Summary
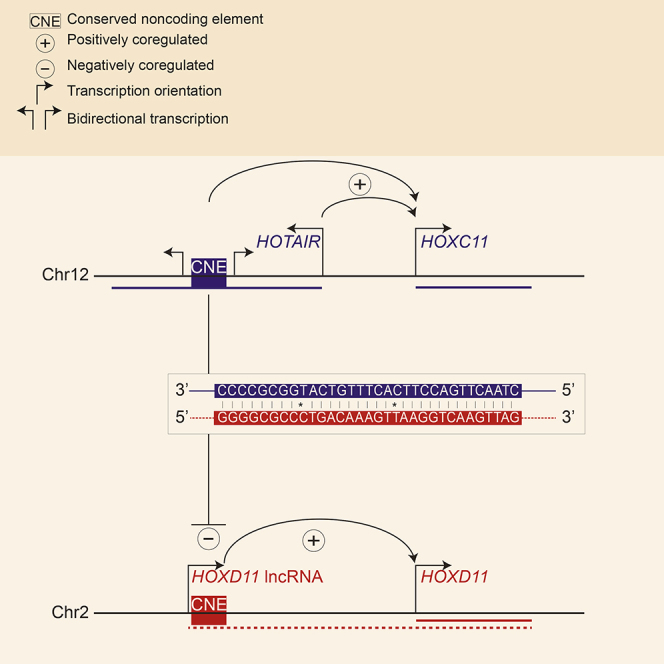
HOTAIR was proposed to regulate either HoxD cluster genes in trans or HoxC cluster genes in cis, a mechanism that remains unclear. We have identified a 32-nucleotide conserved noncoding element (CNE) as HOTAIR ancient sequence that likely originated at the root of vertebrate. The second round of whole-genome duplication resulted in one copy of the CNE within HOTAIR and another copy embedded in noncoding transcript of HOXD11. Paralogous CNEs underwent compensatory mutations, exhibit sequence complementarity with respect to transcripts directionality, and have high affinity in vitro. The HOTAIR CNE resembled a poised enhancer in stem cells and an active enhancer in HOTAIR-expressing cells. HOTAIR expression is positively correlated with HOXC11 in cis and negatively correlated with HOXD11 in trans. We propose a dual modality of HOTAIR regulation where transcription of HOTAIR and its embedded enhancer regulates HOXC11 in cis and sequence complementarity between paralogous CNEs suggests HOXD11 regulation in trans.
Subject Areas: Biological Sciences, Molecular Biology, Molecular Mechanism of Gene Regulation
Graphical Abstract
Highlights
-
•
Two conserved noncoding elements (CNEs) overlap HOTAIR and its paralog on HOXD cluster
-
•
Paralog on HOXD cluster may resolve controversy over cis and trans effects of HOTAIR
-
•
CNEs are positively coregulated with cis HOX genes but negatively with trans cluster
-
•
Transcribed CNEs with coevolved complementarity suggest hybridization-based function
Biological Sciences; Molecular Biology; Molecular Mechanism of Gene Regulation
Introduction
Mammalian genomes are pervasively transcribed, giving rise to thousands of long noncoding RNAs (lncRNAs) (Hon et al., 2017, Iyer et al., 2015). Only a handful of lncRNAs have well-characterized functions, which are attained through diverse mechanisms (chromatin regulation, alternative splicing, gene silencing, trans-regulation) (Guttman and Rinn, 2012, Mercer and Mattick, 2013). Although most early studies showed lncRNAs repress gene expression, some lncRNAs have enhancer-like functions and regulate genes in cis (Orom et al., 2010). Genomic deletion of lncRNA also removes cis-regulatory DNA elements, thus confounding whether the observed phenotype is due to the underlying genomic DNA, the lncRNA transcript itself, or transcription (Bassett et al., 2014, Engreitz et al., 2016, Kaikkonen and Adelman, 2018). As such, transcription blockage and perturbation of the Lockd lncRNA showed that it regulates Cdkn1b transcription through an embedded enhancer sequence, whereas the lncRNA transcript is dispensable for Cdkn1b expression (Paralkar et al., 2016). Deletion of 12 genomic loci encoding various lncRNAs revealed 5 loci whose deletion affected the general process of transcription and enhancer-like activity, but no requirement for the lncRNA transcripts (Engreitz et al., 2016). Lincp21 locus previously thought to function through its RNA transcript was shown to include multiple enhancers and regulate genes in cis (Groff et al., 2016). Moreover, genomic and epigenomic functional annotation have revealed that most intergenic lncRNAs originate from enhancers (Hon et al., 2017). In line with enhancer function overlapping with lncRNAs, the Haunt lncRNA has dual roles (Yin et al., 2015), where its DNA encodes enhancers to activate HoxA genes and Haunt lncRNA prevents aberrant HoxA expression.
HOTAIR is an intergenic lncRNA located between HOXC11 and HOXC12 in chromosome 12. It was proposed to regulate HOXD cluster genes (i.e., HOXD8, HOXD9, HOXD10, and HOXD11; located in chromosome 2) in trans by recruiting the Polycomb Repressive Complex 2 (PRC2) (Rinn et al., 2007). However, this regulatory model was questioned, as PRC2 binding is promiscuous (Davidovich et al., 2013) and PRC2 was found to be dispensable for HOTAIR-mediated transcriptional repression (Portoso et al., 2017). Deletion of the entire Hoxc cluster (including Hotair) in mouse showed limited impact on gene expression and H3K27me3 levels at Hoxd genes (Schorderet and Duboule, 2011). Specific deletion of Hotair produced a phenotype of homeotic transformation and skeletal malformation as well as genome-wide decrease in H3K27me3 levels and upregulation of posterior HoxD genes (i.e., Hoxd10, Hoxd11, and Hoxd13) (Li et al., 2013). These observations were challenged as specific knockouts of the Hotair locus in vivo have shown neither homeotic transformation nor upregulation of HoxD genes, but instead a significant change in HoxC (especially Hoxc11 and Hoxc12) cluster genes (Amandio et al., 2016). This strongly argues in favor of a DNA-dependent effect of the Hotair deletion (Amandio et al., 2016). Whether cis-regulation of HOTAIR is mediated via an unannotated enhancer element within its gene body or through transcription of the HOTAIR promoter remains unknown. Different regulatory mechanisms (cis versus trans) might be explained by tissue origin and changes in developmental stages in distinct genetic backgrounds (Li et al., 2016a). As such, there is no consensus model for HOTAIR-mediated regulation (Selleri et al., 2016). As the two current models suggest fundamentally different modes of HOTAIR function, we decided to revisit the role of HOTAIR by a systematic comparative genomic analysis.
To address whether HOTAIR regulates HOXC cluster genes in cis (Amandio et al., 2016) or HOXD cluster genes in trans (Li et al., 2013, Rinn et al., 2007), we exploited comparative sequence analysis across vertebrates and integrated this with transcriptomic and epigenomic data in human and mouse. The HOXC and HOXD clusters originated from an ancestral HOXC/D cluster during the second round of whole-genome duplication (WGD). We hypothesized that the two clusters may contain previously undetected remnants of an ancestral sequence, which might provide important clues on cis and/or trans interactions. We have identified and characterized a 32-nucleotide conserved noncoding element (CNE) as the HOTAIR ancestral sequence, which is shared by both paralogous loci in HoxC and HoxD clusters, presenting itself in an inverted syntenic position. Strikingly, the paralogous CNEs underwent compensatory mutations during vertebrate evolution, which exhibit sequence complementarity dependent on the transcript orientation. Also, the CNEs have high interaction propensity revealed by microscale thermophoresis (MST). These observations suggest direct hybridization between the two noncoding transcripts. HOTAIR CNE represents either an active or poised enhancer in different cellular contexts. Its expression is positively correlated with HOXC11, whereas negatively correlated with HOXD11, suggesting dual modality of HOTAIR CNE in cis and trans.
Results
Identification of HOTAIR Ancient Sequence and Its Paralog in HoxD Cluster
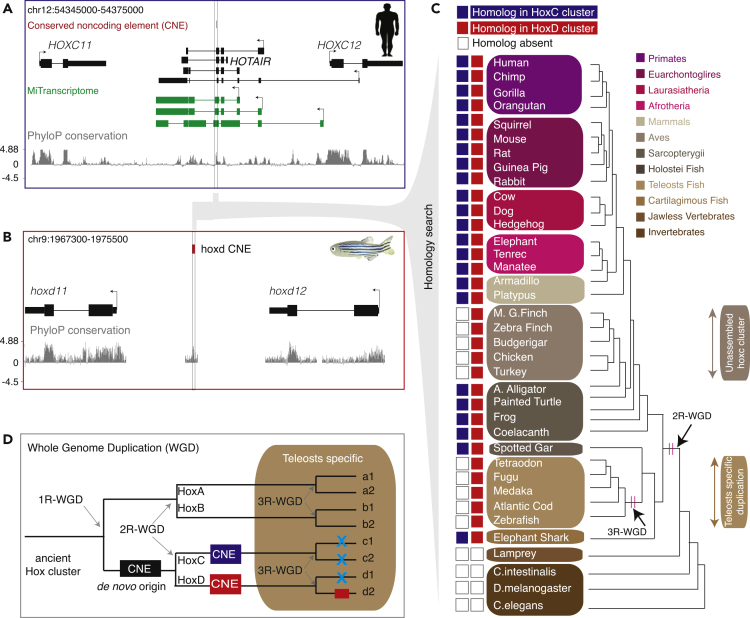
The Hox gene clusters are highly conserved across all vertebrates and contain multiple regulatory elements that often have small stretches of highly conserved noncoding elements (CNEs) (Engstrom et al., 2008, Lee et al., 2006). As HOTAIR is located within the highly conserved HoxC cluster, we asked whether it has small stretches of conserved sequences that were previously overlooked (He et al., 2011). To this end, we analyzed human and zebrafish annotated CNEs from the synteny analysis tool ANCORA (Engstrom et al., 2008) and identified a 32-nucleotide long CNE (Figure 1A) that is conserved across vertebrates (Figures S1A and S1B). Depending upon the transcript models, the CNE sequence is either located in the intron of Ensembl transcripts or in the exon of an intron-retained alternative transcript annotated in the lncRNA catalog (Figure 1A) (Iyer et al., 2015).
Figure 1.
Identification of the HOTAIR Conserved Noncoding Element (CNE) and Its Homolog in HOXD Cluster across Vertebrates
(A) A genome browser view around HOTAIR locus showing CNE from ANCORA browser and UCSC PhyloP conservation track. The CNE highlighted in a rectangular box is located eight nucleotides away from the splice site.
(B) The ortholog of the CNE mapped to the zebrafish hoxd (between hoxd11 and hoxd12) cluster.
(C) Homology search of the CNE across 37 species identified homologous CNEs in only HoxC and HoxD clusters. Homologs of the CNE are undetected in jawless vertebrate and invertebrates. Homologs in HoxC and HoxD clusters are represented by blue and red, respectively. Empty boxes indicate absence of homologs.
(D) Schematic representation for the proposed model of the origin of the CNE. The CNE might have a de novo origin in ancestral HoxC/D cluster where the second round of whole-genome duplication (2R-WGD) resulted two copies in HoxC and HoxD clusters. Teleost-specific duplication might have resulted in loss of CNE from both HoxC clusters and one of the HoxD cluster.
The CNE in zebrafish mapped between hoxd11a and hoxd12a in the hoxd cluster (Figure 1B), but not in the hoxC cluster. To determine whether the CNE is located in the HoxC cluster (the capitalized “HoxC” is used to represent the HoxC cluster across multiple species) or HoxD cluster (Figures S1A and S1B), we systematically mapped CNE sequences across 34 vertebrates and 3 invertebrates (Table S1). Two copies of the CNE were identified in all jawed vertebrates (except in teleosts and birds), but not in the jawless vertebrate lamprey and invertebrates (Figure 1C). The homologous CNEs mapped between HoxD11 and HoxD12 (reported target genes of HOTAIR in trans) in the HoxD cluster and between HoxC11 and HoxC12 (reported target genes of HOTAIR in cis) in the HoxC cluster (Figure 1C) in synteny, suggesting paralogy. The absence of CNE in HoxC cluster in birds might be due to the unassembled HoxC cluster (Table S2). In contrast, teleosts have well-annotated hoxc11 and hoxc12 genes in the same cluster (Table S2), but underwent an additional round of teleost-specific WGD, resulting in lineage-specific loss of the paralog. The basal group of jawed vertebrates, such as elephant shark (cartilaginous fish) and spotted gar (basal ray-finned fish; sister group of teleosts) (Figure 1C), have two copies of the CNE suggesting that it was already present in the ancestral HoxC/D cluster and resulted in two copies following the second round of WGD (Figure 1D). Although CNE and its flanking sequences are duplicated from the common ancestral sequence (Figure S1C), the flanking regions have limited homology (for example, in human and elephant shark; Figure S1D). However, CNE and its flanking sequences aligned separately across HoxC and HoxD clusters and revealed a relatively long stretch of sequence conserved across vertebrates (except teleosts) (Figures S1E and S1F). Thus we conclude that HOTAIR CNE is the ancient sequence and has two paralogous copies in all jawed vertebrates, except teleosts.
Paralogous CNEs Are Transcribed and Embedded in Mature Noncoding Transcripts
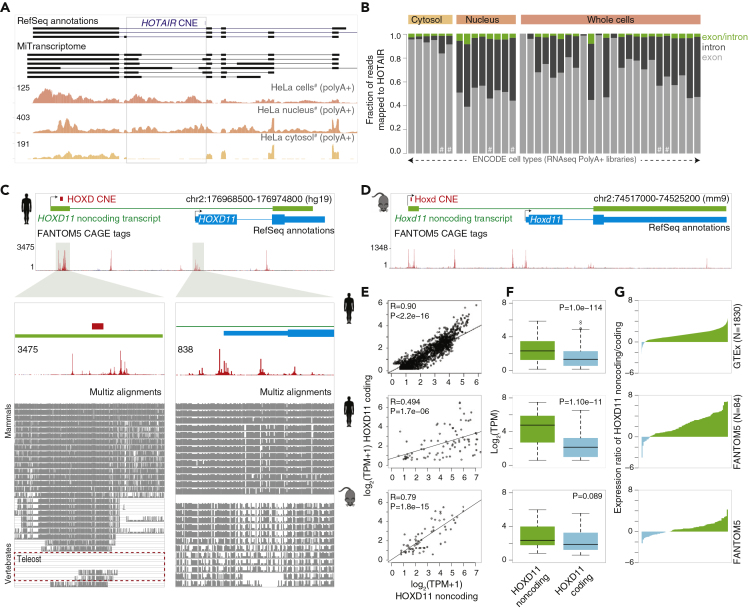
Our findings of paralogous CNE in the HoxD cluster suggest the existence of a HOTAIR homolog transcript overlying the CNE. To understand whether CNEs are embedded in the mature transcript, we first confirmed that HOTAIR CNE can be embedded in the exon by an intron-retained transcript model (Figure 1A). We analyzed long RNA sequencing (RNA-seq) data from ENCODE cell types (Djebali et al., 2012) and observed a large number of reads mapping to introns, particularly the region overlapping CNE, as shown for HeLa S3 cells (Figure 2A). A significant proportion of reads mapped to introns both in whole cell and in nuclear fraction-enriched RNA libraries and was depleted in cytosol-enriched RNA libraries (Figure 2A). We quantified reads mapped to exon, intron, and exon/intron junctions across different cell types and observed a large fraction of reads (relative to exons) mapped to introns in HOTAIR (Figure 2B and Table S3), but not in HOXC11 and HOXD11 genes (Figure S2A). We observed that a similar pattern of reads mapped to the intron in the region overlapping the CNE across different cell types (Yue et al., 2014), additionally confirming that intron retention of Hotair is conserved in mouse (Figures S2B and S2C). Collectively, this suggests that the HOTAIR CNE is embedded in an intron-retained transcript.
Figure 2.
Paralogous CNEs Are Embedded in Mature Noncoding Transcripts
(A) A genome browser view shows HOTAIR transcripts model from RefSeq and MITranscriptome along with RNA-seq coverage tracks from HeLa S3 cells. Large number of reads map to introns in whole cells and nuclear fraction-enriched libraries and are depleted in cytosol fraction-enriched library.
(B) Distribution of reads mapped to HOTAIR exon, intron, and overlapping exon/intron junctions across multiple cell types. “#” denotes the HeLa S3 cells. Cell types are ordered based on increasing number of mapped reads.
(C and D) A genome browser view to show transcription and sequence conservation around the HOXD11 coding (cyan) and HOXD11 noncoding (ncHOXD11) transcripts (green) in human (C) and mouse (D). The zoomed-in promoter regions show lack of sequence conservation of ncHOXD11.
(E) Correlation of expression levels of ncHOXD11 with HOXD11 coding gene across multiple samples in human (from GTEx and FANTOM5 cohorts) and mouse (FANTOM5 cohort).
(F) Expression levels of ncHOXD11 and HOXD11 coding gene across multiple samples from GTEx and FANTOM.
(G) Ratio of expression levels of ncHOXD11 and coding gene across individual cell types. Positive value on y axis indicates higher expression levels of ncHOXD11.
To associate HOXD CNE with the transcript models, we intersected it with Ensembl transcripts and identified that the CNE is embedded in the exon of a previously annotated noncoding transcript sharing the locus with HOXD11 coding gene in human (Figure 2C) and mouse (Figure 2D). The CNE is located in the promoter region of HOXD11 noncoding transcript (referred as ncHOXD11 from here on), which is approximately 55 nucleotides downstream of the dominant transcription start site (denoted by the highest CAGE peak in FANTOM5 data) in human and mouse (Figures 2C and 2D). Given the shared locus, we sought to understand the nature and extent of ncHOXD11 usage in relation to the HOXD11 coding gene. The expression level of ncHOXD11 is positively correlated with HOXD11 coding gene across GTEx (GTEx Consortium, 2013) and FANTOM5 (Arner et al., 2015, FANTOM Consortium and the RIKEN PMI and CLST (DGT) et al., 2014) data in human and mouse (Figure 2E). The expression of ncHOXD11 is significantly higher than that of the HOXD11 coding gene (Figure 2F) in the majority of cell types in human (Figure 2G). However, in mouse, the expression of ncHoxd11 is only marginally higher than that of the Hoxd11 coding gene (Figures 2F and 2G), suggesting a relative gain in the expression of ncHOXD11 in human. Finally, we analyzed RNA-seq transcript models across species (Basu et al., 2016, Hezroni et al., 2015) and detected both transcripts in ferret and dog, whereas only ncHoxD11 in chicken (Figure S2D). The location of HoxD CNE, which is downstream of ncHoxD11 start site, is conserved across species, suggesting that its transcription from ncHoxD11 is an ancient phenomenon. However, ncHoxD11 was undetected in teleosts (zebrafish and tetraodon; Figure S2D), which is further supported by absence of ncHoxD11 promoter sequence (Figure 2A). Collectively, we showed that paralogous CNEs are transcribed and embedded in mature transcripts across multiple species.
Transcribed CNEs Exhibit Conserved Sequence Complementarity across Vertebrates
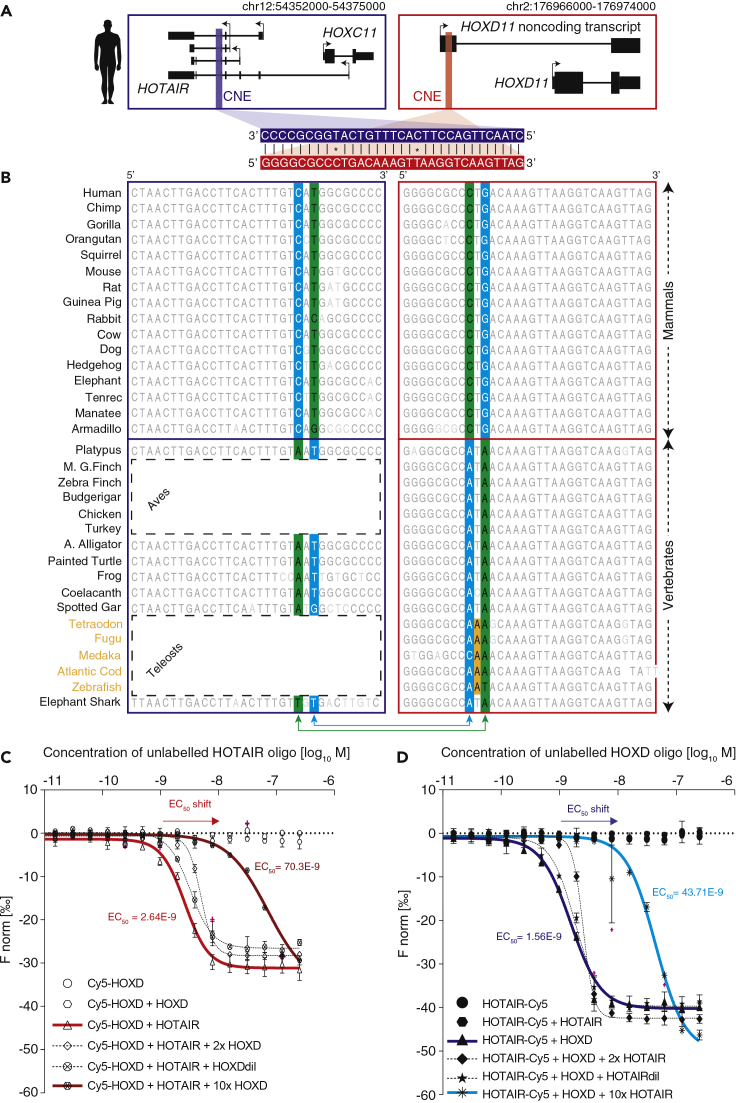
As paralogous CNEs are embedded in mature transcripts, we sought to analyze their directionality with respect to transcript orientation. We observed that human CNEs exhibit sequence complementarity in transcript orientation (Figure 3A). To ensure that the observed sequence complementarity in human is not by chance we analyzed its orientation in other vertebrates. As transcriptional evidence of HOTAIR and ncHOXD11 was limited to a subset of species (Figure S2E), we inferred orientation for missing transcripts (see Methods), as illustrated for chimp and painted turtle (Figures S3A and S3B). We then aligned CNEs in the transcript orientation and observed sequence complementarity across vertebrates (Figures 3B and S3C). This suggests that sequence complementarity between CNEs is an ancient feature that has been under selection pressure for more than 300 million years. It raises an important question as to whether the key function of these transcripts is to provide transcription of the CNE.
Figure 3.
Paralogous CNEs Exhibit Sequence Complementarity in Transcript Orientation
(A) Paralogous HOTAIR CNE (blue bar) and HOXD CNE (red bar) are zoomed and aligned in 5′ to 3′ orientation of respective transcripts.
(B) Alignment of the paralogous CNEs in 5′ to 3′ orientation of respective transcripts reveals sequence complementarity across vertebrates. Genetic substitutions within paralogous CNEs co-occurred at specific positions, which resulted in gain or loss of complementarity, where green represents non-complementary DNA and cyan represents complementary DNA. Teleost-specific change in DNA sequence is shown in orange.
(C and D) Microscale thermophoresis (MST) assay to evaluate the interaction between labeled and unlabeled RNA-oligos at different concentration. MST-on time of 5 s was used for analysis. Baseline-corrected normalized fluoresce (ΔFNorm) was chosen to present data (independent n ≥ 3 measurements; each point on the graphs presents mean ± SD). An extrapolated EC50 ± SD curve is fitted and shown on the graph. The concentration of the labeled RNA-oligo was constant at 5 nM. The concentration of unlabeled RNA-oligo was varied at 250 nM to 7.63 pM. The x axis represents the concentration of titrated unlabeled RNA-oligo. The y axis represents interaction-driven normalized fluorescence change (ΔFnorm[‰]). Measurement of interaction between (C) labeled HOXD CNE (Cy5-HOXD) RNA-oligo and unlabeled HOTAIR CNE RNA-oligo and (D) labeled HOTAIR CNE (HOTAIR-Cy5) RNA-oligo and unlabeled HOXD CNE RNA-oligo.
Interestingly, in addition to the conservation and retention of sequence complementarity, we observed that paralogous CNEs revealed a specific pattern of genetic substitution at two specific positions in both CNEs that co-evolved in two separate waves in vertebrates and mammals (Figure 3B). The sequence pairs that co-evolved at two specific positions are depicted in green (non-complementary) and cyan (complementary) (Figure 3B). The nucleotides “A” colored by green in vertebrates are non-complementary, where both nucleotides co-evolved simultaneously in mammalian lineage resulting in gain of complementarity (highlighted by cyan). On the other hand, nucleotides “A” and “T” highlighted by cyan in vertebrates are complementary, where the nucleotide “A” evolved in mammals resulting in loss of complementarity. In mammals, one substitution resulted in retention of complementarity and the other substitution resulted in loss of complementarity, reflecting that paralogous CNEs underwent compensatory mutations. Unlike vertebrates, the HoxD CNE in teleosts evolved separately in its own lineage (highlighted in orange) reflecting no selection pressure to retain sequence complementarity as its putative binding partner in HoxC cluster is lost. Collectively, the coevolution of CNEs and retention of sequence complementarity in the transcript orientation raises the potential for such hybridization based on trans function.
Hybridization of Paralogous CNEs In Vitro
To verify whether paralogous CNE transcripts hybridize, we designed two Cy5-labeled RNA-oligos (Table S4) for HOXD CNE (Cy5-HOXD) and HOTAIR CNE (HOTAIR-Cy5) and analyzed the interaction propensity using MST (Asmari et al., 2018, Duhr and Braun, 2006a, Duhr and Braun, 2006b, Moon et al., 2018). For labeled Cy5-HOXD RNA-oligo (5 nM), we analyzed the binding with unlabeled HOTAIR CNE RNA-oligo titrated at concentrations ranging between 250 nM and 7.63 pM. Similarly, for labeled HOTAIR-Cy5 RNA-oligo, we analyzed the binding with titrated unlabeled HOXD CNE RNA-oligo (Table S5 and Figures S4A–S4C). The labeled Cy5-HOXD and unlabeled HOTAIR CNE RNA-oligo showed a strong interaction at the nanomolar scale (EC50 = 2.64 × 10−9) (Figure 2C; red line), whereas we observed no binding at control conditions (either labeled oligo alone or mix of labeled and unlabeled counterparts). Similarly, the labeled HOTAIR-Cy5 and unlabeled HOXD CNE RNA-oligo showed a strong interaction at the nanomolar scale (EC50 = 1.56 × 10−9) (Figure 2D; blue line), whereas the control showed no binding. To evaluate if an unlabeled oligo can affect the interaction between labeled Cy5-HOXD RNA-oligo and the unlabeled HOTAIR CNE RNA-oligo, we added unlabeled HOXD RNA-oligo and observed that the interaction (EC50 = 70.3 × 10−9) was sensitive to the presence of unlabeled RNA-oligo (Figure 3C; dark red line). A 10-fold excess of the competitor resulted in a shift of the fluorescent signal resembling depletion of the titrated oligos and correspondingly a shift in EC50 value (Figure 3C; dark red line). Similarly, addition of an unlabeled HOTAIR CNE RNA-oligo affected the interaction of the labeled HOTAIR-Cy5 RNA-oligo and unlabeled HOXD CNE RNA-oligo, resulting in a shift in fluorescent signal (Figure 3D; cyan line). Even at low concentration of the competitor oligo the shift is still clear, confirming that the paralogous CNEs have strong interaction in vitro.
Chromatin Structure of HOTAIR CNE Represents a Poised Enhancer in Stem Cells
CNEs are putative cis-regulatory elements (Bejerano et al., 2004, Harmston et al., 2013, Sandelin et al., 2004), and many of them have been experimentally validated as tissue-specific enhancers (Nobrega et al., 2003, Pennacchio et al., 2006, Woolfe et al., 2005). We analyzed experimentally validated enhancers (Pennacchio et al., 2006) and found that the genomic regions overlapping CNEs were not probed for enhancer activity. However, in the literature, we found that the region overlapping the Hoxd CNE was tested for enhancer activity in mouse and shown to drive expression in a proximal posterior part of the developing forelimbs (Beckers et al., 1996). However, subsequent deletion of Hoxd CNE revealed no phenotype in vivo (Beckers and Duboule, 1998) (see Discussion).
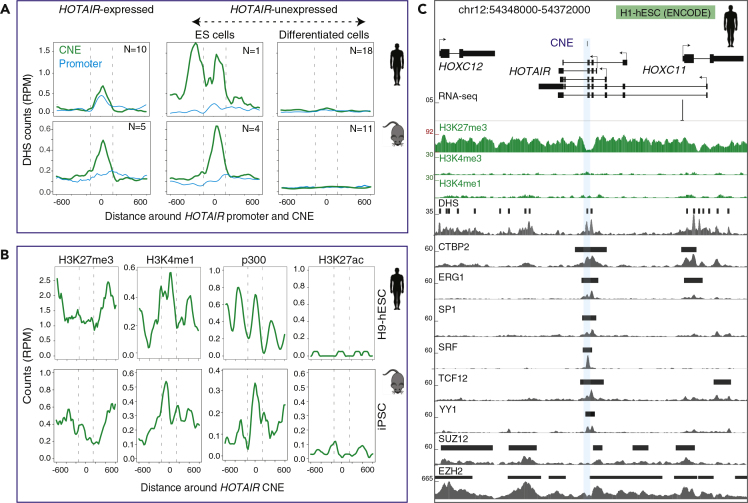
To understand whether chromatin states of CNEs resemble that of enhancers (Andersson et al., 2014, Pundhir et al., 2016, Roadmap Epigenomics Consortium et al., 2015), we selected 29 cell lines (Table S6) from Roadmap Epigenome project. Based on the expression levels of HOTAIR (see Methods), cell lines were classified into HOTAIR-expressing (N = 10) and HOTAIR-non-expressing (N = 19) groups (Figure S5A). The H1-hESC cell line is unique as it is enriched for H3K27me3 and DNase hypersensitive sites (DHSs) (Figure S5B), thus we separately analyzed H1-hESC cells from remaining HOTAIR-non-expressing cells. The HOTAIR CNE has open chromatin in HOTAIR-expressing cells and HOTAIR-non-expressing stem cells and closed chromatin in HOTAIR-non-expressing differentiated cells in both human and mouse (Figure 4A and Table S6). We observed a similar chromatin state around HOXD CNE (Figure S5C). The chromatin state of CNE is dynamically regulated during reprogramming of mouse embryonic fibroblasts to induced pluripotent stem cells (iPSCs) (Chronis et al., 2017) where the CNE has closed chromatin in mouse embryonic fibroblasts and open chromatin in iPSC (Figure S5D). In addition, enrichment of H3K4me1, H3K27me3, and p300 signals at the CNE in human H9-hESC and mouse iPSC (Figure 4B) provides evidence that the HOTAIR CNE represents an embryonic stem cell-specific poised enhancer (Rada-Iglesias et al., 2011). This is further supported by enrichment of enhancer-associated transcription factors (Sigova et al., 2015), such as CTBP2, CHD1, SP1, and YY1 that are exclusively enriched at CNE in H1-hESC (Figure 4C and Table S7). However, p300, H3K4me1, and bimodal H3K27me3 peaks were not enriched around HOXD CNE in hESC (Figures S5E–S5G). As HOXD CNE overlaps with the promoter of ncHOXD11 (Figures 2A and S5G), genomic analyses of chromatin states will be unable to distinguish a putative enhancer from an overlapping promoter. Collectively, these data suggest that HOTAIR CNE resembles a poised enhancer in stem cells in both human and mouse.
Figure 4.
The HOTAIR CNE Represents a Poised Enhancer in HOTAIR-Nonexpressing Stem Cells in Human and Mouse
(A) Average DNase I hypersensitive site (DHS) signals around HOTAIR CNE in HOTAIR-expressing and HOTAIR-nonexpressing cells (embryonic stem cells and differentiated cells). The y axis is normalized DHS coverage in reads per million (RPM). “N” denotes the number of cell lines.
(B) The distribution of H3K4me1, H3K27me3, H3K27ac, and p300 signals around HOTAIR CNE in H9-hESC (human) and iPSC (mouse) cell lines. The y axis is normalized coverage in reads per million (RPM).
(C) A genome browser view with transcription factors, DHS, histone modifications, and RNA-seq tracks from H1-hESC cell line. HOTAIR is not expressed in H1-hESC (as shown by lack of RNA-seq reads) and marked by broad H3K27me3 peak. H3K27me3 signal is depleted around CNE, reflecting a nucleosome-depleted region and bound by multiple transcription factors.
HOTAIR CNE Represents an Active Enhancer RNA
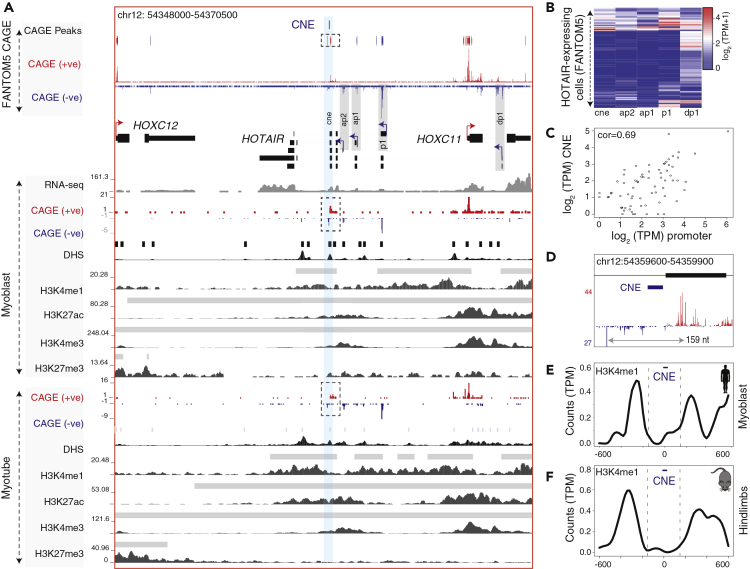
To determine whether the CNE represents an active enhancer in HOTAIR-expressing cells, we analyzed FANTOM5 CAGE data (FANTOM Consortium and the RIKEN PMI and CLST (DGT) et al., 2014) to identify unstable bidirectional transcription, a hallmark of active enhancer RNA (eRNA) (Andersson et al., 2014). We observed bidirectional transcription flanking the CNE (Figure 5A; dashed rectangular box), providing evidence of an active eRNA. Importantly, transcription of HOTAIR primary and alternative promoters is generally co-expressed with bidirectional transcription around the CNE (Figure 5B and Table S8). This is exemplified during myoblast to myotube differentiation (Figure S6A), which suggests coregulation. The expression of CNE is positively correlated with expression from the HOTAIR promoter and alternative promoters (Figure 5C), with the exception of the distal promoter (labeled as dp1) (Figure S6B). Negative correlation of the distal promoter is mostly due to a majority of samples in which only the distal promoter is expressed (Figure 5B). The distance between bidirectional transcription start sites flanking the CNE is about the length of one nucleosome (Figure 5D), which is conserved across vertebrates (Figure S1E), and suggests that the CNE shares an evolutionary conserved typical enhancer structure. On the contrary, no evidence suggests bidirectional transcription around the HOXD CNE, as it overlaps with the promoter region of ncHOXD11 (Figure 2C).
Figure 5.
The CNE Represents an Active Enhancer RNA in HOTAIR-Expressing Cells
(A) A genome browser view with FANTOM5 CAGE tags (combined tracks) and individual tracks on myoblast and myotube along with RNA-seq and histone modifications. Horizontal bars above histone and DHS tracks are annotated peaks. CAGE tags on forward and reverse strand are represented by blue and red, respectively. Bidirectional CAGE tags flanking the CNE are shown in dashed rectangular boxes. Bidirectional CAGE tags overlap with H3K4me1 and H3K27ac peaks in myoblast and myotube.
(B) Expression levels of the HOTAIR CNE and alternative promoters across FANTOM5 samples.
(C) Correlation of the expression levels of the CNE with that of HOTAIR promoter.
(D) Bidirectional transcription (from myoblast and myotube differentiation time points) around the CNE roughly represents the length of a nucleosome.
(E and F) Bimodal H3K4me1 peaks flank the CNE in human myoblast (E) and embryonic day 10.5 hindlimbs in mouse (F).
Next, we focused on myoblast and myotube cell types, for which RNA-seq, histone modifications, and DHS data are available as complements to CAGE tags. The RNA-seq reads mapped on introns and across intron/exon boundary around the CNE (Figure 5A), thus providing evidence for an intron-retained transcript. Furthermore, DHS, H3K4me1, and H3K27ac peaks are enriched around the CNE (Figure 5A) in myoblast and myotube, providing additional evidence for an active enhancer. The observed bimodal H3K4me1 peaks around CNE are a characteristic feature of active enhancers (Figure 5E). However, in mouse, relevant tissues and stages wherein Hotair is expressed (Amandio et al., 2016, Li et al., 2013, Schorderet and Duboule, 2011) were not included in FANTOM5 samples (Figure S6C) and lack CAGE tags around the CNE. However, H3K4me1 and H3K27ac are enriched in mouse embryonic day 10.5 hindlimbs (Andrey et al., 2017) (Figures 5F and S6D) around the CNE. Thus, we showed that HOTAIR CNE resembles an active enhancer in HOTAIR-expressed cells, in both human and mouse. Importantly, transcription of the HOTAIR promoter is tightly linked to enhancer activity of the CNE, suggesting that its transcription might be a contributor to the purifying selection acting on the CNE and further provides support to the notion that the CNE acts as a regulator in cis as previously proposed (Amandio et al., 2016).
HOTAIR Expression Highlights Simultaneous Regulation of Known Target Genes in cis and trans
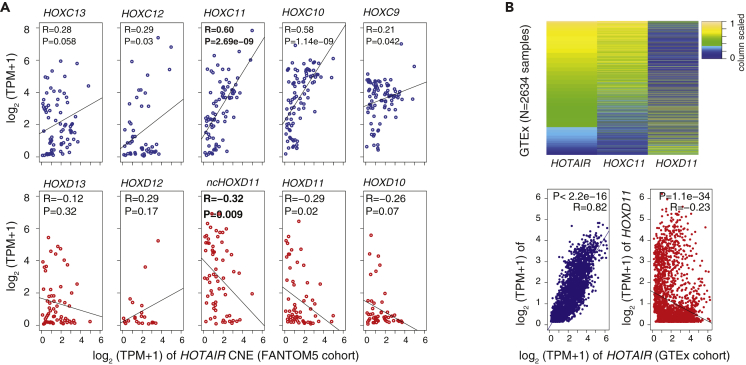
As we showed that transcribed CNEs exhibit sequence complementarity in transcript orientation, we sought to understand whether HOTAIR can simultaneously regulate genes in cis and trans mediated via the CNE. We analyzed transcription levels of the HOTAIR enhancer with HOX clusters genes across 694 cell types from FANTOM5. The HOTAIR was expressed in 104 cell types (Figure S7A), and HOX genes were positively correlated with other genes in the cluster (Figure S7B). The expression of HOTAIR CNE with HOXC/D clusters posterior genes on 104 cell types revealed positive correlation with HOXC cluster genes and a trend toward negative correlation with HOXD cluster genes (Figure S7C). To ensure that the correlations were not driven by missing expression of HOX genes, we reanalyzed data by including only those cell types wherein both HOTAIR and HOX genes are coexpressed. We observed similar correlations wherein HOXC cluster genes are positively correlated and HOXD cluster genes are negatively correlated (Figure 6A). Strikingly, HOXC11 is the most positively correlated (R = 0.60; p value: 2.6 × 10−9) and ncHOXD11 (R = −0.32; p value: 0.009) and coding transcripts (R = −0.29; p value: 0.02) are the most negatively correlated, both of which are previously reported target genes (Amandio et al., 2016, Li et al., 2013, Rinn et al., 2007). This observation was further validated in 2,436 tissue samples from GTEx and 605 patients with breast cancer (see Methods) from The Cancer Genome Atlas (Pereira et al., 2016) where HOXC11 had the most significant positive correlation and HOXD11 had the most significant negative correlation (Figures 6B and S7D).
Figure 6.
Coregulation of HOTAIR with Validated Target Genes HOXC11 and HOXD11
(A) Correlation of expression levels of HOTAIR CNE with HOXC and HOXD cluster posterior genes across FANTOM5 cell types. The x axis represents expression level of HOTAIR CNE, and the y axis represents the expression levels of HOXC and HOXD cluster genes. Expression level is measured as tags per million (TPM). The expression levels of HOXC11 have the highest positive correlation, and those of the HOXD11 coding and noncoding have the highest negative correlation.
(B) Heatmap and correlation of expression levels of HOTAIR with HOXC11 and HOXD11 genes across GTEx cohort.
Therefore, we propose a model to explain the observed correlation between HOTAIR expression and that of HOXC11 and HOXD11, a dual regulatory mechanism mediated via the CNE sequence. The positive correlation between HOTAIR and HOXC11 might be mediated via an active eRNA, the act of transcription of HOTAIR, or the combination of both. We observed positive correlation between ncHOXD11 promoter encoding HOXD CNE and HOXD11 coding gene. Transcriptional activity of the CNE is coupled to HOTAIR transcription, suggesting that a key function of the HOTAIR transcript could be to provide active transcription for the CNE. Paralogous CNEs embedded in intron-retained HOTAIR and ncHOXD11 transcripts have retained sequence complementarity in transcript orientation that might facilitate hybridization between two RNA transcripts. This hybridization between two RNA transcripts downregulates HOTAIR target gene expression. Thus, we propose that HOTAIR can have dual regulatory roles in cis and trans, which is likely mediated by the CNE paralog sequence.
Discussion
We have identified and characterized a 32-nucleotide CNE as the ancestral sequence that probably originated in ancestral HoxC/D cluster, where the second round of WGD gave rise to one copy in the HOTAIR locus and another copy in the ncHOXD11 locus. The paralogous CNEs are only 32 nucleotides, whereas the conserved sequence flanking the HOTAIR CNE is much longer (Figure S1E) and coincides with a region of eRNA, suggesting an ancestral sequence within the HOTAIR locus. The remainder of the HOTAIR sequence has limited homology in vertebrates (Figures S1B and S1E), which evolved rapidly in mammalian lineage (He et al., 2011). This could be indicative of the HOTAIR locus originating from the CNE and evolution favoring the development of its sequence, likely expanding its functionality. Although thousands of CNEs are annotated, only a small minority of them have retained a duplicated copy (McEwen et al., 2006). As such, retention of both copies of HOTAIR CNE had not been reported before. To the best of our knowledge, this is the first instance of reported paralogous CNEs that underwent compensatory mutation and have retained sequence complementarity in their transcribed directionality (Figures 3A and 3B).
Many of the experimentally tested CNEs are validated enhancers (Nobrega et al., 2003, Pennacchio et al., 2006, Woolfe et al., 2005). Genome-wide transcriptomic and epigenomic analyses revealed that enhancers are characterized by distinct transcription and chromatin states (reviewed in Li et al., 2016b), and we used these features to define whether CNEs are enhancers. The HOTAIR CNE region is marked by open chromatin that is flanked by enriched H3K4me1 and H3K27ac peaks along with bidirectional transcription, which collectively meets all characteristic features of an active enhancer. On the other hand, our genomic analyses did not reveal any enhancer features on HOXD CNE, likely because it overlaps with the ncHOXD11 promoter region (Figures 2C and 2D), and it is therefore difficult to entangle overlapping signals. However, the sequence overlapping Hoxd CNE drives expression in a proximal posterior part of the developing forelimbs in mouse (Beckers et al., 1996). Recent findings suggest that some promoters have dual functions as enhancers and influence the expression of a neighboring gene in cis (Engreitz et al., 2016, Paralkar et al., 2016, Yin et al., 2015). Thus, it is plausible that the ncHOXD11 promoter overlapping HOXD CNE has enhancer function and regulates HOXD11 gene in cis.
Multiple enhancers with similar activity provide an effective buffer to prevent deleterious phenotypic consequences upon loss of individual enhancers (Osterwalder et al., 2018). As the HOTAIR CNE has a paralogous copy, how this might affect HOTAIR regulation needs further consideration. Deletion of a sequence overlapping Hoxd CNE revealed no phenotype in vivo (Beckers and Duboule, 1998), which was different from in vitro (Beckers et al., 1996). It was speculated that the difference(s) might be due to other phenotypes that were undetected or might have a redundant copy that masked the effect. In fact, we now have identified that the probed sequence has a paralogous copy in the Hotair locus that might have masked the effect in vivo. Thus, whether paralogous CNEs have redundant functions, such that deletion of one CNE might be compensated by the other, remains unclear. Putting this in the context of deletion of the Hotair locus in vivo (Amandio et al., 2016, Li et al., 2013), it remains unknown whether the effects of Hotair CNE deletion are compensated for, to a certain extent, by paralogous Hoxd CNE.
Transcriptional activity of CNEs is coupled to HOTAIR and ncHOXD11 transcription, suggesting that a key function of these transcripts is to provide active transcription for the CNEs. With respect to transcript orientation, paralogous CNEs exhibit sequence complementarity, which raises the potential for this hybridization principle based on trans function. This is supported by the observed hybridization in vitro (Figures 3C and 3D) and needs future experiments to confirm in vivo. Transcription of HOTAIR CNE is positively correlated with HOXC11 (Figure 6), and transcription of ncHOXD11 is positively correlated with HOXD11. Simultaneously, the transcription of HOTAIR CNE and ncHOXD11 are negatively correlated (Figure 6), which is likely mediated via sequence complementarity between CNEs.
In summary, our analyses suggest that HOTAIR could regulate both HoxC and HoxD cluster genes simultaneously and provide a unifying model of HOTAIR regulation that should clarify ongoing controversies (Amandio et al., 2016, Li et al., 2013, Portoso et al., 2017, Rinn et al., 2007, Schorderet and Duboule, 2011). Our work highlights how an lncRNA locus could possibly function at the DNA and RNA levels to regulate genes both in cis and trans. Unraveling such lncRNAs and determining/validating mechanisms through which they function at the DNA and/or RNA levels is an ongoing challenge. We propose that such integrative analyses bridging evolutionary genomics and comparative transcriptomics/epigenomics could prove a powerful tool for better understanding of lncRNA-dependent regulation processes.
Limitations of the Study
Our conclusion is based on analyses of large-scale genomics data, thus future work is needed to validate the predicted models in vivo. We showed hybridization between paralogous CNEs in vitro, which needs to be validated in vivo. Furthermore, targeted experiments are required to understand how specific deletion of individual CNE(s) along with simultaneous deletion of both CNEs alters HOTAIR-dependent regulation in cis and trans.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We are very grateful to the ENCODE, FANTOM5, GTEx, Roadmap Epigenome consortia, and researchers for making data freely available. Also, the results here are in parts based on data generated by the TCGA Research Network: https://www.cancer.gov/tcga. The authors thank Dr. Colm J. O'Rourke, Dr. Michal Lubas, and Dr. Albin Sandelin for critical comments on the manuscript and Dr. Juan Francisco Lafuente Barquero for help with Illustrator and fruitful discussion. C.N. and A.T. are recipients of a postdoctoral fellowship from the Danish Medical Research Council (6110-00557A) and Lundbeck Foundation (R219-2016-718), respectively. The laboratory of J.B.A. is supported by the Danish Medical Research Council (4183-00118A), Danish Cancer Society (R98-A6446) and Novo Nordisk Foundation (14040). P.M. acknowledges funding from National Science Center (2014/14/E/NZ6/00162, Poland). F.M. and B.L. thank the support of BBSRC (BB/L010488/1) and the Wellcome Trust Investigator Award (106955/Z/15/Z).
Author Contributions
C.N. conceived the story. C.N., A.T., Y.H., and S.P. analyzed data. C.N., A.T., B.L., F.M., and J.B.A. interpreted the results. A.T., Y.H., S.P., P.M., A.T., B.L., F.M., and J.B.A. contributed to critical discussions. C.N., F.M., B.L., and J.B.A. drafted the manuscript, and all authors contributed to revising the manuscript.
Declaration of Interests
Authors declare no conflict of interest and no competing financial interest.
Published: April 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101008.
Contributor Information
Chirag Nepal, Email: chirag.nepal@bric.ku.dk.
Ferenc Müller, Email: f.mueller@bham.ac.uk.
Jesper B. Andersen, Email: jesper.andersen@bric.ku.dk.
Data and Code Availability
Data used in the study were downloaded from ENCODE, mouse ENCODE, GTEx, TCGA, FANTOM5, NIH Roadmap Epigenome project, and additional publicly available datasets mentioned in the methods. All custom code is available upon request.
Supplemental Information
References
- Amandio A.R., Necsulea A., Joye E., Mascrez B., Duboule D. Hotair is dispensible for mouse development. PLoS Genet. 2016;12:e1006232. doi: 10.1371/journal.pgen.1006232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson R., Gebhard C., Miguel-Escalada I., Hoof I., Bornholdt J., Boyd M., Chen Y., Zhao X., Schmidl C., Suzuki T. An atlas of active enhancers across human cell types and tissues. Nature. 2014;507:455–461. doi: 10.1038/nature12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrey G., Schopflin R., Jerkovic I., Heinrich V., Ibrahim D.M., Paliou C., Hochradel M., Timmermann B., Haas S., Vingron M. Characterization of hundreds of regulatory landscapes in developing limbs reveals two regimes of chromatin folding. Genome Res. 2017;27:223–233. doi: 10.1101/gr.213066.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arner E., Daub C.O., Vitting-Seerup K., Andersson R., Lilje B., Drablos F., Lennartsson A., Ronnerblad M., Hrydziuszko O., Vitezic M. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science. 2015;347:1010–1014. doi: 10.1126/science.1259418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmari M., Ratih R., Alhazmi H.A., El Deeb S. Thermophoresis for characterizing biomolecular interaction. Methods. 2018;146:107–119. doi: 10.1016/j.ymeth.2018.02.003. [DOI] [PubMed] [Google Scholar]
- Bassett A.R., Akhtar A., Barlow D.P., Bird A.P., Brockdorff N., Duboule D., Ephrussi A., Ferguson-Smith A.C., Gingeras T.R., Haerty W. Considerations when investigating lncRNA function in vivo. Elife. 2014;3:e03058. doi: 10.7554/eLife.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Hadzhiev Y., Petrosino G., Nepal C., Gehrig J., Armant O., Ferg M., Strahle U., Sanges R., Muller F. The Tetraodon nigroviridis reference transcriptome: developmental transition, length retention and microsynteny of long non-coding RNAs in a compact vertebrate genome. Sci. Rep. 2016;6:33210. doi: 10.1038/srep33210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers J., Duboule D. Genetic analysis of a conserved sequence in the HoxD complex: regulatory redundancy or limitations of the transgenic approach? Dev. Dyn. 1998;213:1–11. doi: 10.1002/(SICI)1097-0177(199809)213:1<1::AID-AJA1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Beckers J., Gerard M., Duboule D. Transgenic analysis of a potential Hoxd-11 limb regulatory element present in tetrapods and fish. Dev. Biol. 1996;180:543–553. doi: 10.1006/dbio.1996.0327. [DOI] [PubMed] [Google Scholar]
- Bejerano G., Pheasant M., Makunin I., Stephen S., Kent W.J., Mattick J.S., Haussler D. Ultraconserved elements in the human genome. Science. 2004;304:1321–1325. doi: 10.1126/science.1098119. [DOI] [PubMed] [Google Scholar]
- Chronis C., Fiziev P., Papp B., Butz S., Bonora G., Sabri S., Ernst J., Plath K. Cooperative binding of transcription factors orchestrates reprogramming. Cell. 2017;168:442–459 e420. doi: 10.1016/j.cell.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidovich C., Zheng L., Goodrich K.J., Cech T.R. Promiscuous RNA binding by Polycomb repressive complex 2. Nat. Struct. Mol. Biol. 2013;20:1250–1257. doi: 10.1038/nsmb.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebali S., Davis C.A., Merkel A., Dobin A., Lassmann T., Mortazavi A., Tanzer A., Lagarde J., Lin W., Schlesinger F. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhr S., Braun D. Optothermal molecule trapping by opposing fluid flow with thermophoretic drift. Phys. Rev. Lett. 2006;97:038103. doi: 10.1103/PhysRevLett.97.038103. [DOI] [PubMed] [Google Scholar]
- Duhr S., Braun D. Why molecules move along a temperature gradient. Proc. Natl. Acad. Sci. U S A. 2006;103:19678–19682. doi: 10.1073/pnas.0603873103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engreitz J.M., Haines J.E., Perez E.M., Munson G., Chen J., Kane M., McDonel P.E., Guttman M., Lander E.S. Local regulation of gene expression by lncRNA promoters, transcription and splicing. Nature. 2016;539:452–455. doi: 10.1038/nature20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engstrom P.G., Fredman D., Lenhard B. Ancora: a web resource for exploring highly conserved noncoding elements and their association with developmental regulatory genes. Genome Biol. 2008;9:R34. doi: 10.1186/gb-2008-9-2-r34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANTOM Consortium and the RIKEN PMI and CLST (DGT), Forrest A.R., Kawaji H., Rehli M., Baillie J.K., de Hoon M.J., Haberle V., Lassmann T., Kulakovskiy I.V., Lizio M. A promoter-level mammalian expression atlas. Nature. 2014;507:462–470. doi: 10.1038/nature13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groff A.F., Sanchez-Gomez D.B., Soruco M.M., Gerhardinger C., Barutcu A.R., Li E., Elcavage L., Plana O., Sanchez L.V., Lee J.C. In vivo characterization of Linc-p21 reveals functional cis-regulatory DNA elements. Cell Rep. 2016;16:2178–2186. doi: 10.1016/j.celrep.2016.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GTEx Consortium The genotype-tissue expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M., Rinn J.L. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmston N., Baresic A., Lenhard B. The mystery of extreme non-coding conservation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20130021. doi: 10.1098/rstb.2013.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S., Liu S., Zhu H. The sequence, structure and evolutionary features of HOTAIR in mammals. BMC Evol. Biol. 2011;11:102. doi: 10.1186/1471-2148-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hezroni H., Koppstein D., Schwartz M.G., Avrutin A., Bartel D.P., Ulitsky I. Principles of long noncoding RNA evolution derived from direct comparison of transcriptomes in 17 species. Cell Rep. 2015;11:1110–1122. doi: 10.1016/j.celrep.2015.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon C.C., Ramilowski J.A., Harshbarger J., Bertin N., Rackham O.J., Gough J., Denisenko E., Schmeier S., Poulsen T.M., Severin J. An atlas of human long non-coding RNAs with accurate 5' ends. Nature. 2017;543:199–204. doi: 10.1038/nature21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer M.K., Niknafs Y.S., Malik R., Singhal U., Sahu A., Hosono Y., Barrette T.R., Prensner J.R., Evans J.R., Zhao S. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015;47:199–208. doi: 10.1038/ng.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaikkonen M.U., Adelman K. Emerging roles of non-coding RNA transcription. Trends Biochem. Sci. 2018;43:654–667. doi: 10.1016/j.tibs.2018.06.002. [DOI] [PubMed] [Google Scholar]
- Lee A.P., Koh E.G., Tay A., Brenner S., Venkatesh B. Highly conserved syntenic blocks at the vertebrate Hox loci and conserved regulatory elements within and outside Hox gene clusters. Proc. Natl. Acad. Sci. U S A. 2006;103:6994–6999. doi: 10.1073/pnas.0601492103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Liu B., Wapinski O.L., Tsai M.C., Qu K., Zhang J., Carlson J.C., Lin M., Fang F., Gupta R.A. Targeted disruption of Hotair leads to homeotic transformation and gene derepression. Cell Rep. 2013;5:3–12. doi: 10.1016/j.celrep.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Helms J.A., Chang H.Y. Comment on "hotair is dispensable for mouse development". PLoS Genet. 2016;12:e1006406. doi: 10.1371/journal.pgen.1006406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Notani D., Rosenfeld M.G. Enhancers as non-coding RNA transcription units: recent insights and future perspectives. Nat. Rev. Genet. 2016;17:207–223. doi: 10.1038/nrg.2016.4. [DOI] [PubMed] [Google Scholar]
- McEwen G.K., Woolfe A., Goode D., Vavouri T., Callaway H., Elgar G. Ancient duplicated conserved noncoding elements in vertebrates: a genomic and functional analysis. Genome Res. 2006;16:451–465. doi: 10.1101/gr.4143406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer T.R., Mattick J.S. Structure and function of long noncoding RNAs in epigenetic regulation. Nat. Struct. Mol. Biol. 2013;20:300–307. doi: 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- Moon M.H., Hilimire T.A., Sanders A.M., Schneekloth J.S., Jr. Measuring RNA-ligand interactions with microscale thermophoresis. Biochemistry. 2018;57:4638–4643. doi: 10.1021/acs.biochem.7b01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega M.A., Ovcharenko I., Afzal V., Rubin E.M. Scanning human gene deserts for long-range enhancers. Science. 2003;302:413. doi: 10.1126/science.1088328. [DOI] [PubMed] [Google Scholar]
- Orom U.A., Derrien T., Beringer M., Gumireddy K., Gardini A., Bussotti G., Lai F., Zytnicki M., Notredame C., Huang Q. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder M., Barozzi I., Tissieres V., Fukuda-Yuzawa Y., Mannion B.J., Afzal S.Y., Lee E.A., Zhu Y., Plajzer-Frick I., Pickle C.S. Enhancer redundancy provides phenotypic robustness in mammalian development. Nature. 2018;554:239–243. doi: 10.1038/nature25461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralkar V.R., Taborda C.C., Huang P., Yao Y., Kossenkov A.V., Prasad R., Luan J., Davies J.O., Hughes J.R., Hardison R.C. Unlinking an lncRNA from its associated cis element. Mol. Cell. 2016;62:104–110. doi: 10.1016/j.molcel.2016.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennacchio L.A., Ahituv N., Moses A.M., Prabhakar S., Nobrega M.A., Shoukry M., Minovitsky S., Dubchak I., Holt A., Lewis K.D. In vivo enhancer analysis of human conserved non-coding sequences. Nature. 2006;444:499–502. doi: 10.1038/nature05295. [DOI] [PubMed] [Google Scholar]
- Pereira B., Chin S.F., Rueda O.M., Vollan H.K., Provenzano E., Bardwell H.A., Pugh M., Jones L., Russell R., Sammut S.J. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat. Commun. 2016;7:11479. doi: 10.1038/ncomms11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portoso M., Ragazzini R., Brencic Z., Moiani A., Michaud A., Vassilev I., Wassef M., Servant N., Sargueil B., Margueron R. PRC2 is dispensable for HOTAIR-mediated transcriptional repression. EMBO J. 2017;36:981–994. doi: 10.15252/embj.201695335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pundhir S., Bagger F.O., Lauridsen F.B., Rapin N., Porse B.T. Peak-valley-peak pattern of histone modifications delineates active regulatory elements and their directionality. Nucleic Acids Res. 2016;44:4037–4051. doi: 10.1093/nar/gkw250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada-Iglesias A., Bajpai R., Swigut T., Brugmann S.A., Flynn R.A., Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470:279–283. doi: 10.1038/nature09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn J.L., Kertesz M., Wang J.K., Squazzo S.L., Xu X., Brugmann S.A., Goodnough L.H., Helms J.A., Farnham P.J., Segal E. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129:1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roadmap Epigenomics Consortium, Kundaje A., Meuleman W., Ernst J., Bilenky M., Yen A., Heravi-Moussavi A., Kheradpour P., Zhang Z., Wang J. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–330. doi: 10.1038/nature14248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelin A., Bailey P., Bruce S., Engstrom P.G., Klos J.M., Wasserman W.W., Ericson J., Lenhard B. Arrays of ultraconserved non-coding regions span the loci of key developmental genes in vertebrate genomes. BMC Genomics. 2004;5:99. doi: 10.1186/1471-2164-5-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorderet P., Duboule D. Structural and functional differences in the long non-coding RNA hotair in mouse and human. PLoS Genet. 2011;7:e1002071. doi: 10.1371/journal.pgen.1002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selleri L., Bartolomei M.S., Bickmore W.A., He L., Stubbs L., Reik W., Barsh G.S. A Hox-embedded long noncoding RNA: is it all hot air? PLoS Genet. 2016;12:e1006485. doi: 10.1371/journal.pgen.1006485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigova A.A., Abraham B.J., Ji X., Molinie B., Hannett N.M., Guo Y.E., Jangi M., Giallourakis C.C., Sharp P.A., Young R.A. Transcription factor trapping by RNA in gene regulatory elements. Science. 2015;350:978–981. doi: 10.1126/science.aad3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolfe A., Goodson M., Goode D.K., Snell P., McEwen G.K., Vavouri T., Smith S.F., North P., Callaway H., Kelly K. Highly conserved non-coding sequences are associated with vertebrate development. PLoS Biol. 2005;3:e7. doi: 10.1371/journal.pbio.0030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Yan P., Lu J., Song G., Zhu Y., Li Z., Zhao Y., Shen B., Huang X., Zhu H. Opposing roles for the lncRNA Haunt and its genomic locus in regulating HOXA gene activation during embryonic stem cell differentiation. Cell Stem Cell. 2015;16:504–516. doi: 10.1016/j.stem.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Yue F., Cheng Y., Breschi A., Vierstra J., Wu W., Ryba T., Sandstrom R., Ma Z., Davis C., Pope B.D. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515:355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in the study were downloaded from ENCODE, mouse ENCODE, GTEx, TCGA, FANTOM5, NIH Roadmap Epigenome project, and additional publicly available datasets mentioned in the methods. All custom code is available upon request.