Abstract
Objective:
The objective of the present work was to study the role of Cxcl1 in cerebral ischemia–reperfusion (I/R) injury and to in-depth explore its pathogenesis.
Methods:
The expression of Cxcl1 based on the public data was analyzed. Then, we constructed an oxygen glucose deprivation/reoxygenation (OGD/R) model in vitro using mice brain microvascular endothelial cells (BMECs) to simulate cerebral I/R in vivo.
Results:
The results of quantitative real-time polymerase chain reaction assay uncovered that Cxcl1 showed higher expression while miR-429 showed lower expression in BMECs damaged by OGD/R, whereas overexpression of Cxcl1 or inhibition of miR-429 expression can strengthen this effect. Hereafter, through dual luciferase reporter assay, we verified that miR-429 directly targets Cxcl1 and negatively regulates Cxcl1 expression. Furthermore, the results also revealed that overexpression of Cxcl1 can reverse the miR-429-mediated effects.
Conclusion:
We concluded that miR-429 exerts protective effects against OGD/R-induce injury in vitro through modulation of Cxcl1 and nuclear factor kinase B pathway, hoping provide a new view on the pathogenesis of cerebral I/R injury and a feasible potential therapeutic target.
Keywords: cerebral I/R injury, Cxcl1, miR-429, OGD/R, NF-κB pathway
Introduction
The most sensitive apparatus of ischemia and hypoxia is brain tissue, and cerebral ischemia leads to impaired function of local brain tissue.1 Restoring the blood supply of brain tissue after cerebral ischemia cannot reinstate the physiologic function of brain tissue but aggravate the encephalon injury, which is called cerebral ischemia–reperfusion (I/R) injury.2,3 Cerebral I/R injury is interrelated with the high disability rate and high death rate of cerebrovascular disease.4 It is an extremely complex pathophysiological process, such as endoplasmic reticulum stress, oxidative stress, inflammation, and autophagy, but its specific pathogenesis is not clear and there is still short of efficacious therapy strategies.5 Cerebral microvascular endothelial cells (CMECs) are special structures of brain microvessels, and one of their important functions is to form blood–brain barrier (BBB).6 Further research found that the initial cause of BBB disruption is CMEC death result from cerebral I/R injury.7,8 Hence, protecting CMEC from cerebral I/R injury is probably an effective treatment to improve the prognosis of cerebral I/R injury.
Chemokines have the function of promoting nerve recovery.9 As a member of the CXC subfamily of chemokines, up to now, numerous reports have been published on the application of Cxcl1 in brain diseases. Cxcl1 can be used as a biomarker for diagnosis of Alzheimer disease in the early stage10 and played a role in promoting the proliferation of neural stem cells in APP/PS1 mice.11 A study in 2005 by Losy et al discovered that Cxcl1 was involved in the inflammatory response in the early stages of ischemic stroke.12 Recently, Cxcl1 has been found to be a central gene after 2, 8, and 24 hours of cerebral I/R injury.13 Additionally, the nuclear factor kinase B (NF-κB) pathway was participated in the regulation of I/R injury,14-16 and activation of this pathway can improve the level of Cxcl1 expression.17 Nevertheless, little work has been done on the application of Cxcl1 as a regulatory gene in cerebral I/R injury.
To date, more than 60% of the genes are considered to be regulated by microRNAs (miRNAs).18 Bioinformatics predictions revealed that miR-429 is a regulatory miRNA on Cxcl1. MiR-429, as an important member of the miR-200 family, has been reported to be induced by hypoxia-inducible factor 1α, which produced in a hypoxic environment, forming a negative feedback cycle in endothelial cells under hypoxic conditions.19 Xiao et al have showed that miR-429 can reduce oxygen glucose deprivation/reoxygenation (OGD/R)-induced neuronal damage via upregulating GATA4.5 However, an miRNA can regulate multiple target genes, and the specific mechanism is still unclear.
In this article, an ODG/R model was established in vitro using mice brain microvascular endothelial cells (BMECs) to simulate cerebral I/R injury. We revealed that miR-429/ Cxcl1 axis acts as a potential regulator to against ODG/R-induced injury through NF-κB pathway, which provided a novel and promising targets for cerebral I/R injury.
Materials and Methods
Data Collection
The samples of I/R injury in wild-type mice were downloaded from the Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/; access number: GSE23160). Four different cases of I/R-2 hours (I/R-2h), I/R-8 hours (I/R-8h), I/R-24 hours (I/R-24h), and Sham were selected for differential gene analysis. The miRNA gene prediction sites miRTarBase (http://mirtarbase.mbc.edu.tw/php/index.php) and TargetScan (http://www.targetscan.org) were used to predict the upstream regulatory miRNA of Cxcl1.
Cell Culture and OGD/R Model Construction
The mice BMECs were cultured in deoxygenated glucose-free Hanks’ Balanced Salt Solution. To simulate I/R, we created the OGD/R cell model. The BMECs were transferred to an anaerobic chamber (5% CO2, 95% N2) and incubated in sugar-free Dulbecco modified Eagle medium at 37°C for 4 hours, followed by replacing with normal glucose level medium and incubated the BMECs for 24 hours at room temperature for reoxygenation (95% air, 5% CO2). Simultaneously, the control cells were cultured under normal conditions.
Cell Transfection
The miR-429 mimic/inhibitor and corresponding negative control (NC) were obtained from Shanghai GenePharma Co, Ltd. (Shanghai, China), and the si-Cxcl1, si-con, and pcDNA3.1-Cxcl1 were designed and synthesized by Shanghai GenePharma Co, Ltd (Shanghai, China). The cells were seeded in a 6-well plate 1 day before transfection. The transfections were carried out with Lipofectamine 2000 (Invitrogen, USA) when the cell fusion achieved 80%, based on the manufacturer’s instructions. After that, the cells were cultured at 37°C with 5% CO2 for 6 hours, followed by replacing the medium into a complete medium. After 48 hours of transfection, follow-up experiments were performed.
Quantitative Real-Time Polymerase Chain Reaction Assay
TRIzol reagent (Invitrogen, Carlsbad, California) was used to extract RNA, and complementary DNA was synthesized by reverse transcriptase using miScript RT Kit (Qiagen, Dusseldorf, Germany) for miRNA or Prime Script RT Kit (RR036A; Takara Biotechnology Co., Ltd, Dalian, China) for messenger RNA (mRNA). Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) assay was performed to test the expression level of miR-429 and Cxcl1 using RT qPCR kit (Kapa Biosystems, Wilmington, Massachusetts), according to the manufacturer’s instructions. The 2−△△Ct method was used to calculate the expression of miRNA and mRNA, while U6 and GAPDH were used as internal controls. The primer sequences were:
miR-429: F: 5′-ATACTGTCTGGTAATGCCG-3′
R: 5′-GAACATGTCTGCGTATCTC -3′;
U6: F: 5′-CTCGCTTCGGCAGCA CATATACT -3′
R: 5′-ACGCTTCACGAATTTGCGTGTC -3′;
CXCL1: F: 5′- TCCAGAGCTTGAAGGTGTTGCC -3′
R: 5′-AACCAAGGGAGCTTCAGGGTCA -3′;
GAPDH: F: 5′- TGTGTCCGTCGTGGATCTGA-3′
R: 5′- CCTGCTTCACCACCTTCTTGA-3′.
Western Blot
Cells were lysed with lysate buffer containing protein inhibitors to extract the total protein, then BCA kit was used to test the protein concentration. Hereafter, 20 µg of protein samples were detached by sodium dodecyl sulfate–polyacrylamide gel electrophoresis. The polyvinylidene fluoride membrane was transferred with the proteins and then sealed with 5% skimmed milk powder for 1 hour. After that, the blotted membrane was incubated with the primary antibody at 4°C overnight, followed by washing it 3 × 5 minutes with TBST. Then the membrane was incubated with the second antibody for 1 hour at room temperature. An ECL detection kit (Sigma Aldrich; Merck KGaA, Darmstadt, Germany) was used to develop the protein signaling after washing the membrane. The protein level was evaluated by using GAPDH as an internal control.
Cell Counting Kit-8 Assay
The transfected cells were prepared into a cell suspension and then seeded the cell suspension in a 96-well plate with the density of 1 × 103/well and then performed OGD/R treatment. Subsequently, cells were conventional cultured in an incubator with CO2 for 24 hours. Using a microplate reader to detect optical density values with 450 nm excitation light. Before detection, 10 µL of CCK8 reagent (Dojindo, Kumamoto, Japan) was added into each well, and incubated the plate for 1.5 hours in a 37°C incubator.
Apoptosis Assay
The cells were stained and quantified with Annexin V-FITC/Propidium iodide (PI) kit (4 A Biotech, Beijing, China) according to the manufacturer’s instructions. The transfected cells were incubated in 200 µL binding buffer containing 10 µL of Annexin V-FITC and 5 µL of PI in the dark at room temperature for 1 hour. Then apoptotic analysis was performed by flow cytometry. Finally, Flowjo software was used to analyze the flow results.
Dual-Luciferase Reporter Assay
In order to further confirm whether miR-429 is an upstream regulatory miRNA of Cxcl1, dual-luciferase reporter assay was carried out. First, wild and mutant Cxcl1-3′-untranslated region (UTR) containing the binding site of miR-429 were cloned into pmirGLO luciferase vector to construct Cxcl1-wild-type (WT) and Cxcl1-mutant (MUT) vectors. Then HEK 239T cells were cotransfected with miR-429 mimic or miR-429 mimic NC and Cxcl1-WT or Cxcl1-MUT using lipofectamine 2000, according to the standard protocol. After 48 hours of transfection, the luciferase activity was detected using the Dual-Luciferase TM system (Promega) based on the standard protocol.
Statistical Analysis
In this article, the data were analyzed by statistical analysis software SPSS22.0 (SPSS, Inc, Chicago, Illinios). Student t test (2 groups) or 1-way analysis of variance analysis with posttest of Dunnett (3 groups and above) was used for comparison between groups. Statistical results were considered to be significantly different when P < .05.
Results
Cxcl1 and MiR-429 Were Differential Expressed in Cerebral I/R Injury
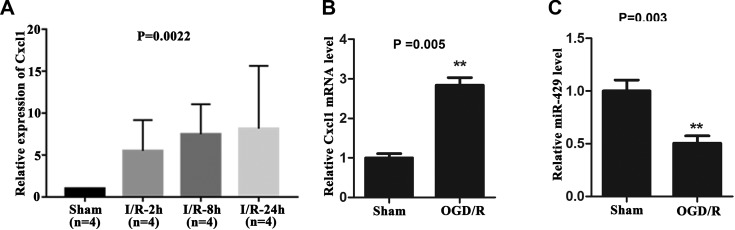
First, the data including I/R-2h (n = 4), I/R-8h (n = 4), I/R-24h (n = 4), and Sham (n = 4) samples were analyzed by GEO Online Analysis Tool GEO2R. It can be seen in Figure 1A that the Cxcl1 expression in I/R-2h group, I/R-8h group, and I/R-24h group was markedly elevated than the sham group (P < .01). Interestingly, it was further found that Cxcl1 level was constantly enhanced with the prolongation of I/R time.
Figure 1.
The expression level of Cxcl1 and miR-429 in OGD/R-treated BMECs. A, The expression level of Cxcl1 in I/R-2h group, I/R-8h group, and I/R-24 h group was significantly increased compared with the sham group, P < .01, which was constantly enhanced with the prolongation of I/R time. B, Cxcl1 was highly expressed in OGD/R treated BMECs. **P < .01. C, MiR-429 expression was low in OGD/R-treated BMECs. **P < .01. BMECs indicates microvascular endothelial cells; I/R, ischemia–reperfusion; OGD/R, oxygen glucose deprivation/reoxygenation.
Based on the target gene prediction web site and literature analysis, we predicted that the upstream regulatory miRNA of Cxcl1 was miR-429. So as to explore the function of Cxcl1 was miR-429 in I/R injury, we constructed an OGD/R model in vitro using BMECs and detected the expression level of Cxcl1 and miR-429 in BMECs suffered to OGD/R using qRT-PCR assay. The results illustrated that the expression level of Cxcl1 was markedly elevated in OGD/R-treated BMECs compared with the sham group (P < .01, Figure 1B). Simultaneously, the expression level of miR-429 showed an opposite result (Figure 1C, P < .01). In summing, these results prompted that Cxcl1 and miR-429 may be involved in cerebral I/R injury.
The Transfection Efficiency of pcDNA3.1-Cxcl1/si-Cxcl1 and MiR-429 Mimic/Inhibitor in BMECs Suffered From OGD/R
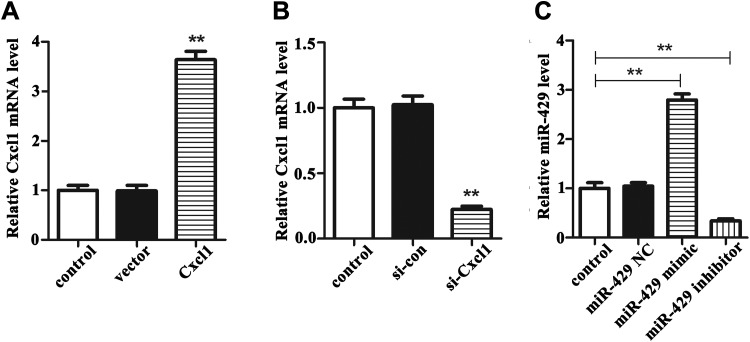
To verified the function of Cxcl1 and miR-429 in BMECs, we divided the cells into Cxcl1 overexpression groups, Cxcl1 knockdown group, and miR-429mimic/inhibitor group. At the same time, the control group in which cells treated with transfection reagent was set up. As can be seen from Figure 2A, the expression of Cxcl1 in cells transfected with pcDNA3.1-Cxcl1 was markedly increased than that in cells transfected with vector and control (P < .01). In the meantime, the results in Figure 2B indicated that the expression of Cxcl1 in cells transected with si-Cxcl1 was significantly decreased than that in cells transected with si-con and control (P < .01). Then, we analyzed the data in the miR-429 mimic/inhibitor group. As described in Figure 2C, the miR-429 expression in cells transected with miR-429 mimic was obviously higher than that in control group (P < .01), and the miR-429 expression in cells transected with miR-429 inhibitor was clearly lower than that in the control group (P < .01). These results indicated that miR-429 and Cxcl1 were significantly decreased or increased in BMECs after transected, laying the foundation for subsequent experiments.
Figure 2.
The transfect ion efficiency of Cxcl1 and miR-429 in OGD/R-treated BMECs. A, The expression of Cxcl1 in cells transected with pcDNA3.1-Cxcl1 or vector compared with control. **P < .01. B, The expression of Cxcl1 in cells transected with si-Cxcl1 or si-con compared with control. **P < .01. C, The miR-429 expression in cells transected with miR-429 mimic, miR-429 inhibitor, or corresponding NC compared with control. **P < .01. BMECs indicates microvascular endothelial cells; OGD/R, oxygen glucose deprivation/reoxygenation.
Knockdown of Cxcl1 Enhances the Viability While Inhibition of MiR-429 Expression Reduced the Viability of OGD/R-Damaged BMECs
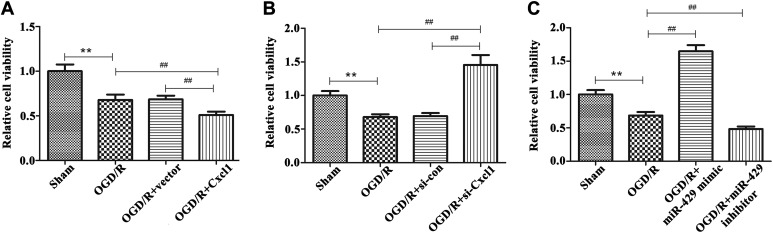
So as to examine the influence of Cxcl1 and miR-429 on the viability of BMECs, CCK-8 assay was carried out. We can see from Figure 3A and B that the cell viability was markedly declined in OGD/R-treated BMECs than that in sham group (P < .01), and this influence was markedly strengthened by overexpression of Cxcl1 and reversed by knocking down of Cxcl1 (P < .01). Furthermore, we found that transfection of 429-mimic can promote the viability of OGD/R-treated BMECs than that of OGD/R group, whereas inhibition of miR-429 showed an opposite effects (Figure 3A, P < .01). These findings demonstrated that Cxcl1 and miR-429 can regulate the cell viability of OGD/R-treated BMECs.
Figure 3.
Effects of Cxcl1 and miR-429 on cell viability in OGD/R-treated BMECs. A, The cell viability in sham group, OGD/R-treated group, vector group, and overexpression of Cxcl1 group, **P < .01 versus the sham group, ## P < .01 versus the OGD/R-treated group. B, The cell viability in sham group, OGD/R-treated group, si-con group, and knockdown of Cxcl1 group, **P < .01, ## P < .01. C, The cell viability in sham group, OGD/R-treated group, miR-429 mimic group, and miR-429 inhibitor group, **P < .01, ## P < .01. BMECs indicates microvascular endothelial cells; OGD/R, oxygen glucose deprivation/reoxygenation.
MiR-429 is an Upstream MiRNA of Cxcl1 and Directly Targets Cxcl1
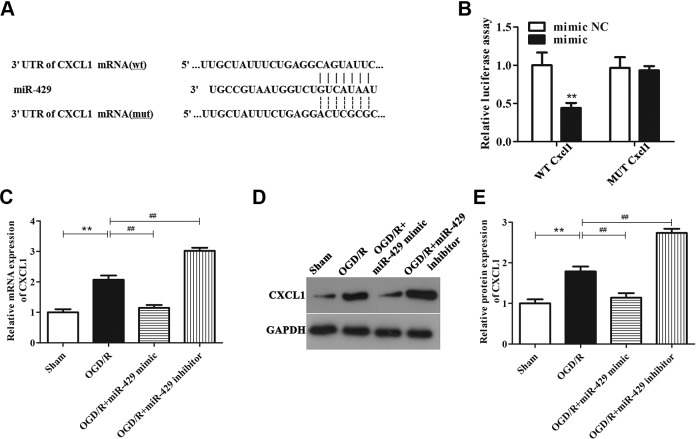
First, through bioinformatics analysis, we predicted that miR-429 was the upstream target gene of Cxcl1, and Cxcl1 has the binding sites for miR-429 in the 3′-UTR region (Figure 4A). To further confirm whether miR-429 directly targets Cxcl1, dual luciferase reporter assay was carried out. WT-Cxcl1 or MUT-Cxcl1 and miR-429 mimic or miR-429 mimic NC were cotransected into HEK 239T cells to determine the luciferase activity. As shown in Figure 4B, luciferase activity in vectors containing WT-Cxcl1 showed a significantly downswing when transected with miR-429 mimic (P < .01), and this influence was hindered in mutations. This result illustrated that miR-429 directly targets Cxcl1.
Figure 4.
MicroRNA-429 directly targets Cxcl1 in OGD/R-treated BMECs. A, Comparison of the sequences of miR-429 and Cxcl1. B, The fluorescence intensity of Cxcl1-WT and miR-429 mimic cotransected cells was markedly declined, **P < .01, while the fluorescence intensity of Cxcl1-MUT and miR-429 mimic cotransected cells was almost unchanged. C, The mRNA level of Cxcl1 in sham group, OGD/R-treated group, miR-429 mimic group, and miR-429 inhibitor group. **P < .01 versus the sham group, ## P < .01 versus the OGD/R-treated group. D and E, The protein level of Cxcl1 in sham group, OGD/R-treated group, miR-429 mimic group, and miR-429 inhibitor group. **P < .01, ## P < .01. BMECs indicates microvascular endothelial cells; mRNA, messenger RNA; MUT, mutant; OGD/R, oxygen glucose deprivation/reoxygenation; WT, wild-type.
To further examine the regulation of miR-429 on Cxcl1, we performed qRT-PCR and Western blot experiments. Figure 4C-E indicate that the mRNA and protein expression levels of Cxcl1 were markedly escalated in BMECs after OGD/R treatment than that in sham group (P < .01), while this effect was reversed after upregulation of miR-429 expression (P < .01). On the contrary, inhibition of miR-429 can markedly increase the mRNA and protein expression levels of Cxcl1 in OGD/R-treated BMECs than that in OGD/R group. These results illustrated that miR-429 can negatively regulate Cxcl1 levels, further confirming that miR-429 was the upstream regulatory miRNA of Cxcl1.
Effect of MiR-429/Cxcl1 Axis on Cell Biological Function
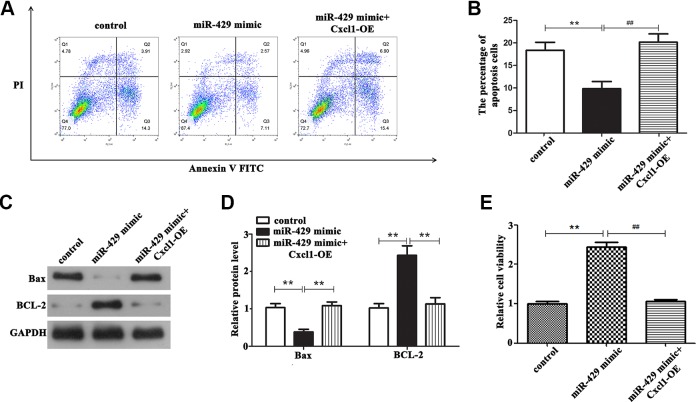
Moreover, we set up a rescue experiment to confirm whether miR-429 affects the biological behavior of OGD/R-treated BMECs by regulating Cxcl1. First, miR-429 mimic and miR-429 mimic plus pcDNA3.1-CXCL1 were transected into OGD/R-treated BMECs, respectively, and the control group was treated with transfect reagent. After that, CCK8 assay was performed to detect cell viability, and flow cytometry was carried out to detect cell apoptosis. The results of flow cytometry assay illustrated that after overexpression of miR-429 in OGD/R-treated BMECs, the number of apoptotic cells was markedly declined compared with the OGD/R group (Figure 5A and B, P < .01), whereas the number of apoptosis was significantly increased in OGD/R-treated BMECs cotransected with miR-429 mimic and pcDNA3.1-Cxcl1 compared with the miR-429 mimic group (P < .01). Simultaneously, we can see the result of CCK-8 from Figure 5E that upregulation of miR-429 expression can markedly induce the cell viability in OGD/R-treated BMECs than that in control (P < .01), while overexpression of Cxcl1 reversed this effect (P < .01). Furthermore, Western blot assay was performed to detect the effect of miR-429/Cxcl1 axis on apoptotic proteins. It can be seen from Figure 5C and D that overexpressed miR-429 can markedly decreased the expressions of proapoptosis protein Bax, while this decrease was hindered when the OGD/R-treated BMECs cotransected with pcDNA3.1-Cxcl1 and miR-429 mimic (P < .01). BCL-2, as an antiapoptosis protein, showed the opposite results. Taken together, these results provided that miR-429 can exert its biological function in OGD/R-treated BMECs by regulating Cxcl1.
Figure 5.
The effect of miR-429/Cxcl1 axis on biological behavior of OGD/R-treated BMECs. A and B, Overexpression of miR-429 can significantly reduce the apoptotic rate of cells compared with the control group, whereas overexpression of Cxcl1 can significantly reverse this effect, **P < .01 versus control, ## P < .01 versus the miR-429 mimic group. C and D, The expression of proapoptosis protein Bax and antiapoptosis protein BCL-2 in different conditions, **P < .01, ## P < .01. E, Overexpression of miR-429 can significantly induce the viability of cells compared with the control group, whereas overexpression of Cxcl1 can significantly reverse this effect, **P < .01, ## P < .01. BMECs indicates microvascular endothelial cells; OGD/R, oxygen glucose deprivation/reoxygenation.
NF-κB Pathway Mediates the Regulation of MiR-429/ Cxcl1 on OGD/R-Induced Injury in BMECs
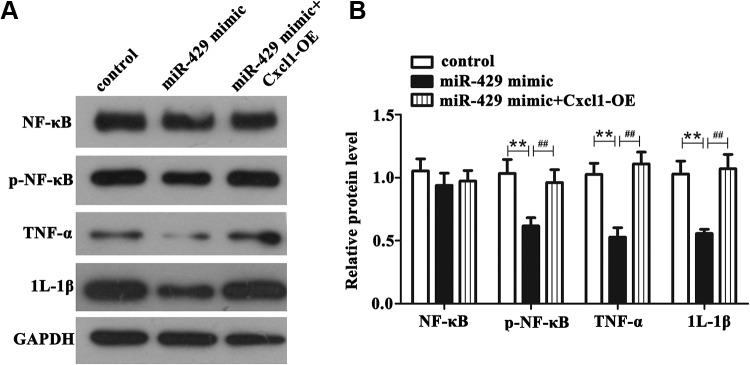
To in-depth study the protective mechanism of miR-429/ Cxcl1 axis on OGD-R-induced injury, we carried out Western blot experiment to detect the protein expression of pathway-related factors and proinflammatory factors. From Figure 6A and B, we can see that the protein level of phosphorylation NF-κB, tumor necrosis factor α (TNF-α), and interleukin (IL) 1β in miR-429 mimic group showed a significantly decrease than those in control group in OGD/R-treated BMECs (P < .01), while upregulation of Cxcl1 hindered the decrease caused by overexpression of miR-429 (P < .01). Contrarily, the protein level of total NF-κB was almost not altered. Overall, these results illustrated that miR-429/Cxcl1 axis can protect OGD/R-induced injury through NF-κB pathway, hoping to provide a new scheme to protect cerebral I/R injury in clinical therapeutics.
Figure 6.
The effect of miR-1293/HAO2 axis on NF-κB pathway in OGD/R-treated BMECs. A and B, The protein level of NF-κB, phosphorylation NF-κB, TNF-α, and IL-1β in different conditions. **P < .01 versus control, ## P < .01 versus the 429-mimic group. BMECs indicates microvascular endothelial cells; IL-1β, interleukin 1β, NF-κB, nuclear factor kinase B; TNF-α, tumor necrosis factor α.
Discussion
In this article, we conclude that Cxcl1 as a novel gene participated in regulating OGD/R-induced injury in vitro, which was highly expressed in OGD/R-treated BMECs, and knocking down of Cxcl1 enhances the cell viability. Then, we identified that miR-429 directly targets Cxcl1, and miR-429 was lowly expressed in BMECs after OGD/R treatment. Further data indicated that miR-429/ Cxcl1 axis can protect BMECs against OGD/R-induced injury, while this protection was achieved by the mediation of the NF-κB pathway.
So far, there have been studies highlighting the role of Cxcl1 in I/R injury. Zheng et al based on bioinformatics analysis indicated that Cxcl1 might be participated in the reinfusion period of liver I/R injury.20 In addition, alveolar type II epithelial cells participate in lung I/R injury by producing strong chemokines such as CXCL1.21 For cerebral I/R injury, Losy et al found that CXCL1 was escalated in the cerebrospinal fluid of patients with stroke at 2, 8, and 24 hours after reperfusion.12 In our study, the data obtained from bioinformatics analysis appear to be similar to the reported earlier by Losy et al that the expression level of Cxcl1 in cerebral I/R group was obviously enhanced than that in the sham group, and its level was constantly enhanced with the prolongation of I/R time. Further experimental results indicated that Cxcl1 was highly expressed in OGD/R-treated BMECs, and knocking down of Cxcl1 enhances the viability of BMECs damaged by OGD/R. These results suggested that Cxcl1 may contribute to protecting cells against the OGD/R-induced injury.
Previous research has shown that most of mRNAs function in diseases through the regulation of miRNAs.18 In the present study, we predicted upstream miRNAs that might regulate Cxcl1, and the results indicated that miR-429 was an upstream molecule of Cxcl1. Moreover, this prediction was further confirmed by the dual luciferase assay. MiR-429 has been reported to function in tumor suppressor genes in multiple cancers, such as nasopharyngeal carcinoma,22 pancreatic cancer,23 thyroid cancer,24 nephroblastoma,25 and so on. Recently, there have also been many reports showed that miR-429 was contributed to the development of various brain diseases. MALAT-1 can accelerate apoptosis of hippocampus neurons through negative regulation of miR-429.26 In addition, upregulation of miR-429 expression can significantly increase the permeability of blood–tumor barrier, so that chemotherapeutic drugs can better act on brain tumors.27 Lee et al found that miR-429 plays a neuron-protective role by targeting prolife hydroxyls 2.28 Our data indicated that miR-429 was downregulated in OGD/R-treated BMECs and overexpression of miR-429 can increase the cell viability while downregulation of miR-429 can decrease the cell viability. Furthermore, the rescue experiment data revealed that overexpression of Cxcl1 can reverse these effects. These findings suggested that miR-429/Cxcl1 axis can against the injury caused by OGD/R in BMECs.
Nuclear factor kinase B can participate in many biological functions of cells,14 and it is activated in a variety of cerebrovascular diseases.29 Nuclear factor kinase B has been proved to be a key factor in the regulation of inflammation,30 which activation can promote the production of extensive inflammatory factors, such as IL-1 and IL-6, thus aggravating the cerebral I/R injury.29 Similarly, a recent study has indicated that baicalein plays a neuron-protective role in mice with cerebral I/R injury by inhibiting NF-κB signal transduction to reduce neuron-inflammation and apoptosis.31 Kim et al have demonstrated that Dexmedetomidine protects neurons from cerebral I/R injury through the anti-inflammatory effect of inactivation of toll-like receptor 4/NF-κB pathway.32 This study demonstrated that miR-429 can reduce the protein expression of pathway-related factor phosphorylation NF-κB and proinflammatory factors TNF-α and IL-1β, whereas upregulation of Cxcl1 expression can block this effect. These results indicated that the protective effect of the miR-429/Cxcl1 axis on OGD/R-induced injury was achieved through NF-κB pathways.
Conclusion
To sum up, on the basis of bioinformatics and experimental data analysis, our results provide substantial evidence for the initial hypothesis that miR-429/ Cxcl1 axis in part acts as a potential regulator to against ODG/R-induced injury through NF-κB pathway in BMECs. It supplied a new opinion for the mechanism of cerebral I/R injury and its clinical treatment. However, the findings of this study are restricted to OGD/R models in vitro, the effects of miR-429/Cxcl1 axis on cerebral I/R injury in vivo need further study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Jun Leng  https://orcid.org/0000-0002-3318-2301
https://orcid.org/0000-0002-3318-2301
References
- 1. Su J, Liu J, Yan XY, Zhang Y, Zhang JJ, Zhang LC, Sun LK. Cytoprotective effect of the UCP2-SIRT3 signaling pathway by decreasing mitochondrial oxidative stress on cerebral ischemia-reperfusion injury. Int J Mol Sci. 2017;18(7):pii: E1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang X, Ji H, Yao Y, et al. Downregulation of circ_008018 protects against cerebral ischemia-reperfusion injury by targeting miR-99a. Biochem Biophys Res Commun. 2018;499(4):758–764. [DOI] [PubMed] [Google Scholar]
- 3. Wang J, Xu Z, Chen X, et al. MicroRNA-182-5p attenuates cerebral ischemia-reperfusion injury by targeting Toll-like receptor 4. Biochem Biophys Res Commun. 2018;505(3):677–684. [DOI] [PubMed] [Google Scholar]
- 4. Liang L, Wang J, Yuan Y, et al. MicRNA-320 facilitates the brain parenchyma injury via regulating IGF-1 during cerebral I/R injury in mice. Biomed Pharmacother. 2018;102:86–93. [DOI] [PubMed] [Google Scholar]
- 5. Xiao J, Kong R, Hu J. Inhibition of microRNA-429 attenuates oxygen-glucose deprivation/reoxygenation-induced neuronal injury by promoting expression of GATA-binding protein 4. Neuroreport. 2018;29(9):723–730. [DOI] [PubMed] [Google Scholar]
- 6. Li Z, Li J, Tang N. Long noncoding RNA Malat1 is a potent autophagy inducer protecting brain microvascular endothelial cells against oxygen-glucose deprivation/reoxygenation-induced injury by sponging miR-26b and upregulating ULK2 expression. Neuroscience. 2017;354:1–10. [DOI] [PubMed] [Google Scholar]
- 7. Lakhan SE, Kirchgessner A, Tepper D, Leonard A. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol. 2013;4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang QY, Wang ZJ, Sun DM, et al. Novel therapeutic effects of leonurine on ischemic stroke: new mechanisms of BBB integrity. Oxid Med Cell Longev. 2017;2017:7150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams JL, Holman DW, Klein RS. Chemokines in the balance: maintenance of homeostasis and protection at CNS barriers. Front Cell Neurosci. 2014;8:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schapiro RC, Kuhn M, Xiong C, et al. Multiplexed immunoassay panel identifies novel CSF biomarkers for Alzheimer’s disease diagnosis and prognosis. PloS One. 2011;6(4):e18850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shang Y, Tian L, Chen T, et al. CXCL1 promotes the proliferation of neural stem cells by stimulating the generation of reactive oxygen species in APP/PS1 mice. Biochem Biophys Res Commun. 2019;515(1):201–206. [DOI] [PubMed] [Google Scholar]
- 12. Losy J, Zaremba J, Skrobański P. CXCL1 (GRO-alpha) chemokine in acute ischaemic stroke patients. Folia Neuropathol. 2005;43(2):97–102. [PubMed] [Google Scholar]
- 13. Shao X, Bao W, Hong X, Jiang H, Yu Z. Identification and functional analysis of differentially expressed genes associated with cerebral ischemia/reperfusion injury through bioinformatics methods. Mol Med Rep. 2018;18(2):1513–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao H, Chen Z, Xie LJ, Liu GF. Suppression of TLR4/NF-κB signaling pathway improves cerebral ischemia-reperfusion injury in rats. Mol Neurobiol. 2018;55(5):4311–4319. [DOI] [PubMed] [Google Scholar]
- 15. Pham C, Sheth SJ, Keeffe JE, Carden SM. New trends in childhood vision impairment in a developed country. J AAPOS. 2017;21(6):496–498. [DOI] [PubMed] [Google Scholar]
- 16. Wu LR, Liu L, Xiong XY, et al. Vinpocetine alleviate cerebral ischemia/reperfusion injury by down-regulating TLR4/MyD88/NF-κB signaling. Oncotarget. 2017;8(46):80315–80324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Listwak SJ, Rathore P, Herkenham M. Minimal NF-kappaB activity in neurons. Neuroscience. 2013;250:282–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Ann Rev Biochem. 2010;79:351–379. [DOI] [PubMed] [Google Scholar]
- 19. Bartoszewska S, Kochan K, Piotrowski A, et al. The hypoxia-inducible miR-429 regulates hypoxia-inducible factor-1alpha expression in human endothelial cells through a negative feedback loop. FASEB J. 2015;29(4):1467–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zheng X, Zhou H, Qiu Z, Gao S, Wang Z, Xiao L. Gene microarray analysis of expression profiles in liver ischemia and reperfusion. Mol Med Rep. 2017;16(3):3299–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sharma AK, Mulloy DP, Le LT, Laubach VE. NADPH oxidase mediates synergistic effects of IL-17 and TNF-alpha on CXCL1 expression by epithelial cells after lung ischemia-reperfusion. Am J Physiol Lung Cell Mol Physiol. 2014;306(1):L69–L79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Z, Zhu Z, Lin Z, et al. Mir-429 suppresses cell proliferation, migration and invasion in nasopharyngeal carcinoma by downregulation of TLN1. Cancer Cell Int. 2019;19:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shen J, Hong L, Yu D, Cao T, Zhou Z, He S. LncRNA XIST promotes pancreatic cancer migration, invasion and EMT by sponging miR-429 to modulate ZEB1 expression. Int J Biochem Cell Biol. 2019;113:17–26. [DOI] [PubMed] [Google Scholar]
- 24. Wu G, Zheng H, Xu J, et al. Mir-429 suppresses cell growth and induces apoptosis of human thyroid cancer cell by targeting ZEB1. Artif Cells, Nanomed Biotechnol. 2019;47(1):548–554. [DOI] [PubMed] [Google Scholar]
- 25. Wang HF, Wang WH, Zhuang HW, Xu M. MiR-429 regulates the proliferation and apoptosis of nephroblastoma cells through targeting c-myc. Eur Rev Med Pharmacol Sci. 2018;22(16):5172–5179. [DOI] [PubMed] [Google Scholar]
- 26. Fang H, Li HF, He MH, et al. Long non-coding RNA MALAT1 sponges microRNA-429 to regulate apoptosis of hippocampal neurons in hypoxic-ischemic brain damage by regulating WNT1. Brain Res Bull. 2019;152:1–10. [DOI] [PubMed] [Google Scholar]
- 27. Chen L, Xue Y, Zheng J, et al. MiR-429 regulated by endothelial monocyte activating polypeptide-II (EMAP-II) influences blood-tumor barrier permeability by inhibiting the expressions of ZO-1, occludin and claudin-5. Front Mol Neurosci. 2018;11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee ST, Chu K, Jung KH, et al. MicroRNAs induced during ischemic preconditioning. Stroke. 2010;41(8):1646–1651. [DOI] [PubMed] [Google Scholar]
- 29. Harari OA, Liao JK. NF-κB and innate immunity in ischemic stroke. Ann N Y Acad Sci. 2010;1207:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54(1):1–15;quiz 16-18. [DOI] [PubMed] [Google Scholar]
- 31. Yang S, Wang H, Yang Y, et al. Baicalein administered in the subacute phase ameliorates ischemia-reperfusion-induced brain injury by reducing neuroinflammation and neuronal damage. Biomed Pharmacother. 2019;117:109102. [DOI] [PubMed] [Google Scholar]
- 32. Kim E, Kim HC, Lee S, et al. Dexmedetomidine confers neuroprotection against transient global cerebral ischemia/reperfusion injury in rats by inhibiting inflammation through inactivation of the TLR-4/NF-kappaB pathway. Neurosci Lett. 2017;649:20–27. [DOI] [PubMed] [Google Scholar]