Abstract
Objective
An expert panel convened to reach a consensus on common misconceptions surrounding buprenorphine, a Schedule III partial µ-opioid receptor agonist indicated for chronic pain. The panel also provided clinical recommendations on the appropriate use of buprenorphine and conversion strategies for switching to buprenorphine from a full µ-opioid receptor agonist for chronic pain management.
Methods
The consensus panel met on March 25, 2019, to discuss relevant literature and provide recommendations on interpreting buprenorphine as a partial µ-opioid receptor agonist, prescribing buprenorphine before some Schedule II, III, or IV options, perioperative/trauma management of patients taking buprenorphine, and converting patients from a full µ-opioid receptor agonist to buprenorphine.
Results
The panel recommended that buprenorphine’s classification as a partial µ-opioid receptor agonist not be clinically translated to mean partial analgesic efficacy. The panel also recommended that buprenorphine be considered before some Schedule II, III, or IV opioids in patients with a favorable risk/benefit profile on the basis of metabolic factors, abuse potential, and tolerability and that buprenorphine be continued during the perioperative/trauma period. In addition, switching patients from a full µ-opioid receptor agonist to buprenorphine should be considered with no weaning period at starting doses that are based on the previous opioid dose.
Conclusions
These recommendations provide a framework for clinicians to address most clinical scenarios regarding buprenorphine use. The overall consensus of the panel was that buprenorphine is a unique Schedule III opioid with favorable pharmacologic properties and a safety profile that may be desirable for chronic pain management.
Keywords: Chronic Pain, Buprenorphine, Partial Agonist, Opioid, µ-Opioid Receptor, Schedule III
Introduction
Chronic pain affects ∼30% of Americans, and this percentage continues to increase as the population ages [1]. The prevalence of chronic pain has led to an increase in the prescription of opioid pain relievers; this is an attempt to minimize the detrimental impacts of this condition on patients’ quality of life, as conservative nonpharmacologic nonopioid therapies are often ineffective [1–3]. Nevertheless, for many practitioners, opioids continue to be a mainstay of chronic pain management despite governmental and social scrutiny [4]. Unfortunately, the improper use and diversion of prescription opioids have contributed to a national opioid crisis, including addiction and opioid overdose–related deaths [5].
Opioids vary in both their ability to produce therapeutic effects and their potential for abuse. Differences in abuse potential are recognized by the Drug Enforcement Administration (DEA) Scheduling criteria; by DEA definition, “Schedule III drugs, substances, or chemicals are defined as drugs with a moderate to low potential for physical and psychological dependence. Schedule III drugs’ abuse potential is less than Schedule I and Schedule II drugs and more than Schedule IV” [6]. Most of the opioids indicated for chronic pain management fall into the Schedule II category [7]. However, the optimal treatment of chronic pain should incorporate an individualized and clinically effective treatment approach on the basis of the patient’s risk/benefit profile with the least potential for physical and psychological dependence.
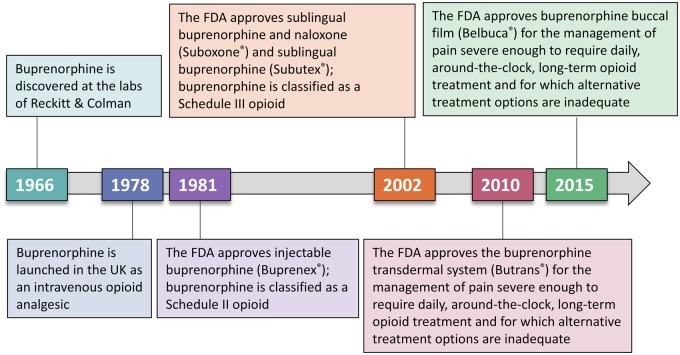
Buprenorphine is a Schedule III opioid analgesic that was approved by the Food and Drug Administration (FDA) in 1981 as an injectable agent (Buprenex) for the treatment of moderate to severe pain (Figure 1) [8]. After the engineering of a sublingual (noninjectable) delivery system, buprenorphine was approved for the treatment of opioid use disorder (OUD) [9]. More recently, novel buprenorphine formulations have been FDA approved for the management of chronic pain [10,11]. Buprenorphine buccal film (Belbuca, BioDelivery Sciences International, Inc, Raleigh, NC, USA) and the buprenorphine transdermal system (Butrans, Purdue Pharma, LP, Stamford, CT, USA) are the formulations indicated for the management of pain severe enough to require daily, around-the-clock, long-term opioid treatment for which alternative treatment options are inadequate [10,11].
Figure 1.
The history of buprenorphine. Buprenorphine was originally developed as an analgesic and was subsequently used for OUD before novel delivery systems allowed for approval in chronic pain management [8,9,12,13]. FDA=Food and Drug Administration; OUD=opioid use disorder.
Unfortunately, buprenorphine’s development history has led to confusion and misunderstandings of its pharmacology and clinical utility. For example, the labeling of buprenorphine as a partial µ-opioid receptor agonist has been misinterpreted as implying “partial” or low efficacy [14] when compared with a full µ-opioid receptor agonist; this is not the case. In addition, although the buprenorphine dose for chronic pain is lower than that for OUD, this does not imply that the analgesic dose is somehow less effective. Buprenorphine is often considered for its indication in patients with OUD; however, it was originally formulated as an analgesic, as data clearly supported its efficacy in providing pain relief [12]. There may also be a lack of awareness regarding conversion strategies to minimize or avoid precipitated withdrawal (e.g., withdrawal caused by opioid switching) when transitioning from a full µ-opioid receptor agonist to buprenorphine [15,16]. Although required to treat OUD in the outpatient setting, clinicians may be under the false impression that a DEA practitioner “X” waiver is required to prescribe or dispense buprenorphine for chronic pain [17]. Overall, it is evident that common misconceptions surround buprenorphine as a treatment option for chronic pain.
The purpose of this publication is to present the opinions of an expert consensus panel regarding these misconceptions and provide clinical recommendations on the appropriate use of buprenorphine for chronic pain, including conversion strategies for switching from a full µ-opioid receptor agonist to buprenorphine (a partial µ-opioid receptor agonist). These recommendations may be a valuable resource for health care providers seeking a potentially safer yet effective analgesic when compared with Schedule II opioids.
Methods
Literature Review
A series of PubMed searches were performed to identify literature relevant to the topics and questions addressed at the Buprenorphine Consensus Panel Meeting held March 25, 2019, in Washington, DC. For interpretation of buprenorphine as a partial µ-opioid receptor agonist, a PubMed search was performed on March 11, 2019, using the following search terms: “partial agonist” AND buprenorphine AND interpret. To research the appropriate use of buprenorphine in chronic pain, a PubMed search was performed on March 11, 2019, with the following search terms: buprenorphine AND “chronic pain” AND “appropriate use.” The clinical use of buprenorphine in perioperative or trauma environments was investigated with a search of PubMed on March 21, 2019, using the following search terms: buprenorphine AND (perioperative OR trauma) AND (continue OR discontinue). For literature detailing the conversion to buprenorphine from another opioid in patients with chronic pain, a PubMed search was performed on March 11, 2019, using the following search terms: buprenorphine AND (conversion OR switching) AND “chronic pain” NOT “opioid use disorder.” Additional relevant references were identified and included at the authors’ discretion on the basis of their experience and manual searches, particularly when literature searches yielded few or no results.
Consensus Development Conference Methodology
The National Institutes of Health has developed the methodology for a consensus conference, whereby a small group of experts is brought together to discuss evidence-based research and reach consensus on an issue [18]. Although the panel is encouraged to reach a consensus, minor or alternative views are also accepted [18,19].
Results
PubMed Literature Searches
The PubMed search for articles discussing the interpretation of buprenorphine as a partial agonist and the appropriate use of buprenorphine for chronic pain did not return any results. The literature search for the clinical use of buprenorphine in a perioperative or trauma environment returned five articles; three articles were considered relevant, whereas one was excluded for being an animal study and another for lack of focus on perioperative or trauma pain (Supplementary Table 1). The search for publications on the conversion from Schedule II opioids to buprenorphine in patients with chronic pain returned 17 articles from PubMed; seven articles were deemed appropriate, whereas five were excluded for not being a primary study and an additional five were excluded for irrelevancy for not including information on opioid-switching to buprenorphine or specifying that all patients were opioid-experienced (Supplementary Table 2).
After critical evaluation of all available information on each topic listed previously, the consensus panel discussed relevant issues or misconceptions and potential solutions and clarification. On the basis of the current literature, the panel’s expertise, and a group discussion, members then drafted consensus recommendations or statements for each topic.
Discussion
1. Interpreting Buprenorphine as a Partial Agonist at the µ-Opioid Receptor
Discussion
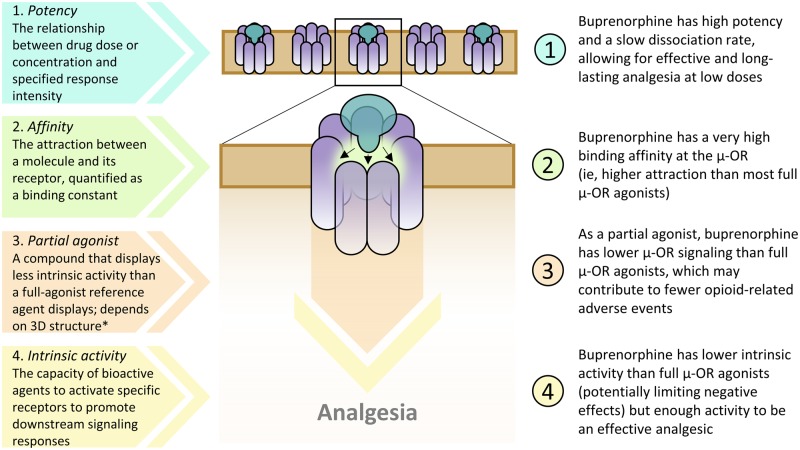
The in vitro classification of buprenorphine as a “partial agonist” at the µ-opioid receptor may lead to the misconception that it is less effective as an analgesic than a reference opioid that is considered a full µ-opioid receptor agonist; however, the definition of partial agonist is context dependent and relative to a reference agent (Figure 2) [14]. For example, some in vitro studies have also classified morphine as a partial µ-opioid receptor agonist [20]. In addition, antinociceptive dose-response curves for buprenorphine [21] and morphine [22–24] have been shown to vary by pain intensity, which highlights that the partial µ-opioid receptor agonism can vary depending on experimental design.
Figure 2.
Receptor/ligand definitions and applications to buprenorphine at the μ-opioid receptor. *Definition of a partial agonist: a compound with an intermediate intrinsic activity that at full receptor saturation produces less than the maximal effect obtainable with full agonists in some specified set of in vitro or clinical circumstances [25]. Buprenorphine is a potent Schedule III opioid with high binding affinity at the μ-opioid receptor that behaves as a partial agonist on the basis of in vitro studies [7,14,26]. Although buprenorphine has less total intrinsic activity (capacity to activate a receptor to induce multiple signaling pathways) than full μ-opioid receptor agonists, it still effectively stimulates the analgesic signaling pathway from the μ-opioid receptor [7,14,25,27,28]. 3D=three-dimensional; OR=opioid receptor.
In some clinical settings, buprenorphine had similar or greater analgesic efficacy and antihyperalgesic effects as full µ-opioid receptor agonists [14,29–32]. Intravenous (IV) buprenorphine was as efficacious as or more efficacious than IV morphine in producing analgesia across various surgical models [14]. The effect size, or correlation between two variables, for change in pain intensity observed in clinical trials of transdermal and buccal buprenorphine vs placebo overlapped with those of several Schedule II opioids across trials of chronic noncancer pain, suggesting similar efficacy [29]. In addition, sublingual buprenorphine was as effective as IV morphine in managing acute renal colic pain [30]. Therefore, the characterization of buprenorphine as a partial µ-opioid receptor agonist does not clinically equate to partial analgesic efficacy.
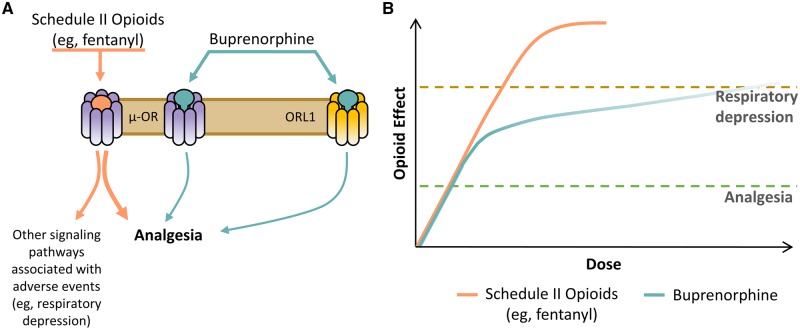
Although the analgesic effects of buprenorphine are well known to be mediated by the µ-opioid receptor, multimechanistic effects at other opioid receptors may also contribute to efficacy and tolerability (Figure 3A) [33]. Buprenorphine is a full agonist at the more recently identified opioid receptor-like 1 (ORL1), which may contribute to analgesia [34], and it is an antagonist at the δ- and κ-opioid receptors, which lessen constipation, dysphoria, and abuse potential and help reduce mental depression [7,35,36]. In fact, buprenorphine has been shown to effectively reduce depressive symptoms and suicidal ideation in patients unresponsive to conventional antidepressant medications [37]. However, these and other complex receptor interactions require further study.
Figure 3.
Efficacy and tolerability of buprenorphine compared with those of other opioids used for chronic pain. (A) Potential mechanism of action for buprenorphine and (B) conceptual representation of possible effects compared with those of Schedule II opioids such as, but not limited to, fentanyl [33,38,39]. OR=opioid receptor; ORL1=opioid receptor-like 1.
In response to µ-opioid receptor binding, buprenorphine has been shown to have low intrinsic signaling activity that is sufficient to reach the analgesic threshold, while exhibiting a relative ceiling effect for respiratory depression (Figure 3B) [40–42]. At therapeutic doses, the risk of respiratory depression appears to be lower than that of analgesic doses of full µ-opioid receptor agonists; nonetheless, there is still a risk of respiratory depression with buprenorphine, which can vary depending on the route of administration, use of concurrent medications, and presence of underlying comorbidity/disease [43].
Consensus Statement
Buprenorphine has higher binding affinity [44] but lower intrinsic activity at the µ-opioid receptor than full µ-opioid receptor agonists [14], with a unique mechanism of action at other receptors (e.g., δ- and κ-opioid receptors and ORL1) that may contribute to analgesia and other favorable clinical properties [7,45–47].
2. When an Opioid Is Considered for a Patient with Chronic Pain, Should Buprenorphine Be Prescribed before a Schedule IV or Schedule II Option?
Discussion
Depending on the patient, buprenorphine may be more favorable for the management of chronic pain than a Schedule IV or II opioid. Compared with Schedule II prescriptions, such as fentanyl and oxycodone, Schedule III medications reduce some of the access barriers faced by patients including, but not limited to, option for refills and the clinician’s ability to call in prescriptions to the pharmacy [48]. In patients whose phenotypic profile is known, buprenorphine or other opioids that exclusively undergo Phase II metabolism may be more appropriate for those who are phenotypic outliers (ultrarapid or poor metabolizers) in producing cytochrome P450 (CYP450) enzymes that induce or inhibit the hepatic isoenzyme CYP2D6 compared with codeine (available as a Schedule III medication when combined with a nonopioid analgesic and/or atropine alkaloids) and certain Schedule IV opioids, such as tramadol [49,50]. With tramadol, ultrarapid CYP2D6 metabolism may increase the risks of toxicity, including respiratory depression, whereas a poor CYP2D6 metabolizer may have limited analgesic benefit upon treatment with tramadol or codeine [49–51]. In addition, an ultrarapid CYP2D6 metabolizer quickly metabolizes codeine into morphine, thereby increasing the risk for respiratory depression [51]. All codeine products now require a black box warning, as life-threatening respiratory depression and death have occurred in children who received codeine, and many of the children had evidence of being ultrarapid metabolizers because of a CYP2D6 polymorphism [51].
When used as directed, buprenorphine appears to have a relatively favorable therapeutic index (safety profile) and a slower onset of tolerance than full µ-opioid receptor agonists on the basis of its pharmacological profile [52–55]; however, patient expectations regarding nausea, which is commonly observed during titration with buprenorphine products in opioid-naïve patients, should be clearly set.
Buprenorphine appears to have a ceiling effect on respiratory depression [40–42], and the Pain Management Best Practices Inter-Agency Task Force recently noted that buprenorphine is expected to have reduced risk for respiratory depression and may be better tolerated than full µ-opioid receptor agonists according to currently available data [56]. As a caveat, respiratory depression is still a concern, especially when buprenorphine is used in combination with nonopioid sedatives/anticonvulsants such as benzodiazepines and gabapentin [57,58], carisoprodol and other muscle relaxants [59], amitriptyline [60], and Z-drugs (nonbenzodiazepine drugs with effects similar to those of benzodiazepines) [61]. Although buprenorphine is generally well tolerated, any relevant contraindications and other safety concerns should be considered when prescribing the medication for chronic pain [10,11]. Combining opioids with other central nervous system suppressants is generally not recommended but may be considered after conducting a thoughtful risk/benefit analysis [62,63].
On the basis of the information above, buprenorphine may also be more suitable than Schedule II opioids in certain patient populations. Patients with chronic pain who may benefit from buprenorphine include those with an increased risk of life-threatening opioid-related adverse events (e.g., high body mass index/obstructive sleep apnea, comorbid psychiatric diagnosis, pulmonary disease, concomitant use of drugs known to increase risk [benzodiazepines, gabapentin, pregabalin, muscle relaxants, alcohol], taking a high morphine milligram equivalent [MME]) [40,41,64–67]. In addition, those with chronic pain and an increased risk of life-threatening opioid-related aberrant behavior (e.g., indications of OUD or substance use disorder, heightened risk of overdose, elevated Opioid Risk Tool [ORT] or other screening tool score [68]) may benefit from buprenorphine [69].
Consensus Recommendation
In patients with chronic pain who have been determined via risk/benefit analysis to benefit from opioid treatment, consider prescribing buprenorphine before Schedule II opioids and perhaps before some Schedule IV opioids, such as tramadol, on the basis of the patient’s underlying conditions.
3. How Should Patients Receiving Buprenorphine Be Managed during the Perioperative/Trauma Period?
Discussion
There is a misconception that buprenorphine will prevent the binding of and compromise the efficacy of concomitantly administered full µ-opioid receptor agonists in the perioperative period. Buprenorphine has been reported to occupy only a subset of µ-opioid receptors, even at high doses [7,70]. Receptor-binding studies utilizing positron emission tomography and radiolabeled (11C) carfentanil in buprenorphine-treated heroin-addicted persons confirmed the availability of µ-opioid receptors in patients maintained on various doses of daily buprenorphine [70]:
With no buprenorphine, 100% of µ-opioid receptors are available.
At 2 mg of buprenorphine (mean 24-hour area under the curve [AUC] of 6.5 ng/mL* h), ∼59% of µ-opioid receptors are available.
At 16 mg of buprenorphine (mean 24-hour AUC of 48.6 ng/mL * h), ∼20% of µ-opioid receptors are available.
At 32 mg of buprenorphine (mean 24-hour AUC of 96.0 ng/mL * h), ∼16% of µ-opioid receptors are available.
For comparison, a single 300-µg dose of buccal buprenorphine has shown a mean AUC0-inf of ∼2.3 ng/mL * h [10], and a single application of modified-release transdermal buprenorphine 10 µg/h has shown a mean AUC0-inf of 27.0 ng/mL * h [11]. Because of variations in methodology, these data cannot be used to compare differences in potency between buprenorphine products. However, these results suggest that adding a full µ-opioid receptor agonist to buprenorphine therapy in the perioperative/trauma period may improve pain relief on the basis of receptor availability. The addition of a full µ-opioid receptor agonist with high binding affinity, such as fentanyl, hydromorphone, or sufentanil, may be preferable in this situation [71,72].
The half-lives of buccal and transdermal buprenorphine are 27 ± 11.2 hours and ∼26 hours, respectively [10,11]. This allows µ-opioid receptors to become available for full agonists to bind and activate. Discontinuation of buprenorphine in a patient receiving stable therapy should be avoided, as discontinuing therapy may confer medical risk, prolong the hospital visit, and increase patient burden [71,73]. Patients who continue their usual buprenorphine dose perioperatively may also benefit by requiring less patient-controlled analgesia [71].
It is important to note that, in general, the use of full µ-opioid receptor agonists may increase the risk of respiratory depression and death, and a superadditive effect may be possible with co-use of buprenorphine and fentanyl, oxycodone, or morphine [7]. In addition, the recommendations provided here do not necessarily apply to immediate-release, high-dose buprenorphine formulations that are not currently approved for chronic pain (e.g., sublingual tablets), as high-dose strengths may result in higher µ-opioid receptor occupancy, which could affect the efficacy of concomitant use with full µ-opioid receptor agonists.
Upon discharge, patients may continue to require a full µ-opioid receptor agonist until their moderate to severe pain subsides, at which point the full µ-opioid receptor agonist can be weaned. A gradual return to baseline analgesic levels through dose titration may help to avoid withdrawal symptoms.
Consensus Recommendation
In patients receiving transdermal or buccal buprenorphine, continue the same buprenorphine dose and add a full µ-opioid receptor agonist, preferably a full µ-opioid receptor agonist with high receptor-binding affinity, for pain relief in the postoperative/trauma period. Treatment should be individualized and should include rescue medication to ensure efficacy. As sublingual buprenorphine is not approved for the treatment of pain, no recommendations are offered here regarding this formulation in the perioperative period.
4. Why Convert from a Full µ-Opioid Receptor Agonist to Buprenorphine?
Discussion
Conversion from another opioid to buprenorphine should occur for the same reasons that any opioid rotation is performed: analgesia or anticipated improved risk/benefit vs current therapy for a given patient. For example, potential safety benefits, such as decreased risk for respiratory depression and use in specific patient populations (e.g., those with OUD), may be provided by switching to buprenorphine [74,75].
Patients with comorbid chronic pain and OUD have reported satisfaction with buprenorphine treatment [75]. However, the dosages and conversion factors recommended for chronic pain are not necessarily the same as those recommended for addiction [76]. Prescription barriers also vary between the two conditions, as the use of buprenorphine for OUD requires a waiver from the DEA, whereas use for chronic pain does not [17].
Consensus Recommendation
If any of the following circumstances are applicable to full µ-opioid receptor agonist treatment, consider switching to buprenorphine:
Lack of efficacy (including tolerance or hyperalgesia)
Previously mentioned reasons to initiate buprenorphine before a Schedule II opioid (e.g., risk of adverse events and OUD)
Concern from health care providers regarding prescription of a Schedule II opioid due to risk of addiction, misuse, and/or overdose death
The limited ability to utilize oral formulations in patients with altered gastrointestinal motility/function (e.g., after bariatric surgery)
A patient is receiving immediate-release treatment and would benefit from a longer-acting analgesic with a relatively favorable safety profile and Schedule III classification
If a patient with OUD is no longer a candidate for any opioid, transitioning to buprenorphine for chronic pain is not recommended.
5. How Should Patients Be Converted from a Full µ-Opioid Receptor Agonist to Buprenorphine?
Discussion
When patients are switched from a full µ-opioid receptor agonist to buprenorphine, maintaining adequate analgesia and avoiding withdrawal symptoms should be the main priorities. The prescribing information for buprenorphine buccal film and the buprenorphine transdermal system suggests that opioid-experienced patients be tapered down from their current daily opioid dose to ≤30 mg of oral MME before initiating therapy [10,11]. This conversion strategy may be impractical and may precipitate withdrawal. The weaning of a full µ-opioid receptor agonist before buprenorphine conversion may help with overall dose reduction, but it is not considered necessary to avoid withdrawal. In addition, precipitated withdrawal is not a clinically significant issue with buprenorphine for patients receiving low doses of full µ-opioid receptor agonists [16].
Consensus Recommendation
Clinical best judgment should be used to individualize any conversion, but the following can be considered while noting that some downward titration may be necessary for patients on high opioid doses:
For patients taking doses below the following amounts (∼≤90 MME):
Fentanyl transdermal: ≤25 µg/h
Oxycodone: ≤60 mg/d
Hydrocodone or morphine: ≤90 mg/d
Hydromorphone: ≤16 mg/d
Oxymorphone: ≤45 mg/d
Tapentadol: Any dose
Discontinue after the last nighttime dose.
Consider initiating an adrenergic α2 agonist (e.g., clonidine, lofexidine) or an immediate-release opioid (e.g., current opioid) to reduce the risk of withdrawal.
Initiate buprenorphine the following morning per the prescribing information, as either 10-µg/h transdermal buprenorphine or 150-µg buccal buprenorphine twice daily. Titrate buprenorphine as needed for pain per recommendations in the prescribing information.
In patients transitioning to buprenorphine from higher doses of opioids (∼>90 MME):
Fentanyl transdermal: >25 µg/h
Oxycodone: >60 mg/d
Hydrocodone or morphine: >90 mg/d
Hydromorphone: >16 mg/d
Oxymorphone: >45 mg/d
Discontinue after the last nighttime dose.
Consider initiating an adrenergic α2 agonist (e.g., clonidine, lofexidine) or an immediate-release opioid (e.g., current opioid) to reduce the risk of withdrawal.
Initiate buprenorphine the following morning as either 20-µg/h transdermal buprenorphine once daily or 300-µg buccal buprenorphine twice daily and follow the recommendations in the prescribing information for upward titration as needed. Note that 20 µg/h is the highest dose of transdermal buprenorphine currently available in the United States. If these doses are ineffective, consider higher doses of the buccal formulation on the basis of risk/benefit analysis.
Short-acting opioids have been suggested to prevent withdrawal during the switch to buprenorphine [77].
Conclusions
An expert panel with preclinical and clinical experience in the use of buprenorphine for chronic pain convened to identify and clarify misinterpretations surrounding buprenorphine and provide clinical recommendations. The overall consensus of this panel was that, in some patients, buprenorphine is an effective and well-tolerated tool in the management of chronic pain; thus, recommendations are provided regarding the definition of a partial agonist, the appropriate use of Schedule III vs Schedule II opioids, and conversion strategies for transitioning from a full µ-opioid receptor agonist to buprenorphine.
The panel identified a prominent misconception regarding the efficacy of buprenorphine on the basis of its in vitro classification as a partial µ-opioid receptor agonist. The panel agreed that for buprenorphine the term partial agonist should not be translated as “partial efficacy.” However, further study of the multimechanistic effects of buprenorphine on opioid receptors and how these effects relate to the unique clinical properties of the molecule is warranted.
Buprenorphine may have safety advantages compared with full µ-opioid receptor agonists. Thus, it is recommended that buprenorphine be considered before Schedule II and perhaps Schedule IV opioids in patients with chronic pain and a favorable risk/benefit ratio for the drug on the basis of abuse potential, tolerability, and other factors. The risk/benefit ratio should also be assessed when considering converting a patient from a full µ-opioid receptor agonist to buprenorphine.
In most patients receiving transdermal or buccal buprenorphine who are undergoing a surgical procedure or who have sustained traumatic injuries, buprenorphine should be continued in the perioperative/trauma period. In these scenarios, a short-acting full µ-opioid receptor agonist with high binding affinity, such as fentanyl, hydromorphone, or sufentanil, or IV buprenorphine can be used in the short term in addition to the previously established buprenorphine regimen. Additional research is recommended on the use of buprenorphine in acute pain management in the postoperative period, in patients with a dual diagnosis of pain and depression, and in combination with full µ-opioid receptor agonists to potentially mitigate tolerance and hyperalgesia.
When switching patients from a full µ-opioid receptor agonist to buprenorphine, converting directly to buprenorphine without a weaning period was advised, with starting doses based on the dose of the previously administered opioid. If withdrawal is a concern, an adrenergic α2 agonist or rescue medication can be used proactively or reactively.
Additional studies are also suggested on the likelihood of precipitated withdrawal during conversion from a full µ-opioid receptor agonist to buprenorphine, on respiratory depression with buprenorphine vs full µ-opioid receptor agonists (with and without benzodiazepines), and on the abuse potential of buprenorphine. The panel also encourages further research on the effectiveness of reversing buprenorphine effects with naloxone or other opioid antagonists.
Overall, the consensus was that, when used appropriately, buprenorphine is a unique opioid with favorable properties for the management of chronic pain. Additional topics, including the off-label use of sublingual buprenorphine in chronic pain and more detailed treatment recommendations for patients with comorbid OUD and chronic pain, were suggested for discussion at future meetings.
Authors’ Contributions
All authors contributed to the drafting and critical revision of the manuscript for scientific accuracy and intellectual content and approved the final manuscript for publication.
Supplementary Material
Acknowledgments
Professional writing and editorial support were provided by MedLogix Communications, LLC, Itasca, Illinois, under the direction of the authors.
Funding sources: The Consensus Meeting and manuscript development were funded by BioDelivery Sciences International, Inc., without any influence over the content.
Disclosure and conflicts of interest: LW has received consultation, advisory board, and travel fees from Charleston Labs, Depomed, Egalet, Insys Therapeutics, Mallinckrodt Pharmaceuticals, Pfizer, Teva, and Trevena; consultation and travel fees from Alcobra, Bonti, Daiichi Sankyo, Elysium, Indivior, KemPharm, Pain Therapeutics, Pernix, and Shionogi; advisory board and travel fees from BioDelivery Sciences International, Inc., Ensysce Biosciences, and Inspirion Pharmaceuticals; travel fees from Cara Therapeutics; and consultation fees from Jefferies, Merck, Trevi, Vallon, and Vector Pharma. JG has served as a consultant for Averitas, Mallinckrodt, Nektar, and Quest Diagnostics; as an advisory board member for AcelRx Pharmaceuticals and GlaxoSmithKline; and as a consultant and part of a speakers’ bureau for BioDelivery Sciences International, Inc., DSI, Salix Pharmaceuticals, and Scilex Pharmaceuticals. RBR was previously an employee of Johnson & Johnson and has received research support or honoraria from multiple pharmaceutical companies involved in analgesics research and development, but he receives no remuneration based on sales of any product. He is a cofounder of CaRafe Drug Innovation and is the CSO of Neumentum. RR has received research funding from BioDelivery Sciences International, Inc., Boston Scientific Corporation, Mainstay Delivery, and Shionogi and is a consultant/speaker for AstraZeneca, Boston Scientific Corporation, Daiichi Sankyo, and Pfizer. TM-S was a part of speakers’ bureau for Scilex, Amgen, and Eli Lilly. JA has received speaker fees from AstraZeneca/Daiichi Sankyo, Collegium Pharmaceuticals, Depomed, Egalet, Pernix, and Scilex and consulting fees from BioDelivery Sciences International, Inc. JK has no relevant disclosures. JF has received advisory board fees from AcelRx Pharmaceuticals, GlaxoSmithKline, Quest Diagnostics, Salix Pharmaceuticals, and Scilex Pharmaceuticals; speaker fees from Acutis Diagnostics, Inc.; consulting fees for BioDelivery Sciences International, Inc., and Firstox Laboratories; advisory board and speakers’ bureau fees from Daiichi Sankyo; and speakers’ bureau fees from Astra-Zeneca.
References
- 1. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67(36):1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dillie KS, Fleming MF, Mundt MP, French MT.. Quality of life associated with daily opioid therapy in a primary care chronic pain sample. J Am Board Fam Med 2008;21(2):108–17. [DOI] [PubMed] [Google Scholar]
- 3. Duenas M, Ojeda B, Salazar A, Mico JA, Failde I.. A review of chronic pain impact on patients, their social environment and the health care system. J Pain Res 2016;9:457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenblum A, Marsch LA, Joseph H, Portenoy RK.. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp Clin Psychopharmacol 2008;16(5):405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Volkow ND, McLellan AT.. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N Engl J Med 2016;374(13):1253–63. [DOI] [PubMed] [Google Scholar]
- 6. United States Drug Enforcement Administration. Drug scheduling. Available at: https://www.dea.gov/drug-scheduling (accessed February 1, 2019).
- 7. Davis MP, Pasternak G, Behm B.. Treating chronic pain: An overview of clinical studies centered on the buprenorphine option. Drugs 2018;78(12):1211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drug Enforcement Administration. Schedules of controlled substances: Rescheduling of buprenorphine from Schedule V to Schedule III. 2002. Available at: https://www.deadiversion.usdoj.gov/fed_regs/rules/2002/fr1007.htm (accessed February 25, 2019).
- 9. Drug Enforcement Administration. Buprenorphine. 2013. Available at: https://www.deadiversion.usdoj.gov/drug_chem_info/buprenorphine.pdf (accessed February 25, 2019).
- 10. Belbuca [Prescribing Information]. Raleigh, NC: BioDelivery Sciences International, Inc; 2018. [Google Scholar]
- 11. Butrans [Prescribing Information]. Stamford, CT: Purdue Pharma L.P; 2018. [Google Scholar]
- 12. Campbell ND, Lovell AM.. The history of the development of buprenorphine as an addiction therapeutic. Ann N Y Acad Sci 2012;1248(1):124–39. [DOI] [PubMed] [Google Scholar]
- 13. Department of Health and Human Services. NDA approval. 2015. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/207932Orig1s000ltr.pdf (accessed April 17, 2019).
- 14. Raffa RB, Haidery M, Huang HM, et al. The clinical analgesic efficacy of buprenorphine. J Clin Pharm Ther 2014;39(6):577–83. [DOI] [PubMed] [Google Scholar]
- 15. Rosado J, Walsh SL, Bigelow GE, Strain EC.. Sublingual buprenorphine/naloxone precipitated withdrawal in subjects maintained on 100mg of daily methadone. Drug Alcohol Depend 2007;90(2–3):261–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glasper A, Reed LJ, Wet CJ, Gossop M, Bearn J.. Induction of patients with moderately severe methadone dependence onto buprenorphine. Addict Biol 2005;10(2):149–55. [DOI] [PubMed] [Google Scholar]
- 17. SAMHSA. Special circumstances for providing buprenorphine. Available at: https://www.samhsa.gov/medication-assisted-treatment/legislation-regulations-guidelines/special (accessed May 13, 2019).
- 18. Kea B, Sun BC.. Consensus development for healthcare professionals. Intern Emerg Med 2015;10(3):373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy MK, Black NA, Lamping DL.. Consensus development methods, and their use in clinical guideline development. Health Technol Assessment 1998;2(3):i–iv; 1–88. [PubMed] [Google Scholar]
- 20. Traynor J. Mu-opioid receptors and regulators of G protein signaling (RGS) proteins: From a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend 2012;121(3):173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raffa RB, Ding Z.. Examination of the preclinical antinociceptive efficacy of buprenorphine and its designation as full- or partial-agonist. Acute Pain 2007;9(3):145–52. [Google Scholar]
- 22. Qi JN, Mosberg HI, Porreca F.. Modulation of the potency and efficacy of mu-mediated antinociception by delta agonists in the mouse. J Pharmacol Exp Ther 1990;254(2):683–9. [PubMed] [Google Scholar]
- 23. Dirig DM, Yaksh TL.. Differential right shifts in the dose-response curve for intrathecal morphine and sufentanil as a function of stimulus intensity. Pain 1995;62(3):321–8. [DOI] [PubMed] [Google Scholar]
- 24. McCormack K, Prather P, Chapleo C.. Some new insights into the effects of opioids in phasic and tonic nociceptive tests. Pain 1998;78(2):79–98. [DOI] [PubMed] [Google Scholar]
- 25. Ariens EJ. Intrinsic activity: Partial agonists and partial antagonists. J Cardiovasc Pharmacol 1983;5(Suppl 1):S8–15. [PubMed] [Google Scholar]
- 26. Gabrielsson J, Peletier LA, Hjorth S.. In vivo potency revisited - keep the target in sight. Pharmacol Ther 2018;184:177–88. [DOI] [PubMed] [Google Scholar]
- 27. McPherson J, Rivero G, Baptist M, et al. Mu-opioid receptors: Correlation of agonist efficacy for signalling with ability to activate internalization. Mol Pharmacol 2010;78(4):756–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buchwald P. A receptor model with binding affinity, activation efficacy, and signal amplification parameters for complex fractional response versus occupancy data. Front Pharmacol 2019;10:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meske DS, Lawal OD, Elder H, Langberg V, Paillard F, Katz N.. Efficacy of opioids versus placebo in chronic pain: A systematic review and meta-analysis of enriched enrollment randomized withdrawal trials. J Pain Res 2018;11:923–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Payandemehr P, Jalili M, Mostafazadeh Davani B, Dehpour AR.. Sublingual buprenorphine for acute renal colic pain management: A double-blind, randomized controlled trial. Int J Emerg Med 2014;7(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Koppert W, Ihmsen H, Korber N, et al. Different profiles of buprenorphine-induced analgesia and antihyperalgesia in a human pain model. Pain 2005;118(1):15–22. [DOI] [PubMed] [Google Scholar]
- 32. Silverman SM. Opioid induced hyperalgesia: Clinical implications for the pain practitioner. Pain Physician 2009;12(3):679–84. [PubMed] [Google Scholar]
- 33. Khanna IK, Pillarisetti S.. Buprenorphine - an attractive opioid with underutilized potential in treatment of chronic pain. J Pain Res 2015;8:859–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamamoto T, Shono K, Tanabe S.. Buprenorphine activates mu and opioid receptor like-1 receptors simultaneously, but the analgesic effect is mainly mediated by mu receptor activation in the rat formalin test. J Pharmacol Exp Ther 2006;318(1):206–13. [DOI] [PubMed] [Google Scholar]
- 35. Stein C, Machelska H.. Modulation of peripheral sensory neurons by the immune system: Implications for pain therapy. Pharmacol Rev 2011;63(4):860–81. [DOI] [PubMed] [Google Scholar]
- 36. Ahmadi J, Jahromi MS, Ehsaei Z.. The effectiveness of different singly administered high doses of buprenorphine in reducing suicidal ideation in acutely depressed people with co-morbid opiate dependence: A randomized, double-blind, clinical trial. Trials 2018;19(1):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Serafini G, Adavastro G, Canepa G, et al. The efficacy of buprenorphine in major depression, treatment-resistant depression and suicidal behavior: A systematic review. Int J Mol Sci 2018;19(8):2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huestis MA. Controlled drug administration studies of high-dose buprenorphine in humans In: Kintz P, Marquet P, eds. Buprenorphine Therapy of Opiate Addiction. Totowa, NJ: Humana Press; 2002:13–27. [Google Scholar]
- 39. Zaveri N, Polgar WE, Olsen CM, et al. Characterization of opiates, neuroleptics, and synthetic analogs at ORL1 and opioid receptors. Eur J Pharmacol 2001;428(1):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dahan A, Yassen A, Bijl H, et al. Comparison of the respiratory effects of intravenous buprenorphine and fentanyl in humans and rats. Br J Anaesth 2005;94(6):825–34. [DOI] [PubMed] [Google Scholar]
- 41. Dahan A, Yassen A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth 2006;96(5):627–32. [DOI] [PubMed] [Google Scholar]
- 42. Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE.. Clinical pharmacology of buprenorphine: Ceiling effects at high doses. Clin Pharmacol Ther 1994;55(5):569–80. [DOI] [PubMed] [Google Scholar]
- 43. van Dorp E, Yassen A, Sarton E, et al. Naloxone reversal of buprenorphine-induced respiratory depression. Anesthesiology 2006;105(1):51–7. [DOI] [PubMed] [Google Scholar]
- 44. Volpe DA, McMahon Tobin GA, Mellon RD, et al. Uniform assessment and ranking of opioid mu receptor binding constants for selected opioid drugs. Regul Toxicol Pharmacol 2011;59(3):385–90. [DOI] [PubMed] [Google Scholar]
- 45. Ding Z, Raffa RB.. Identification of an additional supraspinal component to the analgesic mechanism of action of buprenorphine. Br J Pharmacol 2009;157(5):831–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bliesener N, Albrecht S, Schwager A, Weckbecker K, Lichtermann D, Klingmüller D.. Plasma testosterone and sexual function in men receiving buprenorphine maintenance for opioid dependence. J Clin Endocrinol Metab 2005;90(1):203–6. [DOI] [PubMed] [Google Scholar]
- 47. Coplan PM, Sessler NE, Harikrishnan V, Singh R, Perkel C.. Comparison of abuse, suspected suicidal intent, and fatalities related to the 7-day buprenorphine transdermal patch versus other opioid analgesics in the National Poison Data System. Postgrad Med 2017;129(1):55–61. [DOI] [PubMed] [Google Scholar]
- 48. United States Department of Justice Diversion Control Division. Prescriptions. 2007. Available at: https://www.deadiversion.usdoj.gov/faq/prescriptions.htm (accessed March 1, 2019).
- 49. Pattinson KT. Opioids and the control of respiration. Br J Anaesth 2008;100(6):747–58. [DOI] [PubMed] [Google Scholar]
- 50. Zebula J, Searle S, Webster L, et al. Desmetramadol has the safety and analgesic profile of tramadol without its metabolic liabilities: Consecutive randomized, double-blind, placebo- and active comparator-controlled trials. J Pain 2019;20(10):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dean L. Codeine therapy and CYP2D6 genotype In: Pratt V, McLeod H, Rubinstein W, Dean L, Kattman B, Malheiro A, eds. Medical Genetics Summaries. Bethesda, MD: National Center for Biotechnology Information (US); 2012:1–13. [Google Scholar]
- 52. Cowan A, Lewis JW, Macfarlane IR.. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent. Br J Pharmacol 1977;60(4):537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aiyer R, Gulati A, Gungor S, Bhatia A, Mehta N.. Treatment of chronic pain with various buprenorphine formulations: A systematic review of clinical studies. Anesth Analg 2018;127(2):529–38. [DOI] [PubMed] [Google Scholar]
- 54. Gordon A, Callaghan D, Spink D, Cloutier C, et al. Buprenorphine transdermal system in adults with chronic low back pain: A randomized, double-blind, placebo-controlled crossover study, followed by an open-label extension phase. Clin Ther 2010;32(5):844–60. [DOI] [PubMed] [Google Scholar]
- 55. Sittl R, Nuijten M, Nautrup BP.. Changes in the prescribed daily doses of transdermal fentanyl and transdermal buprenorphine during treatment of patients with cancer and noncancer pain in Germany: Results of a retrospective cohort study. Clin Ther 2005;27(7):1022–31. [DOI] [PubMed] [Google Scholar]
- 56. United States Department of Health and Human Services. Pain Management Best Practices Inter-Agency Task Force report: Updates, gaps, inconsistencies, and recommendations. 2019. Available at: https://www.hhs.gov/sites/default/files/final-pmtf-draft-report-on-pain-management%20-best-practices-2018-12-12-html-ready-clean.pdf (accessed September 17, 2019).
- 57. Reynaud M, Tracqui A, Petit G, Potard D, Courty P.. Six deaths linked to misuse of buprenorphine-benzodiazepine combinations. Am J Psychiatry 1998;155(3):448–9. [PubMed] [Google Scholar]
- 58. Nagappa M, Weingarten TN, Montandon G, Sprung J, Chung F.. Opioids, respiratory depression, and sleep-disordered breathing. Best Pract Res Clin Anaesthesiol 2017;31(4):469–85. [DOI] [PubMed] [Google Scholar]
- 59. Horsfall JT, Sprague JE.. The pharmacology and toxicology of the ‘holy trinity’. Basic Clin Pharmacol Toxicol 2017;120(2):115–9. [DOI] [PubMed] [Google Scholar]
- 60. Saarialho-Kere U, Mattila MJ, Paloheimo M, Seppala T.. Psychomotor, respiratory and neuroendocrinological effects of buprenorphine and amitriptyline in healthy volunteers. Eur J Clin Pharmacol 1987;33(2):139–46. [DOI] [PubMed] [Google Scholar]
- 61. Brandt J, Leong C.. Benzodiazepines and z-drugs: An updated review of major adverse outcomes reported on in epidemiologic research. Drugs R D 2017;17(4):493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lintzeris N, Nielsen S.. Benzodiazepines, methadone and buprenorphine: Interactions and clinical management. Am J Addict 2010;19(1):59–72. [DOI] [PubMed] [Google Scholar]
- 63. Food and Drug Administration. FDA warns about serious risks and death when combining opioid pain or cough medicines with benzodiazepines; requires its strongest warning. Available at: https://www.fda.gov/media/99761/download (accessed September 9, 2019).
- 64. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in Veterans' Health Administration patients. Pain Med 2015;16(8):1566–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gomes T, Greaves S, van den Brink W, et al. Pregabalin and the risk for opioid-related death: A nested case-control study. Ann Intern Med 2018;169(10):732–4. [DOI] [PubMed] [Google Scholar]
- 66. Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W.. Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case-control study. PLoS Med 2017;14(10):e1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nadpara PA, Joyce AR, Murrelle EL, et al. Risk factors for serious prescription opioid-induced respiratory depression or overdose: Comparison of commercially insured and Veterans Health Affairs populations. Pain Med 2018;19(1):79–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Webster LR, Webster RM.. Predicting aberrant behaviors in opioid-treated patients: Preliminary validation of the Opioid Risk Tool. Pain Med 2005;6(6):432–42. [DOI] [PubMed] [Google Scholar]
- 69. Lembke A, Humphreys K, Newmark J.. Weighing the risks and benefits of chronic opioid therapy. Am Fam Physician 2016;93(12):982–90. [PubMed] [Google Scholar]
- 70. Greenwald MK, Johanson CE, Moody DE, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology 2003;28(11):2000–9. [DOI] [PubMed] [Google Scholar]
- 71. Macintyre PE, Russell RA, Usher KA, Gaughwin M, Huxtable CA.. Pain relief and opioid requirements in the first 24 hours after surgery in patients taking buprenorphine and methadone opioid substitution therapy. Anaesth Intensive Care 2013;41(2):222–30. [DOI] [PubMed] [Google Scholar]
- 72. Leighton BL, Crock LW.. Case series of successful postoperative pain management in buprenorphine maintenance therapy patients. Anesth Analg 2017;125(5):1779–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Harrison TK, Kornfeld H, Aggarwal AK, Lembke A.. Perioperative considerations for the patient with opioid use disorder on buprenorphine, methadone, or naltrexone maintenance therapy. Anesthesiol Clin 2018;36(3):345–59. [DOI] [PubMed] [Google Scholar]
- 74. Davis MP. Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. J Support Oncol 2012;10(6):209–19. [DOI] [PubMed] [Google Scholar]
- 75. Streltzer J, Davidson R, Goebert D.. An observational study of buprenorphine treatment of the prescription opioid dependent pain patient. Am J Addict 2015;24(4):357–61. [DOI] [PubMed] [Google Scholar]
- 76. American Society of Addiction Medicine. Public policy statement on morphine equivalent units/morphine milligram equivalents. Available at: https://www.asam.org/docs/default-source/public-policy-statements/2016-statement-on-morphine-equivalent-units-morphine-milligram-equivalents.pdf (accessed April 5, 2019).
- 77. Kornfeld H, Reetz H.. Transdermal buprenorphine, opioid rotation to sublingual buprenorphine, and the avoidance of precipitated withdrawal: A review of the literature and demonstration in three chronic pain patients treated with butrans. Am J Ther 2015;22(3):199–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.