Abstract
US homeland security concerns regarding the potential misuse of some radiation sources used in radiobiological research, for example, cesium-137 (137Cs), have resulted in recommendations by the National Research Council to conduct studies into replacing these sources with suitable X-ray instruments. The objective of this research is to compare the effectiveness of an X-RAD 320 irradiator (PXINC 2010) with a 137Cs irradiator (Gammacell-1000 Unit) using an established bone marrow chimeric model. Using measured radiation doses for each instrument, we characterized the dose–response relationships for bone marrow and splenocyte ablation, using a cytotoxicity-hazard model. Our results show that the X-RAD 320 photon energy spectrum was suitable for ablating bone marrow at the 3 exposure levels used, similar to that of 137Cs photons. However, the 320-kV X-rays were not as effective as the much higher energy γ rays at depleting mouse splenocytes. Furthermore, the 3 X-ray levels used were less effective than the higher energy γ rays in allowing the successful engraftment of donor bone marrow, potentially as a result of the incomplete depletion of the spleen cells. More defined studies are warranted for determining whether bone marrow transplantation in mice can be successfully achieved using 320-kV X-rays. A higher X-ray dose then used is likely needed for transplantation success.
Keywords: Cs-137 γ rays, 320-kV X-rays, bone marrow, spleen
Introduction
Our world is a dangerous place and there is always the threat of terrorism. This includes the possible use by terrorists of a radioactivity dispersal device (eg, dirty bomb) containing radioactive materials such as cesium-137 (137Cs). Such a weapon could cause loss of lives and possibly millions to billions of dollars in damage due to population evacuation, relocation, and area decontamination. Related to homeland security, now there is an ongoing effort to replace 137Cs irradiators (called units) such as the Gammacell-1000 Unit (AECL 1984) used in radiobiological studies with X-ray irradiators with suitable energy spectra. One possible X-ray irradiator is the X-RAD 320 (PXINC 2010). For each type of radiobiological study (eg, in vitro, in vivo) of interest that would be conducted using 137Cs γ rays, it is important to demonstrate that a successful outcome can be achieved using X-rays with a suitable photon energy spectrum.1-5 One such study is bone marrow transplantation in mice after host bone marrow ablation using X-rays from the X-RAD 320 cabinet irradiator, which is the focus of this article.
Bone marrow chimeric mice, which are engrafted with bone marrow of genetically disparate donors, are an important tool in immunological studies.6 Such mice are a valuable tool for evaluating whether bone marrow–derived cells are involved in various immune responses (as they can be distinguished from radio-resistant somatic cells in the recipient). In addition, bone marrow cells that carry or are deficient in specific gene products can be delivered by bone marrow reconstitution of irradiated hosts. They may also be used to study both the immunological mechanisms, such as antitumor immunity and graft-versus-host disease.7 The first step in production of such mice is myeloablation (ie, destruction of some or all of the recipient mouse’s bone marrow).6,8 This step is intended to ensure adequate space is made in the bone marrow compartment of the recipient (host) to allow engraftment of donor marrow, which is delivered shortly after irradiation by intravenous (IV) injection. The donor bone marrow contains pluripotent hematopoietic stem cells as well as more mature hematopoietic cells, such as those of the lymphoid and myeloid lineage.6
Myeloablation is typically achieved using whole-body irradiation of the host mouse. However, there are uncertainties about the appropriate whole-body radiation absorbed dose to use for myeloablation.9 For total-body irradiation, the absorbed dose to the bone marrow is proportional to the whole-body absorbed dose. If the absorbed dose to the bone marrow is too low, the recipient mouse’s residual hematopoietic cells can reconstitute the bone marrow compartment and in doing so prevent the donor bone marrow from engrafting. This can occur either by occupation of the bone marrow niche or by direct host-versus-graft rejection (ie, donor bone marrow rejection and destruction). On the other hand, the use of a whole-body radiation absorbed dose that is too high can cause lethal damage to multiple organs (including the intestines) and early death before any donor bone marrow has time to engraft.9
Radiation damages rapidly dividing cells, such as cells of the hematopoietic system and those of the intestinal epithelium. Intestinal epithelial cells lose their capacity to absorb nutrients, leading to weight loss and diarrhea (ie, radiation sickness). In addition, intestinal contents (bacteria and their toxins) leaking into the bloodstream (due to killed cells) can cause pro-inflammatory cytokines to be released, such as tumor necrosis factor-α, which can lead to septic shock and malaise.9
Self-shielded 137CsCl irradiators with radioactivity levels in the International Atomic Energy Agency Categories 1 and 2 (ie, greater than 27 Ci) have been widely used in 3 major applications (NRC 2008): (1) blood sterilization, (2) biomedical research involving cells in culture and small animals, and (3) calibration. Cesium chloride is highly soluble in water and is dispersible in aerosol form. This led to a homeland security concern (NRC 2008) related to dirty bomb use by terrorists as already indicated. Because of this concern, a report by the National Research Council (NRC/NAS 2008) recommended that careful consideration be given to the possibility of replacing 137Cs irradiators with X-ray irradiators with suitable energy photons.10
Whereas unshielded 137Cs-generated γ rays have an energy of 662 keV, X-rays generated from cabinet irradiators typically have photon energies (variable) <500 keV, thus potentially limiting their ability to penetrate tissues. Radiation photons from 137CsCl and X-ray irradiators have different distribution of energies, with the average energy being higher for γ rays. A filter is used to remove some lower energy X-ray photons (called beam hardening), thereby increasing the average photon energy for the X-ray beam.11
Research reported here relates to possibly replacing 137CsCl irradiators used in radiobiological research involving small animals with an X-RAD 320 Unit X-ray irradiator (PXINC 2010) with energies higher than for a typical X-ray irradiator. The experimental work was performed in a previous project in collaboration with Sandia National Laboratories.12,13 The objective of the research is to demonstrate the feasibility of achieving the same success in bone marrow ablation/transplantation studies (with mice) with an X-RAD 320 Unit photon energy spectrum as has been achieved with higher energy γ-ray photons from a 137CsCl-based irradiator.
Our results, described in detail below, indicate that the X-RAD 320 photon energy spectrum (with filtered lower energy photons) was suitable for ablating mouse bone marrow at either the high, moderate (mid), or low doses used, like was the case for 137Cs γ rays; however, the X-rays were not as effective as γ rays at depleting mouse splenocytes. In addition, X-ray irradiation at the doses used failed to allow for successful engraftment of donor bone marrow of another strain and led instead to outgrowth of immune cells from the host animal’s own hematopoietic system. This likely relates to our highest X-ray dose being too low.
Methods
Irradiators
Gammacell-1000 Unit
A Gammacell-1000 Unit (AECL 1984) was used for whole-body exposure of mice to γ rays. Gammacell-1000 irradiators are self-contained irradiators designed to deliver a large range of radiation doses to small biological samples. They consist of a stationary 137Cs doubly encapsulated radiation source permanently secured within a biological lead shield. The radioactive source consists of an array of 2 137CsCl pencils (Model ISO-1000; AECL 1984). When the emitted 662-keV γ-ray photons penetrate the source encapsulation, a spectrum of photon energies arise, so the irradiated targets are exposed to a variety of photon energies, such as for X-ray irradiators. The biological shield is mounted on a steel frame and is covered with sheet-metal panels.
The biological shield contains the sample chamber rotor. By turning the rotor through an arc of up to 180 degrees, the sample chamber is either exposed to or removed (except for small amount of leakage radiation) from the radiation field and the irradiated target can be rotated during irradiation. The radiation beam is horizontal, unlike for the X-RAD 320 X-ray irradiator discussed below. The Gammacell-1000 Unit used was housed in a highly secure area with access only available for approved individuals.
X-RAD 320 Unit
The X-RAD 320 Unit (PXINC 2010) used is a self-contained X-ray irradiation system that was designed for use in biology and medical research. The X-RAD 320 irradiation system is used in conjunction with the GE ISOVOLT 320 TITAN X-ray Unit. A cathode generator with a power electronics module and anode generator are used to generate the negative and positive high voltages used to excite the X-ray tube. Cooling is achieved via an oil-to-air cooling system, but extra room air cooling is also needed for our facility.
The shielded cabinet includes an adjustable specimen shelf, sample viewing window, and beam hardening filter holder. The 320-kV X-ray tube provides a high output relatively uniform vertical beam with a maximum output of 4000 W allowing for delivery of up to 16 Gy/min at 50-cm source to surface distance. Actual dose rates depend on other factors, including beam filtration and other exposure settings. Our studies employed a filter comprised of 0.75-mm tin + 0.25-mm copper + 1.5-mm aluminum (half value layer of approximately 3.7-mm Cu). The unit is also housed in a secure area with access only available for approved individuals.
Animals
In order to avoid a graft-versus-host response, we chose to use recipient (C.B-17) and donor (B10.D2) mice that were identical at the major histocompatibility region (both strains being H-2d). These 2 strains, however, can be genetically and flow cytometrically distinguished by the cell-surface marker Sca-1, which is expressed on a much higher percentage of hematopoietic cells in the B10.D2 strain than the C.B-17 strain.
Female and male C.B-17 mice (10-18 weeks old) were either bred at Lovelace Respiratory Institute or purchased from Taconic (Germantown, New York). Female and male B10.D2 mice (11-15 weeks old; B10.D2-Hc1 H2d H2-T18c/nSnJ, stock number 000463), used for donor bone marrow, were purchased from Jackson Laboratories (Bar Harbor, Maine). Animals were housed in sterile microisolator caging with autoclaved bedding. They received irradiated food (Teklad Global 18% Protein Rodent Diet 1918 Irradiated; Harlan Laboratories, Madison, Wisconsin) and antibiotic-treated water (enrofloxacin, 175 mg/mL; Baytril 100, Bayer HealthCare LLC, Animal Health Division, Shawnee Mission, KS) for the duration of the experiment. For the first 14 days postirradiation, mice were supplemented with moistened food daily. Lab gel (banana-flavored enrichment gel, no sugar added 71-01-1081, ClearH2O, Portland, Maine) was provided as necessary. All experiments were approved by the Institutional Animal Care and Use Committee and were conducted at Lovelace Respiratory Research Institute, an AAALAC approved facility.
Animal Irradiation
Mice were anesthetized with 75 to 100 mg/kg ketamine (Putney/Dechra Veterinary Products, Overland Park, KS and/or Fort Dodge Animal Health Pharmaceuticals, Overland Park, KS) and 5 mg/kg xylazine (Anased, Akorn, Inc, Lake Forest, IL) delivered in maximum 0.20-mL saline intraperitoneally. Anesthetized mice were placed in 50-mL polypropylene conical centrifuge tubes with the conical end removed to allow a 7-mm hole for breathing. They were secured in the tubes with absorbent wipes placed in front of the tube cap. All target radiation doses were delivered as a split dose, with 50% of the total dose being delivered in each of 2 sessions separated by 4 hours. Using a split-dose design where DNA repair is allowed to occur in the relatively short inter-dose-fraction interval helps to avoid deaths from the hematopoietic and other radiation syndromes.
Target dose rate estimates were 0.9 Gy/min using the X-RAD 320 Unit and 1.0 Gy/min using the Gammacell-1000 Unit. Between the 2 radiation exposure sessions, mice were allowed to wake up from their anesthesia on temperature-controlled warming pads and once awake were placed back in their cages with access to food and water.
Mouse-bearing tubes were secured horizontally on a turntable in the X-RAD 320 Unit because of the vertical beam (from above the target), whereas they were positioned vertically in the stainless-steel sample canister in the Gammacell-1000 Unit because of the horizontal beam. Mice were rotated on turntables for the duration of the exposures in an attempt to achieve as close to a uniform radiation exposure as possible. In addition, as the X-ray source was above the mice, the tubes were positioned with the ventral side of the mouse facing the source during the first irradiation session and during the second session the tubes were positioned such that their dorsal side was facing the source. Mice were monitored closely for weight loss and indications of radiation sickness through the duration of the experiment.
Dosimetry
Radiation-absorbed doses to mice were evaluated using Thermoluminescent Dosimeters (TLD; Quantaflux, Oregonia, Ohio) which were read on-site using a Victoreen 2800 Thermoluminescent Reader. Dose calibration relationships for the X-RAD 320 Unit were established based on Victoreen ionization chamber wands and a Condenser R-Meter Model 570 (Fluke Biomedical, Cleveland, Ohio). Gammacell-1000 Unit exposure readings based on TLDs were checked using nanoDots (Optically Stimulated Luminescence Technology; Landauer, Glenwood, Illinois). The initial dosimetry was performed by inserting TLDs into mouse carcasses in the inguinal area near femurs to estimate the radiation-absorbed dose to the bone marrow. These values were used to set the radiation exposure times for experimental runs. The experimental doses were evaluated based on measurements of TLDs taped to either the center of turntable (X-RAD 320 Unit) or the outside of exposure tubes (Gammacell-1000 Unit).
Cell isolation and transfer
Mice were euthanized and bone marrow and spleen cells isolated as previously described.13 Bone marrow cells from nonirradiated donor animals were further washed in Hanks’ balanced salt solution (HBSS) and resuspended at a concentration of 1 × 107 in 0.2-mL HBSS without fetal bovine serum. Twenty-four hours after irradiation, recipient mice were given an IV transfer of 10 × 106 healthy bone marrow cells from either nonirradiated C.B-17 mice or B10.D2 mice representing syngeneic and allogeneic bone marrow transplantation, respectively. Both of these groups of mice were analyzed 6 weeks later for bone marrow reconstitution.
Flow cytometric analysis
At 6 weeks postirradiation and bone marrow transfer, mice were euthanized and flow cytometric staining of splenocytes was used to assess the percent hematopoietic cell reconstitution and bone marrow engraftment. To determine the extent of allogeneic engraftment, PE anti-Sca-1 (Ly-6A.2 clone CT-6A.2, Caltag/Invitrogen, Carlsbad, CA) was used. This marker distinguishes cells of recipient and donor origin as it is expressed on >50% of spleen lymphocytes from strain B10.D2 and <5% of C.B-17 spleen lymphocytes. Cells were analyzed using a FACS Calibur (BD Biosciences, San Jose, CA) and FlowJo (TreeStar/FlowJo LLC, Becton Dickinson, Ashland, OR) software. Cells were gated on live lymphocyte population as determined by forward scatter and side scatter.
Results
Radiation Doses
Radiation dose information for our split-dose, whole-body irradiation of C.B-17 mice is presented in Table 1 and is arranged by paired low-, mid-, and high-dose groups for X-rays and γ rays. Intended doses (called target dose) based on irradiator unit settings and estimated radiation doses (range) based on dose monitoring (as described in section “Dosimetry”) are presented. The X-ray doses were intended to be about a factor of 1.3 lower than γ-ray doses (X-ray relative biological effectiveness [RBE] of 1.3 assumed).
Table 1.
Target (Intended) and Measured Absorbed Radiation Doses.
| Dose Group | X-Ray (XRAD 320) Target Doses (Range Measured Outside Restrainer), Gy |
γ-Ray (Gammacell-1000) Target Doses (Range Measured Outside Restrainer), Gy |
|---|---|---|
| Low-dose group | 1.56 (1.67-2.01) | 2.03 (2.42-3.14) |
| Mid-dose group | 3.09 (3.10-3.39) | 4.01 (4.49-5.74) |
| High-dose group | 4.62 (4.42-4.86) | 6.00 (6.06-7.11) |
Radiation Cytotoxicity In Vivo
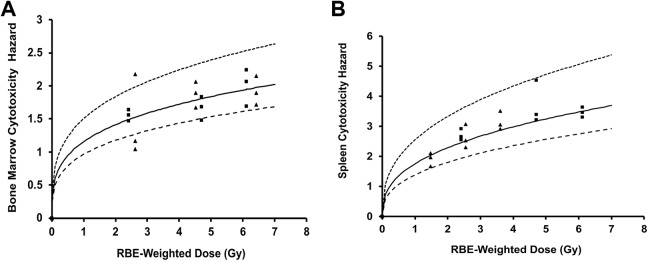
Figure 1A and B shows our in vivo cytotoxicity results evaluated 24 hours after split-dose, whole-body, X-ray or γ-ray exposure of C.B-17 mice, based on bone marrow and spleen cellularity. RBE-weighted total dose in gray (Gy) was used instead of unweighted total dose also in gray,14 to facilitate evaluating whether the doses used for the low-, mid-, and high-exposure groups were equivalent for the 2 radiation sources (X-RAD 320 and Gammacell-1000 irradiators). X-ray RBEs (relative to γ rays) used here for dose weighting are based on an earlier modeling study of our in vivo cytotoxicity data that were published after completion in 2013 of the experiments described in this article.13
Figure 1.
Cytotoxicity hazards (24 hours postirradiation) for split-dose, whole-body X-ray or γ-ray exposure of C.B-17 mice as a function of relative biological effectiveness (RBE)-weighted dose in gray (Gy). (A), bone marrow; (B), splenocytes. Squares indicates γ-ray data; triangles, X-ray data; smooth curves, modeled cytotoxicity hazards (central estimate [mean] via Bayesian analysis) based on both X-ray and γ-ray data and central estimates of v (0.288 for bone marrow; 0.382 for splenocytes) and D 50 (0.17 Gy for bone marrow; 0.0871 Gy for splenocytes)13; upper dashed curves, upper estimates based on modeling results with 5% (percentile) values for D 50 (0.068 Gy for bone marrow and 0.033 Gy for splenocytes); lower dashed curves, lower estimates based on modeling results with 95% (percentile) value for D 50 (0.32 Gy for bone marrow and 0.16 Gy for splenocytes). D 50 is the median effective dose expressed as RBE-weighted dose. See main text for mathematical expression (Equation 2) used to obtain the curves in (A) and (B).
For the γ-ray reference used in this publication, RBE = 1 was used irrespective of the biological target of interest. For bone marrow damage, an X-ray RBE of 1.35 (central estimate) was used and for spleen damage, and an X-ray RBE of 0.76 (central estimate) was used. These RBE values are based on findings in our published modeling study.13 Since for X-rays our experimental study design used X-ray RBE = 1.3 (relative to Cs-137 γ rays), as expected our results for bone marrow cytotoxicity for the 2 radiation sources were consistent when RBE-weighted dose is used. By “consistent,” we mean that the corresponding data points for the low-, mid-, and high-exposure groups are closely spaced along the dose axis and are scattered about the smooth cytotoxicity hazard curve. The curve is the same as our published curve for Cs-137 γ rays, in which RBE-weighted dose was not used.13
For X-ray cytotoxicity to the spleen, we had an unexpected result as paired (X-ray vs γ rays) exposure groups (low, mid, high) based on X-ray RBE = 1.3 used in the study design were not even close to being equally effective, as revealed in our published modeling study.13 Rather than RBE being around 1.3, it was found in the modeling study to be 0.76 (central estimate as indicated above). This means that for spleen damage, γ rays from the Gammacell-1000 Unit were more damaging than X-rays from the X-RAD 320 Unit, unlike for bone marrow cytotoxicity where X-rays were more damaging. The results in Figure 1B for cytotoxicity to splenocytes are based on the use of X-ray RBE = 0.76 for dose weighting rather than RBE = 1.3. Note that with the X-ray RBE used, the corresponding data points for the low-, mid-, and high-exposure groups for X-rays and γ rays are not close together along the dose axis but still scatter about the smooth curve for the cytotoxicity hazard that is based on our published model. The curve is the same as our published curve for Cs-137 γ.13
With our published dose–response model used to obtain results in Figure 1A and B, we can evaluate bone marrow and splenocyte ablation (eg, percentage of the target cells that are ablated). The cytotoxicity hazard curves in Figure 1A and B were used for tissue ablation evaluations as the target cell ablation is related to the cytotoxicity hazard through the following equation13:
| 1 |
The function A(D j) (introduced here for the first time), which takes on values from 0 to 1, is the fraction of the target cells that are expected to be ablated, S(D j) is the fraction that is expected to survive, and H(D j) is the cytotoxicity hazard (a measure of cellular damage) plotted in Figure 1A and B. D j is the dose for radiation type j (“j = x” for X-rays and “j = g” for γ rays). The cytotoxicity hazard is evaluated as a function of D j and 2 parameters D 50, j and v. The parameter D 50, j is the dose that is expected to kill 50% of the target cells. The parameter v (called shape parameter) determines the shape of the dose–response curve for H(D j), which is evaluated based on the following equation13:
| 2 |
The X-ray RBE relative to γ rays is evaluated as the ratio D 50, g/D 50, x and is independent of the X-ray dose since v is modeled as being the same for the different photon radiation sources of interest (X-rays and γ rays). However, the shape parameter v is allowed to be different for different target cell populations and may also be different when comparing high- (eg, α particles, heavy ions, neutrons) and low-LET radiations (eg, γ rays).13 For bone marrow ablation, central estimates of D 50, g and D 50, x are 0.17 and 0.134 Gy, respectively, and the central estimate of v is 0.288.13 For splenocyte ablation, central estimates of D 50, g and D 50, x are 0.0.0871 and 0.112 Gy, respectively, and the central estimate of v is 0.382.13 The central estimates are “means of distributions” obtained using Bayesian inference.13 The central estimate of the X-ray RBE (=1.35) for bone marrow ablation is also the mean of the RBE distribution obtained rather than the ratio of central estimates for D 50, g and D 50, x.
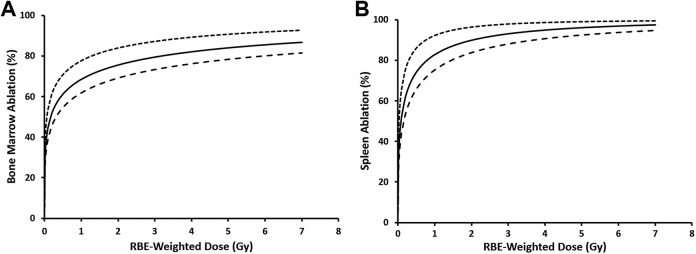
Figure 2 shows central estimates of dose–response relationships for bone marrow and splenocyte ablation as a function of RBE-weighted dose for split-dose, whole-body, γ-ray and X-ray irradiation of C.B-17 mice. The ablation is, however, expressed as a percent (%) rather than the corresponding fraction. Calculations are based on model parameters for γ rays (our reference for RBE evaluation). Note the “steep rise” in the expected target ablation at doses less than 1 Gy. Unfortunately, we did not have experimental data below 1 Gy to confirm the modeling results for low doses (ie, below 1 Gy). Future studies will include radiation doses less than 1 Gy to confirm these modeling results for low doses.
Figure 2.
Central (smooth curve), upper (top dashed curve), and lower (bottom dashed curve) estimates of ablation (%) of bone marrow cells (A) and splenocytes (B) after split-dose, whole-body X-ray or γ-ray irradiation of C.B-17 mice, evaluated 24 hours after irradiation. Dose–response curves are based on the smooth curves in Figure 1A (bone marrow) and B (splenocytes) and Equation 1 in the text that relates the ablation A(D j) to the cytotoxicity hazard H(D j). D j, however, is replaced by the relative biological effectiveness (RBE)-weighted dose as it is the dose that is used since its use applies to both X-rays and γ rays. For γ rays, the RBE-weighted dose and the γ-ray absorbed dose (D g) are the same. Data points are excluded here as the focus is on central risk estimates and the related uncertainties.
Reconstitution of the Hematopoietic System
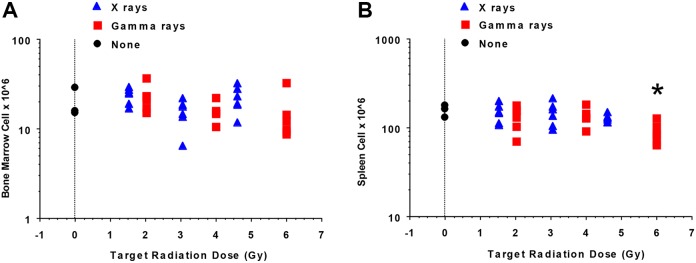
As implicated in Figure 2, radiation doses used for both radiation types spared some bone marrow and spleen cells at all radiation doses used (up to 7 Gy RBE-weighted dose). We, therefore, investigated whether the C.B-17 mice were capable of reconstituting their hematopoietic system without bone marrow transplantation. Figure 3A and B, which is based on exposure groups (target dose is used to represent each exposure group), shows the reconstitution results obtained at 6 weeks postradiation exposure. Figure 3A illustrates that mice irradiated with X-rays or γ rays reconstituted their bone marrow to a similar extent (P = .34). In addition, when comparing the 3 exposure groups for each irradiator, no significant difference in bone marrow cell reconstitution was found for either X-rays or γ rays. In contrast, although spleen cell reconstitution was similar when comparing the 2 radiation types without regard to dose group (P = .09 by 1-way analysis of variance [ANOVA]), when considering the various γ-ray dose groups, a dose association was revealed by 1-way ANOVA (P = .05) with the largest spleen cell reconstitution deficit observed in mice in the high-dose group (Figure 3B: high vs mid group, P = .034; high vs low group, P = .038; results for these and subsequent pairwise comparisons are based on Fisher protected least significant difference [PLSD] post hoc test). This is consistent with findings in our modeling study where one focus was on a possible negative correlation between the cell counts and radiation dose as evaluated using TLD measurements rather than target doses.13 A significant negative correlation (r = −0.712, P < .01) was found between the multiple cell counts for spleen and the corresponding measured γ-ray doses. In addition, a significant negative correlation (r = −0.54, P < .05) was found between the multiple cell counts for bone marrow and the corresponding measured γ-ray doses. The negative correlations implicate reconstitution deficits after the high γ-ray doses used, and a threshold-type, dose–response model was introduced for characterizing the reconstitution deficit.13
Figure 3.
Reconstitution (6 weeks postirradiation) of bone marrow cells (A) and splenocytes (B) of C.B-17 mice after split-dose, whole-body X-ray or γ-ray irradiation (n = 6 mice/dose/irradiator, n = 3 for nonirradiated controls). Target dose (unweighted) is used to indicate exposure groups (controls, low, mid, high) rather than actual doses. The symbol “*” indicates a dose–response relationship existed when comparing the γ-ray exposures as regard to splenocyte reconstitution with doses for the high (6.0 Gy intended dose) exposure group leading to the significantly lower spleen cell reconstitution (P = .05 overall by 1-way analysis of variance; high < mid, P = .034 and high < low, P = .038 [panel B], with all pairwise comparisons being based on the Fisher protected least significant difference post hoc test). See the main text for an explanation of these findings.
No significant radiation exposure-level association was inferred by 1-way ANOVA (P = .29) for mice irradiated with X-rays when considering splenocyte reconstitution. This finding is essentially the same as was obtained using correlation analyses based on TLD measurements of dose as was previously published.13 The absence of a significant radiation effect on reconstitution of cellularity at 6 weeks postradiation exposure may be related to biological effective doses (as reflected by RBE-weighted dose) being too low for the X-ray exposures conducted.
Acute Lethality
Whole-body X-ray doses (split-dose exposure) as high as 4 to 5 Gy (rounded absorbed doses) and γ-ray doses as high as 6 to 7 Gy (rounded absorbed doses) were not lethal, even though most of the irradiated bone marrow cells and splenocytes were killed by such doses (see Figure 2). The C.B-17 mice demonstrated remarkable cellular recovery from such damage even without the benefit of bone marrow transplantation (see Figure 3).
Bone Marrow Transplantation
In spite of the fact that radiation doses used in this study were nonlethal, we performed bone marrow transplantation using the C.B-17 mice as recipients. The indicated mice were irradiated and 1 day later transplanted with approximately 10 × 106 C.B-17 (syngeneic) or B10.D2 (allogeneic) bone marrow cells by IV injection.
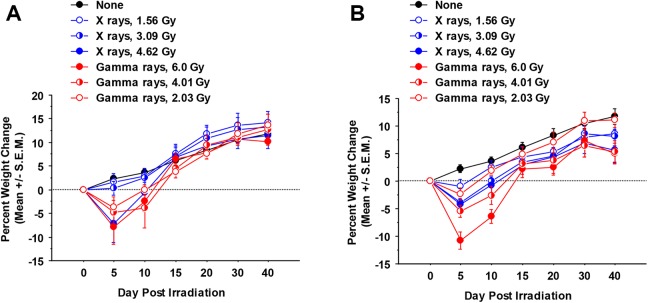
As weight loss is a symptom of both transplant rejection and graft-versus-host disease,15,16 weight changes were monitored. Figure 4A shows the weight changes following syngeneic bone marrow transplantation and Figure 4B shows the changes after allogeneic bone marrow transplantation. When weight changes were analyzed by repeated measures ANOVA, mice irradiated by either irradiator showed significantly more weight loss than unirradiated mice (P < .0001). In addition, significant differences for the different exposure groups (controls, low, mid, high) were revealed (P = .0006 considering syngeneic transplantation and <.0001 considering allogeneic transplantation). In addition, transplantation with syngeneic bone marrow led to greater eventual weight gain than allogeneic bone marrow transplantation, regardless of the type of radiation (P = .01 for X-rays and .05 for γ rays).
Figure 4.
Percent weight change of C.B-17 mice irradiated that were transplanted with either syngeneic (A) or allogeneic (B) bone marrow and monitored for weight loss over the next 6 weeks. (n = 6 mice/dose/irradiator). Weight change was evaluated (± standard error of the mean) relative to from day 0, prior to irradiation. Days indicated on the horizontal axis represent average weight change measured on the day indicated ± 3 days up through day 20 (ie, day 5 represents weights measured on days 2-6, day 10 represents days 7-11, day 15 represents days 12-16, day 20 represents days 17-21). Day 30 weights represent the average of weights measured on days 22 to 31 and day 40 weights represent the average of weights measured on days 32 to 42. See the main text for an explanation of these findings.
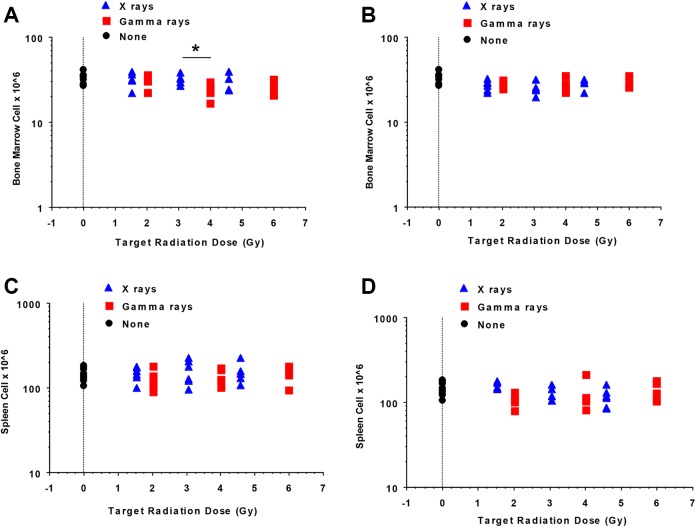
Figure 5A (syngeneic transplant groups) and B (allogeneic transplant groups) shows reconstitution results for bone marrow based on bone marrow cells harvested 6 weeks after split-dose radiation exposure. Target dose is used to indicate exposure group. Corresponding results for spleen are presented in Figure 5C (syngeneic transplant groups) and D (allogeneic transplant groups). Overall, bone marrow cell numbers after either syngeneic or allogeneic bone marrow transplant were significantly increased compared to mice that received no bone marrow transplant (compare Figure 5A and B to Figure 3A; syngeneic, X-rays: P < .0001; syngeneic, γ rays: P = .0006; allogeneic, X-rays, P = .0007; allogeneic, γ rays: P < .0001, all comparisons by 1-way ANOVA). In contrast, bone marrow transplantation did not lead to greater spleen cellularity as compared to when mice were left without a transplant postradiation (compare Figure 5C and D to Figure 3B).
Figure 5.
Bone marrow cells and splenocyte reconstitution (6 weeks postirradiation) in split-dose, whole-body X-ray or γ-ray irradiated C.B-17 mice transplanted after irradiation with either syngeneic (A, C) or allogeneic (B, D) bone marrow (n = 6 mice/dose/irradiator, n = 13 for nonirradiated controls). The symbol “*” indicates significantly less syngeneic bone marrow reconstitution after γ-ray exposure as compared to both X-ray exposure and no radiation exposure controls (A) as revealed by 1-way analysis of variance (P = .03). See the main text for our further explanation of these findings.
Syngeneic bone marrow reconstitution was significantly less after γ-ray exposure as compared to both X-ray exposure and no radiation exposure controls (Figure 5A) as revealed by 1-way ANOVA (P = .03). Allogeneic bone marrow reconstitution after X-ray exposure was significantly less than no radiation exposure control bone marrow levels (Figure 5B, P = .04) but was similar to that observed after γ-ray irradiation. No association with radiation exposure level was revealed when analyzing bone marrow cell numbers after exposure to either type of radiation.
When spleen cell reconstitution was compared between irradiated mice, no significant differences were identified when comparing radiation types in either syngeneic or allogeneic bone marrow recipients. However, a radiation exposure–level association was inferred by 1-way ANOVA when considering mice that were irradiated by X-rays and given an allogeneic bone marrow transplant with higher doses of X-rays leading to less splenocyte reconstitution (P = .004). Overall, however, no differences were observed in splenocyte reconstitution when comparing the 2 types of radiation in either syngeneic or allogeneic bone marrow recipients, and there was no significant difference in the number of splenocytes after irradiation and bone marrow transplant compared to splenocyte number of no radiation exposure controls (Figure 5C and D).
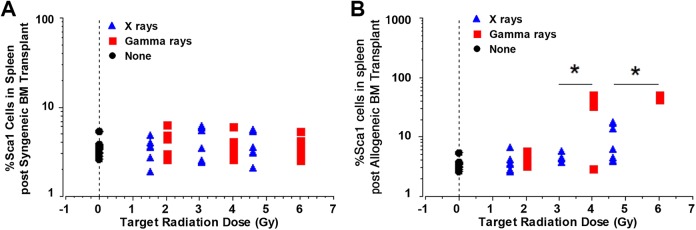
Finally, the effect of radiation source on allogeneic donor bone marrow reconstitution was analyzed using antibodies to the Sca1 cell-surface marker on splenocytes. Sca1 is expressed on >50% of spleen lymphocytes from strain B10.D2 but <5% of C.B-17 spleen lymphocytes. Therefore, the vast majority of Sca1+ cells can be presumed to be of donor (B10.D2) origin when observed in C.B-17 mice receiving an allogeneic bone marrow transplant. Figure 6B shows that target γ-ray doses of either 4.01 or 6.0 Gy resulted in significantly greater spleen cell reconstitution with cells of B10.D2 donor origin as judged by the percentage of Sca1+ cells compared to X-ray irradiation. Figure 6A shows the background Sca1+ expression in spleens 6 weeks after reconstitution of irradiated C.B-17 mice with C.B-17 (syngeneic) bone marrow. Figure 6B shows Sca1 + cell percentage in bone marrow cells after allogeneic (B10.D2) bone marrow transplantation. One-way ANOVA showed that irradiation with γ rays induced significantly different Sca1+ cell percentage as compared to either no irradiation or X-ray irradiation (P < .0001) of C.B-17 mice when analyzed 6 weeks after allogeneic bone marrow transplantation. In addition, a dose–response relationship was implicated for the γ ray target dose levels, with the lowest level used (2.03 Gy) being associated with significantly less Sca1+ cell reconstitution in the spleen compared to the 2 higher levels (4.02 and 6.0 Gy; P < .003 for all pairwise comparisons which are based on the PLSD post hoc test). The effects of the mid (4.02) and high (6.0) γ-ray target dose levels were not significantly different from each other, but both were significantly different than the corresponding target X-ray dose level (percent Sca1+: P = .0012 comparing mid-dose levels and < .0001 comparing high-dose levels).
Figure 6.
C.B-17 mice were irradiated using either the 320-kV X-rays or 137Cs γ rays with the dose groups (categorical variables) indicated on the x-axis, transplanted with either syngeneic (A) or allogeneic (B) bone marrow. Six weeks later, percentage of Sca1+ cells were enumerated by flow cytometry (n = 6 mice/dose/irradiator). Fourteen unirradiated C.B-17 mice were euthanized to obtain unirradiated control cell numbers (none). * indicates significantly higher Sca1+ levels comparing γ rays to X-rays, P < .007 for all comparisons.
We also performed correlation analyses of the posttransplantation cell count data versus TLD measurements of radiation dose for both X-rays and γ rays. Results are presented in Table 2. In these analyses, the cell count for a given organ was divided by the animal’s body weight to adjust for different animal sizes. A significant negative correlation (r = −0.385, P < .05) was found for the syngeneic transplantation group exposed to γ rays, implicating a reconstitution deficit for high doses for this group. No evidence of a reconstitution deficit was found for X-ray-exposed mice. This likely relates to the X-ray doses used not being high enough to cause a reconstitution deficit after bone marrow transplantation.
Table 2.
Correlation r and Significance (P Value) for 6 Weeks Postradiation Exposure Organ Cell Count (Per Unit Body Weight)a Versus the Radiation Dose (in Gray) for C.B-17 Mice Receiving Syngeneic or Allogeneic Bone Marrow Transplantation After Irradiation.
| Organ | Radiation Type | Transplantation Type | Degrees of Freedomb | Correlation r | P |
|---|---|---|---|---|---|
| Bone marrow | γ rays | Syngeneic | 30 | −0.385 | <.05c |
| Bone marrow | X-rays | Syngeneic | 30 | +0.012 | >.5 |
| Bone marrow | γ rays | Allogeneic | 29 | −0.149 | >.2 |
| Bone marrow | X-rays | Allogeneic | 30 | −0.234 | >.1 |
| Spleen | γ rays | Syngeneic | 31 | + 0.115 | >.5 |
| Spleen | X-rays | Syngeneic | 30 | + 0.108 | >.5 |
| Spleen | γ rays | Allogeneic | 30 | −0.013 | >.5 |
| Spleen | X-rays | Allogeneic | 30 | −0.287 | >.1 |
a Cell counts (cellularity) obtained were divided by body weight to adjust for different body sizes.
b Number of data points used minus 2. Controls from γ-ray and X-ray studies were combined.
c A significant dose–response relationship implicating a cell population recovery deficit at high doses even after bone marrow transplantation.
Discussion
This is the first study, to our knowledge, directly comparing the effectiveness of filtered X-RAD 320 X-rays and Gammacell-1000-irradiator γ rays in producing bone marrow chimeric mice. The bone marrow transplantation study was undertaken in order to determine whether the X-RAD 320 irradiator would be a good substitute for solid source, γ-ray irradiators such as the Gammacell-1000 Unit due to the homeland security concerns of the latter. We compared the efficacy of filtered 320-kV X-rays and filtered Cs-137 γ rays in bone marrow transplantation studies focused on ablating bone marrow and spleen cells in recipient mice and reconstitution in bone marrow chimeric mice. A critical question is whether the X-RAD 320 irradiator X-rays have high-enough energy for adequate tissue penetration of experimental study mice to allow for successful bone marrow transplantation study in a split-dose design.
Two factors critical to successful allogeneic bone marrow reconstitution in host mice are (1) sufficient bone marrow ablation to make space for donor bone marrow as well as prohibit bone marrow rejection and (2) adequately suppressed host hematopoietic activity so that the transplanted donor bone marrow can out compete any residual host bone marrow cells and splenocytes in repopulating the host’s hematopoietic cells.16
Our results suggest that unlike filtered high-energy Cs-137 γ-ray photons, X-RAD 320-Unit, X-ray photons (with some low-energy photons removed via filtration) may still not have high-enough energy for successful allogeneic bone marrow transplantation studies in small rodents. The intended doses (target doses) of γ rays were selected based on findings from a literature search and the desired doses of X-rays were chosen based on our preliminary estimate of the X-ray RBE (=1.3) relative to γ rays. The RBE estimate is based on data from several studies for different end points using different energy photons.17-21 Thus, a target X-ray dose of 4.62 Gy (intended rather than measured dose) was chosen to compare to the highest target dose of γ rays (6.0 Gy γ rays; 6.0/1.3 = 4.62 Gy X-rays). Measured radiation doses obtained using TLDs are expected to be proportional to the average absorbed radiation dose to bone marrow. Thus, the shape of dose–response relationships obtained based on TLD-derived doses is expected to reflect the shape that would be obtained if average absorbed dose to bone marrow was used instead. It should be noted that evaluating average absorbed dose to bone marrow of mice, a major undertaking as reflected by the dosimetric studies of Azimi et al22, was beyond the scope of our research. In addition, absorbed radiation doses for the spleen could not be directly evaluated with our experimental design and possibly radiation dose assignments for spleen based on in-air TLD measurements may be overestimated. This would be expected to be less of an issue for the higher energy γ rays than for the lower energy X-rays.
Only mice with intended γ-ray doses (ie, target doses) of 4.01 or 6.0 Gy γ rays (measured dose range: 4.49-7.11 Gy) engrafted donor allogeneic bone marrow as demonstrated by donor-specific (B10.D2) cell phenotype markers in the recipient mice (C.B-17) spleen cell population 6 weeks after bone marrow transplantation (Figure 6B). The failure of filtered 320-kV X-rays at the highest exposure level (target dose 4.62 Gy) to allow for allogeneic bone marrow engraftment was unexpected due to the fact that ablation of bone marrow was quite similar to that for our highest γ-ray dose measured 24 hours after irradiation (Figure 1A) and that the RBE-weighted doses were quite similar for the highest X-ray (RBE = 1.35, central estimate) and γ-ray (RBE = 1) exposure groups.13
Although the bone marrow ablation at 24 hours was similar between doses and radiation types, significantly more splenocytes were spared by X-ray irradiation as compared to corresponding γ-ray irradiation. This may be explained on the basis of the X-ray RBE for ablating splenocytes unexpectedly being found to be significantly less than 1 (RBE = 0.76, central estimate). This can be seen from the results in Figure 1B where X-ray data points are shifted to the left compared to their locations in Figure 1A, where the X-ray RBE was found to be 1.35 (central estimate) for bone marrow cytotoxicity. However, an alternative explanation could be that the RBE < 1 for X-ray effects on splenocytes may be a systematic error related to the absorbed radiation dose to spleen not being directly estimated but rather being based on in-air TLD measurements in air. Stated differently, the absorbed X-ray dose to spleen may be significantly less than the dose assigned based on our TLD measurements. A systematic error of 1.35/0.76 (RBE estimates ratio) ≈1.8 would explain the result; however, such a large systematic error is unlikely, given that for killing bone marrow cells by X-rays the central estimate of the RBE is 1.35, which is quite close to the value of 1.3 used in the study design.13
As murine hematopoiesis has been shown to take place not only in the bone marrow but also in the spleen,23,24 splenocyte survival may be a significant contributing factor in the ability of the X-ray-irradiated mice to have repopulated their hematopoietic system with their own cells, rather than donor bone marrow. In addition, it is interesting to note that the lowest exposure level used for γ rays (target dose 2.03 Gy) spared significantly more splenocytes than the other 2 higher γ-ray exposures and the low γ-ray exposure did not allow for allogeneic bone marrow engraftment.
None of the γ-ray nor X-ray doses used caused acute lethality (no proven deaths from acute radiation effects), suggesting that mice irradiated at the exposure levels used were capable of reconstituting their own hematopoietic system without donor bone marrow. This was borne out by enumerating bone marrow and spleen cell numbers 6 weeks after irradiation in the absence of bone marrow transplantation (Figure 3). Although bone marrow reconstitution was similar using either radiation source, spleen cell reconstitution was significantly lower in mice in the highest γ-ray exposure group (target dose 6.0 Gy). Interestingly, mice in this group were able to accept allogeneic bone marrow. As shown in Figure 2, more than 90% of splenocytes and more than 75% of bone marrow cells would be expected to be ablated by a γ-ray dose of 6.0 Gy or higher (same as RBE-weighted dose of 6.0 Gy or higher).
Figure 4 shows the percentage weight change over time of irradiated C.B-17 mice that were transplanted with either syngeneic (4A) or allogeneic (4B) bone marrow and monitored for weight loss over the following 6 weeks. For weight loss evaluated for 5 days postirradiation (an average of results for days 2-6 postirradiation), the highest exposure levels used for X-rays and γ rays were clearly not equivalent and also indicate an RBE < 1 for X-rays relative to γ rays for this end point. However, a detailed evaluation for RBE for weight loss as a function of radiation dose is beyond the scope of the research conducted as there is no formal dose–response model for weight change profile for a given follow-up time.
Bone marrow transplantation led over time to significantly increased levels of bone marrow cells (Figure 5A and B) as compared to mice that did not receive bone marrow transplantation (Figure 3A and B), but no more spleen cells (Figure 5C and D) than when mice did not receive a bone marrow transplant. For bone marrow, a significant cell population reconstitution deficit after high-dose, γ-ray exposure was implicated even after bone marrow transplantation (Table 2), when evaluated 6 weeks after radiation exposure. This was not the case for high-dose X-rays and likely relates to the highest X-ray exposure level used being too low to cause a deficit. Regarding the reconstitution of specific subsets of lymphocytes in the spleen following bone marrow transplantation, this will be a subject of follow-on research if future support is achieved.
Our findings suggest that C.B-17 mice irradiated with any of the measured X-ray doses involved in this study (1.56-4.62 Gy), or the lowest measured dose of γ rays (2.03 Gy), rejected the donor allogeneic B10.D2 bone marrow and reconstituted their hematopoietic system using their own residual hematopoietic cells. Less than optimal allogeneic engraftment using γ-ray doses lower than 5.5 Gy were found by Down et al.25 However, dose rate, dose fractionation, and interfraction time interval were all considered modifiers of outcome observed.25
Previous studies at our Institute using 8 to 11 Gy X-rays (single-dose) from a 260-kVp Philips Therapy Unit led to widespread mortality even after bone marrow transplantation (unpublished data). Serious damage to multiple organs may have been involved given the dose range indicated and higher linear energy transfer photon radiation source. In addition, it is well known that the mouse strain,26 interval between fractionated dose delivery,25,26 and dose rate25 can all affect the success of bone marrow transplant and subsequent engraftment. Based on the indicated studies, it might be expected that using single doses of X-rays greater than 8 Gy would not allow for successful bone marrow transplantation. But what about split-dose X-rays with total doses exceeding 8 Gy?
Bone marrow chimeric mice were successfully produced by another research group using the split-dose study design and an X-RAD 320 irradiator when the total dose was 8.5 Gy.27 In order to produce bone marrow chimeras, CD45.1 congenic (B6.SJL-Ptprca) mice were first irradiated using the split-dose design (total dose 8.5 Gy from 2 fractions of 4.25 Gy 4 hours apart). The next day, recipients were given 107 bone marrow cells via IV injection into the mouse tail. The mice were held for 8 weeks before evaluating results. For chimeras reconstituted with retrovirally transduced bone marrow cells, recipients each received 5 × 105 transduced bone marrow cells and 106 unmanipulated B6.SJL-Ptprca bone marrow cells. Had we used a similar X-ray dose, our bone marrow transplantation may have also been successful. Based on this, it is reasonable to conclude that the X-RAD 320 Unit is suitable for bone marrow transplantation studies using mice.
Gibson et al compared Cs-137 γ rays (Gammaell 40; MSD Nordion, Ottawa, Ontario, Canada) and X-rays (Rad Source 2000 X-ray Biologic Irradiator; Rad Source Technologies, Alpharetta, Georgia) in bone marrow transplant reconstitution in C57Bl/6J mice. Mainly, 4 radiation doses (0.5, 7, 9, and 11 Gy) were used for each radiation source (ie, X-ray RBE relative to γ rays was not taken into account). Both X-rays and γ rays sufficiently ablated endogenous bone marrow (and likely also spleen) to allow for stem cell engraftment. However, distinct physiologic responses were revealed that need to be considered when selecting the best radiation source. Interestingly, Cs-137 γ rays were found to be associated with lower overall morbidity related to opportunistic infection.5 This finding implicates an X-ray RBE > 1, relative to γ rays, for inducing opportunistic infection-related morbidity in C57Bl/6J mice.
As shown in Figure 2, the cytotoxicity dose–response relationships for bone marrow cells and splenocytes are different and have complex shapes involving negative curvature (ie, decreasing slope as radiation dose increases). The negative curvature may relate to mixed cell populations comprised of large numbers of hypersensitive, modestly radiosensitive, and resistant cells.13 DNA repair capacity differences and bystander effect differences between different cell types that are involved in tissue homeostasis may also be important. Additional studies are needed to resolve key uncertainties related to acute and delayed radiobiological effects in the spleen and bone marrow of 320 kV X-rays from the X-RAD 320 irradiator, especially for somewhat lower and higher radiation doses than we have used. This is the focus of new studies being initiated by our research group, which will involve single rather than split doses. The single-dose design should help with resolving different dose–response relationships for different cell types.
Conclusions
Our highest 2 levels (both sublethal) of Cs-137 γ rays ablated sufficient bone marrow (>75% but <95%) and splenocytes (>80% but <100%) to permit successful syngeneic and allogeneic bone marrow transplantation in C.B-17 mice. In contrast, because the X-ray RBE for ablating splenocytes was unexpectedly <1 (central estimate 0.76) when expected to be about 1.3 (used in study design), bone marrow transplantation was not successful at even the highest 2 levels of 320-kV, X-rays used. However, as demonstrated by another research group, successful transplantation can be achieved using a higher dose. It remains to be determined what subset of bone marrow or spleen cells are hypersensitive or resistant to radiation and new studies are being initiated to address this key uncertainty.
Footnotes
Authors’ Note: This article describes objective technical results and analysis. Any subjective views or opinions that might be expressed in the article do not necessarily represent the views of the US Department of Energy or the U.S. government. Sandia National Laboratories is a multimission laboratory managed and operated by National Technology & Engineering Solutions of Sandia, LLC, a wholly owned subsidiary of Honeywell International Inc, for the US Department of Energy’s National Nuclear Security Administration under contract DE-NA0003525 and SAND2019-15284J.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by the U.S. Department of Energy's National Nuclear Security Administration under Contract DE-NA0003525, SAND2019-15284 J.
ORCID iD: Yong Lin  https://orcid.org/0000-0001-6276-6528
https://orcid.org/0000-0001-6276-6528
References
- 1. Janatpour K, Denning L, Nelson K, Betlach B, Mackenzie M, Holland P. Comparison of X-ray vs. gamma irradiation of CPDA-1 red cells. Vox Sang. 2005;89(4):215–219. [DOI] [PubMed] [Google Scholar]
- 2. Dodd B, Vetter RJ. Replacement of 137Cs irradiators with x-ray irradiators. Health Phys. 2009;96(2 suppl):S27–S30. [DOI] [PubMed] [Google Scholar]
- 3. Potter CA. Kinetics equation replacement function for a particular continuous intake scenario. Health Phys. 2013;104(1):95–96. [DOI] [PubMed] [Google Scholar]
- 4. Scott BR, Potter CA. Stochastic threshold exponential (TE) model for hematopoietic tissue reconstitution deficit after radiation damage. Dose Response. 2014;12(3):415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gibson BW, Boles NC, Souroullas GP, et al. Comparison of cesium-137 and X-ray irradiators by using bone marrow transplant reconstitution in C57BL/6J mice. Comp Med. 2015;65(3):165–172. [PMC free article] [PubMed] [Google Scholar]
- 6. Iwasaki A. The use of bone marrow-chimeric mice in elucidating immune mechanisms. Methods Mol Med. 2006;127:281–292. [DOI] [PubMed] [Google Scholar]
- 7. Dierckx de Casterle I, Fevery S, Rutgeerts O, et al. Reduction of myeloid-derived suppressor cells reinforces the anti-solid tumor effect of recipient leukocyte infusion in murine neuroblastoma-bearing allogeneic bone marrow chimeras. Cancer Immunol Immunother. 2018;67(4):589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sreeramkumar V, Hidalgo A. Bone marrow transplantation in mice to study the role of hematopoietic cells in atherosclerosis. Methods Mol Biol. 2015;1339:323–332. [DOI] [PubMed] [Google Scholar]
- 9. Duran-Struuck R, Dysko RC. Principles of bone marrow transplantation (BMT): providing optimal veterinary and husbandry care to irradiated mice in BMT studies. J Am Assoc Lab Anim Sci. 2009;48(1):11–22. [PMC free article] [PubMed] [Google Scholar]
- 10. National Research Council. Radiation Source Use and Replacement. Washington, DC: The National Academies Press; 2008. [Google Scholar]
- 11. Rana N, Rawat D, Parmar M, Dhawan DK, Bhati AK, Mittal BR. Evaluation of external beam hardening filters on image quality of computed tomography and single photon emission computed tomography/computed tomography. J Med Phys. 2015;40(4):198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Potter CALS, Scott BR, Lin Y, et al. Radiobiological Studies using Gamma and X Rays. Sandia Report SAND2013-0743; 2013. [Google Scholar]
- 13. Scott BR, Gott KM, Potter CA, Wilder J. A Comparison of in vivo cellular responses to Cs-137 gamma rays and 320-kV X rays. Dose Response. 2013;11:444–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kutkov V, Buglova E, McKenna T. Severe deterministic effects of external exposure and intake of radioactive material: basis for emergency response criteria. J Radiol Prot. 2011;31(2):237–253. [DOI] [PubMed] [Google Scholar]
- 15. Thompson JS, Asmis R, Chu Y, Glass J, Nelson B, Brown SA. Amifostine prior to lethal irradiation prevents allogeneic bone marrow engraftment in mice. Bone Marrow Transplant. 2008;41(11):927–934. [DOI] [PubMed] [Google Scholar]
- 16. Koch A, Gulani J, King G, Hieber K, Chappell M, Ossetrova N. Establishment of early endpoints in mouse total-body irradiation model. PLoS One. 2016;11(8):e0161079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kocher DC, Apostoaei AI, Hoffman FO. Radiation effectiveness factors for use in calculating probability of causation of radiogenic cancers. Health Phys. 2005;89(1):3–32. [DOI] [PubMed] [Google Scholar]
- 18. Fabry L, Coton C. Study on the repair of the radioinduced lesions involved in the formation of chromosomal aberrations in G0 human lymphocytes after exposure to gamma-rays and fast neutrons. Mutat Res. 1985;149(3):475–483. [DOI] [PubMed] [Google Scholar]
- 19. Roos H, Schmid E. Analysis of chromosome aberrations in human peripheral lymphocytes induced by 5.4 keV x-rays. Radiat Environ Biophys. 1998;36(4):251–254. [DOI] [PubMed] [Google Scholar]
- 20. Goggelmann W, Jacobsen C, Panzer W, Walsh L, Roos H, Schmid E. Re-evaluation of the RBE of 29 kV x-rays (mammography x-rays) relative to 220 kV x-rays using neoplastic transformation of human CGL1-hybrid cells. Radiat Environ Biophys. 2003;42(3):175–182. [DOI] [PubMed] [Google Scholar]
- 21. Krumrey M, Ulm G, Schmid E. Dicentric chromosomes in monolayers of human lymphocytes produced by monochromatized synchrotron radiation with photon energies from 1.83 keV to 17.4 keV. Radiat Environ Biophys. 2004;43(1):1–6. [DOI] [PubMed] [Google Scholar]
- 22. Azimi R, Alaei P, Spezi E, Hui SK. Characterization of an orthovoltage biological irradiator used for radiobiological research. J Radiat Res. 2015;56(3):485–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Short C, Lim HK, Tan J, O’Neill HC. Targeting the spleen as an alternative site for hematopoiesis. Bioessays. 2019;41(5):e1800234. [DOI] [PubMed] [Google Scholar]
- 24. Poulin LF, Lasseaux C, Chamaillard M. Understanding the cellular origin of the mononuclear phagocyte system sheds light on the myeloid postulate of immune paralysis in sepsis. Front Immunol. 2018;9:823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Down JD, Tarbell NJ, Thames HD, Mauch PM. Syngeneic and allogeneic bone marrow engraftment after total body irradiation: dependence on dose, dose rate, and fractionation. Blood. 1991;77(3):661–669. [PubMed] [Google Scholar]
- 26. Cui YZ, Hisha H, Yang GX, et al. Optimal protocol for total body irradiation for allogeneic bone marrow transplantation in mice. Bone Marrow Transplant. 2002;30(12):843–849. [DOI] [PubMed] [Google Scholar]
- 27. Gardam S, Turner VM, Anderton H, et al. Deletion of cIAP1 and cIAP2 in murine B lymphocytes constitutively activates cell survival pathways and inactivates the germinal center response. Blood. 2011;117(15):4041–4051. [DOI] [PubMed] [Google Scholar]