Abstract
Ebstein’s anomaly (EA) is a rare congenital cardiac anomaly. It is a disease at a minimum of the tricuspid valve (TV) and the right ventricular myocardium. Presentation varies from a severe symptomatic form during the neonatal period to an incidental detection later in life due to the wide morphological variation of the condition. The neonatal presentation can be severe and every attempt should be made at medical management ideally into infancy and early childhood. Neonates not eligible or failing medical management should be surgically managed either with a single ventricle palliative approach or a more desirable biventricular repair with a neonatal TV valvuloplasty. Some neonates initially committed to a single ventricle pathway may be converted to a biventricular repair by a delayed TV valvuloplasty. The da Silva Cone repair has become the valvuloplasty of choice especially beyond the neonatal period and can be applied to a wide morphological variation of the condition with good long-term durability. If the chance of a successful TV valvuloplasty is high, it should be offered early in childhood to prevent further cardiac dilation. Adding a Bidirectional Glenn to a valvuloplasty may help salvage marginal risk patients or marginally repairable valves. If valve replacement is the only option, a bioprosthetic valve should be used as it is less thrombogenic especially with marginal right ventricular function. Heart transplantation should be considered in patients with associated left ventricular dysfunction.
Keywords: Ebstein’s anomaly (EA), Modified Starnes procedure, Knott-Craig procedure, da Silva Cone repair
Introduction
First described by Wilhelm Ebstein in 1866 (1), Ebstein’s anomaly (EA) is a rare congenital cardiac anomaly involving the tricuspid valve (TV) and the right ventricle (RV) (2). It accounts for <1% incidence of all congenital cardiac anomalies. Presentation varies from a severe symptomatic form during the neonatal period to incidental detection later in life including late adulthood (3). This is attributable to the wide morphological variation of the condition.
The following are the features pathognomonic to the EA (4):
Rotational and apical displacement with failure of delamination of the tricuspid valve leaflets, particularly the septal and the inferior leaflets. The anterior valve displacement is limited by the ventriculoinfundibular fold;
A large sail-like anterior leaflet with abnormal chordal attachments to the ventricular wall. Symptomatic neonates are more likely to have a more severe abnormality of the anterior leaflet (5);
Division of the right ventricle proximally into a dilated, non-trabeculated and often thinned out “atrialized right ventricle” (ARV) by the apically displaced TV leaflets attached at the functional annulus and distally the “Functional right ventricle” (FRV). The true anatomic annulus is always dilated;
Varying degrees of infundibular RV obstruction by the redundant anterior tricuspid valvular tissue and its aberrant choral attachments. Failure of the leaflet to separate (delaminate) from the underlying myocardium is present to a variable extent. When the anterior leaflet is involved, in addition to causing TV regurgitation due to non-coaptation from restriction, the right ventricular outflow tract (RVOT) can get obstructed with ventricular outflow confined to the anteroseptal commissure in severely affected cases.
Other abnormalities include:
Either a patent foramen ovale or a Secundum ASD;
Pulmonary valve atresia or stenosis;
Ventricular septal defect;
Wolff-Parkinson—white type of accessory pathway in 10–20% cases. These pathways are commonly around the orifice of the TV;
Varying degree of myopathy of the right ventricle with a decrease in the number of sarcomeric units (2).
While various other congenital cardiac abnormalities have been described including complex conotruncal anomalies, hypoplastic left heart syndrome, all such abnormalities occur with an incidence of <0.5% in all EA cases (6,7). In one series, the following were the associated anomalies with the incidence of >0.5%; ASD/PFO (50%), pulmonary valve stenosis/atresia (4%) and VSD (3%) (6). There is an Ebstein like affection of the left atrioventricular valve in Congenitally corrected transposition of great arteries which is a completely different condition from Classic EA and will not be discussed here.
Classification
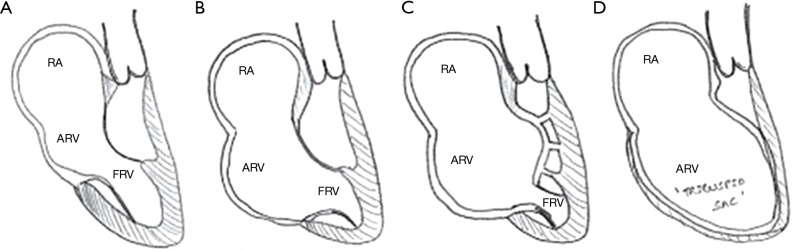
While different morphological classification exists, the most commonly described is the Carpentier classification (4) (Figure 1). It is particularly useful in the initial surgical planning. There are four types as described below.
Figure 1.
Carpentier classification of EA. EA, Ebstein’s anomaly. RA, right atrial; ARV, atrialized right ventricle; FRV, functional right ventricle.
Type A
Mild apical displacement of the leaflets, mildly reduced size of FRV, and normal morphology of the anterior leaflet.
Type B
Moderate apical displacement of the leaflets, a moderate reduction of FRV volume but generally of an adequate in size, anterior leaflet may have abnormal chordal attachments but with normal mobility of the anterior leaflet.
Type C
Severe apical displacement of the leaflets with a small FRV. Anterior leaflet restriction due to abnormal chordal attachments with possible RVOT obstruction.
Type D
“Tricuspid Sac”. Complete non-delamination of the leaflets with an only infundibular portion of the RV remaining.
Much of the variation in the presentation can be explained by the morphological variation in EA. The following morphological determinants are particularly important:
Degree of displacement and extent of delamination of the TV leaflets thus causing non-coaptation leading to regurgitation;
Size of the anterior leaflet of the TV;
Size of the FRV;
RVOT obstruction by abnormal chordal attachments of the anterior leaflet;
Status of the pulmonary valve.
A symptomatic neonate is more likely to have poorly coapting TV leaflets from severe displacement and poor delamination with a small FRV and a restrictive anterior leaflet causing RVOT. There may be functional or anatomical pulmonary valve atresia which further worsens the clinical condition.
Presentation
Presentation varies with age. EA may be picked up as early as during a fetal scan. Such a fetus may survive or may perish due to the development of severe hydrops. A symptomatic neonate is generally cyanotic. Infants are more likely to develop heart failure, whereas, in older children and adults, the presentation may vary from an incidentally detected heart murmur to arrhythmias and exercise intolerance (3). Much of the mortality correlates to an early presentation such as a fetal presentation and echocardiographic severity. Later morbidity is due to gradual hemodynamic deterioration and development of arrythmias (3).
Work-up
A chest radiograph will show varying degrees of cardiomegaly. Cardiomegaly can be particularly massive in a neonate (wall to wall heart or a Box-shaped heart). A cardiothoracic ratio greater than 0.65 is prognostic for poor outcomes in a symptomatic neonate (3). Much of the cardiomegaly is from the dilated RA and the ARV. Resolution of the cardiomegaly after surgical repair can be prognostic and is a useful follow-up tool (8).
EA is primarily diagnosed by echocardiography. Displacement of the septal leaflet of the TV ≥8 mm/m2 from the crux of the heart and presence of an elongated and redundant anterior leaflet of the TV increases the chance of a diagnosis of EA. Otherwise, other conditions such as TV dysplasia should be considered (9). It is also helpful in grading the severity of the EA and has been used for prognostication in the neonatal period using the Celermajer Index (10). To obtain the score, the combined area of the right atrium and ARV is divided by the area of the FRV and the left heart chambers obtained from a four-chamber view at end-diastole (Figure 2). Four grades of increasing severity are obtained: grade 1, ratio <0.5; grade 2, 0.5 to 0.99; grade 3, 1 to 1.49; and grade 4 ≥1.5 with associated early untreated mortality of 0%, 10%, 44%, and 50% respectively in the neonatal period.
Figure 2.
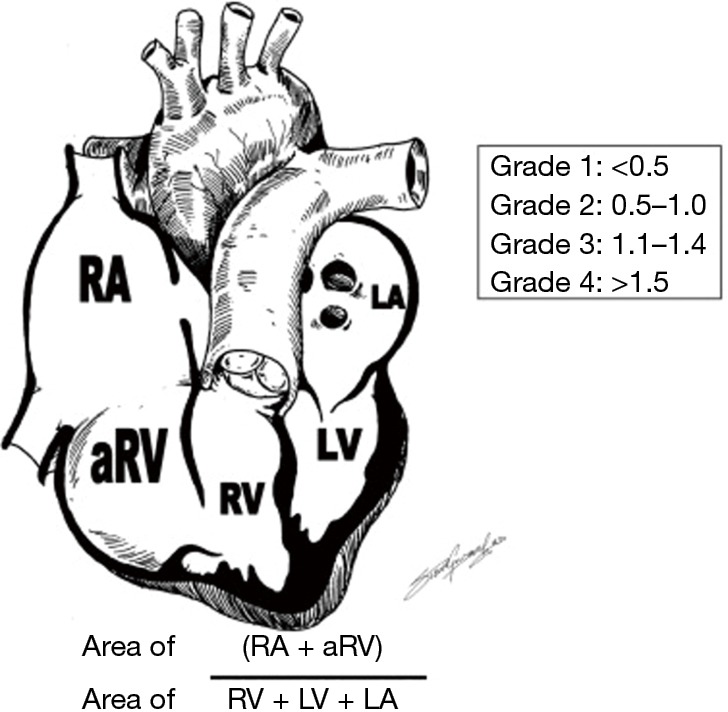
Celermajer index. RA, right atrial; aRV, atrialized right ventricle; RV, right ventricle; LA, left atrium; LV, left ventricle.
Cardiac MRI helps in better visualization of the posterior leaflet of the TV and for better quantitative assessment of the right ventricular size and function as compared to echocardiography. Thus, cardiac MRI is a complementary imaging modality to echocardiography and when combined with echocardiography may help in better risk stratification (11).
Preoperative electrophysiological evaluation should be considered especially in patients with preexcitation, documented arrhythmias or suspected arrhythmias (12). It has both a high diagnostic and therapeutic yield. Therapeutic electrophysiological interventions can be performed both preoperatively and intraoperatively (13). The burden of arrhythmias increases with increasing age. SVT is the most common arrhythmia in the younger age group (14). Atrial fibrillation or flutter is more common in older patients.
Management
Neonates and infants
Pathophysiology
Severely symptomatic neonates have severe right heart failure or are profoundly cyanotic. Neonates with severe morphological forms of EA (Carpentier type C and D) have a small FRV with severe TR, restricted motion of the anterior leaflet and varying degrees of RVOT. With the physiological elevation in the PVR after birth, the FRV is not able to provide for antegrade pulmonary blood flow, especially when the ductus is open leading to a “Functional pulmonary valve atresia”. This condition may be compounded if true “Anatomic pulmonary valve atresia exists”. Another serious condition is when there is pulmonary valve insufficiency with a poorly functioning FRV and severe TR with an ASD and a patent ductus. This is a set-up for a “Circular shunt” with retrograde flow down the RV into the LV via the ASD. It creates a hemodynamically unstable condition with severe hypoxia and diminished systemic cardiac output. Such a presentation may occur spontaneously or occur after the pulmonary valve is opened by an intervention. The gross cardiomegaly may also cause lung hypoplasia by a mass effect. The dilated RV may pancake the LV. The presence of a VSD may further exacerbate an already tenuous condition by creating an LV to RA shunt (15) With the less severe forms of EA (Carpentier type A and B), as the PVR falls or when helped by pulmonary vasodilators such as iNO, the FRV is able to generate an effective output to provide for an antegrade pulmonary blood flow with clinical improvement. Such neonates may be able to get out of the neonatal period without any surgical intervention. However, such neonates should be carefully followed into infancy as the TR may worsen over time leading to worsening cardiomegaly from RA dilation and thinning out of the ARV.
Medical management
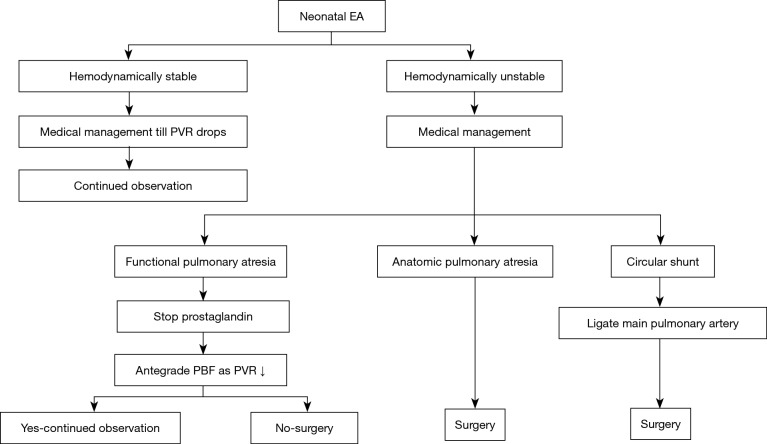
Relatively stable neonates may be observed with supplemental oxygen and prostaglandin infusions. Unstable neonates are managed with intubation, mechanical ventilation, prostaglandin infusion, iNo, and inotropic support. The goal of medical management is to help with the antegrade pulmonary flow by supporting the FRV. This generally improves as the PVR gradually falls. Serial echocardiograms are useful to asses for antegrade pulmonary blood flow. Prostaglandin is important to maintain ductal patency, particularly in neonates with pulmonary atresia. On the contrary, a trial of prostaglandin withdrawal may be needed in functional pulmonary atresia. Similarly, in a circular shunt, early withdrawal may be necessary. In one study, early withdrawal of prostaglandins lead to a mortality benefit once anatomic obstruction was ruled out (16). Some neonates may need ECMO support for stabilization before the surgical intervention (17). Figure 3 describes the management flowchart. Neonates with a circular shunt may be treated with a 2-step procedure. The first step is ligation of the main pulmonary artery with or without branch PA banding followed by a Starnes procedure when stable (18).
Figure 3.
Management algorithm for neonatal EA. EA, Ebstein’s anomaly; PVR, pulmonary vascular resistance; PBF, pulmonary blood flow.
Surgical management
Patients with EA with anatomic pulmonary atresia, a circular shunt, dependency on prostaglandins or mechanical ventilation, worsening cyanosis or heart failure with inability to tolerate feeds due to mesenteric congestion, and generally severe forms such as Carpentier type C/D or GOSE score >3 will require a surgical intervention during the neonatal period. The surgical options include a biventricular repair pathway with a valvuloplasty. Such neonates have a favorable TV morphology such as a large and mobile anterior leaflet, a reasonably sized FRV and are generally of Carpentier type A or B. Anatomic pulmonary atresia is not a contraindication to a biventricular approach as an RV-PA conduit or a transannular patch with a monocusp can be used to establish continuity depending upon how satisfactory the valvuloplasty is (5). Neonates with very small FRV, poor TV morphology, TR jet velocity <3 m/s, and RV systolic pressure <30 mmHg by TR jet are channeled down the single ventricle pathway either with a modified Starnes procedure or a Sano right ventricular exclusion technique (19,20).
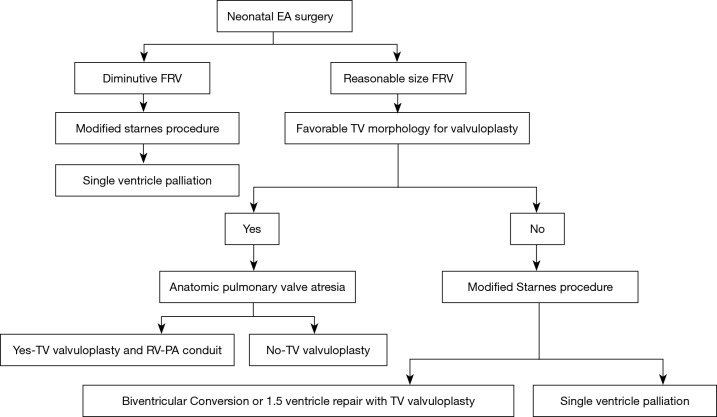
Some neonates who are hemodynamically unstable with some degree of end-organ damage yet have a favorable morphology for a biventricular repair and thus are unlikely to tolerate a prolonged procedure with a valvuloplasty or concerns exist for a possibility of a tenuous repair given the friability of neonatal valvular tissues can be channeled down the single ventricle pathway initially with a modified Starnes procedure (use Gortex patch, as it is more likely to preserve underlying leaflets) with a possibility for reassessment for a Biventricular conversion with a valvuloplasty or a 1.5 ventricle repair at the time of the Glenn procedure (two successful cases of Biventricular conversion at the time of Glenn after modified Starnes procedure by Dr. da Silva, awaiting publication). Similarly, some neonates with a marginal size FRV, who demonstrate interval growth may be reconsidered for a biventricular repair or 1.5 ventricle repair (5) (Figure 4).
Figure 4.
Surgical pathway in neonatal EA. EA, Ebstein’s anomaly; FRV, functional right ventricle; TV, tricuspid valve; RV, right ventricle; PA, pulmonary artery.
The key steps in a biventricular repair are
Mobilization of the TV leaflets. The extent of the leaflet mobilization depends upon the technique of the valvuloplasty used;
Plication and obliteration of the ARV;
Reduction of the true annulus;
Reduction right atrioplasty. This allows for more room for the lungs;
Fenestrated or partial closure of the atrial septum;
Establishment of RV-PA continuity in case of pulmonary valve atresia;
-
Closure of the VSD if present.
The two commonly described neonatal Tricuspid valvuloplasty techniques are described below.
Knott–Craig monocusp technique
This procedure revolutionized the neonatal management of EA by providing a biventricular repair option in a difficult to treat cohort of EA. The repair is centered on the adequate mobilization of the anterior leaflet with a well-supported mobile leading edge to help coapt with the ventricular septum to achieve valvular competency. The septal leaflet is retained as a ventricular septal structure. The following are the key steps:
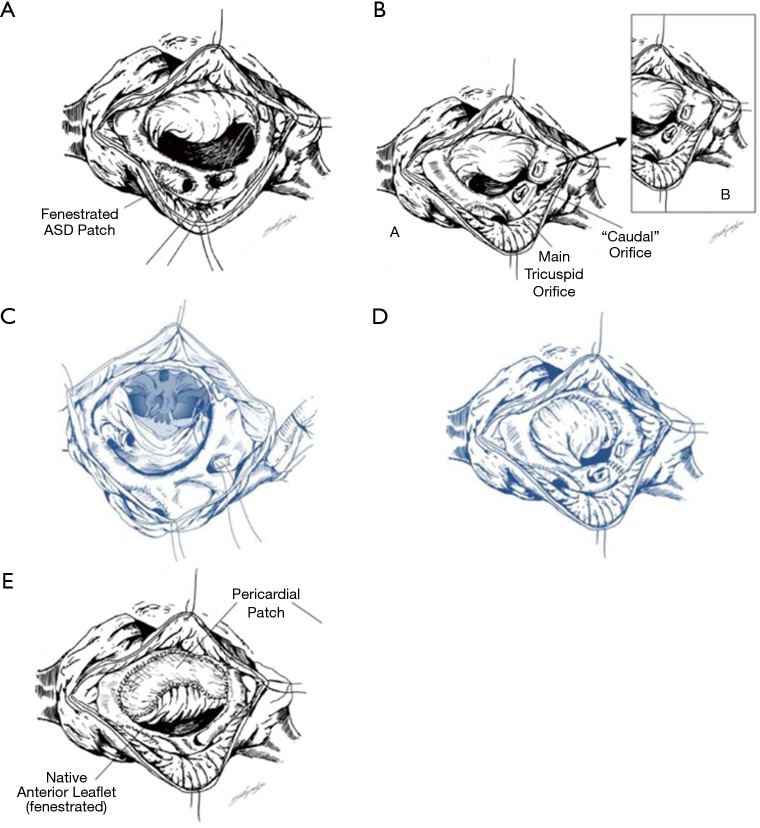
The posterior leaflet is obliterated by plicating it with a stitch that starts at the coronary sinus to the anteroposterior commissure (Figure 5A,B). This creates two orifices, the main tricuspid orifice, and a caudal orifice. The caudal orifice is obliterated with a vertical plication stitch, thus also obliterating the ARV. Care must be taken not to incorporate the right coronary artery during the plication;
The anterior leaflet may need to be delaminated and its body augmented in cases where it is adherent to the ventricular wall and is not of sufficient size to reach the ventricular septum. It is critical to have an adequately mobile and well supported free edge. A modified “Sebening stitch” from the ventricular septum to the anterior leaflet supports it further by proving a better hinge point (21) (Figure 5C,D,E);
The ASD is closed in a fenestrated fashion;
A right atrium reduction atrioplasty is performed (Figure 5A);
In the case of pulmonary atresia, either an RV-PA conduit or a transannular patch with a monocusp is used depending upon how satisfactory the annuloplasty is.
Figure 5.
Knott-Craig monocusp technique.
In a series of 32 consecutive symptomatic neonates with EA described by Knott-Craig, 60% (20/32) had associated pulmonary atresia. The average GOSE score was 1.5. The overall early mortality of the cohort was 28% (9/32). EA with pulmonary atresia had higher early mortality than without (40% vs. 8.3%, P<0.05). However, when an RV-PA conduit was used, the early mortality dropped from 44% to 16%, signifying an easier and stable postoperative course. Also, the biventricular repair rate was only 80% in EA with pulmonary atresia as compared to 100% in patients without it (22). In an earlier report from the same group involving both neonates and infant (23 and 9, respectively) albeit with a longer follow-up to 16 years, the 15-year survival estimate was 74%, with a freedom from reoperation rate of 74% in patients undergoing biventricular repair which included 2 late TV prosthetic valve replacement with a median late post-operative TR grade of 1/4 (23).
da Silva Cone repair
This procedure entails the delamination of all the three leaflets to create a 360 degree of coapting tricuspid valvular tissue to maintain valvular competency (it is described in detail in the next section). Since it is a more extensive procedure than the monocusp technique, concerns exist about the procedure in the context of the friability of neonatal valvular tissues and the more extensive nature of the procedure in an already sick neonate. Nevertheless, there have been successful case reports and case series of the Cone procedure in neonates (15,20). In one such case series of 7 patients with 5 being neonates, rest being young infants a Cone repair was performed successfully. They were either in heart failure or severely cyanotic as expected. Most had type C morphology and the average GOSE score was 1.3, mean CT ratio of 0.92. Sixty percent had some form of RVOT. Early mortality was 14% (1 patient). The ASD was completely closed in two patients (30%) who had no PS. ASD was partially closed in the rest of five patients with PS. In three of those five patients with PS (60%) required an additional BT shunt. The patient who had early mortality at 90 days of the operation had disruption of the posterior leaflet despite a re-repair at 60 days. However, this patient did have a tenuous preoperative presentation with cardiogenic shock and needed post-repair support with ECMO. At a follow-up at a median of 4.5 years, TR was mild in 5 patients, moderate in one patient. PS improved in all but 1 patient who had PA. They did not consider an RV-PA conduit instead opted for a BT shunt due to non-availability (15). There have been reports of successful conversion from another neonatal TV valvuloplasty to Cone repair later in childhood keeping in line with the principle that till the septal and inferior leaflets have been preserved, a delayed cone is possible (24). These case series demonstrate the technical feasibility of the Cone repair in the neonatal period. However, caution should be exercised while considering a very sick neonate.
The key steps in a univentricular repair are:
Ligation of the main pulmonary artery if the valve is incompetent and ligation of the PDA;
Fenestrated patch (modified Starnes procedure) closure of the TV to allow for RV decompression. A Gortex patch with a 4 mm fenestration is more likely to preserve the underlying valvular structures for future valvuloplasty, alternatively, a Sano RV exclusion procedure can be performed. The principle is to create functional tricuspid atresia, which is tolerated better;
Creation of an unrestricted atrial septum;
Creation of Blalock-Taussig shunt;
Reduction of the right atrioplasty to allow for growth of the lungs.
The patients are further channeled down the single ventricle palliation with a Bidirectional Glenn procedure and a Fontan procedure. Again, assessment should be made at each step to entertain the possibility of recruiting the RV, if reasonable as a subpulmonary ventricle with a valvuloplasty/valve replacement. Thus, achieving either a 1.5 ventricle or biventricular repair.
Starnes procedure
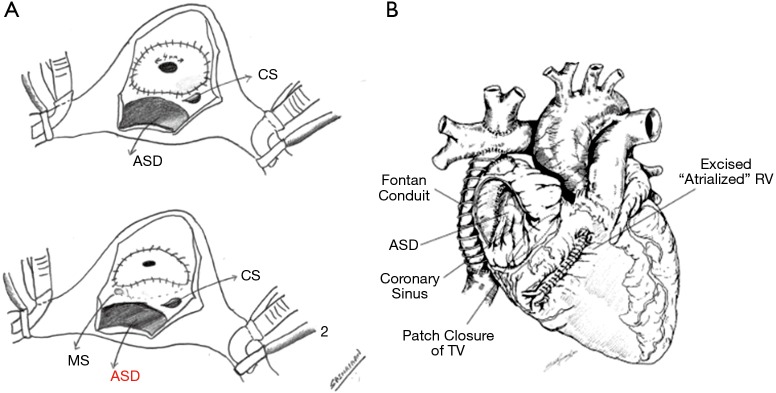
It is a procedure that helped improve survival of an otherwise difficult to treat group with very high mortality. In the original description, glutaraldehyde treated autologous non-fenestrated pericardial patch was used. This patch was sutured at the “anatomic” level of the tricuspid valve and the coronary sinus was left on the excluded RV side to avoid injury to the conduction system. Since then the procedure had been modified with 4 mm Fenestration of the patch with the coronary sinus draining into the right atrium (Figure 6A). The fenestration is particularly important with pulmonary atresia to prevent continued distension of the excluded RV with its attendant compression on the LV. Similarly, the coronary sinus is placed on the RA side to prevent an additional source of blood flow into the excluded RV. In the original series of Starnes procedure of 27 neonates, all the patients were ductal dependent with prostaglandins. Ninety percent of the patients were intubated with mechanical ventilation and were on inotropes. The average mean GOSE score was 1.4 with a mean CT ratio of 0.8. Operative mortality was 19%. There was one mortality after the BDG. Of the survivors, (20/22) 90% underwent a Fontan palliation with one awaiting a BDG. Median follow-up was 7 years. Ten-year survival was 81%. To note survival was only 33% in the cohort where the procedure was done with a non-fenestrated patch as compared to 80% with a fenestrated patch (25,26).
Figure 6.
Right ventricular exclusion procedures. (A) 1: modified Starnes technique, 2: Sano technique of the right AV orifice closure; (B) Sano RV exclusion technique. ASD, atrial septal defect; MS, membranous septum; TV, tricuspid valve; RV, right ventricle.
Sano RV exclusion procedure
Apart from the closure of the TV orifice with a fenestrated Gortex patch, the redundant thinned out right ventricular free wall is excised, and the resultant defect is primarily repaired. This results in the volume reduction of a dilated RV and its effect on LV compression by preventing a late leftward diastolic movement of the ventricular septum. It also helps with the mass effect on the lungs. As compared to the classic Starnes procedure, the coronary sinus is left in the right atrial side (lower pressure chamber) and the patch is skirted distal to the membranous septum to prevent a heart block (Figure 6B). In the series reported by Sano, 7/12 severely symptomatic neonates underwent the right ventricular exclusion with 1 early and late death in this group (19). However, one drawback of the Sano procedure is the inability to convert to a biventricular repair if the excluded RV were to grow.
Neonates with combined LV dysfunction or rare association such as hypoplastic left heart syndrome are better severed by heart transplantation (7).
Children and adults
Observation may be appropriate in asymptomatic patients with no right to left shunting, stable mild cardiomegaly and who have a good exercise tolerance. Operative indications include:
Increasing cardiomegaly. This may be due to an increasing RA size or ARV size. Early incorporation of the ARV into the ventricular side may prevent progressive dilation and interstitial fibrosis of the ARV (15);
Increasing cyanosis;
Symptoms such as exercise intolerance and fatigue;
Paradoxical embolism;
New onset of atrial and ventricular arrhythmias.
Again, the threshold for operative intervention is reduced if the ability to obtain a durable repair is high. In children, operative intervention is recommended between ages 2 to 5 years if the chance of a good repair is likely. This is important to prevent further cardiomegaly, especially from the dilation of ARV (15).
The goal is to achieve a biventricular repair. A 1.5 ventricle repair (valvuloplasty + BDG) may be needed in case there is significant RV dilation or dysfunction, the TV orifice area post valvuloplasty is inadequate to accommodate a full systemic venous return, or to protect a tenuous TV repair (27,28). A BDG by reducing the volume load on the RV by 35% to 45% can mitigate some of the hemodynamic stress on a complex TV valvuloplasty.
The first relatively durable valve repairs for EA were based on the monocusp technique. The prerequisite was a large anterior leaflet with a freely mobile leading edge to help coapt with the ventricular septum for achieving competency. However, the limits of these techniques were either due to a large annulus or severe tethering of the anterior leaflet. In one series described by Danielson, the monocusp technique was applicable in only 58% of the cases with a re-repair rate of 4% (29). In another monocusp technique described by Carpentier, the anterior leaflet was extensively mobilized by detaching it from the annulus. This improved the applicability of the repair by addressing a restricted anterior leaflet (30). These repairs have fallen out of favor for the Cone repair which is described next (31).
da Silva Cone procedure
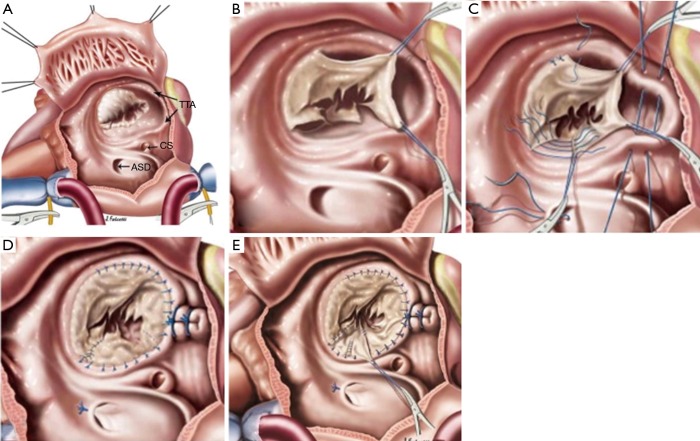
It provides the most anatomic repair with a 360-degree circumferential presence of coapting tricuspid valvular tissue with a central flow of blood as compared to the eccentric flow with a monocusp technique (31-33). There is leaflet to leaflet coaptation rather than a leaflet to ventricular septum coaptation, with a more symmetric distribution of the closing pressure to the leaflets. Since it utilizes all the three leaflets of the TV, its applicability increases across the wide morphological variation seen in EA. The following are the steps described (Figure 7).
Figure 7.
da Silva Cone technique (2.5 times magnified).
Mobilization of the leaflets from the functional annulus: all the three leaflet except certain portions of the anterior leaflet (between 10 to 12 clock—points of divergence of the anterior leaflet from the true annulus) are disconnected from the functional annulus leaving a 1–2 mm rim of valvular tissue retained on the annulus in order to facilitate reattachment. This edge is called the “Cut edge” (Figure 7B).
Delamination of the leaflets: the body of the leaflets are mobilized off the ventricular wall and the septum by dividing fibromuscular adhesions. This is done approximately for two-thirds of the valve body from the annulus. This step gives excellent exposure to the subvalvular apparatus. Small fenestrations may result during this process due to thinning out of the leaflets and should be diligently repaired if they are not part of the coapting surface.
Creation of a freely mobile and supported leading edge: once the body of the leaflets are mobilized, the next step is ensure the “Leading edge” of the leaflets, which is the coapting edge are subtended by existing primary chords or have an autologous neochord from the fibro-muscular tissue off the ventricular wall, ideally arising from a papillary muscle as result of the delamination process. At times, the fibro-muscular tissue subtending the free edge may be very confluent (particularly the posterior and septal leaflets) and longitudinal fenestrations may have to be performed to create discrete neo-chords to provide unrestricted inflow into the ventricle. Again, it is important to have an untethered and well supported leading edge for a successful repair.
Creation of the cone: once all the three leaflets have been mobilized, the cut edge of the posterior leaflet is brought to the cut edge of the septal leaflet by a clockwise rotation to create a 360 degree “Cone” of tricuspid tissue. It is preferable to use interrupted stitches to prevent any distortion. This represents a “Cone”, the base of which is formed by the mobilized cut edges of the leaflet and the apex by the leading edge with the subtending chordal structures (Figure 7C). An attempt must always be made to incorporate the septal leaflet as it lessens the chance of stenosis by increasing the size of the base of the cone (Figure 7E). If it is very diminutive, then the cut edge of the posterior leaflet is brought to the septal edge of the anterior leaflet to create the cone (Figure 7C,D).
Plication of the ARV and reduction of the anatomic annulus: the ARV is plicated longitudinally from an apex to base fashion, which maintains better geometry than the horizontal plication (4). ARV is identified as being the non-trabeculated portion of the RV. This also effectively reduces the true annular size to an extent. Care must be taken not to incorporate or distort the right coronary artery during this step. Also, the annulus may be so dilated that reducing it by just plicating the ARV may not be enough. A more symmetric reduction of the true annulus can be done by plicating it intermittently all along the other portion of the annulus, especially the anterior aspect (Figure 7D).
Attachment of the “Cut edge” to the plicated true annulus: this is either done with interrupted or continuous sutures. A double suture line adds security (Figure 7D).
Closure of ASD: the ASD is closed in a valved or fenestrated fashion when concerns exist about right heart dysfunction, thus acting as a “pop-off” with a right to left shunt.
Reduction atrioplasty: redundant portion of the enlarged right atrium are resected before atrial closure.
Adjunctive procedures:
Augmentation of the height of the deficient anterior and septal leaflets with tissue such as autologous pericardium, bovine pericardium, etc.;
Use of Gortex® neochords when native chords are deficient;
There has been a suggestion to disconnect and mobilize all portion of the leaflets from the annulus and after creating a 360-degree cone of leaflet tissue, to rotate it anti-clockwise by 60 degrees to better align the leaflets and sub-valvular apparatus before attaching the leaflets to the anatomic annulus. This leads to less tension on the leaflets and more symmetric distribution of tension, potentially resulting in better valve function and longevity of repair (33);
Sebening stitch: originally described as the part of the “One-stitch” technique by Sebening as a monocusp repair technique (21). It entails bringing the anterior papillary muscle subtending the anterior leaflet either to the ventricular septum or a modified version to a septal papillary muscle. This reduces the sphericity of the RV and provides a better hinge point for the anterior leaflet of the TV;
Stabilization of the annulus with an annuloplasty ring particularly in patients who have achieved somatic growth. The ring starts from the anteroseptal commissure to the coronary sinus. In children, a partial band can be used selectively for the inferior part of the annulus as it is the most likely part of the annulus to dilate (80% chance of dilation versus 40% for the anterior annulus and 10% for the septal) in the future at the same time not compromising somatic growth (24);
Therapeutic electrophysiological interventions can be performed both preoperatively and intraoperatively (13). When chronic atrial fibrillation is present, it is treated with a minimum of a right-sided Maze procedure with a left-sided lesion set added if stigmata of remodeling exist on the left side. Atrial flutter is treated by ablating the cavotricuspid isthmus. Afib/AF has a 25% chance of late recurrence. Accessory pathway-mediated tachycardia and AVNRT cane be permanently treated, generally with a preoperative electrophysiological procedure (34);
Bidirectional Glenn Procedure (BDG): it is useful in patients with severe RV dilation and more than moderate RV dysfunction. It may also be used in patients with tenuous valve repair or presence of TV stenosis with gradients >5 mmHg to avoid a full systemic venous return through the valve, thus potentially increasing the durability of the repair and lessening the TV stenosis gradients respectively. In a series of 62 patients from the Mayo Clinic, 85% had a planned BDG, 11% needed one due to inability to wean off CPB, and 3% needed it in the post-op period due to hemodynamic instability. Interestingly, 32% of the patients in that cohort were initially referred for a heart transplant. BDG was used both in the setting of a valvuloplasty and a valve replacement. Operative mortality was 1.6%. Intermediate-term survival and quality of life were good to excellent with delay or avoiding transplantation in the majority (27). The caveat to using a BDG is the presence of a low transpulmonary gradient. However, BDG has been successfully performed with LVEF as low as 35% with an eventual improvement of the function on follow-up. In another series of 30 patients, where BDG was exclusively used with a Cone repair, 60% needed a BDG in the context of a preoperative severe RV dilation, complex TV repair due to a diminutive septal leaflet, and hemodynamic instability on separation from CPB. Patients who had BDG were more likely to maintain better TV competency despite the high-risk features (28). In a prospective study of high-risk patients (severe TR, poor RV function), patients who had a BDG along with a valvuloplasty as compared to an isolated valvuloplasty had lower operative mortality, better tolerance of the residual TV dysfunction, and a lower re-operative rate (30). The drawback of a BDG is facial swelling and lack of internal jugular vein access to the heart for EP procedures.
In the original series of 40 consecutive patients with EA by da Silva undergoing the Cone repair with a mean age of 17 years, there was one operative mortality (2.5%). At a mean follow-up of 4 years, most patients had mild residual TR, and most were in NYHA-class I. There were 2 reoperations for re-repair (5%) with no valve replacements or incidence of any heart block (32). The groups further update of 52 patients with longer follow showed long-lasting repair of TR and reverse remodeling of the right heart with sustained clinical improvement (8).
In one of the largest series of 235 consecutive patients with EA with Cone repair from the Mayo Clinic, with a median age of 15 years, there was one early mortality (0.4%). In 80% of the patients, Cone repair was the primary operation. It was used as a re-repair strategy in 12% of the patients. Fourteen patients (5.9%) required an early reoperation after the Cone operation (most were in the earlier part of their experience with the procedure), of which half could be salvaged with a valve re-repair. There was a sustained decrease in TR with a decrease in right ventricular size and an improvement in the function. This series also used other adjunctive measures for valve repair such as leaflet augmentation, neochordae use, Sebening stitch, and annuloplasty band use. Also, 20% of the patients had a BDG (31).
TV re-repair can be performed using the Cone technique particularly when no surgical delamination at the initial operation has been performed. Leaflet augmentation and band annuloplasty is used to stabilize the repair (24).
Tricuspid valve replacement is necessary when a repair is not feasible or has failed. Every attempt should be made to repair the valve especially younger the patient, particularly in infants and young children where valve replacement options are limited. On the contrary, older patients (>60 years old) are served better with a replacement than a complex and tenuous repair with prolonged operative time. A bioprosthetic valve is better suited due to the increased thrombogenicity of the mechanical valves in the tricuspid position. This particularly increased with poor RV function (35). The key principle is to avoid the conduction system on the septal surface of the implant and the right coronary artery on the anterior and inferior aspects of the implant. This may entail placing the suture line more atrially rather than through the annulus. The coronary sinus may have to be left on the RV side. Also, the struts of the bioprosthetic valve should be oriented such that the AV nodal area is not impinged upon and the RVOT is not obstructed. A percutaneous TV valve in valve replacement is a future option, mostly with a melody valve and can be performed with low-risk (36).
Heart transplantation should be given consideration when the LV function is severely depressed. Left-sided abnormalities such as non-compaction cardiomyopathy, systolic and diastolic dysfunction may be seen (37,38). However, older patients with LVEF as low as 30% can be salvaged with a TV repair or replacement with the addition of a BDG (27). Some of these patients show an improvement in their LV function with time. RV end-diastolic index >200 mL/m2, RV EF <40% and age >50 years are increased risk factors for post-op RV failure, suggesting an early intervention in EA may be beneficial and may avoid the need for a heart transplant (39).
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Provenance and Peer Review: This article was commissioned by the Guest Editor (Raghav A. Murthy) for the series “Management of Congenital Heart Disease” published in Journal of Thoracic Disease. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The series “Management of Congenital Heart Disease” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
References
- 1.van Son JA, Konstantinov IE, Zimmermann V. Wilhelm Ebstein and Ebstein's malformation. Eur J Cardiothorac Surg 2001;20:1082-5. 10.1016/S1010-7940(01)00913-7 [DOI] [PubMed] [Google Scholar]
- 2.Anderson KR, Lie JT. The right ventricular myocardium in Ebstein's anomaly: a morphometric histopathologic study. Mayo Clin Proc 1979;54:181-4. [PubMed] [Google Scholar]
- 3.Celermajer DS, Bull C, Till JA, et al. Ebstein's anomaly: presentation and outcome from fetus to adult. J Am Coll Cardiol 1994;23:170-6. 10.1016/0735-1097(94)90516-9 [DOI] [PubMed] [Google Scholar]
- 4.Carpentier A, Chauvaud S, Mace L, et al. A new reconstructive operation for Ebstein's anomaly of the tricuspid valve. J Thorac Cardiovasc Surg 1988;96:92-101. 10.1016/S0022-5223(19)35302-4 [DOI] [PubMed] [Google Scholar]
- 5.Kumar TKS, Boston US, Knott-Craig CJ. Neonatal Ebstein Anomaly. Semin Thorac Cardiovasc Surg 2017;29:331-7. 10.1053/j.semtcvs.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 6.Li B, Sun HS, Pan SW, et al. Outcomes of Ebstein's Anomaly Patients Treated with Tricuspid Valvuloplasty or Tricuspid Valve Replacement: Experience of a Single Center. Chin Med J (Engl) 2018;131:1067-74. 10.4103/0366-6999.230731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammel JM, Danford DA, Spicer RL, et al. Fetal and neonatal imaging and strategy of primary neonatal heart transplantation in hypoplastic left heart with Ebstein's anomaly. Echocardiography 2015;32:598-601. 10.1111/echo.12820 [DOI] [PubMed] [Google Scholar]
- 8.Silva JP, Silva Lda F, Moreira LF, et al. Cone reconstruction in Ebstein's anomaly repair: early and long-term results. Arq Bras Cardiol 2011;97:199-208. 10.1590/S0066-782X2011005000084 [DOI] [PubMed] [Google Scholar]
- 9.Ammash NM, Warnes CA, Connolly HM, et al. Mimics of Ebstein's anomaly. Am Heart J 1997;134:508-13. 10.1016/S0002-8703(97)70088-7 [DOI] [PubMed] [Google Scholar]
- 10.Celermajer DS, Cullen S, Sullivan ID, et al. Outcome in neonates with Ebstein's anomaly. J Am Coll Cardiol 1992;19:1041-6. 10.1016/0735-1097(92)90291-T [DOI] [PubMed] [Google Scholar]
- 11.Attenhofer Jost CH, Edmister WD, Julsrud PR, et al. Prospective comparison of echocardiography versus cardiac magnetic resonance imaging in patients with Ebstein's anomaly. Int J Cardiovasc Imaging 2012;28:1147-59. 10.1007/s10554-011-9923-1 [DOI] [PubMed] [Google Scholar]
- 12.Wackel P, Cannon B, Dearani J, et al. Arrhythmia after cone repair for Ebstein anomaly: The Mayo Clinic experience in 143 young patients. Congenit Heart Dis 2018;13:26-30. 10.1111/chd.12566 [DOI] [PubMed] [Google Scholar]
- 13.Shivapour JK, Sherwin ED, Alexander ME, et al. Utility of preoperative electrophysiologic studies in patients with Ebstein's anomaly undergoing the Cone procedure. Heart Rhythm 2014;11:182-6. 10.1016/j.hrthm.2013.10.045 [DOI] [PubMed] [Google Scholar]
- 14.Delhaas T, Sarvaas GJ, Rijlaarsdam ME, et al. A multicenter, long-term study on arrhythmias in children with Ebstein anomaly. Pediatr Cardiol 2010;31:229-33. 10.1007/s00246-009-9590-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang SC, Wu ET, Chen SJ, et al. Surgical Strategy Toward Biventricular Repair for Severe Ebstein Anomaly in Neonates and Infancy. Ann Thorac Surg 2017;104:917-25. 10.1016/j.athoracsur.2017.01.081 [DOI] [PubMed] [Google Scholar]
- 16.Wald RM, Adatia I, Van Arsdell GS, et al. Relation of limiting ductal patency to survival in neonatal Ebstein's anomaly. Am J Cardiol 2005;96:851-6. 10.1016/j.amjcard.2005.05.035 [DOI] [PubMed] [Google Scholar]
- 17.Aiello S, Loomba RS. Factors Associated with the Need for, and the Impact of, Extracorporeal Membrane Oxygenation in Children with Congenital Heart Disease during Admissions for Cardiac Surgery. Children (Basel) 2017. doi: . 10.3390/children4110101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasegawa M, Iwai S, Yamauchi S, et al. Bilateral Pulmonary Artery Banding in Ebstein's Anomaly With Circular Shunting. Ann Thorac Surg 2019;107:e317-9. 10.1016/j.athoracsur.2018.08.079 [DOI] [PubMed] [Google Scholar]
- 19.Sano S, Fujii Y, Kasahara S, et al. Repair of Ebstein's anomaly in neonates and small infants: impact of right ventricular exclusion and its indications. Eur J Cardiothorac Surg 2014;45:549-55; discussion 555. 10.1093/ejcts/ezt465 [DOI] [PubMed] [Google Scholar]
- 20.Mizuno M, Hoashi T, Sakaguchi H, et al. Application of Cone Reconstruction for Neonatal Ebstein Anomaly or Tricuspid Valve Dysplasia. Ann Thorac Surg 2016;101:1811-7. 10.1016/j.athoracsur.2015.11.029 [DOI] [PubMed] [Google Scholar]
- 21.Augustin N, Schmidt-Habelmann P, Wottke M, et al. Results after surgical repair of Ebstein's anomaly. Ann Thorac Surg 1997;63:1650-6. 10.1016/S0003-4975(97)00090-8 [DOI] [PubMed] [Google Scholar]
- 22.Knott-Craig CJ, Kumar TK, Arevalo AR, et al. Surgical management of symptomatic neonates with Ebstein's anomaly: choice of operation. Cardiol Young 2015;25:1119-23. 10.1017/S1047951114001747 [DOI] [PubMed] [Google Scholar]
- 23.Boston US, Goldberg SP, Ward KE, et al. Complete repair of Ebstein anomaly in neonates and young infants: a 16-year follow-up. J Thorac Cardiovasc Surg 2011;141:1163-9. 10.1016/j.jtcvs.2011.01.029 [DOI] [PubMed] [Google Scholar]
- 24.Dearani JA, Said SM, Burkhart HM, et al. Strategies for tricuspid re-repair in Ebstein malformation using the cone technique. Ann Thorac Surg 2013;96:202-8: discussion 208-10. [DOI] [PubMed]
- 25.Kumar SR, Kung G, Noh N, et al. Single-Ventricle Outcomes After Neonatal Palliation of Severe Ebstein Anomaly With Modified Starnes Procedure. Circulation 2016;134:1257-64. 10.1161/CIRCULATIONAHA.115.021241 [DOI] [PubMed] [Google Scholar]
- 26.Reemtsen BL, Fagan BT, Wells WJ, et al. Current surgical therapy for Ebstein anomaly in neonates. J Thorac Cardiovasc Surg 2006;132:1285-90. 10.1016/j.jtcvs.2006.08.044 [DOI] [PubMed] [Google Scholar]
- 27.Raju V, Dearani JA, Burkhart HM, et al. Right ventricular unloading for heart failure related to Ebstein malformation. Ann Thorac Surg 2014;98:167-73; discussion 173-4. 10.1016/j.athoracsur.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Qiu L, Zhu Z, et al. Cone reconstruction of the tricuspid valve in Ebstein anomaly with or without one and a half ventricle repair. J Thorac Cardiovasc Surg 2011;141:1178-83. 10.1016/j.jtcvs.2011.01.015 [DOI] [PubMed] [Google Scholar]
- 29.Danielson GK, Driscoll DJ, Mair DD, et al. Operative treatment of Ebstein's anomaly. J Thorac Cardiovasc Surg 1992;104:1195-202. 10.1016/S0022-5223(19)34605-7 [DOI] [PubMed] [Google Scholar]
- 30.Chauvaud S, Berrebi A, d'Attellis N, et al. Ebstein's anomaly: repair based on functional analysis. Eur J Cardiothorac Surg 2003;23:525-31. 10.1016/S1010-7940(02)00836-9 [DOI] [PubMed] [Google Scholar]
- 31.Holst KA, Dearani JA, Said S, et al. Improving Results of Surgery for Ebstein Anomaly: Where Are We After 235 Cone Repairs? Ann Thorac Surg 2018;105:160-8. 10.1016/j.athoracsur.2017.09.058 [DOI] [PubMed] [Google Scholar]
- 32.da Silva JP, Baumgratz JF, da Fonseca L, et al. The cone reconstruction of the tricuspid valve in Ebstein's anomaly. The operation: early and midterm results. J Thorac Cardiovasc Surg 2007;133:215-23. 10.1016/j.jtcvs.2006.09.018 [DOI] [PubMed] [Google Scholar]
- 33.Mitchell ME, Hraska V, Kouretas PC. 360-Degree Cone Reconstruction for Ebstein's Anomaly. Ann Thorac Surg 2018;106:e155-8. 10.1016/j.athoracsur.2018.03.073 [DOI] [PubMed] [Google Scholar]
- 34.Khositseth A, Danielson GK, Dearani JA, et al. Supraventricular tachyarrhythmias in Ebstein anomaly: management and outcome. J Thorac Cardiovasc Surg 2004;128:826-33. 10.1016/j.jtcvs.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 35.Said SM, Burkhart HM, Schaff HV, et al. When should a mechanical tricuspid valve replacement be considered? J Thorac Cardiovasc Surg 2014;148:603-8. 10.1016/j.jtcvs.2013.09.043 [DOI] [PubMed] [Google Scholar]
- 36.Taggart NW, Cabalka AK, Eicken A, et al. Outcomes of Transcatheter Tricuspid Valve-in-Valve Implantation in Patients With Ebstein Anomaly. Am J Cardiol 2018;121:262-8. 10.1016/j.amjcard.2017.10.017 [DOI] [PubMed] [Google Scholar]
- 37.Attenhofer Jost CH, Connolly HM, O'Leary PW, et al. Left heart lesions in patients with Ebstein anomaly. Mayo Clin Proc 2005;80:361-8. 10.4065/80.3.361 [DOI] [PubMed] [Google Scholar]
- 38.Kumor M, Lipczynska M, Biernacka EK, et al. Cardiac arrest and ventricular arrhythmia in adults with Ebstein anomaly and left ventricular non-compaction. J Cardiol 2018;71:484-7. 10.1016/j.jjcc.2017.11.012 [DOI] [PubMed] [Google Scholar]
- 39.Mrad Agua K, Burri M, Cleuziou J, et al. Preoperative predictability of right ventricular failure following surgery for Ebstein's anomaly. Eur J Cardiothorac Surg 2019;55:1187-93. 10.1093/ejcts/ezy425 [DOI] [PubMed] [Google Scholar]