Abstract
Background
Hypothyroidism was recently reported to be common and to predict mortality in patients with idiopathic pulmonary fibrosis (IPF). In addition, a high prevalence of hypothyroidism was shown in patients with idiopathic pleuroparenchymal fibroelastosis. However, in idiopathic interstitial pneumonia (IIP), a clinical significance of thyroid function has not been clarified in detail. The goal of this study was to investigate the clinical significance of thyroid function and the presence of thyroid antibodies in IIP.
Methods
We have reviewed IIP patients, and analyzed the positivity of thyroid antibodies at first. Next, the relationship of clinical characteristics with thyroid function and the positivity of thyroid antibodies was analyzed. Lastly, the positivity of thyroid antibodies and other autoantibodies was evaluated.
Results
In IIP patients, thyroglobulin and thyroid peroxidase antibodies were positive in 17 and 16%, respectively, and 22% of patients had either or both antibodies. Subclinical and/or overt hypothyroidism was confirmed in 7% of IIP patients. The free thyrotropin level had a significant positive correlation with vital capacity and a significant negative correlation with the C-reactive protein and surfactant protein-A levels, and erythrocyte sedimentation ratio (ESR). In addition, autoantibodies suggestive of connective tissue diseases (CTDs) were positive in more than two thirds of IIP patients with the thyroid antibody, and the positive rate of antinuclear and proteinase-3 anti-neutrophil cytoplasmic antibodies was significantly higher in IIP patients with thyroid antibodies than those without the antibodies.
Conclusions
Although thyroid dysfunction is not frequent, thyroid hormones and thyroid antibodies are possibly involved in the pathogenesis of IIP and their evaluation may be clinically useful to identify the clinical phenotype of IIP with autoimmune features.
Keywords: Thyroid hormone, thyroid antibodies, interstitial pneumonia, clinical phenotype, autoimmune features
Introduction
Interstitial pneumonia is characterized by inflammation and excess deposition of the extracellular matrix in the pulmonary interstitium. There are some causes of interstitial pneumonia identified such as exposure to occupational dusts and organic substances, and connective tissue diseases (CTDs) are one of the main causes of interstitial pneumonia. However, the exact causes cannot be often identified, and the diagnosis of idiopathic interstitial pneumonia (IIP) is made for such cases. Although IIP is a heterogeneous group of interstitial pneumonia with unknown etiology, growing evidence suggests that IIP with autoimmune features which are not enough for the specific diagnosis of CTD are focused as a clinically distinct phenotype. Several definitions such as undifferentiated CTD (1), lung dominant CTD (2) and autoimmune featured interstitial lung disease (3) have been proposed by research groups for such cases, and the European Respiratory Society/American Thoracic Society Task Force put them together and proposed the tentative clinical entity “interstitial pneumonia with autoimmune features (IPAF)” in 2015 (4). These suggest that autoimmune features are important to identify the different clinical phenotypes among IIPs.
The thyroid and the lung are embryologically derived from the endoderm, and thyroid transcription factor-1 (TTF-1), which is a homeodomain containing transcription factor, is expressed in both organs and involved in their morphogenesis (5). Although these facts suggest a significant similarity between the thyroid and lung, the relationship between the thyroid and interstitial pneumonia has not been elucidated. Even in the definitions of IIP with autoimmune features such as IPAF, neither thyroid function nor thyroid antibodies has been included.
Oldham et al. have showed that hypothyroidism is common and its presence predicts mortality in patients with idiopathic pulmonary fibrosis (IPF) (6). Furthermore, Awano et al. recently reported that hypothyroidism was identified in seven of 13 patients (53.8%) with idiopathic pleuroparenchymal fibroelastosis, another subtype of IIP (7). Although these results suggest the involvement of thyroid hormones in IIP pathogenesis, their clinical importance has yet to be clarified.
The goal of this study was to investigate the role of thyroid hormones in the pathogenesis of IIP and the clinical significance of thyroid function and the presence of thyroid antibodies in IIP.
Methods
Subjects
Patients with IIP, who were hospitalized or followed up in our outpatients’ ward at our department, were randomly included, and a retrospective analysis was performed. Interstitial pneumonia patients with known etiologies such as CTD, drug-induced lung injury, pneumoconiosis, hypersensitivity pneumonitis and sarcoidosis were excluded. IPF was diagnosed using the definition in the 2011 ATS/ERS/JRS/ALAT joint statement (8), and all patients with IIP met the 2013 ATS/ERS Update of the International Multidisciplinary Classification of IIP (9). The ethics committee of Fukushima Medical University approved this work, and all clinical investigations were conducted according to the principles of the Declaration of Helsinki.
Analysis of thyroid function and autoantibodies
Electro chemiluminescence immunoassay was used to analyze the levels of thyroid hormones (FT3: free triiodothyronine, FT4: free thyroxine), thyroid stimulating hormone (TSH) and thyroid antibodies, thyroglobulin (TgAb), thyroid peroxidase (TPOAb) and TSH receptor (TRAb) antibodies. Overt hypothyroidism and hyperthyroidism were defined as an elevated level of TSH in combination with normal or decreased thyroid hormone levels and a decreased level of TSH in combination with normal or elevated thyroid hormone levels, respectively. Subclinical hypothyroidism and hyperthyroidism were confirmed when an elevated level of TSH with normal thyroid hormone levels and a decreased level of TSH with normal thyroid hormone levels were seen, respectively. Low T3 syndrome was defined by the levels of decreased FT3 and normal or decreased FT4 with normal or decreased TSH level. Anti-nuclear antibody (ANA), rheumatoid factor (RF), anti-double stranded DNA antibody (dsDNA), anti-SSA antibody (SSA), anti-SSB antibody (SSB), anti-U1 ribonucleoprotein antibody (U1RNP), anti-cyclic citrullinated peptide antibody (CCP), anti-topoisomerase antibody (Scl70), anti-smith antibody (Sm), anti-aminoacyl tRNA synthetase antibody (ARS), anti-centromere antibody (ACA), myeloperoxidase (MPO)-anti-neutrophil cytoplasmic antibody (ANCA), proteinase-3 (PR3)-ANCA were measured as autoantibodies suggestive of the presence CTD.
The relationship of clinical parameters with thyroid hormone levels and thyroid antibodies
We also examined the relationship between the clinical parameters and thyroid hormone levels. The difference in clinical parameters was evaluated between IIP patients with or without thyroid antibodies.
Statistical analysis
Data are expressed as mean ± standard errors of the mean (SEM), unless otherwise stated. The Mann-Whitney U test or Fisher’s exact test was used to compare two unpaired groups. Correlations between thyroid hormone levels and clinical parameters were analyzed using Spearman’s correlation coefficient. The analysis was performed using IBM SPSS Statistics ver. 25 software (IBM Japan; Tokyo, Japan), and a P value of <0.05 indicated statistical significance.
Results
Clinical characteristics
One hundred IIP patients were included in this study; 30 were patients with IPF and 70 were patients with IIP other than IPF (non-IPF). The patients had a mean age of 70±1 years, and included 72 men and 28 women. The IIP patients had elevated Krebs von den Lungen-6 (KL-6), surfactant protein (SP)-A, and SP-D levels (Table 1). Mean levels of TSH, FT4 and FT3 were within the normal range, and TSH level was more than 10 µIU/mL, which is considered to be a significant risk of progression to overt hypothyroidism (10) in two patients. The positivity of TgAb and TPOAb was 17 and 16%, respectively, and either antibody was positive in 22% of IIP patients. Subclinical hypothyroidism, subclinical hyperthyroidism and overt hypothyroidism were confirmed in six (male: 6.9%; female: 3.6%), two and one percent of IIP patients, respectively. No patient had overt hyperthyroidism as well as TRAb. When IIP was divided into IPF and non-IPF, predominance of male and more smokers were seen in patients with IPF. There was no difference in the frequency of positive TgAb, positive TPOAb, subclinical hypothyroidism, subclinical hyperthyroidism and overt hypothyroidism. Autoantibodies suggestive of the presence CTD were positive in four of seven hypothyroidism patients, one of two subclinical hyperthyroidism patients and five of 11 low T3 syndrome patients.
Table 1. Clinical characteristics of patients with IIP.
| Variable | Total IIP | IPF | Non-IPF | P value |
|---|---|---|---|---|
| Subjects (n) | 100 | 30 | 70 | |
| Age (years) | 70±1 | 70±1 | 71±1 | 0.781 |
| Gender (M/F) | 72/28 | 26/4 | 46/24 | 0.050 |
| Autoantibodies* (+/−) | 51/49 | 15/15 | 35/35 | 1.000 |
| Smoker (+/−) | 68/32 | 25/5 | 43/27 | 0.037 |
| Brinkman index | 602±62 | 739±106 | 544±75 | 0.136 |
| Corticosteroid (%) | 28.0 | 30.0 | 27.1 | 0.810 |
| Duration of corticosteroid treatment (days) | 322±71 | 274±109 | 343±91 | 0.917 |
| Immunosuppressive reagents (%) | 12.0 | 13.3 | 11.4 | 0.749 |
| Duration of immunosuppressive reagent treatment (days) | 149±70 | 116±92 | 163±80 | 0.894 |
| NAC (%) | 11.0 | 23.3 | 5.7 | 0.016 |
| Duration of NAC treatment (days) | 120±44 | 178±86 | 95±51 | 0.015 |
| Raynaud’s phenomenon (%) | 4.0 | 3.3 | 4.3 | 1.000 |
| Arthralgia (%) | 9.0 | 10.0 | 8.6 | 1.000 |
| Morning stiffness (%) | 8.0 | 6.7 | 8.6 | 1.000 |
| Dry mouth/eye (%) | 22.0 | 13.3 | 25.7 | 0.199 |
| Rash (%) | 5.0 | 3.3 | 5.7 | 1.000 |
| Muscle weakness (%) | 4.0 | 0.0 | 5.7 | 0.313 |
| WBC (/μL) | 7,826±285 | 8,253±548 | 7,643±334 | 0.244 |
| LDH (IU/L) | 251±8 | 245±10 | 253±11 | 0.813 |
| CK (U/L) | 118±14 | 99±23 | 127±18 | 0.039 |
| CRP (mg/dL) | 0.60±0.14 | 0.63±0.23 | 0.59±0.18 | 0.279 |
| ESR (mm/h) | 16±1 | 15±2 | 17±2 | 0.428 |
| ALD (U/L) | 5.9±0.4 | 6.0±0.4 | 5.9±0.6 | 0.400 |
| BNP (pg/mL) | 50.7±10.5 | 31.6±5.8 | 61.8±16.0 | 0.555 |
| KL-6 (U/mL) | 1,122±83 | 1,135±99 | 1,117±112 | 0.177 |
| SP-A (ng/mL) | 74.3±4.3 | 76.7±6.8 | 73.3±5.4 | 0.350 |
| SP-D (ng/mL) | 228.8±16.0 | 248.2±22.7 | 220.7±20.6 | 0.083 |
| VC (L) | 2.7±0.1 | 2.7±0.1 | 2.6±0.1 | 0.857 |
| %VC (%) | 82.1±2.3 | 80.1±3.8 | 83.1±2.9 | 0.544 |
| TSH (μIU/mL) | 2.59±0.30 | 2.83±0.75 | 2.49±0.30 | 0.904 |
| FT4 (ng/dL) | 1.17±0.18 | 1.17±0.04 | 1.16±0.02 | 0.609 |
| FT3 (pg/mL) | 2.84±0.04 | 2.95±0.06 | 2.80±0.06 | 0.150 |
| TgAb (IU/mL) | 55.09±13.62 | 42.48±22.83 | 60.57±16.90 | 0.134 |
| TPOAb (IU/mL) | 34.58±9.84 | 27.89±19.84 | 37.45±11.27 | 0.015 |
| TSH (low/high/normal) | 2/7/91 | 1/3/26 | 1/4/65 | 0.600 |
| FT4 (low/normal) | 5/95 | 2/28 | 3/67 | 0.635 |
| FT3 (low/normal) | 11/89 | 0/30 | 11/59 | 0.031 |
| TgAb positive (%) | 17.0 | 16.7 | 17.4 | 1.000 |
| TPOAb positive (%) | 16.0 | 10.0 | 18.6 | 0.379 |
| TRAb positive (%) | 0.0 | 0.0 | 0.0 | 1.000 |
| TgAb or TPOAb positive (%) | 22.0 | 16.7 | 24.3 | 0.445 |
| Subclinical hyperthyroidism (%) | 2.0 | 3.3 | 1.4 | 0.512 |
| Subclinical hypothyroidism (%) | 6.0 | 6.7 | 5.7 | 1.000 |
| Low T3 syndrome (%) | 11.0 | 0.0 | 15.7 | 0.031 |
| Hyperthyroidism (%) | 0.0 | 0.0 | 0.0 | 1.000 |
| Hypothyroidism (%) | 1.0 | 3.3 | 0.0 | 0.300 |
*, anti-nuclear antibody, rheumatoid factor, anti-double stranded DNA antibody, anti-SSA antibody, anti-SSB antibody, anti-U1 ribonucleoprotein antibody, anti-cyclic citrullinated peptide antibody, anti-topoisomerase antibody, anti-smith antibody, anti-aminoacyl tRNA synthetase antibody, anti-centromere antibody, myeloperoxidase-anti-neutrophil cytoplasmic antibody, proteinase 3-anti-neutrophil cytoplasmic antibody. P value: IPF vs. non-IPF. Mean ± SEM. +, positive; −, negative. NAC, inhalation of N-acetylcysteine; WBC, white blood cell; LDH, lactate dehydrogenase; CK, creatine kinase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ALD, aldolase; BNP, brain natriuretic peptide; KL-6, Krebs von den lungen-6; SP-A, surfactant protein-A; SP-D, surfactant protein-D; VC, vital capacity; TSH, thyroid stimulating hormone; FT4, free thyroxine; FT3, free triiodothyronine; TgAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody.
When IPF and non-IPF groups were compared, the frequency of NAC treatment was significantly lower and the levels of TPO-Ab and the frequency of low FT3 were significantly higher in patients with non-IPF.
The relationship between thyroid function and clinical parameters
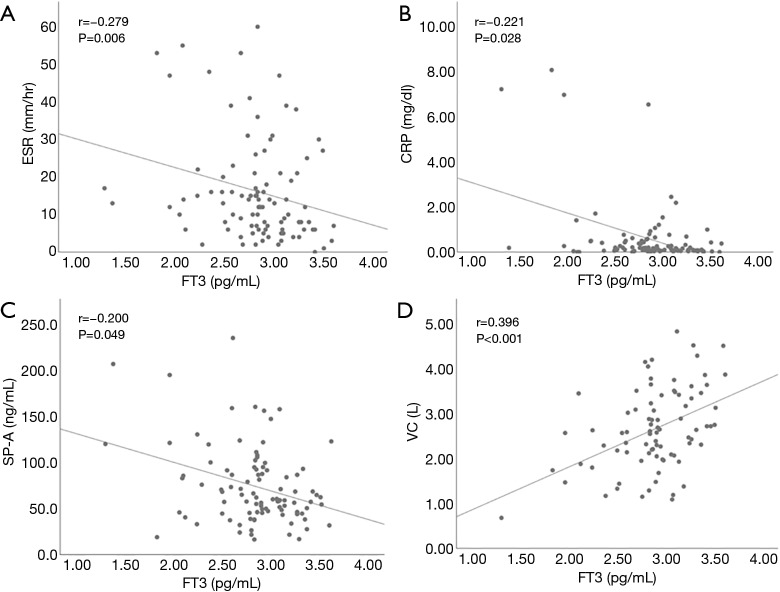
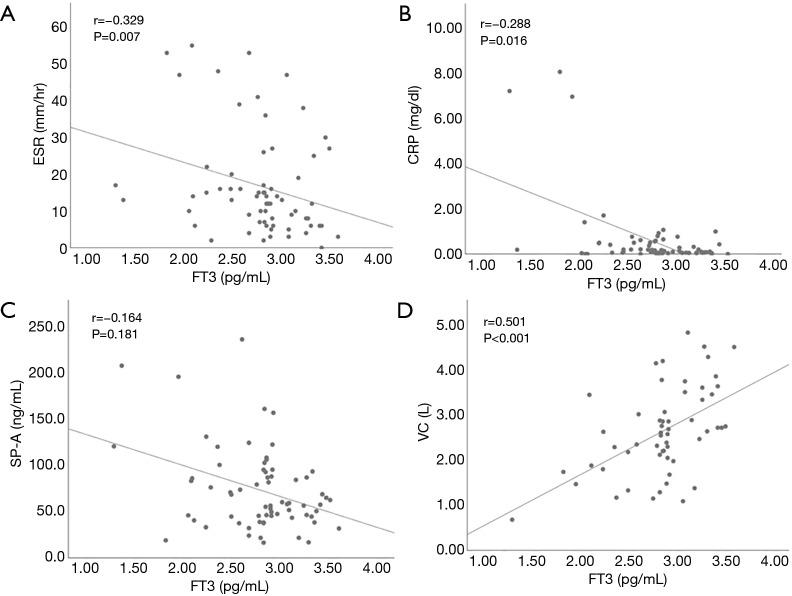
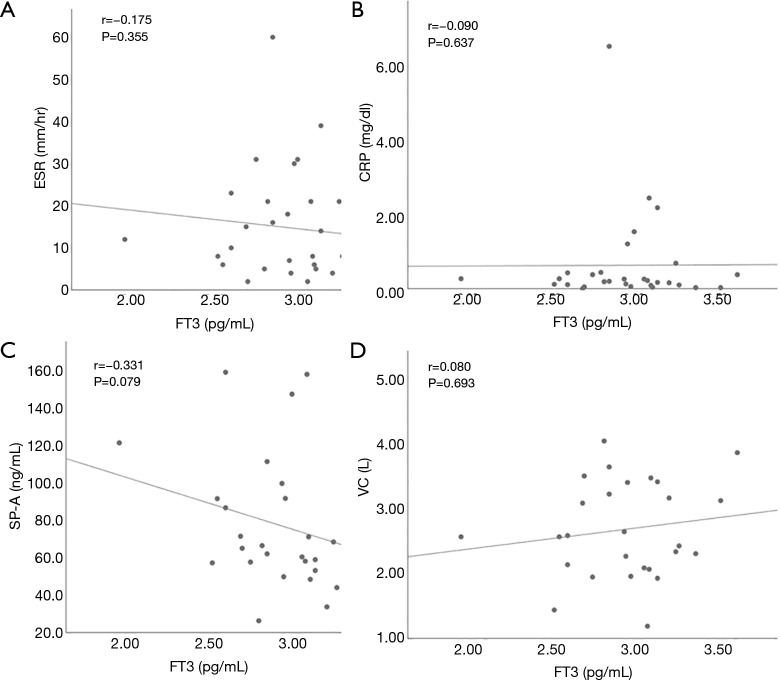
We also examined the relationship between thyroid function and clinical parameters in patients with IIP. Hypothyroidism included overt and subclinical hypothyroidism. Seven hypothyroidism patients and 78 patients with normal thyroid function were compared (Table 2). Corticosteroid and immunosuppressive reagents were given to none of seven hypothyroidism patients, and 23 and 10 patients with normal thyroid function took corticosteroid and immunosuppressive reagents, respectively. There was no difference in the frequency of IPF, positive rate of autoantibodies suggestive of the presence CTD, corticosteroid use, and immunosuppressive reagent use between the two groups. In addition, the levels of lactate dehydrogenase (LDH), KL-6, SP-A, SP-D and vital capacity (VC) were not different. When we focused on FT3 level, it had a significant positive with VC and a significant negative correlation with the levels of CRP and SP-A, and erythrocyte sedimentation ratio (ESR) in all IIP patients (Figure 1). TSH and FT4 did not have any significant correlation with the clinical parameters. Next, we evaluated patients with IPF and non-IPF separately. In IPF patients, there was no difference in clinical parameters except SP-D between patients with hypothyroidism and normal thyroid function. On the other hand, in non-IPF patients, KL-6 levels were significantly higher in patients with hypothyroidism than those with normal thyroid function. In addition, FT3 levels had a significant positive correlation with VC and a significant negative correlation with CRP levels and ESR (Figure 2). These correlations were not found in IPF patients (Figure 3).
Table 2. Comparison of clinical characteristics between IIP patients with subclinical/overt hypothyroidism and euthyroidism.
| Variable | Total IIP | IPF | Non-IPF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Subclinical/overt hypothyroidism | Euthyroidism | P value | Subclinical/overt hypothyroidism |
Euthyroidism | P value | Subclinical/overt hypothyroidism |
Euthyroidism | P value | |||
| Subjects (n) | 7 | 78 | 3 | 25 | 4 | 53 | |||||
| Age (years) | 66.6±4.0 | 69.9±1.1 | 0.481 | 75.3±0.3 | 69.5±1.4 | 0.125 | 60.0±4.8 | 70.0±1.5 | 0.052 | ||
| Gender (M/F) | 5/2 | 60/18 | 0.665 | 2/1 | 23/2 | 0.298 | 3/1 | 37/16 | 1.000. | ||
| Smoker (+/−) | 6/1 | 55/23 | 0.667 | 3/0 | 21/4 | 1.000 | 3/1 | 34/19 | 1.000 | ||
| Brinkman index | 1,120±397 | 598±62 | 0.286 | 1,307±697 | 705±94 | 0.673 | 1,082±541 | 583±80 | 0.459 | ||
| Autoantibodies* (+/−) | 4/3 | 39/39 | 1.000 | 2/1 | 12/13 | 1.000 | 2/2 | 27/26 | 1.000 | ||
| Corticosteroid (+/−) | 0/7 | 23/55 | 0.182 | 0/3 | 9/16 | 0.530 | 0/4 | 14/39 | 0.563 | ||
| Duration of corticosteroid treatment (days) | NA | 343±85 | NA | NA | 329±128 | NA | NA | 350±110 | NA | ||
| Immunosuppressive reagents (+/−) | 0/7 | 10/68 | 0.592 | 0/3 | 4/21 | 1.000 | 0/4 | 6/47 | 1.000 | ||
| Duration of Immunosuppressive reagent treatment (days) | NA | 178±79 | NA | NA | 139±111 | NA | NA | 196±104 | NA | ||
| NAC (+/−) | 1/6 | 8/70 | 0.557 | 1/2 | 6/19 | 1.000 | 0/4 | 2/51 | 1.000 | ||
| Duration of NAC treatment (days) | 80±80 | 99±43 | 0.765 | 187±187 | 192±102 | 0.834 | NA | 55±40 | NA | ||
| Raynaud’s phenomenon (+/−) | 0/7 | 3/75 | 1.000 | 0/3 | 1/24 | 1.000 | 0/4 | 2/51 | 1.000 | ||
| Arthralgia (+/−) | 0/7 | 7/71 | 1.000 | 0/3 | 3/22 | 1.000 | 0/4 | 4/49 | 1.000 | ||
| Morning stiffness (+/−) | 1/6 | 5/73 | 0.413 | 0/3 | 2/23 | 1.000 | 1/3 | 3/50 | 0.259 | ||
| Dry month/eye (+/−) | 1/6 | 16/62 | 1.000 | 0/3 | 4/21 | 1.000 | 1/3 | 12/41 | 1.000 | ||
| Rash (+/−) | 0/7 | 4/74 | 1.000 | 0/3 | 1/24 | 1.000 | 0/4 | 3/50 | 1.000 | ||
| Muscle weakness (+/−) | 0/7 | 3/75 | 1.000 | 0/3 | 0/25 | 1.000 | 0/4 | 3/50 | 1.000 | ||
| WBC (/μL) | 8,600±1,633 | 7,756±278 | 0.981 | 9,800±4,168 | 8,304±458 | 0.622 | 7,700±122 | 7,498±344 | 0.577 | ||
| LDH (IU/L) | 277±27 | 244±8 | 0.217 | 299±50 | 240±10 | 0.192 | 260±33 | 245±10 | 0.598 | ||
| CK (U/L) | 117±17 | 129±18 | 0.289 | 110±37 | 98±28 | 0.391 | 123±19 | 144±23 | 0.470 | ||
| CRP (mg/dL) | 0.21±0.07 | 0.40±0.10 | 0.852 | 0.12±0.07 | 0.74±0.28 | 0.167 | 0.28±0.11 | 0.24±0.04 | 0.451 | ||
| ESR (mm/h) | 18±7 | 15±1 | 0.957 | 17±8 | 14±3 | 0.834 | 19±15 | 15±2 | 0.668 | ||
| ALD (U/L) | 5.1±0.4 | 6.1±0.5 | 0.664 | 5.3±0.7 | 6.2±0.5 | 0.635 | 5.0±0.4 | 6.0±0.8 | 0.826 | ||
| BNP (pg/mL) | 27.2±19.6 | 32.8±4.3 | 0.280 | 58.8±45.7 | 29.7±5.8 | 0.522 | 6.1±6.1 | 35.0±6.2 | 0.049 | ||
| KL-6 (U/mL) | 1,349±230 | 1,149±102 | 0.146 | 983±79 | 1,159±117 | 0.572 | 1,623±352 | 1,144±140 | 0.066 | ||
| SP-A (ng/mL) | 99.5±30.3 | 70.8±4.0 | 0.455 | 82.4±17.3 | 77.2±7.8 | 0.570 | 108.0±46.6 | 67.6±4.5 | 0.696 | ||
| SP-D (ng/mL) | 432.1±125.1 | 220.7±15.4 | 0.140 | 482.9±48.4 | 235.3±21.1 | 0.023 | 406.6±195.1 | 213.7±20.6 | 0.656 | ||
| VC (L) | 2.8±0.3 | 2.7±0.1 | 0.873 | 2.7±0.4 | 2.7±0.2 | 0.906 | 2.9±0.5 | 2.7±0.1 | 0.871 | ||
| %VC (%) | 82.0±6.1 | 83.2±2.6 | 0.722 | 79.5±12.6 | 81.2±3.9 | 0.969 | 83.8±7.1 | 84.2±3.4 | 0.843 | ||
| TSH (μIU/mL) | 10.25±2.94 | 2.15±0.12 | <0.001 | 11.59±5.95 | 1.96±0.17 | 0.001 | 9.24±3.44 | 2.25±0.16 | <0.001 | ||
| FT4 (ng/dL) | 0.99±0.09 | 1.19±0.02 | 0.016 | 0.91±0.18 | 1.22±0.03 | 0.051 | 1.05±0.08 | 1.18±0.02 | 0.083 | ||
| FT3 (pg/mL) | 2.74±0.08 | 2.97±0.04 | 0.050 | 2.87±0.09 | 2.96±0.07 | 0.477 | 2.65±0.11 | 2.98±0.04 | 0.014 | ||
| TgAb (IU/mL) | 134.66±83.56 | 49.40±14.48 | 0.175 | 9.05±9.05 | 49.41±27.25 | 0.889 | 228.86±132.26 | 49.40±17.16 | 0.061 | ||
| TgAb (+/−) | 2/5 | 13/64 | 0.603 | 0/3 | 5/20 | 1.000 | 2/2 | 8/44 | 0.142 | ||
| TPOAb (IU/mL) | 127.39±59.66 | 31.26±11.07 | 0.269 | 5.23±2.68 | 31.44±23.82 | 1.000 | 219.01±76.98 | 31.18±11.99 | 0.037 | ||
| TPOAb (+/−) | 3/4 | 13/65 | 0.120 | 0/3 | 3/22 | 1.000 | 3/1 | 10/43 | 0.034 | ||
*, anti-nuclear antibody, rheumatoid factor, anti-double stranded DNA antibody, anti-SSA antibody, anti-SSB antibody, anti-U1 ribonucleoprotein antibody, anti- cyclic citrullinated peptide antibody, anti-topoisomerase antibody, anti-smith antibody, anti-aminoacyl tRNA synthetase antibody, anti-centromere antibody, myeloperoxidase-anti-neutrophil cytoplasmic antibody, proteinase 3-anti-neutrophil cytoplasmic antibody. Mean ± SEM. +, positive; −, negative. NA, not applicable; IPF, idiopathic pulmonary fibrosis; NAC, inhalation of N-acetylcysteine; WBC, white blood cell; LDH, lactate dehydrogenase; CK, creatine kinase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ALD, aldolase; BNP, brain natriuretic peptide; KL-6, Krebs von den lungen-6; SP-A, surfactant protein-A; SP-D, surfactant protein-D; VC, vital capacity; TSH, thyroid stimulating hormone; FT4, free thyroxine; FT3, free triiodothyronine; TgAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody.
Figure 1.
Correlations between free triiodothyronine and the clinical parameters in patients with IIP. The level of free triiodothyronine (FT3) had significant negative correlations with CRP, ESR and SP-A, and a significant positive correlation with VC. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; SP-A, surfactant protein-A; VC, vital capacity.
Figure 2.
Correlations between free triiodothyronine and the clinical parameters in patients with non-IPF. The level of free triiodothyronine (FT3) had significant negative correlations with CRP and ESR, and a significant positive correlation with VC. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; SP-A, surfactant protein-A; VC, vital capacity.
Figure 3.
Correlations between free triiodothyronine and the clinical parameters in patients with IPF. The level of free triiodothyronine (FT3) had no correlations with CRP and ESR, SP-A and VC. CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; SP-A, surfactant protein-A; VC, vital capacity.
The relationship between thyroid antibodies and clinical parameters
To evaluate whether there is a relationship between thyroid antibodies and clinical parameters, we examined the difference in the clinical parameters between IIP patients with or without positive thyroid antibodies (Table 3). Positive thyroid antibodies were defined to be either or both positive TgAb and TPOAb. The patients with positive thyroid antibodies had a significant less corticosteroid or immunosuppressive reagent use and a tendency to higher rate of positive autoantibodies suggestive of the presence CTD compared to those without positive thyroid antibodies. Although there was no difference in the levels of LDH, KL-6, SP-A, SP-D or VC between the two groups, the creatine kinase (CK) level was significantly higher in IIP patients with positive thyroid antibodies. Interestingly, in non-IPF patients, the rate of positive autoantibodies suggestive of the presence CTD and CK level as well as aldolase level were different between patients with or without positive thyroid antibodies. However, these differences were not found in IPF patients (Table 3).
Table 3. Comparison of clinical characteristics of IIP patients with and without thyroid antibodies.
| Variable | Total IIP | IPF | Non-IPF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TgAb and/or TPOAb positive | TgAb and TPOAb negative | P value | TgAb and/or TPOAb positive | TgAb and TPOAb negative | P value | TgAb and/or TPOAb positive | TgAb and TPOAb negative | P value | |||
| Subjects (n) | 22 | 78 | 5 | 25 | 17 | 53 | |||||
| Age (years) | 69.9±2.2 | 70.7±1.0 | 0.603 | 70.6±2.7 | 70.3±1.4 | 0.957 | 69.7±2.8 | 70.8±1.4 | 0.612 | ||
| Gender (M/F) | 18/4 | 54/24 | 0.293 | 5/0 | 21/4 | 1.000 | 13/4 | 33/20 | 0.383 | ||
| Smoker (+/−) | 15/7 | 53/25 | 1.000 | 5/0 | 20/5 | 0.556 | 10/7 | 33/20 | 1.000 | ||
| Brinkman index | 729±168 | 567±63 | 0.615 | 954±232 | 695±118 | 0.275 | 663±207 | 506±74 | 0.899 | ||
| Autoantibodies* (+/−) | 15/7 | 35/43 | 0.090 | 3/2 | 12/13 | 1.000 | 12/5 | 23/30 | 0.093 | ||
| Corticosteroid (+/−) | 2/20 | 26/52 | 0.031 | 1/4 | 8/17 | 1.000 | 1/16 | 18/35 | 0.028 | ||
| Duration of corticosteroid treatment (days) | 30±29 | 405±89 | 0.019 | 126±126 | 304±128 | 0.706 | 2±2 | 452±116 | 0.019 | ||
| Immunosuppressive reagents (+/−) | 0/22 | 12/66 | 0.063 | 0/5 | 4/21 | 1.000 | 0/17 | 8/45 | 0.185 | ||
| Duration of Immunosuppressive reagent treatment (days) | NA | 191±79 | NA | NA | 139±111 | NA | NA | 215±104 | NA | ||
| NAC (+/−) | 2/20 | 9/69 | 1.000 | 2/3 | 5/20 | 1.565 | 0/17 | 4/49 | 0.566 | ||
| Duration of NAC treatment (days) | 143±108 | 113±48 | 0.783 | 627±442 | 89±48 | 0.385 | NA | 125±66 | 0.247 | ||
| Raynaud’s phenomenon (+/−) | 3/19 | 1/77 | 0.032 | 1/4 | 0/25 | 0.167 | 2/15 | 1/52 | 0.144 | ||
| Arthralgia (+/−) | 3/19 | 6/72 | 0.408 | 1/4 | 2/23 | 0.433 | 2/15 | 4/49 | 0.628 | ||
| Morning stiffness (+/−) | 3/19 | 5/73 | 0.369 | 0/5 | 2/23 | 1.000 | 3/14 | 3/50 | 0.149 | ||
| Dry mouth/eye (+/−) | 4/18 | 18/60 | 0.775 | 0/5 | 4/21 | 1.000 | 4/13 | 14/39 | 1.000 | ||
| Rash (+/−) | 0/22 | 5/73 | 0.583 | 0/5 | 1/24 | 1.000 | 0/17 | 4/49 | 0.566 | ||
| Muscle weakness (+/−) | 3/19 | 1/77 | 0.032 | 0/5 | 0/25 | 1.000 | 3/14 | 1/52 | 0.042 | ||
| WBC (/μL) | 7,409±453 | 7,943±343 | 0.742 | 7,040±520 | 8,496±641 | 0.229 | 7,518±570 | 7,683±404 | 0.681 | ||
| LDH (IU/L) | 260±22 | 248±8 | 0.547 | 222±15 | 249±12 | 0.385 | 271±27 | 247±11 | 0.253 | ||
| CK (U/L) | 182±51 | 100±11 | 0.045 | 81±17 | 102±28 | 0.872 | 211±65 | 99±9 | 0.036 | ||
| CRP (mg/dL) | 0.56±0.29 | 0.62±0.17 | 0.575 | 1.41±1.29 | 0.48±0.13 | 0.627 | 0.31±0.109 | 0.68±0.24 | 0.764 | ||
| ESR (mm/h) | 20±4 | 15±1 | 0.443 | 19±10 | 14±2 | 0.706 | 20±4 | 16±2 | 0.471 | ||
| ALD (U/L) | 7.7±1.7 | 5.5±0.2 | 0.241 | 6.4±1.8 | 5.9±0.4 | 0.737 | 8.0±2.1 | 5.2±0.3 | 0.086 | ||
| BNP (pg/mL) | 75.9±35.6 | 42.8±8.1 | 0.450 | 39.5±23.1 | 30.1±5.7 | 0.811 | 87.1±46.1 | 51.5±12.9 | 0.483 | ||
| KL-6 (U/mL) | 1,152±192 | 1,114±93 | 0.787 | 1,115±180 | 1,139±115 | 0.829 | 1,163±245 | 1,102±126 | 0.758 | ||
| SP-A (ng/mL) | 77.4±7.8 | 73.6±5.0 | 0.336 | 88.3±23.2 | 74.3±6.8 | 0.482 | 73.8±7.4 | 73.2±6.6 | 0.408 | ||
| SP-D (ng/mL) | 237.5±40.5 | 226.5±17.2 | 0.687 | 268.4±89.5 | 244.0±21.5 | 0.933 | 227.9±46.7 | 218.5±23.0 | 0.943 | ||
| VC (L) | 2.8±0.2 | 2.6±0.1 | 0.448 | 2.9±0.3 | 2.6±0.2 | 0.314 | 2.7±0.3 | 2.6±0.1 | 0.698 | ||
| %VC (%) | 82.2±4.9 | 82.1±2.6 | 0.965 | 85.6±5.5 | 78.8±4.5 | 0.739 | 81.0±6.5 | 83.8±3.3 | 0.784 | ||
| TSH (μIU/mL) | 3.39±0.83 | 2.36±0.31 | 0.083 | 2.11±0.50 | 2.97±0.90 | 0.957 | 3.76±1.06 | 2.08±0.18 | 0.057 | ||
| FT4 (ng/dL) | 1.16±0.04 | 1.17±0.02 | 0.835 | 1.13±0.07 | 1.18±0.04 | 0.666 | 1.17±0.05 | 1.16±0.02 | 0.875 | ||
| FT3 (pg/mL) | 2.86±0.09 | 2.84±0.05 | 0.937 | 2.78±0.24 | 2.98±0.06 | 0.552 | 2.89±0.10 | 2.77±0.07 | 0.617 | ||
| TgAb (IU/mL) | 225.93±45.83 | 6.28±0.92 | <0.001 | 235.08±107.16 | 3.96±1.43 | <0.001 | 223.24±52.17 | 7.39±1.16 | <0.001 | ||
| TPOAb (IU/mL) | 131.33±38.69 | 7.29±0.58 | <0.001 | 138.53±115.83 | 5.76±1.13 | 0.074 | 129.21±39.44 | 8.01±0.66 | <0.001 | ||
*, anti-nuclear antibody, rheumatoid factor, anti-double stranded DNA antibody, anti-SSA antibody, anti-SSB antibody, anti-U1 ribonucleoprotein antibody, anti-cyclic citrullinated peptide antibody, anti-topoisomerase antibody, anti-smith antibody, anti-aminoacyl tRNA synthetase antibody, anti-centromere antibody, myeloperoxidase-anti-neutrophil cytoplasmic antibody, proteinase 3-anti-neutrophil cytoplasmic antibody. Mean ± SEM. +, positive; −, negative. NA, not applicable; IPF, idiopathic pulmonary fibrosis; NAC, inhalation of N-acetylcysteine; WBC, white blood cell; LDH, lactate dehydrogenase; CK, creatine kinase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ALD, aldolase; BNP, brain natriuretic peptide; KL-6, Krebs von den lungen-6; SP-A, surfactant protein-A; SP-D, surfactant protein-D; VC, vital capacity; TSH, thyroid stimulating hormone; FT4, free thyroxine; FT3, free triiodothyronine; TgAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody.
The relationship between autoantibodies and clinical parameters
The relationship between autoantibodies suggestive of the presence CTD and the clinical parameters was also analyzed in patients with IIP (Table 4). Fifty patients had at least one positive autoantibody, and the patients had a significantly higher rate of arthralgia and tendencies to higher rates of dry mouth/eye and positive thyroid antibodies than the IIP patients without autoantibodies suggestive of the presence CTD. In addition, the levels of CRP and aldolase were significantly higher and SP-D level was significantly lower in the IIP patients with autoantibodies suggestive of the presence CTD than those without the antibodies. Although these differences were not found in IPF patients, non-IPF patients with autoantibodies suggestive of the presence CTD had significantly higher levels of CRP and aldolase as well as a tendency to higher rate of positive thyroid antibodies than those without the antibodies. The positivity of autoantibodies suggestive of the presence CTD did not affect the frequency of subclinical hypothyroidism and hyperthyroidism. Regarding the type of autoantibodies suggestive of the presence CTD, ANA was positive in about half of IIP patients with thyroid antibodies, and the positive rate of ANCA and PR3-ANCA was significantly higher in IIP patients with thyroid antibodies than those without the antibodies (Table 5).
Table 4. Comparison of clinical characteristics of IIP patients with and without autoantibodies.
| Variable | Total IIP | IPF | Non-IPF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Autoantibody* positive | Autoantibody* negative | P value | Autoantibody* positive | Autoantibody* negative | P value | Autoantibody* positive | Autoantibody* negative | P value | |||
| Subjects (n) | 50 | 50 | 15 | 15 | 35 | 35 | |||||
| Age (years) | 70.5±1.2 | 70.5±1.4 | 0.804 | 72.7±1.4 | 68.0±1.8 | 0.056 | 69.5±1.6 | 71.5±1.9 | 0.245 | ||
| Gender (M/F) | 35/15 | 37/13 | 0.824 | 13/2 | 13/2 | 1.000 | 22/13 | 24/11 | 0.802 | ||
| Smoker (+/−) | 33/17 | 35/15 | 0.830 | 13/2 | 12/3 | 1.000 | 20/15 | 23/12 | 0.624 | ||
| Brinkman index | 614±93 | 591±81 | 0.925 | 808±178 | 669±118 | 0.683 | 531±108 | 557±105 | 0.703 | ||
| Corticosteroid (+/−) | 11/39 | 17/33 | 0.265 | 3/12 | 6/9 | 0.427 | 8/27 | 11/24 | 0.592 | ||
| Duration of corticosteroid treatment (days) | 301±106 | 343±96 | 0.256 | 191±117 | 358±185 | 0.412 | 349±144 | 337±114 | 0.524 | ||
| Immunosuppressive reagents (+/−) | 8/42 | 4/46 | 0.357 | 2/13 | 2/13 | 1.000 | 6/29 | 2/33 | 0.259 | ||
| Duration of Immunosuppressive reagent treatment (days) | 214±110 | 84±57 | 0.222 | 27±22 | 204±184 | 0.967 | 293±155 | 32±23 | 0.131 | ||
| NAC (+/−) | 4/46 | 7/43 | 0.525 | 3/12 | 4/11 | 1.000 | 1/34 | 3/32 | 0.614 | ||
| Duration of NAC treatment (days) | 103±60 | 137±64 | 0.348 | 219±157 | 138±78 | 0.806 | 53±53 | 136±86 | 0.314 | ||
| Raynaud’s phenomenon (+/−) | 4/46 | 0/50 | 0.117 | 1/14 | 0/15 | 1.000 | 3/32 | 0/35 | 0.239 | ||
| Arthralgia (+/−) | 8/42 | 1/49 | 0.031 | 3/12 | 0/15 | 0.224 | 5/30 | 1/34 | 0.198 | ||
| Morning stiffness (+/−) | 5/45 | 3/47 | 0.715 | 1/14 | 1/14 | 1.000 | 4/31 | 2/33 | 0.673 | ||
| Dry eye/mouth (+/−) | 15/35 | 7/43 | 0.090 | 3/12 | 1/14 | 0.598 | 12/23 | 6/29 | 0.171 | ||
| Rash (+/−) | 3/47 | 2/48 | 1.000 | 0/15 | 1/14 | 1.000 | 3/32 | 1/34 | 0.614 | ||
| Muscle weakness (+/−) | 3/47 | 1/49 | 0.617 | 0/15 | 0/15 | 1.000 | 3/32 | 1/34 | 0.614 | ||
| WBC (/μL) | 8,194±396 | 7,458±409 | 0.241 | 8,560±863 | 7,947±697 | 0.838 | 8,037±434 | 7,249±504 | 0.131 | ||
| LDH (IU/L) | 250±11 | 251±12 | 0.680 | 234±9 | 255±18 | 0.967 | 257±15 | 249±16 | 0.581 | ||
| CK (U/L) | 113±18 | 124±23 | 0.933 | 72±9 | 126±45 | 0.436 | 131±25 | 123±26 | 0.573 | ||
| CRP (mg/dL) | 0.80±0.25 | 0.41±0.14 | 0.043 | 0.40±0.15 | 0.87±0.44 | 0.713 | 0.98±0.36 | 0.21±0.05 | 0.007 | ||
| ESR (mm/h) | 18±2 | 15±2 | 0.490 | 13±3 | 16±4 | 0.683 | 20±3 | 14±2 | 0.209 | ||
| ALD (U/L) | 5.9±0.3 | 5.9±0.8 | 0.061 | 5.7±0.5 | 6.3±0.7 | 0.652 | 6.0±0.4 | 5.7±1.1 | 0.008 | ||
| BNP (pg/mL) | 52.0±16.4 | 49.3±12.5 | 0.800 | 42.1±10.3 | 21.0±3.6 | 0.362 | 57.1±24.5 | 67.6±19.6 | 0.315 | ||
| KL-6 (U/mL) | 1,068±107 | 1,177±129 | 0.652 | 928±112 | 1,342±149 | 0.045 | 1,129±145 | 1,106±172 | 0.477 | ||
| SP-A (ng/mL) | 66.5±5.2 | 82.4±6.7 | 0.130 | 64.3±6.8 | 90.1±11.2 | 0.070 | 67.5±6.9 | 79.2±8.3 | 0.377 | ||
| SP-D (ng/mL) | 207.1±22.1 | 250.6±22.8 | 0.057 | 240.6±42.0 | 256.4±15.8 | 0.234 | 192.3±26.0 | 248.2±31.4 | 0.146 | ||
| VC (L) | 2.5±0.1 | 2.8±0.1 | 0.101 | 2.4±0.2 | 2.9±0.2 | 0.085 | 2.5±0.2 | 2.8±0.2 | 0.376 | ||
| %VC (%) | 79.7±3.6 | 84.6±3.0 | 0.387 | 76.8±6.5 | 83.1±4.2 | 0.519 | 81.0±4.3 | 85.4±4.0 | 0.491 | ||
| TSH (μIU/mL) | 2.39±0.21 | 2.79±0.57 | 0.383 | 2.63±0.41 | 3.03±1.47 | 0.106 | 2.29±0.24 | 2.68±0.54 | 0.939 | ||
| FT4 (ng/dL) | 1.17±0.02 | 1.16±0.03 | 0.780 | 1.17±0.04 | 1.17±0.06 | 0.589 | 1.17±0.03 | 1.16±0.03 | 0.537 | ||
| FT3 (pg/mL) | 2.86±0.06 | 2.83±0.06 | 0.524 | 2.88±0.09 | 3.02±0.08 | 0.443 | 2.85±0.08 | 2.75±0.08 | 0.296 | ||
| TgAb (IU/mL) | 76.69±23.40 | 33.05±13.25 | 0.332 | 54.23±42.13 | 30.74±19.09 | 0.744 | 86.31±28.37 | 34.08±17.31 | 0.159 | ||
| TPOAb (IU/mL) | 49.65±17.78 | 19.51±8.09 | 0.141 | 47.02±39.56 | 8.76±3.78 | 0.744 | 50.78±19.36 | 24.12±11.41 | 0.106 | ||
| TSH (low/high/normal) | 1/4/45 | 1/3/46 | 0.926 | 1/2/12 | 0/1/14 | 0.475 | 0/2/33 | 1/2/32 | 0.939 | ||
| FT4 (low/normal) | 1/49 | 4/46 | 0.362 | 0/15 | 2/13 | 0.483 | 1/34 | 2/33 | 1.000 | ||
| FT3 (low/normal) | 5/45 | 6/44 | 1.000 | 0/15 | 0/15 | 1.000 | 5/30 | 6/29 | 1.000 | ||
| TgAb (high/normal) | 12/38 | 5/44 | 0.108 | 3/12 | 2/13 | 1.000 | 9/26 | 3/31 | 0.110 | ||
| TPOAb (high/normal) | 12/38 | 4/46 | 0.054 | 2/13 | 1/14 | 1.000 | 10/25 | 3/32 | 0.062 | ||
| TgAb and/or TPOAb positive (%) | 30.0 | 14.0 | 0.090 | 20.0 | 13.3 | 1.000 | 34.3 | 14.3 | 0.093 | ||
| Subclinical hyperthyroidism (%) | 2.0 | 2.0 | 1.000 | 6.7 | 0.0 | 1.000 | 0.0 | 2.9 | 1.000 | ||
| Subclinical hypothyroidism (%) | 8.0 | 4.0 | 0.678 | 13.3 | 0.0 | 0.483 | 5.7 | 5.7 | 1.000 | ||
| Low T3 syndrome (%) | 10.0 | 112.0 | 1.000 | 0.0 | 0.0 | 1.000 | 14.3 | 17.1 | 1.000 | ||
| Hyperthyroidism (%) | 0.0 | 0.0 | 1.000 | 0.0 | 0.0 | 1.000 | 0.0 | 0.0 | 1.000 | ||
| Hypothyroidism (%) | 0.0 | 2.0 | 1.000 | 0.0 | 6.7 | 1.000 | 0.0 | 0.0 | 1.000 | ||
*, anti-nuclear antibody, rheumatoid factor, anti-double stranded DNA antibody, anti-SSA antibody, anti-SSB antibody, anti-U1 ribonucleoprotein antibody, anti-cyclic citrullinated peptide antibody, anti-topoisomerase antibody, anti-smith antibody, anti-aminoacyl tRNA synthetase antibody, anti-centromere antibody, myeloperoxidase-anti-neutrophil cytoplasmic antibody, proteinase 3-anti-neutrophil cytoplasmic antibody. Mean ± SEM. +, positive; −, negative. IPF, idiopathic pulmonary fibrosis; NAC, inhalation of N-acetylcysteine; WBC, white blood cell; LDH, lactate dehydrogenase; CK: creatine kinase; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ALD, aldolase; BNP, brain natriuretic peptide; KL-6, Krebs von den lungen-6; SP-A, surfactant protein-A; SP-D, surfactant protein-D; VC, vital capacity; TSH: thyroid stimulating hormone; FT4, free thyroxine; FT3, free triiodothyronine; TgAb, thyroglobulin antibody; TPOAb, thyroid peroxidase antibody.
Table 5. Difference in the frequency of autoantibodies between IIP patients with and without thyroid antibodies.
| Variable | TgAb and/or TPOAb positive | TgAb and TPOAb negative | P value |
|---|---|---|---|
| ANA (+/−) | 10/12 | 11/67 | 0.003 |
| RF (+/−) | 9/13 | 20/58 | 0.189 |
| dsDNA (+/−) | 1/21 | 3/75 | 1.000 |
| SSA (+/−) | 2/20 | 2/76 | 0.209 |
| SSB (+/−) | 1/21 | 1/77 | 0.393 |
| U1RNP (+/−) | 0/22 | 3/75 | 1.000 |
| CCP (+/−) | 1/21 | 9/69 | 0.452 |
| Scl70 (+/−) | 0/22 | 2/76 | 1.000 |
| Sm (+/−) | 0/22 | 1/77 | 1.000 |
| ARS (+/−) | 0/22 | 4/74 | 0.573 |
| ACA (+/−) | 0/22 | 0/78 | 1.000 |
| MPO-ANCA (+/−) | 1/21 | 4/74 | 1.000 |
| PR3-ANCA (+/−) | 3/19 | 1/77 | 0.032 |
+, positive; −, negative. ANA, anti-nuclear antibody; RF, rheumatoid factor; dsDNA, anti-double stranded DNA antibody; SSA, anti-SSA antibody; SSB, anti-SSB antibody; U1RNP, anti-U1 ribonucleoprotein antibody; CCP, anti-cyclic citrullinated peptide antibody; Scl70, anti-topoisomerase antibody; Sm: anti-smith antibody; ARS, anti-aminoacyl tRNA synthetase antibody; ACA, anti-centromere antibody; MPO-ANCA, myeloperoxidase-anti-neutrophil cytoplasmic antibody; PR3-ANCA, proteinase 3-anti-neutrophil cytoplasmic antibody.
Discussion
In the present study, we demonstrated that: in patients with IIP: (I) 22% had positive thyroid antibody (either or both TgAb and TPOAb) and 7% had overt or subclinical hypothyroidism in patients with IIP; (II) FT3 level had a significant positive correlation with VC, and a significant negative correlation with the levels of CRP and SP-A, and ESR; (III) autoantibodies suggestive of the presence CTD was positive in more than two thirds of IIP patients with the thyroid antibody; and (IV) CK level was significantly higher, and the positive rates of ANA and PR3-ANCA were more frequent in IIP patients with thyroid antibody; (V) most of the differences which were found in IIP patients were observed in non-IPF patients, however, were not in IPF patients. There are few reports describing the association between the thyroid and lung, and biological analysis was not performed in most patients even in the report by Oldham et al., which analyzed 196 IPF patients (6). This is the first report analyzing the association of thyroid hormone and antibodies with clinical parameters of IIP in detail.
The endoderm gives rise to the respiratory and digestive organs such as the pancreas, liver, lung, stomach, and intestine. The thyroid and the lung are embryologically derived from the endoderm. TTF-1 is a homeodomain containing transcription factor which controls both morphogenesis and gene expression in the thyroid and lung (11). TTF-1 protein binds to DNA consensus sequences which are located in the promoter regions of both thyroid- and lung-specific genes, and activates gene expression (12). In lung epithelial cells, TTF-1 activates the gene expression of SP-A, -B, and -C, which are involved in the fibrotic processes in the lung (12,13). In addition, haploinsufficiency for the TTF-1 gene was recently reported to be the cause of brain-lung-thyroid syndrome that exhibits benign hereditary chorea, hypothyroidism and infant respiratory distress syndrome (14). TTF-1 is also reported to be expressed in chronic thyroiditis as well as interstitial pneumonia (15,16), and increased expression of TTF-1 in respiratory epithelial cells causes pulmonary inflammation (17). These results show the strong association of the thyroid with the lung, and the possible role of TTF-1 on pulmonary inflammation and fibrosis.
Subclinical thyroid dysfunction is more common than overt thyroid disease, and subclinical hypothyroidism is more common than subclinical hyperthyroidism (18). In the US, the national health and nutrition examination survey conducted from 1988 through 1994 showed that 0.3% of the population had overt hypothyroidism, 4.6% had subclinical hypothyroidism, 0.5% had overt hyperthyroidism, and 0.7% had subclinical hyperthyroidism (19). Although prevalence of subclinical hypothyroidism varies based on certain population factors, about 5% of women and 3% of men have subclinical hypothyroidism in the US (18), and 7.5% of females and 2.8% of males have subclinical hypothyroidism in the UK (20). In the Japanese population, the results of health check-ups showed that 4.7% of the population had subclinical hypothyroidism, 0.8% had subclinical hyperthyroidism, and the frequency of positive TgAb and TPOAb was 16.0% and 9.5%, respectively. In addition, 18.0% of the population had either or both positive TgAb and APOAb. The Hisayama study which included 1,251 people from the general Japanese populations, showed that 4.2% of the population had subclinical hypothyroidism, 0.3% had overt hypothyroidism, and thyroid antibody was positive in 7.7% in males and 15.0% in females (21). In the present study, 7% of IIP patients had subclinical or overt hypothyroidism. Although it is not possible to decide whether the prevalence of hypothyroidism is higher in IIP patients than general Japanese population because of the lack of control subjects in this study, hypothyroidism and the frequency of positive thyroid antibody are possibly higher in IIP patients when compared to the results of the previous reports. On the other hand, Oldham et al. reported that hypothyroidism was identified in 16.8% of patients with IPF, which was more than the controls (7.1%). In the cohort, hypothyroidism was recorded only when patients reported the use of thyroid replacement therapy and did not report a history of thyroidectomy or radioactive iodine ablation (6). Although a significant number of patients with subclinical hypothyroidism could have been overlooked (22), the frequency of hypothyroidism was much more common compared to our population. It is difficult to define the exact reason for the difference; however, the difference in race and iodine uptake may have affected the results. On the other hand, Awano et al. reported that seven of 13 (53.8%) Japanese patients with idiopathic pleuroparenchymal fibroelastosis, the rare subgroup of IIP had hypothyroidism (7). Because IIP is a heterogeneous group and includes a couple of subgroups, the frequency of thyroid dysfunction may be different in each specific subgroup of IIP.
The exact mechanism of the involvement of thyroid hormone in the pathogenesis of IIP is not clear. However, growing evidence suggests the important role of thyroid hormone on pulmonary inflammation and fibrosis. In IIP, excess oxidant stress causes inflammation and fibrosis in the lung (23). The association of oxidant stress with both hyperthyroidism and hypothyroidism was reported (24,25). In addition, thyroid hormones were reported to have a protective role during inflammation by controlling macrophage maturation and functions (26), and attenuate fibrotic responses by inhibiting TGF-β signaling (27). Moreover, Yu et al. recently demonstrated that thyroid hormone inhibits lung fibrosis by improving epithelial mitochondrial function (28). Furthermore, the present study showed that FT3 level had a positive correlation with VC and a negative correlation with inflammatory markers such as CRP and ESR in patients with IIP and non-IPF. Their results suggest a critical role of thyroid hormone on pulmonary inflammation and fibrosis.
In autoimmune thyroid disorders, the high prevalence of positive autoantibodies such as ANA, dsDNA and anti-extractable nuclear antigen antibody, was reported (29). Although ANA is the most frequent non-organ-specific antibody, its positive rate is reported to be more than 45% in patients with autoimmune thyroiditis (30). In addition, Elnady et al. also reported the high prevalence of autoantibodies in patients with autoimmune thyroiditis (31). They showed that ANA, RF, CCP, ds-DNA, SSA, and SSB antibodies were positive in 50.8%, 34.4%, 19.7%, 18.0%, 14.8% and 14.8% of their population, respectively. In the present study, thyroid autoantibodies were positive in 22% of IIP patients, and ANA, RF, PR3-ANCA, SSA, CCP, ds-DNA, and SSB antibodies were positive in 45%, 41%, 14%, 9%, 5%, 5% and 5%, respectively. It is not clear whether this fact is clinically significant. However, because there is a high prevalence of CTD such as Sjögren’s syndrome (29), rheumatoid arthritis, and systemic lupus erythematosus (30,31) in patients with autoimmune thyroiditis, a high prevalence of autoimmune antibodies in autoimmune thyroid disorders may indicate the association of autoimmune thyroid disorders with CTD. Thyroid function and autoantibodies are not included in the definition of IPAF (32,33), however, abnormal thyroid dysfunction and the presence of thyroid antibodies may suggest the clinical phenotype of IIP with autoimmune features.
In the point of view of the relationship between thyroid and IIP with autoimmune features, the result of CK levels in the present study are suggestive. We here demonstrated that CK level was significantly higher in IIP patients with thyroid antibodies than those without the antibodies. In patients with non-IPF, the difference was observed, however, it was not in IPF patients. Elevated level of CK is included in the diagnostic criteria for autoimmune featured interstitial lung disease (3), and histological non-IPF patterns such as NSIP is considered to be the findings which suggest the existence of CTD (34). These results suggest the relationship between abnormal thyroid function and/or positive thyroid antibody and IIP with autoimmune features.
This study has some limitations. First, this is a retrospective study of IIP patients, and the number of patients was limited. In addition, the time course of the thyroid and lung functions, as well as the relationship between the levels of thyroid hormones and prognosis, were not analyzed. Second, we could not identify the role of thyroid hormone in the pathogenesis of IIP in this study. Several reports support critical roles of thyroid hormone on pulmonary inflammation and fibrosis as described above; however, the exact role of thyroid hormone might be complex in IIP. Finally, local levels of thyroid hormone in the lung were not clarified in this study. There were relatively a small number of hypothyroidism patients when thyroid hormone levels were evaluated in blood; however, the local levels of thyroid hormone in the lung may be different. Although the correlation between FT3 in blood and clinical parameters was weak, a strong correlation may be found when the relationship between thyroid hormone in the lung and clinical parameters is analyzed. Further studies are necessary to clarify these points.
Conclusions
Although thyroid dysfunction is not frequent, thyroid hormone and thyroid antibodies are possibly involved in the pathogenesis of IIP and evaluation of thyroid function and thyroid antibody is clinically useful to identify the clinical phenotype of IIP with autoimmune features.
Acknowledgments
Funding: This study was partly supported by a grant to the Diffuse Lung Diseases Research Group from the Ministry of Health, Labour and Welfare, Japan.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Data collection and analyses were approved (No. 2425), and the need to obtain written informed consent from each patient was waived by the Institutional Review Board of Fukushima Medical University.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Kinder BW, Collard HR, Koth L, et al. Idiopathic nonspecific interstitial pneumonia: lung manifestation of undifferentiated connective tissue disease? Am J Respir Crit Care Med 2007;176:691-7. 10.1164/rccm.200702-220OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fischer A, West SG, Swigris JJ, et al. Connective tissue disease-associated interstitial lung disease: a call for clarification. Chest 2010;138:251-6. 10.1378/chest.10-0194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vij R, Noth I, Strek ME. Autoimmune-featured interstitial lung disease: a distinct entity. Chest 2011;140:1292-9. 10.1378/chest.10-2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J 2015;46:976-87. 10.1183/13993003.00150-2015 [DOI] [PubMed] [Google Scholar]
- 5.Kimura S, Hara Y, Pineau T, et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev 1996;10:60-9. 10.1101/gad.10.1.60 [DOI] [PubMed] [Google Scholar]
- 6.Oldham JM, Kumar D, Lee C, et al. Thyroid Disease Is Prevalent and Predicts Survival in Patients With Idiopathic Pulmonary Fibrosis. Chest 2015;148:692-700. 10.1378/chest.14-2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awano N, Izumo T, Fukuda K, et al. Is hypothyroidism in idiopathic pleuroparenchymal fibroelastosis a novel lung-thyroid syndrome? Respir Investig 2018;56:48-56. 10.1016/j.resinv.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 8.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733-48. 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper DS, Biondi B. Subclinical thyroid disease. Lancet 2012;379:1142-54. 10.1016/S0140-6736(11)60276-6 [DOI] [PubMed] [Google Scholar]
- 11.Boggaram V. Thyroid transcription factor-1 (TTF-1/Nkx2.1/TITF1) gene regulation in the lung. Clin Sci (Lond) 2009;116:27-35. 10.1042/CS20080068 [DOI] [PubMed] [Google Scholar]
- 12.Bohinski RJ, Di Lauro R, Whitsett JA. The lung-specific surfactant protein B gene promoter is a target for thyroid transcription factor 1 and hepatocyte nuclear factor 3, indicating common factors for organ-specific gene expression along the foregut axis. Mol Cell Biol 1994;14:5671-81. 10.1128/MCB.14.9.5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitsett JA, Glasser SW. Regulation of surfactant protein gene transcription. Biochim Biophys Acta 1998;1408:303-11. 10.1016/S0925-4439(98)00076-3 [DOI] [PubMed] [Google Scholar]
- 14.Kleinlein B, Griese M, Liebisch G, et al. Fatal neonatal respiratory failure in an infant with congenital hypothyroidism due to haploinsufficiency of the NKX2-1 gene: alteration of pulmonary surfactant homeostasis. Arch Dis Child Fetal Neonatal Ed 2011;96:F453-456. 10.1136/adc.2009.180448 [DOI] [PubMed] [Google Scholar]
- 15.Fabro AT, Minatel IO, Rangel MP, et al. Usual interstitial pneumonia and smoking-related interstitial fibrosis display epithelial to mesenchymal transition in fibroblastic foci. Respir Med 2014;108:1377-86. 10.1016/j.rmed.2014.06.008 [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Li X, Lin L, et al. Upregulation of thyroid transcription factor-1 and human leukocyte antigen class I in Hashimoto's disease providing a clinical evidence for possible triggering autoimmune reaction. Eur J Endocrinol 2011;164:795-800. 10.1530/EJE-10-0960 [DOI] [PubMed] [Google Scholar]
- 17.Wert SE, Dey CR, Blair PA, et al. Increased expression of thyroid transcription factor-1 (TTF-1) in respiratory epithelial cells inhibits alveolarization and causes pulmonary inflammation. Dev Biol 2002;242:75-87. 10.1006/dbio.2001.0540 [DOI] [PubMed] [Google Scholar]
- 18.U.S. Preventive Services Task Force . Screening for thyroid disease: recommendation statement. Ann Intern Med 2004;140:125-7. 10.7326/0003-4819-140-2-200401200-00014 [DOI] [PubMed] [Google Scholar]
- 19.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 2002;87:489-99. 10.1210/jcem.87.2.8182 [DOI] [PubMed] [Google Scholar]
- 20.Tunbridge WM, Evered DC, Hall R, et al. The spectrum of thyroid disease in a community: the Whickham survey. Clin Endocrinol (Oxf) 1977;7:481-93. 10.1111/j.1365-2265.1977.tb01340.x [DOI] [PubMed] [Google Scholar]
- 21.Okamura K, Nakashima T, Ueda K, et al. Thyroid disorders in the general population of Hisayama Japan, with special reference to prevalence and sex differences. Int J Epidemiol 1987;16:545-9. 10.1093/ije/16.4.545 [DOI] [PubMed] [Google Scholar]
- 22.Dutt N, Purohit S, Saini L. Idiopathic Pulmonary Fibrosis and Hypothyroidism: Cannot Forget Subclinical Disease and Difficult-to-Eliminate Corticosteroids. Chest 2016;149:600. 10.1016/j.chest.2015.10.076 [DOI] [PubMed] [Google Scholar]
- 23.Fois AG, Paliogiannis P, Sotgia S, et al. Evaluation of oxidative stress biomarkers in idiopathic pulmonary fibrosis and therapeutic applications: a systematic review. Respir Res 2018;19:51. 10.1186/s12931-018-0754-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancini A, Di Segni C, Raimondo S, et al. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediators Inflamm 2016;2016:6757154. [DOI] [PMC free article] [PubMed]
- 25.Resch U, Helsel G, Tatzber F, et al. Antioxidant status in thyroid dysfunction. Clin Chem Lab Med 2002;40:1132-4. 10.1515/cclm.2002.198 [DOI] [PubMed] [Google Scholar]
- 26.Perrotta C, Buldorini M, Assi E, et al. The thyroid hormone triiodothyronine controls macrophage maturation and functions: protective role during inflammation. Am J Pathol 2014;184:230-47. 10.1016/j.ajpath.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 27.Alonso-Merino E, Martin Orozco R, Ruiz-Llorente L, et al. Thyroid hormones inhibit TGF-beta signaling and attenuate fibrotic responses. Proc Natl Acad Sci U S A 2016;113:E3451-3460. 10.1073/pnas.1506113113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu G, Tzouvelekis A, Wang R, et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat Med 2018;24:39-49. 10.1038/nm.4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tektonidou MG, Anapliotou M, Vlachoyiannopoulos P, et al. Presence of systemic autoimmune disorders in patients with autoimmune thyroid diseases. Ann Rheum Dis 2004;63:1159-61. 10.1136/ard.2004.022624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazúrová I, Benhatchi K, Rovensky J, et al. Autoimmune thyroid disease and autoimmune rheumatic disorders: a two-sided analysis. Ann N Y Acad Sci 2009;1173:211-6. 10.1111/j.1749-6632.2009.04809.x [DOI] [PubMed] [Google Scholar]
- 31.Elnady BM, Kamal NM, Shaker RH, et al. Prevalence and clinical significance of nonorgan specific antibodies in patients with autoimmune thyroiditis as predictor markers for rheumatic diseases. Medicine (Baltimore) 2016;95:e4336. 10.1097/MD.0000000000004336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavagna L, Gonzalez Gay MA, Allanore Y, et al. Interstitial pneumonia with autoimmune features: a new classification still on the move. Eur Respir Rev 2018;27:180047. 10.1183/16000617.0047-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambataro G, Sambataro D, Torrisi SE, et al. State of the art in interstitial pneumonia with autoimmune features: a systematic review on retrospective studies and suggestions for further advances. Eur Respir Rev 2018;27:170139. 10.1183/16000617.0139-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholson AG, Colby TV, Wells AU. Histopathological approach to patterns of interstitial pneumonia in patients with connective tissue disorders. Sarcoidosis Vasc Diffuse Lung Dis 2002;19:10-7. [PubMed] [Google Scholar]