Abstract
Anastomotic leak is one of the most feared complications of esophagectomy, leading to prolonged hospital stay, increased postoperative mortality, and additional cost both to the patient and the hospital. Historically, anastomotic leaks have been treated with several techniques including conservative measures, percutaneous or operative drainage, primary surgical repair with buttressing, T-tube drainage, or excision of the esophageal replacement conduit with end esophagostomy. With advances in treatment modalities, including endoscopic stenting, clips and suturing, endoluminal vacuum-assisted closure (EVAC), such leaks increasingly are being managed without operative re-intervention and with salvage of the esophageal replacement conduit. For the purposes of this review, we identified studies analyzing the management of postoperative leak after esophagectomy. We then compared the efficacy of the various newer modalities for closure of anastomotic leaks and gastric conduit defects. We found both esophageal stent and EVAC sponges are effective treatments for closure of anastomotic leak. The chosen treatment modality for salvage of the esophageal replacement conduit is entirely dependent on the patient’s clinical status and the surgeon’s preference and experience. Emerging endoscopic and endoluminal therapies have increased the armamentarium of tools the esophageal surgeon has to facilitate successful resolution of anastomotic leaks following esophagectomy with reconstruction. While some literature suggests that EVACs have a slightly superior result in conduit success, we question this endorsement as EVACs mostly are utilized for contained leaks, many of which may have healed with conservative measures. This poses a challenge as there is clearly a bias given patient selection.
Keywords: Esophagectomy, anastomotic leak, endoluminal stent, endoluminal vacuum-assisted closure (EVAC), over-the-scope clips (OTSCs), endoluminal suturing
Introduction
Esophagectomy with gastric pull up is most commonly performed for the treatment of esophageal cancer, though the operation can be employed in cases of end-stage benign esophageal disease and esophageal rupture. Numerous surgical techniques have been described, with transthoracic (Ivor Lewis, McKeown, thoracoabdominal approaches), transhiatal, and minimally invasive esophagectomies (MIE) being the most common (1). Other less frequently performed operations such as prone esophagectomy with two-lung ventilation and mediastinoscopic esophagectomies also have been described in the literature (2,3). Regardless of surgical approach, esophagectomy is associated with high overall morbidity and significant mortality rates. Leak at the esophagogastric anastomosis is one of the most common and feared complications (4). In the recent literature, the quoted rates of anastomotic leak after cervical and thoracic anastomoses are 6.6–17.2% and 2–15.9%, respectively (5-10). Anastomotic leak after esophagectomy may lead to prolonged length of intensive care and hospital stay, substantial cost to the patient and hospital, increased postoperative mortality, and reduced quality of life, given the potential for post-leak stricture and prolonged dependence on tube feeds (7,10). Various risk factors for the development of anastomotic leak have been reported, including technical errors, a cervical location of the anastomosis, preoperative external beam radiation, and comorbid conditions such as malnutrition, advanced age, diabetes, obesity, active smoking and corticosteroid use (7,8).
Both nonoperative and operative techniques have been utilized for the management of anastomotic leak after esophagectomy. While conventional approach included excising the conduit and diverting the patient with delayed reconstruction, most surgeons now attempt to salvage the conduit whenever possible. When deciding upon optimal treatment strategy, the surgeon typically assesses the overall clinical picture of the patient, the presence or absence of multi-organ failure, the severity of the leak, the size and circumference of the defect, the viability of the conduit, and the extent of contamination. In this review, we concentrate on recent advancements in the management of anastomotic leak after esophagectomy primarily designed to salvage the gastric conduit. These therapies include endoscopic stents, clips and suturing, endoluminal vacuum therapy [endoluminal vacuum-assisted closure (EVAC)], and other novel approaches.
Non-operative management
Traditionally, surgeons had to decide between non-operative, conservative management of anastomotic leaks (consisting of cessation of an oral diet and administration of intravenous antibiotics) versus taking down the conduit with creation of a cervical esophagostomy. Over the last decade, however, surgeons have learned that some patients can be managed non-operatively, even those with intrathoracic anastomosis. Multiple studies comparing non-operative management of anastomotic leaks and surgical intervention (irrigation and debridement of leak site or endoluminal stent) have demonstrated “no statistical difference” in time to closure of leak (11,12). However, one has to read this literature with caution as there is a large component of selection bias amongst these patients. Typically, patients managed conservatively tend not to be septic and indeed have a contained leak versus those who clearly have mediastinal contamination and warrant surgical intervention.
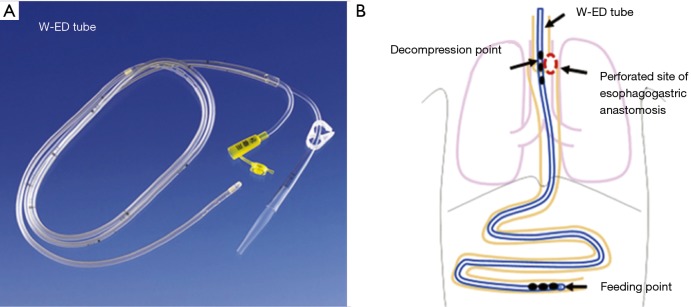
Another minimally invasive intervention to manage patients non-operatively is to place a percutaneous drain either in the neck or chest. As expected, drain placement alone is likely to be more successful in clinically stable patients with isolated cervical anastomotic leak (100%, n=23) compared to patients with thoracic anastomotic leak (41%, 15 out of 37 patients), where there is greater mediastinal contamination with potential leak into the pleural space (13). While a leak can be managed with an external drain, a nasogastric tube to simultaneously decompress the conduit is often used to minimize ongoing contamination from the anastomotic leak. In 2011, Williams et al. presented a novel transabdominal transluminal drainage system that was used on six patients diagnosed with leak with defects up to 10–50% of anastomotic circumference (14). The apparatus essentially was a large-bore 36 Fr reversed nasogastric tube placed retrograde via a gastrotomy about 5 centimeters proximal to the leak (14). Interestingly, there is one other case study illustrating conservative management of an anastomotic leak using a trans nasal double elementary diet tube (W-ED tube, Nippon Sherwood, Tokyo, Japan; Figure 1) (15). The W-ED tube was shown to decompress the perforated site of the anastomosis on one end of the tube while acting as a source for enteral nutrition on the other (15).
Figure 1.
Double elementary tube (W-ED tube) used to manage an anastomotic leak proximally while feeding distally. The tube has two lumens, one that allows for suction and depression of the anastomotic leak site and one that allows the delivery of tube feeds. Reprinted with permission of Dr. Takeyuki Wada and Elsevier publishing.
Endoluminal stent
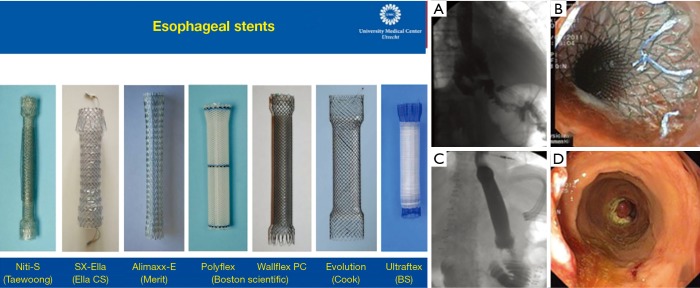
Today, endoluminal stents are widely used in the management of anastomotic leak as they provide for successful closure of large defects while allowing for continued enteral nutrition during recovery. A variety of materials have been studied for this purpose including partially or fully covered plastic and metal stents (Figure 2, left panel). Typically, these stents are left in place for a period of 2–4 weeks after which they are removed, and the area of leak is evaluated endoscopically for closure (Figure 2, right panel labeled A-D). In a review comparing the efficacy of various stent types, clinical success rate was not statistically different between self-expanding plastic stents (84%), fully covered self-expanding metal stents (fcSEMS, 85%) and partially covered self-expanding metal stents (pcSEMS, 86%); however, plastic stents were associated with significantly longer duration of stent placement and exhibited higher rate of migration (16). A well-studied disadvantage of endoluminal stenting is the high rate of stent migration (16–62%), which may occur in addition to complications that arise during stent placement and removal such as tissue overgrowth and erosion when left in place for longer periods of time (17,18). Success of an esophageal stent is dependent on the size of the defect and source control, often requiring concomitant drainage and antibiotics (19).
Figure 2.
Popularly used stents in the management of esophageal strictures, anastomotic leaks, and esophageal perforation. They are either made of plastic (Polyflex) or metal, can be covered or uncovered, and non-absorbable or biodegradable. Reprinted with permission of Peter D. Siersema, MD, PhD, Radboud University, The Netherlands. The panel of four figures on the right demonstrates the detection of an anastomotic leak and endoscopic and fluoroscopic view of the stent to close the leak site. Final completion endoscopy demonstrates complete closure of the leak after stent removal. No stricture is seen here. Reprinted with permission of Dr. Alberto Fernández, POVISA Hospital, Vigo, Spain and Rev Esp Enferm Dig.
The use of endoluminal stents has been explored in detail in the literature. The overall success rate of anastomotic leak closure using stents has improved over time, likely due to improved patient selection and surgical expertise. For example, D’Cunha and colleagues in 2011 identified that only 59% (22/37) of patients who underwent esophageal stent placement for leak and perforation had complete resolution of leak, with non-closure of the leak and stent erosion as the most common reasons for stent failure (18). Another study from 2016 reported that large diameter fcSEMS successfully treated anastomotic leak, perforation, and fistula formation in 50% of cases (17/34), with two of these patients requiring repeat stenting (20). More recently, Liang et al. in 2017 demonstrated that of 81.9% of the patients who underwent SEMS in their study for various indications including perforation, leak, and stricture had native esophageal or conduit salvage (21). The same group also published a case report of two patients with gastric conduit staple line dehiscence below the anastomosis that was salvaged using serial SEMS (22). Historically, these patients would have required operative esophageal diversion.
Biodegradable stents made of woven polydioxanone monofilament (SX-Ella; Milady Horakrove, Hradc Kralove, Czech Republic), which degrade by random hydrolysis accelerated by a low ambient pH have also been used in the management of upper gastrointestinal (GI) anastomotic leaks (23). Multiple studies have reported successful use of these biodegradable stents in closure of anastomotic leak after esophagectomy, diverticulectomy, gastric sleeve and gastrectomy in 80–85% of patients studied (23,24). Although biodegradable stents eliminate complications involved in stent removal and reportedly have a lower migration rate, they are more expensive (24). Additionally, many patients in this study exhibited concerning side effects including drooling, retrosternal pain, sensation of having a foreign object in the body, and aversion to water for up to 2 months (24). Though esophageal stents have proven to be a valuable tool for surgeons in the management of esophageal leak after esophagectomy, the need for serial trips to the operating room and potential major complications such as migration and erosion highlight the importance of judicious patient selection.
EVAC
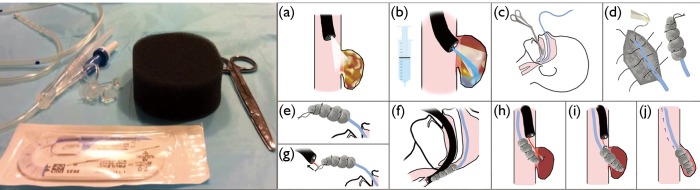
EVAC has gained popularity recently for management of anastomotic leak. The technique was initially shown to be effective in closure of anastomotic leaks in two cases refractory to stent treatment and surgical revision (25). The technology functions by applying negative pressure to the wound via a transnasal gastric tube with an attached vacuum-sealed sponge (Figure 3). The negative pressure system then allows for active drainage of the wound while promoting blood flow and tissue granulation (26). Vacuum-sealed sponges typically are exchanged in the operating room every few days for continued drainage until closure of the defect, with studies showing an average of 5–6 device changes per patient (25,27-29).
Figure 3.
Endovac therapy is based on principles of applying suction to a cavity to allow for granulation tissue to form and thus allow closure of the cavity from inside-out. Left most panel (a, e, g): the apparatus is rather simple and uses a nasogastric tube (NGT) sutured to a black sponge. Reprinted with permission of Dr. Nathan R. Smallwood, Texas Oncology, Sherman, TX and SAGES. Middle panel (b, f): after visualizing the defect, the cavity is cleaned and irrigated. An NGT is passed via the nares and pulled out through the mouth. It is then sutured to a sponge, which is then directed into appropriate place using the endoscope. Negative suction is applied once in position. Reprinted from ANZ Journal of Surgery 2016 with permission granted by John Wiley and Sons. Right most panel (c, d, h, i, j): various mechanisms have been proposed to explain the healing of the leaking bed. Reprinted with permission of Dr. Damien Loh, Melbourne Gastro Oesophageal Surgery, Australia.
While technically challenging, EVAC therapy has been shown to treat anastomotic leakages effectively after a variety of upper and lower GI surgeries. In a study exploring anastomotic leak secondary to esophagectomy (n=30), gastrectomy (n=9), iatrogenic esophageal perforation (n=9) and Boerhaave syndrome (n=4), 94.2% (49 of 52) of patients healed with vacuum therapy (28). A total of 390 polyurethane sponges were used with a median changing interval of 3–5 days and a mean duration of therapy of 22 days, with only 7 of 49 patients requiring over-the-scope clips (OTSCs) in addition to sponge placement (28,29). In this study, there was no comparison group to document how long closure would have taken without the EVAC therapy.
In a study comparing metal or plastic stents to EVAC for treatment of intrathoracic anastomotic leak, the overall closure rate was significantly higher in the EVAC group (84.4%) compared to stent (53.8%) (30). Similar to the Laukoetter et al. study above, this paper did not have a control group. Development of anastomotic stricture was also significantly increased in the stent group (28.2% vs. 9.4% in EVAC, P<0.05) (which may indeed imply larger perforations), however, there was no significant difference in duration of hospitalization (41 vs. 48.5 days in EVAC) and mortality between groups (25.6% vs. 15.6% in EVAC) (30). In contrast, Hwang et al. found that patients undergoing EVAC had a shorter median time to clinical success (19.5 days in EVAC vs. 27 days in SEMS) and shorter overall hospital stay (median of 37.1 days in EVAC) compared to the stent group (median of 87.3 days), which again could be secondary to patient selection (31). Another review found peri-operative complication rates to be significantly higher in endoluminal stenting (35% or 28 of 80 patients included) due to stent migration and difficulty removing stents compared to the EVAC therapy (5.6% or 3 out of 54 patients included) (32).
While complete anastomotic healing and percentage of patients recovered has been documented to be significantly higher in EVAC (93.3%) versus stent (63.3%, P=0.038) group, mortality rates have not differed between the two modalities (33). In another study comparing EVAC to stent, no significant difference between success of treatment, 30-day mortality, or length of hospital stay was found; however, the duration of treatment was significantly longer in the stent (27 days) compared to EVAC treatment (12 days, P<0.001) (34). There have been selected reports of stent-over-sponge technique describing combined EVAC therapy with stent as an effective method to achieve leak resolution (35,36).
While the published literature suggests that EVAC therapy is extremely successful in closing anastomotic leaks, one must also consider the burden of EVAC wound changes both for the patient and the healthcare system. Due to the frequent need for sponge replacement, patients require multiple trips to the operating room. Furthermore, prolonged use of the sponge and vacuum therapy has associated complications of sponge dislocation and potential erosion into neighboring vital structures. Despite these drawbacks, EVAC is emerging as an option for surgeons to employ in the setting of anastomotic leak following esophagectomy. As the technology becomes increasingly widespread, reports of EVAC therapy success following failed attempts at endoluminal stenting are emerging (25,37-39). However, we as authors, question the highly selected nature of the patient cohorts in these studies. There are no prospective randomized trials on this subject and in fact, such trials will likely not be possible as perforations that are not contained are usually not treated with EVAC.
Endoluminal clips and suturing
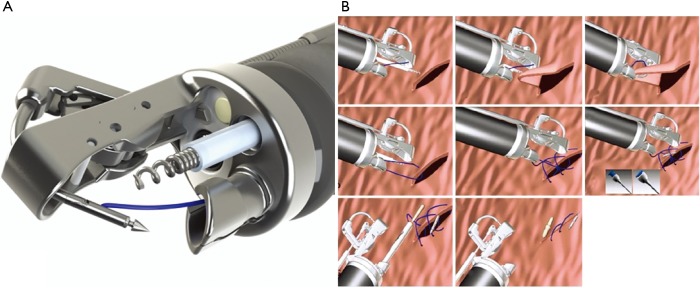
Endoscopic suturing systems have also been used for closure of GI defects. The Overstitch (Apollo Endo-surgery, Austin, Texas, USA) system utilizes a suturing device mounted on the tip of a double-channel endoscope (Figure 4A) that allows for full thickness suture placement and closure of an anastomotic leak or perforation (Figure 4B). These systems have been shown to close staple line leaks after sleeve gastrectomy and mucosal defects after submucosal dissection of stomach and colon lesions (40,41). However, few studies have examined the use of endoluminal suturing on anastomotic leak after esophagectomy alone. Gaur et al. in 2015 showed that a combination of endoscopic suturing plus placement of pcSEMS fixed to the esophageal wall was able to heal an anastomotic leak after esophagectomy (42). However, a notable disadvantage of this technique is lack of longevity. Additional studies are needed to evaluate the use of this technique for management of anastomotic leak and long-term efficacy of treatment.
Figure 4.
Endoluminal suturing (Apollo Endo-surgery, Austin, Texas, panel A) allows for closure of full-thickness defects by using a helix and pulling in tissue into the jaws of the suturing device and then passing the needle from one edge of the perforated site to the other with final cinching of the defect (panel B). Reprinted with permission of Apollo Endosurgery (both panels).
OTSCs (Ovesco Endoscopy GmbH, T übingen, Germany) have also been used for closure of a wide range of GI defects including anastomotic dehiscence, fistula and perforation (43,44). In this technique, an endoscopically mounted clip is deployed in a fashion such that its prongs re-approximate the edges of the leak and allow for closure. When using OTSC, the surgeon is able to close larger GI defects than when clips deployed through the working channel of an endoscope (45). OTSCs have been shown to successfully close GI anastomotic leaks in 73.3% of 32 patients (5 of which were in the esophagus), and long-term success rates have been significantly higher when clips get placed as primary therapy rather than as rescue therapy after a previous failed attempt (46). A recent review article comparing 12 studies analyzing closure of esophageal perforation with endoluminal clips found a clinical success rate of 56–100% (47). Due to the limited number of randomized controlled trials utilizing endoluminal clips for closure of esophageal anastomotic leak, further research is necessary to demonstrate efficacy of this technique.
Novel approaches
Various novel approaches are being explored for management of anastomotic leak after esophagectomy. A thick AlloDerm patch (Lifecell, Branchburg, New Jersey, USA) consisting of human acellular dermis capable of cellular regeneration has been shown to salvage dehisced conduits after esophagectomy in a case report of 3 patients (48). In each of these patients, concomitant stenting was performed after horizontal mattress stitching of the AlloDerm patch into the dehisced area. Although the conduit was successfully healed in each case, long-term complications such as stricture rate were not addressed (48). Finally, one case report demonstrated the use of a “chimera” stent, which essentially took an 18-mm diameter fcSEMS sutured to a 28–34-mm diameter fully covered self-expandable colonic metal stent to successfully treat partial dehiscence of anastomosis after esophagectomy (49). This enlarged “chimera” stent functioned by preventing upstream gastric reflux as the stent diameter was now wide enough to adhere to the tubularized gastric wall; the chimera stent was removed after being in place for 3 weeks (49).
Discussion
Anastomotic leak after esophagectomy will continue to be one of the most feared and costly complications of the operation. Given the significant impact on patient morbidity and mortality, as well as healthcare dollars, surgeons continually evaluate and modify their techniques to avoid an anastomotic leak altogether. The management of such leaks requires considerable skill and judgment on the part of the esophageal surgeon; an extensive knowledge of these emerging treatment modalities helps foster a successful outcome.
After a thorough review of the existing literature on the subject, one of the limitations we recognized was the paucity of data regarding management of anastomotic leaks alone. Indeed, most of the studies discussing the role of endoscopic interventions to salvage the esophagus or gastric conduit included esophagectomy patients for benign and malignant pathology, as well as iatrogenic and spontaneous perforations, fistulae, and esophageal strictures. This impaired our ability to strictly focus on anastomotic leaks, however we tried to center our attention on this specific subset of patients while reviewing the literature to draw our conclusions.
While clinically stable patients with small contained leaks can be managed conservatively with intravenous antibiotics and possible percutaneous drainage, patients with mediastinal and pleural contamination are now often able to have their conduit salvaged using esophageal stents, endoluminal vacuum therapy, and/or other endoluminal suturing and clips. The benefit of continued enteral nutrition with endoluminal stenting must be weighed against higher complication rates and stent migration compared to EVAC therapy. Although EVAC therapy has shown promising results in closure of anastomotic leak, additional studies of its use in patients with complex leak is warranted. As we continue to evolve in medicine, we will come up with more novel ways to address this complication and hopefully, one day obviate anastomotic leaks altogether.
Acknowledgments
Jory Barone, MedStar Washington Hospital Medical Library.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Flanagan JC, Batz R, Saboo SS, et al. Esophagectomy and Gastric Pull-through Procedures: Surgical Techniques, Imaging Features, and Potential Complications. Radiographics 2016;36:107-21. 10.1148/rg.2016150126 [DOI] [PubMed] [Google Scholar]
- 2.Souche R, Nayeri M, Chati R, et al. Thoracoscopy in prone position with two-lung ventilation compared to conventional thoracotomy during Ivor Lewis procedure: a multicenter case-control study. Surg Endosc 2020;34:142-52. 10.1007/s00464-019-06742-w [DOI] [PubMed] [Google Scholar]
- 3.Kuwabara S, Kobayashi K, Kubota A, et al. Comparison of perioperative and oncological outcome of thoracoscopic esophagectomy in left decubitus position and in prone position for esophageal cancer. Langenbecks Arch Surg 2018;403:607-14. 10.1007/s00423-018-1674-1 [DOI] [PubMed] [Google Scholar]
- 4.Raymond D. Surgical Clinics of North America. Surg Clin N Am 2012;92:1299-313. 10.1016/j.suc.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 5.Brown AM, Pucci MJ, Berger AC, et al. A standardized comparison of peri-operative complications after minimally invasive esophagectomy: Ivor Lewis versus McKeown. Surg Endosc 2018;32:204-11. 10.1007/s00464-017-5660-4 [DOI] [PubMed] [Google Scholar]
- 6.Fumagalli U, Baiocchi G, Celotti A, et al. Incidence and treatment of mediastinal leakage after esophagectomy: Insights from the multicenter study on mediastinal leaks. World J Gastroenterol 2019;25:356-66. 10.3748/wjg.v25.i3.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Daele E, Van de Putte D, Ceelen W, et al. Risk factors and consequences of anastomotic leakage after Ivor Lewis oesophagectomy†. Interact Cardiovasc Thorac Surg 2016;22:32-7. 10.1093/icvts/ivv276 [DOI] [PubMed] [Google Scholar]
- 8.Messager M, Warlaumont M, Renaud F, et al. Recent improvements in the management of esophageal anastomotic leak after surgery for cancer. Eur J Surg Oncol 2017;43:258-69. 10.1016/j.ejso.2016.06.394 [DOI] [PubMed] [Google Scholar]
- 9.Schröder W, Raptis DA, Schmidt H, et al. Anastomotic Techniques and Associated Morbidity in Total Minimally Invasive Transthoracic Esophagectomy. Ann Surg 2019;270:820-6. 10.1097/SLA.0000000000003538 [DOI] [PubMed] [Google Scholar]
- 10.Kassis ES, Kosinski AS, Ross P, et al. Predictors of Anastomotic Leak After Esophagectomy: An Analysis of The Society of Thoracic Surgeons General Thoracic Database. Ann Thorac Surg 2013;96:1919-26. 10.1016/j.athoracsur.2013.07.119 [DOI] [PubMed] [Google Scholar]
- 11.Lee DH, Kim HR, Kim SR, et al. Comparison of clinical outcomes after conservative and surgical treatment of isolated anastomotic leaks after esophagectomy for esophageal cancer. Dis Esophagus 2013;26:609-15. 10.1111/dote.12011 [DOI] [PubMed] [Google Scholar]
- 12.Guo J, Chu X, Liu Y, et al. Choice of therapeutic strategies in intrathoracic anastomotic leak following esophagectomy. World J Surg Oncol 2014;12:402. 10.1186/1477-7819-12-402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Rossum PSN, Haverkamp L, Carvello M, et al. Management and outcome of cervical versus intrathoracic manifestation of cervical anastomotic leakage after transthoracic esophagectomy for cancer. Dis Esophagus 2017;30:1-8. [DOI] [PubMed] [Google Scholar]
- 14.Williams RN, Hall AW, Sutton CD, et al. Management of Esophageal Perforation and Anastomotic Leak by Transluminal Drainage. J Gastrointest Surg 2011;15:777-81. 10.1007/s11605-011-1472-3 [DOI] [PubMed] [Google Scholar]
- 15.Wada T, Takeuchi H, Yoshikawa T, et al. Successful Management of Anastomotic Leakage and Lung Fistula After Esophagectomy. Ann Thorac Surg 2014;97:1071-3. 10.1016/j.athoracsur.2013.06.093 [DOI] [PubMed] [Google Scholar]
- 16.van Boeckel PG, Sijbring A, Vleggaar FP, et al. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther 2011;33:1292-301. 10.1111/j.1365-2036.2011.04663.x [DOI] [PubMed] [Google Scholar]
- 17.Speer E, Dunst CM, Shada A, et al. Covered stents in cervical anastomoses following esophagectomy. Surg Endosc 2016;30:3297-303. 10.1007/s00464-015-4661-4 [DOI] [PubMed] [Google Scholar]
- 18.D’Cunha J, Rueth NM, Groth SS, et al. Esophageal stents for anastomotic leaks and perforations. J Thorac Cardiovasc Surg 2011;142:39-46.e1. 10.1016/j.jtcvs.2011.04.027 [DOI] [PubMed] [Google Scholar]
- 19.D’Cunha J. Esophageal Stents for Leaks and Perforations. Semin Thorac Cardiovasc Surg 2011;23:163-7. 10.1053/j.semtcvs.2011.08.003 [DOI] [PubMed] [Google Scholar]
- 20.van den Berg MW, Kerbert AC, van Soest EJ, et al. Safety and efficacy of a fully covered large-diameter self-expanding metal stent for the treatment of upper gastrointestinal perforations, anastomotic leaks, and fistula. Dis Esophagus 2016;29:572-9. 10.1111/dote.12363 [DOI] [PubMed] [Google Scholar]
- 21.Liang DH, Hwang E, Meisenbach LM, et al. Clinical outcomes following self-expanding metal stent placement for esophageal salvage. J Thorac Cardiovasc Surg 2017;154:1145-50. 10.1016/j.jtcvs.2017.03.051 [DOI] [PubMed] [Google Scholar]
- 22.Liang DH, Meisenbach LM, Kim MP, et al. Management of gastric conduit dehiscence with self-expanding metal stents: a case report on salvaging the gastric conduit. J Cardiothorac Surg 2017;12:4. 10.1186/s13019-017-0570-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Černá M, Köcher M, Válek V, et al. Covered biodegradable stent: new therapeutic option for the management of esophageal perforation or anastomotic leak. Cardiovasc Intervent Radiol 2011;34:1267-71. 10.1007/s00270-010-0059-9 [DOI] [PubMed] [Google Scholar]
- 24.Köneş O, Oran E. Self-Expanding Biodegradable Stents for Postoperative Upper Gastrointestinal Issues. JSLS. 2018. doi: . 10.4293/JSLS.2018.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wedemeyer J, Schneider A, Manns MP, et al. Endoscopic vacuum-assisted closure of upper intestinal anastomotic leaks. Gastrointest Endosc 2008;67:708-11. 10.1016/j.gie.2007.10.064 [DOI] [PubMed] [Google Scholar]
- 26.Mennigen R, Senninger N, Laukoetter MG. Novel treatment options for perforations of the upper gastrointestinal tract: Endoscopic vacuum therapy and over-the-scope clips. World J. Gastroenterol 2014;20:7767-76. 10.3748/wjg.v20.i24.7767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Min YW, Kim T, Lee H, et al. Endoscopic vacuum therapy for postoperative esophageal leak. BMC Surg 2019;19:37. 10.1186/s12893-019-0497-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laukoetter MG, Mennigen R, Neumann PA, et al. Successful closure of defects in the upper gastrointestinal tract by endoscopic vacuum therapy (EVT): a prospective cohort study. Surg Endosc 2017;31:2687-96. 10.1007/s00464-016-5265-3 [DOI] [PubMed] [Google Scholar]
- 29.Mennigen R, Senninger N, Laukoetter MG. Endoscopic vacuum therapy of esophageal anastomotic leakage. Gastrointest Endosc 2015;82:397. 10.1016/j.gie.2015.02.027 [DOI] [PubMed] [Google Scholar]
- 30.Brangewitz M, Voigtländer T, Helfritz F, et al. Endoscopic closure of esophageal intrathoracic leaks: stent versus endoscopic vacuum-assisted closure, a retrospective analysis. Endoscopy 2013;45:433-8. 10.1055/s-0032-1326435 [DOI] [PubMed] [Google Scholar]
- 31.Hwang JJ, Jeong YS, Park YS, et al. Comparison of Endoscopic Vacuum Therapy and Endoscopic Stent Implantation With Self-Expandable Metal Stent in Treating Postsurgical Gastroesophageal Leakage. Medicine 2016;95:e3416. 10.1097/MD.0000000000003416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rausa E, Asti E, Aiolfi A, et al. Comparison of endoscopic vacuum therapy versus endoscopic stenting for esophageal leaks: systematic review and meta-analysis. Dis Esophagus 2018. doi: . 10.1093/dote/doy060 [DOI] [PubMed] [Google Scholar]
- 33.Mennigen R, Harting C, Lindner K, et al. Comparison of Endoscopic Vacuum Therapy Versus Stent for Anastomotic Leak After Esophagectomy. J Gastrointest Surg 2015;19:1229-35. 10.1007/s11605-015-2847-7 [DOI] [PubMed] [Google Scholar]
- 34.Berlth F, Bludau M, Plum P, et al. Self-Expanding Metal Stents Versus Endoscopic Vacuum Therapy in Anastomotic Leak Treatment After Oncologic Gastroesophageal Surgery. J Gastrointest Surg 2019;23:67-75. 10.1007/s11605-018-4000-x [DOI] [PubMed] [Google Scholar]
- 35.Gubler C, Schneider P, Bauerfeind P. Complex anastomotic leaks following esophageal resections: the new stent over sponge (SOS) approach. Dis Esophagus 2013;26:598-602. 10.1111/dote.12005 [DOI] [PubMed] [Google Scholar]
- 36.Bludau M, Hölscher A, Herbold T, et al. Management of upper intestinal leaks using an endoscopic vacuum-assisted closure system (E-VAC). Surg Endosc 2014;28:896-901. 10.1007/s00464-013-3244-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weidenhagen R, Hartl WH, Gruetzner KU, et al. Anastomotic Leakage After Esophageal Resection: New Treatment Options by Endoluminal Vacuum Therapy. Ann Thorac Surg 2010;90:1674-81. 10.1016/j.athoracsur.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 38.Smallwood NR, Fleshman JW, Leeds SG, et al. The use of endoluminal vacuum (E-Vac) therapy in the management of upper gastrointestinal leaks and perforations. Surg Endosc 2016;30:2473-80. 10.1007/s00464-015-4501-6 [DOI] [PubMed] [Google Scholar]
- 39.Youn HC, Kwon SH. Endoscopic Vacuum-Assisted Closure (E-VAC) Treatment in a Patient with Delayed Anastomotic Perforation following a Perforated Gastric Conduit Repair after an Ivor-Lewis Esophagectomy. Ann Thorac Cardiovasc Surg 2016;22:363-6. 10.5761/atcs.cr.15-00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai JX, Khashab MA, Okolo PI 3rd, et al. Full-thickness endoscopic suturing of staple-line leaks following laparoscopic sleeve gastrectomy. Endoscopy 2014;46 Suppl 1 UCTN:E623-4. [DOI] [PubMed]
- 41.Kantsevoy SV, Bitner M, Mitrakov AA, et al. Endoscopic suturing closure of large mucosal defects after endoscopic submucosal dissection is technically feasible, fast, and eliminates the need for hospitalization (with videos). Gastrointest Endosc 2014;79:503-7. 10.1016/j.gie.2013.10.051 [DOI] [PubMed] [Google Scholar]
- 42.Gaur P, Lyons C, Malik TM, et al. Endoluminal Suturing of an Anastomotic Leak. Ann Thorac Surg 2015;99:1430-2. 10.1016/j.athoracsur.2014.01.089 [DOI] [PubMed] [Google Scholar]
- 43.Kobara H, Mori H, Nishiyama N, et al. Over-the-scope clip system: A review of 1517 cases over 9 years. J Gastroenterol Hepatol 2019;34:22-30. 10.1111/jgh.14402 [DOI] [PubMed] [Google Scholar]
- 44.Fernandez-Esparrach G, Lautz DB, Thompson CC. Endoscopic repair of gastrogastric fistula after Roux-en-Y gastric bypass: a less-invasive approach. Surg Obes Relat Dis 2010;6:282-8. 10.1016/j.soard.2010.02.036 [DOI] [PubMed] [Google Scholar]
- 45.Robertson C, Savioli F, Shalli K. A novel endoscopic treatment for anastomotic leakage post anterior resection: Padlock over the scope clip. Int J Colorectal Dis 2018;33:91-3. 10.1007/s00384-017-2923-4 [DOI] [PubMed] [Google Scholar]
- 46.Haito-Chavez Y, Law JK, Kratt T, et al. International multicenter experience with an over-the-scope clipping device for endoscopic management of GI defects (with video). Gastrointest Endosc 2014;80:610-22. 10.1016/j.gie.2014.03.049 [DOI] [PubMed] [Google Scholar]
- 47.Watkins JR, Farivar AS. Endoluminal Therapies for Esophageal Perforations and Leaks. Thorac Surg Clin 2018;28:541-54. 10.1016/j.thorsurg.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 48.Thomas M, Allen MS, Shen RK, et al. A Novel Use of Human Acellular Dermis for Conduit Salvage After Esophagectomy. Ann Thorac Surg 2014;97:1459-63. 10.1016/j.athoracsur.2013.08.051 [DOI] [PubMed] [Google Scholar]
- 49.Mutignani M, Dioscoridi L, Manta R, et al. “Chimera” fully covered self-expandable metal stent for refractory esophageal anastomotic leak. Endoscopy 2015;47:E376-7. 10.1055/s-0034-1392238 [DOI] [PubMed] [Google Scholar]