Abstract
Electrical stimulation (ES) is predominantly used as a physical therapy modality to promote tissue healing and functional recovery. Research efforts in both laboratory and clinical settings have shown the beneficial effects of this technique for the repair and regeneration of damaged tissues, which include muscle, bone, skin, nerve, tendons, and ligaments. The collective findings of these studies suggest ES enhances cell proliferation, extracellular matrix (ECM) production, secretion of several cytokines, and vasculature development leading to better tissue regeneration in multiple tissues. However, there is still a gap in the clinical relevance for ES to better repair tissue interfaces, as ES applied clinically is ineffective on deeper tissue. The use of a conducting material can transmit the stimulation applied from skin electrodes to the desired tissue and lead to an increased function on the repair of that tissue. Ionically conductive (IC) polymeric scaffolds in conjunction with ES may provide solutions to utilize this approach effectively. Injectable IC formulations and their scaffolds may provide solutions for applying ES into difficult to reach tissue types to enable tissue repair and regeneration. A better understanding of ES-mediated cell differentiation and associated molecular mechanisms including the immune response will allow standardization of procedures applicable for the next generation of regenerative medicine. ES, along with the use of IC scaffolds is more than sufficient for use as a treatment option for single tissue healing and may fulfill a role in interfacing multiple tissue types during the repair process.
Keywords: Electrical stimulation, Conductive polymers, Ionic conductivity, Tissue engineering, Muscle, Tendon, Ligament, Nerve, Bone and wound healing
Graphical abstract
Highlights
-
•
Electrical stimulation (ES) is used as a physical therapy modality to facilitate functional rehabilitation.
-
•
ES enhances cell proliferation, extracellular matrix (ECM) synthesis, cytokines production, and vasculature development in tissues.
-
•
Beneficial effects of ES in research and clinics is evident for muscle, bone, skin, nerve, tendons and ligaments.
-
•
Ionically conductive (IC) polymers carry charges due to counter flow of ions under ES.
-
•
ES in combination with IC polymeric structures may provide alternative means for tissue repair and regeneration.
1. Introduction
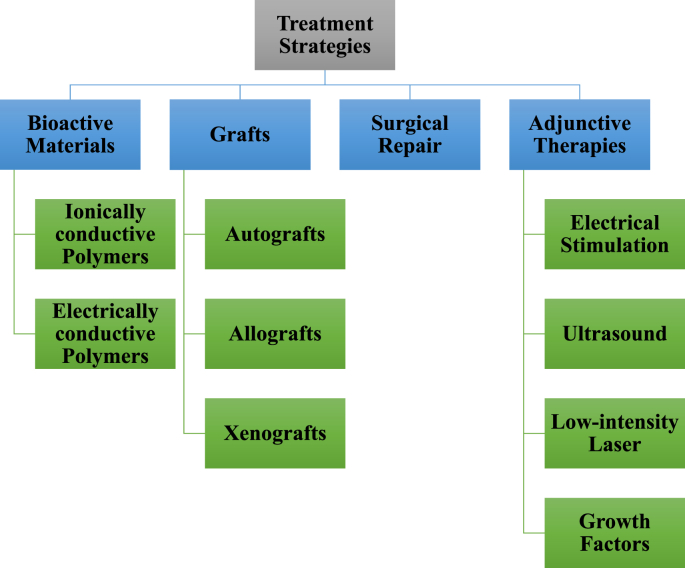
Tissue engineering is a significant field emerging in the world of health care research. This discipline aims to provide new and efficient ways to treat a multitude of clinical injuries, ranging from bone non-unions to nerve transections. Modern treatments for common tissue defects include autografts, using harvested tissue from elsewhere in the patient's body; allografts, using harvested tissue from other patients; or on rare occasions xenografts, using tissues harvested from other species [[1], [2], [3], [4], [5], [6]]. There are many downsides with these approaches, including donor site morbidity, risk of infection, and scarcity of donor material [[6], [7], [8], [9], [10], [11], [12], [13]]. For these reasons, tissue engineering efforts continue to look towards the development of alternative therapeutic options including those summarized in Fig. 1. A common goal of tissue engineering is to develop a scaffold suitable for cell growth, which can then be stimulated and manipulated towards a desired tissue type. The scaffold used must be mechanically competent for the forces present in the relevant tissue, non-immunogenic, biocompatible with the endogenous tissue, and must degrade in the body over time. A variety of cells can be used, including cells derived from natural tissues, specific progenitor cells, as well as various potencies of stem cells [[14], [15], [16]]. An array of stimuli has been used to cause the differentiation and integration of the tissue engineered system into an implantation site. Two examples of external stimuli that remain the focus of ongoing investigations are mechanical and electrical stimulation (ES) [14,15,[17], [18], [19], [20]]. These methods of external stimuli result in differentiation by promoting the release of growth factors and bimolecular signals [16,21].
Fig. 1.
Summary of treatment strategies for musculoskeletal tissues. Most of these modalities are either at various stages of FDA approval or proven effective in pre-clinical models. Biological grafts continue to be the standard treatment modality.
Growth factors play an important role in tissue regeneration. It has been shown that the independent and direct use of exogenous growth factors is an effective means to induce cellular healing and regeneration [22]. While the exogenous use of growth factors has its advantages, adjunctive use of ES for tissue regeneration is perhaps a superior modality, given that side effects are markedly limited for ES. Limitations of growth factors include cost, complications of drug delivery such as avoiding growth factor delivery bolus, and questions of safety [22]. The benefits of ES on excitable tissues, including neurons and muscle tissue, have been studied [23]. ES possesses the ability to promote and direct neurite outgrowth, incite muscle contractions, and promote bone growth [18,[24], [25], [26]]. Moreover, ES has the ability to enhance the maturation and differentiation of cells [17,18]. Numerous methods for delivering ES exist, including surface electrodes and direct tissue stimulation; however, none provide researchers with the ability to deliver ES with high specificity. For this reason, a suitable graft material has been sought that has the ability to conduct and distribute ES to targeted cells while also fulfilling the requirements of a tissue-engineered construct.
Electroactive materials that conduct applied electric potential are appropriate materials for the delivery of ES. Conducting polymers with conjugated pi-bond systems on their backbones lead to a large chain of loosely bound electrons [27]. Once reduced or oxidized by a dopant molecule, this system allows polymers to have high electron mobility [[28], [29], [30]]. As described in Shirakawa et al. iodine vapor was used as a doping agent to oxidize polyacetylene and increase its conductivity 10 million-fold [31,32]. Alternatively, ionically conductive (IC) polymers can be generated on any polymeric biomaterial by introducing ionic functional groups such as carboxylic, sulfonic, phosphonic, and amine, to name a few. This class of materials conducts electricity in the physiologic environment via counter flow of ions upon application of ES [33,34]. Unlike electronically conducting thermoset polymers like polyaniline, IC polymers exhibit properties of the parent polymers chosen for ionic functionalization. Polymers can be chosen to improve overall physicochemical properties leading to improved biocompatibility and degradation profiles [35].
In our previous work, we showed the use of IC polymers and their conductive properties for nerve regeneration [35]. The purpose of this review will be to analyze the current state of conductive materials used in conjunction with ES for tissue regeneration. In addition, this review will provide an in-depth assessment on the effects of ES on major tissues in the body, including how electroactive materials have been used to implement these effects. An analysis of the cellular mechanisms and theories as to how ES works will be discussed, and both in vitro and in vivo studies will be reviewed. It is important to note that other ongoing tissue engineering strategies exist, including ultrasound and laser therapies, which are beyond the scope of this review [36].
2. ES through conductive materials
The production of functional biological substitutes results from the combination of scaffolds, cells and biological molecules, and plays an important role in tissue repair and regeneration in different tissue engineering applications. The properties of biomaterial scaffolds make them to serve as matrices for cell adhesion and it enhances the interactions between biomaterials and cells, as well as further regulate their activities such as proliferation and differentiation. Natural polymers such as chitosan, gelatin, collage, alginate and so on and synthetic polymers including polylactide (PLA), poly(lactic-co-glycolic acid) (PLGA), polycaprolactone (PCL), poly(glycerol sebacate), and polyurethane (PU) are the dominant biomaterials as scaffolds for tissue engineering [37]. Conducting biomaterials include the hybrid biomaterials with aforementioned polymers with carbon nanotubes, carbon nanowires, graphene, and metallic particle (e.g. gold nanoparticle) have been widely investigated in biosensor and bone tissue engineering applications due to their high electrical conductivity [37]. However, drawbacks like non-biodegradability, problems of unknown long-term in vivo toxicity, and inhomogeneous distribution of the conductive particles in the composite system lead to non-reliable conductive properties limited their widespread and successful use.
The use of conductive scaffolds in tissue regeneration provides a unique and attractive new option for delivering localized ES at the implantation site. Their inherent conductance allows for the transmittance of cell based ionic cues during cellular processes that can direct cells to align in specific directions and can promote their differentiation in various tissue types [[38], [39], [40], [41]]. Application of ES has been shown to enhance bone growth and fusion, as well as accelerate axon regeneration [42,43]. The potential of using conductive polymers in conjunction with ES treatment increases the efficiency of ES by better delivering the current to the target damaged tissue. Due to conductive polymers making ES more efficient, a lower stimulus can be applied to the target tissue for the same or greater regenerative effect. While non-conductive biomaterials would still be acceptable as scaffolds for regenerating damaged tissue, they would not have the synergy with amplifying ES treatment that conductive biomaterials have. However, drawbacks like non-biodegradability, problems of unknown long-term in vivo toxicity, and inhomogeneous distribution of the conductive particles in the composite system limited their widespread and successful use.
Conducting polymers such as polypyrrole (PPy), polyaniline (PANI) and polythiophene derivatives are the new generation of organic materials exhibiting electrical and optical properties like metals and inorganic semiconductors, but also displaying properties including ease of synthesis and processing flexibility [44]. The exact potential and advantage of these conductive polymers desired for tissue engineering and regenerative medicine is based on their conductivity, reversible oxidation, redox stability, biocompatibility, hydrophobicity, three-dimensional geometry and surface topography. Also, their ability to control a range of physical and chemical properties electronically by (i) surface functionalization techniques, and (ii) the use of a wide range of molecules that can be trapped or used as dopants [37]. In many regenerative medicine approaches, including neural and cardiac tissue engineering, the importance of conducting polymeric material as nanocomposites could be used to host cell growth so that electrical stimulation can be applied directly to cells through the composite [44]. In comparison to other conductive materials for biological applications, conducting polymers are cheap, simple to synthesize, and flexible. Additionally, conducting polymers provide control over the intensity and length of electrical stimulation in various tissue engineering applications. Table-1 details the properties, conductivity, and doping method for key conductive polymers and relates them to their tissue regenerative applications [44].
Table 1.
Comparison of the conductive polymers used in different tissue regeneration. The Maximum conductivity, doping type, and material properties are listed for the main conductive polymers used in tissue regeneration.
| Polymeric Material | Maximum conductivity (S/cm) | Types of doping | Properties | Applications |
|---|---|---|---|---|
| Polypyrrole (PPy) | 40–200 | P | Highly conductive, opaque, Brittle, Amorphous structure | Nerve, cardiac and Bone tissue engineering application |
| Polythiophene (PT) | 10–100 | P | Good electrical conductivity and optical property | Biosensors and Food Industry |
| Polyaniline (PANI) | 5, bulk films (upto 100 S/m) | n, p | Semiflexible polymer requires simple doping/de-doping chemistry | Nerve and Cardiac tissue engineering |
2.1. Applying ES
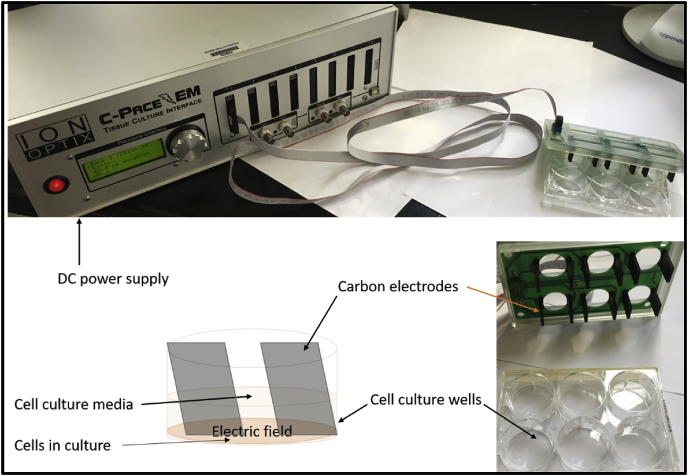
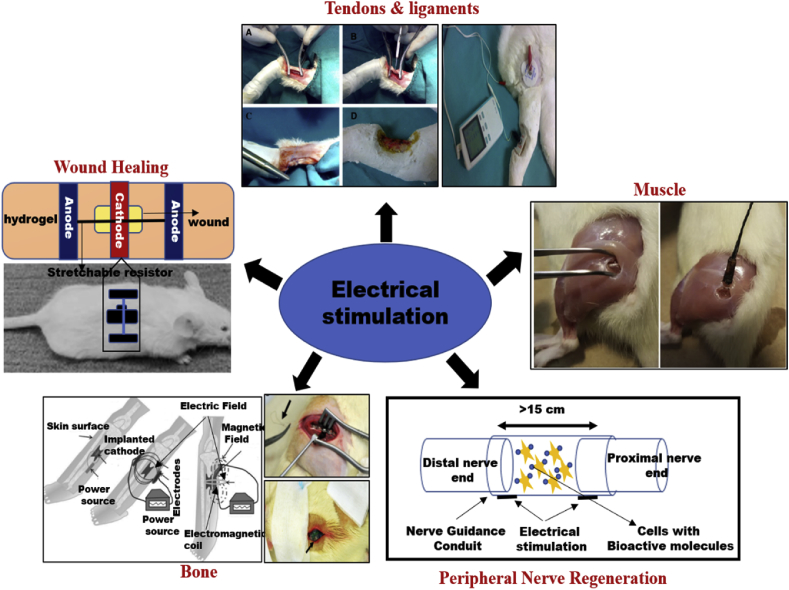
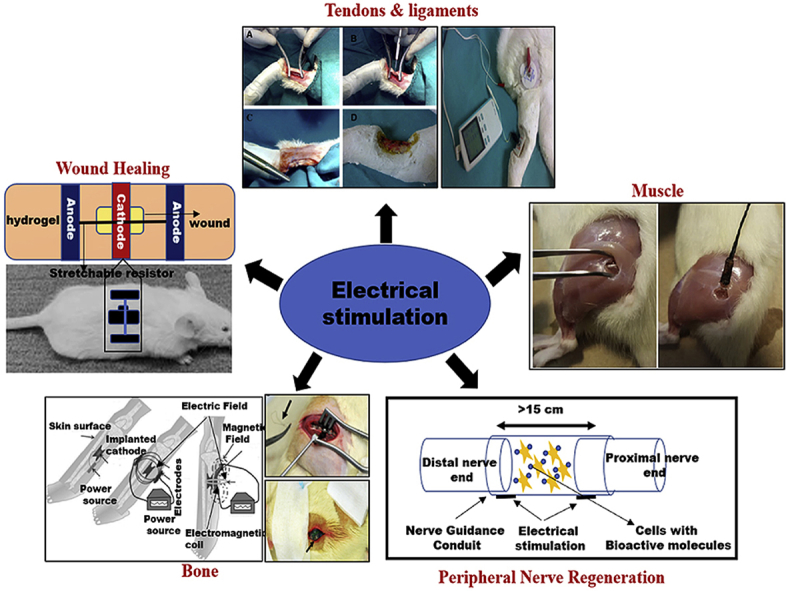
There are various ways to apply ES to both conductive scaffolds and directly on tissues. Fig. 2 displays a schematic for applying ES to study the effect on cells in vitro. Commonly used techniques that can be performed without a scaffold or substrate include capacitive coupling, direct coupling, inductive coupling, or a combination thereof [23]. Fig. 3 summarizes some of the examples of ES application in pre-clinical animal models. Capacitive coupling involves the placement of two cutaneous electrodes on opposite sides of the target tissue. Direct coupling requires the placement of a cathode at the injury site with a complementary anode in surrounding soft tissue. Finally, inductive coupling does not allow for a well-controlled distribution of the ES to desired cell targets, nor do they provide a biocompatible platform for those cells. Further, other limitations exist. Since direct coupling commonly involves the direct placement of an electrode, it can be invasive and cause local imbalances in pH. Other techniques like capacitive coupling require high voltages to work which can be harmful to cells. Polymer scaffolds specifically made with conductive materials are a popular way to counteract these issues.
Fig. 2.
An example of a typical commercially available ES system for in vitro cell culture experiments. Carbon electrodes deploy the applied potential to cells on a substrate in the tissue culture plates and the power module allows different treatment conditions. Many researchers and laboratories continue to use custom built stimulators and parameters to study cell-material interactions and phenotype development under the influence of ES.
Fig. 3.
Summary of pre-clinical studies for the repair and regeneration of musculoskeletal tissues and wound healing [24,35,102,150,151]. Most of the ES studies rely on signal transduction from dermis nerves to applied skin electrodes for stimulation and measurement. Several studies report use of standard percutaneous ES using 10 mm diameter Silver/Silver Chloride (Ag/AgCl) electrodes (3 M Company) to enable ES. The cathode is placed over the implanted graft and the anode is placed over the connective tissue.
2.2. Conductive polymers and other materials
Conductive polymers are those typically defined by their ability to easily move electrons within and between their chains [31]. Typical conductive polymers achieve this through pi-bond conjugation. Electrons bound in pi-bonds are much less tightly held than those in sigma-bonds. Conjugation of these bonds leads to pi-bond orbital overlap which facilitates delocalization of charge, enabling conduction [27]. Further, these polymers must be fabricated with a dopant in order to convert them from their neutral state into a charged and conducting one [26,45,46]. This can come in the form of an n-type, reducing dopant, which donates an electron to the system or a p-type, oxidizing dopant that effectively removes an electron from the system [47,48]. Typically, dopants are integrated into the lattice structure by the use of strong acids containing different aromatic substitutions or the use of amphiphilic dopants such as sulfonic acid [27]. The most studied conductive polymer to date is polypyrrole (PPY) [27,31,38,[49], [50], [51], [52]]. Dopants greatly impact the resulting oxidation state of conductive matrices such as PPY leading to impacts on thermal stability and differences in conductivity up to 102 [53]. Other well-documented conductive polymers include polyaniline (PANI) and polythiophene derivatives, namely poly(3,4-ethylenedioxythiophene) (PEDOT) [26,50,54]. All of these polymers have displayed the ability to effectively deliver ES to target cells. On the other hand, these polymers also share two serious disadvantages. First, conductive polymers are in general thermosets and hence not easily processed after fabrication, not degradable, and often brittle. This greatly limits their versatility and applicability. Non-biodegradable PANI implants have even been shown to cause a degree of inflammation that requires surgical removal [54]. Therefore, newer materials and strategies are required for the effective utilization of ES in biomedical applications.
Polymeric composite structures doped with metallic nanoparticles and carbon fillers offers an attractive means to deliver ES [27]. Doing so imparts the conductivity of the polymer into a system that may already be biodegradable and pliable. Another approach is to modify the backbone of natural polymers with either cationic or anionic functional groups [55]. Among the carbon fillers, carbon nanotubes (CNTs) and graphene have gained much attention as composite approaches to deliver ES. Graphene is used in this application due to the fact that it possesses great conduction and accompanying electron mobility, can be easily dispersed and functionalized, and has good chemical and thermal stability [56,57]. A common method for composite formation is the addition of a conductive filler to a natural and biocompatible hydrogel system [58]. One example of this is a PPY/alginate hydrogel composite created by Yang et al. for the purpose of neural tissue engineering [38]. This system was created by polymerization of PPY with alginate hydrogels. As a result, the hybrid scaffold was shown to promote mesenchymal stem cell adhesion and growth as well as promote neural differentiation of these stem cells. It should be noted that the PPY contained within the system still produced a mild inflammatory effect [38]. Another instance of this motif was a system created by Sayyar et al. that contained conductive graphene filler within a chitosan/lactic acid hydrogel [59]. This system was a classic case that highlighted the purpose of using a composite. Addition of the graphene changed the chitosan-based hydrogel from its base insulating form to a conducting material. Moreover, graphene alone can be toxic to cells as it can penetrate the cell membrane and cause oxidative stress if it accumulates [59]. This negative effect was shown to be mitigated by using low concentrations and suspension in a hydrogel [59].
Composites have proven effective at mitigating the advantages and disadvantages of conductive materials. The use of modified chitosan scaffolds is currently being studied. Sulfonated chitosan in particular generates a lot of interest due to their hemocompatible properties, as they prevent the formation of blood clots by nature of their anti-thrombogenic characteristics and lack of platelet adhesion [60,61]. Sulfonation as a doping mechanism increases conductivity of polymers and maintains the polymers’ conductive properties for longer periods of time. Our previous work investigated the electrical conductivity of sulfonated IC polymers. Table-2 summarizes the ionic conductivity of sulfonated poly (methyl vinyl ether-alt-maleic anhydride)/poly vinyl alcohol (SPMVEMA/PVA), poly (ether ether ketone) (SPEEK), poly (ether sulfone) (SPES) and poly (phenylene oxide) (SPPO) matrices. Ionic conductivity of these matrices is directly proportional to its ion exchange capacity (IEC) and water content [62]. These IC polymers such as ionic hydrogels [chitosan] have an additional utility as a replacement or insulator of conventional electrical electrodes [63]. They could potentially deliver an electric stimulus to the tissue while having less of a negative inflammatory outcome caused by both metal and carbon-based electrodes. These hydrogel-based ionic conductors separate the traditional electrode from the biological tissues. And they also dissipate the heat generated by the current from the electrodes due to their high-water content. This protects the target tissue from negative electrochemical and thermal reactions. Due to their high and sustained conductivity, IC polymers confer overcoming limitations of other materials in ES applications. They function by conducting electrical charges via the flow of counter ions in the physiological environment and can be synthesized to carry positive (cation exchange) or negative (anion exchange) charges, thus increasing the versatility of IC polymers for use as electrodes [64].
Table 2.
Examples of ionically conductive (IC) polymers bearing sulfonic groups previously used by the Kumbar laboratory [62]. IEC = ion exchange capacity; SPEEK = sulfonated poly-ether ether ketone; sulfonated SPES = poly (ether sulfone); SPPO = sulfonated poly (phenylene oxide); SPMVEMA/PVA = sulfonated poly (methyl vinyl ether-alt-maleic anhydride)/poly vinyl alcohol.
| Polymers | % water content | IEC m equiv./g | Membrane conductivity mS/cm |
|---|---|---|---|
| SPEEK | 31.87 | 1.100 | 35.39 |
| SPES | 20.00 | 0.308 | 10.29 |
| SPPO | 48.00 | 1.75 | 53.55 |
| SPMVEMA/PVA | 38.5 | 0.447 | 29.51 |
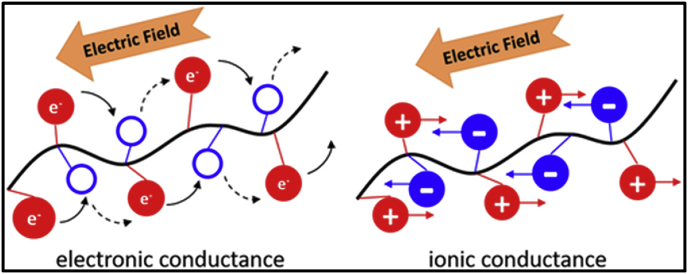
It is also important to note that this conductivity profile remains constant in physiological solutions over a period of several weeks while electronically conducting polymers such as PANI and PPY declines in a few weeks as a result of their reduction [62]. The differential mode of conduction of electricity for both IC and electronically conducting polymers is presented in Fig. 4. For instance, IC polymers conduct electricity through the counter flow of positive and negatively charged ions rather than electrons [33]. The use of electrical stimulation has a mechanistic effect on the alignment, migration and proliferation of cells.
Fig. 4.
Pictorial representation of current flow in electronically conductive and ionically conductive materials. IC polymers rely on the counter flow of ions to conduct electricity in physiological environments as opposed to electron movement in conducting polymers [33].
3. The mechanistic effects of stimulation
3.1. Effects of electrical stimulation on cell alignment
ES direction plays a significant role in aligning and redirecting random cells to the direction in relation to the electric field direction applied. There are various types of cells that align perpendicularly and parallel to electric field vector direction to minimize the field gradient across the cells. Cardiac adipose tissue-derived progenitor cells, endothelial progenitor cells, vascular ECs, BMSCs, adipose-derived stromal cells are the different types of cells that line up themselves perpendicularly. The cells which align parallel to the applied effects of electrical stimulation are the ventricular myocytes, cardiomyocytes, myoblasts, PC-12 cells, and osteoblasts. Typically, the intensity of the ES is < 10V/cm, and the cells aligned better with the intensity of the ES, but the activity of the cells is decreased comparatively [64].
3.2. Effects of electrical stimulation on cell migration
The cell migration also relies on the electrical field applied along with the cell alignment. ES′ guiding effect on cell migration often depends on the type of cell, and electrotaxis is called the method of directing the cells [65]. The following cells are drawn to the cathode and are NSCs, macrophages, mouse neural precursor cells (NPCs), osteoblasts, and endothelial progenitor cells, while the cell types such as BMSCs, human dermal fibroblasts, and SCs are at the anode. The ES intensity can induce cell migration from a minimum of 0.1V/cm to a maximum of 12V/cm and did not result in significant cell damage, did not affect the cell phenotype or the potential for differentiation. At the same time, cells with higher ES intensity showed increasingly increased migration rate and size. It is still uncertain what mechanism contributes to electrotaxis. Other factors may be considered, such as endogenous microenvironment, ion channels, membrane receptors, transportation proteins and competing signal pathways such as Wnt/GSK3β and TGFβ1/ERK/NF-ÿB [66]. In addition to signal pathways, ion channels such as voltage gated Ca2+ channels, during ES, are a critical part of membrane polarization and cell response. The current causes a flood of ions through ion channels and transporters (Na+, Cl−, K+, Ca2+, etc.) In response to ES, intracellular molecular polarization and transport channel polarization, then ion flow occurs, and cytoskeleton changes cause direct cell migration, thus leading to persistent cell migration to cathode.
3.3. Effects of electrical stimulation on cell proliferation
The cell proliferation increases with applied proper electrical stimulation, usually under < 1V/cm of continuous stimulation. Within the range of ES intensity, the rate of cell proliferation increases as the intensity increases. For instance, preosteoblasts, osteoblasts, unregulated human somatic stem cells, human umbilical vein ECs, NSCs, human dermal fibroblasts display 0.2 to 1.5 times proliferation, with cellular metabolic activity increasing, and do not affect cell phenotype. High-intensity ES of >100V/cm is also beneficial for single-stimulation cell proliferation in a short time (<1 ms), but extremely high-intensity results in cell death [67].
3.4. Effects of electrical stimulation on cell differentiation
Stem cell therapy provides a promising approach for regenerative medicine. The cardiac differentiation of pluripotent stem cells and muscle cells induced by humans should encourage electrical stimulation factors of low intensity (several minutes to 0.06~6V/cm) by the short term [68]. Neurons are separated from neural progenitor cells with the ES influence, neural precursor cells rather than being divided into glial cells. The stimulation intensity is basically <2 V/cm and sustain more than 7days. The differential medium can appropriately add with FBS, retinoic acid, and nerve growth factor. ES can induce bone marrow stromal cells, BMSCs, MC3T3-E1 cells osteogenic differentiation instead of cartilage, stimulation intensity should be < 2 V/cm and sustain 14–28 days, the medium need to add dexamethasone in most cases [67]. Therefore, the application of ES could provide a valid approach to induce cell differentiation in tissue engineering. The following sections detail the use of these electroactive materials in the form of scaffolds for a variety of tissue regeneration applications.
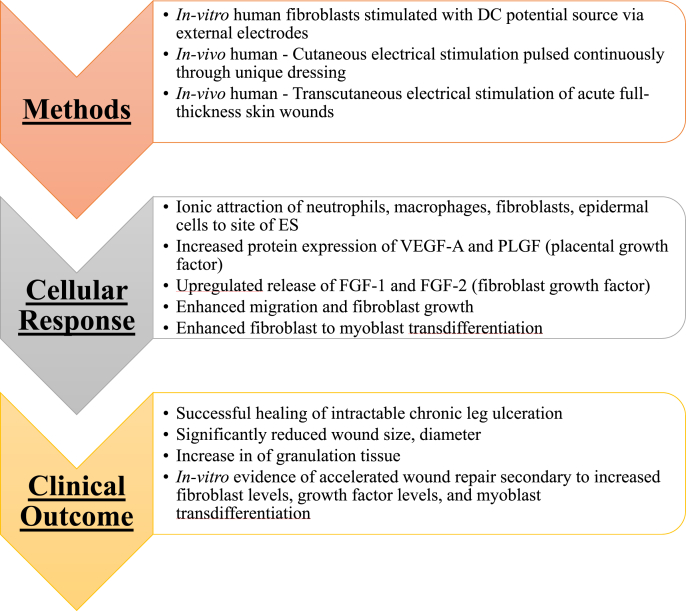
4. Wound healing
4.1. Effects of ES on skin/wound healing
Skin establishes an endogenous electric field following injury. Zhao et al. displayed that following wounds of both a rat cornea and human skin, an outward current from the wound with a magnitude of 1–10 μA/cm2 could be measured at various stages of healing [69]. The electric field variation changed to positive charges at the wound center. Reversing the applied field with an external electric field caused wound opening while the opposite of this significantly increased epithelial cell movement into the wound [69]. When directed towards skin wounds, ES has been shown to accelerate the wound healing process [70]. ES promotes accelerated healing of wounds, ulcers, and breaks in the epidermis [70,71]. It has been shown to increase the presence and activity of human skin fibroblasts [[72], [73], [74]]. The ability of fibroblasts to respond to ES in the dermis is thought to be via an opening of voltage-gated Ca2+ channels found on the surface of fibroblasts, with a subsequent increase in their activity and presence at the site of a wound [42]. A second mechanism by which ES is thought to accelerate wound healing is via ionic attraction. ES can serve to attract cells necessary for the healing process including neutrophils, macrophages, fibroblasts, and epidermal cells, as these all carry a negative charge [71]. Studies have also shown ES promotes accelerated healing, and regenerated tissue exhibits increased tensile properties compared with control [71]. Furthermore, ES is beneficial in providing an increase in temperature to the affected skin, resulting in increased perfusion to the area, promoting tissue healing [75]. ES also has been shown to promote angiogenesis, another important mediator of wound healing [70,76]. Of note, emerging evidence suggests that low frequency ES (<10 Hz) has higher efficacy and faster healing rates than higher frequencies of ES (>50 Hz). The benefits of wound healing and associated changes at the cellular/molecular level under the influence of ES are summarized in Fig. 5. Though initial findings are encouraging, further studies are warranted to fine tune ES parameters for specific wound size and thickness to harness benefits [75]. Specifically, future studies should investigate different voltage intensities, frequencies, and delays from initial injury to adequately assess the most beneficial way that ES could be used in a clinical setting.
Fig. 5.
Application of ES in wound healing and outcome analysis. ES promoted wound healing as a result of increased fibroblast activity, ECM production, and vasculature development [42,70,71].
4.2. Role of conducting polymers with ES
While many studies have investigated the effects of ES on skin healing, very few have done so with a conductive material. An in vitro study by Rouabhia et al. evaluated the viability of this approach through its effects on fibroblasts in culture [72]. The study used a PPY/poly (l-lactic acid) (PLA) membrane supplemented with bioactive heparin. ES was applied to fibroblasts through the conductive membrane they were seeded on. No toxic effects were noted as measured by LDH levels. Further, ES applied to a scratched defect in this culture increased the rate of fibroblast healing [72]. Briefly, after fibroblasts were electrically stimulated on the membrane, they were grown to confluence in 6-well plates at which point a sterile pipette tip was used to create a 0.5 mm defect. Cells initially stimulated through the membrane closed the wound in a 24 h period, outperforming the non-stimulated cells which remained with 30–40% of their initial wound left to heal in the same time frame [72].
5. Nervous tissue
5.1. Action potential
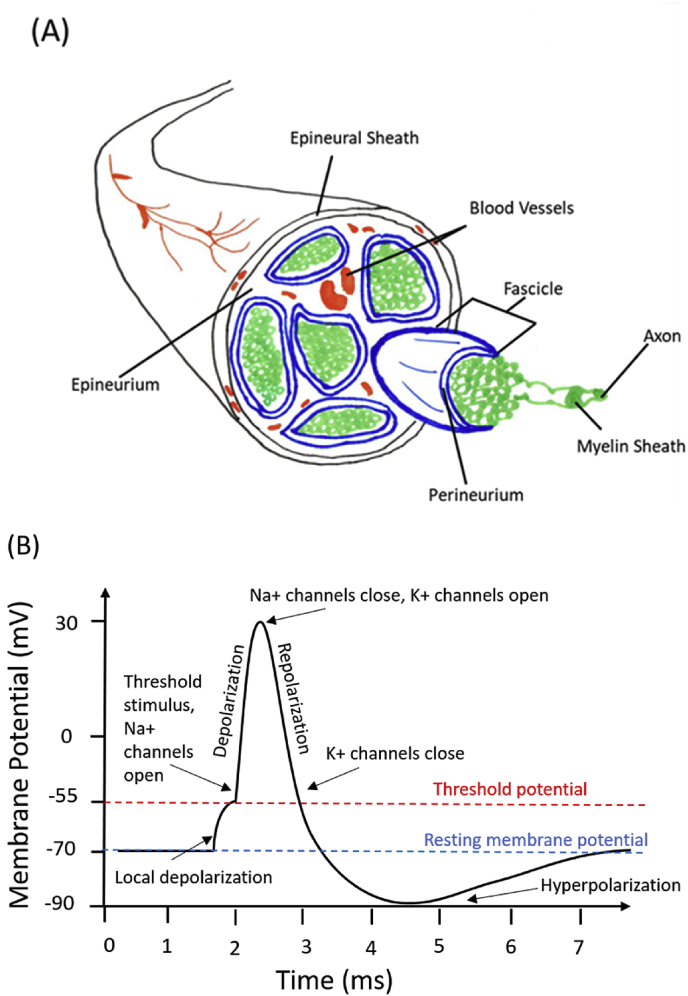
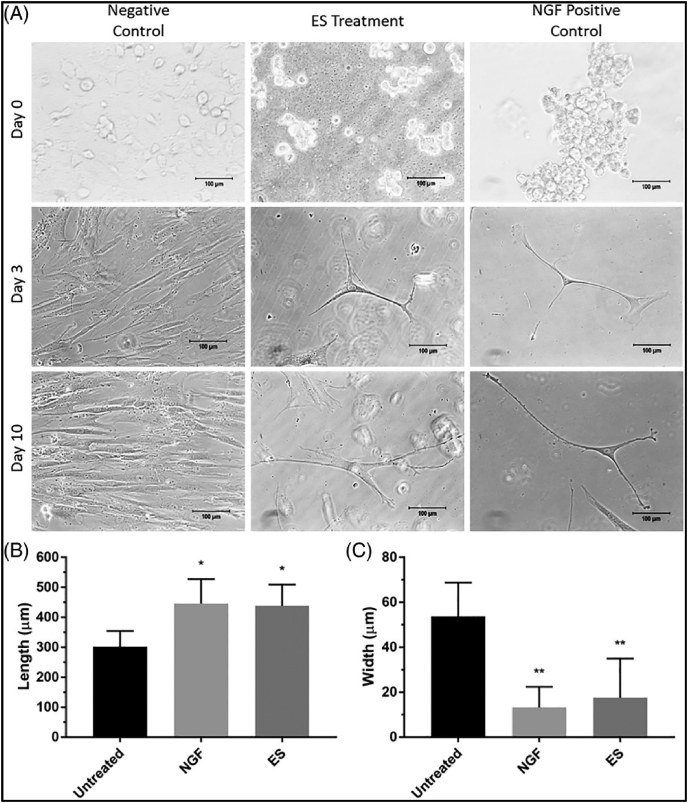
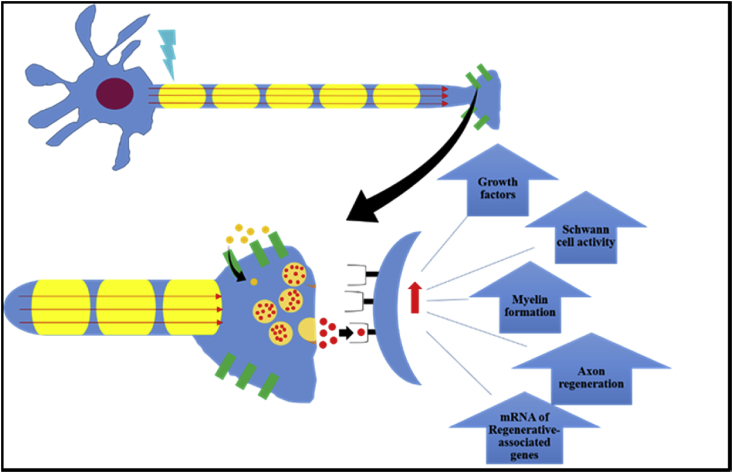
Electrical phenomena play a key role in the function of many anatomical systems, especially the nervous system [77,78]. Nerve cells are prevalent throughout the entire human body, and peripheral nerve injuries are the focus of tissue engineering research, which we have discussed previously [35]. The gross peripheral nerve morphology and action potential response are presented in Fig. 6. Important to the critical functioning of nerve cells, a prevalent electrical potential difference exists between a cell and its environment. Naturally, cells possess a negative charge compared to their surroundings, therefore, the surroundings are considered to be 0 V which results in an average membrane potential of around −70 mV. When there are no net stimuli applied to nerve cells, this potential remains relatively constant and is known as the resting membrane potential. If a stimulus is great enough to cause a depolarization of the membrane to −55 mV an action potential can occur. During an action potential, Na + channels in the cell membrane readily open once −55 mV is reached, when the potential reaches 30 mV Na + channels close and K+ channels open. The opening of K+ channels leads to repolarization. During repolarization, the membrane potential goes below resting potential to around −90 mV, which is termed hyperpolarization [27]. As a result, action potentials can only propagate in one direction, and there is a subsequent latency period during which another action potential cannot occur. Action potentials are the modality in which the nervous system transmits all of its information and messages. These events are caused by differences in electrical potential and demonstrate how integral electricity is to the function of the nervous system. Once the action potential propagates to the nerve terminal, voltage-gated calcium channels are activated. This leads to an influx of Ca2+, which triggers conformational changes in docking proteins, leading to synaptic vesicles (which store neurotransmitters) docking, fusing, and releasing their contents across the synaptic cleft. To add to this, Demerens et al. showed that electrical activity of nearby axons is required for the process of myelination in the central nervous system [79]. Also, an experiment by Schmidt et al. showed how ES increases neurite growth in PC-12 cells from a rat by an average of 8.64 μm [29]. From this, it can be seen that electrical signals can also influence a wide variety of cellular behaviors [80]. Our previous in vitro work, illustrated in Fig. 8, demonstrated that electrically stimulated human mesenchymal stem cells displayed characteristic neuronal cell bodies and dendritic-like processes, again supporting the favorable effects of ES on nerve regeneration [33] (see Fig. 7).
Fig. 6.
Schematics of peripheral nerve and associated action potential. (A) Peripheral Nerve: The epineurium (black), is an outer layer of tough, dense connective tissue surrounding the nerve fascicles and blood vessels. The epineurium provides both support structure and fibroblasts used in maintenance of the nerve sheaths. The perineurium (blue) is the middle protective sheath made of smooth laminar connective tissue, which is used to protect the bundles of axons, called fascicles. The blood vessels (red) are used to supply blood flow to the nerve; capillaries of the blood vessels reach into the perineurium. The axons and their individual surrounding myelin sheaths (green) are the long projections of neurons use to conduct electrical impulses in the form of action potentials. (B) The action potential can be defined as the charge on the membrane of a nerve (membrane potential) over time during an electrical pulse. Sodium and potassium channels enable depolarization and repolarization.
Fig. 8.
Optical micrographs elucidating the changes in cell morphology in response to chemical (nerve growth factor; NGF) and electrical stimulation (ES) in vitro. (A) Human mesenchymal stem cells change into elongated branching cells with ES treatment while untreated cells (negative control) do not. Chemical treatment in the form of NGF also induced an elongated branched morphology (positive control). (B) Quantified length of cells at day 10 showing ES and NGF cells with greater cell length compared to untreated control cells. (C) Quantified width of cells at day 10 showing ES and NGF cells have smaller width than untreated cells [33].
Fig. 7.
Effects of ES on neurons. In response to externally applied ES (blue), an action potential is triggered (red arrows) which propagates along the axon length. Triggering of voltage-gated Ca2+ channels (green rectangles) results in influx of Ca2+, triggering a conformational change in docking proteins, resulting in the fusion of synaptic vesicles (yellow spheres) with the outer membrane and release of neurotransmitter (red spheres) on post-synaptic target cells. Neurotransmitter binds to receptors and results in a variety of increases in cellular and molecular functioning.
5.2. ES effects on nervous tissue
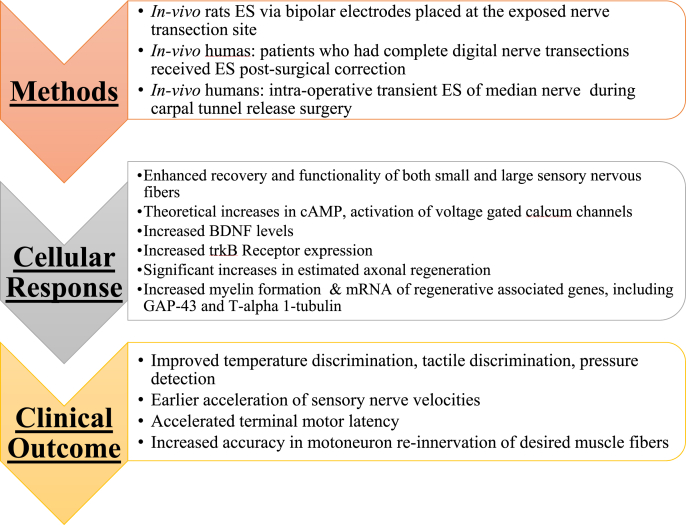
The regenerative capabilities of ES on nervous tissue have been well studied [81]. Most applications involve a low frequency stimulus, around 20 Hz, which is the mean firing frequency of motor neurons in animals and humans [82]. This low frequency stimulus has been shown to benefit nerve regeneration by accelerating axon growth, leading to improved motor and/or sensory functionality, and promoting remyelination [81,[83], [84], [85]]. Several components of the mechanism behind the effects of ES on nervous tissue have been discovered. ES acts on the cell body of nerve cells and requires action potentials to exert its effects. This has been shown in previous studies, including by Al-Majed et al. who demonstrated that the use of tetrodotoxin, a toxin that blocks action potential propagation and transmission to the cell body, blocks the effects of ES [83,86]; Tetrodotoxin blocks voltage-gated sodium channels, which are required for action potential generation and propagation. It has also been noted that ES increases cyclic adenosine monophosphate (cAMP) levels [81,84,86]. Further, ES upregulates the expression of the neurotrophic factors neurotrophin-4/5 (NT-4/5) and brain-derived neurotrophic factor (BDNF) and their receptors [81,[84], [85], [86]]. In addition to neurotrophic factors, ES also increases expression of regenerative and cytoskeletal markers including tubulin, actin, and growth-associated protein 43 (GAP-43) [81,84].
The theory behind this upregulation of various regenerative factors by ES is an influx of calcium ions [81,86]. While the enhanced tissue regrowth properties of ES have been readily studied in animal models, human clinical trials have also been performed and patented devices for ES exist [[86], [87], [88]]. A clinical trial was performed by Gordon et al. to assess the efficacy of 20 Hz ES on the healing process after carpal tunnel release surgery. As a result, the group found that the treatment accelerated and completely re-innervated the targeted thenar muscles leading to earlier and superior functional recovery for patients [87]. Fig. 9 summarizes the effects of ES on neural tissue and associated mechanisms in their regeneration. A pre-clinical study in rat models showed that ES significantly improved sensory and motoneuron axon regeneration and recovery [89]. Brushart and colleagues showed that ES improved re-innervation of neuronal targets after nerve transections, and, importantly, significantly increased the specificity of their target tissue during the regeneration process [89]. Thus, ES both improved the ability of neurons to regenerate, as well as improved their ability to re-innervate the original target cells/tissues, which is critical to the proper functioning of motor and sensory neurons. Another rat study observed after sciatic nerve transections that the ES group experienced higher levels of expression of both BDNF and the TrkB receptor (the receptor for BDNF and NT-3 growth factors) [90]. Additionally, there was a higher level of axonal regeneration in the ES group, but only when ES was applied within 1 week after the transection occurred [90]. A similar study in rat models demonstrated that short-term ES resulted in significant increases in regenerative gene mRNA, including GAP-43 and T-alpha1-tubulin, which correlates with other evidence supporting accelerated and enhanced axonal regeneration after ES [91]. Clinical trials using ES to improve sensory recovery in patients with complete digital nerve transections showed improved patient recovery of cold and heat detection thresholds, pressure sensory function, and 2-point discriminatory ability [86]. Fig. 10 summarizes several in vivo findings of nerve regeneration in conjunction with ES. All these studies are suggestive of beneficial outcomes of neural functions in response to ES and its potential a viable therapeutic option.
Fig. 9.
Summary of peripheral nerve regeneration outcomes in response to applied ES. In general, ES enhanced the secretion of various cytokines and receptor expression that contributed to functional neural tissue regeneration [86,87,90,91].
Fig. 10.
Summary of tendon tissue regeneration in response to ES. Overall, ES contributed to increased cell proliferation; ECM secretion is quite similar to the effects observed in ES-mediated wound healing [36,104].
5.3. Role of conducting polymers and growth factors
Delivery of ES through conductive polymeric scaffolds is an attractive option to relay electrical signals to cellular function. Among several conductive polymers, PPY has been extensively tested for its potential in nervous tissue engineering applications. Shi et al. fabricated a novel dodecylbenzene sulfonic acid (DBSA) doped PPY-cellulose nano-porous scaffolds and seeded them with PC12 cells, a cell line derived from rat adrenal glands, which were further subjected to ES. Increased cell adhesion and proliferation, greater neurite outgrowth, and significant gain in superficial neural phenotypical expression were observed in these scaffolds [92]. Huang et al. tested viability of this approach in 15 mm critical-sized rat sciatic nerve defects. Composite scaffolds of PPY/chitosan were created through longitudinal freeze drying resulted in a micro-channeled structure with honeycomb-like transverse sections. Their work showed ES resulted in numerous positive outcomes: increased size and number of myelinated axons, improved axonal regeneration, greater motor function, faster nerve conducting velocity, and an upregulation of neuronal proteins such as S-100 and BDNF [93]. BDNF is a neuronal protein found in many central and peripheral nervous system cells, and S-100 is a commonly used marker for peripheral nerve myelin, and is present in all Schwann cells. Such neurotrophic growth factors can have a synergistic effect with ES. An example of this was the use of a PPY scaffold seeded with BDNF and neurotrophin-3 (NT-3) via solution during the polymerization reaction [94]. These scaffolds were implanted in rat cochlear neural explants and subjected to ES for 8 h every 24-h period [94]. Coupled with ES, the growth factors were released from the scaffold at an increased rate during the 8-h period. The result of this experiment was a doubling of neurite growth in the implant group, as measured by fluorescence microscopy. Thompson et al. attributed this synergistic relationship to ES increasing Trk receptors on cell membranes leading to a greater effect of the NTs [94]. From these studies, it is clear how much PPY has been investigated, however, it is not the only conductive polymer that shows potential for ES applications.
Few studies have explored PANI-based scaffolds in conjunction with ES. Prabhakam et al. created PLLA-PANI electrospun composite scaffolds at a ratio of 85:15 [77]. Immortalized multipotent C17.2 rat neural progenitor cells seeded onto the scaffold were stimulated for 60 min at 100 mV. Application of ES resulted in increased neurite length measuring 24 ± 4 μm as compared to control 15 ± 3 μm without ES. It was hypothesized that the observed phenomenon was due to changes in local electric fields of ECM proteins with application of ES that increased protein absorption onto scaffolds leading to increased neurite outgrowth [77]. All these studies are suggestive of beneficial effects of ES in enhancing neurite growth. Optimizing ES parameters and conducting scaffolds may benefit the upregulation of neurotrophic factors at the defect site and promote nerve regeneration.
6. Tendons and ligaments
6.1. Tendon and ligament structure
Tendons are a type of connective tissue that function to attach muscle to bone. Tendinous tissue is composed of individual fibers largely made up of type 1 collagen, elastin, and proteoglycans [[95], [96], [97]]. Tendons serve to minimize damage to muscle due to shear stress, and protect from overstretch as well as excessive force and contractions [95,98,99]. Repair of damaged tendons as well as degenerated tendons subjected to repeated stress results in scar tissue formation, predominantly made up of type III collagen. The repair process has been classified in three phases – inflammation, proliferation, and remodeling [100]. The phases involve the migration and proliferation of fibroblasts with eventual collagen formation, as well as an initial increase in capillaries. Eventually, the collagen fibers are remodeled, and the number of capillaries declines [36,100].
Ligaments are structurally similar to tendon, but serve to connect bone to bone [101]. Made up of dense connective tissue, there are a few cellular differences compared to tendon [95]. Ligaments contain fibroblasts which are more active, and ligamentous tissue is comprised of larger proportions of type III collagen and glycosaminoglycans compared to tendinous tissue [95]. Similar to tendon, the repair and regeneration process also results in scar tissue, largely composed of type IIII collagen, however there is a degree of remodeling that occurs, which replaces type III with type I collagen, which has superior mechanical properties [95].
6.2. Effects of ES on tendons/ligaments
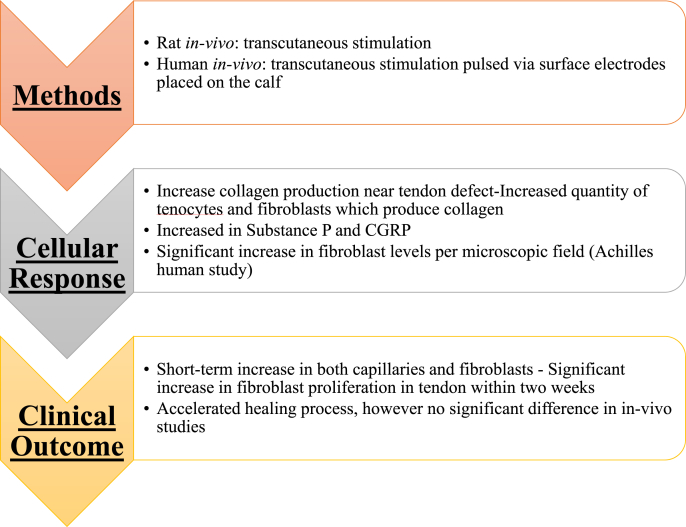
Within tendinous tissue, studies have shown that ES promotes an accelerated and enhanced repair process. Clinical trials have shown that microcurrents applied to damaged tendons enhance the healing process in animal studies, and that the polarity of the microcurrent may play a role in the extent of healing [102]. Studies have shown there is a short-term increase in both capillaries and fibroblasts under the influence of ES within connective tissue [36]. Fibroblasts undergo massive expansion evidenced through increased DNA content and protein expression under the influence of ES [42,103]. An upsurge in capillaries results in an increase of required nutrients that can assist in the regeneration and repair process. A significant rise in collagen synthesis via an increased number of fibroblasts functions to accelerate the repair process in damaged tendon. Scar tissue repair remains the primary way through which tendinous tissue heals, and ES accelerates and enhances this process [36].
Burssens et al. clinical investigation on the use of transcutaneous ES during Achilles tendon repair showed a significant increase in collagen production and organization, as compared to controls [104]. Upregulation of substance P and calcitonin gene-related peptide (CGRP) in the presence of ES resulted in improved tendon healing. These neurotransmitters, released from peripheral nerve terminals are also thought to play important roles in the healing process, facilitating fibroblast function and migration [104]. Fig. 10 summarizes the major ES studies performed on tendon and their clinical outcomes. Further clinical investigations are needed to understand the role of ES in conjunction with conductive polymers for tendon/ligament repair and regeneration as an adjunctive therapy.
7. Muscle
7.1. Muscle structure
Muscle including skeletal, cardiac, and smooth have the ability to respond to ES [105]. Studies have shown that electromagnetic fields affect muscle cell division and signaling [106]. Skeletal muscle receives electrical signals from the nervous system vital to normal function in the body [107]. Electromagnetic fields affect cardiac muscle constantly through transfer of electrical signals that instruct the heart to beat [108]. Electrical stimulus is needed for smooth muscle vasoconstriction and rhythmic contractions in intestines. Thus, use of conductive polymeric structures in conjunction with ES may lead to better muscle repair and regeneration. Skeletal muscle is a highly aligned, multinucleated, and anisotropic tissue [109,110]; Anisotropy means that the cell's properties change based on direction. This anisotropy becomes a challenge when attempting to promote myoblast growth and differentiation into myotubes and eventually functional skeletal muscle. Any successful attempt to regenerate skeletal muscle must direct the growth of myoblasts to create a tissue that retains the anisotropic properties of natural skeletal muscle.
7.2. Implications of ES on muscle tissue
The excitability of muscle tissue can be seen on a macroscopic level. When external ES is applied at a great enough amplitude, a contraction of the muscle can be induced [[111], [112], [113]]. When limbs are immobilized for long periods of time due to injury or recovery from surgeries, a loss of muscle mass and strength can be expected due to atrophy. Using external ES to artificially contract the immobilized muscle has proven effective in preventing this unfortunate muscle wasting [112,114]. While ES can cause changes visible to the eye, it concurrently affects muscle cells on a molecular level. Langelaan et al. used an immortalized myoblast cell lineage, C2C12, and muscle progenitor cells to give insight to the effects of ES on the cells themselves. The results showed an advanced maturation of both cell types subject to a 2 Hz, 4 V/cm signal. This was evidenced by increased levels of α-actinin and cross striations in both samples and an enhanced presence of sarcomere actin in C2C12 cells [115]. Synchronous contractions of C2C12 cells were observed after 3 days of ES due to the propagation of action potentials leading to releases of calcium ions from the sarcoplasmic reticulum [115]. This effect can even be seen in humans where in vivo ES has been shown to increase muscle protein synthesis [116]. ES is currently used as a form of atrophy treatment with muscle tissue and the cellular effects provide insight into its regenerative capacity.
7.3. Effects of ES combined with conductive polymers and growth factors
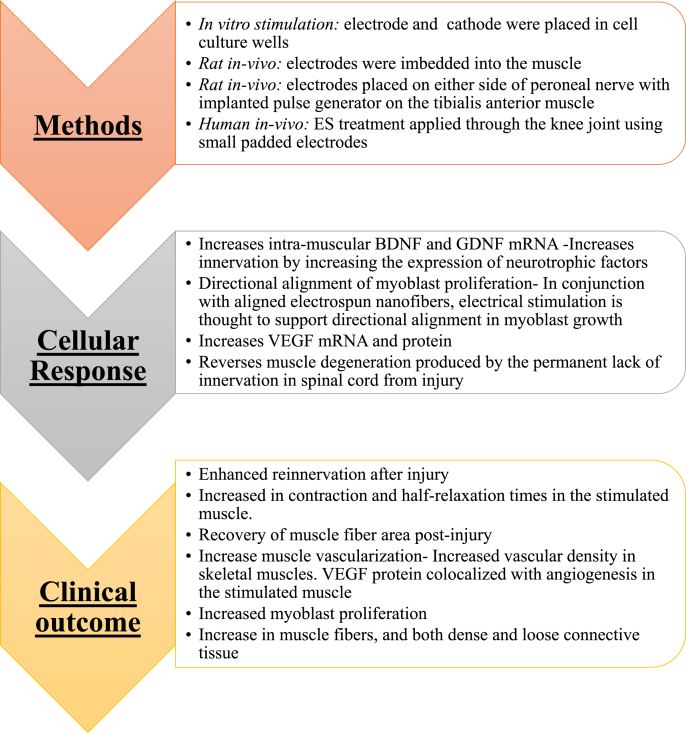
The use of conductive polymers has been shown to have progressive results towards the regeneration of skeletal muscle tissue. In one case, an electrically conductive PPY and polyurethane scaffold with cultured C2C12 mouse myoblasts resulted in increased cell growth myotube formation [117]. Broda et al. attributed this result to increased cell-cell interactions due to the electrically conductive scaffold [117]. It is important to note that no external ES was applied and conductive substrate mediated the cell-cell interaction. Similar observations were also made by Gilmore et al. where mouse primary myoblast resulted in multinucleated myofiber formation [118]. Mouse C2C12 myoblast cells on a poly-ε-caprolactone composite under the influence of ES showed alignment and myotube formation [110]. Although conductive polymers enhance myoblast growth on their own, their effect can be increased once complemented with ES. For instance, multi-walled carbon nanotubes (MWCNTs) have been embedded with para-toluene sulfonic acid-doped PPY. These MWCNTs were then seeded with primary murine myoblasts and underwent various protocols including ES. Compared to other groups, ES with the doped conductive polymer lead to a 38% increase in cell density, more myotubes with 7.5% more having multinucleated structures, and a 12.4% greater myotube differentiation [119]. Measurements were made through immunofluorescent staining for desmin with myotube differentiation defined by the number of nuclei within myotubes out of the total number of nuclei [119]. Interestingly, ES can even improve the function of formed fibers. In a 3D collagen scaffold implanted with C2C12 cells, ES not only improved myofiber formation, but, also promoted the development of contractile apparatuses in cells as well as sarcomeres [120].
Utilizing ES to promote muscle repair clinically has been partially investigated. ES works on muscle fibers by increasing the necessary growth factors that induce recovery and tissue healing. ES has been shown to preserve and promote the healing and recovery of damaged type 1 skeletal muscle fibers [121]. Additionally, studies have shown that stimulation of muscle fibers increases the expression of vascular endothelial growth factor (VEGF), an important mediator of angiogenesis and the subsequent healing process [122]. The mechanism behind this increase is theorized to be a rise in VEGF mRNA transcription due to a transient oxygen deficiency in muscle fibers. Thus, the increased VEGF mRNA leads to an increase in VEGF protein and subsequent blood vessel development, promoting an increased delivery of oxygen and nutrients to affected cells [122]. ES directed at muscle in conjunction with biomaterials can lead to improved clinical outcomes after skeletal muscle injury. The outcomes of each method of ES on muscle and the repair mechanism it induces can be seen in Fig. 11.
Fig. 11.
Summary of muscle regeneration in response to ES. In general, ES enhanced the secretion of various cytokines that lead to increased functionality and muscle regeneration. Increased vasculature and muscle fiber density was the outcome in all these studies [119,121,122,152,153].
While conductive polymer constructs and ES have mostly been applied to skeletal muscle, cardiac muscle has also been investigated. Conductive polymers can cause cardiac myocyte-like fates in human mesenchymal stem cells (hMSCs) [123]. Further, hMSCs have shown early cardiac cell markers through a treatment of 0.15 V/cm for 14 days on carbon nanotube PLA scaffolds. This was determined by increases in markers such as myocyte-specific enhancer factor 2C (MEF2C), cardiac troponin t (CTT), cardiac myosin heavy chain (CMHC), and connexin-43 (C43) [108]. These findings point to the benefits of conductive polymers and ES on muscle regeneration in addition to a desired stem cell differentiation. The effects of ES on smooth muscle have not been as extensively studied as other muscle types. Experiments have noted increased expression in the contractile proteins smooth muscle α-actin and smooth muscle myosin heavy chain [107]. Although further research is needed for the clinical translation of ES in tissue engineering, conducted studies have shown great promise at both tissue and cellular levels.
8. Bone
8.1. Bone bioelectricity
Bone tissue possesses endogenous electrical signals and has a high capacity to regenerate [124]. There are multiple important electrical properties to consider when looking at bone. First, bone is a piezoelectric material, it generates electrical potentials when subjected to stress. This electrical potential is predominantly driven by the sheer force of collagen [124,125]. During stress, parts of the bone under compression become electronegative leading to bone resorption, while those under tension become electropositive resulting in bone production [24]. Second, when bone tissue is damaged, injury potentials are formed which create endogenous currents that aid in the wound healing process [124]. It is important to note that osteoblast cells are responsible for bone growth while osteoclasts facilitate the bone resorption [[126], [127], [128]], and using external ES researchers hope to influence these properties in a way to treat various orthopedic injuries.
8.2. The use of ES for bone regeneration
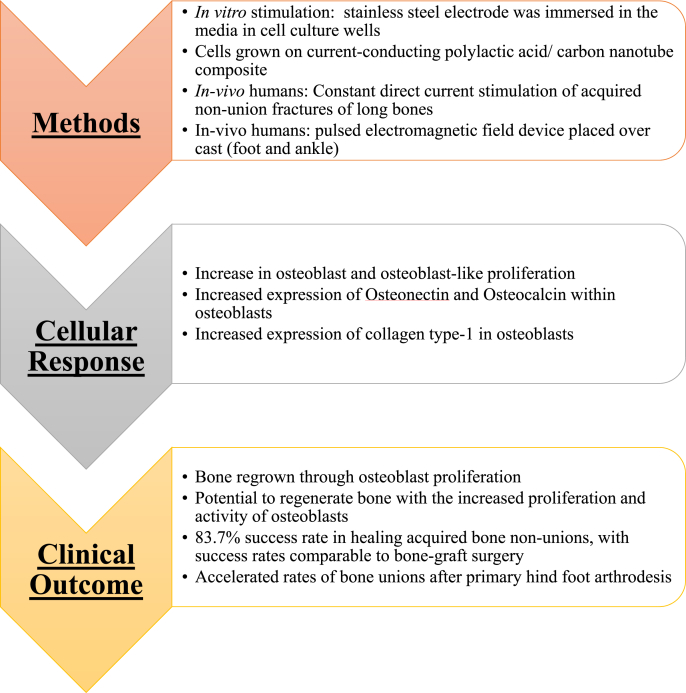
In addition to the endogenous electrical signals in bones, external ES has been shown to cause several osteogenic responses. One such effect is that ion mobility in and out of the cellular membrane increases with applied ES. Such changes in ion concentrations, especially calcium ions, can lead to altered cellular function [129]. Additionally, transduction of weak ES can lead to signal transduction into the cell interior and to coupling proteins. Cellular changes within bone tissue due to ES such as the inhibition of membrane-associated cAMP and increased DNA synthesis have been noted. ES can also be used to control cell migration as cells will naturally move toward the cathode due to their naturally negative charge and a phenomenon in which Ca2+ ions enter the anode side of the cell [124]. For regenerative purposes, ES has been used to heal nonunion, delayed unions, osteotomies, and acute spinal injuries [[130], [131], [132]]. The sites of the electrical stimulators also create environments favorable for bone growth. For example, due to a chemical reaction at the cathode during direct current (DC) ES, hydroxide ion is formed. Increased concentrations of this ion lowers oxygen concentration and heightens the local pH resulting in increased bone formation by osteoblast and a reduction in osteoclast activity [24,133].
Aside from its regenerative benefits on bone tissue, ES can promote osteogenic lineages among various precursor cells. One cell type is the MG63 human osteoblast-like cell. When stimulated by a 15 Hz pulsed electromagnetic field, the number of cellular vesicles with alkaline phosphatase, collagen production, and osteocalcin production increased. These findings all point to improved osteogenic differentiation of MG63 cells [134]. The natural electrical properties and changes caused by external ES make it a promising point of study for bone tissue engineering.
Additionally, ES has been shown to enhance healing and accelerate formation of bone matrix [135]. It works by increasing bone density and promoting the union and healing of damaged bone. As mentioned above, ES increases the proliferation and activity of osteoblasts when cultured in vitro [136]. An additional proposed mechanism for the effects of ES on osseous tissue is via regulation of osteoblast activity. ES increases the expression of both osteonectin and osteocalcin within osteoblasts. Both proteins are critical for increasing the mineralized portion of bone [[135], [136], [137]]. Additionally, ES has been shown to increase mRNA expression of the collagen type-I gene within osteoblasts, leading to a subsequent increase in collagen-I protein levels [136]. Collagen – primarily type I collagen – is the major component of the organic portion of bone. Another study displayed that electrically stimulated hydroxyapatite ceramics used for bone implants displayed significantly improved bone ingrowth compared to controls [138]. The implants had an increase in overall osteoblast activity [138]. ES promotes bone regrowth, regeneration, and increased density by regulating the activity of osteoblasts. In summary, ES enhances bone reformation and healing via pathways that assist both the supporter cells that lay down new bone and remodel old bone (osteoblasts) and by optimizing the function and activity of the osteocytes themselves via ion channel activity altering cellular functions.
8.3. Effect of conductive polymers and ES
Conductive scaffold systems have shown the ability to promote bone growth without the presence of ES. A scaffold containing PPY, alginate, and chitosan was made through a lyophilization process by Sajesh et al. [139]. MG-63 cells on these scaffolds resulted in apatite layer formation [139]. PLA mixed with heparin-containing PPY with Saos-2 osteoblast-like cells resulted in an appetite layer with a Ca/P ratio similar to natural hydroxyapatite and upregulated expression of several osteogenic growth factors [140]. Similarly, a PEDOT:PSS (poly (4-styrene sulfonate)) gelatin bioglass composite induced hMSC display of lamellipodia and filopodia, supporting cell scaffold adhesion and cell-cell interactions [125]. While conductive polymers alone have shown osteogenic-promoting motifs, the addition of ES provides a synergistic effect.
Numerous conductive polymer composite scaffold systems have been used to guide ES for the potential regeneration of bone, such as poly(l-lactide) and heparin-containing PPY scaffolds seeded with Saos-2 cells and stimulated at various DC gradients [141]. A 200 mV/mm of ES resulted in significantly higher alkaline phosphate (ALP) and osteocalcin production than the control untreated group, while a 400 mV/mm of ES lowered the production of these markers, implying that excessive ES can counteract the healing process. The authors showed that excessive stimulation negatively regulates cell growth, which could be through a variety of mechanisms – perhaps excessive activation of voltage-gated Ca+2 channels or depletion of intracellular proteins [141]. This experiment not only showed that osteoblast markers can be controlled by ES, but also that there is an optimal stimulation required to do so [141]. In addition to this, an experiment by Shao et al. coupled the effects of ES with an electrospun poly-dl-lactide (PLA) scaffold embedded with aligned and conductive multiwalled carbon nanotubes [142]. With the presence of a 100 μA DC current, seeded osteoblasts grew along the direction of the current and the conductive fibers. This poses an attractive application for ES as a tool to align cells in order to potentially create naturally anisotropic tissues. However, this experiment again highlighted how ES parameters must be optimized as the application of a 200 μA current inhibited cell proliferation. Fig. 12 summarizes the effects that ES has on bone tissue.
Fig. 12.
Summary of bone regeneration in response to ES. Application of ES resulted in increased osteoblast activity in vitro and bone regeneration in vivo. [135,136,154,155].
9. Immune response to ES
Limited research reports are available that looked at immune responses under the influence of ES. Most of these studies are either clinical trials or pre-clinical models looking at immune cells, secreted enzymes, and inflammatory cytokine production. These preliminary studies have not shown significant immunological responses to ES due to limited test conditions including low to moderate treatment intensity on the healthy patient population. Studies using low-frequency transcutaneous neuromuscular ES showed no significant immune system response as measured by changes in biochemical blood components, white blood cell count, or phagocytosis and oxidative burst function of neutrophil granulocytes and monocytes. One study looked at a group of ten healthy volunteers that underwent neuromuscular ES in the quadriceps femoris muscle on both legs for ten consecutive days [143]. Biochemical serum analyses were performed a day after the last stimulation and revealed that only creatine kinase (an enzyme expressed in the muscles during exercise or damage) levels were increased, although the increase was not significantly different. This finding is attributed to exercise performed by the volunteers as opposed to any immunological response, especially when considering there was no change in white blood cell count and their phagocytosis/oxidative killing functions [143]. Similar outcomes were observed in another study involving twelve healthy volunteers where moderate intensity neuromuscular ES failed to produce significantly different immune responses versus control [144].
While ES doesn't appear to have any negative effects on immunological responses, there is evidence that it can reduce inflammatory responses and suppress cytokine production. When ES is applied to the vagus nerve, the parasympathetic anti-inflammatory pathway is activated, attenuating the release of cytokines [145]. The vagus nerve interfaces with the parasympathetic control of the heart, lungs, and digestive tract, and thus controls systemic inflammatory responses. Rat peripheral vagus nerves that received ES resulted in the prevention of shock and inhibition of inflammatory cytokine release when a lethal amount of endotoxin (a lipopolysaccharide known to activate cytokine release in macrophages) was administered [145]. The mechanism behind this attenuation of inflammatory cytokine release is that the secretion of the neurotransmitter acetylcholine is increased when the vagus nerve is stimulated [145]. However, while many animal studies support ES stimulation of the vagus nerve for the reduction of inflammatory responses, these results have not been duplicated in human clinical trials [146]. In Kox et al. transvenous vagus nerve stimulation did not relieve the inflammatory response induced by endotoxin [146]. In addition, transvenous vagus nerve stimulation did not alter neutrophil phagocytosis capacity or reduce the production of cytokines, in contrast to its effect in animal studies [147]. More studies are needed to determine the potential for ES to reduce inflammatory responses, but current findings suggest that ES does not increase inflammation.
9.1. Limitations of ES
The previous sections point to multiple positive impacts that ES has on many tissues in the body. While emerging evidence suggests that ES could eventually be an adjunctive treatment option for tissue healing, there are notable limitations. Though several pre-clinical studies report on the use of ES for several tissue types, these have not translated into clinical studies. Standardized clinical trials in humans can be difficult, as creating intentional injuries to certain tissues (nerve) and observing the healing process temporally is not realistic or ethical. Goals moving forward should be aimed at considering the use of ES as an adjunctive therapy as early as possible after an injury occurs, and tracking outcomes accordingly. While standardization may be difficult using this method, it will be an important step towards the implementation of ES in clinical settings.
Further studies are needed to clarify which specific parameters to use to optimize tissue regeneration and healing, as there is some variation in the literature. While some studies advocate the use of low frequency stimulation, opinions vary based on the study design as well as which tissue types (e.g., nervous, muscle, skin) are being studied. Additionally, as mentioned above there is a marked dearth of studies investigating ES as an adjunctive therapy for the healing of damaged tendons and ligaments. Although the previous mentioned studies investigating tendon repair have been promising, more research is needed.
Other limitations include the need to travel to the clinic site for every treatment, length of treatment, and costs of the transducer and other equipment. While the procedure for in vivo ES is non-invasive and involves electrodes placed outside the skin for stimulation, the requirement of traveling to a hospital for this procedure may diminish recruitment for clinical trials [116].
10. Future focuses: Built-in electric fields
The future direction for the use of bioactive materials in conjunction with ES is to utilize implants with built-in electric fields. In a study from Ning et al. [148]. a titanium implant was used to induce a microscale electric field (MEF) in order to apply electrical signals to cells in vivo without the need of an external stimulation source. The MEF was created by applying laser irradiation to the anatase titanium dioxide surface film of a titanium implant and convert it to a semiconducting rutile phase. As the anatase titanium dioxide phase is irradiated by the laser, it gains a greater electron density which leads to a transfer of electrons from the greater electron density rutile zone to the anatase zone. The results from the use of this MEF show an induction of osteogenesis BMSCs even in the absence of osteogenic supplements. In addition, there is evidence that the MEF produced by this implant promoted bone regrowth when placed in a rat femur model [148].
This is reiterated in a study from Yu et al. [149] where K0.5Na0.5NbO3 (KNN) ceramics are irradiated with a laser in a similar manner to the method described in Ning et al. [148]. This creates two parallel domains with different piezoelectricity to achieve micro-zone phase transitions. The alternating phases allows the ceramic implant to have microscale electric signals which mimic the natural piezoelectric field in bone and due to this mechanism, the implants are demonstrated to induce bone regeneration in vivo. A third recent use of built-in electric fields to enhance the osseointegration of the implant to the native bone. In the study conducted by Liu et al. an electric field is created between the electropositive implant, coated in a ferroelectric nanofilm, and the electronegative bone [150]. The BiFeO3- coated titanium implant is built to have surface potential that is similar to the amplitude of endogenous electronegative potentials found on the bone defect walls. This is found to increase the adhesion of cells on the implant and increase calcium ion signaling which may be a mechanism for increased osteointegration.
The use of built-in electric field in implants is a new avenue for bone repair and regeneration. It is considered a safe approach as it removes the potential cellular damage caused by applying an external electrical stimulus. Built-in electric field in implants have been proven to be effective on bone repair due to the microscale electric fields mimicking the piezoelectric field of bone tissue. However there have not been any studies to the impact caused by MEF-inducing implant itself. It is not known if the implants or their electric fields cause any negative inflammatory response. In addition, the use of built-in electric fields in implants has only been proven to be effective on osteogenesis and osteointegration. It is unknown how effective it could be to incorporate these built-in electric fields for the repair of other tissue types besides bone [149].
11. Conclusion
ES has been used clinically as a physical therapy modality to achieve better surgical outcomes. In these studies, it has been shown to enhance cell multiplication, ECM production, secretion of several cytokines, and vasculature development leading to better tissue regeneration in multiple tissues. These studies demonstrated an increase in capillary and fibroblast numbers at the wound site that preceded collagen synthesis. Successful tissue engineering requires the maintenance of sufficient vascularization to support tissue growth. Efforts to induce vascular growth into tissue-engineered scaffolds have recently focused on novel strategies to deliver specific biological factors that direct the recruitment of endothelial cell progenitors and their differentiation. For instance, incorporation of VEGF within scaffolds has been a common strategy to vascularize constructs. However, it is challenging to release optimal doses of VEGF to promote vascularization in a biological environment for tissue engineering. ES and exercise have been shown to improve blood vessel growth via expression of VEGF and increased blood flow, which has been suggested to release nitric oxide-like humoral agents that are critical regulatory molecules for angiogenesis. It is important to note that all these studies used ES alone without the involvement of any biomaterial component. However, critical-sized tissue defects require the use of biomaterials and structures for tissue repair. This will allow uniform and controlled ES at the defect site to promote regeneration.
The use of electroactive materials in conjunction with ES provides a promising new approach for the regeneration of musculoskeletal systems. Such biomaterials are necessary for medical implant applications involving long-term electrical recording and stimulation of electrogenic tissues such as brain, spinal cord, nerves, and heart. Conductive materials explored for this application rely on electronic conductivity enabled through polymers containing oxidized pi-bond conjugation states. Typically, these polymers are thermosets, brittle and non-degradable with conductivity that declines in physiologic conditions as they get reduced. Ongoing research efforts are focused on the composite approach where metallic or carbon-based fillers are dispersed into the polymeric structure to address conductivity issues. These polymers raise health concerns in the end as they involve non-degradable metallic components and their body clearance mechanism. Alternatively, IC polymers offer a potential solution to all the aforementioned complications from the biomaterial standpoint. These polymers conduct electrical charges via the flow of counter ions in the physiological environment, a conducting mechanism that is different from electronically-conducting polymers. IC polymers can be synthesized to carry positive (cation exchange) or negative (anion exchange) charges from well-studied biodegradable polymers such as chitosan, silk, PLA, and poly (lactic-co-glycolic acid). Their degradation mechanism and rate depend on the chosen polymer backbone, as well as enhanced surface erosion via ionic group modification-conferred hydrophilicity to the polymer. Recently, we have been exploring IC polymers in conjunction with ES for the repair and regeneration of neural tissue.
Application of ES in regeneration of various tissues provided beneficial effects but underlying mechanisms are poorly understood. A few preliminary studies involving ES have not shown significant immunological responses to ES due to limited test conditions including low to moderate treatment intensity on the healthy patient population. Studies performed on animal models supports these results and may demonstrate a reduction in inflammatory response. However, as the effect of electrical stimulation on the immune system has not been analyzed on its impact on cytokine or chemokine release, effects on cell death, survival or proliferation, or the impact on cell activation. Therefore, additional studies are needed to determine the specific parameters of stimulation that should be applied to enhance regenerative capacity and functional outcomes. Table-3 organizes the parameters used within the studies on each tissue type, thus allowing for standardized protocols for each tissue repair to be established. Establishing standard protocols for each tissue type and condition may further develop this efficient methodology for the use of regenerative therapies. Signal transduction from dermis nerves to applied skin electrodes for stimulation and measurement are currently not reliable in the absence of suitable connecting materials. In a recent opinion article, Professor Adam Heller makes a note on the use of ion-conducting hydrogels as an electrode interface to nerve for signal transduction [63]. Such an approach may enable current or potential transmission via skull to deliver ES. Alternative materials in the form of IC polymers we currently explore may help in building better tissue interfaces with neural prosthetics.
Table 3.
Comparison of electrical stimulation parameters to different tissues. The frequency, current, electric field, treatment time, and polymeric materials used are listed for every studied tissue.
| Tissue Type | Frequency | Current or Electric field | Time of treatment | Polymeric materials used |
|---|---|---|---|---|
| Wound Healing | 10–80 Hz | 0.004 mA 15 mA 50–200 mV/mm |
30 min up to 6 h | PPY/PLA membrane Chitosan, nonconductive Bioactive nanofiber |
| Nervous Tissue | 20 Hz | 100 mV/mm 3 V |
60 min- 2 weeks | PLA with PANi at a ratio of 85:15 PPY-cellulose nano-porous scaffolds PPY/chitosan composite |
| Tendons and Ligaments | 10 Hz internal frequency 100 Hz; burst frequency was set at 2 Hz |
100lA/cm2 | 20–30 min | PCL-cellulose Acetate Micro-Nanostructured Fibrous Scaffolds |
| Muscle | 2–60 Hz | 0.15 V/cm 4 V/cm signal |
60 min- 2 h | PLA scaffolds PCL/PANi nanofibers PPY and polyurethane scaffold multi-walled carbon nanotubes (MWCNTs) embedded with para-toluene sulfonic acid-doped PPY |
| Bone | 15 Hz | 200 mV/mm of ES 100 μA DC current |
2–8 h | PPY, alginate, and chitosan composite PEDOT: PSS (poly (4-styrene sulfonate)) gelatin bioglass composite PPY and PLA scaffolds |
Declaration of competing interest
There is No conflict of interest to publish this review article.
Acknowledgements
The authors acknowledge funding support from the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (R01EB020640) and the Connecticut Regenerative Medicine Research Fund (15-RMBUCHC-08). Thanks to Christopher Bonin for help editing the manuscript.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Ray W.Z., Mackinnon S.E. Management of nerve gaps: autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp. Neurol. 2010;223(1):77–85. doi: 10.1016/j.expneurol.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubernard J.-M., Owen E., Herzberg G., Lanzetta M., Martin X., Kapila H., Dawahra M., Hakim N.S. Human hand allograft: report on first 6 months. Lancet. 1999;353(9161):1315–1320. doi: 10.1016/S0140-6736(99)02062-0. [DOI] [PubMed] [Google Scholar]
- 3.Adachi N., Ochi M., Uchio Y., Sakai Y., Kuriwaka M., Fujihara A. Harvesting hamstring tendons for ACL reconstruction influences postoperative hamstring muscle performance. Arch. Orthop. Trauma Surg. 2003;123(9):460–465. doi: 10.1007/s00402-003-0572-2. [DOI] [PubMed] [Google Scholar]
- 4.Manji R.A., Zhu L.F., Nijjar N.K., Rayner D.C., Korbutt G.S., Churchill T.A., Rajotte R.V., Koshal A., Ross D.B. Glutaraldehyde-fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejection. Circulation. 2006;114(4):318–327. doi: 10.1161/CIRCULATIONAHA.105.549311. [DOI] [PubMed] [Google Scholar]
- 5.Damien C.J., Parsons J.R. Bone graft and bone graft substitutes: a review of current technology and applications. J. Appl. Biomater. 1991;2(3):187–208. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt C.E., Leach J.B. Neural tissue engineering: strategies for repair and regeneration. Annu. Rev. Biomed. Eng. 2003;5(1):293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 7.Kartus J., Movin T., Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthrosc. J. Arthrosc. Relat. Surg. 2001;17(9):971–980. doi: 10.1053/jars.2001.28979. [DOI] [PubMed] [Google Scholar]
- 8.LaPrade R.F., Botker J.C. Donor-site morbidity after osteochondral autograft transfer procedures. Arthrosc. J. Arthrosc. Relat. Surg. 2004;20(7):e69–e73. doi: 10.1016/j.arthro.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Vangsness C.T., Wagner P.P., Moore T.M., Roberts M.R. Overview of safety issues concerning the preparation and processing of soft-tissue allografts. Arthrosc. J. Arthrosc. Relat. Surg. 2006;22(12):1351–1358. doi: 10.1016/j.arthro.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Vangsness C.T., Dellamaggiora R.D. Current safety sterilization and tissue banking issues for soft tissue allografts. Clin. Sports Med. 2009;28(2):183–189. doi: 10.1016/j.csm.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cairns T.D.H., Taube D.H., Stevens N., Binns R., Welsh K.I. Xenografts — future prospects for clinical transplantation. Immunol. Lett. 1991;29(1):167–170. doi: 10.1016/0165-2478(91)90221-u. [DOI] [PubMed] [Google Scholar]
- 12.Allaire E., Bruneval P., Mandet C., Becquemin J.-P., Michel J.-B. The immunogenicity of the extracellular matrix in arterial xenografts. Surgery. 1997;122(1):73–81. doi: 10.1016/s0039-6060(97)90267-1. [DOI] [PubMed] [Google Scholar]
- 13.Tomford W.W. Bone allografts: past, present and future. Cell Tissue Bank. 2000;1(2):105–109. doi: 10.1023/A:1010158731885. [DOI] [PubMed] [Google Scholar]
- 14.Maul T.M., Chew D.W., Nieponice A., Vorp D.A. Mechanical stimuli differentially control stem cell behavior: morphology, proliferation, and differentiation. Biomech. Model. Mechanobiol. 2011;10(6):939–953. doi: 10.1007/s10237-010-0285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nii M., Lai J.H., Keeney M., Han L.-H., Behn A., Imanbayev G., Yang F. The effects of interactive mechanical and biochemical niche signaling on osteogenic differentiation of adipose-derived stem cells using combinatorial hydrogels. Acta Biomater. 2013;9(3):5475–5483. doi: 10.1016/j.actbio.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Schuldiner M., Yanuka O., Itskovitz-Eldor J., Melton D.A., Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc. Natl. Acad. Sci. Unit. States Am. 2000;97(21):11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q., Brus-Ramer M., Martin J.H., McDonald J.W. Electrical stimulation of the medullary pyramid promotes proliferation and differentiation of oligodendrocyte progenitor cells in the corticospinal tract of the adult rat. Neurosci. Lett. 2010;479(2):128–133. doi: 10.1016/j.neulet.2010.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y.J., Wu H.C., Tai N.H., Wang T.W. Carbon nanotube rope with electrical stimulation promotes the differentiation and maturity of neural stem cells. Small. 2012;8(18):2869–2877. doi: 10.1002/smll.201200715. [DOI] [PubMed] [Google Scholar]
- 19.Wu C.-C., Chao Y.-C., Chen C.-N., Chien S., Chen Y.-C., Chien C.-C., Chiu J.-J., Yen B.L. Synergism of biochemical and mechanical stimuli in the differentiation of human placenta-derived multipotent cells into endothelial cells. J. Biomech. 2008;41(4):813–821. doi: 10.1016/j.jbiomech.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Steward A.J., Kelly D.J. Mechanical regulation of mesenchymal stem cell differentiation. J. Anat. 2015;227(6):717–731. doi: 10.1111/joa.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakayama T., Momoki-Soga T., Inoue N. Astrocyte-derived factors instruct differentiation of embryonic stem cells into neurons. Neurosci. Res. 2003;46(2):241–249. doi: 10.1016/s0168-0102(03)00063-4. [DOI] [PubMed] [Google Scholar]
- 22.Chen F.-M., Zhang M., Wu Z.-F. Toward delivery of multiple growth factors in tissue engineering. Biomaterials. 2010;31(24):6279–6308. doi: 10.1016/j.biomaterials.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 23.Balint R., Cassidy N.J., Cartmell S.H. Electrical stimulation: a novel tool for tissue engineering. Tissue Eng. B Rev. 2012;19(1):48–57. doi: 10.1089/ten.TEB.2012.0183. [DOI] [PubMed] [Google Scholar]
- 24.Griffin M., Bayat A. Electrical stimulation in bone healing: critical analysis by evaluating levels of evidence. Eplasty. 2011;11 [PMC free article] [PubMed] [Google Scholar]
- 25.Kern H., Carraro U., Adami N., Biral D., Hofer C., Forstner C., Mödlin M., Vogelauer M., Pond A., Boncompagni S. Home-based functional electrical stimulation rescues permanently denervated muscles in paraplegic patients with complete lower motor neuron lesion. Neurorehabilitation Neural Repair. 2010;24(8):709–721. doi: 10.1177/1545968310366129. [DOI] [PubMed] [Google Scholar]
- 26.Pires F., Ferreira Q., Rodrigues C.A., Morgado J., Ferreira F.C. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim. Biophys. Acta Gen. Subj. 2015;1850(6):1158–1168. doi: 10.1016/j.bbagen.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 27.Ghasemi-Mobarakeh L., Prabhakaran M.P., Morshed M., Nasr-Esfahani M.H., Baharvand H., Kiani S., Al-Deyab S.S., Ramakrishna S. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J. Tissue Eng. Regen. Med. 2011;5(4):e17–e35. doi: 10.1002/term.383. [DOI] [PubMed] [Google Scholar]
- 28.Richard Balint N.J.C., Sarah H. Cartmell Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater. 2014;10 doi: 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt C.E., Shastri V.R., Vacanti J.P., Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl. Acad. Sci. Unit. States Am. 1997;94(17):8948–8953. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guiseppi-Elie A. Electroconductive hydrogels: synthesis, characterization and biomedical applications. Biomaterials. 2010;(31) doi: 10.1016/j.biomaterials.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 31.Balint R., Cassidy N.J., Cartmell S.H. Conductive polymers: towards a smart biomaterial for tissue engineering. Acta Biomater. 2014;10(6):2341–2353. doi: 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 32.Shirakawa H., Louis E.J., MacDiarmid A.G., Chiang C.K., Heeger A.J. Synthesis of electrically conducting organic polymers: halogen derivatives of polyacetylene,(CH) x. J. Chem. Soc., Chem. Commun. 1977;(16):578–580. [Google Scholar]
- 33.Manoukian O.S., Stratton S., Arul M.R., Moskow J., Sardashti N., Yu X., Rudraiah S., Kumbar S.G. Polymeric ionically conductive composite matrices and electrical stimulation strategies for nerve regeneration: in vitro characterization. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107(6):1792–1805. doi: 10.1002/jbm.b.34272. Epub 2018 Nov 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manoukian O.S., Baker J.T., Rudraiah S., Arul M.R., Vella A.T., Domb A.J., Kumbar S.G. Functional polymeric nerve guidance conduits and drug delivery strategies for peripheral nerve repair and regeneration. J. Contr. Release. 2020;317:78–95. doi: 10.1016/j.jconrel.2019.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Moskow J., Ferrigno B., Mistry N., Jaiswal D., Bulsara K., Rudraiah S., Kumbar S.G. 2018. Bioengineering Approach for the Repair and Regeneration of Peripheral Nerve, Bioactive Materials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Araújo R., Franciulli P., Assis R., Souza R., Mochizuki L. Effects of laser, ultrasound and electrical stimulation on the repair of achilles tendon injuries in rats: a comparative study. Braz. J. Morphol. Sci. 2007;24(3):187–191. [Google Scholar]
- 37.Guo B., Ma P.X. Conducting polymers for tissue engineering. Biomacromolecules. 2018;19(6):1764–1782. doi: 10.1021/acs.biomac.8b00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang S., Jang L., Kim S., Yang J., Yang K., Cho S.W., Lee J.Y. Polypyrrole/alginate hybrid hydrogels: electrically conductive and soft biomaterials for human mesenchymal stem cell culture and potential neural tissue engineering applications. Macromol. Biosci. 2016;16(11):1653–1661. doi: 10.1002/mabi.201600148. [DOI] [PubMed] [Google Scholar]
- 39.Yamada M., Tanemura K., Okada S., Iwanami A., Nakamura M., Mizuno H., Ozawa M., Ohyama-Goto R., Kitamura N., Kawano M., Tan-Takeuchi K., Ohtsuka C., Miyawaki A., Takashima A., Ogawa M., Toyama Y., Okano H., Kondo T. Electrical stimulation modulates fate determination of differentiating embryonic stem cells. Stem Cell. 2007;25(3):562–570. doi: 10.1634/stemcells.2006-0011. [DOI] [PubMed] [Google Scholar]
- 40.Tandon N., Goh B., Marsano A., Chao P.-H.G., Montouri-Sorrentino C., Gimble J., Vunjak-Novakovic G. Engineering in Medicine and Biology Society, 2009. EMBC 2009. Annual International Conference of the IEEE. IEEE; 2009. Alignment and elongation of human adipose-derived stem cells in response to direct-current electrical stimulation; pp. 6517–6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe M., Ogata N. Ionic conductivity of polymer electrolytes and future applications. Br. Polym. J. 1988;20(3):181–192. [Google Scholar]
- 42.Aaron R.K., Ciombor D.M. Therapeutic effects of electromagnetic fields in the stimulation of connective tissue repair. J. Cell. Biochem. 1993;52(1):42–46. doi: 10.1002/jcb.240520107. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt C.E., Shastri V.R., Vacanti J.P., Langer R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl. Acad. Sci. Unit. States Am. 1997;94(17):8948–8953. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mozafari M., Mehraien M., Vashaee D., Tayebi L. 2012. Electroconductive Nanocomposite Scaffolds: a New Strategy into Tissue Engineering and Regenerative Medicine. [Google Scholar]
- 45.Wang Y., Shi Y., Pan L., Ding Y., Zhao Y., Li Y., Shi Y., Yu G. Dopant-enabled supramolecular approach for controlled synthesis of nanostructured conductive polymer hydrogels. Nano Lett. 2015;15(11):7736–7741. doi: 10.1021/acs.nanolett.5b03891. [DOI] [PubMed] [Google Scholar]
- 46.Pan L., Yu G., Zhai D., Lee H.R., Zhao W., Liu N., Wang H., Tee B.C.-K., Shi Y., Cui Y. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. Unit. States Am. 2012;109(24):9287–9292. doi: 10.1073/pnas.1202636109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Leeuw D.M., Simenon M.M.J., Brown A.R., Einerhand R.E.F. Stability of n-type doped conducting polymers and consequences for polymeric microelectronic devices. Synth. Met. 1997;87(1):53–59. [Google Scholar]
- 48.Snook G.A., Kao P., Best A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources. 2011;196(1):1–12. [Google Scholar]
- 49.Kai D., Prabhakaran M.P., Jin G., Ramakrishna S. Polypyrrole‐contained electrospun conductive nanofibrous membranes for cardiac tissue engineering. J. Biomed. Mater. Res. 2011;99(3):376–385. doi: 10.1002/jbm.a.33200. [DOI] [PubMed] [Google Scholar]
- 50.Bendrea A.-D., Cianga L., Cianga I. Progress in the field of conducting polymers for tissue engineering applications. J. Biomater. Appl. 2011;26(1):3–84. doi: 10.1177/0885328211402704. [DOI] [PubMed] [Google Scholar]
- 51.Stewart E., Kobayashi N.R., Higgins M.J., Quigley A.F., Jamali S., Moulton S.E., Kapsa R.M., Wallace G.G., Crook J.M. Electrical stimulation using conductive polymer polypyrrole promotes differentiation of human neural stem cells: a biocompatible platform for translational neural tissue engineering. Tissue Eng. C Methods. 2014;21(4):385–393. doi: 10.1089/ten.TEC.2014.0338. [DOI] [PubMed] [Google Scholar]
- 52.Huang Z.-B., Yin G.-F., Liao X.-M., Gu J.-W. Conducting polypyrrole in tissue engineering applications. Front. Mater. Sci. 2014;8(1):39–45. [Google Scholar]
- 53.Goel S., Mazumdar N.A., Gupta A. Synthesis and characterization of polypyrrole nanofibers with different dopants. Polym. Adv. Technol. 2010;21(3):205–210. [Google Scholar]
- 54.Qazi T.H., Rai R., Dippold D., Roether J.E., Schubert D.W., Rosellini E., Barbani N., Boccaccini A.R. Development and characterization of novel electrically conductive PANI–PGS composites for cardiac tissue engineering applications. Acta Biomater. 2014;10(6):2434–2445. doi: 10.1016/j.actbio.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 55.Wan Y., Creber K.A., Peppley B., Bui V.T. Synthesis, characterization and ionic conductive properties of phosphorylated chitosan membranes. Macromol. Chem. Phys. 2003;204(5‐6):850–858. [Google Scholar]
- 56.Dubey N., Bentini R., Islam I., Cao T., Castro Neto A.H., Rosa V. Graphene: a versatile carbon-based material for bone tissue engineering. Stem Cell. Int. 2015;2015 doi: 10.1155/2015/804213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geim A.K., Novoselov K.S. World Scientific; 2010. The Rise of Graphene, Nanoscience and Technology: A Collection of Reviews from Nature Journals; pp. 11–19. [Google Scholar]
- 58.Shelke N.B., Lee P., Anderson M., Mistry N., Nagarale R.K., Ma X.-M., Yu X., Kumbar S.G. Neural tissue engineering: nanofiber-hydrogel based composite scaffolds. Polym. Adv. Technol. 2016;27(1):42–51. [Google Scholar]
- 59.Sayyar S., Murray E., Thompson B., Chung J., Officer D.L., Gambhir S., Spinks G.M., Wallace G.G. Processable conducting graphene/chitosan hydrogels for tissue engineering. J. Mater. Chem. B. 2015;3(3):481–490. doi: 10.1039/c4tb01636j. [DOI] [PubMed] [Google Scholar]
- 60.Dimassi S., Tabary N., Chai F., Blanchemain N., Martel B. Sulfonated and sulfated chitosan derivatives for biomedical applications: a review. Carbohydr. Polym. 2018;202:382–396. doi: 10.1016/j.carbpol.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Manoukian O.S., Arul M.R., Rudraiah S., Kalajzic I., Kumbar S.G. Aligned microchannel polymer-nanotube composites for peripheral nerve regeneration: small molecule drug delivery. J. Contr. Release. 2019;296:54–67. doi: 10.1016/j.jconrel.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.James R., Nagarale R.K., Sachan V.K., Badalucco C., Bhattacharya P.K., Kumbar S.G. Synthesis and characterization of electrically conducting polymers for regenerative engineering applications: sulfonated ionic membranes. Polym. Adv. Technol. 2014;25(12):1439–1445. [Google Scholar]
- 63.Heller A. Searching for new truths of nature and creating people-serving products through bio-electrochemistry: the brain interface. Curr. Opin. Electrochem. 2018;12:3–4. [Google Scholar]
- 64.Zhao S., Tseng P., Grasman J., Wang Y., Li W., Napier B., Yavuz B., Chen Y., Howell L., Rincon J., Omenetto F.G., Kaplan D.L. Programmable hydrogel ionic circuits for biologically matched electronic interfaces. Adv. Mater. 2018;30(25) doi: 10.1002/adma.201800598. [DOI] [PubMed] [Google Scholar]
- 65.Tai G., Reid B., Cao L., Zhao M. Electrotaxis and wound healing: experimental methods to study electric fields as a directional signal for cell migration. Methods Mol. Biol. 2009;571:77–97. doi: 10.1007/978-1-60761-198-1_5. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y., Rouabhia M., Zhang Z. Pulsed electrical stimulation benefits wound healing by activating skin fibroblasts through the TGFbeta1/ERK/NF-kappaB axis. Biochim. Biophys. Acta. 2016;1860(7):1551–1559. doi: 10.1016/j.bbagen.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 67.Chen C., Bai X., Ding Y., Lee I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019;23 doi: 10.1186/s40824-019-0176-8. 25-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leitolis A., Robert A.W., Pereira I.T., Correa A., Stimamiglio M.A. Cardiomyogenesis modeling using pluripotent stem cells: the role of microenvironmental signaling. Front. Cell. Dev. Biol. 2019;7(164) doi: 10.3389/fcell.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao M., Song B., Pu J., Wada T., Reid B., Tai G., Wang F., Guo A., Walczysko P., Gu Y. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature. 2006;442(7101):457. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 70.Ud-Din S., Sebastian A., Giddings P., Colthurst J., Whiteside S., Morris J., Nuccitelli R., Pullar C., Baguneid M., Bayat A. Angiogenesis is induced and wound size is reduced by electrical stimulation in an acute wound healing model in human skin. PloS One. 2015;10(4) doi: 10.1371/journal.pone.0124502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hampton S., King L. Healing an intractable wound using bio-electrical stimulation therapy. Br. J. Nurs. 2005;14(Sup3):S30–S32. [PubMed] [Google Scholar]
- 72.Rouabhia M., Park H., Meng S., Derbali H., Zhang Z. Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PloS One. 2013;8(8) doi: 10.1371/journal.pone.0071660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nada A.A., Arul M.R., Ramos D.M., Kroneková Z., Mosnáček J., Rudraiah S., Kumbar S.G. Bioactive polymeric formulations for wound healing. Polym. Adv. Technol. 2018;29(6):1815–1825. doi: 10.1002/pat.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Manoukian O.S., Ahmad A., Marin C., James R., Mazzocca A.D., Kumbar S.G. 22 - bioactive nanofiber dressings for wound healing. In: Ågren M.S., editor. Wound Healing Biomaterials. Woodhead Publishing; 2016. pp. 451–481. [Google Scholar]
- 75.Machado A.F., Liebano R.E., Furtado F., Hochman B., Ferreira L.M. Effect of high-and low-frequency transcutaneous electrical nerve stimulation on angiogenesis and myofibroblast proliferation in acute excisional wounds in rat skin. Adv. Skin Wound Care. 2016;29(8):357–363. doi: 10.1097/01.ASW.0000488721.83423.f3. [DOI] [PubMed] [Google Scholar]
- 76.Suhail S., Sardashti N., Jaiswal D., Rudraiah S., Misra M., Kumbar S.G. Engineered skin tissue equivalents for product evaluation and therapeutic applications. Biotechnol. J. 2019;14(7):1900022. doi: 10.1002/biot.201900022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Prabhakaran M.P., Ghasemi-Mobarakeh L., Jin G., Ramakrishna S. Electrospun conducting polymer nanofibers and electrical stimulation of nerve stem cells. J. Biosci. Bioeng. 2011;112(5):501–507. doi: 10.1016/j.jbiosc.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 78.Anderson M., Shelke N.B., Manoukian O.S., Yu X., McCullough L.D., Kumbar S.G. Peripheral nerve regeneration strategies: electrically stimulating polymer based nerve growth conduits. Crit. Rev. Biomed. Eng. 2015;43(2–3):131–159. doi: 10.1615/CritRevBiomedEng.2015014015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Demerens C., Stankoff B., Logak M., Anglade P., Allinquant B., Couraud F., Zalc B., Lubetzki C. Induction of myelination in the central nervous system by electrical activity. Proc. Natl. Acad. Sci. Unit. States Am. 1996;93(18):9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCaig C.D., Rajnicek A.M., Song B., Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol. Rev. 2005;85(3):943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- 81.Elzinga K., Tyreman N., Ladak A., Savaryn B., Olson J., Gordon T. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp. Neurol. 2015;269:142–153. doi: 10.1016/j.expneurol.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 82.Macefield V., Gandevia S., Bigland‐Ritchie B., Gorman R., Burke D. The firing rates of human motoneurones voluntarily activated in the absence of muscle afferent feedback. J. Physiol. 1993;471(1):429–443. doi: 10.1113/jphysiol.1993.sp019908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Majed A.A., Neumann C.M., Brushart T.M., Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J. Neurosci. 2000;20(7):2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Willand M.P., Nguyen M.-A., Borschel G.H., Gordon T. Electrical stimulation to promote peripheral nerve regeneration. Neurorehabilitation Neural Repair. 2015;30(5):490–496. doi: 10.1177/1545968315604399. [DOI] [PubMed] [Google Scholar]
- 85.Huang J., Zhang Y., Lu L., Hu X., Luo Z. Electrical stimulation accelerates nerve regeneration and functional recovery in delayed peripheral nerve injury in rats. Eur. J. Neurosci. 2013;38(12):3691–3701. doi: 10.1111/ejn.12370. [DOI] [PubMed] [Google Scholar]
- 86.Wong J.N., Olson J.L., Morhart M.J., Chan K.M. Electrical stimulation enhances sensory recovery: a randomized controlled trial. Ann. Neurol. 2015;77(6):996–1006. doi: 10.1002/ana.24397. [DOI] [PubMed] [Google Scholar]
- 87.Gordon T., Amirjani N., Edwards D.C., Chan K.M. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp. Neurol. 2010;223(1):192–202. doi: 10.1016/j.expneurol.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 88.Syed N., Jullien G. Google Patents; 2006. Electrically Stimulating Nerve Regeneration. [Google Scholar]
- 89.Brushart T.M., Jari R., Verge V., Rohde C., Gordon T. Electrical stimulation restores the specificity of sensory axon regeneration. Exp. Neurol. 2005;194(1):221–229. doi: 10.1016/j.expneurol.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 90.Xu C., Kou Y., Zhang P., Han N., Yin X., Deng J., Chen B., Jiang B. Electrical stimulation promotes regeneration of defective peripheral nerves after delayed repair intervals lasting under one month. PloS One. 2014;9(9) doi: 10.1371/journal.pone.0105045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al-Majed A.A., Tam S.L., Gordon T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell. Mol. Neurobiol. 2004;24(3):379–402. doi: 10.1023/b:cemn.0000022770.66463.f7. [DOI] [PubMed] [Google Scholar]
- 92.Shi Z., Gao H., Feng J., Ding B., Cao X., Kuga S., Wang Y., Zhang L., Cai J. In situ synthesis of robust conductive cellulose/polypyrrole composite aerogels and their potential application in nerve regeneration. Angew. Chem. Int. Ed. 2014;53(21):5380–5384. doi: 10.1002/anie.201402751. [DOI] [PubMed] [Google Scholar]
- 93.Huang J., Lu L., Zhang J., Hu X., Zhang Y., Liang W., Wu S., Luo Z. Electrical stimulation to conductive scaffold promotes axonal regeneration and remyelination in a rat model of large nerve defect. PloS One. 2012;7(6) doi: 10.1371/journal.pone.0039526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thompson B.C., Richardson R.T., Moulton S.E., Evans A.J., O'Leary S., Clark G.M., Wallace G.G. Conducting polymers, dual neurotrophins and pulsed electrical stimulation — dramatic effects on neurite outgrowth. J. Contr. Release. 2010;141(2):161–167. doi: 10.1016/j.jconrel.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 95.Hyman J., Rodeo S.A. Injury and repair of tendons and ligaments. Phys. Med. Rehabil. Clin. 2000;11(2):267–288. [PubMed] [Google Scholar]
- 96.Peach M.S., Ramos D.M., James R., Morozowich N.L., Mazzocca A.D., Doty S.B., Allcock H.R., Kumbar S.G., Laurencin C.T. Engineered stem cell niche matrices for rotator cuff tendon regenerative engineering. PloS One. 2017;12(4) doi: 10.1371/journal.pone.0174789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ramos D., Laurencin C., Kumbar S. TISSUE ENGINEERING PART A, MARY ANN LIEBERT, INC; 140 HUGUENOT STREET, 3RD FL, NEW ROCHELLE, NY 10801 USA: 2015. Peptide Insulin for Tendon Differentiation of Human Mesenchymal Stem Cells; pp. S407–S408. [Google Scholar]
- 98.Koeppen B.M., Stanton B.A. Updated Edition. E-Book, Elsevier Health Sciences; 2009. Berne & Levy Physiology. [Google Scholar]
- 99.Ramos D., Peach M.S., Mazzocca A.D., Yu X., Kumbar S.G. 8 - tendon tissue engineering. In: Nukavarapu S.P., Freeman J.W., Laurencin C.T., editors. Regenerative Engineering of Musculoskeletal Tissues and Interfaces. Woodhead Publishing; 2015. pp. 195–217. [Google Scholar]
- 100.Gross M.T. Chronic tendinitis: pathomechanics of injury, factors affecting the healing response, and treatment. J. Orthop. Sports Phys. Ther. 1992;16(6):248–261. doi: 10.2519/jospt.1992.16.6.248. [DOI] [PubMed] [Google Scholar]
- 101.Ramos D.M., Laurencin C.T., Mazzocca A.D., Kumbar S.G. Frontiers in Bioengineering and Biotechnology Conference Abstracts. 2016. Tendon tissue differentiation with use of fibrous scaffolds and peptide insulin for rotator cuff applications. [Google Scholar]
- 102.Ahmed A.F., Elgayed S.S., Ibrahim I.M. Polarity effect of microcurrent electrical stimulation on tendon healing: biomechanical and histopathological studies. J. Adv. Res. 2012;3(2):109–117. [Google Scholar]
- 103.Ramos D.M., Abdulmalik S., Arul M.R., Rudraiah S., Laurencin C.T., Mazzocca A.D., Kumbar S.G. Insulin immobilized PCL-cellulose acetate micro-nanostructured fibrous scaffolds for tendon tissue engineering. Polym. Adv. Technol. 2019;30(5):1205–1215. doi: 10.1002/pat.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Burssens P., Forsyth R., Steyaert A., Van Ovost E., Praet M., Verdonk R. Influence of burst TENS stimulation on collagen formation after Achilles tendon suture in man. A histological evaluation with Movat's pentachrome stain. Acta Orthop. Belg. 2005;71(3):342–346. [PubMed] [Google Scholar]
- 105.Razal J.M., Kita M., Quigley A.F., Kennedy E., Moulton S.E., Kapsa R.M.I., Clark G.M., Wallace G.G. Wet‐spun biodegradable fibers on conducting platforms: novel architectures for muscle regeneration. Adv. Funct. Mater. 2009;19(21):3381–3388. [Google Scholar]
- 106.Hardy J.G., Lee J.Y., Schmidt C.E. Biomimetic conducting polymer-based tissue scaffolds. Curr. Opin. Biotechnol. 2013;24(5):847–854. doi: 10.1016/j.copbio.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 107.Rowlands A.S., Cooper-White J.J. Directing phenotype of vascular smooth muscle cells using electrically stimulated conducting polymer. Biomaterials. 2008;29(34):4510–4520. doi: 10.1016/j.biomaterials.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 108.Mooney E., Mackle J.N., Blond D.J.P., O'Cearbhaill E., Shaw G., Blau W.J., Barry F.P., Barron V., Murphy J.M. The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials. 2012;33(26):6132–6139. doi: 10.1016/j.biomaterials.2012.05.032. [DOI] [PubMed] [Google Scholar]
- 109.Miklavčič D., Pavšelj N., Hart F.X. Wiley Encyclopedia of Biomedical Engineering, John Wiley & Sons, Inc.; 2006. Electric Properties of Tissues. [Google Scholar]
- 110.Chen M.-C., Sun Y.-C., Chen Y.-H. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 2013;9(3):5562–5572. doi: 10.1016/j.actbio.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 111.Lazzarotto Rucatti A., Boemo Jaenisch R., Dalcin Rossato D., Poletto Bonetto J.H., Ferreira J., Xavier L.L., Sonza A., Lago P.D. Skeletal muscle electrical stimulation improves baroreflex sensitivity and heart rate variability in heart failure rats. Auton. Neurosci. 2015;193:92–96. doi: 10.1016/j.autneu.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 112.Dirks M.L., Wall B.T., Snijders T., Ottenbros C.L.P., Verdijk L.B., Loon L.J.C. Neuromuscular electrical stimulation prevents muscle disuse atrophy during leg immobilization in humans. Acta Physiol. 2014;210(3):628–641. doi: 10.1111/apha.12200. [DOI] [PubMed] [Google Scholar]
- 113.Groah S.L., Lichy A.M., Libin A.V., Ljungberg I. Intensive electrical stimulation attenuates femoral bone loss in acute spinal cord injury. Arch. PM&R (Phys. Med. Rehabil.) 2010;2(12):1080–1087. doi: 10.1016/j.pmrj.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 114.Maffiuletti N.A., Roig M., Karatzanos E., Nanas S. Neuromuscular electrical stimulation for preventing skeletal-muscle weakness and wasting in critically ill patients: a systematic review. BMC Med. 2013;11(1):137. doi: 10.1186/1741-7015-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Langelaan M.L.P., Boonen K.J.M., Rosaria‐Chak K.Y., Schaft D.W.J.v.d., Post M.J., Baaijens F.P.T. Advanced maturation by electrical stimulation: differences in response between C2C12 and primary muscle progenitor cells. J. Tissue Eng. Regen. Med. 2011;5(7):529–539. doi: 10.1002/term.345. [DOI] [PubMed] [Google Scholar]
- 116.Wall B.T., Dirks M.L., Verdijk L.B., Snijders T., Hansen D., Vranckx P., Burd N.A., Dendale P., van Loon L.J.C. Neuromuscular electrical stimulation increases muscle protein synthesis in elderly type 2 diabetic men. Am. J. Physiol. Endocrinol. Metabol. 2012;303(5):E614–E623. doi: 10.1152/ajpendo.00138.2012. [DOI] [PubMed] [Google Scholar]
- 117.Broda C.R., Lee J.Y., Sirivisoot S., Schmidt C.E., Harrison B.S. A chemically polymerized electrically conducting composite of polypyrrole nanoparticles and polyurethane for tissue engineering. J. Biomed. Mater. Res. 2011;98A(4):509–516. doi: 10.1002/jbm.a.33128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gilmore K.J., Kita M., Han Y., Gelmi A., Higgins M.J., Moulton S.E., Clark G.M., Kapsa R., Wallace G.G. Skeletal muscle cell proliferation and differentiation on polypyrrole substrates doped with extracellular matrix components. Biomaterials. 2009;30(29):5292–5304. doi: 10.1016/j.biomaterials.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 119.Quigley A.F., Razal J.M., Kita M., Jalili R., Gelmi A., Penington A., Ovalle‐Robles R., Baughman R.H., Clark G.M., Wallace G.G. Electrical stimulation of myoblast proliferation and differentiation on aligned nanostructured conductive polymer platforms. Adv. Healthc. Mater. 2012;1(6):801–808. doi: 10.1002/adhm.201200102. [DOI] [PubMed] [Google Scholar]
- 120.Park H., Bhalla R., Saigal R., Radisic M., Watson N., Langer R., Vunjak‐Novakovic G. Effects of electrical stimulation in C2C12 muscle constructs. J. Tissue Eng. Regen. Med. 2008;2(5):279–287. doi: 10.1002/term.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Williams H.B. The value of continuous electrical muscle stimulation using a completely implantable system in the preservation of muscle function following motor nerve injury and repair: an experimental study. Microsurgery. 1996;17(11):589–596. doi: 10.1002/(SICI)1098-2752(1996)17:11<589::AID-MICR5>3.0.CO;2-K. Official Journal of the International Microsurgical Society and the European Federation of Societies for Microsurgery. [DOI] [PubMed] [Google Scholar]
- 122.Hang J., Kong L., Gu J., Adair T. VEGF gene expression is upregulated in electrically stimulated rat skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 1995;269(5):H1827–H1831. doi: 10.1152/ajpheart.1995.269.5.H1827. [DOI] [PubMed] [Google Scholar]
- 123.Borriello A., Guarino V., Schiavo L., Alvarez-Perez M.A., Ambrosio L. Optimizing PANi doped electroactive substrates as patches for the regeneration of cardiac muscle. J. Mater. Sci. Mater. Med. 2011;22(4):1053–1062. doi: 10.1007/s10856-011-4259-x. [DOI] [PubMed] [Google Scholar]
- 124.Isaacson B.M., Bloebaum R.D. Bone bioelectricity: what have we learned in the past 160 years? J. Biomed. Mater. Res. 2010;95A(4):1270–1279. doi: 10.1002/jbm.a.32905. [DOI] [PubMed] [Google Scholar]
- 125.Shahini A., Yazdimamaghani M., Walker K.J., Eastman M.A., Hatami-Marbini H., Smith B.J., Ricci J.L., Madihally S.V., Vashaee D., Tayebi L. 3D conductive nanocomposite scaffold for bone tissue engineering. Int. J. Nanomed. 2014;9:167. doi: 10.2147/IJN.S54668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kumar A., Zhang Y., Terracciano A., Zhao X., Su T.-L., Kalyon D.M., Katebifar S., Kumbar S.G., Yu X. Load-bearing biodegradable polycaprolactone-poly (lactic-co-glycolic acid)- beta tri-calcium phosphate scaffolds for bone tissue regeneration. Polym. Adv. Technol. 2019;30(5):1189–1197. doi: 10.1002/pat.4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Miller M.Q., McColl L.F., Arul M.R., Nip J., Madhu V., Beck G., Mathur K., Sahadeo V., Kerrigan J.R., Park S.S., Christophel J.J., Dighe A.S., Kumbar S.G., Cui Q. Assessment of Hedgehog signaling pathway activation for craniofacial bone regeneration in a critical-sized rat mandibular defect. JAMA Facial Plast. Surg. 2019;21(2):110–117. doi: 10.1001/jamafacial.2018.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chang J.H., Chen P.-J., Arul M.R., Dutra E.H., Nanda R., Kumbar S.G., Yadav S. Injectable RANKL sustained release formulations to accelerate orthodontic tooth movement. EJO (Eur. J. Orthod.) 2019;cjz027 doi: 10.1093/ejo/cjz027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Manoukian O.S., Aravamudhan A., Lee P., Arul M.R., Yu X., Rudraiah S., Kumbar S.G. Spiral layer-by-layer micro-nanostructured scaffolds for bone tissue engineering. ACS Biomater. Sci. Eng. 2018;4(6):2181–2192. doi: 10.1021/acsbiomaterials.8b00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Griffin M., Bayat A. Electrical stimulation in bone healing: critical analysis by evaluating levels of evidence. Eplasty. 2011;11:e34. [PMC free article] [PubMed] [Google Scholar]
- 131.Gargiulo P., Helgason T., Reynisson P.J., Helgason B., Kern H., Mayr W., Ingvarsson P., Carraro U. Monitoring of muscle and bone recovery in spinal cord injury patients treated with electrical stimulation using three‐dimensional imaging and segmentation techniques: methodological assessment. Artif. Organs. 2011;35(3):275–281. doi: 10.1111/j.1525-1594.2011.01214.x. [DOI] [PubMed] [Google Scholar]
- 132.Ciombor D.M., Aaron R.K. The role of electrical stimulation in bone repair. Foot Ankle Clin. 2005;10(4):579–593. doi: 10.1016/j.fcl.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 133.Bodamyali T., Kanczler J., Simon B., Blake D., Stevens C. Effect of faradic products on direct current-stimulated calvarial organ culture calcium levels. Biochem. Biophys. Res. Commun. 1999;264(3):657–661. doi: 10.1006/bbrc.1999.1355. [DOI] [PubMed] [Google Scholar]
- 134.Lohmann C.H., Schwartz Z., Liu Y., Guerkov H., Dean D.D., Simon B., Boyan B.D. Pulsed electromagnetic field stimulation of MG63 osteoblast‐like cells affects differentiation and local factor production. J. Orthop. Res. 2000;18(4):637–646. doi: 10.1002/jor.1100180417. [DOI] [PubMed] [Google Scholar]
- 135.Huang C.P., Chen X.M., Chen Z.Q. Osteocyte: the impresario in the electrical stimulation for bone fracture healing. Med. Hypotheses. 2008;70(2):287–290. doi: 10.1016/j.mehy.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 136.Supronowicz P., Ajayan P., Ullmann K., Arulanandam B., Metzger D., Bizios R. Novel current‐conducting composite substrates for exposing osteoblasts to alternating current stimulation. J. Biomed. Mater. Res. 2002;59(3):499–506. doi: 10.1002/jbm.10015. [DOI] [PubMed] [Google Scholar]
- 137.James R., Deng M., Kumbar S.G., Laurencin C.T. Bone regenerative engineering: the influence of micro-and nano-dimension, tissue and organ regeneration. Adv. Micro Nanotechnology. 2014:455. [Google Scholar]
- 138.Itoh S., Nakamura S., Nakamura M., Shinomiya K., Yamashita K. Enhanced bone ingrowth into hydroxyapatite with interconnected pores by electrical polarization. Biomaterials. 2006;27(32):5572–5579. doi: 10.1016/j.biomaterials.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 139.Sajesh K.M., Jayakumar R., Nair S.V., Chennazhi K.P. Biocompatible conducting chitosan/polypyrrole–alginate composite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2013;62:465–471. doi: 10.1016/j.ijbiomac.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 140.Meng S., Zhang Z., Rouabhia M. Accelerated osteoblast mineralization on a conductive substrate by multiple electrical stimulation. J. Bone Miner. Metabol. 2011;29(5):535–544. doi: 10.1007/s00774-010-0257-1. [DOI] [PubMed] [Google Scholar]
- 141.Meng S., Rouabhia M., Zhang Z. Electrical stimulation modulates osteoblast proliferation and bone protein production through heparin‐bioactivated conductive scaffolds. Bioelectromagnetics. 2013;34(3):189–199. doi: 10.1002/bem.21766. [DOI] [PubMed] [Google Scholar]
- 142.Shao S., Zhou S., Li L., Li J., Luo C., Wang J., Li X., Weng J. Osteoblast function on electrically conductive electrospun PLA/MWCNTs nanofibers. Biomaterials. 2011;32(11):2821–2833. doi: 10.1016/j.biomaterials.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 143.Kopitar A.N., Kotnik V., Vidmar G., Ihan A., Novak P., Stefancic M. Therapeutic electric stimulation does not affect immune status in healthy individuals - a preliminary report. Biomed. Eng. Online. 2012;11 doi: 10.1186/1475-925X-11-42. 42-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Novak P., Kopitar A.N., Vidmar G., Ihan A., Štefančič M. Therapeutic electrical stimulation and immune status in healthy men. Int. J. Rehabil. Res. 2018;41(4):349–357. doi: 10.1097/MRR.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 145.Borovikova L.V., Ivanova S., Zhang M., Yang H., Botchkina G.I., Watkins L.R., Wang H., Abumrad N., Eaton J.W., Tracey K.J. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 146.Kwan H., Garzoni L., Liu H.L., Cao M., Desrochers A., Fecteau G., Burns P., Frasch M.G. Vagus nerve stimulation for treatment of inflammation: systematic review of animal models and clinical studies. Bioelectron. Med. 2016;3:1–6. [PMC free article] [PubMed] [Google Scholar]
- 147.Kox M., van Eijk L.T., Verhaak T., Frenzel T., Kiers H.D., Gerretsen J., van der Hoeven J.G., Kornet L., Scheiner A., Pickkers P. Transvenous vagus nerve stimulation does not modulate the innate immune response during experimental human endotoxemia: a randomized controlled study. Arthritis Res. Ther. 2015;17(1) doi: 10.1186/s13075-015-0667-5. 150-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ning C., Yu P., Zhu Y., Yao M., Zhu X., Wang X., Lin Z., Li W., Wang S., Tan G., Zhang Y., Wang Y., Mao C. Built-in microscale electrostatic fields induced by anatase–rutile-phase transition in selective areas promote osteogenesis. NPG Asia Mater. 2016;8(3) doi: 10.1038/am.2016.9. e243-e243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Liu Y., Zhang X., Cao C., Zhang Y., Wei J., Li Y.j., Liang W., Hu Z., Zhang J., Wei Y., Deng X. Built-in electric fields dramatically induce enhancement of osseointegration. Adv. Funct. Mater. 2017;27(47):1703771. [Google Scholar]
- 150.Kai H., Yamauchi T., Ogawa Y., Tsubota A., Magome T., Miyake T., Yamasaki K., Nishizawa M. Accelerated wound healing on skin by electrical stimulation with a bioelectric plaster. Adv. Healthc. Mater. 2017;6(22):1700465. doi: 10.1002/adhm.201700465. [DOI] [PubMed] [Google Scholar]
- 151.Leppik L., Zhihua H., Mobini S., Parameswaran V.T., Eischen-Loges M., Slavici A., Helbing J., Pindur L., Oliveira K.M., Bhavsar M.B. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci. Rep. 2018;8(1):6307. doi: 10.1038/s41598-018-24892-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Annex B.H., Torgan C.E., Lin P., Taylor D.A., Thompson M.A., Peters K.G., Kraus W.E. Induction and maintenance of increased VEGF protein by chronic motor nerve stimulation in skeletal muscle. Am. J. Physiol. Heart Circ. Physiol. 1998;274(3):H860–H867. doi: 10.1152/ajpheart.1998.274.3.H860. [DOI] [PubMed] [Google Scholar]
- 153.Willand M.P., Rosa E., Michalski B., Zhang J.J., Gordon T., Fahnestock M., Borschel G.H. Electrical muscle stimulation elevates intramuscular BDNF and GDNF mRNA following peripheral nerve injury and repair in rats. Neuroscience. 2016;334:93–104. doi: 10.1016/j.neuroscience.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 154.Dhawan S.K., Conti S.F., Towers J., Abidi N.A., Vogt M. The effect of pulsed electromagnetic fields on hindfoot arthrodesis: a prospective study. J. Foot Ankle Surg. 2004;43(2):93–96. doi: 10.1053/j.jfas.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 155.Brighton C.T., Black J., Friedenberg Z., Esterhai J., Day L., Connolly J. A multicenter study of the treatment of non-union with constant direct current. J. Bone Joint Surg. 1981;63(1):2–13. [PubMed] [Google Scholar]