Abstract
Background
The optimal surgical strategy for early-stage non-small cell lung cancer (NSCLC) with visceral pleural invasion (VPI) remains unclear. Due to limited prospective comparative data for these surgical modalities, the objective of the current study was to compare the long-term survival outcomes of sublobectomy (Sub) versus lobectomy (Lob) for NSCLC with a tumor size ≤2 cm and VPI.
Methods
Patients with early-stage NSCLC characterized by VPI diagnosed between 2004 and 2013 were identified from the Surveillance, Epidemiology, and End Results (SEER) program. The baseline demographic and cancer characteristics, treatment information as well as survival outcome data were extracted from the SEER database, and confounders were balanced by propensity score matching (PSM) and inverse probability of treatment weighting (IPTW) analyses. Lung disease-specific survival (DSS) and overall survival (OS) rates were compared with Cox proportional hazards (PH) regression models based on the unmatched cohort, the propensity-based matched cohort, and the IPTW cohort.
Results
Of the 1,386 patients enrolled, 1,000 (72.15%) and 386 (27.85%) underwent lobectomy and sublobectomy, respectively. The 5-year DSS rate was 78.64% for the lobectomy group and 59.47% for the sublobectomy group. Cox regression models demonstrated that the operation type (Sub vs. Lob) was an independent prognostic factor for early-stage NSCLC with VPI based on the three different cohorts. Patients who underwent lobectomy showed better long-term DSS and OS rates than those treated with sublobectomy after PSM [DSS: hazard ratio (HR) 0.689, 95% confidence interval (CI): 0.490–0.968, P=0.032; OS: HR 0.723, 95% CI: 0.549–0.953, P=0.021]. The IPTW analysis yielded similar results.
Conclusions
Lobectomy showed superior long-term survival compared with sublobectomy in patients with early-stage NSCLC with a tumor size ≤2 cm and VPI.
Keywords: Non-small cell lung cancer (NSCLC), SEER database, sublobectomy (Sub), lobectomy (Lob), visceral pleural invasion (VPI)
Introduction
Non-small cell lung cancer (NSCLC) is one of the major causes of cancer-related deaths worldwide, accounting for approximately 85% of all lung cancers (1). Admittedly, with the application of low-dose computed tomography (CT) screening techniques, an increasing number of NSCLC patients in the early stage are being diagnosed (2).
Visceral pleural invasion (VPI), defined as invasion beyond the elastic layer including invasion to the visceral pleural surface (3), has been identified as a non-size-based T2 factor, increasing the T descriptor from T1 to T2a and upstaging a tumor from stage IA to stage IB (4). VPI often serves as an invasive and aggressive indicator of NSCLC (5), and previous studies have demonstrated that VPI correlated with a higher incidence of pleural effusion, poor tumor differentiation, lymph node metastasis, postoperative recurrence, and poor survival (5,6).
Surgical resections are the preferred therapy in the early stable phase of NSCLC (7). Following a randomized controlled trial in 1995, lobectomy became widely adopted as the standard of care and the optimal surgical resection for T1N0 NSCLC (8). However, sublobectomy has been reported as an alternative surgical procedure, especially in patients with significant comorbidities or limited pulmonary function. According to the National Comprehensive Cancer Network (NCCN) Clinical Practice Guideline in Oncology for NSCLC, sublobectomy is acceptable in selected patients with a tumor size ≤2 cm combined with adenocarcinoma in situ (AIS) histology, ≥50% ground-glass appearance on CT or a long doubling radiologic surveillance time (≥400 days). However, controversy still remains about the impact of VPI on the choice of surgical treatment for early-stage NSCLC with VPI. Several prior retrospective studies included only small-sample, single-institution cases, which were subject to selection bias (9-13).
These gaps in knowledge prompted us to use the Surveillance, Epidemiology and End Results (SEER) database to compare the long-term survival outcomes of sublobectomy versus lobectomy in patients with early-stage NSCLC (≤2 cm) characterized by VPI.
Methods
Data source
The SEER program of the National Cancer Institute (NCI) is an authoritative and widely used source of information on patient demographics, primary tumor sites, tumor morphology and pathological stage at diagnosis, initial course of treatment, and survival information, covering approximately 34.6% of the population of the United States (14). With more than 40 years of longitudinal and ongoing data integration, the SEER population is highly representative in terms of geography, socioeconomic status, race, ethnicity and age. The data provided by the SEER database are anonymous, and the requirement for informed consent was therefore waived. The Institutional Review Committee of Changzheng Hospital (Naval Medical University, Shanghai, China) deemed the study to be nonhuman subject research and therefore approved this study to be exempted research.
Population selection
From the SEER database, we identified all patients with histologically confirmed NSCLC (adenocarcinoma, squamous cell carcinoma) diagnosed between 2004 and 2013. The last inclusion was in 2013 to guarantee an adequate follow-up time. The study enrolled patients for whom NSCLC was their only primary malignancy. Moreover, patients with VPI and a maximum tumor diameter of 2 cm or less were included in our cohort. Patients who met the following criteria were excluded: aged <18 years; diagnosed while in a nursing home or hospice; had a clarified diagnosis on autopsy or death certificate data; had tumors ≤2 cm in size but upstaged to T2a disease due to hilar atelectasis or obstructive pneumonia; had tumor stage in T3 or T4 disease; had lymph node (LN) involvement (N1, N2, and N3) or distant metastasis (M1); had a history of preoperative chemotherapy or radiotherapy, without sublobectomy or lobectomy; and missing important information on the VPI status, tumor size, tumor node metastasis (TNM) stage, surgical procedure, and survival outcomes. The whole cohort was classified into two subgroups: the lobectomy (Lob) group and the sublobectomy (Sub) group.
Study covariates
From the SEER database, data on the baseline demographics of patients, including age, sex, race, marital status, insurance status, and cost-of-living index, were extracted. Clinicopathological data on the year of diagnosis, tumor extension, tumor size, TNM stage, histology (adenocarcinoma or squamous carcinoma), lesion location (upper lobe, middle lobe, or lower lobe), tumor differentiation (well, moderate, or poor) were also collected. In addition, treatment strategies including the surgical procedure (Sub vs. Lob), radiotherapy, chemotherapy, and number of LNs sampled were retrieved.
The value of the cost-of-living index is the ratio of the local cost-of-living to the United States population-weighted mean cost-of-living. Tumor location and histology were identified according to the International Classification of Diseases for Oncology, Version 3 (ICD-O-3) in the SEER database. Tumor stage, collected within 4 months of diagnosis or within the completion of the initial course of treatment, was coded according to the sixth [2004–2009] and seventh [2010–2013] editions of the American Joint Committee on Cancer (AJCC) TNM staging systems. Furthermore, due to the staging criteria of our study cohort were based on the sixth and seventh AJCC TNM staging systems, patients were then restaged according to the eighth edition TNM staging criteria.
In the SEER database, the surgery codes were included in ‘RX Summ-Surg Prim Site’, which was defined as the surgical procedure that removed tissue from the primary site performed as part of the initial course of therapy. Surgical procedures were categorized and coded into the sublobectomy (codes 21–22) group and the lobectomy (codes 30–33) group.
The presence of VPI was ascertained using Collaborative Staging Extension (CSE) codes (410, 420, 430, and 450) based on the Collaborative Stage Data Collection System Coding Instructions (Version 02.05). The International Association for the Study of Lung Cancer (IASLC) recommended the classification of the status of VPI as follows: PL0 (CSE code 410), the tumor grows within the parenchyma or does not completely penetrate the elastic layer; PL1 (CSE code 420), the tumor extends beyond the elastic layer; and PL2 (CSE code 430), the tumor invades into the surface of the visceral pleura. However, patients coded CSE 410, indicating no evidence of VPI, were excluded from our cohort. VPI patients prior to 2010 were classified using CSE code 450, which includes both VPI and extension to the pulmonary ligament. In 2010, the SEER program introduced the distinct CSE code 440 for pulmonary ligament involvement (15). However, Lakha et al. (15) reported that the number and proportion of pulmonary ligament cases among patients with CSE code 450 prior to 2010 was negligible compared with those among patients with VPI. Minimal misclassification was also acceptable in our study.
Outcomes
In this study, lung disease-specific survival (DSS) and overall survival (OS) were assessed to evaluate the prognosis and outcomes. Causes of death were coded by the SEER database based on information extracted from the death certificate data. Patients who died of nonlung cancer-related causes were classified as censored at the date of death in the analyses of DSS, while OS was measured from the date of diagnosis to the date of death as a result of any cause. The SEER program is updated annually, including information on follow-up and survival. In this study, the latest patient information was updated in December 2016. Therefore, the survival time was calculated as the number of months from cancer diagnosis until death or the last follow-up (December 31, 2016) for censored observations.
Statistical analysis
In the current study, the baseline characteristics of patients treated with sublobectomy or lobectomy were summarized using conventional statistics, such as the mean ± standard deviation (SD) or the median for continuous variables and frequencies and percentages for categorical variables. X-tile (Version 3.6.1; Yale University, New Haven, CT, USA), a biostatistics tool, was used to determine the optimal cut-off value of age for survival, similar to previous publications (16,17). Pearson’s χ2 test or Fisher’s exact test was performed for categorical covariates, and Student’s t-test was performed for continuous variables, as appropriate.
Given the observational nature of the current study, propensity score matching (PSM) was used to minimize the effect of potential confounders. Baseline characteristics (age, gender, marital status, race, cost-of-living index, year of diagnosis, laterality, lobe, histological type, tumor differentiation, lymphadenectomy/biopsy, radiation, and chemotherapy) were incorporated in the propensity score (PS) analysis. A logistic regression model was constructed to calculate and assign each patient a PS, which was defined as the likelihood of being exposed to an intervention given that the status of particular patient’s measured prognostic factors (18,19). Next, 1:1 matching (Sub vs. Lob) without replacement was performed using a nearest neighbor matching algorithm, with a fixed caliper width of 0.05 (20).
Regarding the second propensity analysis, the stabilized inverse probability of treatment weight (IPTW) was calculated as the inverse PS for patients undergoing lobectomy and as the inverse (1-PS) for patients undergoing sublobectomy. Based on the PS, IPTW allows all patients in the unmatched cohort to obtain unbiased estimates of average treatment effects (21). The subsequent survival analyses were weighted by IPTW.
Survival curves according to the unmatched groups were constructed by the Kaplan-Meier (KM) method, and differences between groups were assessed using the log-rank test. The median survival time and 3- and 5-year DSS and OS rates were also reported in detail among different cohorts. Univariate and multivariate Cox proportional hazards (PH) models were used to estimate the association between surgical approaches and OS or DSS in the unmatched cohort, the 1:1 matched cohort, and the IPTW cohort, with the results reported as hazard ratios (HRs) for mortality with the corresponding 95% confidence intervals (CIs). In our multivariate Cox PH analysis, all the candidate variables with a P<0.1 in the univariate analysis were included a multivariate model. A backward stepwise regression procedure was used. The sublobectomy group served as the reference group in all models.
For all statistical analyses, a two-sided P value of 0.050 was considered statistically significant. The SEER*Stat software program (Version 8.3.5; NCI, Bethesda, MD, USA) was used for data extraction. All statistical analyses were performed using SPSS software (Version 22.0; IBM Corporation, St. Louis, Missouri, USA) and R software (Version 3.6.1; The R Project for Statistical Computing, TX, USA; http://www.r-project.org).
Results
Basic characteristics
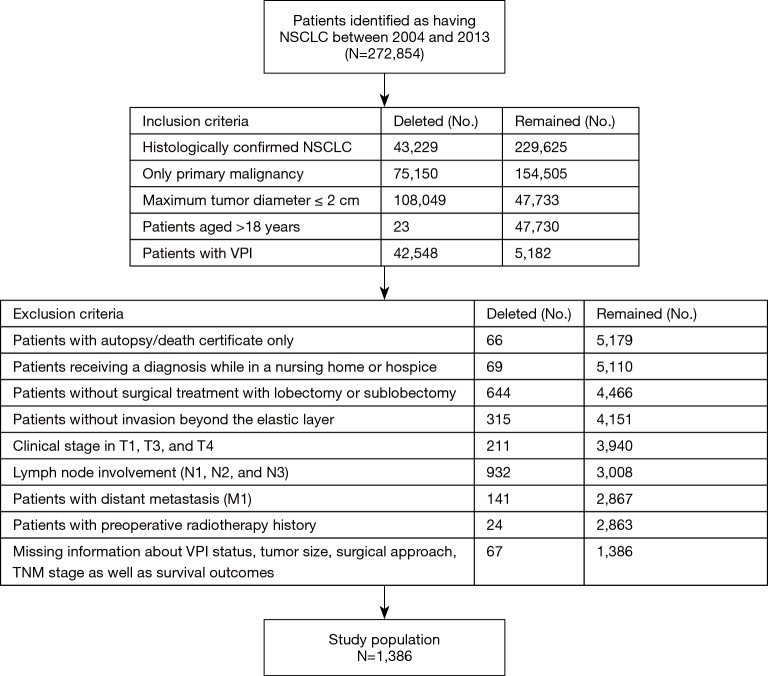
Between January 1, 2004 and December 31, 2013, the SEER database collected data on 272,854 patients diagnosed with NSCLC (adenocarcinoma or squamous carcinoma). After the inclusion and exclusion criteria were applied, the final study cohort consisted of 1,386 patients who had NSCLC with a tumor ≤2 cm in size and VPI. A flow chart of the population selection process is presented in Figure 1. Overall, 386 patients (27.85%) underwent sublobectomy, and 1,000 patients underwent lobectomy (72.15%). The baseline characteristics are reported in Table 1. Lobectomies were more likely to be performed in young (P<0.001), male (P=0.031), and married (P<0.001) patients with middle lobe tumors (P=0.001) and a pathological type of ADC (P=0.019). Patients who underwent lobectomy had more LNs sampled (P<0.001) and were more likely to complete adjuvant radiation therapy (P<0.001). There were no significant differences in the distribution of other baseline characteristics among patients (P>0.050 for all comparisons).
Figure 1.
The Flow Chart of the Study Population Selection Process. SEER, Surveillance, Epidemiology, and End Results; NSCLC, non-small cell lung cancer; VPI, visceral pleural invasion.
Table 1. Baseline characteristics before and after PSM analysis.
| Characteristic | Before matching | After matching | |||||
|---|---|---|---|---|---|---|---|
| Sub | Lob | P value | Sub | Lob | P value | ||
| Total | 386 | 1,000 | 231 | 231 | |||
| Year of diagnosis, n (%) | 0.105 | 0.854 | |||||
| 2004–2006 | 73 (18.91) | 242 (24.20) | 48 (20.78) | 53 (22.94) | |||
| 2007–2009 | 125 (32.38) | 309 (30.90) | 72 (31.17) | 70 (30.30) | |||
| 2010–2013 | 188 (48.70) | 449 (44.90) | 111 (48.05) | 108 (46.75) | |||
| Age group, n (%) | <0.001 | 0.298 | |||||
| 37–64 years | 106 (27.46) | 424 (42.40) | 72 (31.17) | 80 (34.63) | |||
| 65–73 years | 105 (27.20) | 332 (33.23) | 69 (29.87) | 77 (33.33) | |||
| 74–96 years | 175 (45.34) | 244 (24.42) | 90 (38.96) | 74 (32.03) | |||
| Insurance status, n (%) | 0.052* | 0.578* | |||||
| None | 4 (1.04) | 12 (1.20) | 2 (0.87) | 1 (0.43) | |||
| Yes | 307 (79.53) | 733 (73.30) | 180 (77.92) | 173 (74.89) | |||
| Unknown | 75 (19.43) | 255 (25.50) | 49 (21.21) | 57 (24.68) | |||
| Sex, n (%) | 0.031 | 0.924 | |||||
| Male | 143 (37.05) | 434 (43.40) | 93 (40.26) | 94 (40.69) | |||
| Female | 243 (62.95) | 566 (56.60) | 138 (59.74) | 137 (59.31) | |||
| Race, n (%) | 0.092 | 0.611 | |||||
| Black | 37 (9.59) | 88 (8.80) | 21 (9.09) | 25 (10.82) | |||
| White | 326 (84.46) | 816 (81.60) | 195 (84.42) | 187 (80.95) | |||
| Others | 23 (5.96) | 96 (9.60) | 15 (6.49) | 19 (8.23) | |||
| Cost-of-living index, n (%) | 0.441 | 0.300 | |||||
| ≤1 | 166 (43.01) | 453 (45.30) | 103 (44.59) | 92 (39.83) | |||
| >1 | 220 (56.99) | 547 (54.70) | 128 (55.41) | 139 (60.17) | |||
| Marital status, n (%) | <0.001 | 0.631 | |||||
| Single | 36 (9.33) | 102 (10.20) | 22 (9.52) | 31 (13.42) | |||
| Married | 197 (51.04) | 585 (58.50) | 133 (57.58) | 127 (54.98) | |||
| Divorced/separated | 44 (11.40) | 137 (13.70) | 24 (10.39) | 23 (9.96) | |||
| Others | 109 (28.24) | 176 (17.60) | 52 (22.51) | 50 (21.65) | |||
| Laterality, n (%) | 0.330 | 0.849 | |||||
| Right | 226 (58.55) | 614 (61.40) | 139 (60.17) | 141 (61.04) | |||
| Left | 160 (41.45) | 386 (38.60) | 92 (39.83) | 90 (38.96) | |||
| Lobe, n (%) | 0.001 | 0.004 | |||||
| Upper | 248 (64.25) | 643 (64.30) | 153 (66.23) | 149 (64.50) | |||
| Middle | 12 (3.11) | 88 (8.80) | 6 (2.60) | 24 (10.39) | |||
| Lower | 122 (31.61) | 257 (25.70) | 70 (30.30) | 54 (23.38) | |||
| Others/unknown | 4 (1.04) | 12 (1.20) | 2 (0.87) | 4 (1.73) | |||
| Differentiation, n (%) | 0.771 | 0.691 | |||||
| Well | 46 (11.92) | 127 (12.70) | 29 (12.55) | 37 (16.02) | |||
| Moderate | 210 (54.40) | 514 (51.40) | 128 (55.41) | 118 (51.08) | |||
| Poor | 118 (30.57) | 322 (32.20) | 68 (29.44) | 69 (29.87) | |||
| Unknown | 12 (3.11) | 37 (3.70) | 6 (2.60) | 7 (3.03) | |||
| Pathological type, n (%) | 0.019 | 0.905 | |||||
| Adenocarcinoma | 298 (77.20) | 827 (82.70) | 187 (80.95) | 188 (81.39) | |||
| Squamous carcinoma | 88 (22.80) | 173 (17.30) | 44 (19.05) | 43 (18.61) | |||
| Lymphadenectomy/biopsy, n (%) | <0.001 | 0.688 | |||||
| None | 180 (46.63) | 32 (3.20) | 34 (14.72) | 31 (13.42) | |||
| Yes | 206 (53.37) | 968 (96.80) | 197 (85.28) | 200 (86.58) | |||
| Number of LNs sampled, n (%) | <0.001 | <0.001 | |||||
| None/unknown | 208 (53.89) | 108 (10.80) | 61 (26.41) | 72 (31.17) | |||
| 1–3 | 95 (24.61) | 149 (14.90) | 88 (38.10) | 49 (21.21) | |||
| 4–6 | 50 (12.95) | 227 (22.70) | 49 (21.21) | 47 (20.35) | |||
| 7–9 | 12 (3.11) | 213 (21.30) | 12 (5.19) | 30 (12.99) | |||
| 10–12 | 9 (2.33) | 111 (11.10) | 9 (3.90) | 12 (5.19) | |||
| ≥13 | 12 (3.11) | 192 (19.20) | 12 (5.19) | 21 (9.09) | |||
| Radiation, n (%) | <0.001 | 0.190 | |||||
| None/unknown | 348 (90.16) | 982 (98.20) | 217 (93.94) | 223 (96.54) | |||
| Yes | 38 (9.84) | 18 (1.80) | 14 (6.06) | 8 (3.46) | |||
| Chemotherapy, n (%) | 0.019 | 0.141 | |||||
| None/unknown | 352 (91.19) | 866 (86.60) | 210 (90.91) | 200 (86.58) | |||
| Yes | 34 (8.81) | 134 (13.40) | 21 (9.09) | 31 (13.42) | |||
Data is presented as numbers with percentage of study population in brackets. P* is calculated by Fisher’s exact test. PSM, propensity score matching; Sub, sublobectomy; Lob, lobectomy; LN, lymph node.
Survival analysis before matching
The median follow-up time for the entire cohort was 58 months [interquartile range (IQR): 37–89 months]. A total of 634 (45.74%) patients died from any cause, and 400 (28.86%) patients died from NSCLC at the end of the study period (December 2013). The 3- and 5-year DSS rates for the lobectomy group were 84.70% (95% CI: 82.97–87.06%) and 78.64% (95% CI: 75.95–81.42%), respectively, and those for the sublobectomy group were 73.03% (95% CI: 68.56–77.78%) and 59.47% (95% CI: 54.27–65.16%), respectively. The 3- and 5-year OS rates were 81.67% (95% CI: 79.30–84.12%) and 70.13% (95% CI: 67.22–73.16%) in the lobectomy group, and 63.39% (95% CI: 58.76–68.39%) and 46.92% (95% CI: 41.97–52.45%) in the sublobectomy group, respectively.
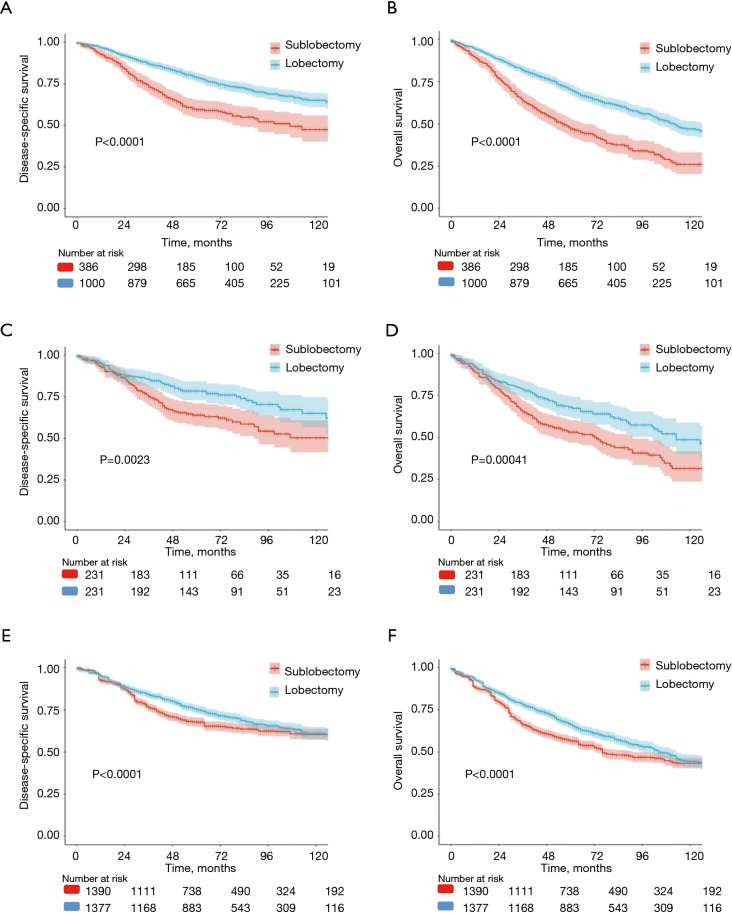
In the univariate analysis of the unmatched cohort, patients treated with lobectomy had better DSS (HR 0.513, 95% CI: 0.418–0.628, P<0.001) and OS (HR 0.507; 95% CI: 0.432–0.597, P<0.001) rates than those treated with sublobectomy (Tables 2,3, Tables S1,S2). After adjusting for other covariates, the multivariate analysis demonstrated that patients undergoing lobectomy had better DSS (HR 0.702; 95% CI: 0.538–0.915, P=0.009) and OS (HR 0.787, 95% CI: 0.629–0.985, P=0.037) rates than those undergoing sublobectomy (Tables 2,3, Tables S1,S2). Figure 2A,B depict the KM curves for DSS and OS in the unmatched cohort (P<0.001).
Table 2. Results of DSS for patients treated with a sublobectomy or lobectomy in three different cohorts.
| Study cohort | Survival model | HR (sub vs. lob) | 95% CIs | P value |
|---|---|---|---|---|
| Unmatched | Univariable | 0.513 | 0.418–0.628 | <0.001 |
| Unmatched | Multivariable | 0.702 | 0.538–0.915 | 0.009 |
| Propensity-based matched | Univariable | 0.597 | 0.427–0.835 | 0.003 |
| Propensity-based matched | Multivariable | 0.689 | 0.490–0.968 | 0.032 |
| IPTW weighted | Univariable | 0.825 | 0.720–0.946 | 0.006 |
| IPTW weighted | Multivariable | 0.765 | 0.665–0.882 | <0.001 |
Survival model was constructed by Cox proportional hazards regression. DSS, disease-specific survival; HR, hazard ratio; CIs, confidence intervals; IPTW, inverse probability of treatment weighting; Sub, sublobectomy; Lob, lobectomy.
Table 3. Results of OS rates for patients treated with a sublobectomy or lobectomy in three different cohorts.
| Study cohort | Survival model | HR (Sub vs. Lob) | 95% CIs | P value |
|---|---|---|---|---|
| Unmatched | Univariable | 0.507 | 0.432–0.597 | <0.001 |
| Unmatched | Multivariable | 0.787 | 0.629–0.985 | 0.037 |
| Propensity-based matched | Univariable | 0.619 | 0.473–0.810 | <0.001 |
| Propensity-based matched | Multivariable | 0.723 | 0.549–0.953 | 0.021 |
| IPTW weighted | Univariable | 0.796 | 0.714–0.888 | <0.001 |
| IPTW weighted | Multivariable | 0.724 | 0.646–0.812 | <0.001 |
Survival model was constructed by Cox proportional hazards regression. OS, overall survival; HR, Hazard ratio; CIs, confidence intervals; IPTW, inverse probability of treatment weighted; Sub, sublobectomy; Lob, lobectomy.
Table S1. Cox regression analysis of associations prognostic factors and DSS in the unmatched cohort.
| Characteristic | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Year of diagnosis (year) | |||||
| 2004–2006 | Reference | ||||
| 2007–2009 | 1.100 (0.858–1.409) | 0.453 | |||
| 2010–2013 | 0.872 (0.671–1.132) | 0.303 | |||
| Age group | |||||
| 37–64 years | Reference | ||||
| 65–73 years | 1.226 (0.963–1.562) | 0.099 | 1.185 (0.930–1.510) | 0.171 | |
| 74–96 years | 1.548 (1.221–1.964) | <0.001 | 1.389 (1.088–1.774) | 0.008 | |
| Insurance status | |||||
| None | Reference | ||||
| Yes | 1.438 (0.461–4.486) | 0.532 | |||
| Unknown | 1.474 (0.468–4.644) | 0.508 | |||
| Sex | |||||
| Male | Reference | ||||
| Female | 0.703 (0.578–0.856) | <0.001 | 0.675 (0.554–0.822) | <0.001 | |
| Race | |||||
| Black | Reference | ||||
| White | 1.163 (0.814–1.663) | 0.407 | |||
| Others | 0.820 (1.351–1.351) | 0.437 | |||
| Cost–of–living index | |||||
| ≤1 | Reference | ||||
| >1 | 0.895 (0.735–1.089) | 0.266 | |||
| Marital status | |||||
| Single | Reference | ||||
| Married | 0.965 (0.686–1.355) | 0.835 | |||
| Divorced/separated | 0.988 (0.652–1.496) | 0.953 | |||
| Others | 1.057 (0.724–1.544) | 0.775 | |||
| Laterality | |||||
| Right | Reference | ||||
| Left | 1.049 (0.859–1.281) | 0.638 | |||
| Lobe | |||||
| Upper | Reference | ||||
| Middle | 0.757 (0.490–1.170) | 0.211 | |||
| Lower | 1.017 (0.815–1.269) | 0.883 | |||
| Others/unknown | 0.896 (0.334–2.405) | 0.827 | |||
| Differentiation | |||||
| Well | Reference | ||||
| Moderate | 1.394 (0.992–1.958) | 0.056 | NS | ||
| Poor | 1.269 (0.886–1.818) | 0.194 | NS | ||
| Unknown | 0.961 (0.492–1.876) | 0.907 | NS | ||
| Pathological type | |||||
| Adenocarcinoma | Reference | ||||
| Squamous carcinoma | 1.205 (0.942–1.541) | 0.139 | |||
| Lymphadenectomy/Biopsy | |||||
| None | Reference | ||||
| Yes | 0.453 (0.360–0.570) | <0.001 | 0.621 (0.464–0.830) | 0.001 | |
| Number of LNs sampled | |||||
| None/unknown | Reference | ||||
| 1–3 | 0.701 (0.527–0.933) | 0.015 | NS | ||
| 4–6 | 0.540 (0.403–0.723) | <0.001 | NS | ||
| 7–9 | 0.569 (0.416–0.777) | <0.001 | NS | ||
| 10–12 | 0.524 (0.355–0.773) | 0.001 | NS | ||
| ≥13 | 0.381 (0.266–0.546) | <0.001 | NS | ||
| Radiation | |||||
| None/unknown | Reference | ||||
| Yes | 2.057 (1.400–3.021) | <0.001 | 1.706 (1.151–2.530) | 0.008 | |
| Chemotherapy | |||||
| None/unknown | Reference | ||||
| Yes | 0.948 (0.704–1.275) | 0.722 | |||
| Surgery | |||||
| Sub | Reference | ||||
| Lob | 0.513 (0.418–0.628) | <0.001 | 0.702 (0.538–0.915) | 0.009 | |
HRs are only reported on multivariate analysis if they remained significant. DSS, disease-specific survival; Sub, sublobectomy; Lob, lobectomy; LN, lymph node; HR, Hazard ratio; CIs, confidence intervals; NS, not significant.
Table S2. Cox regression analysis of associations prognostic factors and OS in the unmatched cohort.
| Characteristic | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Year of diagnosis (year) | |||||
| 2004–2006 | Reference | ||||
| 2007–2009 | 1.022 (0.839–1.245) | 0.831 | |||
| 2010–2013 | 0.901 (0.731–1.110) | 0.326 | |||
| Age group (year) | |||||
| 37–64 | Reference | ||||
| 65–73 | 1.449 (1.186–1.771) | <0.001 | 1.366 (1.116–1.672) | 0.003 | |
| 74–96 | 2.178 (1.799–2.637) | <0.001 | 1.870 (1.532–2.283) | <0.001 | |
| Insurance status | |||||
| None | Reference | ||||
| Yes | 1.288 (0.533–3.111) | 0.574 | |||
| Unknown | 1.343 (0.552–3.268) | 0.516 | |||
| Sex | |||||
| Male | Reference | ||||
| Female | 0.709 (0.606–0.828) | <0.001 | 0.677 (0.578–0.793) | <0.001 | |
| Race | |||||
| Black | Reference | ||||
| White | 1.131 (0.857–1.493) | 0.385 | NS | ||
| Others | 0.619 (0.408–0.939) | 0.024 | NS | ||
| Cost–of–living index | |||||
| ≤1 | Reference | ||||
| >1 | 0.884 (0.756–1.033) | 0.122 | |||
| Marital status | |||||
| Single | Reference | ||||
| Married | 0.884 (0.677–1.154) | 0.365 | |||
| Divorced/separated | 0.932 (0.672–1.294) | 0.675 | |||
| Others | 1.119 (0.835–1.500) | 0.452 | |||
| Laterality | |||||
| Right | Reference | ||||
| Left | 1.000 (0.853–1.174) | 0.996 | |||
| Lobe | |||||
| Upper | Reference | ||||
| Middle | 0.871 (0.630–1.205) | 0.405 | |||
| Lower | 0.969 (0.810–1.158) | 0.726 | |||
| Others/unknown | 1.045 (0.495–2.207) | 0.908 | |||
| Differentiation | |||||
| Well | Reference | ||||
| Moderate | 1.202 (0.930–1.554) | 0.161 | |||
| Poor | 1.109 (0.844–1.457) | 0.457 | |||
| Unknown | 0.880 (0.524–1.476) | 0.627 | |||
| Pathological type | |||||
| Adenocarcinoma | Reference | ||||
| Squamous carcinoma | 1.453 (1.207–1.750) | <0.001 | 1.237 (1.023–1.496) | 0.028 | |
| Lymphadenectomy/biopsy | |||||
| None | Reference | ||||
| Yes | 0.446 (0.371–0.535) | <0.001 | 0.696 (0.500–0.968) | 0.031 | |
| Number of LNs sampled | |||||
| None/unknown | Reference | ||||
| 1–3 | 0.657 (0.522–0.826) | <0.001 | 0.969 (0.706–1.330) | 0.845 | |
| 4–6 | 0.544 (0.433–0.684) | <0.001 | 0.856 (0.620–1.183) | 0.346 | |
| 7–9 | 0.585 (0.459–0.746) | <0.001 | 0.947 (0.675–1.327) | 0.751 | |
| 10–12 | 0.405 (0.289–0.567) | <0.001 | 0.694 (0.461–1.045) | 0.080 | |
| ≥13 | 0.379 (0.286–0.502) | <0.001 | 0.640 (0.444–0.923) | 0.017 | |
| Radiation | |||||
| None/unknown | Reference | ||||
| Yes | 1.578 (1.122–2.219) | 0.009 | NS | ||
| Chemotherapy | |||||
| None/unknown | Reference | ||||
| Yes | 0.793 (0.618–1.019) | 0.070 | NS | ||
| Surgery | |||||
| Sub | Reference | ||||
| Lob | 0.507 (0.432–0.597) | <0.001 | 0.787 (0.629–0.985) | 0.037 | |
HRs are only reported on multivariate analysis if they remained significant. OS, overall survival; Sub, sublobectomy; Lob, lobectomy; LN, lymph node; HR, Hazard ratio; CIs, confidence intervals; NS, not significant.
Figure 2.
Kaplan-Meier (KM) survival curves by type of surgeries (sublobectomy vs. lobectomy). (A,B) KM curves for lung disease-specific survival (DSS) (P<0.0001) and overall survival (OS) (P<0.0001) in the unmatched cohort; (C,D) KM curves for DSS (P=0.0023) and OS (P=0.00041) in the propensity score matching (PSM) cohort; (E,F) KM curves for DSS (P<0.0001) and OS (P<0.0001) in the inverse probability of treatment weighting (IPTW) cohort (P<0.001). P value indicates a log-rank test.
Survival analysis after PSM
PSM produced 231 matched pairs (N=462, 33.33%). Matching was effective in controlling the covariate imbalance. With a total of 15 covariates included in the PS model, 13 covariates were well balanced (P>0.050) between the two groups after matching except for the number of LNs sampled and tumor location. However, choosing stricter caliper widths would reduce the number of matched pairs. A univariate Cox PH analysis was used in the matched sample, and significant differences in survival were observed between the sublobectomy and lobectomy groups (DSS: HR 0.597, 95% CI: 0.427–0.835, P=0.003; OS: HR 0.619, 95% CI: 0.473–0.810, P<0.001) (Tables 2,3, Tables S3,S4). In the multivariate Cox PH analysis, lobectomy was associated with an approximately30% relative increase in the DSS (HR 0.689, 95% CI: 0.490–0.968, P=0.032) and OS (HR 0.723, 95% CI: 0.549–0.953, P=0.021) rates (Tables 2,3, Tables S3,S4). Figure 2C,D depict the KM curves for DSS and OS in the PSM cohort (DSS: P=0.002; OS: P<0.001).
Table S3. Cox regression analysis of associations prognostic factors and DSS in the PSM cohort.
| Characteristic | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Year of diagnosis (year) | |||||
| 2004–2006 | Reference | ||||
| 2007–2009 | 1.184 (0.780–1.797) | 0.427 | |||
| 2010–2013 | 0.764 (0.491–1.189) | 0.233 | |||
| Age group (year) | |||||
| 37–64 | Reference | ||||
| 65–73 | 1.535 (1.004–2.346) | 0.048 | 1.639 (1.065–2.521) | 0.025 | |
| 74–96 | 1.736 (1.145–2.632) | 0.009 | 1.913 (1.245–2.938) | 0.003 | |
| Insurance status | |||||
| None | Reference | ||||
| Yes | / | / | |||
| Unknown | / | / | |||
| Sex | |||||
| Male | Reference | ||||
| Female | 0.675 (0.485–0.938) | 0.019 | 0.694 (0.495–0.973) | 0.034 | |
| Race | |||||
| Black | Reference | ||||
| White | 1.453 (0.783–2.695) | 0.236 | |||
| Others | 1.051 (0.446–2.477) | 0.909 | |||
| Cost-of-living index | |||||
| ≤1 | Reference | ||||
| >1 | 0.832 (0.597–1.161) | 0.279 | |||
| Marital status | |||||
| Single | Reference | ||||
| Married | 1.010 (0.581–1.754) | 0.972 | |||
| Divorced/separated | 1.141 (0.564–2.308) | 0.715 | |||
| Others | 1.044 (0.565–1.929) | 0.890 | |||
| Laterality | |||||
| Right | Reference | ||||
| Left | 1.017 (0.727–1.423) | 0.921 | |||
| Lobe | |||||
| Upper | Reference | ||||
| Middle | 1.046 (0.528–2.073) | 0.898 | |||
| Lower | 1.059 (0.731–1.534) | 0.764 | |||
| Others/unknown | 0.000 (0.000–Inf) | 0.994 | |||
| Differentiation | |||||
| Well | Reference | ||||
| Moderate | 1.693 (0.978–2.931) | 0.060 | NS | ||
| Poor | 1.388 (0.767–2.513) | 0.279 | NS | ||
| Unknown | 0.274 (0.036–2.077) | 0.210 | NS | ||
| Pathological type | |||||
| Adenocarcinoma | Reference | ||||
| Squamous carcinoma | 1.086 (0.714–1.651) | 0.701 | |||
| Lymphadenectomy/biopsy | |||||
| None | Reference | ||||
| Yes | 0.477 (0.324–0.701) | <0.001 | 0.491 (0.331–0.728) | <0.001 | |
| Number of LNs sampled | |||||
| None/Unknown | Reference | ||||
| 1–3 | 0.974 (0.656–1.445) | 0.895 | NS | ||
| 4–6 | 0.569 (0.345–0.940) | 0.028 | NS | ||
| 7–9 | 0.485 (0.230–1.023) | 0.058 | NS | ||
| 10–12 | 0.734 (0.332–1.621) | 0.444 | NS | ||
| ≥13 | 0.358 (0.153–0.837) | 0.018 | NS | ||
| Radiation | |||||
| None/unknown | Reference | ||||
| Yes | 2.431 (1.397–4.231) | 0.001 | 2.275 (1.285–4.028) | 0.005 | |
| Chemotherapy | |||||
| None/unknown | Reference | ||||
| Yes | 1.283 (0.807–2.041) | 0.293 | |||
| Surgery | |||||
| Sub | Reference | ||||
| Lob | 0.597 (0.427–0.835) | 0.003 | 0.689 (0.490–0.968) | 0.032 | |
HRs are only reported on multivariate analysis if they remained significant. PSM, propensity score matching; DSS, disease-specific survival; Sub, sublobectomy; Lob, lobectomy; LN, lymph node; HR, Hazard ratio; CIs, confidence intervals; NS, not significant.
Table S4. Cox regression analysis of associations prognostic factors and OS in the PSM cohort.
| Characteristic | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Year of diagnosis (year) | |||||
| 2004–2006 | Reference | ||||
| 2007–2009 | 1.191 (0.849–1.671) | 0.311 | |||
| 2010–2013 | 0.807 (0.564–1.156) | 0.243 | |||
| Age group (year) | |||||
| 37–64 | Reference | ||||
| 65–73 | 1.538 (1.077–2.198) | 0.018 | 1.744 (1.210–2.514) | 0.003 | |
| 74–96 | 2.177 (1.556–3.046) | <0.001 | 2.219 (1.567–3.142) | <0.001 | |
| Insurance status | |||||
| None | Reference | ||||
| Yes | / | / | |||
| Unknown | / | / | |||
| Sex | |||||
| Male | Reference | ||||
| Female | 0.620 (0.475–0.808) | <0.001 | 0.589 (0.448–0.775) | <0.001 | |
| Race | |||||
| Black | Reference | ||||
| White | 1.620 (0.972–2.700) | 0.064 | NS | ||
| Others | 0.805 (0.374–1.736) | 0.581 | NS | ||
| Cost-of-living index | |||||
| ≤1 | Reference | ||||
| >1 | 1.000 (0.766–1.305) | 1.000 | |||
| Marital status | |||||
| Single | Reference | ||||
| Married | 0.862 (0.568–1.309) | 0.487 | |||
| Divorced/separated | 0.807 (0.452–1.439) | 0.468 | |||
| Others | 0.930 (0.583–1.483) | 0.760 | |||
| Laterality | |||||
| Right | Reference | ||||
| Left | 0.889 (0.676–1.170) | 0.403 | |||
| Lobe | |||||
| Upper | Reference | ||||
| Middle | 1.219 (0.727–2.043) | 0.454 | |||
| Lower | 1.010 (0.744–1.371) | 0.950 | |||
| Others/unknown | 1.063 (0.338–3.338) | 0.917 | |||
| Differentiation | |||||
| Well | Reference | ||||
| Moderate | 1.425 (0.932–2.177) | 0.102 | |||
| Poor | 1.352 (0.859–2.126) | 0.192 | |||
| Unknown | 0.488 (0.148–1.612) | 0.239 | |||
| Pathological type | |||||
| Adenocarcinoma | Reference | ||||
| Squamous carcinoma | 1.171 (0.841–1.631) | 0.349 | |||
| Lymphadenectomy/biopsy | |||||
| None | Reference | ||||
| Yes | 0.524 (0.381–0.721) | <0.001 | 0.575 (0.414–0.798) | 0.001 | |
| Number of LNs sampled | |||||
| None/Unknown | Reference | ||||
| 1–3 | 0.916 (0.661–1.270) | 0.599 | NS | ||
| 4–6 | 0.716 (0.491–1.044) | 0.083 | NS | ||
| 7–9 | 0.6027 (0.2194–1.0491) | 0.073 | NS | ||
| 10–12 | 0.476 (0.219–1.034) | 0.061 | NS | ||
| ≥13 | 0.373 (0.192–0.723) | 0.004 | NS | ||
| Radiation | |||||
| None/unknown | Reference | ||||
| Yes | 1.838 (1.118–3.022) | 0.016 | 2.101 (1.260–3.503) | 0.004 | |
| Chemotherapy | |||||
| None/unknown | Reference | ||||
| Yes | 0.892 (0.583–1.364) | 0.597 | |||
| Surgery | |||||
| Sub | Reference | ||||
| Lob | 0.619 (0.473–0.810) | <0.001 | 0.723 (0.549–0.953) | 0.021 | |
HRs are only reported on multivariate analysis if they remained significant. PSM, propensity score matching; OS, overall survival; Sub, sublobectomy; Lob, lobectomy; LN, lymph node; HR, hazard ratio; CIs, confidence intervals; NS, not significant.
Survival analysis using the IPTW approach
Both univariate (DSS: 0.825, 95% CI: 0.720–0.946, P=0.006; OS 0.796, 95% CI: 0.714–0.888, P<0.001) and multivariate (DSS: HR 0.765, 95% CI: 0.665–0.882, P<0.001; OS: HR 0.724, 95% CI: 0.646–0.812, P<0.001) IPTW Cox PH models showed that lobectomy was associated with decreased mortality compared to sublobectomy (Tables 2,3, Tables S5,S6). Figure 2E,F depict the KM curves for DSS and OS in the IPTW cohort (P<0.001).
Table S5. Cox regression analysis of associations prognostic factors and DSS in the IPTW cohort.
| Characteristic | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Year of diagnosis (year) | |||||
| 2004–2006 | Reference | ||||
| 2007–2009 | 1.079 (0.912–1.277) | 0.374 | 0.92 (0.775–1.101) | 0.3751 | |
| 2010–2013 | 0.845 (0.706–1.010) | 0.065 | 0.776 (0.645–0.935) | 0.008 | |
| Age group (year) | |||||
| 37–64 | Reference | ||||
| 65–73 | 1.385 (1.171–1.638) | <0.001 | 1.371 (1.152–1.631) | <0.001 | |
| 74–96 | 1.364 (1.145–1.625) | <0.001 | 1.481 (1.223–1.794) | <0.001 | |
| Insurance status | |||||
| None | Reference | ||||
| Yes | 2.124 (0.764–5.905) | 0.149 | |||
| Unknown | 2.213 (0.792–6.185) | 0.130 | |||
| Sex | |||||
| Male | Reference | ||||
| Female | 0.573 (0.500–0.656) | <0.001 | 0.553 (0.476–0.643) | <0.001 | |
| Race | |||||
| Black | Reference | ||||
| White | 1.626 (1.283–2.060) | <0.001 | 1.862 (1.443–2.404) | <0.001 | |
| Others | 0.555 (0.388–0.794) | 0.001 | 0.657 (0.442–0.977) | 0.038 | |
| Cost-of-living index | |||||
| ≤1 | Reference | ||||
| >1 | 1.010 (0.879–1.160) | 0.892 | |||
| Marital status | |||||
| Single | Reference | ||||
| Married | 0.824 (0.643–1.056) | 0.126 | |||
| Divorced/separated | 0.773 (0.570–1.048) | 0.098 | |||
| Others | 0.848 (0.642–1.121) | 0.247 | |||
| Laterality | |||||
| Right | Reference | ||||
| Left | 1.072 (0.933–1.233) | 0.326 | |||
| Lobe | |||||
| Upper | Reference | ||||
| Middle | 0.420 (0.288–0.613) | <0.001 | |||
| Lower | 0.712 (0.608–0.835) | <0.001 | |||
| Others/unknown | 0.302 (0.119–0.767) | 0.012 | |||
| Differentiation | |||||
| Well | Reference | ||||
| Moderate | 1.908 (1.499–2.428) | <0.001 | 1.735 (1.350–2.229) | <0.001 | |
| Poor | 1.442 (1.119–1.859) | 0.005 | 1.534 (1.175–2.003) | 0.002 | |
| Unknown | 0.727 (0.396–1.334) | 0.303 | 0.646 (0.349–1.195) | 0.163 | |
| Pathological type | |||||
| Adenocarcinoma | Reference | ||||
| Squamous carcinoma | 1.384 (1.166–1.644) | <0.001 | |||
| Lymphadenectomy/biopsy | |||||
| None | Reference | ||||
| Yes | 0.470 (0.401–0.551) | <0.001 | 0.463 (0.345–0.620) | <0.001 | |
| Number of LNs sampled | |||||
| None/unknown | Reference | ||||
| 1–3 | 0.713 (0.584–0.871) | <0.001 | NS | ||
| 4–6 | 0.540 (0.438–0.666) | <0.001 | NS | ||
| 7–9 | 0.540 (0.438–0.666) | 0.002 | NS | ||
| 10–12 | 0.484 (0.359–0.654) | <0.001 | NS | ||
| ≥13 | 0.447 (0.360–0.556) | <0.001 | NS | ||
| Radiation | |||||
| None/unknown | Reference | ||||
| Yes | 1.059 (0.782–1.436) | 0.710 | |||
| Chemotherapy | |||||
| None/unknown | Reference | ||||
| Yes | 0.813 (0.658–1.004) | 0.054 | NS | ||
| Surgery | |||||
| Sub | Reference | ||||
| Lob | 0.825 (0.720–0.946) | 0.006 | 0.765 (0.665–0.882) | <0.001 | |
HRs are only reported on multivariate analysis if they remained significant. IPTW, inverse probability of treatment weighted; DSS, disease-specific survival; Sub, sublobectomy; Lob, lobectomy; LN, lymph node; HR, hazard ratio; CIs, confidence intervals; NS, not significant.
Table S6. Cox regression analysis of associations prognostic factors and OS in the IPTW cohort.
| Characteristic | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Year of diagnosis (year) | |||||
| 2004–2006 | Reference | ||||
| 2007–2009 | 0.979 (0.849–1.128) | 0.765 | |||
| 2010–2013 | 1.030 (0.890–1.192) | 0.691 | |||
| Age group (year) | |||||
| 37–64 | Reference | ||||
| 65–73 | 1.456 (1.269–1.671) | <0.001 | 1.518 (1.314–1.753) | <0.001 | |
| 74–96 | 1.974 (1.719–2.267) | <0.001 | 1.833 (1.572–2.138) | <0.001 | |
| Insurance status | |||||
| None | Reference | ||||
| Yes | 1.895 (0.865–4.151) | 0.110 | |||
| Unknown | 1.773 (0.806–3.904) | 0.155 | |||
| Sex | |||||
| Male | Reference | ||||
| Female | 0.576 (0.517–0.643) | <0.001 | 0.562 (0.498–0.634) | <0.001 | |
| Race | |||||
| Black | Reference | ||||
| White | 1.960 (1.605–2.393) | <0.001 | 2.112 (1.706–2.613) | <0.001 | |
| Others | 0.450 (0.327–0.620) | <0.001 | 0.561 (0.394–0.800) | 0.001 | |
| Cost-of-living index | |||||
| ≤1 | Reference | ||||
| >1 | 0.737 (0.657–0.827) | <0.001 | NS | ||
| Marital status | |||||
| Single | Reference | ||||
| Married | 0.655 (0.545–0.788) | <0.001 | 0.627 (0.518–0.758) | <0.001 | |
| Divorced/separated | 0.551 (0.434–0.701) | <0.001 | 0.612 (0.475–0.789) | <0.001 | |
| Others | 0.746 (0.606–0.917) | <0.001 | 0.699 (0.560–0.871) | 0.001 | |
| Laterality | |||||
| Right | Reference | ||||
| Left | 0.943 (0.807–1.102) | 0.460 | |||
| Lobe | |||||
| Upper | Reference | ||||
| Middle | 0.545 (0.416–0.713) | <0.001 | 0.659 (0.495–0.879) | 0.005 | |
| Lower | 0.706 (0.621–0.802) | <0.001 | 0.867 (0.758–0.992) | 0.037 | |
| Others/unknown | 0.526 (0.295–0.940) | 0.030 | 0.631 (0.351–1.134) | 0.124 | |
| Differentiation | |||||
| Well | Reference | ||||
| Moderate | 1.570 (1.309–1.883) | <0.001 | 1.420 (1.171–1.721) | <0.001 | |
| Poor | 1.252 (1.034–1.515) | 0.021 | 1.406 (1.145–1.726) | 0.001 | |
| Unknown | 0.944 (0.628–1.419) | 0.781 | 0.842 (0.555–1.277) | 0.419 | |
| Pathological type | |||||
| Adenocarcinoma | Reference | ||||
| Squamous carcinoma | 1.529 (1.337–1.749) | <0.001 | 1.178 (1.023–1.356) | 0.022 | |
| Lymphadenectomy/biopsy | |||||
| None | Reference | ||||
| Yes | 0.511 (0.448–0.583) | <0.001 | 0.569 (0.454–0.713) | <0.001 | |
| Number of LNs sampled | |||||
| None/unknown | Reference | ||||
| 1–3 | 0.687 (0.583–0.810) | <0.001 | NS | ||
| 4–6 | 0.582 (0.493–0.687) | <0.001 | NS | ||
| 7–9 | 0.894 (0.758–1.054) | 0.184 | NS | ||
| 10–12 | 0.376 (0.287–0.493) | <0.001 | NS | ||
| ≥13 | 0.418 (0.350–0.499) | <0.001 | NS | ||
| Radiation | |||||
| None/unknown | Reference | ||||
| Yes | 0.846 (0.647–1.105) | 0.220 | |||
| Chemotherapy | |||||
| None/unknown | Reference | ||||
| Yes | 0.601 (0.499–0.724) | <0.001 | NS | ||
| Surgery | |||||
| Sub | Reference | ||||
| Lob | 0.796(0.714–0.888) | <0.001 | 0.724(0.646–0.812) | <0.001 | |
HRs are only reported on multivariate analysis if they remained significant. IPTW, inverse probability of treatment weighted; OS, overall survival; Sub, sublobectomy; Lob, lobectomy; LN, lymph node; HR, Hazard ratio; CIs, confidence intervals; NS, not significant.
HRs in three different cohorts
In our univariate and multivariate analyses based on three different cohorts (the unmatched cohort, the propensity-based matched cohort, and the IPTW cohort), all 12 Cox PH regression models demonstrated that the type of surgery (Sub vs. Lob) was an independent prognostic factor for early-stage NSCLC with VPI. Lobectomy was associated with a reduced mortality and prolonged survival, with HRs ranging from 0.418 to 0.985. IPTW revealed the relatively more conservative differences between sublobectomy and lobectomy compared with the univariate analysis based on the unmatched cohort.
Discussion
The standard surgical procedure of NSCLC in the early stage, pulmonary lobectomy combined with mediastinal LN dissection, has existed for years and has been considered a curative procedure that for lung cancer. Over the last few decades, the population demographics of NSCLC have changed, with an increasing number of elderly patients with many comorbidities being diagnosed, which has renewed interest in sublobectomy as an alternative therapeutic approach in patients with poor pulmonary function. There are several components regarding NSCLC staging that are able to influence surgeons’ treatment strategies (22). VPI is one of the important elements in the treatment of early-stage resectable NSCLC, and the optimal extent of the surgical procedure for early-stage NSCLC with VPI is still under debate and not deeply understood. No randomized controlled trials comparing survival after sublobectomy or lobectomy have been reported to date. Moreover, the results based on several small single-institution studies were subject to selection bias (10,12).
To address these limitations, we evaluated the survival outcomes of patients undergoing sublobectomy versus lobectomy in three different cohorts by performing a propensity analysis of a SEER sample of 1,386 patients diagnosed with early-stage NSCLC (≤2 cm) with VPI. We found that the extent of the surgical resection (Sub vs. Lob) was an independent prognostic factor by virtue of either the univariate or multivariate Cox PH model. In addition, patients treated with lobectomy experienced better survival outcomes than those who were treated with sublobectomy. This benefit was consistent after PSM and IPTW analyses. To the best of our knowledge, this is the largest cohort examined to dedicatedly compare lobectomy and sublobectomy in small-sized/VPI NSCLC. The SEER program collects data on cancer patients from 18 registries throughout the United States, resulting in a high level of generalizability. Thus, our findings were based on a comprehensive setting and had strong external validity. Moreover, an adequate follow-up time and relatively complete survival data provided sufficient power to assess the efficacy of the extent of surgical resection. Finally, propensity analysis was performed to produce less biased estimates of treatment effects. However, a disadvantage of PSM is the exclusion of many unmatched cases. The IPTW approach was used to verify the results of 1:1 matching based on the entire study population.
There are several studies regarding surgical strategies for early-stage NSCLC with VPI. Xie et al. (10) reported that sublobar resection was an independent risk factor for recurrence in patients with lung adenocarcinomas with sizes of 2 cm or less and VPI positivity. A study by Jiwangga et al. (13) reported that VPI was a significant predictive factor for pleural seeding and bilateral lung metastasis as patterns of recurrence in pathologic stage I lung adenocarcinoma, which might be the main obstacle for long-term survival after resection. However, neither long-term DSS nor OS stratified by the extent of surgical resection was calculated in these two studies. Wo and colleagues (11) analyzed the prognostic value of surgical extent in patients with T1-sized/VPI tumors between 2010 and 2015 based on the SEER database and showed that patients who underwent sublobectomy had slightly shorter survival times than those who underwent lobectomy, but the difference was not statistically significant. The time periods of studies, inclusion and exclusion criteria of the study cohort, and covariates included in the survival models may have led to inconsistent results. However, their study had several limitations, including a limited number of patients who underwent sublobectomy, relatively few outcome events, and a short follow-up due to their data being extracted from the SEER program (2017 update). In contrast to their study, data on the extent of VPI (PL1 and PL2) were not collected in our study for the survival analysis because data were not available before 2010 in the SEER database; however, several studies have confirmed that the extent of VPI may not influence survival outcomes in patients with completely resected NSCLC with VPI (11,23,24). Moon et al. (12) studied the surgical outcomes of 89 NSCLC patients with VPI (N=38) or lymphovascular invasion (N=71) and showed that the OS rate did not differ significantly by the surgical extent (P=0.615), and sublobar resection was not an independent risk factor according to the multivariate analysis. However, their study, a single-center, retrospective review, was limited to a small sample size and few outcome events, which might have affected the performance of regression modeling and could have led to misleading results (25-27). In addition, their study did not distinguish VPI from lymphovascular invasion, which is also a poor prognostic feature of small-sized NSCLC and might have a simultaneous effect on VPI (9,28).
The impact of surgical procedures on survival could be attributed to several underlying reasons. First, VPI is correlated with a high incidence of LN metastasis, and lobectomy shows good lymphatic clearance and sampling. Kudo et al. (29) found that the visceral pleura is phenomenally rich in lymphatic vessels, with an intercommunicating network arranged over the lung surface that penetrates into the lung parenchyma to join the bronchial lymph vessels with drainage to various hilar LNs. Moreover, Imai et al. (30) showed that the lymphatic vessels beneath the pleura might flow directly into the mediastinum without passing through the hilar LNs, which causes skip N2 metastases. Thus, lobectomy, which tends to perform more extensive LN resection, is associated with a better prognosis compared with sublobectomy. Second, there is potentially a concern that sublobectomy is associated with less LN sampling or dissection than lobectomy, resulting in a less precise prediction of nodal staging accuracy (31). Although the current study excluded patients with LN involvement, more undiscovered metastatic LNs might exist in the sublobectomy group due to less extensive lymphadenectomy. In addition, it is quite easy for a sublobectomy to damage the integrity of the LN and disrupt the drainage system, thus reducing lymphatic fluid release during the process of segmental LN dissection. Finally, regardless of how carefully a sublobar resection of NSCLC is performed, the possibility of cancer cells at the surgical margin remains, which is associated with locoregional recurrence and a poor prognosis (32,33). Lobectomy is a more reliable procedure to obtain R0 resection at the surgical margin than sublobectomy. In summary, the focus should be on the quality of the resection—with the surgeon routinely performing hilar and mediastinal LN sampling and ensuring as wide of a resection margin as possible.
In general, lobectomy showed superior OS and DSS rates compared with sublobectomy in our current study. The survival rates reported in our analysis are comparable to those reported in previous studies (10-12,34). No prior studies have reported long-term OS data in patients with small-sized NSCLC accompanied by VPI and treated with sublobectomy or lobectomy. Thus, the OS rates reported in the current study can serve as a benchmark for future comparisons. In addition, the biological characteristics of VPI itself and the extent of LN dissection are the main factors that contributed to the difference in survival outcomes of the two kinds of surgical procedures.
Previous studies demonstrated that patients who underwent sublobectomy were older, and had worse cardiopulmonary reserve and other associated comorbidity (35,36), which might cause treatment selection biases. In the current study, with attempts to correct for selection bias by using the PSM analysis and the IPTW method, multivariate Cox PH models show that lobectomy was associated with decreased mortality compared to sublobectomy (P<0.05). The choice of surgical resections is still associated with the location of tumor (lesion of lobe), its proximity to important blood vessels, and blood vessel invasion (BVI) (37). Based on SEER database, Lin et al. (38) compared the prognosis of patients with stage IA right middle lobe (RML) NSCLC, and concluded that patients undergoing lobectomy had better prognosis than those undergoing sublobectomy. Gabor et al. (37) reported that the BVI should be considered as an important prognostic factor with a higher risk for recurrence in resectable N0M0 patients with NSCLC, which might emphasize the significance of extensive resection.
One of the limitations of the SEER database is the lack of detailed data, such as smoking history, tumor location (central vs. peripheral), type of resection (R0, R1 or R2), and comorbidities. In addition, information about recurrence is not available, and progression-free survival cannot be calculated. However, this current study showed comparable DSS rates to those previously published, and DSS can be assumed to be an indirect extension of progression-free survival. Another limitation is its retrospective nature. Retrospective studies almost always have bias because prognostic factors are unequally distributed between patients exposed or not exposed to an intervention (27,39). PSM analysis and the IPTW method were applied in the current study to control the covariate imbalance and selection bias. Some prognostic factors, however, may still be missing or suboptimally measured, which bias the estimate of the treatment effect (i.e., residual confounding) (39).
In summary, our study analyzed the treatment effect of sublobectomy and lobectomy procedures on the survival of early-stage NSCLC patients with VPI and can conclude that patients treated with lobectomy exhibited better OS and DSS outcomes for the treatment of small-sized (≤2 cm) tumors in the early stage of NSCLC characterized by VPI than those who were treated with sublobectomy. Thus, our findings might provide good surgical guidance for the treatment of patients in the early stage of NSCLC accompanied by VPI in the absence of a randomized clinical trial.
Acknowledgments
Funding: This work was supported by Shanghai Shenkang Medicine Developing Project (Grant No. SHDC12014107), Shanghai Science and Technology Committee Medicine Leading Project (Grant No. 15411960100), and Shanghai Leading Talent Program (Grant No. 44 LJRC-WZN).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. In this study, we use data from surveillance, epidemiology and end results (SEER) database: a publicly available database. The Institutional Review Committee of Changzheng Hospital (Naval Medical University, Shanghai, China) deemed the study to be nonhuman subject research and therefore approved this study to be exempted research.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Church TR, Black WC, Aberle DR, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. 10.1056/NEJMoa1209120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Travis WD, Brambilla E, Rami-Porta R, et al. Visceral pleural invasion: pathologic criteria and use of elastic stains: proposal for the 7th edition of the TNM classification for lung cancer. J Thorac Oncol 2008;3:1384-90. [DOI] [PubMed] [Google Scholar]
- 4.Nitadori JI, Colovos C, Kadota K, et al. Visceral pleural invasion does not affect recurrence or overall survival among patients with lung adenocarcinoma ≤ 2 cm: a proposal to reclassify T1 lung adenocarcinoma. Chest 2013;144:1622-31. 10.1378/chest.13-0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng HY, Li G, Luo J, et al. Novel biologic factors correlated to visceral pleural invasion in early-stage non-small cell lung cancer less than 3 cm. J Thorac Dis 2018;10:2357-64. 10.21037/jtd.2018.03.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agalioti T, Giannou AD, Stathopoulos GT. Pleural involvement in lung cancer. J Thorac Dis 2015;7:1021-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao C, Gupta S, Chandrakumar D, et al. Meta-analysis of intentional sublobar resections versus lobectomy for early stage non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:134-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 22-3. 10.1016/0003-4975(95)00537-U [DOI] [PubMed] [Google Scholar]
- 9.Yip R, Ma T, Flores RM, et al. Survival with Parenchymal and Pleural Invasion of Non-Small Cell Lung Cancers Less than 30 mm. J Thorac Oncol 2019;14:890-902. 10.1016/j.jtho.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 10.Xie H, Su H, Chen D, et al. Use of autofluorescence to intraoperatively diagnose visceral pleural invasion from frozen sections in patients with lung adenocarcinoma 2 cm or less. Am J Clin Pathol 2019;152:608-15. 10.1093/ajcp/aqz081 [DOI] [PubMed] [Google Scholar]
- 11.Wo Y, Zhao Y, Qiu T, et al. Impact of visceral pleural invasion on the association of extent of lymphadenectomy and survival in stage I non-small cell lung cancer. Cancer Med 2019;8:669-78. 10.1002/cam4.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moon Y, Lee KY, Park JK. Prognosis after sublobar resection of small-sized non-small cell lung cancer with visceral pleural or lymphovascular invasion. World J Surg 2017;41:2769-77. 10.1007/s00268-017-4075-7 [DOI] [PubMed] [Google Scholar]
- 13.Jiwangga D, Cho S, Kim K, et al. Recurrence pattern of pathologic stage I lung adenocarcinoma with visceral pleural invasion. Ann Thorac Surg 2017;103:1126-31. 10.1016/j.athoracsur.2016.09.052 [DOI] [PubMed] [Google Scholar]
- 14.Howlader N, Krapcho M, Garshell J, et al. National Cancer Institute: Surveillance, Epidemiology, and End Results. SEER Cancer Statistics Review 1975−2010 2013.
- 15.Lakha S, Gomez JE, Flores RM, et al. Prognostic significance of visceral pleural involvement in early-stage lung cancer. Chest 2014;146:1619-26. 10.1378/chest.14-0204 [DOI] [PubMed] [Google Scholar]
- 16.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res 2004;10:7252-9. 10.1158/1078-0432.CCR-04-0713 [DOI] [PubMed] [Google Scholar]
- 17.Xie JD, Chen F, He YX, et al. Old age at diagnosis increases risk of tumor progression in nasopharyngeal cancer. Oncotarget 2016;7:66170-81. 10.18632/oncotarget.10818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D'Agostino RB, Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265-81. [DOI] [PubMed] [Google Scholar]
- 19.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399-424. 10.1080/00273171.2011.568786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150-61. 10.1002/pst.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med 2015;34:3661-79. 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res 2014;3:242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seok Y, Jeong JY, Lee E. Extent of visceral pleural invasion and the prognosis of surgically resected node-negative non-small cell lung cancer. Thorac Cancer 2017;8:197-202. 10.1111/1759-7714.12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adachi H, Tsuboi M, Nishii T, et al. Influence of visceral pleural invasion on survival in completely resected non-small-cell lung cancer. Eur J Cardiothorac Surg 2015;48:691-7; discussion 7. 10.1093/ejcts/ezu515 [DOI] [PubMed] [Google Scholar]
- 25.Courvoisier DS, Combescure C, Agoritsas T, et al. Performance of logistic regression modeling: beyond the number of events per variable, the role of data structure. J Clin Epidemiol 2011;64:993-1000. 10.1016/j.jclinepi.2010.11.012 [DOI] [PubMed] [Google Scholar]
- 26.Austin PC, Steyerberg EW. The number of subjects per variable required in linear regression analyses. J Clin Epidemiol 2015;68:627-36. 10.1016/j.jclinepi.2014.12.014 [DOI] [PubMed] [Google Scholar]
- 27.Agoritsas T, Merglen A, Shah ND, et al. Adjusted analyses in studies addressing therapy and harm: users' guides to the medical literature. JAMA 2017;317:748-59. 10.1001/jama.2016.20029 [DOI] [PubMed] [Google Scholar]
- 28.Mollberg NM, Bennette C, Howell E, et al. Lymphovascular invasion as a prognostic indicator in stage I non-small cell lung cancer: a systematic review and meta-analysis. Ann Thorac Surg 2014;97:965-71. 10.1016/j.athoracsur.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 29.Kudo Y, Saji H, Shimada Y, et al. Impact of visceral pleural invasion on the survival of patients with non-small cell lung cancer. Lung Cancer 2012;78:153-60. 10.1016/j.lungcan.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 30.Imai K, Minamiya Y, Saito H, et al. Detection of pleural lymph flow using indocyanine green fluorescence imaging in non-small cell lung cancer surgery: a preliminary study. Surg Today 2013;43:249-54. 10.1007/s00595-012-0237-2 [DOI] [PubMed] [Google Scholar]
- 31.Martin JT, Durbin EB, Chen L, et al. Nodal upstaging during lung cancer resection is associated with surgical approach. Ann Thorac Surg 2016;101:238-44; discussion 44-5. 10.1016/j.athoracsur.2015.05.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sawabata N. Locoregional recurrence after pulmonary sublobar resection of non-small cell lung cancer: can it be reduced by considering cancer cells at the surgical margin? Gen Thorac Cardiovasc Surg 2013;61:9-16. 10.1007/s11748-012-0156-6 [DOI] [PubMed] [Google Scholar]
- 33.Sawabata N, Maeda H, Matsumura A, et al. Clinical implications of the margin cytology findings and margin/tumor size ratio in patients who underwent pulmonary excision for peripheral non-small cell lung cancer. Surg Today 2012;42:238-44. 10.1007/s00595-011-0031-6 [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Sun F, Chen L, et al. Prognostic value of visceral pleural invasion in non-small cell lung cancer: A propensity score matching study based on the SEER registry. J Surg Oncol 2017;116:398-406. 10.1002/jso.24677 [DOI] [PubMed] [Google Scholar]
- 35.El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg 2006;82:408-15; discussion 15-6. 10.1016/j.athoracsur.2006.02.029 [DOI] [PubMed] [Google Scholar]
- 36.Berfield KS, Wood DE. Sublobar resection for stage IA non-small cell lung cancer. J Thorac Dis 2017;9:S208-10. 10.21037/jtd.2017.03.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabor S, Renner H, Popper H, et al. Invasion of blood vessels as significant prognostic factor in radically resected T1-3N0M0 non-small-cell lung cancer. Eur J Cardiothorac Surg 2004;25:439-42. 10.1016/j.ejcts.2003.11.033 [DOI] [PubMed] [Google Scholar]
- 38.Lin G, Liu H, Li J. Lobectomy versus sub-lobar resection in patients with stage IA right middle lobe non-small cell lung cancer: a propensity score matched analysis. J Thorac Dis 2019;11:2523-34. 10.21037/jtd.2019.05.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stukel TA, Fisher ES, Wennberg DE, et al. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA 2007;297:278-85. 10.1001/jama.297.3.278 [DOI] [PMC free article] [PubMed] [Google Scholar]