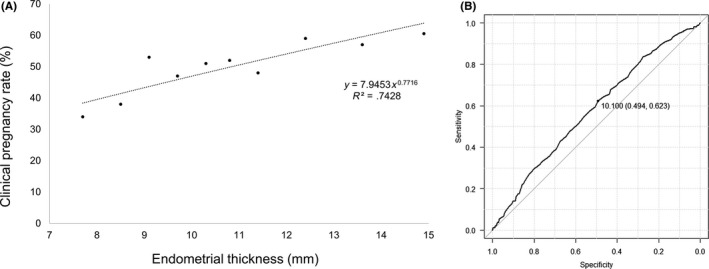
A, Scatter plot and linear correction for clinical pregnancy rates and endometrial thickness showing a positive correlation and a linear trend. B, Receiver operator characteristic curve constructed for the assessment of endometrial thickness and clinical pregnancy by frozen‐thaw embryo transfer. A clinical threshold was calculated when sensitivity plus specificity reached maximum
Figure 2 shows the relationship between the position of the transferred air bubble and clinical PR, which showed a curvilinear relationship. The clinical PR increased as the air bubble position got closer to 6 mm apart from uterine fundus, remained steady until 10 mm, and began decreasing after 10 mm.
Figure 2.
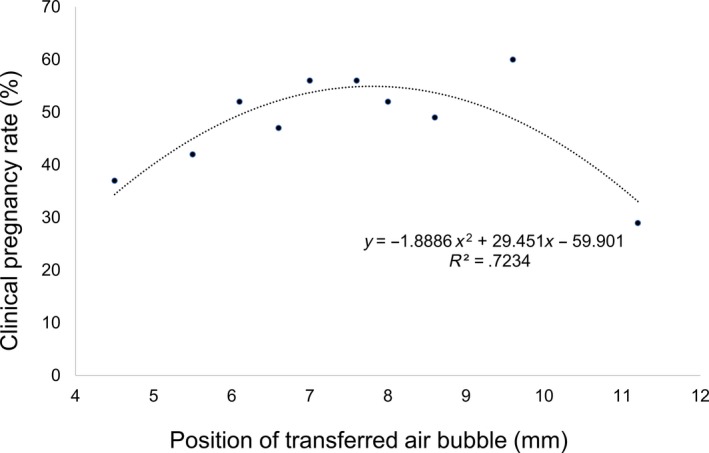
Scatter plot and non‐linear correction describing a curvilinear relationship between the variable position of the transferred air bubble and clinical pregnancy. This graph indicates that the clinical pregnancy rate increased as the air bubble position got closer to 6 mm apart from uterine fundus, remained steady until 10 mm, and began decreasing after 10 mm
The clinical PR was significantly higher in cases with a self‐evaluation rating of A, compared with ratings of B or C (50.2% vs 42.1%, P < .05; Figure 3). Compering the clinical PR based on uterus direction, there was no difference between the anteflexion, retroflexion, and straight groups (59.5%, 47.6%, and 42.9%, respectively, P = .45; Figure 4).
Figure 3.
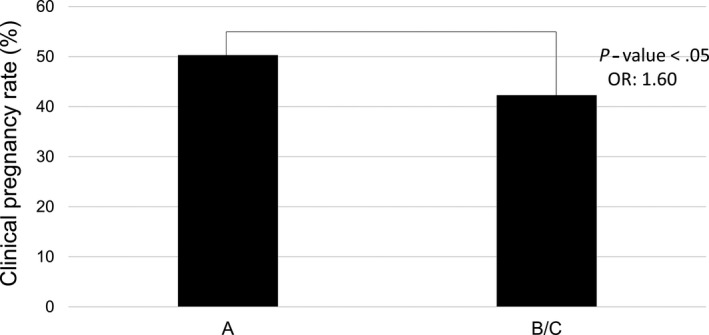
Association between clinical pregnancy rate and self‐assessment relating “A” or “B/C”
Figure 4.
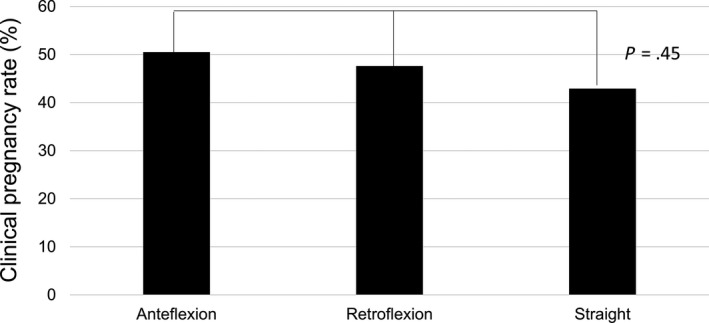
Association between pregnancy rate and uterus direction. Statistical differences are analyzed by chi‐square test of 3 × 2 contingency table
Univariate and multivariate regression analyses were subsequently conducted in order to identify variables related to clinical pregnancy. As shown in Table 2, the univariate analysis identified endometrial thickness, self‐evaluation, and position of the transferred air bubble as significant predictors of clinical pregnancy. The clinical PR increased with patients who had an endometrial thickness ≥10 mm (P < .05, OR: 1.61, 95% CI: 1.20‐2.09), and position of the transferred air bubble was within 6‐10 mm (P < .05, OR: 1.42, 95% CI: 1.08‐1.86) and good self‐evaluation rating group (P < .05, OR: 1.42, 95% CI: 1.08‐1.86). Among these three variables, endometrial thickness and the position of the transferred air bubble appeared to be independently associated with clinical pregnancy in the multivariate analysis.
Table 2.
Univariate and multivariate analyses of several parameters as regulators of clinical pregnancy
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P‐value | Odds ratio | 95% CI | P‐value | |
| Age | 1.01 | 0.99‐1.04 | .33 | ‐ | ‐ | ‐ |
| Endometrial thickness (>10 mm) | 1.61 | 1.24‐2.09 | <.05 | 1.56 | 1.20‐2.03 | <.05 |
| Uterus direction (anteflexion) | 1.17 | 0.89‐1.53 | .28 | ‐ | ‐ | ‐ |
| Self‐evaluation (A) | 1.60 | 1.09‐2.35 | <.05 | 1.35 | 0.95‐2.00 | .13 |
| Position of transferred air bubble (5‐10 mm) | 1.42 | 1.08‐1.86 | <.05 | 1.34 | 1.02‐1.77 | <.05 |
| Hormone replacement cycle or natural cycle | 1.31 | 0.95‐1.31 | .10 | ‐ | ‐ | ‐ |
4. DISCUSSION
In the present study, we attempted to evaluate the outcomes of ET and to identify the parameters influencing pregnancy outcomes. Among variables examined, endometrial thickness and the position of the air bubble were identified as the independent predictors influencing the clinical PR in multivariate analyses.
The relationship between endometrial thickness and PR has been evaluated in multiple studies, but with conflict results. Some investigators have reported endometrial thickness as a significant factor influencing PR, 9 , 10 whereas other studies reported that the influence was only marginal. 11 , 12 However, most studies confirmed that the PR is better in women with a thicker endometrium than those with a thinner endometrium to a greater or lesser extent. The exact mechanisms of how endometrial thickness affects IVF outcome are still unclear, but it has been speculated that high oxygen concentration in the endometrium and protein expression profiles may play a critical role in endometrial receptivity. 25 , 26 Kasius et al reported that although endometrial thickness cannot be used to predict IVF outcome in terms of the occurrence of pregnancy, it does seem to be a factor for the assessment of the probability of conceiving IVF. Indeed, the probability of pregnancy was significantly lower in the group with thin endometrial thickness (endometrial thickness <7 mm: OR 0.42 [95% CI: 0.27‐0.67] P = .0003). 8 Other studies also revealed a positive association between endometrial thickness and clinical pregnancy with different cutoffs. These reports indicated that the probability of pregnancy improves from low cutoff to high cutoff (<6 mm: OR 0.46 [95% CI: 0.20‐1.05], <8 mm: OR 0.56 [95% CI: 0.44‐0.70], <10 mm: OR 0.68 [95% CI: 0.61‐0.76]). 10 , 27 , 28 , 29 The positive correlation between endometrial thickness and clinical pregnancy was also confirmed in the present study, and multivariate analysis confirmed endometrial thickness as the predictive factor independently related to clinical pregnancy after adjusting for confounding variables. In the present study, we used the cutoff level as 10 mm for statistical analysis. Although statistical significance was identified by using this cutoff, the clinical cutoff for canceling ET should be determined more carefully because there is no established treatment for thickening the endometrium. Carolyn et al 30 mentioned that no pregnancies occurred when the endometrial thickness was <6 mm. Conversely, Sundstrom et al 31 reported that a successful pregnancy was induced with IVF despite an endometrial thickness of <4 mm. In the present study, two pregnancies occurred with endometrium of <6 mm out of 8 ET (PR = 25%). These findings indicated that although there is a possibility for pregnancy, the probability was lower with a thin endometrium. Therefore, physicians should pay attention to endometrial thickness when deciding the schedule of ET and proper countermeasures, including canceling ET, should be taken.
Another significant variable was the position of the transferred air bubble, which is often regarded as an indicator for the position of the transferred embryo. It was shown that 94.1% of transferred air bubbles showed no movement even after standing up 32 and that 81% of the embryos that implanted successfully did so in the area where they were initially transferred. 15 Nowadays, many physicians perform ET using ultrasonographic guidance. One of the main advantages is that physicians can recognize the tip of the catheter and the position of the transferred air bubble in real time. In general, the position of the transferred air bubble should be placed a little apart from the fundus. For example, Pope et al 22 reported that PR was higher when the catheter was placed >5.0 mm from the fundus. Similarly, Coroleu et al 21 reported that catheter placement at 1.5‐2.0 cm from the fundus was superior to cases with catheter placement 1.0 cm from the fundus. In the present study, the clinical PR increased as the air bubble position got closer to 6 mm apart from uterine fundus, remained steady until 10 mm, and began decreasing after 10 mm. This indicated that the optimal position was little nearer to the fundus than reported previously. This may be due to the size of the uterus in Japanese women, which is generally considered to be slightly smaller than in the Western population. A recent study also assessed embryo flash position and migration at 1.5 and 60 minutes after ET using 3D ultrasound. 33 They found that the PR and implantation rate in cases with embryo flashes located <15 mm from the fundus at 60 minutes was significantly higher than those with embryo flashes located >15 mm from the fundus. The embryo flash movement from the fundus toward the cervix was also related to significantly lower PR and implantation rate. Fıçıcıoğlu et al 23 evaluated the effect of embryo flash position and movement of the air bubbles at 1 and 60 minutes after ET on PR. They reported that PR was associated with embryo flash movement and migration, and the PR was dramatically reduced when the embryo migrated from its original position toward the cervix at 60 minutes. These findings indicated that kinetic evaluation and the position of air bubbles are important after ET. The movement of the air bubble might be related to undesirable uterine contractions, which can be induced by inadequate stimulation during ET. 34 Further well‐designed studies including the evaluation of kinetic evaluation of the transferred air bubble and uterine contractions are required to optimize the ET procedure.
In the present study, the clinical PR was higher in cases with a good self‐evaluation rating by physicians. However, in the multivariate analysis, the self‐evaluation score was not considered to be a significant predictor influencing the PR. It was speculated that the position of the transferred air bubble should be the main confounding factor that excludes the self‐evaluation rating from the independent predictive factor. Indeed, self‐evaluation rating was low in cases where the position of the air bubble was placed in an unexpected position. This indicated that even though the physician was not satisfied with the procedure through taking a long time for the procedure or using extra‐hard catheter or using uterine forceps, ET was considered successful when the position of the air bubble was an optimal position.
In the present study, the patient's age was not considered to correlate with PR, which was the most frequently mentioned factor known to be negatively associated with PR. 5 , 6 The disagreement of the results can be explained by the embryo quality, which was limited to good‐quality blastocysts (Gardner grade ≥3BB) in this present study. Therefore, the importance of age in ART seemed to be limited to blastocyst formation and not related to the result after ET. Similarly, in the present study, uterus direction was not related to PR, which was considered to relate to PR and miscarriage several decades ago. However, in a recent study, it was considered not to be related to PR, because the ultrasound guidance during ET can minimize the difference due to uterus variation. 35
In the present study, PR was not different between hormone replacement cycle and natural cycle. This result is similar to that of other researchers already reported. For example, Givens et al reported that there was no significant difference in delivery rates for FET in natural vs hormone replacement cycles using both own embryos and donor egg–derived embryos. 35 Similarly, Groenewound et al reported that no differences in the clinical pregnancy rate, ongoing pregnancy rate or live birth rate could be found between the two methods in FET. 35
In this section, we would like to emphasize the limitations of this study. Firstly, although patients with severe complication such as congenital uterine anomalies were excluded from the present study, patients with slight complication such as leiomyoma or hydrosalpinx without surgical indication and adenomyosis were included. Therefore, we could not completely exclude the influence of these factors. Secondly, this study only included Asian populations, mostly Japanese, who have been shown to have different characteristics and different responses to several medications compared with Western populations; therefore, it would be difficult to apply the present findings to the entire cohort of patients receiving ART. Lastly, this study was a non‐randomized, retrospective study, with a limited sample size. Therefore, a larger study would be needed to optimize ET strategy.
In conclusion, endometrial thickness and the position of the air bubble were identified as the independent predictors influencing the clinical PR in FET cycles. We should pay attention to endometrial thickness when deciding the schedule of ET and proper countermeasures, including canceling ET, should be taken. We recommend that the transferred air bubbles, that means a position of the transferred embryo, should be placed between 6 to 10 mm from the fundus.
DISCLOSURES
Conflict of interest: Nao Hayashi, Noritoshi Enatsu, Toshiro Iwasaki, Junko Otsuki, Yukiko Matsumoto, Shoji Kokeguchi, Masahide Shiotani declare that they have no conflict of interest. Human rights statement and informed consent: All patients were well informed, and written informed consent was obtained prior to the treatment period. The treatment investigated in this study has already been discussed in other studies that have shown positive outcomes. Animal studies: This article contains no studies with animal subjects performed by any of the authors. Approval by Ethics Committee: This study was approved by Ethical Committee of Hanabusa Women's Clinic consists of members chosen by our institute and third party medical institute (approval number; 2019‐08).
ACKNOWLEDGMENTS
The authors would like to thank the doctors and laboratory staff at Hanabusa Women's clinic who helped to collect data.
Hayashi N, Enatsu N, Iwasaki T, et al. Predictive factors influencing pregnancy rate in frozen embryo transfer. Reprod Med Biol. 2020;19:182–188. 10.1002/rmb2.12322
REFERENCES
- 1. Roseboom T, Vermeiden J, Schoute E, Lens J, Schats R. The probability of pregnancy after embryo transfer is affected by the age of the patient, cause of infertility, number of embryos transferred and the average morphology score, as revealed by multiple logistic regression analysis. Hum Reprod. 1995;10(11):3035‐3041. [DOI] [PubMed] [Google Scholar]
- 2. Strandell A, Bergh C, Lundin K. Selection of patients suitable for one‐embryo transfer may reduce the rate of multiple births by half without impairment of overall birth rates. Hum Reprod. 2000;15(12):2520‐2525. [DOI] [PubMed] [Google Scholar]
- 3. El‐Toukhy T, Coomarasamy A, Khairy M, et al. The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil Steril. 2008;89(4):832‐839. [DOI] [PubMed] [Google Scholar]
- 4. Kojima K, Nomiyama M, Kumamoto T, Matsumoto Y, Iwasaka T. Transvaginal ultrasound‐guided embryo transfer improves pregnancy and implantation rates after IVF. Hum Reprod. 2001;16(12):2578‐2582. [DOI] [PubMed] [Google Scholar]
- 5. Traub ML, Van Arsdale A, Pal L, Jindal S, Santoro N. Endometrial thickness, caucasian ethnicity, and age predict clinical pregnancy following fresh blastocyst embryo transfer: a retrospective cohort. Reprod Biol Endocrino. 2009;7(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lintsen AME, Eijkemans MJC, Hunault CC, et al. Predicting ongoing pregnancy chances after IVF and ICSI: a national prospective study. Hum Reprod. 2007;22(9):2455‐2462. [DOI] [PubMed] [Google Scholar]
- 7. Munné S, Grifo J, Wells D. Mosaicism:“survival of the fittest” versus “no embryo left behind”. Fertil Steril. 2016;105(5):1146‐1149. [DOI] [PubMed] [Google Scholar]
- 8. Kasius A, Smit JG, Torrance HL, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta‐analysis. Hum Reprod Update. 2014;20(4):530‐541. [DOI] [PubMed] [Google Scholar]
- 9. Al‐Ghamdi A, Coskun S, Al‐Hassan S, Al‐Rejjal R, Awartani K. The correlation between endometrial thickness and outcome of in vitro fertilization and embryo transfer (IVF‐ET) outcome. Reprod Biol Endocrino. 2008;6(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen S‐L, Wu F‐R, Luo C, et al. Combined analysis of endometrial thickness and pattern in predicting outcome of in vitro fertilization and embryo transfer: a retrospective cohort study. Reprod Biol Endocrino. 2010;8(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Geyter C, Schmitter M, De Geyter M, Nieschlag E, Holzgreve W, Schneider HP. Prospective evaluation of the ultrasound appearance of the endometrium in a cohort of 1186 infertile women. Fertil Steril. 2000;73(1):106‐113. [DOI] [PubMed] [Google Scholar]
- 12. Rashidi BH, Sadeghi M, Jafarabadi M, Nejad EST. Relationships between pregnancy rates following in vitro fertilization or intracytoplasmic sperm injection and endometrial thickness and pattern. Eur J Obstet Gynecol Reprod Biol. 2005;120(2):179‐184. [DOI] [PubMed] [Google Scholar]
- 13. Coroleu B, Carreras O, Veiga A, et al. Embryo transfer under ultrasound guidance improves pregnancy rates after in‐vitro fertilization. Hum Reprod. 2000;15(3):616‐620. [DOI] [PubMed] [Google Scholar]
- 14. Matorras R, Urquijo E, Mendoza R, Corcostegui B, Exposito A, Rodriguez‐Escudero F. Ultrasound‐guided embryo transfer improves pregnancy rates and increases the frequency of easy transfers. Hum Reprod. 2002;17(7):1762‐1766. [DOI] [PubMed] [Google Scholar]
- 15. Baba K, Ishihara O, Hayashi N, Saitoh M, Taya J, Kinoshita K. Where does the embryo implant after embryo transfer in humans? Fertil Steril. 2000;73(1):123‐125. [DOI] [PubMed] [Google Scholar]
- 16. Krampl E, Zegermacher G, Eichler C, Obruca A, Strohmer H, Feichtinger W. Air in the uterine cavity after embryo transfer. Fertil Steril. 1995;63(2):366‐370. [DOI] [PubMed] [Google Scholar]
- 17. Lambers MJ, Dogan E, Lens JW, Schats R, Hompes PG. The position of transferred air bubbles after embryo transfer is related to pregnancy rate. Fertil Steril. 2007;88(1):68‐73. [DOI] [PubMed] [Google Scholar]
- 18. Gardner DK, Schoolcraft WB. Culture and transfer of human blastocysts. Curr Opin Obstet Gynecol. 1999;11(3):307‐311. [DOI] [PubMed] [Google Scholar]
- 19. Mukaida T, Oka C. Vitrification of oocytes, embryos and blastocysts. Best Pract Res Clin Obstet Gynaecol. 2012;26(6):789‐803. [DOI] [PubMed] [Google Scholar]
- 20. Enatsu Y, Enatsu N, Kishi K, et al. Effectiveness of high‐dose transvaginal progesterone supplementation for women who are undergoing a frozen‐thawed embryo transfer. Reprod Med Biol. 2018;17(3):242‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Coroleu B, Barri PN, Carreras O, et al. The influence of the depth of embryo replacement into the uterine cavity on implantation rates after IVF: a controlled, ultrasound‐guided study. Hum Reprod. 2002;17(2):341‐346. [DOI] [PubMed] [Google Scholar]
- 22. Pope CS, Cook EK, Arny M, Novak A, Grow DR. Influence of embryo transfer depth on in vitro fertilization and embryo transfer outcomes. Fertil Steril. 2004;81(1):51‐58. [DOI] [PubMed] [Google Scholar]
- 23. Fıçıcıoğlu C, Özcan P, Koçer MG, et al. Effect of air bubbles localization and migration after embryo transfer on assisted reproductive technology outcome. Fertil Steril. 2018;109(2): 310‐314. e1. [DOI] [PubMed] [Google Scholar]
- 24. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’for medical statistics. Bone Marrow Transplant. 2013;48(3):452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Casper RF. It's time to pay attention to the endometrium. Fertil Steril. 2011;96(3):519‐521. [DOI] [PubMed] [Google Scholar]
- 26. Haouzi D, Dechaud H, Assou S, De Vos J, Hamamah S. Insights into human endometrial receptivity from transcriptomic and proteomic data. Reprod Biomed Online. 2012;24(1):23‐34. [DOI] [PubMed] [Google Scholar]
- 27. McWilliams GD, Frattarelli JL. Changes in measured endometrial thickness predict in vitro fertilization success. Fertil Steril. 2007;88(1):74‐81. [DOI] [PubMed] [Google Scholar]
- 28. Singh N, Bahadur A, Mittal S, Malhotra N, Bhatt A. Predictive value of endometrial thickness, pattern and sub‐endometrial blood flows on the day of hCG by 2D doppler in in‐vitro fertilization cycles: a prospective clinical study from a tertiary care unit. J Hum Reprod Sci. 2011;4(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang X, Chen C‐H, Confino E, Barnes R, Milad M, Kazer RR. Increased endometrial thickness is associated with improved treatment outcome for selected patients undergoing in vitro fertilization–embryo transfer. Fertil Steril. 2005;83(2):336‐340. [DOI] [PubMed] [Google Scholar]
- 30. Coulam CB, Bustillo M, Soenksen DM, Britten S. Ultrasonographic predictors of implantation after assisted reproduction. Fertil Steril. 1994;62(5):1004‐1010. [DOI] [PubMed] [Google Scholar]
- 31. Sundstrom P. Establishment of a successful pregnancy following in‐vitro fertilization with an endometrial thickness of no more than 4 mm. Hum Reprod. 1998;13(6):1550‐1552. [DOI] [PubMed] [Google Scholar]
- 32. Woolcott R, Stanger J. Ultrasound tracking of the movement of embryo‐associated air bubbles on standing after transfer. Hum Reprod. 1998;13(8):2107‐2109. [DOI] [PubMed] [Google Scholar]
- 33. Saravelos SH, Wong AWY, Chan CPS, et al. Assessment of the embryo flash position and migration with 3D ultrasound within 60 min of embryo transfer. Hum Reprod. 2016;31(3):591‐596. [DOI] [PubMed] [Google Scholar]
- 34. Mansour RT, Aboulghar MA. Optimizing the embryo transfer technique. Hum Reprod. 2002;17(5):1149‐1153. [DOI] [PubMed] [Google Scholar]
- 35. Henne MB, Milki AA. Uterine position at real embryo transfer compared with mock embryo transfer. Hum Reprod. 2004;19(3):570‐572. [DOI] [PubMed] [Google Scholar]


