Abstract
Crescentic glomerulonephritis (cGN) comprises three main types according to the pathogenesis and immunofluorescence patterns: anti-glomerular basement membrane antibody cGN, vasculitis-associated cGN and post-infectious immune complex cGN. In this brief review of the immune-pathogenesis of cGN, the focus is mainly on the role of CD8+ T cells in the progression of cGN. Under control conditions, Bowman’s capsule (BC) provides a protected immunological niche by preventing access of cytotoxic CD8+ T cells to Bowman’s space and thereby podocytes. Even in experimental nephrotoxic nephritis, leukocytes accumulate around the glomeruli, but remain outside of BC, as long as the latter remains intact. However, when and where breaches in BC occur, the inflammatory cells can gain access to and destroy podocytes, thus converting cGN into rapidly progressive glomerulonephritis (RPGN). These conclusions also apply to human cGN, where biopsies show that loss of BC integrity is associated with RPGN and progression to end-stage kidney disease. We propose a two-hit hypothesis for the role of cytotoxic CD8+ T cells in the progression of cGN. The initial insult occurs in response to the immune complex formation or deposition, resulting in local capillary and podocyte injury (first hit). The injured podocytes release neo-epitopes, eventually causing T-cell activation and migration to the glomerulus. Upon generation of breaches in BC, macrophages and CD8+ T cells can now gain access to the glomerular space and destroy neo-epitope expressing podocytes (second hit), resulting in RPGN. While further investigation will be required to test this hypothesis, future therapeutic trials should consider targeting of CD8+ T cells in the therapy of progressive cGN.
Keywords: ANCA, crescentic glomerulonephritis, glomerulonephritis podocytes, immunology, membranoproliferative
INTRODUCTION
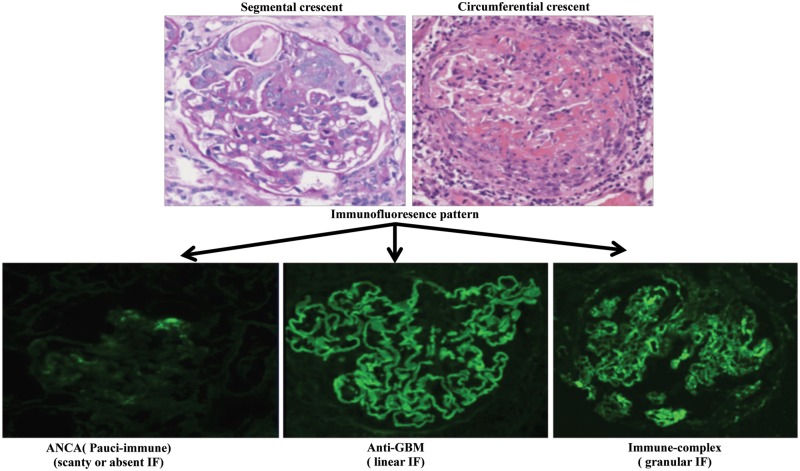
Crescentic glomerulonephritis (cGN) is a severe form of glomerulonephritis (GN), which is also referred to as rapidly progressive GN (RPGN), as it frequently leads to end-stage renal failure within a few months. cGN is classified into three main types according to their pathogenesis and immunofluorescence patterns: (i) anti-neutrophil cytoplasmic antibody (ANCA)-positive and pauci-immune GN, characterized by scanty or absent immune deposits; (ii) anti-glomerular basement membrane (anti-GBM) disease with linear deposition of immunoglobulin G (IgG) on the capillary wall; and (iii) immune complexes- mediated GN with granular deposits of immune complex in the glomeruli (Figure 1). The immune complex GN is mostly due to lupus nephritis (LN), IgA nephropathy (IgAN), Henoch–Schönlein purpura or post-infectious GN [1].
FIGURE 1.
cGN. The top panel shows periodic acid–Schiff stained human kidney sections displaying glomerular crescents. The bottom panel shows the immunofluorescence (IF) images of three types of cGN with their characteristic patterns of IgG deposition (courtesy of Dr Geng Jian from Southern Medical University, Guangzhou, China).
Histologically, glomerular crescents are made up of two or more layers of proliferating cells occupying Bowman’s space. Presence of crescents is a hallmark of glomerular inflammation and of severe glomerular cell injury, such that the severity of the clinical manifestations and the response to treatment in GN patients correlates with the percentage of glomeruli that exhibit crescents [2, 3]. Although the exact mechanism of crescent formation remains unclear, it is generally considered to represent a nonspecific response of the parietal epithelial cells of Bowman’s capsule (BC) to severe injury to the glomerular capillary wall. The initiating event is the development of physical gaps (also called rents or holes) in the glomerular capillary wall [4]. These gaps permit the exudation of coagulation factors that lead to fibrin formation (due to the conversion of fibrinogen to fibrin polymers and delayed fibrinolysis) and cellular elements such as polymorpho-nuclear neutrophils (PMN), all of which may promote crescent formation [2]. In this review, we discuss some of the new insights on the immune-mediated pathogenesis of cGN, focusing mainly on the role of CD8+ T cells in cGN.
Overview of immune-mediated mechanisms in cGN
Immune-mediated GN is a major cause of renal inflammation, leading to terminal kidney failure. B cells and autoantibodies are well known to contribute to the progression of glomerular disease. Antibodies that induce glomerular immune deposits may be directed against the following antigens: (i) normal constituents of the glomerulus, such as the Goodpasture antigen on the noncollagenous domain of the α3 chain of type IV collagen [5, 6]; (ii) nonrenal self antigens localized in the glomeruli, such as DNA nucleosome complexes in LN [7] or abnormally glycosylated IgA in IgAN [8]; and (iii) exogenous antigens or immune aggregates that localize in glomerular capillaries via charge affinity for glomerular structures, passive trapping or local precipitation of macromolecular aggregates [9, 10].
Cellular immunity also plays an important role in the pathogenesis of the cGN. T cells regulate the activities of B cells, other T cells, macrophages and other cells participating in immune responses. They provide help for antibody production by B cells, and they are also the effectors of antigen-specific cell-mediated immunity (CMI). CMI is important in the elimination of cells infected with pathogens that replicate intracellularly (e.g. viruses, mycobacteria and some bacteria) and cells exhibiting aberrant differentiation (e.g. neoplasms). Humoral immunity against a target antigen does not necessarily suggest its pathogenicity. Natural autoantibodies to myeloperoxidase (MPO), proteinase 3 (PR3) and the GBM antigen are present in normal individuals [11]. Strong evidence exists for a primary role for mononuclear cells, particularly PMNs, lymphocytes and macrophages, in the pathogenesis of GN, even in the absence of antibody deposition [12]. Neutrophils, the most abundant immune cells, are first-line responders in inflammation and in a variety of glomerular diseases. In some forms of GN, neutrophils generate neutrophil extracellular traps, web-like structures of the scaffold of decondensed chromatin decorated with proteases, peptides and enzymes that are also likely to be injurious [12]. The amount of activated PMN present within the kidney correlates with renal tissue damage as assessed by serum creatinine levels [13]. In autoimmune kidney disease, both humoral and cell-mediated immune responses use macrophages as effector cells. Macrophage infiltrates were found in the peri-glomerular and intra-glomerular region in human anti-GBM disease and LN [14, 15] and were correlated with the most active glomerular lesions (both segmental necrosis and cellular crescents) in patients with myeloperoxidase-mediated ANCA-associated vasculitis (MPO-AAV) [16]. Interstitial CD68+ macrophage infiltration showed the highest association with the renal function at presentation. Differences in the cellular infiltrate between focal and crescentic ANCA-GN were related to CD68+ macrophages in ANCA-GN patients [17].
The complement system integrates the interactions of leukocytes, platelets and tissues in inflammatory response and can be activated via the classical, lectin or alternative pathways. The classical pathway of the complement cascade is involved in various autoantibody-mediated GN, such as LN, anti-GBM disease, membranoproliferative GN and IgAN [18]. Complement activation via the alternative pathway has been shown to play a key role in a model of AAV in mice [19]. Lectin pathway is activated in some cases of IgAN and is associated with more severe renal damage. The presence of complement component 4d (C4d) in glomeruli indicates the lectin pathway activation and increased C4d staining is associated with increased risk of progression [20].
In patients with RPGN, CD4+ and CD8+ T-cell infiltration are significantly increased in both glomeruli and interstitium [21]. Antigen-specific effector CD4+ T cells are present in the peripheral blood of patients with acute ANCA vasculitis, and the depletion of CD4+ T cell during the effector phase attenuated disease progression [22]. MPO-specific effector CD4+ T cells are important in disease pathogenesis by inducing a delayed-type hypersensitivity-like lesion in the glomeruli [23]. Percentages of circulating effector memory T cells decrease during active disease phase in granulomatosis with polyangiitis (GPA), a type of AAV, and increase with remission, indicating migration of effector memory T cell toward inflamed sites during the disease exacerbation [24]. In line with this finding, effector memory T cells are found in the inflammatory lesions of the kidney in patients with GPA [25]. These findings suggest a role of CD4+ T cell in cGN. However, some studies find that CD8+ T cells are the predominant cells infiltrating the kidney in cGN and in LN [14–16]. The prevalence of CD8+ T cells was correlated with the percentage of glomeruli with ruptured BCs, with the renal activity index and serum creatinine levels [15]. A study by Watanabe et al. [26] indicated that the number of glomerular CD8+ T cells was the most sensitive predictor of disease progression in childhood IgAN. Interstitial CD8+ T cell infiltrates showed a high degree of association with the renal function at presentation in AAV patient [17]. Furthermore, CD8+ T-cell depletion by an anti-CD8 monoclonal antibody can prevent and treat experimental nephrotoxic serum nephritis as a model of autoimmune GN [27]. More recently, our group demonstrated a new role of CD8+ T cells in mediating the progression of cGN [28]. Taken together, these studies indicate an important role of CD8+ T cells in the pathogenesis of cGN.
ANCA-associated vasculitis
AAV are multisystem microvascular forms of vasculitis that cause destructive inflammation of small arterioles and capillaries, leading to several clinically and pathologically defined entities: GPA, microscopic polyangiitis, Churg–Strauss syndrome and renal-limited vasculitis. PR3 and MPO are the major autoantigens in antineutrophil cytoplasmic AAV. Although autoimmunity to either PR3 or MPO can be found in each of these forms of vasculitis, their clinical features, natural history and genetic associations suggest that MPO- and PR3-AAV are different but related autoimmune diseases [16]. The pathogenicity of ANCA is well studied. However, the question of why ANCAs are generated remains to be fully answered. Apart from environmental factors, drugs (the most common of which is propylthiouracil), infections, neoplasm and other factors might contribute to the appearance of ANCA and onset of the diseases [29].
Innate immunity may initiate the process of the AAV, thus playing an important role in this disease. In the pauci-immune necrotizing and cGN (NcGN) model, development of NcGN is accompanied by glomerular accumulation of neutrophils and macrophages. Neutrophil infiltration was most conspicuous at sites of glomerular necrosis and crescent formation, while macrophages were most numerous in crescents. Mice that were depleted of circulating neutrophils with monoclonal antibodies were completely protected from anti-MPO IgG-induced NcGN [30]. Infection, possibly by Staphylococcus aureus, triggers bronchial epithelium and macrophages in a Toll-like receptor (TLR)-dependent manner to release pro-inflammatory cytokines. Then cytokines prime neutrophils to release MPO and PR3, and activate endothelial cells to express adhesion molecules, thus recruiting inflammatory cells (monocytes/macrophages). Neutrophil priming causes upregulation of the expression of neutrophil adhesion molecules (such as CD11b and β2 integrin) and translocation of the ANCA antigens from their lysosomal compartments to the cell surface. Recruited monocytes/macrophages sense TLR ligands and secrete more pro-inflammatory cytokines, including interleukin 23 (IL-23), driving T cells toward a T helper cell 17 (Th17) phenotype. Secreted IL-17 can attract neutrophils and stimulate granuloma formation. In this pro-inflammatory environment, neutrophils are primed and adhere to the endothelium. The release of the autoantigens MPO and PR3 in an environment with antigen-presenting cells, B cells and T cells leads to the local production of ANCA [31]. ANCA bind to their target antigen, thereby co-aggregating and activating Fc gamma receptors on the surface of myeloid cells [32]. The activated PMN producing toxic oxygen metabolites and releasing of lysosomal enzymes are present in renal biopsies from patients with AAV, and the amount of PMNs present within the kidney correlates with renal tissue damage [13]. Activated neutrophils and monocytes primed with pro-inflammatory cytokines and activated by ANCA undergo oxidative burst, degranulation, inflammatory cytokine release and induce damage to endothelial cell walls [33]. ANCA-induced neutrophil activation is also enhanced on priming with pro-inflammatory stimuli, in particular tumour necrosis factor-α [34]. Stimulation of neutrophils by ANCA causes the release of factors that activate complement via the alternative pathway, thus initiating an inflammatory amplification loop that mediates the severe necrotizing inflammation of ANCA disease [19]. Complement component 5 (C5)-inhibiting monoclonal antibody (BB5.1) pretreatment can prevent disease development, as evidenced by the absence of urinary abnormalities, a marked reduction in glomerular neutrophil influx and normal renal morphology [35].
The observations that effector CD4+ T-cell depletion in experimental autoimmune anti-MPO GN attenuated cGN and effector cell influx without altering ANCA titers, and that B cell-deficient mice lacking ANCA still developed severe cGN with accumulation of effector cells [22], indicate that both humoral and cellular arms of the immune system mediate glomerular injury in AAV. Auto-reactive T cells are thought to play a major role in the development and subsequent organ damage of AAV [25, 36]. Patients’ lymphocytes show in vitro reactivity to PR3 or MPO autoantigens and T-cell-directed therapy could be used to treat the disease [32]. CD4+ T cells recognized the planted MPO and PR3 antigen presented by macrophages, which amplified the glomerular injury. Ooi et al. [23] found that transfer of an MPO-specific CD4+ T-cell clone to Rag1-deficient mice could induce focal necrotizing GN when glomerular MPO deposition was lodged in glomeruli, which indicate that MPO-specific CD4+ T cells can mediate experimental necrotizing GN. Patients with AAV have increased numbers of Th17-producing lymphocytes and Th17-associated serum cytokine levels correlate with disease activity [37]. T regulatory (Treg) cells also play role in the AAV [38, 39]. However, both CD4+ and CD8+ T-cell populations were found to be activated in GPA [40]. In the affected kidneys, lymphocyte infiltration was slightly higher for CD8+ than for CD4+ T cells in both glomerular and interstitial compartments. Furthermore, the extent of interstitial infiltration of CD4+ and CD8+ T cells correlated with lower eGFR [16, 41].
Role of CD8+ T cells in AAV
However, the role of CD8+ T cells in vasculitis is less well understood. The proportion of activated CD8+ T cells is increased in the blood of patients with AAV [42]. McKinney et al. [43, 44] showed that CD8+ T-cell exhaustion predicted favorable prognosis in multiple autoimmune and inflammatory diseases such as AAV and systemic lupus erythematosus. mRNA profiling from purified CD8+ T lymphocytes of patients with AAV showed upregulation of the IL-7 receptor (IL-7R) pathway and T-cell receptor (TCR)-mediated signaling, which was associated with poor prognosis in AAV. These data indicate that CD8+ T cell may play a pathogenic injurious in ANCA-associated GN. A recent study from Chang et al. [45] also showed that the depletion of CD8+ T cells attenuates experimental autoimmune anti-MPO GN, while MPO-specific CD8+ T cells could augment kidney injury even in the absence of CD4+ T cells. The effector MPO-specific CD8+ T cells can infiltrate the glomerulus and mediate glomerular injury when MPO is lodged in the glomerulus. These results support a pathogenic injurious role of CD8+ T cell in AAV.
Anti-glomerular basement membrane GN
Anti-GBM GN, also referred to as Goodpasture disease, is an autoimmune disorder characterized by the production of IgG autoantibodies directed against type IV collagen, an abundant type of collagen in alveolar and GBMs. It typically presents with acute renal failure caused by cGN, accompanied by pulmonary vasculitis in 50–60% of cases [32]. Mature GBM collagen forms a lattice-like structure composed of triple helices of α3, α4 and α5 type IV collagens, terminating in short globular non-collagenous domains NC1 and NC2 [46]. α3, α4 and α5 type IV collagen exist in a hexameric structure, and adjacent NC1 domains are cross-linked to form dimers (D-isoform) in the GBM. Under normal conditions, only small amounts of type IV collagen with monomeric NC1 domains (M-isoform) exist. However, conditions such as hydrocarbon or solvent exposure, cigarette smoking and lithotripsy, which potentially could cause damage to the GBM, can result in dissociation of the D-isoform to the M-isoform and exposure of the cryptic epitopes leading to autoimmunity. Once tolerance is lost, the anti-GBM antibodies themselves also dissociate the cross-links of type IV collagen [32]. Autoantibodies to the α3 NC1 monomer and α5 NC1 monomer were found to be bound in the kidneys and lungs in patients with Goodpasture’s disease, indicating roles for the α3 and α5 NC1 monomers as autoantigens. High antibody titers at diagnosis of anti-GBM disease were associated with ultimate loss of renal function [47].
Role of CD4+ T cells in anti-GBM GN
Although direct antibody pathogenicity is established in mouse models of the disease, and plasmapheresis is part of therapies in humans, there is also strong evidence indicating that cell-mediated autoimmunity, and in particular autoreactive T cells, contribute to the manifestations of the disease. CD4+ T-cell infiltration is present around the glomeruli with crescents and was positively correlated with serum creatinine levels [14]. In the animal model of Goodpasture’s disease induced in rats or mice, it was shown that a Th1 response to a planted antigen (sheep anti-mouse GBM globulin) could induce a severe crescentic pattern of GN [48]. Th1 cells specific for planted glomerular antigen-stimulated macrophages that caused kidney damage in nephrotoxic nephritis [49, 50]. There is also evidence that antigen-specific CD4+ T cells per se are sufficient to cause glomerular injury [51]. Studies from experimental autoimmune glomerulonephritis induced in WKY rats by immunization with rat GBM showed that early blockade of the CD154–CD40 T-cell co-stimulatory pathway can prevent the development of crescentic nephritis, and that delayed treatment can reduce the severity of disease [52]. Also, CD28-B7 blockade could reduce autoantibody production and cellular infiltration of glomeruli and prevent target organ injury [53]. All these results highlight the importance of T-cell responses in the disease pathogenesis.
Role of CD8+ T cells in anti-GBM GN
CD8+ T cells may be involved in the pathogenesis of anti-GBM GN at different levels. First, cytotoxic CD8+ T cells may cause direct glomerular damage by infiltrating the glomerulus in human and mouse anti-GBM disease [14, 54, 55]. Second, antigen-specific CD8+ T cells may directly target glomerular antigen to initiate damage [51]. For example, kidney biopsies from patients with Goodpasture’s syndrome are also characterized by the presence of infiltrating CD8+ T cells that recognize the α3 type IV collagen motif [56]. Hu et al. [14] found that CD8+ T cells were the predominant T-cell subtype in human anti-GBM disease and were closely associated with damage to the glomerular structures. A close association between CD8+ T cell infiltration around the glomeruli and the rupture of the BC indicates that kidney structure damage may be mediated by CD8+ effector T cells. In nephrotoxic serum-induced nephritis (NTSN), a common model of human anti-GBM crescentic GN, depletion of CD8+ T cells from WKY rats led to prevention of proteinuria, crescent formation and reduction in infiltration of macrophages in the glomeruli, indicating that CD8+ T cell may play a key role in glomerular injury and crescent formation [57]. Another study in the animal model of Goodpasture’s syndrome with depletion of CD8+ T cells with an anti-CD8 monoclonal antibody showed both preventive and therapeutic effects [27].
Heymann et al. [58] studied the role of CD8+ T cells in disease progression using transgenic mice (NOH mice) that selectively expressed the model antigens ovalbumin (OVA) and hen egg lysozyme in glomerular podocytes. They found that neither activated podocyte-antigen-specific CD8+ T cells (OT-I cells) nor podocyte antigen-specific CD4+ Th cells (OT-II cells) alone caused any immunopathology. Also, a combined injection of naïve OT-I and naïve OT-II cells did not cause nephritis. Co-injection of naïve OT-I cells and activated OT-II cells into NOH mice resulted in periglomerular mononuclear infiltrates and inflammation of parietal epithelial cells, but no intra-glomerular infiltration. When they injected in vitro-activated OT-I cells into NOH mice, they showed evidence that activated cytotoxic T lymphocytes (CTLs) could cause the release of podocyte antigen, which was subsequently cross-presented in the renal lymph nodes but also did not cause a glomerular lesion [58]. Based on these elegant studies, they proposed that podocytes released the neo-antigen OVA into the urine, from where it was taken up by dendritic cells (DCs) in the renal medulla. The DCs then migrated to the regional lymph node where together with helper CD4+ T cells, they activate and expand cytotoxic CD8+ T cells recognizing OVA neo-antigen-expressing cells. The cytotoxic CD8+ T cells then migrate to the kidney and accumulate around the BC of glomeruli with the OVA+ podocytes. However, the cytotoxic CD8+ T cells did not infiltrate the glomerulus or enter Bowman’s space and thereby did not attack their target OVA+ podocytes.
New finding on the role of CD8+ cells in the progression of cGN
CD8+ T cells are not able to access BC space in the normal kidney
In all studies mentioned above, it remained unclear how and with which specific glomerular cell type the CD8+ T cells interacted. We recently took advantage of the two transgenic mouse models to examine this question: (i) Just enhanced green fluorescent protein (EGFP) death-inducing (Jedi) transgenic mice that are engineered to express a TCR that recognizes EGFP [59] and (ii) transgenic mice with a podocyte-restricted expression of the EGFP (pod-EGFP) [60]. Thus, the injection of isolated CD8+ T cells from Jedi mice (CD45.1+) into the pod-EGFP mice would behave as podocyte-specific cytotoxic T cells. However, under normal conditions, we found that the injected Jedi T cells into pod-EGFP mice did not result in any pathological changes in kidney histology, glomerular infiltrates with CD4+ or CD8+ T cells, or alter renal function [28]. Yet in the same mice, we observed a complete elimination of EGFP+ splenocytes transduced by co-injection of EGFP-expressing lentivirus, thus indicating that under basal conditions CD8+ Jedi T cells cannot access EGFP+ podocytes that are within the protected niche of BC.
CD8+ T cells are able to kill podocytes in NTSN model
However, under the conditions of experimental GN (a mild, self-limited form of NTSN), injection of the activated Jedi CD8+ T cells into the pod-EGFP mice aggravated the NTSN, resulting in worse renal function and histopathology resembling RPGN [28]. The renal pathology showed massive lesions with increased crescents, endocapillary lesions, podocyte apoptosis, defects in BC that were associated with infiltration of Jedi CD8+ T cells and macrophage infiltration. We also observed peri-glomerular and interstitial infiltrates in Jedi T cell-injected pod-EGFP mice, when compared with the control NTSN groups. A direct contact of Jedi CD8+ T cells with EGFP+ podocytes was observed in the glomeruli, as well as an inverse relationship between the number of intra-glomerular Jedi CD8+ T cells and the remaining EGFP+ podocytes. Furthermore, breaches in BC were associated with an increased number of CD8+ T cells infiltrating Bowman’s space, which in turn correlated with podocyte destruction. In contrast, we did not observe any infiltration of Jedi CD8+ T cells or destruction of EGFP+ podocytes in glomeruli with intact BC, but a peri-glomerular accumulation of Jedi CD8+ T cells and macrophages outside of BC were present. Thus, breaches in BC with infiltration of CD8+ T cells specific for podocyte antigen were associated with the development of an NTSN into a catastrophic RPGN.
Role of BC integrity in T-cell infiltration in the glomeruli
Previous studies in a rodent model of crescentic NTSN indicated that the activated macrophages and T cells accumulated around BC [4], which was frequently associated with local breaches in BC and with the infiltration by T cells and macrophages in the Bowman’s space [4]. Couzi et al. [15] showed that CD8+ T lymphocytes were the main cells that infiltrated the periglomerular region in the kidneys of experimental LN and that specifically CD8+ T cell infiltrates correlated with the presence of cellular crescents, rupture of BC and a poor response to conventional therapy. Furthermore, patients with nephrotic syndrome with hematuria, whose biopsies show a high histopathologic index, also have more renal CD8+ T-cell infiltrates [42]. In addition, the prevalence of infiltrating CD8+ T cells was associated with ruptured BCs in human anti-GBM disease [14]. Our above-described data also indicated that the breaches in BC might facilitate the entry of CD8+ T cells into Bowman’s space [28]. In order to verify the role of BC integrity in preventing CD8+ T cells infiltration in human forms of crescentic GN, we examined a series of biopsies from patients with anti-GBM nephritis, ANCA-GN, IgAN and acute post-infectious GN [28]. We could confirm that CD8+ T cell aggregated around the glomeruli, particularly around the crescents, irrespective of the underlying etiologies. CD8+ T cells appeared to ‘enter’ through the localized defects in BC and accumulate inside the Bowman’s space, mostly at sites of crescents. These and previous findings by others are consistent with the hypothesis that intact BC prevents CD8+ T cells from gaining access to the glomerular space. However once BC is breached, CD8+ T cells can access the glomerular space in both experimental and human forms of crescentic GN, which results in an acceleration of the glomerular pathology and progressive kidney failure.
A two-hit hypothesis for progression of cGN
Based on all these studies, we propose a hypothetical scheme to incorporate the role of CD8+ T cells in the augmentation and progression of crescentic GN (Figure 2). Our hypothesis does not apply to the initial insult of the various forms of cGN (first hit), which include capillary insults of various etiologies as discussed above. However, we posit that the progression of crescentic GN may in part be due to secondary pathophysiological processes involving the generation of cytotoxic T cells. In the course of GN, the initial insult to the glomerular capillaries may result in secondary damage to podocytes, thereby generating and/or exposing the previously hidden, cryptic, cellular antigen epitopes by the podocytes. Such neo-epitopes could be displayed together with HLA I on the surface of the activated podocyte or be released into the urine, which may subsequently be recognized by renal medullary DCs to generate CD4+ and CD8+ T cells specific for these epitopes. These, in turn, would migrate back from the lymph nodes to the glomerulus, accumulating around BC, likely following a gradient of chemokines generated within the injured glomeruli as detailed by Heymann et al. [58]. This process, similarly to what occurs during vaccination or during an immunological response to tumor neo-antigens, allows that non-immunogenic antigens can be recognized as dangerous by the immune system and elicit an immune response [61]. This response can be further amplified by the cell injury or death concomitant generation and release of Danger Associate Molecular Pattern (DAMP), which activate TLR signaling. We and others have previously shown that DAMPs can accelerate the progression of experimental immune complex nephritis and moderate lupus GN into an RPGN with crescents through the activation of TLRs, resulting in periglomerular macrophages and T cell infiltrates and ruptures of BC and ultimately in the loss of glomerular function [62].
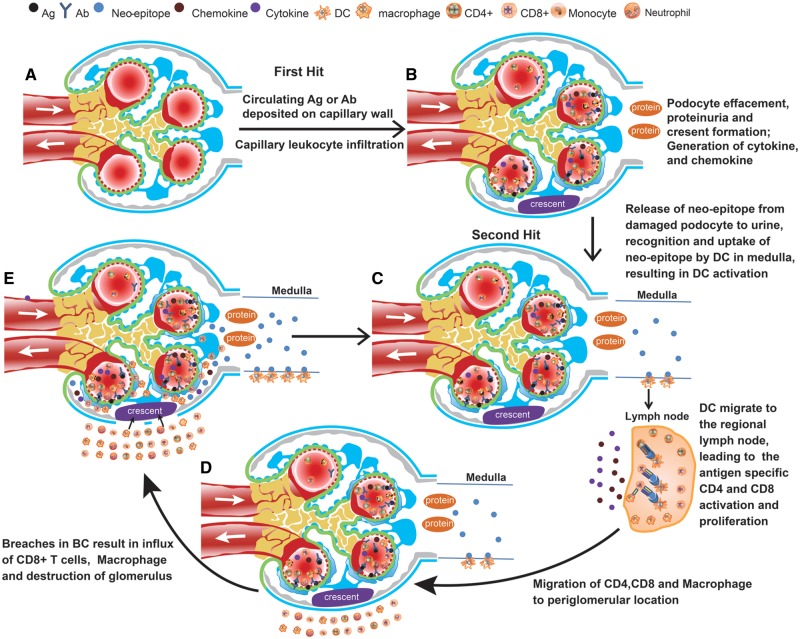
FIGURE 2.
Two-hit hypothesis of cGN progression. (A) Schema of the normal glomerulus. (B) The initial insult to the glomerular capillaries in various forms of cGN result in damage of endothelial cells and podocytes, and proteinuria (first hit). With continued insult to podocytes and the associated proteinuria, parietal epithelial cells of BC will proliferate and generate crescents. Damage to the podocytes will generate pro-inflammatory cytokines and chemokines and neo-epitopes from previously hidden podocyte antigens. (C) The neo-epitope from damaged podocyte (second hit) released into the urine will be recognized and taken up by renal medullary DCs, which will then migrate to the regional lymph nodes. There they will (cross)-present the neo-epitope to CD4+ and CD8+ T cells, causing their activation and expansion specific for these epitopes. (D) The activated T lymphocyte and macrophages migrate to the glomerulus, accumulating around BC, most likely following a gradient of chemokines generated within the injured glomeruli. (E) The activated parietal cells together with the peri-capsular CD8+ T cells, neutrophils and macrophages may create breaches in BC. Through these breaches macrophages and CD8+ T cells can now gain access to the glomerular space, so that CD8+ T cells can now attack and destroy their neo-epitope expressing target podocytes. This CD8+ T cell and macrophage cell-mediated, secondary glomerular injury results in an amplification loop of the cGN and a deleterious RPGN. Ab, antibody; Ag, antigen.
Injured glomerular cells could release cytokines, which subsequently facilitate the proliferation of activated CD8+ T cells and activation of macrophages. At the early stage when the BC is intact, CD8+ T cells and macrophages stay outside the capsule, and the peri-glomerular infiltrate and the crescent formation may potentially be reversible by immune-suppressive therapy. However, as the disease progresses, the activated parietal cells, together with the peri-capsular CD8+ T cells, neutrophils and macrophages, may play an active part in the BC rupture, allowing the entry of macrophages and CD8+ T cells into the glomerular space to destroy the neo-epitope-carrying podocytes. While our [28] and previous studies [4, 15, 17, 45, 63, 64] support the association of breaches in BC with glomerular T cell and macrophage infiltration, the possibility of inflammatory cell access to Bowman’s space via ruptured glomerular capillaries has not been excluded. Nonetheless, we posit that the CD8+ T cell and macrophage cell-mediated, secondary glomerular injury results in an amplification loop of the RPGN, independent of the initial antigen-antibody-mediated process that results in the eventual destruction of the glomeruli and progressive kidney failure. At this point, the usual immune-suppressive therapy would have little therapeutic effect.
Previous animal studies have shown beneficial effects of blocking or eliminating CD8+ T cells in immune-mediated crescentic GN [27, 45, 57, 65], and the role for CD8+ T cells in human autoimmune kidney diseases has been defined recently [15, 43, 44]. Therefore, we propose that future therapeutic trials for crescentic GN should target CD8+ T cells and the destruction of BC to prevent the detrimental action of CD8+ T cells on podocytes and thereby the demise of glomerular and renal function.
Questions and future perspectives
Our recent study and the previous observations on the role of the infiltration of inflammatory cells through breaches in BC raise several new questions, such as: what are the neo-epitopes generated from hidden antigens and released by injured podocytes; which cells and mechanisms are involved in generating breaches in BC; do all cells infiltrating Bowman’s space in cGN enter through breaches in BC or do some transmigrate through the intact BC or the glomerular capillary wall? While there is no evidence at present that podocytes might directly cross-present locally generated neo-epitopes or filtered circulating antigens to CD8+ T cells, this might be considered as an exciting alternative hypothesis. If podocytes could cross-present antigen, then this might also explain why the podocyte is normally protected from access by CD8+ T cells. Exploring this alternative hypothesis might be an exciting subject for future studies.
Hopefully, clarification of these questions will help us to not only better understand the pathogenesis of cGN, but also to develop better treatment for this devastating disease.
FUNDING
This work was supported by the National Natural Science Foundation of China (81800637 to A.C.) and the Fujian Medical Technology Innovation Fund from China (2014-CXB-41 to A.C.); NIH/NIDDK (R01DK117913 to K.L.); the Fujian Medical Technology Innovation Fund from China (2017-CXB-16 to T.-J.G.); and NIH/NIDDK (R01DK078897, R01DK088541, P01DK56492 to J.C.H.) and VA Merit Award (IBX000345C to J.C.H.).
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Sinico RA, Di Toma L, Radice A.. Renal involvement in anti-neutrophil cytoplasmic autoantibody associated vasculitis. Autoimmun Rev 2013; 12: 477–482 [DOI] [PubMed] [Google Scholar]
- 2. Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int 2003; 63: 1164–1177 [DOI] [PubMed] [Google Scholar]
- 3. Singh SK, Jeansson M, Quaggin SE.. New insights into the pathogenesis of cellular crescents. Curr Opin Nephrol Hypertens 2011; 20: 258–262 [DOI] [PubMed] [Google Scholar]
- 4. Lan HY, Nikolic-Paterson DJ, Atkins RC.. Involvement of activated periglomerular leukocytes in the rupture of Bowman’s capsule and glomerular crescent progression in experimental glomerulonephritis. Lab Invest 1992; 67: 743–751 [PubMed] [Google Scholar]
- 5. Hudson BG, Tryggvason K, Sundaramoorthy M. et al. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N Engl J Med 2003; 348: 2543–2556 [DOI] [PubMed] [Google Scholar]
- 6. Hudson BG. The molecular basis of Goodpasture and Alport syndromes: beacons for the discovery of the collagen IV family. J Am Soc Nephrol 2004; 15: 2514–2527 [DOI] [PubMed] [Google Scholar]
- 7. Kalaaji M, Fenton KA, Mortensen ES. et al. Glomerular apoptotic nucleosomes are central target structures for nephritogenic antibodies in human SLE nephritis. Kidney Int 2007; 71: 664–672 [DOI] [PubMed] [Google Scholar]
- 8. Tomana M, Novak J, Julian BA. et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest 1999; 104: 73–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Debiec H, Lefeu F, Kemper MJ. et al. Early-childhood membranous nephropathy due to cationic bovine serum albumin. N Engl J Med 2011; 364: 2101–2110 [DOI] [PubMed] [Google Scholar]
- 10. Gupta A, Quigg RJ.. Glomerular diseases associated with hepatitis B and C. Adv Chronic Kidney Dis 2015; 22: 343–351 [DOI] [PubMed] [Google Scholar]
- 11. Cui Z, Zhao MH, Segelmark M. et al. Natural autoantibodies to myeloperoxidase, proteinase 3, and the glomerular basement membrane are present in normal individuals. Kidney Int 2010; 78: 590–597 [DOI] [PubMed] [Google Scholar]
- 12. Kitching AR, Hutton HL.. The players: cells involved in glomerular disease. Clin J Am Soc Nephrol 2016; 11: 1664–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brouwer E, Huitema MG, Mulder AH. et al. Neutrophil activation in vitro and in vivo in Wegener’s granulomatosis. Kidney Int 1994; 45: 1120–1131 [DOI] [PubMed] [Google Scholar]
- 14. Hu SY, Jia XY, Li JN. et al. T cell infiltration is associated with kidney injury in patients with anti-glomerular basement membrane disease. Sci China Life Sci 2016; 59: 1282–1289 [DOI] [PubMed] [Google Scholar]
- 15. Couzi L, Merville P, Deminiere C. et al. Predominance of CD8+ T lymphocytes among periglomerular infiltrating cells and link to the prognosis of class III and class IV lupus nephritis. Arthritis Rheum 2007; 56: 2362–2370 [DOI] [PubMed] [Google Scholar]
- 16. O’Sullivan KM, Lo CY, Summers SA. et al. Renal participation of myeloperoxidase in antineutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis. Kidney Int 2015; 88: 1030–1046 [DOI] [PubMed] [Google Scholar]
- 17. Kidder D, Bray SE, Fleming S.. Differences in the frequency of macrophage and T cell markers between focal and crescentic classes of anti-neutrophil cytoplasmic antibody (ANCA)-associated glomerulonephritis. J Nephropathol 2016; 6: 97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kościelska-Kasprzak K, Bartoszek D, Myszka M. et al. The complement cascade and renal disease. Arch Immunol Ther Exp (Warsz) 2014; 62: 47–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiao H, Schreiber A, Heeringa P. et al. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol 2007; 170: 52–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cook HT. Complement and kidney disease. Curr Opin Nephrol Hypertens 2013; 22: 295–301 [DOI] [PubMed] [Google Scholar]
- 21. Markovic-Lipkovski J, Muller CA, Risler T. et al. Association of glomerular and interstitial mononuclear leukocytes with different forms of glomerulonephritis. Nephrol Dial Transplant 1990; 5: 10–17 [DOI] [PubMed] [Google Scholar]
- 22. Ruth AJ, Kitching AR, Kwan RY. et al. Anti-neutrophil cytoplasmic antibodies and effector CD4+ cells play nonredundant roles in anti-myeloperoxidase crescentic glomerulonephritis. J Am Soc Nephrol 2006; 17: 1940–1949 [DOI] [PubMed] [Google Scholar]
- 23. Ooi JD, Chang J, Hickey MJ. et al. The immunodominant myeloperoxidase T-cell epitope induces local cell-mediated injury in antimyeloperoxidase glomerulonephritis. Proc Natl Acad Sci USA 2012; 109: E2615–E2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abdulahad WH, van der Geld YM, Stegeman CA. et al. Persistent expansion of CD4+ effector memory T cells in Wegener’s granulomatosis. Kidney Int 2006; 70: 938–947 [DOI] [PubMed] [Google Scholar]
- 25. Komocsi A, Lamprecht P, Csernok E. et al. Peripheral blood and granuloma CD4(+)CD28(−) T cells are a major source of interferon-gamma and tumor necrosis factor-alpha in Wegener’s granulomatosis. Am J Pathol 2002; 160: 1717–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watanabe T, Kawachi H, Ikezumi Y. et al. Glomerular CD8+ cells predict progression of childhood IgA nephropathy. Pediatr Nephrol 2001; 16: 561–567 [DOI] [PubMed] [Google Scholar]
- 27. Reynolds J, Norgan VA, Bhambra U. et al. Anti-CD8 monoclonal antibody therapy is effective in the prevention and treatment of experimental autoimmune glomerulonephritis. J Am Soc Nephrol 2002; 13: 359–369 [DOI] [PubMed] [Google Scholar]
- 28. Chen A, Lee K, D’Agati VD. et al. Bowman’s capsule provides a protective niche for podocytes from cytotoxic CD8+ T cells. J Clin Invest 2018; 128: 3413–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen YX, Chen N.. Pathogenesis of rapidly progressive glomerulonephritis: what do we learn? Contrib Nephrol 2013; 181: 207–215 [DOI] [PubMed] [Google Scholar]
- 30. Xiao H, Heeringa P, Liu Z. et al. The role of neutrophils in the induction of glomerulonephritis by anti-myeloperoxidase antibodies. Am J Pathol 2005; 167: 39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kallenberg CG, Stegeman CA, Abdulahad WH. et al. Pathogenesis of ANCA-associated vasculitis: new possibilities for intervention. Am J Kidney Dis 2013; 62: 1176–1187 [DOI] [PubMed] [Google Scholar]
- 32. Tarzi RM, Cook HT, Pusey CD.. Crescentic glomerulonephritis: new aspects of pathogenesis. Semin Nephrol 2011; 31: 361–368 [DOI] [PubMed] [Google Scholar]
- 33. Coughlan AM, Freeley SJ, Robson MG.. Animal models of anti-neutrophil cytoplasmic antibody-associated vasculitis. Clin Exp Immunol 2012; 169: 229–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huugen D, Xiao H, van Esch A. et al. Aggravation of anti-myeloperoxidase antibody-induced glomerulonephritis by bacterial lipopolysaccharide: role of tumor necrosis factor-alpha. Am J Pathol 2005; 167: 47–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huugen D, van Esch A, Xiao H. et al. Inhibition of complement factor C5 protects against anti-myeloperoxidase antibody-mediated glomerulonephritis in mice. Kidney Int 2007; 71: 646–654 [DOI] [PubMed] [Google Scholar]
- 36. Popa ER, Franssen CF, Limburg PC. et al. In vitro cytokine production and proliferation of T cells from patients with anti-proteinase 3- and antimyeloperoxidase-associated vasculitis, in response to proteinase 3 and myeloperoxidase. Arthritis Rheum 2002; 46: 1894–1904 [DOI] [PubMed] [Google Scholar]
- 37. Summers SA, Steinmetz OM, Gan PY. et al. Toll-like receptor 2 induces Th17 myeloperoxidase autoimmunity while Toll-like receptor 9 drives Th1 autoimmunity in murine vasculitis. Arthritis Rheum 2011; 63: 1124–1135 [DOI] [PubMed] [Google Scholar]
- 38. Morgan MD, Day CJ, Piper KP. et al. Patients with Wegener’s granulomatosis demonstrate a relative deficiency and functional impairment of T-regulatory cells. Immunology 2010; 130: 64–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chavele KM, Shukla D, Keteepe-Arachi T. et al. Regulation of myeloperoxidase-specific T cell responses during disease remission in antineutrophil cytoplasmic antibody-associated vasculitis: the role of Treg cells and tryptophan degradation. Arthritis Rheum 2010; 62: 1539–1548 [DOI] [PubMed] [Google Scholar]
- 40. Schlesier M, Kaspar T, Gutfleisch J. et al. Activated CD4+ and CD8+ T-cell subsets in Wegener’s granulomatosis. Rheumatol Int 1995; 14: 213–219 [DOI] [PubMed] [Google Scholar]
- 41. Weidner S, Carl M, Riess R. et al. Histologic analysis of renal leukocyte infiltration in antineutrophil cytoplasmic antibody-associated vasculitis: importance of monocyte and neutrophil infiltration in tissue damage. Arthritis Rheum 2004; 50: 3651–3657 [DOI] [PubMed] [Google Scholar]
- 42. Iking-Konert C, Vogl T, Prior B. et al. T lymphocytes in patients with primary vasculitis: expansion of CD8+ T cells with the propensity to activate polymorphonuclear neutrophils. Rheumatology (Oxford) 2008; 47: 609–616 [DOI] [PubMed] [Google Scholar]
- 43. McKinney EF, Lyons PA, Carr EJ. et al. A CD8+ T cell transcription signature predicts prognosis in autoimmune disease. Nat Med 2010; 16: 586–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McKinney EF, Lee JC, Jayne DR. et al. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 2015; 523: 612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chang J, Eggenhuizen P, O’Sullivan KM. et al. CD8+ T cells effect glomerular injury in experimental anti-myeloperoxidase GN. J Am Soc Nephrol 2017; 28: 47–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ryan JJ, Mason PJ, Pusey CD. et al. Recombinant alpha-chains of type IV collagen demonstrate that the amino terminal of the Goodpasture autoantigen is crucial for antibody recognition. Clin Exp Immunol 1998; 113: 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pedchenko V, Bondar O, Fogo AB. et al. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med 2010; 363: 343–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang XR, Tipping PG, Shuo L. et al. Th1 responsiveness to nephritogenic antigens determines susceptibility to crescentic glomerulonephritis in mice. Kidney Int 1997; 51: 94–103 [DOI] [PubMed] [Google Scholar]
- 49. Duffield JS, Tipping PG, Kipari T. et al. Conditional ablation of macrophages halts progression of crescentic glomerulonephritis. Am J Pathol 2005; 167: 1207–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tipping PG, Holdsworth SR.. T cells in crescentic glomerulonephritis. J Am Soc Nephrol 2006; 17: 1253–1263 [DOI] [PubMed] [Google Scholar]
- 51. Wu J, Hicks J, Borillo J. et al. CD4(+) T cells specific to a glomerular basement membrane antigen mediate glomerulonephritis. J Clin Invest 2002; 109: 517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Reynolds J, Khan SB, Allen AR. et al. Blockade of the CD154–CD40 costimulatory pathway prevents the development of experimental autoimmune glomerulonephritis. Kidney Int 2004; 66: 1444–1452 [DOI] [PubMed] [Google Scholar]
- 53. Reynolds J, Tam FW, Chandraker A. et al. CD28-B7 blockade prevents the development of experimental autoimmune glomerulonephritis. J Clin Invest 2000; 105: 643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kruger T, Benke D, Eitner F. et al. Identification and functional characterization of dendritic cells in the healthy murine kidney and in experimental glomerulonephritis. J Am Soc Nephrol 2004; 15: 613–621 [DOI] [PubMed] [Google Scholar]
- 55. Fujinaka H, Nameta M, Kovalenko P. et al. Periglomerular accumulation of dendritic cells in rat crescentic glomerulonephritis. J Nephrol 2007; 20: 357–363 [PubMed] [Google Scholar]
- 56. Merkel F, Kalluri R, Marx M. et al. Autoreactive T-cells in Goodpasture’s syndrome recognize the N-terminal NC1 domain on alpha 3 type IV collagen. Kidney Int 1996; 49: 1127–1133 [DOI] [PubMed] [Google Scholar]
- 57. Kawasaki K, Yaoita E, Yamamoto T. et al. Depletion of CD8 positive cells in nephrotoxic serum nephritis of WKY rats. Kidney Int 1992; 41: 1517–1526 [DOI] [PubMed] [Google Scholar]
- 58. Heymann F, Meyer-Schwesinger C, Hamilton-Williams EE. et al. Kidney dendritic cell activation is required for progression of renal disease in a mouse model of glomerular injury. J Clin Invest 2009; 119: 1286–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Agudo J, Ruzo A, Park ES. et al. GFP-specific CD8 T cells enable targeted cell depletion and visualization of T-cell interactions. Nat Biotechnol 2015; 33: 1287–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fu J, Wei C, Lee K. et al. Comparison of glomerular and podocyte mRNA profiles in streptozotocin-induced diabetes. J Am Soc Nephrol 2016; 27: 1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Galluzzi L, Buque A, Kepp O. et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 2017; 17: 97–111 [DOI] [PubMed] [Google Scholar]
- 62. Anders HJ, Banas B, Schlondorff D.. Signaling danger: toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol 2004; 15: 854–867 [DOI] [PubMed] [Google Scholar]
- 63. Boucher A, Droz D, Adafer E. et al. Relationship between the integrity of Bowman’s capsule and the composition of cellular crescents in human crescentic glomerulonephritis. Lab Invest 1987; 56: 526–533 [PubMed] [Google Scholar]
- 64. Rastaldi MP, Ferrario F, Tunesi S. et al. Intraglomerular and interstitial leukocyte infiltration, adhesion molecules, and interleukin-1 alpha expression in 15 cases of antineutrophil cytoplasmic autoantibody-associated renal vasculitis. Am J Kidney Dis 1996; 27: 48–57 [DOI] [PubMed] [Google Scholar]
- 65. Penny MJ, Boyd RA, Hall BM.. Permanent CD8(+) T cell depletion prevents proteinuria in active Heymann nephritis. J Exp Med 1998; 188: 1775–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]