Abstract
Background
An aging United States population profoundly impacts healthcare from both a medical and financial standpoint, especially with an increase in related procedures such as Total Hip Arthroplasty (THA). The Hospital Readmission Reduction Program and Comprehensive Care for Joint Replacement Program incentivize hospitals to decrease post-operative readmissions by correlating reimbursements with smoother care transitions, thereby decreasing hospital burden and improving quantifiable patient outcomes. Many studies have proposed predictive models built upon risk factors for predicting 30-day THA readmissions.
Questions
(1) Are there validated statistical models that predict 30-day readmissions for THA patients when appraised with a standards-based, reliable assessment tool?. (2) Which evidence-based factors are significant and have support across models for predicting risk of 30-day readmissions post-THA?
Methods
Five major electronic databases were searched to identify studies that examined correlations between post-THA readmission and risk factors using multivariate models. We rigorously applied the PRISMA methodology and TRIPOD criteria for assessment of the prognostic studies.
Results
We found 26 studies that offered predictive models, of which two presented models tested with validation cohorts. In addition to the many factors grouped into demographic, administrative, and clinical categories, bleeding disorder, higher ASA status, discharge disposition, and functional status appeared to have broad and significant support across the studies.
Conclusions
Reporting of recent predictive models establishing risk factors for 30-day THA readmissions against the current standard could be improved. Aside from building better performing models, more work is needed to follow the thorough process of undergoing calibration, external validation, and integration with existing EHR systems for pursuing their use in clinical settings. There are several risk factors that are significant in multiple models; these factors should be closely examined clinically and leveraged in future risk modeling efforts.
Keywords: Total hip arthroplasty, Hip replacement, Patient readmission, Risk factors, Statistical models
1. Introduction
1.1. Background
According to the United States Census Bureau's 2017 National Population Projections, the year 2030 marks a demographic milestone by which one in five citizens will be greater than 65 years old.1,2 With this aging population comes a skewed burden on the healthcare system, which is tasked with addressing a dramatic increase in procedures such as primary joint replacements. The most common primary joint replacements are Total Hip Arthroplasty (THA) and Total Knee Arthroplasty (TKA).3 In particular, THA is one of the quickest growing procedures in the U.S., with a projected 520,000 arthroplasties per year by 2030, entailing a 174% increase.4,5 The exponential growth in the number of procedures invites a greater potential for post-surgical complications, such as surgical site infections, sepsis, joint dislocations, and revision arthroplasties. These complications increase hospital length of stay (LOS) and rates of readmission, and thereby have profound medical and financial ramifications for patients and hospital systems. The economic burden on the U.S. healthcare system is enormous, with an average revision THA costing $77,000 and costs compounding with each additional day of hospitalization.6,7
1.2. Rationale
The Affordable Care Act (ACA) was signed into law in 2010 to expand coverage and realign the US healthcare system.8 Section 3205 of the ACA established the Hospital Readmission Reduction Program (HRRP), which incentivizes institutions to improve quality of care by aligning reimbursements with outcome. More specifically, the HRRP utilizes hospital-specific, risk-standardized all-cause 30-day readmission rates as a measure of hospital performance. Underperforming hospitals are penalized by a reduction in Medicare reimbursements for inpatient services.9 Due to these stipulations, it behooves healthcare providers to reduce readmission rates by improving perioperative care transitions and overall patient care. One of the first areas in the field of orthopedics to be affected by these ACA policies was total joint arthroplasty (TJA).8 Focusing on readmission rate reduction is especially appealing because Medicare covers roughly two thirds of THAs in the U.S., making it the largest single payer for hip arthroplasties.10 Moreover, readmissions related to surgical complications are far more expensive than those related to medical complications, with an average cost of $27,979 for surgical complications as compared to $11,682 for medical complications.11 With these fiscal and health considerations, many research teams have proposed statistical models predicting readmission after THA. It is imperative that these models are broadly compared and reconciled in order to maximize clinical usability.
1.3. Questions
-
(1)
Are there validated statistical models that predict 30-day readmissions for THA patients when appraised with a standards-based, reliable assessment tool?
-
(2)
Which evidence-based factors are significant and have support across the models for predicting risk of 30-day readmissions post-THA?
2. Material and methods
2.1. Search strategy and criteria
This study followed criteria set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) statement.12 The paper heading, use of the PRISMA flow diagram shown in Table 1 for study selection,13 and use of the PRISMA-P checklist in Electronic Supplementary Material 1 reflect adherence to the PRISMA-P criteria.14 In addition, a-priori protocol registration and description were done at the PROSPERO registry site (Registration number: CRD42018108571).15
Table 1.
PRISMA 2009 Flow Diagram for THA.13
The initial search was begun with manual exploration of printed articles in the hospital medical library as well as electronically searched articles on Google Scholar. Important and relevant reference articles from the initial search were additionally obtained, accounting for 28 papers during manual search. Analysis of these papers helped establish an automated strategy for searching electronic databases. This systematic review describes readmission prediction models for hip arthroplasty alone; however, the search criteria was formulated for both hip and knee arthroplasty procedures. This strategy was adopted for completeness since our initial search revealed that some studies had used combined hip and knee cohorts for building models. A medical librarian and one author (BA & SM) designed and implemented all the searches in the following databases: (1) PubMed; (2) Embase; (3) Ovid MEDLINE; (4) Cochrane Database of Systematic Reviews; (5) Web of Science. If the database did not take the exact date for search, it was approximated to the nearest month and/or year. We searched for papers between the date of inception of each database and April 2019. Across databases, the search was begun with hospital readmission as the exploded Medical Subject Headings (MeSH) term and the key words readmi*, rehosp*, where * was used as the truncation character. Second, we searched for risk as the exploded MeSH term and the key words model*, predict*, use*, util*, and risk*. Third, we performed a search that used the exploded MeSH term arthroplasty, replacement, total, partial, prosthesis, and knee. Fourth, we performed a search that used the exploded MeSH term arthroplasty, replacement, total, partial, hemiarthroplasty, prosthesis, and hip. Lastly, we combined all the search criteria that identified our final reference set in each database.
2.2. Inclusion and exclusion
Studies were considered eligible if they (a) used readmission as an independent or composite outcome; (b) measured readmission after index hospitalization for THA; (c) examined the association between readmission and predictors using multivariate statistical model; (d) were published in the English language.
2.3. Assessment of study quality
Table 1 shows the steps of article selection in the PRISMA flow diagram. It also shows exclusion criteria at each step of the selection process, starting with 2933 articles and ending with 358 articles for full review.
In the final step, we excluded studies (n = 83) that (1) analyzed readmissions after generic index admission and did not have THA as the discharge diagnosis; (2) created cohorts based on explicit subgroups such as revision arthroplasty, partial- or hemi-arthroplasties, and hip fracture. Each paper selected for the detailed review was appraised for risk of bias using the Transparent Reporting of a multivariate prediction model for Individual Prognosis Or Diagnosis (TRIPOD) quality assessment tool.16 The TRIPOD standard is designed for transparent reporting of studies developing, validating, or updating a multivariate prediction model for prognostic purposes. It is implemented as a 22-item checklist (Electronic Supplementary Material 2) that provides comprehensive criteria for assessing the quality and thoroughness of the reporting provided in predictive modeling studies. Electronic Supplementary Material 3 shows the checklist applied to all the models in the final selection of studies. The TRIPOD tool factored out studies that did not analyze multivariate models (n = 213) and, thus, it reduced the risk of bias in the final selection of studies. During this step, we also found other reviews (n = 10); five of these were related to the postoperative management and interventions after THA discharge and hence were excluded from our analysis. The remaining five studies are noted in the Results section.
2.4. Data collection and abstraction
Our detailed review entailed 26 studies that described 28 risk models (7 combined hip and knee arthroplasty cohort models and 21 THA models). This analysis was carried out by at least two authors separately (AM & SM; CN & SM; JB & SM), and results of the selection process were verified for selection bias and resolution of low-confidence selections by each author. A predictive model was considered to be a statistical construct for this review, created to understand the combined effect of predictors derived from known data sources on readmission as an outcome, using a specific study design. In addition, we extracted the following data items from each study in a tabulated format in three separate documents: (1) research study design, country where study was conducted, data source and timeframe of data cohort, derivation and validation cohort sizes, statistical model used, whether risk score was created (items summarized as research study characteristics and shown in Table 2); (2) readmission as a single or composite outcome measure, whether outcome was THA-specific or all-cause or surrogate readmission measure, timeframe used for readmission, observed readmission rate, and C-statistics or Area Under Curve (AUC) for validation cohort (items summarized as outcome characteristics and shown in Table 3); (3) significant risk factors in each final model (items summarized as risk factors characteristics and shown in Table 4).
Table 2.
Study design characteristics for studies for readmissions for THA.
| Study | Research Study Design | Data Source | Year(s) of Data | Procedures Per Derivation Cohort | Procedures Per Validation Cohort | Statistical Model | Country/Risk Score Creation |
|---|---|---|---|---|---|---|---|
| (Higuera, Elsharkawy et al., 2011)-a (THA subgroup) | Prospective | Single center EHR system | 2008 | 198 THA | NR | HMLR | USA/No |
| (Khan, Hossain et al., 2012) | Retrospective | Single center EHR system | 2009–2010 | 467 THA | NR | MLR | UK/No |
| (Clement, Derman et al., 2013) | Retrospective | Single center EHR system | 2009–2011 | 1583 THA | NR | MLR | USA/No |
| (Pugely, Callaghan et al., 2013)-a (THA subgroup) | Retrospective | ACS NSQIP | 2011 | 8105 THA | NR | MLR | USA/No |
| (Mednick, Alvi et al., 2014) | Retrospective | ACS NSQIP | 2011 | 9441 THA | NR | MLR | USA/No |
| (Schairer, Sing et al., 2014) | Retrospective | Single center EHR system | 2005–2011 | 1415 THA | NR | CPHR | USA/No |
| (Basques, Bohl et al., 2015) | Retrospective | ACS NSQIP | 2011–2012 | 8434 THA | NR | MLR | USA/No |
| (Heyes, Tucker et al., 2015) | Retrospective | Single center EHR system | 2010–2012 | 424 THA | NR | MLR | UK/No |
| (Stavrakis, SooHoo et al., 2015) | Retrospective | OSHPD | 1995–2010 | 202986 THA | NR | MLR | USA/No |
| (Martin, Gao et al., 2016) | Retrospective | ACS NSQIP | 2012–2013 | 15163 THA | NR | MLR | USA/No |
| (Siracuse and Chamberlain 2016) | Retrospective | HCUP for 4 states | 2006–2011 | 268518 THA | 153560 THA | MLR | USA/Yes |
| (Weiss, Garellick et al., 2016) | Retrospective | Swedish hip arthroplasty register | 1992–2012 | 6690 THA | NR | CPHR | Sweden/No |
| (Ali, Loeffler et al., 2017) | Retrospective | NHS ES | 2006–2016 | 514455 THA | NR | MLR | UK/No |
| (Shah, Keswani et al., 2017) | Retrospective | ACS NSQIP | 2011–2014 | 3120 THA | NR | MLR | USA/No |
| (Sofu, Üçpunar et al., 2017) | Retrospective | Single center EHR system | 2010–2014 | 517 THA | NR | MLR | Turkey/No |
| (Cantrell, DeBell et al., 2018) | Retrospective | ACS NSQIP | 2005–2015 | 10032 THA | NR | MLR | US/No |
| (Kimball, Nichols et al., 2018)-a (THA subgroup) | Retrospective | CMS files | 2014–2015 | 26837 THA | NR | CPHR | US/No |
| (White, Sastow et al., 2018)-a (30-day readmissions) | Retrospective | SID (California, Florida, and New York) & HCUP | 2007–2011 | 274851 THA | NR | MLR | USA/No |
| (White, Sastow et al., 2018)-b (90-day readmissions) | Retrospective | SID (California, Florida, and New York) & HCUP | 2007–2011 | 274851 | NR | MLR | USA/No |
| (Zmistowski, Restrepo et al., 2013) | Retrospective | Single center EHR system | 2004–2008 | 5426 THA | NR | MLR | USA/No |
| (Mesko, Bachmann et al., 2014) | Retrospective | Single center EHR system | 2010–2011 | 1291 THKA combined | 1291 (with bootstrapping of 1000 samples) | MLR | USA/Yes |
| (Tiberi, Hansen et al., 2014) (Only for cirrhosis patients undergoing THKA) | Retrospective – 1:2 matched case control | Single center EHR system | 2000–2012 | 230 THKA combined | NR | MLR | USA/Yes |
| (Ricciardi, Oi et al., 2017) | Retrospective – 1:2 matched case control | Single center EHR system | 2010–2014 | 21864 THKA combined | NR | MLR | USA/No |
| (Sher, Keswani et al., 2017) | Retrospective | ACS NSQIP | 2011–2014 | 7474 THKA combined | NR | MLR | USA/No |
| (Yao, Keswani et al., 2017)-a (THA subgroup) | Retrospective | ACS NSQIP | 2011–2014 | 50376 THA | NR | MLR | USA/No |
| (Schroer, Diesfield et al., 2018) | Retrospective | Multi center (5) EHR system | 2014–2015 | 6968 THKA combined | NR | DS | USA/No |
| (Swenson, Bastian et al., 2018)-a (30-day readmissions) | Retrospective | Single center EHR system | 2013–2015 | 622 THKA combined | NR | MLR | USA/No |
| (Swenson, Bastian et al., 2018)-b (90-day readmissions) | Retrospective | Single center EHR system | 2013–2015 | 622 THKA combined | NR | MLR | USA/No |
ACS NSQIP: American College of Surgeons National Surgical Quality Improvement Program; CMS: Center for Medicare and Medicaid Services; CPHR: Cox Proportional Hazards Regression; DS: Descriptive Statistics; EHR: Electronic Health Record; HCUP: Healthcare Cost and Utilization Project of the Agency for Healthcare Research and Quality; HMLR: Hierarchical Logistic Regression Model using Generalized Estimating Equations; MLR: Multivariate Logistic Regression; NHS ES: National Health Service Hospital Episode Statistics database; NR: Not Reported; OSHPD: California Office of Statewide Health Planning and Development; SID: State Inpatient Database; THA: Total Hip Arthroplasty; THKA: Total Hip or Knee Arthroplasty.
Table 3.
Outcome characteristics for studies for readmissions for THA.
| Study | Outcome: Readmission - Single Measure or Composite (Readmission, Mortality, Complications Requiring Readmission) | Readmission type: All-or-any Cause or Other | Readmission Days | Observed Readmission Rate (%) | C-statistics or AUC for Validation Cohort |
|---|---|---|---|---|---|
| (Higuera, Elsharkawy et al., 2011)-a (THA subgroup) | Composite – complications | All | 90 | 9.4 | NR |
| (Khan, Hossain et al., 2012) | Single | All | 28 | 11.8 | NR |
| (Clement, Derman et al., 2013) | Single | All | 30 | 6.5 | NR |
| (Pugely, Callaghan et al., 2013)-a (THA subgroup) | Composite – complications | All | 30 | 4.2 | NR |
| (Mednick, Alvi et al., 2014) | Single | All | 30 | 3.7 | 0.737 |
| (Schairer, Sing et al., 2014) | Composite – complications | All | 30 and 90 | 4.0 and 8.0 | NR |
| (Basques, Bohl et al., 2015) | Single | All | 30 | 10.0 | NR |
| (Heyes, Tucker et al., 2015) | Single | All | 365 | 21.0 | NR |
| (Stavrakis, SooHoo et al., 2015) | Composite – complications | All | 30 | 3.9 | NR |
| (Martin, Gao et al., 2016) | Single | All | 30 | 8.5 | NR |
| (Siracuse and Chamberlain 2016) | Single | All | 30 | 5.9 | NR |
| (Weiss, Garellick et al., 2016) | Composite – readmission, mortality | Other –cardiovascular | 90 | 30.2 | NR |
| (Ali, Loeffler et al., 2017) | Single | All | 30 | 5.9 | NR |
| (Shah, Keswani et al., 2017) | Single | All | 30 | 9.8 | NR |
| (Sofu, Üçpunar et al., 2017) | Single | All | 30 | 12.2 | NR |
| (Cantrell, DeBell et al., 2018) | Single | All | 30 | 8.5 | NR |
| (Kimball, Nichols et al., 2018)-a (THA subgroup) | Single | All | 30 | 6.7 | NR |
| (White, Sastow et al., 2018)-a (30-day readmissions) | Single | All | 30 | 5.6 | 0.681 |
| (White, Sastow et al., 2018)-b (90-day readmissions) | Single | All | 90 | 10.2 | 0.660 |
| (Zmistowski, Restrepo et al., 2013) | Composite – complications | All | 30 and 90 | 3.1 and 5.3 | NR |
| (Mesko, Bachmann et al., 2014) | Single | All | 30 | 3.6 | 0.760 |
| (Tiberi, Hansen et al., 2014) (Only for cirrhosis patients undergoing THKA) | Composite – complications | All | 90 | 10.0 | NR |
| (Ricciardi, Oi et al., 2017) | Single | All | 30 | 0.3 | NR |
| (Sher, Keswani et al., 2017) | Composite – complications | All | 30 | 1.9 | NR |
| (Yao, Keswani et al., 2017)-a (THA subgroup) | Composite – complications | All | 30 | 3.1 | NR |
| (Schroer, Diesfield et al., 2018) | Composite – complications | All | 90 | 8.4 | NR |
| (Swenson, Bastian et al., 2018)-a (30-day readmissions) | Single | All | 90 | 3.4 | NR |
| (Swenson, Bastian et al., 2018)-b (90-day readmissions) | Single | All | 90 | 5.7 | NR |
AUC: Area Under Curve; NR: Not Reported; THA: Total Hip Arthroplasty.
Table 4.
Risk factor characteristics for studies for readmissions for THA.
| Predictors (level) | Unit of measure & comparison | Effect Size [CI] (wrt reference)a,b | Study |
|---|---|---|---|
| Demographics | |||
| Age (patient) | 70-79/≥90 years | OR: 1.35 [1.09–1.67] (≥90 wrt 70-79) | (Basques, Bohl et al., 2015) |
| Age (patient) | ≤55/56-65/66-75/≥76 years | OR: 2.02 [1.14–3.60] (≥76 wrt ≤ 55) | (Clement, Derman et al., 2013) |
| Age (patient) | 65-74/75-84/85+ years | RR: 1.45 [1.08–1.94] (75-84 wrt 65-74) RR: 1.79 [1.08–2.10] (85+ wrt 65-74) |
(Higuera, Elsharkawy et al., 2011)-a |
| Age (patient) | 65-74/75-84/85+ years | RR: 1.43 [1.14–1.80] (75-84 wrt 65-74) RR: 1.25 [0.79–1.98] (85+ wrt 65-74) |
(Higuera, Elsharkawy et al., 2011)-b |
| Age (patient) | Continuous years | OR: 1.06 [1.02–1.06] | (Khan, Hossain et al., 2012) |
| Age (patient) | <45/46-55/56-65/66-75/76-85/>85 years | OR: 2.59 [1.44–4.67] (<45 wrt 56-65) OR: 1.42 [1.08–1.85] (76-85 wrt 56-65) OR: 1.79 [1.09–2.97] (>85 wrt 56-65) |
(Pugely, Callaghan et al., 2013)-b |
| Age (patient) | 50-59/60-69/70-79/>80 | OR: 1.40 [0.72–2.69] (50-59 wrt <50) OR: 1.66 [0.87–3.16] (60-69 wrt <50) OR: 2.33 [1.18–4.59] (70-79 wrt <50) OR: 4.17 [1.18–4.59] (>80 wrt <50) |
(Sher, Keswani et al., 2017) |
| Age (patient) | 21-30/31-40/41-50/51-60/61-70/71-80/81-90/>90 years | OR: 1.46 [1.17–1.82] (21-30 wrt 41-50) OR: 0.99 [0.85–1.15] (31-40 wrt 41-50) OR: 0.91 [0.84–0.98] (51-60 wrt 41-50) OR: 0.98 [0.91–1.06] (61-70 wrt 41-50) OR: 1.37 [1.28–1.47] (71-80 wrt 41-50) OR: 1.97 [1.82–2.13] (81-90 wrt 41-50) OR: 2.22 [1.87–2.63] (>90 wrt 41-50) |
(Siracuse and Chamberlain 2016) |
| Age (patient) | Continuous years | OR: 1.11 [1.07–1.14] | (Sofu, Üçpunar et al., 2017) |
| Age (patient) | <60/60-75/>75 years | HR: 4.2 (60-75 wrt <60) HR: 10.6 (>75 wrt <60) | (Weiss, Garellick et al., 2016) |
| Age (patient) | Continuous years | OR: 1.56 [1.27–1.92] | (Yao, Keswani et al., 2016)-a |
| BMI - obesity (patient) | <30/> = 30 kg/m2 | OR: 1.15 [1.09–1.21] (≥30 wrt <30) | (Siracuse and Chamberlain 2016) |
| BMI (patient) | <18/18-25/25-30/30-35/≥35 kg/m2 | OR: 1.73 [1.24–2.44] (≥35 wrt 18-25) | (Basques, Bohl et al., 2015) |
| BMI (patient) | <25/25-<30/30-<35/> = 35 kg/m2 | OR: 2.28 [1.27–4.09] (≥35 wrt <25) | (Clement, Derman et al., 2013) |
| BMI (patient) | <18.5/25-<30/30-<35/35-40/>40 kg/m2 | OR: 1.94 [1.02–3.70] (≥40 wrt <18.5) | (Mednick, Alvi et al., 2014) |
| BMI (patient) | <35/>35 kg/m2 | OR: 1.47 [1.11–1.97] (>35 wrt <35) | (Pugely, Callaghan et al., 2013)-a |
| BMI (patient) | <40/>40 kg/m2 | OR: 2.11 [1.19–3.72] (>40 wrt <40) | (Shah, Keswani et al., 2017) |
| BMI (patient) | BMI > 40 kg/m2 | OR: 1.25 [0.73–2.16] (BMI >40 wrt no) | (Sher, Keswani et al., 2017) |
| BMI (patient) | BMI > 40 kg/m2 | OR: 1.47 [1.23–1.74] (Yes wrt No) | (Yao, Keswani et al., 2016)-a |
| BMI (patient) | Obesity as BMI > 45 kg/m2 (% readmitted) | 11.3% | (Schroer, Diesfield et al., 2018) |
| Race (patient) | African/White | OR: 0.66 [0.48–0.91] (White wrt African) | (Zmistowski, Restrepo et al., 2013) |
| Race (patient) | White/African/Hispanic-Asian-Native (Other) | OR: 1.23 [1.15–1.31] (African wrt White) OR: 1.02 [0.97–1.08] (Other wrt White) |
(Siracuse and Chamberlain 2016) |
| Race (patient) | White/African/Hispanic/Asian/Native | OR: 1.48 [1.34–1.62] (African wrt White) OR: 0.79 [0.67–0.93] (Asian wrt White) |
(Stavrakis, SooHoo et al., 2015) |
| Race (patient) | Latino/non-Latino | OR: 5.78 [4.04–7.52] (Latino wrt non-Latino) | (Swenson, Bastian et al., 2018)-a |
| Race (patient) | Latino/non-Latino | OR: 9.09 [7.16–11.02] (Latino wrt non-Latino) | (Swenson, Bastian et al., 2018)-b |
| Sex (patient) | Male/Female | OR: 1.36 [1.05–1.75] (Male wrt Female) | (Zmistowski, Restrepo et al., 2013) |
| Sex (patient) | Male/Female | OR: 1.40 [1.20–1.63] (Male wrt Female) | (Basques, Bohl et al., 2015) |
| Sex (patient) | Male/Female | OR: 1.25 [1.03–1.53] (Male wrt Female) | (Pugely, Callaghan et al., 2013)-b |
| Sex (patient) | Male/Female | OR: 0.96 [0.93–0.99] (Female wrt Male) | (Siracuse and Chamberlain 2016) |
| Sex (patient) | Male/Female | OR: 2.78 [1.76–3.80] (Female wrt Male) | (Swenson, Bastian et al., 2018)-b |
| Administrative | |||
| Admission source (system) | Home/Non-home | OR: 2.36 [1.19–4.66] (Non-home wrt Home) | (Khan, Hossain et al., 2012) |
| Disposition (system) | Home/IRF | OR: 1.99 [1.50–2.64] (IRF wrt Home) | (Zmistowski, Restrepo et al., 2013) |
| Disposition (system) | Home/Non-home facility | OR: 1.42 [1.08–1.86] (Non-home facility wrt Home) | (Basques, Bohl et al., 2015) |
| Disposition (system) | Home/SNF/ALF | OR: 1.71 [0.35–0.98] (Non-home wrt Home) | (Heyes, Tucker et al., 2015) |
| Disposition (system) | Other location (than home): Yes/No | OR: 7.72 [5.87–9.57] (Yes wrt No) | (Swenson, Bastian et al., 2018)-a |
| Disposition (system) | Other location (than home): Yes/No | OR: 8.90 [6.74–11.06] (Yes wrt No) | (Swenson, Bastian et al., 2018)-b |
| Distance to facility (patient) | Continuous kilometers | OR: 0.52 [0.40–0.66] | (Zmistowski, Restrepo et al., 2013) |
| Income (patient) | First/Second/Third/Fourth quartile | OR: 1.18 [1.12–1.24] (First wrt Fourth) OR: 1.07 [1.02–1.12] (Second wrt Fourth) OR: 1.03 [0.99–1.08] (Third wrt Fourth) |
(Siracuse and Chamberlain 2016) |
| Insurance (patient) | Private/Medicare/Medicaid/Other | OR: 1.21 [1.15–1.27] (Medicare wrt Private) OR: 1.68 [1.47–1.92] (Medicaid wrt Private) OR: 1.26 [1.04–1.54] (Other wrt Private) | (Stavrakis, SooHoo et al., 2015) |
| LOS (patient) | Continuous days | OR: 10.71 [5.68–20.19] | (Zmistowski, Restrepo et al., 2013) |
| LOS (patient) | Continuous days | OR: 1.09 [1.03–1.16] | (Clement, Derman et al., 2013) |
| LOS (patient) | Continuous days | OR: 0.95 [0.88–1.02] | (Mednick, Alvi et al., 2014) |
| LOS (patient) | ≤5/>5 days | HR: 3.26 [2.1–5.1] (>5 wrt ≤ 5) | (Schairer, Sing et al., 2014) |
| LOS (patient) | <7/> = 7 days | OR: 3.13 [0.12–0.62] (≥7 wrt <7) | (Heyes, Tucker et al., 2015) |
| Preoperative stay >24 h (patient) | Yes/No | OR: 1.45 [0.77–2.71] (Yes wrt No) | (Mednick, Alvi et al., 2014) |
| Time to surgery (system) | <36 h/>36 h | OR: 1.62 [0.16–2.45] (>36 wrt <36) | (Heyes, Tucker et al., 2015) |
| Type of anesthesia (provider) | Spinal/Other | RR: 0.65 [0.51–0.81] (Spinal wrt Other) | (Higuera, Elsharkawy et al., 2011)-b |
| Type of surgery (patient) | THA/TKA | OR: 2.64 [1.84–3.44] (THA wrt TKA) | (Swenson, Bastian et al., 2018)-a |
| Type of surgery (patient) | THA/TKA | OR: 3.04 [2.14–3.94] (THA wrt TKA) | (Swenson, Bastian et al., 2018)-b |
| Type of surgery - revision (provider) | Primary/Revision/AS THA | HR: 1.84 [1.2–2.9] (Revision wrt Primary) HR: 1.85 [1.0–3.5] (AS wrt Primary) |
(Schairer, Sing et al., 2014) |
| Type of surgery - revision (provider) | Primary/Revision THA | OR: 1.82 [1.75–1.90] (Revision wrt Primary) | (Siracuse and Chamberlain 2016) |
| Type of surgery - use of specific prosthesis (provider) | DHS/CN/HA THA | OR: 1.51 [0.41–1.08] (CN wrt DHS) OR: 3.10 [0.19–1.80] (HA or THA wrt DHS) |
(Heyes, Tucker et al., 2015) |
| Type of surgery - procedure (provider) | IMN/HA/ORIF/Total THA | OR: 1.3 [1.1–1.5] (HA wrt IMN) OR: 1.2 [1.1–1.4] (ORIF wrt IMN) OR: 1.4 [1.1–1.9] (Total wrt IMN) |
(Martin, Gao et al., 2016) |
| Clinical | |||
| Fracture etiology (patient) | Yes/No | OR: 1.71 [1.25–2.34] (Yes wrt No) | (Yao, Keswani et al., 2016)-a |
| Hemoglobin level drop (patient) | <2/≥2 g/dL | OR: 1.29 [0.49–1.25] | (Heyes, Tucker et al., 2015) |
| MELD score = 10 x [(0.957 x Ln serum creatinine) + (0.378 x Ln serum total bilirubin) + (1.12 Ln INR) + 0.643] (patient) | Continuous | OR: 2.99 [1.28–7.00] | (Tiberi, Hansen et al., 2014) |
| Preoperative serum albumin level (patient) | Continuous g/dL | OR: 0.69 [0.48–0.99] | (Mednick, Alvi et al., 2014) |
| Transfusion status (patient) | ≥ 2 units: Yes/No | OR: 1.85 [0.49–7.05] (Yes wrt No) | (Heyes, Tucker et al., 2015) |
| Comorbidities | |||
| Alcohol abuse (patient) | None/Alcoholic | OR: 1.52 [0.26–1.67] (Alcoholic wrt None) | (Heyes, Tucker et al., 2015) |
| Anemia (patient) | Hemoglobin <10 g/dL (% readmitted) | 20% | (Schroer, Diesfield et al., 2018) |
| Anemia (patient) | HCT ≤ 36/>36: Yes/No | OR: 1.2 [1.1–1.4] (Yes wrt No) | (Martin, Gao et al., 2016) |
| Anemia (patient) | Yes/No | OR: 1.19 [1.15–1.25] (Yes wrt No) | (Siracuse and Chamberlain 2016) |
| Arrhythmia (patient) | Yes/No | OR: 1.47 [1.36–1.60] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Arthritis (patient) | Yes/No | OR: 1.22 [1.09–1.36] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| ASA class (patient) | Class 1 or 2/Class 3 or 4 | OR: 1.42 [1.01–2.00] (ASA class 3 or 4 wrt ASA class 1 or 2) | (Sher, Keswani et al., 2017) |
| ASA class (patient) | Class 3 or 4 | OR: 1.69 [1.50–1.89] (Yes wrt No) | (Yao, Keswani et al., 2016)-a |
| ASA class (patient) | Class4/Class 3/Class 1-2 | OR: 1.95 [1.48–2.57] (3 wrt 1-2) OR: 2.74 [1.48–5.08] (4 wrt 1-2) |
(Pugely, Callaghan et al., 2013)-a |
| ASA class (patient) | Class 3/Class 4/Class 1-2 | OR: 1.40 [1.09–1.79] (3 wrt 1-2) OR: 1.90 [1.41–2.51] (4 wrt 1-2) |
(Basques, Bohl et al., 2015) |
| ASA class (patient) | 1/2/3/4 | OR: 3.68 [0.06–1.15] (2 wrt 1) OR: 1.95 [0.18–1.48] (3 wrt 1) OR: 2.14 [0.16–1.34] (4 wrt 1) |
(Heyes, Tucker et al., 2015) |
| ASA class (patient) | Class 3/Class4/Class 1-2 | OR: 1.5 [1.2–1.7] (3 wrt 1-2) OR: 1.7 [1.4–2.1] (4 wrt 1-2) |
(Martin, Gao et al., 2016) |
| ASA class (patient) | Class 3-4/not 3-4 | OR: 1.71 [1.19–2.46] (3-4 wrt not 3-4) | (Shah, Keswani et al., 2017) |
| ASA class (patient) | Class 3-4/not 3-4 | OR: 2.85 [1.47–5.52] (3-4 wrt not 3-4) | (Sofu, Üçpunar et al., 2017) |
| Atherosclerosis (patient) | Yes/No | OR: 1.31 [1.18–1.45] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Avascular necrosis etiology (patient) | Relative to osteoarthritis or other etiology: Yes/No | OR: 1.55 [1.25–1.92] (Yes wrt No) | (Yao, Keswani et al., 2016)-a |
| Bleeding disorder (patient) | Yes/No | OR: 1.76 [1.38–2.26] (Yes wrt No) | (Yao, Keswani et al., 2016)-a |
| Bleeding disorder (patient) | Yes/No | OR: 2.10 [1.32–3.37] (Yes wrt No) | (Pugely, Callaghan et al., 2013)-a |
| Bleeding disorder (patient) | Yes/No | OR: 1.51 [0.75–3.03] (Yes wrt No) | (Mednick, Alvi et al., 2014) |
| Bleeding disorder (patient) | Yes/No | OR: 1.3 [1.1–1.5] (Yes wrt No) | (Martin, Gao et al., 2016) |
| Bleeding disorder (patient) | Yes/No | OR: 1.19 [1.08–1.32] (Yes wrt No) | (Siracuse and Chamberlain 2016) |
| Bleeding disorders (patient) | Current bleeding-causing disorder: Yes/No | OR: 2.56 [1.22–5.38] (Yes wrt No) | (Sher, Keswani et al., 2017) |
| Cancer - disseminated (patient) | Yes/No | OR: 1.5 [1.1–2.0] (Yes wrt No) | (Martin, Gao et al., 2016) |
| Cancer - disseminated (patient) | Yes/No | OR: 1.93 [1.01–3.68] (Yes wrt No) | (Shah, Keswani et al., 2017) |
| Cancer (patient) | Yes/No | OR: 1.26 [1.05–1.51] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Cardiac disease (patient) | Chronic heart failure in 30 days prior to surgery, myocardial infarction within 6 months of surgery, previous percutaneous coronary intervention, or history of angina within 1 month of surgery (yes/no) | OR: 1.44 [0.73–2.84] (Yes wrt No) | (Sher, Keswani et al., 2017) |
| Cardiac valve disease (patient) | Yes/No | HR: 2.5[1.3–5.1] (Yes wrt No) | (Schairer, Sing et al., 2014) |
| CCI (patient) | Index between 0 and 8 | RR: 1.17 [1.07–1.28] (Per index point increase) | (Higuera, Elsharkawy et al., 2011)-a |
| CCI (patient) | Index between 0 and 8 | RR: 1.18 [1.11–1.26] (Per index point increase) | (Higuera, Elsharkawy et al., 2011)-b |
| CCI (patient) | 0/2/>2 | HR: 3.4 (>2 wrt 0) | (Weiss, Garellick et al., 2016) |
| Chronic heart failure (patient) | Yes/No | OR: 2.41 [1.30–4.49] (Yes wrt No) | (Yao, Keswani et al., 2016)-a |
| Congestive heart failure (patient) | Yes/No | RR: 2.93 [1.07–8.05] (Yes wrt No) | (Higuera, Elsharkawy et al., 2011)-a |
| Congestive heart failure (patient) | Yes/No | OR: 1.54 [1.39–1.71] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Congestive heart failure (patient) | Yes/No | OR: 1.49 [1.38–1.61] (Yes wrt No) | (Siracuse and Chamberlain 2016) |
| COPD (patient) | Yes/No | OR: 1.36 [0.76–2.44] (Yes wrt No) | (Mednick, Alvi et al., 2014) |
| COPD (patient) | Yes/No | OR: 1.45 [1.34–1.56] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| COPD (patient) | Yes/No | OR: 1.3 [1.1–1.5] (Yes wrt No) | (Martin, Gao et al., 2016) |
| COPD (patient) | Yes/No | OR: 1.33 [1.27–1.39] (Yes wrt No) | (Siracuse and Chamberlain 2016) |
| Decubitus ulcer (patient) | Yes/No | OR: 1.61 [1.18–2.20] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Depression (patient) | Yes/No | OR: 3.48 [1.43–8.51] (Yes wrt No) | (Ricciardi, Oi et al., 2017) |
| Diabetes - uncomplicated (patient) | Yes/No | OR: 1.21 [1.16–1.27] (Yes wrt No) | (Siracuse and Chamberlain 2016) |
| Diabetes - with complications (patient) | Yes/No | HR: 11.55 [3.6–36.8] (Yes wrt No) | (Schairer, Sing et al., 2014) |
| Diabetes (patient) | Yes/No | OR: 1.28 [0.82–2.01] (Yes wrt No) | (Sher, Keswani et al., 2017) |
| Diabetes (patient) | Uncontrolled status > 180 mg/dL blood glucose (% readmitted) | 11% | (Schroer, Diesfield et al., 2018) |
| Diabetes (patient) | Yes/No | OR: 1.21 [1.04–1.40] (Yes wrt No) | (Yao, Keswani et al., 2016)-a |
| Diabetes (patient) | Yes/No | OR: 3.34 [1.54–7.25] (Yes wrt No) | (Khan, Hossain et al., 2012) |
| Diabetes (patient) | Yes/No | OR: 1.24 [0.75–2.06] (Yes wrt No) | (Mednick, Alvi et al., 2014) |
| Diabetes (patient) | Yes/No | OR: 1.28 [1.19–1.38] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Diabetes (patient) | Yes/No | OR: 1.37 [0.98–1.93] (Yes wrt No) | (Shah, Keswani et al., 2017) |
| Dyspnea (patient) | Yes/No | OR: 1.3 [1.1–1.6] (Yes wrt No) | (Martin, Gao et al., 2016) |
| Fluid & electrolyte disorders (patient) | Yes/No | OR: 1.21 [1.14–1.27] (Yes wrt No) | (Siracuse and Chamberlain 2016) |
| Functional status (patient) | Dependency: Yes/No | OR: 1.47 [1.08–2.01] (Yes wrt No) | (Yao, Keswani et al., 2016)-a |
| Functional status (patient) | Dependent/Independent | OR: 1.78 [1.09–2.90] (Dependent wrt Independent) | (Pugely, Callaghan et al., 2013)-a |
| Functional status (patient) | Dependent/Partially Dependent/Independent | OR: 1.31 [1.11–1.54] (Partially Dependent wrt Independent) OR: 1.41 [1.01–1.97] (Dependent wrt Independent) |
(Basques, Bohl et al., 2015) |
| Functional status (patient) | Dependent/Independent | OR: 1.66 [1.10–2.50] (Dependent wrt Independent) | (Shah, Keswani et al., 2017) |
| Hematological disease (patient) | Yes/No | OR: 2.64 [1.88–3.73] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Hypertension (patient) | Yes/No | OR: 1.28 [0.91–1.79] (Yes wrt No) | (Sher, Keswani et al., 2017) |
| Hypertension (patient) | Yes/No | OR: 23.6 [22.4–24.8] (Yes wrt No) | (Swensen, Bastian et al., 2018)-b |
| Hypertension (patient) | Yes/No | OR: 1.22 [1.09–1.36] (Yes wrt No) | (Yao, Keswani et al., 2016)-a |
| Hypertension (patient) | Yes/No | OR: 1.26 [0.88–1.82] (Yes wrt No) | (Mednick, Alvi et al., 2014) |
| Hypertension (patient) | Yes/No | OR: 1.21 [1.02–1.45] (Yes wrt No) | (Basques, Bohl et al., 2015) |
| Hypertension (patient) | Yes/No | OR: 1.2 [1.1–1.4] (Yes wrt No) | (Martin, Gao et al., 2016) |
| Hypertension (patient) | Yes/No | OR: 1.17 [1.12–1.21] (Yes wrt No) | (Siracuse and Chamberlain 2016) |
| Ischemic heart disease/arrhythmia (patient) | Yes/No | RR: 1.60 [1.20–1.40] (Yes wrt No) | (Higuera, Elsharkawy et al., 2011)-a |
| Ischemic heart disease/arrhythmia (patient) | Yes/No | RR: 1.73 [1.36–2.21] (Yes wrt No) | (Higuera, Elsharkawy et al., 2011)-b |
| Ischemic heart disease/arrhythmia (patient) | Yes/No | OR: 1.84 [1.00–3.40] (Yes wrt No) | (Sofu, Üçpunar et al., 2017) |
| Liver disease (patient) | Yes/No | OR: 1.57 [1.39–1.77] (Yes wrt No) | (Siracuse and Chamberlain 2016) |
| Lymphoma (patient) | Yes/No | OR: 23.6 [22.25–24.95] (Yes wrt No) | (Swensen, Bastian et al., 2018)-b |
| Malnutrition (patient) | Albumin <3.4 g/dL (% readmitted) | 8.8% | (Schroer, Diesfield et al., 2018) |
| Narcotic use (patient) | Narcotic prescription filled within 3 months of surgery (% readmitted) | 10.7% | (Schroer, Diesfield et al., 2018) |
| Neurological disorder (patient) | History: Yes/No | OR: 5.66 [2.79–11.47] (Yes wrt No) | (Khan, Hossain et al., 2012) |
| Number of comorbidities (patient) | Continuous | OR: 1.53 [1.17–1.90] | (Swenson, Bastian et al., 2018)-a |
| Pneumonia (patient) | Yes/No | OR: 1.52 [1.08–2.12] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Preoperative corticosteroid use (patient) | Yes/No | OR: 1.64 [1.03–2.61] (Yes wrt No) | (Pugely, Callaghan et al., 2013)-a |
| Preoperative corticosteroid use (patient) | Yes/No | OR: 2.93 [1.73–4.95] (Yes wrt No) | (Mednick, Alvi et al., 2014) |
| Preoperative corticosteroid use (patient) | Yes/No | OR: 1.38 [1.04–1.83] (Yes wrt No) | (Basques, Bohl et al., 2015) |
| Preoperative corticosteroid use (patient) | Yes/No | OR: 1.86 [1.21–2.89] (Yes wrt No) | (Shah, Keswani et al., 2017) |
| Prior cardiac surgery (patient) | Yes/No | OR: 1.92 [0.71–5.22] (Yes wrt No) | (Mednick, Alvi et al., 2014) |
| Protein deficiency (patient) | Yes/No | OR: 1.99 [1.49–2.64] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Psychiatric disease (patient) | Yes/No | OR: 1.97 [1.65–2.35] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Pulmonary disease (patient) | Yes/No | OR: 8.83 [7.20–10.47] (Yes wrt No) | (Swenson, Bastian et al., 2018)-b |
| Pulmonary disease (patient) | Yes/No | OR: 1.36 [0.65–2.86] (Yes wrt No) | (Sher, Keswani et al., 2017) |
| Pulmonary disease (patient) | Yes/No | OR: 1.45 [1.17–1.80] (Yes wrt No) | (Yao, Keswani et al., 2016)-a |
| Pulmonary Disease (patient) | Yes/No | OR: 1.46 [1.22–1.75] (Yes wrt No) | (Basques, Bohl et al., 2015) |
| Renal disease (patient) | Yes/No | OR: 1.57 [1.39–1.76] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Renal failure - end stage disease (patient) | Yes/No | OR: 2.64 [1.81–3.85] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Renal failure (patient) | Yes/No (Creatinine > 1.2/≤1.2) | OR: 1.2 [1.1–1.4] (Yes wrt No) | (Martin, Gao et al., 2016) |
| Renal failure (patient) | Yes/No | OR: 1.26 [1.18–1.36] (Yes wrt No) | (Siracuse and Chamberlain 2016) |
| Rheumatoid arthritis (patient) | Yes/No | OR: 1.19 [1.10–1.29] (Yes wrt No) | (Siracuse and Chamberlain 2016) |
| Severe adverse event pre-discharge (patient) | Yes/No | OR: 1.76 [1.11–2.78] (Yes wrt No) | (Yao, Keswani et al., 2016)-a |
| Severe pre-discharge adverse event (patient) | Wound infection, thromboembolic event, myocardial infarction, wound dehiscence, unplanned intubation, ventilator >48 h, renal insufficiency, renal failure, stroke/CVA, cardiac arrest requiring CPR, sepsis, septic shock, death: Yes/No | OR: 13.13 [5.1–33.79] (Yes wrt No) | (Sher, Keswani et al., 2017) |
| Smoking (patient) | Tobacco use: Yes/No (% readmitted) | 7.6% | (Schroer, Diesfield et al., 2018) |
| Smoking (patient) | Yes/No | OR: 1.62 [1.06–2.46] (Yes wrt No) | (Sher, Keswani et al., 2017) |
| Smoking (patient) | Yes/No | OR: 1.38 [1.20–1.58] (Yes wrt No) | (Yao, Keswani et al., 2016)-a |
| Smoking (patient) | None/Ex-smoker/Smoker | OR: 1.14 [0.64–2.00] (Ex-smoker wrt None) OR: 1.24 [0.56–2.73] (Smoker wrt None) |
(Heyes, Tucker et al., 2015) |
| Steroids (patient) | Yes/No | OR: 1.79 [0.86–3.74] (Yes wrt No) | (Sher, Keswani et al., 2017) |
| Steroids for chronic disease (patient) | Yes/No | OR: 1.43 [1.14–1.79] (Yes wrt No) | (Yao, Keswani et al., 2016)-a |
| Stroke (patient) | Yes/No | 3 OR:.75 [2.66–5.28] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Substance abuse (patient) | Yes/No | HR: 2.05 [0.9–4.8] (Yes wrt No) | (Schairer, Sing et al., 2014) |
| Vascular disease (patient) | Yes/No | OR: 1.48 [1.31–1.67] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Postoperative surgical complications | |||
| Deep venous thrombosis (patient) | Yes/No | OR: 14.37 [5.39–38.29] (Yes wrt No) | (Mednick, Alvi et al., 2014) |
| Infection (patient) | Yes/No | OR: 1.59 [1.05–2.38] (Yes wrt No) | (Stavrakis, SooHoo et al., 2015) |
| Pulmonary embolism (patient) | Yes/No | OR: 19.09 [5.82–62.65] (Yes wrt No) | (Mednick, Alvi et al., 2014) |
| Superficial surgical site infection (patient) | Yes/No | OR: 29.66 [14.43–60.96] (Yes wrt No) | (Mednick, Alvi et al., 2014) |
| Postoperative medical complications | |||
| Number of significant risk factors (patient) | 0/1/2/3/≥4 | OR: 1.22 [0.95–1.57] (1 wrt 0) p > 0.05 OR: 1.90 [1.51–2.40] (2 wrt 0) OR: 3.20 [2.54–4.02] (3 wrt 0) OR: 5.06 [4.01–6.38] (≥4 wrt 0) |
(Yao, Keswani et al., 2016)-a |
| Pneumonia (patient) | Yes/No | OR: 2.72 [0.67–11.05] (Yes wrt No) | (Mednick, Alvi et al., 2014) |
| Postoperative ICU stay (system) | Yes/No | OR: 2.09 [1.12–3.88] | (Sofu, Üçpunar et al., 2017) |
| Sepsis or septic shock (patient) | Yes/No | OR: 9.85 [3.39–28.64] (Yes wrt No) | (Mednick, Alvi et al., 2014) |
| Urinary tract infection (patient) | Yes/No | OR: 2.18 [0.90–5.31] (Yes wrt No) | (Mednick, Alvi et al., 2014) |
a: Effect size reported as Adjusted Odds Ratio (OR) or Hazards Ratio (HR) or Relative Risks Ratio (RR) typically at p < 0.05 (some ratios significant at higher p values reported by some authors); b: Odds ratio for continuous variables is reported as change in readmission odds for unit change in continuous variable; wrt: with respect to; ALF: Assisted Living Facility; AS: Antibiotic Spacer; ASA: American Society of Anesthesiologists patient fitness level before surgery; BMI: Body Mass Index; CCI: Charlson Comorbidity Index; CI: Confidence Interval; CN: Cephalomedullary Nail; COPD: Chronic Obstructive Pulmonary Disease; DHS: Dynamic Hip Screw; HA: Hemiarthroplasty; HCT: Hematocrit; HHC: Home Health Care; ICU: Intensive Care Unit; IMN: Intra-Medullary Nail; IRF: Inpatient Rehabilitation Facility; LOS: Length of Stay; MELD: Model for End-stage Liver Disease; ORIF: Open Reduction Internal Fixation; QOL: Quality of Life; SNF: Skilled Nursing Facility; TKA: Total Knee Arthroplasty; THA: Total Hip Arthroplasty.
3. Results
We found five reviews during our final article selection process that met our initial inclusion criteria: Hofstede et al. (2016) did a systematic review following the PRISMA guidelines that analyzed one-year follow-up and independent factors for patients with osteoarthritis on radiological confirmation in prospective studies. The study, however, focused on only patient related factors and found low quality of evidence across the studies.17 Two reviews focused on total joint arthroplasties and did not adhere to formal systematic review standards; they are literature reviews and described modifiable and non-modifiable risk factors for patients undergoing joint arthroplasties.18,19 One of the two studies (Sveom et al. (2017)) also considered perioperative risk factors including administrative and systemic factors after the discharge and hence did not describe predictive factors for readmissions.19 Podmore et al. (2018) considered short- and long-term impacts of comorbidities in joint arthroplasty patients on many outcome variables, including readmission20; risk factors other than comorbidities were not considered in the review. Thus, none of the reviews has aimed to carry out a systematic review adhering to the specific standards of reporting to understand quantitative impact of the risk factors on readmission post-THA.
To date, we found one validated statistical model that predicts 30-day readmission for THA patients, and one validated model that includes a combined THA and TKA cohort: Siracuse et al. (2016) created and validated a THA model that explained 89.1% variability in readmission in their cohort analysis, with a derivation cohort of 268,518 and a validation cohort of 153,560 patients for the years 2006–2011. Thirty-day readmission rates were 5.89% and 5.82% for the derivation and validation cohorts, respectively. This model is encouraging in terms of applicability to clinical practice, given its large cohort size and validation. However, while the reported R2 of 89.1% explains a great degree of variance in the outcome measure, AUC was not reported. AUC reporting is broadly preferred over R2 reporting, given that AUC measures discriminative capability. The second validated model from Mesko et al. (2014) had a retrospective cohort of 1291 combined THA and TKA patients from 2010 to 2011. Their model exhibited an AUC of 0.76 for the validation cohort, with internal validation done via bootstrapping using 1000 samples. This model is encouraging in terms of reporting and discriminative capability, though validation on an external cohort is preferred as compared to internal validation, given that linear models often suffer from difficulties generalizing to new populations. These two models confirm that there do indeed exist validated statistical models for predicting 30-day readmissions after THA. Nonetheless, these models are an extreme minority of the 28 total models studied, suggesting room for improvement in terms of model construction and validation.
3.1. Study characteristics
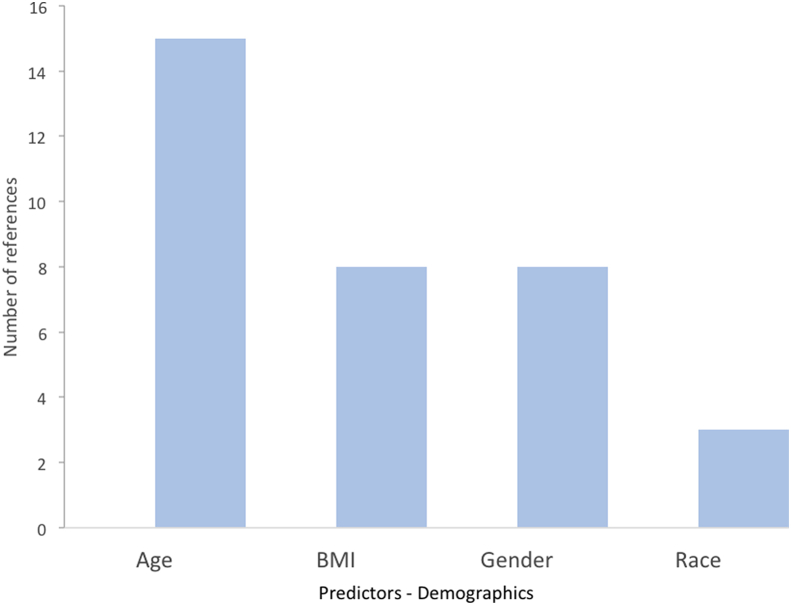
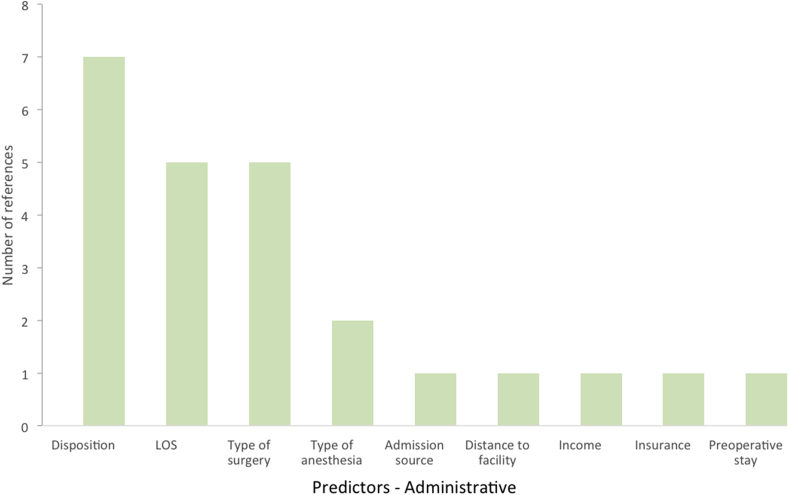
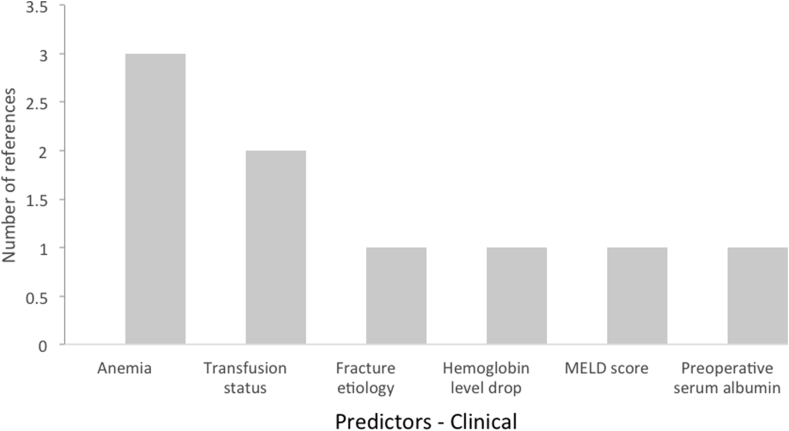
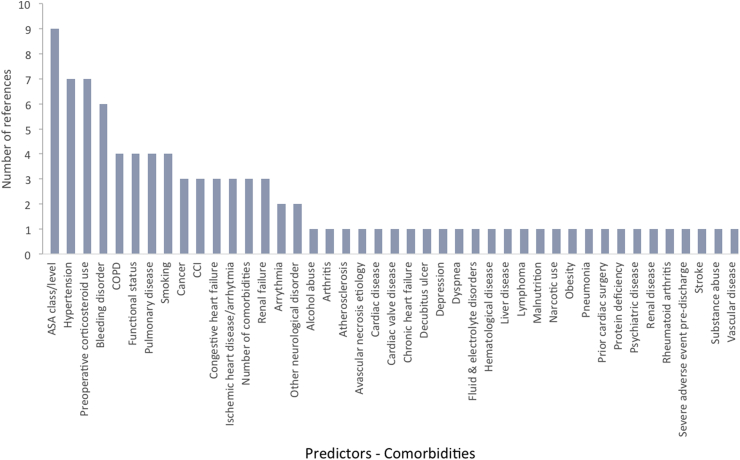
We examined 28 models in our final selection that proposed significant, contributory factors to 30-day readmissions. From a demographic standpoint, age was the leading risk factor for 30-day readmissions, followed by BMI, gender, and race (Fig. 1). From an administrative perspective, disposition, LOS, and type of surgery were the leading risk factors for readmission (Fig. 2). From a clinical standpoint, anemia and transfusion status were the leading risk factors (Fig. 3). In terms of comorbidities contributing to increased readmission rates, ASA class, hypertension, preoperative corticosteroid use, and presence of a bleeding disorder were most cited (Fig. 4). In terms of postoperative complications, severe adverse event pre-discharge, DVT, infection, pneumonia, postoperative ICU stay, pulmonary embolism, sepsis, superficial surgical site infection, and urinary tract infection were referenced once each as risk factors.
Fig. 1.
Demographic risk factors and their support from studies for readmissions post-THA.
Fig. 2.
Administrative risk factors and their support from studies for readmissions post-THA.
Fig. 3.
Clinical risk factors and their support from studies for readmissions post-THA.
Fig. 4.
Comorbidities and their support from studies for readmissions post-THA.
There is high heterogeneity in this study analysis. For example, seven models noted hypertension as a significant predictor of 30-day THA readmission. However, these models do not uniformly delineate the duration or severity of a patient's hypertension, such as if patients are categorized into elevated blood pressure, stage 1 hypertension, or stage 2 hypertension, or when their blood pressure was measured.21 The presence of heterogeneity thus makes it difficult to perform pooled regression—otherwise known as meta-analysis—of effect sizes for various significant risk factors. We therefore analyzed risk factors highlighted as significant by multiple studies. Consensus around significant risk factors provides valuable information regarding directions for future modeling efforts as well as clinical and care practice takeaways.
3.2. TRIPOD analysis
TRIPOD is a global, consensus-based quality assessment tool that defines a gold standard for reporting of predictive models. Examples of specific items to be included in reporting are description of sample size, outcome, limitations, and explanation of statistical models.22 While the TRIPOD standard was published in 2015, prior precedent standards such as the CHARMS checklist23 and CASP tool24 were widely available before then, and none of the studies published before 2015 in our review used those standards for reporting and quality assessment.
4. Discussion
Specific outcome measurements, such as hospital readmissions following surgery, have shown that the increasing demand for aging-related procedures has led to greater expenses for patients and hospitals alike. The majority of primary THA procedures are covered by Medicare, with patients in the age range of 65–84 years.25 Recent changes in payment structure, with a focus on value-based care, have incentivized both patients and healthcare providers to identify risks and improve transition of care and care continuum processes that lead to reductions in readmissions. Therefore, accurate risk assessment tools can have a dramatic impact on both medical and surgical costs and outcomes. This study therefore investigates statistical models that predict 30-day readmission rates for THA patients, evaluates their reporting of performance measures and predictivity statistics using standard TRIPOD criteria, and identifies significant predictors for readmission following primary THA.
4.1. Data synthesis across studies
All the models were published in the last decade from 2011 to 2018, and the majority of them (23 out of 28, or 82%) were built in the USA.26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 Three models were built in the UK47, 48, 49 and one each in Sweden50 and Turkey.51 One model was based on a prospective design,30 and the remaining (96%) were retrospective designs, with two models applying additional case control.37,43 While the creation and implementation of a prospective study design is more difficult than that of a retrospective design, it is greatly preferred: prospective modeling or validation ensures that models are robust to changes in the underlying population over time, thereby allaying concerns about the generalizability of multivariate linear models. For example, the past 15 years have seen a rise in accelerated rehabilitation, the role of orthogeriatricians, and changes to anticoagulation medications. Prospective designs serve to incorporate such changes over time by leveraging developed models in practice and measuring the concurrence between model predictions and clinical outcomes on a case-by-case basis. In turn, these prospectively validated models are significantly more applicable to ongoing policy decisions.
In our review, most studies used either the Electronic Health Records (EHR) database at the facility (13 out of 28, or 46%) or the American College of Surgeons – National Surgical Quality Improvement Program (ACS NSQIP) database (8 out of 28, or 29%) for building models. The other data sources used were: insurance files, health databases, and surgical procedure registers at a national level (3 out of 28, or 11%), and health databases at a state level (4 out of 28, or 14%). The models were built with a minimum of 230 patients to a maximum of 514,455 patients.
4.2. Study reporting
The majority of the studies in our review lacked in-depth descriptions of the models when measured against standard TRIPOD criteria. Four out of 26 studies reported a C-statistic or AUC as a measure of model performance. Heterogeneity or absence of reporting of performance measure renders comparison efforts to assess predictive quality of the models difficult, especially given that some measures describe goodness-of-fit (for example, R2) while others describe discriminatory capability (for example, AUC). Further, it should be noted that the TRIPOD standard was published in 2015 – therefore, it is likely that several of the studies included in this review did not have access to the checklist at the time of analysis. Indeed, the TRIPOD standard was itself designed to combat poor model reporting practices for prediction models. However, certain aspects of the TRIPOD standard were widely accepted before its publication, particularly by standard tools such as the CHARMS checklist (2014) and CASP tool (2006). For example, model discriminatory performance reporting was requested as early as the 2006 version of the CASP tool. Authors of predictive modeling studies should be mindful to check their reporting against whatever prevalent reporting standards exist at the time of publication.
Empirical readmission rates showed a large variance, ranging from 0.3% to 30.2%, with three studies not even reporting the base rate. Such wide variation might be attributable to a multitude of cohort-related factors and facility-specific interventions, procedures, and protocols.
Two of 26 studies used validation cohorts. This practice, requiring to keep 10%–30% of the cohort data aside to measure the performance of the models built using the remaining 70%–90% of the data, is relatively easy to implement but was not adopted by the majority of the studies in our review. This scarcity of validated studies impedes deployment of models in clinical setting.
4.3. Significant risk factors
We identified significant factors correlated to post-THA readmissions along with the number of studies supporting each factor as shown in Fig. 1, Fig. 2, Fig. 3, Fig. 4.
Diabetes has been discussed extensively as a risk factor for THA readmissions amongst various reviews.18, 19, 20,52 Age is another risk factor indicated both in our analysis as well as in prior literature. Specifically, Hofstede et al. (2016) identified patients over the age of 60 to be at risk of readmission following THA,17 while Sveom et al. (2017) specified that patients over the age of 80 are especially at risk.19 Iorio et al. (2017) cited anemia as a risk factor for THA readmissions.52 Siddiqi et al. (2017) identified pre-existing renal insufficiency as a risk factor for readmission due to postoperative acute renal failure.53 Sveom et al. (2017), in addition to corroborating diabetes and age as risk factors, also cited COPD and LOS as risk factors for readmission.19 Podmore et al. (2018) provided support for hypertension and lung disease as risk factors for readmission.20 Among predictors referenced by at least six studies,32,33,36,40,41,45 one of which was a study with a validation cohort, coagulopathy or bleeding disorder had not been referenced in earlier studies.
American Society of Anesthesiologists Physical Status (ASA) class was referenced as a risk factor for readmission by eight different studies.26,32,36,39,40,45,48,51 All eight studies found that ASA class 3 or 4 was a significant risk factor for readmission when compared to ASA class 1 or 2. ASA classes 3 and 4 respectively represent patients with severe systemic disease and with constant threat to life and thus signify higher risk for readmission. While it is likely that surgeons already inform their sickest patients about the risks of surgery and offer help to control chronic medical conditions before opting for THA, it is critical that the entire care team, including anesthesiologists and postoperative nursing teams, are aware of the patient's ASA status and corresponding risks.
Preoperative corticosteroid use was also referenced as a risk factor for readmission by four different studies when compared to no corticosteroid use.26,33,36,39 Corticosteroids act as immunosuppressive agents by decreasing transcription of pro-inflammatory markers. Therefore, use of preoperative corticosteroids dampens the body's ability to mount a robust immune response, leading to an increased probability of surgical site and wound infections given surgical manipulation.54,55 Furthermore, chronic use of corticosteroids predisposes patients to endocrine pathologies such as Cushing's syndrome, which have been shown to negatively affect the wound healing process by inhibiting proliferation of fibroblasts and leading to increased rates of infection given decreased lymphocyte counts and chronically elevated cortisol levels.56 Given the agreement across multiple studies of the significance of preoperative corticosteroid use as a predictive risk factor for post-THA readmission, surgical teams should coordinate with the primary care team to taper corticosteroid use preoperatively.
Discharge disposition was referenced as a risk factor for readmission by four different studies.26,46,48,57 Overall, a discharge to home elicited the best outcomes when compared to other options, such as an inpatient rehabilitation facility and skilled nursing facility. Discharge to a non-home facility likely implies an overall lower health status, impaired functional status, or lack of social support, which may be independent or interrelated risk factors for increased risk of readmission. This system-level risk factor also puts an additional burden on care transition.
Functional status was referenced as a risk factor for readmission by four different studies,26,36,39,45 all of which found that a dependent or partially dependent functional status was a risk factor for readmission when compared to an independent functional status. These degrees of functional status are measured by a patient's ability to independently perform activities of daily living (ADLs). A multitude of parameters could render a patient dependent or partially dependent, such as intense pain, infection, structural abnormalities, comorbidities, frailty, lack of adequate post-operative rehabilitation, or severe obesity. The inability to independently perform ADLs has been shown to increase morbidity and mortality after general, vascular, and orthopedic surgeries such as THA.58 This compromised ability to perform ADLs leads to an increased sedentary state, thereby increasing the risk of deep vein thrombosis, atelectasis, and pneumonia, especially in elderly populations – all of which increase the risk of 30-day readmission.59,60 Lack of autonomous mobility given decreased baseline functional activity can also increase likelihood of physical deconditioning, which may in turn lead to increased risk of readmission via falling or other adverse events.61,62 Functional status is not a risk factor that can be easily resolved preoperatively; however, early perioperative physical therapy may improve patients' physical status and mobility, making them more robust to postoperative risks.
In summary, the limited evidence from the two validated models and corroboration of the risk factors from other studies suggests that researchers have been able to build predictive models for assessing the risk of readmission post-THA with modest predictive capability.
4.4. Limitations
Despite adhering to rigorous process and applying multi-author voting for the article selection process, there is a possibility of selection bias in our review. We might have missed some studies written in non-English languages without known available translations. Since we focused on primary THA cohort studies in our review, some additional nuances related to revision arthroplasties and hip fracture cohorts are missing in our analyses. Since all but one of the studies in our review were retrospective observational cohorts, there is inherent systemic bias in their results, despite the evidence for many motivating predictors. Our review is also limited by the poor adherence to the standard TRIPOD development and reporting criteria and lack of use of procedures such as testing the models with external validation cohorts.
5. Conclusions
Our review of predictive models for the risk of readmission post-THA entailed 28 models from the detailed analyses of 26 studies using the TRIPOD quality assessment criteria. Most models were built within the last eight years in the U.S., either using a facility EHR system or the ACS NSQIP database. Despite two validated models showing predictive capabilities, most studies lacked novel methods for building the models and reporting of performance measurements. These findings suggest that there is ample opportunity for increased rigor and consistency in model reporting. Bleeding disorder, ASA class 3 or 4, preoperative corticosteroid use, and non-home discharge disposition were found to be risk factors of significance for post-THA readmission. Future predictive modeling studies ought to focus on including these significant risk factors to maximize predictivity, and on adhering to standard, TRIPOD-based model reporting and validation practices. These endeavors will increase clinical usability and facilitate meta-analysis of models across studies.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jor.2020.03.045.
Contributor Information
Satish M. Mahajan, Email: satish.mahajan@va.gov.
Amey Mahajan, Email: amey@c2ops.io.
Chantal Nguyen, Email: nguyenct@gwmail.gwu.edu.
Justin Bui, Email: jbui46890@med.lecom.edu.
Bruce T. Abbott, Email: btabbott@ucdavis.edu.
Thomas F. Osborne, Email: thomas.osborne@va.gov.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Knickman J.R., Snell E.K. The 2030 problem: caring for aging baby boomers. Health Serv Res. 2002;37(4):849–884. doi: 10.1034/j.1600-0560.2002.56.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoon R.S., Mahure S.A., Hutzler L.H., Iorio R., Bosco J.A. Hip arthroplasty for fracture vs elective care. One bundle does not fit all. J Arthroplasty. 2017;32(8):2353–2358. doi: 10.1016/j.arth.2017.02.061. [DOI] [PubMed] [Google Scholar]
- 3.Kremers H.M., Larson D.R., Crowson C.S. Prevalence of total hip and knee replacement in the United States. J Bone Jt Surg Am Vol. 2015;97(17):1386. doi: 10.2106/JBJS.N.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Jt Surg Am Vol. 2007;89(4):780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 5.Hutzler L., Williams J. Decreasing the incidence of surgical site infections following joint replacement surgery. Bull Hosp Jt Dis. 2017;75(4):268–273. [PubMed] [Google Scholar]
- 6.Gwam C.U., Mistry J.B., Mohamed N.S. Current epidemiology of revision total hip arthroplasty in the United States: national Inpatient Sample 2009 to 2013. J Arthroplasty. 2017;32(7):2088–2092. doi: 10.1016/j.arth.2017.02.046. [DOI] [PubMed] [Google Scholar]
- 7.Mahure S.A., Hutzler L., Yoon R.S., Bosco J.A. Economic impact of nonmodifiable risk factors in orthopaedic fracture care: is bundled payment feasible? J Orthop Trauma. 2017;31(3):175–179. doi: 10.1097/BOT.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 8.Chambers M.C., Ei-Othmani M.M., Saleh K.J. Health care reform impact on total joint replacement. Orthop Clin N Am. 2016;47(4):645–+. doi: 10.1016/j.ocl.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Kocher R.P., Adashi E.Y. Hospital readmissions and the Affordable Care Act: paying for coordinated quality care. Jama. 2011;306(16):1794–1795. doi: 10.1001/jama.2011.1561. [DOI] [PubMed] [Google Scholar]
- 10.Ong K.L., Mowat F.S., Chan N., Lau E., Halpern M.T., Kurtz S.M. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22–28. doi: 10.1097/01.blo.0000214439.95268.59. [DOI] [PubMed] [Google Scholar]
- 11.Clair A.J., Evangelista P.J., Lajam C.M., Slover J.D., Bosco J.A., Iorio R. Cost analysis of total joint arthroplasty readmissions in a bundled payment care improvement initiative. J Arthroplasty. 2016;31(9):1862–1865. doi: 10.1016/j.arth.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Moher D., Shamseer L., Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1–9. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group T.P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. 1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shamseer L., Moher D., Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350 doi: 10.1136/bmj.g7647. g7647. [DOI] [PubMed] [Google Scholar]
- 15.Dissemination CfRa . NHS: National Institute for Health Research; 2019. PROSPERO: International Prospective Register of Systematic Reviews.https://www.crd.york.ac.uk/prospero/ Published 2011. [Google Scholar]
- 16.Moons K.G., Altman D.G., Reitsma J.B. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162(1):W1–W73. doi: 10.7326/M14-0698. [DOI] [PubMed] [Google Scholar]
- 17.Hofstede S.N., Gademan M.G., Vliet Vlieland T.P., Nelissen R.G., Marang-van de Mheen P.J. Preoperative predictors for outcomes after total hip replacement in patients with osteoarthritis: a systematic review. BMC Muscoskel Disord. 2016;17:212. doi: 10.1186/s12891-016-1070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero J.A., Jones R.E., Brown T. Modifiable risk factors and preoperative optimization of the primary total arthroplasty patient. Curr Orthop Pract. 2017;28(3):272–275. [Google Scholar]
- 19.Sveom D.S., Otteman M.K., Garvin K.L. Improving quality and decreasing cost by reducing Re-admissions in patients undergoing total joint arthroplasty. Curr Rev Musculoskelet Med. 2017;10(3):388–396. doi: 10.1007/s12178-017-9424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podmore B., Hutchings A., van der Meulen J., Aggarwal A., Konan S. Impact of comorbid conditions on outcomes of hip and knee replacement surgery: a systematic review and meta-analysis. BMJ Open. 2018;8(7) doi: 10.1136/bmjopen-2018-021784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whelton P.K., Carey R.M., Aronow W.S. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Collins G., Reitsma J., Altman D., Moons K. Members of Tg. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. Eur Urol. 2015;67(6):1142–1151. doi: 10.1016/j.eururo.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 23.Moons K.G., de Groot J.A., Bouwmeester W. Critical appraisal and data extraction for systematic reviews of prediction modelling studies: the CHARMS checklist. PLoS Med. 2014;11(10) doi: 10.1371/journal.pmed.1001744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CASP E. 2006. Critical appraisal skills programme clinical prediction rule checklist; pp. 1–6.https://casp-uk.net/wp-content/uploads/2018/01/CASP-Clinical-Prediction-Rule-Checklist_2018.pdf [Google Scholar]
- 25.Kim S. Changes in surgical loads and economic burden of hip and knee replacements in the US: 1997-2004. Arthritis Rheum. 2008;59(4):481–488. doi: 10.1002/art.23525. [DOI] [PubMed] [Google Scholar]
- 26.Basques B.A., Bohl D.D., Golinvaux N.S., Leslie M.P., Baumgaertner M.R., Grauer J.N. Postoperative length of stay and 30-day readmission after geriatric hip fracture: an analysis of 8434 patients. J Orthop Trauma. 2015;29(3):e115–120. doi: 10.1097/BOT.0000000000000222. [DOI] [PubMed] [Google Scholar]
- 27.Cantrell C.K., DeBell H.A., Lehtonen E.J. Risk factors for readmission within thirty days following revision total hip arthroplasty. J Clin Orthop Trauma. 2018;11(1):38–42. doi: 10.1016/j.jcot.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clement R.C., Derman P.B., Graham D.S. Risk factors, causes, and the economic implications of unplanned readmissions following total hip arthroplasty. J Arthroplasty. 2013;28(8 Suppl):7–10. doi: 10.1016/j.arth.2013.04.055. [DOI] [PubMed] [Google Scholar]
- 29.French D.D., Bass E., Bradham D.D., Campbell R.R., Rubenstein L.Z. Rehospitalization after hip fracture: predictors and prognosis from a national veterans study. J Am Geriatr Soc. 2008;56(4):705–710. doi: 10.1111/j.1532-5415.2007.01479.x. [DOI] [PubMed] [Google Scholar]
- 30.Higuera C.A., Elsharkawy K., Klika A.K., Brocone M., Barsoum W.K. 2010 Mid-America Orthopaedic Association Physician in Training Award: predictors of early adverse outcomes after knee and hip arthroplasty in geriatric patients. Clin Orthop Relat Res. 2011;469(5):1391–1400. doi: 10.1007/s11999-011-1804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kimball C.C., Nichols C.I., Nunley R.M., Vose J.G., Stambough J.B. Skilled nursing facility star rating, patient outcomes, and readmission risk after total joint arthroplasty. J Arthroplasty. 2018;33(10):3130–3137. doi: 10.1016/j.arth.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 32.Martin C.T., Gao Y., Pugely A.J. Incidence and risk factors for 30-day readmissions after hip fracture surgery. Iowa Orthop J. 2016;36:155–160. [PMC free article] [PubMed] [Google Scholar]
- 33.Mednick R.E., Alvi H.M., Krishnan V., Lovecchio F., Manning D.W. Factors affecting readmission rates following primary total hip arthroplasty. J Bone Jt Surg Am Vol. 2014;96(14):1201–1209. doi: 10.2106/JBJS.M.00556. [DOI] [PubMed] [Google Scholar]
- 34.Mesko N.W., Bachmann K.R., Kovacevic D., LoGrasso M.E., O'Rourke C., Froimson M.I. Thirty-day readmission following total hip and knee arthroplasty - a preliminary single institution predictive model. J Arthroplasty. 2014;29(8):1532–1538. doi: 10.1016/j.arth.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 35.Molina C.S., Thakore R.V., Blumer A., Obremskey W.T., Sethi M.K. Use of the national surgical quality improvement Program in orthopaedic surgery. Clin Orthop Relat Res. 2015;473(5):1574–1581. doi: 10.1007/s11999-014-3597-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pugely A.J., Callaghan J.J., Martin C.T., Cram P., Gao Y. Incidence of and risk factors for 30-day readmission following elective primary total joint arthroplasty: analysis from the ACS-NSQIP. J Arthroplasty. 2013;28(9):1499–1504. doi: 10.1016/j.arth.2013.06.032. [DOI] [PubMed] [Google Scholar]
- 37.Ricciardi B.F., Oi K.K., Daines S.B., Lee Y.Y., Joseph A.D., Westrich G.H. Patient and perioperative variables affecting 30-day readmission for surgical complications after hip and knee arthroplasties: a matched cohort study. J Arthroplasty. 2017;32(4):1074–1079. doi: 10.1016/j.arth.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Schairer W.W., Sing D.C., Vail T.P., Bozic K.J. Causes and frequency of unplanned hospital readmission after total hip arthroplasty. Clin Orthop Relat Res. 2014;472(2):464–470. doi: 10.1007/s11999-013-3121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah C.K., Keswani A., Chi D., Sher A., Koenig K.M., Moucha C.S. Nonelective primary total hip arthroplasty: the effect of discharge destination on postdischarge outcomes. J Arthroplasty. 2017;32(8):2363–2369. doi: 10.1016/j.arth.2017.03.042. [DOI] [PubMed] [Google Scholar]
- 40.Sher A., Keswani A., Yao D.H., Anderson M., Koenig K., Moucha C.S. Predictors of same-day discharge in primary total joint arthroplasty patients and risk factors for post-discharge complications. J Arthroplasty. 2017;32(9):S150–+. doi: 10.1016/j.arth.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Siracuse B.L., Chamberlain R.S. A preoperative scale for determining surgical readmission risk after total hip replacement. JAMA Surg. 2016;151(8):701–709. doi: 10.1001/jamasurg.2016.0020. [DOI] [PubMed] [Google Scholar]
- 42.Stavrakis A.I., SooHoo N.F., Lieberman J.R. Bilateral total hip arthroplasty has similar complication rates to unilateral total hip arthroplasty. J Arthroplasty. 2015;30(7):1211–1214. doi: 10.1016/j.arth.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 43.Tiberi J.V., 3rd, Hansen V., El-Abbadi N., Bedair H. Increased complication rates after hip and knee arthroplasty in patients with cirrhosis of the liver. Clin Orthop. 2014;472(9):2774–2778. doi: 10.1007/s11999-014-3681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White R.S., Sastow D.L., Gaber-Baylis L.K., Tangel V., Fisher A.D., Turnbull Z.A. Readmission rates and diagnoses following total hip replacement in relation to insurance payer status, race and ethnicity, and income status. J Racial Ethn Health Disparities. 2018;5(6):1202–1214. doi: 10.1007/s40615-018-0467-0. [DOI] [PubMed] [Google Scholar]
- 45.Yao D.H., Keswani A., Shah C.K., Sher A., Koenig K.M., Moucha C.S. Home discharge after primary elective total joint arthroplasty: postdischarge complication timing and risk factor Analysis. J Arthroplasty. 2017;32(2):375–380. doi: 10.1016/j.arth.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Zmistowski B., Restrepo C., Hess J., Adibi D., Cangoz S., Parvizi J. Unplanned readmission after total joint arthroplasty: rates, reasons, and risk factors. J Bone Jt Surg Am Vol. 2013;95(20):1869–1876. doi: 10.2106/JBJS.L.00679. [DOI] [PubMed] [Google Scholar]
- 47.Ali A.M., Loeffler M.D., Aylin P., Bottle A. Factors associated with 30-day readmission after primary total hip arthroplasty analysis of 514 455 procedures in the UK national health service. Jama Surg. 2017;152(12) doi: 10.1001/jamasurg.2017.3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heyes G.J., Tucker A., Marley D., Foster A. Predictors for readmission up to 1 Year following hip fracture. Arch Plus. 2015;4(2) doi: 10.5812/atr.4(2)2015.27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khan M.A., Hossain F.S., Dashti Z., Muthukumar N. Causes and predictors of early re-admission after surgery for a fracture of the hip. J Bone Joint Surg. 2012;94(5):690–697. doi: 10.1302/0301-620X.94B5.28933. [DOI] [PubMed] [Google Scholar]
- 50.Weiss R.J., Garellick G., Kärrholm J., Hailer N.P. Total hip arthroplasty in 6690 patients with inflammatory arthritis: effect of medical comorbidities and age on early mortality. J Rheumatol. 2016;43(7):1320–1327. doi: 10.3899/jrheum.151287. [DOI] [PubMed] [Google Scholar]
- 51.Sofu H., Üçpunar H., Çamurcu Y. Predictive factors for early hospital readmission and 1-year mortality in elder patients following surgical treatment of a hip fracture. Ulusal Travma ve Acil Cerrahi Dergisi. 2017;23(3):245–250. doi: 10.5505/tjtes.2016.84404. [DOI] [PubMed] [Google Scholar]
- 52.Iorio R., Osmani F.A. Strategies to prevent periprosthetic joint infection after total knee arthroplasty and lessen the risk of readmission for the patient. J Am Acad Orthop Surg. 2017;25:S13–S16. doi: 10.5435/JAAOS-D-16-00635. [DOI] [PubMed] [Google Scholar]
- 53.Siddiqi A., White P.B., Etcheson J.I. Acute kidney injury after total knee arthroplasty: a clinical review. Surg Technol Int. 2017;31:243–252. [PubMed] [Google Scholar]
- 54.Boddapati V., Fu M., Su E., Sculco P., Bini S., Mayman D. Preoperative corticosteroid use for medical conditions is associated with increased postoperative infectious complications and readmissions after total hip arthroplasty: a propensity-matched study. Am J Orthoped. 2018;47(12) doi: 10.12788/ajo.2018.0104. [DOI] [PubMed] [Google Scholar]
- 55.Durant S., Duval D., Homo-Delarche F. Factors involved in the control of fibroblast proliferation by glucocorticoids: a review. Endocr Rev. 1986;7(3):254–269. doi: 10.1210/edrv-7-3-254. [DOI] [PubMed] [Google Scholar]
- 56.Aubert C., Folly A., Mancinetti M., Hayoz D., Donzé J. Association of stress biomarkers with 30-day unplanned readmission and death. J Hosp Med. 2017;12(7):523–529. doi: 10.12788/jhm.2766. [DOI] [PubMed] [Google Scholar]
- 57.Swenson E.R., Bastian N.D., Nembhard H.B., Davis C.M. Reducing cost drivers in total joint arthroplasty: understanding patient readmission risk and supply cost. Health Syst. 2018;7(2):135–147. doi: 10.1080/20476965.2017.1397237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scarborough J.E., Bennett K.M., Englum B.R., Pappas T.N., Lagoo-Deenadayalan S.A. The impact of functional dependency on outcomes after complex general and vascular surgery. Ann Surg. 2015;261(3):432. doi: 10.1097/SLA.0000000000000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hatipoğlu U., Wells B.J., Chagin K., Joshi D., Milinovich A., Rothberg M.B. Predicting 30-day all-cause readmission risk for subjects admitted with pneumonia at the point of care. Respir Care. 2018;63(1):43–49. doi: 10.4187/respcare.05719. [DOI] [PubMed] [Google Scholar]
- 60.Woodfield J.C., Jamil W., Sagar P.M. Incidence and significance of postoperative complications occurring between discharge and 30 days: a prospective cohort study. J Surg Res. 2016;206(1):77–82. doi: 10.1016/j.jss.2016.06.073. [DOI] [PubMed] [Google Scholar]
- 61.Slyepchenko A., Maes M., Jacka F.N. Gut microbiota, bacterial translocation, and interactions with diet: pathophysiological links between major depressive disorder and non-communicable medical comorbidities. Psychother Psychosom. 2017;86(1):31–46. doi: 10.1159/000448957. [DOI] [PubMed] [Google Scholar]
- 62.Britteon P., Cullum N., Sutton M. Association between psychological health and wound complications after surgery. Br J Surg. 2017;104(6):769–776. doi: 10.1002/bjs.10474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.