Abstract
Background
Retinal degeneration causes irreversible blindness. Human retinal progenitor cells (hRPCs) have the potential to treat retinal diseases. The vitreous cavity is a relatively immune-privileged site that is suitable for stem cell transplantation in the treatment of retinal diseases. This study aimed to evaluate the therapeutic efficacy and safety of intravitreal injection of hRPCs in retinal degeneration therapy.
Material/Methods
hRPCs were primary-cultured and injected into the vitreous cavity of RCS rats. To determine whether hRPCs formed teratomas in immune-deficient mice, hRPCs at different passages were transplanted into BALB/c-nu mice. The visual function was detected by electroretinography recording. Changes in the outer nuclear layer (ONL) were analyzed by histological testing and cell counting. The protective mechanism was further assessed by cytokine antibody array.
Results
Intravitreal transplantation of hRPCs maintained retinal function and preserved retinal morphology. Importantly, grafted cells in the vitreous cavity were well tolerated, with no adverse effects. Teratoma was not formed in BALB/c-nu mice after hRPCs transplantation. The number of hRPCs-injected eyes and thickness of ONL in the hRPCs-treated group were higher than those in the untreated group and HBSS injection group. The cytokine antibody array revealed that hRPCs expressed GDF-15, PDGF-AA, EGF, and NT-4.
Conclusions
Our findings show that intravitreal injection of hRPCs is effective and safe in protecting photoreceptor cells in RCS rats, but were no longer effective at 12 weeks after transplantation. Moreover, hRPCs released multiple neurotrophic factors that may be involved in treating retinal disease.
MeSH Keywords: Intravitreal Injections, Retinal Degeneration, Stem Cells, Transplantation
Background
Retinal degeneration, such as age-related macular degeneration (AMD) and retinitis pigmentosa (RP), is characterized by progressive loss of retinal photoreceptors and eventual blindness. Conservative treatment can delay the process of degeneration, but its efficacy is very limited. This ineffectiveness is due to the heterogeneity of the retinal degeneration process, which makes the single-therapeutic approach very challenging. Cell therapy is a promising strategy for regeneration of injured retinal cells. Various sources of stem cells that can replace retinal cells are being investigated, including embryonic stem cells (ESCs) [1], induced pluripotent stem cells (iPSCs) [2,3], bone marrow-derived mesenchymal stem cells (BMSCs), and adipose tissue-derived mesenchymal stem cells (ADMSCs). These cells are expected to differentiate into retinal cells, slowing the rate of retinal degeneration [4,5].
Human retinal progenitor cells (hRPCs), derived from well-defined fetal retinas (at 16–20 weeks of gestational age), have the ability to self-renew and differentiate into all retinal cell types under appropriate conditions [6]. Subretinal transplantation of hRPCs can repair injured retinal cells, contributing to the differentiation of retinal cells and paracrine neuroprotective factors. Mounting evidence suggests that retinal progenitor cells are a feasible and preferred candidate for cell-based treatment of retinal degeneration [7–9]. However, Satarian et al. demonstrated that subretinal transplantation is associated with a relatively high incidence of complications such as cataract, retinal hemorrhage, and retinal detachment [10]. Moreover, the subretinal space is not immune-privileged and is prone to immune rejection [11]. As an immune-privileged site, the vitreous cavity allows the use of standard intravitreal injection for stem cell delivery [4]. A number of preclinical animal studies and clinical studies have verified the good tolerability and effects of intravitreally transplanted BMSCs and neuroprotective factors [12]. However, there are few reports about hRPCs transplantation in the vitreous cavity.
The present study aimed to evaluate the efficacy and safety of intravitreal injection of hRPCs in a model of retinal degeneration of Royal College of Surgeons (RCS) rats. Our data not only demonstrated the importance of hRPCs in delaying progression, but also show it to be a safe cell source. hRPCs can also release a variety of neuroprotective factors in the conditioned medium.
Material and Methods
Cell culture
hRPCs isolated from donated neural retinas of 16 to 18 weeks gestational age were obtained from the Eye Bank of He Eye Hospital in Shenyang, China. The donor signed the informed consent form. All works with human samples were performed after obtaining approval from the Institutional Review Board (IRB) of He Eye Hospital of He University (approval K007.01, March 2019). The protocol used a previously reported approach [13]. Briefly, the whole neuroretina was taken, then minced and digested with TrypLE express (Invitrogen) for 2 min. The cell pellet was resuspended using Ultraculture media (Lonza), supplemented with 1% N2 neural supplement, L-glutamine (2 mM), penicillin-streptomycin (100 U/ml), and recombinant human epidermal growth factor (EGF, 20 ng/ml) and basic fibroblast growth factor (bFGF, 20 ng/ml) (all purchased from Invitrogen), followed by inoculation in fibronectin pre-coated cell flasks in an incubator (37°C, 5% CO2). The hRPCs were passaged when the confluence reached 80%. The hRPCs at passage 6 were used for the experiments.
Characterization of hRPCs
For flow cytometry analysis, hRPCs at passage 6 were collected and analyzed using Nestin, Ki67, Pax6, and Chx10 antibodies. For immunofluorescence testing, the hRPCs were cultured with medium containing 10% serum for induction. After hRPCs differentiation, the cells were seeded on a coated slide, fixed with 4% paraformaldehyde, and permeated with 0.2% Triton-X100 (Sigma-Aldrich) for 10 min. Subsequently, the cells were blocked with 5% BSA (Sigma-Aldrich) at room temperature for 1 h, and incubated overnight after adding the primary antibody, followed by staining with TRITC-conjugated secondary antibody and incubation at room temperature for 30 min. Nuclei were stained with DAPI. Information on the antibodies is shown in Table 1.
Table 1.
The information of the antibodies used for immunofluorescence.
| Antibody | Working dilution | Source |
|---|---|---|
| Map2 | 1: 1000 | Abcam |
| GFAP | 1: 100 | Abcam |
| NF-200 | 1: 1000 | Abcam |
| Recoverin | 1: 200 | Abcam |
| Rhodopsin | 1: 200 | Abcam |
Teratoma formation
For teratoma formation analysis, BALB/c-nu mice (Shenyang Medicine Certification Center, China) at 6 weeks of age were used for cell transplantation. The mice were randomly divided into 4 groups: a negative control group (MRC-5, 1×107), a positive control group (Hela, 1×106), an hRPCs 1 group (Passage 6 of hRPCs, 1×107), and an hRPCs 2 group (Passage 12 of hRPCs, 1×107) (n=10 per group). Intraperitoneal injection of cell suspension in 200 μl of PBS was given to each group. Mice were euthanized, and their organs were fixed with 10% formalin and stained with hematoxylin and eosin (H&E) for teratoma evaluation. The ethics approval number was 2018081501.
Intravitreal transplantation of hRPCs
The intravitreal transplantation was conducted with the approval and regulation of the Animal Care Committee of He Eye Hospital. RCS rats, which are the classical model of retinal degeneration, were used in this study. A total of 48 RCS rats were divided into 3 groups at 3 weeks after birth (8 males and 8 females in each). The hRPCs-treated group received an intravitreal injection of 3 ul of 2×105 hRPCs solution in the right eyes with a Hamilton 30-gauge needle; the vehicle group was intravitreally injected with the same volume of HBSS in all right eyes; the untreated group was used as the baseline control. All left eyes in the 3 groups were untreated and served as a control group. All operations were performed under a surgical microscope (Topcon OMS-800).
Electroretinography (ERG) recording
To evaluate the protective efficacy of hRPCs, ERG recordings were performed every 2 weeks for both eyes until the 12th week after transplantation. All the RCS rats were dark-adapted for 12 h prior to start of ERG recordings. Then, RCS rats were anesthetized with pentobarbital sodium (8 mg/ml) at a dose of 25–50 mg/kg, and 1% topical tropicamide was used to dilate the pupils. Following this, each RCS rat was tested in a fixed position, with a contact lens electrode placed on each cornea, a subcutaneous ground needle electrode positioned in the tail, and the reference electrodes placed subcutaneously in the head region. The b wave amplitudes were recorded at intensities of 0.01, 1.4, 3.0, and 10.0 cd.s/m2. The recordings were analyzed using Espion e2 system software. The entire process was carried out in the dark.
Retinal histological analysis
The rats were sacrificed by CO2 overdose after the ERG recordings were made. Their eyes were immediately dissected out and put in Davidson’s fixative for 2 h, and then embedded in paraffin to prepare retinal sections. Retinal sections with whole optic nerves were selected and stained with hematoxylin and eosin (H&E). The number of outer nuclear layer cells was counted in 10 random fields at the nasal and temporal sides of the optic nerve head.
Cytokine antibody array
hRPCs were cultured to reach 90% confluence in T75 flasks. After washing 3 times in DPBS, the Ultraculture media was replaced to culture hRPCs. Subsequently, the culture supernatant and cell lysates were collected after 48 h, centrifuged at 300 g for 5 min, and filtered through a 0.22-μm syringe filter. Cytokines secreted by hRPCs were assessed using a Quantibody (R) Human Cytokine Antibody Array 9000 chip, performed according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed with GraphPad Prism software using the unpaired t test or one-way analysis of variance (ANOVA), following Bonferroni’s test, unless otherwise stated. P value <0.05 indicates a statistically significant difference. Data are presented as mean ± SEM. Experiments were repeated at least 3 times.
Results
Identification of hRPCs
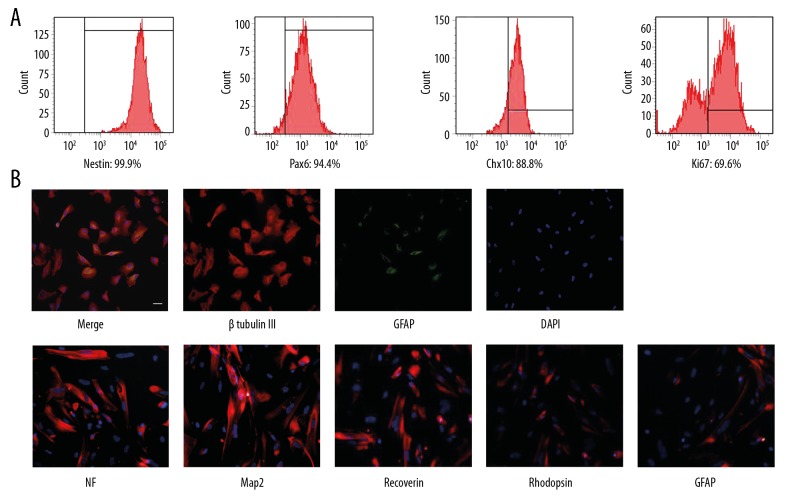
The hRPCs were analyzed for the expression of their proliferation and differentiation markers. Flow cytometry analysis showed that hRPCs highly expressed Nestin, Pax 6, Chx 10, and Ki67 (Figure 1A). Nestin is a specific neural stem cell marker, Pax 6 and Chx 10 are markers of retinal progenitor cells, and Ki67 is a proliferation marker. After induction with medium containing 10% serum, the differentiated hRPCs expressed high levels of retinal markers, including microtubule-associated protein 2 (Map2), neurofilament-200 (NF), rhodopsin (the rod photoreceptor marker), recoverin (a marker for both rod and cone photoreceptors, and for cone bipolar cells), and glial fibrillary acidic protein (GFAP, an astroglia marker). Most cells expressing β Tubulin III did not simultaneously express GFAP, while only a few hRPCs showed double-label for β Tubulin III and GFAP (Figure 1B). These results indicated that hRPCs were the neural progenitor cells and could potentially differentiate into various retinal cell types.
Figure 1.
Characterization of hRPCs in vitro. (A) The markers of Nestin, Ki67, Pax6, and Chx10 were used to identify the characteristics of hRPCs by flow cytometry analysis. (B) After serum induction of hRPCs, double-staining for β Tubulin III and GFAP markers. Red refers to β Tubulin III; green refers to GFAP; blue (nucleus) refers to DAPI. Immunofluorescence of retinal markers with antibodies against Map2, GFAP, NF, recoverin, and rhodopsin in hRPCs were detected. Red refers to positive cells; blue refers to DAPI. Scale bars: 50 μm.
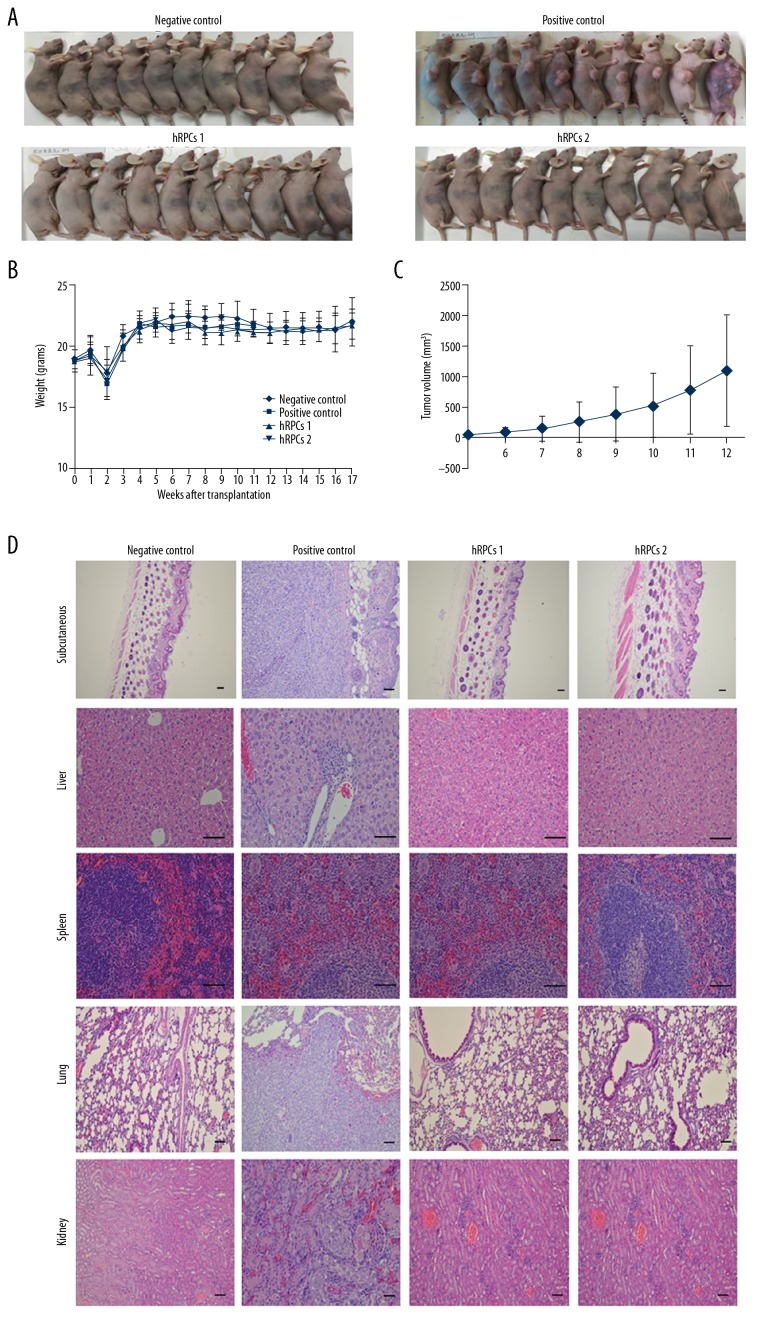
To further evaluate teratoma formation capability, hRPCs at different passages were transplanted into BALB/c-nu mice. Sixteen weeks after transplantation, teratoma formation induced by cancer cell transplantation was obvious, while no teratoma formation was observed in the hRPCs injection groups (Figure 2).
Figure 2.
The hRPCs injection groups were unable to develop teratoma. (A) Teratoma formation in BALB/c-nu mice (n=10) at 16 weeks after cell transplantation. (B) Weight change curves of animals in different groups. (C) Tumor size was monitored weekly when the tumor growth became visible. The graph represents the tumor growth volume observed in the positive control group at different time points before tumor resection. (D) Hematoxylin and eosin (H&E) staining of different sections of the animals in different groups. hRPCs in passage 6 and passage 12 had no subcutaneous, liver, spleen, lung, or kidney tumor formation, which was the same as in the negative control group, but the positive control group had tumor formation at these sites. Scale bars: 50 μm.
Intravitreal transplantation of hRPCs improves ERG b wave amplitude in RCS rats
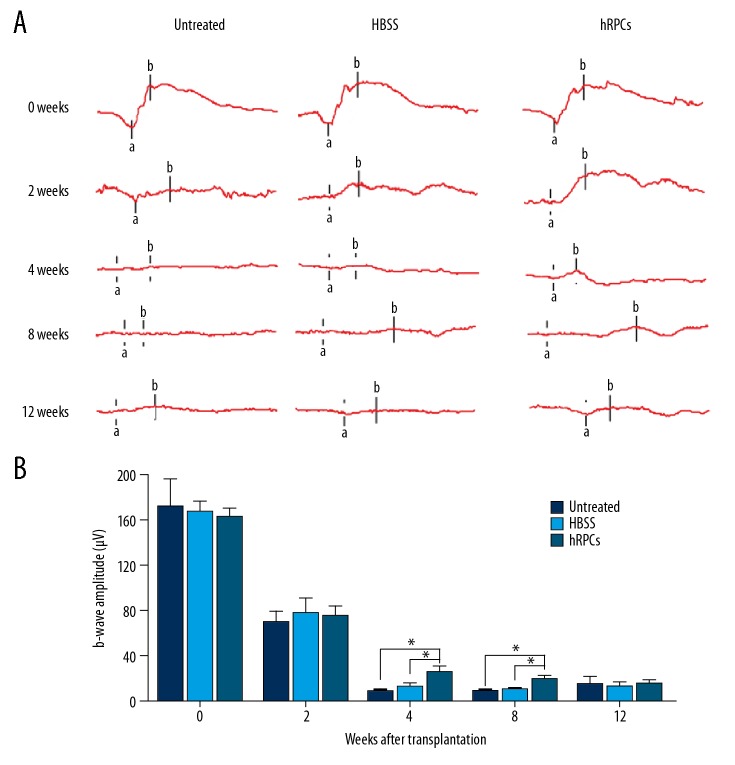
To determinate the effect of hRPCs transplantation, we monitored the visual function using sensitive ERG up to 12 weeks after transplantation. The b wave amplitude is one of the vital clinical indicators and is related to the number of photoreceptor cells. The mean values of b wave amplitudes of all RCS rats at 2, 4, 8, and 12 weeks after intravitreal injections were recorded. As shown in Figure 3, hRPCs injection displayed a significant improvement of the amplitude of b waves at 4 weeks and 8 weeks compared to those in the untreated group and HBSS injection group. Notably, there was no significant difference among the 3 groups at 2 weeks. However, the amplitude of b waves in the hRPCs-treated group at 4 weeks was significantly higher than that in the HBSS group and untreated group after transplantation (P=0.0418 and P=0.026, respectively). At 8 weeks, the amplitude of b waves in the hRPCs-treated group decreased, but was still remarkably higher than that in the HBSS transplantation group and untreated group (P=0.0386 and P=0.0395, respectively). Nevertheless, at 12 weeks after hRPCs injection, there was no significant difference among the 3 groups, and no significant difference was found between the untreated group and HBSS injection group at any time points.
Figure 3.
Electroretinography (ERG) recording analysis. (A) The ERG waveforms were recorded weekly in the 3 groups. (B) ERG amplitudes of scotopic b waves of the 3 groups. Data are shown as mean±SEM (n ≥4 at each time point; * P<0.05).
Intravitreal injection of hRPCs protects photoreceptors
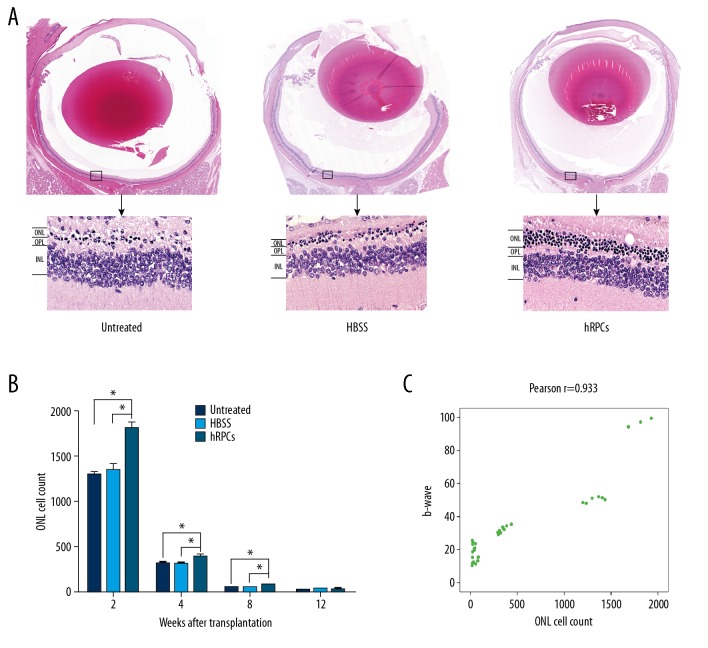
Because retinal function was remarkably improved after intravitreal injection of hRPCs, we hypothesized that intravitreal injection of hRPCs would protect photoreceptors in model of retinal degeneration-RCS rats. This was assessed by counting the number of adjacent photoreceptor cell nuclei located in the outer nuclear layer (ONL) and measuring the thickness of the ONL. Over time, all photoreceptors exhibited gradual degeneration. The pathological slices showed that the hRPCs-treated group had significantly thicker ONL than the untreated group and HBSS injection group at 8 weeks after transplantation. There were more than 5 layers of photoreceptors in the ONL in the hRPCs-treated group, whereas there were only 1–2 layers of photoreceptors in the HBSS-injected group and untreated group (Figure 4A). The number of adjacent photoreceptor cell nuclei in the ONL was counted at 2, 4, 8, and 12 weeks after transplantation. Figure 4B shows that the number of hRPCs-injected eyes was significantly higher than in the other groups at 2, 4, and 8 weeks after treatment (P=0.0106, P=0.0479, P=0.0446, respectively). We also demonstrated a correlation between the number of photoreceptor cell nuclei in the ONL and the ERG b wave amplitude (Figure 4C). The above results indicate that intravitreal injection of hRPCs plays a protective role in the maintenance of photoreceptors and slows retinal degeneration.
Figure 4.
Intravitreal injection of hRPCs protected photoreceptors. (A) The eyeball was embedded and stained with H&E at 8 weeks after transplantation. In the hRPCs-injected eyes, there were at least 5 layers of photoreceptor cells in the ONL, while only 1–2 layers of photoreceptors were present in HBSS-injected and untreated eyes. (B) The cell count was significantly higher at 2, 4, and 8 weeks in the hRPCs-treated group (* P<0.05). (C) Correlation coefficient analysis showed that the b wave response was correlated with the ONL cell count.
The conditioned medium of hRPCs contains neurotrophic factors
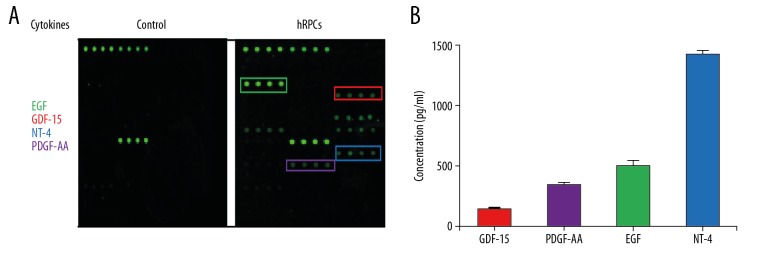
It has been reported that mesenchymal stem cells can protect retinal tissue by enhancing neurotrophic factors [14]. To the best of our knowledge, no published study has reported neurotrophic factors secreted by hRPCs. Therefore, cytokines secreted by hRPCs were detected via Quantibody Human Cytokine Antibody Array 9000 chip. The experimental results showed that hRPCs expressed a panel of factors, including GDF-15, PDGF-AA, EGF, and NT-4 (Figure 5), which play an important role in protecting the retina and inhibiting neural cell apoptosis [15–18].
Figure 5.
hRPCs expressed neurotrophic factors. The supernatants of the hRPCs were collected and detected by Quantibody Human Cytokine Antibody Array 9000. (A) The boxes show the upregulated cytokines. (B) The mean values of the 4 upregulated cytokines were: GDF-15 was 133.0 pg/ml, PDGF-AA was 336.2 pg/ml, EGF was 490 pg/ml, and NT-4 was 1420.8 pg/ml. Each value represents the mean of 3 experiments.
Discussion
Retinal degeneration results in severe visual impairment, primarily due to the gradual death of retinal photoreceptors in the retina. Currently, there is no effective treatment to inhibit cell death. Therefore, it is urgent to discover effective approaches to treat retinal degeneration. In our study, we selected RCS rats as the animal model of Merk gene mutation, which is widely used in studies of inherited retinal degeneration [19].
In recent years, various studies have used subretinal injection to investigate the therapeutic effect in treatment of retinal disease. However, the subretinal injection procedure is considered to be a complicated and risky operation, which is prone to retinal detachment, cataracts, vitreous hemorrhage, recurrence of submacular hemorrhage, and postoperative development of CNV and other incurable injuries [20]. In contrast, the intravitreal injection procedure is established and widely used in clinical treatment of retinal diseases [4,21–24]. Additionally, the vitreous cavity is a relevantly immune-privileged site with a low risk of immune rejection. Jonas et al. proved that intravitreal injection is technically feasible and safe in patients [25]. With the increasing incidence of retinal diseases, safe and effective treatments are urgently needed. Because of the obvious advantages of hRPCs, we chose intravitreal injection of hRPCs to evaluate its effect on the treatment of retinal degeneration. Our results revealed that the hRPCs highly expressed retinal precursor cells markers, including Nestin, Ki67, Pax6, and Chx10. The expression of Pax6 is required for the multipotent state of retinal progenitor cells [26], and the co-expression of Pax6 and Chx10 is known as a hallmark of neural and retinal progenitor cells [27]. Our cultured hRPCs were able to differentiate into mature retinal cell types by expression of mature retinal markers such as Map2, NF, recoverin, and rhodopsin, and a small amount of GFAP. Thus, these findings indicate that the hRPCs have potential for stem cell therapy.
After the injection of hRPCs into the vitreous cavity, we investigated the change of amplitude of b wave among the 3 groups. The amplitude of b waves in the hRPCs-treated group at 4 weeks was significantly higher than that in the HBSS group and untreated groups, and the protective function was maintained to week 8. We found that intravitreal injection of hRPCs was well tolerated and without adverse effects, and did not develop teratoma in immune-deficient mice after 16 weeks of observation. Ezquer et al. showed that intravitreal transplantation of adipose-derived mesenchymal stem cells can prevent the loss of neurosensory retina via secreted neurotrophic factors in the vitreous cavity, and that mesenchymal stem cells can remain in the vitreous cavity for up to 12 weeks [28]. The diameter of hMSCs is about 15–19 μm [29], while the mean size of the diameter of hRPCs is 16–25 μm. In this study, we did not find cell integration into the retina. Therefore, hRPCs mainly survived in the vitreous cavity after intravitreal transplantation. We speculated that the function of delaying retinal degeneration may be due to the paracrine effect of hRPCs, since it is difficult for cells to move to the retina and replace the dead photoreceptors through the vitreous cavity.
Previous studies have revealed that stem cells can rescue dying retinal tissue, mainly through their own secretion of nutrients and anti-inflammatory factors rather than by replacement [30–33]. However, there is a paucity of data showing that hRPCs can secrete neurotrophic factors. Here, we reported for the first time a variety of neurotrophic factors expressed by hRPCs in vitro, including EGF, GDF-15, NT-4, and PDGF-AA. GDF-15 is a member of the TGF-β superfamily, which is widely expressed in various tissues; it plays an important role in neurotrophic effects on mid-brain dopaminergic neurons. Strelau et al. [15] showed that GDF-15 has a positive function in the maintenance of adult motor and sensory neurons. NT-4 is a member of the NT family and is essential for neuronal survival, development, and functional maintenance of CNS. Machalinska et al. [18] found that the NT-4 sustainably released from MSCs can protect damaged retinal cells when the MSCs are injected into the vitreous cavity of mice with acute retinal injury. The present study further shows that paracrine-mediated anti-apoptotic neurotrophic factors from hRPCs may be involved in the underlying mechanism of the protective effects on the degenerative retina.
In our study, hRPCs were intravitreally transplanted for treatment of RCS rats. Our data showed promising protective effects by intravitreal injection of hRPCs on retinal degeneration therapy; the neurotrophic factors secreted from hRPCs could rescue the dying photoreceptors, and the underlying mechanisms require further investigation. Intravitreal transplantation is a safe and easy procedure for retinal treatment, and should be performed at an early stage before severe damage begins.
Conclusions
We demonstrated that intravitreal injection of hRPCs is an effective and safe method to protect photoreceptors and delay the process of retinal degeneration, but this method is not effective at 12 weeks after transplantation. The mechanisms may be via the paracrine function of transplantation of hRPCs into the vitreous cavity. Overall, intravitreal injection appears to be an effective way to delivery stem cells and preserve photoreceptor cells and retinal tissue.
Footnotes
Conflict of interests
None.
Source of support: This work was supported by grants from the National High Technology Research and Development Program of China (2013CB967500) and the Natural Science Fund of Liaoning Province (201602581). Wei He was the recipient of the funding and designed the research
References
- 1.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4(1):73–79. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamba DA, Andrew MU, Hirata RK, et al. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS One. 2010;5(1):e8763. doi: 10.1371/journal.pone.0008763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou L, Wang W, Liu Y, et al. Differentiation of induced pluripotent stem cells of swine into rod photoreceptors and their integration into the retina. Stem Sells. 2011;29(6):972–80. doi: 10.1002/stem.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moisseiev E, Smit-McBride Z, Oltjen S, et al. Intravitreal administration of human bone marrow CD34+ stem cells in a murine model of retinal degeneration. Invest Ophthalmol Vis Sci. 2016;57(10):4125–35. doi: 10.1167/iovs.16-19252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y, Ng TK, Chen CB, et al. Notch signaling activation enhances human adipose-derived stem cell retinal differentiation. Stem Cells Int. 2018;2018 doi: 10.1155/2018/9201374. 9201374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baranov PY, Tucker BA, Young MJ. Low-oxygen culture conditions extend the multipotent properties of human retinal progenitor cells. Tissue Eng Part A. 2014;20(9–10):1465–75. doi: 10.1089/ten.tea.2013.0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo J, Baranov P, Patel S, et al. Human retinal progenitor cell transplantation preserves vision. J Biol Chem. 2014;289(10):6362–71. doi: 10.1074/jbc.M113.513713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Chen SJ, Li SY, et al. Long-term safety of human retinal progenitor cell transplantation in retinitis pigmentosa patients. Stem Cell Res Ther. 2017;8(1):209. doi: 10.1186/s13287-017-0661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semo M, Haamedi N, Stevanato L, et al. Efficacy and safety of human retinal progenitor cells. Transl Vis Sci Technol. 2016;5(4):6. doi: 10.1167/tvst.5.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satarian L, Nourinia R, Safi S, et al. Intravitreal injection of bone marrow mesenchymal stem cells in patients with advanced retinitis pigmentosa; A safety study. J Ophthalmic Vis Res. 2017;12(1):58–64. doi: 10.4103/2008-322X.200164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennelly KP, Holmes TM, Wallace DM, et al. Early subretinal allograft rejection is characterized by innate immune activity. Cell Transplant. 2017;26(6):983–1000. doi: 10.3727/096368917X694697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss JN, Levy S. Stem Cell Ophthalmology Treatment Study (SCOTS): Bone marrow derived stem cells in the treatment of Usher syndrome. Stem Cell Investig. 2019;6:31. doi: 10.21037/sci.2019.08.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang M, Zhang F, Sun J, et al. The condition medium of mesenchymal stem cells promotes proliferation, adhesion and neuronal differentiation of retinal progenitor cells. Neurosci Lett. 2017;657:62–68. doi: 10.1016/j.neulet.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Lu B, Girman S, et al. Non-invasive stem cell therapy in a rat model for retinal degeneration and vascular pathology. PLos One. 2010;5(2):e9200. doi: 10.1371/journal.pone.0009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strelau J, Strzelczyk A, Rusu P, et al. Progressive postnatal motoneuron loss in mice lacking GDF-15. J Neurosci. 2009;29(43):13640–48. doi: 10.1523/JNEUROSCI.1133-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahama S, Adetunji MO, Zhao T, et al. Retinal astrocytes and GABAergic wide-field amacrine cells express PDGFRα: Connection to retinal ganglion cell neuroprotection by PDGF-AA. Invest Ophthalmol Vis Sci. 2017;58(11):4703–11. doi: 10.1167/iovs.21783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang D, Shen B, Zhang Y, et al. Betacellulin regulates the proliferation and differentiation of retinal progenitor cells in vitro. J Cell Mol Med. 2018;22(1):330–45. doi: 10.1111/jcmm.13321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machalinska A, Kawa M, Pius-Sadowska E, et al. Long-term neuroprotective effects of NT-4 – engineered mesenchymal stem cells injected intravitreally in a mouse model of acute retinal injury. Invest Ophthalmol Vis Sci. 2013;54(13):8292–305. doi: 10.1167/iovs.13-12221. [DOI] [PubMed] [Google Scholar]
- 19.D’Cruz PM, Yasumura D, Weir J, et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9(4):645–51. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- 20.Peng Y, Tang L, Zhou Y. Subretinal injection: A review on the novel route of therapeutic delivery for vitreoretinal diseases. Ophthalmic Res. 2017;58(4):217–26. doi: 10.1159/000479157. [DOI] [PubMed] [Google Scholar]
- 21.Zhu M, Jiang L, Yuan Y, et al. Intravitreal Ets1 siRNA alleviates choroidal neovascularization in a mouse model of age-related macular degeneration. Cell Tissue Res. 2019;376(3):341–51. doi: 10.1007/s00441-019-03001-1. [DOI] [PubMed] [Google Scholar]
- 22.Solomon SD, Lindsley K, Vedula SS, et al. Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2014;(8):CD005139. doi: 10.1002/14651858.CD005139.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartman RR, Kompella UB. Intravitreal, subretinal, and suprachoroidal injections: Evolution of microneedles for drug delivery. J Ocul Pharmacol Ther. 2018;34(1–2):141–53. doi: 10.1089/jop.2017.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang JD, An Y, Zhang JS, et al. Retinal vascular injuries and intravitreal human embryonic stem cell-derived haemangioblasts. Acta Ophthalmol. 2017;95(6):e468–76. doi: 10.1111/aos.13477. [DOI] [PubMed] [Google Scholar]
- 25.Jonas JB, Mathias WH, Lubomir A, Ho AD. Intravitreal autologous bone marrow-derived mononuclear cell transplantation: A feasibility report. Acta Ophthalmol. 2009;88(4):e131–32. doi: 10.1111/j.1755-3768.2009.01564.x. [DOI] [PubMed] [Google Scholar]
- 26.Marquardt T, Ashery-Padan R, Andrejewski N, et al. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105(1):43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 27.Dixit R, Tachibana N, Touahri Y, et al. Gene expression is dynamically regulated in retinal progenitor cells prior to and during overt cellular differentiation. Gene Expr Patterns. 2014;14(1):42–54. doi: 10.1016/j.gep.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Ezquer M, Urzua CA, Montecino S, et al. Intravitreal administration of multipotent mesenchymal stromal cells triggers a cytoprotective microenvironment in the retina of diabetic mice. Stem Cell Res Ther. 2016;7(1):42. doi: 10.1186/s13287-016-0299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker PA, Jimenez F, Aroom K, et al. CSC: Effect of needle diameter and flow rate on mesenchymal stromal cell characterization and viability. Tissue Eng Part C Methods. 2010;16(5):989–97. doi: 10.1089/ten.tec.2009.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu B, Wanga S, Mcgill T, Ragaglia V, Lund R. Human adult bone marrow-derived somatic cells rescue vision in a rodent model of retinal degeneration. Exp Eye Res. 2010;91(3):449–55. doi: 10.1016/j.exer.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 31.Johnson TV, Bull ND, Hunt DP, et al. Neuroprotective effects of intravitreal mesenchymal stem cell transplantation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;51(4):2051–59. doi: 10.1167/iovs.09-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar A, Kumar V, Rattan V, et al. Secretome cues modulate the neurogenic potential of bone marrow and dental stem cells. Mol Neurobiol. 2017;54(6):4672–82. doi: 10.1007/s12035-016-0011-3. [DOI] [PubMed] [Google Scholar]
- 33.Huang L, Li Z, Tian H, et al. Adult human periodontal ligament-derived stem cells delay retinal degeneration and maintain retinal function in RCS rats. Stem Cell Res Ther. 2017;8(1):290. doi: 10.1186/s13287-017-0731-y. [DOI] [PMC free article] [PubMed] [Google Scholar]