Abstract
Hereditary spastic paraplegia (HSP) is a neurodegenerative disorder characterized by pyramidal weakness and spasticity of the lower limbs. SPG46, one of autosomal recessive HSP, is clinically characterized by spasticity and pyramidal weakness of the lower limbs, mental retardation, congenital bilateral cataract, thin corpus callosum, and hypogonadism in males. Mutations in the nonlysosomal glucosylceramidase β2 (GBA2) gene have been identified in patients with SPG46. A Japanese woman was identified with bilateral cataracts when she was in an elementary school. She felt falling easily, speaking unclearness, and difficulty in walking and raising her left leg in her 30s. Her neurological examination at the age of 44 revealed dysarthria, spasticity in the upper and lower extremities, increased jaw jerk and tendon reflexes in the extremities, bilateral extensor plantar reflexes, ataxia, and pollakiuria. Magnetic resonance imaging showed thinning of the corpus callosum body as well as atrophy in the pons and cerebellum. A novel homozygous c.1838A > G (p.D613G) missense mutation was detected at exon 12 in GBA2. We diagnosed her illness as an autosomal-recessive form of hereditary SPG46. The clinical features matched previously reported phenotype of SPG46. This is the first report of a Japanese patient with SPG46 with a novel mutation in GBA2. We presume that the novel GBA2 missense mutation found in our patient would cause loss of GBA2 activity, resulting in the neurological manifestations of SPG46.
Keywords: Spastic paraplegia, Cataract, Corpus callosum, Spastic paraplegia gene 46, GBA2
Abbreviations: GBA2, glucosylceramidase β2; HSP, Hereditary spastic paraplegia; SPG, spastic paraplegia gene
Highlights
-
•
SPG46 is a rare autosomal recessive hereditary spastic paraplegia.
-
•
In Japan, clinical cases of SPG46 have never been reported.
-
•
We report a case of a Japanese patient with SPG46 with a novel mutation in GBA2.
-
•
She showed cataracts, mild cognitive impairment, spasticity, and cerebellar ataxia.
1. Introduction
Hereditary spastic paraplegia (HSP) is a neurodegenerative disorder which is clinically characterized by pyramidal weakness and spasticity of the lower limbs. Inheritance of HSP has been reported to be autosomal dominant, autosomal recessive, or X-linked. To date, the causative genes or gene loci of HSPs have been assigned to SPGs 1–80 [2]. The most frequent form of HSP is SPG4 [1,2].
SPG46, one of autosomal recessive HSP, is clinically characterized by spasticity and pyramidal weakness of the lower limbs, mental retardation, congenital bilateral cataract, thin corpus callosum, and hypogonadism in males [3,4]. Mutations in the nonlysosomal glucosylceramidase β2 (GBA2) gene have been identified in patients with SPG46 [4]. To date, patients with SPG46 have been reported to be from Tunisia, Belgium, Turkey, Portugal, Italy, and China [[3], [4], [5], [6]]. In Japan, four mutations in GBA2 have been reported [1], but details of individual cases have not been fully described. Here, we report the first case of a Japanese patient with SPG46 with a novel mutation in GBA2 (c.1838A > G, p.D613G).
2. Case report
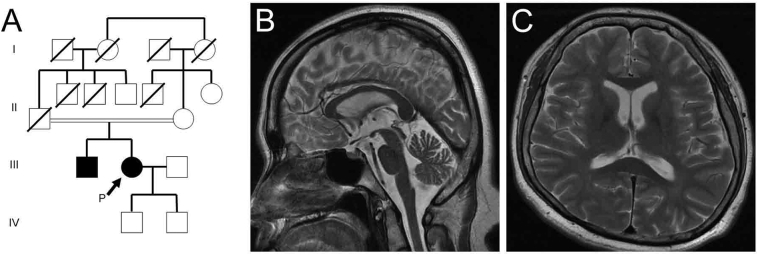
A Japanese woman was identified with bilateral cataracts when she was in an elementary school. She received surgery for bilateral cataract at age 26. She felt falling easily, speaking unclearness, and difficulty in walking and raising her left leg in her 30s. At the age of 44, she felt bilateral leg spasticity and began to limp. Her elder brother, who also had bilateral cataract surgery in his 20s, recognized gait disturbances with spasticity and delusional ideations in his 30s. Her parents were cousins, and neither they nor any other relatives had these symptoms (Fig. 1A).
Fig. 1.
Family tree (A), and T2-weighted brain MRI of the patient (B, C). A sagittal section showing thinning of the corpus callosum body and atrophy of the pons and cerebellum (B). Neither brain atrophy nor white matter lesions were apparent in this axial section (C).
Her neurological examination at the age of 44 revealed dysarthria, spasticity in the upper and lower extremities, increased jaw jerk and tendon reflexes in the extremities, bilateral extensor plantar reflexes, ataxia in the trunk and lower limbs, and pollakiuria. Neither weakness of extremities nor sensory disturbances were apparent.
Haematological and cerebrospinal fluid examinations revealed no abnormalities. She scored 30 on the Mini-Mental Status Examination; however, the Wechsler Adult Intelligence Scale (third edition) showed that her full intelligence quotient was 70, verbal intelligence quotient was 78, and performance intelligence quotient was 68. Her Frontal Assessment Battery score was 16 out of 18. Magnetic resonance imaging (MRI) showed thinning of the corpus callosum body as well as atrophy in the pons, cerebellar hemispheres, and vermis (Fig. 1B). Neither brain atrophy nor white matter lesions were apparent (Fig. 1C).
We carried out whole-exome analysis of genomic DNA from the patient. Exome capture was performed using a SureSelect All Exon V5 + UTRs Kit (Agilent Technologies, Santa Clara, USA) followed by massively parallel sequencing using a Hiseq2500 (Illumina, San Diego, USA) [1]. A novel homozygous c.1838A>G (p.D613G) missense mutation was detected at exon 12 in GBA2. This homozygous mutation was confirmed by Sanger sequencing. This variant does not appear in the Exome Aggregation Consortium online database which is derived from various disease-specific and population-genetics studies and based on gene sequences from 60,706 unrelated individuals [7]. In silico analyses using Sorting Intolerant from Tolerant [8] predicted the mutation as damaging, and Combined Annotation Dependent Depletion score [9] was 28.7. We diagnosed her illness as an autosomal-recessive form of hereditary SPG46 based on the characteristic clinical symptoms, a family history in her older brother, MRI findings, and the mutation in GBA2.
3. Discussion
We described the first case of a Japanese patient with SPG46 with a novel mutation in GBA2 (c.1838A>G, p.D613G). She showed bilateral cataract at a young age, mild cognitive impairment, spasticity with pyramidal signs, and cerebellar ataxia. These findings were similar to the previous reports of SPG46.
GBA2 is localized in the endoplasmic reticulum and Golgi membranes, where it cleaves glucosylceramide to glucose and ceramide [10]. It is predominantly expressed in neurons, and the highest level of GBA2 is found in cerebellar cortex [10]. Mutations in GBA2 (missense and nonsense) identified in patients with SPG46 cause GBA2 activity loss [10].
GBA2 protein is composed of an N-terminal glucosyl-hydrolase family 116 domain and a C-terminal catalytic domain [10]. The majority of missense mutations previously identified in SPG46 patients are located in the C-terminal catalytic domain of GBA2, and these patients commonly revealed pyramidal sign, spasticity, ataxia, or cataract [10]. The novel mutation detected in our patient also localized to the C-terminal catalytic domain of GBA2. Her clinical features matched previously reported phenotype of SPG46 and those in patients with missense mutations located in the C-terminal catalytic domain of GBA2 [3,4,10]. We presume that the novel GBA2 missense mutation found in our patient would also cause loss of GBA2 activity, resulting in the neurological manifestations of SPG46.
4. Conclusion
We report a Japanese patient with SPG46 with a novel mutation in GBA2. The clinical features in our patient matched previously reported phenotype of SPG46.
Declaration of interest
This work was supported by Grants-in-Aid from the Research Committee for Ataxic Disease (Y.T.), the Ministry of Health, Labor and Welfare, Japan, and JSPS KAKENHI Grant Number JP18K07495 (Y.T.) from the Ministry of Education, Culture, Sports, and Technology, Japan.
Acknowledgements
The authors would like to thank Yukari Yamaguchi and Yumiko Kakuda from Kanazawa University for providing technical assistance.
References
- 1.Koh K., Ishiura H., Tsuji S., Takiyama Y. JASPAC: Japan spastic paraplegia research consortium. Brain Sci. 2018;8 doi: 10.3390/brainsci8080153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nan H., Ichinose Y., Tanaka M., Koh K., Ishiura H., Mitsui J. UBAP1 mutations cause juvenile-onset hereditary spastic paraplegias (SPG80) and impair UBAP1 targeting to endosomes. J. Hum. Genet. 2019;64:1055–1065. doi: 10.1038/s10038-019-0670-9. [DOI] [PubMed] [Google Scholar]
- 3.Boukhris A., Feki I., Elleuch N., Miladi M.I., Boland-Augé A., Truchetto J. A New locus (SPG46) maps to 9p21.2-q21.12 in a Tunisian family with a complicated autosomal recessive hereditary spastic paraplegia with mental impairment and thin corpus callosum. Neurogenetics. 2010;11:441–448. doi: 10.1007/s10048-010-0249-2. [DOI] [PubMed] [Google Scholar]
- 4.Martin E., Schüle R., Smets K., Rastetter A., Boukhris A., Loureiro J.L. Loss of function of Glucocerebrosidase GBA2 is responsible for motor neuron defects in hereditary spastic paraplegia. Am. J. Hum. Genet. 2013;92:238–244. doi: 10.1016/j.ajhg.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y.J., Zhou Z.F., Liao X.X., Luo Y.Y., Zhan Z.X., Huang M.F. SPG46 and SPG56 are rare causes of hereditary spastic paraplegia in China. J. Neurol. 2016;263:2136–2138. doi: 10.1007/s00415-016-8256-3. [DOI] [PubMed] [Google Scholar]
- 6.Coarelli G., Romano S., Travaglini L., Ferraldeschi M., Nicita F., Spadaro M. Novel homozygous GBA2 mutation in a patient with complicated spastic paraplegia. Clin. Neurol. Neurosurg. 2018;168:60–63. doi: 10.1016/j.clineuro.2018.02.042. [DOI] [PubMed] [Google Scholar]
- 7.ExAC Browser http://exac.broadinstitute.org/ [accessed 5 November 2019]
- 8.SIFT-Predict Effects of Nonsynonmous/Missense Variants. https://sift.bii.a-star.edu.sg/ [accessed 22 November 2019]
- 9.CADD-Combined Annotation Dependent Depletion. https://cadd.gs.washington.edu/ [accessed 22 November 2019]
- 10.Woeste M.A., Stern S., Raju D.N., Grahn E., Dittmann D., Gutbrod K. Species-specific differences in nonlysosomal glucosylceramidase GBA2 function underlie locomotor dysfunction arising from loss-of-function mutations. J. Biol. Chem. 2019;294:3853–3871. doi: 10.1074/jbc.RA118.006311. [DOI] [PMC free article] [PubMed] [Google Scholar]