Low back pain (LBP) is a common issue in the musculoskeletal system. Over half a billion people worldwide suffer from LBP, and it may affect up to 80% of adults at different stages of their life; it is the leading cause of years lived with disability [1]. However, the pathogenesis of LBP is still not clear. Intervertebral disk degeneration (IDD) has been recognized as a major factor associated with LBP [2]. Although IDD is thought to be an age-related condition, it may appear as early as the 20 s [3]. Besides the influence of genetics, aberrant loading is considered to be the main cause of non-aging IDD. Compression is the major form of loading that intervertebral discs receive. Studies have reported that excessive compression may induce extracellular matrix (ECM) metabolism (anabolism & catabolism) imbalance and apoptosis in disk cells [4]; however the mechanism is still to be explored.
In the current issue of EBioMedicine, Xiang and colleagues focused on the role of circular RNAs (circRNAs) in compression induced IDD [5]. They isolated nucleus pulposus (NP) cells from human subjects and put them into a custom apparatus to deliver 1.0 MPa static compression, which may mimic the pathological condition of discs in vivo. They performed a microarray to analyze the differential expression of circRNAs. The results showed that 286 circRNAs were up-regulated and 1498 were down-regulated in compression-treated human NP cells. They focused on the down-regulated -, which were the majority of the differentially expressed circRNAs - and found that Hsa_circ_0044722, was the most significantly down-regulated.. They named it circRNA-CIDN, which stands for Compression-Induced Damage of NP cells.
Functional study demonstrated that administration of circRNA-CIDN in compression treated NP cells may lead to increased ECM anabolism (as indicated by the expression of Aggrecan and Collagen II) and decreased ECM catabolism (as indicated by the expression of MMP-1 and MMP-13); meanwhile, the expression of Bax, BCL-2 and cleaved-caspase 3 as well as Annexin V staining showed inhibition of apoptosis by circRNA-CIDN in compression treated NP cells. Knock-down of circRNA-CIDN in the compression treated NP cells, displayed the opposite effects of administration. Thus, circRNA-CIDN is shown to be beneficial in compression treated NP cells through ECM homeostasis promotion and apoptosis suppression.
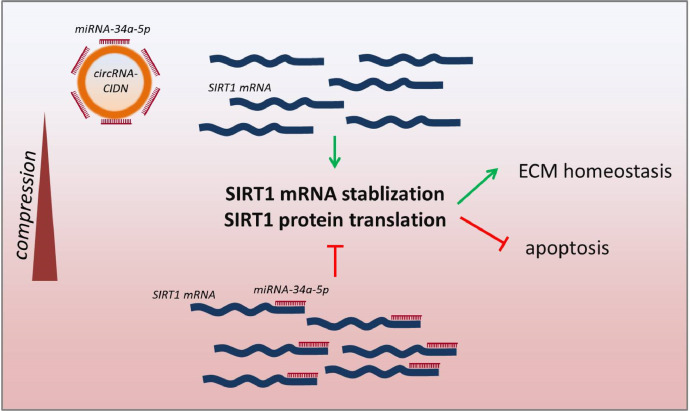
Next, the authors aimed to elucidate the mechanism of circRNA-CIDN in compression induced IDD. Bioinformatic analysis suggested that circRNA-CIDN possesses complementary sequences to miRNA-34a-5p, and follow up study verified that circRNA-CIDN acts as a sponge of miRNA-34a-5p in NP cells. The expression and function of miRNA-34a-5p was assessed in compression treated NP cells, with the results proving that miRNA-34a-5p is the down-stream effector. Further study revealed that miRNA-34a-5p may repress SIRT1 expression by targeting 3′-UTR of SIRT1. At this point, they proposed that circRNA-CIDN may mitigate compression loading-induced damage in human NP cells via miRNA-34a-5p/SIRT1 axis (illustrated in Fig. 1).
Fig. 1.
Mechanism of circRNA-CIDN in compression induced IDD. CircRNA-CIDN may serve as a sponge of miRNA-34a-5p to suppress its binding to the mRNA of SIRT1, which in turn promote mRNA stabilization and protein translation of SIRT1; while SIRT1 is responsible for the extracellular matrix (ECM) homeostasis and apoptosis suppression in nucleus pulposus cells. Pathological compression may lead to decreased expression of circRNA-CIDN and suppress ECM homeostasis and promote apoptosis through miRNA-34a-5p/SIRT1.
Although circRNAs have been investigated in IDD [6], [7], [8], [9], [10], Xiang et al.’s study is the first to screen the differentially expressed circRNAs in compression induced IDD; they also illuminated the function and working mechanism of circRNA-CIDN in compression induced IDD. Most importantly, they showed in rat disk organ culture that circRNA-CIDN may ameliorate IDD ex vivo, suggesting circRNA-CIDN could be a potential therapeutic agent for IDD. Xiang's study may help to better understand how compression induces IDD. They suggest circRNAs may play a role in compression induced IDD, which is a new perspective of the pathogenesis of compression induced IDD. Also, the differentially expressed circRNAs identified in their microarray study represent a pool of circRNAs, which may facilitate further study of circRNAs in compression induced IDD.
However, more basic and preclinical studies are needed prior to clinical application of circRNA-CIDN in IDD therapy. 1784 circRNAs were differentially expressed; however, only circRNA-CIDN was studied; the role of other circRNAs in compression induced IDD is unknown. The therapeutic effect of circRNA-CIDN was only investigated in rat disk ex vivo model, its effect in vivo as well as in large animal models is still not clear. The key point for the in vivo application of circRNA-CIDN in IDD is the administration regimen. Puncture of the discs is known to induce IDD, while systematic administration may reduce the local distribution of circRNA in discs; therefore, the administration regimen should be specifically designed. It is likely that administration of long term slow released circRNA will be an option; perhaps with a more intelligent delivery system developed for further application.
In summary, Xiang's study identifies circRNAs in compression induced IDD and illustrates that circRNA-CIDN, may promote ECM homeostasis and suppress apoptosis through miRNA-34a-5p/SIRT1 axis. This study provides a new perspective of the pathogenesis of compression induced IDD and establishes a pool of differentially expressed circRNAs which may facilitate further studies. It also suggests that circRNA-CIDN could be a therapeutic strategy for IDD, although it is only studied ex vivo and its effect in vivo is still to be investigated.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
The authors apologize to the researchers whose works are not specifically referenced due to space limitation. The authors are supported by Lin He's New Medicine and Clinical Translation Academician Workstation Research Fund (No. 18331213).
References
- 1.Hurwitz EL, Randhawa K, Yu H, Cote P, Haldeman S. The Global Spine Care Initiative: a summary of the global burden of low back and neck pain studies. Eur Spine J. 2018;27(Suppl 6):796–801. doi: 10.1007/s00586-017-5432-9. [DOI] [PubMed] [Google Scholar]
- 2.Vergroesen PP, Kingma I, Emanuel KS. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage. 2015;23(7):1057–1070. doi: 10.1016/j.joca.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Samartzis D, Karppinen J, Mok F, Fong DY, Luk KD, Cheung KM. A population-based study of juvenile disc degeneration and its association with overweight and obesity, low back pain, and diminished functional status. J Bone Joint Surg Am. 2011;93(7):662–670. doi: 10.2106/JBJS.I.01568. [DOI] [PubMed] [Google Scholar]
- 4.Chan SC, Ferguson SJ, Gantenbein-Ritter B. The effects of dynamic loading on the intervertebral disc. Eur Spine J. 2011;20(11):1796–1812. doi: 10.1007/s00586-011-1827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiang Q, Kang L, Wang J. CircRNA-CIDN mitigated compression loading-induced damage in human nucleus pulposus cells via miR-34a-5p/SIRT1 axis. EBioMed. 2020 doi: 10.1016/j.ebiom.2020.102679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng X, Zhang L, Zhang K. Circular RNA VMA21 protects against intervertebral disc degeneration through targeting miR-200c and X linked inhibitor-of-apoptosis protein. Ann Rheum Dis. 2018;77(5):770–779. doi: 10.1136/annrheumdis-2017-212056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo W, Zhang B, Mu K. Circular RNA GRB10 as a competitive endogenous RNA regulating nucleus pulposus cells death in degenerative intervertebral disk. Cell Death Dis. 2018;9(3):319. doi: 10.1038/s41419-017-0232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song J, Wang HL, Song KH. CircularRNA_104670 plays a critical role in intervertebral disc degeneration by functioning as a ceRNA. Exp Mol Med. 2018;50(8):94. doi: 10.1038/s12276-018-0125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, He P, Pan H. Circular RNA circ-4099 is induced by TNF-alpha and regulates ECM synthesis by blocking miR-616-5p inhibition of Sox9 in intervertebral disc degeneration. Exp Mol Med. 2018;50(4):27. doi: 10.1038/s12276-018-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Wang B, Zou M. CircSEMA4B targets miR-431 modulating IL-1beta-induced degradative changes in nucleus pulposus cells in intervertebral disc degeneration via Wnt pathway. Biochim Biophys Acta Mol Basis Dis. 2018;1864(11):3754–3768. doi: 10.1016/j.bbadis.2018.08.033. [DOI] [PubMed] [Google Scholar]