Abstract
Patients with chronic kidney disease are at increased risk of cardiovascular disease and this often manifests clinically like heart failure. Conversely, patients with heart failure frequently have reduced kidney function. The links between the kidneys and cardiovascular system are being elucidated, with blood pressure being a key risk factor. Patients with heart failure have benefitted from many trials which have now established a strong evidence based on which to base management. However, patients with advanced kidney disease have often been excluded from these trials. Nevertheless, there is little evidence that the benefits of such treatments are modified by the presence or absence of kidney disease, but more direct evidence among patients with advanced kidney disease is required. Neprilysin inhibition is the most recent treatment to be shown to improve outcomes among patients with heart failure. The UK HARP-III trial assessed whether neprilysin inhibition improved kidney function in the short- to medium-term and its effects on cardiovascular biomarkers. Although no effect (compared to irbesartan control) was found on kidney function, allocation to neprilysin inhibition (sacubitril/valsartan) did reduce cardiac biomarkers more than irbesartan, suggesting that this treatment might improve cardiovascular outcomes in this population. Larger clinical outcomes trials are needed to test this hypothesis.
Keywords: blood pressure, cardiovascular, CKD, heart failure, renin-angiotensin system
CKD AND STRUCTURAL HEART DISEASE ARE CLOSELY ASSOCIATED
Chronic kidney disease (CKD) and heart failure (HF) frequently coexist and both are associated with high morbidity and mortality [1, 2]. Numerous studies have shown that there is an inverse association between kidney function and cardiovascular risk [3, 4]. Structural heart disease, which may manifest clinically as HF, is a leading cause of cardiovascular disease in CKD patients and its prevalence increases with declining kidney function [2, 5]. A cross-sectional echocardiographic observational study reported an increasing prevalence of left ventricular hypertrophy (LVH) with decreasing estimated glomerular filtration rate (eGFR) (from 32% among patients with eGFR ≥60 mL/min/1.73 m2 to 75% among patients with eGFR <30 mL/min/1.73 m2) [6, 7]. Studies using cardiac magnetic resonance imaging with gadolinium enhancement have found that diffuse late gadolinium enhancement is associated with the degree of LVH [8] and indicates myocyte disarray and interstitial fibrosis histologically [9]. Although overt systolic dysfunction is not common (affecting only 8% of patients in the above cross-sectional echocardiographic study) and not clearly associated with kidney function [7], more subtle disturbances in ventricular function (such as reduced left ventricular deformation, early myocardial relaxation velocity or reduction in global longitudinal strain that may contribute to diastolic dysfunction) are more common and are present even in the early stages of CKD [10, 11]. These abnormalities provide the anatomical substrate for the excess risk of symptomatic HF, arrhythmia and sudden cardiac death observed among patients with advanced CKD. Conversely, in large HF registries, 20–68% of patients with HF have moderate to severe kidney disease [1]. The presence of CKD is associated with poor prognosis in HF and can be used to stratify the risk of patients with HF [6, 12, 13].
PATHOPHYSIOLOGY OF HF IN CKD
The pathophysiological relationship between the heart and the kidneys involves many different pathways. CKD may disturb homoeostasis in ways that may be directly damaging to the cardiovascular system [i.e. ‘direct’ risk factors such as high blood pressure (BP) or vascular calcification] or the kidneys and circulation may both be subject to ‘indirect’ risk factors (e.g. diabetes mellitus and smoking). In addition, HF may worsen CKD by decreasing renal perfusion, causing renal venous congestion and activation of the sympathetic nervous system and renin–angiotensin–aldosterone system (RAS, which may in turn cause inflammation and oxidative stress). Treatment for HF in CKD can be divided into two broad types: (i) treatments that intervene on pathophysiological links between CKD and HF to prevent HF and (ii) treatments known to improve prognosis in established HF among people without CKD.
TREATMENT TO PREVENT HF IN CKD
CKD is commonly associated with high BP, due to salt and water retention, activation of the sympathetic nervous and other neurohormonal systems and accumulation of endogenous vasopressors [14]. Studies of living kidney donors suggest that reducing GFR by 10 mL/min as a consequence of donor nephrectomy leads to a 5 mmHg increase in systolic BP [15]. BP is positively associated with the risk of death from HF [16] and randomized trials have demonstrated that this association is causal [17]. Meta-analysis of all the major BP-lowering trials has shown that a 10 mmHg reduction in systolic BP lowers the risk of HF by 28% [95% confidence interval (CI) 22–33] [18]. Most classes of antihypertensive treatments have similar effects, with the exception of calcium channel blockers (which may have a smaller benefit) and diuretics (which may have a larger benefit) [18]. A subgroup analysis within this meta-analysis (which included 13 trials involving nearly 38 000 participants, of whom 6000 had CKD) suggested that the effect of BP lowering on HF was larger among patients without CKD [relative risk (RR) 0.48 (95% CI 0.38–0.62)] than among patients with CKD [RR 0.95 (95% CI 0.70–1.04); P for interaction <0.001] [18]. Nevertheless, the benefits of lowering BP on other cardiovascular outcomes remain clear even among patients with CKD.
Anaemia is a well-recognized complication of CKD and has been proposed as a direct cause of HF in patients with CKD following observational and non-randomized interventional studies, suggesting that anaemia is associated with LVH and correcting the anaemia reverses the LVH [19, 20]. However, randomized trials have shown that full or partial correction of anaemia with erythropoiesis-stimulating agents (ESAs) does not reduce left ventricular mass nor the risk of HF and may even increase the risk of other cardiovascular outcomes such as stroke [21].
Reducing parathyroid hormone concentrations with calcimimetic therapy might reduce the risk of non-atherosclerotic cardiovascular events (such as HF) among haemodialysis patients [22, 23]. Such treatment also reduces fibroblast growth factor 23 (FGF23; see below). Unfortunately, the randomized data on other interventions that target CKD-specific mechanisms of HF are much less robust. For example, although there is evidence that hyperphosphataemia (i) can cause vascular smooth muscle cells to adopt an osteoblastic phenotype and cause vascular calcification (which in turn increases cardiac afterload) [24] and (ii) is associated with LVH [25], no sufficiently large trials of phosphate reduction have been conducted to elucidate whether these associations are causal. Although FGF23 has been found to induce LVH after direct intracardiac injection in mice [26], the totality of the observational evidence does not suggest that FGF23 is a cause of cardiovascular disease (and no trials of FGF23 reduction in CKD exist) [27].
TREATMENT TO IMPROVE PROGNOSIS IN ESTABLISHED HF IN THE GENERAL POPULATION
The main objectives of HF therapy in CKD (as well as in non-CKD) patients are to decrease the preload and afterload and to reduce LVH, treat myocardial ischaemia and inhibit neurohumoral hyperactivity, especially the sympathetic nervous system and RAS [28]. However, the optimum treatment of HF in patients with CKD remains unclear, as there is little direct evidence to support any recommendations. Most of the pivotal randomized trials that guide the management of HF define CKD as a baseline eGFR <60 mL/min/1.73 m2 but have excluded patients with more advanced stages of CKD (i.e. eGFR <30 mL/min/1.73 m2).
Many pharmacological and device treatments are recommended for HF with reduced ejection fraction (HFrEF) [29]. The mainstays of such treatment are angiotensin-converting enzyme inhibitors (ACEis) and β-blockers. The largest trial of ACEis in HFrEF was Studies of Left Ventricular Dysfunction (SOLVD)-Treatment, which compared enalapril 10 mg twice daily with placebo among 2569 patients with HFrEF and demonstrated a 16% (95% CI 5–26) reduction in mortality (primary outcome) [30]. This effect was similar in patients with and without CKD [31]. Similarly, in the four large trials of β-blockers in HFrEF, there was no good evidence that the benefits of β-blocker therapy were modified by baseline kidney function. The results of these trials (and their published effects by baseline kidney function) are summarized in Table 1.
Table 1.
Effect of kidney function on the efficacy of established treatments for chronic HFrEF
| Trial (ref) | Intervention (sample size) | Main eligibility criteria | Follow-up (years) | Primary outcome | Overall treatment effect (95% CI) | CKD subgroups (eGFR, mL/min/ 1.73 m2) | Treatment effect in CKD | P for treatment × CKD interaction |
|---|---|---|---|---|---|---|---|---|
| ACEi | ||||||||
| SOLVD-TREATMENT [31] | Enalapril versus placebo (n = 2569) | LVEF ≤35%; NYHA I–IV; creatinine <177 μmol/L | 3.5 | All-cause mortality | 0.84 (0.74–0.95) | ≥60 (n = 1466) | 0.82 (0.69–0.98) | 0.62 |
| <60 (n = 1036) | 0.88 (0.73–1.06) | |||||||
| β-blocker | ||||||||
| CIBIS-II [32] | Bisoprolol versus placebo (n = 2647) | LVEF ≤35%; NYHA III–IV; creatinine <300 μmol/L | 1.3 | All-cause mortality | 0.66 (0.54–0.81) | <45 (n = 450) | 0.71 (0.48–1.05) | 0.81 |
| ≥45 <60 (n = 669) | 0.69 (0.46–1.04) | |||||||
| ≥60 <75 (n = 640) | 0.53 (0.34–0.82) | |||||||
| >75 (n = 863) | 0.64 (0.42–0.99) | |||||||
| MERIT-HF [33, 34] | Metoprolol versus placebo (n = 3991) | LVEF ≤40%; NYHA II–IV; ‘significant’ kidney disease | 1 | All-cause mortality | 0.66 (0.53–0.81) | <45 (n = 493) | 0.41 (0.25–0.68) | 0.095 |
| ≥45–≤60 (n = 976) | 0.68 (0.45–1.02) | |||||||
| >60 (n = 2496) | 0.71 (0.54–0.95) | |||||||
| SENIORS [35, 36] | Nebivolol versus placebo (n = 2128) | LVEF <35% or hospitilization for decompensated HF; NYHA II–IV; creatinine <250 μmol/L | 1.75 | All-cause mortality or CV hospital admission | 0.86 (0.74–0.99) | <55.5 (n = 704) | 0.84 (0.67–1.07) | 0.442 |
| 55.5–72.8 (n = 704) | 0.79 (0.60–1.04) | |||||||
| >72.8 (n = 704) | 0.86 (0.65–1.14) | |||||||
| Mineralocorticoid receptor antagonist | ||||||||
| RALES [37, 38] | Spironolactone versus placebo (n = 1663) | LVEF <35%; NYHA III–IV; creatinine ≤221 μmol/L | 2 | All-cause mortality | 0.70 (0.60–0.82) | <60 (n = 792) | 0.68 (0.56–0.84) | N/A |
| ≥60 (n = 866) | 0.71 (0.57–0.90) | |||||||
| EMPHASIS-HF [39] | Eplerenone versus placebo (n = 2737) | LVEF ≤35%; NYHA II; eGFR ≥30 mL/min/1.73 m2 | 1.75 | CV death or hospitalization for HF | 0.63 (0.54–0.74) | <60 (n = 912) | N/A | 0.50 |
| ≥60 (n = 1821) | N/A | |||||||
| Angiotensin receptor neprilysin inhibitor | ||||||||
| PARADIGM-HF [40] | Sacubitril/valsartan versus enalapril (n = 8442) | LVEF ≤40%; NYHA II–IV; eGFR ≥30 mL/min/1.73 m2 | 2.25 | CV death or hospitalization for HF | 0.80 (0.73–0.87) | <60 (n = 3061) | N/A | 0.91 |
| ≥60 (n = 5338) | N/A | |||||||
| ICD | ||||||||
| MADIT II [41] | Prophylactic ICD versus conventional medical therapy (n = 1232) | LVEF ≤30%; NYHA III; eGFR ≥15 mL/min/1.73 m2 | 2.67 | All-cause mortality | 0.69 (0.51–0.93) | <35 (n = 80) | 1.09 (0.49–2.43) | 0.29 |
| 35–59 (n = 387) | 0.74 (0.48–1.15) | |||||||
| ≥60 (n = 756) | 0.66 (0.43–1.02) | |||||||
| CRT | ||||||||
| CARE-HF [42] | CRT versus conventional medical therapy (n = 813) | LVEF ≤35%; NYHA III–IV; | 1.5 | Death from any cause or unplanned hospitalization for a major CV event | 0.63 (0.51–0.77) | <60 (n = 369) | 0.67 (0.50–0.89) | N/A |
| ≥60 (n = 370) | 0.57 (0.40–0.80) | |||||||
Data extracted from large trials where subgroup analysis by kidney function is available. NYHA, New York Heart Association; CV, cardiovascular; N/A, not available.
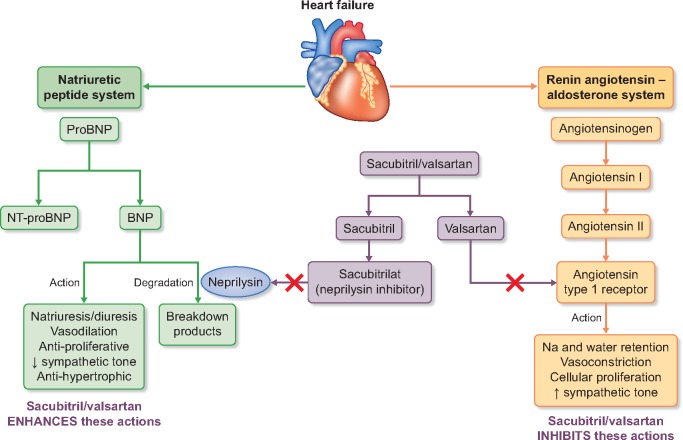
FIGURE 1.
Effects of sacubitril/valsartan on vasoactive peptides.
For patients with HFrEF [with a left ventricular ejection fraction (LVEF) <35%] who remain symptomatic after optimization of ACEi and β-blocker therapy, guidelines recommend a mineralcorticoid receptor antagonist (MRA). This recommendation follows two large trials (see Table 1). Again, the effect of treatment on the primary outcome was not modified by baseline kidney function. However, these trials highlight the importance of safety as a consideration in the treatment of patients with CKD. Patients with CKD are at higher risk of hyperkalaemia (due to the reduced ability of their kidneys to excrete potassium), which is associated with an increased risk of hospitalization and death [43]. The trials had stringent monitoring of serum potassium and developed criteria for reducing the dose or stopping the MRA, such that there was no excess death due to hyperkalaemia in the trials. The importance of such monitoring is highlighted by population-based studies, which demonstrate increased rates of hospitalization for hyperkalaemia since the publication of these trials [44]. Device therapies [implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy (CRT)] also improve prognosis in selected patients with HFrEF). A meta-analysis of the trials of ICDs has raised the hypothesis that worse kidney function might attenuate the benefit of these devices [45], but this is not the case for CRT devices. Intravenous iron has been shown to improve functional capacity among patients with HFrEF and results of clinical outcomes trials are needed [46]. Indeed, the PIVOTAL trial among haemodialysis patients suggests that intravenous iron may reduce cardiovascular morbidity in this population [47]. This finding may alter the interpretation of the placebo-controlled ESA trials in which participants allocated to placebo received more iron.
However, as noted above, few patients with CKD have HFrEF, whereas structural substrates for diastolic dysfunction are common among patients with CKD. In contrast with HFrEF, no treatment has yet demonstrated convincing benefit (in terms of morbidity and mortality) in patients with HF with moderately reduced EF (HFmrEF: LVEF ≥40–<50%) or HF with preserved EF (HFpEF: LVEF ≥50%). The Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist trial tested spironolactone (15–45 mg daily) versus placebo in 3445 patients with LVEF ≥45% and observed a non-significant 11% (95% CI −4–23) reduction in the primary outcome of cardiovascular death, aborted cardiac arrest or hospitalization for HF [37]. There was again no modification of the treatment effect by baseline kidney function. However, post hoc analyses have suggested that patients recruited from certain geographic regions had significantly worse adherence to treatment (when measured biochemically), which may have made the overall result a ‘false negative’ [48].
NEPRILYSIN INHIBITION
Neprilysin [also known as neutral endopeptidase (NEP)] degrades natriuretic and other vasoactive peptides (including bradykinin, substance P, endothelin and angiotensin II) and therefore neprilysin inhibition (NEPi) enhances the activity of the natriuretic peptide system leading to natriuresis, diuresis, BP reduction and inhibition of RAS and the sympathetic nervous system [49]. Isolated NEPi causes reflex activation of the RAS, so development of NEPi has always been combined with ACEi or ARB. The potential of NEPi in HFrEF was suggested in the Omapatrilat versus Enalapril Randomized Trial of Utility in Reducing Events trial, which compared omapatrilat (a combined ACEi and NEPi) to enalapril in 5770 patients with HF and found a non-significant 6% (95% CI −3–14) reduction in the primary outcome of all-cause mortality or hospitalization for HF [50]. However, development of omapatrilat was stopped when the Omapatrilat Cardiovascular Treatment Assessment Versus Enalapril trial (in 25 302 patients with hypertension) found an excess risk of angioedema compared with enalapril (2.17 versus 0.68%; P < 0.005) [51]. This was thought to be due to excessive bradykinin concentrations (as both ACE and NEP degrade bradykinin) and led to the development of a new class of drug called an angiotensin receptor neprilysin inhibitor (ARNI), which combines NEPi with an ARB.
Sacubitril/valsartan is a first-in-class ARNI that is rapidly metabolized after ingestion to the NEPi pro-drug sacubitril and the ARB valsartan. Sacubitril/valsartan reduces BP more than equivalent doses of valsartan alone [52]. The Prospective Comparison of ARNI with ACEi to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial randomized 8442 participants with HFrEF to treatment with sacubitril/valsartan or enalapril and was terminated earlier than planned based on the recommendation by the Data Monitoring Committee after interim efficacy analysis showed overwhelming evidence of benefit at a median follow-up duration of 27 months. Compared with those assigned to enalapril, participants assigned to sacubitril/valsartan in PARADIGM-HF experienced a 20% (95% CI 13–27) reduction in the primary composite endpoint of cardiovascular death or HF hospitalization. This effect was again similar among participants with and without CKD. Sacubitril/valsartan is now recommended in the European Society of Cardiology guidelines as a replacement for ACEi (or ARB) in patients who have symptomatic HF with a reduced LVEF ≤35% and who remain symptomatic despite maximum-tolerated evidence-based treatment [29, 40].
Sacubitril/valsartan has also been tested among patients with HFpEF. The PARAMOUNT trial compared sacubitril/valsartan with valsartan in 301 patients with change in NT-proBNP as the primary outcome [53]. At 12 weeks, among participants assigned sacubitril/valsartan, NT-proBNP was 23% (95% CI 8–36) lower compared with participants assigned valsartan. The PARAGON-HF trial has recruited 4822 participants with HFpEF to compare sacubitril/valsartan with valsartan and is scheduled to be completed in mid-2019 [54]. The primary outcome is the composite of cardiovascular death and total (first and recurrent) hospitalizations for HF.
In addition to its known benefits in HFrEF (and potential for benefit in HFpEF), NEPi might also have beneficial effects on the kidney. Experiments using 5/6 nephrectomy models suggested that NEPi reduces proteinuria and histological markers of kidney damage more than ACE inhibition alone [55, 56]. In addition, sacubitril/valsartan appeared to slow the deterioration of kidney function in the PARADIGM-HF [57] and PARAMOUNT trials [58]. However, it also modestly increased albuminuria in both trials (although baseline levels were very low in these HF populations) [59].
The UK Heart and Renal Protection (HARP)-III trial was designed to investigate the short- to medium-term effects of sacubitril/valsartan 97/103 mg twice daily versus irbesartan 300 mg once daily on kidney function among patients with established CKD [60]. Patients were eligible for the UK HARP-III trial if either their eGFR was ≥20–<45 mL/min/1.73 m2 or their eGFR was ≥45–<60 mL/min/1.73 m2 and the urine albumin:creatinine ratio was >20 mg/mmol. Other pre-specified outcomes included albuminuria, BP and cardiac biomarkers. A total of 414 participants were randomized and the average eGFR was 35 mL/min/1.73 m2 and median urine albumin:creatinine ratio was 54 mg/mmol. Only 4 and 13% reported HF and coronary heart disease, respectively, at baseline.
The primary outcome of measured GFR at 12 months did not differ between the two groups: the difference in means was −0.1 (standard error 0.7) mL/min/1.73 m2 [61]. Albuminuria was not significantly reduced [9% (95% CI −1–18) among those assigned sacubitril/valsartan] despite an additional 5.4/2.1 (both P < 0.001) mmHg reduction in BP. Despite the apparent lack of an effect on short to medium-term kidney function, allocation to sacubitril/valsartan did reduce both NT-proBNP and troponin I compared with allocation to irbesartan. Study average concentations of NT-proBNP and troponin I were 18% (95% CI 11–25) and 16% (95% CI 8–23) lower, respectively.
Although the effects on kidney function are not encouraging, they do not exclude a benefit on long-term progression of CKD (although any effect would not be large). However, the effects on BP and cardiac biomarkers support the hypothesis that sacubitril/valsartan might reduce the risk of cardiovascular events (and in particular those related to HF) among patients with CKD, irrespective of whether they have known cardiac disease. The neutral effects on tolerability and safety outcomes in the UK HARP-III trial would also support further investigation of this hypothesis.
CONCLUSION
The burden of HF among patients with CKD is considerable and contributes significantly to the excess of cardiovascular morbidity and mortality observed in this growing population. The anatomical substrates of HF develop early in the progression of CKD and strategies to prevent it have not been rigorously tested in the CKD population. Furthermore, trials among patients with known HF have usually excluded patients with moderate or advanced CKD, so the efficacy and—importantly—the safety of these treatments in the CKD population are uncertain. NEPi looks promising as a treatment that could reduce the risk of HF safely among patients with CKD, but clinical outcome trials are required. Newer treatments for HF, such as sodium glucose co-transporter-2 inhibitors, are being tested in large trials in both HF and CKD populations [62–64] and may be the first treatments that have proven efficacy for HF among patients with a wide-spectrum of kidney disease. Nevertheless, further trials of established and future interventions are required that allow doctors to confidently reduce excess risk of cardiovascular disease in CKD.
FUNDING
RH, PK, WH and CB work at the Clinical Trial Service Unit, University of Oxford which received a grant from Novartis Pharma AG to conduct the UK HARP-III trial. CTSU has also received grants from Merck, Pfizer and Boehringer-Ingelheim for research in kidney disease. CTSU has a staff policy of not accepting honoraria or other payments from the pharmaceutical industry, except for the reimbursement of costs to participate in scientific meetings (www.ctsu.ox.ac.uk).
CONFLICT OF INTEREST STATEMENT
The UK HARP-III trial was funded by a grant to the University of Oxford from Novartis Pharma. The trial was conducted, analysed and published independently of the funder. See www.ctsu.ox.ac.uk.
REFERENCES
- 1. Ahmed A, Campbell RC.. Epidemiology of chronic kidney disease in heart failure. Heart Fail Clin 2008; 4: 387–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Segall L, Nistor I, Covic A.. Heart failure in patients with chronic kidney disease: a systematic integrative review. Biomed Res Int 2014; 2014: 937398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mafham M, Emberson J, Landray MJ. et al. Estimated glomerular filtration rate and the risk of major vascular events and all-cause mortality: a meta-analysis. PLoS One 2011; 6: e25920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsushita K, van der Velde M, Astor BC. et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bagshaw SM, Cruz DN, Aspromonte N. et al. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant 2010; 25: 1406–1416 [DOI] [PubMed] [Google Scholar]
- 6. Heywood JT, Fonarow GC, Costanzo MR. et al. High prevalence of renal dysfunction and its impact on outcome in 118,465 patients hospitalized with acute decompensated heart failure: a report from the ADHERE database. J Card Fail 2007; 13: 422–430 [DOI] [PubMed] [Google Scholar]
- 7. Park M, Hsu CY, Li Y. et al. Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 2012; 23: 1725–1734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mark PB, Johnston N, Groenning BA. et al. Redefinition of uremic cardiomyopathy by contrast-enhanced cardiac magnetic resonance imaging. Kidney Int 2006; 69: 1839–1845 [DOI] [PubMed] [Google Scholar]
- 9. Aoki J, Ikari Y, Nakajima H. et al. Clinical and pathologic characteristics of dilated cardiomyopathy in hemodialysis patients. Kidney Int 2005; 67: 333–340 [DOI] [PubMed] [Google Scholar]
- 10. Rakhit DJ, Zhang XH, Leano R. et al. Prognostic role of subclinical left ventricular abnormalities and impact of transplantation in chronic kidney disease. Am Heart J 2007; 153: 656–664 [DOI] [PubMed] [Google Scholar]
- 11. Edwards NC, Hirth A, Ferro CJ. et al. Subclinical abnormalities of left ventricular myocardial deformation in early-stage chronic kidney disease: the precursor of uremic cardiomyopathy? J Am Soc Echocardiogr 2008; 21: 1293–1298 [DOI] [PubMed] [Google Scholar]
- 12. Campbell RC, Sui X, Filippatos G. et al. Association of chronic kidney disease with outcomes in chronic heart failure: a propensity-matched study. Nephrol Dial Transplant 2009; 24: 186–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamaguchi S, Tsuchihashi-Makaya M, Kinugawa S. et al. Chronic kidney disease as an independent risk for long-term adverse outcomes in patients hospitalized with heart failure in Japan. Report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD). Circ J 2009; 73: 1442–1447 [DOI] [PubMed] [Google Scholar]
- 14. Edmunds MR. Hypertension in renal failure In: Swales JD. (ed). Textbook of Hypertension. Oxford: Blackwell Scientific, 1994 [Google Scholar]
- 15. Boudville N, Prasad GV, Knoll G. et al. Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med 2006; 145: 185–196 [DOI] [PubMed] [Google Scholar]
- 16. Lewington S, Clarke R, Qizilbash N. et al. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913 [DOI] [PubMed] [Google Scholar]
- 17. Turnbull F; Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet 2003; 362: 1527–1535 [DOI] [PubMed] [Google Scholar]
- 18. Ettehad D, Emdin CA, Kiran A. et al. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016; 387: 957–967 [DOI] [PubMed] [Google Scholar]
- 19. Levin A, Thompson CR, Ethier J. et al. Left ventricular mass index increase in early renal disease: impact of decline in hemoglobin. Am J Kidney Dis 1999; 34: 125–134 [DOI] [PubMed] [Google Scholar]
- 20. Macdougall IC, Lewis NP, Saunders MJ. et al. Long-term cardiorespiratory effects of amelioration of renal anaemia by erythropoietin. Lancet 1990; 335: 489–493 [DOI] [PubMed] [Google Scholar]
- 21. Palmer SC, Navaneethan SD, Craig JC. et al. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med 2010; 153: 23–33 [DOI] [PubMed] [Google Scholar]
- 22. Chertow GM, Block GA, Correa-Rotter R. et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med 2012; 367: 2482–2494 [DOI] [PubMed] [Google Scholar]
- 23. Wheeler DC, London GM, Parfrey PS. et al. Effects of cinacalcet on atherosclerotic and nonatherosclerotic cardiovascular events in patients receiving hemodialysis: the EValuation Of Cinacalcet HCl Therapy to Lower CardioVascular Events (EVOLVE) trial. J Am Heart Assoc 2014; 3: e001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jono S, McKee MD, Murry CE. et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 2000; 87: E10–E17 [DOI] [PubMed] [Google Scholar]
- 25. Chue CD, Edwards NC, Moody WE. et al. Serum phosphate is associated with left ventricular mass in patients with chronic kidney disease: a cardiac magnetic resonance study. Heart 2012; 98: 219–224 [DOI] [PubMed] [Google Scholar]
- 26. Faul C, Amaral AP, Oskouei B. et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marthi A, Donovan K, Haynes R. et al. Fibroblast growth factor-23 and risks of cardiovascular and noncardiovascular diseases: a meta-analysis. J Am Soc Nephrol 2018; 29: 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang AY, Sanderson JE.. Treatment of heart failure in long-term dialysis patients: a reappraisal. Am J Kidney Dis 2011; 57: 760–772 [DOI] [PubMed] [Google Scholar]
- 29. Ponikowski P, Voors AA, Anker SD. et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200 [DOI] [PubMed] [Google Scholar]
- 30. Yusuf S, Pitt B, Davis CE. et al. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med 1991; 325: 293–302 [DOI] [PubMed] [Google Scholar]
- 31. Bowling CB, Sanders PW, Allman RM. et al. Effects of enalapril in systolic heart failure patients with and without chronic kidney disease: insights from the SOLVD Treatment trial. Int J Cardiol 2013; 167: 151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Castagno D, Jhund PS, McMurray JJ. et al. Improved survival with bisoprolol in patients with heart failure and renal impairment: an analysis of the cardiac insufficiency bisoprolol study II (CIBIS-II) trial. Eur J Heart Fail 2010; 12: 607–616 [DOI] [PubMed] [Google Scholar]
- 33. MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353: 2001–2007 [PubMed] [Google Scholar]
- 34. Ghali JK, Wikstrand J, Van Veldhuisen DJ. et al. The influence of renal function on clinical outcome and response to beta-blockade in systolic heart failure: insights from Metoprolol CR/XL Randomized Intervention Trial in Chronic HF (MERIT-HF). J Card Fail 2009; 15: 310–318 [DOI] [PubMed] [Google Scholar]
- 35. Cohen-Solal A, Kotecha D, van Veldhuisen DJ. et al. Efficacy and safety of nebivolol in elderly heart failure patients with impaired renal function: insights from the SENIORS trial. Eur J Heart Fail 2009; 11: 872–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Flather MD, Shibata MC, Coats AJ. et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur Heart J 2005; 26: 215–225 [DOI] [PubMed] [Google Scholar]
- 37. Pitt B, Pfeffer MA, Assmann SF. et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392 [DOI] [PubMed] [Google Scholar]
- 38. Vardeny O, Wu DH, Desai A. et al. Influence of baseline and worsening renal function on efficacy of spironolactone in patients with severe heart failure: insights from RALES (Randomized Aldactone Evaluation Study). J Am Coll Cardiol 2012; 60: 2082–2089 [DOI] [PubMed] [Google Scholar]
- 39. Zannad F, McMurray JJ, Krum H. et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364: 11–21 [DOI] [PubMed] [Google Scholar]
- 40. McMurray JJ, Packer M, Desai AS. et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004 [DOI] [PubMed] [Google Scholar]
- 41. Goldenberg I, Moss AJ, McNitt S. et al. Relations among renal function, risk of sudden cardiac death, and benefit of the implanted cardiac defibrillator in patients with ischemic left ventricular dysfunction. Am J Cardiol 2006; 98: 485–490 [DOI] [PubMed] [Google Scholar]
- 42. Cleland JG, Daubert JC, Erdmann E. et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352: 1539–1549 [DOI] [PubMed] [Google Scholar]
- 43. Thomsen RW, Nicolaisen SK, Hasvold P. et al. Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes—a Danish population-based cohort study. Nephrol Dial Transplant 2018; 33:1610–1620 [DOI] [PubMed] [Google Scholar]
- 44. Juurlink DN, Mamdani MM, Lee DS. et al. Rates of hyperkalemia after publication of the Randomized Aldactone Evaluation Study. N Engl J Med 2004; 351: 543–551 [DOI] [PubMed] [Google Scholar]
- 45. Pun PH, Al-Khatib SM, Han JY. et al. Implantable cardioverter-defibrillators for primary prevention of sudden cardiac death in CKD: a meta-analysis of patient-level data from 3 randomized trials. Am J Kidney Dis 2014; 64: 32–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Anker SD, Comin Colet J, Filippatos G. et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 2009; 361: 2436–2448 [DOI] [PubMed] [Google Scholar]
- 47. Macdougall IC, White C, Anker SD. et al. Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med 2019; 380: 447–458 [DOI] [PubMed] [Google Scholar]
- 48. de Denus S, O’Meara E, Desai AS. et al. Spironolactone metabolites in TOPCAT—new insights into regional variation. N Engl J Med 2017; 376: 1690–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Judge P, Haynes R, Landray MJ. et al. Neprilysin inhibition in chronic kidney disease. Nephrol Dial Transplant 2015; 30: 738–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Packer M, Califf RM, Konstam MA. et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 2002; 106: 920–926 [DOI] [PubMed] [Google Scholar]
- 51. Kostis JB, Packer M, Black HR. et al. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 2004; 17: 103–111 [DOI] [PubMed] [Google Scholar]
- 52. Ruilope LM, Dukat A, Bohm M. et al. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet 2010; 375: 1255–1266 [DOI] [PubMed] [Google Scholar]
- 53. Solomon SD, Zile M, Pieske B. et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012; 380: 1387–1395 [DOI] [PubMed] [Google Scholar]
- 54. Solomon SD, Rizkala AR, Gong J. et al. Angiotensin receptor neprilysin inhibition in heart failure with preserved ejection fraction: rationale and design of the PARAGON-HF trial. JACC Heart Fail 2017; 5: 471–482 [DOI] [PubMed] [Google Scholar]
- 55. Taal MW, Nenov VD, Wong W. et al. Vasopeptidase inhibition affords greater renoprotection than angiotensin-converting enzyme inhibition alone. J Am Soc Nephrol 2001; 12: 2051–2059 [DOI] [PubMed] [Google Scholar]
- 56. Cao Z, Burrell LM, Tikkanen I. et al. Vasopeptidase inhibition attenuates the progression of renal injury in subtotal nephrectomized rats. Kidney Int 2001; 60: 715–721 [DOI] [PubMed] [Google Scholar]
- 57. Packer M, Claggett B, Lefkowitz MP. et al. Effect of neprilysin inhibition on renal function in patients with type 2 diabetes and chronic heart failure who are receiving target doses of inhibitors of the renin–angiotensin system: a secondary analysis of the PARADIGM-HF trial. Lancet Diabetes Endocrinol 2018; 6: 547–554 [DOI] [PubMed] [Google Scholar]
- 58. Voors AA, Gori M, Liu LC. et al. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur J Heart Fail 2015; 17: 510–517 [DOI] [PubMed] [Google Scholar]
- 59. Ruggenenti P, Remuzzi G.. Combined neprilysin and RAS inhibition for the failing heart: straining the kidney to help the heart? Eur J Heart Fail 2015; 17: 468–471 [DOI] [PubMed] [Google Scholar]
- 60. Judge PK, Haynes R, Herrington WG. et al. Randomized multicentre pilot study of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease: United Kingdom Heart and Renal Protection (HARP)-III—rationale, trial design and baseline data. Nephrol Dial Transplant 2017; 32: 2043–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Haynes R, Judge PK, Staplin N. et al. Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation 2018; 138: 1505–1514 [DOI] [PubMed] [Google Scholar]
- 62. Zannad FF, Filippatos G, Butler J. et al. Design and rationale of the EMPagliflozin outcome trial in patients with chronic heart failure (EMPEROR-reduced). Eur J Heart Fail 2018; 20: 441–441 [Google Scholar]
- 63. Butler J, Packer M, Filippatos G. et al. Design and rationale of the EMPagliflozin outcomE tRial in patients with chrOnic heaRt failure (EMPEROR-preserved). Eur J Heart Fail 2018; 20: 232–232 [Google Scholar]
- 64. Herrington WG, Preiss D, Haynes R. et al. The potential for improving cardio-renal outcomes by sodium–glucose co-transporter-2 inhibition in people with chronic kidney disease: a rationale for the EMPA-KIDNEY study. Clin Kidney J 2018; 11: 749–761 [DOI] [PMC free article] [PubMed] [Google Scholar]