Abstract
Background
Camptocormia is an axis symptom of Parkinson disease. It remains uncertain whether treatment with medications and surgery are effective. In this study, we assessed the efficacy of subthalamic nucleus deep brain stimulation (STN DBS) in Parkinson disease-associated camptocormia and explored some of its mechanisms.
Material/Methods
Parkinson disease-associated camptocormia was diagnosed by the following procedures. All patients underwent bilateral STN DBS. The patents’ camptocormia was rated by degree and MDS Unified Parkinson’s Disease Rating Scale (UPDRS) item 3.13 before and after DBS surgery. Rehabilitation and psychological interventions were used after surgery, in addition to adjustments of medication and stimulus parameters. The treatment effects on camptocormia were assessed comparing medication-off (presurgery) versus stimulation-on (post-surgery). Ethical approval for this study was provided through the Center of Human Research Ethics Committee (No. 2019-35). This study trial was registered in Chinese Clinical Trial Registry (No. ChiCTR1900022655). All the participants provided written informed consent.
Results
After DBS surgery, all of study patients’ symptoms were improved, with different levels of improvement. The minimum and maximum improvement rates were 20% and 100% respectively. The score of item 3.13 of the MDS-UPDRS III and the degree of camptocormia were found to be obviously improved (P<0.05).
Conclusions
STN DBS can improve Parkinson disease-associated camptocormia; STN DBS assisted with rehabilitation and psychological intervention appears to be more effective.
MeSH Keywords: Deep Brain Stimulation, Parkinson Disease, Subthalamic Nucleus
Background
Among progressive neurodegenerative diseases, Parkinson disease (PD) is the most common, followed by Alzheimer disease; and PD concern over PD has been increasing [1]. Although tremors, rigidity, akinesia, and gait disorders are common symptoms of PD, abnormal posture is also recognized as a concern because it causes serious inconveniences in the daily life and work of patients with PD. Abnormal postures include camptocormia and Pisa syndrome. Pisa syndrome, also known as pleurothotonus, is characterized by tonic lateral flexion, accompanied by a slight rotation of the trunk in the sagittal plane, which results in an abnormal posture, reminiscent of the leaning tower of Pisa [2]. Camptocormia is a posture abnormality, described as involuntary flexion of the thoracolumbar spine, which worsens when walking, sitting, or standing for long periods of time, but completely disappears in the recumbent position. “Camptocormia” is from the Greek “kamptein”, which means bend, and “kormos”, which means trunk [3].
Most motor symptoms can initially be controlled with levodopa and other dopaminergic drugs. However, after a few years, motor fluctuations and dyskinesia typically develop due to progressive motor dysfunction, and quality of life deteriorates as well. At this stage, deep brain stimulation (DBS) therapy should be considered. The physiological mechanisms that underlie the effectiveness of DBS remain to be clarified [4]. Nevertheless, DBS is a highly efficient, evidence-based therapy used for a number of neurodegenerative diseases, particularly for movement disorders such as PD, essential tremors, and dystonia [5].
In 2002, DBS of the subthalamic nucleus (STN) and globus pallidus internus (GPi) were both approved by the US Food and Drug Administration (FDA) as a safe, effective treatment options for patients with advanced PD. And in 2015, the FDA approval the use of deep brain stimulation (DBS) therapy in people with PD “of at least 4 years duration and with recent onset motor complications, or motor complications of longer-standing duration that are not adequately controlled with medication” [6]. Since then, DBS in the STN has been widely applied to improve patient quality of life and the motor and non-motor symptoms of PD. However, more difficult issues arise as the condition progresses; these issues are known as the axis symptoms of PD. Some axis symptoms can be treated with anti-PD medications and DBS surgery. But the effects of most of these treatments cannot be predicted with absolute certainty [7–9].
Camptocormia is an axis symptom of PD. It remains uncertain whether treatment with medications and surgery are effective; preliminary findings must be confirmed. Some authors have postulated that camptocormia is responsive to STN DBS in some, but not all patients with PD [10–12]. Here, we aimed to explore the clinical characteristics of patients with PD-associated camptocormia that were responsive or unresponsive to DBS surgery, and we endeavored to reveal the underlying disease mechanisms.
Material and Methods
Patients
Between March 2017 and March 2019, 15 patients with PD that exhibited camptocormia underwent bilateral STN DBS in our hospital. All 15 patients (7 males and 8 females) presented with camptocormia in the absence of medications. We defined camptocormia as an involuntary anterior flexion of the thoracolumbar spine greater than 15° or more, when the patient was standing or walking, but reversible by lying in a recumbent position [13,14].
All patients had been diagnosed with PD based on the clinical diagnostic criteria for PD established by the Movement Disorder Society (MDS). Screening for DBS surgery was carried out according to MDS guidelines. Patients were considered eligible for DBS treatment when, in levodopa challenge tests, they showed a sufficient response to levodopa (i.e., >30% improvement) assessed with the Unified PD Rating Scale III (UPDRS III). The inclusion criteria for study participation were the following: a substantial reduction in the curative effect of levodopa or the appearance of a serious movement fluctuation or dyskinesia, which affected quality of life. Exclusion criteria were the following: obvious cognitive impairment, severe anxiety and depression, coagulation disorders, and any other severe illness that might affect surgery [15–17].
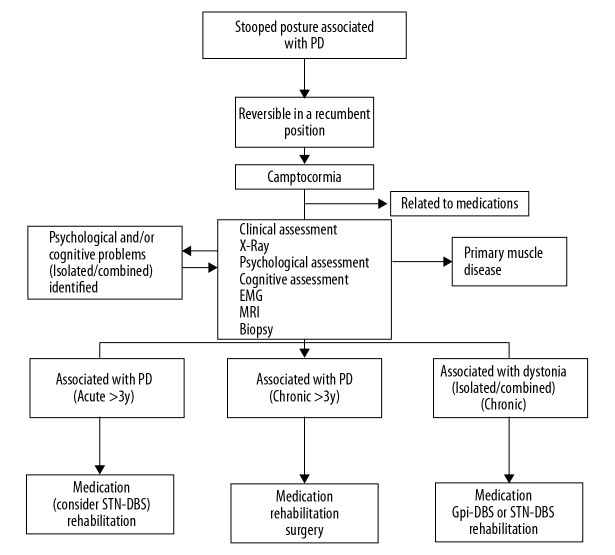
To confirm that all camptocormia conditions were related to PD, we conducted a series of procedures (Figure 1). First, the stooped posture had to disappear completely in the recumbent position. Second, it should be ruled out as a medication side-effect. Third, primary muscle disease had to be excluded with magnetic resonance imaging (MRI) scans and other examinations. Next, psychological and cognitive assessments were conducted to identify psychological elements that should be managed peri- and intra-operationally [18,19]. Then, after confirming that the camptocormia was related to PD, it was very important to estimate the duration of camptocormia. For durations less than 3 years, STN DBS surgery, followed by medication and rehabilitation, is considered a good choice. If the durations are more than 3 years, the efficacy of treatment can be obtained only through medication and rehabilitation, sometimes plastic surgery is a reluctantly choice [11,20]. When dystonia is the main cause of camptocormia, either the Gi or STN should be considered for the DBS target.
Figure 1.
Flow diagram shows the procedures for diagnosing and treating Parkinson disease-associated camptocormia. PD – Parkinson disease; STN – subthalamic nucleus; DBS – deep brain stimulation; GPi – globus pallidus internus; EMG – electromyography; MRI – magnetic resonance imaging.
Surgery
After the patients and their families fully understood the risks and benefits of DBS, we conducted the surgery, in accordance with good clinical practices. All patients were treated by the same surgical team. All patients underwent bilateral STN DBS. We used the Leksell Stereotactic System (Elekta, Stockholm, Sweden) and the Frame Link planning system (Medtronic, Minneapolis, Minnesota, USA) for the operation. According to the Schaltenbrand-Wahren atlas, the tentative target site was 2 mm posterior to the midpoint of the anterior-posterior commissure (AC-PC) line, 12 mm lateral to the AC-PC line, and 4 mm ventral to the AC-PC line. Target sites were corrected, based on T2-weighted MRIs. The target was reconfirmed physiologically with an intraoperative microelectrode recording, just prior to performing the test stimulation studies. After all procedures were progressing smoothly, Quadripolar DBS electrodes (Activa 3389s, Medtronic) were implanted bilaterally, with stereotactic guidance. After inducing general anesthesia, implantable pulse generators (Activa RC Medtronic) were implanted subcutaneously in the subclavian pockets of the chest wall; then, they were subcutaneously connected to the DBS leads. The accuracy of electrode placement in the STN was confirmed by superimposing images from postoperative computed tomography and preoperative MRI, with the Frame Link planning system [21,22].
All patients started stimulations at 1 month after the operation, and they were first treated with a unipolar stimulation. The parameters were: frequency: 130–170 Hz; pulse width: 60–90 μs; and stimulation amplitude: 1.5–3.5 V. These parameters were the same on both sides [23,24].
Assessment
We assessed age at onset, sex, PD duration, age at the time of operation, MDS-UPDRS III, degree of camptocormia, and the duration of camptocormia prior to surgery. The levodopa equivalent daily dose (LEDD, mg/day) was obtained by adding together the L-dopa equivalent dose for each anti-Parkinsonian drug, by multiplying the total daily dose of each drug by its potency relative to a standard levodopa dose. LEDD of dopamine agonist was calculated in the same way. According to the latest literature, we calculated the LEDDs as follows: for a dose of 100 mg L-dopa, the equivalent doses were: entacapone-levodopa×0.33, 5 mg ropinirole, 3.3 mg rotigotine, 100 mg piribedil, 1 mg pramipexole, 10 mg selegiline, 1 mg rasagiline, 100 mg amantadine, and 10 mg apomorphine [25].
All patients were evaluated preoperatively with the MDS-UPDRS-III, in the presence and absence of their daily medication. Typically, for this preoperative assessment, we administered a levodopa challenge test, where a levodopa dose of 1.5×the LEDD was given as the first dose in the morning. The standard indication for DBS surgery was that the levodopa test dose provided >30% improvement, assessed with item 3.13 in the UPDRS III [21]. We also used the android app (Max Protractor ver. 1.1.2, an open source software by Maxcom) to measure the degree of camptocormia in patients, based on the angle between the long axis of the femur and the upper thoracic plane (bending angle). This measurement was performed preoperatively and at 1 month and 6 months after surgery.
The responsiveness of camptocormia to levodopa was calculated preoperatively, based on the change in the bending angle between the off-medication (med-off) and on-medication (med-off) states. Similarly, the postoperative improvement rate was calculated as the difference between the preoperative and postoperative bending angles, divided by the preoperative bending angle.
Postoperative management
Postoperative management of PD was very important. It included adjusting the stimulation parameters and medications as well as providing rehabilitation, a psychological intervention, and so on. Electrical stimulation usually began 1 month after surgery. Stimulation parameters were adjusted to produce the maximal clinically beneficial effect on cardinal PD symptoms with minimal side effects. In most cases, we used a monopolar electrode setting. When it was apparent that stimulations had induced side effects or when PD symptoms had not improved sufficiently, we applied a different stimulation paradigm. For example, bipolar stimulation, double negative stimulation, cross pulse stimulation, and so forth. Conventional stimulation parameters were pulse width at 90 ms, pulse rate at 130 Hz, and amplitude at 2–3 V [23,24].
After surgery, the PD medication was adjusted based on stimulation-induced improvements in PD symptoms and any side effects such as dyskinesia. In general, PD medications were reduced by nearly 50% after surgery. The sequence for reducing PD medications was: cholinesterase inhibitor (ChEI), CMOTi, MAOBi, dopamine receptor agonists, and levodopa [26].
Rehabilitation is very important for PD-associated camptocormia. DBS surgery can improve PD symptoms such as rigidity, akinesia, and tremors; however, camptocormia cannot be improved immediately for patients that have experienced a stooped posture for a long time. Rehabilitation training is necessary to correct abnormal posture [27–30]. All rehabilitation exercises were performed once per day for 8 weeks, as follows:
Stretching exercises: Hold every stretch for 10 seconds. Repeat 5 times for each stretch. The total stretching time is 10 minutes.
Stretching the neck muscles: Look down, look up, tilt the head to the sides, rotate the head and neck in a circle.
Stretching the shoulders and chest: Stand facing a wall with the feet about a foot from the wall. Place the hands as high up on the wall as possible and hold this position for at least 10 seconds.
Back extension: Lie on the stomach, lift the upper body off the surface, supporting the body weight on the forearms, hold this position for 5 seconds.
Stretching the waist muscles: Assume a sitting position and twist at the waist, both directions.
Strengthening exercises: Perform at least 1 set of 10 to 15 repetitions for each exercise.
Back muscle strength: Lying on a bed or on a mat on the floor, with your arms at your side, use your back muscles to lift your shoulders and head
Shoulder blade squeeze: Sit tall on the edge of chair; open the arms out to the sides, pull the arms back, and squeeze the shoulder blades together; hold for 5 seconds.
Gluteal muscle strength: Lie on the stomach with the knees bent; kick backwards, lifting the knees off the bed, and hold this pose for 5 seconds.
Bridge exercise: Lie on the back with the knees bent and feet flat; raise the hips and squeeze the buttocks; hold this pose for 5 seconds.
Patients were told to make good posture a habit.
Check your posture every day. Stand against a wall and be sure the lower back and shoulder blades are touching the wall. Try to pull the back of the head towards the wall. Try to maintain this posture when walking or doing other things.
A psychological intervention should not be avoided. Most patients with PD have psychological problems, mainly anxiety and depression. However, camptocormia can present with a somatic symptom of anxiety and depression. Therefore, we first determined whether the anxiety and depression were primary or secondary to the camptocormia. When secondary, the psychiatric symptoms can be ameliorated by improvements in motor symptoms achieved with DBS surgery. When primary, psychiatric symptoms should be treated with psychological behavioral therapy and antidepressant and anxiolytic medications [18].
Statistics
Preoperative levodopa responsiveness was determined by measuring the change in each score from the med-off to the med-on state. The postoperative improvement rate was calculated as the difference between the preoperative and postoperative scores, divided by the preoperative score.
Descriptive statistics are reported as the mean±standard deviation (SD). Within-group comparisons (i.e., preoperative versus postoperative and med-on versus med-off) were performed with the paired t-test, or with Wilcoxon’s signed-rank test, when the normality assumption was violated. We analyzed the degree of camptocormia and MDS-UPDRS item 3.13 with one-way repeated measures ANOVAs, which compared baseline data with data measured med-off and med-on at 1-month post DBS and 6-months post DBS, during the stimulated state. The on-stimulation data was compared to the off-stimulation data with a paired t-test. The relationships between the PDQ39 score, LEDD, LEDD of dopamine agonist and the degree of camptocormia and Hoehn and Yahr staging were examined with correlation analyses. All statistical measurements were performed with IBM SPSS software (IBM Inc, USA, version 22.0.0.1). The level of statistical significance was set at 0.05.
Results
The study cohort included 15 patients (7 males) with PD who underwent bilateral STN DBS between March 2017 and March 2019 (Table 1). The mean age was 62.5±8.05 years (range 45 to 77 years) and the PD duration was 10.5±4.47 years (range 5 to 21 years). The median Hoehn and Yahr score was 2.5. The duration of camptocormia was ≤3 years for all of patients. The scores for item 3.13 of the MDS-UPDRS III ranged from 2 to 4 in the med-off condition, and from 0 to 2, in the med-on condition. The degree of camptocormia ranged from 15° to 60° in the med-off condition and from 0° to 24° in the med-on condition.
Table 1.
Characteristics of patients with PD-associated camptocormia.
| Characteristic | Patients | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||
| Age, years | 64 | 59 | 60 | 64 | 77 | 66 | 67 | 61 | 66 | 61 | 77 | 60 | 45 | 54 | 57 | |
| Gender | M | M | M | F | M | F | F | F | F | M | F | M | M | F | F | |
| H&Y preop | 4 | 3 | 3 | 3 | 3 | 3 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | |
| H&Y postop | 3 | 2 | 2 | 1 | 3 | 3 | 2 | 1 | 2 | 2 | 2 | 2 | 1 | 2 | 3 | |
| Duration of PD | 21 | 10 | 7 | 7 | 15 | 8 | 14 | 6 | 9 | 8 | 12 | 5 | 7 | 12 | 16 | |
| Duration of camptocormia | 3 | 2 | 2.5 | 1 | 3 | 2.5 | 3 | 0.5 | 2 | 1.5 | 3 | 0.5 | 1.5 | 2 | 3 | |
| LEDD preop | 2800 | 760 | 1600 | 400 | 875 | 500 | 500 | 200 | 600 | 600 | 550 | 1000 | 1000 | 2700 | 400 | |
| LEDD postop | 400 | 760 | 400 | 0 | 450 | 600 | 375 | 0 | 400 | 400 | 500 | 700 | 800 | 700 | 400 | |
| LEDD of DA preop | 0 | 75 | 75 | 37.5 | 75 | 150 | 75 | 37.5 | 150 | 150 | 75 | 150 | 150 | 150 | 75 | |
| LEDD of DA postop | 0 | 75 | 37.5 | 0 | 75 | 150 | 0 | 0 | 75 | 75 | 75 | 75 | 0 | 0 | 75 | |
| Med-off | MDS-UPDRS item 3.13 score | 2 | 3 | 3 | 2 | 3 | 4 | 4 | 2 | 3 | 2 | 3 | 2 | 3 | 2 | 4 |
| Degree of camptocormia (°) | 35 | 41 | 36 | 30 | 42 | 60 | 55 | 37 | 40 | 35 | 43 | 31 | 45 | 37 | 63 | |
| Med-on | MDS-UPDRS item 3.13 score | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 2 |
| Degree of camptocormia (°) | 16 | 15 | 23 | 10 | 11 | 23 | 17 | 13 | 17 | 12 | 12 | 11 | 0 | 15 | 24 | |
| Post DBS 6-months (med-off) | MDS-UPDRS item 3.13 score | 1 | 1 | 1 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 2 |
| Degree of camptocormia (°) | 12 | 5 | 15 | 0 | 10 | 20 | 0 | 0 | 15 | 12 | 5 | 8 | 0 | 5 | 20 | |
| Post DBS 6-months (med-on) | MDS-UPDRS item 3.13 score | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 2 |
| Degree of camptocormia (°) | 10 | 5 | 14 | 0 | 8 | 15 | 0 | 0 | 13 | 12 | 0 | 5 | 0 | 5 | 19 | |
| Post DBS 1-month | MDS-UPDRS item 3.13 score | 2 | 2 | 2 | 1 | 3 | 3 | 0 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 3 |
| Degree of camptocormia (°) | 25 | 25 | 29 | 5 | 35 | 45 | 0 | 10 | 25 | 12 | 8 | 10 | 0 | 5 | 35 | |
| PDQ-39 preop | 98 | 58 | 101 | 76 | 50 | 123 | 131 | 54 | 46 | 43 | 50 | 80 | 121 | 73 | 68 | |
| PDQ-39 postop | 57 | 20 | 10 | 46 | 77 | 90 | 56 | 9 | 44 | 1 | 34 | 54 | 18 | 14 | 43 | |
| MDS-UPDRS III med-off preop | 67 | 47 | 53 | 52 | 59 | 70 | 45 | 39 | 37 | 67 | 65 | 36 | 43 | 44 | 102 | |
| MDS-UPDRS III med-off postop | 32 | 14 | 13 | 10 | 21 | 41 | 14 | 6 | 24 | 37 | 30 | 23 | 21 | 30 | 49 | |
MDS-UPDRS, item 3.13 – Movement Disorder Society-Unified Parkinson Disease Rating Scale, item 3.13, which indicates the fractional response to a levodopa test dose (i.e., 1.00=no response). PD – Parkinson disease; preop – preoperative; postop – postoperative; H&Y – Hoehn-Yahr grading; LEDD – levodopa equivalent daily dose; DA – dopamine agonist; DBS – deep brain stimulation; PDQ-39 – Parkinson Disease Questionnaire-39; med-on – on medication; med-off – off medication.
After DBS surgery, all patients displayed symptom improvements, but at different levels. The minimum and maximum improvement rates were 20% and 100%, respectively. The score for item 3.13 of the MDS-UPDRS III and the degree of camptocormia improved significantly, both in off-medication and on-medication states (P<0.05) (Tables 2, 3). The mean degree of camptocormia and mean MDS-UPDRS item 3.13 scores among patients with PD-associated camptocormia were improved most obviously after 6 months (Figures 2, 3). The scores of MDS-UPDRS III in off-medication and PDQ-39 scores also improved significantly. The total LEDD and the LEDD of dopamine agonist decreased significantly after undergoing DBS surgery (Table 4), but the changes about LEDD of dopamine agonist were not associated with improvement of camptocormia(P=0.774).
Table 2.
Comparisons of MDS-UPDRS, Item 3.13, and the degree of camptocormia in patients with PD in med-off pre-DBS and 6-months of post-DBS.
| Measure | Med-off pre-DBS | Med-off 6-months post-DBS | P-value |
|---|---|---|---|
| MDS-UPDRS, item 3.13 (mean±SD) | 2.80±0.77 | 0.86±0.64 | <0.001 |
| Degree of camptocormia (°) | 46.20±8.79 | 8.46±7.08 | <0.001 |
MDS-UPDRS, item 3.13 – Movement Disorder Society-Unified Parkinson Disease Rating Scale, item 3.13, which indicates the fractional response to a levodopa test dose (i.e., 1.00=no response). PD – Parkinson disease; med-off – off medication; med-on – on medication; DBS – deep brain stimulation, SD – standard deviation.
Table 3.
Comparisons of MDS-UPDRS, Item 3.13, and the degree of camptocormia in patients with PD in med-on pre-DBS and 6-months of post-DBS.
| Measure | Med-on pre-DBS | Med-on 6-months post-DBS | P-value |
|---|---|---|---|
| MDS-UPDRS, item 3.13 (mean±SD) | 1.13±0.52 | 0.87±0.64 | <0.05 |
| Degree of camptocormia (°) | 14.60±6.09 | 8.47±7.08 | <0.001 |
MDS-UPDRS, item 3.13 – Movement Disorder Society-Unified Parkinson Disease Rating Scale, item 3.13, which indicates the fractional response to a levodopa test dose (i.e., 1.00=no response). PD – Parkinson disease; med-off – off medication; med-on – on medication; DBS – deep brain stimulation.
Figure 2.
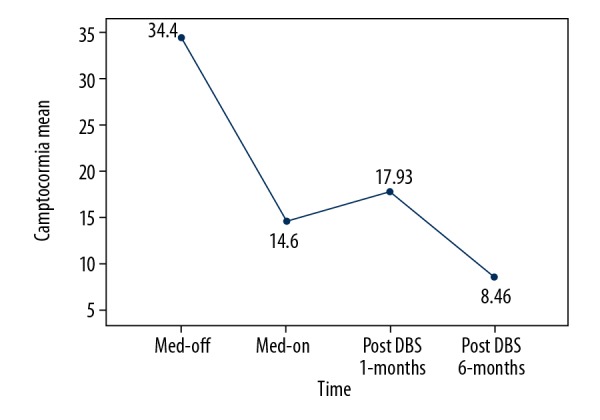
Modifications in the mean degree of camptocormia among patients with Parkinson disease-associated camptocormia. The mean angle of camptocormia is shown at different times in the study. Numbers display the exact angles (°). Med-off – off medication; Med-on – on medication; DBS – deep brain stimulation.
Figure 3.
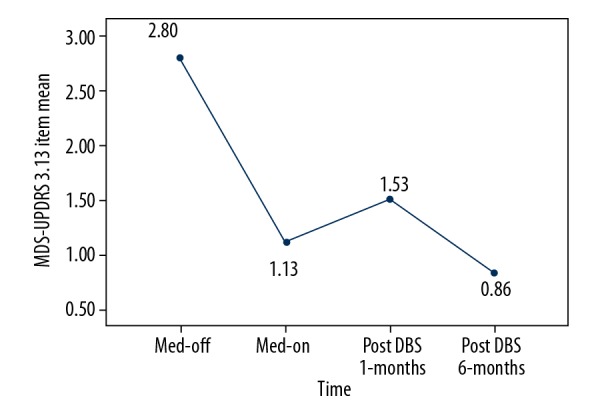
Modifications in the mean MDS-UPDRS item 3.13 scores of patients with Parkinson disease-associated camptocormia. The mean response of camptocormia to the levodopa test dose is shown at different times in the study. Numbers display the fractional response score (i.e., 1.00=no response). MDS-UPDRS – Movement Disorder Society-Unified Parkinson Disease Rating Scale; Med-off – off medication; Med-on – on medication; DBS – deep brain stimulation.
Table 4.
Responses to DBS surgery for camptocormia in patients with PD.
| Measures | Preop | Postop 6-months | t | P-value |
|---|---|---|---|---|
| MDS-UPDRS III, med-off score | 55.07±17.36 | 24.33±12.18 | 10.51 | <0.001 |
| PDQ-39 score | 78.13±29.93 | 38.20±26.19 | 4.68 | <0.001 |
| H&Y score | 3.20±0.41 | 2.07±0.70 | 6.86 | <0.001 |
| LEDD | 965.67±798.57 | 459.00±237.62 | −2.60 | 0.020 |
| LEDD of DA | 95.00±50.84 | 47.50±45.85 | 3.54 | 0.003 |
Values are the mean±standard deviation, except where indicated. DBS – deep brain stimulation; PD – Parkinson disease; preop – preoperative measurement; postop – postoperative measurement; t – t-test; MDS-UPDRS – Movement Disorder Society-Unified Parkinson Disease Rating Scale; med-off – off medication; PDQ-39 – Parkinson Disease Questionnaire-39; H&Y – Hoehn-Yahr grading; LEDD – levodopa equivalent daily dose; DA – dopamine agonist.
Discussion
Previous studies on the incidences of camptocormia in PD have provided diverse estimates, ranging from 3% to 17% among individuals with idiopathic PD [31–33]. In the early stages of PD, camptocormia does not appear to impact the quality of a patient’s life. However, as the disease continues to advance, it can cause difficulties in patients with PD; indeed, it could even aggravate depression and anxiety.
An abnormal posture (stoop) is not sufficient evidence to diagnose camptocormia. Some studies [18,19,34,35] define camptocormia with clinical diagnostic criteria. Essentially, there are 6 criteria for screening patients with PD that experience camptocormia. These criteria are: 1) Involuntary flexion of the thoracolumbar spine. 2) The flexion must be reversible; it must disappear in a recumbent position. 3) There must be some forward-bending angle. There are different opinions about the bending angle of camptocormia. In this study, we used more than 15° as the standard. 4) Camptocormia must be an individual complaint. 5) Most patients experience various degrees of back pain. 6) Hardening of the paraspinal muscle is typical in most patients.
Although the mechanism underlying PD-associated camptocormia is complicated, it is important and necessary to explore putative mechanisms meticulously. This information will have positive effects on future treatment. Currently, the underlying cause of camptocormia in PD remains controversial. We have concluded and inferred several hypotheses about the cause. First, it is related to neural circuitry. Dr. Delong first introduced the notion that dopaminergic neurons formed a network, which included a cortico-basal ganglia-thalamo-cortical circuit. In idiopathic PD, dopamine signaling is reduced in the substantia nigra through the D1 and D2 receptors and through direct and indirect pathways. Signaling is over-active in the STN and GPi nuclei; and signaling is inhibited the thalamus. These disturbances induce the movement disorders characteristic of PD. The pedunculopontine nucleus also participates in the circuitry through the STN, GPi, and thalamus [36]. The famous circuit theory (Dr. Delong and Dr. Benabid received the Larkes Pride award for their studies on PD [37]) could explain the instability/gait difficulties experienced by patients with PD. However, for tremor dominant PD, there is another theory, although it is currently a hypothesis, which is accepted by most neurologists [38]. This theory postulates the involvement of a cerebellum-dentate nucleus-red nucleus-basal ganglia-thalamo-cortical circuit [39]. For camptocormia-related symptoms, the pedunculopontine nuclei play a very important role. In this network, the pedunculopontine nuclei and the midbrain locomotor region regulate postural muscle tone [40]. This regulation might explain why patients with PD-associated camptocormia typically recover in a recumbent position.
Another interpretation of the evidence, which might explain camptocormia, is the presence of trunk dystonia [41]. Many authors think that some dystonia is observed in patients with PD, including strum hand/foot, Pisa syndrome [42], and camptocormia. In some patients with PD who experience camptocormia, a sensory trick has been observed [11]. For example, a simple action such as touching the hip could release the camptocormia. However, this explanation is not very popular because in many patients with these symptoms a sensory trick cannot be observed, and no overactivity can be observed in the paraspinal muscles with electromyography (EMG). Toxin therapy is usually useless, so another theory should be considered. Some authors have accepted trunk rigidity [43] as a plausible mechanism of camptocormia. This hypothesis holds that a reduction in the interaction between the abdominal muscles and the paraspinal muscles can induce flexion in the thoracolumbar spine and some degree of spine rotation. Ruttiman et al.[44] emphasized the possibility that both dystonia and myopathy might play a role in the pathophysiology of PD-associated camptocormia, and that peripheral mechanisms are indeed very important factors.
Other studies have suggested that peripheral mechanisms could be involved in the pathophysiology of camptocormia. In the early stages, swelling and edema in the paraspinal muscles could contribute to the disease. As the disease continues to advance, fat degeneration might contribute to the later stages [45,46]. However, almost all the peripheral mechanisms are related to the equilibrium of the central mechanisms. Therefore, the main mechanisms underlying camptocormia are factors related to dystonia, trunk rigidity, weak signaling in the neuronal circuitry, including in the pedunculopontine nuclei, and some peripheral mechanisms.
Different mechanisms might be dominant in the development of camptocormia in different patients. Thus, the treatment of choice might be STN DBS. Studies have confirmed that DBS surgery could improve PD symptoms, such as tremor, rigidity, and akinesia [47], and DBS was also effective for treating dystonia [48]. Those findings might partly explain why DBS could improve camptocormia. It is well known that the pedunculopontine nucleus is a key nucleus in the neuronal circuit that also includes the STN and GPi [49]. Therefore, STN DBS might also influence the pedunculopontine nucleus and participate in the regulation of neural conduction and signal integration [50]. All the mechanisms aforementioned indicate that STN DBS is a very important treatment for PD-associated camptocormia; however, a DBS treatment might not be sufficient due to the complicated mechanisms involved in camptocormia. Based on our clinical observations, we found that DBS surgery, followed by rehabilitation and combined with a psychological intervention, could improve PD-associated camptocormia more effectively than DBS alone.
It is well known that rehabilitation is very important for PD [29]. DBS surgery typically improves Parkinsonian symptoms, such as tremor, rigidity, and akinesia. However, DBS was only partly effective for ameliorating a freezing gait or difficulties in maintaining balance, and only in some patients, DBS had no effect on these symptoms [51,52]. For these symptoms, rehabilitation is a good treatment choice. For PD-associated camptocormia, although STN DBS is effective, rehabilitation plays an important role in the treatment [53,54]. In our study, all patients had experienced PD-associated camptocormia for less than 3 years. According to the literature, camptocormia that has lasted less than 3 years should respond to DBS treatment. However, our patients displayed different degrees of improvement [20].
Based on the known mechanisms underlying PD-associated camptocormia, stretching exercises and strengthening exercises have been specially designed to ameliorate the rigidity and dystonia. Stretching exercises can relieve muscle tension, and strengthening exercises can increase the strength of the paravertebral muscles to counteract the contraction in the rectus abdominis [27,54]. These rehabilitative exercises can further improve PD-associated camptocormia after DBS surgery.
Psychological interventions are important for nearly all kinds of surgery, particularly for DBS. Most patients with PD have psychological disorders [55], mainly anxiety and depression [56–58]. Indeed, camptocormia can be a kind of somatic symptom of anxiety and depression. As a secondary psychiatric symptom, camptocormia can be ameliorated with improvements in motor symptoms after DBS surgery (when camptocormia has not lasted for 3 years or more). However, when it is a primary disorder, DBS surgery is not an effective solution for camptocormia.
Compared to results reported in previous studies [10,45,59,60], our patients seemed to achieve better performance with treatment, not only in off-medication but also in on-medication states. This might be explained by several reasons. First, we selected patients that had experienced camptocormia for less than 3 years. This time frame is important, because paraspinal muscles may become hardened through atrophy; thus, after 3 years, it is very difficult to recover with any kind of treatment. In a previous study, Schulz-Schaeffer et al. [11] confirmed that the duration of camptocormia was very important. A second explanation for our study results might be the timeliness of surgical treatment, which we considered very important to provide rehabilitation training and psychological treatments. Although the DBS surgery improved tremors, rigidity, and akinesia, the rehabilitation improved incorrect or abnormal posture using stretching exercises, strengthening exercises, and an emphasis on making good posture a habit. To be specific, rehabilitation is considered one of the core reasons for improvement in patients in med-on state [61]. STN DBS surgery is considered to mainly improves PD symptoms in med-off states, especially when looking at long-term efficacy [62–64]. Thus, rehabilitation combined with DBS should be a good choice for improvement both in med-on and med-off states. A third explanation for our study results is that psychology also plays an important role. It is well known that most patients with PD experience depression and anxiety. Moreover, camptocormia could be a somatic symptom caused by these psychological disorders. In most patients, when PD symptoms improved after DBS surgery, the depression and anxiety also improved; however, in a few patients, psychological symptoms were not improved satisfactorily. Those patients required a psychological intervention and rational drug-treatment.
We chose the STN as the target of DBS because it was suitable for ameliorating rigidity and dystonia related to camptocormia. Moreover, a recent study by Ruijin Hospital of Shanghai showed that STN DBS was an effective treatment for dystonia [48]. In addition, we selected STN DBS for this study, because it would provide results that would be convenient in comparative observations.
Previous studies have found that dopamine agonist can cause abnormal posture, such as camptocormia in PD patients, or make it worse [65–68]. However, we found that dopamine agonist did not influence the changes of degree of camptocormia during the perioperative period when using DBS surgery in our study, possibly because the dosage of this medicine was relatively small as is typical in China.
In this study, we found that all patients achieved improvements in PDQ39 scores. Improving PDQ49 scores is important for the study of camptocormia [12], because it indicates that STN DBS could enhance the quality of life of patients with PD. However, we did not find any relevance between camptocormia and the PDQ39 score. Camptocormia is very painful in patients with PD, and pain can impact anxiety and depression; thus, any improvement in camptocormia would be expected to improve the quality of life in patients with PD.
Finally, we identified another very important factor that influenced the recovery of patients with PD. This factor was a humanistic concern for patients. Although the study included psychological intervention, the humanistic concern expressed from everyone on our team seemed to affect the patient’s state, even illness progression.
Conclusions
Although it could be argued whether STN DBS can improve PD-axis symptoms, in our opinion, STN DBS, combined with rehabilitation and psychological interventions, was very effective in treating camptocormia that had lasted less than 3 years.
Acknowledgments
We thank the patients with Parkinson disease who participated in this study; without them, this work would not have been possible.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016;15(12):1257–72. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 2.Barone P, Santangelo G, Amboni M, et al. Pisa syndrome in Parkinson’s disease and parkinsonism: Clinical features, pathophysiology, and treatment. Lancet Neurol. 2016;15(10):1063–74. doi: 10.1016/S1474-4422(16)30173-9. [DOI] [PubMed] [Google Scholar]
- 3.Tatu L, Bogousslavsky J. Camptocormia: New signs in an old syndrome. Front Neurol Neurosci. 2018;42:87–95. doi: 10.1159/000475683. [DOI] [PubMed] [Google Scholar]
- 4.Herrington TM, Cheng JJ, Eskandar EN. Mechanisms of deep brain stimulation. J Neurophysiol. 2016;115(1):19–38. doi: 10.1152/jn.00281.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wichmann T, DeLong MR. Deep brain stimulation for movement disorders of basal ganglia origin: Restoring function or functionality? Neurotherapeutics. 2016;13(2):264–83. doi: 10.1007/s13311-016-0426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabrera LY, Goudreau J, Sidiropoulos C. Critical appraisal of the recent US FDA approval for earlier DBS intervention. Neruology. 2018;91(3):133–36. doi: 10.1212/WNL.0000000000005829. [DOI] [PubMed] [Google Scholar]
- 7.Fasano A, Aquino CC, Krauss JK, et al. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol. 2015;11(2):98–110. doi: 10.1038/nrneurol.2014.252. [DOI] [PubMed] [Google Scholar]
- 8.Brandmeir NJ, Brandmeir CL, Carr D, et al. Deep brain stimulation for Parkinson disease does not worsen or improve postural instability: A prospective cohort trial. Neurosurgery. 2018;83(6):1173–82. doi: 10.1093/neuros/nyx602. [DOI] [PubMed] [Google Scholar]
- 9.Debû B, De Oliveira Godeiro C, Lino JC, et al. Managing gait, balance, and posture in Parkinson’s disease. Curr Neurol Neurosci Rep. 2018;18(5):23. doi: 10.1007/s11910-018-0828-4. [DOI] [PubMed] [Google Scholar]
- 10.Chieng LO, Madhavan K, Wang MY. Deep brain stimulation as a treatment for Parkinson’s disease related camptocormia. J Clin Neurosci. 2015;22(10):1555–61. doi: 10.1016/j.jocn.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 11.Schulz-Schaeffer WJ, Margraf NG, Munser S, et al. Effect of neurostimulation on camptocormia in Parkinson’s disease depends on symptom duration. Mov Disord. 2015;30(3):368–72. doi: 10.1002/mds.26081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roediger J, Artusi CA, Romagnolo A, et al. Effect of subthalamic deep brain stimulation on posture in Parkinson’s disease: A blind computerized analysis. Parkinsonism Relat Disord. 2019;62:122–27. doi: 10.1016/j.parkreldis.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Djaldetti R, Mosberg-Galili R, Sroka H, et al. Camptocormia (bent spine) in patients with Parkinson’s disease – characterization and possible pathogenesis of an unusual phenomenon. Mov Disord. 1999;14(3):443–47. doi: 10.1002/1531-8257(199905)14:3<443::aid-mds1009>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 14.Abe K, Uchida Y, Notani M. Camptocormia in Parkinson’s disease. Parkinsons Dis. 2010;2010 doi: 10.4061/2010/267640. pii: 267640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fox SH, Katzenschlager R, Lim SY, et al. The Movement Disorder Society evidence-based medicine review update: Treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(Suppl 3):S2–41. doi: 10.1002/mds.23829. [DOI] [PubMed] [Google Scholar]
- 16.Okun MS, Fernandez HH, Pedraza O, et al. Development and initial validation of a screening tool for Parkinson disease surgical candidates. Neurology. 2004;63:161–63. doi: 10.1212/01.wnl.0000133122.14824.25. [DOI] [PubMed] [Google Scholar]
- 17.Moro E, Schüpbach M, Wächter T, et al. Referring Parkinson’s disease patients for deep brain stimulation: A RAND/UCLA appropriateness study. J Neurol. 2016;263(1):112–19. doi: 10.1007/s00415-015-7942-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margraf NG, Wrede A, Deuschl G, et al. Pathophysiological concepts and treatment of camptocormia. J Parkinsons Dis. 2016;6(3):485–501. doi: 10.3233/JPD-160836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Margraf NG, Granert O, Hampel J, et al. Clinical definition of camptocormia in Parkinson’s disease. Mov Disord Clin Pract. 2016;4(3):349–57. doi: 10.1002/mdc3.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margraf NG, Rohr A, Granert O, et al. MRI of lumbar trunk muscles in patients with Parkinson’s disease and camptocormia. J Neurol. 2015;262(7):1655–64. doi: 10.1007/s00415-015-7726-3. [DOI] [PubMed] [Google Scholar]
- 21.Bronstein JM, Tagliati M, Alterman RL, et al. Deep brain stimulation for Parkinson disease: An expert consensus and review of key issues. Arch Neurol. 2011;68(2):165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grupo de Cirugía Funcional Sociedad Española de Neurocirugía (SENEC); Grupo de Trastornos del Movimiento Sociedad Española de Neurología (SEN) [Consensus statement on deep brain stimulation in Parkinson’s disease]. Rev Neurol. 2009;49(6):327–31. [in Spanish] [PubMed] [Google Scholar]
- 23.Picillo M, Lozano AM, Kou N, et al. Programming deep brain stimulation for Parkinson’s disease: The Toronto Western Hospital algorithms. Brain Stimul. 2016;9(3):425–37. doi: 10.1016/j.brs.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Volkmann J, Moro E, Pahwa R. Basic algorithms for the programming of deep brain stimulation in Parkinson’s disease. Mov Disord. 2006;21(14):S284–89. doi: 10.1002/mds.20961. [DOI] [PubMed] [Google Scholar]
- 25.Tomlinson CL, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25(15):2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 26.Fasano A, Appel-Cresswell S, Jog M, et al. Medical management of Parkinson’s disease after initiation of deep brain stimulation. Can J Neurol Sci. 2016;43(5):626–34. doi: 10.1017/cjn.2016.274. [DOI] [PubMed] [Google Scholar]
- 27.Gandolfi M, Tinazzi M, Magrinelli F, et al. Four-week trunk-specific exercise program decreases forward trunk flexion in Parkinson’s disease: A single-blinded, randomized controlled trial. Parkinsonism Relat Disord. 2019;64:268–74. doi: 10.1016/j.parkreldis.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 28.Lee KH, Kim JM, Kim HS. Back extensor strengthening exercise and backpack wearing treatment for camptocormia in Parkinson’s disease: A retrospective pilot study. Ann Rehabil Med. 2017;41(4):677–85. doi: 10.5535/arm.2017.41.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borrione P, Tranchita E, Sansone P, et al. Effects of physical activity in Parkinson’s disease: A new tool for rehabilitation. World J Methodol. 2014;4(3):133–43. doi: 10.5662/wjm.v4.i3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroeteler FE, Fietzek UM, Ziegler K, et al. Upright posture in parkinsonian camptocormia using a high-frame walker with forearm support. Mov Disord. 2011;26(8):1560–61. doi: 10.1002/mds.23585. [DOI] [PubMed] [Google Scholar]
- 31.Song W, Guo X, Chen K, et al. Camptocormia in Chinese patients with Parkinson’s disease. J Neurol Sci. 2014;337(1–2):173–75. doi: 10.1016/j.jns.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Seki M, Takahashi K, Koto A, et al. Camptocormia in Japanese patients with Parkinson’s disease: A multicenter study. Mov Disord. 2011;26(14):2567–71. doi: 10.1002/mds.23955. [DOI] [PubMed] [Google Scholar]
- 33.Tatu L, Bogousslavsky J. Camptocormia: New signs in an old syndrome. Front Neurol Neurosci. 2018;42:87–95. doi: 10.1159/000475683. [DOI] [PubMed] [Google Scholar]
- 34.Mano T. Camptocormia induced by a dopaminergic agonist. Clin Neuropharmacol. 2018;41(2):70–72. doi: 10.1097/WNF.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 35.Galbusera F, Bassani T, Stucovitz E, et al. Surgical treatment of spinal disorders in Parkinson’s disease. Eur Spine. 2018;27(Suppl 1):101–8. doi: 10.1007/s00586-018-5499-y. [DOI] [PubMed] [Google Scholar]
- 36.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64(1):20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 37.Jackson S. Alim-Louis Benabid and Mahlon DeLong win the 2014 Lasker~DeBakey Clinical Medical Research Award. J Clin Invest. 2014;124(10):4143–47. doi: 10.1172/JCI78491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah VV, Goyal S, Palanthandalam-Madapusi HJ. A Possible explanation of how high-frequency deep brain stimulation suppresses low-frequency tremors in Parkinson’s disease. IEEE Trans Neural Syst Rehabil Eng. 2017;25(12):2498–508. doi: 10.1109/TNSRE.2017.2746623. [DOI] [PubMed] [Google Scholar]
- 39.Helmich RC. The cerebral basis of Parkinsonian tremor: A network perspective. Mov Disord. 2018;33(2):219–31. doi: 10.1002/mds.27224. [DOI] [PubMed] [Google Scholar]
- 40.Takakusaki K, Chiba R, Nozu T, et al. Brainstem control of locomotion and muscle tone with special reference to the role of the mesopontine tegmentum and medullary reticulospinal systems. J Neural Transm (Vienna) 2016;123(7):695–729. doi: 10.1007/s00702-015-1475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ponfick M, Gdynia HJ, Ludolph AC, et al. Camptocormia in Parkinson’s disease: A review of the literature. Neurodegener Dis. 2011;8(5):283–88. doi: 10.1159/000324372. [DOI] [PubMed] [Google Scholar]
- 42.Tinazzi M, Geroin C, Gandolfi M, et al. Pisa syndrome in Parkinson’s disease: An integrated approach from pathophysiology to management. Mov Disord. 2016;31(12):1785–95. doi: 10.1002/mds.26829. [DOI] [PubMed] [Google Scholar]
- 43.Lenoir T, Guedj N, Boulu P, et al. Camptocormia: The bent spine syndrome, an update. Spine J. 2010;19(8):1229–37. doi: 10.1007/s00586-010-1370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruttiman R, Eltorai AEM, Daniels AH. Etiology and management of spinal deformity in patients with Parkinson’s disease. Int J Spine Surg. 2018;12(1):15–21. doi: 10.14444/5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakai W, Nakane S, Urasaki E, et al. The cross sectional area of paraspinal muscles predicts the efficacy of deep drain stimulation for camptocormia. J Parkinsons Dis. 2017;7(2):247–53. doi: 10.3233/JPD-160948. [DOI] [PubMed] [Google Scholar]
- 46.Wrede A, Margraf NG, Goebel HH, et al. Myofibrillar disorganization characterizes myopathy of camptocormia in Parkinson’s disease. Acta Neuropathol. 2012;123(3):419–32. doi: 10.1007/s00401-011-0927-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bronstein JM, Tagliati M, Alterman RL, et al. Deep brain stimulation for Parkinson disease: An expert consensus and review of key issues. Arch Neurol. 2011;68(2):165. doi: 10.1001/archneurol.2010.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin S, Wu Y, Li H, et al. Deep brain stimulation of the globus pallidus internus versus the subthalamic nucleus in isolated dystonia. J Neurosurg. 2019;8:1–12. doi: 10.3171/2018.12.JNS181927. [DOI] [PubMed] [Google Scholar]
- 49.Takakusaki K, Tomita N, Yano M. Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. J Neurol. 2008;255(Suppl 4):19–29. doi: 10.1007/s00415-008-4004-7. [DOI] [PubMed] [Google Scholar]
- 50.Takakusaki K. Functional neuroanatomy for posture and gait control. J Mov Disord. 2017;10(1):1–17. doi: 10.14802/jmd.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fasano A, Aquino CC, Krauss JK, et al. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat Rev Neurol. 2015;11(2):98–110. doi: 10.1038/nrneurol.2014.252. [DOI] [PubMed] [Google Scholar]
- 52.Brandmeir NJ, Brandmeir CL, Carr D, et al. Deep brain stimulation for Parkinson disease does not worsen or improve postural instability: A prospective cohort trial. Neurosurgery. 2018;83(6):1173–82. doi: 10.1093/neuros/nyx602. [DOI] [PubMed] [Google Scholar]
- 53.Lee KH, Kim JM, Kim HS. Back extensor strengthening exercise and backpack wearing treatment for camptocormia in Parkinson’s disease: A retrospective pilot study. Ann Rehabil Med. 2017;41(4):677–85. doi: 10.5535/arm.2017.41.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Capecci M, Serpicelli C, Fiorentini L, et al. Postural rehabilitation and Kinesio taping for axial postural disorders in Parkinson’s disease. Arch Phys Med Rehabil. 2014;95(6):1067–75. doi: 10.1016/j.apmr.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 55.Swalwell C, Pachana NA, Dissanayaka NN. Remote delivery of psychological interventions for Parkinson’s disease. Int Psychogeriatr. 2018;30(12):1783–95. doi: 10.1017/S1041610218000340. [DOI] [PubMed] [Google Scholar]
- 56.Schrag A, Taddei RN. Depression and anxiety in Parkinson’s disease. Int Rev Neurobiol. 2017;133:623–55. doi: 10.1016/bs.irn.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 57.Egan SJ, Laidlaw K, Starkstein S. Cognitive behaviour therapy for depression and anxiety in Parkinson’s disease. J Parkinsons Dis. 2015;5(3):443–51. doi: 10.3233/JPD-150542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cui SS, Du JJ, Fu R, et al. Prevalence and risk factors for depression and anxiety in Chinese patients with Parkinson disease. BMC Geriatr. 2017;17(1):270. doi: 10.1186/s12877-017-0666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Atsushi U, Yuichi O, Kenji O, et al. Effect of subthalamic deep brain stimulation on postural abnormality in Parkinson disease. J Neurosurg. 2010;112:1283–88. doi: 10.3171/2009.10.JNS09917. [DOI] [PubMed] [Google Scholar]
- 60.Artusi CA, Zibetti M, Romagnolo A, et al. Subthalamic deep brain stimulation and trunk posture in Parkinson’s disease. Acta Neurol Scand. 2018;137(5):481–87. doi: 10.1111/ane.12889. [DOI] [PubMed] [Google Scholar]
- 61.Kim SJ, Udupa K, Ni Z, et al. Effects of subthalamic nucleus stimulation on motor cortex plasticity in Parkinson disease. Neurology. 2015;85(5):425–32. doi: 10.1212/WNL.0000000000001806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Debû B, De Oliveira Godeiro C, Lino JC, et al. Managing gait, balance, and posture in Parkinson’s disease. Curr Neurol Neurosci Rep. 2018;18(5):23. doi: 10.1007/s11910-018-0828-4. [DOI] [PubMed] [Google Scholar]
- 63.Limousin P, Foltynie T. Long-term outcomes of deep brain stimulation in Parkinson disease. Nat Rev Neurol. 2019;15(4):234–42. doi: 10.1038/s41582-019-0145-9. [DOI] [PubMed] [Google Scholar]
- 64.Kim R, Kim HJ, Shin C, et al. Long-term effect of subthalamic nucleus deep brain stimulation on freezing of gait in Parkinson’s disease. J Neurosurg. :2019. doi: 10.3171/2018.8.JNS18350. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 65.Kashihara K, Ohno M, Tomita S. Dropped head syndrome in Parkinson’s disease. Mov Disord. 2006;21(8):1213–16. doi: 10.1002/mds.20948. [DOI] [PubMed] [Google Scholar]
- 66.Mano T. Camptocormia induced by a dopaminergic agonist. Clin Neuropharmacol. 2018;41(2):70–72. doi: 10.1097/WNF.0000000000000266. [DOI] [PubMed] [Google Scholar]
- 67.Tinazzi M, Gandolfi M, Ceravolo R, et al. Postural abnormalities in Parkinson’s disease: An epidemiological and clinical multicenter study. Mov Disord Clin Pract. 2019;6(7):576–85. doi: 10.1002/mdc3.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taguchi Y, Takashima S, Tanaka K. Pramipexole-induced dropped head syndrome in Parkinson’s disease. Intern Med. 2008;47(22):2011–12. doi: 10.2169/internalmedicine.47.1579. [DOI] [PubMed] [Google Scholar]