Abstract
It is an ugly fact that a significant amount of the world's population will contract SARS-CoV-II infection with the current spreading. While a specific treatment is not yet coming soon, individual risk assessment and management strategies are crucial. The individual preventive and protective measures drive the personal risk of getting the disease. Among the virus-contracted hosts, their different metabolic status, as determined by their diet, nutrition, age, sex, medical conditions, lifestyle, and environmental factors, govern the personal fate toward different clinical severity of COVID-19, from asymptomatic, mild, moderate, to death. The careful individual assessment for the possible dietary, nutritional, medical, lifestyle, and environmental risks, together with the proper relevant risk management strategies, is the sensible way to deal with the pandemic of SARS-CoV-II.
Keywords: COVID-19, SARS-CoV-II, Diet, Nutrition, Lifestyle, Smoking, Herbs, Antiviral medication
Highlights
-
•
A significant amount of the world's population will contract COVID-19 infection.
-
•
Individual risk assessment and management strategies are crucial.
-
•
Metabolic status determines the clinical severity of COVID-19, from asymptomatic to death.
-
•
Important factors include diet, nutrition, age, sex, health, lifestyle, and environment.
1. Introduction
In December 2019, several unidentified pneumonia cases occurred in Wuhan, China. On 30 January 2020, this led the World Health Organization (WHO) to declare a public health emergency of international concern. On 12 March 2020, the WHO declared the outbreak of the 2019 novel coronavirus, a global pandemic [[1], [2], [3]]. The WHO suggested the official name for the disease from this virus as the coronavirus disease 2019 (COVID-19). The Coronaviridae Study Group of the International Committee on Taxonomy of Viruses proposed the name of the virus as ‘severe acute respiratory syndrome coronavirus 2 (SARS-CoV-II)’, designated by its phylogeny and taxonomy [4]. Up to 4 April 2020, there are registered 1,117,942 confirmed cases and 59,201 deaths worldwide [5].
COVID-19 is the third-known zoonotic disease from coronavirus after severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) [6]. SARS-CoV-II belongs to the family of coronaviridae and the genus of betacoronavirus, which includes SARS-CoV and MERS-CoV [[7], [8], [9]]. The current data suggest the mortality rate from COVID-19 at 2–5%, which is substantially lower than the mortality rate of 10% and 40% in SARS and MERS, respectively [10]. However, the concerning high transmissibility of SARS-CoV-II, with the basic reproduction number (R0) at 1.4–5.5, make it a rapidly spreading disease, as compared to the R0 of SARS-CoV and MERS-CoV at 2–5 and less than 1, respectively [11,12].
The viral genome analysis suggested that the SARS-CoV-II was a recombinant virus between the bat coronavirus and a coronavirus of unknown origin [13,14]. While the human transmission is presumably from the animals, it is still inconclusive whether the animal origins in the human transmission chain are from bats, snakes, or others [11,15,16]. However, there were positive virus findings in the environmental samples from the seafood industry and the seafood markets. The respiratory droplets from coughing or sneezing are the primary mediums for human-to-human transmission [17]. The frequent symptoms of respiratory illnesses, i.e., a fever higher than 38.1 °C (98% of patients), coughing fits (76% of patients), and finally severe fatigue or myalgia (44% of patients) have been reported in several patients [18]. Dyspnea (55% of patients) appears after eight days and is the first severe complication of the disease. However, headache, diarrhea, hemoptysis, and dyspnea have been reported as clinical manifestations of COVID-19 [18,19]. A study from China also reported that the majority of patients (80.9%) were considered to have mild pneumonia or being asymptomatic, which posed big challenges for the spreading of COVID-19 [20]. The close contact to infected individuals, either asymptomatic or clinical COVID-19 cases, increase the risk of infection. The monitor of a cluster of COVID-19 cases in China suggested the possibility of indirect viral transmission without a history of close physical connection to the infected individuals. The viral transfer is possible through the common contaminated objects, viral aerosolization in confined space, or from the asymptomatic viral carriers [21].
The general preventive guidelines include frequent hand washing, mouth and nose covering during coughing, sneezing, and cooking. Social distancing helps to avoid close contact with symptomatic and asymptomatic individuals [17]. Rapid identification, isolation, and treatment of the patients impact the spreading of SARS-CoV-II. The host metabolic conditions also determine the clinical course and outcomes of COVID-19. As there is no specific treatment for COVID-19, most of the case management is supportive and symptomatic measures.
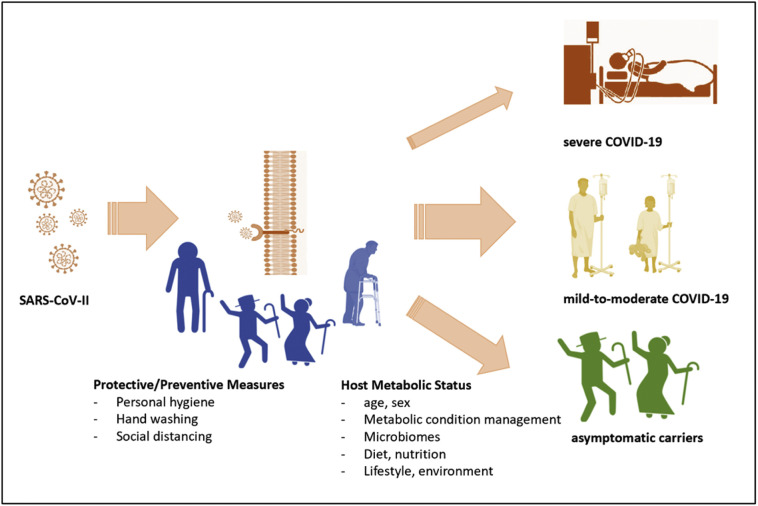
With global pandemic spreading, most of us may get the SARS-CoV-II infection at a certain period. Therefore, individual actions that minimize the infection risk and modulate the severity of the clinical courses are crucial amidst all public healthcare measures. Fig. 1 represents this conceptual framework. In this article, we reviewed the personal preventive measures, the predisposing host factors, some potential therapeutically options, and suggested a comprehensive approach for COVID-19 management.
Fig. 1.
The schematic representation of individual risk management strategies to reduce the risk of SARS-CoV-II infection and moderate the severity of COVID-19.
2. Personal hygiene and social distancing determine the individual risk of SARS-CoV-II infection
While the SARS-CoV-II remains viable in the aerosols for only three hours, it can live on the different surface materials up to three days on polypropylene plastic, couple days on stainless steel, twenty-four hours on cardboard, and four hours on copper [22]. The increased temperature and humidity can reduce the transmission of COVID-19 for a certain degree [23]. The surface disinfectants, including 62–71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite, can efficiently inactivate SARS-CoV-II within one minute. In contrast, other biocidal agents, such as 0.05–0.2% benzalkonium chloride, or 0.02% chlorhexidine digluconate, are less effective [24]. The avoidance of these contaminated mediums is, therefore, the critical preventive measure, together with the social distancing from the possible infected individual.
Personal hygiene could reduce individual exposure to SARS-CoV-II contaminated surfaces. These measures include regular hand washing, particularly after sneezing, coughing, exposure to the public washroom, or before the meal preparation. The regular cleaning of the public-touch surfaces and the common tools and utensils with disinfectants is also necessary. The avoidance of face, eyes, nose, and mouth touching reduces the introduction of contaminated hands to the respiratory mucosal surfaces [25].
Social distancing prevents contact with the aerosol droplets from infected individuals or asymptomatic carriers. The face mask may not be necessary for a healthy person. While the face-covering provides a sense of protection in people, the inappropriate use and disposal of the mask may increase their infection risks. Hence, there is a recommendation to use a face mask only in individuals with symptoms or under quarantines [26]. With the spreading of SARS-CoV-II, one can reduce their viral contracting risk through these preventive measures.
3. The host metabolic status determines the clinical course of COVID-19
Reports from China and Italy suggested a high mortality rate of COVID-19 in older male patients who had multiple metabolic comorbidities [19,27]. The host metabolic status, as influenced by age, sex, medical conditions, and lifestyle factors such as cigarette smoking, determine the clinical severity of COVID-19 [28,29].
3.1. Chronic disease
A new study is refining our knowledge of the symptoms caused by the COVID-19 epidemic, which affects the world's population. It also highlights the presence of aggravating factors in the most severe cases. When looking at critically ill patients, two prevalent illnesses appear to worsen COVID-19 infection. The first is hypertension, since 23.7% of patients in critical condition are suffering from it. In the second position comes diabetes, without a distinction of the type being made, which affects 16.2% of the most severe cases [30,31].
Moreover, the comorbidities of coronary heart disease (5·8%), and cerebrovascular disease (2·3%) have been reported in severe cases [32]. Bad lifestyle habits, such as smoking, can also play a role. While 85.6% of the infected patients are non-smokers, 16.9% of severe cases declared that they used tobacco (compared to 11.8% of less severe cases) [33]. Co-existing metabolic diseases include type 2 diabetes, hypertension, heart disease, a history of stroke, and cancers. Therefore, the host predisposing factors significantly determine the illness course, the progression, and the outcome of COVID-19 [10,17].
3.2. Age and sex
The elderly are more prone to infection, including respiratory diseases than young people, while accompanied by the increased mortality rate. These age-related immunologic changes are probably the results of primary or secondary structural and functional changes of bone marrow, thymus, lymphoid organs, and immune cells [34]. Despite the multifaceted process of aging, the elders commonly have a decreased ability to fight infection, diminished response to the vaccine, increased prevalence of autoimmunity, and constitutive low-grade inflammation [34]. Conforming to this fact, the mortality rate from COVID-19 is increasing in older adults in the clinical settings [19,27,28].
The study of blood mononuclear cells suggested that the epigenomic signature of declining naïve T cells and increasing monocyte and cytotoxic cell functions were higher in male than female elders [35]. The age-related immunologic changes and sex differences first occurred around the age of late-thirties, with the second-biggest spike after the age of sixty-five. The older man is more prone to infectious diseases with high pro-inflammatory immune responses and low adaptive immune responses than an older woman [35,36]. The COVID-19 cases in both China and Italy reported higher fatality in males than women [19,27].
There are connections between age, diet, nutrients, and immunity in the elders [37]. The clinical or subclinical micronutrient deficiency is common in older adults, which contributes to several age-related diseases and decreased immune functions [38,39]. This prevalence is probably the consequence of the low appetite and the nature of little diversification of their dietary patterns in the elders. The nutritional assessment and proper management are, therefore, essential to determine the risk of infection, the illness course, and the outcome of COVID-19 in older adults [25].
3.3. Microbiota
The diverse intestinal microbiota shapes the immune system and promotes the host well-being [40,41]. The respiratory tract microbiota also influences the host immune responses to the virus [42]. While the immune responses to viral infection determine the efficacy of a vaccine, the disrupted gut microbiota contribute to vaccine failures and other inflammatory conditions [43]. The acute respiratory viral infections disrupt the host-microbiota interactions and create the intestinal dysbiosis with the post-viral immune responses, that contribute to pneumonia development by the secondary bacterial infection [44]. The healthy, diverse intestinal and respiratory tract microbiota is then another critical determinant for the clinical courses of COVID-19 [42,45].
Interferons (IFNs) are the first line of immune defense against viral infection, particularly the type I IFNs and the type III IFNs, or IFN-λs [46]. Despite the preliminary understanding of their roles, IFN-λs are probably the critical antiviral cytokines in the respiratory epithelial surfaces during the early stages of viral infection. While the type I IFN signaling during acute viral infection increase the pro-inflammatory responses, their signaling in the persistent infection modulates the counter-regulatory immune responses [47,48]. Some gut microbiomes mediate the IFN responses to viral infection through their metabolites, such as the Clostridium orbiscindens-derived desaminotyrosine [49]. Certain strains of Lactobacillus also influence the IFN responses following the influenza infection [50].
Host dietary pattern is the pivotal determinant of gut microbiota community, structure, and function [51,52]. In general, the balanced diet with a variation of the prebiotic fibers, probiotics, and polyphenols, promote the healthy, diverse microbiota [53]. Improving the diet quality in susceptible individuals for COVID-19 might alleviate their risk of severe infection [54]. Despite the inconclusive pieces of evidence, oral probiotics are expecting to be the rational adjunctive option in various viral disease management [[55], [56], [57]].
4. The host macro- and micronutrient status as the preventive measures for COVID-19
Diet and nutrition invariably influence the immune system competence and determine the risk and severity of infections. There are bi-directional relationships among diet, nutrition, infection, and immunity. The changes in one component have an impact on the others [58]. The macro-, micronutrients, and phytonutrients in diet, mainly the fruits and colorful vegetables, generally promote healthy immune responses. These micro- and phytonutrients provide the antioxidants and the anti-inflammatory nutrients, including beta-carotene, vitamin C, vitamin E, and polyphenolic compounds, which modulate the immune functions [30,59]. The anti-inflammatory strategy, either by foods, nutrients, or medicines, is a viable option for COVID-19 management [60,61]. Apart from the age-related micronutrient insufficiency, as previously mentioned, the nutritional status of an individual affects the risk of SARS-CoV-II infection, the clinical course, and the outcomes of COVID-19. Therefore, the maintenance of host macro- and micronutrient status is an important preventive measure for COVID-19.
Numerous micronutrients are essential for immunocompetence, particularly vitamin A, C, D, E, Bs, iron, selenium, and zinc. Dietary pattern is vital for maintaining the individual nutritional status. However, diet alone may not be sufficient in certain metabolic and lifestyle conditions, including advancing age, co-existing medical condition, cigarette smoking, or occupational exposure to environmental toxins [58]. We herein reviewed some micronutrients that require attention for risk reduction and clinical course modulation of COVID-19.
4.1. Vitamin D
Several meta-analysis and systematic reviews supported the protective role of vitamin D supplementation for the prevention of acute respiratory tract infection [[62], [63], [64], [65]]. The effective supplementation needs to start before the onset of respiratory tract infection. However, there is inconclusive evidence regarding the underlying mechanisms of vitamin D deficiency and viral disease development [66]. The potential mechanisms include the antiviral immune induction, the modulation of immunoregulatory defense, induction of autophagy and apoptosis, and genetic or epigenetic regulation [66]. Furthermore, the risk of viral infections can be reduced by vitamin D. The related mechanisms comprised of stimulation of defensins and cathelicidins that can decrease the replication of virus and increase levels of anti-inflammatory cytokines, as well as decreasing concentrations of pro-inflammatory cytokines that induce inflammation-related pneumonia [67]. Supportive data for the effective role of vitamin D in decreasing risk of COVID-19 could be highlighted by increased case-fatality rates with chronic disease comorbidity and age, in which lower concentrations of 25(OH)D have been reported. Vitamin D deficiency is globally prevalent, particularly in the elders [68]. Over half of the hospitalized elders and nursing home residents in the U.S. had vitamin D deficiency [69,70]. This high prevalence probably contributes to the first outbreak COVID-19 during winter and the high mortality rate in older adults [[71], [72], [73], [74]]. While the natural source of vitamin D is from sunlight exposure, some dietary sources can provide a certain amount of vitamin D, including the fortified cereals and milk. However, for people at risk of COVID-19, the goal should be to raise the concentrations of 25(OH)D above 40–60 ng/ml (100–150 nmol/l) by considering taking 10,000 IU/d of vitamin D3 for a few weeks to rapidly raise 25(OH)D concentrations, followed by 5000 IU/d. [67,75].
4.2. Vitamin A
Despite the established roles of vitamin A in supporting the immune functions, there is inadequate evidence to support the supplementation benefit in healthy individuals for the prevention of acute viral respiratory infection [76]. However, vitamin A deficient people are prone to the increased risk, the high severity, and the impaired immune responses to viral infections, including the respiratory syncytial virus, measle virus, and the influenza virus [77,78]. The immune-supporting roles of vitamin A include the promotion of mucins and keratins, lymphopoiesis, apoptosis, cytokine expression, antibody production, and the enhanced functions of neutrophils, natural killer cells, monocytes or macrophages, T cells, and B cells [79,80].
4.3. Vitamin C
Vitamin C deficiency is associated with pneumonia in several pieces of literature in the early days [81,82]. The immune-modulating effects in respiratory infection of vitamin C are also well-documented [[83], [84], [85], [86]]. Nevertheless, the supporting evidence of vitamin C supplementation in the prevention and treatment of acute respiratory diseases are inconclusive [[87], [88], [89], [90]]. In alignment with the evidence of other micronutrients, the supplementation could benefit the vitamin C deficient individual but not in the healthy subjects [87,88]. Moreover, it has been reported that megadoses administration of Vitamin C before or after the appearance of flu symptoms could prevent and relieve the flu symptoms in the test population regarding the control group [83,86,91,92]. Based on 31 study comparisons with 9745 common cold episodes, it has been revealed that the regular supplementation of Vitamin C had a modest but consistent effect in decreasing the duration of common cold symptoms [89,90]. Furthermore, five trials with 598 participants showed that vitamin C decreased the risk of common cold without any adverse effects [93]. Vitamin C supplementation is thus the sensible option to prevent and support the immune responses in the micronutrient-deficit individual at risk for COVID-19.
4.4. Selenium
As the integral part of several selenoproteins, including the glutathione peroxidases and thioredoxin reductases, selenium has a critical role in the defense against viral infection through its antioxidant, redox signaling, and redox homeostatic contributions [94]. Selenium deficiency is associated with increased pathogenicity of several virus infections [[95], [96], [97]]. In the deficient state, the selenium supplementation is helpful for the prevention and treatment of viral infections [[97], [98], [99], [100]]. Recently, it has been reported that a mild strain of influenza virus, also shows increased virulence in selenium-deficient mice. Increased virulence is related to several modifications in the viral genome [95,101]. Furthermore, the immune response, such as proinflammatory chemokines, can be increased in selenium-deficient mice. Moreover, the mRNA expression of macrophage inflammatory protein-1α and -1β, monocyte chemotactic protein-1, and RANTES (regulated upon activation, normal T cell expressed and secreted) were changed in selenium-deficient mice. The mRNA levels of cytokine were also modified in the selenium-deficient mice. IL-4, IL-5, IL-10, and IL-13 were increased, whereas γ-interferon and Interleukin (IL)-2 were decreased, which suggests a modification toward a pattern of T-helper-2-like in the Se-deficient mice regarding the pattern of T-helper-1-like in the Se-adequate mice [95]. Therefore, selenium intake differentially affects numerous types of immune responses and related mechanisms, revealing an effective role of selenium-supplementation in viral diseases.
4.5. Zinc
Zinc is an essential micronutrient with the crucial contributions to most enzymatic functions and the transcription regulations in the human body [30,59]. Zinc is essential for normal function and development of cells regulating nonspecific immunity, including natural killer cells and neutrophils. Zinc is the main structural component of around 750 zinc-finger transcription factors [102]. The deficiency of zinc also modifies the development of acquired immunity by limiting both the certain and outgrowth functions of T lymphocytes, including the production and activation of Th1 cytokine [103]. The function of macrophage also is adversely affected by the deficiency of zinc through the dysregulation of cytokine production, intracellular killing, and phagocytosis [103]. Zinc deficiency is surprisingly common in modern-day lifestyle [104]. Zinc deficiency impairs the antiviral immunity, particularly to herpes simplex, common cold, herpes simplex virus, hepatitis C, and the human immunodeficiency virus (HIV) [104,105]. A meta-analysis of oral zinc supplementation studies suggested beneficial effects on the shortened of symptoms and duration of common cold infection [[106], [107], [108]]. Zinc supplementation was also helpful against hepatitis C virus infection through the induction of metallothionein expressions [109,110]. Moreover, research has shown that zinc has antiviral effects; it improves immune responses and suppresses viral replication. Therefore, the consumption of up to 50 mg zinc per day may provide a protective role against the COVID-19 pandemic, likely by improving the host's resistance against viral infection [102]. However, these studies did not account for the underlying zinc status in the studied participants.
With the established role of nutritional status on host immunity, the individual nutritional evaluation is probably essential to prepare someone for the SARS-CoV-II pandemic. When improving the nutritional status, either through dietary modifications or nutritional supplementation, it is pivotal to determine the clinical course of COVID-19, particularly in nutrient-deficient individuals.
5. Potential therapeutic options for COVID-19
There is yet no specific treatment for COVID-19. Therefore, physicians are trying to fight the coronavirus with existing treatments. Patients admitted to the hospitals are administered intravenous antibiotics (57.5% of cases), prescribe oseltamivir, an oral antiviral (35.8% of cases), and corticosteroids (18.6% of cases). This protocol is accompanied by oxygen therapy and non-invasive ventilation for the most severely affected patients [111]. Even with all those preventive and protective measures, there are still the chances of getting the SARS-CoV-II infection. Without the specific treatment for COVID-19, we here explore some potential therapeutic options of some prescribed medications and herbs.
5.1. Antiviral medications and herbs
The antiviral medications target several components of the SARS-CoV-II lifecycle. These molecular targets include the viral entry into the host cells, the viral RNA synthesis, and the viral replication [112]. There are high sequence similarities in the genomes of SARS-CoV-II, SARS-CoV, and MERS-CoV. It is possible for the shared effectiveness of the previously approved medications in these conditions for the treatment of COVID-19.
5.2. The blockages of the virus entry into the host cells
5.2.1. Chloroquine and hydroxychloroquine
These viral entry blockages include chloroquine, hydroxychloroquine umifenovir, and interferon [112]. A cell line study reported that chloroquine significantly decreased the human coronavirus-229E replication at a lower concentration than the clinical dosage [113]. A systematic review suggested the rationale, pre-clinical supporting evidence of the effectiveness against SARS-CoV-II, and the clinical safety profiles, that justify future clinical research of chloroquine and hydroxychloroquine in patients with COVID-19 [114].
There are currently several clinical trials of chloroquine for COVID-19, either as monotherapy or in combination with other medications such as azithromycin [112]. A non-randomized clinical trial reported the reduction of the viral load from the hydroxychloroquine-azithromycin combination in twenty COVID-19 patients but failed to report the critical clinical outcomes, including death [115]. Chloroquine and hydroxychloroquine are the immunomodulatory drugs with potential antiviral effects. However, there are some long-known clinical side-effects and interactions with other medications. It is still premature to conclude the role of chloroquine and hydroxychloroquine in COVID-19, while several clinical trials are on their ways [116].
5.2.2. The transmembrane protease, serine 2 (TMPRSS2) inhibitor
SARS-CoV -II enters the target cells through the angiotensin-converting enzyme 2 (ACE2) receptor and the transmembrane protease, serine 2 (TMPRSS2). The TMPRSS2 inhibitors block the cellular entry of the SARS-CoV-II virus through the downregulated priming of the SARS-CoV-II spike protein [117,118]. There is a known TMPRRSS2 inhibitor in the market, i.e., camostat mesylate. Machine learning algorithms have been used to predict the dynamics of this pathway, including Janus-associated kinase (JAK) inhibitors through baricitinib, ruxolitinib, and imatinib [119,120]. Currently, some of these drugs are being tested in clinical trials in the treatment of COVID-19.
5.3. The inhibitors of viral RNA synthesis
5.3.1. Remdesivir
The medications that inhibit viral RNA synthesis include remdesivir, favipiravir, and ribavirin. Remdesivir is a novel nucleotide analog with the broad-spectrum antiviral activities against the single-stranded RNA viruses, including the Ebola virus, Marburg virus, respiratory syncytial virus, Junin virus, Lassa fever virus, Nipah virus, Hendra virus, and the coronaviruses [[121], [122], [123], [124]]. Remdesivir inhibits the RNA-dependent RNA polymerase, which crucially replicates copies of viral RNA in the host cells. The animal models and cell line studies suggested the effectiveness of remdesivir to selectively inhibit the infection and pathology of MERS-CoV and SARS-CoV-II [125,126]. While the proofreading exoribonuclease hampers the effects of most nucleotide-base antiviral treatment, remdesivir inhibits coronavirus with the intact proofreading, thus renders its superior antiviral efficacy [127]. The experimental treatment of intravenous remdesivir in the first COVID-19 patient in the U.S. showed an impressive response [128]. There is a current randomized, placebo-controlled, double-blind, multicenter, phase III clinical trial to determine the efficacy and safety of remdesivir in COVID-19 [129].
5.3.2. Favipiravir
Favilavir is a guanine analog with the broad-spectrum antiviral activities through its selective inhibition of viral RNA-dependent RNA polymerase [130]. Favilavir has efficacy against various RNA viruses, including influenza, ebola, yellow fever, chikungunya, norovirus, and enterovirus [131,132]. A recent cell line study suggested its efficacy against the SARS-CoV-II [133]. While it got the approval for novel influenza treatment, favipiravir is currently on the clinical trials for COVID-19 treatment by the National Infectious Diseases Scientific Science Research Center and the Shenzhen Third People's Hospital [134]. The preliminary results in eighty patients reported the superior efficacy of favipiravir than the lopinavir/ritonavir combination without the significant adverse reactions [135,136].
5.4. The viral replication inhibitors
5.4.1. The lopinavir-ritonavir combination
The medications that block the virus replication include lopinavir-ritonavir combination and darunavir-cobicistat combination. The lopinavir-ritonavir combination is a fixed-dose medication for the prevention and treatment of HIV infection [137]. The cytochrome P450 inhibitory effects of ritonavir prolonged the half-life of Lopinavir and extended its protease inhibitory action on the HIV replications. The in-vitro studies suggested that the lopinavir/ritonavir combination can inhibit coronavirus replication.
There were case reports from China, Japan, and Thailand for the effectiveness of the lopinavir-Ritonavir combination in COVID-19 [138]. However, the recent clinical trial reported no benefit of the lopinavir-ritonavir combination treatment beyond the standard care in hospitalized adults with severe COVID-19 [139]. Nonetheless, there are several ongoing clinical trials of COVID-19 and the lopinavir-Ritonavir combination, either alone or together with other drugs. The potential synergetic treatments include the combinations with interferons, guanosine-analog RNA synthesis inhibitors, reverse transcriptase inhibitors, or influenza drugs, such as baloxavir marboxil, oseltamivir, and umifenovir [112,125,[140], [141], [142]].
5.4.2. Darunavir
Darunavir is another anti-retroviral protease inhibitor that often uses in combination with other cytochrome P450 inhibitors, such as ritonavir or cobicistat, for the treatment of HIV infection [143]. The in-vitro studies suggested the inhibitory action of SARS-CoV-II replication [144]. It is still under the evaluation trials for the efficacy against COVID-19 [112].
5.4.3. Viperin, emodin, and promazine
There was the in-vitro evidence of inhibitory action on the viral replication of viperin among a broad spectrum of DNA and RNA viruses, including herpes viruses, West Nile virus, dengue virus, sindbis virus, influenza A virus, dendai virus, and HIV-1 [145]. A traditional Chinese medicinal herb, emodin, is an anthraquinone compound that exhibits the interaction of the SARS-CoV-II spike proteins and ACE2 in the cell line studies [146,147]. The antipsychotic drug, promazine, has a similar structure to emodin on its spike protein binding site structure that contributes to the replication suppression of SARS-CoV. Promazine displayed the more potent spike-protein-mediated ACE2 binding inhibition than emodin [30,148]. Despite all these in-vitro evidence, there is still no confirming evidence of these compounds in the clinical COVID-19 patients [149].
5.4.4. Herbs with 3 chymotrypsin-like protease inhibitory activities
The 3 chymotrypsin-like protease (3CLpro) is essential for the replication of coronavirus, including MERS-CoV and SARS-COV, which lead to the potential therapeutic benefit of its inhibitors [150,151]. The Chinese herb, cinanserin, is a serotonin receptors antagonist that may inhibit the 3CLpro and inhibit the SARS-CoV replication [152,153]. Some polyphenol compounds also exhibit the 3CLpro inhibitory effect, such as the antioxidant flavonoids. The in-vitro studies demonstrated that various flavonoids suppress the hepatitis C virus, MERS-CoV, and SARS-CoV, through their 3CLpro inhibitory effects. These flavonoids include herbacetine, isobavachalcone, quercetin, and helichrysetin, rhoifolin, and pectolinarin, [[154], [155], [156]]. With the upregulated expression of 3CLpro during COVID-19, the 3CLpro inhibitory herbs can be the sensible options in the COVID-19management [157].
5.5. Other potential therapeutic options
5.5.1. The human convalescent plasma
The human convalescent plasma from the recovered patients can be another option for COVID-19 management [158,159]. The passive immunoglobulin-containing plasma can provide immediate immunity to the susceptible individual. There is a long history of this passive antibody treatment in various infective diseases beyond the era of antimicrobial development [160,161]. A meta-analysis suggested the beneficial role of early administration of convalescent plasma on the mortality reduction during influenza epidermic in 1918 [159]. There is no report of serious adverse effects of the treatment up to now. The convalescent plasma from the patients who have recovered from the viral infection is thus another rational option for COVID-19 management [160,161].
5.6. The anticipating options
5.6.1. The monoclonal antibodies
The monoclonal antibodies are the well-recognized passive immunotherapeutic options in many diseases. This human-made antibody can specifically bind to the designated target, thus involves in its molecular mechanisms and provides the desirable effects, which can either inhibit or enhance those molecular pathways [162]. With the updated knowledge of the SARS-CoV-II molecular mechanisms, there are several studies on monoclonal antibody and their trials for COVID-19, conducted by many pharmaceutical companies. Some previously approved drugs for other conditions and several novel drugs target various molecular targets of SARS-CoV II infection, with the promising therapeutic outcome for COVID-19 management soon [163,164]. These clinical trials include the monoclonal antibodies that target the pathogenic and pathophysiologic processes of COVID-19. These trials comprise the tocilizumab, which targets the interleukin-6 receptor and possibly mediates the SARS-CoV II-mediated inflammation and modulates the cytokine storms, and several neutralized monoclonal antibodies targeting the SARS-CoV and MERS-CoV molecular mechanism [163,165].
5.6.2. Vaccines
Several companies and research groups initiate the development of potential vaccines for COVID-19. These companies include Pfizer, GlaxoSmithKline, Johnson & Johnson, and many others. However, these trials are still in their early stages and require a certain period until their potential clinical launches. This anticipating option will not come soon [[166], [167], [168]].
6. Concluding remarks
It is a great tragedy for the ugly fact that a lot of world population will contract SARS-CoV infection. While specific treatment is not yet coming soon, individual preventive and protective measures drive the personal risk of getting the disease. Among the virus-infected hosts, their different metabolic status, as determined by their diet, nutrition, age, sex, medical conditions, lifestyle, and environmental factors, govern the personal fate toward different clinical severity of COVID-19. The individual assessment for the possible dietary, nutritional, lifestyle, and environmental risks, together with the proper risk management, is the sensible way to deal with the pandemic of SARS-CoV-II.
References
- 1.World Health Organization International Health Regulations Emergency Committee on novel coronavirus in China. https://www.who.int/news-room/events/detail/2020/01/30/default-calendar/international-health-regulations-emergency-committee-on-novel-coronavirus-in-china (Accessed: 7 April 2020)
- 2.Peeri N.C., Shrestha N., Rahman M.S., Zaki R., Tan Z., Bibi S., Baghbanzadeh M., Aghamohammadi N., Zhang W., Haque U. The SARS, MERS and novel coronavirus (COVID-19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int. J. Epidemiol. 2020 doi: 10.1093/ije/dyaa033. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization WHO announces COVID-19 outbreak a pandemic. http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic (Accessed: 7 April 2020)
- 4.Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J., V. Coronaviridae Study Group of the International Committee on Taxonomy of The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020 doi: 10.1038/s41564-020-0695-z. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Worldometer. Covid-19 Coronavirus Pandemic. https://www.worldometers.info/coronavirus (Accessed: 7 April 2020).
- 6.Dhama K., Sharun K., Tiwari R., Dadar M., Malik Y.S., Singh K.P., Chaicumpa W. COVID-19, an emerging coronavirus infection: advances and prospects in designing and developing vaccines, immunotherapeutics, and therapeutics. Hum. Vaccin Immunother. 2020:1–7. doi: 10.1080/21645515.2020.1735227. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phan M.V.T., Ngo Tri T., Hong Anh P., Baker S., Kellam P., Cotten M. Identification and characterization of Coronaviridae genomes from Vietnamese bats and rats based on conserved protein domains. Virus Evol. 2018;4:vey035. doi: 10.1093/ve/vey035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J. Med. Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Y.C., Chen C.S., Chan Y.J. The outbreak of COVID-19: an overview. J. Chin. Med. Assoc. 2020;83:217–220. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benvenuto D., Giovanetti M., Ciccozzi A., Spoto S., Angeletti S., Ciccozzi M. The 2019-new coronavirus epidemic: evidence for virus evolution. J. Med. Virol. 2020;92:455–459. doi: 10.1002/jmv.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khachfe H., Chahrour M., Sammouri J., Salhab H., Makki B., Fares M. An epidemiological study on COVID-19: a rapidly spreading disease. Cureus. 2020;12:e7313. doi: 10.7759/cureus.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji W., Wang W., Zhao X., Zai J., Li X. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020;92:433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malik Y.S., Sircar S., Bhat S., Sharun K., Dhama K., Dadar M., Tiwari R., Chaicumpa W. Emerging novel coronavirus (2019-nCoV)-current scenario, evolutionary perspective based on genome analysis and recent developments. Vet. Q. 2020;40:68–76. doi: 10.1080/01652176.2020.1727993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee A., Kulcsar K., Misra V., Frieman M., Mossman K. Bats and coronaviruses. Viruses. 2019;11:41. doi: 10.3390/v11010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun P., Lu X., Xu C., Sun W., Pan B. Understanding of COVID-19 based on current evidence. J. Med. Virol. 2020 doi: 10.1002/jmv.25722. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cascella M., Rajnik M., C. A, Features . StatPearls Publishing; Treasure Island (FL): 2020. Evaluation and Treatment Coronavirus (COVID-19), Updated 2020 Mar 8 ed.https://www.ncbi.nlm.nih.gov/books/NBK554776/ [PubMed] [Google Scholar]
- 18.Adhikari S.P., Meng S., Wu Y.-J., Mao Y.-P., Ye R.-X., Wang Q.-Z., Sun C., Sylvia S., Rozelle S., Raat H. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect. Dis. Poverty. 2020;9:29. doi: 10.1186/s40249-020-00646-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J.a., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J. Med. Virol. 2020 doi: 10.1002/jmv.25748. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai J., Sun W., Huang J., Gamber M., Wu J. Indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2606.200412. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J., Tang K., Feng K., Lv W. High Temperature and High Humidity Reduce the Transmission of COVID-19 (March 9, 2020) Available at SSRN: (Accessed: 7 April 2020) [DOI]
- 24.Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104:246–251. doi: 10.1016/j.jhin.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19—studies needed. N. Engl. J. Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 26.Hellewell J., Abbott S., Gimma A., Bosse N.I., Jarvis C.I., Russell T.W., Munday J.D., Kucharski A.J., Edmunds W.J., Sun F. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob. Health. 2020;8:e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020 doi: 10.1001/jama.2020.4683. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 28.Weiss P., Murdoch D.R. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395:1014–1015. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vardavas C.I., Nikitara K. COVID-19 and smoking: A systematic review of the evidence. Tob. Induc. Dis. 2020;18:20. doi: 10.18332/tid/119324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020 doi: 10.1038/s41569-020-0360-5. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020;8:e21. doi: 10.1016/S2213-2600(20)30116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir. Med. 2020;8:e20. doi: 10.1016/S2213-2600(20)30117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sadighi Akha A.A. Aging and the immune system: an overview. J. Immunol. Methods. 2018;463:21–26. doi: 10.1016/j.jim.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Márquez E.J., Chung C.-h., Marches R., Rossi R.J., Nehar-Belaid D., Eroglu A., Mellert D.J., Kuchel G.A., Banchereau J., Ucar D. Sexual-dimorphism in human immune system aging. Nat. Commun. 2020;11:751. doi: 10.1038/s41467-020-14396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaillon S., Berthenet K., Garlanda C. Sexual dimorphism in innate immunity. Clin. Rev. Allergy Immunol. 2019;56:308–321. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y., Li W., Zhang Q., Zhang L., Cheung T., Xiang Y.-T. Mental health services for older adults in China during the COVID-19 outbreak. Lancet Psychiatry. 2020;7:e19. doi: 10.1016/S2215-0366(20)30079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffman R. Micronutrient deficiencies in the elderly - could ready meals be part of the solution? J. Nutr. Sci. 2017;6:e2. doi: 10.1017/jns.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conzade R., Koenig W., Heier M., Schneider A., Grill E., Peters A., Thorand B. Prevalence and predictors of subclinical micronutrient deficiency in German older adults: results from the population-based KORA-age study. Nutrients. 2017;9 doi: 10.3390/nu9121276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy M., Thaiss C.A., Elinav E. Metabolites: messengers between the microbiota and the immune system. Genes Dev. 2016;30:1589–1597. doi: 10.1101/gad.284091.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y., Hooper L.V. Immune control of the microbiota prevents obesity. Science. 2019;365:316–317. doi: 10.1126/science.aay2057. [DOI] [PubMed] [Google Scholar]
- 42.Kumpitsch C., Koskinen K., Schöpf V., Moissl-Eichinger C. The microbiome of the upper respiratory tract in health and disease. BMC Biol. 2019;17:87. doi: 10.1186/s12915-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vlasova A.N., Takanashi S., Miyazaki A., Rajashekara G., Saif L.J. How the gut microbiome regulates host immune responses to viral vaccines. Curr. Opin. Virol. 2019;37:16–25. doi: 10.1016/j.coviro.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanada S., Pirzadeh M., Carver K.Y., Deng J.C. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front. Immunol. 2018;9:2640. doi: 10.3389/fimmu.2018.02640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Samuelson D.R., Welsh D.A., Shellito J.E. Regulation of lung immunity and host defense by the intestinal microbiota. Front. Microbiol. 2015;6:1085. doi: 10.3389/fmicb.2015.01085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou J., Wang Y., Chang Q., Ma P., Hu Y., Cao X. Type III interferons in viral infection and antiviral immunity. Cell. Physiol. Biochem. 2018;51:173–185. doi: 10.1159/000495172. [DOI] [PubMed] [Google Scholar]
- 47.Teijaro J.R. Type I interferons in viral control and immune regulation. Curr. Opin. Virol. 2016;16:31–40. doi: 10.1016/j.coviro.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murira A., Lamarre A. Type-I interferon responses: from friend to foe in the battle against chronic viral infection. Front. Immunol. 2016;7:609. doi: 10.3389/fimmu.2016.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vouloumanou E.K., Makris G.C., Karageorgopoulos D.E., Falagas M.E. Probiotics for the prevention of respiratory tract infections: a systematic review. Int. J. Antimicrob. Agents. 2009;34:197.e1–197.e10. doi: 10.1016/j.ijantimicag.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 50.Hori T., Kiyoshima J., Shida K., Yasui H. Augmentation of cellular immunity and reduction of influenza virus titer in aged mice fed Lactobacillus casei strain Shirota. Clin. Diagn. Lab. Immunol. 2002;9:105–108. doi: 10.1128/CDLI.9.1.105-108.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kolodziejczyk A.A., Zheng D., Elinav E. Diet–microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019;17:742–753. doi: 10.1038/s41579-019-0256-8. [DOI] [PubMed] [Google Scholar]
- 52.Zmora N., Suez J., Elinav E. You are what you eat: diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019;16:35–56. doi: 10.1038/s41575-018-0061-2. [DOI] [PubMed] [Google Scholar]
- 53.Singh R.K., Chang H.-W., Yan D., Lee K.M., Ucmak D., Wong K., Abrouk M., Farahnik B., Nakamura M., Zhu T.H., Bhutani T., Liao W. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017;15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trompette A., Gollwitzer E.S., Pattaroni C., Lopez-Mejia I.C., Riva E., Pernot J., Ubags N., Fajas L., Nicod L.P., Marsland B.J. Dietary fiber confers protection against flu by shaping Ly6c− patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity. 2018;48:992–1005.e8. doi: 10.1016/j.immuni.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 55.Kanauchi O., Andoh A., AbuBakar S., Yamamoto N. Probiotics and paraprobiotics in viral infection: clinical application and effects on the innate and acquired immune systems. Curr. Pharm. Des. 2018;24:710–717. doi: 10.2174/1381612824666180116163411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eguchi K., Fujitani N., Nakagawa H., Miyazaki T. Prevention of respiratory syncytial virus infection with probiotic lactic acid bacterium Lactobacillus gasseri SBT2055. Sci. Rep. 2019;9:4812. doi: 10.1038/s41598-019-39602-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lehtoranta L., Pitkäranta A., Korpela R. Probiotics in respiratory virus infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1289–1302. doi: 10.1007/s10096-014-2086-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maggini S., Pierre A., Calder P.C. Immune function and micronutrient requirements change over the life course. Nutrients. 2018;10 doi: 10.3390/nu10101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Calder P.C., Carr A.C., Gombart A.F., Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. https://www.preprints.org/manuscript/202003.0199/v1 Preprints. Retrieved from. (Accessed: 7 April 2020) [DOI] [PMC free article] [PubMed]
- 60.Kritas S., Ronconi G., Caraffa A., Gallenga C., Ross R., Conti P. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/20-Editorial-Kritas. [DOI] [PubMed] [Google Scholar]
- 61.Conti P., Ronconi G., Caraffa A., Gallenga C.E., Ross R., Frydas I., Kritas S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents. 2020;34 doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 62.Bergman P., Lindh A.U., Björkhem-Bergman L., Lindh J.D. Vitamin D and respiratory tract infections: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 2013;8 doi: 10.1371/journal.pone.0065835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charan J., Goyal J.P., Saxena D., Yadav P. Vitamin D for prevention of respiratory tract infections: a systematic review and meta-analysis. J. Pharmacol. Pharmacother. 2012;3:300–303. doi: 10.4103/0976-500X.103685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., Goodall E.C., Grant C.C., Griffiths C.J., Janssens W., Laaksi I., Manaseki-Holland S., Mauger D., Murdoch D.R., Neale R., Rees J.R., Simpson S., Stelmach I., Kumar G.T., Urashima M., Camargo C.A. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martineau A.R., Jolliffe D.A., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., Goodall E.C., Grant C.C., Janssens W., Jensen M.E., Kerley C.P., Laaksi I., Manaseki-Holland S., Mauger D., Murdoch D.R., Neale R., Rees J.R., Simpson S., Stelmach I., Kumar G. Trilok, Urashima M., Camargo C.A., Griffiths C.J., Hooper R.L. Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta-analysis. Health Technol. Assess. (Winchester, England) 2019;23:1–44. doi: 10.3310/hta23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Teymoori-Rad M., Shokri F., Salimi V., Marashi S.M. The interplay between vitamin D and viral infections. Rev. Med. Virol. 2019;29 doi: 10.1002/rmv.2032. [DOI] [PubMed] [Google Scholar]
- 67.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., Bhattoa H.P. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12 doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nair R., Maseeh A. Vitamin D: the “sunshine” vitamin. J. Pharmacol. Pharmacother. 2012;3:118–126. doi: 10.4103/0976-500X.95506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Elliott M.E., Binkley N.C., Carnes M., Zimmerman D.R., Petersen K., Knapp K., Behlke J.M., Ahmann N., Kieser M.A. Fracture risks for women in long-term care: high prevalence of calcaneal osteoporosis and hypovitaminosis D. Pharmacotherapy. 2003;23:702–710. doi: 10.1592/phco.23.6.702.32182. [DOI] [PubMed] [Google Scholar]
- 70.Kennel K.A., Drake M.T., Hurley D.L. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin. Proc. 2010;85:752–757. doi: 10.4065/mcp.2010.0138. (quiz 757-758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Holick M.F. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am. J. Clin. Nutr. 2004;80:1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 72.Nonnecke B., McGill J., Ridpath J., Sacco R., Lippolis J., Reinhardt T. Acute phase response elicited by experimental bovine diarrhea virus (BVDV) infection is associated with decreased vitamin D and E status of vitamin-replete preruminant calves. J. Dairy Sci. 2014;97:5566–5579. doi: 10.3168/jds.2014-8293. [DOI] [PubMed] [Google Scholar]
- 73.Greiller C.L., Martineau A.R. Modulation of the Immune response to respiratory viruses by vitamin D. Nutrients. 2015;7:4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Science M., Maguire J.L., Russell M.L., Smieja M., Walter S.D., Loeb M. Low serum 25-hydroxyvitamin D level and risk of upper respiratory tract infection in children and adolescents. Clin Infect. Dis. 2013;57:392–397. doi: 10.1093/cid/cit289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wimalawansa S.J. Global epidemic of coronavirus: What can we do to minimize the risks. Eur. J. Biomed. Pharm. Sci. 2020;7:432–438. https://www.ejbps.com/ejbps/abstract_id/6656 [Google Scholar]
- 76.Semba R.D. Vitamin A and immunity to viral, bacterial and protozoan infections. Proc. Nutr. Soc. 1999;58:719–727. doi: 10.1017/s0029665199000944. [DOI] [PubMed] [Google Scholar]
- 77.McGill J.L., Kelly S.M., Guerra-Maupome M., Winkley E., Henningson J., Narasimhan B., Sacco R.E. Vitamin A deficiency impairs the immune response to intranasal vaccination and RSV infection in neonatal calves. Sci. Rep. 2019;9:15157. doi: 10.1038/s41598-019-51684-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel N., Penkert R.R., Jones B.G., Sealy R.E., Surman S.L., Sun Y., Tang L., DeBeauchamp J., Webb A., Richardson J., Heine R., Dallas R.H., Ross A.C., Webby R., Hurwitz J.L. Baseline serum vitamin A and D levels determine benefit of oral vitamin A & D supplements to humoral immune responses following pediatric influenza vaccination. Viruses. 2019;11:907. doi: 10.3390/v11100907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jee J., Hoet A.E., Azevedo M.P., Vlasova A.N., Loerch S.C., Pickworth C.L., Hanson J., Saif L.J. Effects of dietary vitamin A content on antibody responses of feedlot calves inoculated intramuscularly with an inactivated bovine coronavirus vaccine. Am. J. Vet. Res. 2013;74:1353–1362. doi: 10.2460/ajvr.74.10.1353. [DOI] [PubMed] [Google Scholar]
- 80.Kańtoch M., Litwińska B., Szkoda M., Siennicka J. Importance of vitamin A deficiency in pathology and immunology of viral infections. Rocz. Panstw. Zakl. Hig. 2002;53:385–392. https://www.ncbi.nlm.nih.gov/pubmed/12664666 [PubMed] [Google Scholar]
- 81.Hess A.F. Diet, nutrition and infection. N. Engl. J. Med. 1932;207:637–648. [Google Scholar]
- 82.Robertson E.C. The vitamins and resistance to infection. Medicine. 1934;13:123–206. [Google Scholar]
- 83.Hemilä H. Vitamin C and the common cold. Br. J. Nutr. 1992;67:3–16. doi: 10.1079/bjn19920004. [DOI] [PubMed] [Google Scholar]
- 84.Manning J., Mitchell B., Appadurai D.A., Shakya A., Pierce L.J., Wang H., Nganga V., Swanson P.C., May J.M., Tantin D., Spangrude G.J. Vitamin C promotes maturation of T-cells. Antioxid. Redox Signal. 2013;19:2054–2067. doi: 10.1089/ars.2012.4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Field C.J., Johnson I.R., Schley P.D. Nutrients and their role in host resistance to infection. J. Leukoc. Biol. 2002;71:16–32. doi: 10.1189/jlb.71.1.16. [DOI] [PubMed] [Google Scholar]
- 86.Hemilä H. Vitamin C and SARS coronavirus. J. Antimicrob. Chemother. 2003;52:1049–1050. doi: 10.1093/jac/dkh002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hemilä H. Vitamin C and infections. Nutrients. 2017;9:339. doi: 10.3390/nu9040339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Driel M.L., Beller E.M., Thielemans E., Deckx L., Price-Haywood E., Clark J., De Sutter A.I.M. Oral vitamin C supplements to prevent and treat acute upper respiratory tract infections. Cochrane Database Syst. Rev. 2019;2019(3):CD013292. doi: 10.1002/14651858.CD013292. [DOI] [Google Scholar]
- 89.Hemilä H. Vitamin C supplementation and the common cold - was Linus Pauling right or wrong? Int. J. Vitam. Nutr. Res. 1997;67:329–335. https://www.ncbi.nlm.nih.gov/pubmed/9350474 [PubMed] [Google Scholar]
- 90.Hemilä H., Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database Syst. Rev. 2013;(1):CD000980. doi: 10.1002/14651858.CD000980.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Colunga Biancatelli R.M.L., Berrill M., Marik P.E. The antiviral properties of vitamin C. Expert Rev. Anti Infect. Ther. 2020;18:99–101. doi: 10.1080/14787210.2020.1706483. [DOI] [PubMed] [Google Scholar]
- 92.Gorton H.C., Jarvis K. The effectiveness of vitamin C in preventing and relieving the symptoms of virus-induced respiratory infections. J. Manipulative Physiol. Ther. 1999;22:530–533. doi: 10.1016/s0161-4754(99)70005-9. [DOI] [PubMed] [Google Scholar]
- 93.Maggini S., Beveridge S., Suter M. A combination of high-dose vitamin C plus zinc for the common cold. J. Int. Med. Res. 2012;40:28–42. doi: 10.1177/147323001204000104. [DOI] [PubMed] [Google Scholar]
- 94.Guillin O.M., Vindry C., Ohlmann T., Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11:2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beck M.A., Nelson H.K., Shi Q., Van Dael P., Schiffrin E.J., Blum S., Barclay D., Levander O.A. Selenium deficiency increases the pathology of an influenza virus infection. FASEB J. 2001;15:1481–1483. doi: 10.1096/fj.00-0721fje. [DOI] [PubMed] [Google Scholar]
- 96.Beck M.A., Kolbeck P.C., Rohr L.H., Shi Q., Morris V.C., Levander O.A. Benign human enterovirus becomes virulent in selenium-deficient mice. J. Med. Virol. 1994;43:166–170. doi: 10.1002/jmv.1890430213. [DOI] [PubMed] [Google Scholar]
- 97.Beck M.A., Shi Q., Morris V.C., Levander O.A. Rapid genomic evolution of a non-virulent coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat. Med. 1995;1:433–436. doi: 10.1038/nm0595-433. [DOI] [PubMed] [Google Scholar]
- 98.Harthill M. Review: micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011;143:1325–1336. doi: 10.1007/s12011-011-8977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rayman M.P. Selenium and human health. Lancet. 2012;379:1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 100.Shojadoost B., Kulkarni R.R., Yitbarek A., Laursen A., Taha-Abdelaziz K., Alkie T.N., Barjesteh N., Quinteiro-Filho W.M., Smith T.K., Sharif S. Dietary selenium supplementation enhances antiviral immunity in chickens challenged with low pathogenic avian influenza virus subtype H9N2. Vet. Immunol. Immunopathol. 2019;207:62–68. doi: 10.1016/j.vetimm.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 101.Beck M.A., Levander O.A., Handy J. Selenium deficiency and viral infection. J. Nutr. 2003;133:1463S–1467S. doi: 10.1093/jn/133.5.1463S. [DOI] [PubMed] [Google Scholar]
- 102.Razzaque M. COVID-19 pandemic: Can maintaining optimal zinc balance enhance host resistance? 2020. Preprints. 2020040006 (Accessed: 7 April 2020) [DOI] [PubMed]
- 103.Shankar A.H., Prasad A.S. Zinc and immune function: the biological basis of altered resistance to infection. Am. J. Clin. Nutr. 1998;68:447S–463S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 104.Read S.A., Obeid S., Ahlenstiel C., Ahlenstiel G. The role of zinc in antiviral immunity. Adv. Nutr. 2019;10:696–710. doi: 10.1093/advances/nmz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maares M., Haase H. Zinc and immunity: an essential interrelation. Arch. Biochem. Biophys. 2016;611:58–65. doi: 10.1016/j.abb.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 106.Science M., Johnstone J., Roth D.E., Guyatt G., Loeb M. Zinc for the treatment of the common cold: a systematic review and meta-analysis of randomized controlled trials. Cmaj. 2012;184:E551–E561. doi: 10.1503/cmaj.111990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saigal P., Hanekom D. Does zinc improve symptoms of viral upper respiratory tract infection? Evid. Based Pract. 2020;23:37–39. [Google Scholar]
- 108.Awotiwon A.A., Oduwole O., Sinha A., Okwundu C.I. Zinc supplementation for the treatment of measles in children. Cochrane Database Syst. Rev. 2017;6:CD011177. doi: 10.1002/14651858.CD011177.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Read S.A., Parnell G., Booth D., Douglas M.W., George J., Ahlenstiel G. The antiviral role of zinc and metallothioneins in hepatitis C infection. J. Viral Hepat. 2018;25:491–501. doi: 10.1111/jvh.12845. [DOI] [PubMed] [Google Scholar]
- 110.Matsuoka S., Matsumura H., Nakamura H., Oshiro S., Arakawa Y., Hayashi J., Sekine N., Nirei K., Yamagami H., Ogawa M., Nakajima N., Amaki S., Tanaka N., Moriyama M. Zinc supplementation improves the outcome of chronic hepatitis C and liver cirrhosis. J. Clin. Biochem. Nutr. 2009;45:292–303. doi: 10.3164/jcbn.jcbn08-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Y., Zhu L.Q. Pharmaceutical care recommendations for antiviral treatments in children with coronavirus disease 2019. World J. Pediatr. 2020 doi: 10.1007/s12519-020-00353-5. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harrison C. Coronavirus puts drug repurposing on the fast track. Nat. Biotechnol. 2020 doi: 10.1038/d41587-020-00003-1. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 113.Kono M., Tatsumi K., Imai A.M., Saito K., Kuriyama T., Shirasawa H. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antivir. Res. 2008;77:150–152. doi: 10.1016/j.antiviral.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020 doi: 10.1016/j.jcrc.2020.03.005. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V., Dupont H., Honoré S., Colson P., Scola B., Rolain J.-M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Groome M.J., Page N., Cortese M.M., Moyes J., Zar H.J., Kapongo C.N., Mulligan C., Diedericks R., Cohen C., Fleming J.A. Effectiveness of monovalent human rotavirus vaccine against admission to hospital for acute rotavirus diarrhoea in south African children: a case-control study. Lancet Infect. Dis. 2014;14:1096–1104. doi: 10.1016/S1473-3099(14)70940-5. [DOI] [PubMed] [Google Scholar]
- 117.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Matsuyama S., Nao N., Shirato K., Kawase M., Saito S., Takayama I., Nagata N., Sekizuka T., Katoh H., Kato F., Sakata M., Tahara M., Kutsuna S., Ohmagari N., Kuroda M., Suzuki T., Kageyama T., Takeda M. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. U.S.A. 2020;117:7001–7003. doi: 10.1073/pnas.2002589117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020 doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020 doi: 10.1016/j.apsb.2020.02.008. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R., Ray A.S., Cihlar T., Siegel D., Mackman R.L., Clarke M.O., Baric R.S., Denison M.R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio. 2018;9 doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C., Larson N., Strickley R., Wells J., Stuthman K.S., Van Tongeren S.A., Garza N.L., Donnelly G., Shurtleff A.C., Retterer C.J., Gharaibeh D., Zamani R., Kenny T., Eaton B.P., Grimes E., Welch L.S., Gomba L., Wilhelmsen C.L., Nichols D.K., Nuss J.E., Nagle E.R., Kugelman J.R., Palacios G., Doerffler E., Neville S., Carra E., Clarke M.O., Zhang L., Lew W., Ross B., Wang Q., Chun K., Wolfe L., Babusis D., Park Y., Stray K.M., Trancheva I., Feng J.Y., Barauskas O., Xu Y., Wong P., Braun M.R., Flint M., McMullan L.K., Chen S.-S., Fearns R., Swaminathan S., Mayers D.L., Spiropoulou C.F., Lee W.A., Nichol S.T., Cihlar T., Bavari S. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lo M.K., Jordan R., Arvey A., Sudhamsu J., Shrivastava-Ranjan P., Hotard A.L., Flint M., McMullan L.K., Siegel D., Clarke M.O., Mackman R.L., Hui H.C., Perron M., Ray A.S., Cihlar T., Nichol S.T., Spiropoulou C.F. GS-5734 and its parent nucleoside analog inhibit filo-, pneumo-, and paramyxoviruses. Sci. Rep. 2017;7:43395. doi: 10.1038/srep43395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sheahan T.P., Sims A.C., Leist S.R., Schäfer A., Won J., Brown A.J., Montgomery S.A., Hogg A., Babusis D., Clarke M.O., Spahn J.E., Bauer L., Sellers S., Porter D., Feng J.Y., Cihlar T., Jordan R., Denison M.R., Baric R.S. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 2020;11:222. doi: 10.1038/s41467-019-13940-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Amirian E.S., Levy J.K. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health. 2020;9:100128. doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. First Case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brown A.J., Won J.J., Graham R.L., Dinnon K.H., III, Sims A.C., Feng J.Y., Cihlar T., Denison M.R., Baric R.S., Sheahan T.P. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res. 2019;169:104541. doi: 10.1016/j.antiviral.2019.104541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jin Z., Smith L.K., Rajwanshi V.K., Kim B., Deval J. The ambiguous base-pairing and high substrate efficiency of T-705 (Favipiravir) Ribofuranosyl 5′-triphosphate towards influenza A virus polymerase. PLoS One. 2013;8 doi: 10.1371/journal.pone.0068347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Furuta Y., Takahashi K., Shiraki K., Sakamoto K., Smee D.F., Barnard D.L., Gowen B.B., Julander J.G., Morrey J.D. T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antivir. Res. 2009;82:95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.De Clercq E. New nucleoside analogues for the treatment of hemorrhagic fever virus infections. Chem. Asian J. 2019;14:3962–3968. doi: 10.1002/asia.201900841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 135.Wang Y., Fan G., Salam A., Horby P., Hayden F.G., Chen C., Pan J., Zheng J., Lu B., Guo L. Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically ill patients with influenza virus infection. J. Infect. Dis. 2019 doi: 10.1093/infdis/jiz656. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 136.Elfiky A.A. Anti-HCV, nucleotide inhibitors, repurposing against COVID-19. Life Sci. 2020 doi: 10.1016/j.lfs.2020.117477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chandwani A., Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther. Clin. Risk Manag. 2008;4:1023–1033. doi: 10.2147/tcrm.s3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J. Gen. Intern. Med. 2020 doi: 10.1007/s11606-020-05762-w. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2001282. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Chu C., Cheng V., Hung I., Wong M., Chan K., Chan K., Kao R., Poon L., Wong C., Guan Y. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kim U.J., Won E.-J., Kee S.-J., Jung S.-I., Jang H.-C. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-α for Middle East respiratory syndrome. Antivir. Ther. 2016;21:455–459. doi: 10.3851/IMP3002. [DOI] [PubMed] [Google Scholar]
- 142.Arabi Y.M., Asiri A.Y., Assiri A.M., Jokhdar H.A. Aziz, Alothman A., Balkhy H.H., AlJohani S., Al Harbi S., Kojan S., Al Jeraisy M., Deeb A.M., Memish Z.A., Ghazal S., Al Faraj S., Al-Hameed F., AlSaedi A., Mandourah Y., Al Mekhlafi G.A., Sherbeeni N.M., Elzein F.E., Almotairi A., Al Bshabshe A., Kharaba A., Jose J., Al Harthy A., Al Sulaiman M., Mady A., Fowler R.A., Hayden F.G., Al-Dawood A., Abdelzaher M., Bajhmom W., Hussein M.A., the Saudi Critical Care Trials Treatment of Middle East respiratory syndrome with a combination of lopinavir/ritonavir and interferon-β1b (MIRACLE trial): statistical analysis plan for a recursive two-stage group sequential randomized controlled trial. Trials. 2020;21:8. doi: 10.1186/s13063-019-3846-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Balayan T., Horvath H., Rutherford G.W. Ritonavir-boosted darunavir plus two nucleoside reverse transcriptase inhibitors versus other regimens for initial antiretroviral therapy for people with HIV infection: a systematic review. AIDS Res. Treat. 2017:2345617. doi: 10.1155/2017/2345617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 145.Seo J.-Y., Yaneva R., Cresswell P. Viperin: a multifunctional, interferon-inducible protein that regulates virus replication. Cell Host Microbe. 2011;10:534–539. doi: 10.1016/j.chom.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 2007;74:92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schwarz S., Wang K., Yu W., Sun B., Schwarz W. Emodin inhibits current through SARS-associated coronavirus 3a protein. Antivir. Res. 2011;90:64–69. doi: 10.1016/j.antiviral.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang X.W., Yap Y.L. Old drugs as lead compounds for a new disease? Binding analysis of SARS coronavirus main proteinase with HIV, psychotic and parasite drugs. Bioorg. Med. Chem. 2004;12(10):2517–2521. doi: 10.1016/j.bmc.2004.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16:1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Needle D., Lountos G.T., Waugh D.S. Structures of the Middle East respiratory syndrome coronavirus 3C-like protease reveal insights into substrate specificity. Acta Crystallogr. D Biol. Crystallogr. 2015;71:1102–1111. doi: 10.1107/S1399004715003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lee H., Mittal A., Patel K., Gatuz J.L., Truong L., Torres J., Mulhearn D.C., Johnson M.E. Identification of novel drug scaffolds for inhibition of SARS-CoV 3-chymotrypsin-like protease using virtual and high-throughput screenings. Bioorg. Med. Chem. 2014;22:167–177. doi: 10.1016/j.bmc.2013.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen L., Gui C., Luo X., Yang Q., Günther S., Scandella E., Drosten C., Bai D., He X., Ludewig B. Cinanserin is an inhibitor of the 3C-like proteinase of severe acute respiratory syndrome coronavirus and strongly reduces virus replication in vitro. J. Virol. 2005;79:7095–7103. doi: 10.1128/JVI.79.11.7095-7103.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Panche A., Diwan A., Chandra S. Flavonoids: an overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jo S., Kim S., Shin D.H., Kim M.-S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzyme Inhib. Med. Chem. 2020;35:145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jo S., Kim H., Kim S., Shin D.H., Kim M.S. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem. Biol. Drug Des. 2019;94:2023–2030. doi: 10.1111/cbdd.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ryu Y.B., Jeong H.J., Kim J.H., Kim Y.M., Park J.-Y., Kim D., Naguyen T.T.H., Park S.-J., Chang J.S., Park K.H. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg. Med. Chem. 2010;18:7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Prajapat M., Sarma P., Shekhar N., Avti P., Sinha S., Kaur H., Kumar S., Bhattacharyya A., Kumar H., Bansal S., Medhi B. Drug targets for corona virus: A systematic review. Indian J. Pharmacol. 2020;52:56–65. doi: 10.4103/ijp.IJP_115_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J. Clin. Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Casadevall A., Scharff M.D. Return to the past: the case for antibody-based therapies in infectious diseases. Clin. Infect. Dis. 1995;21:150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Casadevall A., Dadachova E., Pirofski L.A. Passive antibody therapy for infectious diseases. Nat. Rev. Microbiol. 2004;2:695–703. doi: 10.1038/nrmicro974. [DOI] [PubMed] [Google Scholar]
- 162.Golub E.S. Monoclonal antibodies. In: Brenner S., Miller J.H., editors. Encyclopedia of Genetics. Academic Press; New York: 2001. pp. 1235–1237. [Google Scholar]
- 163.Shanmugaraj B., Siriwattananon K., Wangkanont K., Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for coronavirus disease-19 (COVID-19) Asian Pac. J. Allergy Immunol. 2020;38:10–18. doi: 10.12932/AP-200220-0773. [DOI] [PubMed] [Google Scholar]
- 164.del Rio C., Malani P.N. COVID-19—new insights on a rapidly changing epidemic. JAMA. 2020 doi: 10.1001/jama.2020.3072. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 165.F. Hoffmann-La Roche Ltd. Roche initiates Phase III clinical trial of Actemra/RoActemra in hospitalised patients with severe COVID-19 pneumonia. https://www.roche.com/media/releases/med-cor-2020-03-19.htm (Accessed: 7 April 2020)
- 166.Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 168.National Institutes of Health NIH clinical trial of investigational vaccine for COVID-19 begins: Study enrolling Seattle-based healthy adult volunteers. https://www.nih.gov/news-events/news-releases/nih-clinical-trial-investigational-vaccine-covid-19-begins (Accessed: 7 April 2020)