Abstract
Purpose
To describe a case of unilateral retinal arteriolar occlusion following multiple intravitreal brolucizumab injections for neovascular age-related macular degeneration (nAMD).
Observations
A 92-year-old Caucasian woman presented with blurry vision in her left eye (OS) after receiving the third dose of intravitreal brolucizumab. At the time of presentation, visual acuity (VA) was 20/40 in her right eye (OD) and had decreased from 20/150 to count finger (CF) at 1-foot OS. On examination, there was no evidence of active inflammation in the anterior chamber OU. Dilated fundus examination showed no vitritis in OD and 1+ vitreous cells OS, flame-shaped hemorrhage at the superior optic disc margin, and retinal whitening surrounding the proximal portion of the supero-temporal branch of the central retinal artery. There were drusen in OS and retinal pigment epithelial (RPE) changes in the maculae of OU. Intra-arteriolar greyish deposits were seen OS. Fluorescein angiography (FA) showed hyper-fluorescence in the maculae corresponding to fibrovascular pigment epithelial detachments (PED) OU. No peri-vascular leakage was noted OU. Delayed filling of multiple arterioles in early and late phases OS was observed on FA. The patient was diagnosed with retinal arteriolar occlusion associated with repeated intravitreal brolucizumab administrations.
Conclusion
Retinal arteriolar occlusion with severe vision loss, possibly secondary to inflammatory responses, can occur after subsequent intravitreal brolucizumab injections, even if no inflammation occurred after initial administrations. Vaso-occlusive disease should be considered as a potential ocular complication, with acute as well as delayed onset, following intravitreal brolucizumab therapy.
Keywords: Age-related macular degeneration, Brolucizumab, Intravitreal, Neovascular, Retinal vasculitis, Vaso-occlusion, Retinal occlusive vasculitis
1. Introduction
Intravitreal vascular endothelial growth factor (VEGF) inhibitors are currently the preferred treatment for choroidal neovascularization (CNV) secondary to neovascular age-related macular degeneration (nAMD), which is a major cause of vision loss in the elderly in developed countries.1, 2, 3 Ranibizumab, approved by Food and Drug Administration (FDA) in 2004, and aflibercept, approved by FDA in 2011 in the United States, have been well established as effective and safe anti-VEGF therapies for nAMD. In addition, bevacizumab, is an “off-label” VEGF inhibitor widely used in nAMD.
Brolucizumab is a rabbit derived humanized, single-chain variable fragment (scFv) antibody with a molecular mass of ~26kDa that inhibits VEGF-A. The phase 3 clinical trials, HAWK and HARRIER4,5 demonstrated non-inferiority in BCVA with brolucizumab (dosed every 8 or 12 weeks) compared to aflibercept (dosed every 8 weeks). In addition, brolucizumab treated eyes had greater reductions in retinal thickness compared to aflibercept treated eyes. HAWK and HARRIER reported that potentially serious adverse events associated with brolucizumab include hypersensitivity, endophthalmitis and retinal detachments, increased intraocular pressure, and systemic arterial thromboembolic events.6 While uveitis was noted as ocular adverse events (AEs) of interest in these studies, these AEs occurred at an incidence of 2.2% and 0.8% for brolucizumab 6 mg versus 0.3% and 0% for aflibercept, respectively, in HAWK and HARRIER. Approximately 90% of the uveitis cases were described and considered mild to moderate and were treated with a course of topical corticosteroids and/or topical antibiotics.5 In addition, there were 3 cases of either retinal artery “embolism, occlusion, or thrombosis” with 6 mg brolucizumab in the two studies versus 1 case with aflibercept.5 Based on the efficacy and safety outcomes from the pivotal clinical trials, on October 7, 2019, the United State Food and Drug Administration (FDA) approved brolucizumab for the treatment of nAMD.
On February 23, 2020, the American Society of Retinal Specialists (ASRS) alerted members to reported cases of ocular inflammation after brolucizumab treatment. In the statement, the ASRS indicated that “it has received reports of inflammation which included more than a dozen cases of vasculitis, of which greater than two-third were designated as occlusive retinal vasculitis by the reporting providers.”7
We herein describe a case of multiple retinal arteriolar occlusions associated with intravitreal brolucizumab injection that led to severe loss of vision in a patient with nAMD.
1.1. Case report
A 92-year-old Caucasian woman with nAMD in both eyes (OU) returned to the retina clinic because of significantly decreased vision in the left eye (OS). The patient's underlying systemic diseases included hypertension, arthritis, and hyperlipidemia.
The patient had been treated with different types of anti-VEGF therapy for her nAMD. In OD, the patient had received multiple intravitreal injections of bevacizumab with an incomplete response to treatment and persistent intraretinal fluid which resolved when treatment was switched to intravitreal aflibercept and remained as such when patient was transitioned back to bevacizumab. In OS, there was persistent CNV activity despite multiple injections of bevacizumab, ranibizumab, and aflibercept. There was no evidence of inflammation in OD or OS after intravitreal bevacizumab, ranibizumab, and aflibercept administrations. Because of the persistent nAMD activity in OS, intravitreal brolucizumab (6 mg) was recommended. After her first intravitreal brolucizumab injection, complete resolution of retinal fluid was noted, but there was no change in visual acuity (VA). No evidence of intraocular inflammation was noted after the first and second intravitreal brolucizumab injections. At the time of the third administration of intravitreal brolucizumab OS (February 13, 2020), the VA was 20/30 OD and 20/150 OS. No treatment was rendered OD at that visit. On February 29, 2020, the patient developed sudden blurry vision and noted floaters without eye pain or redness OS. However, she did not communicate the symptoms with the physician or the office until her follow-up appointment.
The patient returned to the clinic on March 5, 2020 with no changes in symptoms since onset five days prior. On ophthalmic examination, VA had decreased to count fingers (CF) at 1-foot OS and was stable OD. Intraocular pressure (IOP) was normal OU and slit-lamp examination did not reveal any cell or flare in either eye. Dilated fundus examination of OD showed no vitreous cells, disc with normal borders, and RPE changes in the macula. In OS, there was 1+ vitreous cells/debris. A flame-shape hemorrhage was noted at the superior disc border along with retinal whitening in the supero-nasal macula following the course of a retinal arteriole as well as whitening in the papillo-macular bundle adjacent to the optic nerve (Fig. 1E). In addition, there were intra-arteriolar greyish deposits (Fig. 1E) in multiple arterioles. Spectral-domain optical coherence tomography (SD-OCT) showed pigment epithelial detachments (PED) OU with no intraretinal or subretinal fluid (Fig. 2). The patient was given another intravitreal injection of bevacizumab OD and started on topical 1% prednisolone acetate four times daily OS.
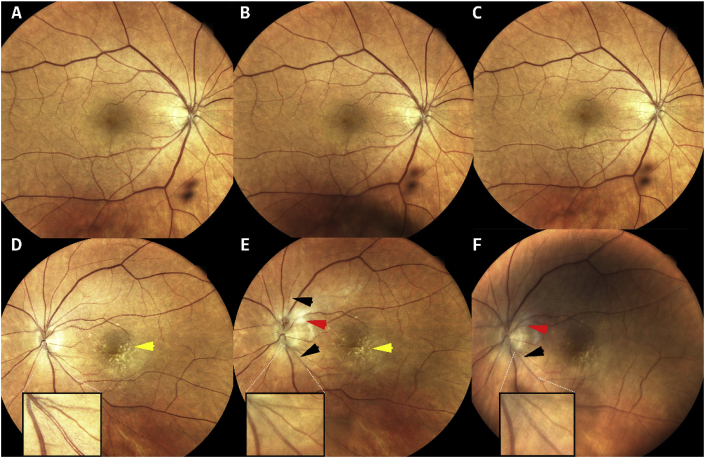
Fig. 1.
Fundus photograph (FP) of both eyes. FP of the right eye before (1A) and after (1B–1C) bevacizumab injection are within normal limits. Left eye before (1D) the third intravitreal brolucizumab injection showing drusen (yellow arrow); three weeks post-third intravitreal brolucizumab injection (1E) showing drusen (yellow arrow), whitish lesions near the disc (red arrow), small intra-arteriolar greyish depositions (black arrow); and four weeks post-third intravitreal brolucizumab injection (1F) showing whitish lesions near disc (red arrow), small intra-arteriolar greyish depositions (black arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
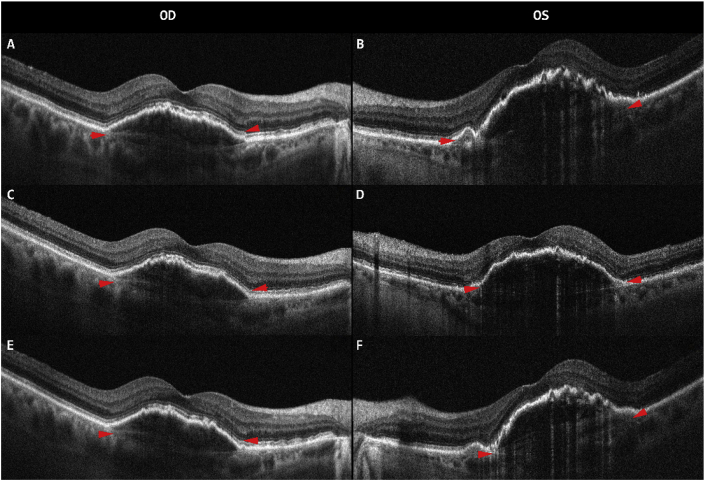
Fig. 2.
Optical coherence tomography (Spectralis, Heidelberg Engineering, Heidelberg, Germany) of the right (OD) and left (OS) eyes. OCT of OD after multiple intravitreal bevacizumab injections (2A, 2C, 2E). Before the third intravitreal brolucizumab injection in OS (2B), three weeks post-third intravitreal brolucizumab injection (2D) and four weeks post-third intravitreal brolucizumab injection (2F). Pigmented epithelial detachment can be seen at all visits in both OD and OS (red arrow). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
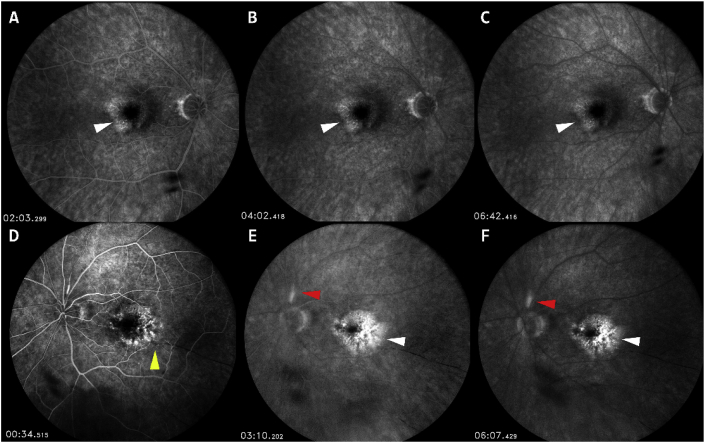
The patient returned to the clinic on March 12, 2020. VA remained CF at 1-foot OS. Slit-lamp examination revealed no cells or flare in the anterior chamber OU. Dilated fundus examination revealed RPE changes OU. SD-OCT remained unchanged from the previous visit on March 5, 2020 (Fig. 2E–F). Fluorescein angiography (FA) was performed and demonstrated hyper-fluorescence in the maculae corresponding to fibrovascular PED OU (Fig. 3). In addition, OS demonstrated segmental peri-vascular staining (less likely leakage) of an arteriole. In addition, there was delayed filling of multiple arterioles in the early phase demonstrating arteriolar occlusion (Fig. 3D) as well as areas of focal choroidal infarcts OS (not shown). There was staining of the disc border but no disc leakage OU.
Fig. 3.
Fluorescein angiography of right eye (OD) post-bevacizumab injection (top row) and post-third intravitreal brolucizumab injection (bottom row) in left eye (OS). No leakage of optic nerve and peri-vasculature can be seen in OD (3A-3C). Hyper-fluorescence in the peri-foveal region with no active leakage can be seen OU (white arrows). Delayed arteriolar filling in early phase (yellow arrow) can be seen in early phase FA in OS (3D). Hyper-fluorescence of the superior retinal arteriole near the optic disc (red arrows) can be seen without leakage in mid to late FA in OS (3E-3F). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Because of the ocular inflammation and multiple arteriolar occlusions OS, laboratory evaluations (CBC with differential, erythrocyte sedimentation rate, and C-reactive protein) along with a thorough history and review of systems were obtained, both were negative for giant cell arteritis (GCA). Evaluation for autoimmune/inflammatory diseases were also normal or negative.
Based on the negative laboratory testing, we suspected that the patient had multiple retinal arteriolar occlusions secondary to ocular inflammation attributed to brolucizumab OS. Given the presentation, the time of events post intravitreal administration of pharmacologic agents, clinical and angiographic findings, and the negative laboratory evaluation for associated systemic diseases, a diagnosis of multiple retinal arteriolar occlusions secondary to brolucizumab appears logical, especially in light of other cases reported by the ASRS. As the vision has decreased to CF, and there was no evidence of active inflammation on FA, we have decided to observe the patient rather than initiating any therapy such as corticosteroid.
2. Discussion
We have presented a case of retinal arteriolar occlusions in an eye with nAMD treated with brolucizumab that has led to severe loss of vison. The left eye in the index patient has been treated previously with multiple other anti-VEGF agents (bevacizumab, ranibizumab, and aflibercept) without incidents. Although previous therapies did not lead to complete resolution of active nAMD, no ocular inflammation was previously detected by the retina specialist. Improvement in retinal fluid was noted after two intravitreal injections of brolucizumab without an associated improvement in VA. Unfortunately, delayed vitreous cells and retinal occlusions occurred in OS after the third administration of brolucizumab, leading to significant loss of vision. The index case is among the first published reports in the literature to describe in detail retinal arteriolar occlusions that occur after brolucizumab.
Brolucizumab (~26 kDa) is the smallest of the anti-VEGF antibodies, significantly smaller than bevacizumab (149 kDa), aflibercept (97–115 kDa), and ranibizumab (48 kDa). Therefore, higher concentrations of brolucizumab molecules can be delivered in the same volume, resulting in an anti-VEGF binding capacity 11-times greater than aflibercept and 22-times than ranibizumab.8
Ocular and non-ocular AEs of the two phase 3 studies were comparable between the patients treated with brolucizumab and aflibercept. Ocular AEs reported in more than 5% of the study patients included conjunctival hemorrhage, eye pain, vitreous floaters, and reduced VA.2 Intraocular inflammation was reported to be higher in brolucizumab 6 mg versus aflibercept at week 48 in HAWK and HARRIER.5
At the 43rd Annual Meeting of the Macula Society in San Diego, February 19–22, 2020, additional safety data of brolucizumab in the HAWK and HARRIER studies were presented.9 There were six (6) cases of retinal artery occlusion (including terms of retinal artery thrombosis, retinal artery occlusion, and retinal artery embolism) in the brolucizumab 6 mg patients in the HAWK and HARRIER studies based on the assessment by an independent Safety Review Committee (SRC). The SRC examined the clinical and imaging data of all cases of inflammation in the HAWK and HARRIER studies. These findings were different than the published Phase 3 data of the HAWK and HARRIER studies which reported only 3 cases of retinal artery occlusion/thrombosis based on the data reported by the investigators. Subsequently, on February 23, 2020, the American Society of Retina Specialists (ASRS) announced to its members that it has received reports of 14 cases of vasculitis following commercially available brolucizumab administration. Of these, 11 cases were designated as occlusive retinal vasculitis by the reporting providers. On March 30, 2020, the ASRS updated the number that it had received to be 25 cases of vasculopathy following intravitreal injections of brolucizumab, with 21 cases being occlusive.7 Recent publication has also provided additional information about retinal vaso-occlusion that occurs after brolucizumab administration.10
In our index case, the patient developed 1+ vitreous cells/debris and multiple retinal arteriolar occlusions after her 3rd brolucizumab injection OS. The fundus photograph before the administration of the third dose of brolucizumab demonstrated RPE changes but no signs of retinal vasculitis or arteriolar occlusion. However, subsequent funduscopic examination and photographs demonstrated the presence of intra-arteriolar greyish substances, which most likely contributed to the occlusive process. It could be theorized that the observed adverse event is attributed to the more potent VEGF blockade, owing to the properties of the brolucizumab molecule. However, studies using various anti-VEGF pharmacologic agents in the past have not reported similar events which could be attributed to hi-dose VEGF antagonists and VEGF blockade. In both the HARBOR Study for nAMD and the READ-3 Study for diabetic macular edema, ranibizumab 2.0 mg, which is at least quadrupled of the current approved standards, was evaluated.11,12 There were no reported cases of retinal arterial or venous occlusion. Additionally, numerous phase 2 and phase 3 clinical trials, with thousands of enrolled subjects, conducted to assess the safety and efficacy of ranibizumab and aflibercept for treatment of diabetic macular edema and nAMD also did not report such a finding.12, 13, 14, 15, 16, 17 As of March 2020, retinal arterial occlusion or retinal vasculitis has been reported at a rate of less than 1 out of every 6 million intravitreal injections of aflibercept, and such cases have appeared in association with culture positive infectious endophthalmitis.18 In the index patient, the retinal arteriolar occlusions were observed without any underlying ocular inflammation or infection. As of March 13, 2020, more than 65,000 vials of brolucizumab for intravitreal injection have been shipped to prescribing physicians in the US; the incidence of adverse events reported to Novartis as retinal artery occlusion, vasculitis or severe vision loss is 10 per 10,000 injections.19
Possibly, a type IV hypersensitivity reaction after intravitreal brolucizumab for the treatment of nAMD in the left eye has occurred, leading to an inflammatory response inside the vessels (such as accumulation of immune complexes) along the vessel wall where the plasma is in contact with the foreign protein (brolucizumab) and subsequent vascular occlusion. The presence of intra-arteriolar greyish material OS (Fig. 1E) suggests that there may indeed be intravascular immune complexes.
Should local, including sustained-release devices, or systemic corticosteroids have been initiated to control the inflammation? Careful consideration was made regarding therapy to salvage the vision. However, the FA showed near-complete vascular occlusion and the vision OS had already decreased to CF. In addition, the lack of leakage of the optic disc or peri-vasculature on FA did not support active inflammation. Furthermore, an afferent pupillary defect was present. The acute inflammatory process likely has already occurred and passed, and the sequelae of such inflammation were seen. Given the age of patient, the prognosis of visual recovery, and the risks and benefits of corticosteroid therapy (or other anti-inflammatory therapy), local or systemic, were considered and discussed with the patient. Close observation was recommended and agreed upon.
3. Conclusion
We reported a case of retinal arteriolar occlusions that are postulated to result from intra-arteriolar deposition of immune complexes and retinal vasculitis, leading to severe visual loss following multiple intravitreal brolucizumab injections. Such a significant adverse event did not occur after the first intravitreal brolucizumab administration, as presented in some cases, but rather after the third. Therefore, unfortunately, it is not always possible to guarantee safety even if the patient has tolerated previous brolucizumab treatments without incidents. We suggest that ophthalmologists and retina specialists discuss thoroughly with patients the potential risks and benefits of therapy with brolucizumab and other anti-VEGF therapies. Patients treated with brolucizumab and other anti-VEGF therapy should be examined carefully and monitored closely for signs of inflammatory reaction in their anterior as well as posterior segments. Such reactions to the agent can occur not just after the first but also after subsequent intravitreal administrations of brolucizumab. Anti-inflammatory therapy may be considered depending on the presence or absence of active inflammation, the visual prognosis, and the overall status of and risks and benefits for the patient.
Patient consent
Consent has been obtained from the patient.
Funding
A Research to Prevent Blindness Department Challenge Award, a National Eye Institute of the National Institutes of Health P30 Award (EY026877), and an unrestricted educational grant from the Ocular Imaging Research and Reading Center (OIRRC), among others, have been awarded to the Byers Eye Institute at Stanford University.
Authorship
All authors attest that they met the current ICMJE criteria.
Declaration of competing interest
The authors declare that there are no conflicts of interest related to this manuscript.
References
- 1.Hernandez-Pastor L.J., Ortega A., Garcia-Layana A., Giraldez J. Ranibizumab for neovascular age-related macular degeneration. Am J Health Syst Pharm: Offc J Am Soc Health Sys Pharama. 2008;65:1805–1814. doi: 10.2146/ajhp070342. [DOI] [PubMed] [Google Scholar]
- 2.Morris B., Imrie F., Armbrecht A.M., Dhillon B. Age-related macular degeneration and recent developments: new hope for old eyes? Postgrad Med. 2007;83:301–307. doi: 10.1136/pgmj.2006.052944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong W.L., Su X., Li X. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. [DOI] [PubMed] [Google Scholar]
- 4.Dugel P.U., Jaffe G.J., Sallstig P. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: a randomized trial. Ophthalmology. 2017;124:1296–1304. doi: 10.1016/j.ophtha.2017.03.057. [DOI] [PubMed] [Google Scholar]
- 5.Dugel P.U., Koh A., Ogura Y. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127:72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Markham A. Brolucizumab: first approval. Drugs. 2019;79:1997–2000. doi: 10.1007/s40265-019-01231-9. [DOI] [PubMed] [Google Scholar]
- 7.American Society of Retina Specialists Beovu update for ASRS members. https://www.asrs.org/clinical/clinical-updates Published February 23, 2020 and March 30, 2020.
- 8.Holz F.G., Dugel P.U., Weissgerber G. Single-chain antibody fragment VEGF inhibitor RTH258 for neovascular age-related macular degeneration: a randomized controlled study. Ophthalmology. 2016;123:1080–1089. doi: 10.1016/j.ophtha.2015.12.030. [DOI] [PubMed] [Google Scholar]
- 9.Dugel P.U. Expanded week 96 safety outcomes: analysis of pooled data from HAWK & HARRIER studies. Presented at the 2020 Meeting of the Macula Society, March 19 to 22, San Diego, California.
- 10.Nguyen Q.D., Das A., Do D.V. Brolucizumab: evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Ophthalmology. 2020 doi: 10.1016/j.ophtha.2019.12.031. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Busbee B.G., Ho A.C., Brown D.M. Twelve-month efficacy and safety of 0.5 mg or 2.0 mg ranibizumab in patients with subfoveal neovascular age-related macular degeneration. Ophthalmology. 2013;120:1046–1056. doi: 10.1016/j.ophtha.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 12.Sepah Y.J., Sadiq M.A., Boyer D. Twenty-four-month outcomes of the ranibizumab for edema of the macula in diabetes - protocol 3 with high dose (READ-3) study. Ophthalmology. 2016;123:2581–2587. doi: 10.1016/j.ophtha.2016.08.040. [DOI] [PubMed] [Google Scholar]
- 13.Heier J.S., Korobelnik J.F., Brown D.M. Intravitreal aflibercept for diabetic macular edema: 148-week results from the VISTA and VIVID studies. Ophthalmology. 2016;123:2376–2385. doi: 10.1016/j.ophtha.2016.07.032. [DOI] [PubMed] [Google Scholar]
- 14.Kaiser P.K., Brown D.M., Zhang K. Ranibizumab for predominantly classic neovascular age-related macular degeneration: subgroup analysis of first-year ANCHOR results. Am J Ophthalmol. 2007;144:850–857. doi: 10.1016/j.ajo.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell P., McAllister I., Larsen M. Evaluating the impact of intravitreal aflibercept on diabetic retinopathy progression in the VIVID-DME and VISTA-DME studies. Ophthalmol Retina. 2018;2:988–996. doi: 10.1016/j.oret.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen Q.D., Brown D.M., Marcus D.M. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 17.Heier J.S., Brown D.M., Chong V. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Data on File. Regeneron Pharmaceuticals, Inc. Tarrytown, NY 10591.
- 19.Data on File. Internal Sales Data. Novartis Pharmaceuticals Corporation; March 2020. https://www.novartis.com [Google Scholar]