Key Points
Question
Is celiac disease associated with increased mortality?
Findings
In this population-based cohort study of 49 829 patients in Sweden with celiac disease followed up for a median of 12.5 years, the mortality rate compared with general population controls was 9.7 vs 8.6 deaths per 1000 person-years, a difference that was statistically significant.
Meaning
In a Swedish population, celiac disease was associated with a small but statistically significant increased mortality risk.
Abstract
Importance
Celiac disease may be associated with a modest but persistent increased long-term mortality risk. It is uncertain whether this risk has changed in the era of wider diagnosis rates, less severe clinical disease, and more widespread availability of gluten-free food.
Objective
To evaluate the association between celiac disease and mortality risk in a population-based cohort in Sweden.
Design, Setting, and Participants
All individuals in Sweden with celiac disease diagnosed between 1969 and 2017 were identified through the Epidemiology Strengthened by histoPathology Reports in Sweden (ESPRESSO) cohort. Participants (n = 49 829) were observed starting on the day of the biopsy. The final date of follow-up was December 31, 2017.
Exposures
Celiac disease was defined by the presence of small intestinal villus atrophy on histopathology specimens during the years 1969-2017 from Sweden’s 28 pathology departments. Each individual was matched with as many as 5 control participants in the general population by age, sex, county, and calendar period.
Main Outcomes and Measures
The primary outcome was all-cause mortality, and the secondary outcome was cause-specific mortality. Patients with celiac disease were compared with controls using stratified Cox proportional modeling, stratifying by year of diagnosis.
Results
There were 49 829 patients with celiac disease, including 24% who were diagnosed between the years 2010 and 2017. The mean (SD) age at diagnosis was 32.2 (25.2) years and 62.4% were women. During a median follow-up time of 12.5 years, 13.2% (n = 6596) died. Compared with controls (n = 246 426), overall mortality was increased in those with celiac disease (9.7 vs 8.6 deaths per 1000 person-years; absolute difference, 1.2 per 1000 person-years; hazard ratio [HR], 1.21 [95% CI, 1.17-1.25]). The relative increase in mortality risk was present in all age groups and was greatest in those diagnosed in the age range of 18 to 39 years (1.9 vs 1.1 per 1000 person-years; HR, 1.69 [95% CI, 1.47-1.94]; P values for heterogeneity comparing 18-39 years with 40-59 years and with ≥60 years were both <.001). Individuals with celiac disease were at increased risk of death from cardiovascular disease (3.5 vs 3.4 per 1000 person-years; HR, 1.08 [95% CI, 1.02-1.13]), cancer (2.7 vs 2.2 per 1000 person-years; HR, 1.29 [95% CI, 1.22-1.36]), and respiratory disease (0.6 vs 0.5 per 1000 person-years; HR, 1.21 [95% CI, 1.08-1.37]). When compared with controls, the overall mortality risk was greatest in the first year after diagnosis (15.3 vs 6.5 per 1000 person-years; HR, 2.34 [95% CI, 2.14-2.55]) but persisted beyond 10 years after diagnosis (10.5 vs 10.1 per 1000 person-years; HR, 1.15 [95% CI, 1.10-1.20]). The mortality risk was likewise present for patients diagnosed during the years 2010-2017 (7.5 vs 5.5 per 1000 person-years; HR, 1.35 [95% CI, 1.21-1.51]).
Conclusions and Relevance
In a Swedish population studied between 1969 and 2017, a diagnosis of celiac disease compared with the general population was associated with a small but statistically significant increased mortality risk.
This population epidemiology study used Swedish histopathology registry data to estimate mortality risk in patients with vs without celiac disease.
Introduction
Celiac disease is an immune-based enteropathy characterized by malabsorption, small intestinal villus atrophy, and antibodies to transglutaminase, triggered by the ingestion of gluten, a protein found in wheat, rye, and barley.1 Morbidity and mortality have been documented in undiagnosed celiac disease,2 and clinical, serologic, and histologic normalization typically occurs after institution of the gluten-free diet; however, most studies have shown that even after diagnosis, celiac disease is associated with increased mortality.3,4,5 This increased risk has been attributed to the multiple morbidities associated with celiac disease including lymphoproliferative malignancy,6 osteoporotic fracture,7 type 1 diabetes,8 and other conditions.9,10
Large population-based studies of celiac disease have reported a 1.3- to 2-fold mortality risk increase,11 but these studies largely predate the increase in diagnosis rates that have occurred since the year 2000, when awareness of celiac disease, widespread availability of serologic testing, and a rise in seroprevalence all contributed to an increased incidence of celiac disease.2,12 Moreover, other studies have shown no increased overall mortality risk,13 a risk that is declining in more recent years,14 or a long-term risk that is restricted to those with a diagnosis in childhood.15 It is uncertain whether patients diagnosed in this era have a similar mortality increase, as higher diagnosis rates may result in a less severe clinical phenotype.
The objective of this study was to evaluate the association between celiac disease and mortality risk in a population-based cohort in Sweden. This analysis also includes siblings as a secondary comparator group and the assessment of mucosal healing as a modulator of mortality risk.
Methods
Study Design
This was a population-based, retrospective cohort study. The study was approved by the Stockholm Ethics Review Board (No. 2014/1287-31/4) on August 27, 2014. The ethics review board waived the requirement for informed consent.
Participants
The Epidemiology Strengthened by histoPathology Reports in Sweden (ESPRESSO) cohort consists of histopathology specimens submitted from patients to all (N = 28) pathology departments in Sweden.16 Between 2015 and 2017, these pathology departments provided all gastrointestinal histopathology records including personal identity number, topography within the gastrointestinal tract, and morphology.
Exposure
We identified those specimens submitted during the years spanning 1969-2017 with topography codes corresponding to the small intestine (but not ileum) and Systematized Nomenclature of Medicine (SNOMED) codes corresponding to villus atrophy (eTable in the Supplement). A validation study of a previous query of these data in 2006-2008 with medical record review found that this methodology yielded patients with a clinical diagnosis of celiac disease with a positive predictive value of 95%17 (see Sensitivity Analyses regarding recent guidelines now allowing the diagnosis of celiac disease to be made in select cases of children without an intestinal biopsy). Each individual with celiac disease was then matched to 5 control participants from the general population by Statistics Sweden using the following matching parameters: age, sex, calendar year, and county within Sweden.
Outcomes
The primary outcome was all-cause mortality. Individuals with celiac disease and controls were linked to the Swedish Cause of Death Register.18 The secondary outcome was cause-specific mortality, based on diagnosis codes, to classify causes of death as due to cardiovascular disease, cancer, respiratory disease, and other causes (eTable 2 in the Supplement). We subsequently performed stratified analyses by follow-up time, age, sex, country of birth, educational attainment, and calendar period. For the latter category, we additionally compared those diagnosed on or after January 1, 2010, to the overall cohort, restricting all individuals to the first 3 years of follow-up time after diagnosis; this restricted time frame was chosen to allow for comparability, given the changing risk over time after diagnosis.
Statistical Analysis
We used Cox regression conditioned on matching factors (age, sex, county, and calendar year) to estimate the association between celiac disease and mortality. The proportional hazard assumption was tested by including an interaction term of the exposure and follow-up time in the model. The proportional hazard assumption was violated for overall follow-up less than 1 year and 1 to less than 5 years, but not for 5 to less than 10 years, 10 years or greater, or for 1 year or greater. As such, we report overall hazard ratios (HRs), as well as HRs stratified by follow-up time: less than 1 year, 1 year to less than 5 years, 5 years to less than 10 years, 10 years or greater, and 1 year or less. Testing for heterogeneity between age groups was performed by including an interaction term of the exposure and age in the Cox regression model. Patients were followed-up until death, emigration, or December 31, 2017 (patients were censored on December 31, 2016, for the cause-specific mortality analysis, given that cause-specific mortality data were only available until this date). At a 2-sided significance level of 0.05, we had 80% power to detect a 1.04-fold increased risk of death. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Sensitivity Analyses
We performed the following sensitivity analyses to test the robustness of the findings.
Narrowing the Definition of Celiac Disease
So as to reduce the chance that the mortality risk is driven by individuals with villus atrophy but without celiac disease, we repeated the analysis but excluded individuals with inflammatory bowel disease and any gastrointestinal cancer at the time of the intestinal biopsy, and we limited the sample of cases to those who had a celiac disease–associated visit recorded in the national patient register within 6 months of villus atrophy diagnosis.
Further Adjustment for Confounders
Because educational attainment, country of birth, and additional immune-based morbidity may confound the association between celiac disease and mortality, we performed further adjustment, including the following variables to the model: educational attainment, country of birth (Nordic country or elsewhere), type 1 diabetes, autoimmune thyroid disease, rheumatoid arthritis, and inflammatory bowel disease. Educational attainment was classified into the following categories: compulsory school (0-9 years); upper secondary (10-12 years), and college or university (≥13 years). For individuals diagnosed when younger than 18 years, we instead included the highest educational attainment of the 2 parents. Missing information on education (<5%) and country of birth (<0.01%) were replaced by using a missing indicator category. Type 1 diabetes, autoimmune thyroid disease, rheumatoid arthritis, and inflammatory bowel disease were identified via their corresponding International Classification of Diseases codes (see eTable 3 in the Supplement) and were included in the model as time-dependent variables.
Seropositive Patients
For a subset of the cohort (n = 3307), serologic data were available, indicating a positive celiac disease–related serology (antibodies to tissue transglutaminase, endomysium, native gliadin, or deamidated gliadin). We repeated the main analysis, restricted to known seropositive patients.
Siblings as Comparators
Using the Swedish Total Population Register with data on families,19 we identified a subset of patients with celiac disease who had at least 1 sibling without celiac disease who was alive at the time of the index case’s celiac disease diagnosis. We then compared the patient with celiac disease with his or her sibling(s) with regard to all-cause mortality, conditioned on matching set within family, and further adjusting for all of the previous covariates in addition to age and gender.
Era of Nonbiopsy Diagnosis
In 2012, the European Society for Paediatric Gastroenterology Hepatology and Nutrition published guidelines that allow for a pediatric diagnosis of celiac disease to be made without an intestinal biopsy, if certain clinical and laboratory criteria are met.20 To determine whether this change had an effect on the prognosis of those with biopsy-diagnosed celiac disease, we repeated the overall analysis, now limited to children (younger than 18 years) diagnosed in the years 2010-2011, and again among those children diagnosed in the years 2012-2014. In this analysis, follow-up was censored at 3 years to equalize the relative contribution of the first year after diagnosis to the overall risk estimate.
Follow-up Histology and Mortality
Previous analyses of an older subset of this cohort found an association between persistent villus atrophy and risk of lymphoproliferative malignancy21 and osteoporotic fracture22 but not mortality.23 We tested for an association between persistent villus atrophy (corresponding to a Marsh score of ≥3; 0, preinfiltrative stage-4, most-advanced stage)24 and all-cause mortality, comparing these patients with those showing healed villi on follow-up biopsy. For this analysis we included all patients with celiac disease who had a follow-up biopsy between 6 months and 5 years after their initial biopsy. We adjusted for age, sex, year of diagnosis, follow-up duration, educational attainment, country of birth, and autoimmune comorbidity (type 1 diabetes, autoimmune thyroid disease, rheumatoid arthritis, and inflammatory bowel disease).
Results
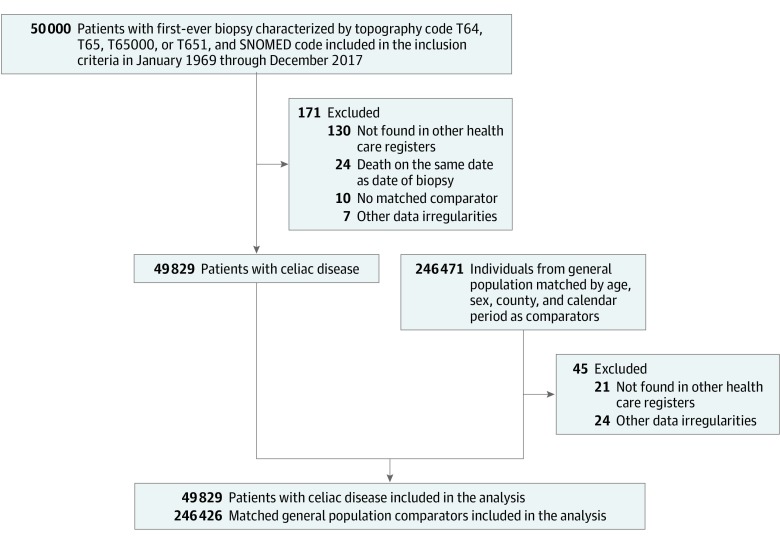
Among 50 000 individuals identified with a relevant histology and SNOMED code pertaining to celiac disease, 171 were excluded due to data irregularities or a lack of corroborating information in other health care records. The remaining 49 829 individuals with celiac disease were matched to 246 426 controls (Figure).
Figure. Flowchart of Identified Patients and Their Matched Comparators.
SNOMED indicates Systematized Nomenclature of Medicine.
Baseline characteristics of celiac disease patients and controls are shown in Table 1. The mean age at diagnosis was 32 years and 62% were female. Among celiac disease patients, 39% were younger than 18 years at the time of diagnosis, and 64% were diagnosed after January 1, 2000, including 24% who were diagnosed after January 1, 2010. Autoimmune comorbidity, including type 1 diabetes, autoimmune thyroid disease, rheumatoid arthritis, and inflammatory bowel disease, were more common in patients with celiac disease than in controls (see eTable 4 in the Supplement).
Table 1. Baseline Characteristics of Study Cohort.
| Characteristic | No. (%) | |
|---|---|---|
| Celiac disease (n = 49 829) |
Matched comparators (n = 246 426) |
|
| Female sex | 31 086 (62.4) | 154 056 (62.5) |
| Male sex | 18 743 (37.6) | 92 370 (37.5) |
| Age at diagnosis, y | ||
| Mean (SD) | 32.2 (25.2) | 31.9 (25.1) |
| Median (interquartile range) | 28.5 (8.4-53.5) | 28.0 (8.3-53.0) |
| Range | 0.0-95.4 | 0.0-95.8 |
| Age categories | ||
| <18 y | 19 632 (39.4) | 98 080 (39.8) |
| ≥18-<40 y | 10 913 (21.9) | 54 212 (22.0) |
| ≥40-<60 y | 9946 (20.0) | 49 268 (20.0) |
| ≥60 y | 9338 (18.7) | 44 866 (18.2) |
| Country of birth | ||
| No. of participants | 49 827 (100.0) | 246 414 (100.0) |
| Nordic country | 47 765 (95.9) | 226 192 (91.8) |
| Other country | 2062 (4.1) | 20 222 (8.2) |
| Level of education | ||
| No. of participants | 28 496 (57.2) | 139 930 (56.8) |
| ≤9 y | 8617 (30.2) | 44 048 (31.5) |
| 10-≤12 y | 11 856 (41.6) | 58 570 (41.9) |
| >12 y | 8023 (28.2) | 37 312 (26.7) |
| Parents’ highest level of education (used when patient’s level of education is missing | ||
| No. of applicable participants | 47 682 (95.7) | 235 572 (95.6) |
| ≤9 y | 9798 (20.5) | 50 987 (21.6) |
| 10-≤12 y | 20 090 (42.1) | 99 391 (42.2) |
| >12 y | 17 794 (37.3) | 85 194 (36.2) |
| Start year of follow-up | ||
| 1969-1989 | 4339 (8.7) | 21 604 (8.8) |
| 1990-1999 | 13 585 (27.3) | 67 331 (27.3) |
| 2000-2009 | 19 914 (40.0) | 98 232 (39.9) |
| 2010-2017 | 11 991 (24.1) | 59 259 (24.0) |
| Disease history before start of follow-up | ||
| Type 1 diabetes | 1565 (3.1) | 667 (0.3) |
| Inflammatory bowel disease | 1401 (2.8) | 365 (0.1) |
| Autoimmune thyroid disease | 1126 (2.3) | 1852 (0.8) |
| Rheumatoid arthritis | 423 (0.8) | 1096 (0.4) |
| Deaths | ||
| Days after index date | ||
| ≤30 | 114 (0.2) | 121 (<0.1) |
| ≤90 | 280 (0.6) | 401 (0.2) |
| ≤365 | 757 (1.5) | 1599 (0.6) |
| All follow-up time | 6596 (13.2) | 28 890 (11.7) |
During a median follow-up time of 12.5 years, 6596 (13.2%) patients with celiac disease died, yielding a mortality rate of 9.7 deaths per 1000 person-years, compared with 8.6 deaths per 1000 person-years in controls, an absolute risk difference of 1.2 per 1000 person-years (Table 2). The risk of mortality was increased in those with celiac disease (HR, 1.21 [95% CI, 1.17-1.25]). When considering cause-specific mortality, the increase was present for death from cardiovascular disease (3.5 vs 3.4 per 1000 person-years; HR, 1.08 [95% CI, 1.02-1.13]), cancer (2.7 vs 2.2 per 1000 person-years; HR, 1.29 [95% CI, 1.22-1.36]), respiratory disease (0.6 vs 0.5 per 1000 person-years; HR, 1.21 [95% CI, 1.08-1.37]), and other causes (2.9 vs 2.3 per 1000 person-years; HR, 1.35 [95% CI, 1.28-1.43]). When further adjusting for educational attainment, region of birth, and the presence of type 1 diabetes, autoimmune thyroid disease, rheumatoid arthritis, and inflammatory bowel disease, the overall mortality increase was diminished but remained elevated compared with controls (HR, 1.14 [95% CI, 1.11-1.18]). Cause-specific mortality estimates were likewise attenuated after adjustment for these covariates (Table 2).
Table 2. Risk of All-Cause Mortality (Follow-up Until December 31, 2017) and Cause-Specific Mortality (Follow-up Until December 31, 2016) in Patients With Celiac Disease and Matched General Population Comparators.
| All-cause mortality | Cardiovascular disease | Cancer | Respiratory disease | Other cause of death | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Celiac disease | Comparators | Celiac disease | Comparators | Celiac disease | Comparators | Celiac disease | Comparators | Celiac disease | Comparators | |
| No. | 49 829 | 246 426 | 49 744 | 246 009 | 49 744 | 246 009 | 49 744 | 246 009 | 49 744 | 246 009 |
| Deaths, no (%) | 6596 (13.2) | 28 890 (11.7) | 2246 (4.5) | 10 892 (4.4) | 1736 (3.5) | 7117 (2.9) | 406 (0.8) | 1713 (0.7) | 1837 (3.7) | 7319 (3.0) |
| Follow-up y | ||||||||||
| Total | 678 990 | 3 377 760 | 636 806 | 3 169 268 | 636 806 | 3 169 268 | 636 806 | 3 169 268 | 636 806 | 3 169 268 |
| Mean (SD) | 13.6 (8.4) | 13.7 (8.4) | 12.8 (8.3) | 12.9 (8.3) | 12.8 (8.3) | 12.9 (8.3) | 12.8 (8.3) | 12.9 (8.3) | 12.8 (8.3) | 12.9 (8.3) |
| Median (IQR) | 12.5 (6.8-19.4) |
12.6 (6.8-19.6) |
11.6 (6.0-18.6) |
11.7 (6.0-18.7) |
11.6 (6.0-18.6) |
11.7 (6.0-18.7) |
11.6 (6.0-18.6) |
11.7 (6.0-18.7) |
11.6 (6.0-18.6) |
11.7 (6.0-18.7) |
| Incidence rate per 1000 person-years (95% CI) | 9.7 (9.5-9.9) |
8.6 (8.5-8.7) |
3.5 (3.4-3.7) |
3.4 (3.4-3.5) |
2.7 (2.6-2.9) |
2.2 (2.2-2.3) |
0.6 (0.6-0.7) |
0.5 (0.5-0.6) |
2.9 (2.8-3.0) |
2.3 (2.3-2.4) |
| HR (95% CI) | ||||||||||
| Conditioneda | 1.21 (1.17-1.25) | 1.08 (1.02-1.13) | 1.29 (1.22-1.36) | 1.21 (1.08-1.37) | 1.35 (1.28-1.43) | |||||
| Adjustedb | 1.14 (1.11-1.18) | 1.03 (0.98-1.08) | 1.22 (1.15-1.29) | 1.16 (1.03-1.32) | 1.23 (1.16-1.31) | |||||
Abbreviations: HR, hazard ratio; IQR, interquartile range.
Indicates conditioned on matching set (age, sex, county, and calendar period).
Indicates conditioned on matching set and further adjusted for education, Nordic country of birth, and time-dependent medical comorbidities (type 1 diabetes, autoimmune thyroid disease, rheumatoid arthritis, and inflammatory bowel disease).
Stratified analyses by age, sex, year, country of birth, educational attainment, and comorbidity are shown in Table 3. The mortality risk associated with celiac disease remained elevated throughout the study period, was present in males and females, and all age strata. The increase in risk was greatest in those diagnosed as young adults (aged 18-39 years, 1.9 vs 1.1 per 1000 person-years; HR, 1.69 [95% CI, 1.47-1.94]; P value for heterogeneity comparing 18-39 years with 40-59 years and with ≥60 years was <.001). Mortality risk was most elevated in the first year after diagnosis compared with controls (15.3 vs 6.5 per 1000 person-years; HR, 2.34 [95% CI, 2.14-2.55]) and diminished thereafter. Among individuals followed for 10 years or more, the mortality risk remained elevated (10.5 vs 10.1 per 1000 person-years; HR, 1.15 [95% CI, 1.10-1.20]). When restricted to individuals diagnosed on or after January 1, 2010, compared with controls, the association between celiac disease and mortality remained significant (7.5 vs 5.5 per 1000 person-years; HR, 1.35 [95% CI, 1.21-1.51]) When comparing those diagnosed on or after January 1, 2010, to the overall cohort, restricting all individuals to the first 3 years of follow-up time after diagnosis, those diagnosed in the most recent time period had the same short-term mortality risk (8.2 vs 5.1 per 1000 person-years; HR, 1.58 [95% CI, 1.35-1.83]) as that of the overall cohort (10.4 vs 6.8 per 1000 person-years; HR, 1.58 [95% CI, 1.49-1.68]).
Table 3. Risk of All-Cause Mortality Overall and by Subgroups in Patients With Celiac Disease and Matched General Population Comparators.
| Group | No. (%) | Time at risk, y | No. (%) events | Mortality rate (95% CI) per 1000 person-years |
HR (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Celiac disease | Comparators | Celiac disease | Comparators | Celiac disease | Comparators | Celiac disease | Comparators | Conditioneda | Conditioned and adjustedb |
|
| Overall | 49 829 (100) | 246 426 (100) | 678 990 | 3 377 760 | 6596 13.2) | 28 890 (11.7) | 9.7 (9.5-9.9) | 8.6 (8.5-8.7) | 1.21 (1.17-1.25) | 1.14 1.11-1.18) |
| Follow-up, y | ||||||||||
| 0-<1 | 49 829 (100) | 246 426 (100) | 49 322 | 244 945 | 757 (1.5) | 1599 (0.6) | 15.3 (14.3-16.4) | 6.5 (6.2-6.8) | 2.34 (2.14-2.55) | 2.14 (1.92-2.39) |
| ≥1-<5 | 48 899 (98.1) | 243 135 (98.7) | 182 394 | 905 577 | 1414 (2.9) | 6323 (2.6) | 7.8 (7.3-8.2) | 7.0 (6.8-7.2) | 1.14 (1.07-1.21) | 1.10 (1.03-1.17) |
| ≥5-<10 | 41 516 (83.3) | 205 882 (83.5) | 179 030 | 887 869 | 1602 (3.9) | 7382 (3.6) | 8.9 (8.5-9.4) | 8.3 (8.1-8.5) | 1.09 (1.02-1.15) | 1.02 (0.96-1.08) |
| ≥10 | 30 251 (60.7) | 149 993 (60.9) | 268 241 | 1 339 368 | 2823 (9.3) | 13 586 (9.1) | 10.5 (10.1-10.9) | 10.1 (10.0-10.3) | 1.15 (1.10-1.20) | 1.09 (1.04-1.14) |
| ≥1 | 48 899 (98.1) | 243 135 (98.7) | 629 667 | 3 132 815 | 5839 (11.9) | 27 291 (11.2) | 9.3 (9.0-9.5) | 8.7 (8.6-8.8) | 1.13 (1.09-1.16) | 1.07 (1.04-1.11) |
| Female sex | 31 086 (62.4) | 154 056 (62.5) | 429 231 | 2 127 213 | 3308 (10.6) | 14 663 (9.5) | 7.7 (7.4-8.0) | 6.9 (6.8-7.0) | 1.20 (1.15-1.25) | 1.13 (1.08-1.18) |
| Male sex | 18 743 (37.6) | 92 370 (37.5) | 249 758 | 1 250 547 | 3288 (17.5) | 14 227 (15.4) | 13.2 (12.7-13.6) | 11.4 (11.2-11.6) | 1.22 (1.17-1.27) | 1.16 (1.11-1.21) |
| Age, y | ||||||||||
| <18 | 19 632 (39.4) | 98 080 (39.8) | 301 673 | 1 486 310 | 125 (0.6) | 398 (0.4) | 0.4 (0.3-0.5) | 0.3 (0.2-0.3) | 1.56 (1.27-1.91) | 1.35 (1.08-1.68) |
| ≥18-<40 | 10 913 (21.9) | 54 212 (22.0) | 144 239 | 712 441 | 277 (2.5) | 818 (1.5) | 1.9 (1.7-2.1) | 1.1 (1.1-1.2) | 1.69 (1.47-1.94) | 1.36 (1.16-1.60) |
| ≥40-<60 | 9946 (20.0) | 49 268 (20.0) | 146 049 | 734 633 | 1467 (14.7) | 6207 (12.6) | 10.0 (9.5-10.6) | 8.4 (8.2-8.7) | 1.20 (1.13-1.27) | 1.12 (1.05-1.19) |
| ≥60 | 9338 (18.7) | 44 866 (18.2) | 87 028 | 444 373 | 4727 (50.6) | 21 467 (47.8) | 54.3 (52.8-55.9) | 48.3 (47.7-49.0) | 1.18 (1.14-1.22) | 1.13 (1.09-1.17) |
| Year | ||||||||||
| 1969-1989 | 4339 (8.7) | 21 604 (8.8) | 114 629 | 575 538 | 1198 (27.6) | 5536 (25.6) | 10.5 (9.9-11.0) | 9.6 (9.4-9.9) | 1.18 (1.10-1.26) | 1.10 (1.02-1.18) |
| 1990-1999 | 13 585 (27.3) | 67 331 (27.3) | 267 823 | 1 341 976 | 2862 (21.1) | 12 428 (18.5) | 10.7 (10.3-11.1) | 9.3 (9.1-9.4) | 1.27 (1.22-1.33) | 1.20 (1.14-1.25) |
| 2000-2009 | 19 914 (40.0) | 98 232 (39.9) | 239 014 | 1 175 498 | 2105 (10.6) | 9354 (9.5) | 8.8 (8.4-9.2) | 8.0 (7.8-8.1) | 1.12 (1.07-1.18) | 1.07 (1.01-1.12) |
| 2010-2017 | 11 991 24.1) | 59 259 (24.0) | 57 523 | 284 746 | 431 (3.6) | 1572 (2.7) | 7.5 (6.8-8.2) | 5.5 (5.2-5.8) | 1.35 (1.21-1.51) | 1.29 (1.15-1.45) |
| Country of birth | ||||||||||
| Nordic | 47 765 (95.9) | 226 192 (91.8) | 657 328 | 3 167 915 | 6407 (13.4) | 27 571 (12.2) | 9.7 (9.5-10.0) | 8.7 (8.6-8.8) | 1.20 (1.17-1.24) | 1.14 (1.10-1.17) |
| Other | 2062 (4.1) | 20 222 (8.2) | 21 650 | 209 739 | 189 (9.2) | 1318 (6.5) | 8.7 (7.5-10.0) | 6.3 (5.9-6.6) | 1.69 (1.11-2.58) | 1.60 (1.01-2.51) |
| Level of education, y | ||||||||||
| ≤9 | 9706 (19.5) | 50 073 (20.3) | 126 965 | 666 678 | 2744 (28.3) | 12 657 (25.3) | 21.6 (20.8-22.4) | 19.0 (18.7-19.3) | 1.19 (1.13-1.26) | 1.14 (1.08-1.20) |
| 10-12 | 18 441 (37.0) | 90 913 (36.9) | 275 979 | 1 346 506 | 1621 (8.8) | 7014 (7.7) | 5.9 (5.6-6.2) | 5.2 (5.1-5.3) | 1.18 (1.10-1.27) | 1.11 (1.03-1.20) |
| >12 | 14 827 (29.8) | 70 702 (28.7) | 209 099 | 1 020 551 | 673 (4.5) | 2624 (3.7) | 3.2 (3.0-3.5) | 2.6 (2.5-2.7) | 1.31 (1.14-1.50) | 1.20 (1.04-1.38) |
| Missing | 6855 (13.8) | 34 738 (14.1) | 66 946 | 344 024 | 1558 (22.7) | 6595 (19.0) | 23.3 (22.1-24.4) | 19.2 (18.7-19.6) | 1.18 (1.09-1.26) | 1.11 (1.03-1.20) |
| Comorbidity | ||||||||||
| Type 1 diabetes | 1565 (3.1) | 667 (0.3) | 19 239 | 7553 | 87 (5.6) | 57 (8.5) | 4.5 (3.6-5.5) | 7.5 (5.6-9.5) | ||
| Autoimmune thyroid disease | 1126 (2.3) | 1852 (0.8) | 10 230 | 15 199 | 240 (21.3) | 456 (24.6) | 23.5 (20.5-26.4) | 30.0 (27.2-32.8) | 1.23 (0.65-2.31) | 1.58 (0.73-3.42) |
| Rheumatoid arthritis | 423 (0.8) | 1096 (0.4) | 4101 | 10 021 | 142 (33.6) | 379 (34.6) | 34.6 (28.9-40.3) | 37.8 (34.0-41.6) | 2.50 (0.49-12.89) | 2.87 (0.47-17. 1) |
| Inflammatory bowel disease | 1401 (2.8) | 365 (0.1) | 20 167 | 4803 | 260 (18.6) | 67 (18.4) | 12.9 (11.3-14.5) | 13.9 (10.6-17.3) | 6.00 (0.72-49.84) | |
Conditioned on matching set (age, sex, county, and calendar period).
Conditioned on matching set and further adjusted for education, Nordic country of birth, and time dependent medical comorbidities (type 1 diabetes, autoimmune thyroid disease, rheumatoid arthritis, and inflammatory bowel disease.
Sensitivity Analyses
Narrowing the Definition of Celiac Disease
When excluding individuals with inflammatory bowel disease and gastrointestinal cancer at the time of the intestinal biopsy (eTable 5 in the Supplement), the overall HR for mortality in celiac disease was 1.13 (95% CI, 1.09-1.16; 9.4 vs 8.5 per 1000 person-years). When limiting the analysis to those whose diagnosis of celiac disease was confirmed via the Swedish National Patient Register (n = 22 115 [44.4% of the initial cohort]), the overall HR was 1.17 (95% CI, 1.09-1.24; 5.7 vs 5.0 per 1000 person-years).
Positive Serologies
When restricting the celiac disease population to those with positive serologies (among participants with available serologic data) there were 3307 patients with celiac disease. The mean age of these patients was 25 years (eTable 6 in the Supplement). The mortality rate was 5.5 deaths per 1000 person-years among patients with celiac disease vs 4.7 per 1000 person-years in matched controls. When compared with matched controls (n = 15 853) the mortality risk was not significantly different (HR, 1.02 [95% CI, 0.89-1.17]; eTable 7 in the Supplement).
Siblings as Comparators
The analysis was then restricted to individuals with celiac disease who had at least 1 sibling alive at the time of diagnosis who did not have celiac disease (eTable 8 in the Supplement). When comparing individuals with celiac disease (n = 32 558) with their siblings (n = 56 442), conditioned on matching set (within each family) and additionally adjusting for age and sex, those with celiac disease had an increased mortality risk (HR, 1.26 [95% CI, 1.17-1.35]). The cause-specific mortality associations were present as in the main analysis (Table 4).
Table 4. Risk of All-Cause (Follow-up Until December 31, 2017) and Cause-Specific (Follow-up Until December 31, 2016) Mortality in Patients With Celiac Disease and Their Siblings.
| All-cause mortality | Cardiovascular disease | Cancer | Respiratory disease | Other cause of death | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Celiac disease | Siblings | Celiac disease | Siblings | Celiac disease | Siblings | Celiac disease | Siblings | Celiac disease | Siblings | |
| No. | 32 558 | 56 442 | 32 494 | 56 340 | 32 494 | 56 340 | 32 494 | 56 340 | 32 494 | 56 340 |
| Deaths, No. (%) | 1580 (4.9) | 2696 (4.8) | 310 (1.0) | 643 (1.1) | 564 (1.7) | 939 (1.) | 79 (0.2) | 124 (0.2) | 486 (1.5) | 759 (1.3) |
| Follow-up, y | ||||||||||
| Total | 445 951 | 771 317 | 415 745 | 720 669 | 415 745 | 720 669 | 415 745 | 720 669 | 415 745 | 720 669 |
| Mean (SD) | 13.7 (8.2) | 13.7 (8.3) | 12.8 (8.2) | 12.8 (8.3) | 12.8 (8.2) | 12.8 (8.3) | 12.8 (8.2) | 12.8 (8.3) | 12.8 (8.2) | 12.8 (8.3) |
| Median (IQR) | 12.5 (7.0-19.3) |
12.6 (6.8-19.4) |
11.6 (6.1-18.4) |
11.7 (5.9-18.5) |
11.6 (6.1-18.4) |
11.7 (5.9-18.5) |
11.6 (6.1-18.4) |
11.7 (5.9-18.5) |
11.6 (6.1-18.4) |
11.7 (5.9-18.5) |
| Incidence rate per 1000 person-years (95% CI) | 3.5 (3.4-3.7) |
3.5 (3.4-3.6) |
0.7 (0.7-0.8) |
0.9 (0.8-1.0) |
1.4 (1.2-1.5) |
1.3 (1.2-1.4) |
0.2 (0.1-0.2) |
0.2 (0.1-0.2) |
1.2 (1.1-1.3) |
1.1 (1.0-1.1) |
| Hazard ratio (95% CI) | ||||||||||
| Conditioneda | 1.30 (1.22-1.39) | 1.09 (0.94-1.26) | 1.36 (1.22-1.53) | 1.55 (1.14-2.11) | 1.32 (1.17-1.49) | |||||
| Adjusted Ib | 1.21 (1.13-1.30) | 1.05 (0.91-1.23) | 1.27 (1.13-1.43) | 1.40 (1.01-1.95) | 1.16 (1.01-1.32) | |||||
| Adjusted IIc | 1.26 (1.17-1.35) | 1.13 (0.96-1.33) | 1.26 (1.11-1.42) | 1.64 (1.15-2.34) | 1.24 (1.08-1.43) | |||||
Indicates conditioned on family.
Indicates conditioned on family and further adjusted for education, Nordic country of birth, and time-dependent medical comorbidities (type 1 diabetes, autoimmune thyroid disease, rheumatoid arthritis, and inflammatory bowel disease).
Indicates conditioned on Adjusted I criteria and further adjusted for age and sex.
Era of Nonbiopsy Diagnosis
Among children younger than 18 years with villus atrophy, 3682 were diagnosed with celiac disease between January 1, 2010, and December 31, 2014. When comparing children diagnosed in 2010-2011 (n = 1770) with those diagnosed in 2012-2014 (n = 1912) when small intestinal biopsy might be omitted in selected cases, the risk estimates for mortality were the same, but the CIs were wide (2010-2011, 0.4 vs 0.2 per 1000 person-years; HR, 1.67 [95% CI, 0.34-8.26]; vs 2012-2014, 0.2 vs 0.1 per 1000 person-years; HR, 1.67 [95% CI, 0.17-16.02]; see eTable 9 in the Supplement).
Persistent Villus Atrophy
Among patients with celiac disease who underwent a follow-up biopsy between 6 months and 5 years after their initial diagnosis (n = 9843) persistent villus atrophy was present in 2919 (29.7%; eTable 10 in the Supplement). Those with persistent villus atrophy had a mean age of 44.0 years at the time of follow-up biopsy, compared with 32.8 years among those with mucosal healing. Although the unadjusted mortality risk was greater among patients with persistent villus atrophy (12.5 vs 5.8 per 1000 person-years), after adjusting for age, sex, year of diagnosis, follow-up duration, educational attainment, country of birth, and autoimmune comorbidity, there was no significant association between persistent villus atrophy and overall mortality when compared with celiac disease patients with mucosal healing (HR, 0.99 [95% CI, 0.87-1.13]; see eTable 11 in the Supplement).
Discussion
In this study, patients diagnosed with celiac disease had a small but statistically significant increased mortality risk compared with the general population. The mechanism by which celiac disease is associated with increased mortality risk is unknown, though it is plausible that chronic inflammation, a driver of mortality in other contexts,25 may be the underlying cause in this population.
Though mortality was increased across age strata, the risk was greater in those diagnosed as young adults (aged 18-39 years) compared with those aged 40 to 59 years (P for heterogeneity <.001) and those aged 60 and older (P for heterogeneity <.001). These younger patients have longer time periods to bear the effects of the disease compared with individuals diagnosed as older adults, who may have developed the disease later in life or had a more mild clinical phenotype that did not rise to the threshold of diagnosis until later in life.
To our knowledge, this study has the largest number of deaths (n = 6596) in an investigation of mortality risk in celiac disease to date. An analysis of a subset of this cohort, including 29 096 individuals with celiac disease and 3049 deaths, had previously found an increased mortality risk with an overall hazard ratio of 1.39 (95% CI, 1.33-1.45).5 The present estimate (HR, 1.21 [95% CI, 1.17-1.25]) does not overlap with this previous estimate, raising the possibility that the mortality increase is somewhat attenuated but is nevertheless present. However, the lack of a clear decline over time in the era-stratified risk estimates in the present analysis suggests that there has not been a substantial mitigation of risk. The persistent increased mortality risk in the most recent time period (2010-2017) is also congruent with a study in the United Kingdom that included 1092 individuals with celiac disease and 142 deaths. In that study, the overall standardized mortality ratio (1.37) was similar among patients diagnosed before 1990 (correlating with the advent of serologic testing), 1990-1999 (the serologic era), and 2000 onward (a time period of increasing awareness of the protean clinical manifestations of celiac disease).4 As such, despite the improved diagnosis rates that have been documented in the United States12 and Europe,26 this expansion has not been accompanied by a substantial dilution of risk.
The excess risk of death associated with respiratory disease raises the possibility that the increased rates of pneumococcal disease27,28 and death from respiratory disease4 previously described in celiac disease may be contributing to mortality. Vaccination against pneumococcus has been advocated in this population, though prospective studies are lacking.29 Still, the results regarding mortality from respiratory disease should be interpreted with caution; given the small proportion of patients with celiac disease who died of respiratory causes (0.8%), only a small subset of whom may have had a vaccine-preventable illness.
On sensitivity analysis, when the sample was restricted to those patients with celiac disease who had elevated serologies, there was no significantly increased mortality compared with controls. There are a number of plausible explanations for this finding. Those individuals whose serologies were drawn after their biopsy are subject to immortal time bias (ie, follow-up during a period when they cannot be at risk of death, since their inclusion was determined by subsequent seropositivity), which would diminish the overall HR; this is suggested by the finding of zero deaths in the celiac disease group in the first 30 days after diagnosis (see eTable 6 in the Supplement). Moreover, those with seronegative disease (which is associated with increased mortality30) were all excluded from that analysis. However, it is also possible that this null finding was due to chance, particularly given the relatively small sample size and number of deaths (n = 289) in this subanalysis.
In the current study, celiac disease was linked to a 26% increased risk of death in comparison between individuals with celiac disease vs their siblings. This is notable since sibling analyses reduce residual confounding in taking shared genetics and early environmental factors into account.
The overall risk estimate (HR, 1.21 [95% CI, 1.17-1.25]) was similar to that computed by Tio et al in a systematic review and meta-analysis of mortality in celiac disease published in 2012 (odds ratio, 1.24 [95% CI, 1.19-1.30]).31 However, that study did not consider change in mortality risk over time. In contrast, an analysis of patients in the United Kingdom with celiac disease diagnosed during the years spanning 1998-2012 found no increased risk of death compared with age-matched controls.13,32 That analysis, based on claims codes for office visits and limited to primary care practices, may not have encompassed the full spectrum of patients with celiac disease. Furthermore, when restricting their analysis to incident cases of celiac disease, there were fewer than 100 deaths in each category of cause-specific mortality (cardiovascular disease, cancer, and respiratory disease), limiting their ability to conduct subanalyses with sufficient precision. Another analysis of mortality risk in celiac disease, set in the Lothian region of Scotland and including 237 deaths, found that beyond 25 years, mortality was no longer increased among those diagnosed with celiac disease as adults (standardized mortality ratio, 0.97 [95% CI 0.74-1.24]), but was persistently increased among those diagnosed in childhood (standardized mortality ratio, 2.24 [95% CI, 1.45-3.30]).15 In contrast, in this study, mortality risk was present across age strata, including children. This mortality risk diminished after diagnosis and institution of the gluten-free diet but remained modestly elevated in the long term. The mortality risk in celiac disease persisted over time, despite changes in diagnosis rates, diagnostic modalities, and access to gluten-free food choices. Though mortality decreased in the years after diagnosis and may reflect the beneficial effect of the gluten-free diet on mortality, the lack of a substantial change in mortality risk in recent years, characterized by improved access to diagnosis and treatment, is consistent with the notion that mucosal healing does not modify the risk of death.23
Limitations
This study has several limitations. First, the reliance on histopathology codes raises the possibility of misclassification, though the algorithm that was used has been shown to have a high positive predictive value for celiac disease, as noted above.17 Given the possibility that mortality risk may be driven by patients without celiac disease who were misclassified as having this condition, the analysis was repeated excluding those patients with inflammatory bowel disease and gastrointestinal cancer (2 prominent conditions associated with villus atrophy in a prior validation study that affect mortality).17 The fact that the mortality risk was present after excluding these patients and was again present when restricting the analysis to those with a register-confirmed diagnosis of celiac disease suggests that celiac disease, and not its mimickers or accompanying conditions, was driving mortality.
Second, the partial data on serology, together with the fact that celiac disease may be diagnosed without a duodenal biopsy in symptomatic children, according to 2012 guidelines,20 raises the possibility of selection bias. But in additional sensitivity analysis, mortality estimates were not significantly different in the periods preceding and following this recommendation.
Third, the mechanism by which celiac disease is associated with increased mortality risk could not be ascertained in this study. On cause-specific mortality analysis, the risk of death from cardiovascular causes was markedly diminished after adjustment for type 1 diabetes, autoimmune thyroid disease, rheumatoid arthritis, and inflammatory bowel disease; it is possible that as-yet unidentified confounders, if adjusted for, would result in similar diminution of risk of mortality from other causes.
Conclusions
In a Swedish population studied between 1969 and 2017, a diagnosis of celiac disease compared with the general population was associated with a small but statistically significant increased mortality risk.
eTable 1. Definitions of Celiac Disease and Normal Mucosa Using SNOMED Codes
eTable 2. Definitions of Cause-Specific Mortality
eTable 3. Definitions of Baseline Comorbidities Ever Before Start of Follow-up
eTable 4. Comorbidity at Baseline, During Follow-up and at Baseline or Follow-up, by Index Year in Celiac Disease and Matched Comparators
eTable 5. Narrowing the Definition of Celiac Disease
eTable 6. Celiac Disease With Positive Celiac Disease Serology vs Matched Comparators
eTable 7. Risk of All-Cause (Follow-up Until 31 Dec, 2017) and Cause-Specific (Follow-up Until 31 Dec, 2016) Mortality in Patients With Celiac Disease and Positive Celiac Disease Serology and Matched General Population Comparators
eTable 8. Celiac Disease Compared to Siblings: Baseline Characteristics of Study Cohort
eTable 9. Risk of All-Cause Mortality in Children (<18 Years) Diagnosed in 2010-2011 and in 2012-2014, Restricted to 3 Years of Follow-up
eTable 10. Baseline Characteristics of Study Cohort of Those With Mucosal Healing on Follow-up Biopsy Compared to Those With Persistent Villus Atrophy
eTable 11. Risk of All-Cause (Follow-up Until 31 Dec, 2017) and Cause-Specific (Follow-up Until 31 Dec, 2016) Mortality in Patients With Celiac Disease and Mucosal Healing vs Persistent Villus Atrophy
References
- 1.Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391(10115):70-81. doi: 10.1016/S0140-6736(17)31796-8 [DOI] [PubMed] [Google Scholar]
- 2.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. . Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137(1):88-93. doi: 10.1053/j.gastro.2009.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corrao G, Corazza GR, Bagnardi V, et al. . Mortality in patients with coeliac disease and their relatives. Lancet. 2001;358(9279):356-361. doi: 10.1016/S0140-6736(01)05554-4 [DOI] [PubMed] [Google Scholar]
- 4.Grainge MJ, West J, Card TR, Holmes GKT. Causes of death in people with celiac disease spanning the pre- and post-serology era. Am J Gastroenterol. 2011;106(5):933-939. doi: 10.1038/ajg.2010.506 [DOI] [PubMed] [Google Scholar]
- 5.Ludvigsson JF, Montgomery SM, Ekbom A, Brandt L, Granath F. Small-intestinal histopathology and mortality risk in celiac disease. JAMA. 2009;302(11):1171-1178. doi: 10.1001/jama.2009.1320 [DOI] [PubMed] [Google Scholar]
- 6.Catassi C, Fabiani E, Corrao G, et al. . Risk of non-Hodgkin lymphoma in celiac disease. JAMA. 2002;287(11):1413-1419. doi: 10.1001/jama.287.11.1413 [DOI] [PubMed] [Google Scholar]
- 7.Ludvigsson JF, Michaelsson K, Ekbom A, Montgomery SM. Coeliac disease and the risk of fractures—a general population-based cohort study. Aliment Pharmacol Ther. 2007;25(3):273-285. doi: 10.1111/j.1365-2036.2006.03203.x [DOI] [PubMed] [Google Scholar]
- 8.Elfström P, Sundström J, Ludvigsson JF. Systematic review with meta-analysis: associations between coeliac disease and type 1 diabetes. Aliment Pharmacol Ther. 2014;40(10):1123-1132. doi: 10.1111/apt.12973 [DOI] [PubMed] [Google Scholar]
- 9.Ludvigsson JF, Lindelöf B, Zingone F, Ciacci C. Psoriasis in a nationwide cohort study of patients with celiac disease. J Invest Dermatol. 2011;131(10):2010-2016. doi: 10.1038/jid.2011.162 [DOI] [PubMed] [Google Scholar]
- 10.Zingone F, Swift GL, Card TR, Sanders DS, Ludvigsson JF, Bai JC. Psychological morbidity of celiac disease: a review of the literature. United European Gastroenterol J. 2015;3(2):136-145. doi: 10.1177/2050640614560786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludvigsson JF. Mortality and malignancy in celiac disease. Gastrointest Endosc Clin N Am. 2012;22(4):705-722. doi: 10.1016/j.giec.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 12.Choung RS, Unalp-Arida A, Ruhl CE, Brantner TL, Everhart JE, Murray JA. Less hidden celiac disease but increased gluten avoidance without a diagnosis in the United States. Mayo Clin Proc. Published online December 5, 2016. doi: 10.1016/j.mayocp.2016.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdul Sultan A, Crooks CJ, Card T, Tata LJ, Fleming KM, West J. Causes of death in people with coeliac disease in England compared with the general population. Gut. 2015;64(8):1220-1226. doi: 10.1136/gutjnl-2014-308285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes GKT, Muirhead A. Mortality in coeliac disease. BMJ Open Gastroenterol. 2018;5(1):e000201. doi: 10.1136/bmjgast-2018-000201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quarpong W, Card TR, West J, Solaymani-Dodaran M, Logan RF, Grainge MJ. Mortality in people with coeliac disease. United European Gastroenterol J. 2019;7(3):377-387. doi: 10.1177/2050640618814662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ludvigsson JF, Lashkariani M. Cohort profile: ESPRESSO (Epidemiology Strengthened by histoPathology Reports in Sweden). Clin Epidemiol. 2019;11:101-114. doi: 10.2147/CLEP.S191914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludvigsson JF, Brandt L, Montgomery SM, Granath F, Ekbom A. Validation study of villous atrophy and small intestinal inflammation in Swedish biopsy registers. BMC Gastroenterol. 2009;9:19. doi: 10.1186/1471-230X-9-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooke HL, Talbäck M, Hörnblad J, et al. . The Swedish cause of death register. Eur J Epidemiol. 2017;32(9):765-773. doi: 10.1007/s10654-017-0316-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludvigsson JF, Almqvist C, Bonamy A-KE, et al. . Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125-136. doi: 10.1007/s10654-016-0117-y [DOI] [PubMed] [Google Scholar]
- 20.Husby S, Koletzko S, Korponay-Szabó IR, et al. . European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54(1):136-160. doi: 10.1097/MPG.0b013e31821a23d0 [DOI] [PubMed] [Google Scholar]
- 21.Lebwohl B, Granath F, Ekbom A, et al. . Mucosal healing and risk for lymphoproliferative malignancy in celiac disease: a population-based cohort study. Ann Intern Med. 2013;159(3):169-175. doi: 10.7326/0003-4819-159-3-201308060-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebwohl B, Michaëlsson K, Green PHR, Ludvigsson JF. Persistent mucosal damage and risk of fracture in celiac disease. J Clin Endocrinol Metab. 2014;99(2):609-616. doi: 10.1210/jc.2013-3164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lebwohl B, Granath F, Ekbom A, et al. . Mucosal healing and mortality in coeliac disease. Aliment Pharmacol Ther. 2013;37(3):332-339. doi: 10.1111/apt.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsh MN. Gluten, major histocompatibility complex, and the small intestine: a molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’). Gastroenterology. 1992;102(1):330-354. doi: 10.1016/0016-5085(92)91819-P [DOI] [PubMed] [Google Scholar]
- 25.Kaptoge S, Di Angelantonio E, Lowe G, et al. . C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality. Lancet. 2010;375(9709):132-140. doi: 10.1016/S0140-6736(09)61717-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lohi S, Mustalahti K, Kaukinen K, et al. . Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26(9):1217-1225. doi: 10.1111/j.1365-2036.2007.03502.x [DOI] [PubMed] [Google Scholar]
- 27.Thomas HJ, Wotton CJ, Yeates D, Ahmad T, Jewell DP, Goldacre MJ. Pneumococcal infection in patients with coeliac disease. Eur J Gastroenterol Hepatol. 2008;20(7):624-628. doi: 10.1097/MEG.0b013e3282f45764 [DOI] [PubMed] [Google Scholar]
- 28.Zingone F, Abdul Sultan A, Crooks CJ, Tata LJ, Ciacci C, West J. The risk of community-acquired pneumonia among 9803 patients with coeliac disease compared to the general population: a cohort study. Aliment Pharmacol Ther. 2016;44(1):57-67. doi: 10.1111/apt.13652 [DOI] [PubMed] [Google Scholar]
- 29.Ludvigsson JF, Bai JC, Biagi F, et al. ; BSG Coeliac Disease Guidelines Development Group; British Society of Gastroenterology . Diagnosis and management of adult coeliac disease: guidelines from the British Society of Gastroenterology. Gut. 2014;63(8):1210-1228. doi: 10.1136/gutjnl-2013-306578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiepatti A, Biagi F, Fraternale G, et al. . Short article: mortality and differential diagnoses of villous atrophy without coeliac antibodies. Eur J Gastroenterol Hepatol. 2017;29(5):572-576. doi: 10.1097/MEG.0000000000000836 [DOI] [PubMed] [Google Scholar]
- 31.Tio M, Cox MR, Eslick GD. Meta-analysis: coeliac disease and the risk of all-cause mortality, any malignancy and lymphoid malignancy. Aliment Pharmacol Ther. 2012;35(5):540-551. doi: 10.1111/j.1365-2036.2011.04972.x [DOI] [PubMed] [Google Scholar]
- 32.Biagi F, Corazza GR. Do different patients with coeliac disease have different mortality rates? Gut. 2015;64(8):1187-1188. doi: 10.1136/gutjnl-2014-308567 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definitions of Celiac Disease and Normal Mucosa Using SNOMED Codes
eTable 2. Definitions of Cause-Specific Mortality
eTable 3. Definitions of Baseline Comorbidities Ever Before Start of Follow-up
eTable 4. Comorbidity at Baseline, During Follow-up and at Baseline or Follow-up, by Index Year in Celiac Disease and Matched Comparators
eTable 5. Narrowing the Definition of Celiac Disease
eTable 6. Celiac Disease With Positive Celiac Disease Serology vs Matched Comparators
eTable 7. Risk of All-Cause (Follow-up Until 31 Dec, 2017) and Cause-Specific (Follow-up Until 31 Dec, 2016) Mortality in Patients With Celiac Disease and Positive Celiac Disease Serology and Matched General Population Comparators
eTable 8. Celiac Disease Compared to Siblings: Baseline Characteristics of Study Cohort
eTable 9. Risk of All-Cause Mortality in Children (<18 Years) Diagnosed in 2010-2011 and in 2012-2014, Restricted to 3 Years of Follow-up
eTable 10. Baseline Characteristics of Study Cohort of Those With Mucosal Healing on Follow-up Biopsy Compared to Those With Persistent Villus Atrophy
eTable 11. Risk of All-Cause (Follow-up Until 31 Dec, 2017) and Cause-Specific (Follow-up Until 31 Dec, 2016) Mortality in Patients With Celiac Disease and Mucosal Healing vs Persistent Villus Atrophy