Abstract
Background & Aims
High IgG levels are considered a hallmark of autoimmune hepatitis (AIH). A subgroup of patients with AIH has IgG within the normal range despite evidence of clinical disease activity. The clinical significance of this biomarker has not been explored.
Methods
In a European multicentre study we compared biochemical, clinical and histological features from patients with AIH and normal IgG-values at diagnosis to an age- and sex-matched control group of patients with typical AIH presenting with elevated IgG. Data were assessed at diagnosis, after 12 months of therapy and at last follow-up.
Results
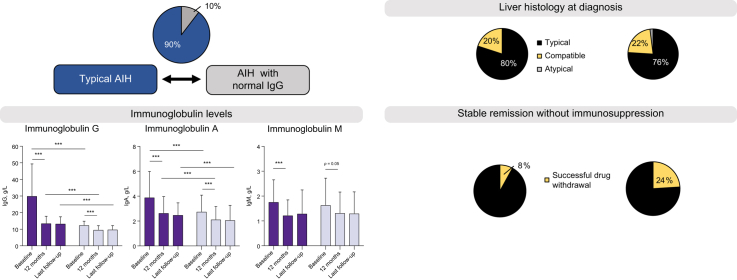
Out of 1,318 patients with AIH, 130 (10%) had normal IgG at presentation. Histological and biochemical parameters at diagnosis, as well as treatment response, showed no difference between groups. Stable remission off treatment was achieved more commonly in the normal IgG group than in the typical AIH group (24 vs. 8%; p = 0.0012). Patients of the control group not only had higher IgG levels (29.5 ± 5.8 vs. 12.5 ± 3.2 g/L; p <0.0001), but also a higher IgG/IgA ratio (9.3 ± 6.9 vs. 5.4 ± 2.4; p <0.0001) at diagnosis. The IgG/IgA ratio only declined in patients with typical AIH and was no longer different between groups after 12 months (6.3 ± 4.3 vs. 5.5 ± 2.2; p = 0.1), indicating a selective increase of IgG in typical AIH and its suppression by immunosuppression. Autoantibody titres were higher in the typical AIH group, but not when controlled for IgG levels.
Conclusions
Compared to AIH with typical biochemical features, patients with normal IgG levels at diagnosis (i) show similar biochemical, serological and histological features and comparable treatment response, (ii) appear to lack the selective elevation of serum IgG levels observed in typical active AIH disease, (iii) may represent a subgroup with a higher chance of successful drug withdrawal.
Lay summary
A characteristic feature of autoimmune hepatitis (AIH) is an elevation of immunoglobulin G (IgG), which is therefore used as a major diagnostic criterion, as well as to monitor treatment response. Nevertheless, normal IgG does not preclude the diagnosis of AIH. Therefore, we herein assessed the features of patients with AIH and normal IgG in a large multicentre study. This study demonstrates that about 10% of all patients with AIH have normal IgG; these patients are indistinguishable from other patients with AIH with respect to biochemical markers, liver histology, disease severity and treatment response, but might represent a subgroup with a higher chance of remission after drug withdrawal.
Keywords: autoimmune hepatitis, immunoglobulin G, drug withdrawal, immunoglobulins, hypergammaglobulinemia
Abbreviations: AIH, autoimmune hepatitis; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AMA, anti-mitochondrial antibody; ANA, anti-nuclear antibody; Anti-SLA/LP, anti-soluble liver antigen and anti-liver-pancreas antibodies; AST, aspartate aminotransferase; INR, international normalized ratio; LKM, liver kidney microsomal antigen; SMA, smooth muscle antibody
Graphical abstract
Highlights
-
•
Patients with AIH and normal IgG comprise around 10% of all patients with AIH.
-
•
These patients are indistinguishable from patients with typical AIH by biochemical markers or liver histology.
-
•
They have no selective IgG elevation, with lower IgG and IgA levels than patients with typical AIH.
-
•
These patients might represent a subgroup in whom there is a high chance of successful drug withdrawal.
Introduction
Autoimmune hepatitis (AIH) is a chronic non-resolving disorder of the immune system characterized by loss of immunological tolerance against hepatocytes inducing chronic inflammatory destruction of liver parenchyma.1,2 In keeping with other autoimmune disorders, AIH predominately affects women at any age and its onset may range from asymptomatic to acute presentation with liver failure.3,4 If left untreated, AIH is a devastating disease, however, the first controlled trails demonstrated that immunosuppression prolonged survival.[5], [6], [7] Despite the outlook generally being good for treated AIH,[8], [9], [10], [11] it is usually a chronic disease and relapse after drug withdrawal occurs in the vast majority of patients.12,13
The diagnosis of AIH is based on descriptive criteria assembled in a scoring system first issued in 1993,14 revised in 1999,15 and proposed in a simplified manner for routine clinical use in 2008.16 Selective elevation of serum IgG levels is considered a characteristic feature of AIH which is found in up to 85% of patients[14], [15], [16] and therefore has found its way into diagnostic scores.[17], [18], [19] In fact, apart from characteristic autoantibodies, findings on liver histology and the exclusion of viral hepatitis, elevated IgG levels are the diagnostic backbone in the simplified diagnostic scoring system proposed by the IAIHG in 2008.19
Moreover, it has been demonstrated that elevated IgG levels indicate ongoing inflammatory activity in treated AIH.20,21 Consequently, most recent practice guidelines have defined complete biochemical remission as repeatedly normal serum aminotransferases and IgG levels,2,22,23 which is now accepted as major treatment goal in AIH.
It is estimated that about 15%[14], [15], [16] of patients with AIH present with IgG levels within the normal range. However, the clinical characteristics at presentation and the treatment response of patients who are lacking such a distinctive feature of AIH, have never been explored in detail. Therefore, we compared the biochemical, clinical and histological features of patients with AIH and normal IgG-values at diagnosis to an age- and sex-matched control group of patients with typical AIH presenting with elevated IgG.
Patients and methods
Patient population
This is a retrospective multicentre study including patients from 5 European high-volume centres: Hamburg (HH), London (LON), Nijmegen (NIJ), Birmingham (BHAM), Larissa (LA).
Each centre was invited to include patients with AIH and normal serum IgG levels at diagnosis (“normal IgG group”) and a 1:1 age- and sex-matched control group of patients with AIH presenting with elevated IgG at diagnosis. Patients in the control AIH group are referred to as patients with typical AIH (“typical AIH group”) throughout the manuscript, since high serum IgG levels are considered a very distinctive feature of AIH.
Only patients who had undergone a liver biopsy at diagnosis and in whom autoantibody titres (anti-nuclear antibody [ANA], smooth muscle antibody [SMA], liver kidney microsomal antigen [LKM], anti-mitochondrial antibody [AMA]) at diagnosis were available were considered eligible for this study. Each centre was asked to report the total number of patients with AIH who fulfilled these criteria.
The diagnosis of AIH relied on clinical, biochemical, serological and histopathological findings.2 In order to further assure a valid diagnosis of AIH in patients with normal IgG, a crude assessment of the revised original diagnostic score from 1999 was performed.18 Due to the retrospective study design, no detailed information on alcohol intake nor on HLA DR3 and DR4 was available. These factors were given zero points. In patients in whom liver histology had been classified according to the simplified score from 2008 as “typical AIH” or “compatible with AIH” but in whom a more detailed specification of histological criteria was not available, liver histology was scored as follows: Patients with “typical AIH” were given +4 points, since classification as “typical AIH” requires interface hepatitis (+3) and rosettes (+1) as well as emperipolesis; patients with “compatible AIH” were given +1 point,18,19 since it is unclear which characteristic histological feature was present.
Patients with overlap with primary biliary cholangitis or sclerosing cholangitis and with follow-up of less than 2 years were not included.
Concomitant autoimmune diseases
The presence of the following concomitant autoimmune diseases was assessed: rheumatoid arthritis, diabetes mellitus type 1, sicca syndrome, coeliac disease, scleroderma, thyroid disease, multiple sclerosis, vitiligo, inflammatory bowel disease.
Biochemical parameters
Data on biochemical and serological parameters, outcome and treatment were assessed at diagnosis, after 12 months under immunosuppression and at last follow-up. The following biochemical and serological markers were assessed: ANA, SMA, LKM, AMA, anti-soluble liver antigen and anti-liver-pancreas antibodies, g-globulin, IgG, IgM, IgA, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma glutamyltransferase, albumin, bilirubin, creatinine, platelet count and international normalized ratio (INR).
Liver histology
Only patients in whom liver biopsy had been performed to establish the diagnosis of AIH were included. Histological staging and grading according to Desmet and Scheuer classification were assessed.24 A more detailed histological work-up was hampered by the retrospective, multicentre study design. Therefore, each centre was asked to send liver specimens to a reference pathologist (M.R.) Histological re-examination involved using Ishak's score,25 the METAVIR scoring system26 and simplified criteria for AIH diagnosis.19 We assessed the presence of biliary lesions (cholangitis, bile duct loss, ductular reaction, periductular fibrosis), the presence of steatosis (if present we used the NAFLD activity score27) and fibrosis according to Kleiner.28
The study has been approved by the local ethics committees. Written informed consent was obtained from all participants.
Statistical analysis
Summary statistics for categorical variables are expressed as numbers (percentages). Quantitative variables are described as means with their standard deviations or as medians with their range if not normally distributed. Depending on the distribution, parametric and non-parametric tests including paired/unpaired t test and Wilcoxon signed rank test were used to test for differences between groups.
Comparison of categorical data between groups was performed using the chi-square test or fisher's exact test as appropriate. All p values were 2-tailed. P values <0.1 were reported, the significance level was set at p <0.05. Statistical analysis was performed with GraphPad PRIMS (GraphPad Prism) Version 8.
Results
Out of a total of 1,318 eligible patients with AIH, 130 patients with normal IgG were identified. Hence, the overall proportion of patients with AIH and normal IgG was 10% (130/1,250), and this percentage was similar in all centres (London: 51/481 (10%), Hamburg: 31/352 (9%), Larissa: 30/317 (10%), Birmingham: 9/89 (10%), Nijmegen: 9/79 (13%)).
In all patients with AIH and normal IgG, the crude assessment of the original diagnostic score18 reached at least a score corresponding to “probable diagnosis of AIH”, ranging from 10 to 19. Each centre included an age- and sex-matched control group of patients with elevated IgG (typical AIH). Median age at diagnosis was 51 years (range: 17–85) and 77% were female. Concomitant autoimmune diseases were equally common in both groups (normal IgG group: 20% vs. typical AIH group: 23%).
Clinical characteristics of patients with AIH and normal IgG, and of the control group, are displayed in Table 1.
Table 1.
Biochemical, histological and clinical features at diagnosis, after 12 months of treatment and at last follow-up.
| Normal IgG group (n = 130) | Typical AIH group (control group) (n = 130) | p value (if <0.1)∗ | |
|---|---|---|---|
| At diagnosis | |||
| Female | 77% | 77% | |
| Age, years | 51 (17-85) | 51 (21-82) | |
| AST, U/L | 590 ± 229 (529) | 564 ± 345 (534) | |
| ALT, U/L | 701 ± 448 (265) | 688 ± 523 (480) | |
| ALP, U/L | 193 ± 159 (143) | 185 ± 120 (154) | |
| Albumin, g/L | 37 ± 11.9 (35) | 35 ± 6.8 (35) | |
| INR | 1.2 ± 0.2 (1.2) | 1.4 ± 1.1 (1.2) | |
| Platelet count Mrd/L | 228 ± 89 (210) | 208 ± 83 (200) | |
| Concomitant autoimmune disease∗∗ | 23% | 20% | |
| Histological staging∗∗∗ | 2.3 ± 1.3 (2) | 2.3 ± 1.2 (2) | |
| Histological grading∗∗∗ | 3.0 ± 0.9 (3) | 3.0 ± 1.0 (3) | |
| Severe fibrosis (>2) | 50% | 50% | |
| Liver cirrhosis | 20% | 20% | |
| After 12 months of treatment | |||
| AST, U/L | 36 ± 18 (28) | 32 ± 23 (30) | |
| ALT, U/L | 45 ± 31 (28) | 34 ± 27 (25) | |
| Normal transaminases | 75% | 75% | |
| At last follow-up | |||
| AST, U/L | 29 ± 15 (24) | 29 ± 13 (25) | |
| ALT, U/L | 28 ± 13 (22) | 28 ± 14 (21) | |
| Normal transaminases | 77% | 84% | |
| Immunosuppressive treatment | 76% | 92% | 0.0012 |
| Follow-up, years | 7.4 (2-10) | 8.4 (2-15) | |
| Adverse outcome | 2 | 3 |
Mean values are presented with median in brackets.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; INR, international normalized ratio.
Continuous variables were compared using Wilcoxon singed rank rest. Fisher's exact was used for comparing percentages.
Rheumatoid arthritis, diabetes mellitus type 1, sicca syndrome, coeliac disease, scleroderma, thyroid disease, multiple sclerosis, vitiligo, inflammatory bowel disease.
According to Desmet and Scheuer classification.24
Patient characteristics at diagnosis
Immunoglobulins
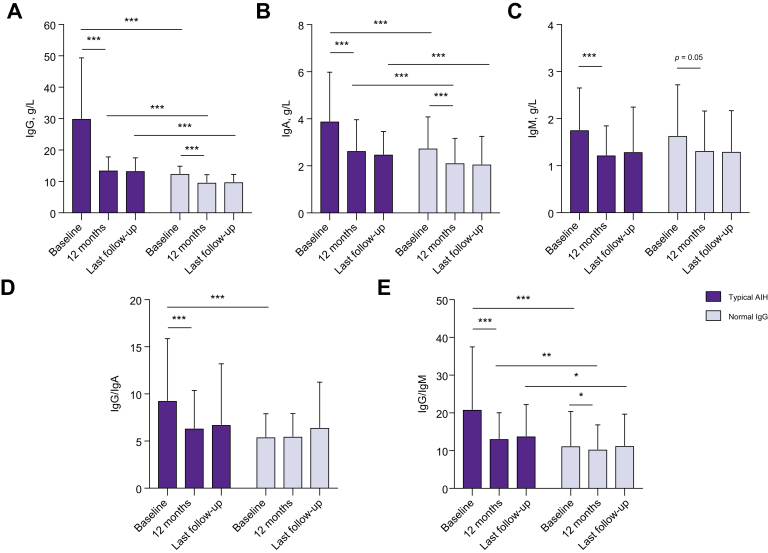
At diagnosis, average serum IgG levels were 12.5 ± 3.2 g/L in the “normal IgG group” and 29.5 ± 5.8 g/L (p <0.0001) in patients with typical AIH. Serum IgG levels decreased from 29.5 ± 5.8 g/L to 13.6 ± 4.9 g/L (−55 ± 24%) within the first 12 months of treatment in patients with typical AIH. Interestingly, patients with normal IgG at diagnosis also showed a significant drop of IgG within the normal range (Fig. 1). In these patients, IgG declined from 12.5 ± 3.2 g/L to 9.7 ± 3.2 g/L within 12 months, which corresponds to an average decrease of −22 ± 16% from baseline (p <0.0001). A decrease of IgG within the normal range could be observed in virtually all patients and IgG levels remained lower than in the “typical AIH group” throughout the study (p <0.0001) (Table 2).
Fig. 1.
Immunoglobulin levels at diagnosis, after 12 months of treatment and at last follow-up.
(A) IgG, (B) IgA, and (C) IgM levels, as well as (D) IgG/IgA ratio and (E) IgG/IgM ratio were compared betweein AIH with normal IgG at diagnosis and typicial AIH with elevated IgG. ∗∗∗p <0.0001, ∗∗p <0.001, ∗p <0.05. AIH, autoimmune hepatitis.
Table 2.
Serum immunoglobulin levels at diagnosis, after 12 months of treatment and at last follow-up.
| Normal IgG group (n = 130) | Control group (typical AIH) (n = 130) | p value (if <0.1)∗ | |
|---|---|---|---|
| At diagnosis | |||
| IgG | 12.5 ± 3.2 (12.8) | 29.5 ± 5.8 (25.9) | <0.00001 |
| IgA | 2.8 ± 0.8 (2.4) | 3.9 ± 2.1 (3.4) | 0.000025 |
| IgM | 1.64 ± 0.3 (1.5) | 1.8 ± 0.3 (1.6) | |
| IgG/IgA | 5.4 ± 2.8 (5.0) | 9.3 ± 3.6 (7.9) | <0.00001 |
| IgG/IgM | 11.3 ± 5.9 (9.0) | 20.9 ± 16.2 (16.9) | <0.00001 |
| ANA (≥1:40) | 70% | 84% | 0.012 |
| SMA (≥1:40) | 70% | 50% | 0.0015 |
| ANA, titre | 1:80 (1:40-1:1,280) | 1:160 (1:40-1:5,120) | |
| SMA, titre | 1:80 (1:20-1:2,560) | 1:40 (1:20-1:5120) | |
| Anti-SLA/LP (≥20 units/ml)∗∗ | 17% | 13% | |
| LKM | 6% | 3% | |
| At 12 months of treatment | |||
| IgG | 9.7 ± 3.2 (9.8) | 13.6 ± 4.9 (13.1) | <0.00001 |
| IgA | 2.1 ± 0.6 (1.9) | 2.6 ± 0.8 (2.5) | 0.0045 |
| IgM | 1.32 ± 0.2 (1.1) | 2.0 ± 0.4 (1.9) | |
| IgG/IgA | 5.5 ± 2.2 (5.2) | 6.3 ± 4.2 (5.4) | 0.094 |
| IgG/IgM | 10.3 ± 6.2 (8.4) | 13.1 ± 6.5 (11.1) | 0.006 |
| At last follow-up | |||
| IgG | 9.9 ± 3.0 (9.8) | 13.4 ± 4.7 (12.6) | <0.00001 |
| IgA | 2.1 ± 1.1 (1.9) | 2.5 ± 0.98 (2.4) | 0.013 |
| IgM | 1.3 (1.1) | 1.4 (1.2) | |
| IgG/IgA | 6.3 ± 3.4 (5.3) | 6.6 ± 4.3 (5.6) | |
| IgG/IgM | 11.4 ± 5.3 (9.0) | 13.8 ± 5.9 (12.8) | 0.05 |
Mean values are presented with median in brackets.
AIH, autoimmune hepatitis; AMA, anti-mitochondrial antibody; ANA, anti-nuclear antibody; Anti-SLA/LP, anti-soluble liver antigen and anti-liver-pancreas antibodies; LKM, liver kidney microsomal antigen; SMA, smooth muscle antibody.
Continuous variables were compared using Wilcoxon singed rank rest. Fisher's exact was used for comparing percentages.
Anti-SLA/LP antibodies were available in 85% of patients with normal IgG, and in 90% of patients with typical AIH.
Immunoglobulin A levels were available in 95 (73%) patients with normal IgG and in 107 patients (82%) with typical AIH. Patients with normal IgG at diagnosis had not only a lower IgG, but also lower IgA-levels (at diagnosis: 3.9 ± 2.1 g/L vs. 2.8 ± 0.8 g/L, p <0.0001) (Table 2). Moreover, a significant but similar drop of IgA could be observed in the “typical AIH group” (−29 ± 18%) as well as in the “normal IgG group” (−22 ± 17%) within the first 12 months of immunosuppression (Table 2).
Since both IgG and IgA were higher in the “typical AIH group”, we compared the IgG/IgA ratio in order to unveil a selective IgG elevation, which is considered a serological hallmark of AIH. Patients with typical AIH had a significantly higher IgG/IgA ratio at diagnosis than patients in the “normal IgG group” (9.3 ± 3.6 vs. 5.4 ± 2.8, p <0.00001) (Table 2). Moreover, while the IgG/IgA ratio remained stable in patients with normal IgG during follow-up (p = 0.47), it decreased in patients with typical AIH. On month 12 (typical AIH group: 6.3 ± 4.2 vs. normal IgG group 5.5 ± 2.2; p = 0.1) and at the end of follow-up (6.6 ± 4.3 vs. 6.3 ± 3.4), the IgG/IgA ratio was no longer different between groups (Table 2). These findings underline the selective elevation of IgG in typical AIH, while IgG and IgA dropped to the same extent in the “normal IgG group”.
Immunoglobulin M levels were available in 95 (73%) patients with normal IgG and in 107 patients (82%) with typical AIH. In both groups, IgM levels showed a slight decrease upon immunosuppression (Fig. 1C). However, in contrast to IgG and IgA, IgM levels were not significantly different between the normal IgG and typical AIH group. Consequently, the IgG/IgM ratio was higher in the typical AIH group at diagnosis and during follow-up (p <0.001), and moreover, the IgG/IgM ratio decreased in both patients with typical AIH (p <0.0001) and patients with normal IgG at diagnosis (p = 0.02) (Fig. 1E).
Hence, while the hypergammaglobulinemia typically seen in AIH is predominantly attributable to IgG, IgA but not IgM also seems to contribute.
Immunoglobulin levels at diagnosis and during follow-up are displayed in Table 2 and Fig. 1.
Since some centres measure gamma-globulins instead of/in addition to IgG, we also aimed to compare gamma-globulin levels between groups. Gamma-globulins were only routinely assessed by 2/5 participating centres (UKE and LA). In addition, the mode of measurement was different between centres. Gamma-globulins at diagnosis and during follow-up were available in 50 patients of the “normal IgG group” and 50 patients of the “typical AIH group”. Despite IgG levels within the normal range, 14 (28%) patients of the “normal IgG group” had elevated gamma-globulins at diagnosis. However, in those patients, gamma-globulins were only slightly above the upper range of normal (<21% [normal range: <18.8%] or <3.5 g/L [<3.2 g/L]). In contrast, all patients within the “typical AIH group” had considerably elevated gamma-globulins (range 24-37% or 3.5–6.6 g/L).
Autoantibodies
Median ANA and SMA titres in the “typical AIH group” were twice as high as in patients with normal IgG at diagnosis (ANA 1:160 (range 1:40–1:5,120) vs. 1:80 (1:40–1:1,280), p = 0.001; SMA 1:80 (range 1:20–1:5,120) vs. 1:40 (1:20–1:2,560), p <0.0001). However, IgG levels at diagnosis were also 2-fold higher in the control group than in patients with normal IgG (29.5 ± 5.8 g/L vs. 12.5 ± 3.2 g/L); correcting for this factor, ANA and SMA levels were similar between groups. The higher IgG levels in the control group may also explain why ANA and SMA could be detected more frequently in the control group (ANA: 84% vs. 70%, p = 0.012; SMA: 70% vs. 50%, p = 0.0015). These results suggest that lower cut-offs for autoantibodies should be considered in patients without elevated IgG levels at presentation.
Histological parameters
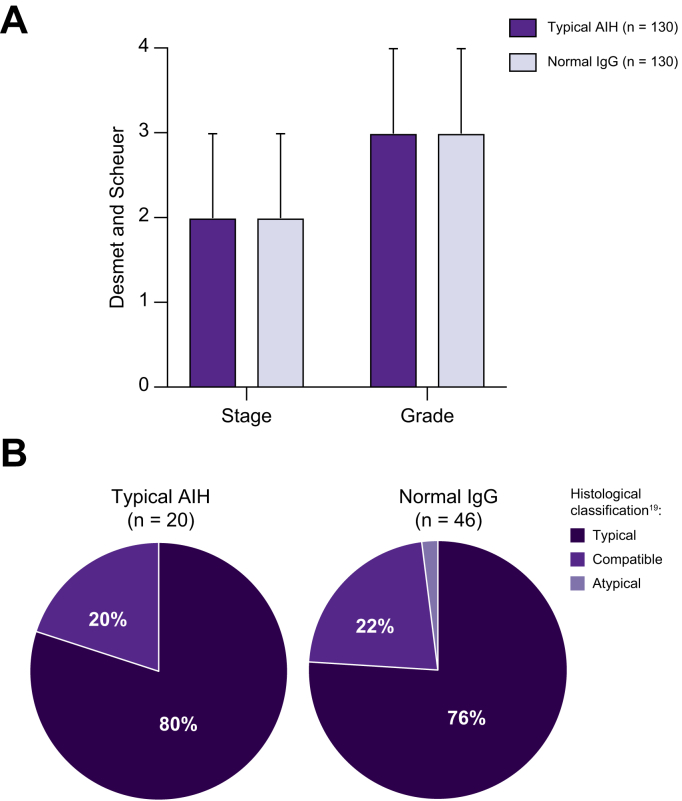
Average fibrosis stage (2.3 ± 1.2 vs. 2.3 ± 1.3) and grade (3.0 ± 0.9 vs. 3.0 ± 23) according to Desmet and Scheuer classification were not different between patients with normal IgG and typical AIH at the time of diagnosis (Fig. 2). Moreover, patients with severe fibrosis (fibrosis stage ≥3) or cirrhosis at diagnosis were equally distributed between groups (50%, and 20%, respectively).
Fig. 2.
Liver histology at the time of diagnosis.
(A) Histological staging and grading according to Desmet and Scheuer classification. (B) Histological classification according to the simplified diagnostic scoring system19 of liver biopsies that were re-assessed by a reference pathologist.
Liver histology was available for a detailed re-assessment by a reference pathologist in 46 patients of the normal IgG group and in 20 patients with typical AIH.
In patients with normal IgG at diagnosis, re-assessment according to the simplified diagnostic criteria revealed histological features atypical for the diagnosis of AIH in only a single patient and this patient was consequently not included in this study.
In all other patients, liver histology was classified as typical (76%) or compatible (22%) with AIH (Fig. 2). Interface hepatitis, hepatocyte rosettes and emperipolesis were observed in 93%, 91%, and 78%, respectively. All liver specimens of the control group were classified as typical (80%) or compatible (20%) with AIH (Fig. 2).
Inflammatory activity according to Ishak's modified activity index at diagnosis did not differ between groups (typical AIH group: 11 (median) vs. normal IgG: 11), and there was no difference in components of the METAVIR system (Table S1).
Biliary lesions were frequently observed in both study groups, but generally minimal and focal. While steatosis was seen in one-fifth of patients in both groups, the degree of steatosis was generally mild and only a single patient with normal IgG was classified as AIH with non-alcoholic steatohepatitis.
A summary of the detailed histological assessment of re-examined liver specimens is given in Table S1.
Biochemical parameters and treatment response
Except for IgG, no difference in biochemical markers could be observed at diagnosis, after 12 months of immunosuppression or at last follow-up. In fact, serum aminotransferases, biochemical parameters of cholestasis, albumin and INR were almost identical in both groups (Table 1).
Average serum aminotransferases after 12 months of treatment were 36 ± 18 U/L and 32 ± 23 U/L in patients with normal IgG and in classical AIH, respectively. Moreover, the proportion of patients with normalized serum aminotransferases was not significantly different between groups (at last follow-up: “normal IgG group”: 76% vs. “typical AIH group”: 84%, p = 0.16).
All patients received prednisolone to induce remission. Median steroid dosage at diagnosis was 40 mg/d in both groups. In 23% (normal IgG) vs. 20% (control group) of patients, prednisolone could be tapered off within the first 12 months, while 26% vs. 30% required dual immunosuppressive treatment on the long-term (usually steroids/azathioprine or steroids/mycophenolate mofetil) to maintain biochemical remission. Taken together, these findings indicate that there was no difference in treatment response in patients with normal IgG at diagnosis and those of the control AIH group.
We next assessed whether lower immunoglobulin levels reflect different success rates in inducing immunological tolerance. At last follow-up, only 8% of patients in the control group were in stable biochemical remission without immunosuppression, while in 24% of patients with normal IgG at diagnosis immunosuppression had been successfully withdrawn (p = 0.0012). It its well-known that most relapses occurs within the first months after drug withdrawal,13 therefore, only patients who remained in remission off treatment for at least 2 years were considered in long-term remission after drug withdrawal.
In the cohort of patients with normal IgG, the 31 patients who remained in remission without treatment were indistinguishable from the 99 patients under immunosuppression at last follow-up by age, sex, biochemical markers at diagnosis or after 12 months of treatment. Moreover, on histological assessment there was no difference in hepatic inflammation at diagnosis (grading: 3.1 ± 1.4 [median: 3] vs. 3.0 ± 1.3 [median: 3]). Regarding fibrosis stage, liver cirrhosis was less frequently present in patients with successful drug withdrawal (4% vs. 25%, p = 0.026).
Likewise, no patient with successful drug withdrawal in the typical AIH group had cirrhosis on liver histology, while cirrhosis was present at diagnosis in 25% of patients who were under immunosuppression at last-follow-up (p = 0.1). Interestingly, the only significant difference between the 17 patients with successful drug withdrawal and the rest of the control group were lower IgG levels after 12 months of treatment (11.1 vs. 14.1 g/L, p = 0.03), confirming previous reports29,30 and pointing to the value of immunoglobulin levels as a marker of hepatic immune tolerance in AIH.
All patients in this study with successful drug withdrawal had AIH type I (presence of ANA and/or SMA).
Patient outcome
Follow-up time did not differ between study groups (normal IgG: 7.4 years, range 2–10, vs. control group: 8.4 years, range 2–15; p = 0.82) and there was no difference in the severity of liver disease at diagnosis as assessed by frequency of liver cirrhosis, histological staging or grading, serum aminotransferases, and INR (Table 1). During follow-up, an adverse event defined as de novo signs of hepatic decompensation (ascites, hepatic encephalopathy, variceal bleeding), liver transplantation or liver-related death was documented in 2 patients in the “normal IgG group” and in 3 patients in the control group. Thus, there was no difference in outcome/prognosis between patients with normal IgG concentration at diagnosis and those with typical AIH.
Discussion
Selective elevation of serum IgG is a very distinctive feature of AIH, which has been incorporated as a major criterion in diagnostic scores.[17], [18], [19] Nevertheless, we found that every tenth patient with AIH presented with normal IgG, which is in line with previous reports.[14], [15], [16] The clinical significance of this finding has so far never been explored in detail.
This study highlights that patients with normal IgG are indistinguishable from patients with typical AIH, based on the frequency of concurrent immune diseases, laboratory indices, histological features including grading and staging at diagnosis, as well as treatment response. In fact, it was striking how similar biochemical and histological parameters, as well as treatment responses, were in both groups.
Patients with normal IgG at diagnosis might represent a subtype with a higher chance of long-term remission after drug withdrawal. Despite reports of relapse rates as high as 95% after stopping immunosuppression,13 24% of patients with normal IgG at diagnosis were in stable biochemical remission off treatment at last follow-up, while this was only the case in 8% of patients with typical AIH. This finding is in line with a former report, which observed that lower gamma-globulin levels at diagnosis were associated with successful drug withdrawal.29 Moreover, in the typical AIH group, those with stable remission off treatment differed from those who were on immunosuppression at last follow-up by lower IgG levels after 12 months of treatment. Accordingly, it has been reported that IgG levels in month 6 of treatment were an independent predictor of maintenance of remission after treatment withdrawal30 and low IgG (below 12 g/L) at the time of tapering off medication were predictive of successful drug withdrawal.31 Thus, these results indicate that low IgG might reflect success rates of inducing hepatic immune tolerance, highlighting the pathogenic role of plasma cells in AIH.32,33
It remains unclear why some patients with AIH develop no IgG elevation despite evidence of ongoing inflammatory disease activity. One explanation might be a shorter exposure to the immunologic trigger of AIH, e.g. an offending drug or food components. On the other hand, it was assumed that these patients in general have lower IgG levels because of their genetic predisposition,34 and therefore, show a relative elevation considering their naturally very low IgG. Along with this line, it was suggested that even in patients with normal IgG at diagnosis, variations of IgG could be used to monitor treatment response.2 In support of this assumption, we observed a significant fall of IgG (−22%) upon immunosuppression, and a decrease of IgG, sometimes below the limit of normal, was observed in virtually all patients. Moreover, IgG levels remained significantly lower in the normal IgG group throughout the study. Nonetheless, the fall of IgG might not be related to disease activity, but to an unspecific effect of immunosuppressive treatment.35 For instance, IgG and IgA decreased to the same extent under immunosuppression in the normal IgG group. Hence, it remains unclear whether variations of IgG can be used as a surrogate marker of disease activity in patients with normal IgG at diagnosis. An assessment of histological activity according to IgG levels would be required in order to clarify this question. Of note, IgM levels were not different between groups, consequently the IgG/IgM ratio decreased in both study groups after initiation of treatment.
The hypergammaglobulinemia typically seen in AIH is considered to be attributable to increased IgG. Contrary to this assumption, our findings suggest that IgA also contributes to the hypergammaglobulinemia in typical AIH: most patients with active AIH had IgA within the upper limit of normal or slightly elevated IgA. In addition, IgA was higher in patients in the typical AIH group than in those with normal IgG at diagnosis. IgA is a crucial factor in mucosal immunity and the predominant antibody class in mucosal surfaces.36 Therefore, our findings on IgA might be seen in context with the emerging data linking alterations of gut microbiota (dysbiosis) and increased intestinal permeability with experimental and human AIH.[37], [38], [39]
Besides increased IgG and gamma-globulins, autoantibodies are a serological hallmark of AIH and are a crucial part of diagnostic scores. ANA and SMA antibodies were slightly less frequently detected in patients with normal IgG than in the control group. Presumably the detection of autoantibodies has been missed in some patients with low IgG, since antibody titres were not corrected for IgG. Likewise, patients with typical AIH had higher autoantibody titres, however, not when controlled for the higher IgG levels in these patients. These data indicate that autoantibody titres and cut-offs when using ELISA testing should be adjusted to serum IgG levels, otherwise seropositivity may be missed in patients with low IgG.
Our study has the obvious limitations of a retrospective study design. For instance, we could only assess the number of patients in stable remission off treatment at last follow-up, but no information on the number of attempts to withdraw medication was available. The participating centres are prominent referral centres, hence a referral bias of atypical and/or more advanced cases of AIH is possible. Moreover, patients without dependence on immunosuppression to maintain remission and without IgG elevation in active disease lack 2 characteristic features of AIH. Hence, a definite diagnosis in these patients may be difficult to obtain. In particular, the differential diagnosis between immunoallergic drug-induced liver injury and AIH poses a diagnostic dilemma in these patients.
Liver histology is considered a prerequisite in the diagnostic work-up.2 Therefore, a strength of this study may be that only patients who had undergone a liver biopsy to establish the diagnosis were included. Moreover, liver specimens of 46 patients in the normal IgG group could be located and sent to a reference pathologist for detailed re-assessment. Histological hallmark features of AIH such as interface hepatitis, hepatocyte rosettes and emperipolesis were observed in 93%, 91%, and 78% of cases, respectively. Consequently, liver histology was considered atypical for AIH in only a single patient, while it was classified as typical (76%) or compatible (22%) in all others (Fig. 2). Moreover, to further assure a valid diagnosis of AIH in patients with normal IgG, a crude assessment of the revised diagnostic criteria from 1999 were applied,18 and all patients reached a score corresponding to at least a probable diagnosis of AIH.
In conclusion, compared to patients with typical AIH, patients with normal IgG at diagnosis, show strikingly similar biochemical, serological and histological features as well as comparable treatment responses, but might represent a subgroup with a higher chance of successful drug withdrawal. Serum IgG levels might be used as a predictor of hepatic immune tolerance.
Financial support
The project was supported by “Deutsche Forschungsgemeinschaft” (DFG SFB841), “YAEL Foundation”, “Helmut and Hannelore Greve Foundation”.
Authors' contributions
Hartl Johannes: First author and corresponding authors, substantial contribution to the design, data acquisition and analysis, interpretation of data, drafting of the articled. Zachou Kalliopi, Wong Guan-Wee, Ashgar Asma, Pape Simon, Drenth Joost P.H., Oo Ye, Dalekos George Nikolaos, Heneghan Michael: Data acquisition, critical revision. Sebode Marcial, Peiseler Moritz, Zenouzi Roman, Ehlken Hanno, Weiler-Normann Christina: Study design, critical revision. Krech Till, Miquel Rosa: Histological evaluation of liver specimens. Christoph Schramm, Ansgar Wilhelm Lohse: Senior authors, substantial contribution to the design, interpretation of data, drafting of the article, critical revision for important intellectual content.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2020.100094.
Supplementary data
References
- 1.Krawitt E.L. Autoimmune hepatitis. N Engl J Med. 2006;354:54–66. doi: 10.1056/NEJMra050408. [DOI] [PubMed] [Google Scholar]
- 2.EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol. 2015;63:971–1000. doi: 10.1016/j.jhep.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 3.Ngu J.H., Bechly K., Chapman B.A., Burt M.J., Barclay M.L., Gearry R.B. Population-based epidemiology study of autoimmune hepatitis: a disease of older women? J Gastroenterol Hepatol. 2010;25:1681–1686. doi: 10.1111/j.1440-1746.2010.06384.x. [DOI] [PubMed] [Google Scholar]
- 4.Van Gerven N.M.F., Verwer B.J., Witte B.I., van Erpecum K.J., van Buuren H.R., Maijers I. Epidemiology and clinical characteristics of autoimmune hepatitis in The Netherlands. Scand J Gastroenterol. 2014;49:1245–1254. doi: 10.3109/00365521.2014.946083. [DOI] [PubMed] [Google Scholar]
- 5.Cook G.C., Mulligan R., Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med. 1971;40:159–185. doi: 10.1093/oxfordjournals.qjmed.a067264. [DOI] [PubMed] [Google Scholar]
- 6.Murray-Lyon I.M., Stern R.B., Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet. 1973;1:735–737. doi: 10.1016/s0140-6736(73)92125-9. [DOI] [PubMed] [Google Scholar]
- 7.Soloway R.D., Summerskill W.H., Baggenstoss A.H., Gitnick G.L., Elveback L.R., Schoenfield L.J. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology. 1972;63:820–833. [PubMed] [Google Scholar]
- 8.Kanzler S., Lohr H., Gerken G., Galle P.R., Lohse A.W. Long-term management and prognosis of autoimmune hepatitis (AIH): a single center experience. Z Gastroenterol. 2001;39:339–341. doi: 10.1055/s-2001-13708. [DOI] [PubMed] [Google Scholar]
- 9.Roberts S.K., Therneau T.M., Czaja A.J. Prognosis of histological cirrhosis in type 1 autoimmune hepatitis. Gastroenterology. 1996;110:848–857. doi: 10.1053/gast.1996.v110.pm8608895. [DOI] [PubMed] [Google Scholar]
- 10.Hoeroldt B., McFarlane E., Dube Basumani P., Karajeh M., Campbell M.J., Gleeson D. Long-term outcomes of patients treated for autoimmune hepatitis at non-tertiary care centers. Gastroenterology. 2011;140:1980–1989. doi: 10.1053/j.gastro.2011.02.065. [DOI] [PubMed] [Google Scholar]
- 11.Grønbæk L., Vilstrup H., Jepsen P. Autoimmune hepatitis in Denmark: incidence, prevalence, prognosis, and causes of death. A nationwide registry-based cohort study. J Hepatol. 2014;60:612–617. doi: 10.1016/j.jhep.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Hegarty J.E., Nouri Aria K.T., Portmann B., Eddleston A.L., Williams R. Relapse following treatment withdrawal in patients with autoimmune chronic active hepatitis. Hepatology. 1983;3:685–689. doi: 10.1002/hep.1840030510. [DOI] [PubMed] [Google Scholar]
- 13.van Gerven N.M., Verwer B.J., Witte B.I., van Hoek B., Coenraad M.J., van Erpecum K.J. Relapse is almost universal after withdrawal of immunosuppressive medication in patients with autoimmune hepatitis in remission. J Hepatol. 2013;58:141–147. doi: 10.1016/j.jhep.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Al-Chalabi T., Underhill J.A., Portmann B.C., McFarlane I.G., Heneghan M.A. Impact of gender on the long-term outcome and survival of patients with autoimmune hepatitis. J Hepatol. 2008;48:140–147. doi: 10.1016/j.jhep.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Floreani A., Niro G., Rosa Rizzotto E., Antoniazzi S., Ferrara F., Carderi I. Type I autoimmune hepatitis: clinical course and outcome in an Italian multicentre study. Aliment Pharmacol Ther. 2006;24:1051–1057. doi: 10.1111/j.1365-2036.2006.03104.x. [DOI] [PubMed] [Google Scholar]
- 16.Muratori P., Granito A., Quarneti C., Ferri S., Menichella R., Cassani F. Autoimmune hepatitis in Italy: the Bologna experience. J Hepatol. 2009;50:1210–1218. doi: 10.1016/j.jhep.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Johnson P.J., McFarlane I.G. Meeting report: International Autoimmune Hepatitis Group. Hepatology. 1993;18:998–1005. doi: 10.1002/hep.1840180435. [DOI] [PubMed] [Google Scholar]
- 18.Alvarez F., Berg P.A., Bianchi F.B., Bianchi L., Burroughs A.K., Cancado E.L. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol. 1999;31:929–938. doi: 10.1016/s0168-8278(99)80297-9. [DOI] [PubMed] [Google Scholar]
- 19.Hennes E.M., Zeniya M., Czaja A.J., Pares A., Dalekos G.N., Krawitt E.L. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169–176. doi: 10.1002/hep.22322. [DOI] [PubMed] [Google Scholar]
- 20.Lüth S., Herkel J., Kanzler S., Frenzel C., Galle P.R., Dienes H.P. Serologic markers compared with liver biopsy for monitoring disease activity in autoimmune hepatitis. J Clin Gastroenterol. 2008;42:926–930. doi: 10.1097/MCG.0b013e318154af74. [DOI] [PubMed] [Google Scholar]
- 21.Hartl J., Ehlken H., Sebode M., Peiseler M., Krech T., Zenouzi Usefulness of biochemical remission and transient elastography in monitoring disease course in autoimmune hepatitis. J Hepatol. 2018;68:754–763. doi: 10.1016/j.jhep.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Chapman R., Fevery J., Kalloo A., Nagorney D.M., Boberg K.M., Schneider B. AASLD practice guidelines: diagnosis and management of primary sclerosing cholangitis. Hepatology. 2009;51:660–678. doi: 10.1002/hep.23294. [DOI] [PubMed] [Google Scholar]
- 23.Gleeson D., Heneghan M.A., British Society of Gastroenterology British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut. 2011;60:1611–1629. doi: 10.1136/gut.2010.235259. [DOI] [PubMed] [Google Scholar]
- 24.Desmet V.J., Gerber M., Hoofnagle J.H., Manns M., Scheuer P.J. Classification of chronic hepatitis: Diagnosis, grading and staging. Hepatology. 1994;19:1513–1520. [PubMed] [Google Scholar]
- 25.Ishak K., Baptista A., Bianchi L., Callea F., De Groote J., Gudat F. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 26.Bedossa P., Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 27.Brunt E.M. Nonalcoholic steatohepatitis: definition and pathology. Semin Liver Dis. 2001;21:3–16. doi: 10.1055/s-2001-12925. [DOI] [PubMed] [Google Scholar]
- 28.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 29.Czaja A.J., Menon K.V., Carpenter H.A. Sustained remission after corticosteroid therapy for type 1 autoimmune hepatitis: a retrospective analysis. Hepatology. 2002;35:890–897. doi: 10.1053/jhep.2002.32485. [DOI] [PubMed] [Google Scholar]
- 30.Zachou K., Gatselis N.K., Arvaniti P., Gabeta S., Rigopoulou E.I., Koukoulis G.K. A real-world study focused on the long-term efficacy of mycophenolate mofetil as first-line treatment of autoimmune hepatitis. Aliment Pharmacol Ther. 2016;43:1035–1047. doi: 10.1111/apt.13584. [DOI] [PubMed] [Google Scholar]
- 31.Hartl J., Ehlken H., Weiler-Normann C., Sebode M., Kreuels B., Pannicke N. Patient selection based on treatment duration and liver biochemistry increases success rates after treatment withdrawal in autoimmune hepatitis. J Hepatol. 2015;62:642–646. doi: 10.1016/j.jhep.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Beland K., Marceau G., Labardy A., Bourbonnais S., Alvarez F. Depletion of B cells induces remission of autoimmune hepatitis in mice through reduced antigen presentation and help to T cells. Hepatology. 2015;62:1511–1523. doi: 10.1002/hep.27991. [DOI] [PubMed] [Google Scholar]
- 33.Burak K.W., Swain M.G., Santodomingo-Garzon T., Lee S.S., Urbanski S.J., Aspinall A.I. Rituximab for the treatment of patients with autoimmune hepatitis who are refractory or intolerant to standard therapy. Can J Gastroenterol. 2013;27:273–280. doi: 10.1155/2013/512624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jonsson S., Sveinbjornsson G., de LapuentePortilla A.L., Swaminathan B., Plomp R., Dekkers G. Identification of sequence variants influencing immunoglobulin levels. Nat Genet. 2017;49:1182–1191. doi: 10.1038/ng.3897. [DOI] [PubMed] [Google Scholar]
- 35.Settipane G.A., Pudupakkam R.K., McGowan J.H. Corticosteroid effect on immunoglobulins. J Allergy Clin Immunol. 1978;62:162–166. doi: 10.1016/0091-6749(78)90101-x. [DOI] [PubMed] [Google Scholar]
- 36.Cerutti A., Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28(6):740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yuksel M., Wang Y., Tai N., Peng J., Guo J., Beland K. A novel “humanized mouse” model for autoimmune hepatitis and the association of gut microbiota with liver inflammation. Hepatology. 2015;62:1536–1550. doi: 10.1002/hep.27998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin R., Zhou L., Zhang J., Wang B. Abnormal intestinal permeability and microbiota in patients with autoimmune hepatitis. Int J Clin Exp Pathol. 2015;8:5153–5160. [PMC free article] [PubMed] [Google Scholar]
- 39.Manfredo Vieira S., Hiltensperger M., Kumar V., Zegarra-Ruiz D., Dehner C., Khan N. Translocation of a gut pathobiont drives autoimmunity in mice and humans. Science. 2018;359:1156–1161. doi: 10.1126/science.aar7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.