Highlights
-
•
The role of thoracic control for lung cancer has become increasingly important with improvements in lung cancer treatment and patient survival. Thoracic re-irradiation became feasible with the advance and adaptation of radiation technology.
-
•
Our study retrospectively collected data related to clinicopathological features and treatment outcomes of 50 patients who had two courses of thoracic RT in at least 50% overlapping fields. A promissing local control with median time to local progression of 18 months and a median overall survival of 25.1 months were found.
-
•
We also found that subsequent chemotherapy with taxanes- or gemcitabine-based regimen following thoracic re-irradiation was significantly related to lethal lung events.
-
•
These results helped prove the feasibility of re-irradiation with conventionally fractionated RT and increase awareness regarding underestimated lung toxicity resulting from subsequent chemotherapy following thoracic re-irradiation.
Keywords: Lung cancer, Locoregional recurrence, Thoracic radiotherapy, Re-irradiation
Abstract
Background and purpose
Thoracic re-irradiation may be an alternative treatment for lung cancer patients who develop intrathoracic locoregional recurrence without systemic progression. This study aimed to retrospectively assess locoregional control, clinical outcomes, and toxicities in lung cancer patients who received thoracic re-irradiation.
Materials and methods
We retrospectively reviewed 50 lung cancer patients who received thoracic re-irradiation using conventional photon radiotherapy (RT) and stereotactic body radiotherapy (SBRT) between 2009 and 2017. The correlations of clinicopathologic factors, treatment factors, and dosimetric factors of RT with time to local progression (TTLP), progression-free survival (PFS), and overall survival (OS) after starting thoracic re-irradiation were calculated using log-rank tests and Cox regression models.
Results
The median re-irradiation dose in equivalent dose in 2-Gy fractions was 51.1 Gy, and the mean re-irradiation planning target volume was 201.58 ml. The median mean lung dose (MLD) was 4.18 Gy, and the total lung volumes receiving a dose of 5 Gy (lung V5) and of 20 Gy (V20) were 19.8% and 5.85%, respectively. The TTLP, PFS, and OS were 18.0, 5.9, and 25.1 months, respectively. Lung V5 (p < 0.001), V20 (p = 0.011), and MLD (p = 0.002) were significantly associated with grade ≥2 lung toxicity. Seven (14%) patients developed lethal lung events. Subsequent chemotherapy following thoracic re-irradiation was significantly correlated with lethal lung events (p = 0.009).
Conclusion
Promising local control can be achieved with thoracic re-irradiation in lung cancer patients with locoregional recurrence. However, unexpected lethal lung events may occur, especially in patients receiving systemic therapy following thoracic re-irradiation.
1. Introduction
Thoracic radiotherapy (RT) has been shown to play an important role in the treatment of both non-small-cell lung cancer (NSCLC) and small-cell lung cancer (SCLC) [1], [2]. RT can serve as a definitive treatment for early-stage NSCLC in the form of stereotactic body radiation therapy (SBRT) [3]. Chemoradiotherapy (CCRT) is also the standard definitive treatment for locally advanced NSCLC and limited SCLC [4], [5], [6], [7]. In addition to curative treatment for localized disease, recent investigations revealed that thoracic RT can improve progression-free survival (PFS) after systemic control for stage IV NSCLC [8], [9] and even prolong overall survival (OS) for extensive-stage SCLC [10].
Although the effectiveness of thoracic RT has been established for lung cancer, around 35% of patients with locally advanced lung cancer experienced locoregional recurrence after definitive CCRT [11]. Furthermore, 25% of patients with lung cancer have isolated locoregional recurrence [12] and are considered potentially curable without distant disease failure. However, most patients who experience locoregional recurrences are not eligible for surgical resection due to comorbidities and mediastinal lymphadenopathies. With improvements in RT techniques in the modern era, including intensity-modulated radiation therapy (IMRT), SBRT, and proton therapy, thoracic re-irradiation is now a possible choice for local treatment of patients with intrathoracic recurrence after thoracic RT who are not candidates for surgical approaches [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. De Bari et al. [16] reported that thoracic re-irradiation with SBRT provided good control rates of 70–90% at 2 years as well as an acceptable toxicity profile of 3%–28% of grade ≥3 pulmonary toxicities. Nevertheless, SBRT is generally administered to limited patients with small tumor volumes and relative peripheral lesions and may not suitable for regional nodal recurrence.
Considering the institutional feasibility, tumor recurrence patterns, and patient characteristics, re-irradiation with traditional photon therapy is more common in daily practice; however, reports of the results of this treatment are scarce. Although several retrospective studies with small cohorts have reported the clinical outcomes of photon thoracic re-irradiation [23], [24], [25], the optimal RT dose, toxicities, and clinical outcomes remain unclear.
We retrospectively analyzed the toxicities and clinical outcomes of patients with NSCLC or SCLC who received thoracic re-irradiation using photon therapy. We also assessed the relationship between clinical features, RT dose distributions in lung and treatment factors, and “lethal lung events”, defined as death due to a lung-related event which might not be indicated by the common definitions of bacteria-associated pneumonitis and disease progression. Furthermore, we evaluated the association between clinicopathological features; treatment factors; dosimetric factors of RT; and time to local progression (TTLP), PFS, and OS of patients after thoracic re-irradiation.
2. Materials and methods
2.1. Patients
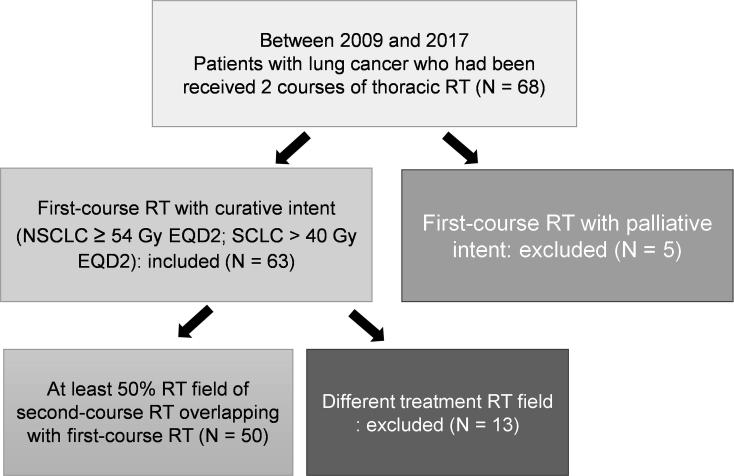
This retrospective study was approved by the Institutional Research Ethics Committee of our hospital (Institutional review board (IRB) number: 201707036RIND). The patients’ medical data were anonymized before access and analysis. Patients with lung cancer, including NSCLC and SCLC, who had received thoracic RT with curative intent and experienced locoregional recurrence and subsequently received a second course of thoracic irradiation between April 2009 and February 2017 were enrolled. In this study, patients who received neoadjuvant, definitive, and adjuvant RT had all been enrolled. The first course of definitive RT with curative intent was defined as an RT dose at least 54 Gy for NSCLC and 40 Gy for SCLC in 2-Gy dose equivalent (EQD2) with an alpha-beta ratio of 10 Gy. At least 50% field overlap of RT was required for the two courses. Patient selection is illustrated in Fig. 1.
Fig. 1.
Flowcharts of patients with lung cancer receiving thoracic re-irradiation selected for data analysis (RT, radiotherapy; N, number; NSCLC, non-small cell lung cancer; SCLC, small cell lung cancer; EQD2, equivalent total doses in 2-Gy fractions).
After excluding ineligible patients, 50 patients who met the inclusion criteria were enrolled for further analysis. All 50 patients underwent chest, abdomen, and brain computed tomography (CT) to detect local recurrence and exclude extra-thoracic progression before receiving thoracic re-irradiation. Furthermore, 17 patents underwent PET and 11 underwent biopsy to confirm intrathoracic recurrence. Age, gender, presence of extra-thoracic disease, treatment details of both RT courses, and use of systemic therapy (type and timing) were recorded. Concurrent systemic therapy was defined as an overlap between systemic treatment and RT. Subsequent systemic therapy was defined as further medical anti-cancer treatment within 3 months of completing thoracic re-irradiation.
3. Radiation treatment
For all radiotherapy plans, CT images and dosimetric data for both tumor and the organs at risk were required for assessment. For treatment techniques, three-dimensional (3D) conformal, IMRT, or volumetric arc therapy (VMAT)/rapid arc were allowed for both courses of RT. Patients who received RT with a 2D plan were excluded due to the lack of dosimetric information. For dose comparison, RT dose was transformed to EQD2, as mentioned above. For patients receiving 3D conformal RT, IMRT, and VMAT/rapid arc, gross tumor volume (GTV) was defined as the volume encompassing the gross lung tumor and/or involved lymph nodes. In this retrospective study, the definition of clinical target volume (CTV) was according to the physician’s opinion and practice. Generally, the range of CTV was defined as GTV plus 0.5 to 1 cm margin (included subclinical involved region), with and without regional (selective) lymph nodes. Patients who received elective nodal irradiation were not included in this study. The planning target volume (PTV) was defined as the CTV plus a margin of 0.5–0.8 cm for set-up uncertainty and respiratory motion. For patients receiving SBRT, the PTV was the internal target volume (ITV) plus a 0.3-cm margin.
4. Response and toxicity evaluation
After the second course of RT, the patients received follow-up chest imaging every 2 to 3 months or when clinically indicated. Treatment response was assessed according to the revised Response Evaluation Criteria in Solid Tumors (RECIST) guidelines, version 1.1 [26]. Acute and late toxicities were evaluated using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [27].
This study defined lethal lung events as unexpected lung-related deaths caused by possible post-treatment toxicity, including events which might not have been recorded by CTCAE. A lethal lung event was defined as death within 1 year post-re-irradiation caused by radiation pneumonitis, lung events related to non-confirmed disease progression, or suspicious pneumonia without definite culture results. Deaths caused by definite disease progression (solitary lesion enlargement meeting RECIST criteria, or biopsy-proven) or pathogen-proven bacteria-associated pneumonia were excluded from this definition.
4.1. Statistical analysis
The chi-square or Fisher’s exact test was used for the analysis of categorical data. Independent t-tests were used to compare continuous variables, while Mann–Whitney U-tests were applied to ordinal, nonparametric data. TTLP was defined as the time from the date of starting thoracic re-irradiation to locoregional thoracic failure, recurrence, or any cause of death. PFS was calculated from the date of re-irradiation to locoregional recurrence, distant metastases, or any cause of death. OS was defined as the time from the date of starting thoracic re-irradiation until death. The Kaplan–Meier method was used to estimate survival curves, and differences between patient or treatment characteristics were assessed by log-rank tests. Hazard analyses of TTLP, OS, and PFS were performed through forward stepwise regression using the Cox regression model. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY). Two-sided p-values less than 0.05 were considered statistically significant.
5. Results
5.1. Patients and treatment characteristics
The median follow-up time was 7.9 months (range, 0.4 to 106.0 months) and the median interval between the first- and second-course of RT was 13 months (range 4.3 to 53.3 months). Squamous cell carcinoma and adenocarcinoma accounted for 46% and 32% of cases, while 8% of the patients were diagnosed with SCLC. Most lesions (86%) were characterized as central lesions, defined as within 2 cm around the proximal bronchial tree [28]. At the time of re-irradiation, 68% of patients had no extra-thoracic disease. Concurrent systemic therapy with re-irradiation and subsequent systemic therapy were administered to 18% (n = 9) and 42% (n = 21) of patients, respectively. The patient characteristics are summarized in Table 1.
Table 1.
Patient characteristics receiving thoracic re-irradiation.
| All patients N = 50 | Number | Percentage (%) |
|---|---|---|
| Median age | 65 (years) | |
| Gender | ||
| Male | 40 | 80% |
| Female | 10 | 20% |
| Histology | ||
| Adenocarcinoma | 16 | 32% |
| Squamous cell carcinoma | 23 | 46% |
| Small cell carcinoma | 4 | 8% |
| Others | 7 | 14% |
| Tumor location | ||
| Central | 43 | 86% |
| Peripheral | 7 | 14% |
| Interval between 2 RT courses (median months) | 13 | |
| Previous lung surgery | ||
| Yes | 20 | 40% |
| No | 30 | 60% |
| Presence of extra-thoracic disease | ||
| Yes | 16 | 32% |
| No | 34 | 68% |
| Systemic therapy | ||
| Concurrent systemic therapy (C/T/Target therapy/Immunotherapy) | 9 (7/1/1) | 18% |
| Subsequent systemic therapy (C/T/Target therapy/Immunotherapy) | 21 (18/3/1) | 42% |
Abbreviations: N, number of patients; C/T, chemotherapy.
The median re-irradiation dose in EQD2 for first and second courses of RT were 60.0 and 51.1 Gy, respectively. Regarding the treatment volume and field-location in the first course of thoracic RT, 26 of 50 patients received upper-lung field irradiation, 9 received pure lower-lung field irradiation, and the remaining 15 received mainly hilum or mediastinal irradiation, or mixed upper and lower-lung field irradiation. For the first course thoracic RT, the mean PTV volume was 529.24 ml (range 104.1–1690.8 ml), 24 out of 50 patients received 3DRT, and the rest received IMRT/VMAT (Supplementary Table 1). All patients had conventional radiation treatment at the first course (range, 1.8 to 2.2 Gy).
Among 50 patients who received thoracic re-irradiation, 15 received RT for lung tumor alone, 22 received RT for lung tumor with mediastinal and ipsilateral supraclavicular lymphatics, 12 received RT for regional lymphadenopathies only, and one received RT for mediastinal lymphadenopathies and adjacent chest wall lesions. The median re-irradiation fraction size was 2.84 Gy (range, 2 to 15 Gy). The mean PTV of re-irradiation was 201.58 ml (range, 12.8–1180.0 ml) and the median mean lung dose of re-irradiation was 4.18 Gy (range, 0.50 to 13.71 Gy). The detailed RT technique, RT dose, and lung dose for re-irradiation and the cumulative EQD2 for two RT courses are listed in Table 2. An example of treatment response of patients who received thoracic re-irradiation is shown in Fig. 2. The examples of two cases showing the accumulated dose EQD2 to the PTV and the accumulated dose of lung, heart, and mediastinum are illustrated in Supplementary Fig. 1.
Table 2.
Radiation treatment details for thoracic re-irradiation.
| All patients N = 50 | Values (range) |
|---|---|
| Radiation dose | |
| Second RT median dose in EQD2 (Gy) | 51.1 (16.2–125.0) |
| Median fraction size of re-irradiation | 2.84 (2–15) |
| Conventional/hypofractionated/SBRT | 11/31/8 |
| Cumulative dose in EQD2 (Gy) (median) | 106.1 (74.2–195.0) |
| Radiation technique | |
| 3D conformal RT | 7 |
| Modern technique | 43 |
| IMRT/VMAT or Rapid Arc/Tomotherapy | 15/23/5 |
| PTV volume (mean, ml) | 201.58 (12.8–1180.0) |
| Lung dose (Median value) of re-irradiation (Gy) | |
| Mean dose | 4.18 (0.5–13.71) |
| V5 | 19.80 (5.0–68.0) |
| V20 | 5.85 (0–30.0) |
| Mean heart dose (median) | 3.32 (0.06–19.83) |
Abbreviations: N, number of patients; EQD2, dose in equivalent dose in 2 Gy fractions; RT, radiotherapy; SBRT, stereotactic body radiotherapy; IMRT, intensity modulated radiation therapy; VMAT, volumetric arc therapy; Vdose (V5 and V20), percentage of total normal lung volume receiving equal to or greater than the designated dose (Gy) of radiation.
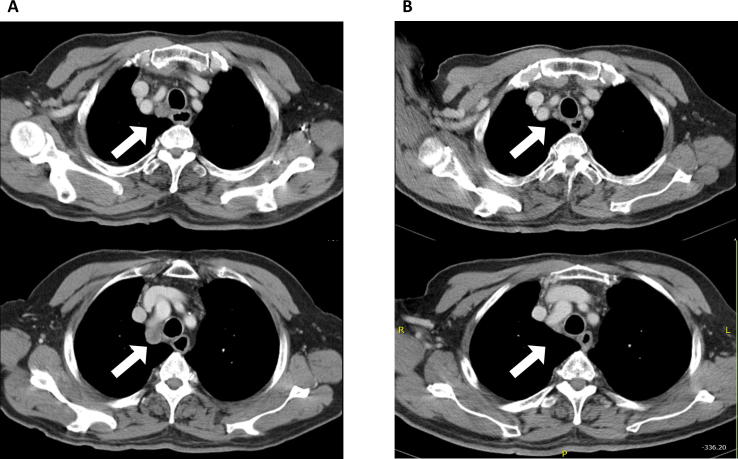
Fig. 2.
An example of treatment response of thoracic re-irradiation in a 83-year-old man with lung adenocarcinoma who experienced recurrences of mediastinal lymphadenopathies 11 months after receiving thoracic irradiation for his right lower lung, right hilum and mediastinal lymphatics (A) Pre-treatment chest computed tomography (CT) scan of re-irradiation showed mediastinal lymphadenopathies of this patient. (B) Two months after completing thoracic re-irradiation with 50 Gy in 20 fractions, this patient achieved near complete remission via chest CT scan images.
6. Clinical treatment outcomes
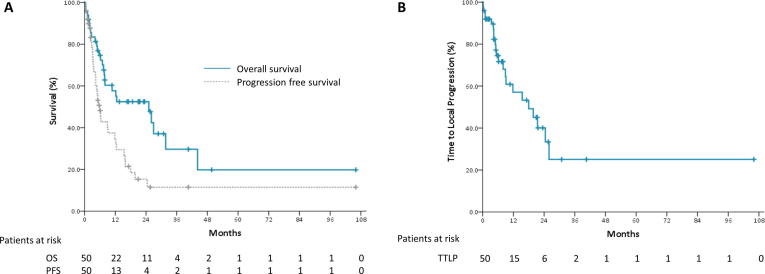
The median TTLP, PFS, and OS were 18.0, 5.9, and 25.1 months, respectively (Fig. 3). The OS and PFS are shown in Fig. 3A and TTLP is shown in Fig. 3B. Evaluation of the factors associated with local control showed that tumor histology (p = 0.955), re-irradiation dose (p = 0.619), and concurrent or subsequent systemic therapies (p = 0.825 and p = 0.767, respectively) were not associated with TTLP. Patients with local disease progression had a non-significant larger PTV than patients without local progression (264.05 vs. 150.68 ml, p = 0.124). A worse OS was significantly correlated with the presence of extra-thoracic disease (p = 0.014) at the time of re-irradiation and was not related to other clinical and dosimetric factors.
Fig. 3.
Clinical outcomes of patients after starting thoracic re-irradiation. (A) Progression-free survival (PFS) and overall survival (OS). (B) Time to local progression (TTLP).
Multivariate analysis showed that no specific prognostic factors were associated with TTLP (Table 3). Furthermore, the presence of extra-lung disease was significantly associated with poor PFS (hazard ratio [HR] = 2.854, 95% confidence interval [CI] = 1.198–6.801, p = 0.018) but not OS (p = 0.200). However, histologic subtype, RT technology, patient gender, concurrent chemotherapy, and PTV volumes were not associated with PFS or OS. In the multivariate analyses (Table 3), subsequent chemotherapy used was marginally associated with poor OS (HR = 2.055, 95% CI = 0.886–4.770, p = 0.094), whereas the higher re-irradiation dose was marginally associated with better OS (HR = 0.965, 95% CI = 0.926–1.006, p = 0.093). However, both subsequent chemotherapy used and re-irradiation dose were not correlated with PFS (Table 3).
Table 3.
Multivariate Analysis for factors associated with time to local progression, progression free survival, and overall survival.
| Time to Local Progression |
Progression-Free Survival |
Overall Survival |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | CI | p-value | HR | CI | p-value | HR | CI | p-value | |
| Extra-Lung disease (Presence/Absence) | 0.660 | 0.186–2.348 | 0.521 | 2.854 | 1.198–6.801 | 0.018 | 1.935 | 0.705–5.312 | 0.200 |
| Histology (Others/Adenocarcinoma) | 0.957 | 0.319–2.877 | 0.938 | 0.654 | 0.387–1.813 | 0.654 | 0.684 | 0.240–1.947 | 0.477 |
| RT technology (Modern/3D) | 0.397 | 0.074–2.145 | 0.283 | 1.178 | 0.356–3.897 | 0.788 | 0.971 | 0.223–2.230 | 0.969 |
| Gender (Female/Male) | 1.378 | 0.422–4.501 | 0.596 | 1.119 | 0.446–2.811 | 0.810 | 1.551 | 0.515–4.673 | 0.435 |
| Age (years) | 0.979 | 0.944–1.015 | 0.252 | 1.000 | 0.973–1.027 | 0.974 | 0.999 | 0.962–1.037 | 0.952 |
| Concurrent chemotherapy (Yes/No) | 0.773 | 0.200–2.987 | 0.709 | 0.989 | 0.407–2.404 | 0.980 | 2.452 | 0.794–7.575 | 0.119 |
| Subsequent chemotherapy (Yes/No) | 0.873 | 0.328–2.323 | 0.786 | 1.368 | 0.714–2.623 | 0.345 | 2.055 | 0.886–4.770 | 0.094 |
| Re-irradiation dose (Gy) | 0.982 | 0.945–1.019 | 0.332 | 0.979 | 0.951–1.008 | 0.149 | 0.965 | 0.926–1.006 | 0.093 |
| Second RT PTV volume (ml) | 1.001 | 0.999–1.004 | 0.205 | 0.999 | 0.997–1.001 | 0.178 | 0.999 | 0.996–1.001 | 0.163 |
Abbreviations: HR, hazard ratio; CI, confidence interval; RT, radiotherapy; Gy, gray.
7. Lung toxicity and lethal lung events
We evaluated dosimetric and clinical factors associated with lung toxicity. Twenty-four patients (48%) experienced grade ≥ 2 lung toxicity, among which 13 patients (26%) developed grade ≥ 3 toxicities. Higher lung V5 (p < 0.001), V20 (p = 0.011), and mean lung dose (p = 0.002) were significantly associated with higher incidences of grade ≥ 2 lung toxicity, respectively. However, RT interval, age, re-irradiation dose, fraction size, and PTV volume for the first or second course RT were not related to grade 2 and above lung toxicity. In addition, the location of lower-lobe irradiation (n = 17) did not increase the risk of grade ≥ 2 lung toxicity (56.3% vs. 31.3%, p = 0.102) compared with the upper-lobe irradiation (n = 33) in patients who received thoracic re-irradiation.
Seven of 50 (14%) patients had “lethal lung events” by definition. Subsequent chemotherapy following thoracic re-irradiation was significantly related to lethal lung events (p = 0.009) while re-irradiation dose, previous lung surgery, lung dose (mean lung dose, V5, and V20), tumor location, RT interval between the two RT courses, PTV volume for the first or second course RT, and RT prescription dose were not (Table 4). However, patients with lethal lung events had paradoxically lower cumulative EQD2 of two RT courses compared with those without lethal lung events (96.1 vs. 112.8, p = 0.095) (Table 4), indicating that there was no a threshold for cumulative dose of RT associated with incidence of lethal lung events in our cohort.
Table 4.
Factors associated with lethal lung events.
| All patients N = 50 | Patients with lethal lung events (N = 7) | Patients without lethal lung events (N = 43) | P-value |
|---|---|---|---|
| Gender (Male/Female) | 5/2 | 35/8 | 0.616 |
| Age (mean) | 64.4 | 65.6 | 0.818 |
| Previous lung surgery (Yes/No) | 2/5 | 18/25 | 0.687 |
| Interval between 2 courses RT (months) | 18.7 m | 18.4 m | 0.949 |
| Tumor location (Central/Peripheral) | 7/0 | 36/7 | 0.573 |
| 1st RT PTV volume (ml) | 475.3 | 540.7 | 0.675 |
| 2nd RT PTV volume (ml) | 294.1 | 186.2 | 0.269 |
| Lung V5 (%) | 29.4% | 22.1% | 0.174 |
| Lung V20 (%) | 11.1% | 7.5% | 0.234 |
| Mean lung dose (Gy) | 6.3 Gy | 4.7 Gy | 0.184 |
| Concurrent C/T (Yes/No) | 2/5 | 7/36 | 0.595 |
| Subsequent C/T (Yes/No) | 6/1 | 13/30 | 0.009 |
| Cumulative EQD2 for two course RT (Gy) | 96.1 | 112.8 | 0.095 |
Abbreviations: N, number of patients; RT, radiotherapy; Vdose (V5 and V20), percentage of total normal lung volume receiving equal to or greater than the designated dose (Gy) of radiation; C/T. chemotherapy.
Among the 18 patients who received subsequent chemotherapy after thoracic re-irradiation, 8 received taxotere or paclitaxel containing regimen, 3 received gemcitabine, and 7 received pemetrexed or platinum-based chemotherapy. The taxanes- and gemcitabine-based chemotherapy regimens were reported to have radiation-enhancing effects and were also associated with a higher risk of radiation toxicity of lung [29], [30]. We compared the incidence of lethal lung events for patients who received subsequent chemotherapy with and without taxanes-based regimen. We found that patients receiving taxanes-based subsequent chemotherapy had higher rates of lethal lung event than those not receiving taxanes-based regimens; however, a statistical significance was not achieved (37.5% vs. 20%, p = 0.608). If we considered gemcitabine, 36.4% of patients who received taxanes- or gemcitabine-based subsequent chemotherapy had lethal lung events compared to 14.3% of patients who received other chemotherapy regimens (p = 0.596). Three patients received subsequent EGFR tyrosine kinase inhibitor (TKI) and one patient received subsequent pembrolizumab. Both of the aforementioned regimens were not related to toxicity or outcome. Concurrent chemotherapy was not related to lethal lung events (Table 4). Subsequent systemic status was not related to extra-thoracic disease status at the time of re-irradiation.
8. Discussion
With prolonged survival of lung cancer patients, loco-regional control has become as important as distant control since the lungs are vital organs and symptomatic lung lesions might be life-threating. In patients with locoregional recurrence after thoracic RT, thoracic re-irradiation has become a challenging issue with improvements in locoregional control and subsequent survival benefits. In this study, we demonstrated that thoracic re-irradiation using modern RT techniques, including 3DRT, IMRT, VMAT/Arc, and SBRT, provided a median TTLP of 18.0 months, a median OS of 25.1 months, and acceptable pulmonary toxicities (26% grade ≥ 3 toxicities). Our results are concordant with those of other studies, which showed a median time to progression of 10 months (ranged 4.5 to 16 months) and mean OS of 17.7 months (ranged 11.1 to 24 months) [22]. In another study evaluating the high-dose thoracic re-irradiation of 24 patients with lung cancer, Griffioen et al. [25] reported that conventionally fractionated RT resulted in a median event-free survival (EFS) of 8.4 months and median OS of 13.5 months.
Considering the physical and radiobiological advantages, proton beam therapy (PBT) reduces treatment toxicity and allows dose intensification in the treatment of lung cancer [31], [32], [33]. Chang et al. [33] reported convincing results, including an objective low pneumonitis rate and late grades 2 (16%) and 3 pneumonitis (12%) rates in a phase II trial to test the feasibility of PBT concurrent with chemotherapy. Regarding thoracic re-irradiation using PBT, McAvoy et al. [34] reported that 7 (21%) of 22 patients developed grade ≥ 3 toxicities with a median dose of 66 Gy (relative biological effectiveness, RBE) divided in 33 fractions. In addition, Ho et al. [35] reported that only two (7%) patients experienced grade ≥ 3 lung toxicity among 27 patients receiving intensity-modulated proton therapy (IMPT) for re-irradiation of thoracic malignancies, indicating that IMPT may be a potential way to further reduce treatment toxicity. In the silico trial comparing the RT dose to the target volume and organ at risk of 24 NSCLC patients who received thoracic re-irradiation using different photon and proton technique, Troost et al. [36] demonstrated that IMPT provided similar high dose to target lesions compared with IMRT, VMAT, tomotherapy, and cyberknife, but significantly less dose to organ at risk.
SBRT is another method for thoracic re-irradiation. SBRT has been proven to be effective and is the standard of care in inoperable early-stage lung cancer [3], [37]. However, in re-irradiation for lung cancer, late toxicity of SBRT, varying from 3% to 28%, is a concern [17], [18], [19], [20], [21], [23]. Cumulative dose in corresponding critical organs has been used to predict the pulmonary toxicity of SBRT in re-irradiation of thoracic malignancies [18], [24]. Liu et al. [18] reported that V20 above 30% in patients administered SBRT was associated with a risk of grade ≥3 radiation pneumonitis. Binkley et al. [24] conducted a comprehensive planning study to evaluate the correlation between toxicity and RT planning factors, reporting that only the tumor location (central or peripheral) was associated with grade ≥2 pulmonary toxicity in patients treated with photon re-irradiation, including conventional RT and SBRT. However, the limitation of SBRT in lung cancer is tumor size and location. In addition, patient characteristics and indications and treatment decisions to perform SBRT might differ among institutions. These differences might explain the varying reports of toxicity for re-irradiation with SBRT in retrospective series.
As discussed above, SBRT re-irradiation is confined to limited patient groups, and PBT is not available in every institution. Most patients with locoregional recurrence face challenge with re-irradiation with conventional photon therapy. Data on thoracic re-irradiation with conventional RT are scarce and usually have limited numbers of few patients [22], [23], [24], [25], [38], [39], [40], [41]. In one Japanese series treated by 3DRT, 21% of patients experienced grade ≥3 lung toxicity [38]. Another single-institution study reported that 1 (4.8%) of 21 patients experienced grade 3 acute radiation pneumonitis after re-irradiation and that the V5 of the composite plans (HR = 7.398, p = 0.023) and overlapping V5 area in two courses of RT (p = 0.041) were independent predictors for grade ≥3 radiation pneumonitis [39]. In our series, 24 (48%) out of 50 patients developed grade 2 and above lung toxicity associated with mean lung dose, lung V5, and V20, comparable to previous reports.
Consolidative chemotherapy after definitive chemoradiation for NSCLC was reported to associated with higher rates of lung toxicity [42], [43]. However, there was no strong evidence suggesting the impact of subsequent chemotherapy on post thoracic re-irradiation-related lung toxicity. To our knowledge, this is the first study to focus on systemic treatment and its relation to lung toxicity in patients administered thoracic re-irradiation. In the present study, chemotherapy following re-irradiation was significantly correlated with lethal lung-related events (p = 0.009), while the dosimetric factors and patient’s factors were not (Table 3). Taxanes or gemcitabine containing regimens were associated with non-significant higher chancer of lethal lung event (36.4% vs. 14.3%, p = 0.596) compared to other chemotherapy regimens. Since this is a retrospective investigation, the concern was raised that disease progression and worse underlying disease status at the time of thoracic irradiation might be correlated to patient survival. To eliminate the bias, we evaluated the correlation between subsequent chemotherapy and TTLP and PFS after starting re-irradiation. The results showed no significant correlation. These findings suggested that unexpected lung-related death post-re-irradiation might be related to the subsequent use of chemotherapy.
The main limitation of this study was the retrospective setting and limited number of patients. We were unable to perform composite plans for two courses of RT as some patients had incomplete RT planning data. In addition, the anatomical changes between two RT courses made the summation of planning information unreliable. We compromised by selecting patients administered high-dose RT (median EQD2 at least 60 Gy) as the first course to reduce baseline heterogeneity. These patients were treated in a tertiary medical center; thus, our results may not be extrapolated to other patient groups. The number of patients receiving targeted therapy and immunotherapy was low and not analyzed in this study.
9. Conclusion
The results of this study demonstrated that photon thoracic re-irradiation using modern RT techniques provided good local control, with a median TTLP of 18 months and survival benefit with a median OS of 25.1 months. However, toxicity requires caution, with 14% of patients dying because of unexpected lung-related deaths. The administration of thoracic re-irradiation should be carefully considered, especially with the use of subsequent taxanes- or gemcitabine-based chemotherapy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the Cancer Registry, Office of Medical Records, National Taiwan University Hospital, for providing the necessary patient information. This research was funded by Ministry of Science and Technology, Taiwan, No. MOST 107-2314-B-002-217-MY3; and National Taiwan University Hospital, Taiwan, No. NTUH 109-S4716.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2020.03.008.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Postmus P.E., Kerr K.M., Oudkerk M., Senan S., Waller D.A., Vansteenkiste J. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv1-iv21. doi: 10.1093/annonc/mdx222. [DOI] [PubMed] [Google Scholar]
- 2.Früh M, De Ruysscher D, Popat S, Crinò L, Peters S, Felip E, et al. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi99-105. [DOI] [PubMed]
- 3.Timmerman R., Paulus R., Galvin J., Michalski J., Straube W., Bradley J. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aupérin A., Le Péchoux C., Pignon J.P., Koning C., Jeremic B., Clamon G. Concomita nt radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol. 2006;17:473–483. doi: 10.1093/annonc/mdj117. [DOI] [PubMed] [Google Scholar]
- 5.Turrisi A.T., 3rd, Kim K., Blum R., Sause W.T., Livingston R.B., Komaki R. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 6.Bradley J.D., Paulus R., Komaki R., Masters G., Blumenschein G., Schild S. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faivre-Finn C., Snee M., Ashcroft L., Appel W., Barlesi F., Bhatnagar A. Concurrent once-daily versus twice-daily chemoradiotherapy in patients with limited-stage small-cell lung cancer (CONVERT): an open-label, phase 3, randomised, superiority trial. Lancet Oncol. 2017;18:1116–1125. doi: 10.1016/S1470-2045(17)30318-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyengar P., Wardak Z., Gerber D.E., Tumati V., Ahn C., Hughes R.S. Consolidative radiotherapy for limited metastatic non-small-cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2017.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang Y., Yang J.C., Hsu F.M., Chen Y.H., Shih J.Y., Lin Z.Z. The response, outcome and toxicity of aggressive palliative thoracic radiotherapy for metastatic non-small cell lung cancer patients with controlled extrathoracic diseases. PLoS ONE. 2015;10 doi: 10.1371/journal.pone.0145936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slotman B.J., van Tinteren H., Praag J.O., Knegjens J.L., El Sharouni S.Y., Hatton M. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015;385:36–42. doi: 10.1016/S0140-6736(14)61085-0. [DOI] [PubMed] [Google Scholar]
- 11.Aupérin A., Le Péchoux C., Rolland E., Curran W.J., Furuse K., Fournel P. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 12.Curran WJ, Jr., Paulus R, Langer CJ, Komaki R, Lee JS, Hauser S, et al. Sequential vs. concurrent chemoradiation for stage III non-small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst; 103: 1452–1460. [DOI] [PMC free article] [PubMed]
- 13.Chao H.H., Berman A.T., Simone C.B., 2nd, Ciunci C., Gabriel P., Lin H. Multi-institutional prospective study of reirradiation with proton beam radiotherapy for locoregionally recurrent non-small cell lung cancer. J Thorac Oncol. 2017;12:281–292. doi: 10.1016/j.jtho.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 14.McAvoy S., Ciura K., Wei C., Rineer J., Liao Z., Chang J.Y. Definitive reirradiation for locoregionally recurrent non-small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: predictors of high-grade toxicity and survival outcomes. Int J Radiat Oncol Biol Phys. 2014;90:819–827. doi: 10.1016/j.ijrobp.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 15.Trakul N., Harris J.P., Le Q.T., Hara W.Y., Maxim P.G., Loo B.W., Jr Stereotactic ablative radiotherapy for reirradiation of locally recurrent lung tumors. J Thorac Oncol. 2012;7:1462–1465. doi: 10.1097/JTO.0b013e31825f22ce. [DOI] [PubMed] [Google Scholar]
- 16.De Bari B., Filippi A.R., Mazzola R., Bonomo P., Trovò M., Livi L. Available evidence on re-irradiation with stereotactic ablative radiotherapy following high-dose previous thoracic radiotherapy for lung malignancies. Cancer Treat Rev. 2015;41:511–518. doi: 10.1016/j.ctrv.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Kilburn J.M., Kuremsky J.G., Blackstock A.W., Munley M.T., Kearns W.T., Hinson W.H. Thoracic re-irradiation using stereotactic body radiotherapy (SBRT) techniques as first or second course of treatment. Radiother Oncol. 2014;110:505–510. doi: 10.1016/j.radonc.2013.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H., Zhang X., Vinogradskiy Y.Y., Swisher S.G., Komaki R., Chang J.Y. Predicting radiation pneumonitis after stereotactic ablative radiation therapy in patients previously treated with conventional thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:1017–1023. doi: 10.1016/j.ijrobp.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meijneke T.R., Petit S.F., Wentzler D., Hoogeman M., Nuyttens J.J. Reirradiation and stereotactic radiotherapy for tumors in the lung: dose summation and toxicity. Radiother Oncol. 2013;107:423–427. doi: 10.1016/j.radonc.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 20.Peulen H., Karlsson K., Lindberg K., Tullgren O., Baumann P., Lax I. Toxicity after reirradiation of pulmonary tumours with stereotactic body radiotherapy. Radiother Oncol. 2011;101:260–266. doi: 10.1016/j.radonc.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Trovo M., Minatel E., Durofil E., Polesel J., Avanzo M., Baresic T. Stereotactic body radiation therapy for re-irradiation of persistent or recurrent non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;88:1114–1119. doi: 10.1016/j.ijrobp.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 22.De Ruysscher D., Faivre-Finn C., Le Pechoux C., van de Ven P.M., Slotman B.J., Senan S. High-dose re-irradiation following radical radiotherapy for non-small-cell lung cancer. Lancet Oncol. 2014;15:e620–e624. doi: 10.1016/S1470-2045(14)70345-6. [DOI] [PubMed] [Google Scholar]
- 23.Kruser T.J., McCabe B.P., Mehta M.P., Khuntia D., Campbell T.C., Geye H.M. Reirradiation for locoregionally recurrent lung cancer: outcomes in small cell and non-small cell lung carcinoma. Am J Clin Oncol. 2014;37:70–76. doi: 10.1097/COC.0b013e31826b9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Binkley M.S., Hiniker S.M., Chaudhuri A., Maxim P.G., Diehn M., Loo B.W., Jr Dosimetric factors and toxicity in highly conformal thoracic reirradiation. Int J Radiat Oncol Biol Phys. 2016;94:808–815. doi: 10.1016/j.ijrobp.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Griffioen G.H., Dahele M., de Haan P.F. High-dose, conventionally fractionated thoracic reirradiation for lung tumors. Lung Cancer. 2014;83:356–362. doi: 10.1016/j.lungcan.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIS guideline (version 1.1). Eur J Cancer. 2009; 45: 228-47. [DOI] [PubMed]
- 27.Bethesda, MD, US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. National Cancer Institute (2009) Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed 28 May 2009.
- 28.Timmerman R., McGarry R., Yiannoutsos C., Papiez L., Tudor K., DeLuca J. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833–4839. doi: 10.1200/JCO.2006.07.5937. [DOI] [PubMed] [Google Scholar]
- 29.Palma D.A., Senan S., Tsujino K., Barriger R.B., Rengan R., Moreno M. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeter M.D., Jänne P.A., Brooks S., Burstein H.J., Wen P., Fuchs C.S. Gemcitabine-induced radiation recall. Int J Radiat Oncol Biol Phys. 2002;53:394–400. doi: 10.1016/s0360-3016(02)02773-6. [DOI] [PubMed] [Google Scholar]
- 31.Higgins K.A., O'Connell K., Liu Y., Gillespie T.W., McDonald M.W., Pillai R.N. National cancer database analysis of proton versus photon radiation therapy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2017;97:128–137. doi: 10.1016/j.ijrobp.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Chang J.Y., Jabbour S.K., De Ruysscher D., Schild S.E., Simone C.B., 2nd, Rengan R. Consensus statement on proton therapy in early-stage and locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2016;95:505–516. doi: 10.1016/j.ijrobp.2016.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang J.Y., Verma V., Li M., Zhang W., Komaki R., Lu C. Proton beam radiotherapy and concurrent chemotherapy for unresectable stage III non-small cell lung cancer: final results of a phase 2 study. JAMA Oncol. 2017;3 doi: 10.1001/jamaoncol.2017.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAvoy S.A., Ciura K.T., Rineer J.M., Allen P.K., Liao Z., Chang J.Y. Feasibility of proton beam therapy for reirradiation of locoregionally recurrent non-small cell lung cancer. Radiother Oncol. 2013;109:38–44. doi: 10.1016/j.radonc.2013.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Ho J.C., Nguyen Q.N., Li H., Allen P.K., Zhang X., Liao Z. Reirradiation of thoracic cancers with intensity modulated proton therapy. Pract Radiat Oncol. 2018;8:58–65. doi: 10.1016/j.prro.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Troost E.G.C., Wink K.C.J., Roelofs E., Simone C.B., 2nd, Makocki S., Löck S. Photons or protons for reirradiation in (non-)small cell lung cancer: results of the multicentric ROCOCO in silico study. Br J Radiol. 2020;93:20190879. doi: 10.1259/bjr.20190879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Videtic G.M., Hu C., Singh A.K., Chang J.Y., Parker W., Olivier K.R. A randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer: NRG oncology RTOG 0915 (NCCTG N0927) Int J Radiat Oncol Biol Phys. 2015;93:757–764. doi: 10.1016/j.ijrobp.2015.07.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okamoto Y., Murakami M., Yoden E., Sasaki R., Okuno Y., Nakajima T. Reirradiation for locally recurrent lung cancer previously treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2002;1(52):390–396. doi: 10.1016/s0360-3016(01)02644-x. [DOI] [PubMed] [Google Scholar]
- 39.Sumita K., Harada H., Asakura H., Ogawa H., Onoe T., Murayama S. Re-irradiation for locoregionally recurrent tumors of the thorax: a single-institution, retrospective study. Radiat Oncol. 2016;11:104. doi: 10.1186/s13014-016-0673-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ren C., Ji T., Liu T., Dang J., Li G. The risk and predictors for severe radiation pneumonitis in lung cancer patients treated with thoracic reirradiation. Radiat Oncol. 2018;13:69. doi: 10.1186/s13014-018-1016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshitake T., Shioyama Y., Nakamura K., Sasaki T., Ohga S., Shinoto M. Definitive fractionated re-irradiation for local recurrence following stereotactic body radiotherapy for primary lung cancer. Anticancer Res. 2013;33:5649–5653. [PubMed] [Google Scholar]
- 42.Hanna N., Neubauer M., Yiannoutsos C., McGarry R., Arseneau J., Ansari R. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol. 2008;26:5755–5760. doi: 10.1200/JCO.2008.17.7840. [DOI] [PubMed] [Google Scholar]
- 43.Ahn J.S., Ahn Y.C., Kim J.H., Lee C.G., Cho E.K., Lee K.C. Multinational randomized phase III trial with or without consolidation chemotherapy using docetaxel and cisplatin after concurrent chemoradiation in inoperable stage III non-small-cell lung cancer: KCSG-LU05-04. J Clin Oncol. 2015;33:2660–2666. doi: 10.1200/JCO.2014.60.0130. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.