Highlights
-
•
We examined prefrontal and frontotemporal brain activity using fNIRS.
-
•
We investigated individuals at risk for psychosis and bipolar disorder.
-
•
Individuals at risk for psychosis showed slower reaction times.
-
•
Decreased frontotemporal activity suggests an unspecific dysfunction.
-
•
Hypofrontality was observed in all at-risk individuals.
Keywords: Early recognition, At-risk stage, Dorsolateral prefrontal cortex (dlpfc), Emotion processing, Emotional interference
Abstract
Objectives
: The emotional Stroop effect is defined as increased reaction times to emotional stimuli compared to neutral ones. It has been often reported in the literature, on both behavioral and neurophysiological level. The goal of this study was to investigate the frontal brain activation in individuals at risk for schizophrenic psychosis and bipolar disorder during an emotional Stroop task. We expected to observe decreased activation in the at-risk individuals compared to the healthy controls.
Methods
: Individuals at high risk for psychosis (HR), at ultra-high risk for psychosis (UHR), at risk for bipolar disorder (BIP) and healthy controls (HC) performed an emotional Stroop task, which included positively, negatively and neutrally valenced words. Functional near-infrared spectroscopy (fNIRS) was used to measure levels of oxygenated hemoglobin (O2Hb) representing brain activity in the dorsolateral prefrontal and frontotemporal cortex.
Results
: Results showed significantly decreased levels of O2Hb in the right dorsolateral prefrontal cortex (DLPFC) in the HR and UHR groups compared to the HC, indicating lower activity. Even though the decrease was independent from the valence of the words, it was the most visible for the negative ones. Moreover, significantly lower O2Hb levels in the frontotemporal cortex (FTC) were observed in all at risk groups compared to the HC.
Conclusions
: Lower activity in the FTC in groups at risk for psychosis and bipolar disorder reflects unspecific dysfunctions. Decreased activity in the DLPFC in the HR and UHR groups indicates that hypofrontality can be found already in individuals at risk for schizophrenic psychosis.
1. Introduction
Schizophrenia and bipolar disorder often start in adolescence or early adulthood but remain undiagnosed for an extended period of time (Angst et al., 2005; Beiser et al., 1993; Drancourt et al., 2013; Schimmelmann et al., 2008). It has been shown that a delay of diagnosis has a negative influence on the progression of the disorder, whereas an early and appropriate treatment has beneficial effects (Cadenhead et al., 2005; Penttila et al., 2014; Schimmelmann et al., 2008). Therefore, extensive research to identify individuals at risk for schizophrenic psychosis and bipolar disorder has been conducted (Angst et al., 2005; Fusar-Poli et al., 2013; Metzler et al., 2014). Furthermore, biological markers linking neurophysiological and behavioral symptoms of psychosis have been explored (Bender et al., 2007; Ehlis et al., 2011; Ferrarelli, 2013; Fusar-Poli et al., 2012; Stöber et al., 2009). The goal of this study is to investigate differences in frontal brain activity in individuals at risk for schizophrenic psychosis and bipolar disorder during an emotional Stroop task, using functional near-infrared spectroscopy (fNIRS). fNIRS is a non-invasive neuroimaging method, which is gaining popularity in psychiatric research (Ehlis et al., 2014). In a comprehensive review, Koike et al. (2013) describe recent findings of the fNIRS studies in schizophrenia research, which showed decreased activation of prefrontal and frontoparietal cortices during various neuropsychological tasks such as: verbal fluency task, tower of London, continuous performance test and kana Stroop task. fNIRS uses light from the near-infrared spectrum to measure relative concentrations of both oxygenated (O2Hb) and deoxygenated (HHb) hemoglobin representing brain activity (Hoshi, 2003; Obrig and Villringer, 2003). The time course and shape of the hemodynamic response measured by fNIRS is comparable to the blood oxygenation level-dependent (BOLD) signal of functional magnetic resonance imaging (fMRI) (Logothetis and Wandell, 2004). fNIRS possesses many advantages. Its biggest advantage is insensitivity to motion, which makes it very suitable for studies with psychiatric or nervous patients (e.g., Thome et al., 2012). Moreover, unlike with other imaging methods, the device is small, mobile and it is easy to apply. This accounts especially for portable and wireless fNIRS-systems (Ayaz et al., 2013). Also it does not require use the of radioactive tracers like position emission tomography (PET). However, the depth of penetration of near-infrared light is rather low (~30 mm), limiting the measurement only to the cortical activity.
The emotional Stroop task is a modification of the classic Stroop task, assessing the impact of emotional stimuli on attentional processes (Williams et al., 1996). During the task, individuals are asked to name the ink color of emotionally valenced and neutral words (see Fig. 1). The emotional Stroop effect is a difference between the mean response time to the emotional and neutral words, thus indicating the so-called emotional interference. A stable emotional Stroop effect has been observed in patients with anxiety disorders and non-clinical anxious individuals. (Bar-Haim et al., 2007). Furthermore, the emotional Stroop effect has been observed in other psychiatric patient groups, when negative words, related to their specific symptoms, were used (Williams et al., 1996).
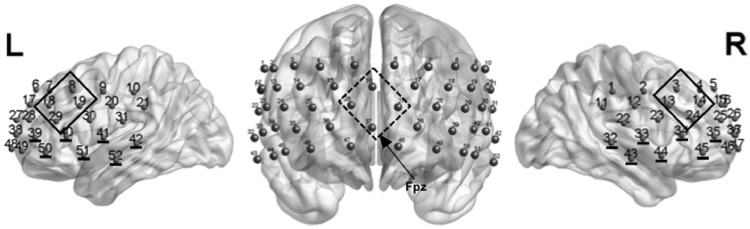
Fig. 2.
Positioning of the fNIRS probe set with measurement channels. Channels included in the regions of interests (ROIs): bilateral dorsolateral prefrontal cortex (DLPFC, continuous frames; channels 8,18,19,29 left and 3,13,14,24 right), bilateral frontotemporal cortex (FTC; underlined channel numbers; channels 32,33,34,35,43,44,45 left and 39,40,41,42,50,51,52 right) and medial prefrontal cortex (MPFC; dashed frame; channels 16,26,27,37). The position Fpz refers to the international 10–20 system of electrodes placement (Jasper, 1958).
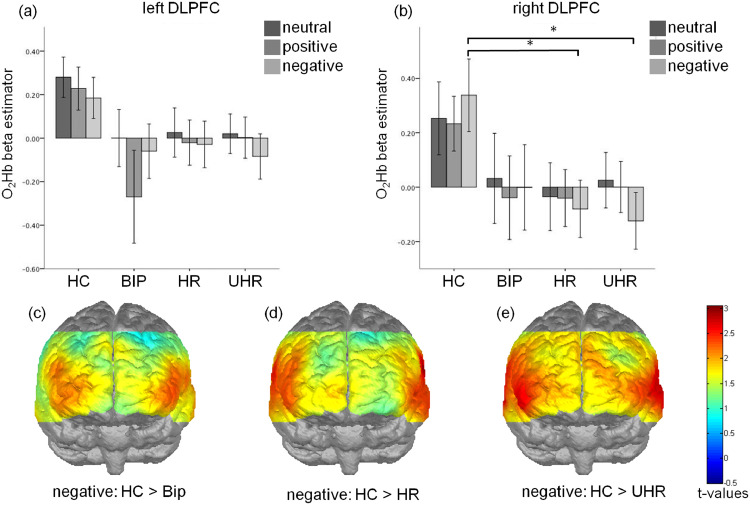
Fig. 3.
Effects of the group and valence on the dorsolateral prefrontal cortex (DLPFC) activation measured by the oxygenated hemoglobin levels (O2Hb) in the left (a) and right (b) hemisphere. T-maps comparing the prefrontal activation elicited by the negative words for the HC and BIP (c), HR (d) and UHR (e) groups. * p<0.05; error bar: 2 standard deviations.
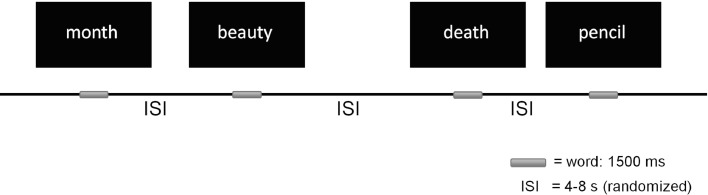
Fig. 1.
Schema of the emotional Stroop paradigm.
Emotional interference studies with schizophrenia patients showed rather heterogeneous results. In an emotional Stroop study, Demily et al. (2010) showed no difference in reaction times for negatively and positively valenced words (e.g. slaughter, failure and holidays, success) in schizophrenia patients. Contrary to these findings other studies showed a connection between the emotional interference and symptom-specific words. Besnier et al. (2011) reported increased emotional interference in schizophrenia patients, which also correlated with positive symptoms. However, negatively valenced, emotional words used in this study were related to paranoid symptoms (e.g. mad, invaded), and substantially differed from the negatively valenced words used by Demily et al. (2010). Anxiety-related words (e.g. panic, fearful) also seem to have an influence on the emotional processing of individuals suffering from psychosis. Fear and Healy (1996) assessed emotional interference in delusional disorder patients elicited by words related to depression, threat and anxiety. They reported significantly reduced interference in individuals on antipsychotic medication compared to medication naive ones. Only a few studies have investigated the emotional Stroop effect in bipolar patients. Still, the interference caused by depression and mania related words (e.g. sad, depressed or happy, agitation) has been observed in manic bipolar patients and their relatives (Besnier et al., 2011, 2009).
Most of the studies investigating neural correlates of the emotional Stroop task employed the fMRI technique. In healthy subjects Compton et al. (2003) discovered an increase of activity in dorsolateral prefrontal cortex (DLPFC), frontal cortex dorsal to anterior cingulate cortex (ACC), orbitofronal cortex, bilateral inferior parietal lobes and bilateral superior temporal gyri as a response to color naming of the negative words compared to the neutral words. Furthermore, in an fNIRS study, Tupak et al. (2013) showed decreased levels of oxygenated hemoglobin (O2Hb) in DLPFC and medial prefrontal cortex (MPFC) in healthy individuals carrying the T allele of the neuropeptide S gene. These individuals showed increased anxiety symptoms and therefore, the obtained results are considered to reflect a less efficient prefrontal regulation system. Another fMRI study reported increased BOLD signal in the left inferior frontal gyrus as a response to panic related words in panic disorder patients (Dresler et al., 2012).
Until now, not many studies have investigated the emotional Stroop effect in individuals at risk for psychoses and bipolar disorder. In the study presented here, we want to investigate whether changes in attention, dorsolateral prefrontal and frontotemporal brain activity can be observed in individuals at-risk for these disorders, before the manifest symptoms appear. Since increased anxiety is a common symptom found in individuals at risk for psychosis and in bipolar disorder patients (Simon et al., 2004; Yung and McGorry, 1996a, b) and the emotional Stroop effect has been described in clinical and non-clinical groups, the results of the current study could be similar to those of anxious individuals described by Tupak et al. (2013). Based on previous behavioral studies (e.g. Besnier et al., 2011; Fear and Healy, 1996) and imaging findings (e.g. Tupak et al., 2013) we hypothesized that the groups at risk for psychosis and bipolar disorder will show a lower DLPFC activity compared to healthy controls. Since previous studies have reported the strongest emotional interference for the negatively valenced words, the difference should be especially prominent in this category. Furthermore, we analyzed activity in the bilateral frontotemporal cortex, as this structure is responsible for recognizing visual objects and reading (Turkeltaub et al., 2002). We expected the FTC to be activated during this visual processing task and to see significantly lower frontotemporal activity reflected in smaller O2Hb measures in the individuals at-risk for psychosis.
2. Materials and methods
2.1. Participants
169 participants between 13 and 35 years of age were recruited from individuals, who contacted any of the four Early Recognition Centers in the Canton of Zurich, Switzerland. The current study was a part of a prospective longitudinal study on early recognition of psychoses, which was a part of the “Zurich Program for Sustainable Development of Mental Health Services” (ZInEP, Zürcher Impulsprogram zur Entwicklung der Psychiatrie) The full study design is described elsewhere (Theodoridou et al., 2014).
The participants were assigned to one of four groups – at high risk for psychosis (HR), at ultra high risk for psychosis (UHR), at risk for bipolar disorder (BIP) or to a healthy control group (HC). Both the groups at risk for psychosis (HR and UHR) consisted of clinical high-risk individuals. By naming the groups HR and UHR we wanted to emphasize the different diagnostic approaches. The HR group was defined based on the basic symptoms, whereas the UHR group was defined based on early psychotic symptoms, which usually appear after the basic symptoms.
The assignment to the respective at-risk groups was based on the following inclusion criteria. The participants were included in the HR group if they experienced at least one cognitive-perceptive (COPER) basic symptom or at least two cognitive disturbances (COGDIS) assessed with the adult or children-youth versions of the Schizophrenia Proneness Interview (SPI-A; Schultze-Lutter et al., 2007, SPI-CY; Schultze-Lutter and Koch, 2010). The inclusion criteria for the UHR group were at least one attenuated psychotic symptom or at least one brief limited intermittent psychotic symptom assessed with Structured Interview for Prodromal Syndromes (SIPS; McGlashan et al., 2001). Further UHR inclusion criteria were >30% reduction in Global Assessment of Functioning (GAF; Endicott et al., 1976) and either schizotypal personality disorder or a first degree relative with psychosis. In the BIP group, participants had to score ≥14 points in the Hypomania Checklist (HCL; Angst et al., 2005) or ≥12 points on the Hamilton Depression Rating Scale (HAMD; Williams, 1988), or had a first degree relative with a history of bipolar disorder as well as a reduction of more than 30% in GAF during the past year. Furthermore, in addition to the clinical scales mentioned, the Positive Negative Symptoms Scale (PANSS; Kay et al., 1987) and Beck Anxiety Inventory (Steer and Beck, 1997) were used. The IQ was measured with multiple choice vocabulary test MWT-B (Lehrl et al., 1995).
The HC group was composed of individuals without history of any mental disorder, which was assessed with a face-to-face MINI Interview (Sheehan et al., 1998). Furthermore, individuals were not included from the HC group if they reported a family history of psychotic disorders,
The participants were excluded from the study if they presented with a bipolar disorder, manifest schizophrenia or organic or substance-induced psychosis. Moreover, substance or alcohol abuse, or IQ <80 were further exclusion criteria. From the initial fNIRS sample of 169 individuals, 18 were excluded due to insufficient quality caused by bad scalp contacts of the optodes and severe muscle artifacts. Furthermore, 6 participants were excluded from the behavioral analysis because of an error rate exceeding 33%. The detailed characteristics of the groups are presented in Table 1.
Table 2.
Emotional Stroop performance of the study groups.
| HC | BIP | HR | UHR | P | ||
|---|---|---|---|---|---|---|
| Reaction times (ms) | ||||||
| Positive words | 542.0 ± 88 | 742.7 ± 104 | 758.7 ± 95 | 780.2 ± 140 | <0.05 | |
| Negative words | 538.1 ± 95 | 748.3 ± 88 | 767.2 ± 94 | 759.7 ± 140 | ns | |
| Neutral words | 555.3 ± 92 | 738.1 ± 94 | 769.8 ± 87 | 777.0 ± 120 | <0.05 | |
| Error rate | ||||||
| Positive words | 2.5 ± 2.1 | 2.6 ± 2.3 | 2.9 ± 3.0 | 6.0 ± 6.0 | <0.05 | |
| Negative words | 2.7 ± 2.1 | 3.3 ± 3.3 | 2.9 ± 3.1 | 5.6 ± 6.3 | ns | |
| Neutral words | 2.8 ± 2.1 | 3.4 ± 2.6 | 3.1 ± 3.6 | 5.38 ± 5.6 | ns |
Table 1.
Demographic characteristic and emotional Stroop performance of the study groups.
| HC | BIP | HR | UHR | P | ||
|---|---|---|---|---|---|---|
| n | 46 | 16 | 41 | 48 | ||
| Gender (F:M) | 22:24 | 4:12 | 14:27 | 19:29 | ns. | |
| Pre-morbid verbal IQ | 107.7 ± 12.5 | 105.8 ± 10.2 | 102.4 ± 11.4 | 100.1 ± 13.3 | <0.01 | |
| Age | 21.2 ± 5.3 | 23.3 ± 5.9 | 23.6 ± 5.6 | 18.3 ± 3.5 | <0.05 | |
| SPI-A/CY | ||||||
| COPER | – | 0 | 39 | 36 | ||
| COGDIS | – | 0 | 27 | 30 | ||
| SIPS | ||||||
| APS | – | 0 | 0 | 43 | ||
| BLIPS | – | 0 | 0 | 8 | ||
| State-trait criteria | – | 0 | 0 | 6 | ||
| GAF | – | 65.3 ± 8.1 | 57.7 ± 19.9 | 53.2 ± 14.6 | <0.05 | |
| PANSS positive | – | 8.2 ± 1.1 | 9.3 ± 1.8 | 15.5 ± 4.0 | <0.01 | |
| PANSS negative | – | 12.0 ± 5.6 | 11.5 ± 4.4 | 16.0 ± 6.1 | <0.01 | |
| PANSS global | – | 23.7 ± 4.5 | 23.7 ± 4.9 | 29.7 ± 6.2 | <0.01 | |
| BAI | – | 17.5 ± 11.1 | 18.0 ± 11.7 | 19.3 ± 10.3 | ns | |
| HAMD | – | 10.5 ± 5.4 | 14.0 ± 6.4 | 16.8 ± 8.4 | <0.01 | |
| HCL | – | 16.5 ± 6.0 | 17.0 ± 5.8 | 18.0 ± 5.3 | ns |
All participants gave written informed consent after being presented with the complete description of the study. For underage participants, parent's or guardian's informed consent was obtained additionally. The study was approved by the ethics committee of the Canton of Zurich and was conducted in accordance with the declaration of Helsinki.
Values given as mean ± standard deviation or number, and Bonferroni corrected p-values of χ2-test (gender), one-way measures analysis of variance (all other variables) testing for group differences.
2.2. Emotional Stroop task
The emotional Stroop task consisted of a total of 10 neutral (e.g. month, pencil), 10 positively (e.g. beauty, spring) and 10 negatively valenced (e.g. nightmare, death) words unrelated to any specific psychopathology. Each word was presented in red, green, blue and yellow on a black computer screen using Presentation software (Neurobehavioral Systems, Albany, CA). The words in all categories did not differ regarding the number of letters and syllables, and their frequency in German language was similar. The words were presented randomly in an event-related design with the inter stimulus intervals varying between 4 and 8 s. Presentation of each word took 1500 ms and was preceded by a white fixation cross shown for 500 ms (Fig. 1). After the stimulus presentation the participants had to name the color of the word as fast as possible by pressing a corresponding color key on a keyboard. An error was recorded when a person pressed a key corresponding to a color other then the one presented on the screen. Before the actual task, the participants practiced the color-button correspondence by naming colors of 20 meaningless strings of letter X.
2.3. fNIRS
Changes in oxygenated (O2Hb) and deoxygenated hemoglobin (HHb) were measured using the 52-channel ETG-4000 Optical Topography System (Hitachi Medical Corporation, Tokyo, Japan). A 3 × 11 channel probe set was placed on the participants’ foreheads, so that the middle probe in the lowest row was placed a the Fpz position according to the international 10–20 System for electrode placement (Jasper, 1958). The near-infrared light was emitted in two wavelengths (695 ± 20 nm and 830 ± 30 nm) by 17 laser diodes and the relative changes of the reflected light were measured by 16 photo detectors. The sampling frequency of the recording was set to 10 Hz. A modified Beer-Lambert Law (Delpy et al., 1988) was applied to transform the measured signal into the relative O2Hb and HHb changes. A correlational based signal improvement (CBSI; Cui et al., 2010) and a band pass filter between 0.015 and 0.25 Hz were applied. Subsequently, channels containing artifacts or showing a flat line were interpolated. Moreover, after a visual inspection, segments with low signal to noise ratio were removed from further analysis. Estimated beta weights for corrected O2Hb measures were determined using the ordinary least squares regression analysis. The peak of the hemodynamic response was set to 6.5 s after the stimulus onset following the analysis by Tupak et al. (2013).
Based on the previous fNIRS literature about the emotional Stroop (Tupak et al., 2013) we defined regions of interests (ROIs) located in the bilateral dorsolateral and medial prefrontal cortex (DLPFC, MPFC). Channels 8, 18, 19, 29 and 3, 13, 14, 24 were pooled in order to compute the parameters for the left and right DLPFC respectively. For the MPFC parameter channels 16, 26, 27, 37 were pooled. Furthermore, activation in bilateral frontotemporal cortices (FTC; pooled channels 32,33,34,35,43,44,45 and 39,40,41,42,50,51,52) was investigated, as these regions are involved in reading (Turkeltaub et al., 2002). The current analysis focused on CBSI corrected O2Hb, as it is more sensitive to changes in the blood flow compared to HHb and it has been shown to deliver more stable results.
2.4. Statistical analysis
A valence (positive, negative, neutral) × group (HR, UHR, BIP, HC) repeated measures ANOVA was applied to investigate the reaction times (RTs) differences. Since the error rate (ER) was not normally distributed a non-parametric Kruskal-Wallis test was applied. The fNIRS CBSI corrected O2Hb data were each entered into a separate valence × group repeated measures analysis of variance (ANOVA). Subsequently, to compare differences between the groups for each condition and ROI separately, planned contrasts comparing the at-risk groups against HC were conducted. Bonferroni correction was applied to correct for multiple comparisons. Furthermore, Pearson's correlations between the O2Hb and behavioral data for all the regions of interests were computed. The significance level was set to p < 0.05 and trend-results were reported for p < 0.1.
The statistical analysis was performed using Matlab 2012b (The Math Works, Natick, MA) and SPSS 22.0 (IBM, SPSS Statistics, Munich, Germany).
3. Results
3.1. Task performance
The descriptive statistics of the performance data are depicted in the Table 1. The 3 × 4 repeated measures ANOVA revealed a significant main effect of the group for the RTs (F(3141) = 4.97, p = 0.003). Planned contrasts revealed significantly slower RTs in UHR group compared to HC (p < 0.001) for all valences. Furthermore, results of planned contrasts for the RTs approached significance for the differences between the HR and HC groups (p < 0.1). The ER showed a group difference only for positively valenced words (H(3)=11.25, p < 0.05). Planned contrast revealed a significant difference between HC and UHR groups (p < 0.05).
Values given as mean ± standard deviation or number, and Bonferroni corrected p-values of one-way measures analysis of variance (reaction times) and Kruskal-Wallis test (error rates) testing for group differences.
3.2. Mean corrected O2Hb changes in DLPFC & MPFC
The valence (positive, negative, neutral) × group (HC, HR, UHR, BIP) repeated measures ANOVA revealed a significant main effect of the group (F(3147) = 2.792, p = 0.43) in the right DLPFC. Furthermore, main effect of the group approaching significance was observed in the left DLPFC and MPFC (F(3, 147) = 2.438, p = 0.067 and F(3, 147) = 2.256, p = 0.084, respectively). However, no significant group × valence interactions were found.
The planned contrasts showed significantly lower O2Hb measures in HR and UHR groups compared to HC in the right DLPFC, indicating lower brain activity (p = 0.014 and p = 0.016 for both HC—HR and HC-UHR comparisons). Moreover, significantly lower O2Hb measures between all the at-risk groups and HC were found in the left DLPFC (p = 0.038, p = 0.049 and p = 0.031 for HC—HR, HC-UHR and HC-BIP comparisons respectively). In the MPFC planned contrasts revealed significantly lower O2Hb measures for UHR compared to HC (p = 0.014).
A further post-hoc analysis revealed the group differences in the right DLPFC only for the negative words at a trend level for the HC—HR comparison (p = 0.071) and at the significant level for the HC-UHR comparison (p = 0.023).
Significant negative correlations (r < −0.200, p < 0.05) between O2Hb measures and RTs were found in left and right DLPFC but only for neutral and positively valenced words. Furthermore, no significant correlations between O2Hb measures and ER were observed.
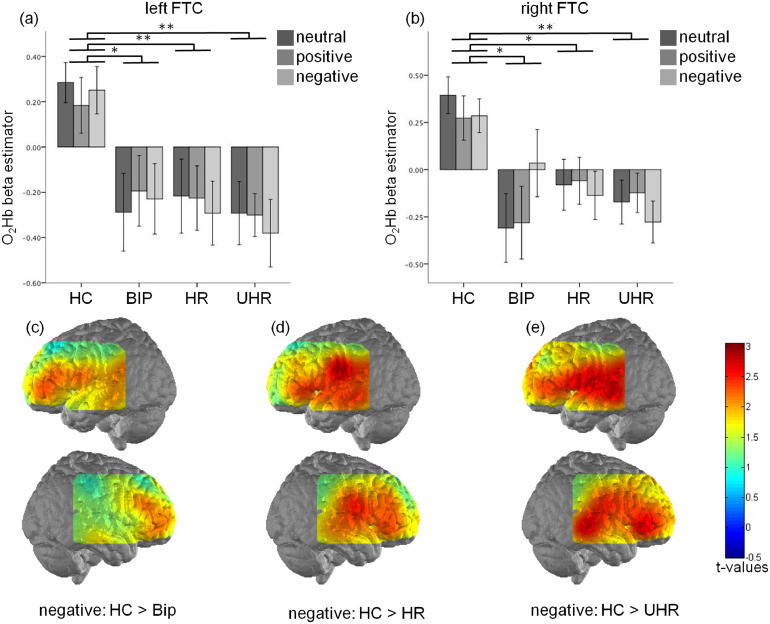
3.3. Mean corrected O2Hb changes in FTC
Significant main group effects were observed in left and right FTC (F(3147) = 6.058, p = 0.001 and F(3147) = 6.775, p = 0.001, respectively). Post-hoc tests revealed bilateral, significantly lower O2Hb differences in all of the at-risk groups compared to the healthy controls (p = 0.02 for BIP-HC and p = 0.001 for HR-HC and UHR-HC comparisons respectively). This finding was observed in all experimental conditions (Fig. 4). Furthermore, no differences between the at-risk groups were observed.
Fig. 4.
Effects of the group and valence on the frontotemporal cortex (FTC) activation measured by the oxygenated hemoglobin levels (O2Hb) in the left (a) and right (b) hemisphere. Activation map showing higher levels of O2Hb elicited by the negative words in the left and right hemisphere for the healthy controls compared to BIP (a), HR (b), UHR (c) groups. * p<0.05; ** p<0.005; error bar: 2 standard deviations.
Pearson's correlations between the O2Hb measures and RTs revealed significant associations (r < −0.200, p < 0.05) in the left and right FTC for all types of the words. ER revealed inconsistent results as, O2Hb measures correlated only with positively valenced words in left and right FTC and with negatively valenced words in left FTC.
4. Discussion
The goal of the study presented here was to investigate the processing of emotionally valenced and neutral words via the frontal and frontotemporal brain activity in individuals at-risk for psychosis and bipolar disorder. Presented results support the initial hypothesis of lower DLPFC activity, measured by changes in mean corrected beta estimators for the O2Hb responses, in HR and UHR compared to the HC group. Significant differences were observed in the left and approached significance in the right DLPFC. Furthermore, lower activation in the bilateral FTC was observed in all of the at-risk groups compared to HC. These results are in line with our initial hypothesis, expecting lower FTC activation in the individuals at risk for psychosis. General deficits in attentional processing, reflected in the longer RTs, were observed on a behavioral level in the individuals at risk for psychosis. It is also visible in the correlations between RTs and the FTC as well as the DLPFC activity. These findings could indicate that the deficits observed in the at-risk individuals of both behavioral and of neurological nature.
The hypofrontality concept developed by Ingvar and Franzen (1974) describes a reduction of the cerebral blood flow and through that a reduction of the functionality of the frontal brain in schizophrenia patients. This dysfunctionality has been widely demonstrated using various imaging techniques and during various neuropsychological tasks (e.g., Fallgatter and Müller, 2001; Perlstein, et al., 2003). Furthermore, hypofrontality has been observed in first episode schizophrenia patients (e.g. Schneider, et al., 2007). The results reported here are in line with previous findings, as we observed lower DLPFC activity in the HR and UHR groups compared to HC group. The BIP group showed only slightly decreased activity in the left DLPFC compared to the HC. These findings reflect a deficit in the prefrontal cortex during the emotional Stroop task, which could be related to deficits in performance. This assumption is supported by negative correlations between brain activation and RTs, which were present for positively and neutrally valenced words. Possibly, the results could indicate a general attention deficit, since the PFC is associated with attentional processes (Dresler et al., 2012). This could indicate that the individuals in the HR and UHR groups actively read the words and through that needed more time to process (Dresler et al., 2012).
Anxiety is a frequent symptom of the prodromal phase of schizophrenia, occurring often as a response to psychotic and other early symptoms (Yung and McGorry, 1996a, b). Therefore, we were particularly interested in the influence of the negative words on the brain activation. Based on the previous research, we expected to obtain similar results as those reported for anxiety prone individuals or patients with anxiety disorders. In an fNIRS study with anxiety prone individuals Tupak et al. (2013) assumed that lower DLPFC activity reflects an inability to inhibit the amygdala response to fear-related stimuli. A similar explanation could also be applied to our findings showing a decreased right DLPFC activity in HR and UHR groups compared to HC, as a response to negative words. However, since fNIRS can only measure cortical activity, this interpretation remains theoretical.
Frontal and temporal activation during the emotional Stroop task has been reported in various studies with healthy individuals and patients with anxiety disorders (Compton et al., 2003; Dresler et al., 2012). The temporal cortex has been associated with semantic processing (Whitney et al., 2011) as well as reading and language (Fallgatter and Strik, 1998; Perani et al., 1999; Schneider et al., 2015; Tyler et al., 2001). Since lower FTC activity was found in all the at-risk individuals compared to the HC group, it could suggest that all of them have reading or language related deficits. It is possible that these deficits go beyond the verbal IQ assessed in this study, since only the UHR group showed significantly lower IQ compared to the others. This explanation is supported by negative correlations between FTC activation and the performance measures (RTs). Correlations between RTs and FTC activation seem to be stable across different types of words, which further indicates a valence-independent processing deficit.
All the findings have to be interpreted with caution as this study poses several limitations. Firstly, the definition of the at-risk stage for bipolar disorder is not well researched and no consistent description has been developed (Martin and Smith, 2013). The definition used in the present study is based on a continuum from depression to mania, focusing on the whole spectrum of the disorder (Angst et al., 2005). Secondly, the UHR individuals differed from the other groups regarding their age and IQ. It is regarded as a limitation, since the task involved the frontal cortex, which is still developing at young age. Furthermore, since the task required processing of written words, it is possible that the IQ differences could have influenced the results. However, because of these significant group differences an analysis of covariance (ANCOVA) including IQ and age is not the appropriate solution, as the assumption of the independence of covariate and independent variable is violated. In this situation, part of the variance attributed to the independent variable is wrongly explained by the covariate. This issue is discussed in a greater detail by Miller and Chapman (2001). A possible explanation for the lower age and IQ in the UHR group is that individuals with the highest risk for psychosis have more severe underlying deficits causing them to exhibit the symptoms earlier compared to other individuals. Nevertheless, most of the statistically significant results showed that both at-risk for psychosis groups differ from the HC group, indicating that the results are related to the underlying pathology rather than age, as only the UHR individuals were significantly younger. Furthermore, a minor limitation is connected to the depth of penetration of the near-infrared light, as it only allows investigating cortical processes. Therefore, activation of the subcortical structures, such as amygdala involved in the emotional processing, or anterior cingulate cortex involved in attentional processes have not been investigated.
Future research will include a follow-up study investigating various ways of progression of the at-risk individuals in relation to emotional processing and brain activity. This could not only advance our understanding of the PFC and FTC functioning but also help establishing a diagnostic tool for the identification of individuals in the prodromal phase of a psychosis. Moreover, it would be interesting to examine, if processing of emotional words related to the symptoms of particular disorder could be more distinctive for a disorder than when just positive and negative words are used.
5. Conclusions
There are few studies investigating frontal brain activation elicited by the emotional Stroop task using fNIRS in individuals with psychosis (e.g. Quaresima et al., 2009, Taniguchi et al., 2012) and bipolar disorder (e.g. Matsubara et al., 2014), especially few concerning the at risk state (e.g. Iwashiro et al., 2016, Koike 2017). The HR and UHR groups differed from the HC group regarding lower bilateral DLPFC and FTC activity as well as longer RTs, whereas individuals in the BIP group only differed with the respect to left DLPFC and FTC activity. These findings show that the neurophysiological differences regarding processing appear as early as the at-risk state for mental disorders. Moreover, prolonged RTs to all three types of words, found in the HR and UHR groups, indicate general processing deficits only in the individuals at risk for psychosis. Nevertheless, follow-up research investigating the transition from the at-risk phase to manifest disorder is needed to further analyze the neurophysiological and neuropsychological changes.
CRediT authorship contribution statement
Aleksandra Aleksandrowicz: Data curation, Formal analysis, Writing - original draft. Florence Hagenmuller: Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Helene Haker: Data curation, Investigation, Project administration, Writing - original draft. Karsten Heekeren: Data curation, Formal analysis, Writing - original draft. Anastasia Theodoridou: Data curation, Formal analysis, Investigation, Writing - original draft. Susanne Walitza: Methodology, Supervision, Writing - original draft. Ann-Christine Ehlis: Formal analysis, Writing - original draft. Andreas Fallgatter: Formal analysis, Writing - original draft. Wulf Rössler: Methodology, Conceptualization, Project administration, Resources, Supervision, Validation, Writing - original draft. Wolfram Kawohl: Methodology, Conceptualization, Project administration, Validation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors would like to thank Jacqueline Lutz, Magali Meier and Lukas Moll for the help in data acquisition.
Acknowledgments
ZInEP was supported by a private donation. The donor had no further role in the experimental design, collection, analysis, interpretation of data, writing and submitting this paper for publication. The authors declare no existing financial conflicts of interests.
References
- Angst J., Adolfsson R., Benazzi F., Gamma A., Hantouche E., Meyer T.D., Skeppar P., Vieta E., Scott J. The HCL-32: towards a self-assessment tool for hypomanic symptoms in outpatients. J. Affect. Disord. 2005;88:217–233. doi: 10.1016/j.jad.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Ayaz H., Onaral B., Izzetoglu K., Shewokis P.A., McKendrick R., Parasuraman R. Continuous monitoring of brain dynamics with functional near infrared spectroscopy as a tool for neuroergonomic research: empirical examples and a technological development. Front. Hum. Neurosci. 2013;7:1–13. doi: 10.3389/fnhum.2013.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y., Lamy D., Pergamin L., Bakermans-Kranenburg M.J., van Ijzendoorn M.H. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol. Bull. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beiser M., Erickson D., Fleming J.A., Iacono W.G. Establishing the onset of psychotic illness. Am. J. Psychiatry. 1993;150:1349–1354. doi: 10.1176/ajp.150.9.1349. [DOI] [PubMed] [Google Scholar]
- Bender S., Weisbrod M., Resch F. Which perspectives can endophenotypes and biological markers offer in the early recognition of schizophrenia? J. Neural Transm. 2007;114:1199–1215. doi: 10.1007/s00702-007-0742-4. [DOI] [PubMed] [Google Scholar]
- Besnier N., Kaladjian A., Mazzola-Pomietto P., Adida M., Fakra E., Jeanningros R., Azorin J.-.M. Differential responses to emotional interference in paranoid schizophrenia and bipolar mania. Psychopathology. 2011;44:1–11. doi: 10.1159/000322097. [DOI] [PubMed] [Google Scholar]
- Besnier N., Richard F., Zendjidjian X., Kaladjian A., Mazzola-Pomietto P., Adida M., Azorin J.-.M. Stroop and emotional Stroop interference in unaffected relatives of patients with schizophrenic and bipolar disorders: distinct markers of vulnerability? World J. Biol. Psychiatry. 2009;10:809–818. doi: 10.1080/15622970903131589. [DOI] [PubMed] [Google Scholar]
- Cadenhead K.S., Light G.A., Shafer K.M., Braff D.L. P50 suppression in individuals at risk for schizophrenia: the convergence of clinical, familial, and vulnerability marker risk assessment. Biol. Psychiatry. 2005;57:1504–1509. doi: 10.1016/j.biopsych.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Compton R.J., Banich M.T., Mohanty A., Milham M.P., Herrington J., Miller G.A., Scalf P.E., Webb A., Heller W. Paying attention to emotion: an fMRI investigation of cognitive and emotional Stroop tasks. Cog. Affect., Behav. Neurosci. 2003;3:81–96. doi: 10.3758/cabn.3.2.81. [DOI] [PubMed] [Google Scholar]
- Cui X., Bray S., Reiss A.L. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. Neuroimage. 2010;49:3039–3046. doi: 10.1016/j.neuroimage.2009.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpy D.T., Cope M., van der Zee P., Arridge S., Wray S., Wyatt J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 1988;33:1433–1442. doi: 10.1088/0031-9155/33/12/008. [DOI] [PubMed] [Google Scholar]
- Demily C., Attala N., Fouldrin G., Czernecki V., Ménard J.F., Lamy S., Dubois B., Thibaut F. The emotional Stroop task: a comparison between schizophrenic subjects and controls. Eur. Psychiatry. 2010;25:75–79. doi: 10.1016/j.eurpsy.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Drancourt N., Etain B., Lajnef M., Henry C., Raust A., Cochet B., Mathieu F., Gard S., Mbailara K., Zanouy L., Kahn J.P., Cohen R.F., Wajsbrot-Elgrabli O., Leboyer M., Scott J., Bellivier F. Duration of untreated bipolar disorder: missed opportunities on the long road to optimal treatment. Acta. Psychiatr Scand. 2013;127:136–144. doi: 10.1111/j.1600-0447.2012.01917.x. [DOI] [PubMed] [Google Scholar]
- Dresler T., Attar C.H., Spitzer C., Löwe B., Deckert J., Büchel C., Ehlis A.-.C., Fallgatter A.J. Neural correlates of the emotional Stroop task in panic disorder patients: an event-related fMRI study. J Psychiatr Res. 2012;46:1627–1634. doi: 10.1016/j.jpsychires.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Ehlis A.-.C., Pauli P., Herrmann M.J., Plichta M.M., Zielasek J., Pfuhlmann B., Stöber G., Ringel T., Jabs B., Fallgatter A.J. Hypofrontality in schizophrenic patients and its relevance for the choice of antipsychotic medication: an event-related potential study. World J. Biol. Psychiatry. 2011;13:188–199. doi: 10.3109/15622975.2011.566354. [DOI] [PubMed] [Google Scholar]
- Ehlis A.-.C., Schneider S., Dresler T., Fallgatter A.J. Application of functional near-infrared spectroscopy in psychiatry. Neuroimage. 2014;85(1):478–488. doi: 10.1016/j.neuroimage.2013.03.067. Part. [DOI] [PubMed] [Google Scholar]
- Endicott J., Spitzer R.L., Fleiss J.L., Cohen J. The global assessment scale: a procedure for measuring overall severity of psychiatric disturbance. Arch. Gen. Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Fallgatter A.J., Strik W.K. Frontal brain activation during the Wisconsin card sorting test assessed with two-channel near-infrared spectroscopy. Eur. Arch. Psychiatry Clin. Neurosci. 1998;248:245–249. doi: 10.1007/s004060050045. [DOI] [PubMed] [Google Scholar]
- Fear C.F., Healy D. The use of the emotional stroop test to establish the onset and efficacy of antipsychotic activity. Hum. Psychopharmacol. 1996;11:373–377. [Google Scholar]
- Ferrarelli F. Endophenotypes and biological markers of schizophrenia: from biological signs of illness to novel treatment targets. Curr. Pharm. Design. 2013;19:6462–6479. doi: 10.2174/13816128113199990554. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Borgwardt S., Bechdolf A. . the psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–120. doi: 10.1001/jamapsychiatry.2013.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusar-Poli P., Smieskova R., Serafini G., Politi P., Borgwardt S. Neuroanatomical markers of genetic liability to psychosis and first episode psychosis: a voxelwise meta-analytical comparison. J. Biol. Psychiatry. 2012;15:219–228. doi: 10.3109/15622975.2011.630408. [DOI] [PubMed] [Google Scholar]
- Hoshi Y. Functional near-infrared optical imaging: utility and limitations in human brain mapping. Psychophysiology. 2003;40:511–520. doi: 10.1111/1469-8986.00053. [DOI] [PubMed] [Google Scholar]
- Ingvar D.H., Franzen G. Abnormalities of cerebral blood flow distribution in patients with chronic schizophrenia. Acta. Psychiatr Scand. 1974;50:425–462. doi: 10.1111/j.1600-0447.1974.tb09707.x. [DOI] [PubMed] [Google Scholar]
- Iwashiro N., Koike S., Satomura Y., Suga M., Nagai T., Natsubori T., Tada M., Gpnoi W., Takizawa R., Kunimatsu A., Yamasue H., Kasai K. Association between impaired brain activity and volume at the sub-region of Broca’s area in ultra-high risk and first-episode schizophrenia: a multi-modal neuroimaging study. Schizophr. Res. 2016;172:9–15. doi: 10.1016/j.schres.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Jasper H. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalogr. Clin. Neurophysiol. 1958;10:370–375. [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Koike S., Nishimura Y., Takizawa R., Yahata N., Kasai K. Near-Infrared spectroscopy in schizophrenia: a possible biomarker for predicting clinical outcome and treatment response. Front. Psychiatry. 2013;4:145. doi: 10.3389/fpsyt.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrl S., Triebig G., Fischer B. Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta. Neurol. Scand. 1995;91:335–345. doi: 10.1111/j.1600-0404.1995.tb07018.x. [DOI] [PubMed] [Google Scholar]
- Logothetis N.K., Wandell B.A. Interpreting the bold signal. Annu. Rev. Physiol. 2004;66:735–769. doi: 10.1146/annurev.physiol.66.082602.092845. [DOI] [PubMed] [Google Scholar]
- Martin D.J., Smith D.J. Is there a clinical prodrome of bipolar disorder? A review of the evidence. Expert Rev. Neurother. 2013;13:89–98. doi: 10.1586/ern.12.149. [DOI] [PubMed] [Google Scholar]
- Matsubara T., Matsuo K., Nakashima M., Nakano M., Harada K., Watanuki T., Egashira K., Watanabe Y. Prefrontal activation in response to motional words in patients with bipolar disorder and major depressive isorder. Neuroimage. 2014;85:489–497. doi: 10.1016/j.neuroimage.2013.04.098. [DOI] [PubMed] [Google Scholar]
- McGlashan T.H., Miller T.J., Woods S.W., Hoffman R.E., Davidson L. Instrument for the assessment of prodromal symptoms and states. In: Miller T., Mednick S.A., McGlashan T.H., Libiger J., Johannessen J.O., editors. Early Intervention in Psychotic Disorders. Springer; Netherlands: 2001. pp. 135–149. [Google Scholar]
- Metzler S., Dvorsky D., Wyss C., Müller M., Traber-Walker N., Walitza S., Theodoridou A., Rössler W., Heekeren K. Neurocognitive profiles in help-seeking individuals: comparison of risk for psychosis and bipolar disorder criteria. Psychol. Med. 2014;44:3543–3555. doi: 10.1017/S0033291714001007. [DOI] [PubMed] [Google Scholar]
- Miller G.A., Chapman J.P. Misunderstanding analysis of covariance. J. Abnorm. Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Obrig H., Villringer A. Beyond the visible-imaging the human brain with light. J. Cerebral Blood Flow Metabolism. 2003;23:1–18. doi: 10.1097/01.WCB.0000043472.45775.29. [DOI] [PubMed] [Google Scholar]
- Penttila M., Jaaskelainen E., Hirvonen N., Isohanni M., Miettunen J. Duration of untreated psychosis as predictor of long-term outcome in schizophrenia: systematic review and meta-analysis. Br. J. Psychiatry. 2014;205:88–94. doi: 10.1192/bjp.bp.113.127753. [DOI] [PubMed] [Google Scholar]
- Perani D., Cappa S.F., Schnur T., Tettamanti M., Collina S., Rosa M.M., Fazio F. The neural correlates of verb and noun processing a pet study. Brain. 1999;122:2337–2344. doi: 10.1093/brain/122.12.2337. [DOI] [PubMed] [Google Scholar]
- Quaresima V., Giosuè P., Roncone R., Casacchia M., Ferrari M. Prefrontal cortex dysfunction during cognitive tests evidenced by functional near-infrared spectroscopy. Psychiatry Res. 2009;171:252–257. doi: 10.1016/j.pscychresns.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Schimmelmann B.G., Huber C.G., Lambert M., Cotton S., McGorry P.D., Conus P. Impact of duration of untreated psychosis on pre-treatment, baseline, and outcome characteristics in an epidemiological first-episode psychosis cohort. J. Psychiatr Res. 2008;42:982–990. doi: 10.1016/j.jpsychires.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Schneider S., Wagels L., Haeussinger F.B., Fallgatter A.J., Ehlis A.-.C., Rapp A.M. Haemodynamic and electrophysiological markers of pragmatic language comprehension in schizophrenia. World J. Biol. Psychiatry. 2015:1–13. doi: 10.3109/15622975.2015.1019359. [DOI] [PubMed] [Google Scholar]
- Schultze-Lutter F., Addington J., Ruhrmann S., Klosterkötter J. Schizophrenia proneness instrument, adult version (SPI-A) Giovanni Fioriti Editore, Rome. 2007 [Google Scholar]
- Schultze-Lutter F., Koch E. Schizophrenia proneness instrument: child and youth version (SPI-CY) Giovanni Fioriti Editore, Rome. 2010 doi: 10.1016/j.schres.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for Dsm-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Simon N.M., Otto M.W., Wisniewski S.R., Fossey M., Sagduyu K., Frank E., Sachs G.S., Nierenberg A.A., Thase M.E., Pollack M.H. Anxiety disorder comorbidity in bipolar disorder patients: data from the first 500 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Am. J. Psychiatry. 2004;161:2222–2229. doi: 10.1176/appi.ajp.161.12.2222. [DOI] [PubMed] [Google Scholar]
- Steer R.A., Beck A.T. In: Zalaquett C.P., Wood R.J., editors. Evaluating stress: A book of resources. Scarecrow Education; Lanham, MD, US: 1997. pp. 23–40. [Google Scholar]
- Stöber G., Ben-Shachar D., Cardon M., Falkai P., Fonteh A.N., Gawlik M., Glenthoj B.Y., Grünblatt E., Jablensky A., Kim Y.-.K., Kornhuber J., McNeil T.F., Müller N., Oranje B., Saito T., Saoud M., Schmitt A., Schwartz M., Thome J., Uzbekov M., Durany N., Riederer P. Schizophrenia: from the brain to peripheral markers. a consensus paper of the WFSBP task force on biological markers. World J. Biol. Psychiatry. 2009;10:127–155. doi: 10.1080/15622970902898980. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Sumitani S., Watanabe Y., Akiyama M., Ohmori T. Multi-channel near-infrared spectroscopy reveals reduced prefrontal activation in schizophrenia patients during performance of the kana Stroop task. J. Med. Invest. 2012;59:45–52. doi: 10.2152/jmi.59.45. [DOI] [PubMed] [Google Scholar]
- Theodoridou A., Heekeren K., Dvorsky D., Metzler S., Franscini M., Haker H., Kawohl W., Ruesch N., Walitza S., Rössler W. Early recognition of high risk of bipolar disorder and psychosis: an overview of the ZINEP “early recognition” study. Front. Public Health. 2014;2:166. doi: 10.3389/fpubh.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome J., Ehlis A.-.C., Fallgatter A.J., Krauel K., Lange K.W., Riederer P., Romanos M., Taurines R., Tucha O., Uzbekov M., Gerlach M. Biomarkers for attention-deficit/hyperactivity disorder (ADHD). A consensus report of the WFSBP task force on biological markers and the world federation of ADHD. World J. Biol. Psychiatry. 2012;13:379–400. doi: 10.3109/15622975.2012.690535. [DOI] [PubMed] [Google Scholar]
- Tupak S.V., Reif A., Pauli P., Dresler T., Herrmann M.J., Domschke K., Jochum C., Haas E., Baumann C., Weber H., Fallgatter A.J., Deckert J., Ehlis A.-.C. Neuropeptide S receptor gene: fear-specific modulations of prefrontal activation. Neuroimage. 2013;66:353–360. doi: 10.1016/j.neuroimage.2012.10.033. [DOI] [PubMed] [Google Scholar]
- Turkeltaub P.E., Eden G.F., Jones K.M., Zeffiro T.A. Meta-snalysis of the functional neuroanatomy of single-word reading: method and validation. Neuroimage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Tyler L.K., Russell R., Fadili J., Moss H.E. The neural representation of nouns and verbs: pet studies. Brain. 2001;124:1619–1634. doi: 10.1093/brain/124.8.1619. [DOI] [PubMed] [Google Scholar]
- Whitney C., Kirk M., O'Sullivan J., Lambon Ralph M.A., Jefferies E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cereb. Cortex. 2011;21:1066–1075. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J.M.G., Mathews A., MacLeod C. The emotional Stroop task and psychopathology. Psychol. Bull. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Williams J.W. A structured interview guide for the hamilton depression rating scale. Arch. Gen. Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]
- Yung A.R., McGorry P.D. The initial prodrome in psychosis: descriptive and qualitative aspects. Aus. New Zealand J. Psychiatry. 1996;30:587–599. doi: 10.3109/00048679609062654. [DOI] [PubMed] [Google Scholar]
- Yung A.R., McGorry P.D. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr. Bull. 1996;22:353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]